Introduction
Natural products are valuable resources for the
screening of anti-cervical cancer drugs, and include compounds such
as artesunate (1), resveratrol
(2), betulinic acid (3) and Timosaponin A-III (4). Reineckia carnea (Liliaceae),
a traditional Chinese herb containing the saponin (1β, 3β, 5β,
25S)-spirostan-1, 3-diol
1-[α-L-rhamnopyranosyl-(1→2)-β-D-xylopyranoside] (RCE-4; Fig. 1A) as a biologically active
component, has been used to treat diseases including rheumatism,
coughs and hepatitis (5,6). To the best of our knowledge, the
steroidal saponins primarily exert anti-inflammatory and antitumor
activity, with toxicity towards both normal and tumor cells
(7). However, RCE-4 exhibited a
significant anti-inflammatory (8)
and notable cytotoxic effects on cancer CaSki, HeLa, HT-29 and
CNE-2 cell lines, whereas it exerted relatively weak cytotoxic
effects in normal Marc-145 and MDCK cells (9). Furthermore, CaSki cell xenograft
experiments using nude mice demonstrated that RCE-4 inhibited tumor
growth and had notably low levels of toxicity in normal tissues,
including those of the liver and the uterus (10).
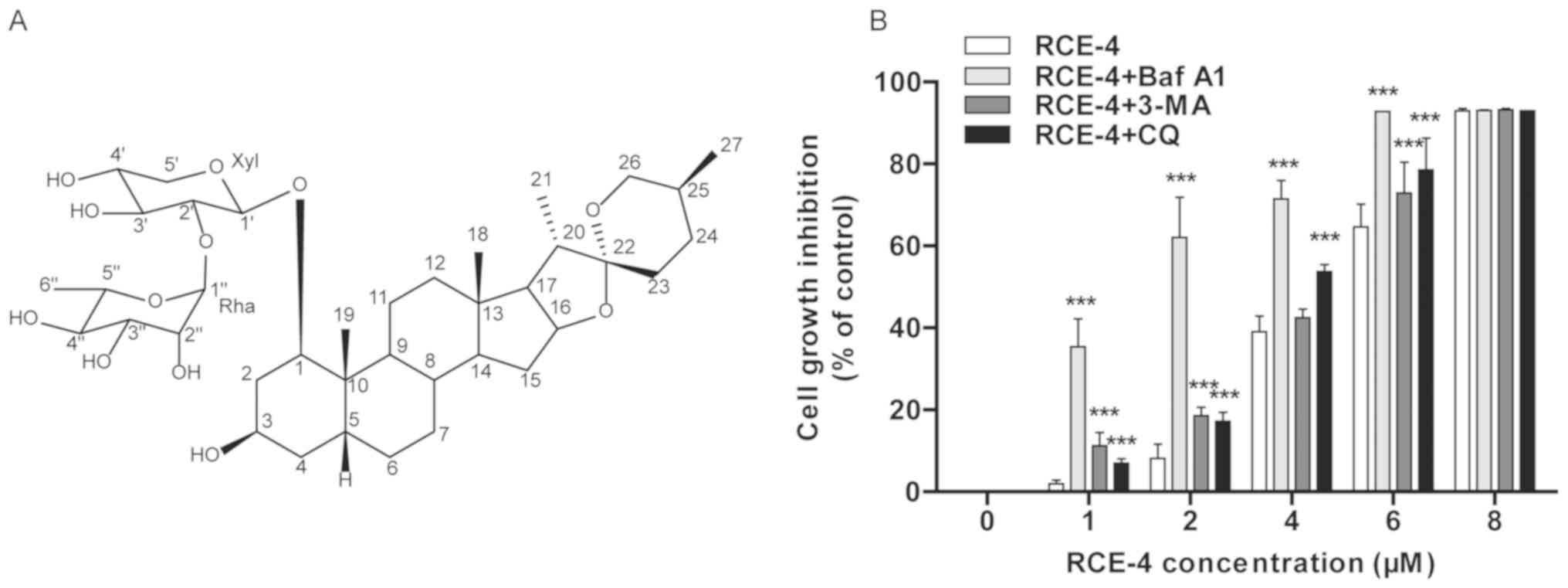 | Figure 1Autophagy inhibitors 3-MA, CQ and Baf
A1 enhance the ability of RCE-4 to inhibit CaSki cell
proliferation. (A) Chemical structure of RCE-4. (B) CaSki cells
were treated with RCE-4 for 48 h, following pretreatment with
autophagy inhibitors for 6 h, and proliferation inhibition was
assessed by MTT assay. Values for each RCE-4 concentration were
generated from 3 independent experiments, and are presented as the
mean ± standard deviation. ***P<0.001 vs. control
group (the corresponding concentration of RCE-4 without autophagy
inhibitor treatment). 3-MA, 3-methyladenine; CQ, chloroquine; Baf
A1, bafilomycin A1; RCE-4, (1β, 3β, 5β, 25S)-spirostan-1, 3-diol
1-[α-L-rhamnopyranosyl-(1→2)-β-D-xylopyranoside]. |
Drug-induced tumor-cell death is a complex process
that includes various modes of programmed cell death, including
apoptosis, autophagy and necrosis (11). Cross-talk has been observed
between autophagy (type II programmed cell death), a process in
which newly formed membrane-encapsulated vesicles phagocytose and
consume cellular components including damaged organelles and
misfolded protein aggregates, and apoptosis, which share various
cell death mediators, such as Bcl-2 and Beclin-1 (12). Normally, autophagic
self-degradation not only removes damaged organelles and misfolded
proteins (13,14), but also recycles nutrients.
However, deregulation of autophagy may induce tumor cell death, and
may therefore serve as a cell survival pathway to inhibit
apoptosis, either in combination with apoptosis or as a secondary
mechanism when the former is ineffective (15-17). Mechanistically, numerous studies
have shown that the PI3K/AKT and Ras/Raf/dual specificity
mitogen-activated protein kinase kinase (MEK)/ERK pathways serve an
important role in autophagy by inhibiting the expression of mTOR,
which is a key homeostatic regulator of cellular proliferation
(18). In addition, the
AMP-activated protein kinase (AMPK) signaling pathway, which is
closely associated with energy metabolism, has also been indicated
to activate autophagy (19-21).
Conversely, cross-talk between the Ras/Raf/MEK/Erk
and PI3K/Akt pathways in regulating tumor cell proliferation,
differentiation, apoptosis and senescence has been identified. This
cross-talk may provide the focus for further studies investigating
drug therapies that inhibit both signaling networks (22-24). Specifically, activation of the
PI3K/Akt pathway promotes cell survival by inhibiting cell cycle
progression; additionally, high activity levels of Erk, which is
regulated by the Ras/Raf/MEK/Erk pathway, promote cyclin D1
expression and cellular proliferation. Moreover, activation of the
PI3K/Akt or Ras/Raf/MEK/Erk pathways inhibits apoptosis by
phosphorylating and blocking apoptosis-associated targets including
Bcl-2, Bad and caspase-9 (3,25).
These studies indicate that the ERK and the PI3K pathways promote
cell proliferation and survival. However, inhibitors of each single
pathway have limited anti-tumor activity, as the inhibition of one
pathway results in the compensatory activation of the other
(18). This may also partially
explain why cancer treatment often results in resistance to
chemotherapeutic drugs. For example, the MEK inhibitor PD98059
blocks the ERK pathway, leading to an abnormal increase in AKT
activation levels (26). In
addition, in breast cancer cells, the activity of an PI3K inhibitor
was demonstrated to be enhanced, resulting in a decreased level of
proliferation and enhanced anti-tumor activity by co-treatment with
an ERK pathway inhibitor (27).
These examples suggest that simultaneous inhibition of the ERK and
PI3K pathways in the treatment of certain tumor cells may
contribute to the improved anti-tumor activity of chemotherapeutic
drugs.
In previous studies, RCE-4, as a potential
anti-cervical cancer chemotherapy drug, was demonstrated to induce
mitochondria-mediated apoptosis, which was considered to be the
mechanism for inhibiting the proliferation of CaSki and HeLa cells
(5,9). The aim of the present study was to
clarify whether RCE-4-induced autophagy is a survival or death
mechanism during cervical cancer treatment. Subsequently, the
levels of autophagy were assessed by treating cells with different
concentrations of RCE-4, and to determine the degree of autophagy
at different treatment exposure times. Given the key roles of the
AMPK, PI3K and ERK pathways in cancer cell proliferation and
autophagy, it was necessary to additionally identify whether RCE-4
treatment affected the expression and phosphorylation statuses of
proteins involved in these pathways. Collectively, the aim of the
present study was to improve the understanding of the anti-tumor
mechanisms of RCE-4.
Materials and methods
Reagents and antibodies
The RCE-4 preparation used in the present study was
isolated from R. carnea and its structure is demonstrated in
Fig. 1A. A 50-mM stock solution
of purified RCE-4 was prepared in DMSO, and diluted to the desired
concentration with RPMI-1640 medium prior to use. Rabbit monoclonal
antibodies against β-actin (cat. no. 4970), AMPKα (cat. no. 5831),
phosphorylated (p)-AMPKα (cat. no. 2535), the
serine/threonine-protein kinase ULK1 (ULK1; cat. no. 8054), p-ULK1
(cat. no. 14202), Beclin-1 (cat. no. 3495), p-Beclin-1 (cat. no.
14717), microtubule-associated proteins 1A/1B light chain 3B (LC3;
cat. no. 12741), sequestosome 1 (p62; cat. no.8052), mTOR (cat. no.
2983), p-mTOR (cat. no. 5536), PI3K (cat. no. 4257), p-PI3K (cat.
no. 4228), Akt (cat. no. 4691), p-Akt (cat. no. 4060), Ras (cat.
no. 3339), c-Raf (cat. no. 9422), p-c-Raf (cat. no. 9431), MEK1/2
(cat. no. 8727), p-MEK1/2 (cat. no. 9154), p-Erk1/2 (cat. no. 4370)
and Erk1/2 (cat. no. 4695), all purchased from Cell Signaling
Technology, Inc., were used at a dilution of 1:1,000. The
horseradish peroxidase-labeled secondary antibody (anti-rabbit
IgG), which was purchased from Cell Signaling Technology, Inc.
(cat. no. 7074V), was used at a dilution of 1:5,000. The CYTO-ID
Autophagy Detection kit (cat. no. ENZ-51031) was purchased from
Enzo Life Sciences Inc., and the autophagy inhibitors bafilomycin
A1 (Baf A1), 3-methyladenine (3-MA) and chloroquine (CQ) were
obtained from MedChemExpress. The monomeric red fluorescent protein
(mRFP)-green fluorescent protein (GFP)-LC3 tandem fluorescent
protein kit was purchased from Han heng Biotechnology Co., Ltd.
Cell culture
The human cervical cancer CaSki cell line was
obtained from the Cell Bank of the Shanghai Institutes for
Biological Sciences, Chinese Academy of Sciences. The cells were
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum (FBS: Gibco; Thermo Fisher
Scientific, Inc.) and 0.2% HEPES at 37°C and 5% CO2.
Growth inhibitory assays
CaSki cells (2×104 cells/well) were
seeded into 96-well plates, incubated at 37°C for 12 h and
subsequently treated with 1, 2, 4, 6 and 8 µM RCE-4 for an
additional 48 h. For the autophagy inhibition assay, the CaSki
cells were pretreated with the selective autophagy inhibitors 3-MA
(8 mM), CQ (30 µM) and Baf A1 (0.4 µM) for 6 h, and
then treated with RCE-4 (0, 1, 2, 4, 6 and 8 µM) for an
additional 48 h. Subsequently, 20 µl 5 mg/ml MTT
(Sigma-Aldrich; Merck KGaA) reagent was added to each well, and the
cells were cultured for another 4 h; the medium was gently removed,
and the adherent cells were lysed in 150 µl DMSO solution
for 10 min with shaking. The absorbance at 490 nm was measured
using a microplate reader (Tecan Group, Ltd.), and the inhibition
rate was calculated according to the following formula: Inhibition
rate = [1 - optical density (OD) value of the administration
group/OD value of blank control group] x 100%.
CYTO-ID autophagy detection assay
The CYTO-ID Autophagy Detection kit was used to
selectively label pre-autophagosomes, autophagosomes and
autolysosomes; bright fluorescence is detectable in autophagic
vacuoles, thereby providing a quantitative method for autophagy
detection (28). The CYTO-ID
autophagy detection assay was performed according to the
manufacturer's kit protocol. CaSki cells were treated with RCE-4 at
concentrations of 0, 8, 12 and 16 µM for 6, 12 and 24 h
each. Following the collection of the adherent cells and those in
solution, the cells were resuspended in 1X assay buffer (provided
by the CYTO-ID Autophagy Detection kit) and centrifuged (1,000 × g)
at room temperature for 5 min to remove residual supernatant
impurities, including cell growth medium and serum. After carefully
removing the buffer, cells were suspended and stained with 500
µl of 0.1% (v/v) CYTO-ID Green staining solution (1
µl CYTO-ID Green Detection Reagent diluted with 1 ml 1X
Assay buffer) for 30 min at room temperature in the dark. After
treatment, stained cells were collected via centrifugation and
washed with 1X Assay Buffer. The cell pellets were then resuspend
in 500 µl of fresh 1X Assay Buffer to analyze autophagy
status. To detect autophagic vesicles, the green fluorescence
emission (530 nm) of ≥1×104 cells was detected using
flow cytometry (BD FACSVerse; BD Biosciences), and the data were
analyzed using BD FACSuite™ software (version 1.0.0.1477; BD
Biosciences).
Assessment of autophagic flux
To determine the autophagy levels in living cells,
mRFP-GFP-LC3 adenoviruses or plasmids have been widely used to
identify autophagosomes and autoly-sosomes (29,30). However, this method is only able
to indicate a specific point within the process, rather than the
autophagic status of the cells at different points following drug
treatment. Following culture of the mRFP-GFP-LC3
adenovirus-transfected CaSki cells in cell culture dishes (Wuxi
NEST Biotechnology Co., Ltd.) for 6 h at a multiplicity of
infection of 200, 12 µM RCE-4 was added for an additional 6,
12, 24 or 30 h. The fluorescence values of the green dots,
indicating autophagosomes, and red dots, indicating autophagosomes
and autolysosomes, were observed using laser confocal fluorescence
microscopy (magificatin, ×1,000; LCFM; Olympus FV1200; Olympus
Corporation). The number of red and yellow dots (indicating
autophagosomes) in the merged images was then recorded.
Western blot analysis
CaSki cells were exposed to RCE-4 at a concentration
of 0, 8, 12 and 16 µM for 6, 12 or 24 h. Following
incubation, the cells lysed in radioimmunoprecipitation assay
buffer (containing 1% phosphorylated protease inhibitor and 1%
PMSF) were centrifuged (12,000 × g) at 4°C for 10 min, and the
total protein was quantified using a bicinchoninic (BCA) protein
concentration acid assay kit (Beyotime Institute of Biotechnology).
The protein samples were adjusted to a uniform concentration with
5X buffer and water, and denatured by boiling at 100°C for 10 min.
The proteins (60 µg/well) were separated using SDS-PAGE with
a 6-15% polyacrylamide gel, and transferred onto a PVDF membrane
(EMD Millipore). Following blocking at room temperature for 2 h
with 5% skim milk, the membrane was incubated with the
corresponding primary antibodies at 4°C overnight. The membrane was
washed 5 times with TBS + 0.05% Tween-20 (TBST) and the secondary
antibody was subsequently added prior to further incubation for 1.5
h at 37°C. An enhanced chemiluminescent kit (Beyotime Institute of
Biotechnology) was used to visualize the protein bands, and the
results were recorded with Kodak Film or a Luminescence Imaging
System (Tanon 5200; Tanon Science and Technology Co., Ltd.). The
grey values were analyzed with β-actin as a loading control.
Densitometry was analyzed using Image J version 2.1 software
(National Institutes of Health).
Statistical analysis
The data are expressed as the mean ± standard
deviation. GraphPad prism v.5.0 (GraphPad Software, Inc.) was used
for all statistical analyses, and the differences between the
treatment groups and control group were assessed by one-way
analysis of variance followed by Dunnett's post hoc tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Autophagy inhibition increases the level
of RCE-4-induced CaSki cell death
To investigate the effects of RCE-4 on the growth of
human cervical cancer cells, an MTT assay was performed using CaSki
cells. The results revealed that RCE-4 treatment significantly
decreased the viability of CaSki cells after 48 h of treatment,
which was compared with an RCE-4-untreated control group (Fig. 1B). In addition, the RCE-4-induced
inhibition was enhanced following pretreatment with the autophagy
inhibitors 3-MA, CQ or Baf A1 for 6 h. The half maximal inhibitory
concentration values were 4.18, 3.36, 3.32 and 1.54 µM for
the RCE-4 control, the 3-MA+RCE-4, the CQ+RCE-4 and the Baf
A1+RCE-4 groups, respectively. It was demonstrated that the
inhibition of autophagy significantly sensitized CaSki cells to
RCE-4-induced cell death, inferring that RCE-4 activated autophagy
in anti-cervical cancer cells (Fig.
1B).
RCE-4 triggers autophagy in cervical
cancer cells
Using the CYTO-ID autophagy assay, mRFP-GFP-LC3
tandem fluorescent protein kit and western blot analysis, RCE-4 was
determined to induce autophagy in CaSki cells. The CYTO-ID
autophagy assay kit was used to demonstrate the presence of
autophagosomes in RCE-4 treated CaSki cells; the results indicated
an increase in the formation of autophagosomes following treatment
with 8, 12 and 16 µM RCE-4 for 6, 12 and 24 h (Fig. 2A).
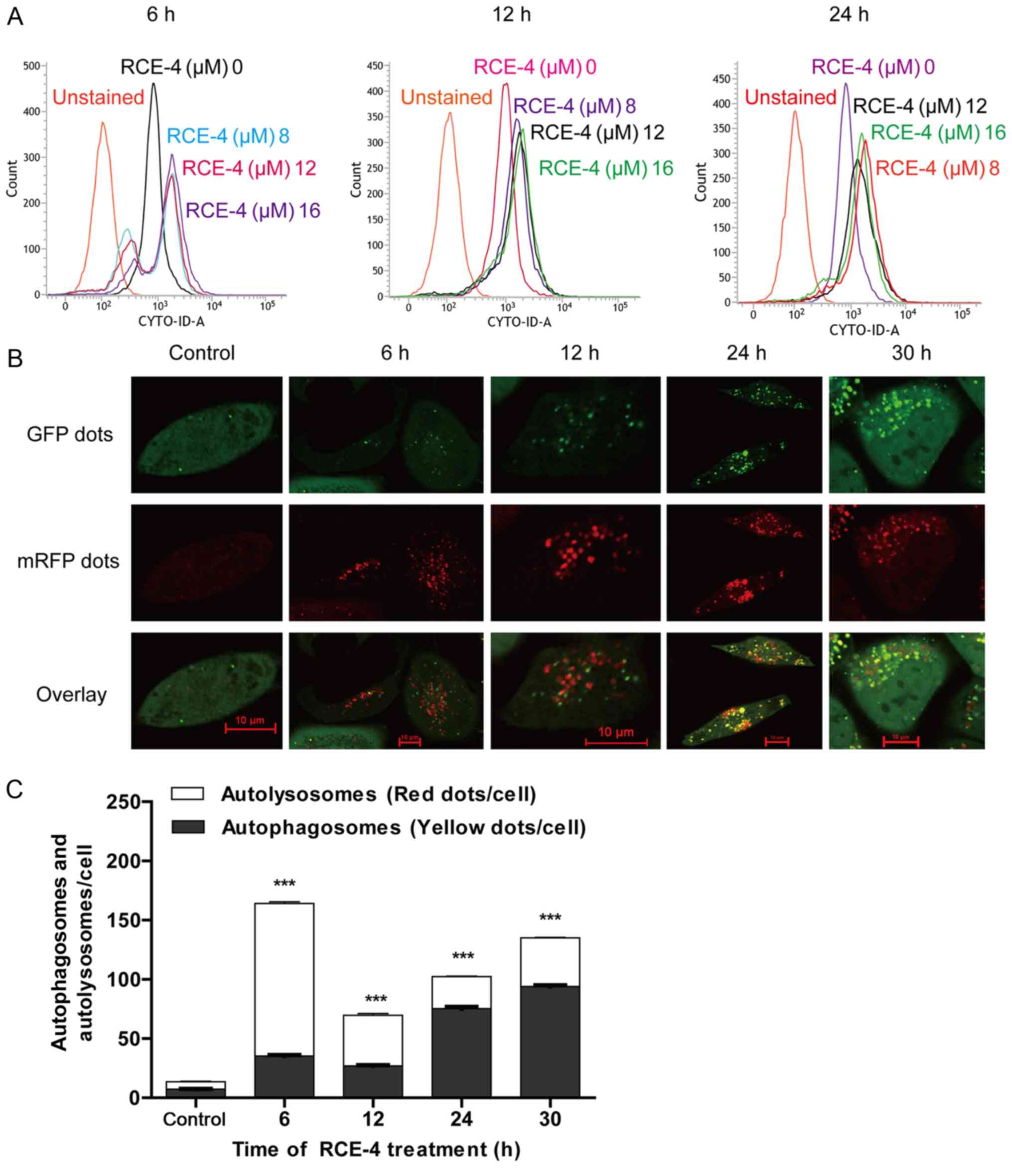 | Figure 2RCE-4 promotes autophagy in CaSki
cells. (A) CYTO-ID histograms of RCE-4-treated cells indicated a
shift to the right in comparison with the RCE-4-untreated group
(control group) after an exposure period of 6, 12 or 24 h,
indicating an increase in the mean fluorescence intensity of the
RCE-4-treated group. (B) Cells were transfected with mRFP-GFP-LC3
adenoviruses for 6 h, and treated with RCE-4 for a further 6, 12,
24 or 30 h. The formation of autophagosomes (yellow dots) and
autolysosomes (red dots) in each cell was observed and quantified
by laser confocal fluorescence microscopy. A total of 50 cells for
each condition were counted. Scale bars=10 µm. (C) Changes
in the number and percentage of autophagosomes and autolysosomes in
the control and RCE-4-treated groups are presented at different
RCE-4 treatment times. ***P<0.001 vs. RCE-4-untreated
group. GFP, green fluorescent protein; mRFP, monomeric red
fluorescent protein; RCE-4, (1β, 3β, 5β, 25S)-spirostan-1, 3-diol
1-[α-L-rhamnopyranosyl-(1→2)-β-D-xylopyranoside]. |
The location and number of autophagosomes and
autolysosomes in RCE-4-treated cells was visualized using LCFM
after CaSki cell transfection with mRFP-GFP-LC3 adenoviruses. mRFP
is stably present in autolysosomes in an acidic environment, while
GFP fluorescence is easily quenched. The emission of red and green
fluorescence from one cell was recorded and merged; consequently,
yellow dots, indicating autophagosomes, and red dots, indicating
autolysosomes, appear in the merged images. When CaSki cells were
treated with RCE-4 at a concentration of 12 µM for 6 or 12
h, an increased percentage of red fluorescent dots in the combined
images was observed compared with the control group, representing
autophagy activation. Conversely, when treated for 24 or 30 h,
autophagy may have been inhibited, based on a decreased percentage
of red fluorescent dots (Fig. 2B and
C).
As indicated in Fig.
3, RCE-4 increased ULK1, p-ULK1, p-Beclin-1 and lipid-modified
LC3 (LC3II) expression levels, and downregulated p62 protein
expression levels when used for 12 h, compared with the control
group. There were no significant alterations between the expression
levels of autophagy-associated proteins, with the exception of p62,
in CaSki cells treated with RCE-4 for 6 and 12 h. Upregulation of
the p62 expression level may be due to a compensatory increase in
the number of autophagosomes at the initial stages of autophagy
(31). Furthermore, following
treatment with RCE-4 for 24 h, the expression levels of ULK1 and
p-Beclin-1 were downregulated, and that of p62 was upregulated,
which further supported the inhibition of autophagy after prolonged
treatment. Notably, it was expected that RCE-4 would induce an
upregulation in the expression levels of Beclin-1, as it promotes
autophagy; however, Beclin-1 expression was not upregulated in the
RCE-4-treated cells.
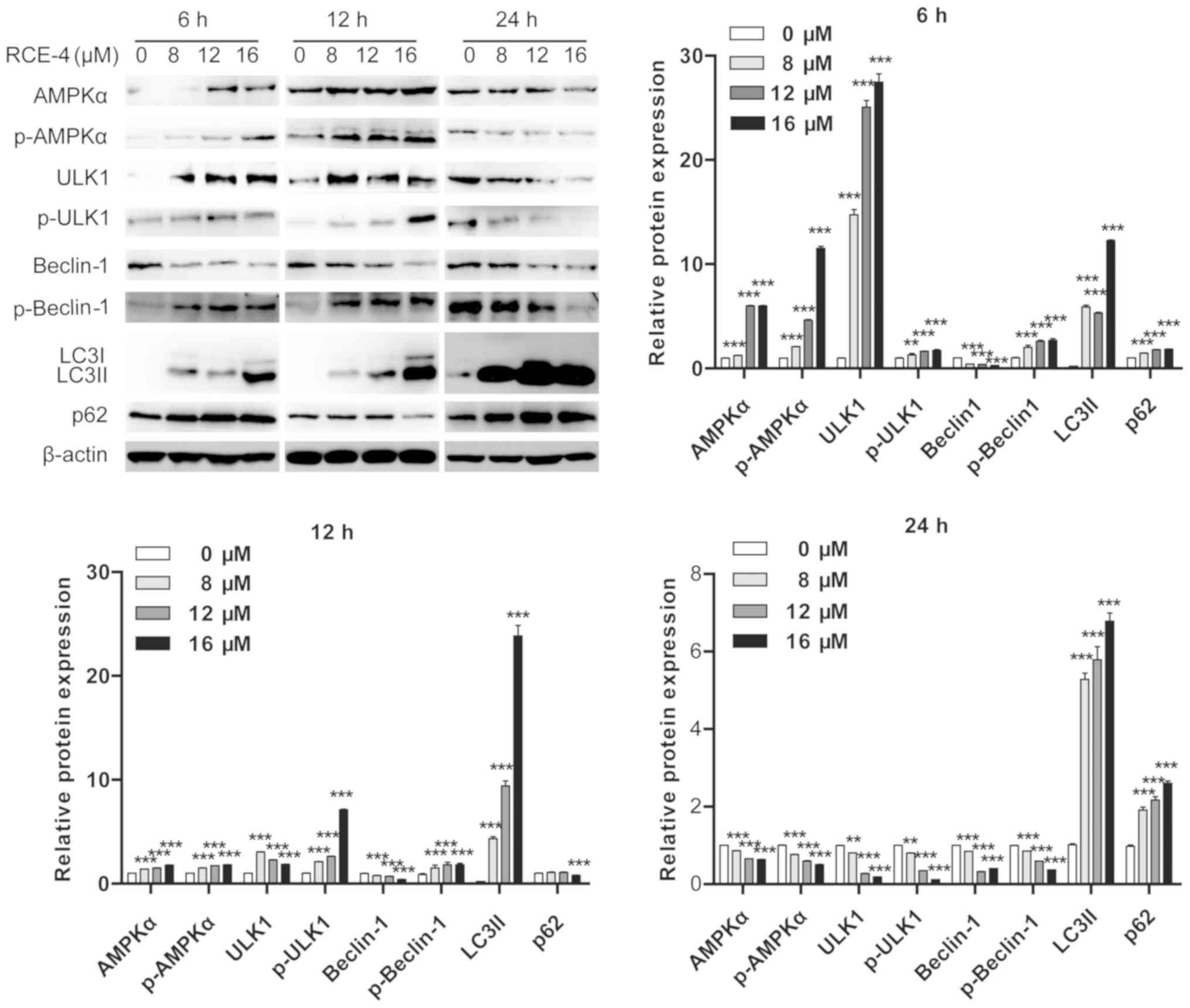 | Figure 3Expression levels of autophagy-
(ULK1, p-ULK1, Beclin-1, p-Beclin-1, LC3II and p62) and AMPK
pathway-associated proteins (AMPK and p-AMPK) in CaSki cells
following treatment with different concentrations of RCE-4 for 6,
12 or 24 h. Bar graphs represent the western blot grey values,
presented as the mean ± standard deviation. **P<0.01
and ***P<0.001 vs. 0 µM RCE-4 group. ULK1,
serine/threonine-protein kinase ULK1; p-, phosphorylated; LC3,
microtubule-associated proteins 1A/1B light chain 3B; AMPK,
AMP-activated protein kinase; p62, sequestosome 1; RCE-4, (1β, 3β,
5β, 25S)-spirostan-1, 3-diol
1-[α-L-rhamnopyranosyl-(1→2)-β-D-xylopyranoside]. |
Conversely, the increase in the LC3II protein
expression level may be due to increased autophagosome formation
following autophagy activation, or incomplete autolysosome
clearance. Following pretreatment with autophagy inhibitors 3-MA,
Baf A1 or CQ for 6 h, the LC3II expression level in the 3-MA+RCE-4
group was decreased, compared with that of the RCE-4 group.
However, the LC3II expression levels of the Baf A1+RCE-4 and the
CQ+RCE-4 groups were increased following autophagy inhibition
(Fig. 4A). Differences in LC3II
relative protein expression were analyzed via densitometric
analysis and presented in Fig.
4B. These results support the hypothesis that RCE-4 increases
autophagic flux rather than inhibits the degradation of autophagic
components. In conclusion, RCE-4 induced autophagy after 6 and 12
h, though such autophagy may be inhibited or ineffective after 24 h
of treatment.
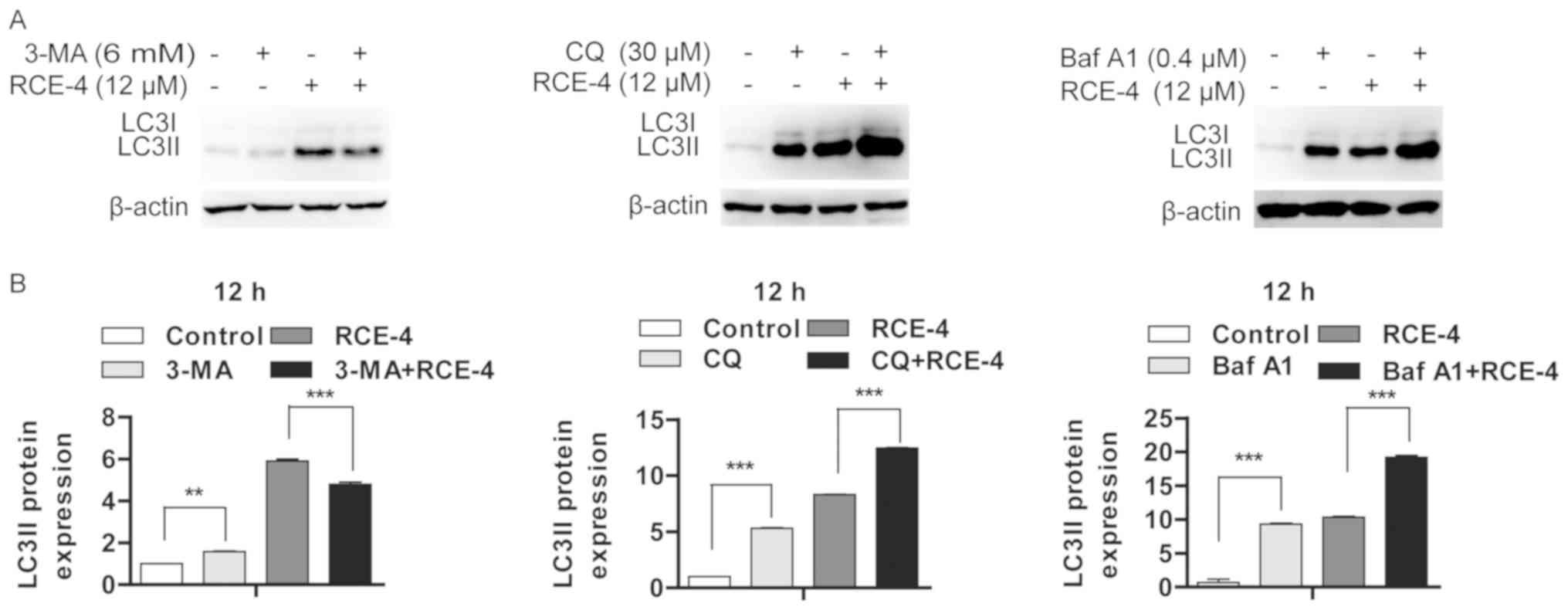 | Figure 4RCE-4 promotes the formation of
autophagosomes in CaSki cells. (A) Effect of RCE-4 treatment for 12
h, with or without autophagy inhibitors, on the protein expression
level of LC3II. (B) Bar graphs represent the western blot grey
values, presented as the mean ± standard deviation.
**P<0.01 and ***P<0.001 vs. control
group (corresponding concentration of RCE-4 without autophagy
inhibitor treatment). RCE-4, (1β, 3β, 5β, 25S)-spirostan-1, 3-diol
1-[α-L-rhamno pyranosyl-(1→2)-β-D-xylopyranoside]; LC3,
microtubule-associated proteins 1A/1B light chain 3B; LC3II,
lipid-modified LC3; 3-MA, 3-methyladenine; CQ, chloroquine; Baf A1,
bafilomycin A1. |
RCE-4 regulates autophagy by altering the
protein expression level of mTOR
mTOR, which forms two distinct signaling complexes
[mTOR complex (C)1 and mTORC2] by binding to multiple accompanying
proteins, is a major regulator of cellular metabolism and serves a
key role in autophagy regulation. The PI3K/AKT, Ras/Raf/MEK/Erk and
AMPK pathways, activated by a high AMP/ATP ratio, are upstream
signal cascades of mTOR (32).
Western blot analysis revealed that AMPK and p-AMPK protein
expression levels were upregulated at 6 and 12 h, and downregulated
at 24 h post-RCE-4 treatment in CaSki cells (Fig. 3). After a 24-h period, RCE-4 had
also downregulated the expression of Ras, c-Raf, p-c-Raf, MEK1/2,
p-MEK1/2 and p-Erk1/2 at all of the concentrations examined
(Fig. 5). In addition, the
expression levels of PI3K, p-PI3K, p-Akt, and p-mTOR were inhibited
at 6 h post-RCE-4 treatment at concentrations of 12 and 16
µM; the protein expression levels of p-PI3K, p-Akt, mTOR and
p-mTOR were suppressed at 12 h after post-RCE-4 treatment; the
protein expression levels of Akt, p-Akt, mTOR and p-mTOR were also
suppressed at 24 h after post-RCE-4 treatment (Fig. 6).
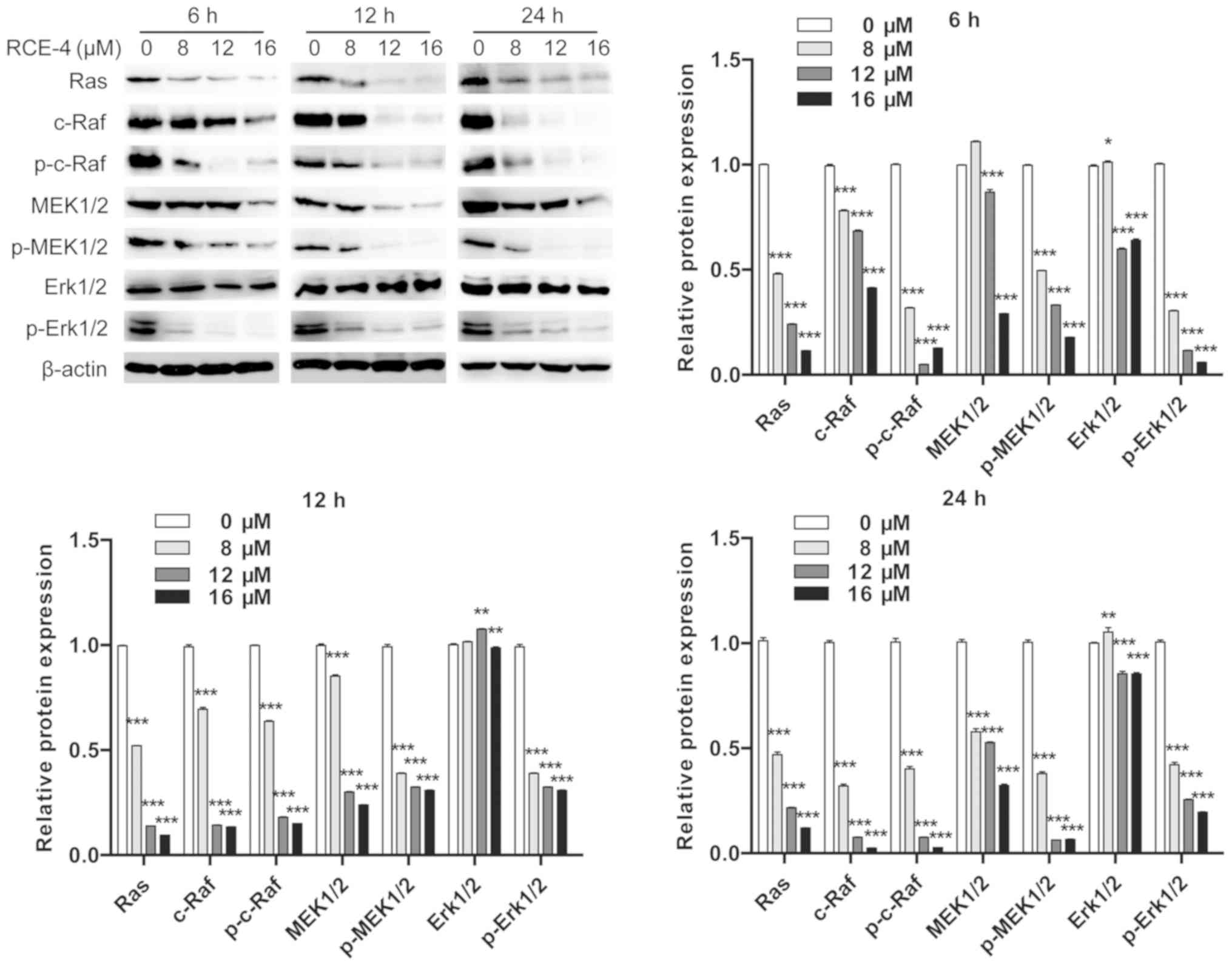 | Figure 5RCE-4 suppresses the Erk pathway
signaling. Total proteins were collected from CaSki cells following
treatment with various concentrations of RCE-4 (0, 8, 12 and 16
µM) for 6, 12 or 24 h. Expression levels of β-actin, Ras,
total c-Raf, p-c-Raf, total MEK1/2, p-MEK1/2, total Erk1/2 and
Erk1/2 were determined by western blot analysis. Bar graphs
represent the western blot grey values, presented as the mean ±
standard deviation. *P< 0.05, **P<0.01
and ***P<0.001 vs. 0 RCE-4 group. RCE-4, (1β, 3β, 5β,
25S)-spirostan-1, 3-diol
1-[α-L-rhamnopyranosyl-(1→2)-β-D-xylopyranoside]; MEK,
mitogen-activated protein kinase kinase; Erk, extracellular
signal-regulated kinase; p-, phosphorylated. |
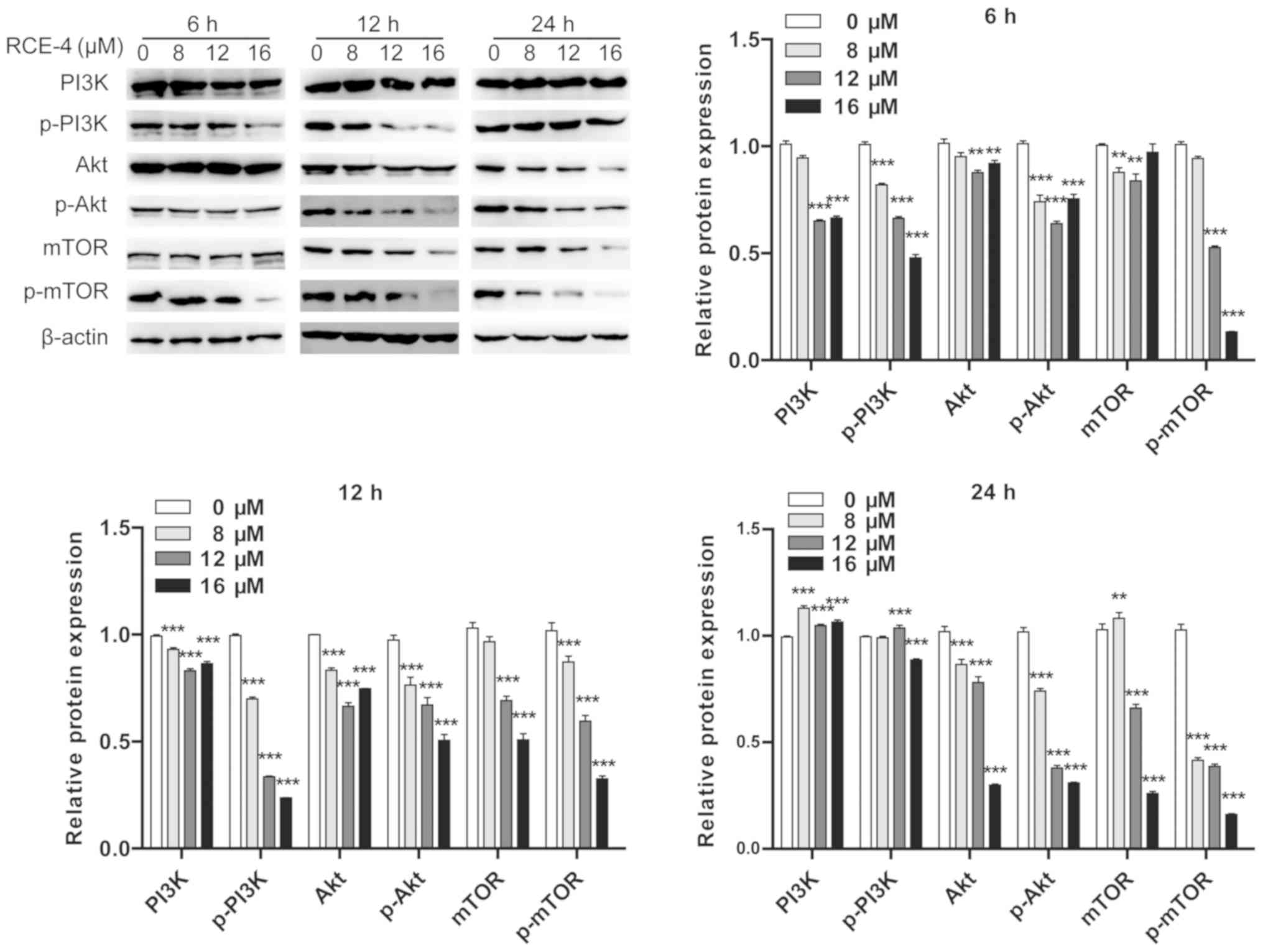 | Figure 6RCE-4 suppresses PI3K pathway
signaling. Total proteins were collected from CaSki cells following
treatment with various concentrations of RCE-4 (0, 8, 12 and 16
µM) for 6, 12 or 24 h. Expression levels of β-actin, total
PI3K, p-PI3K, total Akt, p-Akt, total mTOR and p-mTOR were analyzed
by western blot analysis. Bar graphs represent the western blot
grey values, presented as the mean ± standard deviation.
**P<0.01 and ***P<0.001 vs. 0 RCE-4
group. RCE-4, (1β, 3β, 5β, 25S)-spirostan-1, 3-diol
1-[α-L-rhamnopyranosyl-(1→2)-β-D-xylopyranoside]; p-,
phosphorylated. |
Discussion
It is well known that cervical cancer is a serious
health threat to the female population worldwide. Due to the
negative effects of radiotherapy on ovarian function, chemotherapy
as a treatment for cervical cancer has become increasingly valued
by clinicians (33). RCE-4, a
potential chemotherapeutic drug for cervical cancer, was
demonstrated to induce mitochondria-mediated apoptosis (5,9).
However, not all apoptosis-inducing agents are effective in complex
in vivo systems; for example, it has been reported that have
demonstrated that the tumor necrosis factor-associated
apoptosis-inducing ligand was unable to initiate apoptosis in tumor
cells due to compensatory inhibition by autophagy (34,35).
Autophagy and apoptosis may determine the fate of
cells through cooperative or competitive pathways, depending on
which process predominates (36).
The aim of the present study was to assess the role of
RCE-4-induced autophagy in proliferation inhibition, and the level
and duration of autophagy in the CaSki cervical cancer cell line.
To improve the understanding of the anti-tumor mechanisms induced
by RCE-4, the molecular mechanisms of RCE-4-induced autophagy and
the specific cellular targets or signaling pathways associated with
its anti-proliferative activity, were additionally elucidated. It
was demonstrated that RCE-4 was potently cytotoxic to CaSki cells,
which was characterized by decreased levels of cell proliferation
and survival. Furthermore, the use of autophagy inhibitors enhanced
proliferative inhibition following RCE-4 treatment, suggesting that
autophagy promoted cell survival (37). In addition, it has been reported
that have also demonstrated that autophagy inhibitors restrict the
pro-survival function of autophagy, restore treatment sensitivity
and promote tumor cell death (38-40). This indicates that RCE-4, combined
with autophagy inhibitors, may be a promising strategy for cervical
cancer treatment. Furthermore, the results of the present study
support the role of RCE-4-induced autophagy and its contribution to
the formation of chemoresistance.
Autophagy is a fundamental process of energy
metabolism, where autophagosomes encapsulate dysfunctional cellular
components, fuse with lysosomes to form autolysosomes, and
facilitate degradation. Several important autophagy-associated
proteins, including LC3II and p62, are involved in this process.
LC3II, a classical autophagosomal marker, is located on the inner
and outer membranes of autophagosomes and is often used to indicate
autophagy. p62, which is inversely associated with selective
autophagic degradation, serves as a bridge between LC3II and the
ubiquitinated substrate to be degraded (41). Therefore, p62 and LC3II expression
levels are important indicators of autophagy flux.
In the present study, it was determined that RCE-4
induced autophagy in CaSki cells. CYTO-ID staining revealed an
increase in the number of autophagosomes in stained cells following
an exposure period of 6, 12 or 24 h. Furthermore, using
mRFP-GFP-LC3 adenoviruses and LCFM an increase in autophagosome and
autolysosome formation in CaSki cells following RCE-4 treatment was
revealed. These data were supported by the results from the western
blot analysis, which indicated that p62 was downregulated and LC3II
was upregulated after 12 h of RCE-4 treatment. Furthermore, the use
of 3 autophagy inhibitors (3-MA, CQ and Baf A1) with different
targeting effects altered the expression level of LC3II following
12 h of RCE-4 treatment. LC3II was downregulated in the 3-MA+RCE-4
group, but upregulated in the CQ+RCE-4 and Baf A1+RCE-4 groups,
compared with the RCE-4 control group. These results indicated that
the increase in LC3II protein expression level was due to increased
autophagosome formation following autophagy activation, which was
consistent with harmine-induced autophagy (42). To summarize, the present study
demonstrated that RCE-4 induced autophagy and the formation of
autolysosomes, which were subsequently degraded.
Growth factors activate downstream signaling
cascades, including the PI3K and ERK pathways, and thereby suppress
the tuberous sclerosis (TSC) tumor suppressor complex TSC1/TSC2. As
a GTPase-activating protein, the TSC complex is the most critical
upstream negative regulator of mTORC1 (43). By contrast, AMPK activates TSC
complexes under stress conditions, indirectly inhibiting the
activity of mTORC1. AMPK also directly suppresses mTORC1 by
phosphorylating regulatory-associated protein of mTOR, which itself
binds to mTOR (44). In addition,
mTORC1 not only suppresses autophagy by inhibiting ULK1/2 and
vacuolar protein sorting-34 complex, but also decreases the
transcriptional activity of lysosomal and autophagy-associated
genes by phosphorylating transcription factor EB (32). mTORC1 and AMPK are upstream
signaling components of ULK1, an autophagy-initiating kinase, and
autophagy is regulated by the kinase activities of AMPK, mTOR and
ULK1 (45). A previous study
suggested that various AMPK and mTORC1-dependent phosphorylation
events overlapped at ULK1 (20),
indicating that both AMPK and mTORC1 tightly controlled the
function of ULK1 through protein phosphorylation. To enhance
phosphatidylinositol 3-kinase catalytic subunit type 3 (PIK3C3)
activity in complexes containing UV radiation resistance-associated
gene protein (UVRAG) or Beclin-1-associated autophagy-related key
regulator (ATG14), AMPK phosphorylates Beclin-1 at Ser91 and Ser94;
these ATG14- or UVRAG-containing complexes are involved in
autophagy initiation. Activation of PIK3C3 complexes containing
ATG14 by Beclin-1 phosphorylation was demonstrated to be necessary
for autophagy induction (46). In
the present study, it was demonstrated that following 12 h of RCE-4
treatment, the expression levels of AMPK, p-AMPK, ULK1, p-ULK1,
p-Beclin-1 and LC3II were upregulated, and mTOR and p-mTOR
expression levels were downregulated, indicating that autophagy had
been initiated. It was also revealed that when treated with RCE-4,
the expression levels of p-PI3K, p-Akt, mTOR, p-mTOR, Ras, c-Raf,
p-c-Raf, MEK1/2, p-MEK1/2 and p-Erk1/2 were decreased. Therefore,
RCE-4-induced autophagy was concluded to be closely associated with
the AMPK pathway, and the dual blockade of the ERK and PI3K
pathways.
Notably, in the present study, after 24 h of
treatment, RCE-4-mediated autophagy was inhibited, or RCE-4 may
have prevented normal lysosomal functioning, which was indicated by
a percentage decrease in autolysosome staining, the down-regulation
of AMPK, p-AMPK, ULK1, p-ULK1 and p-Beclin-1, and the upregulation
of p62 expression levels. Wang et al (29) and Feng et al (30) reported that the inhibition of
autophagy was associated with a decreased percentage of
autolysosomes and an upregulated expression of p62. p-AMPK, p-ULK1
and p-Beclin-1 exhibited similar time- and concentration-dependent
changes. This is supported by the previous observation of direct
phosphorylation and activation of ULK1 and Beclin-1 kinases by AMPK
(47). Although the results from
the present study demonstrated that the activation of autophagy was
regulated by the AMPK pathway, the RCE-4-induced protective
autophagy in CaSki cells was not conducive to the anti-cervical
cancer effect of RCE-4; however, autophagy may have been inhibited
by prolonged treatment times. Furthermore, the downregulation of
Beclin-1 levels indicated that crosstalk and feedback mechanisms
between RCE-4-induced apoptosis and autophagy may be involved
(48).
Activation of the ERK and PI3K pathways promotes
tumor cell proliferation and inhibits apoptosis (3,18,25). RCE-4 has a stronger inhibitory
effect on CaSki cells by inducing the dual blockade of the
Ras/Raf/MEK/Erk and PI3K/Akt/mTOR pathways, compared with a single
pathway inhibitor. These pathways are the most frequently
dysregulated kinase cascades in human cancer (49), and both PI3K/Akt/mTOR and
Ras/Raf/MEK/Erk cascade inhibitors have been studied for their
possible chemotherapeutic use (50). The present study suggested that
the cytotoxicity of RCE-4 was closely associated with the
suppression of PI3K/AKT and Ras/Raf/MEK/Erk signaling. However, it
is unclear how RCE-4 mediates both pathways, and this requires
further investigation.
To conclude, the present study revealed that RCE-4
inhibited the proliferation of cervical cancer cells, and activated
protective autophagy. In addition, RCE-4 activated the AMPK
pathway, and inhibited PI3K and ERK signaling pathways by
regulating the associated proteins involved in autophagy. These
data may provide novel insights into the molecular mechanisms of
the potential anti-cervical cancer effects of RCE-4, and assist in
developing this potential candidate for cervical cancer
treatment.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81773952) and the
Traditional Chinese Medicine Research Foundation of the Health
Commission of Hubei Province (grant no. ZY2019M028).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon request.
Authors' contributions
JFC conceived and designed the research, supplied
reagents, materials, analysis tools and designed certain
experimental methods. WXiang performed the experiments and wrote
the manuscript. GLJ, XFX and WXi collected Reineckia carnea
herbs in the field and completed their extraction and separation.
FC and JZW purified and identified RCE-4 compounds. RJZ, LT and SJT
assisted in the analysis of data and provided key commentary. All
authors read the results and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to the Medical College of
China Three Gorges University for the provision of experimental
equipment.
References
|
1
|
Jiang F, Zhou JY, Zhang D, Liu MH and Chen
YG: Artesunate induces apoptosis and autophagy in HCT116 colon
cancer cells, and autophagy inhibition enhances the artesunate
induced apoptosis. Int J Mol Med. 42:1295–1304. 2018.PubMed/NCBI
|
|
2
|
García-Zepeda SP, García-Villa E,
Díaz-Chávez J, Hernández-Pando R and Gariglio P: Resveratrol
induces cell death in cervical cancer cells through apoptosis and
autophagy. Eur J Cancer Prev. 22:577–584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu T, Pang Q, Wang Y and Yan X: Betulinic
acid induces apoptosis by regulating PI3K/Akt signaling and
mitochondrial pathways in human cervical cancer cells. Int J Mol
Med. 40:1669–1678. 2017.PubMed/NCBI
|
|
4
|
Sy LK, Yan SC, Lok CN, Man RY and Che CM:
Timosaponin A-III induces autophagy preceding mitochondria-mediated
apoptosis in HeLa cancer cells. Cancer Res. 68:10229–10237. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bai C, Yang X, Zou K, He H, Wang J, Qin H,
Yu X, Liu C, Zheng J, Cheng F, et al: Anti-proliferative effect of
RCE-4 from Reineckia carnea on human cervical cancer HeLa cells by
inhibiting the PI3K/Akt/mTOR signaling pathway and NF-κB
activation. Naunyn Schmiedebergs Arch Pharmacol. 389:573–584. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Q, Hou Q, Guo Z, Zou K, Xue Y, Huang
N, Cheng F and Zhou Y: Three new steroidal glycosides from roots of
Reineckia carnea. Nat Prod Res. 27:85–92. 2013. View Article : Google Scholar
|
|
7
|
Tang Y, Li N, Duan JA and Tao W:
Structure, bioactivity, and chemical synthesis of OSW-1 and other
steroidal glycosides in the genus Ornithogalum. Chem Rev.
113:5480–5514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fu XJ, Zou K, Wang GP and Zhang X:
Anti-inflammatory effect and mechanism of ethyl acetate extract
from Reineckia carnea. Lishizhen Med Mater Med Res. 24:822–825.
2013.In Chinese.
|
|
9
|
Wang G, Huang W, He H, Fu X, Wang J, Zou K
and Chen J: Growth inhibition and apoptosis-inducing effect on
human cancer cells by RCE-4, a spirostanol saponin derivative from
natural medicines. Int J Mol Med. 31:219–224. 2013. View Article : Google Scholar
|
|
10
|
Yang XJ, Bai CH, Zou K, He HB, Yu XQ, Qin
HL, Zhang YF and Wang JZ: Steroidal saponin RCE-4 from Reineckia
carnea (Andr.) Kunth inhibits growth of human cervical cancer
xenograft in nude mice. J Third Mil Med Univ. 38:476–482. 2016.In
Chinese.
|
|
11
|
Chaabane W, User SD, El-Gazzah M, Jaksik
R, Sajjadi E, Rzeszowska-Wolny J and Los MJ: Autophagy, apoptosis,
mitoptosis and necrosis: Interdependence between those pathways and
effects on cancer. Arch Immunol Ther Exp (Warsz). 61:43–58. 2013.
View Article : Google Scholar
|
|
12
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pham DC, Chang YC, Lin SR, Fuh YM, Tsai MJ
and Weng CF: FAK and S6K1 inhibitor, neferine, dually induces
autophagy and apoptosis in human neuroblastoma cells. Molecules.
23:3110–3125. 2018. View Article : Google Scholar
|
|
14
|
Russo M and Russo GL: Autophagy inducers
in cancer. Biochem Pharmacol. 153:51–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: Role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16(Suppl 2):
S17–S27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shang L, Chen S, Du F, Li S, Zhao L and
Wang X: Nutrient starvation elicits an acute autophagic response
mediated by Ulk1 dephosphorylation and its subsequent dissociation
from AMPK. Proc Natl Acad Sci USA. 108:4788–4793. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song L, Wang Z, Wang Y, Guo D, Yang J,
Chen L and Tan N: Natural cyclopeptide RA-XII, a new autophagy
inhibitor, suppresses protective autophagy for enhancing apoptosis
through AMPK/mTOR/P70S6K pathways in HepG2 cells. Molecules.
22:1934–1950. 2017. View Article : Google Scholar
|
|
22
|
Vakifahmetoglu-Norberg H, Xia HG and Yuan
J: Pharmacologic agents targeting autophagy. J Clin Invest.
125:5–13. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sooro MA, Zhang N and Zhang P: Targeting
EGFR-mediated autophagy as a potential strategy for cancer therapy.
Int J Cancer. 143:2116–2125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chappell WH, Steelman LS, Long JM, Kempf
RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone
P, et al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors:
Rationale and importance to inhibiting these pathways in human
health. Oncotarget. 2:135–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar
|
|
26
|
Normanno N, De Luca A, Maiello MR,
Campiglio M, Napolitano M, Mancino M, Carotenuto A, Viglietto G and
Menard S: The MEK/MAPK pathway is involved in the resistance of
breast cancer cells to the EGFR tyrosine kinase inhibitor
gefitinib. J Cell Physiol. 207:420–427. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Serra V, Scaltriti M, Prudkin L, Eichhorn
PJA, Ibrahim YH, Chandarlapaty S, Markman B, Rodriguez O, Guzman M,
Rodriguez S, et al: PI3K inhibition results in enhanced HER
signaling and acquired ERK dependency in HER2-overexpressing breast
cancer. Oncogene. 30:2547–2557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mqoco T and Joubert A:
2-Methoxyestradiol-bis-sulphamate induces apoptosis and autophagy
in an oesophageal carcinoma (SNO) cell line. Biomed Res-India.
23:469–474. 2012.
|
|
29
|
Wang Y, Nie H, Zhao X, Qin Y and Gong X:
Bicyclol induces cell cycle arrest and autophagy in HepG2 human
hepatocellular carcinoma cells through the PI3K/AKT and
Ras/Raf/MEK/ERK pathways. BMC Cancer. 16:742–757. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng X, Zhou J, Li J, Hou X, Li L, Chen Y,
Fu S, Zhou L, Li C and Lei Y: Tubeimoside I induces accumulation of
impaired autopha-golysosome against cervical cancer cells by both
initiating autophagy and inhibiting lysosomal function. Cell Death
Dis. 9:1117–1133. 2018. View Article : Google Scholar
|
|
31
|
Zheng Q, Su H, Ranek MJ and Wang X:
Autophagy and p62 in cardiac proteinopathy. Circ Res. 109:296–308.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim YC and Guan KL: mTOR: A pharmacologic
target for autophagy regulation. J Clin Invest. 125:25–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jemal A, Simard EP, Dorell C, Noone AM,
Markowitz LE, Kohler B, Eheman C, Saraiya M, Bandi P, Saslow D, et
al: Annual Report to the Nation on the Status of Cancer, 1975-2009,
featuring the burden and trends in human papillomavirus
(HPV)-associated cancers and HPV vaccination coverage levels. J
Natl Cancer Inst. 105:175–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nazim UM and Park SY: Attenuation of
autophagy flux by 6-shogaol sensitizes human liver cancer cells to
TRAIL-induced apoptosis via p53 and ROS. Int J Mol Med. 43:701–708.
2019.
|
|
35
|
Thorburn A, Behbakht K and Ford H: TRAIL
receptor-targeted therapeutics: Resistance mechanisms and
strategies to avoid them. Drug Resist Updat. 11:17–24. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun Y, Liu JH, Jin L, Lin SM, Yang Y, Sui
YX and Shi H: Over-expression of the Beclin1 gene upregulates
chemosensitivity to anti-cancer drugs by enhancing therapy-induced
apoptosis in cervix squamous carcinoma CaSki cells. Cancer Lett.
294:204–210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lima RT, Sousa D, Paiva AM, Palmeira A,
Barbosa J, Pedro M, Pinto MM, Sousa E and Vasconcelos MH:
Modulation of Autophagy by a Thioxanthone Decreases the Viability
of Melanoma Cells. Molecules. 21:1343–1358. 2016. View Article : Google Scholar
|
|
38
|
Maycotte P, Aryal S, Cummings CT, Thorburn
J, Morgan MJ and Thorburn A: Chloroquine sensitizes breast cancer
cells to chemotherapy independent of autophagy. Autophagy.
8:200–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rao R, Balusu R, Fiskus W, Mudunuru U,
Venkannagari S, Chauhan L, Smith JE, Hembruff SL, Ha K, Atadja P,
et al: Combination of pan-histone deacetylase inhibitor and
autophagy inhibitor exerts superior efficacy against
triple-negative human breast cancer cells. Mol Cancer Ther.
11:973–983. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Palmeira dos Santos C, Pereira GJS,
Barbosa CMV, Jurkiewicz A, Smaili SS and Bincoletto C: Comparative
study of autophagy inhibition by 3MA and CQ on Cytarabine induced
death of leukaemia cells. J Cancer Res Clin Oncol. 140:909–920.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin YT, Wang HC, Hsu YC, Cho CL, Yang MY
and Chien CY: Capsaicin induces autophagy and apoptosis in human
nasopha-ryngeal carcinoma cells by downregulating the PI3K/AKT/mTOR
πathway. Int J Mol Sci. 18:1343–1359. 2017. View Article : Google Scholar
|
|
42
|
Zou N, Wei Y, Li F, Yang Y, Cheng X and
Wang C: The inhibitory effects of compound Muniziqi granule against
B16 cells and harmine induced autophagy and apoptosis by inhibiting
Akt/mTOR pathway. BMC Complement Altern Med. 17:517–528. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Inoki K, Zhu T and Guan KL: TSC2 mediates
cellular energy response to control cell growth and survival. Cell.
115:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gwinn DM, Shackelford DB, Egan DF,
Mihaylova MM, Mery A, Vasquez DS, Turk BE and Shaw RJ: AMPK
phosphorylation of raptor mediates a metabolic checkpoint. Mol
Cell. 30:214–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dunlop EA and Tee AR: mTOR and autophagy:
A dynamic relationship governed by nutrients and energy. Semin Cell
Dev Biol. 36:121–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim J, Kim YC, Fang C, Russell RC, Kim JH,
Fan W, Liu R, Zhong Q and Guan KL: Differential regulation of
distinct Vps34 complexes by AMPK in nutrient stress and autophagy.
Cell. 152:290–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Russell RC, Yuan HX and Guan KL: Autophagy
regulation by nutrient signaling. Cell Res. 24:42–57. 2014.
View Article : Google Scholar :
|
|
48
|
Pattingre S, Tassa A, Qu X, Garuti R,
Liang XH, Mizushima N, Packer M, Schneider MD and Levine B: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Su CC: Tanshinone IIA can inhibit
MiaPaCa-2 human pancreatic cancer cells by dual blockade of the
Ras/Raf/MEK/ERK and PI3K/AKT/mTOR pathways. Oncol Rep.
40:3102–3111. 2018.PubMed/NCBI
|
|
50
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Montalto G, Cervello M, Nicoletti F, Fagone P, Malaponte
G, Mazzarino MC, et al: Mutations and deregulation of
Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades which alter therapy
response. Oncotarget. 3:954–987. 2012. View Article : Google Scholar : PubMed/NCBI
|




















