Introduction
As a type of systemic inflammatory response syndrome
induced by the imbalanced pro- and anti-inflammatory mechanism,
sepsis normally develops as a result of severe infections, burns,
brain injury, trauma, shock and major surgery, causing serious
pathophysiological changes in vital organs (1). The mortality of patients with
sepsis-induced brain injury combined with sepsis shock and multiple
organ dysfunction has been reported to be as high as 80-90%
worldwide (2,3).
The NLR family pyrin domain containing 3 (NLRP3)
pathway has been demonstrated to play an important role in the
pathogenesis and development of sepsis lung injury, and the
inhibition of the NLRP3 pathway could not only block the aggressive
inflammatory response of sepsis, but also suppress its excessive
anti-inflammatory response, thereby protecting important organ
functions (4). Multiple cytokines
such as tumor necrosis factor (TNF)-α, interleukin (IL)-6 and
growth factors can induce signal transduction by activating the
NLRP3 pathway (5). NLRP3 protein
is downstream of Janus kinase 2, which is critically involved in
the inflammatory mediator response and immunoregulation of sepsis
(6). NLRP3 can be phosphorylated
and activated through the NLRP3 pathway, subsequently transmitting
the extracellular signal to the cell nucleus, binding with the
target gene promotor in the nucleus, inducing the transcription of
the target gene, and thereby initiating the release of a series of
cell cytokines (4).
MicroRNAs (miRNAs/miRs), the complete or incomplete
matching on the 3'-untranslated region (UTR) of the target gene
mRNAs, can degrade mRNA or inhibit its translation and regulate
protein expression at the post-transcriptional level (7). Bioinformatics prediction has
discovered that miRNAs act on an extensive range of target genes
and can regulate the expression of ~30% of human genes (7). It has also been shown that the host
cell could produce miRNA immediately after being stimulated by the
pathogenic microorganism, thereby participating in the innate
immune response, promoting the release of inflammatory factors and
inducing immune hyperfunction (7,8).
In addition, miRNAs could induce cell apoptosis or degrade
inflammatory factors, leading to immunosuppression (8). A previous study has verified that
miRNAs are involved in the immunoregulation of sepsis, and exert
important regulatory effects (8).
miRNAs act on a wide range of target genes and participate in the
regulation of multiple biological processes, including immunity,
metabolism, differentiation, proliferation and carcinogenesis
(8). Zhang et al (9) revealed that miR-200a-3p promotes
β-amyloid-induced neuronal apoptosis in Alzheimer's disease. The
results of the present study indicated the possible molecular
mechanisms underlying the effect of miRNA-200a-3p on inflammation
during sepsis.
Materials and methods
Ethics statement and mouse model of cecal
ligation and puncture (CLP)
Male C57BL/6J mice (5-6 weeks, 18-20 g; n=12) were
obtained from Animal Research Center of Xiamen University and
housed under standard laboratory conditions at 21-23°C with 55-60%
humidity, a 12-h light/dark cycle and free access to food and
water. Mice of sepsis model were anesthetized intraperitoneally
with 35 mg/kg pentobarbital sodium. Once under anesthetization, the
abdominal area of sepsis model mice was shaved and disinfected. The
cecum was exposed for 15 min and ligatured at 2/3, and punctured
with a 27-gauge needle. The wound was then sterilized and closed by
applying a simple suture. Sham group mice were only anesthetized
intraperitoneally with 35 mg/kg pentobarbital sodium. The present
study was approved by The First Affiliated Hospital of Xiamen
University on Animal Care.
Reverse transcription-quantitative PCR
(RT-qPCR)
After 24 h of CLP, mice were anesthetized
intraperitoneally with 35 mg/kg pentobarbital sodium, and
peritoneal fluid was extracted using a sterile syringe and cultured
in RPMI-1640 supplemented with 2% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.). The single-cell suspensions were washed
with PBS three times and peritoneal macrophages (PMs) were
collected at 800 x g for 20 min at 4°C. Total RNA was extracted
with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions. Total RNA (2 μg)
was reverse transcribed into cDNA using the RealMasterMix First
Strand cDNA Synthesis kit (Tiangen Biotech Co., Ltd.) at 42°C for
60 min and 82°C for 10 sec. RT-qPCR was performed in the Applied
Biosystems 7500 Real-time PCR system using SYBR Premix ExTag™
(Takara Biotechnology Co., Ltd.) according to the manufacturer's
protocols. Data were presented as the relative expression level by
comparing Cq values (10). The
thermocycling conditions were as follows: Denaturation at 94°C for
5 min, amplification for 40 cycles at 94°C for 30 sec, annealing at
60°C for 30 sec and extension at 72°C for 1 min. qPCR was performed
using the following primers: miR-200a-3p forward
5′-AACACTGTCTGGTAACGATGTCGT-3′ and reverse,
5′-CATCTTACCGGACAGTGCTGGA-3′; U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′.
Behavior assessment
The white open field consisted of an open
square-shaped area with 30 cm high walls. Mice were placed in the
center of the field, and were observed for 5 min to measure their
locomotor activity. Testing videos were analyzed using Any-maze™
software 4.99 (Stoelting Co.). The field was filled with water to
150×50 cm in depth at 23°C and mice where then placed in the water
in different quadrants and allowed to search for the hidden
platform (1 min); if mice failed to find the platform they were
guided to it. Mice underwent 6 training trials a day with 25 min
inter-trial intervals. After 5 days of training, the mice were
given a probe test with the platform removed. Mice were released
from the west quadrant, and allowed to swim for 1 min and their
swimming paths recorded.
Gene microarray
Total RNA was extracted using the mirVana™ miRNA
Isolation kit (cat. no. AM1561; Ambion; Thermo Fisher Scientific,
Inc.). Total RNA was then amplified using the Low Input Quick Amp
WT Labeling kit (cat. no. 5190-2943; Agilent Technologies, Inc.)
according to the manufacturer's instructions. The slides were
scanned using a Agilent Microarray Scanner (cat. no. G2565CA;
Agilent Technologies, Inc.) and analyzed using Feature Extraction
software 10.7 (Agilent Technologies, Inc.).
Cell culture and transfection
Human brain microvascular endothelial cells (HBMECs)
were purchased from ScienCell Research Laboratories, Inc. and were
maintained in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a 5% CO2. incubator. HBMEC
cells (70%) were plated in 6-well plates 24 h before transfection
and were then transfected with 100 ng of miRNA-200a-3p plasmid
(5′-TAACACTGTCTGGTAACGATGT-3′ and 5′-GCGGGTCACCTTTGAACATC-3′), 100
ng of small interfering RNA (si)-NLRP3 (cat. no. sc-45469; Santa
Cruz Biotechnology, Inc.) plasmid and 100 ng of negative plasmid
(5′-TTCTCCGAACGTGTCACGT-3′ and 5′-TTCTCTAGAACGTGTCAT-3′; Sangon
Biotech Co., Ltd.) in Opti-MEM using Lipofectamine 2000™
(Invitrogen; Thermo Fisher Scientific, Inc.). The medium was
removed after 6 h. Then, at 24 h post-transfection HBMECs were
treated with 50 ng/ml lipopolysaccharide (LPS) for 2 h at 37°C, as
described previously (11).
Then, 100 ng of si-miRNA-200a-3p plasmids
(5′-ATTGTGACAGACCATTGCTACA-3′) and 100 ng of si-Keap1
(5′-TGGGGTCGTCGGTCTAGGG-3′; cat. no. sc-43878; Santa Cruz
Biotechnology, Inc.), 100 ng of si-Nrf2
(5′-AGTAGTACTACCTGAACCTC-3′; cat. no. sc-37030; Santa Cruz
Biotechnology, Inc.) or 100 ng of si-HO-1
(5′-CCTACCGCAGTAGTGATGGTAA-3′; cat. no. sc-35554; Santa Cruz
Biotechnology, Inc.) were co-transfected into cells using
Lipofectamine 2000™ (Invitrogen; Thermo Fisher Scientific, Inc.).
After 24 h of transfection, HBMECs were treated with 50 ng/ml LPS
for 2 h at 37°C.
miRNA-200a-3p plasmids were transfected into cells
using Lipofectamine 2000™ (Invitrogen; Thermo Fisher Scientific,
Inc.). After 6 h of transfection, 3 mmol/l of tempol (ROS
inhibitor; MedChemExpress) was added to cells for 18 h at 37°C and
then HBMECs were treated with 50 ng/ml LPS for 2 h at 37°C.
Measurements of intracellular reactive
oxygen species (ROS)
HBMECs were washed twice with PBS and then incubated
in PBS containing 10 μM of dichlorodihydrofluorescein
diace-tate for 30 min at 37°C. ROS levels were measured using a
microplate reader (Tecan Group, Ltd.) at 450 nm and were visualized
with an Olympus IX2-SL epifluorescence microscope (magnification,
×200; Olympus Corporation).
Luciferase reporter assay
TargetScanHuman 7.2 (www.targetscan.org) was employed to evaluate the
miR-200a-3p network signaling pathway, which indicated that NLRP3
and Keap1 expression may be regulated by miR-200a-3p. NLRP3-3′UTR
and Keap1-3′UTR were constructed and purchased from GeneCopoeia,
Inc. Cells (1×106 cell) were co-transfected with 100 ng
of the reporter constructs, NLRP3-3′UTR or Keap1-3′UTR, with 100 ng
of the aforementioned miR-200a-3p mimic or control mimic using
Lipofectamine 2000™ (Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Luciferase activity was measured
using a dual luciferase reporter assay kit (GeneCopoeia, Inc.)
after 48 h of transfection. Normalization was performed via
comparisons with Renilla luciferase activity.
Western blotting
HBMECs, after transfection, were lysed using
radioimmunoprecipitation assay (RIPA) buffer (Beyotime Institute of
Biotechnology) and phosphatase inhibitors (Beyotime Institute of
Biotechnology). The supernatants were collected after
centrifugation at 10,000 x g for 15 min at 4°C and used to
quantitate protein concentration using BCA (Beyotime Institute of
Biotechnology). An equal amount (50 μg) of protein was
applied to 8-12% SDS-PAGE and transferred onto nitrocellulose
filter membranes (Thermo Fisher Scientific, Inc.). Membranes were
blocked with 0.1% TBST with 5% skim milk powder for 1 h at room
temperature and incubated with primary antibodies against NLRP3
(cat. no. 13158; 1:1,000; Cell Signaling Technology, Inc.),
caspase-1 (cat. no. 24232; 1:1,000; Cell Signaling Technology,
Inc.), Keap1 (cat. no. 8047; 1:1,000; Cell Signaling Technology,
Inc.), Nrf2 (cat. no. 12721, 1:1,000; Cell Signaling Technology,
Inc.), HO-1 (cat. no. 86806; 1:1,000; Cell Signaling Technology,
Inc.) and GAPDH (cat. no. 5174; 1:2,000; Cell Signaling Technology,
Inc.) at 4°C overnight. Membranes were washed with TBST and then
incubated with anti-rabbit immunoglobulin G horseradish
peroxidase-linked secondary antibody (cat. no. D110058; 1:5,000;
Sangon Biotech Co., Ltd.) for 1 h at 37°C. Protein bands were
visualized using enhanced chemiluminescence (cat. no. C500044;
Sangon Biotech Co., Ltd.) and analyzed using the Odyssey™ Infrared
Imaging System (version 3.0; Gene Company, Ltd.). GAPDH protein
expression was used as an internal control to show equal loading of
the protein bands.
Enzyme-linked immunosorbent assay
(ELISA)
HBMECs after transfection were lysed with RIPA
buffer (Beyotime Institute of Biotechnology) and phosphatase
inhibitors (Beyotime Institute of Biotechnology). The supernatants
were collected after centrifugation at 10,000 × g for 15 min at 4°C
and protein concentration was measured by BCA (Beyotime Institute
of Biotechnology). An equal amount (10 μg) was then used to
measure the cytokine concentrations of TNF-α (cat. no. H052), IL-1β
(cat. no. H002), IL-6 (cat. no. H007) and IL-18 (cat. no. H015) by
ELISA according to the manufacturer's instructions (Nanjing
Jiancheng Bioengineering Institute).
Statistical analysis
Data are presented as the mean ± standard error of
the mean for each group of 3 experimental repeats using SPSS 17.0
(SPSS, Inc.). Differences between groups were compared by Student's
t-test or one-way analysis of variance with Tukey's post hoc test
for multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miRNA-200a-3p in the sepsis
model
The present study first explored whether the
expression of miRNA-200a-3p was different between the sepsis model
and the normal group. The results revealed that the expression of
TNF-α, IL-1β, IL-6 and IL-18 were significantly increased in the
sepsis model compared with sham group (Fig. 1A-D). The escape latency was
markedly longer in the sepsis model group when compared with that
of the sham group (Fig. 1E).
However, the number of crossings and the time in the target
quadrant was lower in the sepsis model group than in the sham group
(Fig. 1F-H). In addition, the
number of neuronal cells was lower in the hippocampal tissue of the
sepsis model compared with the sham group (Fig. 1I). As shown in Fig. 1J and K, the expression of
miRNA-200a-3p was significantly higher in the sepsis model group
than in the sham group.
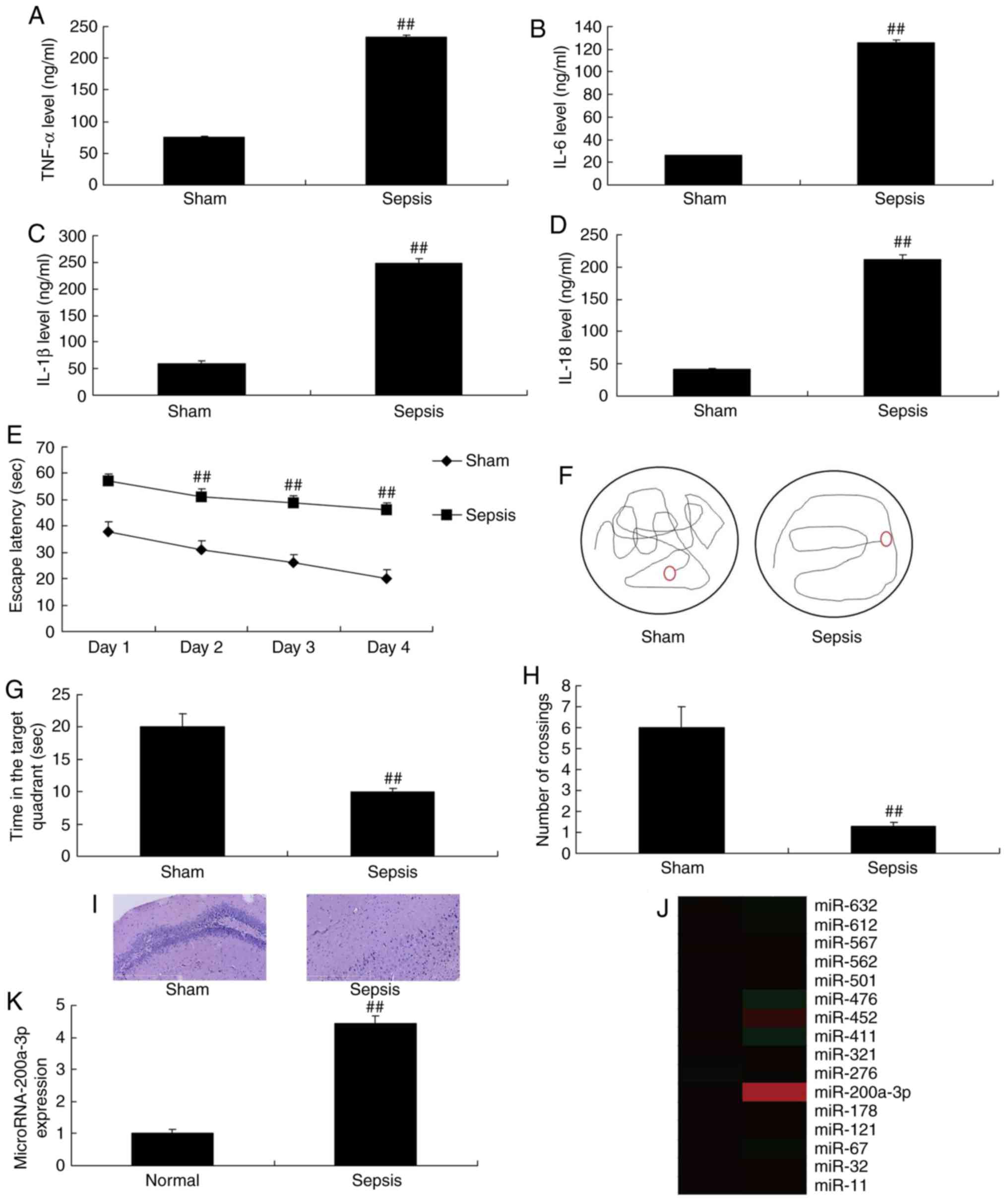 | Figure 1Expression of miRNA-200a-3p in sepsis
model. (A) TNF-α, (B) IL-6, (C) IL-1β and (D) IL-18 levels were
determined by ELISA. The (E) escape latency, (F) tracking maps, (G)
the time in the target quadrant and (H) the number of crossings
were analyzed via behavior assessments. (I) Evaluation of the
number of neuronal cells in hippocampal tissues (magnification,
×100), (J) gene chip analysis and (K) reverse
transcription-quantitative PCR for miRNA-200a-3p expression were
also conducted. Data are presented as the mean ± standard error of
the mean. ##P<0.01 vs. sham group. Sham, sham group;
Sepsis, sepsis model group; TNF-α, tumor necrosis factor-α; IL,
interleukin; miRNA/miR, microRNA. |
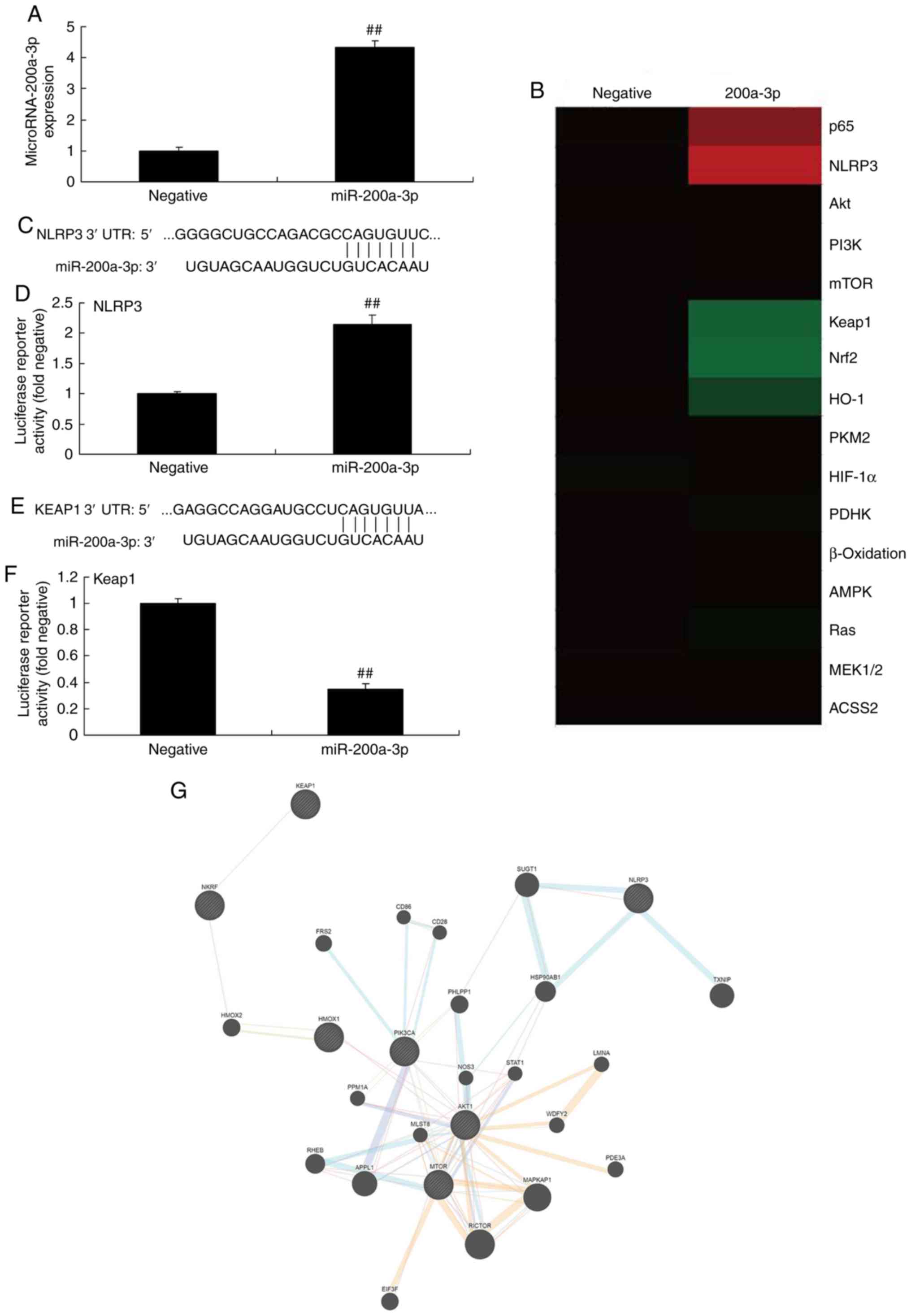
miRNA-200a-3p regulates NLRP3 and Keap1
expression in vitro
The present study examined the mechanism of
miRNA-200a-3p in vitro. miRNA-200a-3p mimics were used to
increase the expression of miRNA-200a-3p, compared with the
negative group (Fig. 2A). The
results of the heat map revealed that the overexpression of
miRNA-200a-3p increased the expression of p65 and NLRP3, but
reduced that of Keap1, Nrf2 and HO-1 in vitro, when in
comparison with the negative group (Fig. 2B). The 3'-UTR of NLRP3 mRNA was
the binding site of miRNA-200a-3p, and the luciferase assay
activity levels were increased in the miRNA-200a-3p group, compared
with the negative group (Fig. 2C
and D). Moreover, the 3′-UTR of Keap1 mRNA was the binding site of
miRNA-200a-3p, and the luciferase assay activity levels were
reduced in the miRNA-200a-3p group when compared with the negative
group (Fig. 2E and F). The
network signaling pathway suggested that miRNA-200a-3p may regulate
the expression of NLRP3 and Keap1 in vitro (Fig. 2G). Overexpression of miRNA-200a-3p
significantly suppressed the protein expression of Keap1, Nrf2 and
HO-1, and significantly induced that of NLRP3 and caspase-1 in
vitro, when in comparison with negative group (Fig. 3A-F). Overexpression of
miRNA-200a-3p significantly increased the levels of ROS, IL-1β and
IL-18 in vitro, compared with the negative group (Fig. 3G-J).
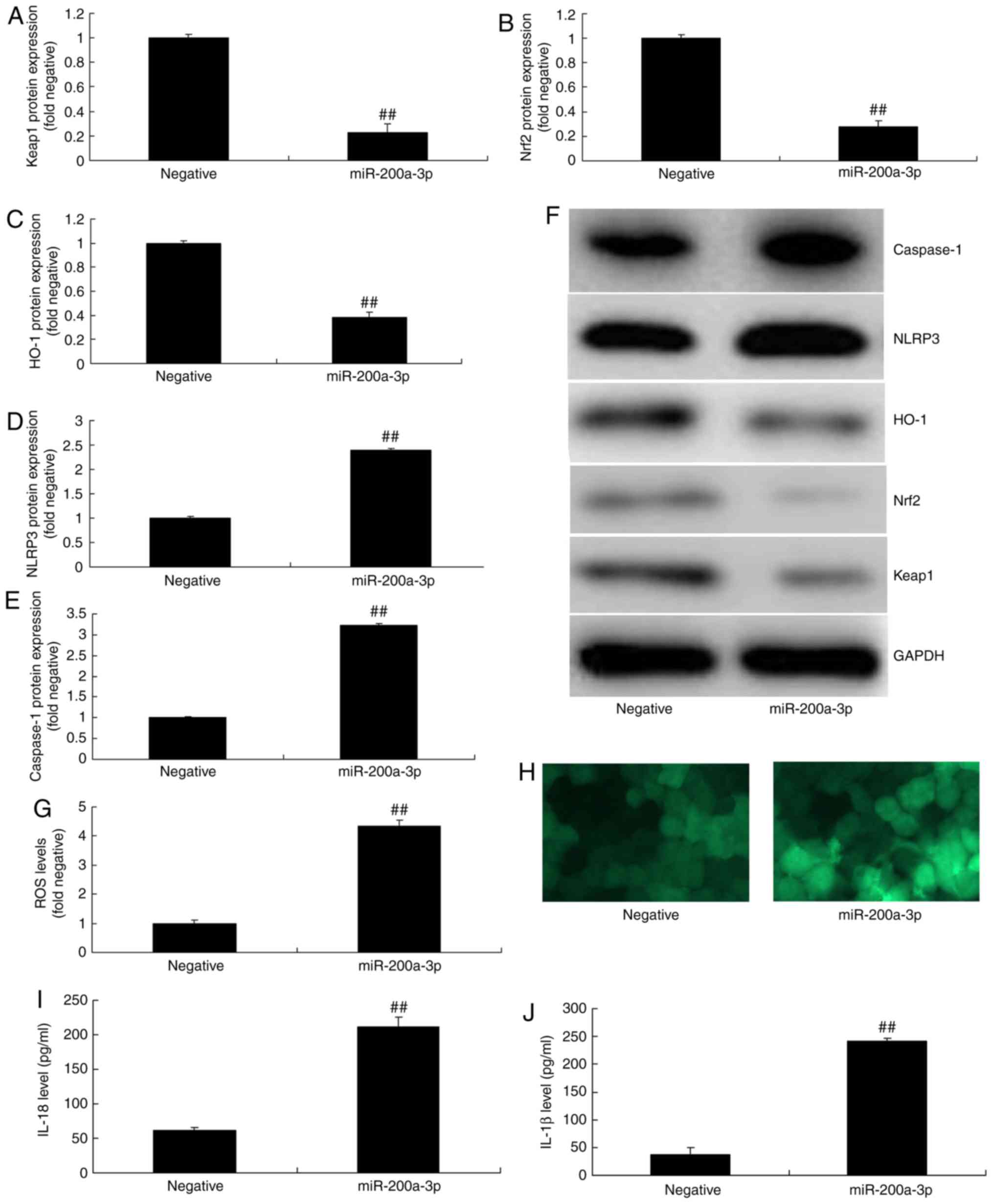 | Figure 3Effect of miRNA-200a-3p overexpression
on inflammation in vitro. (A) Keap1, (B) Nrf2, (C) HO-1, (D)
NLRP3 and (E) caspase-1 protein expressions were determined by (F)
western blotting analysis. (G and H) ROS levels (magnification,
×200), and (I) IL-18 and (J) IL-1β levels were also assessed. Data
are presented as the mean ± standard error of the mean.
##P<0.01 vs. negative group. Negative, negative
group; miR-200a-3p, miR-200a-3p overexpression group; miRNA/miR,
microRNA; NLRP3, NLR family pyrin domain containing 3; Keap1, Kelch
like ECH associated protein-1; Nrf2, nuclear factor erythroid 2
like 2; HO-1, heme oxygenase; ROS, reactive oxygen species; IL,
interleukin. |
Downregulation of miRNA-200a-3p
expression on inflammation in vitro
To further explore the effects and mechanism of
anti-miRNA-200a-3p on inflammation in vitro,
anti-miRNA-200a-3p mimics was utilized to reduce the expression of
miRNA-200a-3p in vitro, when compared with negative group
(Fig. 4A). In addition, the
protein expression of Keap1, Nrf2 and HO-1 was significantly
increased, while that of NLRP3 and caspase-1 were significantly
decreased in vitro following the downregulation of
miRNA-200a-3p, compared with negative group (Fig. 4B-G). Downregulation of
miRNA-200a-3p also reduced significantly ROS levels and inhibited
the levels of IL-1β and IL-18 in vitro, when compared with
the negative group (Fig.
4H-K).
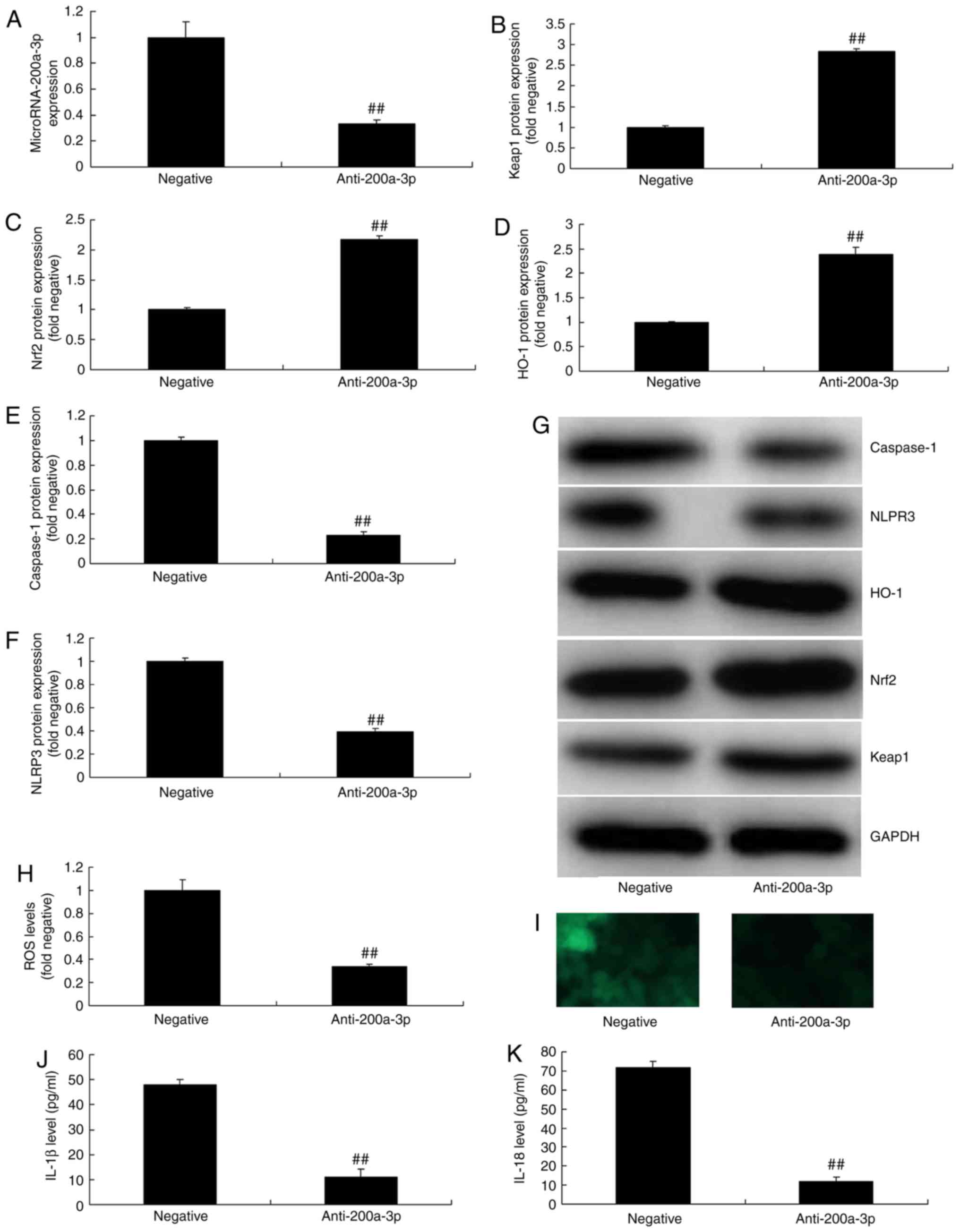 | Figure 4Effect of miRNA-200a-3p downregulation
on inflammation in vitro. (A) miRNA-200a-3p expression
following anti-miRNA-200a-3p plasmid trans-fection. (B) Keap1, (C)
Nrf2, (D) HO-1, (E) caspase-1 and (F) NLRP3 protein expressions
were determined by (G) western blotting analysis. (H and I) ROS
levels (magnification, ×200), and (J) IL-1β and (K) IL-18 levels
were also assessed. Data are presented as the mean ± standard error
of the mean. ##P<0.01 vs. negative group. Negative,
negative group; anti-200a-3p, miR-200a-3p downregulation group;
miRNA/miR, microRNA; NLRP3, NLR family pyrin domain containing 3;
Keap1, Kelch like ECH associated protein-1; Nrf2, nuclear factor
erythroid 2 like 2; HO-1, heme oxygenase; ROS, reactive oxygen
species; IL, interleukin. |
Inhibition of Keap1 attenuates the
effects of anti-miRNA-200a-3p on inflammation in vitro
In addition, the present study investigated whether
Keap1 was a key signaling mediator and if it played important roles
in the effect of miRNA-200a-3p on sepsis. si-Keap1 significantly
suppressed the protein expression of Keap1, Nrf2 and HO-1, and
significantly induced that of NLRP3 and caspase-1 in vitro
following the downregulation of miRNA-200a-3p when compared with
the miRNA-200a-3p downregulation group (Fig. 5A-F). Moreover, si-Keap1
significantly increased ROS levels and significantly promoted the
levels of IL-1β and IL-18 in vitro following the
downregulation of miRNA-200a-3p, compared with the miRNA-200a-3p
downregulation group (Fig.
5G-J).
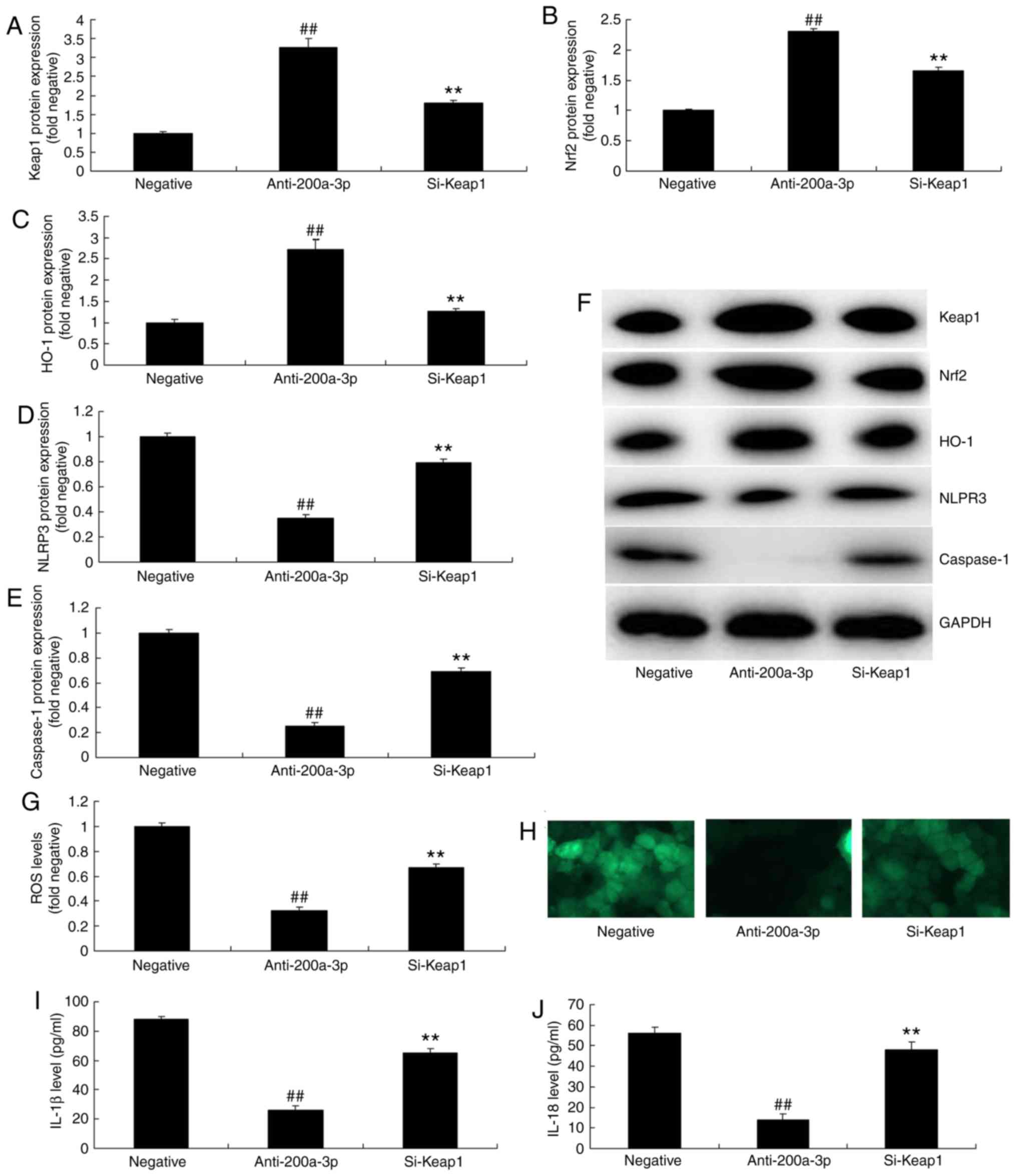 | Figure 5Inhibition of Keap1 reduces the
effects of anti-miRNA-200a-3p on inflammation in vitro. (A)
Keap1, (B) Nrf2, (C) HO-1, (D) NLRP3 and (E) caspase-1 protein
expressions were determined by (F) western blotting analysis. The
levels of (G and H) ROS (magnification, x200), (I) IL-1β and (J)
IL-18 were also assessed. Data are presented as the mean ± standard
error of the mean. ##P<0.01 vs. negative group;
**P<0.01 vs. miR-200a-3p downregulation group.
Negative, negative group; anti-200a-3p, miR-200a-3p downregulation
group; si-Keap1, downregulation of miR-200a-3p and si-Keap1 group;
si-, small interfering RNA; miRNA/miR, microRNA; NLRP3, NLR family
pyrin domain containing 3; Keap1, Kelch like ECH associated
protein-1; Nrf2, nuclear factor erythroid 2 like 2; HO-1, heme
oxygenase; ROS, reactive oxygen species; IL, interleukin. |
Inhibition of Nrf2 attenuates the effects
of anti-miRNA- 200a-3p on inflammation in vitro
To elucidate the role of Nrf2 in the effects of
anti-miRNA-200a-3p on inflammation in vitro, si-Nrf2 was
administered, which suppressed the protein expression of Nrf2 and
HO-1, and induced that of NLRP3 and caspase-1 in vitro
following the downregulation of miRNA-200a-3p, in comparison with
the miRNA-200a-3p downregulation group (Fig. 6A-E). si-Nrf2 also significantly
promoted ROS levels and significantly increased the levels IL-1β
and IL-18 in vitro following the downregulation of
miRNA-200a-3p, compared with the miRNA-200a-3p downregulation group
(Fig. 6F-I).
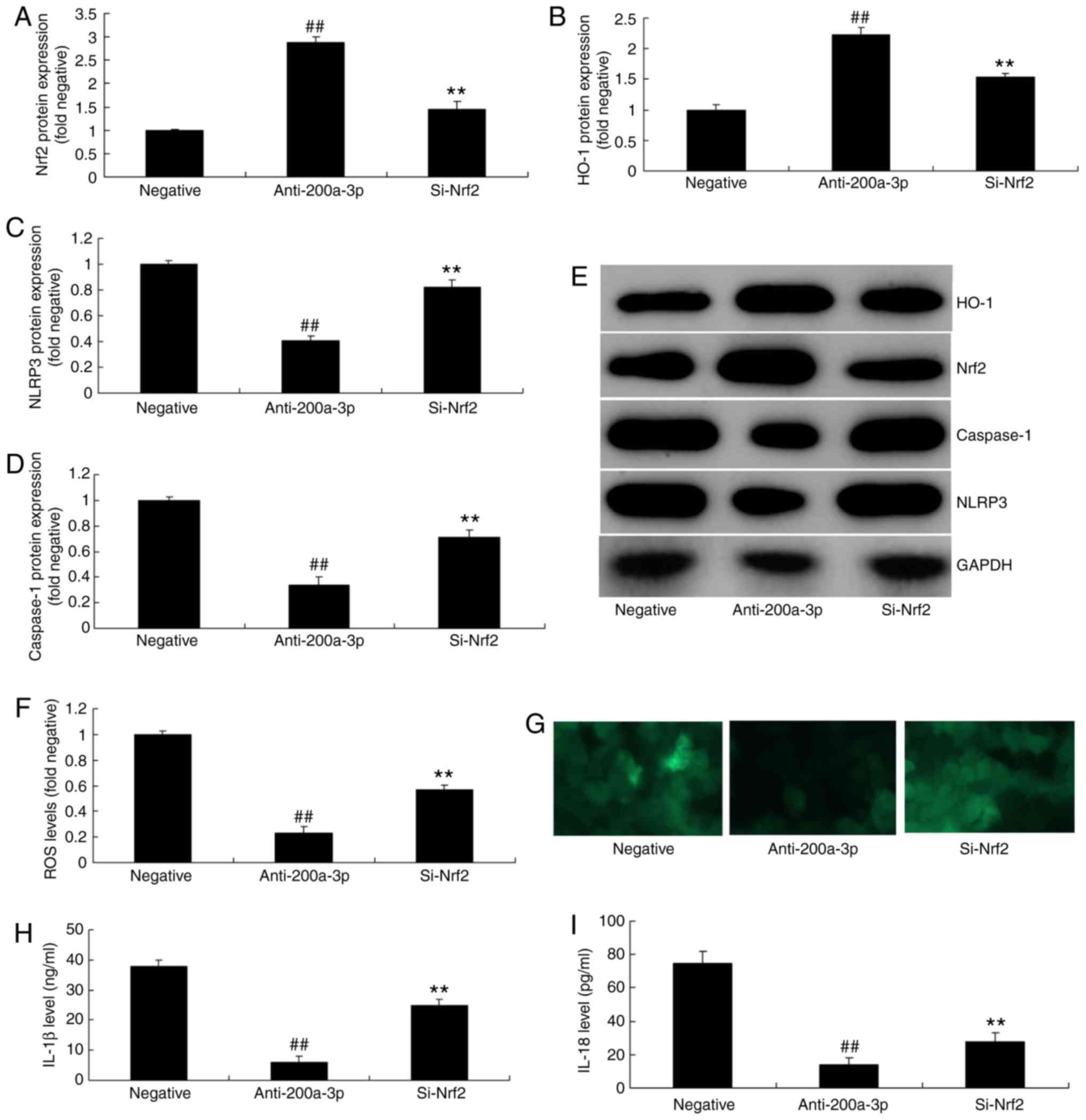 | Figure 6Inhibition of Nrf2 reduces the effects
of anti-miRNA-200a-3p on inflammation in vitro. (A) Nrf2,
(B) HO-1, (C) NLRP3 and (D) caspase-1 protein expressions were
determined by (E) western blot analysis. The levels of (F and G)
ROS (magnification, x200), (H) IL-1β and (I) IL-18 were also
assessed. Data are presented as the mean ± standard error of the
mean. ##P<0.01 vs. negative group;
**P<0.01 vs. miR-200a-3p downregulation group.
Negative, negative group; anti-200a-3p, miR-200a-3p downregulation
group; si-Nrf2, downregulation of miR-200a-3p and si-Nrf2 group;
si-, small interfering RNA; miRNA/miR, microRNA; NLRP3, NLR family
pyrin domain containing 3; Keap1, Kelch like ECH associated
protein-1; Nrf2, nuclear factor erythroid 2 like 2; HO-1, heme
oxygenase; ROS, reactive oxygen species; IL, interleukin. |
Inhibition of HO-1 attenuates the effects
of anti-miRNA-200a-3p on inflammation in vitro
To further elucidate the roles of HO-1 in the
effects of anti-miRNA-200a-3p on inflammation in vitro,
si-HO-1 was applied to reduce the protein expression of HO-1.
si-HO-1 administration induced the expression of NLRP3 and
caspase-1 in vitro following the downregulation of
miRNA-200a-3p, in comparison with the miRNA-200a-3p downregulation
group (Fig. 7A-F). The
application of si-HO-1 significantly promoted ROS levels, and
increased IL-1β and IL-18 levels in vitro following the
downregulation of miRNA-200a-3p, compared with the miRNA-200a-3p
downregulation group (Fig.
7E-H).
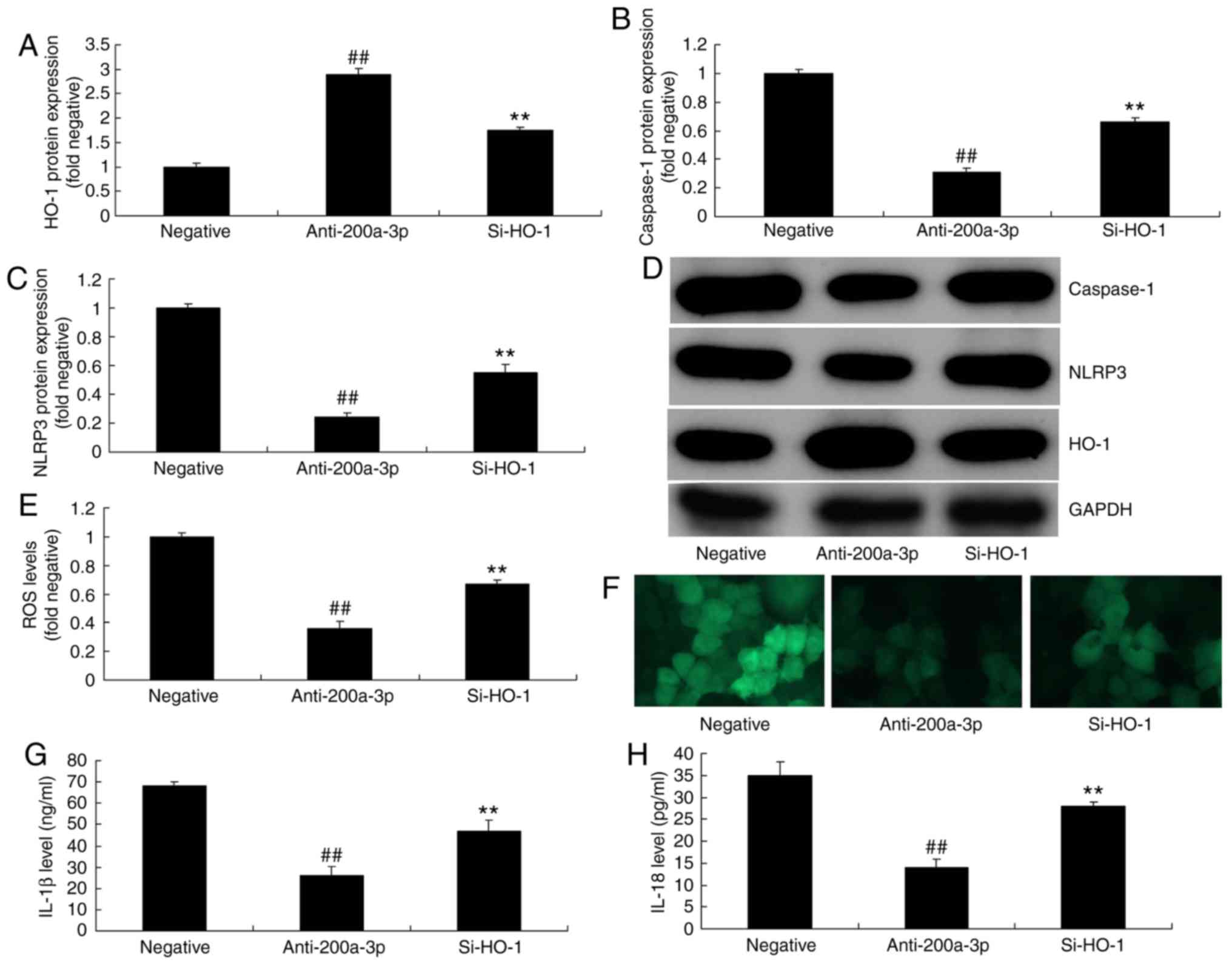 | Figure 7Inhibition of HO-1 reduces the effects
of anti-miRNA-200a-3p on inflammation in vitro. (A) HO-1,
(B) caspase-1 and (C) NLRP3 protein expressions were determined by
(D) western blot analysis. The levels of (E and F) ROS
(magnification, x200), (G) IL-1β and (H) IL-18 were also assessed.
Data are presented as the mean ± standard error of the mean.
##P<0.01 vs. negative group; **P<0.01
vs. miR-200a-3p downregulation group. Negative, negative group;
anti-200a-3p, miR-200a-3p downregulation group; si-HO-1,
downregulation of miR-200a-3p and si-HO-1 group; si-, small
interfering RNA; miRNA/miR, microRNA; NLRP3, NLR family pyrin
domain containing 3; Keap1, Kelch like ECH associated protein-1;
Nrf2, nuclear factor erythroid 2 like 2; HO-1, heme oxygenase; ROS,
reactive oxygen species; IL, interleukin. |
ROS is involved in the effect of
miRNA-200a-3p on inflammation in vitro
The present study further investigated the
mechanisms of miRNA-200a-3p in ROS-regulated inflammation in
vitro. As shown in Fig. 8A-E,
the administration of ROS inhibitor (3 mmol/l tempol) reduced ROS
levels, and significantly suppressed the protein expression of
caspase-1 and NLRP3 in vitro following the overexpression of
miRNA-200a-3p, compared with the miRNA-200a-3p over-expression
group. The inhibition of ROS also attenuated the effect of
miRNA-200a-3p on IL-1β and IL-18 levels in vitro, when
compared with the miRNA-200a-3p overexpression group (Fig. 8F-G).
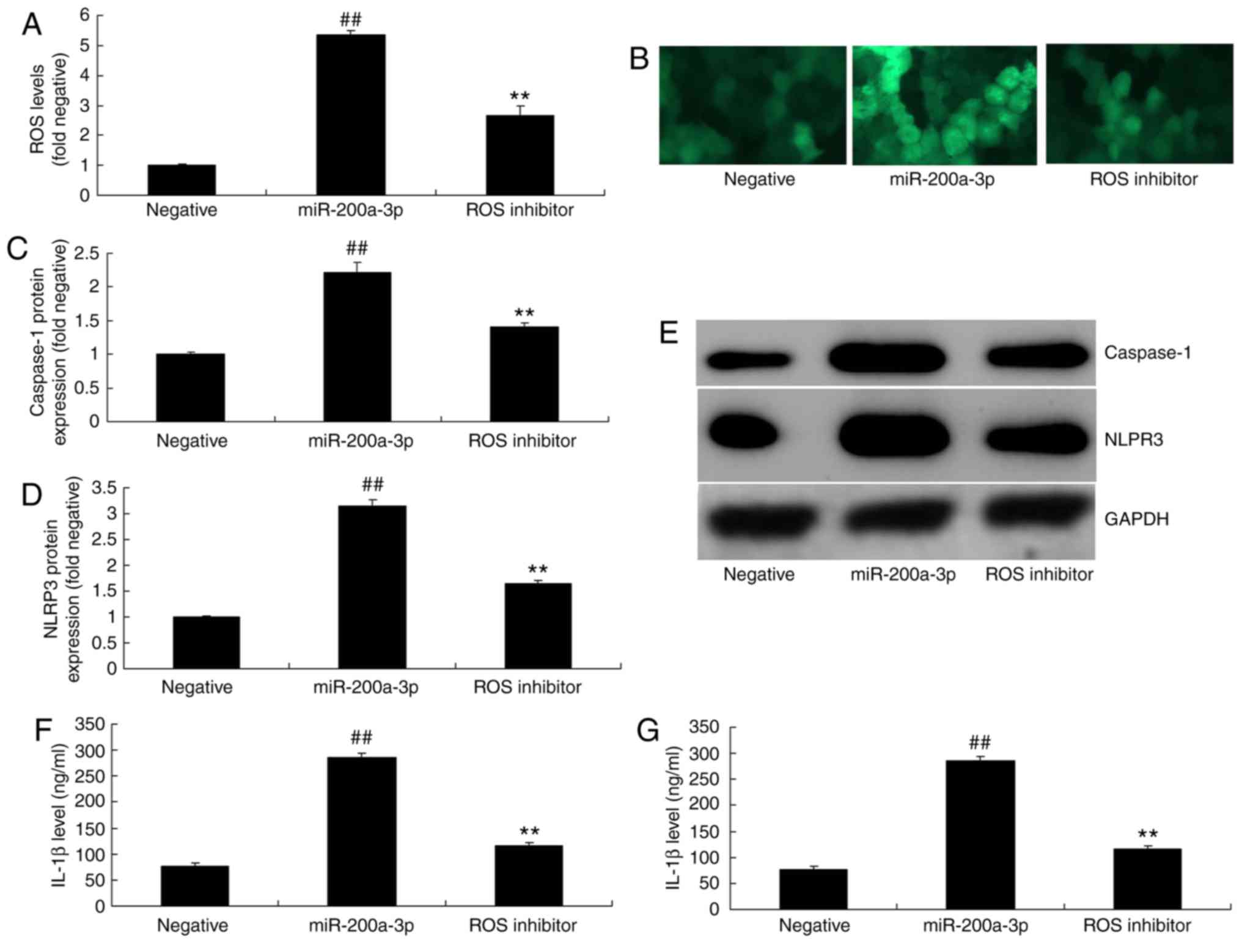 | Figure 8ROS participates in the effect of
miRNA-200a-3p on inflammation in vitro. (A and B) ROS levels
were assessed in cells treated with miR-200a-3p plasmid and ROS
inhibitor (magnification, ×200). (C) Caspase-1 and (D) NLRP3
protein expressions were determined by (E) western blotting
analysis. (F) IL-1β and (G) IL-18 levels were also determined. Data
are presented as the mean ± standard error of the mean.
##P<0.01 vs. negative group; **P<0.01
vs. miR-200a-3p overexpression group. Negative, negative group;
miR-200a-3p, miR-200a-3p overexpression group; ROS inhibitor,
overexpression of miR-200a-3p and ROS inhibitor group; miR,
microRNA; NLRP3, NLR family pyrin domain containing 3; Keap1, Kelch
like ECH associated protein-1; Nrf2, nuclear factor erythroid 2
like 2; HO-1, heme oxygenase; ROS, reactive oxygen species; IL,
interleukin. |
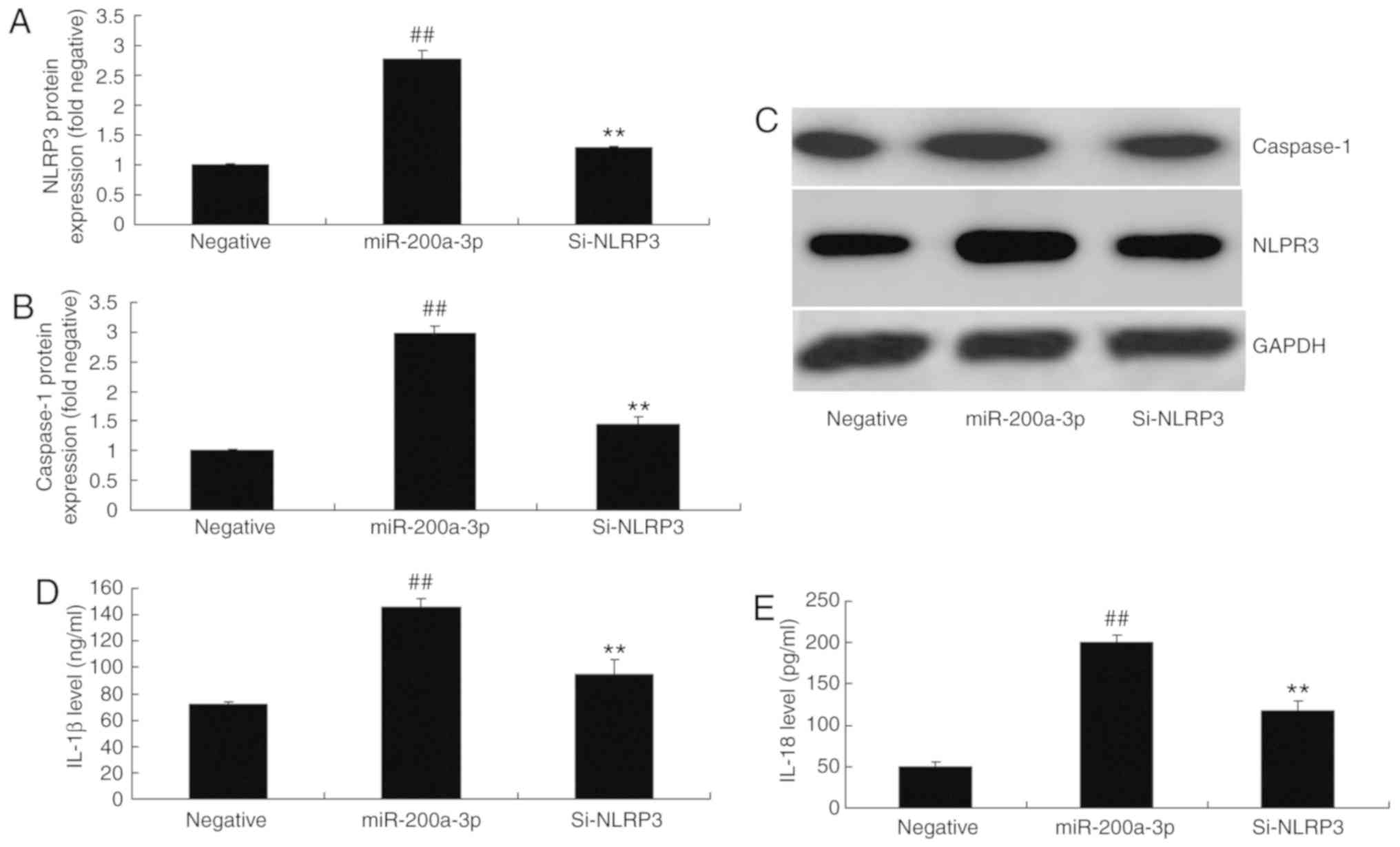
Inhibition of NLRP3 attenuates the
effects of miRNA-200a-3p on inflammation in vitro
To further elucidate the roles of NLRP3 in the
effects of miRNA-200a-3p on inflammation in vitro, si-NLRP3
was employed to reduce the protein expression of NLRP3 and
caspase-1 in vitro following the overexpression of
miRNA-200a-3p, when in comparison with the miRNA-200a-3p
overexpression group (Fig. 9A-C).
si-NLRP3 also reduced IL-1β and IL-18 levels in vitro
following the overexpression of miRNA-200a-3p, compared with the
miRNA-200a-3p overexpression group (Fig. 9D and E).
Discussion
As a type of systemic inflammatory response syndrome
induced by the imbalance between the pro- and anti-inflammatory
mechanism, sepsis is generally caused by severe infection, burns,
trauma, shock and major surgery (12). The major pathophysiological
process of sepsis is the intense self-destructive systemic
inflammatory response caused by the excessive expression of the
pro-inflammatory factors and other inflammatory mediators (13). Sepsis often leads to serious
pathophysiological alterations in the vital organs (14). The mortality of sepsis patients
with sepsis shock and multiple organ dysfunction has been suggested
to be as high as 80-90% (2). To
the best of our knowledge, the present study demonstrated for the
first time that the expression of miRNA-200a-3p was higher in the
sepsis model group than in the sham group.
miRNAs can enhance the immunocompetence of the body
mainly by regulating the synthesis and release of inflammatory
factors (15). In the case of
sepsis, miRNAs can upregulate the expression of miRNA-155, enhance
the release of inflammatory factors and inhibit the synthesis of
anti-inflammatory factors (15).
In the pathophysiological process of sepsis, miRNAs can promote the
synthesis of inflammatory factors, enhance immune function, induce
the apoptosis of immune cells and induce immunosuppression
(16). miRNAs are involved in the
precise regulation of the genesis, development and outcome of
sepsis at various levels, such as the inflammatory response, immune
cell differentiation and apoptosis. It is speculated that these
miRNAs may possess regulatory effects on the differentiation of the
effector T-cell (16). Thus, the
present study next determined that overexpression of miRNA-200a-3p
promoted the levels of IL-1β and IL-18 in vitro.
ROS, key cytokines in the inflammatory response, are
produced early in the inflammatory response, reaching a peak
rapidly, and in turn inducing the production of downstream
cytokines, such as NLRP1, NLRP3, IL-1β, IL-6 and IL-8, thereby
mediating a series of inflammatory cascade reactions (17). ROS, which are considered to be
advanced stage inflammatory factors, play a key role in the lethal
process of severe sepsis, which is also characterized by delayed
secretion and long release time in relation to NLRP3 (18). In addition, the present study
demonstrated that overexpression of miRNA-200a-3p increased ROS
levels and promoted IL-1β and IL-18 levels in vitro. Xiao
et al (19) indicated that
the p38/p53/miR-200a-3p feedback loop promotes oxidative
stress-mediated liver cell death. The present study revealed only
the effects of miRNA-200a-3p on inflammation, and as ROS also
regulates oxidative stress, this is experiment alone is
insufficient; we will analyze the effects of miRNA-200a-3p on
oxidative stress in a future study.
NLRP3 is an important factor for amplifying and
continuing inflammation as it can promote the activation,
differentiation and infiltration of macrophages, upregulate the
expression of adhesion molecules, enhance the inflammatory
reaction, promote the activation and aggregation of neutrophils,
release a large amount of elastase and oxygen free radicals (which
result in lung capillary endothelial cell and alveolar epithelial
cells injury), vessel extracellular matrix destruction, increased
pulmonary vascular permeability, and they can also induce severe
alveoli and pulmonary interstitial edema (4,20).
NLRP3 is an important factor that results in acute respiratory
distress syndrome, which can be used to evaluate the degree of
inflammatory response in patients with systemic infection and can
serve as a monitoring index for inflammation treatment (4). The present data revealed that the
overexpression of miRNA-200a-3p suppressed the protein expression
of Keap1, Nrf2 and HO-1 and induced that of NLRP3 and caspase-1
in vitro. The inhibition of NLRP3 attenuated the effects of
miRNA-200a-3p on inflammation in vitro. Furthermore, Ding
et al (21) reported that
curcumin and allopurinol ameliorated fructose-induced hepatic
inflammation via miR-200a-mediated thioredoxin interacting
protein/NLRP3 inflammasome inhibition in rats.
In recent years, an increasing amount of attention
has been paid to the signal transduction mechanism during the
pathogenesis and development of sepsis (22). A recent discovery has indicated
that the Keap1/Nrf2/HO-1 signaling pathway played an important role
in ROS transduction (22). The
Keap1/Nrf2/HO-1 pathway can mediate multiple-cytokine-regulated
cell growth, differentiation, proliferation and apoptosis, thereby
playing a key role in the process of sepsis, driving ROS
production; Keap1/Nrf2/HO-1 also plays a critical role in the ROS
mediator response (22). In
addition, the Keap1/Nrf2/HO-1 signaling pathway plays an important
role in the development of the multiple-organ dysfunction of sepsis
(23). In the present study, the
inhibition of Keap1, Nrf2 or HO-1 attenuated the effects of
anti-miRNA-200a-3p on inflammation in vitro. Wei et
al (24) suggested that
miR-200a-3p/141-3p coordinated Keap1-Nrf2 signaling in renal
mesangial cells and the renal cortex of diabetic mice. Therefore,
these results indicated that Keap1/Nrf2/HO-1-regulated
miRNA-200a-3p in sepsis.
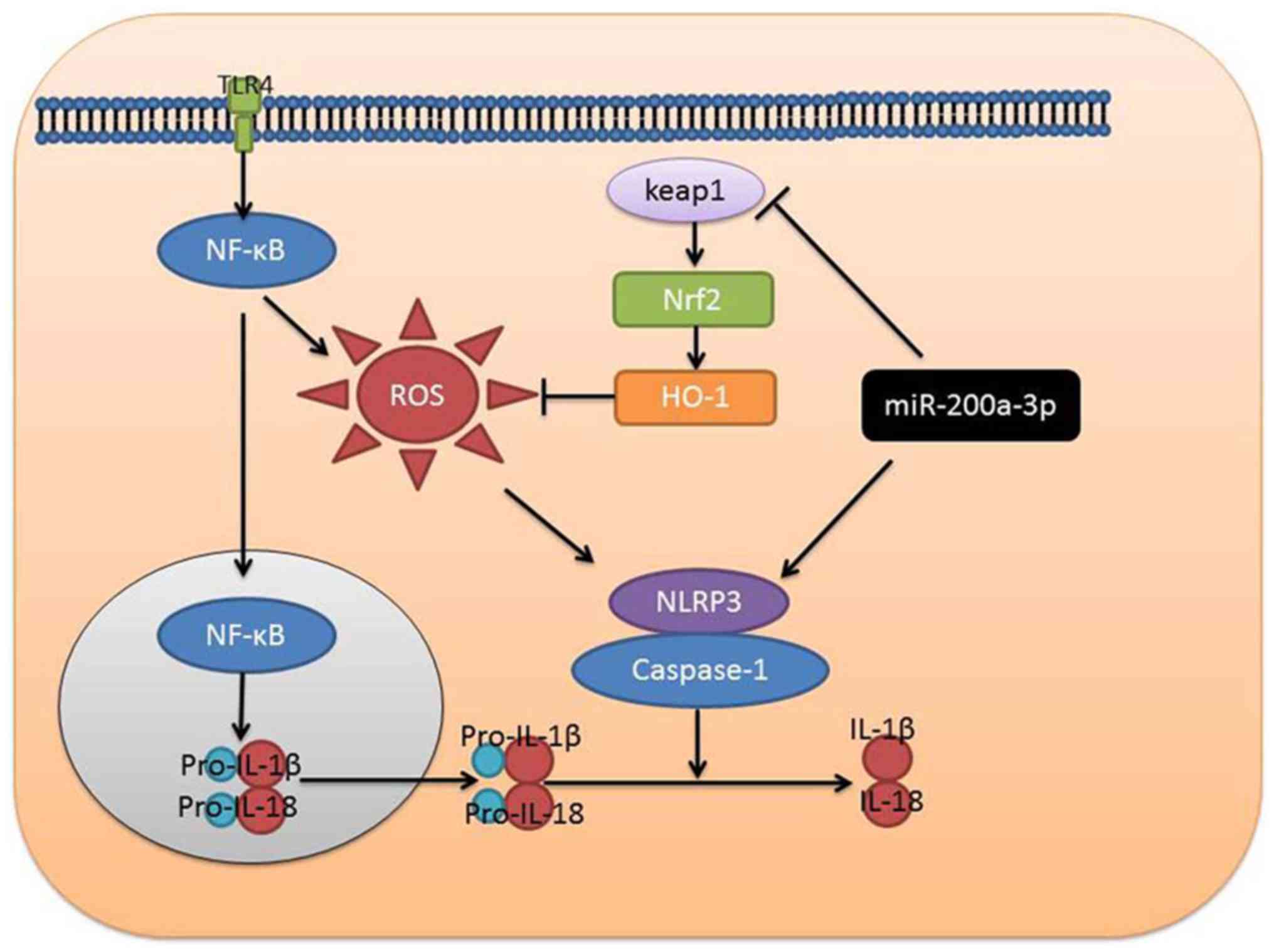
In conclusion, the present study examined the roles
of miRNA-200a-3p in sepsis-induced brain injury. The results
demonstrated that miRNA-200a-3p promoted sepsis through
Keap1/Nrf2/HO-1/ROS-induced NLRP3 in sepsis-induced brain injury
(Fig. 10). Therefore, the
present findings indicate that miRNA-200a-3p may provide a better
understanding of sepsis-induced brain injury and help to identify
potential therapeutic targets for sepsis-induced brain injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data sets generated and/or analyzed during the
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW designed the experiment, analyzed the data and
wrote the manuscript. JY, JC, SC and HY performed the experiments.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The First
Affiliated Hospital of Xiamen University on Animal Care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Xie J, Liu JH, Liu H, Liao XZ, Chen Y, Lin
MG, Gu YY, Liu TL, Wang DM, Ge H and Mo SL: Tanshinone IIA combined
with adriamycin inhibited malignant biological behaviors of NSCLC
A549 cell line in a synergistic way. BMC Cancer. 16:8992016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang XT, Lu YX, Sun YH, He WK, Liang JB
and Li L: TAK-242 Protects against apoptosis in coronary
microem-bolization-induced myocardial injury in rats by suppressing
TLR4/NF-kappaB signaling pathway. Cell Physiol Biochem.
41:1675–1683. 2017. View Article : Google Scholar
|
|
3
|
Trojsi F, Christidi F, Migliaccio R,
Santamaria-Garcia H and Santangelo G: Behavioural and cognitive
changes in neurodegenerative diseases and brain injury. Behav
Neurol. 2018.4935915:2018.
|
|
4
|
Huang Z, Shi T, Zhou Q, Shi S, Zhao R, Shi
H, Dong L, Zhang C, Zeng K, Chen J and Zhang J: miR-141 Regulates
colonic leukocytic trafficking by targeting CXCL12beta during
murine colitis and human Crohn's disease. Gut. 63:1247–1257. 2014.
View Article : Google Scholar
|
|
5
|
Saito S, Thuc LC, Teshima Y, Nakada C,
Nishio S, Kondo H, Fukui A, Abe I, Ebata Y, Saikawa T, et al:
Glucose fluctuations aggravate cardiac susceptibility to
Ischemia/Reperfusion injury by modulating micrornas expression.
Circ J. 80:186–195. 2016. View Article : Google Scholar
|
|
6
|
Roberts RL, Topless RK, Phipps-Green AJ,
Gearry RB, Barclay ML and Merriman TR: Evidence of interaction of
CARD8 rs2043211 with NALP3 rs35829419 in Crohn's disease. Genes
Immun. 11:351–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong YF, Chen ZZ, Zhao Z, Yang DD, Yan H,
Ji J and Sun XL: Potential role of microRNA-7 in the
anti-neuroinflammation effects of nicorandil in astrocytes induced
by oxygen-glucose deprivation. J Neuroinflammation. 13:602016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu HL, Wei YJ, Jin ZG, Zhang J, Ding P,
Yang SL, Luo JP, Ma DX, Liu Y and Han W: Design and rationale of
the APELOT trial: A randomized, open-label, multicenter, phase IV
study to evaluate the antiplatelet effect of different loading dose
of ticagrelor in patients with non-st acute coronary syndrome
undergoing percutaneous coronary intervention. Medicine
(Baltimore). 95:e37562016. View Article : Google Scholar
|
|
9
|
Zhang QS, Liu W and Lu GX: miR-200a-3p
promotes b-Amyloid-induced neuronal apoptosis through
down-regulation of SIRT1 in Alzheimer's disease. J Biosci.
42:397–404. 2017. View Article : Google Scholar
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
11
|
Yang ML, Li JJ, So KF, Chen JY, Cheng WS,
Wu J, Wang ZM, Gao F and Young W: Efficacy and safety of lithium
carbonate treatment of chronic spinal cord injuries: A
double-blind, randomized, placebo-controlled clinical trial. Spinal
Cord. 50:141–146. 2012. View Article : Google Scholar
|
|
12
|
Lin C, Liu Z, Lu Y, Yao Y, Zhang Y, Ma Z,
Kuai M, Sun X, Sun S, Jing Y, et al: Cardioprotective effect of
Salvianolic acid B on acute myocardial infarction by promoting
autophagy and neovascularization and inhibiting apoptosis. J Pharm
Pharmacol. 68:941–952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai L, Wang H and Yang Q: CRKL
overexpression promotes cell proliferation and inhibits apoptosis
in endometrial carcinoma. Oncol Lett. 13:51–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh SK, Banerjee S, Acosta EP, Lillard
JW and Singh R: Resveratrol induces cell cycle arrest and apoptosis
with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and
p27KIP1 pathway. Oncotarget. 8:17216–17228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee Y, Kim E, Kim BK and Shin JH: A case
of successful reperfusion through a combination of intracoronary
thrombolysis and aspiration thrombectomy in ST-segment elevation
myocardial infarction associated with an ectatic coronary artery.
BMC Cardiovasc Disord. 17:942017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gwag HB, Kim EK, Park TK, Lee JM, Yang JH,
Song YB, Choi JH, Choi SH, Lee SH, Chang SA, et al:
Cardioprotective effects of intracoronary morphine in ST-Segment
elevation myocardial infarction patients undergoing primary
percutaneous coronary intervention: A prospective, randomized
trial. J Am Heart Assoc. 6:e0054262017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dalmau Llorca MR, Goncalves AQ and
Forcadell Drago E: Fernández-Sáez J, Hernández Rojas Z, Pepió
Vilaubí JM, Rodríguez Cumplido D, Morral Parente RM and Aguilar
Martín C: A new clinical decision support tool for improving the
adequacy of anticoagulant therapy and reducing the incidence of
stroke in nonvalvular atrial fibrillation: A randomized clinical
trial in primary care. Medicine (Baltimore). 97:e95782018.
View Article : Google Scholar
|
|
18
|
Khan MT, Ikram A, Saeed O, Afridi T, Sila
CA, Smith MS, Irshad K and Shuaib A: Deep vein thrombosis in acute
stroke-a systemic review of the literature. Cureus.
9:e19822017.
|
|
19
|
Xiao Y, Yan W, Lu L, Wang Y, Lu W, Cao Y
and Cai W: p38/p53/miR-200a-3p feedback loop promotes oxidative
stress-mediated liver cell death. Cell Cycle. 14:1548–1558. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Costa CL, Spilborghs GM, Martins MA,
Saldiva PH and Mauad T: Nitric acid-induced bronchiolitis in rats
mimics childhood Bronchiolitis obliterans. Respiration. 72:642–649.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding XQ, Wu WY, Jiao RQ, Gu TT, Xu Q, Pan
Y and Kong LD: Curcumin and allopurinol ameliorate fructose-induced
hepatic inflammation in rats via miR-200a-mediated TXNIP/NLRP3
inflammasome inhibition. Pharmacol Res. 137:64–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sadaka F, Jadhav A, O'Brien J and Trottier
S: Do all acute stroke patients receiving tPA require ICU
Admission? J Clin Med Res. 10:174–177. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alhazzani AA, Mahfouz AA, Abolyazid AY,
Awadalla NJ, Aftab R, Faraheen A and Khalil SN: Study of stroke
incidence in the aseer region,. Southwestern Saudi Arabia Int J
Environ Res Public Health. 15:E2152018. View Article : Google Scholar
|
|
24
|
Wei J, Zhang Y, Luo Y, Wang Z, Bi S, Song
D, Dai Y, Wang T, Qiu L, Wen L, et al: Aldose reductase regulates
miR-200a-3p/141-3p to coordinate Keap1-Nrf2, Tgfb1/2, and Zeb1/2
signaling in renal mesangial cells and the renal cortex of diabetic
mice. Free Radic Biol Med. 67:91–102. 2014. View Article : Google Scholar
|