Introduction
Lung cancer is a highly prevalent malignancy and the
leading cause of cancer-related mortality worldwide. Non-small-cell
lung cancer (NSCLC) accounts for nearly 80% of all lung cancer
casesω1). Current chemotherapies have been found to be only
marginally effective in prolonging overall survival. Natural
products have been used as the main sources of therapeutic agents
in ancient times and, in modern medicine, they remain a major
source of new drug development (2,3).
Isolation of anticancer compounds from traditional herbs may offer
new options for lung cancer treatment.
Brucea javanica (L.) Merr. (Fructus Bruceae)
and its oil emulsion have long been used for the treatment of
various types of cancer in China (4). Quassinoids are characteristic
metabolites of B. javanica and are well-known for their
anticancer properties (5).
Bruceine D is an abundant naturally occurring active tetracyclic
triterpene quassinoid in B. javanica. Our previous studies
have demonstrated that bruceine D exerts potent antiproliferative
effects on cultured human pancreatic adenocarcinoma cells through
induction of apoptosis involving the activation of the p38-MAPK,
NF-κB, and reactive oxygen species (ROS)-associated PI3K/Akt
signaling pathways (6-8). By contrast, bruceine D was shown to
exert only mild cytotoxic effects on human gastric mucosal
epithelial GES-1 cells, human foreskin fibroblast Hs68 cells and
the WRL68 human hepatocyte cell line (8-10).
Furthermore, bruceine D effectively reduced the rate of xeno-graft
human pancreatic tumor and orthotopic xenograft in nude mice, with
no overt toxicity (7,8).
Although a number of studies have predominantly
focused on the anti-pancreatic cancer activity of bruceine D, its
potential effect and mechanism of action in other types of cancer,
including lung cancer, remain elusive. As part of the ongoing
investigation of natural sources of anticancer treatments, the
present study was initiated to investigate the potential inhibitory
effect of bruceine D on NSCLC cells in vitro and elucidate
the underlying mechanism. In the present study, the effects of
bruceine D on the proliferation of four NSCLC cell lines, including
wild-type (A549 and H1650) and epidermal growth factor receptor
(EGFR)-mutant (PC-9 and HCC827) cell lines, were assessed. The
mechanism of action of bruceine D was also evaluated through
investigation of colony formation, migratory ability, cellular
apoptosis induction, cell cycle arrest, oxidative status,
mitochondrial membrane potential disruption and
apoptosis-associated protein expression. The aim was to investigate
the cytotoxic activity and elucidate the underlying mechanism of
action of bruceine D in NSCLC cells, in order to improve our
understanding of the role of B. javanica and its
commercially available derivatives in lung cancer therapy, and
determine whether bruceine D may be of value as a naturally
occurring candidate for the treatment of NSCLC.
Materials and methods
Plant materials and reagents
The dried ripe fruits of B. javanica were
purchased from Zhixin Pharmaceutical Co. and were authenticated by
Professor ZXL of Guangdong Provincial Key Laboratory of New Drug
Development and Research of Chinese Medicine, Mathematical
Engineering Academy of Chinese Medicine, Guangzhou University of
Chinese Medicine, according to the methods specified in the Chinese
Pharmacopoeia (11). The voucher
specimen (Pan-Ca. 01) was deposited in the Herbarium of School of
Chinese Medicine, The Chinese University of Hong Kong. Antibodies
against procaspase-3 (cat. no. sc-7148), procaspase-8 (cat. no.
sc-5263), X-linked inhibitor of apoptosis (XIAP; cat. no.
sc-55550), Bcl-2 (cat. no. sc-492), Bcl-xL (cat. no. sc-8392), Bax
(cat. no. sc-493), Bak (cat. no. sc-517390), β-actin (cat. no.
sc-47778) and horseradish peroxidase (HRP)-conjugated secondary
antibodies were purchased from Santa Cruz Biotechnology, Inc.
CM-H2DCFDA (cat. no. C6827) and Rhodamine 123 (cat. no. R302) were
purchased from Invitrogen; Thermo Fisher Scientific, Inc. FxCycle™
PI/RNase staining solution (cat. no. F10797) was obtained from
Molecular Probes; Thermo Fisher Scientific, Inc. Dead Cell
Apoptosis kit with Annexin V Alexa Fluor® 488 &
Propidium Iodide (cat. no v13245) was acquired from Invitrogen;
Thermo Fisher Scientific, Inc. All other chemicals were obtained
from Sigma-Aldrich; Merck KGaA, unless otherwise stated.
Isolation and identification of bruceine
D
Bruceine D was isolated from B. javanica (5
kg) in our laboratory, as described previously (12), with a yield of 1 g. Bruceine D
(C20H26O9, CAS: 21499-66-1) was
obtained as a colorless amorphous solid with a melting point of
290-292°C, in agreement with a previous report (13); UV (methanol, λmax, nm): 208, 244,
315. ESI-MS (m/z): 411.4 [M+H]+, 433.4
[M+Na]+, 393.5, 381.6. Nuclear magnetic resonance (NMR)
spectra were recorded in CD3OD on a Bruker AC 400 MHz FT
NMR spectrometer using tetra-methylsilane as the internal standard.
1H NMR (CD3OD) δ 5.21 (s, H-1), 6.03 (m,
H-3), 2.93 (d, J=13 Hz, H-5), 2.37 (dd, J=1.5 and 5
Hz, H-6), 1.83 (m, H-6), 5.09 (t, H-7), 2.32 (dt, J=3 and 15
Hz, H-9), 4.57 (d, J=5 Hz, H-11), 3.75 (d, J=1 Hz,
H-12), 4.22 (s, H-15), 3.82 (dd, J=2 and 7 Hz, H-17), 4.52
(d, J=7 Hz, H-17), 1.96 (m, H-18), 1.41 (s, H-19), 1.17 (s,
H-20). 13C NMR (CD3OD) δ 83.1 (C-1), 199.8
(C-2), 125.2 (C-3), 165.6 (C-4), 44.4 (C-5), 28.7 (C-6), 81.2
(C-7), 50.8 (C-8), 46.3 (C-9), 49.6 (C-10), 75.5 (C-11), 81.5
(C-12), 85.0 (C-13), 82.4 (C-14), 70.7 (C-15), 176.2 (C-16), 70.4
(C-17), 22.5 (C-18), 11.5 (C-19), 18.4 (C-20) (Figs. S1 and S2). The compound was
identified as bruceine D by comparing its ESI-MS, 1H and
13C NMR spectra with the published literature (13,14). The purity of bruceine D was
>98.0%, as determined by high-performance liquid chromatography
with a diode-array detector (Fig.
S3).
Cell lines and culture
Human NSCLC cells, including wild-type cell lines
(A549 and H1650) and the EGFR-mutant PC-9 cell line, were kindly
provided by the Guangdong Provincial Academy of Chinese Medical
Sciences. The EGFR-mutant HCC827 cell line was obtained from the
Institute of Basic Medical Sciences, Chinese Academy of Medical
Sciences. All cells were maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), penicillin (100 U/ml) and streptomycin (100
µg/ml) in a humidified incubator with an atmosphere of 95%
air and 5% CO2 at 37°C.
Cell viability assay
Cell viability was assessed by the MTT assay
(Invitrogen; Thermo Fisher Scientific, Inc.), as described
previously (6). bruceine D was
dissolved in dimethyl sulfoxide (DMSO) and stored at 2-8°C. Culture
media containing different concentrations of bruceine D (0.05, 0.5,
1, 5, 25 and 50 µg/ml) were freshly prepared at the time of
each experiment. The final concentration of DMSO was <0.1% in
all experiments. Cells grown in medium containing an equivalent
amount of DMSO without bruceine D were used as control.
Colony formation assay
Colony formation assay was performed according to
the protocol previously described, with minor modifications
(15). A549 NSCLC cells were
seeded in 6-well plates at a density of 200 cells per well.
Different concentrations of bruceine D (1, 2.5 and 5 µg/ml)
were added to the wells and incubated for 24 h. Cells were allowed
to form colonies for 2 weeks post-treatment. After a 2-week
incubation, all cells were fixed with 4% paraformaldehyde and
stained with 0.1% crystal violet solution (Beyotime Institute of
Biotechnology). After 15 min, the cells were washed with distilled
water at least three times and then dried at room temperature.
Images were obtained using an ordinary Nikon camera (Nikon
Corporation).
Wound healing assay
A549 cells were cultured under standard conditions,
as mentioned above, and plated onto 60-mm2 dishes.
Scratch wounds were created into the confluent monolayers after
attaining ~90% confluence. The cell monolayer was scratched using a
sterile 200-µl pipette tip and the cells were washed with
PBS to remove the debris. Cells were incubated in regular culture
media with 1, 2.5 and 5 µg/ml bruceine D or vehicle. Wound
closure was monitored over time and photographed using an Olympus
IX71 microscope (Olympus Corporation). Wound closure was quantified
by measuring the remaining open area using ImageJ software, version
1.52 (National Institutes of Health). The wound open area was
calculated as follows: Wound open area as percentage of original =
(unmigrated area/original wound area) ×100%.
Cell cycle analysis
Following incubation for 48 h, cells were harvested
and fixed overnight in cold 75% ethanol at 4°C. Next, cells were
washed again with pre-cooled PBS and resuspended in 400 µl
FxCycle™ PI/RNase staining solution (Molecular Probes; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The samples were incubated for 30 min at room temperature in the
dark, and then analyzed using a CytomicsTM FC500 flow cytometer
(Beckman Coulter, Inc.). Cell cycle distribution was calculated
with ModFit LT 4.1 software (Becton Dickinson and Company).
Annexin V-PI staining apoptosis
assay
A549 cells were seeded onto 6-well plates at a
seeding density of 5×105 cells/well and allowed to
adhere overnight. Bruceine D was added to the culture media to the
specified final concentrations (1, 2.5 and 5 µg/ml). Vehicle
alone was added to the culture medium, serving as the untreated
control. After 48 h, the cells were harvested, rinsed twice with 1X
PBS, and resuspended in 100 µl 1X Annexin binding buffer.
Apoptosis was analyzed by flow cytometry (Beckman Coulter, Inc.)
using Dead Cell Apoptosis kit with Annexin V Alexa
Fluor® 488 & Propidium Iodide (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Fluorescence minus one controls were used to set the
positive/negative cell gates and validate the flow cytometric
results.
Measurement of ROS
The ROS level was measured using the CM-H2DCFDA
probe (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol, followed by analysis using CXP
cytometer software (version 2.0) and a flow cytometer (Beckman
Coulter, Inc.).
To eliminate ROS generation, N-acetylcysteine (NAC;
20 µM) was dissolved in PBS and incubated with the cells 1 h
prior to bruceine D treatment. A549 cells were treated with
vehicle, 20 µM NAC, 20 µM NAC + 5 µg/ml
bruceine D, or 5 µg/ml bruceine D for 48 h at 37°C in
96-well microplates. Cell viability was assessed by the MTT assay,
as described above.
Glutathione (GSH) and malondialdehyde
(MDA) assays
Following treatment, A549 cells were washed twice
with ice-cold PBS, harvested by centrifugation at 1,000 × g for 4
min at 4°C, pooled in 0.5 ml PBS and homogenized. The homogenate
was centrifuged at 4,000 × g for 15 min at 4°C, and the supernatant
was collected for GSH and MDA assays.
GSH content was measured as previously described
(16). The GSH level was
normalized to the protein concentration of each sample and
expressed as fold change compared with the control group.
The MDA content was determined using the
thiobarbituric acid method, as previously described (16). The MDA level was normalized to the
protein concentration of each sample and expressed as fold change
compared with the control group.
Measurement of mitochondrial membrane
potential (ΔΨm)
The ΔΨm of cells exposed to 1, 2.5 and 5
µg/ml bruceine D or vehicle alone was measured using the
fluorescent cationic dye Rhodamine 123, according to the
manufacturer's protocol (Molecular Probes; Thermo Fisher
Scientific, Inc.). Cells were analyzed using a CytomicsTM FC500
flow cytometer (Beckman Coulter, Inc.).
Western blotting
A549 cells were seeded in culture dishes (100
mm2; 5×106 cells/dish) and treated with
bruceine D (1, 2.5 and 5 µg/ml) for 24 h at 37°C after
seeding. The cells were lysed with RIPA buffer containing protease
inhibitor cocktail (Roche Molecular Biochemicals), 1 mM PMSF and 1
mM Na3NO4. The protein concentration was
determined by the BCA assay (BCA kit; Sigma-Aldrich; Merck KGaA).
Protein aliquots (50-µg) were placed in each lane, separated
by 10% SDS-PAGE and electrotransferred to a PVDF membrane (EMD
Millipore). After four washes, the membranes were incubated with 5%
skimmed milk for 2 h at room temperature, and then incubated
overnight at 4°C with primary antibodies (1:200, Santa Cruz
Biotechnology, Inc.) against Bax, Bad, Bcl-2, Bcl-xL, X-linked
inhibitor of apoptosis (XIAP), pro-caspase-3 and pro-caspase-8.
After washing three times with TBST (0.1% v/v Tween-20 in TBS), the
membranes were incubated for 2 h at room temperature with
species-specific HRP-conjugated secondary antibodies (1:2,000;
Santa Cruz Biotechnology, Inc.) for 1.5 h. Immunoreactive bands
were visualized using the ECL chemiluminescent substrate reagent
kit (NOVEX). β-actin (1:500, Santa Cruz Biotechnology, Inc.) served
as the loading control. ImageJ software, version 1.52 (National
Institutes of Health) was used to quantify the intensity of the
immunoreactive bands.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. The experiments were repeated three times. Multiple-group
comparisons were performed using one-way analysis of variance
followed by Fisher's least significant difference test to detect
differences between treatment groups and the control. P<0.05 was
considered to indicate a statistically significant difference. All
statistical tests were performed using SPSS software, version 17.0
(SPSS, Inc.).
Results
Bruceine D inhibits the proliferation of
wild-type and EGFR-mutant NSCLC cells
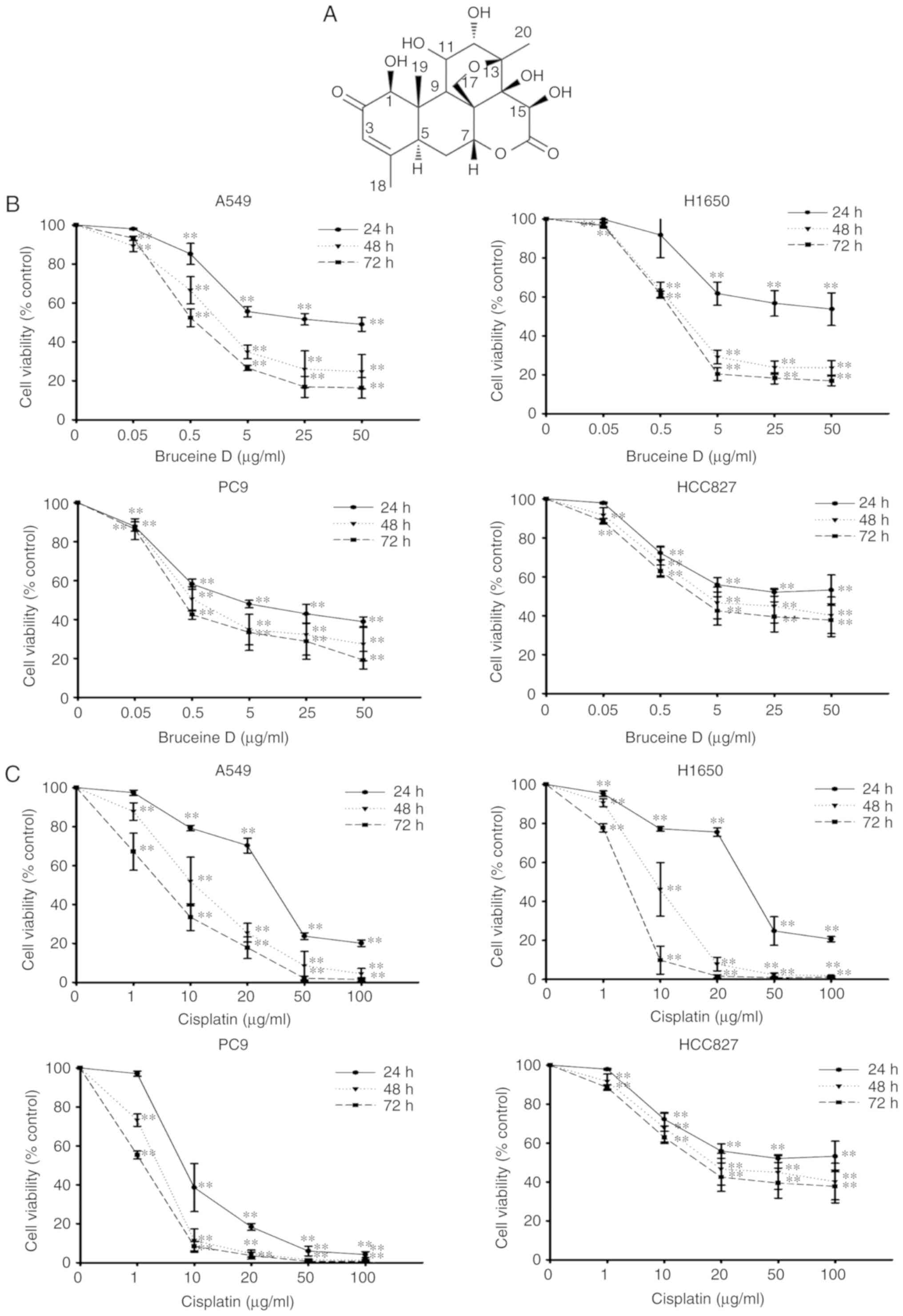
To evaluate the effect of bruceine D on the
viability of NSCLC cells, wild-type A549 and H1650 cells, and
EGFR-mutant PC-9 and HCC827 cells were treated with various
concentrations of bruceine D (0, 0.05, 0.5, 5, 25 and 50
µg/ml) for different time periods (24, 48 and 72 h), and the
cell viability was evaluated by the MTT assay. The results revealed
that bruceine D treatment inhibited the proliferation of the four
NSCLC cells in a concentration- and time-dependent manner (Fig. 1B). Following treatment with
bruceine D for 72 h, the IC50 values were 1.01±0.11,
1.19±0.07, 2.28±1.54 and 6.09±1.83 µg/ml for A549, H1650,
PC-9 and HCC827 cells, respectively. In addition, the
IC50 values were 2.26±0.48, 1.76±0.15, 1.14±0.03 and
3.48±0.10 µg/ml for cisplatin in A549, H1650, PC-9 and
HCC827 cells, respectively (Fig.
1C). These results indicated that bruceine D exerted either a
similar or superior anti-NSCLC effect compared with cisplatin.
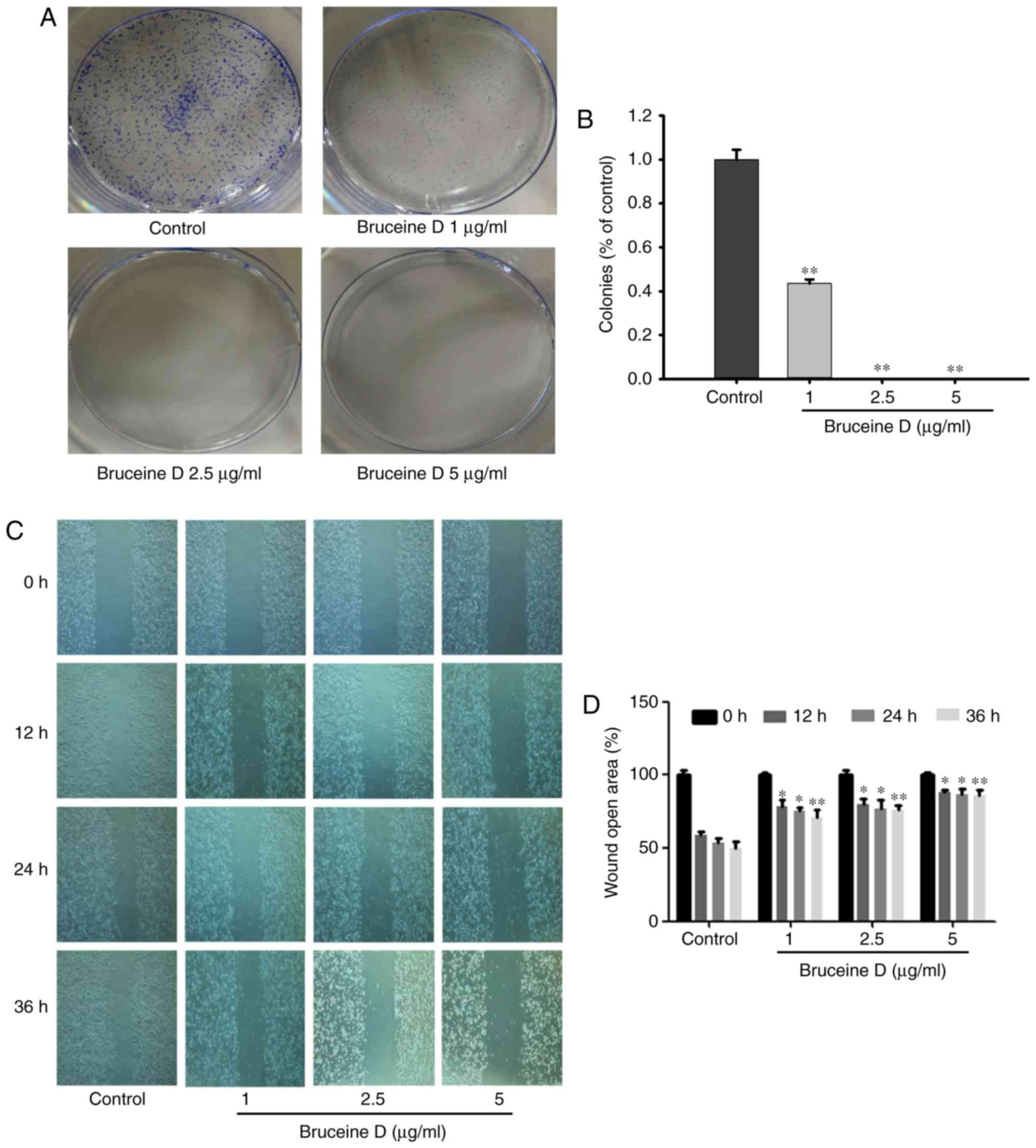
Bruceine D inhibits colony formation and
migration of A549 NSCLC cells
Considering that bruceine D exhibited potent
cytotoxic activity against A549 cells, this cell line was used in
the subsequent experiments. To investigate the inhibitory potential
of bruceine D on the colony-forming ability of A549 NSCLC cells, a
colony formation assay was performed. As shown in Fig. 2A, the number of colonies makedly
decreased in a dose-dependent manner following treatment with
bruceine D, suggesting that bruceine D exerts an anti-colony
forming effect on A549 cells. This result was consistent with the
results of the MTT assay, indicating that bruceine D exhibits
antiproliferative activity against A549 cells.
To elucidate the potential anti-migratory effect of
bruceine D on A549 cells, a would healing assay was performed. As
shown in Fig. 2B, bruceine
D-treated cells migrated at a slower rate compared with the control
group in a time- and dose-dependent manner, indicating that
bruceine D exerts a potent suppressive effect on A549 cell
migration.
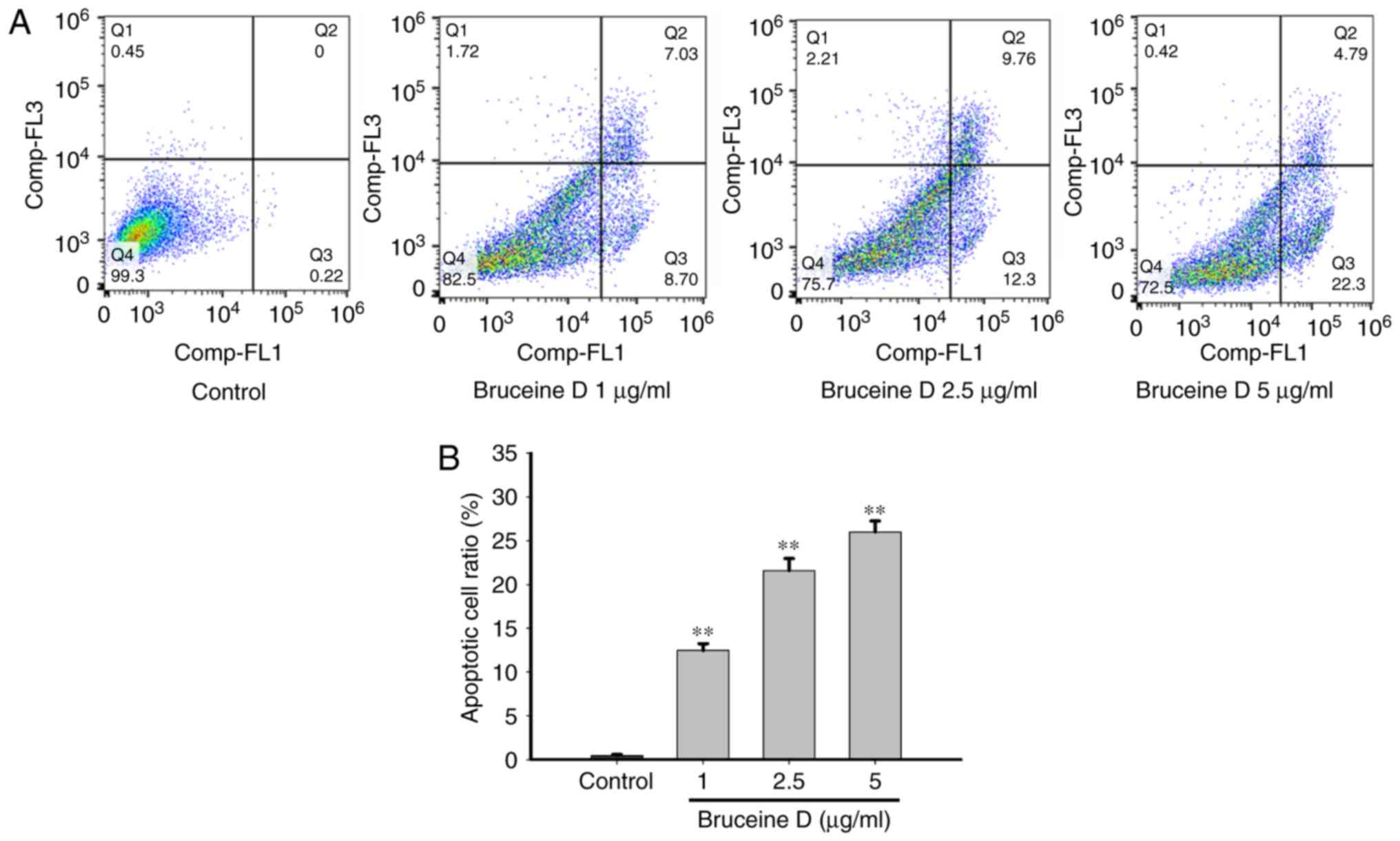
Bruceine D increases apoptosis of A549
NSCLC cells
To further verify whether decreased viability was
associated with an induction of apoptosis, flow cytometric analysis
was performed. As shown in Fig.
3A, bruceine D treatment induced early and late apoptosis in a
concentration-dependent manner compared with the untreated control
cells. In the untreated group there was a small percentage
(0.44±0.05%) of Annexin V-positive cells. By contrast, the
percentage of apop-totic cells significantly increased to
12.5±0.28, 21.58±0.50 and 25.98±0.44% (all P<0.01) in a
concentration-dependent manner following treatment with bruceine D
(1, 2.5 and 5 µg/ml, respectively) (Fig. 3B). These results suggest that the
reduction in the viability of A549 cells is associated with
apoptosis induced by bruceine D.
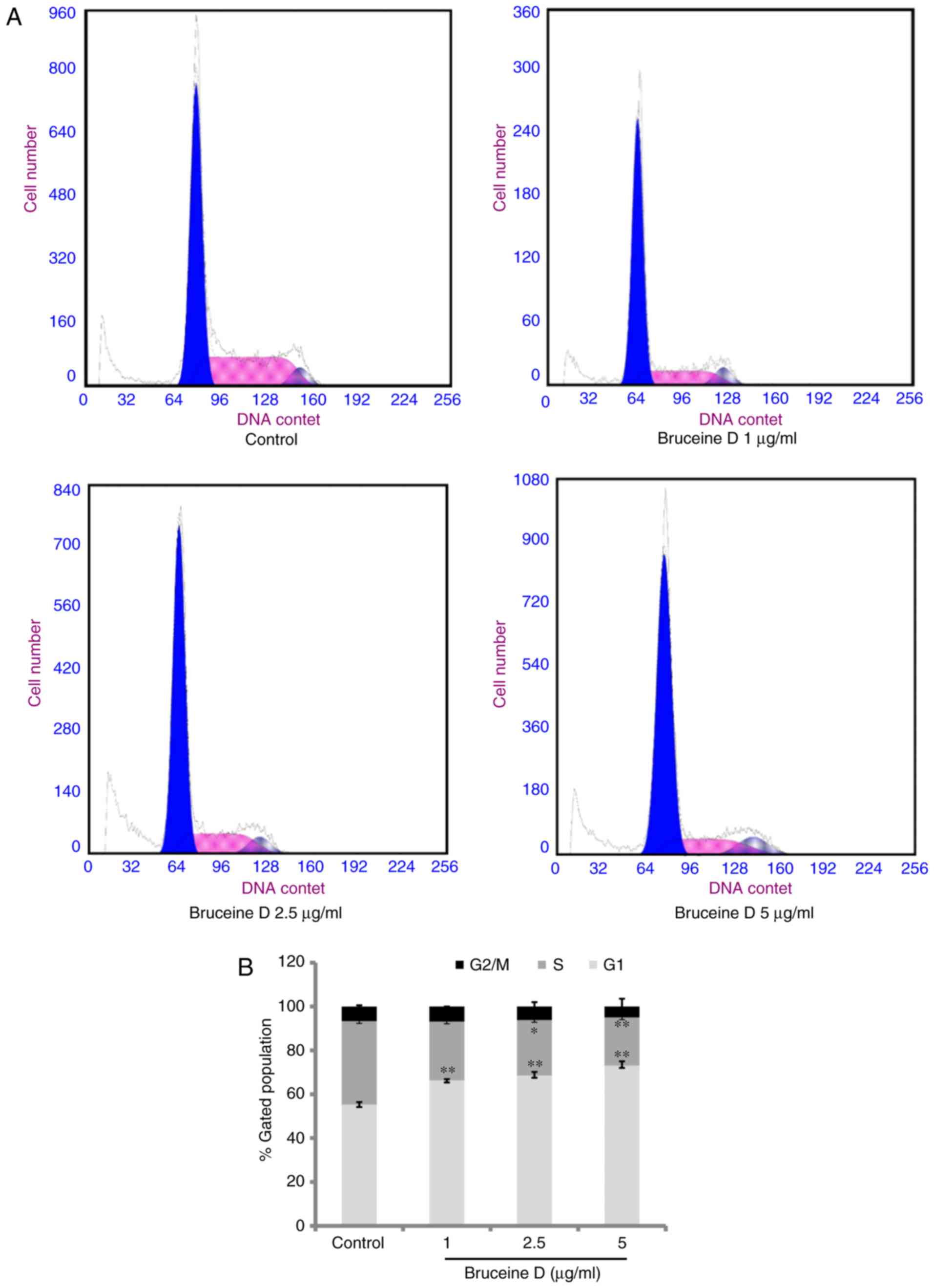
Bruceine D induces G0/G1 phase cell cycle
arrest in A549 cells
Cell cycle regulation is a critical event in
cellular division and is considered a valuable target in anticancer
therapies (17). To investigate
the potential mechanism underlying the anti-proliferative activity
of bruceine D, flow cytometry was used to determine the cell cycle
distribution. As shown in Fig. 4,
bruceine D at 1, 2.5 and 5 µg/ml significantly increased the
percentage of cells in the G0/G1 phase (all P<0.01) and
significantly decreased the ratio of cells in the S phase
(P>0.05, P<0.05 and P<0.01, respectively). However, the
percentage of cells in the G2/M phase remained almost unchanged
following bruceine D treatment. These results indicated that
bruceine D may inhibit the proliferation of A549 cells, at least
partially by inducing cell cycle arrest at the G0/G1 phase.
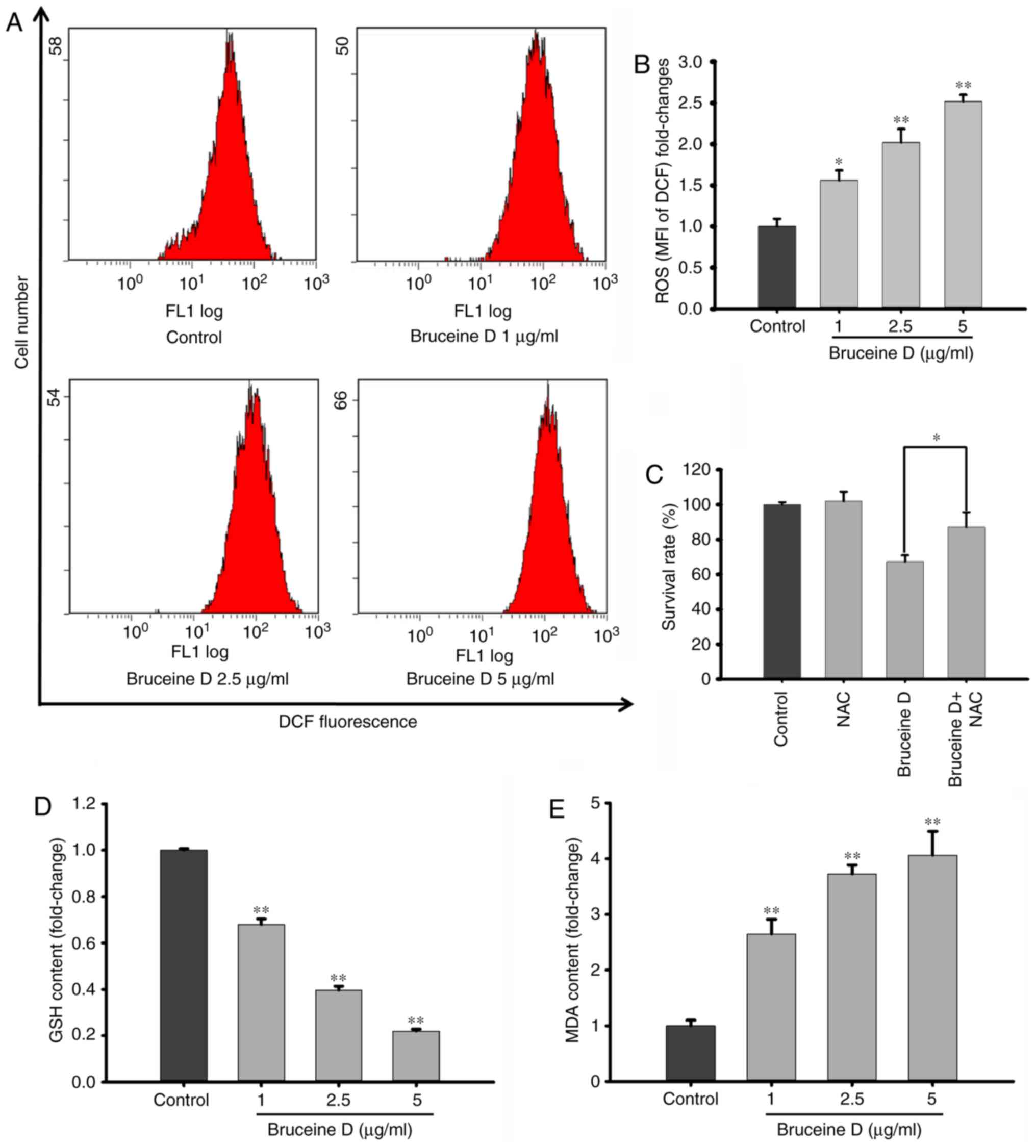
Bruceine D induces ROS overproduction,
GSH depletion and MDA accumulation in A549 cells
Redox disequilibrium has been reported to play a
pivotal role in apoptosis (18).
To investigate whether ROS generation is involved in bruceine
D-induced apoptosis of A549 cells, the fluorescence probe DCFH-DA
was used to measure the intracellular ROS levels. As shown in
Fig. 5A, flow cytometry revealed
that bruceine D markedly induced ROS generation in A549 cells.
After treatment with 1, 2.5 and 5 µg/ml bruceine D, the
level of intracellular ROS significantly and dose-dependently
increased from 100±5.36 to 156.18±6.83, 202.11±9.43 and
251.90±4.55%, respectively (all P<0.01; Fig. 5B).
To verify whether ROS generation is associated with
the bruceine D-induced inhibition, cells were treated with bruceine
D in the presence or absence of NAC, a typical ROS scavenger. It
was observed that the cell survival rate was significantly
increased by NAC pre-treatment compared with that of cells
subjected to bruceine D treatment alone (P<0.05; Fig. 5C). This result further confirms
that the accumulation of ROS is involved in the suppressive effect
of bruceine D on A549 cells.
As shown in Fig.
5D, the GSH level significantly decreased following bruceine D
treatment, from 100±0.07 at 0 µg/ml to 67.9±2.49, 39.7±1.67
and 22.0±0.01% at 1, 2.5 and 5 µg/ml, respectively (all
P<0.01). By contrast, the cellular MDA levels in the 1, 2.5 and
5 µg/ml bruceine D treatment groups were significantly
higher compared with the control group (2.6-, 3.7- and 4.1-fold,
respectively; all P<0.01; Fig.
5E). These findings indicate that a redox disequilibrium may
contribute to the bruceine D-induced apoptosis of A549 NSCLC
cells.
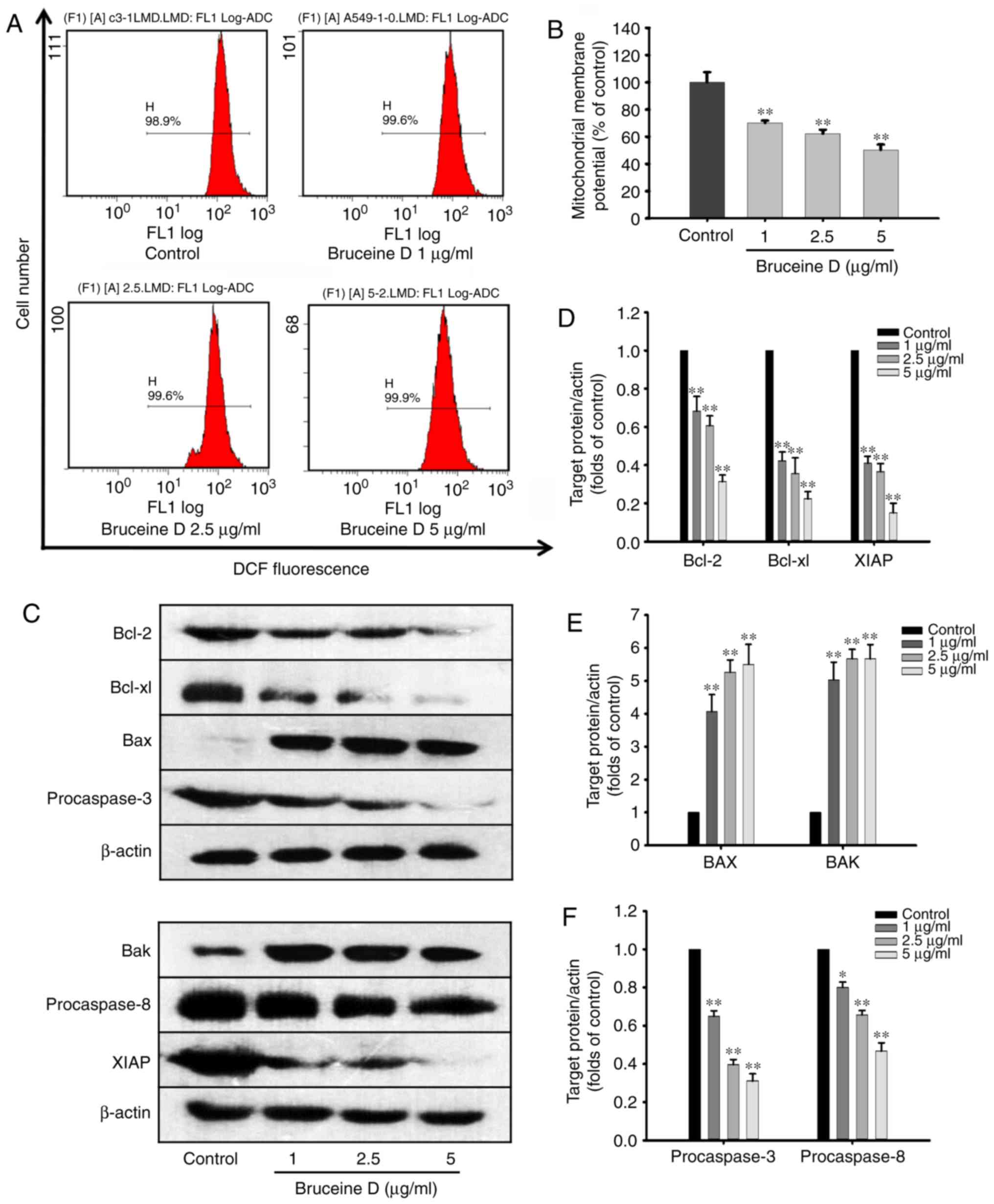
Bruceine D decreases the Δψm and triggers
the mitochondrial signaling pathway in A549 cells
Mitochondria play a key role in the regulation of
ROS and apoptosis, and alternations in the Δψm may reflect
mitochondrial dysfunction, redox status imbalance and apoptotic
events (19). Therefore, in the
present study, the Δψm was detected in A549 cells by flow cytometry
following staining with Rhodamine 123 (Fig. 6A and B). The results revealed
that, following treatment with bruceine D (1, 2.5 and 5
µg/ml), the mean fluorescence intensity of Rhodamine 123
significantly decreased from 100±3.74 to 70.13±0.88, 62.23±1.48 and
50.33±1.97%, respectively (all P<0.01; Fig. 6B). These data indicate that
apoptosis induced by bruceine D is associated with an alteration of
the Δψm.
The Bcl-2 family, which includes both anti- and
proapoptotic members, constitutes a key checkpoint in the intrinsic
mitochondrial pathway of apoptosis (20). To investigate whether
mitochondrial apoptotic events are involved in bruceine D-induced
apoptosis, the present study analyzed changes in the levels of
Bcl-2 family proteins and XIAP. As shown in Fig. 6C, bruceine D significantly
downregulated the expression levels of Bcl-2, Bcl-xL and XIAP, and
markedly upregulated the expression levels of Bax and Bak in A549
cells. Therefore, changes in the expression levels of Bcl-2 family
proteins and XIAP in A549 cells may play an important role in
bruceine D-induced apoptosis.
The sequential activation of caspases is a key step
in the execution phase of cell apoptosis. Pro-caspase-3 and its
activator, pro-caspase-8, are two important executioners of
apoptosis in the extrinsic pathway, and their inhibition may play a
key role in inducing apoptosis (21). Therefore, the role of
pro-caspase-3 and pro-caspase-8 in bruceine D-induced A549
apoptosis was investigated by western blotting. The results
demonstrated that bruceine D markedly downregulated the expression
of pro-caspase-3 and pro-caspase-8 in a dose-dependent manner.
These results suggest that the caspase family may be involved in
bruceine D-induced apoptosis of A549 cells.
Discussion
Quassinoids, a family of molecules with potent
anticancer properties, have been established as the major category
of anticancer phytochemicals of B. javanica, which is
commonly used for the treatment of cancer in Southeast Asia
(4). bruceine D, one of the major
active quassinoids isolated from B. javanica, has been
reported to exert an inhibitory effect against several types of
cancer (8,22,23). In our previous research, bruceine
D was found to exhibit a prominent suppressive effect on the
proliferation of various pancreatic cancer cells, displaying only
modest cytotoxicity against normal tissue cell lines, including
GES-1 cells, human pancreatic progenitor cells and the WRL68 human
hepatocyte cell line (6,8-10).
Furthermore, bruceine D administration at a high dose (3 mg/kg) was
associated with no obvious toxicity or distant organ metastasis in
mice (8). Therefore, this
naturally occurring tetracyclic triterpene quassinoid is considered
to be promising and may be further developed into an effective and
less toxic candidate for the treatment of cancer. In the present
study, it was demonstrated that bruceine D-induced inhibition of
NSCLC A549 cells was associated with reduced migration and colony
formation, increased apoptosis, induced G0/G1 cell cycle arrest,
and a disruption of the intracellular redox equilibrium and Δψm.
bruceine D induced pro-apoptotic signaling at least partially via
triggering the mitochondrial ROS-mediated death signaling
pathway.
Migration is a key characteristic of cancer
progression and metastasis (24),
and suppression of cancer cell migration ability may be an
effective mechanism for arresting cancer metastasis. Therefore, the
present study evaluated the effect of bruceine D on the migratory
ability of A549 cells. A wound healing assay revealed that bruceine
D significantly and dose-dependently reduced the migration of A549
cells. Furthermore, the anticlonogenic effects of bruceine D were
evaluated, and bruceine D was shown to significantly inhibit colony
formation by decreasing the number and size of colonies of A549
cells. In summary, these results indicate that bruceine D exerts
both anti-migratory and anticlonogenic effects on A549 NSCLC
cells.
Apoptosis plays a key role in cell proliferation,
differentiation, senescence and death (25). Therefore, the ability to induce
cancer cell apoptosis has been established as a valuable anticancer
strategy. Bruceine D was previously found to exert potent apoptotic
effects on hepatocellular carcinoma (22), pancreatic adenocarcinoma (8) and human chronic myeloid leukemia
K562 cells (23). Using
fluorescence microscopy and an Annexin V-FITC assay, the present
study demonstrated that bruceine D induces apoptosis of A549 NSCLC
cells. Following bruceine D exposure, the viability of A549 cells
decreased rapidly, and the majority of the cells were shrunken and
detached from the substratum of the culture dish (Data S1 and Fig. S4A). Typical apoptotic
morphological changes, including condensation of chromatin, nuclear
fragmentation and apoptotic bodies, were observed in bruceine
D-treated A549 cells (Data S1 and
Fig. S4B). Flow cytometric analysis indicated that bruceine D
treatment significantly induces both early and late apoptosis in
A549 cells, confirming that bruceine D is an effective inducer of
apoptosis in A549 cells.
Cell cycle regulation has been hypothesised to be a
critical step in cancer chemoprevention (26) and is considered as a feasible
strategy for slowing tumor growth (17). Following bruceine D treatment,
G0/G1 cell cycle arrest was observed. In addition, the proportion
of cells in the S phase was significantly decreased, which
indicates inhibition of DNA synthesis. By contrast, the proportion
of cells in the G2/M phase was less affected. Therefore, treatment
of A549 cells with bruceine D inhibited the cell cycle transition
from the G1 to the S phase, suggesting that inhibition of cell
cycle progression may be one of the mechanisms through which
bruceine D inhibits the proliferation of A549 NSCLC cells. The
present results are consistent with those of previous studies
demonstrating that bruceine D induces G1 phase arrest in cancer
cell lines (6,10).
The generation of intracellular ROS and depletion of
GSH are closely associated with cellular apoptosis and disruption
of the Δψm (18). ROS are vital
for cell proliferation, differentiation, apoptosis and survival
(27). ROS at low concentrations
are crucial for maintaining redox equilibrium and cell
proliferation (28). However,
overaccumulation of intracellular ROS leads to mitochondrial
dysfunction, which may reciprocally increase ROS production,
leading to oxidative stress, lipid peroxidation, depletion of GSH
and consequent cell apoptosis or death (17). GSH is a major non-enzymatic
antioxidant that participates in the maintenance of the cellular
redox status (29). Low GSH
levels are associated with mitochondrial dysfunction and induction
of apoptosis, thereby increasing sensitivity to anticancer drugs
(30). The level of MDA, an
intermediate product of lipid peroxidation, directly reflects the
oxidative damage of cell membranes. In the present study, increased
intracellular ROS and MDA levels associated with depleted GSH
levels were observed in bruceine D-treated A549 cells. Furthermore,
the inhibitory effect was significantly reversed by pretreating the
cells with the ROS scavenger NAC prior to bruceine D treatment,
suggesting that oxidative stress caused by ROS accumulation is
involved in the anti-NSCLC effect of bruceine D. These results
suggest that bruceine D-induced overproduction of ROS and redox
disequilibrium may play a key role in the inhibition of A549
cells.
Mitochondria play an important role in the
alteration of the intracellular redox state and induction of
apoptosis (31). Disruption of
Δψm is an initial and irreversible step of apoptosis. Mitochondrial
dysfunction resulting from a decrease of Δψm may cause defects in
ROS production and lipid homeostasis, leading to DNA damage and
apoptosis (32). In the present
study, when A549 cells were exposed to bruceine D, a change in Δψm
was detected, indicating that mitochondrial dysfunction contributes
to the anti-NSCLC effect of bruceine D.
Apoptosis usually occurs via the mitochondrial
intrinsic pathway and/or the death receptor extrinsic pathway
(33). The intrinsic pathway of
apoptosis is regulated by the Bcl-2 family of proteins (34). Anti-apoptotic proteins, including
Bcl-2 and Bcl-xL, and pro-apoptotic proteins, including Bad, Bax
and Bak, which exert opposing effects on mitochondria, are two
subgroups of the Bcl-2 family (35). Enhancement of pro-apoptotic
protein expression compared with anti-apoptotic proteins may
increase the permeability of the mitochondrial membrane, which in
turn results in the release of apoptogenic factors (36). In the present study, bruceine D
treatment substantially downregulated the expression of Bcl-2 and
Bcl-xL, whereas it markedly upregulated the expression levels of
Bad, Bax and Bak, resulting in of A549 cell apoptosis. These
experimental findings suggest that bruceine D-induced apoptosis is
associated with the regulation of the mitochondrial pathway.
Caspases are known to be activated during apoptosis
in numerous cell types and play key roles in the initiation and
execution of mitochondria-mediated apoptosis (34). Pro-caspase-3 activation is a
hallmark of apoptotic induction through both the extrinsic and
intrinsic apoptotic pathways. In the extrinsic pathway,
pro-caspase-3 is activated by pro-caspase-8 (37). In the present study, western
blotting demonstrated that bruceine D simultaneously decreased the
protein expression of pro-caspase-3 and pro-caspase-8, suggesting
that a mitochondria-associated pathway is involved in the induction
of apoptosis by bruceine D. However, further investigations are
required to improve our understanding of the detailed underlying
mechanism.
The results of the present demonstrate that bruceine
D exerts its anti-NSCLC effects at least partially via triggering
the mitochondrial ROS-mediated death signaling pathway. bruceine D
appears to be a potent antiproliferative and apoptosis-inducing
component of B. javanica, and may contribute to the
anticancer activity of this medicinal herb. These findings may
improve our understanding of the molecular mechanisms underlying
the anticancer properties of bruceine D, further elucidate the
pharmacodynamics of bruceine D, and support the ethnomedical
application of B. javanica and its preparations against
NSCLC. This may provide a basis for future in vivo studies
and the potential clinical application of bruceine D in the
treatment of lung cancer. Further research is required to identify
other pivotal signaling pathways induced by bruceine D in A549
NSCLC cells. Future animal studies are also warranted to fully
elucidate the value of bruceine D as a treatment option for
NSCLC.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Shenzhen University General Hospital (grant
nos. SUGH2018QD070 and SUGH2018QD035), the Youth Innovative Talents
Project of Colleges and Universities of Guangdong Province (grant
no. 2018KQNCX216), the Science and Technology Planning Project of
Guangdong Province, China (grant no. 2017A050506044), the Science
and Technology Planning Project of Guangzhou, Guangdong, China
(grant no. 201704030028), the Natural Science Foundation of
Guangdong Province, China (grant no. 2018A030313408), the Science
and Technology Research Project of Guangdong Provincial Hospital of
Chinese Medicine (grant no. YN2018ZD02), the National Natural
Science Foundation of China (grant no. 81503458), the Science and
Technology Planning Project of Guangdong Province, China (grant
nos. 2016A020226036 and 2017B030314166), the General Research Fund
from the Research Grants Council of Hong Kong (grant no. 469912),
the Science and Technology Planning Project of Guangdong Province
(grant no. 2017B030314166), the Characteristic Cultivation Program
for Subject Research of Guangzhou University of Chinese Medicine
(grant no. XKP2019007), and the Key Program for Subject Research of
Guangzhou University of Chinese Medicine (grant no. XK2018016 and
XK2019002).
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YLX, JNC, ZRS, ZXL and XBY were involved in
developing the concept and design of the study, and are guarantors
of the integrity of the study. JHX and ZQL performed the
experiments, prepared and revised the manuscript. XHZ and YFX were
responsible for the literature review and assisted with data
analysis. QL and SPI performed the experiments and revised the
manuscript. All authors have read and approved the final version of
the manuscript submitted for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Segal RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar
|
|
2
|
Huang CY, Ju DT, Chang CF, Muralidhar
Reddy P and Velmurugan BK: A review on the effects of current
chemotherapy drugs and natural agents in treating non-small-cell
lung cancer. Biomedicine (Taipei). 7:232017. View Article : Google Scholar
|
|
3
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao L, Li C, Zhang Y, Wen Q and Ren D:
Phytochemical and biological activities of an anticancer plant
medicine: Brucea javanica. Anticancer Agents Med Chem. 14:440–458.
2014. View Article : Google Scholar
|
|
5
|
Lichota A and Gwozdzinski K: Anticancer
activity of natural compounds from plant and marine environment.
Int J Mol Sci. 19:pii: E3533. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lau ST, Lin ZX, Liao Y, Zhao M, Cheng CH
and Leung PS: Brucein D induces apoptosis in pancreatic
adenocarcinoma cell line PANC-1 through the activation of
p38-mitogen activated protein kinase. Cancer Lett. 281:42–52. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lau ST, Lin ZX and Leung PS: Role of
reactive oxygen species in Brucein D-mediated p38-mitogen-activated
protein kinase and nuclear factor kappaB signalling pathways in
human pancreatic adenocarcinoma cells. Br J Cancer. 102:583–593.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lai ZQ, Ip SP, Liao HJ, Lu Z, Xie JH, Su
ZR, Chen YL, Xian YF, Leung PS and Lin ZX: Bruceine D, a naturally
occurring tetracyclic triterpene quassinoid, induces apoptosis in
pancreatic cancer through ROS-associated PI3K/Akt signaling
pathway. Front Pharmacol. 22:9362017. View Article : Google Scholar
|
|
9
|
Lau ST, Lin ZX, Zhao M and Leung PS:
Brucea javanica fruit induces cytotoxicity and apoptosis in
pancreatic adenocarcinoma cell lines. Phytother Res. 22:477–486.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu L, Lin ZX, Leung PS, Chen LH, Zhao M
and Liang J: Involvement of the mitochondrial pathway in bruceine
D-induced apoptosis in Capan-2 human pancreatic adenocarcinoma
cells. Int J Mol Med. 30:93–99. 2012.PubMed/NCBI
|
|
11
|
Chinese Pharmacopoeia Commission (CP):
Pharmacopoeia of the People's Republic of China. China Medical
Science Press; Beijing: pp. 254–255. 2015
|
|
12
|
Liu JH, Qin JJ, Jin HZ, Hu XJ, Chen M,
Shen YH, Yan SK and Zhang WD: A new triterpenoid from Brucea
javanica. Arch Pharm Res. 32:661–666. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee KH, Imakura Y, Sumida Y, Wu RY, Hall
IH and Huang HC: Antitumor agents. 33. Isolation and structural
elucidation of bruceoside-A and -B, novel antileukemic quassinoid
glycosides, and brucein-D and -E from Brucea javanica. J Org Chem.
44:2180–2185. 1979. View Article : Google Scholar
|
|
14
|
Ablat A, Halabi MF, Mohamad J, Hasnan MH,
Hazni H, Teh SH, Shilpi JA, Mohamed Z and Awang K: Antidiabetic
effects of Brucea javanica seeds in type 2 diabetic rats. BMC
Complement Altern Med. 17:942017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou L, Wei E, Zhou B, Bi G, Gao L, Zhang
T, Huang J, Wei Y and Ge B: Antiproliferative benefit of curcumol
on human bladder cancer cells via inactivating EZH2 effector.
Biomed Pharmacother. 104:798–805. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xian YF, Lin ZX, Zhao M, Mao QQ, Ip SP and
Che CT: Uncaria rhynchophylla ameliorates cognitive deficits
induced by D-galactose in mice. Planta Med. 77:1–7. 2011.
View Article : Google Scholar
|
|
17
|
Otto T and Sicinski P: Cell cycle proteins
as promising targets in cancer therapy. Nat Rev Cancer. 17:93–115.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Redza-Dutordoir M and Averill-Bates DA:
Activation of apoptosis signalling pathways by reactive oxygen
species. Biochim Biophys Acta. 1863:2977–2992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zorova LD, Popkov VA, Plotnikov EY,
Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD,
Balakireva AV, Juhaszova M, et al: Mitochondrial membrane
potential. Anal Biochem. 552:50–59. 2018. View Article : Google Scholar :
|
|
20
|
Hata AN, Engelman JA and Faber AC: The
BCL-2 family: Key mediators of the apoptotic response to targeted
anticancer therapeutics. Cancer Discov. 5:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pfeffer CM and Singh ATK: Apoptosis: A
target for anticancer therapy. Int J Mol Sci. 19:pii E448.
2018.
|
|
22
|
Cheng Z, Yuan X, Qu Y, Li X, Wu G, Li C,
Zu X, Yang N, Ke X, Zhou J, et al: Bruceine D inhibits
hepatocellular carcinoma growth by targeting β-catenin/jagged1
pathways. Cancer Lett. 403:195–205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang JY, Lin MT, Tung HY, Tang SL, Yi T,
Zhang YZ, Tang YN, Zhao ZZ and Chen HB: Bruceine D induces
apoptosis in human chronic myeloid leukemia K562 cells via
mitochondrial pathway. Am J Cancer Res. 6:819–826. 2016.PubMed/NCBI
|
|
24
|
Wei SC and Yang J: Forcing through tumor
metastasis: The interplay between tissue rigidity and
epithelial-mesenchymal transition. Trends Cell Biol. 26:111–120.
2016. View Article : Google Scholar :
|
|
25
|
Cerella C, Grandjenette C, Dicato M and
Diederich M: Roles of apoptosis and cellular senescence in cancer
and aging. Curr Drug Targets. 17:405–415. 2016. View Article : Google Scholar
|
|
26
|
Rather RA and Bhagat M: Cancer
chemoprevention and piperine: Molecular mechanisms and therapeutic
opportunities. Front Cell Dev Biol. 6:102018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumari S, Badana AK, G MM, G S and Malla
R: Reactive oxygen species: A key constituent in cancer survival.
Biomark Insights. 13:11772719187553912018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan SWS, Lee QY, Wong BSE, Cai Y and Baeg
GH: Redox homeostasis plays important roles in the maintenance of
the drosophila testis germline stem cells. Stem Cell Reports.
9:342–354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Couto N, Wood J and Barber J: The role of
glutathione reductase and related enzymes on cellular redox
homoeostasis network. Free Radic Biol Med. 95:27–42. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Traverso N, Ricciarelli R, Nitti M,
Marengo B, Furfaro AL, Pronzato MA, Marinari UM and Domenicotti C:
Role of glutathione in cancer progression and chemoresistance. Oxid
Med Cell Longev. 2013:9729132013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Georgieva E, Ivanova D, Zhelev Z, Bakalova
R, Gulubova M and Aoki I: Mitochondrial dysfunction and redox
imbalance as a diagnostic marker of 'free radical diseases'.
Anticancer Res. 37:5373–5381. 2017.PubMed/NCBI
|
|
32
|
Kim YM, Youn SW, Sudhahar V, Das A,
Chandhri R, Cuervo Grajal H, Kweon J, Leanhart S, He L, Toth PT, et
al: Redox regulation of mitochondrial fission protein Drp1 by
protein disulfide isomerase limits endothelial senescence. Cell
Rep. 23:3565–3578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baig S, Seevasant I, Mohamad J, Mukheem A,
Huri HZ and Kamarul T: Potential of apoptotic pathway-targeted
cancer therapeutic research: Where do we stand? Cell Death Dis.
7:e20582016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kale J, Osterlund EJ and Andrews DW: BCL-2
family proteins: Changing partners in the dance towards death. Cell
Death Differ. 25:65–80. 2018. View Article : Google Scholar
|
|
36
|
Castelli G, Pelosi E and Testa U: Emerging
therapies for acute myelogenus leukemia patients targeting
apoptosis and mitochondrial metabolism. Cancers (Basel). 11:pii:
E260. 2019. View Article : Google Scholar
|
|
37
|
Kiraz Y, Adan A, Kartal Yandim M and Baran
Y: Major apoptotic mechanisms and genes involved in apoptosis.
Tumour Biol. 37:8471–8486. 2016. View Article : Google Scholar : PubMed/NCBI
|