Introduction
Inflammation is a protective mechanism activated by
pathogenic stimuli, which removes harmful factors and repairs
damaged tissue through associated signaling pathways (1). Chronic inflammation is associated
with multiple diseases, such as asthma, atherosclerosis, rheumatoid
arthritis (RA), colitis and inflammatory bowel diseases (IBD)
(2). Lipopoly-saccharide (LPS) is
expressed at the cell wall of gram-negative bacteria and it acts as
an endotoxin (3). The LPS
stimulus initiates the differentiation of macrophages and promotes
inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2)
expression (4,5). Nitric oxide (NO) is a signaling
molecule that has multiple molecular targets and regulates several
responses in mammalian cells. Nitric oxide synthases (NOSs) employ
L-arginine as a substrate; NOS initiates to oxidize L-arginine to
NO and L-citrulline (6). Notably,
iNOS is controlled by activated cytokines and induces
transcriptional activity in macrophage cells (7). The expression levels of COX-2 have
been reported to be increased during inflammation (8). Tumor necrosis factor-α (TNF-α),
interleukin (IL)-1β and IL-6 are regarded as pro-inflammatory
cytokines that regulate the inflammatory responses (9,10).
The production of iNOS, COX-2 and pro-inflammatory
cytokines are controlled by NF-κB, which is an essential
transcription factor in the inflammatory mechanism (11). In the cytoplasm, NF-κB is combined
with inhibitor of κB (IκB) proteins and maintained in an inactive
state (12). Following an
inflammatory stimulus, IκB is phosphorylated by the IκB kinase
(IKK) protein, resulting in the degradation of IκB, and the
subsequent release of NF-κB; free NF-κB then translocates to the
nucleus and it starts its transcriptional activity (13).
Mitogen-activated protein kinase (MAPK) pathways are
activated as a response to numerous signal transductions, including
inflammatory signaling (14).
MAPK pathways are stimulated by inflammatory cytokines or chemical
stress (15). LPS stimulation
also activates MAPKs; therefore, multiple studies have investigated
the involvement of NF-κB and MAPK pathways in inflammatory
responses (16-18).
Kaempferol is widely known for its anti-inflammatory
activity through the NF-κB pathway and kaempferol-3-O-ru-tinoside
is isolated from tartary buckwheat (Fagopyrum tatricum)
(19,20). The bioactivity or mechanism of
kaempferol-3-O-β-rutinoside has not been extensively
studied; therefore, the present study used
kaempferol-3-O-β-rutinoside to evaluate its potential
anti-inflammatory effect and mechanism in LPS-stimulated RAW 264.7
macrophage cells.
Materials and methods
Chemicals and antibodies
Kaempferol-3-O-β-rutinoside (cat. no. 90242),
kaempferol (cat. no. K0133) and LPS from Escherichia coli
(cat. no. L2630) were purchased from Sigma-Aldrich (Merck KGaA).
Kaempferol-3-O-β-rutinoside was dissolved in DMSO (100 mM),
aliquoted and stored at -80°C. Prior to each experiment,
kaempferol-3-O-β-rutinoside was diluted fresh in warm
Dulbecco's modified Eagle's medium (DMEM). After checking that
there was no precipitate, RAW264.7 cells were treated.
Monoclonal antibodies directed against GAPDH (cat.
no. 2118), iNOS (cat. no. 13120), COX-2 (cat. no. 12282), TNF-α
(cat. no. 3707), members of the NF-κB pathway [phosphorylated (p-)
IKKα, IKKα, p-IKKβ, IKKβ, p-IκBα, IκBα, p-NF-κB and NF-κB; NF-κB
sampler kit; cat. no. 9936), p-p38 (Thr180/Tyr 182; cat. no. 9215),
p38 (cat. no. 9212), p-p44/42 [also known as extracellular
signal-regulated kinase (ERK) 1/2; Thr202/Tyr204; cat. no. 4377),
p44/42 (cat. no. 4695), stress-activated protein kinase (SAPK, also
known as JNK2; cat. no. 9252) and p-SAPK (Thr183/Tyr185; cat. no.
4671) were purchased from Cell Signaling Technology, Inc.
Antibodies against IL-1β (cat. no. sc-12742) and IL-6 (cat. no.
sc-57315) were purchased from Santa Cruz Biotechnology, Inc. All
antibodies were diluted 1:1,000 with 5% bovine serum albumin.
Cell culture
RAW 264.7 and 293 cells were obtained from the
American Type Culture Collection. Both cell lines were grown in
DMEM (Cellgro; Corning, Inc.) containing 10% heat-inactivated fetal
bovine serum (Cellgro; Corning, Inc.), penicillin (100 U/ml) and
streptomycin (100 µg/ml; PAA Laboratories GmbH; GE
Healthcare). Cells were maintained in a humidified incubator under
5% CO2 at 37°C and the medium was refreshed every 2
days. All experiments were performed with healthy cells obtained
between passages 3 and 5.
Cell viability assay
The cytotoxicity of
kaempferol-3-O-β-rutinoside was assayed as reported
previously (21). Briefly, cells
were treated with kaempferol-3-O-β-rutinoside for 24 h. The
medium was replaced with fresh DMEM, and WST-1 solution (Daeil Lab
Services Company, Ltd.) was added for a further 3 h. The absorbance
was then measured at 460 nm using a microplate reader (Molecular
Devices LLC).
NO assay
To measure the inhibitory activity of kaempferol-3-
O-β-rutinoside on NO production, the protocol from a
previous study was followed (21). Briefly, the cells were treated
with kaempferol-3-O-β-rutinoside for 2 h, and then LPS (1
µg/ml) was added for 18 h. Supernatants were mixed with the
same volume of Griess reagent (Sigma-Aldrich; Merck KGaA) and
incubated for 15 min. The absorbance of the mixture was measured at
540 nm using a microplate reader (Molecular Devices LLC).
Western blot analysis
Western blot analysis was performed according to a
previous study (21). Whole-cell
protein was lysed with cell lysis buffer and supernatants were
obtained and quantified using the Bradford reagent (Biosesang Co.,
Ltd.). A total of 50 µg from each sample was denatured with
sample buffer at 95°C for 5 min, separated by 12% SDS-PAGE,
transferred to a nitrocellulose membrane, and blocked with 5% skim
milk in PBS with 1% Tween-20 at room temperature (RT) for 1 h.
After blocking, the membrane was incubated with specific antibodies
at 4°C overnight, followed by incubation with horseradish
peroxidase-conjugated secondary antibodies [anti-rabbit
immunoglobulin (Ig) G, cat. no. 7074; anti-mouse IgG, cat. no.
7076; and anti-rat IgG, cat. no. 7077; all 1:20,000; Cell Signaling
Technology, Inc.] at RT for 1 h. The membranes were developed on
X-ray films using an enhanced chemiluminescence
solution® (AbFrontier Co., Ltd.).
Reverse transcription (RT)-PCR
The mRNA expression levels of the cytokines TNF-α,
IL-6 and IL-1β were analyzed by RT-PCR, according to previous
studies (22,23). Total RNA was isolated using the
RNeasy® Plus Mini kit (Qiagen GmbH), and then cDNA was
synthesized using AccuPower® RT premix (Bioneer
Corporation). Each cDNA was amplified by PCR with Prime Tag Premix
(Genetbio Co., Ltd.) using specific primers. The specific primers
were as follows: TNF-α, 5′-CCC CTC AGC AAA CCA CCA AGT-3′ (forward
primer) and 5′-CTT GGG CAG ATT GAC CTC AGC-3′ (reverse primer);
IL-6, 5′-GGA GGC TTA ATT ACA CAT GTT-3′ (forward primer) and 5′-TGA
TTT CAA GAT GAA TTG GAT-3′ (reverse primer); IL-1β, 5′-AAT CTC ACA
GCAGCA CAT CAA-3′ (forward primer) and 5′-AGC CCA TAC TTT AGG AAG
ACA-3′ (reverse primer); and GAPDH, 5′-AAC TTT GGC ATT GTG GAA
GG-3′ (forward primer) and 5′-CAC ATT GGG GGT AGG AAC AC-3′
(reverse primer). PCR was performed under the following conditions:
denaturation (94°C, 1 min), annealing (TNF-α, 59.8°C; IL-1b, 55°C;
IL-6, 48°C; and GAPDH, 55°C; 1 min) and extension (72°C, 1 min) for
30 cycles. The amplified PCR products were analyzed by 2% agarose
gel electrophoresis with ethidium bromide. The band intensity was
measured using ImageJ 1.48v (National Institutes of Health).
Immunofluorescence staining of NF-κB
protein
Immunofluorescence staining was performed according
to a previous study (21). Cells
were stained with DAPI and fixed with 4% formaldehyde at RT for 15
min. After fixation, samples were blocked with 5% rabbit serum
(cat. no. sc-2338; Santa Cruz Biotechnology, Inc.) and
permeabilized with 0.3% Triton X-100 at RT for 1 h in the dark.
Cells were then incubated with a NF-κB p65 antibody (0.1
µg/ml; cat. no. 8242; Cell Signaling Technology, Inc.) at RT
for 2 h, followed by incubation with Alexa Fluor®
488-conjugated secondary antibody (1:2,000; cat. no. 4412; Cell
Signaling Technology, Inc.) at RT for 1 h. Samples were mounted
using the Prolong® Gold Anti-fade reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and examined under a Carl Zeiss LSM
700 fluorescence microscope (Carl Zeiss AG). The translocation
results were captured and processed with Zen 2011 SP2 software
(Carl Zeiss AG).
Statistical analysis
All the experiments were performed in triplicate and
the results are expressed as the mean ± standard error of the mean.
Statistical analyzes were performed using a one-way analysis of
variance, followed by Dunnett's multiple comparison post hoc
test for multigroup comparisons. The statistical significance of
the results was analyzed by the R 3.5.2 program for Windows
(24). P<0.05 was considered
to indicate a statistically significant difference.
Results
Cytotoxicity of
kaempferol-3-O-β-rutinoside on RAW264.7 macrophage cells
To determine the cytotoxicity of
kaempferol-3-O-β-rutinoside on RAW 264.7 and 293 cells, cell
viability assays were performed. The viability of the treated cells
was compared with untreated cells as a negative control, and with
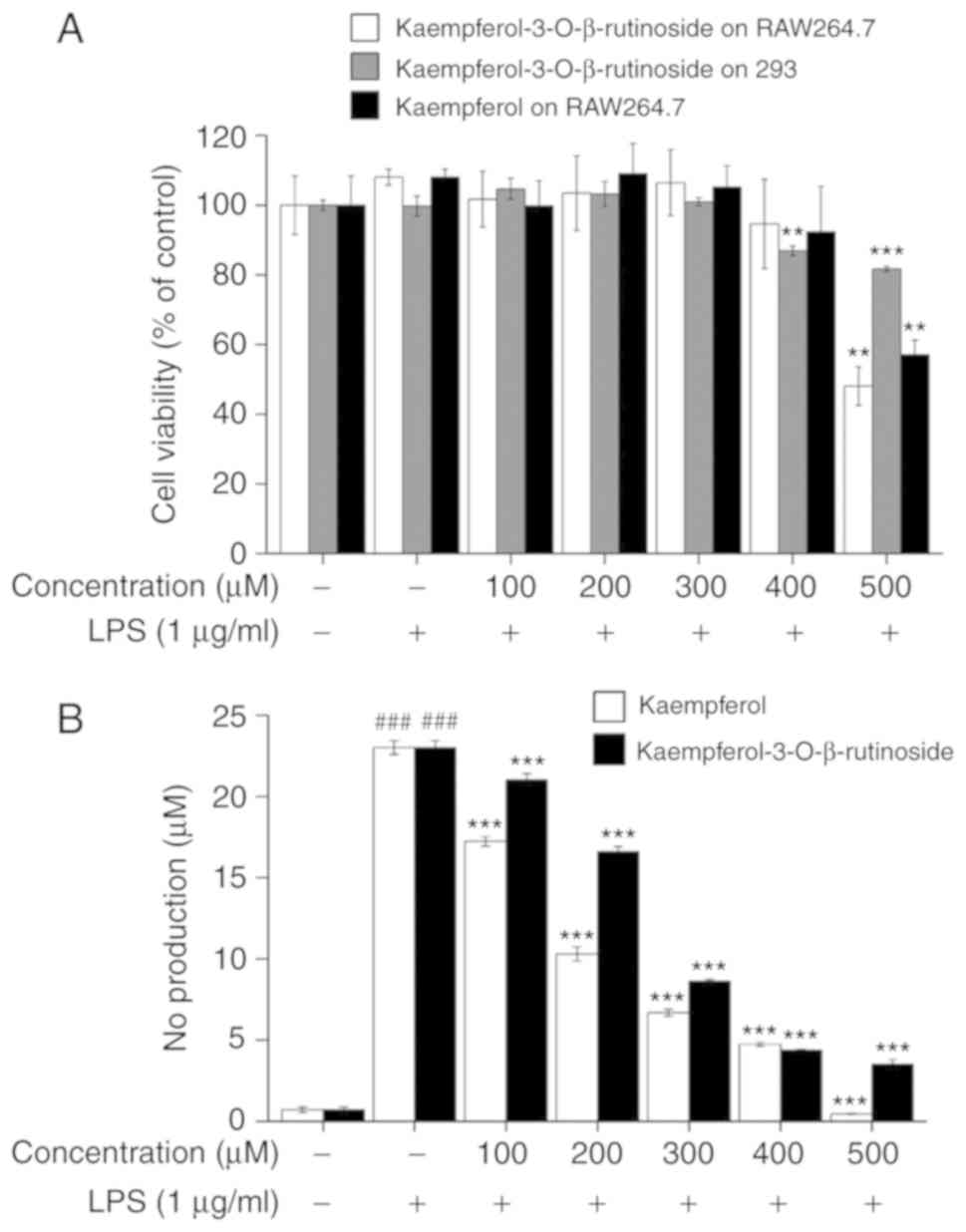
kaempferol-terated cells as a positive control. As Fig. 1A indicates,
kaempferol-3-O-β-rutinoside and kaempferol in doses up to
300 µM did not exhibit any toxic effects in both the
RAW264.7 and 293 cell lines. Over 90% of RAW264.7 cells survived at
400 µM kaempferol and kaempferol-3-O-β-rutinoside,
and 86% of 293 cells survived at 400 µM of
kaempferol-3-O-β-rutinoside. Approximately 50% of RAW264.7
cells survived at 500 µM of kaempferol and
kaempferol-3-O-β-rutinoside, and 81% of 293 cells survived
at 500 µM of kaempferol-3-O-β-rutinoside. Therefore,
the results of cell viability assays demonstrated that a
concentration up to 400 µM of
kaempferol-3-O-β-rutinoside was not toxic to RAW264.7 and
293 cells; thus, concentrations <400 µM were used for
further experiments.
Kaempferol-3-O-β-rutinoside suppresses NO
production
To determine whether
kaempferol-3-O-β-rutinoside has an anti-inflammatory effect,
Griess reagent was used to measure NO production, a known
inflammatory mediator (21,25). Fig.
1B shows that as the concentration of
kaempferol-3-O-β-rutinoside was increased, NO production was
significantly suppressed in LPS-stimulated RAW264.7 cells. Over 50%
of NO production was suppressed at 300 µM of
kaempferol-3-O-β-rutinoside treatment. Therefore,
concentrations of up to 300 µM of
kaempferol-3-O-β-rutinoside were used for further
experiments, based on the observations that they did not affect
cell viability but inhibited NO production.
Kaempferol-3-O-β-rutinoside downregulates
the expression of inflammation-related factors
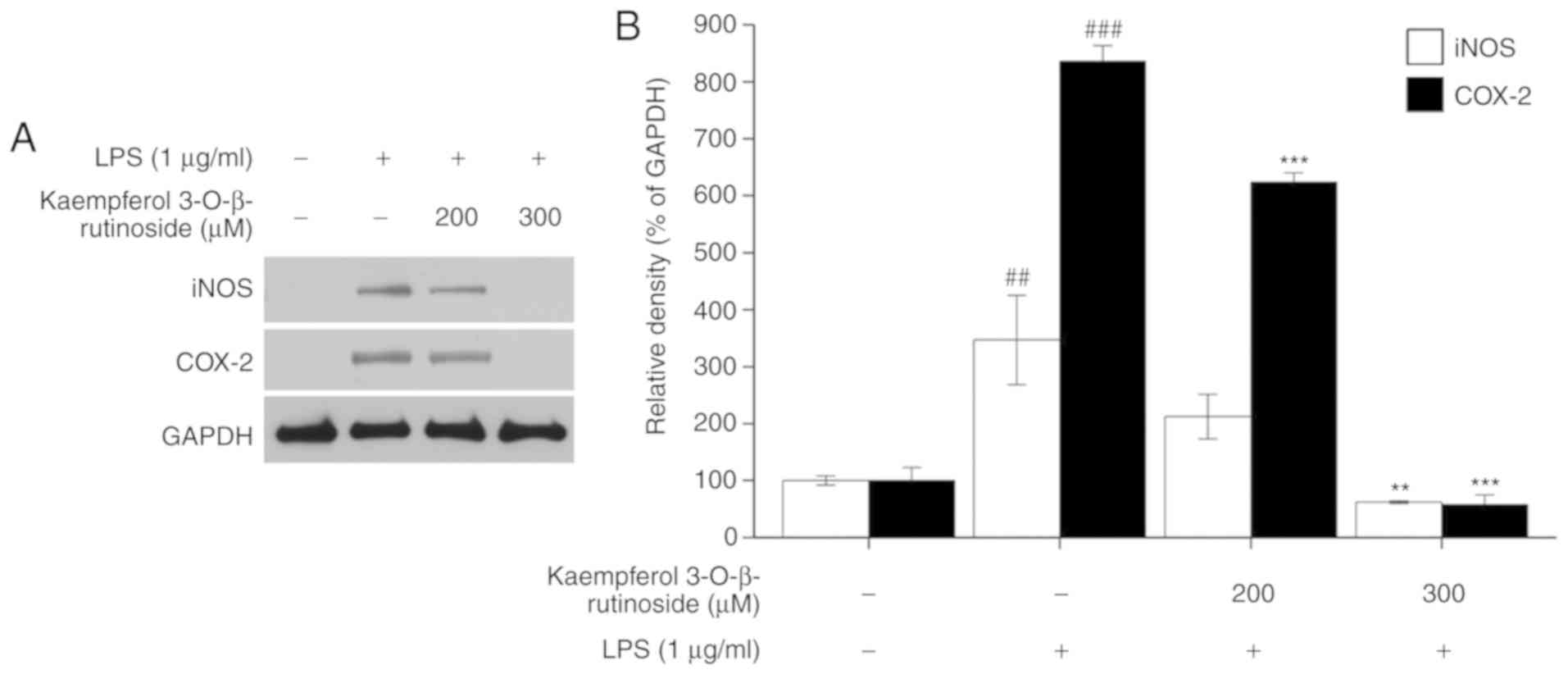
Next, the present study investigated whether
kaempferol-3-O-β-rutinoside inhibited the expressions of the
key inflammation-related proteins iNOS and COX-2. When iNOS is
upregulated, it causes a high level of NO production, which affects
the expression of pro-inflammatory cytokines (7); COX-2 is expressed during
inflammation in response to infection or invasion of pathogens
(5). Fig. 2 shows that following
kaempferol-3-O-β-rutinoside treatment, the expression levels
of iNOS and COX-2 were significantly suppressed in LPS-stimulated
RAW264.7 cells, in a dose-dependent manner.
Kaempferol-3-O-β-rutinoside treatment completely reversed
the LPS-induced iNOS and COX-2 expression at 300 µM
(Fig. 2).
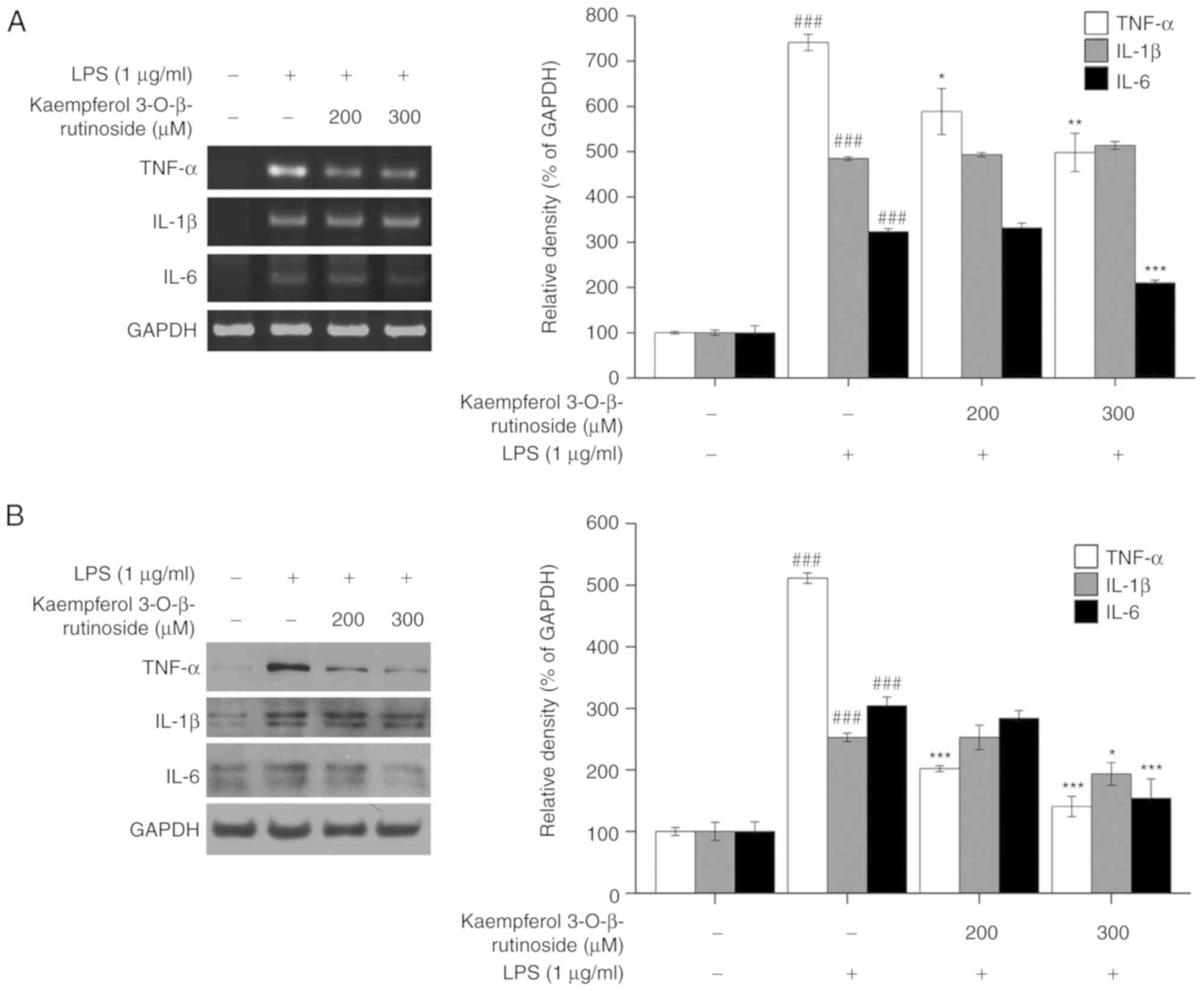
In addition, the expression levels of TNF-α, IL-β
and IL-6 were analyzed at the mRNA (Fig. 3A) and protein level (Fig. 3B). The results demonstrated that
kaempferol-3-O-β-rutinoside downregulated the expression
levels of the pro-inflammatory cytokines (Fig. 3).
Kaempferol-3-O-β-rutinoside decreased the TNF-α mRNA
expression levels, and it almost completely inhibited its protein
expression in LPS-stimulated RAW264.7 cells. In addition,
kaempferol-3-O-β-rutinoside suppressed IL-6 expression at
both mRNA and protein level in LPS-stimulated RAW264.7 cells.
However, IL-1β expression was not markedly changed by
kaempferol-3-O-β-rutinoside in LPS-stimulated RAW264.7
cells. The present data suggested that
kaempferol-3-O-β-rutinoside had an effect on the expression
of inflammation-related factors iNOS, COX-2, TNF-α and IL-6.
Kaempferol-3-O-β-rutinoside suppresses
the NF-κB pathway
NF-κB is a transcription factor and the most
significant modulator of inflammation-related gene expression,
including iNOS, COX-2, TNF-α, IL-1β and IL-6. Therefore, the NF-κB
pathway is considered as the main target for investigations of
inflammation (12,26). When IKK is activated, it leads to
the phosphorylation of IκB. IκB binds to NF-κB under normal
conditions; however, phosphorylated IκB is ubiquitinated and
degraded, resulting in the activation and nuclear translocation of
NF-κB (12). In the present
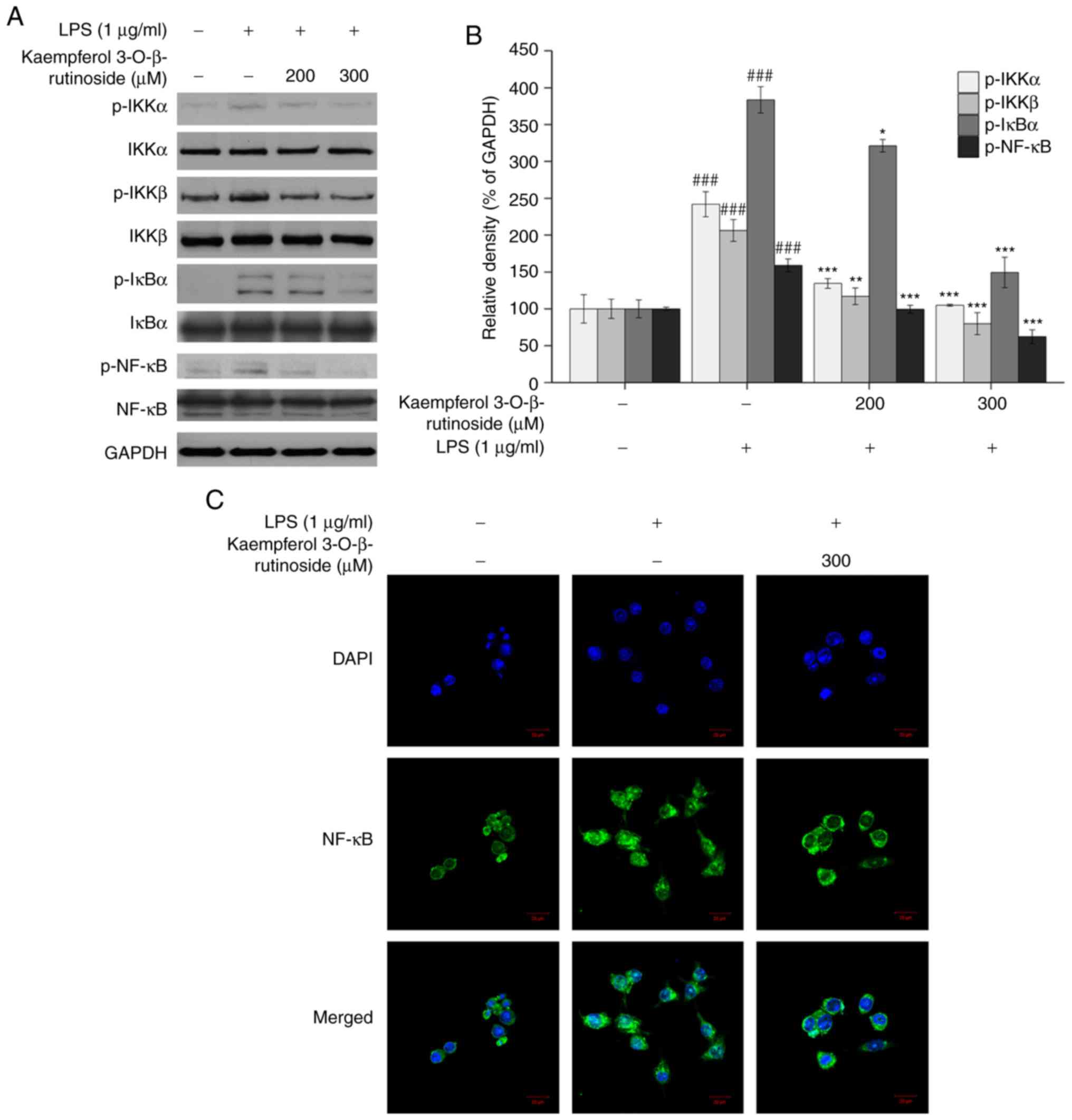
results, as the kaempferol-3-O-β-rutinoside concentration
was increased, the phosphorylation of NF-κB, IκBα, IKKα and IKKβ
were decreased (Fig. 4A). In
addition, the subcellular localization of NF-κB was examined by
confocal microscopy. Compared with the LPS group,
kaempferol-3-O-β-rutinoside treatment inhibited the nuclear
translocation of NF-κB (Fig. 4C).
These data demonstrated that kaempferol-3-O-β-rutinoside
suppressed the phosphorylation of NF-κB by controlling the
activities of upstream signaling factors. Furthermore,
kaempferol-3-O-β-rutinoside treatment reduced NF-κB nuclear
translocation, potentially resulting in the iNOS, COX-2 and
cytokine downregulation.
Kaempferol-3-O-β-rutinoside inhibits the
MAPK pathway
MAPK pathways are considered as the main pathways of
the inflammatory mechanism and are well studied in inflammatory
diseases, including RA, pancreatitis, hepatitis, IBD and psoriasis
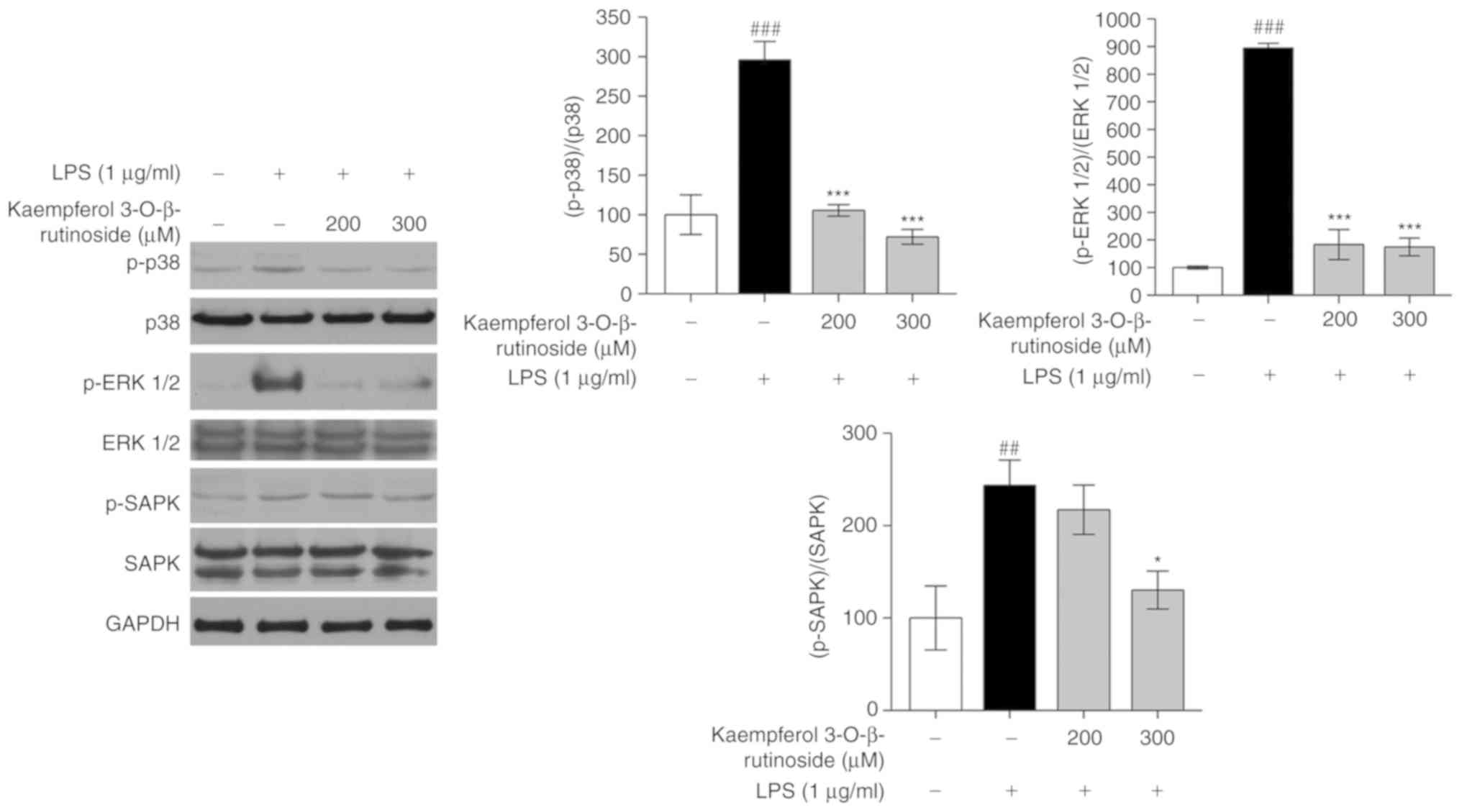
(15). As shown in Fig. 5,
kaempferol-3-O-β-rutinoside treatment decreased the
phosphorylation of p38, ERK, and SAPK in a dose-dependent manner.
Among the key MAPK factors, kaempferol-3-O-β-rutinoside
appeared to inhibit the phosphorylation of ERK1/2 the most
(Fig. 5). Hence,
kaempferol-3-O-β-rutinoside exhibited significant inhibitory
activity on MAPK pathway signaling.
Discussion
Kaempferol-3-O-rutinoside was isolated from tartary
buckwheat (F. tatricum) (19,20). The photochemistry of tartary
buckwheat has been investigated, including quercetin, rutin and
kaempferol, and several studies have suggested that flavonoids from
tartary buckwheat may be used in preparing novel pharmaceuticals
(27,28). Therefore, the present study
investigated whether kaempferol-3-O-β-rutinoside affects
inflammation in LPS-stimulated RAW 264.7 cells.
In the immune response, the overexpression of NO
indicates that the body is affected by pathological symptoms, such
as endotoxemia, allograft rejection and diabetes (29). It also affects pro-inflammatory
responses that cause multiple side effects, including cytotoxicity,
vasodilation and edema (7).
Therefore, in order to investigate whether
kaempferol-3-O-β-rutinoside has an anti-inflammatory
function, first its effect on NO production was detected. NO is
produced by NOSs; iNOS is especially expressed by several stimuli,
including LPS or pro-inflammatory cytokines, and then large amount
of NO is produced (7).
Kaempferol-3-O-β-rutinoside inhibited the production of NO
in LPS-stimulated RAW 264.7 cells in a dose-dependent manner; the
IC50 was ~260±2.5 µM (Fig. 1B). The results of the
kaempferol-3-O-β-rutinoside cytotoxicity assay revealed that
kaempferol-3-O-β-rutinoside was cytotoxic at 500 µM
in macrophage RAW264.7 cells (Fig.
1A). No cytotoxicity effect was observed however at the dose of
300 µM. There are several substances which are effective
even at high concentration. For example, 5-aminosalicylic acid
(5-ASA), known as mesalazine and used to treat IBS safely in
pregnancy and breastfeeding, is routinely applied in in
vitro experiments at 500 µM to 20 mM (30-32). Aspirin is also tested at high
concentrations, for example, 1- 5 mM (33). The concentration of 5-ASA or
aspirin is high in in vitro experiments, however, they are
both currently used as drugs in humans and are extensively studied.
Therefore, the presents study considered the results of the NO and
cytotoxicity assays, and doses up to 300 µM
kaempferol-3-O-β-rutinoside were selected for further
experiments aiming to investigate its molecular mechanisms.
iNOS and COX-2 are widely known as the main
regulatory factors in inflammatory responses; thus, iNOS and COX-2
expression levels have been examined by many studies for the
development of anti-inflammatory agents (21,25,34). As shown in Fig. 2, as the concentration of
kaempferol-3-O-β-rutinoside was increased, iNOS and COX-2
expression was decreased. Pro-inflammatory cytokines are involved
in inflammatory reactions, stimulate acute phase inflammation and
act as endogenous pyrogens (35).
The pro-inflammatory cytokines first activate macrophage cells,
then initiate iNOS and COX-2 expression, followed by NO production
(7). The reduction of
pro-inflammatory cytokine expression is considered an effective way
to treat inflammation-related diseases. The present study examined
the pro-inflammatory cytokine mRNA and protein expression levels by
RT-PCR and western blotting, respectively. Fig. 3 shows
kaempferol-3-O-β-rutinoside to be effective in reducing both
mRNA and protein expressions of IL-6 and TNF-α in LPS-stimulated
RAW 264.7 cells. Kaempferol-3-O-β-rutinoside was slightly
effective at inhibiting the IL-1β protein expression in
LPS-stimulated RAW 264.7 cells. Multiple studies have revealed that
IL-6 gene expression is controlled by inflammation triggers and
performs important functions in the immune response (10,36). NF-κB is reported to regulate IL-6
gene expression following LPS stimulation in human monocytic cells
and human cervical carcinoma cells (10). Therefore, the NF-κB pathway was
explored in order to elucidate the mechanism of the
anti-inflammatory activity of
kaempferol-3-O-β-rutinoside.
In the normal state, the NF-κB protein is associated
with the IκBα protein in the cytoplasm, thereby maintained in an
inactivate state. When the inflammatory response is initiated, IKK
phosphorylates IκBα, leading to its degradation. Therefore, the
NF-κB protein is free to translocate to the nucleus and to initiate
the transcription of inflammation-related genes (13). NF-κB is associated with multiple
human inflammation-related disorders, including asthma, RA, IBD and
atherosclerosis, and leads to the transcription of pro-inflammatory
cytokines, chemokines, iNOS and COX-2 (12). It has been reported that
constitutive NF-κB is highly expressed in the inflamed colonic
tissue in patients with IBD (37). In addition, NF-κB controls the
induction of pro-inflammatory cytokines and chemokines, and
promotes the recruitment of monocytes and the progression of
atherosclerosis (38). The
present results demonstrated that
kaempferol-3-O-β-rutinoside suppressed the phosphorylation
of IKKα, IKKβ, IκBα and NF-κB and reduced the NF-κB translocation
to the nucleus.
MAPK pathways are also considered as targets for
drug development (15). Both
NF-κB and MAPK pathways are associated with various important
biological processes, including inflammation, cell survival and
apoptosis, in macrophage cells (39). MAPK pathways are involved in
regulating inflammatory-related gene transcription, leading to the
overproduction of pro-inflammatory cytokines (40,41). LPS is known as one of the potent
activators of MAPK pathway proteins (16). Following LPS stimulation, MAPKs
are phosphorylated or activated to produce several transcription
factors and inflammatory mediators in human monocytic cells
(3). Thus, regulating the MAPK
pathway is also contemplated as an approach in anti-inflammation
treatment. The present data suggested that
kaempferol-3-O-β-rutinoside significantly downregulated the
phosphorylation levels of p38 and ERK, and slightly decreased the
phosphorylation of SAPK. These findings indicated that
kaempferol-3-O-β-rutinoside exerted an anti-inflammatory
activity by inhibiting the MAPK pathways.
In conclusion, the present study explored the anti-
inflammatory effect of kaempferol-3-O-β-rutinoside in
LPS-stimulated macrophages via the NF-κB and MAPK pathways.
Kaempferol-3-O-β-rutinoside substantially reduced NO
production at 300 µM; this concentration did not show
cytotoxicity on RAW264.7 and 293 cells.
Kaempferol-3-O-β-rutinoside inhibited the expression of
inflammation-related factors, such as iNOS, COX-2, TNF-α and IL-6.
In addition, kaempferol-3-O-β-rutinoside suppressed the
phosphorylation of IKKα, IKKβ, IκBα and NF-κB, as well as MAPK
phosphorylation, including p38, ERK1/2 and SAPK. Therefore, these
data demonstrated that kaempferol-3-O-β-rutinoside inhibited
the inflammation-related gene expression in LPS-induced macrophages
via the NF-κB and MAPK pathways. The present study may be used as
basic pharmacological information in the field of anti-inflammation
reagent development.
Acknowledgments
Not applicable.
Funding
This study was supported by the Ministry of Trade,
Industry, and Energy, under the 'Regional Specialized Industry
Development Program' (grant no. R0003893), supervised by the Korea
Institute for Advancement of Technology.
Availability of data and materials
The analyzed datasets during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
CK and GK conceptualized the experiments. DH and CK
collected the data. DH and MK performed the experiments. DH
analyzed the data and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Medzhitov R: Origin and physiological
roles of inflammation. Nature. 454:428–435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guzik TJ, Korbut R and Adamek-Guzik T:
Nitric oxide and superoxide in inflammation and immune regulation.
J Physiol Pharmacol. 54:469–487. 2003.
|
|
3
|
Kim YS and Joh TH: Microglia, major player
in the brain inflammation: Their roles in the pathogenesis of
Parkinson's disease. Exp Mol Med. 38:333–347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Korhonen R, Lahti A, Kankaanranta H and
Moilanen E: Nitric oxide production and signaling in inflammation.
Curr Drug Targets Inflamm Allergy. 4:471–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rouzer CA and Marnett LJ: Cyclooxygenases:
Structural and functional insights. J Lipid Res. 50(Suppl):
S29–S34. 2009. View Article : Google Scholar :
|
|
6
|
Förstermann U and Sessa WC: Nitric oxide
synthases: Regulation and function. Eur Heart J. 33:829–837. 2012.
View Article : Google Scholar :
|
|
7
|
Paradise WA, Vesper BJ, Goel A, Waltonen
JD, Altman KW, Haines GK and Radosevich JA: Nitric oxide:
Perspectives and emerging studies of a well known cytotoxin. Int J
Mol Sci. 11:2715–2745. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sautebin L: Prostaglandins and nitric
oxide as molecular targets for anti-inflammatory therapy.
Fitoterapia. 71(Suppl 1): S48–S57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wink DA, Hines HB, Cheng R, Switzer CH,
Flores-Santana W, Vitek MP, Ridnour LA and Colton CA: Nitric oxide
and redox mechanisms in the immune response. J Leukoc Biol.
89:873–891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka T, Narazaki M and Kishimoto T: IL-6
in inflammation, immunity, and disease. Cold Spring Harb Perspect
Biol. 6:a0162952014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christman JW, Blackwell TS and Juurlink
BH: Redox regulation of nuclear factor kappa B: Therapeutic
potential for attenuating inflammatory responses. Brain Pathol.
10:153–162. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wullaert A, Bonnet MC and Pasparakis M:
NF-κB in the regulation of epithelial homeostasis and inflammation.
Cell Res. 21:146–158. 2011. View Article : Google Scholar
|
|
14
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hommes DW, Peppelenbosch MP and van
Deventer SJ: Mitogen activated protein (MAP) kinase signal
transduction pathways and novel anti-inflammatory targets. Gut.
52:144–151. 2003. View Article : Google Scholar
|
|
16
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schorey JS and Cooper AM: Macrophage
signalling upon mycobacterial infection: The MAP kinases lead the
way. Cell Microbiol. 5:133–142. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saqib U, Sarkar S, Suk K, Mohammad O, Baib
MS and Savai R: Phytochemicals as modulators of M1-M2 macrophages
in inflammation. Oncotarget. 9:17937–17950. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kadioglu O, Nass J, Saeed ME, Schuler B
and Efferth T: Kaempferol is an anti-inflammatory compound with
activity towards NF-κB pathway proteins. Anticancer Res.
35:2645–2650. 2015.PubMed/NCBI
|
|
20
|
Li D, Li X and Ding X: Composition and
antioxidative properties of the flavonoid-rich fractions from
tartary buckwheat grains. Food Sci Biotechnol. 19:711–716. 2010.
View Article : Google Scholar
|
|
21
|
Hwang D, Kang MJ, Jo MJ, Seo YB, Park NG
and Kim GD: Anti-inflammatory activity of β-thymosin peptide
derived from Pacific oyster (Crassostrea gigas) on NO2
and PGE production by down-regulating NF-κB in LPS-induced RAW264.7
macrophage cells. Mar Drugs. 17:E1292019. View Article : Google Scholar
|
|
22
|
Seong YA, Hwang D and Kim GD: The
anti-inflammatory effect of Gnaphalium affine through inhibition of
NF-κB and MAPK in lipopolysaccharide-stimulated RAW264.7 cells and
analysis of its phytochemical components. Cell Biochem Biophys.
74:407–417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim EJ, Kang MJ, Seo YB, Nam SW and Kim
GD: Acer okamotoanum Nakai leaf extract inhibits adipogenesis via
suppressing expression of PPAR γ and C/EBP α in 3T3-L1 cells. J
Microbiol Biotechnol. 28:1645–1653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
R Core Team: R: A language and environment
for statistical computing. R Foundation for Statistical Computing;
Vienna: 2018
|
|
25
|
Karabay AZ, Koc A, Gurkan-Alp AS,
Buyukbingol Z and Buyukbingol E: Inhibitory effects of indole
α-lipoic acid derivatives on nitric oxide production in LPS/IFNγ
activated RAW 264.7 macrophages. Cell Biochem Funct. 33:121–127.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi HW, Shin PG, Lee JH, Choi WS, Kang
MJ, Kong WS, Oh MJ, Seo YB and Kim GD: Anti-inflammatory effect of
lovastatin is mediated via the modulation of NF-κB and inhibition
of HDAC1 and the PI3K/Akt/mTOR pathway in RAW264.7 macrophages. Int
J Mol Med. 41:1103–1109. 2018.
|
|
27
|
Jing R, Li HQ, Hu CL, Jiang YP, Qin LP and
Zheng CJ: Phytochemical and pharmacological profiles of three
Fagopyrum buckwheats. Int J Mol Sci. 17:E5892016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang S, Liu Q, Xie Y, Zeng H, Zhang L,
Jiang X and Chen X: Separation of five flavonoids from tartary
buckwheat (Fagopyrum tataricum (L.) Gaertn) grains via off-line two
dimensional high-speed counter-current chromatography. Food Chem.
186:153–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Groves JT and Wang CC: Nitric oxide
synthase: Models and mechanisms. Curr Opin Chem Biol. 4:687–695.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Joo K, Lee Y, Choi D, Han J, Hong S, Kim
YM and Jung Y: An anti-inflammatory mechanism of taurine conjugated
5-aminosalicylic acid against experimental colitis: Taurine
chlo-ramine potentiates inhibitory effect of 5-aminosalicylic acid
on IL-1beta-mediated NFkappaB activation. Eur J Pharmacol.
618:91–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qu T, Wang E, Jin B, Li W, Liu R and Zhao
ZB: 5-Aminosalicylic acid inhibits inflammatory responses by
suppressing JNK and p38 activity in murine macrophages.
Immunopharmacol Immunotoxicol. 39:45–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Serra D, Rufino AT, Mendes AF, Almeida LM
and Dinis TC: Resveratrol modulates cytokine-induced Jak/STAT
activation more efficiently than 5-aminosalicylic acid: An in vitro
approach. PLoS One. 9:e1090482014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin MJ, Yamamoto Y and Gaynor RB: The
anti-inflammatory agents aspirin and salicylate inhibit the
activity of I(kappa)B kinase-beta. Nature. 396:77–80. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JH, Kang BS, Hwang KH and Kim GH:
Evaluation for anti-inflammatory effects of Siegesbeckia
glabrescens extract in vitro. Food Agric Immunol. 22:145–160. 2011.
View Article : Google Scholar
|
|
35
|
Sprague AH and Khalil RA: Inflammatory
cytokines in vascular dysfunction and vascular disease. Biochem
Pharmacol. 78:539–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Taniguchi K and Karin M: IL-6 and related
cytokines as the critical lynchpins between inflammation and
cancer. Semin Immunol. 26:54–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schreiber S, Nikolaus S and Hampe J:
Activation of nuclear factor kappaB inflammatory bowel disease.
Gut. 42:477–484. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2:170232017. View Article : Google Scholar
|
|
39
|
Karin M: Inflammation-activated protein
kinases as targets for drug development. Proc Am Thorac Soc.
2:386–390. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choi WS, Jeong JW, Kim SO, Kim GY, Kim BW,
Kim CM, Seo YB, Kim WY, Lee SY, Jo KH, et al: Anti-inflammatory
potential of peat moss extracts in lipopolysaccharide-stimulated
RAW 264.7 macrophages. Int J Mol Med. 34:1101–1109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jeong SH, Kim J and Min H: In vitro
anti-inflammatory activity of the Artemisia montana leaf ethanol
extract in macrophage RAW 264.7 cells. Food Agric Immunol.
29:688–698. 2018. View Article : Google Scholar
|