Introduction
Abdominal aortic aneurysm (AAA), a progressive
pathological dilatation of the aortic wall, occurs mostly
asymptomatic, but may become life threatening in case of rupture.
The pathophysiology of AAA is a complex multifactorial process that
remains incompletely understood. Common risk factors for this
disease are male gender, age, smoking and hypertension (1,2).
In addition, familial clustering points to inheritance of
predisposing genetic alterations (3,4).
Histologically, AAA is characterized by chronic inflammation, loss
of vascular smooth muscle cells, and degradation of elastic fibers
and extracellular matrix (ECM) (5,6).
For many years, atherosclerosis was considered a dominant cause for
AAA progression. However, increasing evidence suggests that
inflammatory reactions and autoimmunity across the tunica
media/adventitia junction are important factors for aortic
destruction and dilatation (7,8).
Inflammatory infiltrates were described even 30 years ago in the
adventitial layer of AAA without any clinical signs of inflammation
(9). A few years later, an
immune-mediated response was suggested, after characterization of
the inflammatory cells present in the aortic wall of AAA specimens
(10). Since that time, many
others have confirmed the presence of T and B lymphocytes,
macrophages, mast cells and neutrophils (11-15). The presence of B and T lymphocytes
points to an antibody-mediated humoral immunity and potentially an
autoimmune response driving AAA progression (14,15). In addition, the different
inflammatory/immune cells release proinflammatory cytokines,
including tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β
and interferon (IFN)-γ, all of which have been detected in the wall
of AAA specimens (16).
Regardless of the increasing knowledge on
inflammatory cells and mediators, the initial trigger for
inflammation and the precise order of the process, resulting in a
destructive autoimmune response to the aortic wall, is unknown. A
very early event in response to danger signals on cells is
activation of inflammasomes, sensing many environmental and
pathogen/host-derived factors (17,18). The inflammasomes are a family of
cytoplasmic multiprotein complexes required for maturation of the
IL-1β and IL-18 cytokines, which are key regulators of immune
response and tissue homoeostasis (19). Because deregulated activity of
IL-1 is linked to autoimmune and inflammatory diseases (20), inflammasomes are tightly regulated
at both the transcriptional and protein level. Depending on the
initial sensor, several subfamilies are distinguished: The NOD-like
receptors (NLR), such as NLR family pyrin domain containing 3
(NLRP3), act as sensors for intracellular damage-associated
signals, including cholesterol crystals, nanoparticles and reactive
oxygen species. A second subfamily, that includes absent in
melanoma 2 (AIM2), acts as sensors for intracellular dsDNA
(17). Upon sensing of the danger
signal, both subfamilies induce assembly of a multiprotein complex
consisting of the apoptosis-associated speck-like protein
containing a caspase recruitment domain (ASC) and one of the
inflammatory caspases, Caspase-1 or Caspase-5. In the present
study, therefore, NLRP3, AIM2, ASC, Caspase-1 and Caspase-5 were
defined as relevant inflammasome markers for investigation.
Although initially detected in mono-cytes, it is now evident that
inflammasomes are expressed and assembled in response to different
stimuli in many myeloid and non-myeloid cell types, including
epithelial, mesenchymal and neuronal cells (21,22).
In a recent pilot study, our group demonstrated high
levels of inflammasome components in lymphocytic infiltrates of AAA
(23). The present study aimed to
characterize inflammation in different AAA stages in more detail,
according to the composition of the infiltrating immune cells and
according to the expression of NLRP3, AIM2 and ASC in these immune
cells. Additionally, the hypothesis that the inflammasome activity
may differ between different stages of AAA progression was
examined.
Materials and methods
Tissue sampling and patient
selection
Formalin-fixed and paraffin-embedded (FFPE) biopsies
derived from AAA patients were provided by the Vascular Biobank
Heidelberg (VBBH). A total of 104 aortic wall specimens from 48
patients (AAA group) were collected during open surgery between
February 2013 and April 2014 at the Department of Vascular and
Endovascular Surgery, Heidelberg. Tissue sampling from these
patients was based on preoperative finite element analysis (FE-A),
identification of AAA wall regions with highest and lowest peak
wall rupture index (PWRI, see below) and extraction according to
clockwise orientation from FE-A measurement protocols. In addition,
40 aortic- and visceral artery samples (control group), free of
macroscopic disease, were obtained during organ transplantations
from anonymous individuals. All patients gave their written
informed consent to the study and all samples were processed
immediately and stored in the VBBH for further histological and
immunohisto-chemical analysis.
Patient characteristics
Laboratory parameters [C-reactive protein (CRP)
plasma level, leukocyte count, cholesterol plasma level] and
patient specific characteristics (smoking history, diabetes type II
medication, hypercholesterinemia, hypertension, and statin therapy)
of the AAA group were collected from the patient information system
of the University Hospital Heidelberg (ISH-MED). No clinical or
personal data were available from the control group. The patient
characteristics of the AAA group are summarized in Table I.
 | Table IPatient characteristics of the AAA
cohort. |
Table I
Patient characteristics of the AAA
cohort.
| Parameter | AAA (n=46) |
|---|
| Mean age, years
(range) | 62.2 (47-87) |
| Male, n (%) | 40 (87.6) |
| Current smoker, n
(%) | 24 (52.2) |
| Non-smoker, n
(%) | 9 (19.6) |
| Medication for | |
| Type 2 diabetes, n
(%) | 4 (10.4) |
|
Hypercholesterinemia, n (%) | 39 (87.2) |
| Hypertension, n
(%) | 42 (91.7) |
FE-A
Preoperative FE-A was performed for every patient of
the AAA group by a single investigator using the commercially
available A4-Clinics software (VASCOPS GmbH). For analysis, Digital
Imaging and Communications in Medicine (DICOM) data were used from
computer tomography angiography (CT-A; in plane solution 0.33 mm,
slice thickness 0.7-3.3 mm) to detect AAA wall regions between the
renal arteries and aortic bifurcation. Semiautomatic FE-A
calculation is based on the subsequent steps of luminal and
exterior AAA surface recognition, three dimensional mesh generation
and computation of biomechanical parameters. The later step
incorporates patient specific (blood pressure, sex, smoking
history) and anatomical boundary conditions (thrombus, vessel
morphology) for calculating peak wall stress (PWS; in kPascal),
peak wall rupture risk index (PWRI) and intra-luminal thrombus size
(ILT; in cm3). The three dimensional analysis report,
marking regions with highest and lowest PWRI values, was used to
extract samples during open surgery based on clockwise orientation.
FE-A data included morphological (AAA diameter, thrombus volume)
and biomechanical parameters (PWS and PWRI).
Immunohistochemistry
Processing and immunohistochemical staining was
performed following standard procedures by using the following
primary antibodies: Mouse-anti-human CD45 (1:1,000; #3575,
pan-leukocyte marker; Cell Signaling Technology, Inc.), rabbit
anti-human CD3 (1:400; #85061, T-cell marker; Cell Signaling
Technology, Inc.), rabbit anti-human NLRP3 (1:500; #13158; Cell
Signaling Technology, Inc.), rabbit anti-human Caspase-1 (1:50;
#85061; Cell Signaling Technology, Inc.), mouse-anti-human CD68
(1:2,000; #M0876, macrophage marker; Dako; Agilent Technologies,
Inc.), rabbit anti-human AIM2 (1:200; #HPA031365; Sigma-Aldrich;
Merck KGaA), rabbit anti-human ASC (#ADI-905-173; Enzo Life
Sciences, Inc.), and rabbit anti-human Caspase-5 (1:200; #3029,
BioVision, Inc.). Detailed staining protocols are available from
the authors upon request. Briefly, 4 µm sections were
deparaffinized, rehydrated and incubated in 100 mM citrate buffer
pH 6.0 for antigen retrieval, prior to incubation with the primary
antibody at 4°C overnight. After washing, detection was performed
by using the Dako REAL Detection System Peroxidase/AEC rabbit/mouse
(Dako; Agilent Technologies, Inc.), according to the
recommendations of the manufacturer. All sections were
counterstained with hematoxylin. Immunohistochemical staining for
the B-cell marker CD20 was performed by the Institute of Pathology
of the University Hospital Heidelberg, according to standard
diagnostic procedures.
Grading of inflammatory lesions in aortic
samples
Histopathological grading of inflammatory lesions
and aortic samples was performed based on the intramural location
of CD45+ cells. Briefly, grade 0 describes a healthy
vessel wall without inflammation and few, isolated leukocyte
infiltrates. A Grade 1 lesion describes a mild chronic inflammation
with leukocyte infiltrations located to atherosclerotic plaques. A
Grade 2 lesion describes a moderate chronic inflammation with
localized and diffuse lymphocyte infiltrates within the tunica
adventitia. A Grade 3 lesion refers to a severe chronic vessel wall
inflammation with lobular arrangement of lymphocyte infiltrates at
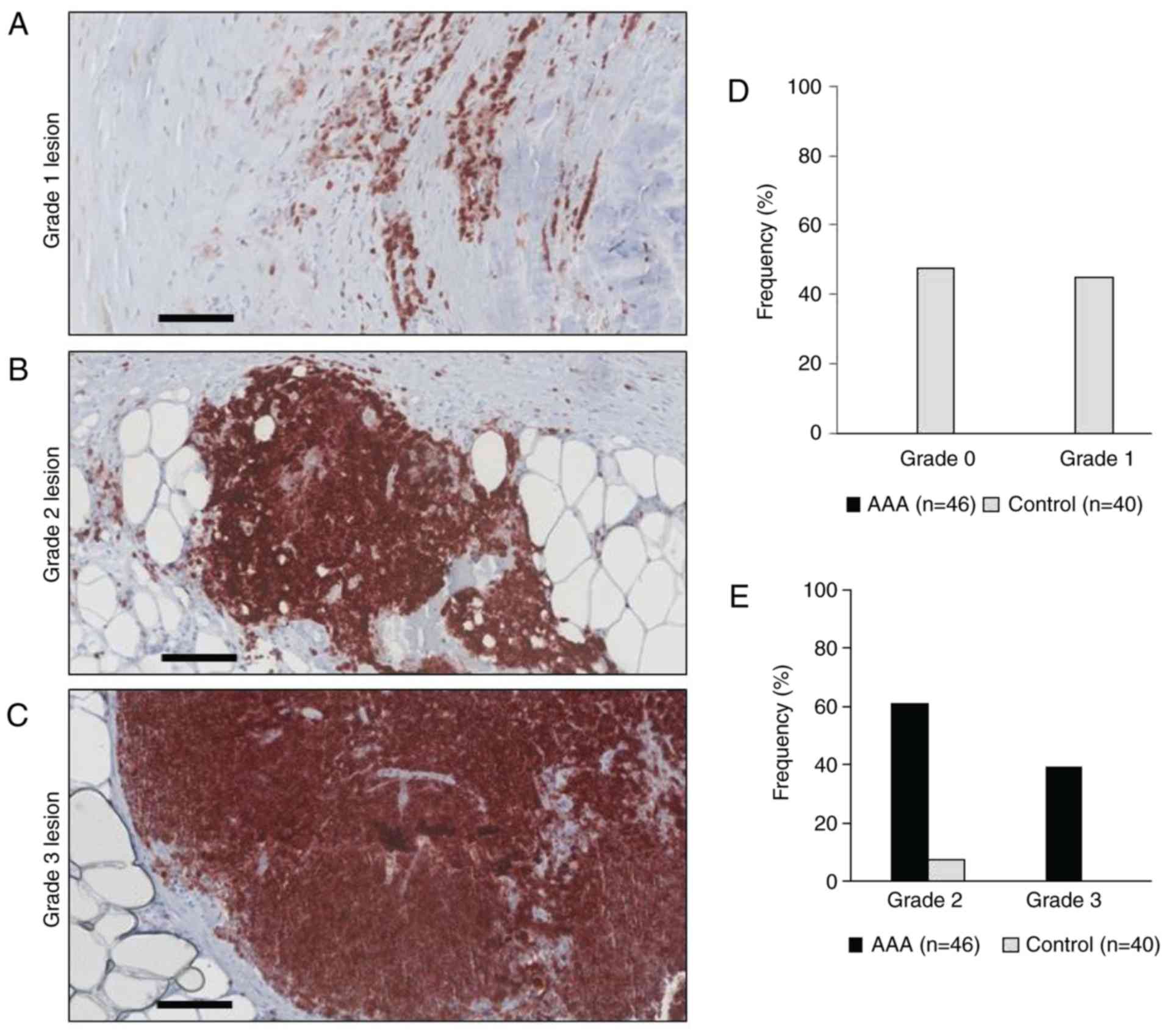
the media-adventitia border. Examples of the grades are presented
in Fig. 1. Two to three samples
from each aneurysm were used for analysis and the highest lesion
grade of inflammation in the sample of each patient was used to
categorize the total inflammation grade of this aneurysm (patient
sample).
Statistical analysis
Only informative samples, i.e. sections showing the
lesion of interest with a technically reliable staining quality,
were used for statistical analysis. Therefore, the total number of
samples included in the statistical analysis was sometimes lower
than the total number of AAA or control tissues.
Immunohistochemically identified differences in the grade of
inflammation between AAA and control patient groups, as well as
differences in expression of inflammasome components between grades
and between AAA and control groups, were tested for significance by
Barnard's unconditional test for 2×2 contingency tables (24), using CRAN-R package version 1.8
(https://github.com/kerguler/Barnard).
Global heterogeneity of inflammasome component expression across
the grades of inflammation (I-III) was calculated with the Fisher
exact 2×3 test. Patient characteristics and FEA data were compared
with histopathological findings (inflammation grading and
inflammasome component expression) using the same test. For the
comparison of the histological data with the clinical features of
the patients, student's t-test was applied using SPSS (version 25;
IBM Corp.).
Results
Categorization of samples according to
the CD45+ cell infiltration pattern
To categorize the inflammation grade and to locate
intramural inflammatory cells in AAA and control tissues, FFPE
sections of all samples were initially analyzed for CD45 expression
by immunohistochemistry. In agreement with our previous pilot study
(23), all examined AAA specimens
exhibited visible signs of vessel wall inflammation. None of the 46
AAA samples was categorized as grade 0 or grade 1, 28/46 (60.8%)
were categorized as grade 2 and 18/46 (39.1%) as grade 3 (Table II and Fig. 1). By contrast, none of the 40
control samples exhibited profound inflammatory cell infiltration
(grade 3) and only 3/40 samples were categorized grade 2 (Table II and Fig. 1). Because samples categorized as
grade 3 (lobular arrangement of CD45+ cells) did
additionally show grade 2 and 1 infiltrates, and samples
categorized as grade 2 (diffuse infiltration) additionally
displayed grade 1 lesions (isolated CD45+ cells located
to atherosclerotic plaque), the number of individual lesions was
higher than the total number of samples (Table III).
 | Table IICategorization of samples according
to the CD45+ cell infiltration pattern. |
Table II
Categorization of samples according
to the CD45+ cell infiltration pattern.
| Sample | Grade 0, n (%) | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) |
|---|
| AAA (n=46) | 0 (0.0) | 0 (0.0) | 28 (60.8) | 18 (39.1) |
| Control (n=40) | 19 (47.5) | 18 (45) | 3 (7.5) | 0 (0.0) |
 | Table IIIGrading of individual lesions within
the samples according to the CD45+ cell infiltration
pattern. |
Table III
Grading of individual lesions within
the samples according to the CD45+ cell infiltration
pattern.
| Sample | Grade 0 lesions
n/total samples | Grade 1 lesions
n/total samples | Grade 2 lesions
n/total samples | Grade 3 lesions
n/total samples |
|---|
| AAA | 0/46 | 46/46 | 44/46 | 18/46 |
| Control | 19/40 | 21/40 | 3/40 | 0/40 |
The pattern of inflammatory cell
phenotypes within the same inflammation grade differs between AAA
samples and controls
Next, the composition of different leukocyte
phenotypes in each inflammation grade of healthy and AAA tissue
samples was analyzed. Macrophages (CD68+ cells), B
lymphocytes (CD20+ cells) and T cells (CD3+
cells) have been identified in AAA for decades (14). The present study aimed to compare
the leukocyte pattern in the different inflammatory lesions and
total inflammation grades between control and AAA tissue, as
identified in Table II.
Macrophages (CD68+ cells) were detected
in a similar frequency in grade 1 lesions of both AAA and control
samples [43/44 (97.7%) vs. 20/21 (95.2%), respectively, of
informative samples], and were predominantly located around the
atherosclerotic plaque (Fig. 2A and
J). By contrast, grade 1 lesions of AAA samples were
significantly more infiltrated with T lymphocytes (CD3+
cells) than control samples [38/46 (82.6%) vs. 5/21 (23.8%),
respectively; P<0.001; Fig. 2B and
K]. Likewise, B lymphocytes (CD20+ cells) were more
frequent in grade 1 lesions of AAA samples than in control samples
[32/46 (69.6%) vs. 2/21 (9.5%); P<0.001; Fig. 2C and L].
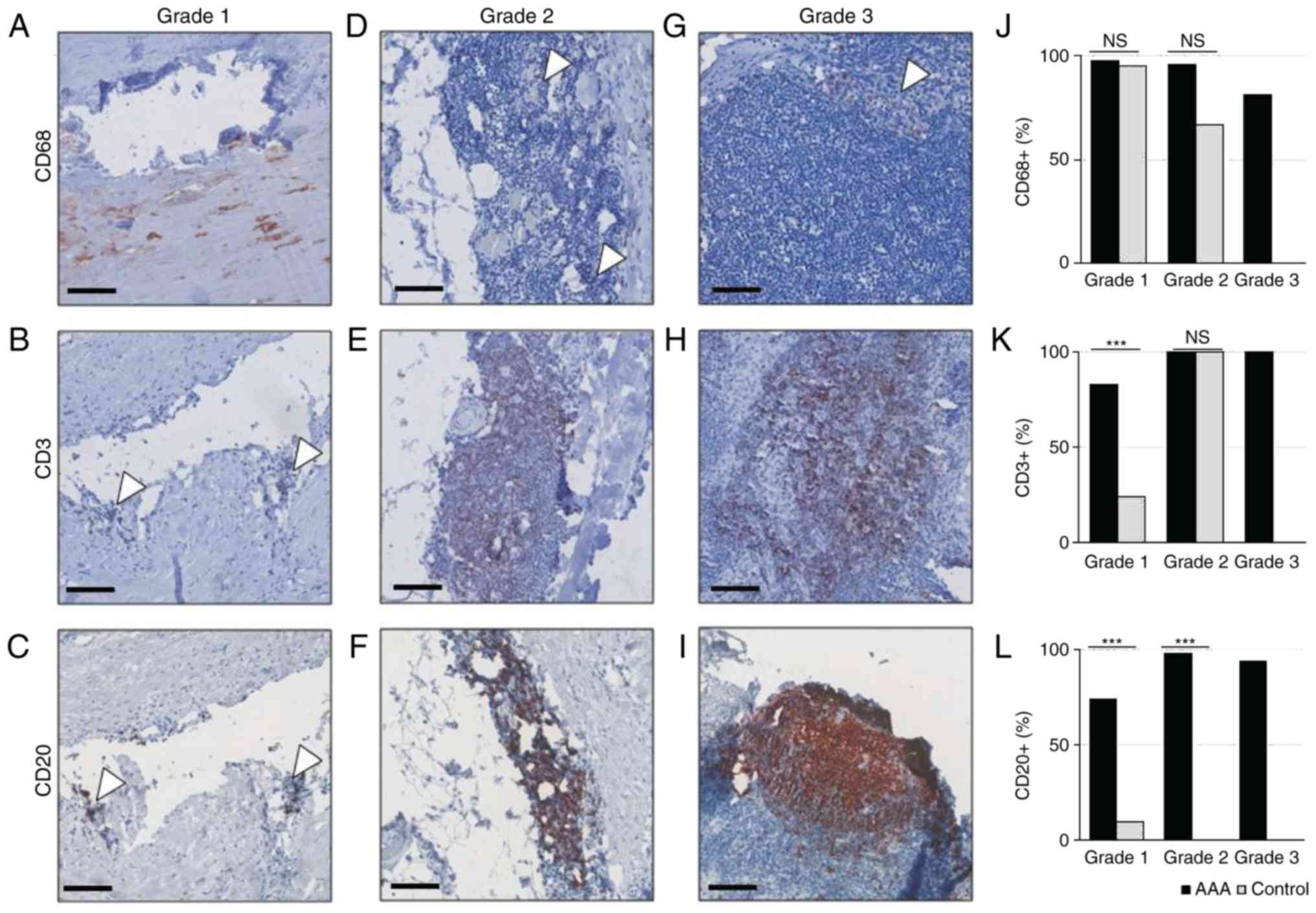 | Figure 2Immunohistochemical analysis of
inflammatory infiltrates in AAA and healthy controls. (A-C) Grade
1, (D-F) grade 2 and (G-I) grade 3 lesions were stained with
anti-CD68 to detect macrophages, with anti-CD3 to detect T
lymphocytes, or with anti-CD20 to detect B- lymphocytes, as
indicated. Scale bar, 100 µm. (J) Distribution of
CD68+ cells in grade 1, 2 and 3 lesions of AAA and
control samples. (K) Distribution of CD3+ cells in grade
1,2 and 3 lesions of AAA and control samples. (L) Distribution of
CD20+ cells in grade 1,2 and 3 lesions of AA and control
samples. Arrows point to positive isolated cell clusters.
***P<0.001, comparisons are indicated by lines (Barnard's test).
AAA, abdominal aortic aneurysm; ns, not significant. |
In grade 2 lesions (diffusely clustered leukocytes
in the tunica media and adventitia), macrophages were detected at
similar frequencies in both AAA samples and controls [41/43 (95,4%)
vs. 2/3 (66.7%), respectively; P=0.093; Fig. 2D and J]. In addition, AAA and
control samples were equally infiltrated with T lymphocytes (100%
of samples; Fig. 2E and K). By
contrast, B lymphocytes were exclusively found in grade 2 lesions
of AAA samples, and not in controls [44/45 (97.8%) vs. 0/3 (0.0%),
respectively; P<0.001; Fig. 2F and
L].
Grade 3 lesions (lobular accumulations of
leukocytes) were exclusively detected in AAA samples (Fig. 2G and L) and heavily infiltrated
with macrophages [13/16 (81.3%)], T lymphocytes [14/14 (100%)] and
B lymphocytes [14/15 (93.3%)].
The inflammasome expression pattern
within the same inflammation grade differs between AAA samples and
controls
To refine the findings of our previous pilot study
(23), the present study compared
next the frequencies of inflammasome components in each lesion
grade between AAA and control samples. In AAA samples, grade
1-associated expression of NLRP3, AIM2, ASC and Caspase-1 was
detected in 100% of samples, whereas 80.4% of AAA samples were
positive for Caspase-5 expression (Fig. 3A-E and P-T). Similarly, ASC and
Caspase-1 were expressed in 100% of grade 1 lesions in control
samples (Fig. 3R and S). The
expression frequencies of NLRP3 and AIM2 were significantly lower
in grade 1 lesions of control samples [57.1% (12/21), P<0.001
and 17/21 (81.0%), P=0.008, respectively; Fig. 3P and Q].
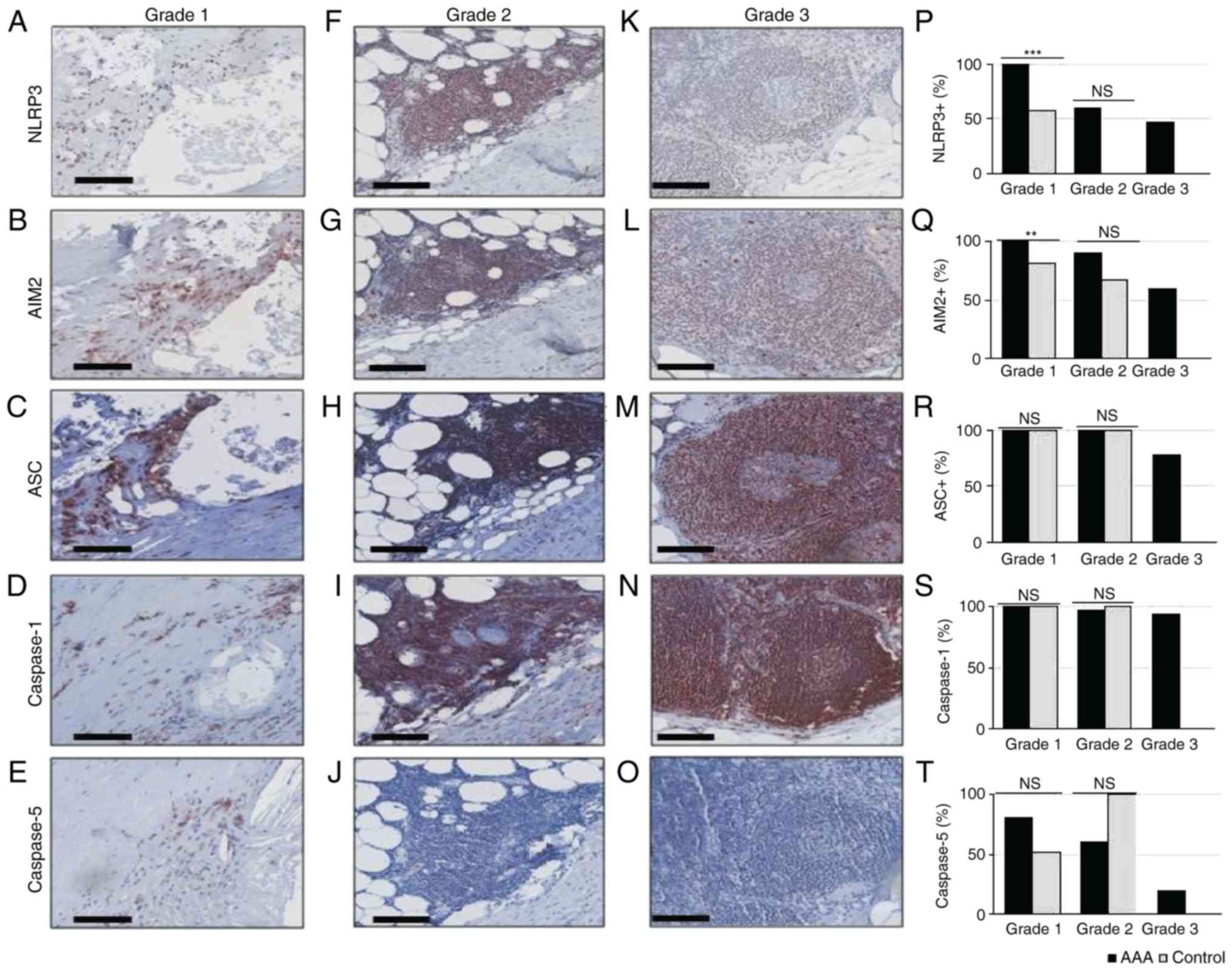 | Figure 3Immunohistochemical analysis of
inflammatory infiltrates in AAA and healthy controls. (A-E) Grade
1, (F-J) grade 2 and (K-O) grade 3 lesions were stained with
anti-NLRP3, anti-AIM2, anti-ASC, anti-Caspase-1 or anti-Caspase-5,
as indicated. Scale bar, 100 µm. (P) Distribution of
NLRP3+ cells, (Q) AIM2+ cells, (R)
ASC+ cells, (S) Caspase-1+ cells, and (T)
Caspase-5+ cells in grade 1, 2 and 3 lesions of AAA and
control samples. **P<0.01 and
***P<0.001, comparisons are indicated by lines
(Barnard's test). AAA, abdominal aortic aneurysm; NLRP3, NLR family
pyrin domain containing 3; AIM2, absent in melanoma 2; ASC,
apoptosis-associated speck-like protein containing a caspase
recruitment domain; ns, not significant. |
The grade 2-associated expression frequency of NLRP3
and AIM2 appeared higher in AAA samples compared with control
samples [59.9% (26/44) vs. 0% (0/3), P=0.082; and 90.7% (39/43) vs.
66.7% (2/3), P=0.298, respectively; Fig. 3P and Q], although these
differences were not statistically significant, most likely due to
the low amount of grade 2 lesions in control samples. ASC,
Caspase-1 and Caspase-5 expression frequencies did not differ
between the two groups [100% (44/44) vs. 100% (3/3), P=1; 97.8%
(45/46) vs. 100% (2/2), P=1; and 60.9% (28/46) vs. 100% (3/3),
P=0.239, respectively; Fig.
3R-T].
In grade 3 lesions that were exclusively detected in
AAA samples, NLRP3 was expressed in 6/13, AIM2 in 9/15, ASC in
11/14, Caspase-1 in 15/16 and Caspase-5 in 3/15 informative
samples.
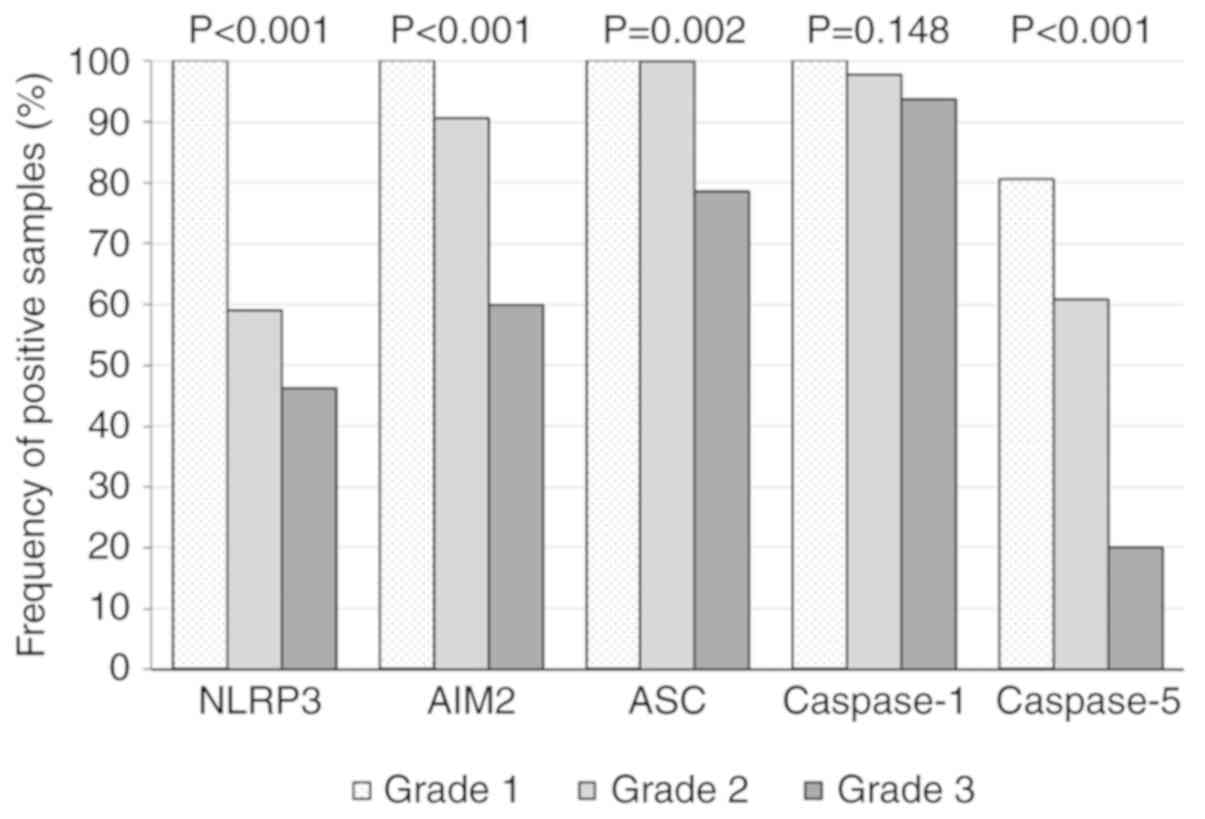
Inflammasome expression decreases from
grade 1 to grade 3 lesions in human AAA tissues
For testing of heterogeneity of inflammasome
component expression across different inflammation grades (I-III)
the Fisher test was used. Based on our previous pilot study, the
present study hypothesized that the expression of inflammasome
components might change during AAA progression. As presented in
Fig. 4, the frequencies of all
inflammasome components, except Caspase-1, were significantly
different across the inflammation grades (NLRP3, P<0.001; AIM2,
P<0.001; ASC, P=0.002; Caspase-1, P=0.148; and Caspase-5,
P<0.001). These results suggested a different innate immune
response in inflammatory regions around atherosclerotic areas
compared with the immune response at the media/adventitia border.
In addition, AIM2, ASC and Caspase-5 expressions indicated a
gradual decrease of inflammasome positive leukocytes during AAA
progression (Fig. 4). An overall
comparison of expression frequencies of leukocyte cell markers and
inflammasome proteins for AAA and control samples is detailed in
Table IV.
 | Table IVExpression frequencies of leukocyte
markers and inflammasome proteins in grades 0, 1, 2 and 3 of AAA
and control samples. |
Table IV
Expression frequencies of leukocyte
markers and inflammasome proteins in grades 0, 1, 2 and 3 of AAA
and control samples.
|
Immunohistochemistryresults | Grade 0
| Grade 1
| Grade 2
| Grade 3
|
|---|
| ++ | + | − | n | ++ | + | − | n | ++ | + | − | n | ++ | + | − | n |
|---|
| CD68 | | | | | | | | | | | | | | | | |
| AAA | 0 | 0 | 0 | 0 | 0 | 43 | 1 | 44 | 0 | 41 | 2 | 43 | 0 | 13 | 3 | 16 |
| AAA (%) | - | - | - | | 0 | 97,73 | 2,27 | | 0 | 95,35 | 4,65 | | 0 | 81,25 | 18,75 | |
| Control | 0 | 12 | 6 | 18 | 0 | 20 | 1 | 21 | 0 | 2 | 1 | 3 | 0 | 0 | 0 | 0 |
| Control (%) | 0 | 66,67 | 33,33 | | 0 | 95,24 | 4,76 | | 0 | 66,67 | 33,33 | | - | - | - | |
| CD3 | | | | | | | | | | | | | | | | |
| AAA | 0 | 0 | 0 | 0 | 0 | 38 | 8 | 46 | 22 | 46 | 0 | 46 | 8 | 14 | 0 | 14 |
| AAA (%) | - | - | - | | 0 | 82,61 | 17,39 | | 47,83 | 100 | 0 | | 57,14 | 100 | 0 | |
| Control | 0 | 2 | 15 | 17 | 0 | 5 | 16 | 21 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 |
| Control (%) | 0 | 11,76 | 88,24 | | 0 | 23,81 | 76,19 | | 0 | 100 | 0 | | - | - | - | |
| CD20 | | | | | | | | | | | | | | | | |
| AAA | 0 | 0 | 0 | 0 | 0 | 34 | 12 | 46 | 33 | 44 | 1 | 45 | 10 | 14 | 1 | 15 |
| AAA (%) | - | - | - | | 0 | 73,91 | 26,09 | | 73,33 | 97,78 | 2,22 | | 66,67 | 93,33 | 6,67 | |
| Control | 0 | 0 | 17 | 17 | 0 | 2 | 19 | 21 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 |
| Control (%) | 0 | 0 | 100 | | 0 | 9,52 | 90,48 | | 0 | 0 | 100 | | - | - | - | |
| NLRP3 | | | | | | | | | | | | | | | | |
| AAA | - | 0 | 0 | 0 | - | 45 | 0 | 45 | - | 26 | 18 | 44 | - | 6 | 7 | 13 |
| AAA (%) | - | - | - | | - | 100 | 0 | | - | 59,09 | 40,91 | | - | 46,15 | 53,85 | |
| Control | - | 0 | 18 | 18 | - | 12 | 9 | 21 | - | 0 | 3 | 3 | - | 0 | 0 | 0 |
| Control (%) | - | 0 | 100 | | - | 57,14 | 42,86 | | - | 0 | 100 | | - | - | - | |
| AIM2 | | | | | | | | | | | | | | | | |
| AAA | - | 0 | 0 | 0 | - | 45 | 0 | 45 | - | 39 | 4 | 43 | - | 9 | 6 | 15 |
| AAA (%) | - | - | - | | - | 100 | 0 | | - | 90,7 | 9,3 | | - | 60 | 40 | |
| Control | - | 9 | 8 | 17 | - | 17 | 4 | 21 | - | 2 | 1 | 3 | - | 0 | 0 | 0 |
| Control (%) | - | 52,94 | 47,06 | | - | 80,95 | 19,05 | | - | 66,67 | 33,33 | | - | - | - | |
| ASC | | | | | | | | | | | | | | | | |
| AAA | - | 0 | 0 | 0 | - | 45 | 0 | 45 | - | 44 | 0 | 44 | - | 11 | 3 | 14 |
| AAA (in %) | | - | - | - | | - | 100 | 0 | - | 100 | 0 | | - | 78,57 | 21,43 | |
| Control | - | 8 | 8 | 16 | - | 20 | 0 | 20 | - | 3 | 0 | 3 | - | 0 | 0 | 0 |
| Control (%) | - | 50 | 50 | | - | 100 | 0 | | - | 100 | 0 | | - | - | - | |
| Caspase-1 | | | | | | | | | | | | | | | | |
| AAA | - | 0 | 0 | 0 | - | 46 | 0 | 46 | - | 45 | 1 | 46 | - | 15 | 1 | 16 |
| AAA (%) | - | - | - | | - | 100 | 0 | | - | 97,83 | 2,17 | | - | 93,75 | 6,25 | |
| Control | - | 11 | 6 | 17 | - | 21 | 0 | 21 | - | 2 | 0 | 2 | - | 0 | 0 | 0 |
| Control (%) | - | 64,71 | 35,29 | | - | 100 | 0 | | - | 100 | 0 | | - | - | - | |
| Caspase-5 | | | | | | | | | | | | | | | | |
| AAA | - | 0 | 0 | 0 | - | 37 | 9 | 46 | - | 28 | 18 | 46 | - | 3 | 12 | 15 |
| AAA (%) | - | - | - | | - | 80,43 | 19,57 | | - | 60,87 | 39,13 | | - | 20 | 80 | |
| Control | - | 2 | 17 | 19 | - | 11 | 10 | 21 | - | 3 | 0 | 3 | - | 0 | 0 | 0 |
| Control (%) | - | 10,53 | 89,47 | | - | 52,38 | 47,62 | | - | 100 | 0 | | - | - | - | |
The immune response within the AAA tissue
is not associated with plasma inflammatory markers, the maximal AAA
diameter or predicted rupture risk
Using FE-A, our group has previously analyzed
whether histological features of AAA correlate with predicted
rupture risk and clinical parameters of AAA patients (25,26). To investigate, whether the
intramural immune response analyzed in the present study may be
reflected by any diagnostic marker that is clinically used for AAA
disease control, different parameters were tested for association.
Neither clinical inflammatory characteristics (CRP levels,
leukocyte count and plasma cholesterol levels), nor parameters from
FE-A (PWS, PWRR and maximal diameter), were associated with the
respective inflammatory grade, predominant leukocyte phenotype or
inflammasome protein expressions within individual lesions (data
not shown). Thus, a higher grade of inflammation within the AAA
group was not necessarily associated to a higher systemic
inflammatory response (CRP, leucocyte count) or AAA vessel wall
regions with a higher calculated rupture risk from FE-A.
Discussion
Emerging evidence suggests that chronic intramural
inflammation, involving a variety of inflammatory cell types, is
the major driving force of AAA progression (14,27). The presence of T and B lymphocytes
argues for autoimmunity as an etiological component of AAA
pathophysiology, which involves both adaptive and innate immunity
(7). Given that activation of
inflammasomes in hematopoietic cells is an initiating step in
innate immunity, and sterile inflammation is implicated in
cardiovascular diseases (28,29), the present study addressed the
leukocyte composition and the inflammasome expression pattern in
different inflammatory grades of AAA compared with control
(apparently healthy) tissues. The results demonstrated that: i)
Whereas in control samples, grade 1 (atherosclerotic) lesions were
predominantly infiltrated with macrophages, the majority of AAA
grade 1 lesions were additionally infiltrated with B and T
lymphocytes; ii) The expression frequencies of the inflammasome
components NLRP3, AIM2 and Caspase-5 were significantly higher in
grade 1 lesions of AAA samples compared with grade 1 lesions in
control samples; and iii) AIM2, ASC, and Caspase-5 displayed
significantly lower expression frequencies in grade 3 compared with
grade 2 AAA specimens, and all inflammasome components were less
frequently detected in grade 3 compared with grade 1 lesions of
AAA.
The detection of different infiltrating cell types
in histologically similar areas (grade 1 lesion, atherosclerotic
plaque) between AAA and non-AAA samples suggests different
pathological mechanisms. B and T lymphocytes do not only infiltrate
the adventitial layer of AAA, as described previously (10,30,31), but are also frequent in
atherosclerotic areas of AAA, in contrast to atherosclerotic areas
of non-AAA arteries. The reasons for this imbalance remain unclear.
The present findings support the hypothesis of
inflammasome-mediated innate immunity in AAA pathology. The
expression patterns of NLRP3, AIM2, ASC, and Caspase-1 overlap
particularly with that of T and B lymphocytes, suggesting that
these cell types, rather than macrophages, display inflammasome
activity in AAA tissues. Double-staining by using
immunofluorescence on cryosections will help to allocate
inflammasome expressions to certain cell types more precisely.
Irrespective of the cell type, the expression of
inflammasome components appears to decline during AAA progression,
according to the present results. Assuming that well-organized
lobular lymphocyte infiltrations (grade 3 lesions) develop later in
AAA than diffuse lymphocyte infiltrations (grade 2 lesions) and
infiltration of atherosclerotic areas (grade 1 lesions), the
decline of inflammasome expression frequencies from grade 1 to
grade 3 lesions argues for a change of the immune response during
AAA progression. It is thus tempting to speculate that signaling by
inflammasomes is an early event of the inflammatory response by
lymphocytes, whereas later these lymphocytes perform other
mechanisms in AAA pathology. Alternatively, the different
inflammation grades occur independently within AAA tissues and
represent different etiologies. However, the simultaneous presence
of different infiltration grades within the same sample argues for
the hypothesis of a progressive change from single cells, over
diffuse aggregates, and finally lymphoid follicles with B-cells
forming germinative centers.
The role of inflammasomes in AAA has been
extensively studied in animal experiments. Genetic deletion of
NLRP3, ASC or Caspase-1 protected ApoE-deficient mice from
angiotensin II-induced aortic aneurysms (29). The incidence, maximal diameter and
severity of AAA, as well as adventitial fibrosis and inflammatory
responses, were significantly reduced in inflammasome-deficient
mice (29). In addition, genetic
and pharmacological disruption of IL-1β, the major product of
inflammasome activity, has been demonstrated to inhibit
experimental aortic aneurysm formation (32,33). In humans, the CANTOS trial, a
randomized, double-blind, placebo-controlled trial of canakinumab,
an IL-1β neutralizing antibody, in 10,061 patients, was shown to
reduce cardiovascular event rates (34-36). This was the first study
demonstrating that targeting an inflammasome-activated cytokine can
prevent cardiovascular events in high risk patients. Unfortunately,
data on AAA development or progression are not available from this
trial. The current data, demonstrating inflammasome expression
particularly in grade 1 and 2 AAA lesions, supports the hypothesis
that therapeutic inhibition of IL-1β or certain inflammasome
components might also be effective in attenuating AAA development
and progression.
A limitation of the present study was that
insufficient clinical information was available for the anonymous
organ transplantation patients of the control group. Antidiabetic
(glyburide) or antihypertensive medication could influence
inflammasome activity, and may thus affect the comparability of the
study groups.
In conclusion, the present findings suggested that
different inflammatory areas in the aortic wall of AAA consisted of
lymphocytes and macrophages at different states of activity. The
current results might help to identify molecular targets for
development of therapeutic drugs targeting AAA growth.
Acknowledgments
We thank Anja Spieler for excellent technical
assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PE contributed to the conception and design of the
study, data analysis and interpretation, and writing of the
manuscript. SC contributed to data analysis and interpretation,
data collection, writing and revising the manuscript. CG-G, MH and
DB contributed to data analysis and interpretation, and manuscript
revision. SD contributed to data analysis and interpretation, data
collection, writing and revising the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Tissue sampling protocol was approved by the Medical
Ethics Committee of the University Heidelberg, Germany (reference
numbers S-301/2013: Addendum 23.09.2013, 05.07.2016; S-412/2013:
Addendum 04.11.2015, 05.01.2016; S-462/2017). All patients gave
their written consent prior to the study inclusion.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kent KC, Zwolak RM, Egorova NN, Riles TS,
Manganaro A, Moskowitz AJ, Gelijns AC and Greco G: Analysis of risk
factors for abdominal aortic aneurysm in a cohort of more than 3
million individuals. J Vasc Surg. 52:539–548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Michel JB, Martin-Ventura JL, Egido J,
Sakalihasan N, Treska V, Lindholt J, Allaire E, Thorsteinsdottir U
and Cockerill G: Novel aspects of the pathogenesis of aneurysms of
the abdominal aorta in humans. Cardiovasc Res. 90:18–27. 2011.
View Article : Google Scholar :
|
|
3
|
Golledge J and Kuivaniemi H: Genetics of
abdominal aortic aneurysm. Curr Opin Cardiol. 28:290–296. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim HW and Stansfield BK: Genetic and
epigenetic regulation of aortic aneurysms. Biomed Res Int.
2017:72685212017.PubMed/NCBI
|
|
5
|
Kuivaniemi H, Ryer EJ, Elmore JR and Tromp
G: Understanding the pathogenesis of abdominal aortic aneurysms.
Expert Rev Cardiovasc Ther. 13:975–987. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nordon IM, Hinchliffe RJ, Loftus IM and
Thompson MM: Pathophysiology and epidemiology of abdominal aortic
aneurysms. Nat Rev Cardiol. 8:92–102. 2011. View Article : Google Scholar
|
|
7
|
Jagadesham VP, Scott DJ and Carding SR:
Abdominal aortic aneurysms: An autoimmune disease? Trends Mol Med.
14:522–529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tilson MD: Decline of the atherogenic
theory of the etiology of the abdominal aortic aneurysm and rise of
the autoimmune hypothesis. J Vasc Surg. 64:1523–1525. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beckman EN: Plasma cell infiltrates in
atherosclerotic abdominal aortic aneurysms. Am J Clin Pathol.
85:21–24. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koch AE, Haines GK, Rizzo RJ, Radosevich
JA, Pope RM, Robinson PG and Pearce WH: Human abdominal aortic
aneurysms. Immunophenotypic analysis suggesting an immune-mediated
response. Am J Pathol. 137:1199–1213. 1990.PubMed/NCBI
|
|
11
|
Cohen JR, Keegan L, Sarfati I, Danna D,
Ilardi C and Wise L: Neutrophil chemotaxis and neutrophil elastase
in the aortic wall in patients with abdominal aortic aneurysms. J
Invest Surg. 4:423–430. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimizu K, Mitchell RN and Libby P:
Inflammation and cellular immune responses in abdominal aortic
aneurysms. Arterioscler Thromb Vasc Biol. 26:987–994. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsuruda T, Kato J, Hatakeyama K, Kojima K,
Yano M, Yano Y, Nakamura K, Nakamura-Uchiyama F, Matsushima Y,
Imamura T, et al: Adventitial mast cells contribute to pathogenesis
in the progression of abdominal aortic aneurysm. Circ Res.
102:1368–1377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dale MA, Ruhlman MK and Baxter BT:
Inflammatory cell phenotypes in AAAs: Their role and potential as
targets for therapy. Arterioscler Thromb Vasc Biol. 35:1746–1755.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L and Wang Y: B lymphocytes in
abdominal aortic aneurysms. Atherosclerosis. 242:311–317. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rizas KD, Ippagunta N and Tilson MD III:
Immune cells and molecular mediators in the pathogenesis of the
abdominal aortic aneurysm. Cardiol Rev. 17:201–210. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Latz E, Xiao TS and Stutz A: Activation
and regulation of the inflammasomes. Nat Rev Immunol. 13:397–411.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kono H, Kimura Y and Latz E: Inflammasome
activation in response to dead cells and their metabolites. Curr
Opin Immunol. 30:91–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo H, Callaway JB and Ting JP:
Inflammasomes: Mechanism of action, role in disease, and
therapeutics. Nat Med. 21:677–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vasanthakumar A and Kallies A: Interleukin
(IL)-33 and the IL-1 family of cytokines-regulators of inflammation
and tissue homeostasis. Cold Spring Harb Perspect Biol.
11:a0285062019. View Article : Google Scholar
|
|
21
|
Yazdi AS, Drexler SK and Tschopp J: The
role of the inflamma-some in nonmyeloid cells. J Clin Immunol.
30:623–627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei-Leston AC, Murphy AG and Maloy KJ:
Epithelial cell inflammasomes in intestinal immunity and
inflammation. Front Immunol. 8:11682017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dihlmann S, Erhart P, Mehrabi A,
Nickkholgh A, Lasitschka F, Bockler D and Hakimi M: Increased
expression and activation of absent in melanoma 2 inflammasome
components in lympho-cytic infiltrates of abdominal aortic
aneurysms. Mol Med. 20:230–237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barnard GA: A new test for 2×2 tables.
Nature. 156:1771945. View
Article : Google Scholar
|
|
25
|
Erhart P, Grond-Ginsbach C, Hakimi M,
Lasitschka F, Dihlmann S, Bockler D and Hyhlik-Dürr A: Finite
element analysis of abdominal aortic aneurysms: Predicted rupture
risk correlates with aortic wall histology in individual patients.
J Endovasc Ther. 21:556–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Erhart P, Schiele S, Ginsbach P,
Grond-Ginsbach C, Hakimi M, Bockler D, Lorenzo-Bermejo J and
Dihlmann S: Gene expression profiling in abdominal aortic aneurysms
after finite element rupture risk assessment. J Endovasc Ther.
24:861–869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Bai S, Ao Q, Wang X, Tian X, Li X,
Tong H, Hou W and Fan J: Modulation of Immune-Inflammatory
responses in abdominal aortic aneurysm: Emerging molecular targets.
J Immunol Res. 2018:72137602018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Deroide N and Mallat Z: The role of
the inflammasome in cardiovascular diseases. J Mol Med (Berl).
92:307–319. 2014. View Article : Google Scholar
|
|
29
|
Usui F, Shirasuna K, Kimura H, Tatsumi K,
Kawashima A, Karasawa T, Yoshimura K, Aoki H, Tsutsui H, Noda T, et
al: Inflammasome activation by mitochondrial oxidative stress in
macrophages leads to the development of angiotensin II-induced
aortic aneurysm. Arterioscler Thromb Vasc Biol. 35:127–136. 2015.
View Article : Google Scholar
|
|
30
|
Bobryshev YV and Lord RS:
Vascular-associated lymphoid tissue (VALT) involvement in aortic
aneurysm. Atherosclerosis. 154:15–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ocana E, Bohorquez JC, Perez-Requena J,
Brieva JA and Rodriguez C: Characterisation of T and B lymphocytes
infiltrating abdominal aortic aneurysms. Atherosclerosis.
170:39–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Johnston WF, Salmon M, Su G, Lu G, Stone
ML, Zhao Y, Owens GK, Upchurch GR Jr and Ailawadi G: Genetic and
pharmacologic disruption of interleukin-1β signaling inhibits
experimental aortic aneurysm formation. Arterioscler Thromb Vasc
Biol. 33:294–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Isoda K, Akita K, Kitamura K,
Sato-Okabayashi Y, Kadoguchi T, Isobe S, Ohtomo F, Sano M, Shimada
K, Iwakura Y and Daida H: Inhibition of interleukin-1 suppresses
angiotensin II-induced aortic inflammation and aneurysm formation.
Int J Cardiol. 270:221–227. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ridker PM, Thuren T, Zalewski A and Libby
P: Interleukin-1β inhibition and the prevention of recurrent
cardiovascular events: Rationale and design of the canakinumab
anti-inflammatory thrombosis outcomes study (CANTOS). Am Heart J.
162:597–605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shah SR, Abbasi Z, Fatima M, Ochani RK,
Shahnawaz W, Asim Khan M and Shah SA: Canakinumab and
cardiovascular outcomes: Results of the CANTOS trial. J Community
Hosp Intern Med Perspect. 8:21–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weber C and von Hundelshausen P: CANTOS
trial validates the inflammatory pathogenesis of Atherosclerosis:
Setting the stage for a new chapter in therapeutic targeting. Circ
Res. 121:1119–1121. 2017. View Article : Google Scholar : PubMed/NCBI
|