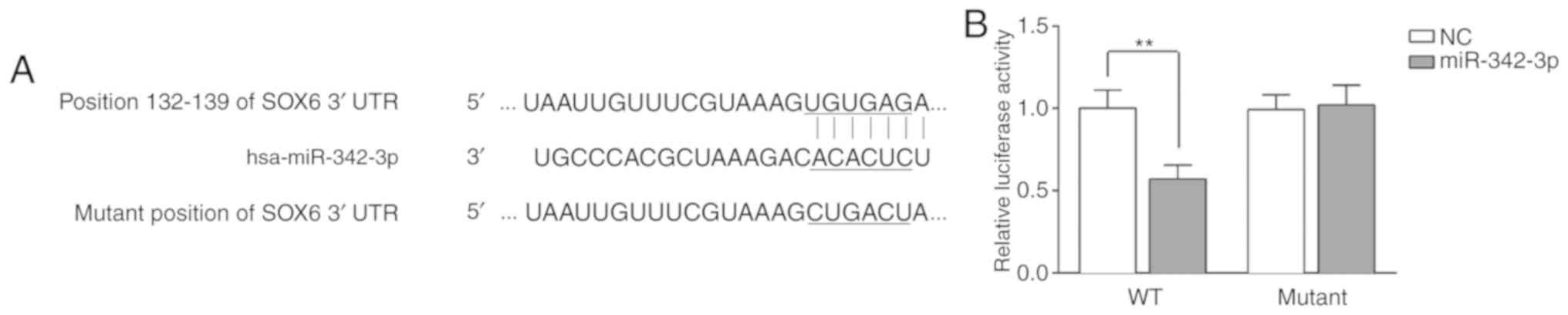Introduction
Diabetic kidney disease (DKD), one of the major
microvascular complications in patients with type 1 and/or type 2
diabetes, and is the primary cause of end-stage renal disease
(ESRD) in several countries (1).
The use of renin/angiotensin axis inhibitors is a widely accepted
treatment strategy for DKD, as it decreases the blood glucose
levels and blood pressure (2).
However, the pathogenesis of DKD and the mechanisms through which
it leads to ESRD have not yet been fully elucidated.
MicroRNAs (miRNAs or miRs) are small endogenous,
non-coding RNAs (~17-23 nucleotides in length) that bind to the 3′
non-coding region (3′-UTR) of their target genes (3). miRNAs negatively regulate the
expression of their target genes by inducing the degradation of
messenger RNAs (mRNAs) and/or inhibiting the translation of the
proteins from the mRNAs (4).
Increasing evidence has indicated that the dysregulation of miRNAs
may be involved in the pathogenesis and development of DKD
(5,6). Previous studies have also revealed
that miR-342-3p is highly expressed in mouse kidney tissues;
however, whether miR-342-3p is associated with the progression of
DKD remains unknown.
SRY-box 6 (SOX6) is an important member of the
Sry-related high mobility group box (Sox) family of transcription
factors, which is highly conserved in various mammalian species. It
has been shown that SOX6 may play an important role in cell
differentiation, apoptosis and proliferation (7,8).
Recent studies have demonstrated that SOX6 is involved in the
mechanisms underlying diabetes mellitus. For example, Iguchi et
al suggested that SOX6 downregulation induces pancreatic β-cell
proliferation (9). Pleskovič
et al also indicated that the SOX6 gene polymorphism
rs16933090 accelerated the progression of subclinical
atherosclerosis in patients with type 2 diabetes mellitus (10). However, the effects of SOX6 on the
pathogenesis of DKD have not yet been fully elucidated.
In the present study, it was found that miR-342-3p
expression was downregulated in the kidneys of mice with DKD,
whereas SOX6 was highly expressed. In vitro experiments
revealed a decreased miR-342-3p and an increased SOX6 expression in
mesangial cells (MCs) cultured under high glucose conditions.
Furthermore, it was found that the overexpression of miR-342-3p
promoted cell proliferation, and inhibited cell apoptosis and
fibrosis. It was also verified that miR-342-3p suppressed the
activation of the PTEN/Akt axis by downregulating SOX6 expression.
Finally, it was demonstrated that SOX6 is a target gene of
miR-342-3p by prediction using bioinformatics and validation using
luciferase activity assay. Overall, it was demonstrated that
miR-342-3p attenuated the progression of DKD procession by
targeting SOX6.
Materials and methods
Animal model and cell culture
This study was approved by the Animal Ethics
Committee of Tianjin Medical University (Tianjin, China). All
experimental mice (db/db mice, 5-6 weeks old, weighing 20-40 g, all
male) were cage-fed in a specific-pathogen-free grade environment
(temperature, 23±2°C; relative humidity, 55±5%) and fed standard
feed and were provide with access to clean drinking water. The
db/db mice fed a high-fat and sugar diet (10% fat, 20% sugars, 2.5%
cholesterol, 1.0% cholate, 66.5% conventional forage; Specialty
Feeds Pty, Ltd.) for 12-16 weeks were used as a model for DKD, and
db/m mice fed normally served as the non-DKD controls (n=10 each
group). Each strain was randomized and 3 mice were used for
independent experiments. After the mice were sacrificed, the mouse
kidney tissue was removed and washed with precooled normal saline
for further analysis. Mouse renal MCs were provided by the National
Infrastructure of Cell Line Resource of China.
Cell culture and treatment
Mouse renal mesangial cells (MCs) were cultured in
low-glucose DMEM medium supplemented with 10% FBS and incubated at
37°C with 5% CO2. For high glucose induction, MCs were
treated with 100 µM glucose for 12, 24 and 36 h,
respectively. MCs were also treated with various concentrations of
glucose (0, 50, 100 and 200 µM) for 24 h.
Plasmid and mimic transfection
miR-342-3p mimics, miRNA mimics control, plasmid
SOX6 and plasmid control were synthesized by Shanghai GenePharma
Co., Ltd. Lipofectamine 2000 reagent (Thermo Fisher Scientific,
Inc.) was used for transient transfection, following the
manufacturer's instructions. The cells were harvested at 48 h
following transfection.
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the mouse kidney
tissues and MCs using TRIzol solution (Thermo Fisher Scientific,
Inc.). The RNA sample was stored in a -80°C freezer. Reverse
transcription was performed according to the instructions provided
with the PrimeScript RT reagent (Takara Bio, Inc.). RT-qPCR was
performed using SYBR-Green Master Mix II (Takara Bio, Inc.). The
thermocycling conditions were conducted as follows: Initial
denaturation 95°C for 5 min, followed by 40 cycles of denaturation
at 95°C for 15 sec and annealing/elongation at 60°C for 30 sec. All
miRNA samples were normalized to U6. Each experiment was repeated 2
times. The 2−ΔΔCq method (11) was used for miR-342-3p expression
analysis. Specific gene primer sequences were purchased from Sangon
Biotech, and the sequences of the primers are presented in Table SI.
Western blot analysis
Subconfluent cells were washed 3 times with PBS, and
the proteins were then harvested with radioim-munoprecipitation
assay buffer. The protein concentration was quantified using a
BCATM Protein Assay kit (Pierce, Thermo Fisher Scientific, Inc.).
Protein (30 µg protein/lane) from each sample was separated
by 12% SDS-polyacrylamide gel electrophoresis and western blot
analysis were performed following standard protocols. The separated
proteins were transferred onto polyvinylidene fluoride membranes
and blocked with 5% skim milk at room temperature for 1 h. The
membranes were then incubated with primary antibodies at 4°C
overnight, followed by incubation with anti-mouse IgG, HRP-linked
antibody (cat no. 7076; dilution: 1:2,000) or anti-rabbit IgG,
HRP-linked antibody (cat no. 7074; dilution: 1:2,000) (both from
Cell Signaling Technology) at room temperature for 2 h. The primary
antibodies used were as follows: Anti-SOX6 (cat. no. 14010-1-AP;
dilution, 1:2,000), anti-transforming growth factor (TGF)-β1 (cat.
no. 21898-1-AP; dilution, 1:1,000), anti-fibronectin (FN; cat. no.
15613-1-AP; dilution, 1:2,000), anti-type-Ⅳ-collagen (referred to
as C-IV; cat. no. 55131-1-AP; dilution, 1:2,000), anti-phosphatase
and tensin homolog (PTEN; cat. no. 22034-1-AP; dilution, 1:2,000),
anti-Akt (cat. no. 10176-2-AP; dilution, 1:2,000), anti-p-Akt (cat.
no. 66444-1-Ig; dilution, 1:1,000), GADPH (cat. no. 20536-1-AP;
dilution, 1:2,000) (ProteinTech Group, Inc.). Protein expression
levels for each sample were normal-ized to β-actin. Densitometry
was performed by using the ImageJ software (version 1.38X; National
Institutes of Health).
CCK-8 assay
The proliferative ability of the cells was examined
using the Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.), as per the manufacturer's instructions. The
MCs transfected with miR-342-3p mimics, miRNA mimics control,
miR-342-3p mimics/plasmid SOX6 and miR-342-3p mimics/plasmid
control were plated in 96-well plates. Subsequently, CCK-8 solution
(10% of the medium, 10 µl) was added to each well followed
by incubation for 4 h at 4°C prior to analysis. The absorbance was
then measured at 450 nm using a FLUOstar® Omega
Microplate Reader (BMG Labtech GmbH).
Apoptosis assay
The Annexin V-fluorescein isothio-cyanate
(FITC)/propidium iodide (PI) apoptosis detection kit (cat. no.
C1062M; Beyotime) was used to assess cell apoptosis. The MCs were
placed into 6-well plates (6×105 cells/well). At 48 h
following transfection with miR-342-3p mimics, miRNA mimics
control, miR-342-3p mimics/plasmid SOX6 and miR-342-3p
mimics/plasmid control, flow cytometry (BDFACSCelesta; BD
Biosciences) was performed to determine the apoptosis of the
transfected cells by measuring the amount of Annexin V-positive and
PI-negative cells. FlowJo software (version 7.2.4; FlowJo LLC) was
used to analyze the data.
Dual-luciferase activity assay
Bioinformatics software TargetScan (http://www.targetscan.org/vert_72/) was used to
predict the bindings sites of miR-342-3p in the 3′UTR of SOX6, and
the results indicated the binding sites between the 3′UTR of SOX6
and miR-342-3p. Subsequently, to confirm the binding sites between
SOX6 and miR-342-3p, dual-luciferase activity assay was performed.
The 3′-UTR of SOX6, which contained the putative target site
of miR-342-3p, was ligated into the pGL3 construct (Promega Corp.).
The pGL3-SOX6-3′-UTR (wild-type) or pGL3-SOX6-3′-UTR (mutant) (200
ng) and pRL-TK (80 ng; Promega Corp.) constructs were
co-transfected into the cells with 60 pmol miR-342-3p mimic or
miRNA mimic control using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.). Primers were synthesized by Genscript Nanjing
Inc., and the sequences are presented in Table SI. At 24 h following
transfection, the Dual Luciferase Reporter Assay System (Promega
Corp.) was used to measure the luciferase activity and the
luciferase activity was normalized to Renilla luciferase
activity.
Statistical analysis
Statistical analysis was performed using SPSS 25.0
software (IBM Corp.). The data were analyzed using the Student's
t-test or one-way ANOVA followed by Dunnett's multiple post-hoc
tests. All data are presented as the means ± SD of 3 independent
experiments. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
High SOX6 expression is inversely
associated with miR-342-3p expression in mice with DKD
First, to confirm the successful establishment of
the mouse model of DKD, RT-qPCR and western blot analysis were
performed to detect the levels of biomarkers of kidney injury,
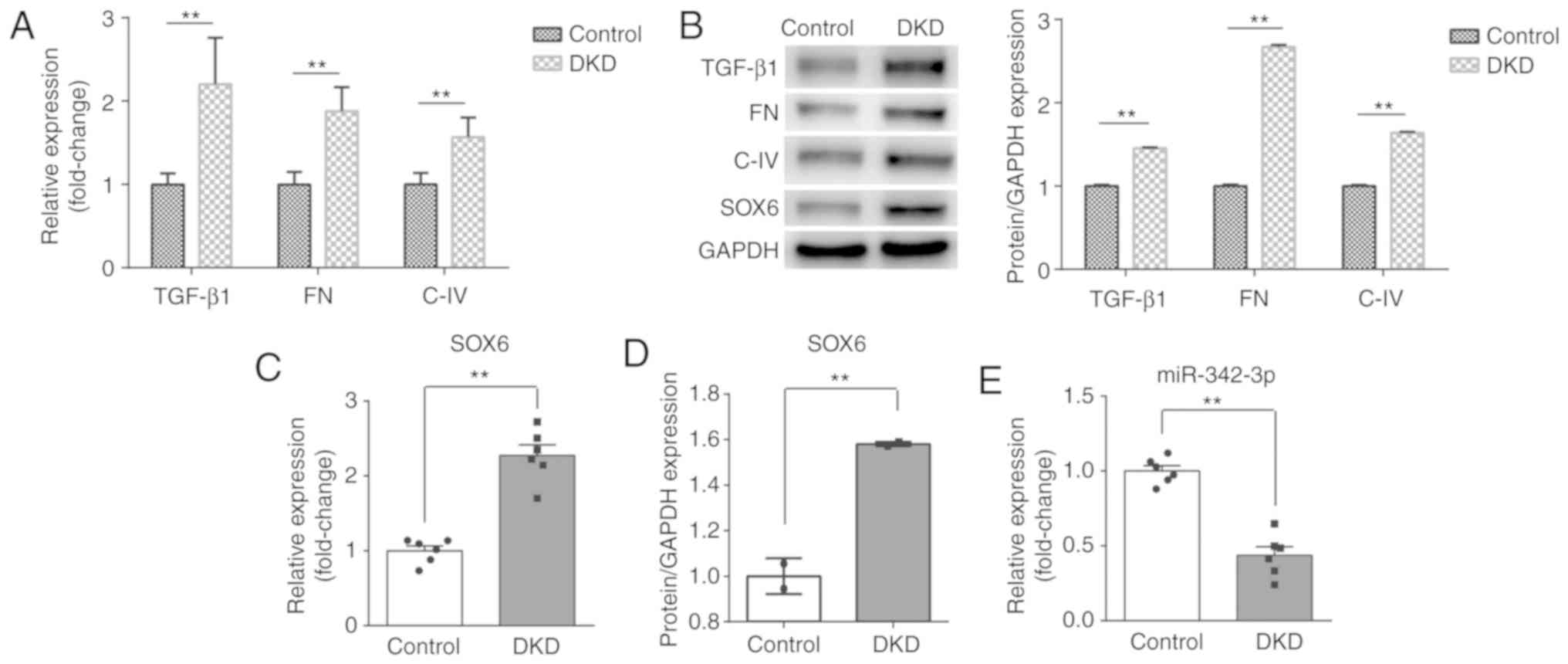
including TGF-β1, FN and C-IV. As shown in Fig. 1A and B, the expression of TGF-β1,
FN and C-IV was significantly increased at both the mRNA and
protein level in the DKD group, as compared with the control group.
Following the successful establishment of the DKD model, the SOX6
and miR-342-3p expression levels were measured in mice with DKD. As
expected, the expression of SOX6 was higher than that in the
control group at both the mRNA and protein level (Fig. 1B-D). However, the expression of
miR-342-3p was inversely associated with that of SOX6 and was
significantly decreased in the DKD group (Fig. 1E).
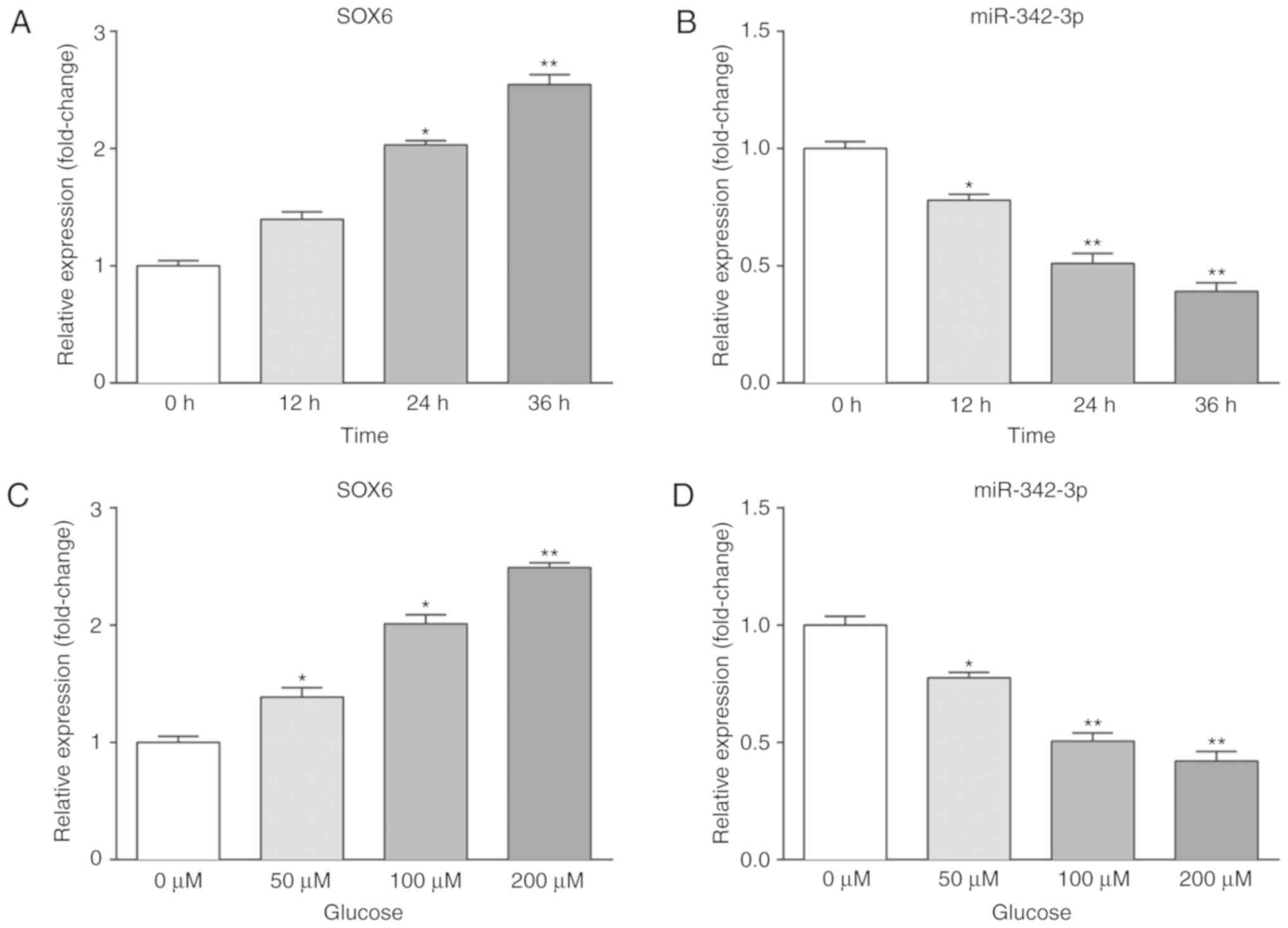
High glucose induces a high SOX6 and low
miR-342-3p expression
We further examined the effects of high glucose on
the expression of SOX6 and miR-342-3p in vitro. MCs were
placed in pre-configured high-glucose DMEM and allowed to grow to
the logarithmic growth phase. To evaluate the time-dependent
effects of high glucose, the MCs were cultured with 100 µM
glucose and harvested at 0, 12, 24 and 36 h. To evaluate the
dose-dependent effects of high glucose, the MCs were cultured with
various concentrations of glucose (0, 50, 100 and 200 µM)
and harvested at 24 h. Subsequently, RT-qPCR and western blot
analysis were performed to measure the expression levels of SOX6
and miR-342-3p. As shown in Fig.
2, SOX6 expression was upregulated, while that of miR-342-3p
was downregulated in the high-glucose environment.
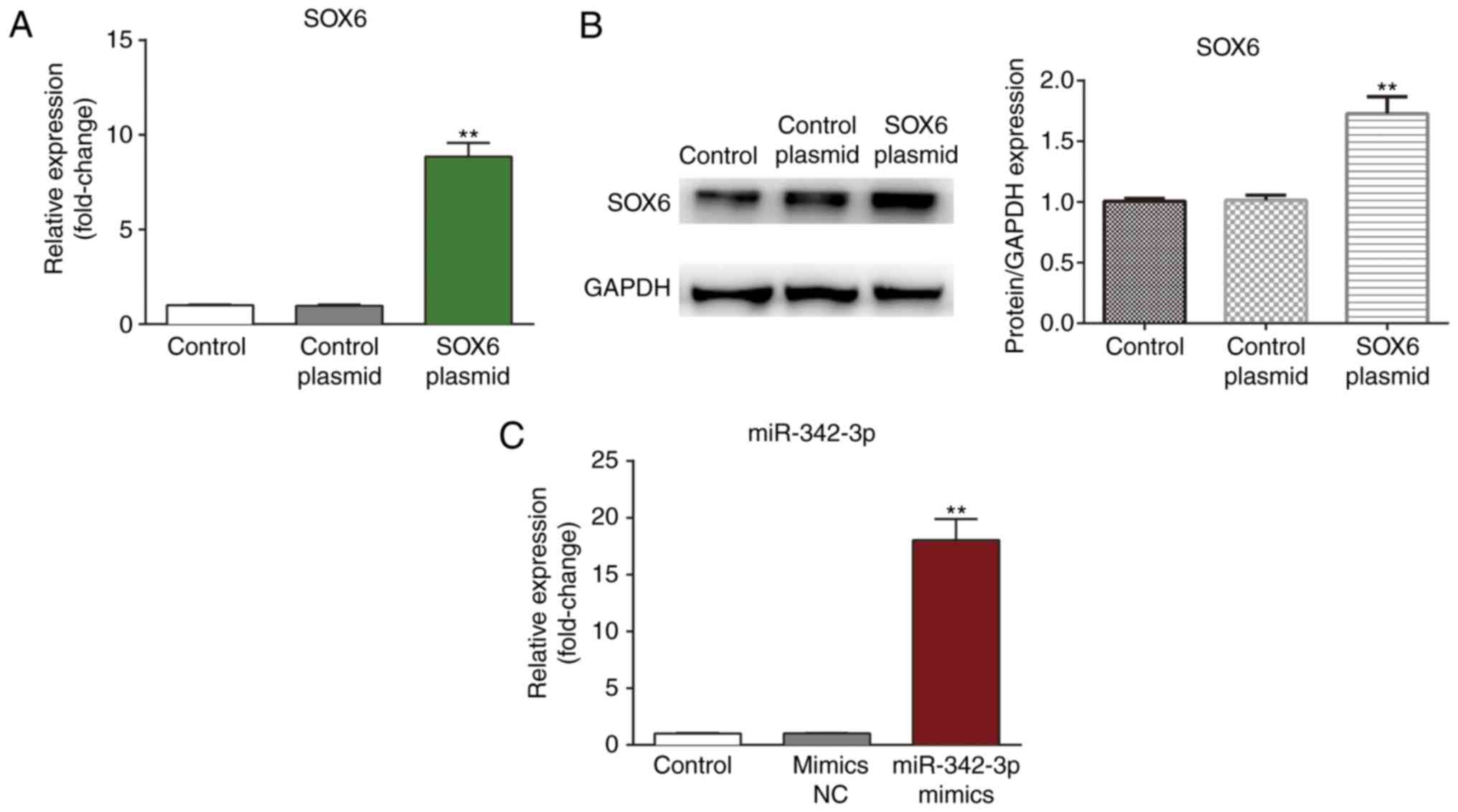
Confirmation of transfection efficiency
in mouse MCs
The MCs were transfected with SOX6 plasmid and
miR-342-3p and the transfection efficiency was confirmed by
measuring mRNA expression by RT-qPCR and protein expression by
western blot analysis (Fig. 3).
As shown in Fig. 3, a notable
increase was observed in SOX6 expression in the MCs at both the
mRNA and protein level following transfection with the SOX6 plasmid
(Fig. 3A and B). In addition, the
expression of miR-342-3p was significantly elevated in the MCs
following transfection with the miR-342-3p mimics.
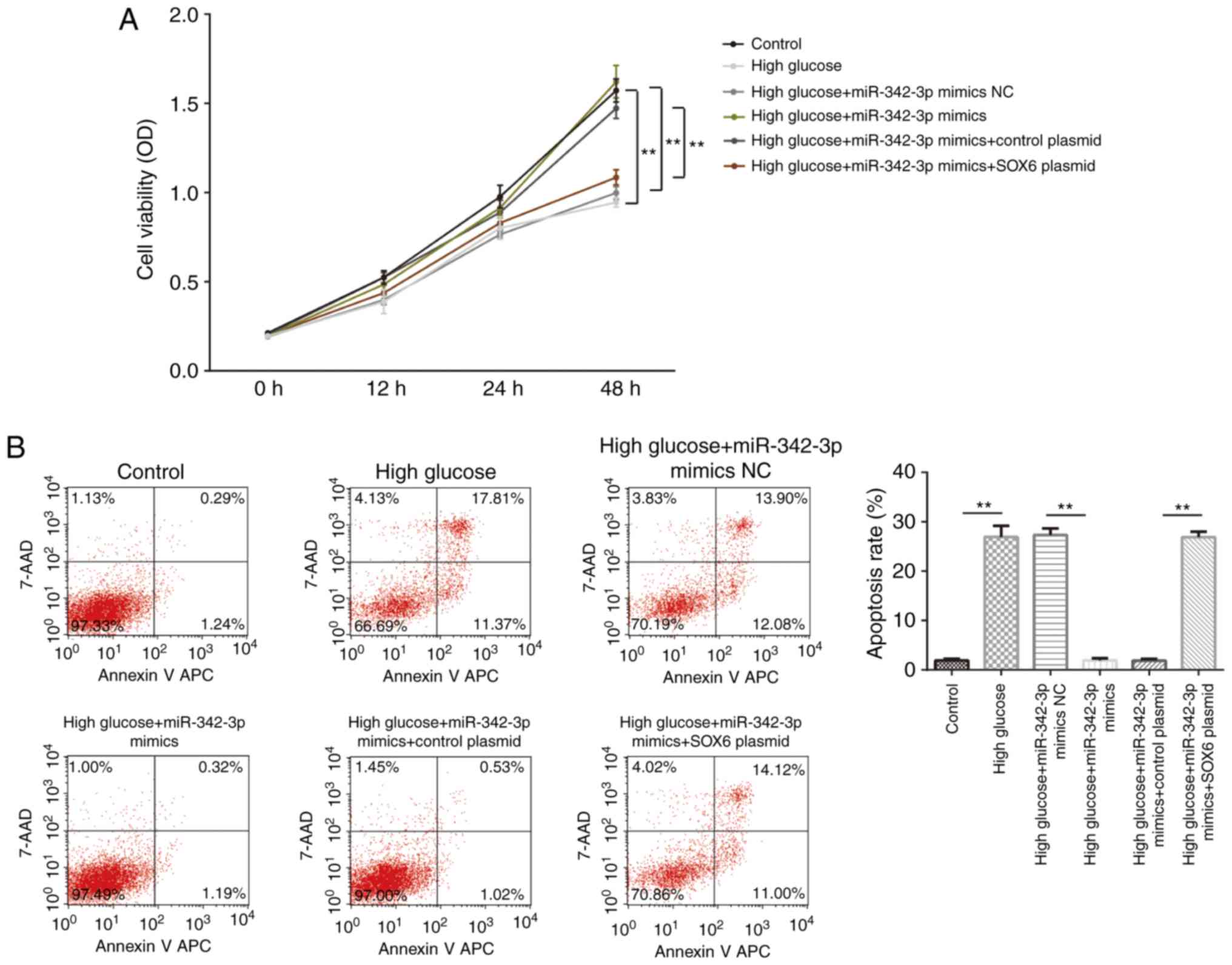
Overexpression of miR-342-3p promotes
cell proliferation and inhibits cell apoptosis
We then evaluated the role of miR-342-3p in the
cellular behavior of MCs. The results of CCK-8 assay revealed that
miR-342-3p overexpression suppressed high glucose-induced cell
proliferation, and this effect was reversed by the exogenous
expression of SOX6 (Fig. 4A).
Furthermore, miR-342-3p overexpression suppressed high
glucose-induced cell apoptosis, and again, this effect was partly
reversed by SOX6 overexpression (Fig.
4B). Collectively, these findings suggested that high
glucose-induced cellular behavioral changes were suppressed by
miR-342-3p overexpression in the MCs.
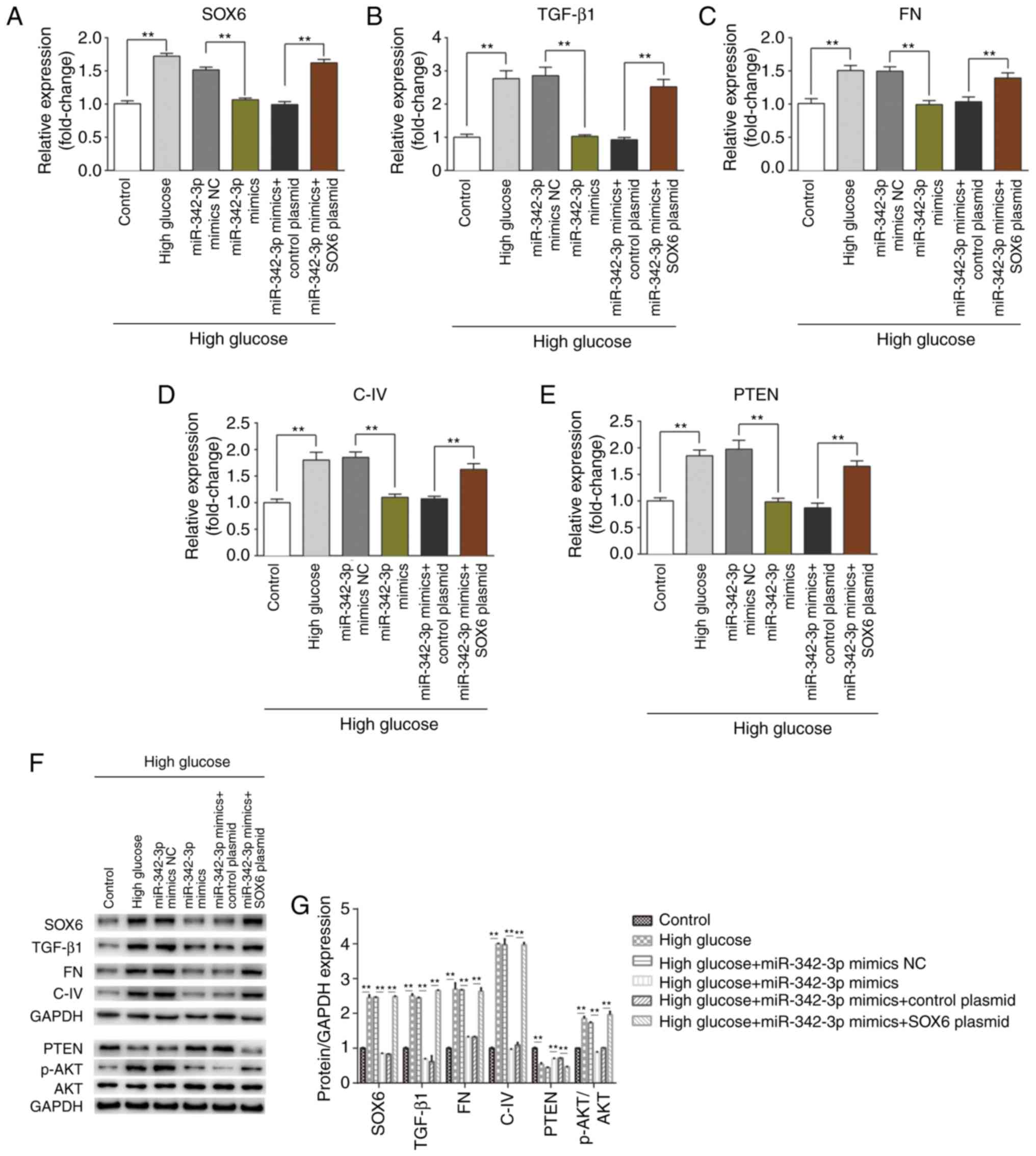
miR-342-3p overexpression suppresses high
glucose-induced renal interstitial fibrosis
Since miR-342-3p expression was downregulated in
mice with DKD and in the MCs cultured under high glucose
conditions, and the overexpression of miR-342-3p inhibited high
glucose-induced cellular behavioral changes, we hypothesized that
miR-342-3p may suppress renal interstitial fibrosis, a crucial
procedure in high glucose-induced kidney injury. RT-qPCR and
western blot analysis were performed to detect the expression
levels of biomarkers of fibrosis (TGF-β1, FN, C-IV and PTEN). As
expected, miR-342-3p overexpression notably suppressed the
expression of TGF-β1, FN, C-IV, and enhanced PTEN expression at
both the mRNA and protein level (Fig.
5); however, SOX6 overexpression partly impaired the
miR-342-3p-induced suppression of renal interstitial fibrosis, as
the levels of the above-mentioned biomarkers increased following
SOX6 overexpression. Overall, these data suggested that miR-342-3p
suppressed renal interstitial fibrosis that involves the
downregulation of SOX6.
miR-342-3p directly targets SOX6
As described above, it was found that a high SOX6
expression was inversely associated with miR-342-3p expression in
the progression of DKD in vivo, and that SOX6 overexpression
partly reversed the protective effects of miR-342-3p on MCs. We
therefore hypothesized that miR-342-3p may directly target SOX6.
The results of bioinformatics analysis using TargetScan revealed
the binding sites between 3′-UTR of SOX6 and miR-342-3p (Fig. 6A). The data indicated that SOX6
was a potential target of miR-342-3p. Subsequenlty, to further
verify the binding of miR-342 and SOX6, luciferase activity assay
was performed by inserting the wild-type and mutant sites of SOX6
into the pGL3 lucif-erase reporter vector. The pGL3 luciferase
reporter vector and miR-342-3p mimics or NC were co-transfected
into the MCs. After 48 h, the fluorescence intensity of the
wild-type group was notably decreased, as compared to that of the
mutant type group (Fig. 6B). The
above-mentioned results demonstrated that SOX6 is the target gene
of miR-342-3p.
Discussion
To date, multi-factor intervention controlling blood
glucose, blood lipids and blood pressure, including drugs that
block renin angiotensin, remains the main treatment option for
diabetic nephropathy (12,13).
However, with the development of molecular biology techniques, the
therapeutic value of molecular targets in diabetic nephropathy is
gradually becoming evident. SOX6, an important member of the SOX
family of transcription factors, has been shown to limit the growth
and invasion of tumor cells (14-16). However, studies focusing on the
function of SOX6 in the progression of DKD are limited. Herein, a
high expression of SOX6 was observed in the kidneys of mice with
DKD, and SOX6 expression was found to be inversely associated with
miR-342-3p expression. Cellular and molecular assays revealed that
SOX6 suppressed the miR-342-3p-induced inhibition of DKD
progression.
The dysregulated expression of miRNAs has been shown
to be involved in various mechanisms of DKD progression (6,12,17,18). miR-342-3p has been reported to
participate in the progression of DKD (19). However, the potential mechanisms
of action of miR-342-3p in DKD have not been fully assessed. In the
present study, it was found that the expression of miR-342-3p was
significantly decreased in mice with DKD. The pathological changes
of diabetic nephropathy mainly include the abnormal proliferation
of MCs and the excessive accumulation of extracellular matrix (ECM)
components. MCs have been widely used for the study of diabetic
nephropathy in vitro (20-22). For example, Xiang et al
suggested that fork-head box protein P1 (FOXP1) prevented high
glucose-induced oxidative stress and ECM accumulation in MCs by
inhibiting the activation of the Akt/mammalian target of rapamycin
(mTOR) signaling pathway; thus, FOXP1 may be a therapeutic target
for the treatment of diabetic nephropathy (20). Li et al reported that
miR-379-5p agomir suppressed MC proliferation and the accumulation
of ECM components by regulating the LIN28B/let-7 pathway,
indicating that miR-379-5p may be a potential effective therapeutic
target for DN (21). Zhang et
al also indicated that long non-coding RNA Rpph1 promoted
inflammation and the proliferation of MCs cells in diabetic
nephropathy via an interaction with Gal-3 (22). Thus, mouse renal MCs were used for
the study of diabetic nephropathy in vitro in the present
study. Following the in vitro experiments performed to
create a high glucose environment, it was revealed that miR-342-3p
inhibited SOX6 expression, promoted cell proliferation, and
inhibited the apoptosis of the MCs. Moreover, the overexpression of
miR-342-3p also suppressed high glucose-induced renal interstitial
fibrosis. These biochemical and molecular biological findings
suggest that miR-342-3p is likely to function as an inhibitor of
the progression of DKD.
In this study, a high SOX6 expression was found to
be inversely associated with miR-342-3p expression in the
progression of DKD in vivo, and SOX6 overexpression was
shown to partly reverse the miR-342-3p-induced protective effects
on MCs. The TargetScan database (http://www.targetscan.org) indicated that SOX6 is a
potential target of miR-342-3p (Fig.
S1). We therefore hypothesized that SOX6 is the target gene of
miR-342-3p. Based on the assumption that miR-342-3p directly
targets SOX6, a dual-luciferase activity assay was carried out to
confirm the targeted association between miR-342-3p and SOX6. Using
dual-luciferase activity assays, SOX6 was identified as a target
gene of miR-342-3p. The targeting association between miR-342-3p
and SOX6 represents a key step in an unknown mechanism of DKD
progression. SOX6 is a transcription factor that may bind with the
promoters of TGF-β1, FN, C-IV and PTEN and regulate the
transcription of the aforementioned genes (TGF-β1, FN, C-IV and
PTEN). Herein, it was revealed that miR-342-3p targets SOX6 and
downregulates its expression. miR-342-3p may regulate the
expression of TGF-β1, FN, C-IV and PTEN via SOX6. However, in the
present study, we did not examine the role of SOX6 in cell
proliferation, apoptosis and extracellular matrix in MCs cultured
under high glucose conditions, which would have made our
conclusions more convincing. This may be a limitation of the
present study.
In conclusion, the present study demonstrated that
miR-342-3p exerts an inhibitory effect on the progression of DKD
in vitro and in vivo. Moreover, miR-342-3p inhibits
the progression of renal interstitial fibrosis by targeting SOX6.
Overall, the results of the present study revealed that the
miR-342-3p/SOX6 axis may serve as a potential therapeutic target
for DKD. However, this study is only a preliminary study of the
role of miR-342-3p in diabetic nephropathy. In order to confirm our
findings on the role of miR-342-3p in diabetic nephropathy, further
extensive in-depth studies are required. For example, the role of
SOX6 alone in MCs cultured under high glucose conditions also
warrants investigation. The role of the miR-342-3p/SOX6 axis in
diabetic nephropathy in vivo also requires further analysis.
Furthermore, there is a vast difference between the conditions in
in vitro studies and in real-life human diabetic
nephropathy. Thus, we aim to perform further studies to elucidate
these issues in the future.
Supplementary Data
Acknowledgments
Not applicable.
Funding
This study was supported by grants from the National
Science Foundation for Young Scholars of Tianjin Medical University
(grant no. 2011KY24; Tianjin, China), the Natural Science
Foundation of China (grant no. 81600628; China) and the Natural
Science Foundation of Tianjin (grant no. 16JCYBJC25700; Tianjin,
China).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZHJ contributed to the study design, data
collection, statistical analysis, data interpretation and
manuscript preparation. YZT HNS, MY and BL contributed to data
collection and statistical analysis. CLN contributed to the study
design, data collection and manuscript preparation. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Tianjin Medical University (Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lu Y, Stamm C, Nobre D, Pruijm M, Teta D,
Cherpillod A, Halabi G, Phan O, Fumeaux Z, Bullani R, et al:
Changing trends in end-stage renal disease patients with diabetes.
Swiss Med Wkly. 147:w144582017.PubMed/NCBI
|
|
2
|
Rahimi Z: The role of renin angiotensin
aldosterone system genes in diabetic nephropathy. Can J Diabetes.
40:178–183. 2016. View Article : Google Scholar
|
|
3
|
Xiong H, Yan T, Zhang W, Shi F, Jiang X,
Wang X, Li S, Chen Y, Chen C and Zhu Y: miR-613 inhibits cell
migration and invasion by downregulating Daam1 in triple-negative
breast cancer. Cell Signal. 44:33–42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beltrami C, Simpson K, Jesky M, Wonnacott
A, Carrington C, Holmans P, Newbury L, Jenkins R, Ashdown T, Dayan
C, et al: Association of elevated urinary miR-126, miR-155, and
miR-29b with diabetic kidney disease. Am J Pathol. 188:1982–1992.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang WB, Zheng L, Yan R, Yang J, Ning J,
Peng L, Zhou Q and Chen L: miR302a-3p may modulate renal
epithelial-mesen-chymal transition in diabetic kidney disease by
targeting ZEB1. Nephron. 138:231–242. 2018. View Article : Google Scholar
|
|
7
|
Hamada-Kanazawa M, Ogawa D, Takano M and
Miyake M: Sox6 suppression induces RA-dependent apoptosis mediated
by BMP-4 expression during neuronal differentiation in P19 cells.
Mol Cell Biochem. 412:49–57. 2016. View Article : Google Scholar :
|
|
8
|
Han Y, Xu H, Cheng J, Zhang Y, Gao C, Fan
T, Peng B, Li B, Liu L and Cheng Z: Downregulation of long
non-coding RNA H19 promotes P19CL6 cells proliferation and inhibits
apoptosis during late-stage cardiac differentiation via
miR-19b-modulated Sox6. Cell Biosci. 6:582016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iguchi H, Urashima Y, Inagaki Y, Ikeda Y,
Okamura M, Tanaka T, Uchida A, Yamamoto TT, Kodama T and Sakai J:
SOX6 suppresses cyclin D1 promoter activity by interacting with
beta-catenin and histone deacetylase 1, and its down-regulation
induces pancreatic beta-cell proliferation. J Biol Chem.
282:19052–19061. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pleskovič A, Šantl Letonja M, Cokan
Vujkovac A, Kruzliak P and Petrovič D: SOX6 gene polymorphism
(rs16933090) and markers of subclinical atherosclerosis in patients
with type 2 diabetes mellitus. Int Angiol. 35:552–556. 2016.
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
12
|
Liu F, Zhang ZP, Xin GD, Guo LH, Jiang Q
and Wang ZX: miR-192 prevents renal tubulointerstitial fibrosis in
diabetic nephropathy by targeting Egr1. Eur Rev Med Pharmacol Sci.
22:4252–4260. 2018.PubMed/NCBI
|
|
13
|
Rossing P, Persson F and Frimodt-Møller M:
Prognosis and treatment of diabetic nephropathy: Recent advances
and perspectives. Nephrol Ther. 14(Suppl 1): S31–S37. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Zheng X, Chen LJ, Xu B and Jiang
JT: microRNA-181b suppresses the metastasis of lung cancer cells by
targeting sex determining region Y-related high mobility group-box
6 (Sox6). Pathol Res Pract. 215:335–342. 2019. View Article : Google Scholar
|
|
15
|
Jiang W, Yuan Q, Jiang Y, Huang L, Chen C,
Hu G, Wan R, Wang X and Yang L: Identification of Sox6 as a
regulator of pancreatic cancer development. J Cell Mol Med.
22:1864–1872. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kurtsdotter I, Topcic D, Karlén A, Singla
B, Hagey DW, Bergsland M, Siesjö P, Nistér M, Carlson JW, Lefebvre
V, et al: SOX5/6/21 prevent oncogene-driven transformation of brain
stem cells. Cancer Res. 77:4985–4997. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia Y, Zheng Z, Guan M, Zhang Q, Li Y,
Wang L and Xue Y: Exendin-4 ameliorates high glucose-induced
fibrosis by inhibiting the secretion of miR-192 from injured renal
tubular epithelial cells. Exp Mol Med. 50:562018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xue M, Cheng Y, Han F, Chang Y, Yang Y, Li
X and Chen L, Lu Y, Sun B and Chen L: Triptolide attenuates renal
tubular epithelial-mesenchymal transition via the
MiR-188-5p-mediated PI3K/AKT pathway in diabetic kidney disease.
Int J Biol Sci. 14:1545–1557. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Assmann TS, Recamonde-Mendoza M, de Souza
BM, Bauer AC and Crispim D: MicroRNAs and diabetic kidney disease:
Systematic review and bioinformatic analysis. Mol Cell Endocrinol.
477:90–102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiang H, Xue W, Wu X, Zheng J, Ding C, Li
Y and Dou M: FOXP1 inhibits high glucose-induced ECM accumulation
and oxidative stress in mesangial cells. Chem Biol Interact.
313:1088182019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li N, Wang LJ, Xu WL, Liu S and Yu JY:
MicroRNA-379-5p suppresses renal fibrosis by regulating the
LIN28/let-7 axis in diabetic nephropathy. Int J Mol Med. 2019.
View Article : Google Scholar
|
|
22
|
Zhang P, Sun Y, Peng R, Chen W, Fu X,
Zhang L, Peng H and Zhang Z: Long non-coding RNA Rpph1 promotes
inflammation and proliferation of mesangial cells in diabetic
nephropathy via an interaction with Gal-3. Cell Death Dis.
10:5262019. View Article : Google Scholar : PubMed/NCBI
|