Introduction
Using mechanical devices, mechanical ventilation
replaces or controls autonomous breathing movements (1). It is an indispensable part of
general anesthesia and one of the main methods in treating
respiratory failure (2). However,
studies found that the incidence of ventilator-induced lung injury
(VILI) was high, which seriously affected the health and quality of
life of patients (3-5). The prevention and mitigation of the
occurrence of VILI have also attracted much research attention.
At present, the pathogenesis of VILI still remains
unclear. A previous study has suggested that VILI was mainly caused
by mechanical injury (6). It was
discovered that mechanical damage could act on signal transduction
in effector cells, thereby affecting the expression of target genes
and subsequently cell apoptosis, damage, migration or various
phenotypic changes, which triggered a series of inflammatory
responses, cascades of inflammatory mediators and caused
inflammatory lesions in the lungs. Previous studies have shown that
VILI could cause the release of inflammatory factors such as tumor
necrosis factor (TNF-α) and interleukin (IL-6), aggravating lung
injury (7-9).
In the process of lung inflammation, the body's
innate immune system plays an important role. Alveolar macrophages
are lung-resident cells (10).
Macrophages can be divided into 2 major subtypes, that is, Ml
(pro-inflammatory) and M2 (anti-inflammatory) (11). M1 type macrophages induce
secretion of TNF-α and promote inflammation, while M2 type
macrophages have anti-inflammatory effects and promote tissue
repair (12,13). A previous study found that
mechanical stretch stimulation can activate macrophages and
macrophages recruit peripheral neutrophils to the lungs and
participate in the inflammatory response when activated (14). Another study showed that alveolar
macrophages played an important regulatory role in the mechanism of
VILI production (15). However,
the mechanism of activation and regulation of alveolar macrophage
activation in mechanical ventilation has not been fully
elucidated.
Notch receptors are mainly distributed on the
surface of stem cells or protocells and are also widely expressed
on the surface of various immune cells such as macrophages, T cells
and dendritic cells (16). A
previous study has suggested that the activation of Notch signaling
pathway would cause macrophage polarization and promote the
secretion of IL-6 (17). Han
et al (18) have shown
that blocking the Notch signaling pathway could reduce the
inflammatory response and tissue damage. It has also been found
that inhibiting the Notch signaling pathway could reduce the
expression of TNF-α (19). In
addition, the use of the Notch signaling pathway inhibitor
N-[N-(3,5-difluorophenylacetyl)-1-alanyl] phenylglycine t-butyl
ester (DAPT) can improve arthritis symptoms and joints in arthritic
damaged mice (20,21). Therefore, the present study
hypothesized that the Notch signaling pathway was involved in the
regulation of pulmonary inflammatory response after mechanical
ventilation and participation in the occurrence and development of
VILI.
The present study mainly investigated the
relationship between VILI and Notch signaling pathways in
regulating macrophage polarization and studied the pathogenesis of
VILI. The present findings provide evidence for and new approaches
to VILI prevention and treatment.
Materials and methods
Animals and establishment of mechanical
ventilation lung injury model
A total of 60 male Sprague-Dawley rats (weight:
250-300 g; age: ~8 weeks) were purchased from the Laboratory Animal
Center. Modeling and follow-up experimental programs had been
approved by the Institutional Animal Care and Use Committee and
China Council on Animal Care. The rats were fed with a normal diet
with water ad libitum before the treatment and kept on a
12-h light/dark cycle in a controlled room at 22±2°C
temperature in 60-70% humidity. The rats were fasted 12 h before
surgery but were allowed to have free access to drinking water. The
rats were anesthetized with an intraperitoneal injection of 10%
chloral hydrate 300 mg/kg in a supine position and fixed on an
adjustable warming pad (Shanghai Alcott Biotech, Co., Ltd.), and
the body temperature of the rats was maintained at 37±1°C.
Peritonitis was not observed in rats after anesthesia. After
anesthesia, the trachea was exposed by cutting along the midline
and the rats were subjected to tracheal intubation. Ophthalmic
scissors were used to cut a small opening between the tracheal
cartilage and a 22-gauge sterile intravenous indwelling needle
cannula was inserted slowly. The casing was fixed with surgical
wire and connected to a small animal ventilator (Inspira ASV;
Harvard Apparatus, Ltd.). The tidal volume was adjusted according
to the weight, with reference to previous studies (22,23). The tidal volume parameter of the
normal tidal volume group (LVT group; n=10) was 8 ml/kg and in the
high tidal volume group (HVT; n=10) it was 40 ml/kg. The rats in
the control group (Con; n=10) were subjected to a tracheotomy and
tracheal intubation, and spontaneous breathing was retained. The
ventilator parameters was as follows: The inspiration and
expiration ratio was 1:2, the respiratory rate was 80 times/min,
the oxygen concentration was 40%, and the positive end-expiratory
pressure was 0. The high frequency oscillating ventilation time was
4 h. The rats in each group were alive during mechanical
ventilation.
Other rats were modeled by the same method and
divided into the Con, HVT and DAPT groups. The DAPT group received
intraperitoneal injection of DAPT 100 mg/kg 3 h before mechanical
ventilation, while the other groups received the same dose of
DMSO.
Humane endpoints were defined in case of unexpected
or major change in the behavior (quantified using a scoring
system). In short, the endpoints included: Weight loss >15%
between two adjacent body weighing sessions (three times a week);
Signs of severe dyspnea, defined as an inability of the rats to
keep oxygen saturation >90%; Signs indicating encephalitis,
e.g., nystagmus, paresis, or head tilt.
Hematoxylin and eosin (H&E)
staining
H&E staining was applied to observe the lung
tissues in each group. After modeling, lung tissues from rats were
immediately fixed in formalin for 24 h at room temperature and were
then dehydrated with alcohol and embedded in paraffin. The sample
was cut into 4-µm thick-uniform flakes and placed on
APES-coated glass slides. The sections were deparaffinized using
xylene and hydrated and stained with the H&E reagent
(Sigma-Aldrich; Merck KGaA). Hematoxylin was added and the sample
was incubated at room temperature for 5 min. After washing, eosin
was added and the sample was incubated at room temperature for ~2
min. After being washed with gradient ethanol, a neutral gel was
used for sealing. The alveolar morphology was observed under a
light microscope and assessed by the lung injury score.
Lung wet/dry ratio
All rats were sacrificed by intraperitoneal
injection of sodium pentobarbital (200 mg/kg). The sound of
breathing and heartbeat (respiratory arrest and cardiac arrest)
could not be heard through the stethoscope and this confirmed the
death of the rats. After the rats were sacrificed, the pulmonary
circulation was washed with PBS and the left lung was separated and
weighed as the wet lung weight. The wet lungs were placed in a 65°C
oven and allowed to be dried for 48 h and weighed again as the dry
lung weight. The lung wet/dry ratio=wet lung weight/dry lung
weight.
Bicinchoninic acid (BCA) assay
The protein concentration in the bronchoalveolar
lavage fluids (BALF) was measured using the BCA assay. After the
rats were sacrificed, PBS was injected into the lungs via the
trachea, the liquid was aspirated slowly and the procedure was
repeated 3 times. The supernatant of the aspirated liquid was
separated by centrifugation at 12,000 × g for 10 min at 4°C and the
protein concentration in the supernatant was measured. BCA kit was
purchased from Biomiga, Inc., and the reagents were added according
to the manufacturer's protocol. Absorbance at 570 nm was measured
by a microplate reader (FilterMax F3/F5; Molecular devices, LLC)
and the protein concentrations were calculated from the standard
curve concentration.
ELISA
The concentrations of TNF-α (CSB-E11987r), IL-6
(CSB-E04640r) and IL-10 (CSB-E04595r) in BALF were measured by
ELISA. The kit was purchased from Cusabio Biotech Co., Ltd. The
enzyme labeling reagent and developer were added following the
protocol, and the reaction was terminated by adding a stop
solution. Optical densities at 450 nm were determined using an
ELISA reader (Model 680; Bio-Rad Laboratories, Inc.).
Flow cytometry
Flow cytometry was used to detect the polarity of
macrophages in lung tissues and the stain buffer (BSA) was
purchased from BD Pharmingen; Becton, Dickson and Company. Lung
tissues were lysed in a lysis buffer containing 10 mM Tris-Cl, 160
mM KCl and 1.5 mM MgCl2, 250 mM sucrose, 0.5% Nonidet P
40 Substitute (Fluka), 3 mM β-mercaptoethanol, 2 mM
phenylmethanesulfonylflouride, 10 µg/ml leupeptin and 10
µg/ml aprotinin, and the cells were resuspended to a
concentration of 4×105 cells/ml. F4/80, Cmaf and
inducible nitric oxide synthase (iNOS) antibodies (eBioscience;
Thermo Fisher Scientific, Inc.) were added, respectively. The
samples were incubated at room temperature in the dark for 10 min.
A flow cytometer (Becton, Dickson and Company) was applied to
analyze the cell apoptosis, and the data was analyzed using BD
CellQuest™ Pro version 1.2 software (BD Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression levels of Notch intracellular
domain (NICD), Hes1, Hes5 and Hey1 were determined by RT-qPCR.
Total RNA was extracted using the TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and the reverse transcription kit
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to synthesize
cDNA at 37°C for 15 min, followed by a reverse transcriptase
inactivation at 85°C for 15 sec. RT-qPCR was carried out to
determine NICD, Hes1, Hes5 and Hey1, and the amplification reaction
procedures were as follows: At 95°C for 5 min, followed by 40
cycles (at 95°C for 15 sec, at 60°C for 1 min, at 72°C for 1 min)
and a final extension at 72°C for 10 min and held at 4°C. All
primers were obtained from Takara Bio, Inc. and are listed in
Table I. GAPDH was used as
reference gene. The formula 2−ΔΔCq (24) was implemented to analyze the mRNA
expression levels.
 | Table IThe sequences of primers. |
Table I
The sequences of primers.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| NICD |
TGGCCTCAATGGATACAAATG |
GGGCCAACACCACCTCAC |
| Hes-1 CC |
AGCCAGTGTCAACACGA |
AATGCCGGGAGCTATCTTTCT |
| Hes-5 |
AGTCCCAAGGAGAAAAACCGA |
GCTGTGTTTCAGGTAGCTGAC |
| Hey 1 |
AAAGACGGAGAGGCATCATCG |
GCAGTGTGCAGCATTTTCAGG |
| GAPDH CC |
TTCCGTGTTCCTACCCC |
GCCCAGGATGCCCTTTAGTG |
Western blotting
Western blotting was applied to detect Notch
signaling pathway-associated proteins, which included NICD, Hes1,
Hes5 and Hey1. The cells were lysed with RIPA (Abcam) and the
supernatant was collected by a centrifugation at 12,000 × g at 4°C
for 15 min. A BCA assay was used to determine the protein
concentration. Protein lysates (25 µg/lane) were separated
by 12% SDS-PAGE. The PVDF membrane (Bio-Rad Laboratories, Inc.) was
transferred by a Trans-Blot Transfer Slot (Bio-Rad Laboratories,
Inc.) and blocked with 5% fat-free milk for 2 h at room
temperature. The primary antibodies (anti-NICD; Abcam; cat. no.
ab8925; 1:800; anti-Hes1; Abcam; cat. no. ab71559; 1:700;
anti-Hes5; Abcam; cat. no. ab25374; 1:600; anti-Hey1; Abcam; cat.
no. ab22614; 1:800) were added following the kit protocol and
shaken at room temperature for 2 h and then incubated at 4°C for 12
h. The HRP-conjugated secondary antibodies (rabbit anti-human IgG;
Abcam; cat. no. ab6759; 1:8,000; rabbit anti-goat IgG; Abcam; cat.
no. ab6741; 1:10,000; goat anti-rabbit IgG; Abcam, ab6721; 1:8,000)
were added and incubated at room temperature for 1 5 h.
Chemiluminescence detection was carried out using ECL reagent
(SignalFire, cat. no. 6883, Cell Signaling Technology, Inc.).
Densitometry was performed using Quantity One software version 2.4
(Bio-Rad Laboratories, Inc.)
Statistical analyses
Data from three independent and repetitive
experiments were shown as the mean ± standard deviation.
Differences between the experimental groups were assessed by
analysis of variance, followed by Dunnett's post hoc test.
Differences were analyzed by GraphPad Prism 6 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
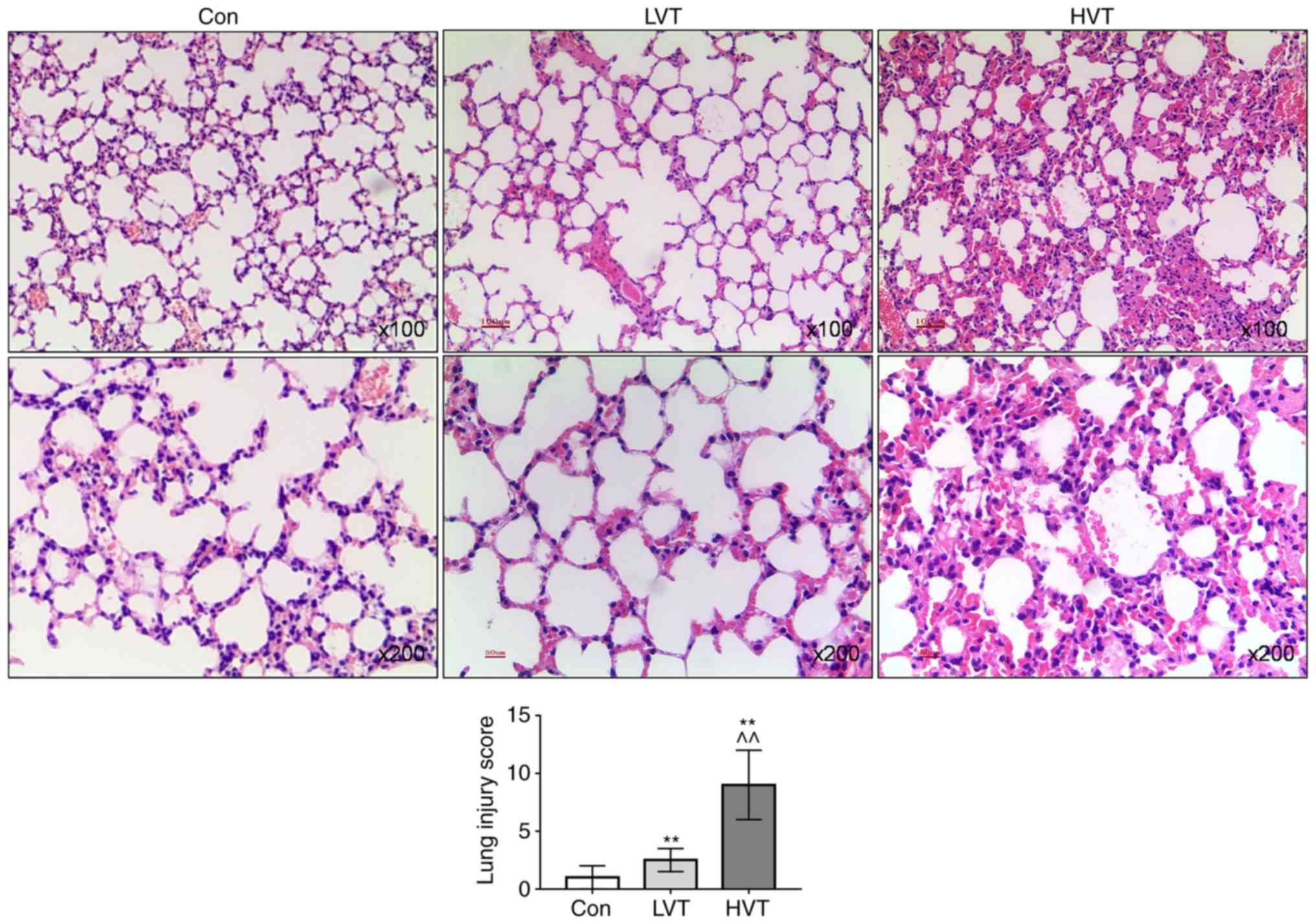
Effects of mechanical ventilation on lung
tissue
The H&E staining results showed that the
alveolar morphology of the lung tissue from the Con group was
normal and there was no inflammatory cell infiltration. The
alveolar structure of the LVT group was normal and a mild
inflammatory infiltration was observed. In the HVT group, the
alveolar structure was disordered, the alveolar space was thickened
and a large number of inflammatory cells infiltrated in the lung
tissue, and the interstitial hemorrhage showed changes in
congestion and hemorrhage and the lung injury score significantly
increased (P<0.01; Fig.
1).
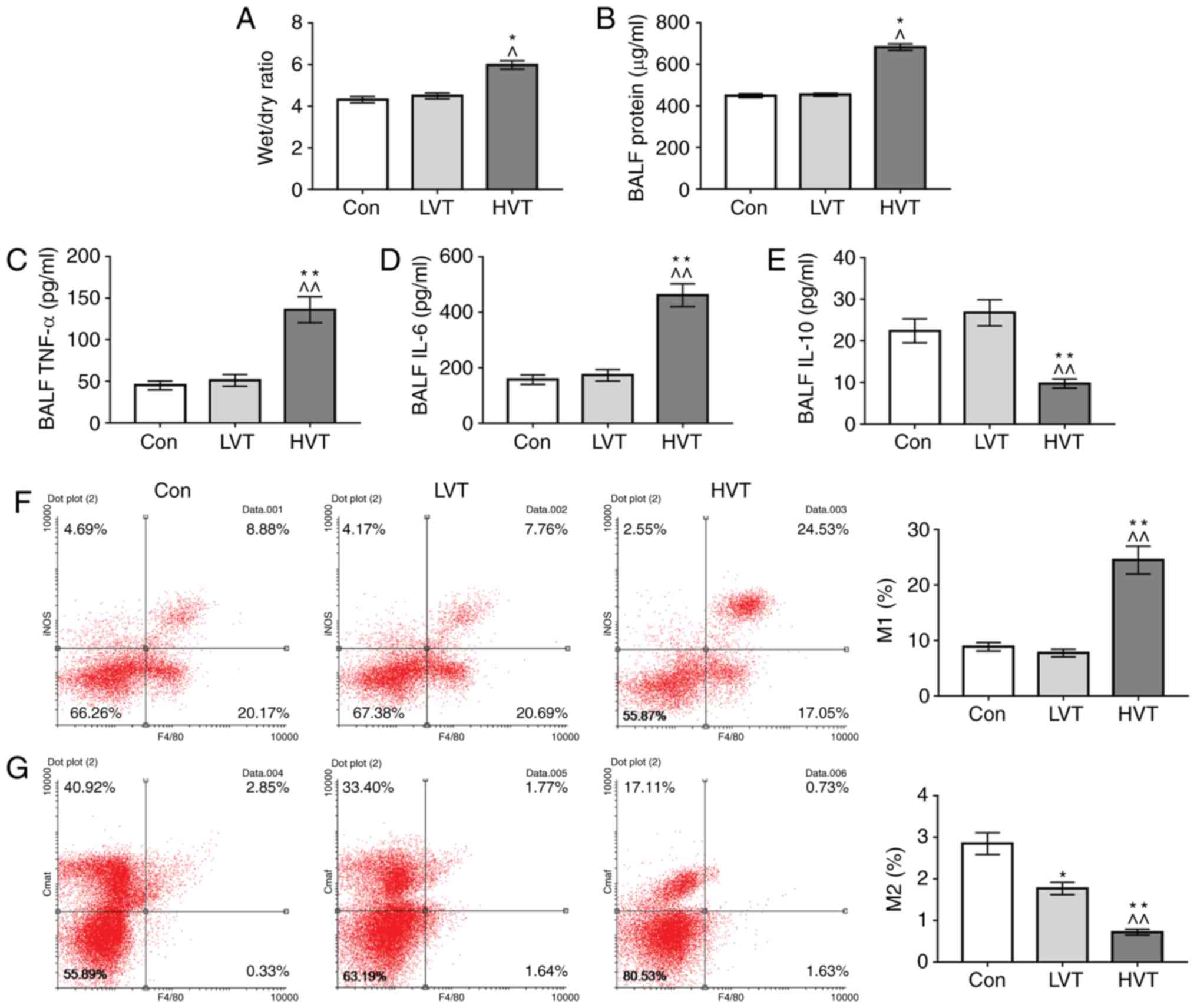
Effects of mechanical ventilation on lung
and inflammatory factors in BALF
To investigate the effects of mechanical ventilation
on pulmonary edema and the inflammatory response in lung tissue,
lung wet/dry ratio, protein, TNF-α, IL-6 and IL-10 in BALF were
determined. The results showed no difference in lung wet/dry ratio
between the Con group and the LVT group, and that the lung wet/dry
ratio of the HVT group was increased significantly (P<0.05;
Fig. 2A). No significant
difference was detected in protein content, TNF-α, IL-6 and IL-10
levels in BALF between the Con group and LVT group. The protein
content, TNF-α and IL-6 in BALF in HVT group was significantly
increased compared with the Con group and the LVT group
(P<0.01), and the IL-10 level in BALF in HVT group was
significantly decreased compared with the Con group and the LVT
group (P<0.01; Fig. 2B-E).
This suggested that high-frequency mechanical ventilation could
damage lung tissue and lead to pulmonary edema inflammatory
reactions in the lungs.
Effects of mechanical ventilation on the
polarization of macrophages
To explore the causes to inflammatory reactions in
the lungs, flow cytometry was used to detect the polarization
levels of macrophages. The results showed no significant change in
M1 macrophage levels in the Con group and those in the LVT group,
and that the proportion of M1 macrophages in the HVT group was
significantly increased compared with the Con group and the LVT
group (P<0.01; Fig. 2F). For
M2 macrophages, the proportions of M2 macrophages in the LVT group
and the HVT group were decreased, and the level of M2 macrophages
in the HVT group was decreased compared with the LVT group
(Fig. 2G), suggesting that
mechanical ventilation might induce polarization of macrophages in
lung tissue to M1.
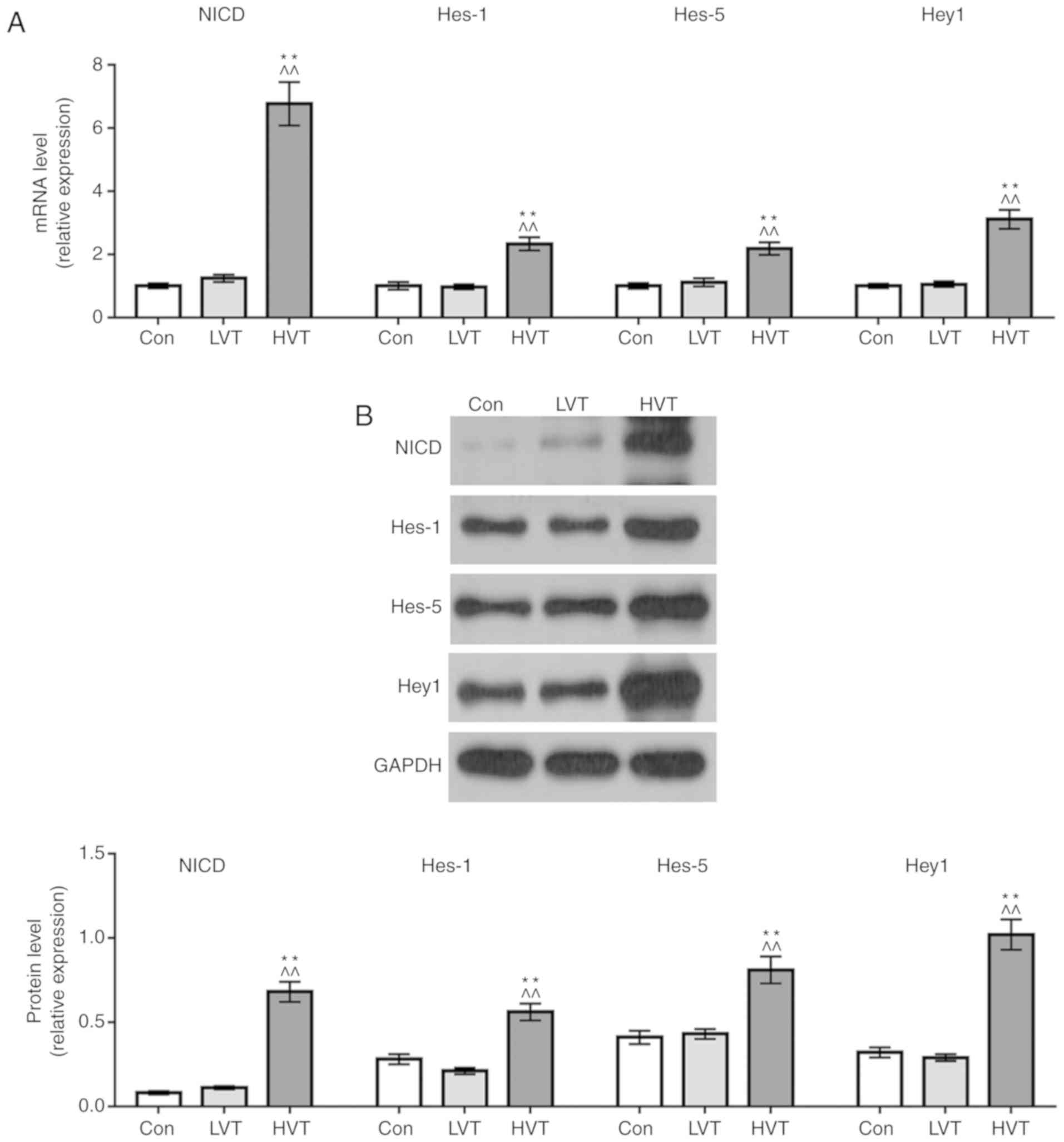
Effects of mechanical ventilation on the
expression of Notch pathway associated proteins
NICD, Hes1, Hes5 and Hey1 mRNA and protein
expression levels were determined by RT-qPCR and western blotting
to explore the mechanism of macrophage polarization. The results
showed no significant difference in the mRNA and protein expression
levels of the 4 proteins between the control group and the LVT
group. The mRNA and protein expression levels of NICD, Hes1, Hes5
and Hey1 in HVT group were significantly increased compared with
the Con group and the LVT group (P<0.01; Fig. 3A and B). This suggested that
high-frequency mechanical ventilation could promote the expression
of Notch signaling pathway-associated proteins.
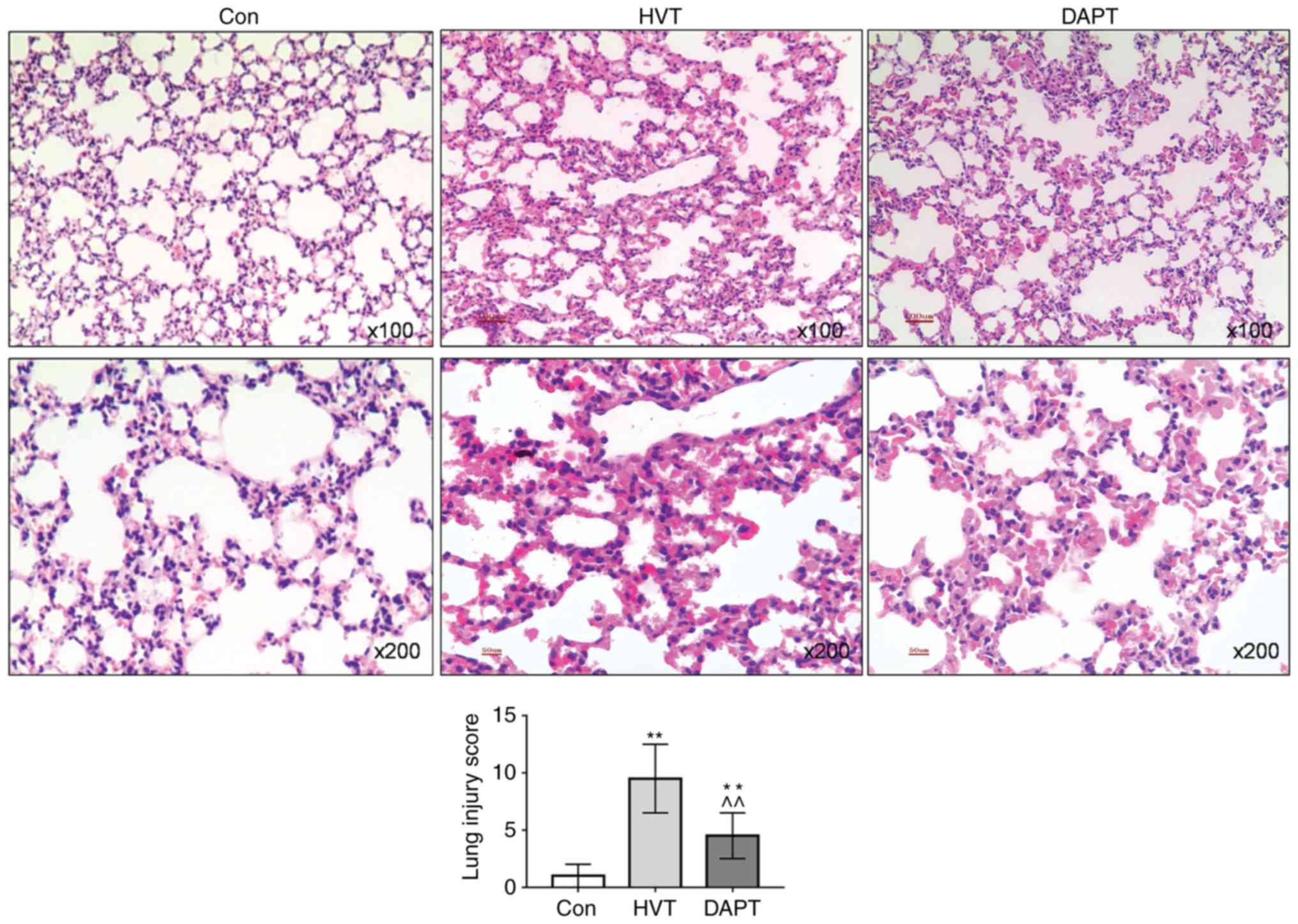
Effects of inhibition of Notch pathway on
lung injury in rats
To explore the effects of inhibition of the Notch
pathway on lung injury caused by mechanical ventilation, the Notch
pathway was inhibited using DAPT. The H&E staining results
showed that the alveolar morphology of the lung tissue in the Con
group was normal. In the HVT group, the alveolar structure was
disordered, the alveolar space was significantly thickened and a
large number of inflammatory cells infiltrated in the lung tissue
and the interstitial hemorrhage showed changes in congestion and
hemorrhage. The alveolar structure of the DAPT group was basically
normal, however, some inflammatory infiltration occurred. The lung
injury score of the DAPT group declined significantly (P<0.01;
Fig. 4).
Effects of inhibition of Notch pathway on
lung and inflammatory factors in BALF
To investigate the effects of inhibition of Notch
pathway on pulmonary edema and the inflammatory response in lung
tissue, lung wet/dry ratio, protein, TNF-α, IL-6 and IL-10 in BALF
were determined. The results showed that lung wet/dry ratio,
protein content, TNF-α and IL-6 levels in BALF in the HVT group
were increased compared with the Con group and DAPT group, and that
no significant difference on above indicators between the Con group
and the DAPT group was observed. The IL-10 levels in the HVT group
and DAPT group were decreased compared with the Con group, however,
the IL-10 level in the DAPT group was increased compared with the
HVT group (Fig. 5A-E). This
suggested that the inhibition of the Notch pathway by DAPT could
attenuate lung injury and the inflammatory response in the lungs of
mechanical ventilation.
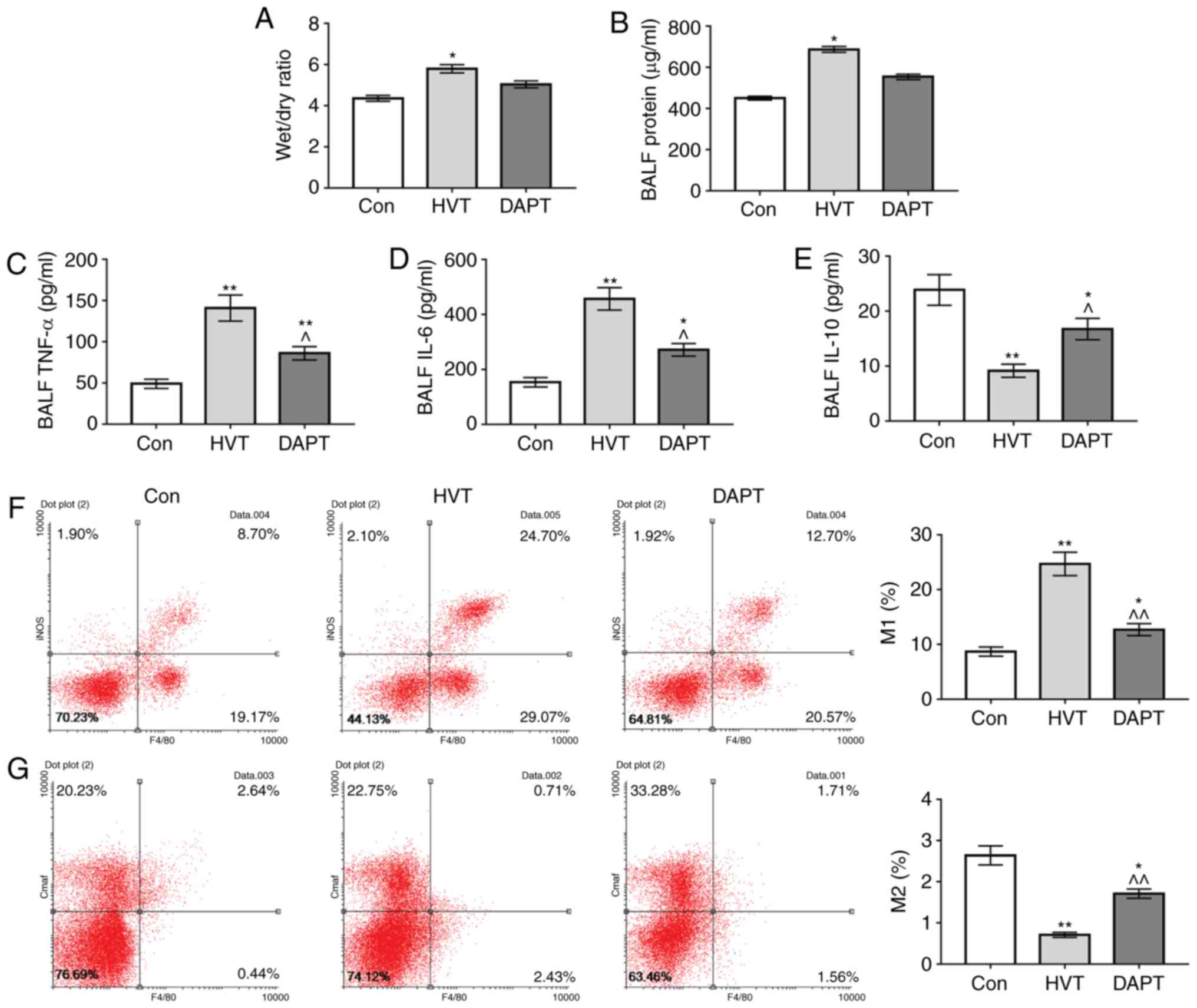 | Figure 5Effects of DAPT on wet/dry ratio
inflammatory factors in BALF and macrophage polarization. (A) The
lung wet/dry ratios of the Con group, HVT group and DAPT group were
weighed and compared. BCA assay and ELISA were used to test (B)
protein, (C) TNF-α, (D) IL-6 and (E) IL-10 levels in BALF,
respectively. Alveolar macrophage polarization [(F) M1 and (G) M2]
was detected by flow cytometry. *P<0.05 and
**P<0.01 vs. control group; ^P<0.05 and
^^P<0.01 vs. HVT group. LVT, normal tidal volume;
HVT, high volume tidal; Con, control; DAPT,
N-[N-(3,5-difluorophenylacetyl)-1-alanyl] phenylglycine t-butyl
ester; BALF, broncho alveolar lavage fluid; IL, interleukin; TNF,
tumor necrosis factor. |
Effects of inhibition of Notch pathway on
the polarization of macrophages
The results showed that the proportions of M1
macrophages in the HVT group and DAPT group were significantly
increased compared with the Con group (P<0.01), while the
proportion of M1 macrophages in DAPT group was significantly
decreased compared with in the HVT group (P<0.01). The
proportions of M2 macrophages in the HVT group and DAPT group were
decreased compared with the Con group, while the proportion of M2
macrophages in DAPT group was significantly increased compared with
the HVT group (P<0.01; Fig. 5F and
G). This suggested that the inhibition of Notch pathway by DAPT
could downregulate macrophage M1 polarization caused by mechanical
ventilation.
Inhibition of Notch pathway by DAPT
To demonstrate the inhibitory effect of DAPT on
Notch pathway, RT-qPCR and western blotting were performed to
detect NICD, Hes1, Hes5 and Hey1 mRNA and protein expression levels
in each group. The results showed that the NICD, Hes1, Hes5 and
Hey1 mRNA and protein expression levels in the DAPT group were
decreased compared with the HVT group (Fig. 6A and B), indicating that using
DAPT could inhibit the levels of Notch pathway-related protein.
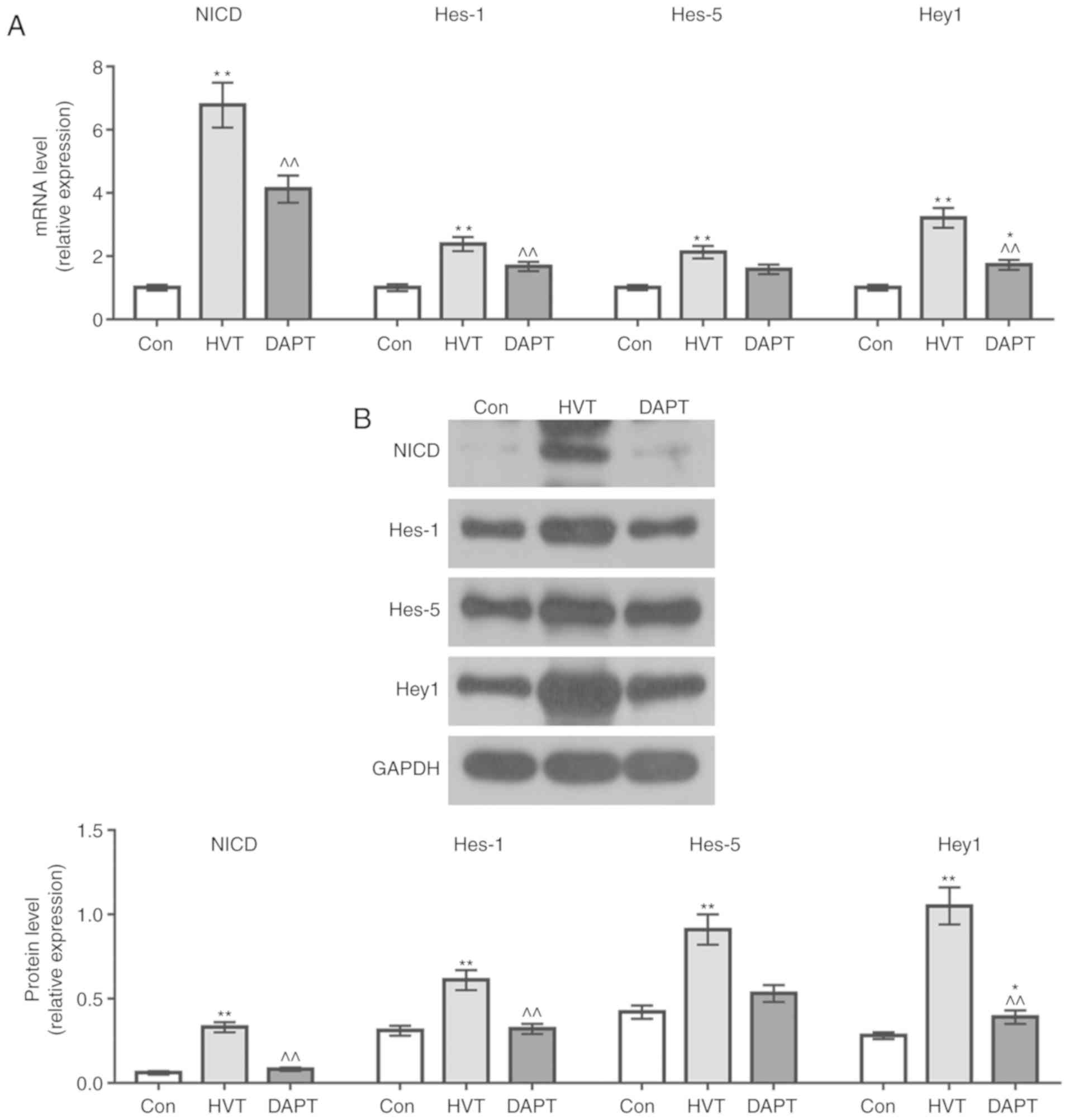 | Figure 6Effects of mechanical ventilation on
the Notch pathway of macrophages. (A) The mRNA expression of the
NICD, Hes1, Hes5 and Hey1 in the Con, HVT and DAPT group were
detected using reverse transcription-quantitative PCR. (B) The
protein expressions of the NICD, Hes1, Hes5 and Hey1 in Con, HVT
and DAPT group were detected by western blotting.
*P<0.05 and **P<0.01 vs. Con group;
^^P<0.01 vs. HVT group. HVT, high volume tidal; Con,
control; DAPT, N-[N-(3,5-difluorophenylacetyl)-1-alanyl]
phenylglycine t-butyl ester; NICD, Notch intracellular domain. |
Discussion
Excessive alveolar expansion or excessive
intrapulmonary pressure caused by mechanical ventilation can lead
to damage to lung tissue, interstitial structures and alveolar
membrane damage, and such damage is characterized by pulmonary
edema and oxygen dysfunction (25). The main pathological change in
VILI is the increased permeability of the alveolar capillary
membrane, which is a result of the release of inflammatory cells
and inflammatory factors. As demonstrated by previous studies, the
immunoinflammatory response caused by an early activation of
macrophages was found in VILI (26,27).
Previous studies have found that high-frequency
mechanical ventilation led to elevated levels of TNF-α and IL-6 in
serum and lung BALF (27,28). To investigate the effects of
mechanical ventilation on lung and alveolar macrophages, rats were
used to establish a mechanical ventilation model and detected
changes in lung tissue and in inflammatory factors in BALF. The
results showed that high-frequency mechanical ventilation changed
the alveolar structure and caused inflammatory cell infiltration
and pulmonary edema, suggesting that the VILI rat model was
successfully established. The results also showed that
high-frequency mechanical ventilation also increased the protein
content, TNF-α and IL-6 levels in BALF, and decreased the IL-10
level, therefore causing an inflammatory response. It has been
shown that the release of inflammatory factors was not only
secondary to non-cell damage, but also could be induced by
mechanical ventilation, which induces macrophage activation,
recruits inflammatory cells to the lungs and amplifies the
inflammatory response (29-31).
In order to study the causes of lung inflammation in
VILI rats, the polarization of macrophages in lung tissue was
measured. The results showed that high-frequency mechanical
ventilation caused polarization of rat macrophages to M1. In
previous years, a study also found abnormal polarization of
alveolar macrophages in VILI animals, moreover, TNF-α also induces
macrophage polarization to M1 and M1 macrophages release TNF-α and
IL-6 to aggravate the inflammatory response (13). In addition, macrophage
polarization will also recruit a large number of peripheral
neutrophils to the lungs, aggravating inflammatory lesions of the
lungs (15,32). This suggested that high-frequency
mechanical ventilation could aggravate lung injury in VILI rats by
inducing polarization of macrophages to M1.
To investigate the mechanism of polarization of
macrophages in VILI rats, the levels of Notch pathway-associated
proteins were examined in rat macrophages. The results showed that
high-frequency mechanical ventilation significantly upregulated
NICD, Hes1, Hes5 and Hey1 mRNA and protein expression levels. The
Notch signaling pathway plays an important role in immune
regulation (33). After the Notch
receptor binds to the ligand, the extracellular fragment is
released and the rest is cleaved by γ-secretase and releases NICD
(34). NICD binds to the
recombination signal binding protein J in the nucleus to form
transcriptional activators, which affects the expression of the
target genes Hes1, Hes5 and Hey1. Hes1, Hes5 and Hey1 gene
expression products interact with other signaling pathways to
affect cell proliferation, differentiation and apoptosis (35-38). A previous study has shown that
upregulation of macrophage Notch-1 gene expression level could
promote macrophage activation and activated Notch signaling pathway
can affect mature macrophage function by upregulating levels of
interferon (IFN)-γ (38-40). Wongchana et al (41) also demonstrated that LPS and IFN-γ
could stimulate macrophage expression of NICD-1, while NICD-1 can
promote the secretion of IL-6 via the nuclear factor-κB pathway to
induce macrophage polarization to M1. The results of this study
showed that the NICD, Hes1, Hes5 and Hey1 protein expression levels
were increased in the lung macrophages of the HVT group, indicating
that high-frequency mechanical ventilation promoted the activation
of the Notch pathway, thereby inducing macrophages to polarize to
M1 and aggravating the inflammatory response.
To further explore the mechanism of macrophage
polarization in VILI rats, the effect of the Notch pathway on
macrophage polarization was investigated. A previous study found
that subcutaneous injection of DAPT effectively inhibited the
activity of the Notch pathway and that DAPT lowered the level of
NICD (18). In addition, a
different study also found that DAPT did not affect the level of
TNF-α in arthritic mice (42).
The results of the present study showed that the macrophage NICD,
Hes1, Hes5 and Hey1 mRNA and protein expression levels in the lung
tissue of DAPT mice were decreased, suggesting that the Notch
signaling pathway in the DAPT group was successfully inhibited by
intraperitoneal injection of DAPT. The results of this study also
showed that inhibition of the Notch pathway could reduce pulmonary
edema and pulmonary inflammatory factor release caused by
high-frequency mechanical ventilation and could inhibit the
polarization of macrophages to M1. The study by Sun et al
(39) has shown that in
rheumatoid arthritis, activation of the Notch pathway has the
effect of activating macrophages and that the M1 macrophage was the
most abundant cells with Notch activation. Notch signaling
inhibitor Thapsigargin can attenuate joint tissue damage by
switching M1 to M2 macrophages. It has also been found that in
human Crohn's disease cells, inhibition of Notch signaling caused
macrophages to polarize to M2, thereby reducing intestinal
epithelial cell damage (43). In
tumor cells, activation of the Notch pathway also causes macrophage
activation and polarization to M1 (38,44), indicating that in VILI rats,
inhibition of Notch pathway could reduce inflammatory response by
inhibiting M1 polarization of macrophages, therefore protecting
lung tissue.
However, there were some limitations in the present
study, for example, at multiple time points it was necessary to
further explore the effects of different mechanical ventilation
durations on rat lung tissue. In the current study, F4/80 and INOS
were used to assess the M1 and M2 macrophages, however, using more
macrophage markers to gate the macrophage and M1/M2 phenotype may
help reach a more convincing conclusion. The issue will be further
analyzed comprehensively. In the present study, the polarization
levels of M1 and M2 macrophages were detected by flow cytometry,
which should be confirmed by performing immunofluorescence
analysis.
In conclusion, the present study demonstrated that
high-frequency mechanical ventilation could cause inflammation and
macrophages were polarized toward M1. The activation of the Notch
pathway in macrophages was also found. After using DAPT to inhibit
the Notch pathway, the lung damage caused by mechanical ventilation
was reduced and the level of inflammatory factors was also reduced,
which may be explained by that the inhibition of the Notch pathway
could inhibit macrophage polarization to M1.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Dean's Fund
of Jinan Military General Hospital in 2015 (grant. no.
2015QN02).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
Substantial contributions to conception and design;
data acquisition, data analysis and interpretation, drafting the
article or critically revising it for important intellectual
content, final approval of the version to be published and
agreement to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of the work are
appropriately investigated and resolved: All authors.
Ethics approval and consent to
participate
All animal procedures were approved by the
Institutional Animal Care and Use Committee and China Council on
Animal Care. All animal experiments were performed in compliance
with the Guidelines for Proper Conduct of Animal Experiments,
established by the Science Council.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sutherasan Y, Vargas M and Pelosi P:
Protective mechanical ventilation in the non-injured lung: Review
and meta-analysis. Crit Care. 18:2112014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schnell D, Timsit JF, Darmon M, Vesin A,
Goldgran-Toledano D, Dumenil AS, Garrouste-Orgeas M, Adrie C,
Bouadma L, Planquette B, et al: Noninvasive mechanical ventilation
in acute respiratory failure: Trends in use and outcomes. Intensive
Care Med. 40:582–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Unroe M, Kahn JM, Carson SS, Govert JA,
Martinu T, Sathy SJ, Clay AS, Chia J, Gray A, Tulsky JA and Cox CE:
One-year trajectories of care and resource utilization for
recipients of prolonged mechanical ventilation: A cohort study. Ann
Intern Med. 153:167–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Müller-Redetzky HC, Felten M, Hellwig K,
Wienhold SM, Naujoks J, Opitz B, Kershaw O, Gruber AD, Suttorp N
and Witzenrath M: Increasing the inspiratory time and I:E ratio
during mechanical ventilation aggravates ventilator-induced lung
injury in mice. Crit Care. 19:232015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamlington KL, Smith BJ, Allen GB and
Bates JH: Predicting ventilator-induced lung injury using a lung
injury cost function. J Appl Physiol 1985. 121:106–114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reddy SP, Hassoun PM and Brower R: Redox
imbalance and ventilator-induced lung injury. Antioxid Redox
Signal. 9:2003–2012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Curley GF, Contreras M, Higgins B, O'Kane
C, McAuley DF, O'Toole D and Laffey JG: Evolution of the
inflammatory and fibroproliferative responses during resolution and
repair after ventilator-induced lung injury in the rat.
Anesthesiology. 115:1022–1032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bertok S, Wilson MR, Morley PJ, de Wildt
R, Bayliffe A and Takata M: Selective inhibition of intra-alveolar
p55 TNF receptor attenuates ventilator-induced lung injury. Thorax.
67:244–251. 2012. View Article : Google Scholar :
|
|
9
|
Wilson MR, Wakabayashi K, Bertok S, Oakley
CM, Patel BV, O'Dea KP, Cordy JC, Morley PJ, Bayliffe AI and Takata
M: Inhibition of TNF receptor p55 By a domain antibody attenuates
the initial phase of acid-induced lung injury in mice. Front
Immunol. 8:1282017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Opitz B, van Laak V, Eitel J and Suttorp
N: Innate immune recognition in infectious and noninfectious
diseases of the lung. Am J Respir Crit Care Med. 181:1294–1309.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martinez FO and Gordon S: The M1 and M2
paradigm of macrophage activation: Time for reassessment. F1000
Prime Rep. 6:132014. View
Article : Google Scholar
|
|
12
|
Jablonski KA, Amici SA, Webb LM,
Ruiz-Rosado Jde D, Popovich PG, Partida-Sanchez S and
Guerau-de-Arellano M: Novel markers to delineate murine M1 and M2
macrophages. PLoS One. 10:e01453422015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jaecklin T, Otulakowski G and Kavanagh BP:
Do soluble mediators cause ventilator-induced lung injury and
multi-organ failure? Intensive Care Med. 36:750–757. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai H, Pan L, Lin F, Ge W, Li W and He S:
Mechanical ventilation modulates Toll-like receptors 2, 4, and 9 on
alveolar macrophages in a ventilator-induced lung injury model. J
Thorac Dis. 7:616–624. 2015.PubMed/NCBI
|
|
16
|
Kopan R and Ilagan MX: The canonical Notch
signaling pathway: Unfolding the activation mechanism. Cell.
137:216–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Z, Guo L, Liu D, Sun L, Chen H, Deng
Q, Liu Y, Yu M, Ma Y, Guo N and Shi M: Acquisition of resistance to
trastuzumab in gastric cancer cells is associated with activation
of IL-6/STAT3/Jagged-1/Notch positive feedback loop. Oncotarget.
6:5072–5087. 2015.PubMed/NCBI
|
|
18
|
Han H, Gong G, Bai X, Lin YC, Sun J, Wang
W, Zhao Y, Yang L, Wang X, Zhang Z, et al: Inhibition of Notch
signaling protects mouse lung against zymosan-induced injury.
Shock. 40:312–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsao PN, Wei SC, Huang MT, Lee MC, Chou
HC, Chen CY and Hsieh WS: Lipopolysaccharide-induced Notch
signaling activation through JNK-dependent pathway regulates
inflammatory response. J Biomed Sci. 18:562011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiao Z, Wang W, Hua S, Liu M, Wang H, Wang
X, Chen Y, Xu H and Lu L: Blockade of Notch signaling ameliorates
murine collagen-induced arthritis via suppressing Th1 and Th17 cell
responses. Am J Pathol. 184:1085–1093. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JS, Kim SH, Kim K, Jin CH, Choi KY,
Jang J, Choi Y, Gwon AR, Baik SH, Yun UJ, et al: Inhibition of
Notch signalling ameliorates experimental inflammatory arthritis.
Ann Rheum Dis. 74:267–274. 2015. View Article : Google Scholar
|
|
22
|
Huang C, Pan L, Lin F, Dai H and Fu R:
Monoclonal antibody against Toll-like receptor 4 attenuates
ventilator-induced lung injury in rats by inhibiting MyD88- and
NF-κB-dependent signaling. Int J Mol Med. 39:693–700. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whitehead TC, Zhang H, Mullen B and
Slutsky AS: Effect of mechanical ventilation on cytokine response
to intratracheal lipopolysaccharide. Anesthesiology. 101:52–58.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Silva PL, Negrini D and Rocco PR:
Mechanisms of ventilator-induced lung injury in healthy lungs. Best
Pract Res Clin Anaesthesiol. 29:301–313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cressoni M, Gotti M, Chiurazzi C, Massari
D, Algieri I, Amini M, Cammaroto A, Brioni M, Montaruli C, Nikolla
K, et al: Mechanical power and development of Ventilator-induced
lung injury. Anesthesiology. 124:1100–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borges JB, Costa EL, Suarez-Sipmann F,
Widström C, Larsson A, Amato M and Hedenstierna G: Early
inflammation mainly affects normally and poorly aerated lung in
experimental ventilator-induced lung injury*. Crit Care Med.
42:e279–e287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu YH and Guo NL: USP14 inhibitor IU1
prevents ventilator-induced lung injury in rats. Cell Mol Biol
(Noisy-le-grand). 60:50–54. 2014.
|
|
29
|
Malaviya R, Sunil VR, Venosa A, Verissimo
VL, Cervelli JA, Vayas KN, Hall L, Laskin JD and Laskin DL:
Attenuation of nitrogen mustard-induced pulmonary injury and
fibrosis by anti-tumor necrosis factor-α antibody. Toxicol Sci.
148:71–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Halbertsma FJ, Vaneker M, Scheffer GJ and
van der Hoeven JG: Cytokines and biotrauma in ventilator-induced
lung injury: A critical review of the literature. Neth J Med.
63:382–392. 2005.PubMed/NCBI
|
|
31
|
Ko YA, Yang MC, Huang HT, Hsu CM and Chen
LW: NF-κB activation in myeloid cells mediates ventilator-induced
lung injury. Respir Res. 14:692013. View Article : Google Scholar
|
|
32
|
Huang C, Pan L, Lin F, Qian W and Li W:
Alveolar macrophage TLR4/MyD88 signaling pathway contributes to
ventilator-induced lung injury in rats. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 31:182–185. 1892015.In Chinese.
|
|
33
|
Morimoto M, Nishinakamura R, Saga Y and
Kopan R: Different assemblies of Notch receptors coordinate the
distribution of the major bronchial Clara, ciliated and
neuroendocrine cells. Development. 139:4365–4373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nagase H and Nakayama K:
γ-Secretase-regulated signaling typified by Notch signaling in the
immune system. Curr Stem Cell Res Ther. 8:341–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Katoh M and Katoh M: Integrative genomic
analyses on HES/HEY family: Notch-independent HES1, HES3
transcription in undifferentiated ES cells, and Notch-dependent
HES1, HES5, HEY1, HEY2, HEYL transcription in fetal tissues, adult
tissues, or cancer. Int J Oncol. 31:461–466. 2007.PubMed/NCBI
|
|
36
|
Ma Y, Bian J and Zhang F: Inhibition of
perillyl alcohol on cell invasion and migration depends on the
Notch signaling pathway in hepatoma cells. Mol Cell Biochem.
411:307–315. 2016. View Article : Google Scholar
|
|
37
|
Liu X, Cong N, Cheng X, Ma R, Wang J,
Huang YB, Zhao M, Wang XW, Chi FL and Ren DD: The role of the Notch
signal pathway in mucosal cell metaplasia in mouse acute otitis
media. Sci Rep. 7:45882017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang YC, He F, Feng F, Liu XW, Dong GY,
Qin HY, Hu XB, Zheng MH, Liang L, Feng L, et al: Notch signaling
determines the M1 versus M2 polarization of macrophages in
antitumor immune responses. Cancer Res. 70:4840–4849. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun W, Zhang H, Wang H, Chiu YG, Wang M,
Ritchlin CT, Kiernan A, Boyce BF and Xing L: Targeting
notch-activated M1 macrophages attenuates joint tissue damage in a
mouse model of inflammatory arthritis. J Bone Miner Res.
32:1469–1480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang F, Zhao JL, Wang L, Gao CC, Liang
SQ, An DJ, Bai J, Chen Y, Han H and Qin HY: miR-148a-3p mediates
Notch signaling to promote the differentiation and M1 activation of
macrophages. Front Immunol. 8:13272017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wongchana W and Palaga T: Direct
regulation of interleukin-6 expression by Notch signaling in
macrophages. Cell Mol Immunol. 9:155–162. 2012. View Article : Google Scholar
|
|
42
|
Zhang H, Hilton MJ, Anolik JH, Welle SL,
Zhao C, Yao Z, Li X, Wang Z, Boyce BF and Xing L: NOTCH inhibits
osteoblast formation in inflammatory arthritis via noncanonical
NF-κB. J Clin Invest. 124:3200–3214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ortiz-Masia D, Cosin-Roger J, Calatayud S,
Hernández C, Alós R, Hinojosa J, Esplugues JV and Barrachina MD: M1
macrophages activate Notch signalling in epithelial cells:
Relevance in crohn's disease. J Crohns Colitis. 10:582–592. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao JL, Huang F, He F, Gao CC, Liang SQ,
Ma PF, Dong GY, Han H and Qin HY: Forced activation of Notch in
macrophages represses tumor growth by upregulating miR-125a and
disabling tumor-associated macrophages. Cancer Res. 76:1403–1415.
2016. View Article : Google Scholar : PubMed/NCBI
|