Introduction
Allergic asthma is a chronic inflammatory disease
and a major health issue, and its prevalence is increasing
worldwide (1). The major features
of asthma pathophysiology include airway inflammation and mucus
hypersecretion (2,3). It is well known that the increased
levels of eosinophil recruitment and T helper lymphocytes 2 (Th2)
cytokines, such as interleukin-4 (IL-4), IL-5 and IL-13, are
closely associated with sustained airway inflammation (4). Macrophages-derived chemokines such
as monocyte chemoattractant protein-1 (MCP-1) increased the
recruitment of inflammatory cells including eosinophils in asthma
pathogenesis (5,6) The increased concentration of
immunoglobulin E (IgE) has a pivotal role in allergic reactions and
is much higher in asthmatic patients (7). Changes in the number of goblet cells
and production of mucus are key to airway inflammation and
obstruction (8). The
mitogen-activated protein kinase (MAPK) signaling pathways have an
important role in the inflammatory processes of allergic asthma
(9). The activation of c-Jun
N-terminal kinase (JNK) has been implicated in IgE class switching
(10). Extracellular
signal-regulated kinase (ERK) and p38 have been reported to play a
role in the production of cytokines, including IL-5 (11). Nuclear factor (NF)-κB plays an
important role in inflammatory cell influx, Th2 cytokine levels and
inflammatory molecules in allergic asthma (12,13).
In recent years, the approaches to improve the side
effects of medicine have focused on research into allergic asthma
(14) and natural herbal extracts
are attracting increased attention due to their prominent
biological activities and minimal side effects (15). Pistacia weinmannifolia (PW)
is used as a herbal medicine in China (16,17) and its major metabolites possess
biological activities, such as inhibitory activities against
histamine release (16,18,19). In a previous study, it was
confirmed that the anti-inflammatory activities of P.
weinmannifolia root extract (PWRE) in PMA/tumour necrosis
factor-α-stimulated airway epithelial cells and in pulmonary
inflammatory response induced by cigarette smoke and
lipopolysaccharide (LPS) (20).
Based on these results and those of other studies (16-20), which reflect the anti-inflammatory
activities of PWRE on pulmonary inflammation, it was hypothesized
that PWRE could exert a protective effect against ovalbumin
(OVA)-induced lung inflammation. Therefore, the aim of the present
study was to evaluate the regulatory effects of PWRE against
eosinophil recruitment and Th2 cytokines, IgE and mucus
overproduction, which are the major characteristics of allergic
asthma.
Materials and methods
Preparation of PWRE
PWRE was prepared as previously described (20). P. weinmannifolia roots
(PWRs) were collected from the Yunnan province of China. A voucher
specimen recorded as D180305001 was deposited at the International
Biological Material Research Center, Korea Research Institute of
Bioscience and Biotechnology. The active substance of PWR was
extracted by the processing method described in the International
Conference on Harmonisation and Ministry of Food and Drug Safety
guidelines (20). The collected
roots were dried immediately following sampling and then ground to
a powder. The raw materials were then packed in laminated bags and
delivered to Korea. The PWREs were provided by the BTC Corporation.
The powdered samples were extracted with 50% ethanol at 80°C and
the product was dried in a freeze dryer (-70°C) to produce dried
extracts (~19%) [Korea Good Manufacturing Practice (KGMP), lot no.
BTC-PWE-180118].
Induction of ovalbumin (OVA) and
alum-induced lung inflammation in murine models
Healthy female BALB/c mice (n=30, 6 weeks old; body
weight, 16-18 g) were purchased from Koatech Co., Ltd., and used
after 1 week of acclimatization with free access to food and water
in specific pathogen-free conditions (22-23°C; 55-60% humidity;
12-h light/dark cycle). The experimental procedure was performed
according to the methods described by Park et al (21). Briefly, the mice were sensitized
twice intraperitoneally on day 0 and 14 with 30 µg OVA and 3
mg Alums (Thermo Fisher Scientific, Inc.) dissolved in a solution
of 0.2 ml PBS. On days 21-23, the mice were aerosol challenged with
1% OVA (alum-free saline solution, 60 min/day) with a nebulizer
(NE-U12; OMRON Corp.). The PWRE or montelukast (MON) was given by
oral gavage for 6 consecutive days (from day 18 to 23). The mice
were sacrificed on day 25. The mice were randomly divided into 4
groups (n=6 per subgroup) as follows: i) The normal control (NC)
group; ii) the OVA group (intraperitoneally sensitized with
OVA-Alum); iii) the MON group (intraperitoneally sensitized with
OVA-Alum) + MON (30 mg/kg, per os); and iv) the PW group
(intraperitoneally sensitized with OVA-Alum) + PWRE (7.5 or 15.0
mg/kg, per os). MON was used as a positive control. All animal
experiments were approved by the Institutional Animal Care and Use
Committee of the Korea Research Institute of Bioscience and
Biotechnology and performed in compliance with the National
Institutes of Health Guidelines for the care and use of laboratory
animals and Korean national laws for animal welfare. The humane
endpoints are the condition of rapid loss of weight (>20% of
normal body weight) and/or rapid or labored breathing.
Counting the inflammatory cells
BALF collection was performed in order to count the
inflammatory cells and evaluate the levels of inflammatory
cytokines as previously described (22). The mice were anesthetized with
Zoletil 50® (30-50 mg/kg IP; Virbac) and Xylazine (5-10
mg/kg IP; Bayer Korea) on day 25 based on prior anesthesia
condition (20). Briefly, on day
25, the trachea was cannulated and infused with 0.7 ml PBS for the
collection of BALF (infusion was performed twice with a total
volume of 1.4 ml) and blood was collected for the detection of IgE.
Mice were sacrificed under Zoletil/Xylazine anaesthesia and
exsanguinated. In order to distinguish the different cells, 0.1 ml
of BALF was centrifuged at 246 × g for 5 min at room temperature to
transfer the cells to the glass slide and then the glass slide was
stained with Diff-Quik® solution (IMEB, Inc.) according
to the manufacturer's protocol.
Measuring the Th2 cytokines and IgE
production
The levels of IL-4, IL-5 and IL-13 in the BALF were
determined using ELISA kits (R&D Systems, Inc.; IL-4, cat. no.
M4000B; IL-5, cat. no. M5000; IL-13, cat. no. M1300CB) according to
the manufacturer's protocol. Blood (0.4 ml) was collected in order
to determine the serum IgE levels. The concentration of the total
or OVA-specific IgE in the serum was determined using an ELISA
(Biolegend, Inc.; Total IgE, cat. no. 432404; R&D Systems,
Inc., OVA-specific IgE, cat. no. 439807). The absorbance was
measured at 450 nm with a Spark™ 10 M multimode microplate reader
(Tecan System Inc.).
Western blot analysis
Lung tissues were removed 48 h after the final OVA
inhalation and incubated in CelLytic™ MT Cell Lysis reagent (cat.
no. c3228; Sigma-Aldrich; Merck KGaA) containing protease and
phosphatase inhibitors (cat. nos. 11836153001 and 04906837001;
Roche Diagnostics) in order to obtain the proteins. The protein
concentration was measured with the Pierce bicinchoninic acid
Protein assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The proteins (50
µg/lane) were separated via SDS-PAGE (10-12% gels) and then
transferred to PVDF membranes (EMD Millipore). The membranes were
blocked in 5% skimmed milk dissolved in TBS and 0.05% Tween-20
(TBST) for 1 h at room temperature and probed overnight with
primary antibodies at 4°C. The primary antibodies used were as
follows: Anti-phosphorylated (p)-extracellular signal-regulated
kinase (ERK; 1:1,000; cat. no. 9101; 1:1,000; Cell Signaling
Technology, Inc.), anti-p-p38 (cat. no. 9211; 1:1,000; Cell
Signaling Technology, Inc.), anti-p-NF-κB p65 (cat. no. 3033;
1:1,000; Cell Signaling Technology, Inc.), anti-p-inhibitor of
NF-κB (p-IκBα; cat. no. 2859; 1:1,000; Cell Signaling Technology,
Inc.), anti-β-actin (1:2,500; cat. no. 4967; 1:1,000; Cell
Signaling Technology, Inc.), anti-ERK (cat. no. sc-154; 1:1,000;
Santa Cruz Biotechnology, Inc.), anti-p-c-Jun N-terminal kinase
(JNK; cat. no. sc-6254; 1:1,000; Santa Cruz Biotechnology, Inc.),
anti-JNK (cat. no. sc-474; 1:1,000; Santa Cruz Biotechnology,
Inc.), anti-p38 (cat. no. sc-7149; 1:1,000; Santa Cruz
Biotechnology, Inc.), anti-MCP-1 (cat. no. sc-28879; 1:1,000; Santa
Cruz Biotechnology, Inc.), anti-NF-κB p65 (cat. no. sc-372;
1:1,000; Santa Cruz Biotechnology, Inc.) and anti-IκBα (cat. no.
MA5-15132; 1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.).
The membranes were washed five times with TBST for 10 min and
developed with horseradish peroxidase-conjugated secondary
antibodies (goat anti-mouse & anti-rabbit; 1:2,000; cat. nos.
115-035-003 and 111-035-003; Jackson ImmunoResearch Laboratories,
Inc.) at room temperature (RT) for 1 h. The membranes were
developed with an ECL kit (Thermo Fisher Scientific, Inc.). All
bands were visualized using a LAS-4000 luminescent image analyzer
(Fujifilm) and quantified by densitometry using Fuji Multi Gauge
software version 3.0 (Fujifilm).
Histological analysis of lung tissue
A total of 24 h after the final administration of
PWRE and MON, the mice were sacrificed, and the lung tissues were
collected. For histological evaluation, the lung tissues were fixed
in 10% (v/v) neutral-buffered formalin solution at room temperature
for 48 h and embedded in paraffin. The lung tissues were then
sliced into 4-µm thick sections with a rotary microtome and
stained with hematoxylin (BBC Biochemical Inc.) and eosin (Thermo
Fisher Scientific Inc.; H&E) solutions at RT for 30 sec each to
estimate the inflammatory response. The lung sections then were
visualized using a light microscope (magnification, ×100; scale
bar, 50 µm) to estimate the recruitment of inflammatory
cells. Periodic acid-Schiff (PAS) staining (IMEB, Inc., cat. no.
K7308) was performed to estimate the mucus secretion. The degree of
inflammatory score and mucus production in each group was assessed
by two independent observers in the laboratory using a
semi-quantitative scope. The H&E staining was scored as
follows: 0, no recruitment of inflammatory cells; 1, small amount
of recruitment; 2, moderate recruitment; 3, large amount of
recruitment. The PAS staining was scored as follows: 0, no mucus
production; 1, mild mucus production; 2, moderate mucus production;
3, distinct mucus production; 4, severe mucus production.
Cell culture
The macrophage cell line RAW264.7 was obtained from
the American Type Culture Collection. The cells were grown in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences), 100 U/ml penicillin and 100 µg/ml
streptomycin and were incubated at 37°C in a humidified chamber
with 5% CO2. The cells were activated with
lipopolysaccharide (LPS; 0.5 µg/ml) 1 h after PWRE treatment
(1.25, 2.5 and 5 µg/ml). The dose of LPS was based on a
previous study (23). The level
of MCP-1 in the culture supernatant was determined by ELISA.
Statistical analysis
All values are expressed as the mean ± standard
deviation of at least three independent experiments. The
statistical significance was determined by a two-tailed Student's
t-test for comparisons between two groups. One-way analysis of
variance followed by Dunnett's multiple groups. Data were analyzed
using SPSS 20.0 (IBM Corp.). P<0.05 was considered to indicate a
statistically significant result.
Results
Effect of PWRE on alleviating the
eosinophil numbers in the BALF
The significant increase in eosinophils and
macrophages has been well established in OVA-induced pulmonary
inflammatory response (24,25). Therefore, the present study
focused on the inhibitory effect of PWRE on the cell numbers. To
distinguish the inflammatory cells and count the cell numbers,
Diff-Quik® staining was performed according to the
manufacturer's protocol. As presented in Fig. 1, the numbers of eosinophil and
macrophages were significantly increased in the OVA-exposed group
compared with the NC group (P<0.05). Conversely, this increase
in inflammatory cell numbers was significantly decreased in the
PWRE-treated group (P<0.05; Fig.
1A and B).
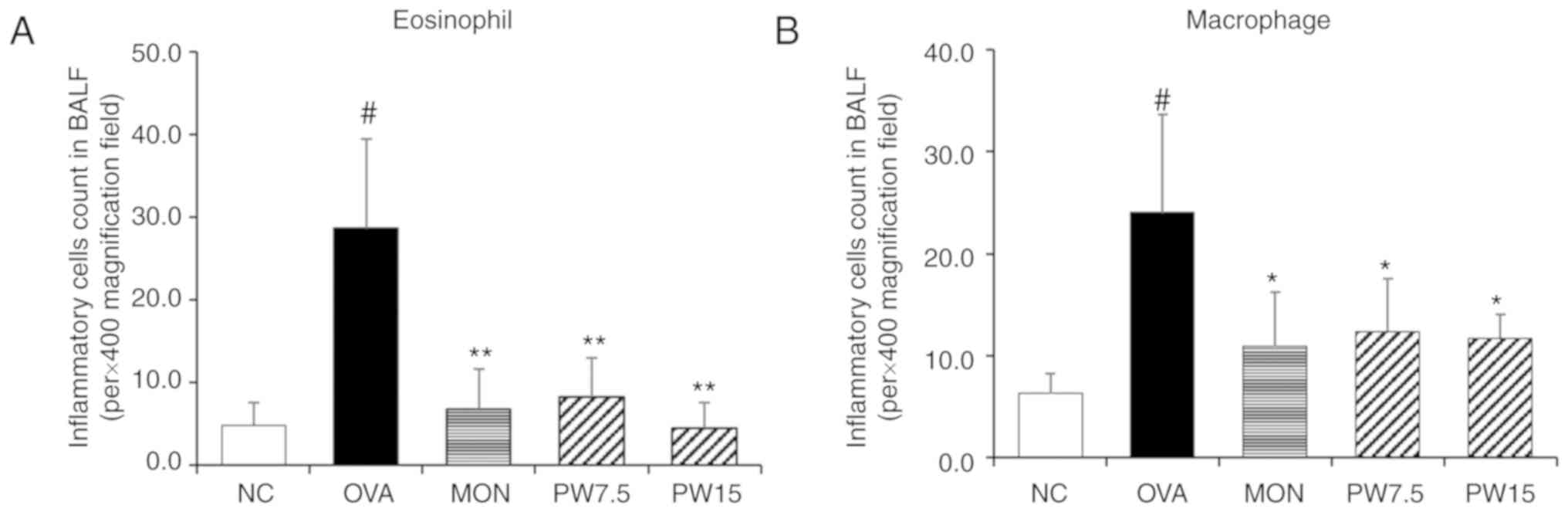 | Figure 1Effect of PWRE on the numbers of
inflammatory cells in the BALF of OVA-challenged mice. The count of
(A) eosinophils and (B) macrophages in the BALF was determined by
Diff-Quik® staining. Data are expressed as the mean ±
standard deviation (n=6). #P<0.05 vs. NC group;
*P<0.05 and **P<0.01 vs. OVA-induced
group. NC, normal control mice; OVA group, mice administered
ovalbumin; MON group, mice administered MON (30 mg/kg) + OVA; PW
7.5, mice administered P. weinmannifolia root extract (7.5
mg/kg) + OVA. OVA, ovalbumin; MON, montelukast; PWRE, P.
weinmannifolia root extract; PW 7.5, 7.5 mg/kg PW + OVA, PW15,
15 mg/kg PW + OVA; BALF, bronchoalveolar lavage fluid; NC, negative
control. |
Effect of PWRE on attenuating Th2
cytokines in the BALF
The present study next investigated the regulatory
effect of PWRE on the production of Th2 cytokines that are deeply
associated with the pathophysiology of asthma. ELISAs were
performed in order to evaluate the levels of Th2 cytokines. It was
revealed that IL-4, IL-5 and IL-13 were significantly increased in
the OVA group when compared with the NC group (P<0.05; Fig. 2A-C). However, treatment with PWRE
decreased the levels of these cytokines induced by OVA. In
particular, the inhibitory effects of 15 mg/kg PWRE on the
production of cytokines were similar to those of 30 mg/kg MON,
which was used as a positive control.
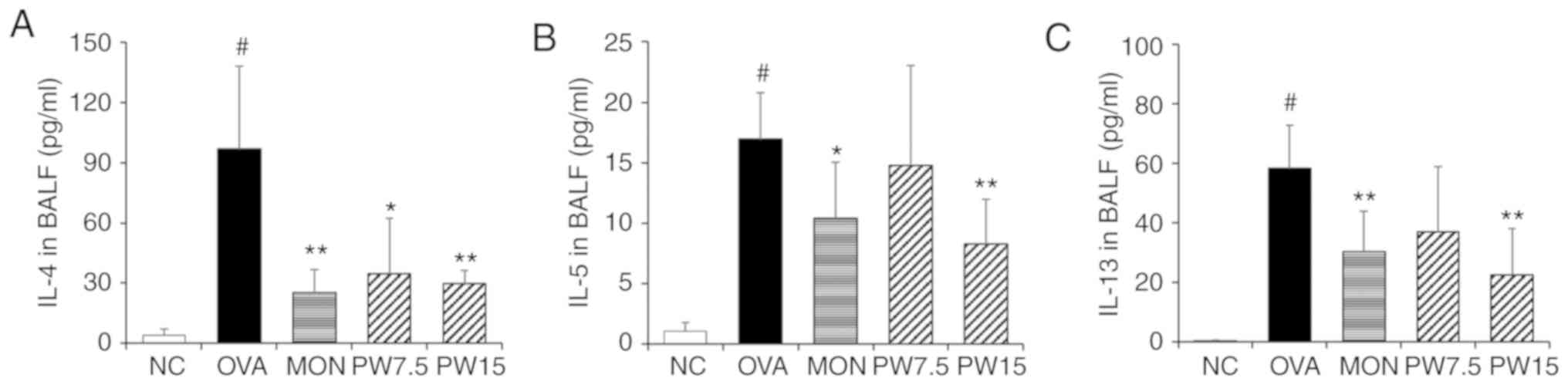 | Figure 2Effect of PWRE on the production of
Th2 cytokines in the BALF. The levels of Th2 cytokines, such as (A)
IL-4, (B) IL-5 and (C) IL-13, were determined by ELISA kits. The
absorbance was measured at 450 nm with a microplate reader.
#P<0.05 vs. NC group; *P<0.05 and
**P<0.01 vs. OVA-induced group. PWRE, P.
weinmannifolia root extract; IL, interleukin; OVA, ovalbumin;
MON, montelukast; BALF, bronchoalveolar lavage fluid; NC, negative
control; Th2, T-helper 2. |
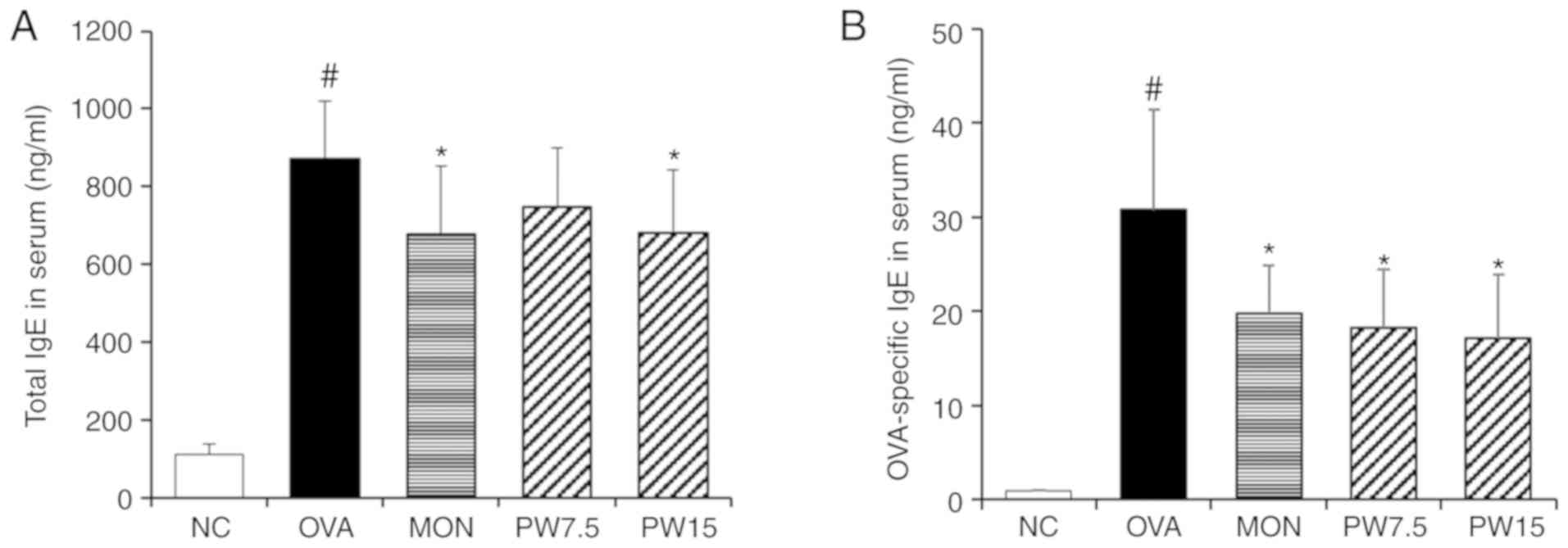
Effect of PWRE on downregulating IgE
production
The serum total IgE level is highly elevated in
allergic patients such as bronchial asthma and is known to increase
with the onset and aggravation of the disease (26,27). A specific IgE test is needed
together with total IgE for proper evaluation of allergic diseases
(28). Based on the importance of
the IgE-mediated immune response in asthma (29), the present study investigated the
inhibitory activity of PWRE on OVA-induced IgE production. As
presented in Fig. 3, the
concentration of total IgE or OVA-specific IgE in the serum were
significantly increased in the asthmatic group compared with those
in the NC group (P<0.05), whereas treatment with PWRE
effectively decreased the levels of total IgE and OVA-specific IgE
(Fig. 3).
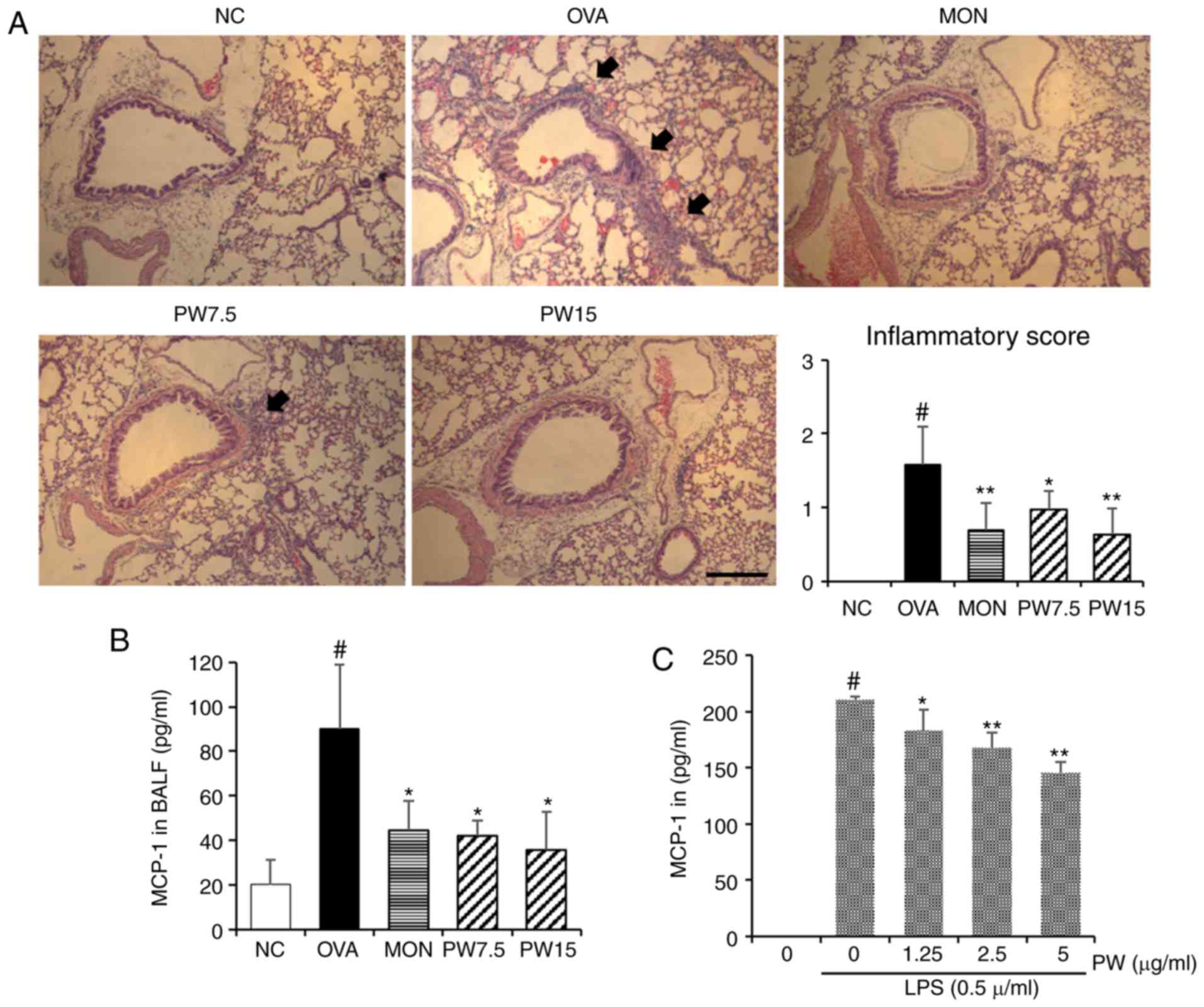
Effect of PWRE on inhibiting inflammatory
cell influx and mucus hypersecretion
In order to investigate whether PWRE suppresses the
OVA-induced inflammatory cell influx into the lungs, paraffin lung
sections were stained with H&E in the present study. A
significantly increased level of inflammatory cell influx was
observed in the OVA group compared with the NC group (P<0.05;
Fig. 4A). Notably, this level was
down-regulated in the PWRE-treated group. The arrows point to the
influx of inflammatory cells. The increased secretion of MCP-1 is
closely associated with airway inflammation by inducing the influx
of inflammatory cells (5,30). Therefore, the present study next
assessed the inhibitory effect of PWRE on OVA-induced MCP-1
secretion. As presented in Fig.
4B, the marked increase in MCP-1 was observed in the BALF of
the OVA group, whereas treatment with PWRE inhibited this
secretion. In order to further investigate the regulatory effect of
PWRE on MCP-1 secretion, the inhibitory effect of PWRE on MCP-1 was
assessed in LPS-stimulated RAW264.7 macrophages. As presented in
Fig. 4C, the administration of
LPS significantly increased the MCP-1 secretion (P<0.05).
However, pretreatment with PWRE significantly downregulated this
secretion (P<0.05; Fig. 4C).
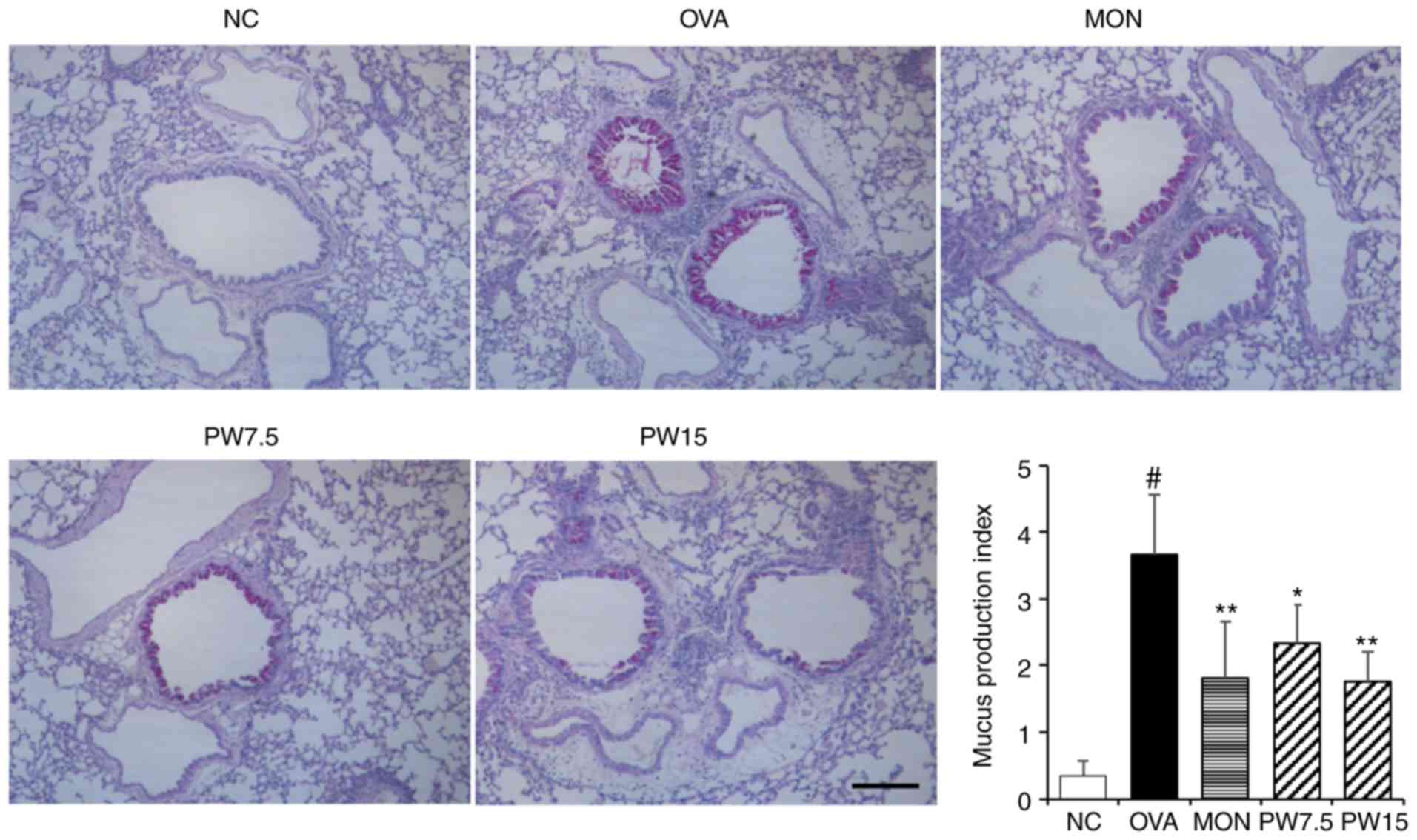
Mucus hypersecretion is an prominent characteristic in the
pathophysiology of allergic asthma (31). Therefore, the present study
assessed whether PWRE led to an attenuation of the OVA-induced
mucus overproduction. The paraffin lung sections were stained with
the PAS staining reagent to measure the mucus production around the
airways. As presented in Fig. 5,
the levels of mucus production were significantly increased in the
OVA group when compared with the NC group (P<0.05). However, a
decrease in this level was observed in the PWRE group (Fig. 5). The mucus was stained a purple
color by the PAS staining reagent.
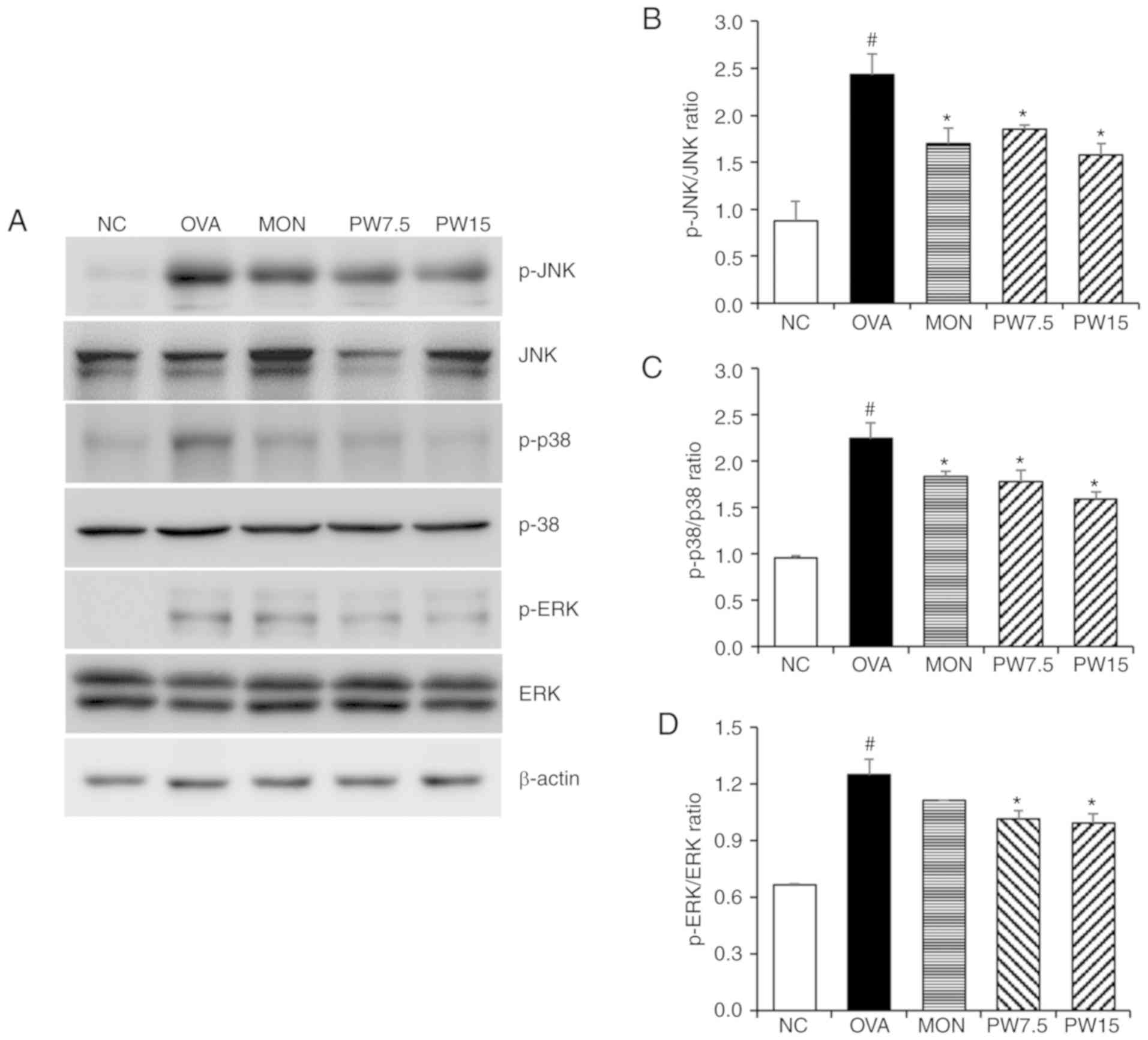
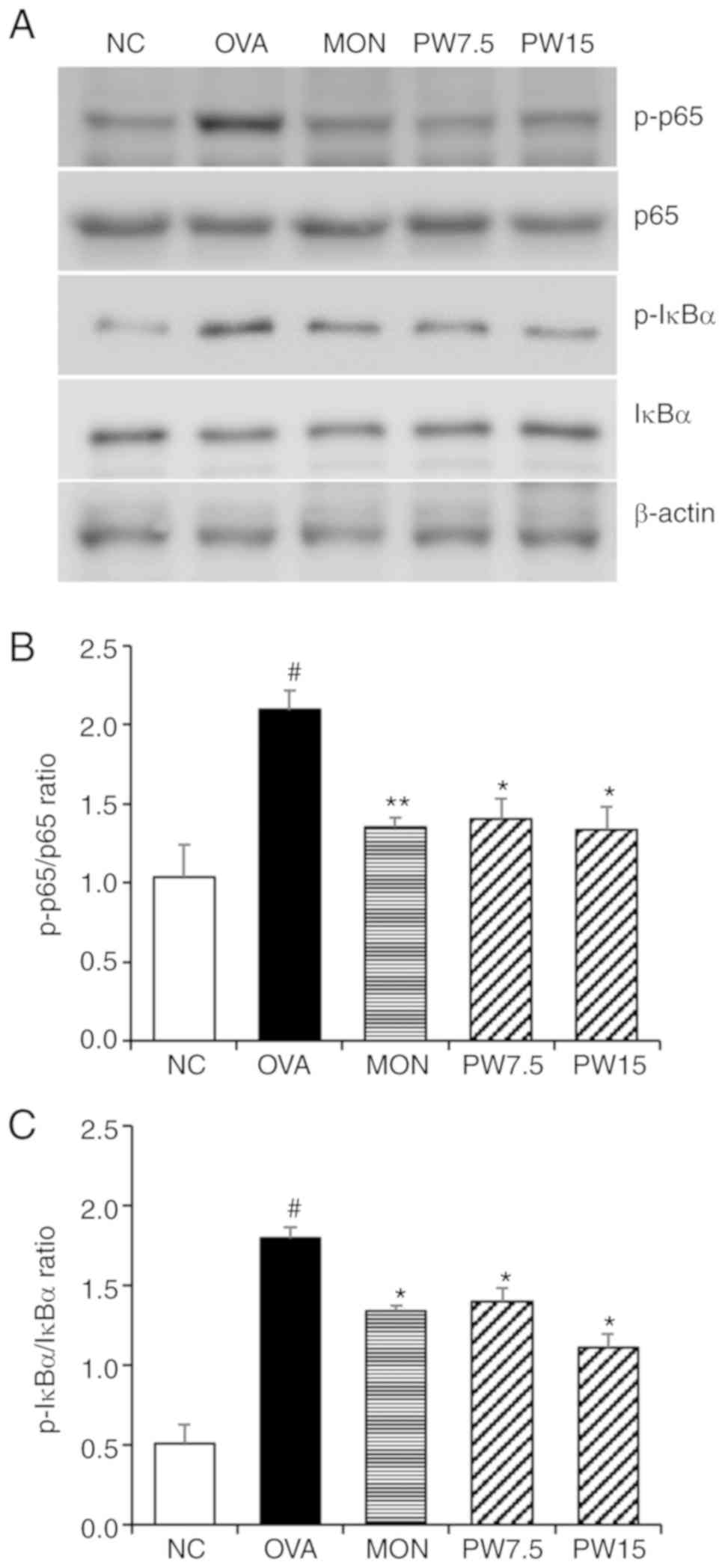
Effect of PWRE on decreasing MAPKs and
NF-κB activation in the lungs
In order to investigate whether the airway
inflammatory response was mediated by MAPK-responsive mechanisms,
the present study evaluated the levels of ERK, JNK and p38
phosphorylation. As presented in Fig.
6, the levels of JNK, p38 and ERK were significantly
upregulated in the OVA group compared with the NC group
(P<0.05). However, 15 mg/kg PWRE significantly downregulated the
enhanced activation of JNK, p38 and ERK in the lungs (P<0.05;
Fig. 6). In order to further
investigate the mechanism of PWRE, the NF-κB signaling pathway was
assessed in the present study. As presented in Fig. 7, the activation of NF-κB p65 and
IκBα was significantly upregulated in the OVA-exposed group
compared with the NC group. However, this increase was effectively
blocked by the PWRE treatment.
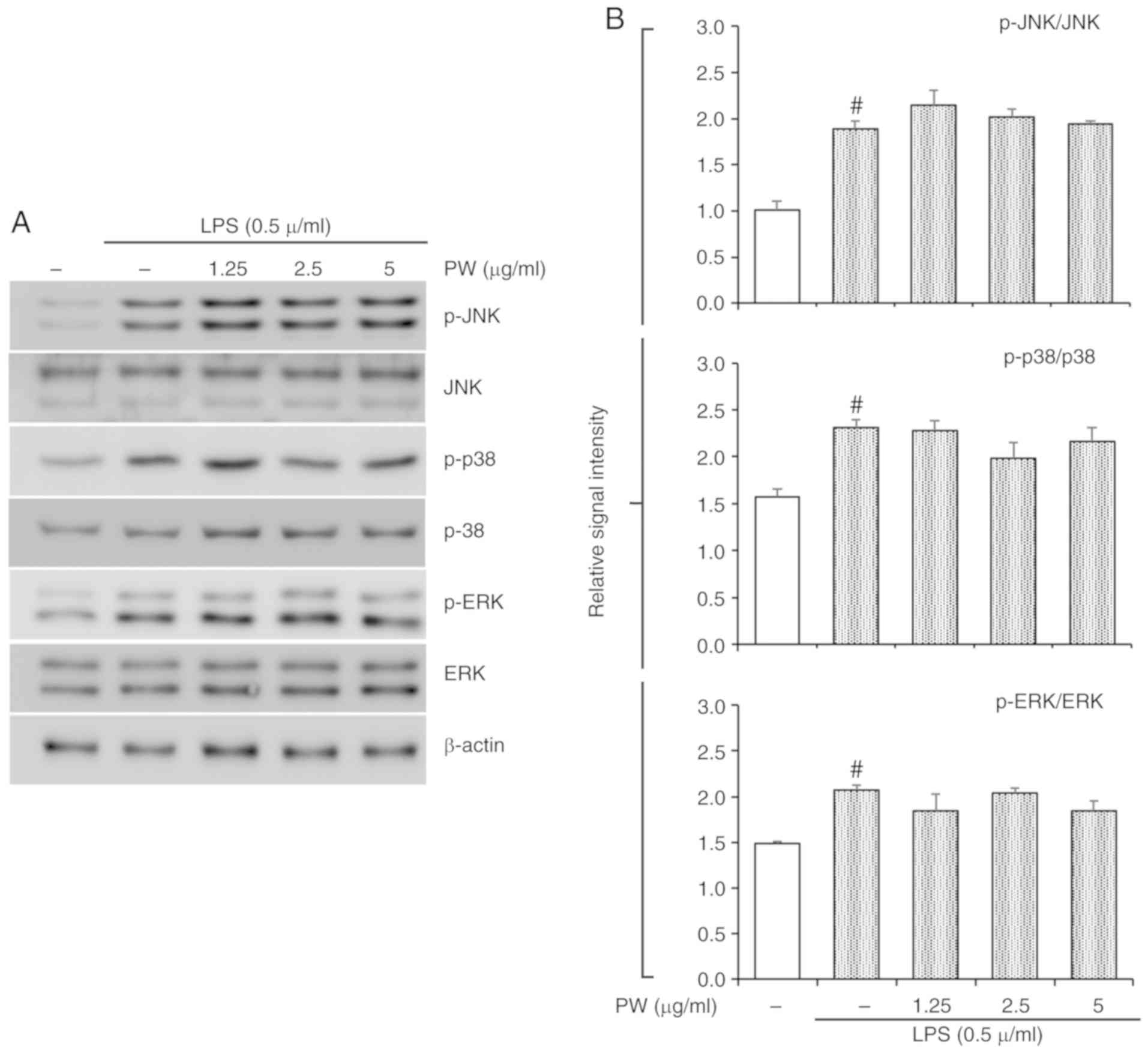
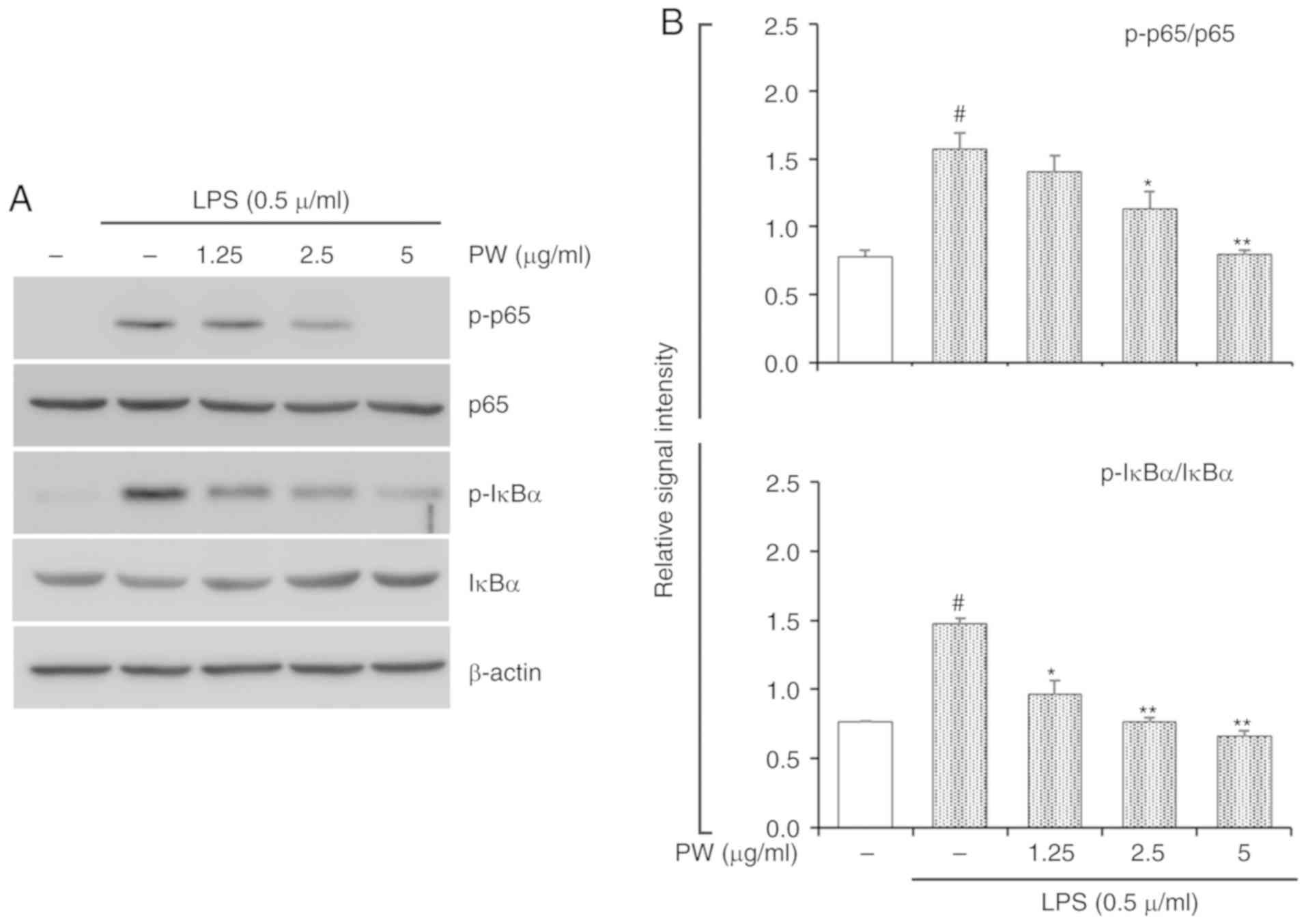
Effect of PWRE on LPS-stimulated MAPKs
and NF-κB activation in RAW264.7 macrophages
In the present study, PWRE exerted a protective
effect in pulmonary inflammation in OVA-exposed mice. Its effects
were accompanied by MAPK and NF-κB inactivation (Figs. 6 and 7). In particular, NF-κB activation was
effectively downregulated upon PWRE administration. The results
from the present study also demonstrated that PWRE regulates MCP-1
production in the BALF of OVA-exposure mice and in LPS-stimulated
RAW264.7 macrophages (Fig. 4B and
C). The regulatory effect of PWRE on LPS-stimulated MAPKs and
NF-κB activation was therefore investigated in RAW264.7
macrophages. The administration of LPS significantly upregulated
the activation of MAPKs and NF-κB (P<0.05; Figs. 8 and 9). However, the levels of JNK, p38 and
ERK activation was not significantly downregulated by PWRE
pretreatment (Fig. 8). Similar to
those results presented in Fig.
7, the activation of NF-κB p65 and IκBα was significantly
downregulated by ≥2.5 µg/ml PWRE pretreatment (P<0.05;
Fig. 9).
Discussion
Previously, studies have demonstrated that PWRE
exerts anti-inflammatory effects via downregulation of inflammatory
molecules including IL-6 and IL-8, which are important parameters
in chronic obstructive pulmonary disease (16,18,20). The present study extended the
results of these previous publications, which demonstrate the
protective effects of PWRE in OVA-induced pulmonary
inflammation.
The airway inflammatory response is well known as a
major cause of allergic asthma and is caused by a variety of
inflammatory cells and molecules. IL-4 has been reported to
differentiate native T cells into Th2 cells and induce class
switching in B cells to IgE production (32,33). IL-5 has an important role in the
maturation and recruitment of eosinophils, and IL-13 is recognized
as a dominant factor for IgE class switching, eosinophil
inflammation and mucus production (9). Eosinophil infiltration is well known
as an indispensable indicator in airway inflammation and the
increase of eosinophil cationic proteins leads to airway
hyper-responsiveness (34). The
high level of macrophages is also well known as a characteristic of
the allergic asthma murine model and macrophage-derived MCP-1 is
known as a potent eosinophil chemoattractant (5,30).
Therefore, the regulation of eosinophil influx, Th2 cytokine
secretion and IgE production are important therapeutic approaches
in the treatment of asthma. OVA has been used as an allergen in
asthma animal models and the utility of OVA-induced asthma model
has been well established and this model has been widely used to
evaluate anti-asthmatic effects and immunological mechanisms
involved in the pathogenesis of asthma (35). In this study an allergic asthma
mouse model, in which the levels of Th2 cytokines, IgE and mucus
production were successfully upregulated by OVA compared with the
NC control was established. In the present study, it was confirmed
that PWRE administration attenuated OVA-induced eosinophils and
macrophage recruitment. OVA-induced IL-4, IL-5, IL-13 and IgE were
suppressed by the treatment of PWRE. In addition, the increased
levels of MCP-1 were downregulated following PWRE treatment in both
in vivo and in vitro studies. Therefore, the results
from the present study suggest that PWRE has a protective role
against OVA-induced pulmonary inflammation.
In normal circumstances, goblet cell-derived mucus
exerts protective roles against harmful agents. However, the
excessive production of mucus could easily obstruct breathing
(36,37). Therefore, the regulation of mucus
hypersecretion may be a valuable therapeutic strategy in
alleviating airway obstruction. MUC5AC is a major oligomeric mucin
in airway mucus and its level is upregulated in patients with
asthma (38). The inhibitory
activities of PWRE on MUC5AC secretion in PMA-stimulated airway
epithelial cells have already been confirmed (20). Therefore, the regulatory effect of
PWRE on mucus overproduction was expected in the present study and
it was observed that PWRE ameliorated the OVA-induced mucus
hypersecretion.
The MAPK and NF-κB signaling pathways are known as
key mediators in allergic asthma, and are closely associated with
the activation of various immune cells (39,40). Accumulating evidence emphasizes
the importance of the inhibition of the MAPK pathway in airway
inflammatory diseases such as asthma (9). Accordingly, the inhibitory effect of
PWRE on MAPKs activation was assessed in the present study. It was
subsequently confirmed that OVA-induced MAPKs activation was
significantly decreased by PWRE treatment. In LPS-stimulated
RAW264.7 macrophages, PWRE did not exert any inhibitory effects on
MAPKs activation. It is well established that the activation of IκB
leads to airway inflammation by inducing NF-κB activation and
production of inflammatory molecules (41-43); therefore, the
present study next investigated the ability of PWRE to inactivate
NF-κB and IκB. Notably, PWRE exerted an inhibitory effect on
OVA-induced NF-κB p65 and IκBα activation. Similar to the results
presented, the inhibitory effect of PWRE was observed in IκBα and
NF-κB activation in LPS-stimulated RAW264.7 macrophages. Therefore,
the results from the present study suggest that the molecular
mechanism underlying the protective effects of PWRE on pulmonary
inflammation primarily regard the downregulation of NF-κB
activation.
In the present study, PWRE inhibited the pulmonary
inflammatory response by diminishing the recruitment of
inflammatory cells and the concentration of IL-4, IL-5, IL-13 and
IgE. PWRE also downregulated the levels of MCP-1 and mucus
production. Notably, the effects of PWRE were accompanied by MAPKs
and NF-κB inactivation. Abnormal weight changes and toxicological
changes (such as intraperitoneal changes) were not observed after
administration of PWRE. Therefore, the results from the present
study suggest that PWRE may ameliorate airway inflammation and
mucus hypersecretion in allergic asthma as a potential
anti-inflammatory adjuvant. However, there was no evaluation of
accurate count of inflammatory cells using flow cytometry. The
levels of T-cell activation and eotaxin production in the
pathogenesis of OVA-induced pulmonary inflammation have also not
been investigated. It is necessary to confirm the inhibitory effect
of PWER on MCP-1 in alveolar macrophages. These limitations should
be addressed in the near future. In addition, the present study has
limitations on the efficacy of PWRE in OVA-induced pulmonary
inflammation. Therefore, clinical trials should be performed to
elucidate this efficacy.
Abbreviations:
|
OVA
|
ovalbumin
|
|
BALF
|
bronchoalveolar lavage fluid
|
|
IL-4
|
interleukin-4
|
|
IL-5
|
interleukin-5, IL-13,
interleukin-13
|
|
IgE
|
immunoglobulin E
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
MAPKs
|
mitogen-activated protein kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
p38
|
ERK, extracellular-signal-regulated
kinase
|
|
NF-κB
|
nuclear factor-κB
|
|
IκB
|
inhibitor of NF-κB
|
Acknowledgments
Not applicable.
Funding
The present study was supported by the Ministry of
Trade, Industry and Energy and the Korea Institute for the
Advancement of Technology (grant no. N0002410, 2017).
Availability of data and materials
All data generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
JWL performed the in vivo experiments and
wrote the manuscript. JHM, MGK and SMK performed the in vivo
experiments and contributed to the interpretation of the results.
OKK performed the in vitro experiments. TKO, JKL and TYK
contributed to the acquisition of data. SWL, SC, WYL, HWR and KSA
made substantial contributions to the conception and design of the
present study, acquisition of data, and the analysis and
interpretation of data. SRO designed the present study and was
involved in revising it critically for important intellectual
content. All authors discussed the results and read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Institutional
Animal Care and Use Committee of the Korea Research Institute of
Bioscience and Biotechnology (permit no. KRIBB-AEC-18054).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xiong J, Liu S, Pan Y, Zhang B, Chen X and
Fan L: Combination of fish oil and ethanol extracts from Spirulina
platensis inhibits the airway inflammation induced by ovalbumin in
mice. J Funct Foods. 40:707–714. 2018. View Article : Google Scholar
|
|
2
|
Ye P, Yang XL, Chen X and Shi C:
Hyperoside attenuates OVA-induced allergic airway inflammation by
activating Nrf2. Int Immunopharmacol. 44:168–173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barnes PJ: Pathophysiology of asthma. Br J
Clin Pharmacol. 42:3–10. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gu X, Zhang Q, Du Q, Shen H and Zhu Z:
Pinocembrin attenuates allergic airway inflammation via inhibition
of NF-κB pathway in mice. Int Immunopharmacol. 53:90–95. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schneider D, Hong JY, Bowman ER, Chung Y,
Nagarkar DR, McHenry CL, Goldsmith AM, Bentley JK, Lewis TC and
Hershenson MB: Macrophage/epithelial cell CCL2 contributes to
rhinovirus-induced hyperresponsiveness and inflammation in a mouse
model of allergic airways disease. Am J Physiol Lung Cell Mol
Physiol. 304:L162–L169. 2013. View Article : Google Scholar :
|
|
6
|
Kim MG, Kim SM, Min JH, Kwon OK, Park MH,
Park JW, Ahn HI, Hwang JY, Oh SR, Lee JW and Ahn KS:
Anti-inflammatory effects of linalool on ovalbumin-induced
pulmonary inflammation. Int Immunopharmacol. 74:1057062019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mizusaki A, Nishi K, Nishiwaki H, Ishida
M, Tamamoto T and Sugahara T: Suppressive effect of ethanol extract
from passion fruit seeds on IgE production. J Funct Foods.
32:176–184. 2017. View Article : Google Scholar
|
|
8
|
Zhu X, Li Q, Hu G, Wang J, Hu Q, Liu Z, Wu
G and Zhong Y: BMS-345541 inhibits airway inflammation and
epithelial-mesen-chymal transition in airway remodeling of
asthmatic mice. Int J Mol Med. 42:1998–2008. 2018.PubMed/NCBI
|
|
9
|
Liang Z, Nie H, Xu Y, Peng J, Zeng Y, Wei
Y, Wen X, Qiu J, Zhong W, Deng X and He J: Therapeutic effects of
rosmarinic acid on airway responses in a murine model of asthma.
Int Immunopharmacol. 41:90–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jabara HH and Geha RS: Jun N-terminal
kinase is essential for CD40-mediated IgE class switching in B
cells. J Allergy Clin Immunol. 115:856–863. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Subhashini, Chauhan PS, Dash D, Paul BN
and Singh R: Intranasal curcumin ameliorates airway inflammation
and obstruction by regulating MAPKinase activation (p38, Erk and
JNK) and prostaglandin D2 release in murine model of asthma. Int
Immunopharmacol. 31:200–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi IW, Kim DK, Ko HM and Lee HK:
Administration of antisense phosphorothioate oligonucleotide to the
p65 subunit of NF-kappaB inhibits established asthmatic reaction in
mice. Int Immunopharmacol. 4:1817–1828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zou XL, Pei DA, Yan JZ, Xu G and Wu P: A20
overexpression inhibits lipopolysaccharide-induced NF-κB
activation, TRAF6 and CD40 expression in rat peritoneal mesothelial
cells. Int J Mol Sci. 15:6592–6608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li S, Miao Z, Tian Y, Wang H, Wang S, He
T, Yang Y, Wang P, Ma M, Yang T, et al: Limethason reduces airway
inflammation in a murine model of ovalbumin-induced chronic asthma
without causing side effects. Exp Ther Med. 15:2269–2276.
2018.PubMed/NCBI
|
|
15
|
Haslam E: Natural polyphenols (vegetable
tannins) as drugs: Possible modes of action. J Nat Prod.
59:205–215. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao X, Sun H, Hou A, Zhao Q, Wei T and
Xin W: Antioxidant properties of two gallotannins isolated from the
leaves of Pistacia weinmannifolia. Biochim Biophys Acta.
1725:103–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen S, Wu X, Ji Y and Yang J: Isolation
and characterization of microsatellite loci in Pistacia
weinmannifolia (Anacardiaceae). Int J Mol Sci. 12:7818–7823. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Minami K, Nakasugi T, Sun HD, Hou AJ,
Ihara M, Morimoto M and Komai K: Isolation and identification of
histamine-release inhibitors from Pistacia weinmannifolia J. Pisson
ex Franch J Nat Med. 60:138–140. 2006. View Article : Google Scholar
|
|
19
|
Ci X, Chu X, Chen C, Li X, Yan S, Wang X,
Yang Y and Deng X: Oxytetracycline attenuates allergic airway
inflammation in mice via inhibition of the NF-κB pathway. J Clin
Immunol. 31:216–227. 2011. View Article : Google Scholar
|
|
20
|
Lee JW, Ryu HW, Lee SU, Kim MG, Kwon OK,
Kim MO, Oh TK, Lee JK, Kim TY, Lee SW, et al: Pistacia
weinmannifolia ameliorates cigarette smoke and
lipopolysaccharide-induced pulmonary inflammation by inhibiting
interleukin-8 production and NF-κB activation. Int J Mol Med.
44:949–959. 2019.PubMed/NCBI
|
|
21
|
Park HA, Kwon OK, Ryu HW, Min JH, Park MW,
Park MH, Paik JH, Choi S, Paryanto I, Yuniato P, et al: Physalis
peru- viana L. inhibits ovalbumin-induced airway inflammation by
attenuating the activation of NF-κB and inflammatory molecules. Int
J Mol Med. 43:1830–1838. 2019.PubMed/NCBI
|
|
22
|
Lee JW, Seo KH, Ryu HW, Yuk HJ, Park HA,
Lim Y, Ahn KS and Oh SR: Anti-inflammatory effect of stem bark of
Paulownia tomentosa Steud. in lipopolysaccharide (LPS)-stimulated
RAW264.7 macrophages and LPS-induced murine model of acute lung
injury. J Ethnopharmacol. 210:23–30. 2018. View Article : Google Scholar
|
|
23
|
Yuk HJ, Lee JW, Park HA, Kwon Ok, Seo KH,
Ahn KS, Oh SR and Ryu HW: Protective effects of coumestrol on
lipopolysaccharide-induced acute lung injury via the inhibition of
proinflammatory mediators and NF-κB activation. J Funct Foods.
34:181–188. 2017. View Article : Google Scholar
|
|
24
|
He J, Lv L, Wang Z, Huo C, Zheng Z, Yin B,
Jiang P, Yang Y, Li J, Gao Y and Xue J: Pulvis Fellis Suis extract
attenuates ovalbumin-induced airway inflammation in murine model of
asthma. J Ethnopharmacol. 207:34–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kao ST, Wang SD, Lin CC and Lin LJ: Jin
Gui Shen Qi Wan, a traditional Chinese medicine, alleviated
allergic airway hypersensitivity and inflammatory cell infiltration
in a chronic asthma mouse model. J Ethnopharmacol. 227:181–190.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hizawa N, Yamaguchi E, Jinushi E, Konno S,
Kawakami Y and Nishimura M: Increased total serum IgE levels in
patients with asthma and promoter polymorphisms at CTLA4 and
FCER1B. J Allergy Clin Immunol. 108:74–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia Z, Zhang Y, Li C, Xu Y, Dong J, Wang
L, He Q, Zou X, Wu H, Han J, et al: Traditional Tibetan medicine
Anzhijinhua San attenuates ovalbumin-induced diarrhea by regulating
the serotonin signaling system in mice. J Ethnopharmacol.
236:484–494. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang N and Shang YX: Epigallocatechin
gallate ameliorates airway inflammation by regulating Treg/Th17
imbalance in an asthmatic mouse model. Int Immunopharmacol.
72:422–428. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paiva Ferreira LKD, Paiva Ferreira LAM,
Alves AF, Leite FC, de Araújo Silva LA, Vieira GC, Rodrigues LC and
Piuvezam MR: MHTP, 2-Methoxy-4-(7-methoxy-
1,2,3,4-tetrahydroiso-quinolin-1-yl) phenol, a synthetic alkaloid,
induces IFN-γ production in murine model of ovalbumin-induced
pulmonary allergic inflammation. Inflammation. 41:2116–2128. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nguyen TH, Maltby S, Simpson JL, Eyers F,
Baines KJ, Gibson PG, Foster PS and Yang M: TNF-α and macrophages
are critical for respiratory syncytial virus-induced exacerbations
in a mouse model of allergic airways disease. J Immunol.
196:3547–3558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bakar NA, Anyanji VU, Mustapha NM, Lim SL
and Mohamed S: Seaweed (Eucheuma cottonii) reduced inflammation,
mucin synthesis, eosinophil infiltration and MMP-9 expressions in
asthma-induced rats compared to Loratadine. J Funct Foods.
19:710–722. 2015. View Article : Google Scholar
|
|
32
|
Alvaro M, Sancha J, Larramona H, Lucas JM,
Mesa M, Tabar AI and Martinez-Cañavate A; Immunotherapy Working
Group; Sociedad Española de Inmunología Clínica y Alergia
Pediátrica (SEICAP): Allergen-specific immunotherapy: Update on
immunological mechanisms. Allergol Immunopathol (Madr). 41:265–272.
2013. View Article : Google Scholar
|
|
33
|
Xu W, Hu M, Zhang Q, Yu J and Su W:
Effects of anthraqui-nones from Cassia occidentalis L. on
ovalbumin-induced airways inflammation in a mouse model of allergic
asthma. J Ethnopharmacol. 221:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
SAun MV, Bonamichi-Santos R, Arantes-Costa
FM, Kalil J and Giavina-Bianchi P: Animal models of asthma: Utility
and limitations. J Asthma Allergy. 10:293–301. 2017. View Article : Google Scholar
|
|
35
|
Wang L, Wang M, Li S, Wu H, Shen Q, Zhang
S, Fang L and Liu R: Nebulized lidocaine ameliorates allergic
airway inflammation via downregulation of TLR2. Mol Immunol.
97:94–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen Y, Huang S, Kang J, Lin J, Lai K, Sun
Y, Xiao W, Yang L, Yao W, Cai S, et al: Management of airway mucus
hypersecretion in chronic airway inflammatory disease: Chinese
expert consensus (English edition). Int J Chron Obstruct Pulmon
Dis. 13:399–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Huang L, Wang N, Yi H and Wang H:
Sulfur dioxide exposure enhances Th2 inflammatory responses via
activating STAT6 pathway in asthmatic mice. Toxicol Lett.
285:43–50. 2018. View Article : Google Scholar
|
|
38
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang CQ, Li W, Wu B, Chen WM, Chen LH, Mo
GW, Zhang QF, Gong L, Li J, Zhang HC, et al: Pheretima aspergillum
decoction suppresses inflammation and relieves asthma in a mouse
model of bronchial asthma by NF-κB inhibition. J Ethnopharmacol.
189:22–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Q, Wang L, Chen B, Zhuo Q, Bao C and
Lin L: Propofol inhibits NF-κB activation to ameliorate airway
inflammation in ovalbumin (OVA)-induced allergic asthma mice. Int
Immunopharmacol. 51:158–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee JW, Chun W, Kwon OK, Park HA, Lim Y,
Lee JH, Kim DY, Kim JH, Lee HK, Ryu HW, et al:
3,4,5-Trihydroxycinnamic acid attenuates lipopolysaccharide
(LPS)-induced acute lung injury via downregulating inflammatory
molecules and upregulating HO-1/AMPK activation. Int
Immunopharmacol. 64:123–130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee K, Choi J, Choi BK, Gu YM, Ryu HW, Oh
SR and Lee HJ: Picroside II Isolated from Pseudolysimachion
rotundum var. subintegrum inhibits glucocorticoid refractory serum
amyloid A (SAA) expression and SAA-induced IL-33 secretion.
Molecules. 24:2019.
|