Introduction
The genus Artemisia argyi H. (A.
argyi), a perennial herb that belongs to the Asteraceae
family, is ubiquitously distributed in the northern hemisphere
(1). The plant has been used for
various purposes, such as spring greens, spices, native tea or
ornamentation (2). In addition,
it has been traditionally used in the Far East to treat or prevent
a variety of diseases, which include eczema, diarrhea,
inflammation, hemostasis, menstruation-related symptoms and
tuberculosis (3). Pharmacological
research has demonstrated that A. argyi exhibits a variety
of biological activities, such as anti-diabetic (4), anti-oxidant (5), anti-cancer (6,7),
anti-microbial (8),
anti-inflammatory (9), anti-ulcer
(10), and anti-allergic
(11) activities.
Among a number of components (fatty acid, amino
acid, vitamin C, coumarins, glycosides, polyphenols,
polyacetylenes, terpenes, sterols, sesquiterpene lactones and
essential oils) identified from A. argyi (12,13). Polyphenols usually detected in
plants can be classified into two groups of flavonoids and
non-flavonoids, and may be notably responsible for various
pharmacological activities (14).
Polyphenols including flavo-noids serve a role as powerful
anti-oxidants that are capable of scavenging reactive oxygen
species, thereby suppressing the pathogenesis of age-related
degenerative diseases, such as diabetes, cardiovascular disease,
cancer, and neurodegenera-tive diseases (15,16). Scientists have been shown great
interest in the study of plant flavonoids, as of their potential
application in the fields of nutrition and pharmacology, where
A. argyi could be an attractive research target (17).
Inflammation is a defensive immune response of the
human body that is conferred by the host against harmful stimuli,
such as foreign pathogens, damaged cells or irritants (18). Prolonged and uncontrolled
inflammation hyperactivates macrophage and nuclear factor-κB
(NF-κB), which up regulates pro-inflammatory mediators, such as
inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2)
and and cytokines which include tumor necrosis factor-α (TNF-α),
interleukin-1β (IL-1β), and IL-6 (19). This inflammation-related process
may result in the pathogenesis of various chronic diseases, such as
hay fever, periodontitis, atherosclerosis, rheumatoid arthritis,
Alzheimer's disease, and even cancer, such as gallbladder carcinoma
(20). Therefore, inhibiting
NF-κB and/or various pro-inflammatory mediators could be considered
a promising strategy for drug discovery in relation to the
treatment of various chronic disease (21). Widely used pharmaceuticals, such
as indo-methacin, naproxen and etanercept have been designed for
the inhibition of specific inflammatory mediators (22). Despite their benefits, such
synthetic medicines may cause serious adverse effects (23). Therefore, scientific trials that
have been intensively concentrated on plant substances to find more
effective and less deleterious therapeutic agents are required. It
has been scientifically demonstrated that various compounds of
plant species show anti-inflammatory potential (24). The polyphenols are a group of
secondary metabolites that are ubiquitously found in the plant
kingdom, and exhibit various pharmaceutical effects (25). It is well-known that the
polyphenols of oriental herbs can inhibit inflammatory pathways and
mediators, such as NF-κB, TNF-α, iNOS, and COX-2, IL-1β and IL-6
(26).
Seomae mugwort (SM) is a Korean variety of
A. argyi H. Lév. & Vaniot that is exclusively cultivated
on Namhae Island, and has been registered under protection as a
local-specific resource by the Korea Forest Service (registration
no. 42, 2013, 09. 27) (27). Few
studies have been conducted to investigate its chemical and
biological characteristics (12).
Therefore, further analysis of its chemical composition and
biological activities is required. Though previous studies have
been conducted on the anti-inflammatory effect of Artemisia
polyphenols (28), no information
regarding the anti-inflammatory properties of SM polyphenols has
been obtained at the molecular level.
In the present study, we aimed to comprehensively
char-acterize the SM polyphenolic metabolomes, such as flavonoid
using high-performance liquid chromatography coupled with tandem
mass spectrometry (HPLC-MS/MS), and investigated their
anti-inflammatory effects.
Materials and methods
Experimental extraction of polyphenols
Standards and reagents
Caffeic acid, apigenin, kaempferol, amentoflavone,
ferulic acid, quercetin and flavone were purchased from
Sigma-Aldrich (Merck KGaA), and used as external standards, after
recrystallization in ≥99.9% HPLC-grade methanol. The purities of
all standards were confirmed by HPLC to be >99%. All other
chemicals and solvents were of analytical grade, and were obtained
from Duksan Pure Chemical Co., Ltd.
Collection and preparation of plant
materials
The SM plants were collected in May 2017 from Namhae
Island. The Animal Bioresource Research Bank authenticated the
plants as taxonomically homozygous. The voucher specimens (ref nos.
2012M3A9B8019303 and 2017R1A2B4003974) were deposited at the
herbarium of the Research Institute of Life Science, Gyeongsang
National University. The plants were washed with water, lyophilized
and stored at -20°C, till extraction.
Extraction and purification
The isolation of polyphenols from the plant was
carried out based on the technique described by Song et al
with minor modifications (29).
The lyophilized plants (90 g) were refluxed in 70% methanol (1.5 l)
for 20 h at room temperature. The mixture was filtered through a
Büchner funnel, and concentrated to ~300 ml at reduced pressure at
35°C, using a rotary evaporator with 80 rpm. The concentrated
filtrate was washed with n-hexane (300 ml) three times to
remove nonpolar impurities. Subsequently, the filtrate was
extracted using ethyl acetate (100 ml) three times, and dried over
anhydrous MgSO4. The solvent was removed under reduced
pressure. The sticky residue was placed on the top of a silica gel
solvent (40×2.5 cm), and eluted with ethyl acetate to eliminate
highly polar impurities. The solvent was then removed to yield
solids of polyphenol mixture (1.34 g, 1.5% of the lyophilized
plants). The mixture was stored at -20°C, pending analysis.
HPLC-MS/MS analysis
HPLC-MS/MS were conducted according to the method
previously reported by Song et al (29). HPLC-MS/MS was performed on a 1,100
series HPLC system (Agilent Technologies, Inc.) and 3200 QTrap
tandem mass system (Sciex LLC) operated in negative ion mode (spray
voltage set at -4.5 kV). The exception being only that the gradient
system comprised 0.5% aqueous formic acid (A) and 100% methanol (B)
at a flow rate of 0.5 ml/min. The gradient conditions used in the
mobile phase were 0-10 min, 15% B; 10-15 min, 15-20% B; 15-25 min,
20% B; 25-30 min, 20-25% B; 30-60 min, 25-45% B; 60-65 min, 45-70%
B; 65-70 min, 70-15% B.
Quantification of polyphenol
Polyphenol samples were quantified using LC-UV
chromatograms (at 280 nm) with seven selected standards.
Calibration curves were plotted for each using five concentration
levels (n=5; 50, 100, 200, 500, and 1,000 μg/ml) and
polyphenol contents were determined in terms of peak area ratios
with the analyte vs. analyte concentrations using a 1/x (x,
concentration) weighted linear regression (n=5). Quantification of
each polyphenol can be conducted using a standard with the same
chromophore. Thus, the standard caffeic acid was used to quantify
compounds 1, 6, 10, 11 and 14; apigenin was used to quantify
compounds 2 and 3; kaempferol was used for compounds 8 and 9;
amentoflavone was applied for compounds 5 and 7, followed by
ferulic acid, quercetin, and flavone was used to quantify compounds
4, 12 and 13, respectively. And all samples were repeated three or
more times.
Anti-inflammatory experiments Cell
culture
The mouse macrophage cells, RAW 264.7 (American Type
Culture Collection), were cultured in Dulbecco's Modified Eagles
medium (HyClone; GE Healthcare Life Sciences) supplemented with 10%
fetal bovine serum and 100 U/ml streptomycin at 37°C in humidified
5% CO2 incubator.
Cell viability assay
Cell viability was evaluated with MTT assay. RAW
264.7 cells were seeded at a density of 5×104 per well
in 48-well culture plates. The cells were stimulated with LPS at 1
μg/ml for 1 h and then incubated with SM polyphenols at the
indicated concentration (2.5-30 μg/ml) for 24 h at 37°C.
Control and LPS-only treated groups were treated with the same
volume of the solvent dimethyl sulfoxide. After washing the cells,
0.05% MTT solution was added to each well, and then incubated for 3
h at 37°C. The formazan crystals in live cells were dissolved in
dimethyl sulfoxide. The absorbance of each well was measured at 570
nm using PowerWave HT microplate spectrophotometry (BioTek, Inc.).
The cell viability was expressed as a percentage of viable cells
compared with the control group consisting of untreated cells.
Measurement of nitric oxide (NO)
expression
The quantitation of NO expression in biological
systems was analyzed using Griess reagent kit (Promega Corporation,
TB229), according to the manufacturer's instructions. RAW 264.7
cells were seeded at a density of 1.5×104 per well in
96-well culture plates. RAW 264.7 cells were then pretreated with 1
μg/ml LPS for 1 h, followed by treatment with SM polyphenols
at a concentration of 2.5 and 5 μg/ml for 24 h at 37°C. The
mixture of 50 μl of cell culture supernatant and 50
μl of sulfanilamide solution (1% sulfanilamide in 5%
phosphoric acid) was incubated in 96-well plate for 10 min at room
temperature, protected from light. After incubation, 50 μl
of N-(1-naphthyl) ethylene diamine hydrochloride solution was added
to each mixture, and incubated for 10 min at room temperature,
protected from light. The absorbance of each well was measured at a
wavelength of 520 nm using PowerWave HT microplate
spectrophotometer (BioTek Instruments, Inc.). To calibrate the
amount of NO, sodium nitrite (0, 1.56, 3.13, 6.25, 12.5, 25, 50,
and 100 μM) was used as the nitrate standard.
Enzyme-linked immunosorbent assay
(ELISA)
RAW 264.7 cells were seeded at a density of
5×104 per well in 48-well culture plates. The cells were
pretreated with 1 μg/ml LPS for 1 h and then incubated with
SM polyphenols for 24 h at 37°C. The cytokine IL-1 β levels were
quantified using the mouse IL-1β kit (ADI-900-132A, Enzo Life
Sciences) according to the manufacturer's instructions.
Western blot analysis
Whole cell lysates were prepared using
radioimmunoprecipitation assay buffer (RIPA; iNtRON; 50 mM
Tris-HCl, pH 7.5, 150 mM sodium chloride, 0.5% sodium deoxycholate,
1% Triton X-100, 0.1% SDS, 2 mM EDTA) containing protease inhibitor
cocktail (Thermo Fisher Scientific, Inc.) and phosphatase inhibitor
(Thermo Fisher Scientific, Inc.). The concentration of proteins was
determined by a BCA protein assay kit (Thermo Fisher Scientific,
Inc.). An equal amount of protein (20 μg) was separated
using 8-15% SDS-PAGE, and transferred onto a polyvinylidene
difluoride membrane. The membranes were blocked with 5% non-fat
milk or 5% bovine serum albumin (Gibco; Thermo Fisher Scientific,
Inc.) in Tris-buffered saline with Tween-20 (TBS-T; 25 mM Tris, 137
mM NaCl, 2.7 mM KCl, and 0.1 % Tween-20) at room temperature for 1
h, and incubated with the respective primary antibodies at 4°C for
16 h. Primary antibodies against iNOS (cat. no. 13120S; 1:1,000),
COX-2 (cat. no. 12282S; 1:1,000), phosphorylated (p)-p65 (Ser536;
3033S; 1:1,000), p65 (8242S; 1:1,000), p-IκBα (Ser32; cat. no.
2859S; 1:1,000), IκBα (cat. no. 4812S; 1:1,000), p-JNK1/2
(Thr183/Tyr185; cat. no. 4671S, 1:1,000), JNK (cat. no. 9258S,
1:1,000), p-p38 (Thr180/Tyr182; cat. no. 9216S, 1:1,000), p38 (cat.
no. 8690S; 1:1,000), p-ERK1/2 (Thr202/Tyr204; cat. no. 4370S,
1:1,000), ERK1/2 (cat. no. 4695S; 1:1,000), and β-actin (cat. no.
3700S, 1:10,000) were purchased from Cell Signaling Technology,
Inc. After washing with TBS-T more than five times, the membranes
were incubated with the anti-rabbit or anti-mouse (cat. nos.
A120-101P and A90-116P, respectively, Bethyl Laboratory, Inc.)
secondary antibodies (1:2,000) conjugated with horseradish
peroxidase for 3 h at room temperature, followed by visualization
with an enhanced chemiluminescence kit (Bio-Rad Laboratories,
Inc.). The images were acquired by the ChemiDoc imaging system
(Bio-Rad Laboratories, Inc.). The β-actin protein was used as a
loading control. Western blot images were quantified using the
ImageJ 1.50i software (National Institutes of Health).
Reverse transcription-quantitative
polymerase chain reaction analysis (RT-qPCR)
After treatment, total RNA was extracted from RAW
264.7 cells by using TRIzol reagent (Thermo Fisher Scientific,
Inc.). cDNA was reverse-transcribed from 1 μg of RNA with
iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc.), according
to the manufacturer's instructions, and used as templates in
quantitative real-time PCR using AccuPower® 2X
GreenstarTM qPCR Master (Bioneer, Daejeon, Republic of Korea). The
sequence of primers were as follows: iNOS, 5′-TCC TAC ACC ACA CCA
AAC-3′ (forward) and 5′-CTC CAA TCT CTG CCT ATC-3′ (reverse),
IL-1β, 5′-TGC AGA GTT CCC CAA CTG GTA CAT C-3′ (forward) and 5′-GTG
CTG CCT AAT GTC CCC TTG AAT C-3′ (reverse), TNFα, 5′-TGG AGT CAT
TGC TCT GTG AAG GGA-3′ (forward) and 5′-AGT CCT TGA TGG TGG TGC ATG
AGA-3′ (reverse), β-actin, 5′-TAC TGC CCT GGC TCC TAG CA-3′
(forward), and 5′-TGG ACA GTG AGG CCA GGA TAG-3′ (reverse)
(30-32). Thermocycling conditions consisted
of an pre-denaturation for 2 min at 95°C, followed by 40 cycles at
95°C for 5 sec, 58°C for 30 sec, and 95°C for 5 sec; qPCR was
conducted with a CFX384 Real-Time PCR Detection System (Bio-Rad
Laboratories, Inc.). All data was investigated with the Bio-Rad CFX
Manager Version 3.1 software. Relative quantitation was analyzed on
taking the difference (ΔCq). The mRNA expression levels were
normal-ized using the expression of β-actin.
Statistical analysis
All the results of the anti-inflammatory experiment
were presented as the mean ± standard error of the mean of
triplicate samples. Statistical analyses were conducted using
GraphPad Prism software version 5.02 (GraphPad Software, Inc.).
Significant differences between groups were calculated by one-way
factorial analysis of variance, followed by a Dunnett's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Separation and characterization of SM
polyphenols
The mixture of polyphenols isolated from SM by 70%
methanol extraction was followed by the elution of ethyl acetate
over a silica gel column. Each polyphenol was characterized via
HPLC using a C18 column, MS/MS in negative ion mode, and a
comparison with the reported data was performed. The polyphenols
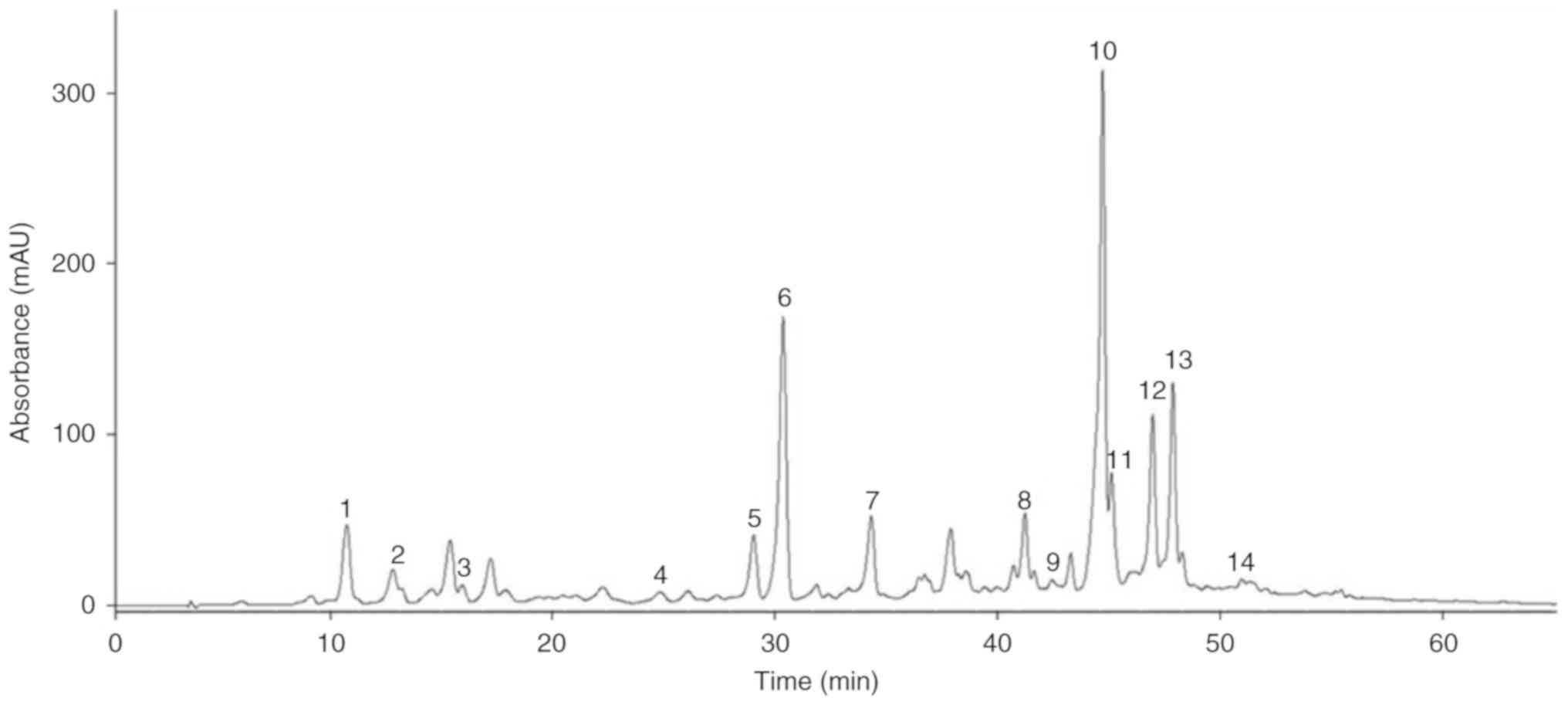
were labeled according to their retention time (tR) order in
the 10‑60 min absorbance segment of chromatogram recorded at 280 nm
(Table I). A total of fourteen
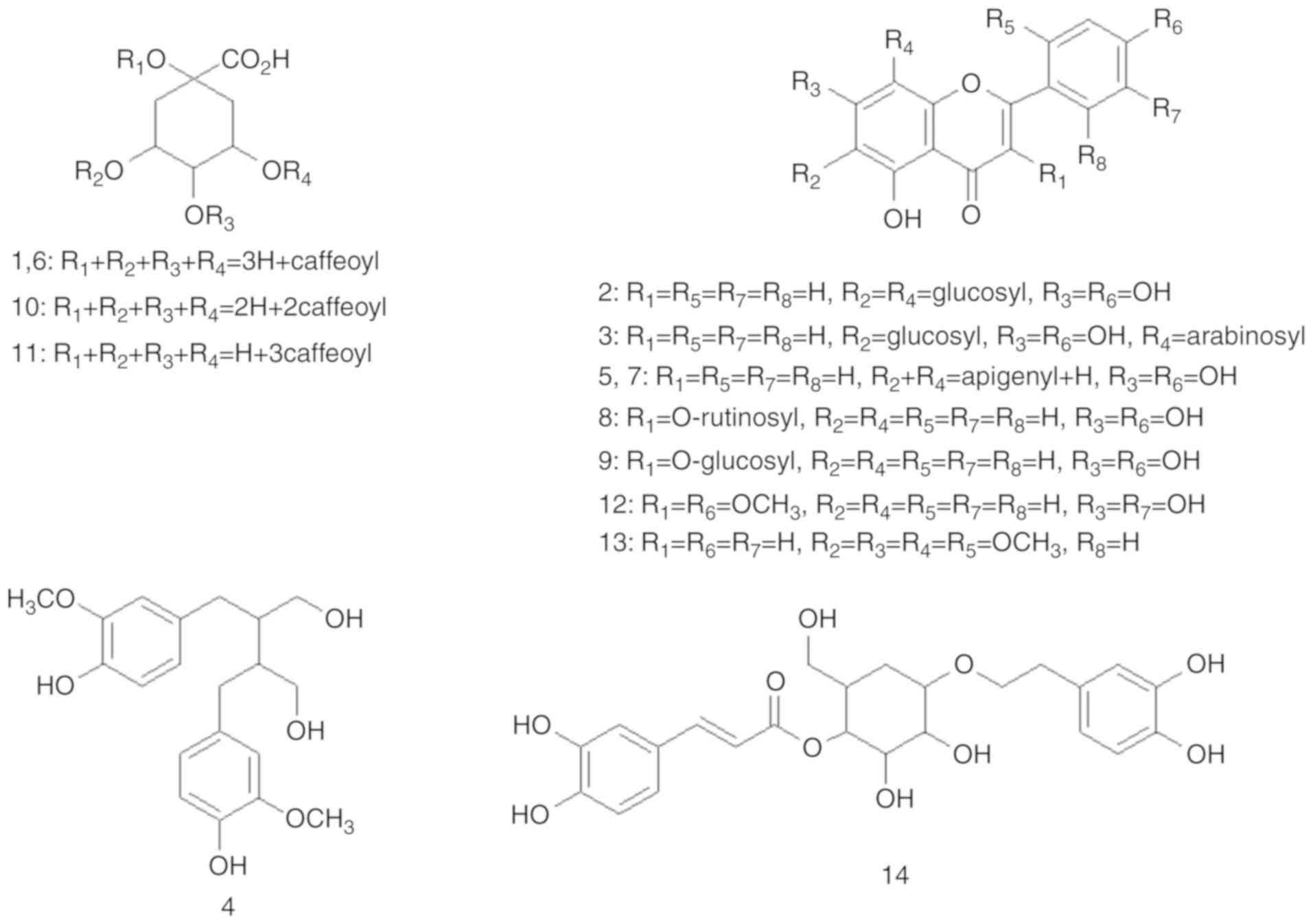
polyphenols were characterized, which are composed of five
hydroxycinnamates (1, 6, 10, 11 and 14), eight flavonoids (2, 3, 5,
7, 8, 9, 12 and 13) and one lignan (4). As shown in Figs. 1 and 2, and Table I, the structures and HPLC‑ MS/MS
data of the polyphenols were presented. Hydroxycinnamates (1, 6,
10, and 11) and flavonoids (2 and 3) were recently reported in
Chinese and Korean A. argyi (33). To the best of our knowledge, we
are the first to characterize one hydroxycinnamate (14), six flavonoids (5, 7, 8, 9, 12 and
13), and lignan (4) in the SM
variety of A. argyi. It has been known that plant bioactive
components may vary in accordance with the plant variety based on
geographical location, which is attributed mainly to climatic
variation and nutrient availability (12). The novel polyphenols were
identified on the basis of their molecular ions and mass
fragmentation patterns in comparison with the literature data.
Thus, phenolic component 4 (tR=24.02 min) yield
[M‑H]‑ at m/z 361, which was fragmented to ions m/z 346 [M‑H‑C
H3]‑, 313 [M‑H‑C H2O]‑, 179
[C6H4(O)(OCH3)CH=CHCH2OH]‑,
and 165
[C6H3(CO)2CH2CH2CH2Ox]‑,
was identified as secoisolariciresinol (34). Flavonoid 5
(tR=28.70 min) was identified as amentoflavone
isomer, which has shown [M‑H]‑ at m/z 537, and
fragmented to generate an ion at m/z 443 [M‑H‑C
6H5OH]‑, 417 [C ring 0,2 cleavage,
M‑H‑C 6H4(O)CO]‑, 399 [C' ring
0',3' cleavage, M‑H‑C
6H2(OH)2CO‑2H]‑,
375 [C ring 0,4 retro‑D iels Alder fragment, M‑H‑C
6H4(OH)C(O)CHCO]‑, 331 [M‑H‑(A' +
C') rings‑C O]‑ and 117 [C6H4(OH)C=C]‑
(35). Flavonoid 7
(tR=34.17 min) was also identified as an
amentoflavone isomer, which showed the similar fragmentation
pattern [M‑H]‑ as flavonoid 5. Three amentoflavone type
isomers (molecular weight=538, amentoflavone, robustaflavone and
hinokiflavone) have been reported until now (36). However at present, it is unclear
which of the three isomers should be assigned to 5 or 7, because
the MS/MS data are not sufficient to exactly characterize the
atomic connectivity of the isomers. Therefore, for exact
identification and confirmation, further spectroscopic
investigation should be performed. Flavonoid 8
(tR=41.69 min) yielded [M‑H]‑ at m/z
593 with additional peaks observed at m/z 447
[M‑H‑rhamnosyl]‑ and 285 [kampferol aglycon]‑, and this
component was identified as kaempferol 3‑O‑rutinoside
(37). Flavonoid 9
(tR=42.83 min) yielded [M‑H]‑ at m/z
461, and showed an additional fragmented peak at 285 [kampferol
aglycon, M‑H‑glucuronyl]‑, which was identified as
kaempferol-3-O-glucuronide (38). Flavonoid 12
(tR=46.49 min) yielded [M-H]- at m/z
329, which was fragmented to ions at m/z 314
[M-H-CH3]-, 299 [quercetin aglycon,
M-H-2CH3 ]-, and 271 [quercetin aglycon-CO]
-, and was identified as quercetin dimethyl ether
(39). Flavonoid 13
(tR=48.12 min) was identified as skullcapflavone
II, which showed [M-H]- at m/z 373, which was fragmented
to generate an ion at m/z 358 [M-H-CH3]- and
343 [M-H-2CH3]- (40). Hydroxycinnamate (14) (tR=51.73 min) was
identified as calce-larioside A, and its MS/MS showed
[M-H]- at m/z 477, which was fragmented to 315
[M-H-caffeoyl]-, 179 [caffeic acid-H]- and
161 [glucosyl-H2O]- (41).
 | Table IHigh-performance liquid
chromatography MS/MS data of polyphenols from Seomae
mugwort. |
Table I
High-performance liquid
chromatography MS/MS data of polyphenols from Seomae
mugwort.
| Author, year | No. | Compound |
tR (min) |
[M-H]- | MS/MS (m/z) | (Refs.) |
|---|
| Dou et al,
2007; | 1 | Caffeoylquinic acid
isomer | 10.29 | 353 | 353, 191, 179,
135 | (53,54) |
| Del Rio et
al, 2004 | | | | | | |
| Simirgiotis et
al, 2013 | 2 |
6,8-di-C-glucosylapigenin (vicenin
II) | 12.19 | 593 | 593, 503, 473, 413,
383, 353 | (55) |
| Dou et al,
2007 | 3 |
6-C-Arabinosyl-8-C-glucosylapigenin | 16.60 | 563 | 563, 545, 503, 473,
443, 383, 353 | (53) |
| Fischer et
al, 2012 | 4 |
Secoisolariciresinol | 24.02 | 361 | 361, 346, 313, 179,
165 | (34) |
| Yao et al,
2017 | 5 | Amentoflavone
isomer | 28.70 | 537 | 537, 443, 417, 399,
375, 357, 335, 331, 201, 178, 161, 117 | (35) |
| Dou et al,
2007; | 6 | Caffeoylquinic acid
isomer | 30.89 | 353 | 353, 191, 179,
135 | (53,54) |
| Del Rio et
al, 2004 | | | | | | |
| Yao et al,
2017 | 7 | Amentoflavone
isomer | 34.17 | 537 | 537, 375, 357, 179,
134 | (35) |
| Ahmed et al,
2016 | 8 |
Kaempferol-3-O-rutinoside | 41.69 | 593 | 593, 447, 285 | (56) |
| Ahmed et al,
2016 | 9 |
Kaempferol-3-O-glucuronide | 42.83 | 461 | 461, 323, 285,
160 | (56) |
| Bastos et
al, 2007 | 10 | Dicaffeoylquinic
acid | 44.79 | 515 | 515, 353, 191, | (57) |
| | | | | 179, 173, 134 | |
| Gouveia and
Castilho, | 11 |
3,4,5-O-Tricaffeoylquinic acid | 45.01 | 677 | 677, 515, 353, | (58) |
| 2012 | | | | | 335, 299, 191,
173 | |
| Li et al,
2018 | 12 | Quercetin dimethyl
ether | 46.49 | 329 | 329, 314, 299,
271 | (59) |
| Luo et al,
2012 | 13 | Skullcapflavone
II | 48.12 | 373 | 373, 358, 343 | (40) |
| Sanz et al,
2012 | 14 | Calcelarioside
A | 51.73 | 477 | 477, 179, 161 | (41) |
Validation and quantification of SM
polyphenols
Quantification of the individual polyphenols was
performed using calibration curves obtained from structurally
related external standards as described above. As presented in
Table II, satisfactory
validation data were obtained for the parameters considered. The
calibration curve (R2) was found to be ≥0.9714. The
limits of detection and limits of quantitation were between
1.00-2.96 and 3.01-8.95 mg/l, respectively. Recoveries at 50 and
100 mg/ml were between 80.9-98.1 and 80.3-100.3%, respectively. The
relative standard deviation values were in the ranges of 3.4-7.5
and 2.3-9.1%, respectively.
 | Table IICalibration curves and validation
data for quantification of polyphenols in Seomae
mugwort. |
Table II
Calibration curves and validation
data for quantification of polyphenols in Seomae
mugwort.
| Standard | Calibration
curve | R2 | LOD (mg/l) | LOQ (mg/l) | Recovery (%) ±
RSD |
|---|
| 50 mg/ml | 100 mg/ml |
|---|
| Catechin | y=13.58x+147 | 0.9962 | 2.95 | 8.95 | 80.9±7.1 | 98.5±6.2 |
| Caffeic acid | y=67.66x+80.2 | 0.9954 | 1.15 | 3.47 | 90.9±6.4 | 90.3±4.4 |
| Ferulic acid | y=59.25x+93.3 | 0.9914 | 1.00 | 3.01 | 98.1±6.1 | 100.3±5.7 |
| Apigenin | y=33.87x+31.8 | 0.9998 | 2.96 | 8.95 | 93.7±3.4 | 93.3±2.3 |
| Quercetin | y=44.86x-133 | 0.9975 | 1.81 | 5.49 | 85.7±7.1 | 88.1±5.3 |
| Kaempferol | y=43.96x-58.4 | 0.9983 | 1.10 | 3.34 | 90.8±7.5 | 80.6±9.1 |
| Amentoflavone | y=39.64x+70.9 | 0.9911 | 1.08 | 3.24 | 91.7±5.9 | 89.7±4.2 |
| Flavone | y=55.20x+201 | 0.9714 | 1.67 | 5.07 | 81.4±5.1 | 80.3±3.8 |
The contents of the individual components were
listed in Table III. The total
hydroxycinnamate content was ~2-folds higher than that of
flavonoids. Among the hydroxycinnamates, caffeoyl quinates (1, 6,
10 and 11) were predominant, and in the case of flavonoids,
amentoflavones (5 and 7) were found to be abundant. Additionally,
>90% of the total isolated compounds were found to be caffeoyl
quinates and amentoflavones derivatives.
 | Table IIIContent of polyphenols in
Seomae mugwort. |
Table III
Content of polyphenols in
Seomae mugwort.
| Compounds | Amount (mg/kg of
dried plant) |
|---|
| 1 | 7.25±0.41 |
| 6 | 58.91±0.54 |
| 10 | 19.90±0.31 |
| 11 | 0.84±0.11 |
| 14 | 0.65±0.04 |
| Total
hydroxycinnamates | 87.55±0.75 |
| 2 | 0.92±0.03 |
| 3 | 0.30±0.02 |
| 5 | 12.18±0.51 |
| 7 | 15.83±1.17 |
| 8 | 0.21±0.01 |
| 9 | 0.11±0.01 |
| 12 | 3.67±0.11 |
| 13 | 3.59±0.29 |
| Total
flavonoids | 36.81±1.31 |
| 4 | 0.97±0.01 |
| Total | 125.34±1.31 |
Anti-inflammatory effects of SM
polyphenols on RAW 264.7 macrophage cells
Our results indicated that >90% of the SM
polyphenols were composed of caffeoyl quinates and amentofla-vones,
which have been known to possess various pharmacological
activities, including anti-oxidant, anti-viral, anti-depressant and
anti-inflammatory effects (36,42). The alcoholic extract of A.
argyi was recently studied for its anti-inflammatory effects
(9), but that of A. argyi
polyphenols remains unclear. Thus, we further investigated this
property in SM.
Cytotoxicity of SM polyphenols on RAW
264.7 cells
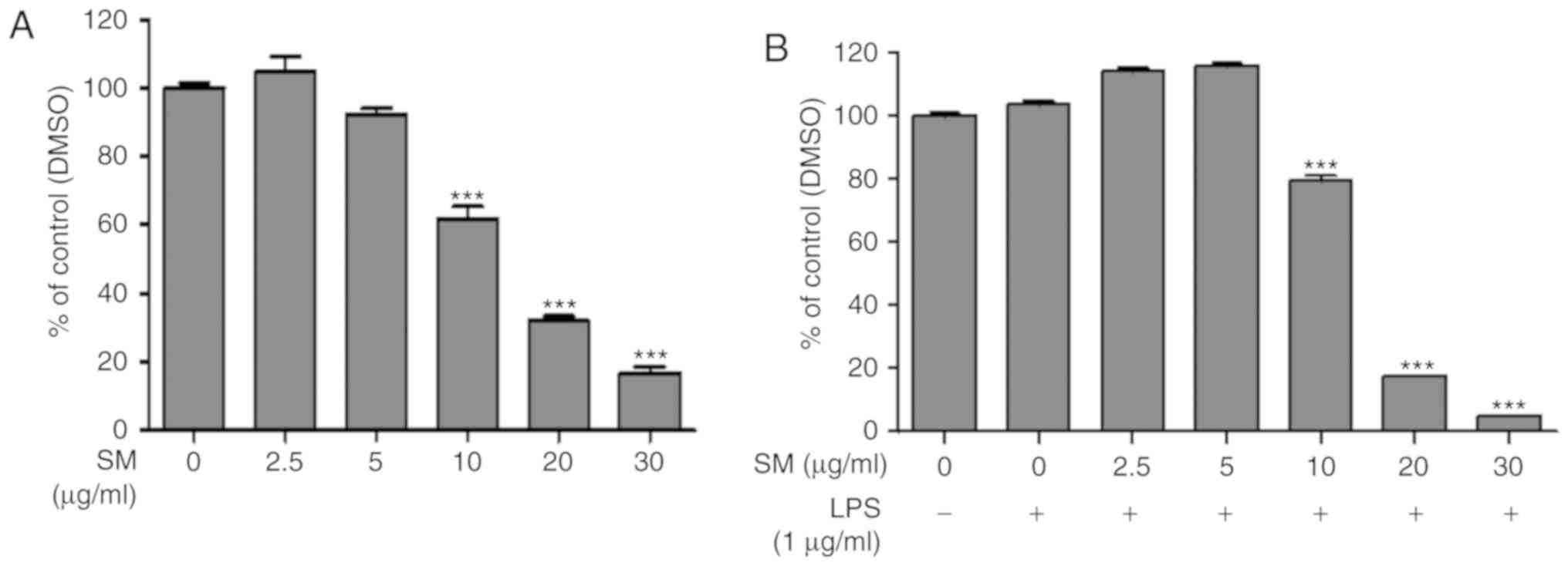
An MTT assay was used to investigate the potential
cytotoxic effects of SM polyphenols. RAW 264.7 macrophage cells
were treated with SM polyphenols at a concentration range of 2.5-30
μg/ml with or without LPS (1 μg/ml) for 24 h. SM
polyphenols at a concentration of 2.5 and 5 μg/ml did not
exhibit significant cytotoxicity to RAW 264.7 macrophages in both
LPS-treated and untreated cell group of cells. On the contrary,
significant cytotoxicity was reported for cells treated with ≥10
μg/ml SM in the presence or absence of LPS, compared with
the control group of cells (Fig.
3A and B).
Effects of SM polyphenols on LPS-induced
NO production and protein expression of iNOS in RAW 264.7
cells
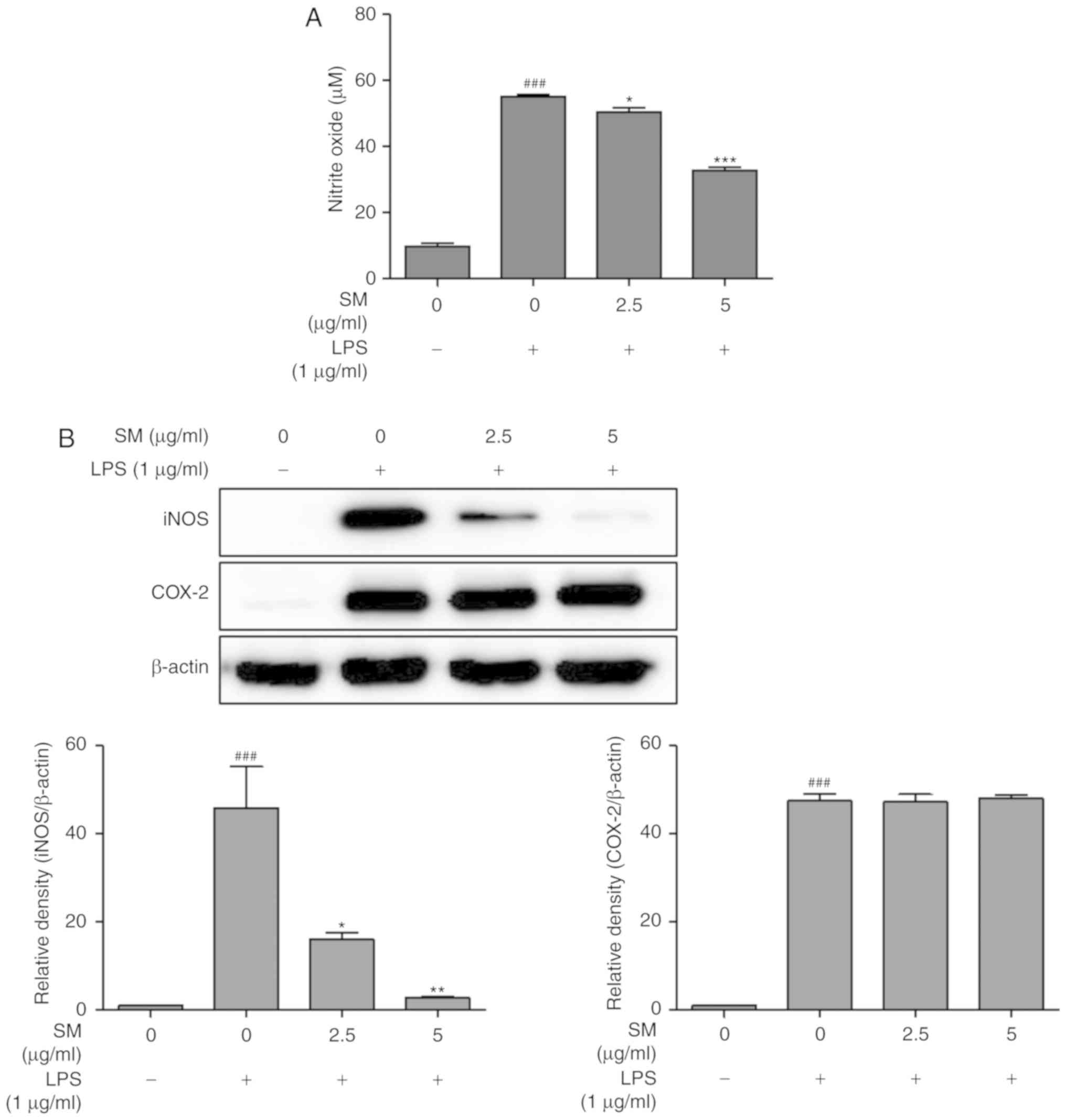
In order to investigate the inhibitory effects of SM
polyphenols on NO formation in LPS-induced RAW 264.7 cells,
NO2- production was measured by a Griess
assay. As shown in Fig. 4A, NO
production followed by LPS-treatment was increased significantly by
5-fold, compared with that of non-induced cells. SM polyphenols
were determined to significantly inhibit LPS-induced NO production
in a dose-dependent manner. The protein expression of iNOS and
COX-2 were investigated by a western blot assay. The proteins iNOS
and COX-2 are involved in NO and prostaglandin-endoperoxide
synthesis, respectively. LPS could significantly increase the
expression of iNOS in LPS-stimulated RAW 264.7 cells, compared with
that of unstimulated cells. Treatment with SM polyphenols revealed
a significant decrease in LPS-stimulated iNOS expression in a
dose-dependent manner (Fig. 4B).
On the contrary, SM polyphenols did not notably affect the protein
expression of COX-2; LPS induced a significant upregulation in
COX-2 expression compared with the control.
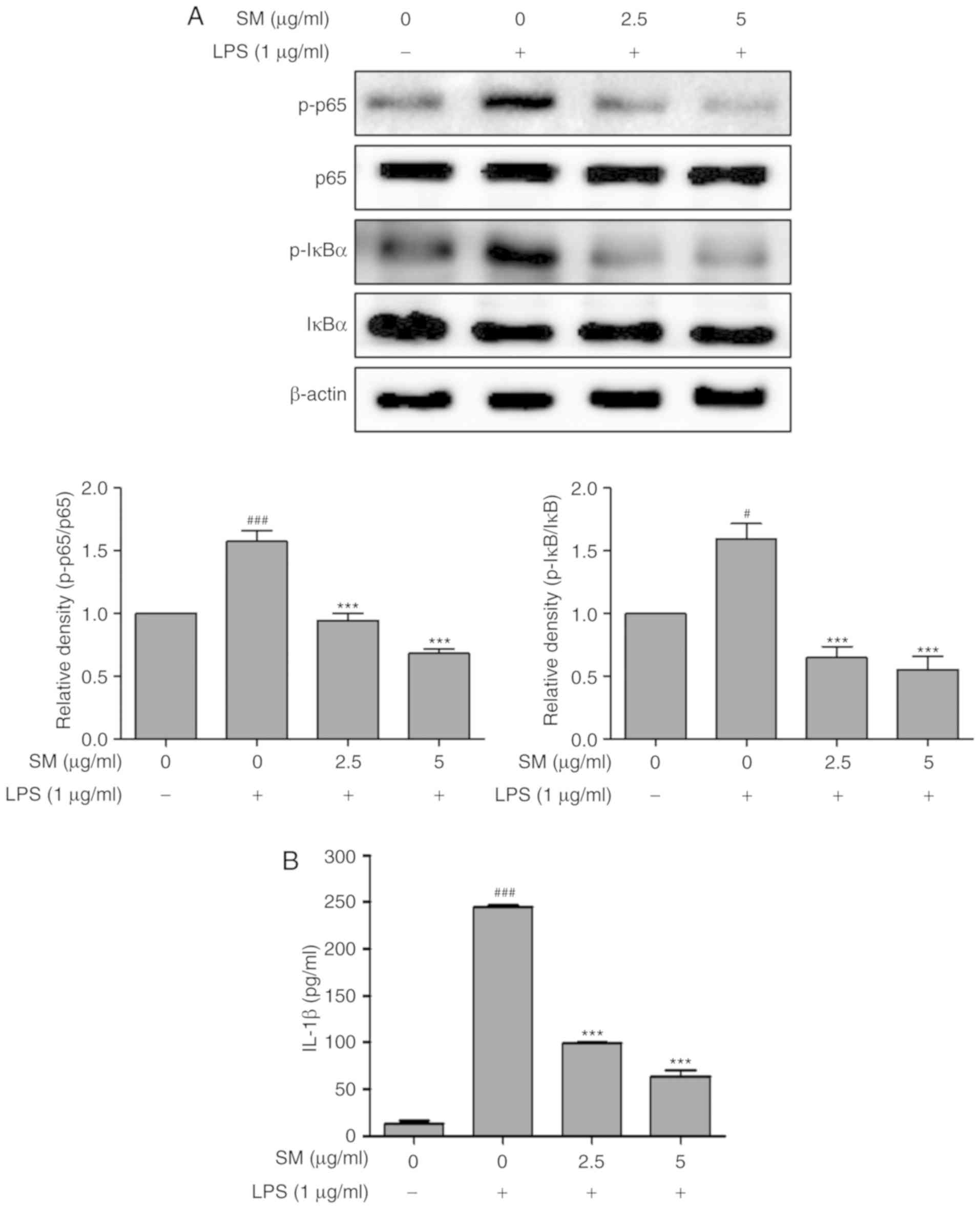
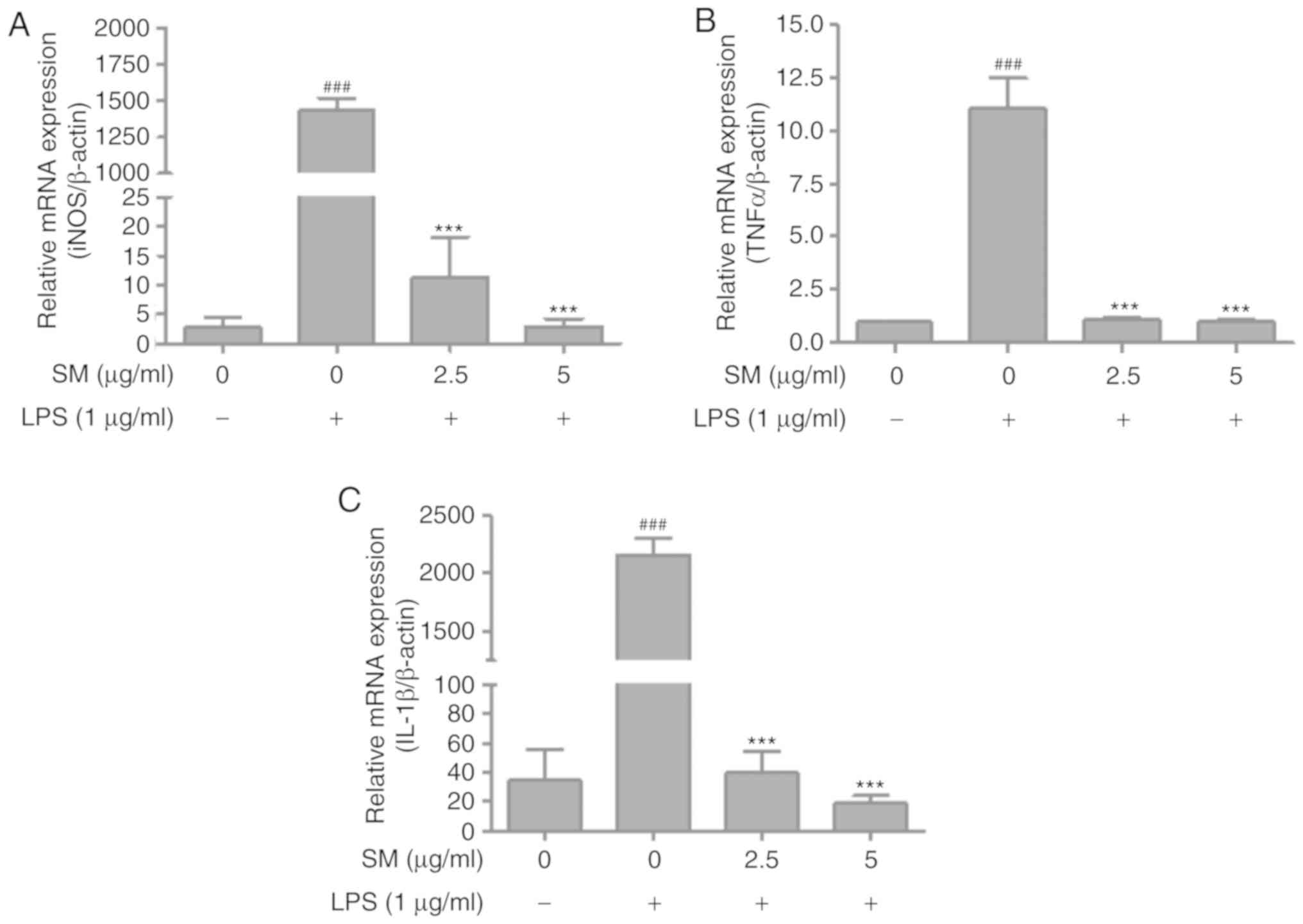
Effects of SM polyphenols on NF-κB and
inflammatory cytokines in LPS-stimulated RAW 264.7 cells
The ability of SM polyphenols to induce the
expression of the cytokines via iNOS was examined in LPS-induced
RAW 264.7 macrophage cells. The activation of NF-κB and the mRNA
levels of cyto-kines following LPS treatment were investigated by
western blotting and RT-qPCR, respectively. The expression levels
of IL-1β were measured using ELISA. The SM polyphenols
significantly attenuated the upregulation of IL-1β, p-NF-κB (p-p65
and p-IκBα) induced by LPS in a dose-dependent manner, also
decreased the mRNA expression of iNOS, TNFα, and
IL-1β (Figs. 5 and
6). These results indicate that
SM polyphenols effectively inhibited the NF-κB pathway, and the
protein and mRNA expression of cytokines involved in the
inflammatory process.
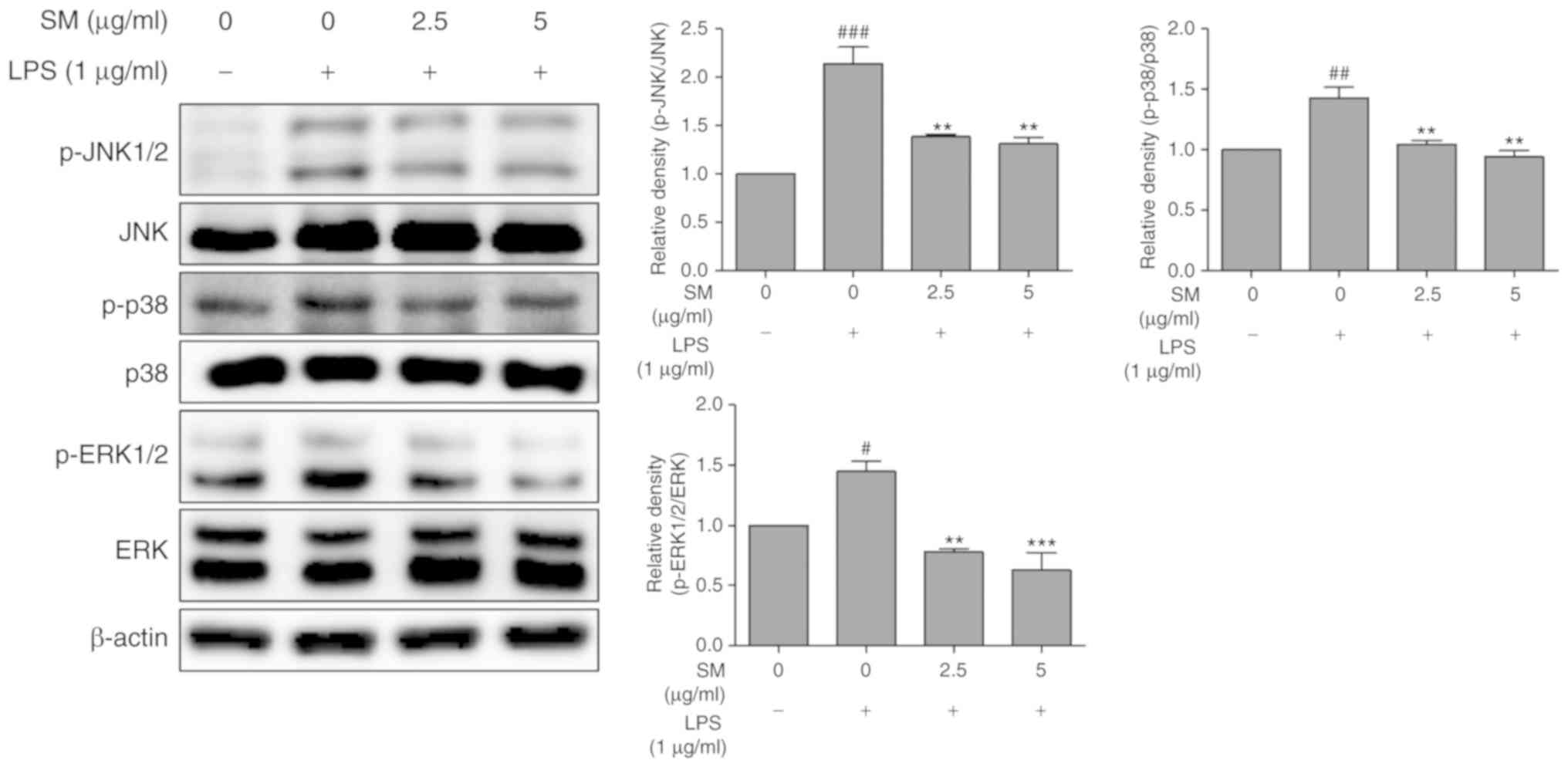
Effects of SM on the phosphorylation of
MAPKs in LPS-stimulated RAW 264.7 cells
To determine the relevance of the MAPK pathway with
SM polyphenols, we examined the effects of the SM polyphenols on
the phosphorylation of JNK, p38 and ERK. Treatment with SM
polyphenols significantly suppressed the phosphorylation of the
JNK, p38 and ERK in a dose-dependent manner when induced by LPS
(Fig. 7). These findings suggest
that the SM polyphenols exhibit anti-inflammatory effects by
regulating the activation of NF-κB and MAPK pathways.
Discussion
A. argyi is a traditional Asian medicinal
remedy for diarrhea, hemostasis and inflammation. SM is a Korean
variety of A. argyi harvested in Namhae, Korea. The
alcoholic extract of A. argyi was recently studied for its
anti-inflammatory effect (9), but
that of A. argyi polyphenols has remained unknown until now.
In the present study, the anti-inflammatory effects of polyphenol
extract form of SM on LPS-stimulated RAW 264.7 mouse macrophage
cells were investigated. Among the 14 components profiled via a
single HPLC-MS/MS, one hydroxy-cinnamate, six flavonoids and one
lignan were reported for the first time in A. argyi. Our
quantification analysis based on the validated methods indicated
that >90% of the total compounds comprised caffeoyl quinates and
amentoflavones, which have been known to possess various
pharmacological activities, including anti-oxidant, anti-viral,
anti-depressant and anti-inflammatory effects (36,42). In the present study, we
investigated the anti-inflammatory effect of SM polyphenols.
Additionally, the total content (125.34 mg/kg) was
calculated by the sum of each isolated phenol compound and
identified by HPLC analysis. As the lyophilized sample was
subjected to liquid-liquid extraction to remove lipophilic
impurities, such as fatty acids, the silica column was subjected to
the removal of sugars and proteins before the HPLC analysis.
Therefore, there may be a difference in the content between the
substances purified by the gel column of raw samples and some
phenolic compounds detected by their HPLC analysis at 280 nm.
Nitric oxide synthases (NOSs) are composed of three
types of enzyme (iNOS, endothelial NOS and neuronal NOS) that
catalyze the production of NO from L-arginine (43). iNOS is involved in the immune
response-like secretion of pro-inflammatory cytokines through the
increased production of NO (43).
NO radicals serve a crucial role in regulating inflammation and
immune responses in asthma and inflammatory bowel diseases
(44). In the process of
inflammation, cellular NO is immediately oxidized to
NO2- (45).
As expected, treatment with SM polyphenols inhibited NO production
in our study. In addition, the protein expression of iNOS was
decreased, but no significant reduction in the expression level of
COX-2 was observed by SM polyphenols in LPS-stimulated RAW 264.7
cells. This discordant result may be attributed to the degree of
reliance of the transcription factors required for the expression
of iNOS and COX-2 (46).
Activation of transcription factor NF-κB has been
considered as the pivotal factor to regulate the expression of
inflammatory enzymes and cytokines, such as iNOS, TNFα and IL-1β,
which comprise the NF-κB binding sites in their promoters (47). Therefore, the proper regulation of
NF-κB could be useful in the treatment of many inflammation-related
disorders, such as allergies, cancer, dermatitis and arthritis
(48-50). The activation of NF-κB and the
increased mRNA levels of cytokines following LPS treatment were
investigated by western blot and RT-qPCR analyses, respectively.
The levels of IL-1β were measured using ELISA. SM polyphenols were
observed to attenuate the upregulation of IL-1β, phosphory-lated
NF-κB (p-p65 and p-IκBα), and increases in the mRNA expression of
iNOS, TNFα and IL-1β in a dose-dependent manner. These results
indicated that SM polyphenols effectively inhibit the NF-κB
pathway, and the protein and mRNA expression of cytokines involved
in the inflammatory process.
During the inflammatory process, the phosphorylation
of MAPKs, in which serine and threonine protein kinases perform a
crucial role in the transcriptional regulation, mediates
LPS-stimulated iNOS and COX2 expression through NF-κB activation
(51,52). To determine the relevance of the
MAPK pathway with SM polyphenols, we examined the effects of the SM
polyphenols on JNK, ERK and the p38 phosphorylation levels.
Treatment with SM polyphenols significantly suppressed the
phosphorylation of JNK, p38, and ERK in a dose-dependent manner in
the current study.
These findings confirm the anti-inflammatory effects
of SM polyphenols, which may be accomplished by regulating the
activation of NF-κB and MAPKs signaling pathways. Therefore, SM
polyphenols have great potential to be developed into a therapeutic
drug for inflammatory-related disorders. In the future, we may also
perform animal experiment to further validate our findings. The
present study proposed that SM could be effective for treating
chronic inflammatory related diseases, including diabetes, cancer
and arthritis due to its significant effects on the suppression of
the inflammatory response.
Acknowledgments
Not applicable.
Funding
The present study was supported partly by the
National Research Foundation of Korea (NRF) funded by the Ministry
of Science, ICT and Future Planning (grant nos. 2012M3A9B8019303
and 2017R1A2B4003974).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SMK and SJL conceived and designed the experiments,
performed the experiments, organized focus group discussion,
collected, analyzed all study data; VVGS made contributions to the
analysis of data and prepared the final manuscript; SEH and PV
revised the study design and prepared the final manuscript; KTD and
SCS performed the extraction of polyphenols. JYC, WSL and GSK and
revised the study design, revised the results and final revision of
manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torrell M, Cerbah M, Siljak-Yakovlev S and
Vallès J: Molecular cytogenetics of the genus Artemisia
(Asteraceae, Anthemideae): Fluorochrome banding and fluorescence in
situ hybridization. I. Subgenus Seriphidium and related taxa. Plant
Syst Evol. 239:141–153. 2003. View Article : Google Scholar
|
|
2
|
Lee MY, Doh EJ, Park CH, Kim YH, Kim ES,
Ko BS and Oh SE: Development of SCAR marker for discrimination of
Artemisia princeps and A. argyi from other Artemisia herbs. Biol
Pharm Bull. 29:629–633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shin NR, Ryu HW, Ko JW, Park SH, Yuk HJ,
Kim HJ, Kim JC, Jeong SH and Shin IS: Artemisia argyi attenuates
airway inflammation in ovalbumin-induced asthmatic animals. J
Ethnopharmacol. 209:108–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adams JD, Garcia C and Garg G: Mugwort
(Artemisia vulgaris, Artemisia douglasiana, Artemisia argyi) in the
treatment of menopause, premenstrual syndrome, dysmenorrhea and
attention deficit hyperactivity disorder. Chin Med. 3:116–123.
2012. View Article : Google Scholar
|
|
5
|
Ferreira JF, Luthria DL, Sasaki T and
Heyerick A: Flavonoids from Artemisia annua L. as antioxidants and
their potential synergism with artemisinin against malaria and
cancer. Molecules. 15:3135–3170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adams M, Efferth T and Bauer R:
Activity-guided isolation of scopoletin and isoscopoletin, the
inhibitory active principles towards CCRF-CEM leukaemia cells and
multi-drug resistant CEM/ADR5000 cells, from Artemisia argyi.
Planta Med. 72:862–864. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seo JM, Kang HM, Son KH, Kim JH, Lee CW,
Kim HM, Chang SI and Kwon BM: Antitumor activity of flavones
isolated from Artemisia argyi. Planta Med. 69:218–222. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JH, Kim HK, Jeon SB, Son KH, Kim EH,
Kang SK, Sung ND and Kwon BM: New sesquiterpene-monoterpene
lactone, artemisolide, isolated from Artemisia argyi. Tetrahedron
Lett. 43:6205–6208. 2002. View Article : Google Scholar
|
|
9
|
Yun C, Jung Y, Chun W, Yang B, Ryu J, Lim
C, Kim JH, Kim H and Cho SI: Anti-inflammatory effects of artemisia
leaf extract in mice with contact dermatitis in vitro and in vivo.
Mediators Inflamm. 2016.8027537:2016.
|
|
10
|
Yoon KD, Chin YW, Yang MH and Kim J:
Separation of anti-ulcer flavonoids from Artemisia extracts by
high-speed countercurrent chromatography. Food Chem. 129:679–683.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji HY, Kim SY, Kim DK, Jeong JH and Lee
HS: Effects of eupatilin and jaceosidin on cytochrome p450 enzyme
activities in human liver microsomes. Molecules. 15:6466–6475.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JK, Shin EC, Lim HJ, Choi SJ, Kim CR,
Suh SH, Kim CJ, Park GG, Park CS, Kim HK, et al: Characterization
of nutritional composition, antioxidative capacity, and sensory
attributes of seomae mugwort, a native Korean variety of artemisia
argyi H. Lév. & Vaniot. J Anal Methods Chem.
2015.916346:2015.
|
|
13
|
Bao X, Yuan H, Wang C, Liu J and Lan M:
Antitumor and immu-nomodulatory activities of a polysaccharide from
Artemisia argyi. Carbohydr Polym. 98:1236–1243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Libro R, Giacoppo S, Soundara Rajan T,
Bramanti P and Mazzon E: Natural phytochemicals in the treatment
and prevention of dementia: An overview. Molecules. 21:5182016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seo ON, Kim GS, Park S, Lee JH, Kim YH,
Lee WS, Lee SJ, Kim CY, Jin JS, Choi SK and Shin SC: Determination
of poly-phenol components of Lonicera japonica Thunb. using liquid
chromatography-tandem mass spectrometry: Contribution to the
overall antioxidant activity. Food Chem. 134:572–577. 2012.
View Article : Google Scholar
|
|
16
|
Lee CY: Challenges in providing credible
scientific evidence of health benefits of dietary polyphenols. J
Funct Foods. 5:524–526. 2013. View Article : Google Scholar
|
|
17
|
Carvalho IS, Cavaco T and Brodelius M:
Phenolic composition and antioxidant capacity of six artemisia
species. Industr Crops Prod. 33:382–388. 2011. View Article : Google Scholar
|
|
18
|
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng
J, Li Y, Wang X and Zhao L: Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget.
9:7204–7218. 2018.PubMed/NCBI
|
|
19
|
Hayes JB, Sircy LM, Heusinkveld LE, Ding
W, Leander RN, McClelland EE and Nelson DE: Modulation of
macrophage inflammatory nuclear factor κB (NF-κB) signaling by
intracellular cryptococcus neoformans. J Biol Chem.
291:15614–15627. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar :
|
|
21
|
Niu N, Li B, Hu Y, Li X, Li J and Zhang H:
Protective effects of scoparone against lipopolysaccharide-induced
acute lung injury. Int Immunopharmacol. 23:127–133. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li P, Zheng Y and Chen X: Drugs for
autoimmune inflammatory diseases: From small molecule compounds to
anti-TNF biologics. Front Pharmacol. 8:4602017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Lai L, Teng L, Li Y, Cheng J, Chen
J and Deng C: Mechanism of the anti-inflammatory activity by a
polysaccharide from Dictyophora indusiata in
lipopolysaccharide-stimulated macrophages. Int J Biol Macromol.
126:1158–1166. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gautam R and Jachak SM: Recent
developments in anti-inflammatory natural products. Med Res Rev.
29:767–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin D, Xiao M, Zhao J, Li Z, Xing B, Li X,
Kong M, Li L, Zhang Q, Liu Y, et al: An overview of plant phenolic
compounds and their importance in human nutrition and management of
type 2 diabetes. Molecules. 21:2016. View Article : Google Scholar
|
|
26
|
Giugliano D, Ceriello A and Esposito K:
The effects of diet on inflammation: Emphasis on the metabolic
syndrome. J Am Coll Cardiol. 48:677–685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ha GJ, Lee DS, Seung TW, Park CH, Park SK,
Jin DE, Kim NK, Shin HY and Heo HJ: Anti-amnesic and
neuroprotective effects of Artemisia argyi H. (Seomae mugwort)
extracts. Korean J Food Sci Technol. 47:380–387. 2015. View Article : Google Scholar
|
|
28
|
Chen LL, Zhang HJ, Chao J and Liu JF:
Essential oil of Artemisia argyi suppresses inflammatory responses
by inhibiting JAK/STATs activation. J Ethnopharmacol. 204:107–117.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song Y, Desta KT, Kim GS, Lee SJ, Lee WS,
Kim YH, Jin JS, Abd El-Aty AM, Shin HC, Shim JH and Shin SC:
Polyphenolic profile and antioxidant effects of various parts of
Artemisia annua L. Biomed Chromatogr. 30:588–595. 2016. View Article : Google Scholar
|
|
30
|
Ji G, Zhang Y, Yang Q, Cheng S, Hao J,
Zhao X and Jiang Z: Genistein suppresses LPS-induced inflammatory
response through inhibiting NF-κB following AMP kinase activation
in RAW 264.7 macrophages. PLoS One. 7:pp. e531012012, View Article : Google Scholar
|
|
31
|
Oh YC, Jeong YH, Cho WK, Ha JH, Gu MJ and
Ma JY: Anti-inflammatory and analgesic effects of pyeongwisan on
LPS-stimulated murine macrophages and mouse models of acetic
acid-induced writhing response and xylene-induced ear edema. Int J
Mol Sci. 16:1232–1251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Scarfi S, Magnone M, Ferraris C, Pozzolini
M, Benvenuto F, Benatti U and Giovine M: Ascorbic acid pre-treated
quartz stimulates TNF-alpha release in RAW 264.7 murine macrophages
through ROS production and membrane lipid peroxidation. Respir Res.
10:252009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han B, Xin Z, Ma S, Liu W, Zhang B, Ran L,
Yi L and Ren D: Comprehensive characterization and identification
of antioxidants in Folium Artemisiae Argyi using high-resolution
tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life
Sci. 1063:84–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fischer UA, Jaksch AV, Carle R and
Kammerer DR: Determination of lignans in edible and nonedible parts
of pomegranate (Punica granatum L.) and products derived therefrom,
particularly focusing on the quantitation of isolar-iciresinol
using HPLC-DAD-ESI/MSn. J Agric Food Chem. 60:283–292. 2012.
View Article : Google Scholar
|
|
35
|
Yao H, Chen B, Zhang Y, Ou H, Li Y, Li S,
Shi P and Lin X: Analysis of the total biflavonoids extract from
selaginella doeder-leinii by HPLC-QTOF-MS and its in vitro and in
vivo anticancer effects. Molecules. 22:2017. View Article : Google Scholar
|
|
36
|
Zhang YX, Li QY, Yan LL and Shi Y:
Structural characterization and identification of biflavones in
Selaginella tamariscina by liquid chromatography-diode-array
detection/electrospray ionization tandem mass spectrometry. Rapid
Commun Mass Spectrom. 25:2173–2186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pandey R, Chandra P, Arya KR and Kumar B:
Development and validation of an ultra high performance liquid
chromatography electrospray ionization tandem mass spectrometry
method for the simultaneous determination of selected flavonoids in
Ginkgo biloba. J Sep Sci. 37:3610–3618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kajd ža noska M: HPLC-DAD-ESI-MSn
identification of phenolic compounds in cultivated strawberries
from Macedonia. Macedonian J Chem Chem Eng. 29:2010.
|
|
39
|
Bertrams J, Kunz N, Müller M, Kammerer D
and Stintzing FC: Phenolic compounds as marker compounds for
botanical origin determination of German propolis samples based on
TLC and TLC-MS. J Appl Bot Food Qual. 86:143–153. 2013.
|
|
40
|
Luo JL, Lu FL, Liu YC and Lo CF:
Identification of scutel-laria baicalensis in traditional Chinese
medicine preparations by LC/MS/MS fingerprinting method. J Food
Drug Anal. 20:887–899. 9842012.
|
|
41
|
Sanz M, de Simón BF, Cadahía E, Esteruelas
E, Muñoz AM, Hernández T, Estrella I and Pinto E: LC-DAD/ESI-MS/MS
study of phenolic compounds in ash (Fraxinus excelsior L. and F.
americana L.) heartwood. Effect of toasting intensity at cooperage.
J Mass Spectrom. 47:905–918. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miyamae Y, Kurisu M, Han J, Isoda H and
Shigemori H: Structure-activity relationship of caffeoylquinic
acids on the accelerating activity on ATP production. Chem Pharm
Bull (Tokyo). 59:pp. 502–507. 2011, View Article : Google Scholar
|
|
43
|
Green SJ, Scheller LF, Marletta MA, Seguin
MC, Klotz FW, Slayter M, Nelson BJ and Nacy CA: Nitric oxide:
Cytokine-regulation of nitric oxide in host resistance to
intracellular pathogens. Immunol Lett. 43:87–94. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guzik TJ, Korbut R and Adamek-Guzik T:
Nitric oxide and superoxide in inflammation and immune regulation.
J Physiol Pharmacol. 54:469–487. 2003.
|
|
45
|
Wu C, Zhao W, Zhang X and Chen X:
Neocryptotanshinone inhibits lipopolysaccharide-induced
inflammation in RAW264.7 macrophages by suppression of NF-κB and
iNOS signaling pathways. Acta Pharm Sin B. 5:pp. 323–329. 2015,
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee JA, Song HY, Ju SM, Lee SJ, Kwon HJ,
Eum WS, Jang SH, Choi SY and Park JS: Differential regulation of
inducible nitric oxide synthase and cyclooxygenase-2 expression by
superoxide dismutase in lipopolysaccharide stimulated RAW 264.7
cells. Exp Mol Med. 41:629–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lawrence T, Willoughby DA and Gilroy DW:
Anti-inflammatory lipid mediators and insights into the resolution
of inflammation. Nat Rev Immunol. 2:787–795. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee JH, Cho DH and Park HJ: IL-18 and
cutaneous inflammatory diseases. Int J Mol Sci. 16:29357–29369.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang Y, Kim SC, Yu T, Yi YS, Rhee MH, Sung
GH, Yoo BC and Cho JY: Functional roles of p38 mitogen-activated
protein kinase in macrophage-mediated inflammatory responses.
Mediators Inflamm. 2014.352371:2014.
|
|
53
|
Dou J, Lee VS, Tzen JT and Lee MR:
Identification and comparison of phenolic compounds in the
preparation of oolong tea manufactured by semifermentation and
drying processes. J Agric Food Chem. 55:7462–7468. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Del Rio D, Stewart AJ, Mullen W, Burns J,
Lean ME, Brighenti F and Crozier A: HPLC-MSn analysis of phenolic
compounds and purine alkaloids in green and black tea. J Agric Food
Chem. 52:2807–2815. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Simirgiotis MJ, Schmeda-Hirschmann G,
Bórquez J and Kennelly EJ: The Passiflora tripartita (Banana
Passion) fruit: A source of bioactive flavonoid C-glycosides
isolated by HSCCC and characterized by HPLC-DAD-ESI/MS/MS.
Molecules. 18:1672–1692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ahmed H, Moawad A, Owis A, AbouZid S and
Ahmed O: Flavonoids of Calligonum polygonoides and their
cytotoxicity. Pharm Biol. 54:2119–2126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bastos DH, Saldanha LA, Catharino RR,
Sawaya AC, Cunha IB, Carvalho PO and Eberlin MN: Phenolic
antioxidants identified by ESI-MS from Yerba maté (Ilex
paraguariensis) and green tea (Camelia sinensis) extracts.
Molecules. 12:423–432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gouveia S and Castilho PC: Helichrysum
monizii Lowe: Phenolic composition and antioxidant potential.
Phytochem Anal. 23:72–83. 2012. View Article : Google Scholar
|
|
59
|
Li W, Pang X, Han LF, Zhou Y and Cui YM:
Chemcial constituents of Eclipta prostrata. Zhongguo Zhong Yao Za
Zhi. 43:3498–3505. 2018.In Chinese. PubMed/NCBI
|