Introduction
As a main complication of diabetes mellitus,
diabetic nephropathy (DN) is a leading cause of end-stage renal
disease, accounting for >35% of all new cases requiring dialysis
therapy globally (1). Clinically,
DN is characterized by the development of proteinuria followed by
decreased glomerular filtration (2). Pathologically, DN is characterized
by the thickening of the basement membrane, renal tubal
epithelial-mesenchymal transition (EMT), the accumulation of
extracellular matrix (ECM) components, glomerular sclerosis and
tubulointerstitial fibrosis (3).
However, there is limited acknowledge available on the pathological
mechanisms involved in DN, and thus there is also a lack of
effective treatments for DN. Therefore, it is of utmost importance
to study its precise molecular mechanisms and to develop novel
effective therapeutic strategies for DN.
MicroRNAs (miRNAs or miRs) are small non-coding
single-stranded RNAs which have been confirmed to play critical
roles in cell proliferation, apoptosis and differentiation,
contributing to the pathogenesis of a number of human deceases,
such as cancer and diabetes (4,5).
Researches have indicated that miRNAs are highly involved in DN,
which suggests the great potential of miRNAs in DN detection, as
well as therapy (6,7). It has been found that urinary miRNAs
related to histopathological lesions and kidney damage can be
applied to the sensitive, specific and non-invasive detection of DN
(8). Furthermore, miRNAs have
been successfully applied to DN therapy. The addition of the agomir
of the downregulated miRNAs (i.e., miR-455-3p) or antagomir of the
commonly upregulated miRNAs (i.e., miR-192 and miR-377) in DN has
been shown to induce a decrease in albuminuria and mesangial matrix
accumulation in animal models (9-11).
miR-379-5p has been reported to be downregulated in
spontaneous type 2 diabetic KKay mice (12). In this study, we investigated the
role of miR-379-5p in DN both in vitro and in vivo.
Glomerular hypertrophy, mainly caused by glomerular mesangial cell
(MC) proliferation and the accumulation of ECM components, is
considered to be one of the earliest pathological characteristics
of DN. Of the three types of constitutive cells in the glomerulus,
MCs are the main source of ECM proteins. Therefore, we isolated and
cultured mouse glomerular MCs (MMCs) for experiments in
vitro. LIN28B, the direct target of miR-379-5p, was identified
by the database, TargetScan. LIN28B has been shown to be closely
related to various types of cancer (13). It has been reported that in MMCs
treated with transforming growth factor (TGF)-β, the LIN28/let-7
pathway can affect the accumulation of ECM components by targeting
collagen type 1-α2 (Col1a2) and collagen type 4-α1 (Col4a1)
(14). In this study, we
attempted to investigate whether miR-379-5p regulates the
LIN28/let-7 axis in DN, in an aim to provide a promising approach
for the clinical treatment of DN.
Materials and methods
Cell culture and transfection
MMCs were isolated from 8-week-old male C57BL/6 mice
(n=6) as previously described (15). The mice were euthanized by an
intraperitoneal injection of sodium pentobarbital (100 mg/kg). All
experiments were approved by the Animal Care and Use Committee of
the Nanjing University of Chinese Medicine and complied with the
Declaration of the National Institutes of Health Guide for the Care
and Use of Laboratory Animals. MMCs were obtained from the renal
cortex of the 8-week-old male mice using sterile techniques. After
washing with RPMI-1640 medium (Beyotime Biotechnology), the
cortices were separated from the medullas, pooled, minced and the
glomeruli were isolated. The glomeruli were then pre-treated with
collagenase for 15 min and plated in RPMI-1640 medium supplemented
with 10% fetal bovine serum (FBS) and 20 µg/ml insulin at 37°C with
5% CO2. Cells at passages between 3 and 7 were used in
all the experiments.
The MMCs were treated with normal glucose (5 mmol/l)
plus mannitol (25 mmol/l) for the control group and high glucose
(30 mmol/l) for the high glucose (HG) group. miR-379-5p mimics,
inhibitors, agomir and siRNA against LIN28B and the negative
controls were purchased from RiboBio (Guangzhou). All of the
transient transfections were conducted using Lipofectamine 2000
reagent (Invitrogen; Thermo Fisher Scientific). 293T cells were
acquired from the Chinese Academy of Sciences Cell Bank and were
cultured in Dulbecco's modified Eagle's medium (DMEM) containing
10% FBS.
Luciferase reporter assay
The LIN28B transcript sequences were acquired
from NCBI resources (gene ID, 380669). The 3′ UTR of LIN28B with
predicted target sites for miR-379-5p seed sequence was amplified
and cloned into the PGL-3 vector (Promega) using
KpnI/HindIII sites. The primers used were as follows:
Sense, 5′-GGTACCGCCTTTGATTCAGAAACGG-3′ and antisense,
5′-AAGCTTCTATAAAACATGACACCCGC-3′. The mutated 3′ UTR of LIN28B was
generated using the QuikChange II Site-Directed Mutagenesis kit
(Stratagene). At 48 h following the co-transfection of miR-379-5p
mimics (100 nM) and the wild-type or mutated 3′ UTR of LIN28B, the
luciferase activity of the 293T cells was measured using the
dual-luciferase assay kit (Promega) with Renilla luciferase
as the internal control.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the MMCs and renal
tissues (see below) using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific). cDNA was synthesized using the PrimeScript®
RT reagent kit (Takara). qPCR was performed using SYBR Premix
ExTaq™ (Takara) on the platform of Applied Biosystems 7500. U6 was
used as an endogenous normalization control. The total reaction
system of 20 μl volume was as follows: 1 μl cDNA, 10
μl SYBR Premix EX Taq, 1 μl each of the primers (10
μM) and 7 μl ddH2O. The PCR cycling
conditions were as follows: 95°C for 5 min, followed by 40 cycles
of 95°C for 15 sec and 60°C for 30 sec. The primers used were as
follows: miR-379-5p forward, 5′-GCGCTGGTAGACTATGGAA-3′ and reverse,
5′-GTG CAG GGT CCG AGG T-3′; U6 forward, 5′-CTC GCT TCG GCA GCA CAT
ATA CT-3′ and reverse, 5′-ACG CTT CAC GAA TTT GCG TGT C-3′; Lin28B
forward, 5′-GTC AAT ACG GGT AAC AGG AC-3′ and reverse, 5′-TTC TTT
GGC TGA GGA GGT AG-3′; Let-7b forward, 5′-TGA GGT AGT AGG TTG
TGT-3′ and reverse, 5′-GTC GTA TCC AGT GCA GGG TCC GAG GT-3′; Col-1
forward, 5′-CCA CCC CAG CCG CAA AGA GTC-3′ and reverse, 5′-GTC ATC
GCA CAC AGC CGT GC-3′; Col-4 forward, 5′-ATC TCT GCC AGG ACC AAG
TG-3′ and reverse, 5′-CGG GCT GAC ATT CCA CAA T-3′; GAPDH forward,
5′-GAA ATC CCA TCA CCA TCT TCC AGG-3′ and reverse, 5′-GAG CCC CAG
CCT TCT CCA TG-3′. Data analyses were performed using the
comparative Cq (ΔΔCq) method for calculating relative gene
expression (16).
Western blot analysis
Renal tissue (see below) and MMCs were lysed in
radioimmunoprecipitation buffer. Approximately 80 μg of
total proteins were separated by 12% or 10% SDS-PAGE gel and then
transferred onto PVDF membranes (Millipore Corp.) by
electroblotting. After being blocked for 60 min with 5% non-fat
milk, the membranes were incubated at 4°C overnight with primary
antibodies against Col4 (ab6586; 1:1,000), fibronectin (FN; ab2413;
1:1,000), p21 (ab109199; 1:1,000), cyclin D1 (ab134175; 1:1,000)
(all from Abcam) and LIN28B (#5422; 1:1,000; Cell Signaling
Technology). GAPDH (ab9485; 1:2,000; Abcam) was served as the
loading control. The membranes were then incubated with the
secondary antibodies anti-rabbit IgG, HRP-linked antibody (1:1,000,
#7074, Cell Signaling Technology) for 1 h at room temperature.
Subsequently, they were visualized with ECL detection reagent
(Millipore), and the gray values of the bands were calculated
automatically (ImageJ software, version 4.3).
Cell proliferation
Cell viability was assayed using the Cell Counting
kit-8 (Dojindo) following the manufacturer's instructions. The
cells were seeded in 96-well plates at a density of
2×103 cells per well. CCK-8 solution (10 μl) was
added to each well for 1 h following 48 h of incubation, and OD450
was determined using a microplate reader (ELx×808, BioTek).
Moreover, 5-ethynyl-2′-deoxyuridine (EdU) assay (Beyotime
Biotechnology) was performed to detect the proliferation of the
MMCs. Following incubation with 10 μM EdU for 2 h at 37°C,
the cells were fixed in 4% paraformaldehyde. Subsequently, Hoechst
33342 (Beyotime Biotechnology) was used to stain the nuclei at room
temperature for 30 min. Finally, the cells were visualized under a
fluorescence microscopy (Leica).
Cell cycle analysis
For cell cycle analysis, the MMCs were harvested,
suspended in 500 μl of phosphate-buffered saline (PBS),
fixed with 70% ethanol and subjected to PI/RNase (Beyotime
Biotechnology) staining for 30 min at 4°C. The MMCs were then
analyzed by flow cytometry (FACSCanto II, BD Biosciences).
Animal experiments
A total of 18 C57BL/KsJ type 2 diabetic db/db male
mice (aged 4 weeks, weighing 18-22 g) and a total of 6 heterozygote
db/m male mice (aged 4 weeks, weighing 18-22 g) used in this study
were purchased from the Model Animal Research Center of Nanjing
University. The mice were housed in a clean environment with 50-60%
humidity, 20-22°C and a 12-h dark/light cycle, and fed a standard
diet during a 1-week adaptation period. When the mice were 8 weeks
old, they were randomly divided into the control group (untreated
db/m mice, n=6); the DN group (untreated db/db mice, n=6); an
agomir NC (agomir NC-treated db/db, n=6) group and an agomir-379-5p
(agomir-379-5p-treated db/db, n=6) group. Every 2 days, the db/db
mice were injected with miR-379-5p agomir or agomir NC (10 mg/kg)
(GenePharma) via the tail vein. Four weeks later, the mice were
sacrificed by an intraperitoneal injection of sodium pentobarbital
(100 mg/kg) and the kidneys were harvested for analysis. During the
experiments, animal health and behavior were monitored every 2
days. All experiments were approved by the Animal Care and Use
Committee of the Nanjing University of Chinese Medicine and
complied with the Declaration of the National Institutes of Health
Guide for the Care and Use of Laboratory Animals.
Measurement of urine protein levels
Metabolic cages were used to collect urine samples
in a 24-h period, and urinary protein levels were evaluated using
the mouse albumin ELISA quantitation set (Bethyl Laboratories).
Histology and immunostaining assays
Fresh renal tissues were fixed with 4%
paraformaldehyde and embedded in paraffin. Paraffin-embedded
specimens were sectioned (4 μm thicknesses) and stained with
PAS, Masson's trichrome staining (Beyotime Biotechnology) at room
temperature for 15 min and immunohistochemistry. The antibodies
included LIN28B (ab71415; 1:50), Ki-67 (ab15580; 1:500) and normal
rabbit IgG (ab6728, 1:1,000) (all from Abcam) were incubated for 2
h at 37°C.
In situ hybridization (ISH)
To detect the presence of miR-379-5p in the renal
tissues, in situ hybridization was performed. The frozen
renal tissues sections of mice from control group (untreated db/m
mice), DN group (untreated db/db mice) and agomir-379-5p
(agomir-379-5p-treated db/db mice) group were fixed in
paraformaldehyde followed by rinsing in PBS. A digoxin-labeled
oligonucleotide probe (5′-TGG TAG ACT ATG GAA CGT AGG-3′) (0.5
μg/ml) was synthesized by Dingguo BioTechnology Co. Ltd.
In situ hybridization was performed with the ISH kit
according to the protocol (Dingguo BioTech). The slides were
treated with 3,3′-diaminobenzidine (DAB) for 5 min, and then
counterstained with hematoxylin for 1-2 min at room temperature.
All images were acquired by using a light microscope (Olympus) at
the magnification of ×400.
Bioinformatics analysis
TargetScan (http://www.targetscan.org/) was used to predict the
putative target genes for miR-379-5p.
Statistical analysis
GraphPad Prism (version 5.0; GraphPad Software,
Inc.) was used to conduct all statistical analyses. Measurement
data are presented as the means ± standard deviation. One-way ANOVA
analysis followed by a Tukey's post hoc test were used to compare
the difference between multiple groups. Values of P<0.05 and
P<0.01 were considered to indicate statistically significant and
highly statistically significant differences, respectively.
Results
miR-379-5p is downregulated by high
glucose treatment in MMCs
To explore the potential role of miR-379-5p in DN,
we analyzed the expression of miR-379-5p in MMCs stimulated with
HG. As shown in Fig. 1A and B,
miR-379-5p expression was significantly decreased in the cells
treated with high glucose in a dose- and time-dependent manner, and
its expression was approximately one third of that of the control
following stimulation of the cells with 30 mM glucose for 48 h.
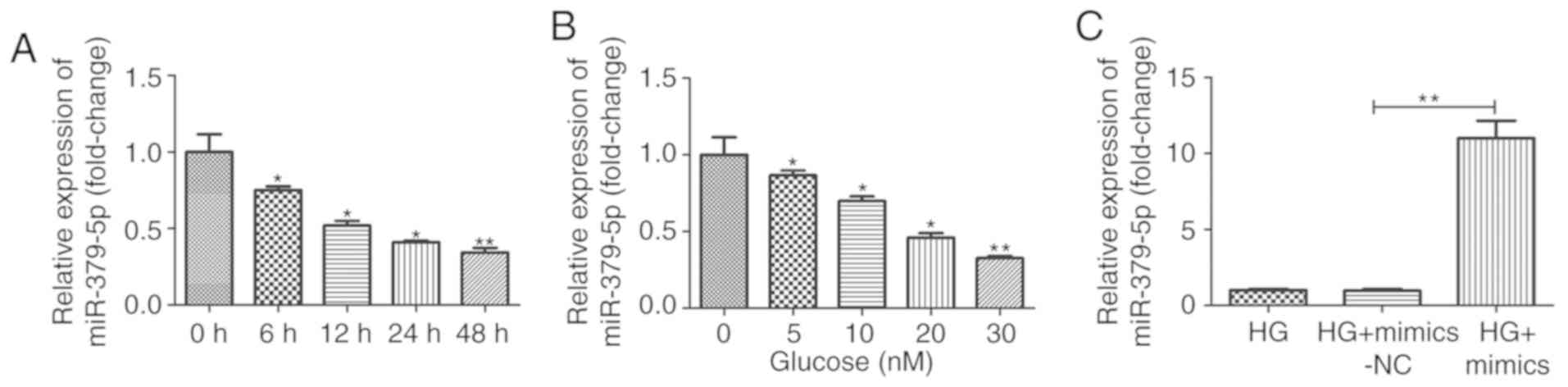 | Figure 1miR-379-5p expression is
downregulated by HG treatment in MMCs. Expression of miR-379-5p was
detected by RT-qPCR in MMCs treated with (A) 30 mM glucose for 0,
6, 12, 24 and 48 h or (B) with various concentration of glucose (0,
5, 10, 20 and 30 mM) for 24 h. (C) Following transfection with
mimics-NC or miR-379-5p mimics, the cells were treated with HG (30
mmol/l) for 24 h, and miR-379-5p expression was then detected by
RT-qPCR. Data are expressed as the mean ± SEM.
*P<0.05 and **P<0.01 compared with the
control group or HG group. HG, high glucose; MMCs, mouse mesangial
cells. |
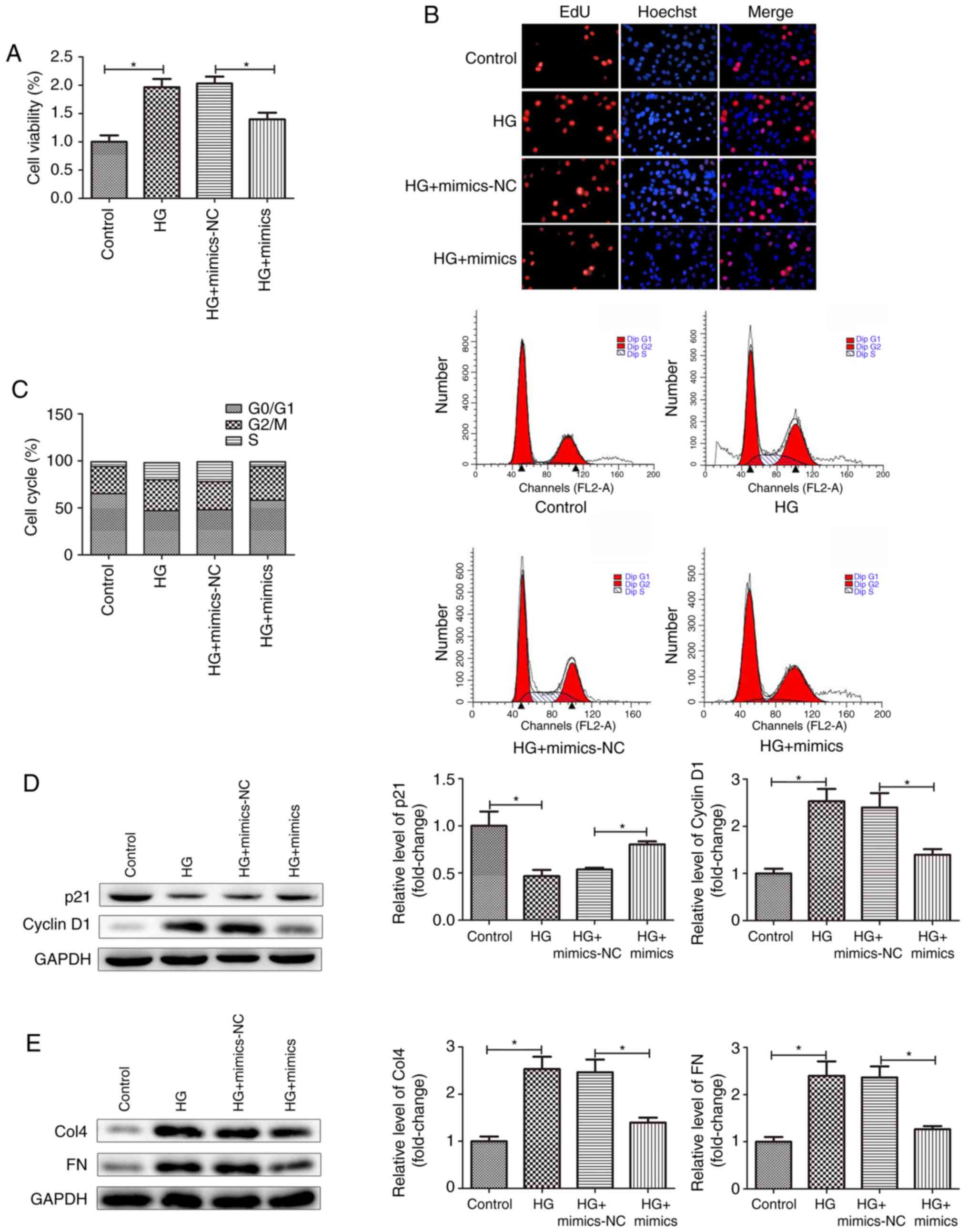
miR-379-5p suppresses the proliferation
and accumulation of ECM components in MMCs
Mesangial cell proliferation and the accumulation of
ECM components are important pathological features of DN (17). In this study, to explore the
effects of miR-379-5p on cell proliferation and the accumulation of
ECM components, the MMCs were transfected with miR-379-5p mimics.
The results revealed that miR-379-5p expression was markedly
increased in the MMCs transfected with miR-379-5p mimics (Fig. 1C). The results of CCK-8 assay also
revealed that HG stimulation markedly promoted the viability of the
MMCs. However, the promoting effects of HG stimulation on cell
viability were reversed following transfection of the MMCs with
miR-379-5p mimics (Fig. 2A). The
results of EdU assays yielded similar conclusions, in that
transfection with miR-379-5p mimics attenuated the effects of HG
stimulation on cell proliferation (Fig. 2B). Furthermore, cell cycle
analysis indicated that there was a decrease in the percentage of
cells in the G0/G1 phase, as well as an
increase in the percentage of cells in the S phase in the HG group
compared with the control group. MMCs transfected with miR-379-5p
mimics exhibited an increase in the percentage of cells in the
G0/G1 phase and a decrease in the percentage
of cells in the S phase compared with the HG group (Fig. 2C). This suggested that the
overexpression of miR-379-5p prevented MMCs cell cycle
progression.
p21, a cyclin-dependent kinase inhibitor, almost
inhibits all cyclin/CDK complexes. The expression level of p21 and
cyclin D1 can reflect the proliferation of MMCs (18). In this study, the results of
western blot analysis revealed that transfection with miR-379-5p
mimics attenuated the decrease and increase in the expression level
of p21 and cyclin D1, respectively induced by HG stimulation
(Fig. 2D). The cell cycle
analysis and the related protein experimental data suggested that
the overexpression of miR-379-5p suppressed the proliferation of
MMCs following HG stimulation. Tubulointerstitial fibrosis is
always involved with the increased deposition of ECM proteins, such
as collagens and FN (19,20). In this study, we detected Col4 and
FN expression by western blot analysis. As shown in Fig. 2E, Col4 and FN expression levels
were markedly promoted in the HG group and the mimics-NC group
compared to the control group, and decreased in the mimics group
compared to the mimics-NC group. Taken together, these results
indicated that miR-379-5p suppressed the proliferation and
accumulation of ECM components in MMCs.
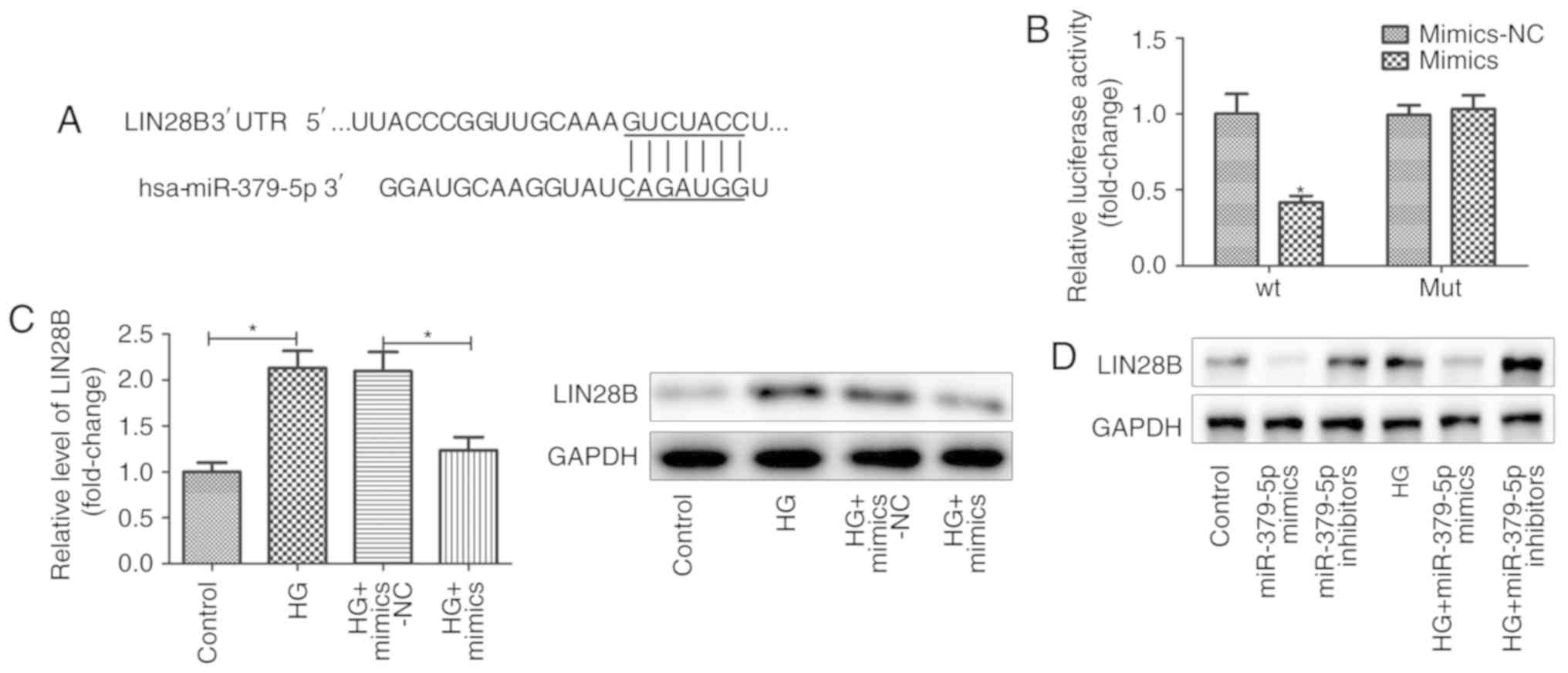
LIN28B is a target of miR-379-5p
The TargetScan database was used to identify the
targets of miR-379-5p. It was suggested that miR-379-5p had a
conserved binding site in the 3′ UTR of LIN28B (Fig. 3A). To verify the association
between miR-379-5p and LIN28B, luciferase reporter assays were
performed. The results revealed that the luciferase activity was
significantly decreased in the MMCs co-transfected with miR-379-5p
mimics and the wild-type 3′ UTR reporter gene other than the
mutated 3′ UTR reporter gene, indicating that miR-379-5p directly
binds to the 3′ UTR of LIN28B (Fig.
3B).
LIN28B, a RNA-binding protein belongs to LIN28
family, mediates diverse biological functions (21). In this study, to examine the
regulatory association between miR-379-5p and LIN28B, the
expression levels of LIN28B and let-7b were detected in MMCs
transfected with miR-379-5p mimics. As shown in Fig. 3C, the overexpression of miR-379-5p
markedly suppressed the LIN28B expression level, which was induced
by HG. The results of western blot analysis also revealed that
miR-379-5p inactivated LIN28B expression in a HG-independent manner
(Fig. 3D).
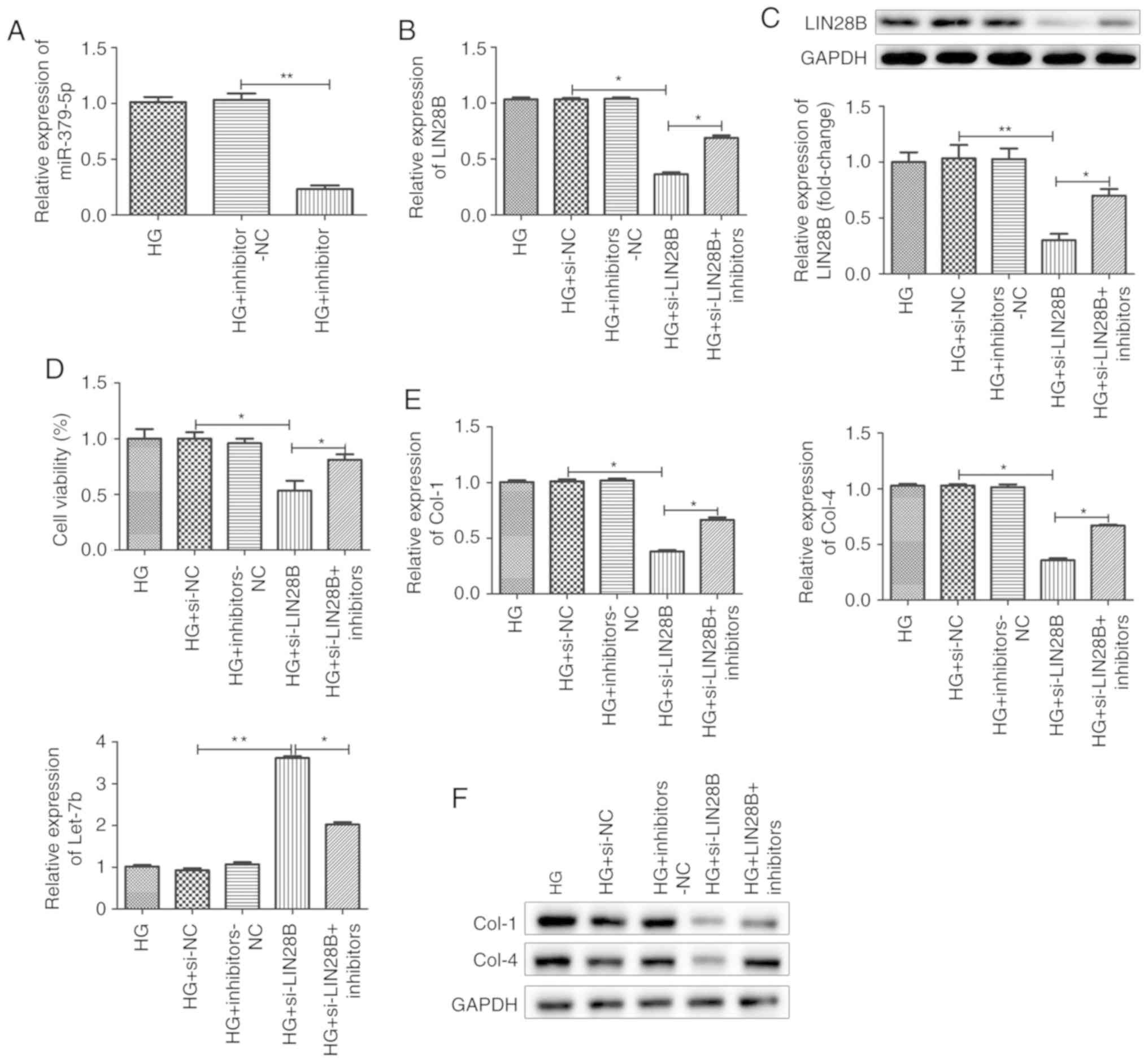
miR-379-5p suppresses the proliferation
and accumulation of ECM components by regulating LIN28B
expression
Previously, Kim et al demonstrated that
LIN28B increased the expression of ECM proteins, such as Col1a2 and
Col4a1, by decreasing let-7 levels (22). In this study, firstly, the
transfection efficiency of miR-379-5p inhibitor was evaluated by
RT-qPCR. The results revealed that the cells transfected with
miR-379-5p inhibitor exhibited lower levels of miR-379-5p
expression compared with the negative control group, indicating
that miR-379-5p inhibitor was transfected into the cells
successfully (Fig. 4A). To
explore whether miR-379-5p suppresses the proliferation and
accumulation of ECM components by regulating the LIN28/let-7 axis,
a si-LIN28B vector was constructed and transfected into the MMCs
(Fig. 4B and C). Transfection
with si-LIN28B decreased LIN28B expression; however, miR-379-5p
inhibitor partly reversed the decrease in LIN28B induced by
si-LIN28B in the cells treated with HG (Fig. 4B and C). CCK-8 assay was performed
in the HG group, si-NC group, inhibitors-NC group, si-LIN28B group
and si-LIN28B + miR-379-5p inhibitors group to examine the effects
of LIN28B on MMC viability. The results revealed that the knockdown
of LIN28B suppressed MMC viability (Fig. 4D). Transfection with si-LIN28B
decreased the mRNA expression levels of Col1 and Col4, and
increased the let-7 expression level, as shown by RT-qPCR (Fig. 4E). Western blot analysis for Col-1
and Col-4 revealed that si-LIN28B decreased the accumulation of the
ECM components, Col-1 and Col-4, compared to si-NC group (Fig. 4F). However, MMCs co-transfected
with miR-379-5p inhibitors and si-LIN28B exhibited a higher
proliferation rate and a greater accumulation of ECM components
compared to the corresponding si-LIN28B group (Fig. 4D-F). Taken together, these results
suggested that miR-379-5p suppressed the proliferation and
accumulation of ECM components by regulating the LIN28/let-7
axis.
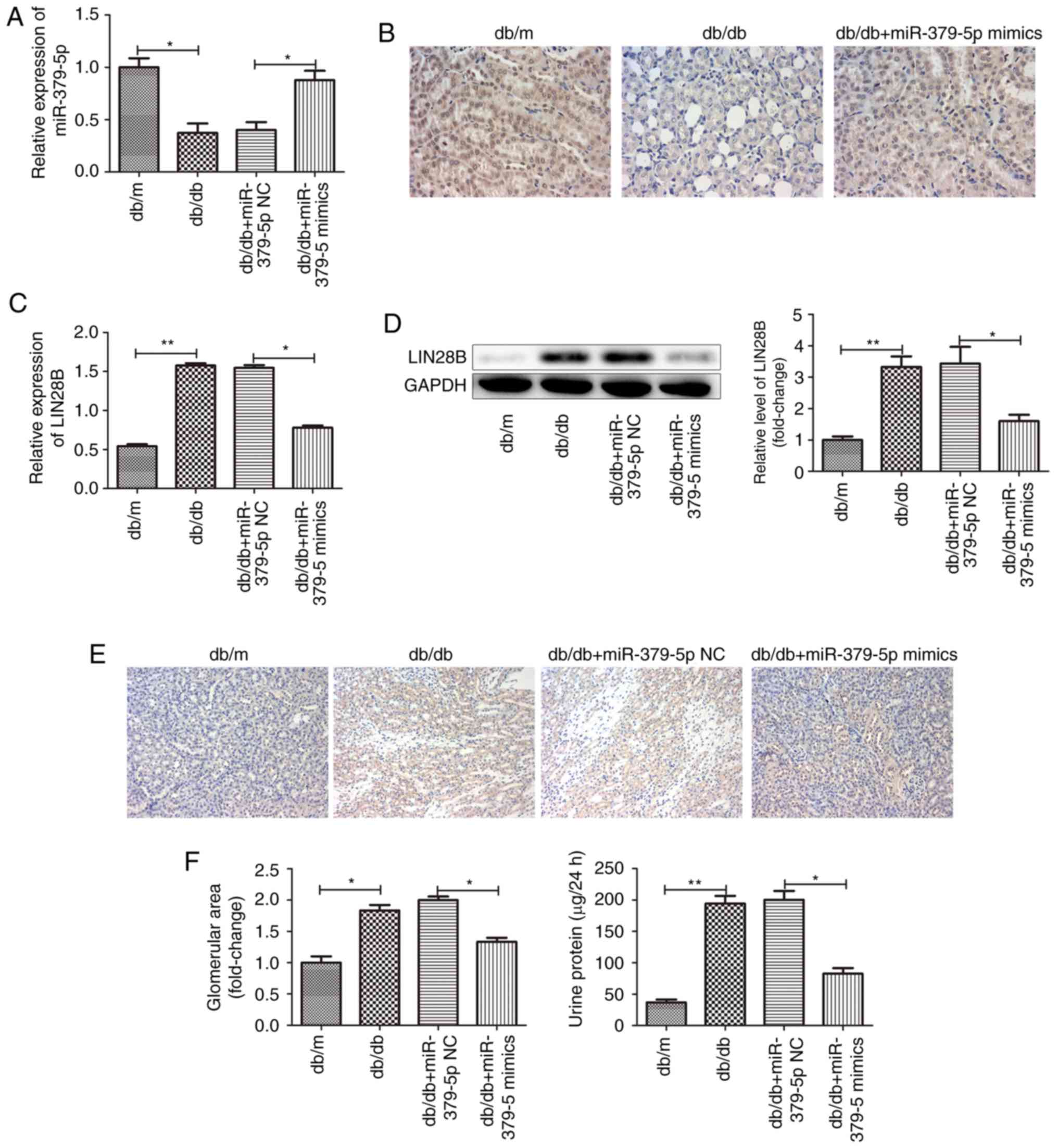
miR-379-5p suppresses renal fibrosis in
vivo
To examine the effects of miR-379-5p on renal
fibrosis in DN, we used type 2 diabetic db/db mice for research
in vivo with db/m mice as a normal control. miR-379-5p
expression levels in the kidney tissues were significantly
downregulated in the db/db mice, as detected by RT-qPCR and in
situ hybridization assays. miR-379-5p agomir injection markedly
promoted miR-379-5p expression (Fig.
5A and B). Consequently, LIN28B expression was upregulated in
the kidney tissues of the db/db mice and downregulated after the
injection of miR-379-5p agomir (Fig.
5C-E). Moreover, glomerular hypertrophy with an increased
glomerular area was observed in the db/db mice. However, miR-379-5p
agomir attenuated glomerular hypertrophy. As is well-known, the
earliest pathological characteristic of DN is glomerular
hypertrophy, which is mainly caused by glomerular MC proliferation
and ECM accumulation. In this study, we wished to determine the
role of the miR-379-5p/LIN28/let-7 axis in glomerular MCs during
DN. Physiological markers, such as urine protein and blood glucose
were also measured. It was found that urine protein levels were
significantly increased in the db/db mice, and urine protein levels
were decreased in the miR-379-5p agomir group (Fig. 5F).
In order to examine the effects of miR-379-5p on
renal fibrosis in DN, some pathological markers, such as PAS and
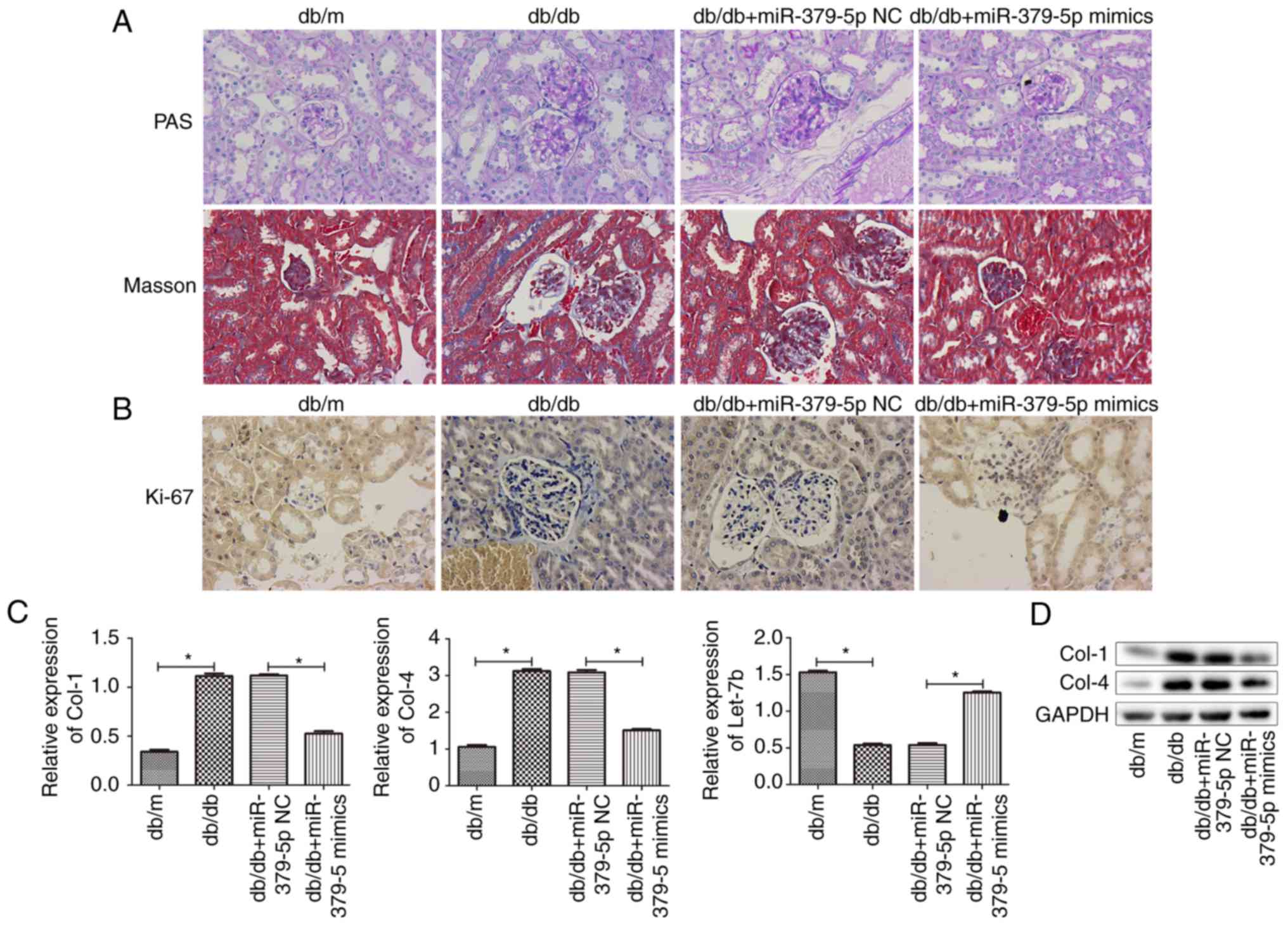
Masson's trichrome staining were used (Fig. 6A). The results indicated that
miR-379-5p alleviated pathological changes, such as glomerular
hypertrophy, mesangial amplification and renal fibrosis in the
diabetic mice. Furthermore, mesangial cell proliferation detected
by Ki-67 staining is presented in Fig. 6B, and ECM accumulation was
measured by RT-qPCR and western blot analysis for let-7b, Col-4 and
Col-1 expression (Fig. 6C and D).
The results revealed that miR-379-5p agomir decreased mesangial
cell proliferation and accumulation of ECM components that were
increased in the db/db mice, which was consistent with the data
obtained from the in vitro assays.
Discussion
Recently, increasing evidence has indicated that the
dysregulation of miRNAs, such as miR-214, miR-15a, miR-29a and
miR-34a plays an important role in the pathogenesis of DN (23-31). In this study, we found that
miR-379-5p was downregulated and acted as a suppressor of renal
fibrosis by regulating LIN28B in diabetic nephropathy.
miR-379 has been found to participate in a number of
biological processes. It acts as a tumor suppressor and is
downregulated in human cancers (32-34). In hepatocel-lular carcinoma and
non-small-cell lung cancer, miR-379 is associated with
chemosensitivity (35,36). Moreover, miR-379 has been shown to
be downregulated in patients with athero-sclerotic coronary artery
disease and acute myocardial infraction (37,38).
LIN28B is an oncogene that has been reported to be
overexpressed in numerous malignant tumors (39-42). LIN28A AND LIN28B selectively block
the expression of let-7 miRNAs and promote tumorigenesis (43). On the other hand, the LIN28B/let-7
axis has been reported to be involved in controlling TGF-β-induced
collagen accumulation in DN (14). In addition, LIN28B has been
reported to be associated with fibrosis lesions. For instance,
LIN28B can participate in the progression of organ damage and
fibrosis in metabolic diseases, such as diabetes (14). LIN28B can ameliorate liver
fibrosis by inhibiting mesenchymal signaling pathways (44). The upregulation of LIN28B has been
shown to contribute to idiopathic pulmonary fibrosis (45). It is well known that transforming
growth factor-β1 (TGF-β1) expression is increased in glomerular MCs
that lead to an increased accumulation of ECM components and
hypertrophy during the progression of DN (46). TGF-β1 can induce LIN28B expression
which results in the suppression of let-7 and the upregulation of
collagen expression in glomerular mesangial cells under diabetic
conditions (14). Moreover, the
LIN28/let-7 axis plays an important role in regulating glucose
metabolism (47). Let-7a
regulates glucose metabolism and insulin synthesis/secretion in
type 2 diabetes mellitus by targeting the LIN28 pathway (48).
However, there are some limitations to this study.
Mesangial cells have been used to study glomerular fibrosis in a
number of studies (49,50). The LIN28B/let-7 signaling pathway
has been reported to be involved in cell energy metabolism in some
cells. Further, we aim to examine the effects of miR-379-5p on
podocytes in metabolism, not through fibrosis, particularly the
influence of miR-379-5p in the process of podocyte diminution and
disappearance in nephropathy in future studies.
In this study, another miRNA, miR-379-5p, was found
to suppress renal fibrosis by regulating the LIN28B/let-7 axis in
DN. miR-379-5p expression was downregulated and that of LIN28B was
upregulated both in MMCs treated with HG and in glomeruli of db/db
mice. However, miR-379-5p mimics and agomir suppressed the
expression of LIN28B in vitro and in vivo,
respectively. The TargetScan database and luciferase reporter assay
indicated that miR-379-5p directly bound to the 3′ UTR of LIN28B
and suppressed the expression of LIN28B. We verified that
miR-379-5p regulated LIN28B in a HG-independent manner. In
addition, let-7b, a target of LIN28B, was upregulated when the
expression of miR-379-5p was increased. As the results of the
overexpression of miR-379-5p, MMC proliferation and collagen
protein accumulation were alleviated. It is thus suggested that
miR-379-5p suppressed renal fibrosis by the regulating LIN28/let-7
axis in DN, which may lay the foundation for clinical treatment in
the future.
In conclusion, the findings of this study suggested
a coherent mechanism for the role of miR-379-5p in renal fibrosis
during DN, and provide new insight into the mechanisms of renal
protection associated with miR-379-5p and LIN28/let-7, which may
lead to a novel therapeutic strategy for DN.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the General
Program of National Natural Science Foundation of China (grant no.
81774117); Postgraduate Research and Practice Innovation Program of
Jiangsu Province (Grant no. KYCX18_1574) and National Youth
Foundation of China (grant no. 81804027).
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
JYY designed research and revised the manuscript; NL
and LJW performed the research analysis and wrote the manuscript;
NL, LJW, WLX and SL analyzed the data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Animal Care and
Use Committee of the Nanjing University of Chinese Medicine and
complied with the Declaration of the National Institutes of Health
Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Incidence and prevalence of ESRD: United
States Renal Data System. Am J Kidney Dis. 32(2 Suppl 1): pp.
S38–S49. 1998, View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun YM, Su Y, Li J and Wang LF: Recent
advances in understanding the biochemical and molecular mechanism
of diabetic nephropathy. Biochem Biophys Res Commun. 433:359–361.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herbach N: Pathogenesis of diabetes
mellitus and diabetic complications. Studies on diabetic mouse
models. Pathologe. 33(Suppl 2): pp. S318–S324. 2012, In German.
View Article : Google Scholar
|
|
4
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gholaminejad A, Abdul Tehrani H and
Gholami Fesharaki M: Identification of candidate microRNA
biomarkers in diabetic nephropathy: A meta-analysis of profiling
studies. J Nephrol. 31:813–831. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allison SJ: Diabetic nephropathy: A lncRNA
and miRNA mega-cluster in diabetic nephropathy. Nat Rev Nephrol.
12:7132016. View Article : Google Scholar
|
|
7
|
Alvarez ML and DiStefano JK: Towards
microRNA-based therapeutics for diabetic nephropathy. Diabetologia.
56:444–456. 2013. View Article : Google Scholar
|
|
8
|
Cardenas-Gonzalez M, Srivastava A,
Pavkovic M, Bijol V, Rennke HG, Stillman IE, Zhang X, Parikh S,
Rovin BH, Afkarian M, et al: Identification, confirmation, and
replication of novel urinary MicroRNA biomarkers in lupus nephritis
and diabetic nephropathy. Clin Chem. 63:1515–1526. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu F, Zhang ZP, Xin GD, Guo LH, Jiang Q
and Wang ZX: miR-192 prevents renal tubulointerstitial fibrosis in
diabetic nephropathy by targeting Egr1. Eur Rev Med Pharmacol Sci.
22:4252–4260. 2018.PubMed/NCBI
|
|
10
|
Peng J, Wu Y, Deng Z, Zhou Y, Song T, Yang
Y, Zhang X, Xu T, Xia M, Cai A, et al: miR-377 promotes white
adipose tissue inflammation and decreases insulin sensitivity in
obesity via suppression of sirtuin-1 (SIRT1). Oncotarget.
8:70550–70563. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Wu J, Ding J, Zhu Y, Lu M, Zhou K, Xie
J, Xu X, Shen Y, Chen XY, et al: miR-455-3p suppresses renal
fibrosis through repression of ROCK2 expression in diabetic
nephropathy. Biochem Biophys Res Commun. 503:977–983. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu X, Zhang C, Fan Q, Liu X, Yang G,
Jiang Y and Wang L: Inhibiting microRNA-503 and microRNA-181d with
losartan ameliorates diabetic nephropathy in KKAy mice. Med Sci
Monit. 22:3902–3909. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balzeau J, Menezes MR, Cao S and Hagan JP:
The LIN28/let-7 pathway in cancer. Front Genet. 8:312017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park JT, Kato M, Lanting L, Castro N, Nam
BY, Wang M, Kang SW and Natarajan R: Repression of let-7 by
transforming growth factor-β1-induced Lin28 up-regulates collagen
expression in glomerular mesangial cells under diabetic conditions.
Am J Physiol Renal Physiol. 307:F1390–F1403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim YS, Reddy MA, Lanting L, Adler SG and
Natarajan R: Differential behavior of mesangial cells derived from
12/15-lipoxygenase knockout mice relative to control mice. Kidney
Int. 64:1702–1714. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Shao Y, Lv C, Wu C, Zhou Y and Wang Q:
Mir-217 promotes inflammation and fibrosis in high glucose cultured
rat glomerular mesangial cells via Sirt1/HIF-1α signaling pathway.
Diabetes Metab Res Rev. 32:534–543. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Wang S, Qian W, Ji D, Wang Q,
Zhang Z, Wang S, Ji B, Fu Z and Sun Y: uc.338 targets p21 and
cyclin D1 via PI3K/AKT pathway activation to promote cell
proliferation in colorectal cancer. Oncol Rep. 40:1119–1128.
2018.PubMed/NCBI
|
|
19
|
Kolset SO, Reinholt FP and Jenssen T:
Diabetic nephropathy and extracellular matrix. J Histochem
Cytochem. 60:976–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou SX, Huo DM, He XY, Yu P, Xiao YH, Ou
CL, Jiang RM, Li D and Li H: High glucose/lysophosphatidylcholine
levels stimulate extracellular matrix deposition in diabetic
nephropathy via plateletactivating factor receptor. Mol Med Rep.
17:2366–2372. 2018.
|
|
21
|
McWhorter ES, West RC, Russ JE, Ali A,
Winger QA and Bouma GJ: LIN28B regulates androgen receptor in human
trophoblast cells through Let-7c. Mol Reprod Dev. Jun 19–2019, Epub
ahead of print. View Article : Google Scholar : 2019, PubMed/NCBI
|
|
22
|
Kim CW, Vo MT, Kim HK, Lee HH, Yoon NA,
Lee BJ, Min YJ, Joo WD, Cha HJ, Park JW and Cho WJ: Ectopic
over-expression of tristetraprolin in human cancer cells promotes
biogenesis of let-7 by down-regulation of Lin28. Nucleic Acids Res.
40:3856–3869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bera A, Das F, Ghosh-Choudhury N,
Mariappan MM, Kasinath BS and Ghosh Choudhury G: Reciprocal
regulation of miR-214 and PTEN by high glucose regulates renal
glomerular mesangial and proximal tubular epithelial cell
hypertrophy and matrix expansion. Am J Physiol Cell Physiol.
313:C430–C447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen D, Li Y, Mei Y, Geng W, Yang J, Hong
Q, Feng Z, Cai G, Zhu H, Shi S, et al: miR-34a regulates mesangial
cell proliferation via the PDGFR-β/Ras-MAPK signaling pathway. Cell
Mol Life Sci. 71:4027–4042. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jee YH, Wang J, Yue S, Jennings M, Clokie
SJ, Nilsson O, Lui JC and Baron J: Mir-374-5p, mir-379-5p, and
mir-503-5p regulate proliferation and hypertrophic differentiation
of growth plate chondrocytes in male rats. Endocrinology.
159:1469–1478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang Y, Zhao G, Tang L, Zhang J, Li T and
Liu Z: miR-1003p and miR-8773pregulate overproduction of IL-8 and
IL-1β in mesangial cells activated by secretory IgA from IgA
nephropathy patients. Exp Cell Res. 347:312–321. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun T, Yang J, Dong W, Wang R, Ma P, Kang
P, Zhang H, Xie C, Du J and Zhao L: Down-regulated miR-15a mediates
the epithelial-mesenchymal transition in renal tubular epithelial
cells promoted by high glucose. Biosci Biotechnol Biochem.
78:1363–1370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang B, Yao K, Wise AF, Lau R, Shen HH,
Tesch GH and Ricardo SD: miR-378 reduces mesangial hypertrophy and
kidney tubular fibrosis via MAPK signalling. Clin Sci (Lond).
131:411–423. 2017. View Article : Google Scholar
|
|
29
|
Wang X, Shen E, Wang Y, Jiang Z, Gui D,
Cheng D, Chen T and Wang N: miR-196a regulates high glucose-induced
mesangial cell hypertrophy by targeting p27kip1. J Lab Autom.
20:491–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du B, Ma LM, Huang MB, Zhou H, Huang HL,
Shao P, Chen YQ and Qu LH: High glucose down-regulates miR-29a to
increase collagen IV production in HK-2 cells. FEBS Lett.
584:811–816. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu H, Zhao J and Lv J: Inhibitory effects
of miR-101 overexpression on cervical cancer SiHa cells. Eur J
Gynaecol Oncol. 38:236–240. 2017.PubMed/NCBI
|
|
32
|
Laddha SV, Nayak S, Paul D, Reddy R,
Sharma C, Jha P, Hariharan M, Agrawal A, Chowdhury S, Sarkar C and
Mukhopadhyay A: Genome-wide analysis reveals downregulation of
miR-379/miR-656 cluster in human cancers. Biol Direct. 8:102013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khan S, Brougham CL, Ryan J, Sahrudin A,
O'Neill G, Wall D, Curran C, Newell J, Kerin MJ and Dwyer RM:
miR-379 regulates cyclin B1 expression and is decreased in breast
cancer. PLoS One. 8:pp. e687532013, View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nayak S, Aich M, Kumar A, Sengupta S,
Bajad P, Dhapola P, Paul D, Narta K, Purkrait S, Mehani B, et al:
Novel internal regulators and candidate miRNAs within
miR-379/miR-656 miRNA cluster can alter cellular phenotype of human
glioblastoma. Sci Rep. 8:76732018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang DJ, Huang JZ, Yang J, Li YH, Luo YC,
He HY and Huang HJ: Bioinformatic identification of IGF1 as a hub
gene in hepatocellular carcinoma (HCC) and in-vitro analysis of the
chemosensitizing effect of miR-379 via suppressing the IGF1/IGF1R
signaling pathway. Eur Rev Med Pharmacol Sci. 20:5098–5106.
2016.
|
|
36
|
Hao GJ, Hao HJ, Ding YH, Wen H, Li XF,
Wang QR and Zhang BB: Suppression of EIF4G2 by miR-379 potentiates
the cisplatin chemosensitivity in nonsmall cell lung cancer cells.
FEBS Lett. 591:636–645. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yi J and An Y: Circulating miR-379 as a
potential novel biomarker for diagnosis of acute myocardial
infarction. Eur Rev Med Pharmacol Sci. 22:540–546. 2018.PubMed/NCBI
|
|
38
|
Xu Z, Han Y, Liu J, Jiang F, Hu H, Wang Y,
Liu Q, Gong Y and Li X: miR-135b-5p and miR-499a-3ppromote cell
proliferation and migration in atherosclerosis by directly
targeting MEF2C. Sci Rep. 5:122762015. View Article : Google Scholar
|
|
39
|
Cheng SW, Tsai HW, Lin YJ, Cheng PN, Chang
YC, Yen CJ, Huang HP, Chuang YP, Chang TT, Lee CT, et al: Lin28B is
an oncofetal circulating cancer stem cell-like marker associated
with recurrence of hepatocellular carcinoma. PLoS One. 8:pp.
e800532013, View Article : Google Scholar : PubMed/NCBI
|
|
40
|
King CE, Cuatrecasas M, Castells A,
Sepulveda AR, Lee JS and Rustgi AK: LIN28B promotes colon cancer
progression and metastasis. Cancer Res. 71:4260–4268. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alajez NM, Shi W, Wong D, Lenarduzzi M,
Waldron J, Weinreb I and Liu FF: Lin28b promotes head and neck
cancer progression via modulation of the insulin-like growth factor
survival pathway. Oncotarget. 3:1641–1652. 2012. View Article : Google Scholar
|
|
42
|
Kugel S, Sebastian C, Fitamant J, Ross KN,
Saha SK, Jain E, Gladden A, Arora KS, Kato Y, Rivera MN, et al:
SIRT6 suppresses pancreatic cancer through control of lin28b. Cell.
165:1401–1415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Piskounova E, Polytarchou C, Thornton JE,
LaPierre RJ, Pothoulakis C, Hagan JP, Iliopoulos D and Gregory RI:
Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct
mechanisms. Cell. 147:1066–1079. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McDaniel K, Huang L, Sato K, Wu N, Annable
T, Zhou T, Ramos-Lorenzo S, Wan Y, Huang Q, Francis H, et al: The
let-7/Lin28 axis regulates activation of hepatic stellate cells in
alcoholic liver injury. J Biol Chem. 292:11336–11347. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liang H, Liu S, Chen Y, Bai X, Liu L, Dong
Y, Hu M, Su X, Chen Y, Huangfu L, et al: miR-26a suppresses EMT by
disrupting the Lin28B/let-7d axis: Potential cross-talks among
miRNAs in IPF. J Mol Med (Berl). 94:pp. 655–665. 2016, View Article : Google Scholar
|
|
46
|
Hills CE and Squires PE: TGF-beta1-induced
epithelial-to-mesenchymal transition and therapeutic intervention
in diabetic nephropathy. Am J Nephrol. 31:68–74. 2010. View Article : Google Scholar
|
|
47
|
Zhu H, Shyh-Chang N, Segre AV, Shinoda G,
Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG,
et al: The Lin28/let-7 axis regulates glucose metabolism. Cell.
147:81–94. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Frost RJ and Olson EN: Control of glucose
homeostasis and insulin sensitivity by the Let-7 family of
microRNAs. Proc Natl Acad Sci USA. 108:21075–21080. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ji J, Tao P and He L: Kangxianling
decoction prevents renal fibrosis in rats with 5/6 nephrectomy and
inhibits Ang II-induced ECM production in glomerular mesangial
cells. J Pharmacol Sci. 139:367–372. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee EJ, Kang MK, Kim DY, Kim YH, Oh H and
Kang YH: Chrysin inhibits advanced glycation end products-induced
kidney fibrosis in renal mesangial cells and diabetic kidneys.
Nutrients. 10:E8822018. View Article : Google Scholar : PubMed/NCBI
|