Introduction
Aging is characterized by a gradual loss of the
body's structural integrity and physiological functions. Unlike
other organs, the human skin is subjected to both intrinsic and
extrinsic aging processes (1).
Extrinsic aging can be caused by ultraviolet (UV) radiation,
stress, or smoking and is characterized by wrinkles, slackness,
dryness, and a rough skin tone. Exposure to UV radiation (UV-A,
UV-B, and UV-C) is a major cause of skin aging (2). UV-B penetrates the epidermis and the
upper part of the dermis, damaging keratinocytes in particular and
causing sunburn, photoaging, and skin cancer (3). Exposure of the skin to UV-B
radiation increases the production of inflammatory cytokines and
matrix metalloproteinases (MMPs) in keratinocytes through the
action of activator protein-1 (AP-1) and nuclear factor-κB (NF-κB)
(4). The inflammatory cytokines
produced in response to UV-B irradiation stimulate fibroblasts to
increase the production of MMPs that degrade the extracellular
matrix (ECM) (5).
UV-B radiation also promotes the production of
reactive oxygen species (ROS) in cells. ROS induce a number of
deleterious effects, such as DNA damage, inflammatory responses,
and damage to the integrity of the ECM (6). ROS production therefore also plays
an important role in skin aging. UV-B-induced ROS production
regulates signaling through growth factor and cytokine receptors,
many of which lead to the activation of MAPK pathways namely the
c-Jun N terminal kinase (JNK), extracellular signal-regulated
kinase (ERK), and p38 kinase pathways (7). AP-1, a heterodimer composed of c-Jun
and c-Fos, is regulated by c-Jun phosphorylation through the JNK
pathway as well as through the expression levels of c-Fos. The
resulting increased AP-1 activity increases the production of
MMP-1, leading to a decrease in type I procollagen levels and
resulting in the breakdown of the ECM of the dermis, which leads to
skin aging (8).
The field of cosmetics has been trying to prevent
skin aging by studying the mechanisms that regulate the production
of type I collagen and/or regulate MMP-1 expression levels.
Cosmeceuticals, a compound word for 'cosmetics' and 'medicines'
that are able to medically improve the skin's condition, are
gaining more and more attention in the cosmetics industry (9). Using medically proven ingredients
such as antioxidants [e.g. vitamin C and E (10-12), coenzyme Q10 (13), and phenolic acid derivatives
(14)] cosmetics can delay the
skin aging caused by physiological environmental factors such as
exposure to UV radiation. More recently, the use of human-derived
stem cell cultures to regenerate the skin has been proposed
(15). The concept is that stem
cells can be induced to differentiate into skin cells that can then
be used to treat patients with skin damage or to improve the damage
caused by skin aging (16). Other
approaches including the use of growth factors, natural products,
and biologically active peptides have been proposed as treatments
to prevent and/or reverse the effects of skin aging (17).
Natural killer cells (NK cells) account for 10-15%
of human peripheral blood lymphocytes (18). NK cells are functionally defined
by their ability to destroy target cells without restriction by
major histocompatibility antigens. The cytokines secreted by NK
cells mediate the eradication of pathogens and infected cells and
regulate the adaptation of the immune response, providing a means
for the dynamic interaction between innate immunity and adaptive
immunity (19). The cytokines
secreted by NK cells include IL-1β, IL-6, IL-10, IL-12, TNF-α,
interferon (IFN)-γ inducible factor, also referred to as
transforming growth factor β (TGF-β), IL-15, and IL-18. NK cells
can also produce cytokines with antiviral functions such as IFN-γ
and TNF (20).
In this study, the anti-wrinkle effects of natural
killer cell conditioned medium (NK-CdM) on elastin synthesis,
collagen synthesis, and the abundance of MMP-1 and TIMP-1
transcripts and proteins were evaluated using neonatal human dermal
fibroblasts (NHDFs). The present study demonstrated that NK-CdM may
be a possible candidate anti-skin aging agent.
Materials and methods
Ethics statement
All study samples were obtained after the
acquisition of written informed consent from the study
participants, in accordance with the Declaration of Helsinki. The
research protocol was reviewed and approved by the Institutional
Review Board of Seoul National University Hospital (permit no.
H-1811-023-985).
NK cell enrichment and expansion
Peripheral blood mononuclear cells were collected
from healthy donors (n=3; 2 males and 1 female; average age, 36.6)
via lymphapheresis for 2-4 batch productions in February 2019 from
Seoul National University Hospital (Seoul, Korea). NK cells present
in the mononuclear cells were enriched and expanded with Cellgro
SCGM medium (CellGenix) containing human plasma and interleukin-2
for approximately three weeks as previously described (21,22). When the NK cell cultivation was
completed, the NK-CdM was harvested by centrifugation at 400 × g
for 3 min at 4˚C to remove the NK cells. NK cell concentration at
the end of the cultivation process was
~0.5×106-2.0×106 cells/ml. The NK-CdM
collection and production were performed at GC LabCell.
Neonatal human dermal fibroblast
culture
The neonatal human dermal fibroblast cell line
(NHDF; C0045C) was purchased from Thermo Fischer Scientific, Inc.
The cells were grown in a humidified incubator at 37°C with a 5%
CO2 atmosphere in Medium 106 (Thermo Fisher Scientific,
Inc.) supplemented with 10% (v/v) fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) and 0.5% (v/v) penicillin-streptomycin and
used up to passage seven. When they were 80% confluent, the cells
were sub-cultured by trypsinization. The culture medium was
replenished every 48 h.
CCK-8 assay
NHDFs were plated at a density of 5×103
cells/well in 96-well plates, and their proliferation was measured
using a Cell Counting Kit-8 (CCK)-8 assay (Dojindo Molecular
Technologies, Inc.). Cells were treated, serum-starved for 24 h,
and then treated with various concentrations of NK-CdM (2.5, 5, and
10%) for 24 and 72 h. CCK-8 solution (10 μl) was added to
the cells in 1 ml DMEM (Thermo Fisher Scientific, Inc.), and
incubated for 2 h at 37°C. Absorbance was measured at 450 nm using
a microplate reader (SpectraMax 340; Molecular Devices, Inc.).
Quantitative determination of type I
collagen secretion
Type I collagen secretion was assessed using a
pro-collagen type 1 C-peptide (PIP) enzyme immunoassay (MK101;
Takara Bio Inc.). Briefly, NHDFs were plated into microtiter plates
(96 wells) at a density of 1×105 cells/well,
serum-starved for 24 h, and incubated with NK-CdM at concentrations
of 2.5, 5, and 10% for 48 h. TGF-β (10 nM) was employed as a
positive control. After the incubation period, 100 μl an
antibody-peroxidase conjugate solution was added to each well,
followed by the addition of 20 μl diluents or standard
solution. After incubation for 3 h at 37°C, the well contents were
removed by aspiration, and all the wells were washed four times
with 400 μl PBS. Following this, 100 μl substrate
solution was added to each well and the plates were incubated at
room temperature for 15 min. The reaction was stopped by the
addition of 100 μl stop solution. Absorbance was measured at
450 nm using an ELISA reader (SpectraMax 340; Molecular Devices,
LLC).
MMP-1 inhibition assay
MMP-1 activity was determined using an MMP-1
immunoassay kit (DY901; R&D Systems, Inc.). NHDFs were seeded
in microtiter plates (96 wells) at a density of 1×105
cells per well. The cells were pretreated, serum starved for 24 h,
and then treated with NK-CdM prior to UV-B irradiation (30
mJ/cm2) using a Biospectra (Vilber Lourmat) and
harvested after 48 h. Before UV-B irradiation, the cultures were
rinsed with PBS and irradiated at the indicated intensity in PBS to
avoid absorption by the phenol-red present in the culture medium.
All cell treatment groups were similarly cultured in PBS at room
temperature during the experimental procedure to ensure equal
treatment conditions. The activity of MMP-1 in the cell supernatant
was determined using the method described in the MMP-1 assay
kit.
Quantification of elastin
Supernatants from NHDF cultures were collected,
serum starved for 24 h, and then treated with NK-CdM for 48 h.
Supernatants were analyzed using the Fastin Elastin Assay kit
(Biocolor Ltd.) as recommended by the manufacturer. The Fastin
Elastin Assay uses a dye reagent that binds to the 'basic' and
'non-polar' amino acid sequences found in mammalian elastins. The
recovered dye-bound elastins from each sample and the standards
were detected by reading the absorption at 513 nm. All measurements
were performed in triplicate. The measured amount of each elastin
protein was normalized to the corresponding cell number.
Antibody array
Growth factor levels in the NK-CdM were assessed
using an antibody array kit (C1; RayBiotech) according to the
manufacturer's protocol. Unconditioned NK cell medium was used as
the negative control. The membranes were blocked by incubation with
the blocking buffer at room temperature for 30 min and incubated
with the sample at room temperature for 3 h. Membranes were then
washed three times with Wash Buffer I and two times with Wash
Buffer II at room temperature for 5 min per wash and incubated with
biotin-conjugated antibodies at room temperature for 2 h. Finally,
the membranes were washed, incubated with HRP conjugated
streptavidin at room temperature for 2 h and with detection buffer
for 1 min, and exposed to X-ray film for 40 sec (Kodak). The
exposed films were digitized and the relative growth factor levels
were compared after densitometry analysis (Scion NIH Image 1.63).
The relative protein levels were obtained by subtracting the
background staining and normalizing to the positive controls on the
same membrane.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from the dorsal skin tissues
and isolated using the Trizol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol cDNA synthesis was performed according to the protocol
provided by the Takara PrimeScript™ RT Master Mix (Takara Bio,
Inc.). The temperature protocol for RT was as follows: 37°C for 15
min, followed by 85°C for 5 sec. For quantitative PCR, TB
Green® Premix Ex Taq™ II (Takara Bio, Inc.) and CFX96TM
Real-Time System (Bio-Rad Laboratories, Inc.) were used, and all
reactions were repeated three times under the following conditions:
Initial denaturation at 95°C for 30 sec, followed by 40 cycles at
95°C for 5 sec and 60°C for 30 sec. Relative gene expression values
were determined using the 2−ΔΔCq method (23). The primers used for q-PCR are
listed in Table I.
 | Table IPrimer sequence information used in
reverse transcription-quantitative PCR. |
Table I
Primer sequence information used in
reverse transcription-quantitative PCR.
| Gene | Sequences |
|---|
| Type 1
collagen | |
| Forward |
5′-AGACTGGCAACCTCAAGAAG-3′ |
| Reverse |
5′-TTCGGTTGGTCAAAGATAAA-3′ |
| Type 3
collagen | |
| Forward |
5′-ATGGTTGCACGAAACACACT-3′ |
| Reverse |
5′-CTTGATCAGGACCACCAATG-3′ |
| MMP-1 | |
| Forward |
5′-CTGGCCACAACTGCCAAAT-3′ |
| Reverse |
5′-CTGTCCCTGAACAGCCCAGTACTTA-3′ |
| MMP-2 | |
| Forward |
5′-TCTCCTGACATTGACCTTGGC-3′ |
| Reverse |
5′-CAAGGTGCTGGCTGAGTAGATC-3′ |
| MMP-3 | |
| Forward |
5′-ACACCGGATCTGCCAAGAGA-3′ |
| Reverse |
5′-CTGGAGAACGTGAGTGGAGTCA-3′ |
| MMP-9 | |
| Forward |
5′-TTGACAGCGACAAGAAGTGG-3′ |
| Reverse |
5′-GCCATTCACGTCGTCCTTAT-3′ |
| TIMP-1 | |
| Forward |
5′-TCTGCAATTCCGACCTCGTCATCA-3′ |
| Reverse |
5′-AAGGTGGTCTGGTTGACTTCTGGT-3′ |
| Tropoelastin | |
| Forward |
5′-CGGAATTCACCTCTTAAGCCAGTTCCCG-3′ |
| Reverse |
5′-CCCAAGCTTCGGGAACACCTCCGACACTA-3′ |
| GAPDH | |
| Forward |
5′-AGGGCTGCTTTTAACTCTGGT-3′ |
| Reverse |
5′-CCCCACTTGATTTTGGAGGGA-3′ |
Western blot analysis
The harvested tissues were homogenized in Pro-prep
solution (iNtRON Biotechnology) and lysates were centrifuged at
12,000 × g for 30 min at 4°C. Total protein (40 μg) was
separated by electrophoresis on 6-8% SDS-polyacrylamide gels and
transferred to polyvinylidene fluoride membranes (EMD Millipore).
After transfer, the membranes were blocked with 5% fat-free
milk-TBST buffer for 1 h at room temperature and washed with TBS
containing 0.05% Tween-20 (TBS-T). The membranes were incubated
overnight at 4°C with a 1:1,000 dilution of primary rabbit
monoclonal antibodies against proCOL1A1 (Santa Cruz Biotechnology,
Inc.; cat. no. sc-30136), collagen type I (Novus Biologicals, LLC;
cat. no. NB600-408), MMP-3 (Abcam; cat. no. ab52915), TIMP-1 [Cell
Signaling Technology, Inc. (CST); cat. no. 8946], phospho-p38 (CST;
cat. no. 4511), phospho-c-Fos (CST; cat. no. 5348), c-Fos (CST;
cat. no. 2250), primary rabbit polyclonal antibodies against
collagen type III (Abcam; cat. no. ab7778), α-elastin (Abcam; cat.
no. ab21607), MMP-1 (Abcam, cat. no. ab137332), MMP-2 (CST; cat.
no. 4022), MMP-9 (Abcam; cat. no. ab38898), p38 (CST; cat. no.
9212), phospho-ERK (CST; cat. no. 9101), ERK (CST; cat. no. 9102),
phospho-JNK (CST; cat. no. 9251), JNK (CST; cat. no. 9252),
phospho-c-Jun (CST; cat. no. 9164), c-Jun (CST; cat. no. 9165), and
mouse monoclonal antibodies against β-actin (Santa Cruz
Biotechnology, Inc.; cat. no. sc-47778). For pathway determination,
the following inhibitors were added and incubated for 30 min at
37°C: 20 μM of PD98059 (ERK inhibitor; cat. no. 167869-21-8;
EMD Millipore), 20 μM of SP600125 (JNK inhibitor; cat. no.
129-56-6; EMD Millipore), 15 μM of SB203580 (p38 inhibitor;
cat. no. 152121-47-6; EMD Millipore), 20 μM of LY294002
(PI3K inhibitor, cat. no. 942289-87-4; EMD Millipore). Samples were
then treated with 1.25% NK-CdM. After washing the blots three times
with TBST, bound primary antibodies were detected by the addition
of HRP-conjugated anti-mouse (1:1,000; cat. no. P0447; Dako;
Agilent Technologies, Inc.) or anti-rabbit secondary antibodies
(1:1,000; cat. no. P0448; Dako; Agilent Technologies, Inc.) for 1 h
at room temperature. The transferred proteins were visualized with
a Pierce ECL western blotting substrate (Thermo Fisher Scientific,
Inc.) and quantified by scanning densitometry using Image-Pro Plus
6.0 (Media Cybernetics, Inc.).
Total antioxidant capacity assay
An antioxidant assay (709001; Cayman Chemical
Company) based on the oxidation of
2,2′-azino-di-(3-ethylbenzthiazoline sulfonate), was used to
measure the total antioxidant capacity according to the
manufacturer's protocol. Values were compared to a standard curve
of known concentrations of Trolox (a tocopherol/vitamin E
antioxidant analogue).
Immunocytochemistry
NHDFs were seeded into a chamber slide, serum
starved for 24 h, and then treated with NK-CdM for 48 h. Following
treatment with 4% paraformaldehyde for 10 min at 4°C and 0.1%
Triton X-100 for 5 min, the cultured NHDFs were incubated with an
anti-type I collagen antibody (1:500; cat. no. ab34710; Abcam) at
4°C overnight and then with fluorescein isothiocyanate-labeled goat
anti-rabbit IgG (1:1,000; cat. no. NB7182; Novus Biologicals, LLC).
A 4′6-diamidino-2-phenylindole mounting medium kit (OriGene
Technologies, Inc.) was used to counterstain the nuclei for 10 min
at room temperature, and stained cells were visualized using an
Olympus FLUOVIEW FV10i confocal microscope (Olympus Optical Co.,
Ltd.).
3D reconstructed human full skin model
(Keraskin-FT™) and UV-B irradiation
A Keraskin-FT™ and Keraskin-FT™ culture media were
purchased from Biosolution Co., Ltd. After shipment, the tissues
were transferred into 6-well plates filled with 0.9 ml culture
media per well, and pre-incubated at 37°C in 5% CO2 for
24 h. The tissues were irradiated with UV-B (125 mJ/cm2)
and then test materials were applied to the surface of the insert
with NK-CdM (50 and 100%) diluted in PBS containing 0.1% DMSO for
48 h. The culture medium and tissues were recovered for analysis
using ELISA kits for Pro-collagen type 1 C-peptide (PIP) enzyme
immunoassay (cat. no. MK101; Takara Bio, Inc.) and MMP-1
immunoassay kits (cat. no. DY901; R&D Systems, Inc.) and
picrosirius red staining, respectively.
Picrosirius red staining
The KeraSkin™-FT model (Biosolution) was fixed with
4% formaldehyde for 10 min at 4°C, embedded with paraffin, and
prepared as 4 μm sections using a microtome (Leica
Microsystems, Inc.). Paraffin sections were de-paraffinized in
xylene, hydrated through a decreasing ethanol concentration, and
fixed with Bouin's solution for 1 h at room temperature. The
sections were counterstained with 0.1% Fast Green in distilled
water for 10 min at room temperature, and collagen was stained
using 0.1% Sirius red in saturated aqueous picric acid for 30 min
at room temperature. After dehydration and mounting, the tissues
were examined under a microscope (Olympus Corporation). Each
pathologist assigned each section a score according to the
following scale: 0=negative control, +=moderately increased
staining, ++=considerably increased staining), based on the
percentage of stained cells in each category.
Statistical analyses
Statistical analyses of data were performed using
the Student's t-test or multivariate analysis of variance by post
hoc Tukey method for direct comparison. Data were analyzed using
SPSS software version 12.0 (SPSS, Inc.). The results are expressed
as the mean ± standard deviation of at least three independent
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
Detection of active biomolecules in the
conditioned medium derived from NK cells
The growth factors present in NK-CdM were first
profiled using a human growth factor antibody array capable of
detecting 41 growth factors. A map of the array is shown in
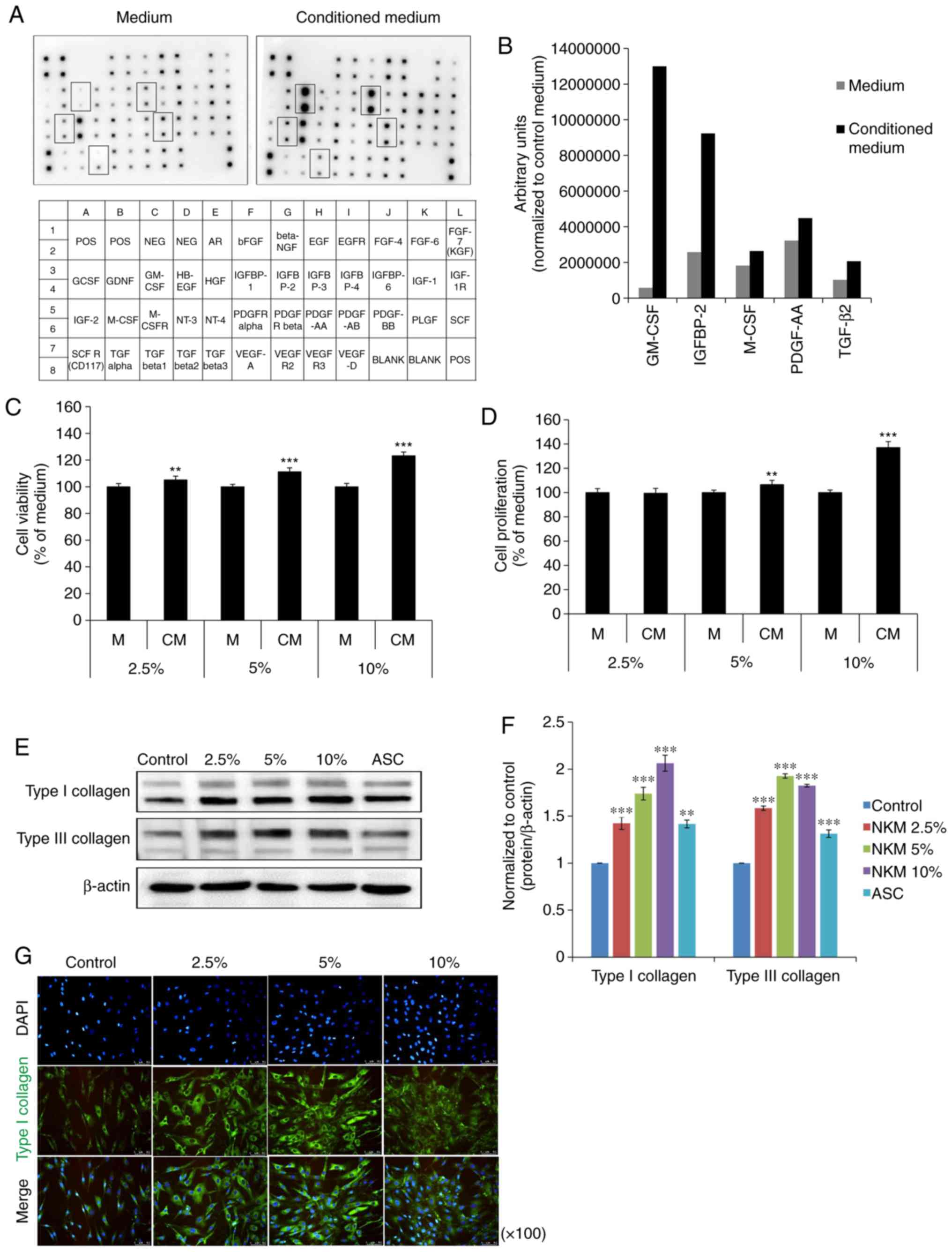
Fig. 1A. Compared with the NK
culture medium, NK-CdM showed a strong increase in the levels of
five individual growth factors. These were granulocyte-macrophage
colony-stimulating factor (GM-CSF), insulin-like growth factor
binding protein 2 (IGFBP), macrophage colony-stimulating factor
(M-CSF), platelet-derived growth factor-AA (PDGF-AA), and TGF-β2
(Fig. 1B).
Effect of NK-CdM on proliferation and
collagen synthesis in NHDFs
Next, the effects of NK-CdM on NHDF proliferation
and their ability to synthesize collagen were evaluated. Treatment
of NHDFs with NK-CdM (2.5, 5 and 10%) for up to 24 h did not induce
cell toxicity (Fig. 1C). At 72 h,
the cell viability increased in a concentration-dependent manner.
The ability of NK-CdM to increase NHDF proliferation was measured
at 24 and 72 h using a CCK-8 assay. At 24 h, NK-CdM caused a
dose-dependent increase in NHDF proliferation, with a maximal
increase of 1.37-fold observed at 10% NK-CdM (Fig. 1D). The expression levels of
proteins related to collagen synthesis were also analyzed by
western blotting. NHDFs were treated with NK-CdM (2.5, 5 and 10%)
for 48 h, using ascorbic acid (200 μM) as the positive
control. The expression of type I collagen and type III collagen
was increased in a dose-dependent manner in NK-CdM treated cells
(Fig. 1E and F). Fig. 1G shows that both intracellular
collagen production and extracellular collagen secretion levels
were increased after 48 h of treatment with NK-CdM. Therefore,
NK-CdM increases both type I collagen production and proliferation
in NHDFs.
NK-CdM inhibits the collagen degradation
induced by UV-B irradiation of NDHFs
Next, the effect of NK-CdM treatment (2.5, 5 and
10%) on proliferation in UV-B treated NK-CdM cells was evaluated.
As a result, the cytotoxicity induced by UV-B exposure was reduced
over time (Fig. 2A). Next, to
investigate the effect of NK-CdM on collagen synthesis and/or
secretion by NHDF cells, the amount of PIP was measured in the
culture supernatants of NHDF cells treated with NK-CdM after UV-B
irradiation. As shown in Fig. 2B,
NHDF cells treated with 2.5% NK-CdM effectively produced PIP
compared with the TGF-β1 treated group. In addition, the effect of
NK-CdM on UV-B-induced MMP-1 secretion was also determined. The PIP
levels in the culture supernatants accumulated significantly within
48 h after the addition of 5% NK-CdM (P<0.05), with the MMP-1
levels decreased by 2.5% NK-CdM (P<0.05). Moreover, treatment
with NK-CdM significantly stimulated elastin synthesis in a
dose-dependent manner, whereas the other group did not (P<0.05;
Fig. 2D). However, measuring the
protein expression level of elastin is not sufficient to examine
the level of elastin deposition in the ECM. Changes in
intracellular type collagen I expression levels were detected by
immunofluorescent staining. After 48 h of NK-CdM treatment (2.5, 5
and 10%), NHDF cells showed a markedly increased level of type I
collagen compared to that in untreated cells, and NK-CdM was also
effective in increasing the recovery of the expression of type I
collagen after UV-B irradiation (Fig.
2E). However, quantitative determination of type I collagen
secretion does not indicate deposition of collagen. These results
demonstrate that NK-CdM treatment increases both total collagen and
type I procollagen secretion, as well as total elastin
synthesis.
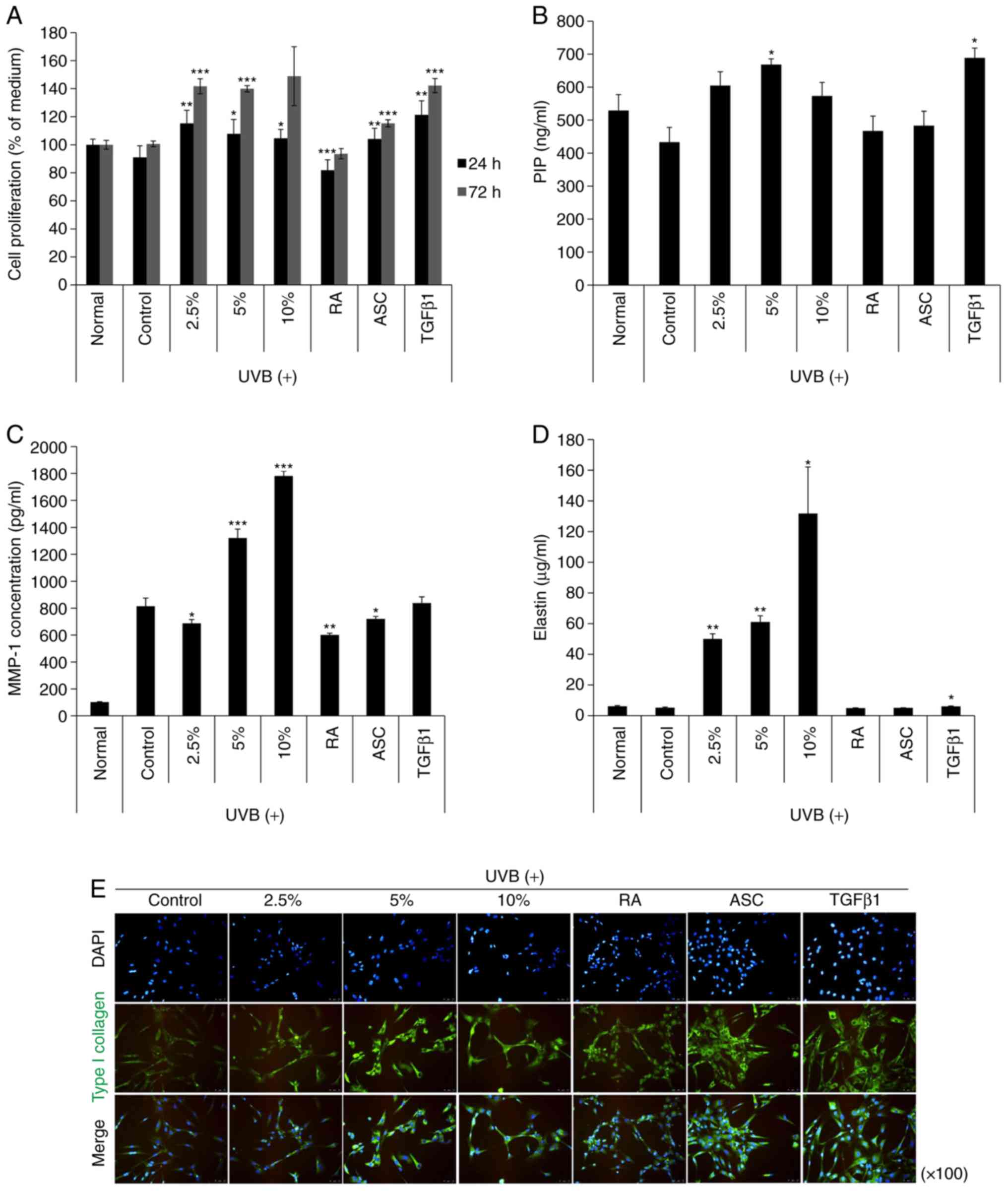 | Figure 2The effects of NK-CdM on UV-B-induced
MMP-1 and type I procollagen secretion in NHDFs. NHDFs were
irradiated with 30 mJ/cm2 UV-B, followed by treatment
with the indicated concentrations of NK-CdM (2.5, 5 and 10%), RA
(0.03 μg/ml), ASC (200 μM), and TGF-β1 (10 ng/ml).
(A) Time course of NHDF proliferation up to 24 and 72 h. (B) PIP
secretion, (C) MMP-1 levels, and (D) Elastin levels in harvesting
culture media at 48 h. MMP-1, PIP, and elastin levels were measured
using a commercially available ELISA kit, as described in the
Methods section. The data are the mean ± standard deviation values
of three individual experiments. (E) Immunocytochemical analysis
showing an inhibition of type 1 collagen induction in
NK-CdM-treated NHDF cells. Magnification, ×100. *P<0.05,
**P<0.01 and ***P<0.001 vs. Control
(UV-B only group). MMP, matrix metalloproteinase; PIP, pro-collagen
type 1 C-peptide; NK-CdM, natural killer cell conditioned medium;
NHDF, neonatal human dermal fibroblasts; UV, ultraviolet; RA,
retinoic acid; ASC, ascorbic acid; TGF, transforming growth
factor. |
NK-CdM inhibits UV-B-induced collagen
degradation via inactivation of ROS/JNK signaling in NHDFs
The effect of UV-B treatment on the expression of
collagen and MMP family members in NHDFs was examined by both
western blot analysis and RT-qPCR. The western blot analysis
indicated that UV-B irradiation of NHDF cells increased the levels
of MMPs and decreased collagen expression. NK-CdM treatment
(1.25-5%) completely blocked the upregulation of MMPs and promoted
collagen synthesis, essentially reversing the effects of UV-B
irradiation (Fig. 3A-D). RT-qPCR
analysis revealed that UV-B-irradiation decreased the levels of
mRNAs encoding type I collagen and type III collagen in NHDFs and
that treatment with NK-CdM enhanced the levels of these collagens
in a time- and dose-dependent manner (Fig. 3E and F). In addition, the mRNA
expression levels of MMP-1, MMP-3, MMP-9, TIMP-1 were markedly
increased in UV-B-exposed NHDF cells, but these increases were
inhibited by treatment with 1.25% NK-CdM for 48 h. However, these
increases were not dose-dependently inhibited (Fig. 3E).
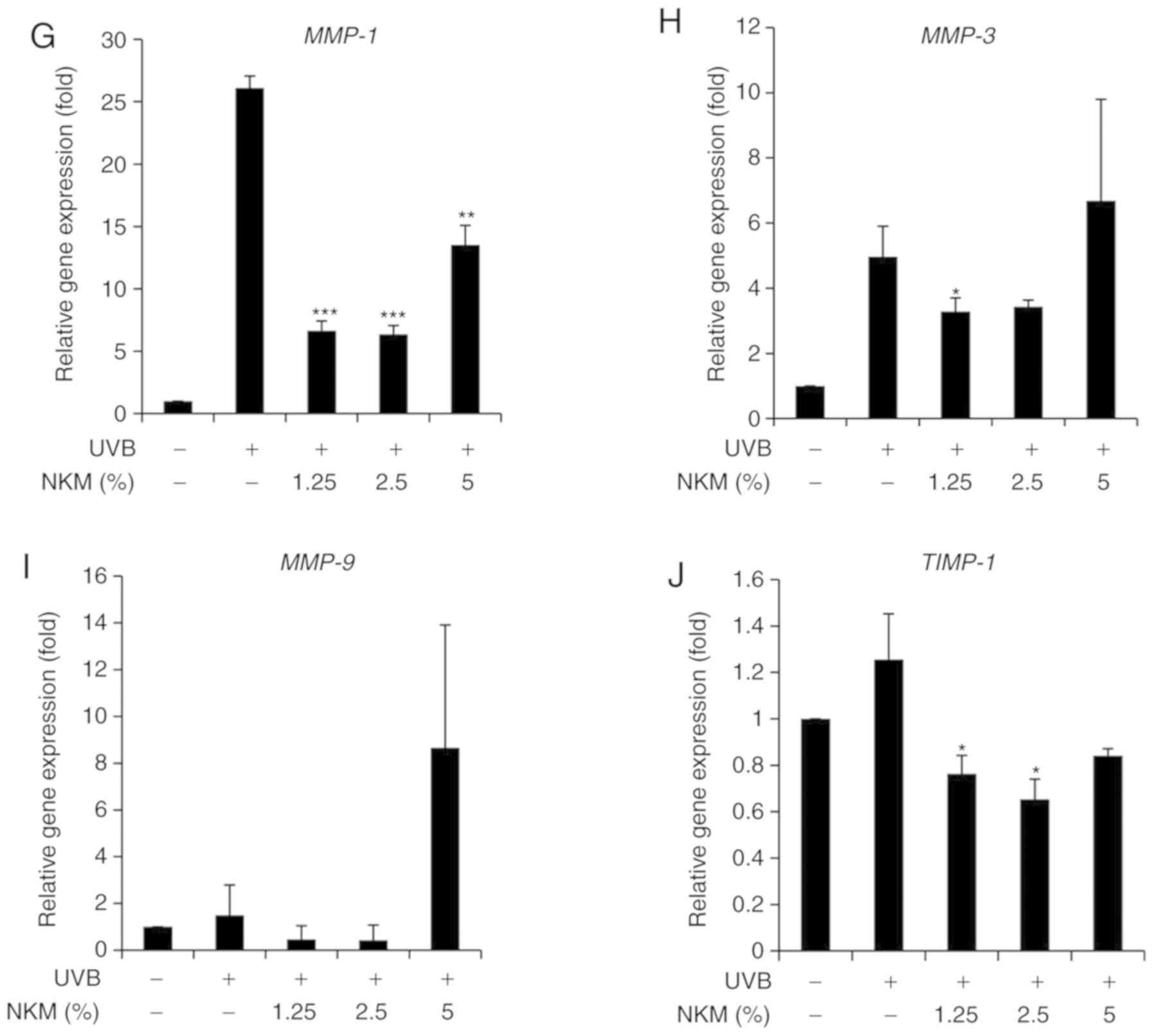 | Figure 3Modulation of the expression of
MMPs/collagen in UV-B-irradiated NHDFs by NK-CdM. After being
serum-starved for 24 h, NHDFs were irradiated with UV-B (30
mJ/cm2), and further incubated with NK-CdM at the
indicated concentrations for 48 h. (A) The expression levels of
procollagen I, type I collagen, type III collagen, α-elastin and
(B) a quantitative densitometric analysis of the upper bands. (C)
MMP-1, -2, -3, -9, and TIMP-1 were evaluated by western blotting.
(D) The bar graph (mean ± standard deviation of the mean; n=3)
represent a quantitative densitometric analysis of the upper bands.
Changes in the mRNA expression of (E) type I collagen and (F) type
III collagen were determined using quantitative PCR at 0, 6, 12, 24
and 48 h following culture in NK-CdM after UV-B exposure. The
expression of photoaging-related genes such as (G) MMP-1, (H)
MMP-3, (I) MMP-9, and (J) TIMP-1 was evaluated by quantitative PCR.
Equal protein and mRNA loading were verified by normalization with
β-actin and GAPDH, respectively. *P<0.05,
**P<0.01 and ***P<0.001 vs. with the
UV-B-irradiated control. #P<0.05,
##P<0.01 and ###P<0.001 vs. the Normal
(non-irradiated control). MMP, matrix metalloproteinase; NK-CdM,
natural killer cell conditioned medium; NHDF, neonatal human dermal
fibroblasts; UV, ultraviolet; TGF, transforming growth factor;
TIMP, tissue inhibitor of matrix metalloproteinases. |
To further understand the molecular mechanisms
underlying these effects of NK-CdM in UV-B-exposed NHDF cells, the
effect of NK-CdM on c-Jun phosphorylation levels and c-Fos
expression levels was examined. Since AP-1 is activated by MAPK
signaling, the effects of NK-CdM on the different MAPK signaling
pathways were studied further in UV-B-irradiated NHDFs. As shown in
Fig. 4, UV-B radiation elevated
the phosphorylation of the different MAPK molecules namely, ERK,
JNK, and p38. Interestingly, NK-CdM treatment was found to
partially inhibit the phosphorylation of ERK, JNK, and p38 induced
by UV-B exposure, respectively. It was confirmed that 1.25% NK-CdM
recovered UV-aB-induced decrease in collagen. Based on these
results, inhibition experiments were performed using 1.25% NK-CdM;
it was not necessary to conduct experiments with increasing
concentrations of inhibitors.
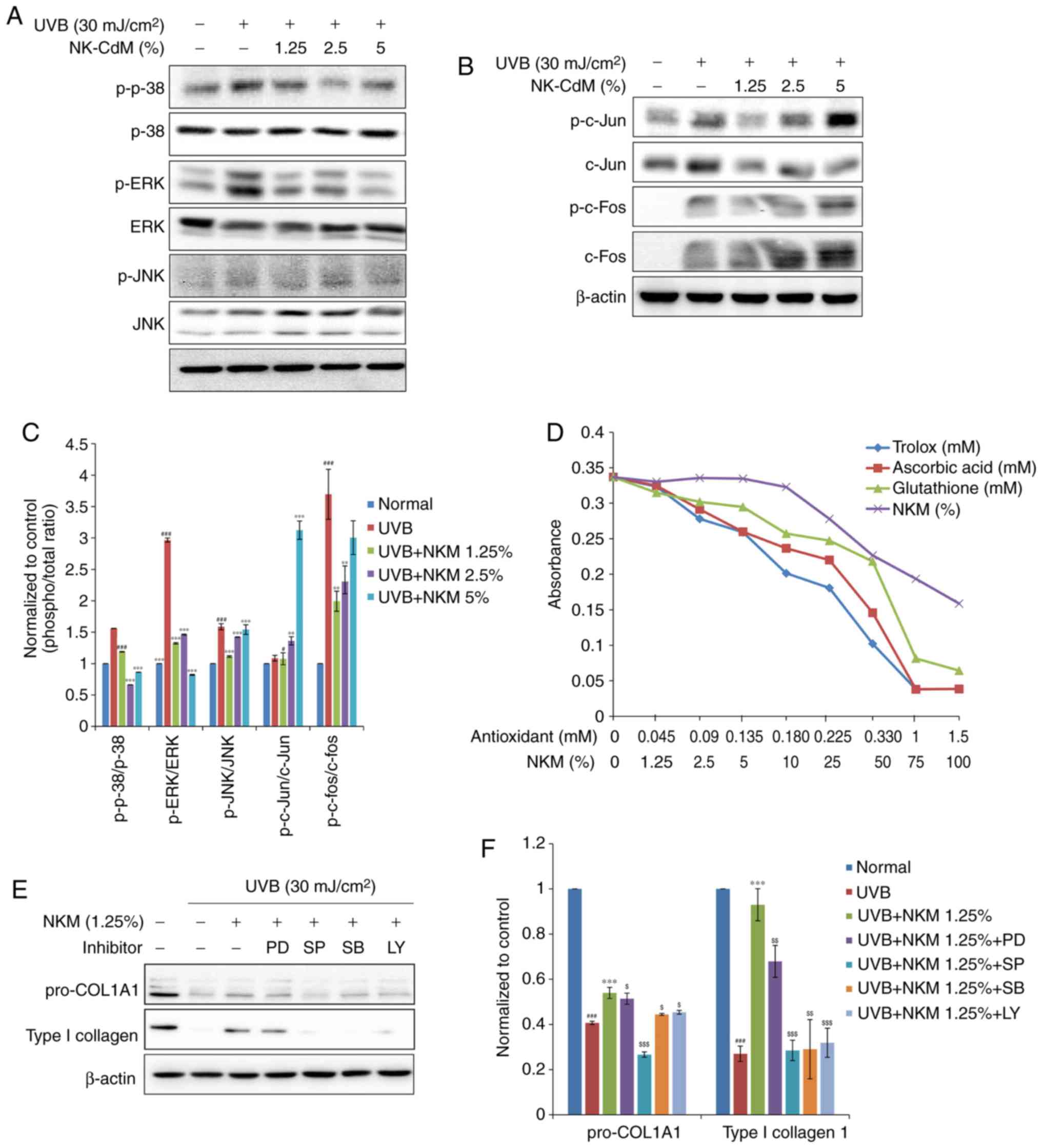 | Figure 4Inhibitory effect of the
phosphorylation of the JNK, p38 MAPK, and ERK1/2, and the
transcription factors c-Jun and c-Fos, in UV-B-exposed and
NK-CdM-treated NHDFs. After being serum-starved for 24 h, NHDFs
were irradiated with UV-B (30 mJ/cm2), and further
incubated with NK-CdM at the indicated concentrations for 48 h.
Total cell protein extracts were prepared and separated by
electrophoresis on SDS-PAGE gels followed by a western blot
analysis with primary antibodies capable of detecting (A)
phospho-JNK, -p38 MAPK, and -ERK1/2 and (B) phospho-c-Jun, -c-Fos.
β-actin was used as the loading control. (C) Equal protein loading
was verified by analyzing β-actin levels. (D) The antioxidant
activity of NK-CdM, at the indicated concentration, was measured
using an Antioxidant Assay kit. (E) NHDFs were treated with 10
μM of the ERK1/2 inhibitor PD, 10 μM of the JNK
inhibitor SP, 10 μM of the p38 MAPK inhibitor SB, or 10
μM of the PI3K inhibitor LY and then irradiated. The levels
of procollagen I and type I collagen at 48 h after irradiation were
measured by western blot analysis. (F) The bar graphs (mean ±
standard error of the mean; n=3) represent a quantitative
densitometric analysis of the bands. #P<0.05 and
###P<0.001 vs. the Normal (non-irradiated control).
**P<0.01 and ***P<0.001 vs. the
UV-B-irradiated control. $P<0.05,
$$P<0.01 and $$$P<0.001 vs. the UV-B
irradiated and 1.25% NK-CdM treated group. MMP, matrix
metalloproteinase; ERK, extracellular signal regulated kinase;
NK-CdM, natural killer cell conditioned medium; NHDF, neonatal
human dermal fibroblasts; UV, ultraviolet; MAPK, mitogen associated
protein kinase; PI3K, phosphatidylinositol 3 kinase; SP, SP600125;
LY, LY294002; SB, SB203580; PD, PD98059; phosphor, phosphorylated;
JNK, c-Jun N terminal kinase. |
As shown in Fig.
4, UV-B irradiation increased c-Jun phosphorylation levels as
well as c-Fos expression levels in NHDFs. The upregulation of c-Jun
phosphorylation observed after UV-B exposure was not
dose-dependently reduced by NK-CdM treatment (Fig. 4B).
The antioxidant activity of NK-CdM was also directly
measured using an antioxidant assay kit. The antioxidative capacity
of NK-CdM, expressed relative to the antioxidative capacity of
Trolox, increased in a dose-dependent manner (Fig. 4D). Interestingly, NK-CdM (>50%)
had a similar anti-oxidative effect to that of glutathione (0.33
mM).
Next, PD98059 (an ERK inhibitor), SP600125 (a JNK
inhibitor), SB203580 (a p38 inhibitor), and LY294002 (a PI3K
inhibitor) were used to further investigate the activation of the
different MAPK pathways. Consistently, SP600125, SB203580, and
LY294002, but not PD98059, partially blocked the NK-CdM-induced
increase in collagen levels (Fig.
4E). Taken together, these results suggest that NK-CdM has an
antioxidative activity, protecting NHDFs by scavenging free
radicals and reducing UV-B-induced MMP-1 and type I collagen
expression by modulating the ROS-mediated JNK signaling
pathway.
Effects of NK-CdM on UV-B-induced
photoaging in a 3D reconstructed human full skin model
To further assess the effects of NK-CdM on collagen
production after exposure to UV-B, type I collagen expression was
examined in the biopsied tissue irradiated with UV-B. As shown in
Fig. 5A and B, the UV-B-induced
increases in MMP-1 expression and secretion were inhibited by
treatment with NK-CdM (50 and 100%) for 48 h, while at the same
time, NK-CdM caused a dramatic increase in collagen-1 levels. To
visually examine the anti-photoaging effects of NK-CdM,
UV-B-treated Keraskin-FT™ sections were stained with a picrosirius
red stain. As a result, NK-CdM treatment increased the diameter of
the collagen fibers (Fig. 5C).
Therefore, the various growth factors present in NK-CdM appear to
cause the proliferation of fibroblasts and the production of ECM,
resulting in the formation of thick mature collagen.
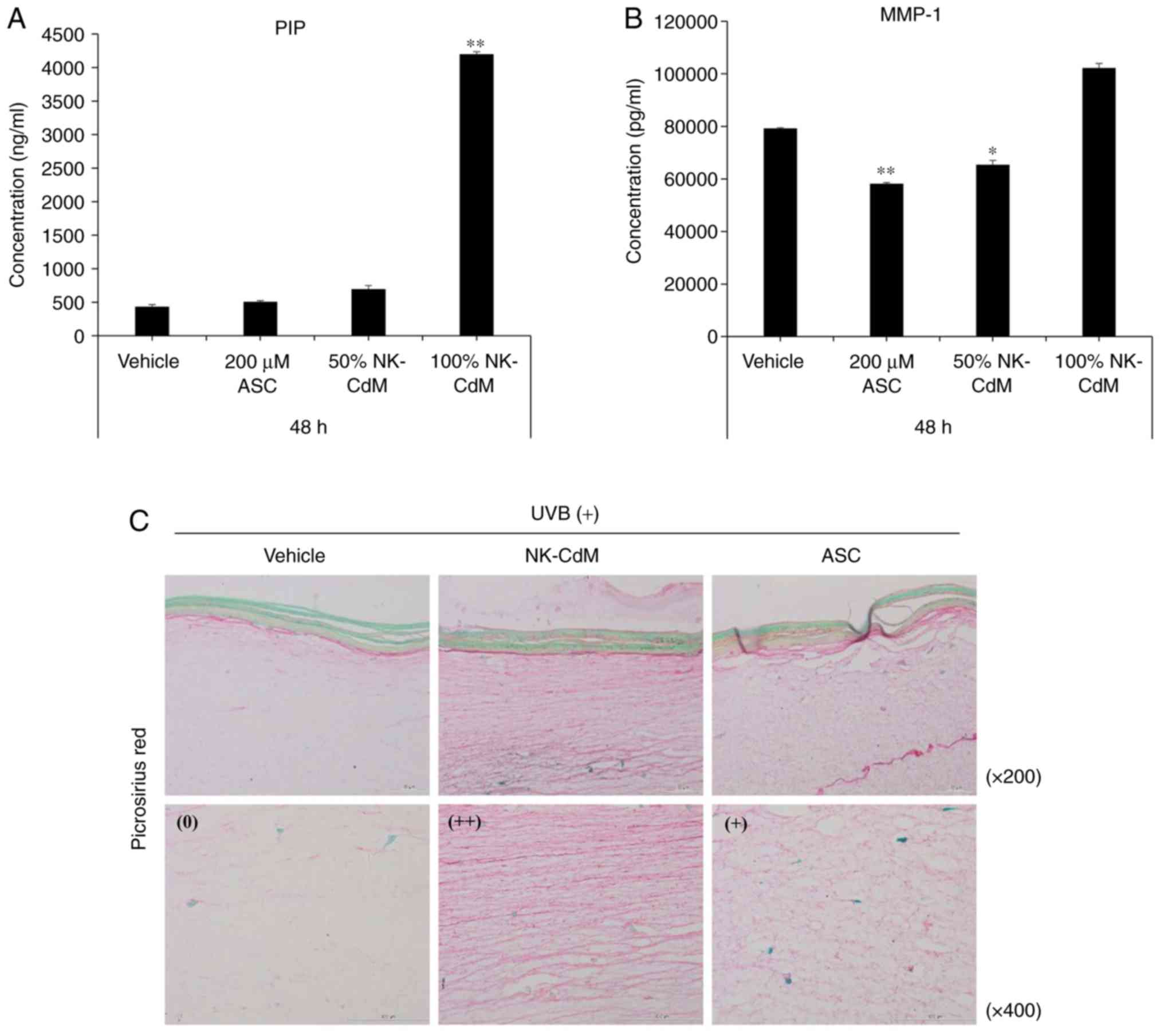 | Figure 5Inhibitory effects of NK-CdM on
UV-B-induced collagen degradation in a 3D reconstructed human full
skin model. Tissues were irradiated with UV-B (125
mJ/cm2), and further incubated with vehicle (PBS
containing 0.1% DMSO) and NK-CdM (50 and 100%) and ascorbic acid
(200 μM) at the indicated concentrations for 48 h. The
levels of (A) type I procollagen and (B) MMP-1 in the culture media
were measured using a commercially available ELISA kit, as
described in the Methods. The data are the mean ± standard
deviation values of three individual experiments.
*P<0.05 and **P<0.01 vs.
UV-B-irradiated vehicle control. (C) To confirm the inhibitory
effects of NK-CdM on UV-B-induced collagen degradation in skin
model, the Keraskin-FT™ was stained with PSR, which stains collagen
red. The visual assessments of collagen degradation represent the
average of the scores in each PSR staining intensity category (++
indicates pronounced findings, + moderate findings, and 0 no/scant
findings) identified for each score. NK-CdM, natural killer cell
conditioned medium; NHDF, neonatal human dermal fibroblasts; UV,
ultraviolet; PSR, picrosirius red; ASC, ascorbic acid; MMP, matrix
metalloproteinase; PIP, pro-collagen type 1 C-peptide. |
Discussion
Conditioned medium (CM) from various sources,
containing numerous growth factors and cytokines, is known to
promote the regeneration of damaged tissue and so might be of use
in regenerating aged skin (24).
Growth factors present in CM could reduce the signs of skin aging
as a result of their ability to stimulate the proliferation of
dermal fibroblasts and keratinocytes and induce the production of
extracellular matrix components, including collagen (17). Therefore, NK-CdM can stimulate
both collagen synthesis and the migration of fibroblasts during the
wound healing process and so is of potential utility in
regenerating aged skin.
The antibody array results were compared between
unconditioned NK cell media and conditioned NK cell media. For the
remaining experiments, diluted NK-CdM was used for treating
fibroblasts. This experiment was aimed to characterize NK-CdM, and
it was confirmed that no specific cytokine and growth factor were
generated in Medium 106, a free medium for fibroblasts. The present
study found that NK-CdM contains factors that are anti-apoptotic
such as IGFBP, GM-CSF, SCF; angiogenic such as VEGF;
anti-inflammatory such as TGFβ; and also factors that promote
proliferation and migration of progenitor cells such as IGF-1,
PDGF, and GM-CSF. Interestingly, TGF-β appears to modulate the
action of growth factors on the expression of MMPs and TIMPs
(25). Thus, TGF-β and PDGF are
important players in maintaining the proper balance between the
tissue modification and repair processes that involve many
cytokines (26). Kim et al
(27) also reported that the
effect PDGF-AA on the production of type I collagen in human
fibroblasts, suggesting its function as a key factor in skin
remodeling and skin aging.
Type I collagen is the most abundant protein found
in skin connective tissue and along with other types of collagen
(III, V and VII), elastin, proteoglycans, fibronectin, and other
ECM proteins, it helps maintain the skin structure (28). Fibrous (type I and III) collagen
is characteristic of chronologically aged or damaged skin. Type I
collagen levels are regulated by the activity of MMPs, a family of
zinc-requiring endoproteases that can degrade all the components of
the ECM. Of particular importance, MMP-1 initiates the degradation
of collagen type I and III, whereas MMP-9 further degrades the
collagen fragments produced by MMP-1 (29). All of the known MMPs are inhibited
by the four homologous TIMP proteins. Of particular importance,
TIMP-2 inhibits ECM proteolysis in a number of tissues by directly
inhibiting the activity of metalloproteinases, including MMP-2.
TIMP-2 is also known to be required for the activation of MMP-2. In
this study, NK-CdM inhibited the expression of MMP-1, as well as
MMP-2 and MMP-9, in NHDFs. Moreover, the expression of TIMP-2 was
generally increased by NK-CdM treatment. Overall, these results
suggest that the increased levels of type I procollagen induced by
NK-CdM in UV-B treated NHDFs may be caused by a decrease in MMP-1,
MMP-2, and MMP-9 expression/activity levels and an increase in
TIMP-2 levels. At the mRNA level, low-levels of the mRNA encoding
MMP-9 were detected, but MMP-9 expression levels were considerably
changed. Treatment with NK-CdM inhibited the mRNA expression of
MMP-9. When high concentration of medium is directly applied to the
cells, various growth factors and cytokines are secreted in excess,
thereby inhibiting cell proliferation. Although it may be used
without dilution, toxicity due to the substance itself may appear
at a high concentration; even if it is not toxic, it may not be
cost effective. Therefore, the experiment was carried out by
diluting NK-CdM, in order to confirm that it is effective at low
concentrations.
When UV-A and UV-B are applied to the skin, UV-A
acts on the dermis while UV-B acts directly on the epidermis. The
penetration of UV-B into the dermis is limited by its wavelength.
Since UV-A penetrates deeper, it seems to be more important.
Although their targets are different, both UV-A and UV-B generate
ROS, and the mechanism of photoaging is the same for both.
Moreover, UV-B has a lower penetration rate into the dermis owing
to its shorter wavelength than UV-A; however, its energy is
stronger than that of UV-A, which is more suitable for visualizing
the photoaging mechanism of ROS.
Excessive production of ROS, induced by UV-B
radiation, may be the cause of UV-B-induced toxicity (30). Therefore, the use of antioxidants
that interfere with ROS production may be preferable for the
prevention of photoaging and skin cancer. ROS also plays an
important role in collagen metabolism (31). ROS not only directly destroys
collagen, but also inactivates TIMPs, while at the same time
inducing the synthesis and activation of matrix-degrading
metalloproteases.
In addition to increasing MMP production and
decreasing collagen levels, UV irradiation also reduces the
expression of TGF-β2 (32). Since
TGF-β2 promotes collagen formation, a decrease in its expression
levels also leads to a reduction in collagen production. As
described earlier, UV light exposure and the subsequent generation
of ROS result in the decreased synthesis of collagens type I and
III and an increase in MMP-1 activity in the dermis. Oxidative
stress induces the phosphorylation of proteins in the different
MAPK signaling pathways, namely, ERK, JNK, and p38, as well as the
activation of the protein kinase B pathway (33). In this study, the UV-B-induced
phosphorylation of ERK, JNK, and p38 was attenuated by NK-CdM
treatment. Since the activation of JNK phosphorylates c-Jun to
increase AP-1 complex transcriptional activity, NK-CdM appears to
inhibit UV-B-induced AP-1 activation by reducing c-Jun
phosphorylation and c-Fos expression, thereby reducing MMP-1
levels.
3D reconstructed human tissue models are widely used
to examine the effects of cosmetic ingredients and their safety in
an in vivo-like condition (34). In the present study, the
anti-aging effects of NK-CdM were also examined using a 3D
reconstructed human skin model. It was observed that similar to the
NHDFs, UV irradiation decreased collagen levels and increased MMP
levels, and this could be prevented by NK-CdM. Importantly, to
confirm that NK-CdM prevents UV-B-induced photoaging, NK-CdM was
treated at different concentrations and the effect was confirmed at
low concentrations. Therefore, the present study concluded that
there is a relationship between the biological effect and the
NK-CdM concentration used to confirm its various effects.
The present study demonstrates that NK-CdM inhibits
UV-B-induced MMP expression. The mechanisms underlying the
anti-photoaging effect of NK-CdM involve its actions on
UV-B-induced ROS generation and inhibition of MAPK activation.
Therefore, the authors suggest that NK-CdM is an effective
therapeutic candidate for preventing skin photoaging.
Acknowledgements
The authors would like to thank Green Cross LabCell
Co., Ltd. for providing the conditioned medium of the natural
killer cells.
Funding
The present study was supported by Green Cross
WellBeing Corporation of Korea (grant no. 201809).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
SEL, TRK, CTO and BJK designed experiments. SEL,
TRK, JHK and CTO performed the experiments. MI, BCL, YKH, SHP, SH,
JYK and CTO analyzed data. YKH, SHP and SH prepared the materials.
SEL and TRK prepared the figures. TRK wrote the manuscript. BJK and
TRK edited the manuscript for final content. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All study samples were obtained after the
acquisition of written informed consent from the study
participants, in accordance with the Declaration of Helsinki. The
research protocol was reviewed and approved by the Institutional
Review Board of Seoul National University Hospital (permit no.
H-1811-023-985).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Makrantonaki E, Vogel M,
Scharffetter-Kochanek K and Zouboulis CC: Skin aging: Molecular
understanding of extrinsic and intrinsic processes. Hautarzt.
66:730–737. 2015.Article in German. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gonzaga ER: Role of UV light in
photodamage, skin aging, and skin cancer: Importance of
photoprotection. Am J Clin Dermatol. 10(Suppl 1): pp. S19–S24.
2009, View Article : Google Scholar
|
|
3
|
Wenk J, Brenneisen P, Meewes C, Wlaschek
M, Peters T, Blaudschun R, Ma W, Kuhr L, Schneider L and
Scharffetter- Kochanek K: (UV-induced oxidative stress and
photoaging). Curr Probl Dermatol. 29:83–94. 2001. View Article : Google Scholar
|
|
4
|
Berneburg M, Plettenberg H and Krutmann J:
Photoaging of human skin. Photodermatol Photoimmunol Photomed.
16:239–244. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fisher GJ: The pathophysiology of
photoaging of the skin. Cutis. 75(2 Suppl): pp. S8–S9. 2005
|
|
6
|
Scharffetter-Kochanek K, Brenneisen P,
Wenk J, Herrmann G, Ma W, Kuhr L, Meewes C and Wlaschek M:
Photoaging of the skin from phenotype to mechanisms. Exp Gerontol.
35:307–316. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishigori C: (Cellular aspects of
photocarcinogenesis). Photochem Photobiol Sci. 5:208–214. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brenneisen P, Sies H and
Scharffetter-Kochanek K: (Ultraviolet-B irradiation and matrix
metalloproteinases: From induction via signaling to initial
events). Ann N Y Acad Sci. 973:31–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vermeer BJ and Gilchrest BA:
(Cosmeceuticals. A proposal for rational definition, evaluation,
and regulation). Arch Dermatol. 132:337–340. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Geesin JC, Darr D, Kaufman R, Murad S and
Pinnell SR: (Ascorbic acid specifically increases type I and type
III procol-lagen messenger RNA levels in human skin fibroblast). J
Invest Dermatol. 90:420–424. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Conti M, Couturier M, Lemonnier F and
Lemonnier A: (Antioxidant properties of vitamin E and membrane
permeability in human fibroblast cultures). Adv Exp Med Biol.
264:125–128. 1990. View Article : Google Scholar
|
|
12
|
Cho HS, Lee MH, Lee JW, No KO, Park SK,
Lee HS, Kang S, Cho WG, Park HJ, Oh KW and Hong JT: (Anti-wrinkling
effects of the mixture of vitamin C, vitamin E, pycnogenol and
evening primrose oil, and molecular mechanisms on hairless mouse
skin caused by chronic ultraviolet B irradiation). Photodermatol
Photoimmunol Photomed. 23:155–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hernández-Camacho JD, Bernier M,
López-Lluch G and Navas P: (Coenzyme Q10 supplementation in aging
and disease). Front Physiol. 9:442018. View Article : Google Scholar
|
|
14
|
Cos P, Rajan P, Vedernikova I, Calomme M,
Pieters L, Vlietinck AJ, Augustyns K, Haemers A and Vanden Berghe
D: In vitro antioxidant profile of phenolic acid derivatives. Free
Radic Res. 36:711–716. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kwon TR, Oh CT, Choi EJ, Kim SR, Jang YJ,
Ko EJ, Yoo KH and Kim BJ: (Conditioned medium from human bone
marrow-derived mesenchymal stem cells promotes skin moisturization
and effacement of wrinkles in UVB-irradiated SKH-1 hairless mice).
Photodermatol Photoimmunol Photomed. 32:120–128. 2016. View Article : Google Scholar
|
|
16
|
Jayaraman P, Nathan P, Vasanthan P, Musa S
and Govindasamy V: (Stem cells conditioned medium: A new approach
to skin wound healing management). Cell Biol Int. 37:1122–1128.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sundaram H, Mehta RC, Norine JA, Kircik L,
Cook-Bolden FE, Atkin DH, Werschler PW and Fitzpatrick RE:
Topically applied physiologically balanced growth factors: A new
paradigm of skin rejuvenation. J Drugs Dermatol. 8(5): Suppl Skin
Rejuenation. pp. S4–S13. 2009
|
|
18
|
Lotzová E: (Definition and functions of
natural killer cells). Nat Immun. 12:169–176. 1993.
|
|
19
|
Vivier E, Tomasello E, Baratin M, Walzer T
and Ugolini S: (Functions of natural killer cells). Nat Immunol.
9:503–510. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Robertson MJ and Ritz J: (Biology and
clinical relevance of human natural killer cells). Blood.
76:2421–2438. 1990.PubMed/NCBI
|
|
21
|
Lim O, Lee Y, Chung H, Her JH, Kang SM,
Jung MY, Min B, Shin H, Kim TM, Heo DS, et al: GMP-compliant,
large-scale expanded allogeneic natural killer cells have potent
cytolytic activity against cancer cells in vitro and in vivo. PLoS
One. 8:pp. e536112013, View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Min B, Choi H, Her JH, Jung MY, Kim HJ,
Jung MY, Lee EK, Cho SY, Hwang YK and Shin EC: Optimization of
large-scale expansion and cryopreservation of human natural killer
cells for anti-tumor therapy. Immune Netw. 18:pp. e312018,
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: (Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method). Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Park BS, Jang KA, Sung JH, Park JS, Kwon
YH, Kim KJ and Kim WS: (Adipose-derived stem cells and their
secretory factors as a promising therapy for skin aging). Dermatol
Surg. 34:1323–1326. 2008.PubMed/NCBI
|
|
25
|
Beanes SR, Dang C, Soo C and Ting K: (Skin
repair and scar formation: The central role of TGF-beta). Expert
Rev Mol Med. 5:1–22. 2003. View Article : Google Scholar
|
|
26
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: (Growth factors and cytokines in wound
healing). Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar
|
|
27
|
Kim MS, Song HJ, Lee SH and Lee CK:
(Comparative study of various growth factors and cytokines on type
I collagen and hyaluronan production in human dermal fibroblasts).
J Cosmet Dermatol. 13:44–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Werb Z: (ECM and cell surface proteolysis:
Regulating cellular ecology). Cell. 91:439–442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lukashev ME and Werb Z: (ECM signalling:
Orchestrating cell behaviour and misbehaviour). Trends Cell Biol.
8:437–441. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pillai S, Oresajo C and Hayward J:
(Ultraviolet radiation and skin aging: Roles of reactive oxygen
species, inflammation and protease activation, and strategies for
prevention of inflammation-induced matrix degradation-a review).
Int J Cosmet Sci. 27:17–34. 2005. View Article : Google Scholar
|
|
31
|
Kammeyer A and Luiten RM: (Oxidation
events and skin aging). Ageing Res Rev. 21:16–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rittié L and Fisher GJ: (UV-light-induced
signal cascades and skin aging). Ageing Res Rev. 1:705–720. 2002.
View Article : Google Scholar
|
|
33
|
Muthusamy V and Piva TJ: (The UV response
of the skin: A review of the MAPK, NFkappaB and TNFalpha signal
transduction pathways). Arch Dermatol Res. 302:5–17. 2010.
View Article : Google Scholar
|
|
34
|
Brohem CA, Cardeal LB, Tiago M, Soengas
MS, Barros SB and Maria-Engler SS: (Artificial skin in perspective:
Concepts and applications). Pigment Cell Melanoma Res. 24:35–50.
2011. View Article : Google Scholar
|