Introduction
Functional dyspepsia (FD) is a common functional
gastrointestinal disorder marked chiefly by postprandial fullness,
early satiation, epigastric pain and epigastric burning (1). The pathogenic mechanisms of FD are
complex, and include gastric emptying, impaired gastric
accommodation, duodenal inflammation, mucosal permeability and
psychosocial factors (2). The
role of the duodenum in the pathogenesis of FD is well known, and
the integrity of the duodenal mucosal barrier is significant to the
maintenance of normal duodenal function (3). Previous findings have shown that
impaired intestinal barrier function and low-grade inflammation are
apparent in the duodenum of patients with FD (4,5).
Interactions between the brain and the gut serve an
important role in functional gastrointestinal diseases (FGIDs),
including FD, and there is evidence to suggest that the central
nervous system and gut interact bi-directionally in FD patients
(6). Corticotropin-releasing
factor (CRF) is one of the brain peptides that co-ordinates the
behavioral, endocrine, autonomic and visceral responses to stress
(7). Research indicates that the
binding of CRF to its receptors, primarily CRF receptor 1 (CRF-R1)
and CRF-R2, results in mast cell (MC) degranulation, which
contributes to the characteristic low-grade inflammation observed
in FD patients (8). MCs are one
of the key contributing factors to duodenal micro-inflammation,
which can further influence epithelial barrier integrity via the
tryptase/proteinase-activated receptor 2 (PAR-2) pathway (9,10).
Sini San (SNS) is a traditional Chinese medicine
that has been used for thousands of years. In the clinic, SNS is
widely used to treat FGIDs, particularly FD (11,12). SNS is composed of an equal ratio
of four different herbs: i) Chaihu (Radix Bupleuri Chinensis); ii)
Baishao (Radix Paeoniae Alba); iii) Zhishi (Fructus Aurantii
Immaturus); and iv) and Gancao (Radix Glycyrrhizae). It has been
confirmed that SNS can improve depression by regulating the
expression of central neurotransmitters (13), and improve duodenal tight junction
integrity in FD rats (14).
However, the protective mechanism of SNS on low-grade inflammation
and duodenal mucosal barrier integrity have not been extensively
researched.
The aim of the present study was to determine
whether SNS restores mucosal barrier integrity and suppresses
low-grade inflammation in the duodenum via the CRF signaling
pathway.
Materials and methods
SNS preparation
SNS, which consisted of Chaihu (Radix Bupleuri
Chinensis), Baishao (Radix Paeoniae Alba), Zhishi (Fructus Aurantii
Immaturus) and Gancao (Radix Glycyrrhizae) was purchased from
Beijing Xinglin Pharmaceutical Industry Co., Ltd., and identified
as eligible medicinal material according to the Pharmacopoeia of
the People's Republic of China (2015 edition) (15). SNS was decocted by the Beijing
Hospital of Traditional Chinese Medicine, Capital Medical
University (Beijing, China). Briefly, the raw herbs were combined
to a total weight of 200 g and impregnated in 1,000 ml distilled
water for 30 min at room temperature. After boiling for 30 min, 200
ml SNS was harvested and set aside. Another 200-ml distilled water
was added to the original herb mixture, which was boiled for a
further 30 min, and both decoctions were combined to generate a
400-ml SNS preparation at a concentration of 0.5 g/ml. The
preparation was filtered through a 100-mesh sieve to remove
particulate material.
Animals
A total of 36, 7-day-old (12-15 g), male Sprague
Dawley rats were purchased from SPF (Beijing) Biotechnology Co.,
Ltd. The rats were housed in a standardized environment with a 12-h
light/dark cycle, at a temperature of 20-26°C (humidity, 50±5%) and
free access to food and water. In the present study, the
iodoacetamide (IA)-treated and tail-squeezed methods were combined
to generate a modified rat model of functional dyspepsia that
better simulated pathogenesis of FD patients. Previous findings
revealed that the combined model rats exhibited obvious symptoms of
FD, such as the reduction of gastric accommodation and gastric
emptying (16). After acclimation
for 3 days prior to the experiment, 10-day-old rats were randomly
divided into 3 groups of 12 rats each: The control, model and SNS
groups. The FD model rats received 0.2 ml 0.1% IA in 2% sucrose by
oral gavage, every day for 6 days, and the control group received
an equal volume of 2% sucrose only. At 7 weeks old, tail-clamping
was conducted on the FD rats for a further 7 days, and surgical
forceps were used to clamp the distal third of the tail, every 4 h
for 30 min, 3 times per day. Then, the FD rats were randomly
divided into two groups that received intragastric administration
of SNS (1 ml/100 g) or an equal volume of water as a vehicle
control, for 7 consecutive days. The rats received euthanasia by
intraperitoneal injection of sodium pentobarbital (100 mg/kg of
body weight).
The present study was performed in accordance with
the Guide for the Care and Use of Laboratory Animals published by
the National Institutes of Health, and was approved by the Animal
Care and Use Committee of Beijing Institute of Traditional Chinese
Medicine (no. 2018030101).
Western blot analysis
Rats were decapitated for hypothalamus and duodenum
tissues after euthanasia, and samples were fixed immediately in
liquid nitrogen, and stored at -80°C in ultra-low temperature
freezer. The hypothalamus and duodenum tissues were homogenized
with an appropriate volume of RIPA buffer with protease inhibitor
cocktail and 1 mM PMSF (Beijing Solarbio Science & Technology
Co., Ltd.). The protein concentration was determined using a
bicinchoninic acid (BCA) assay kit and the protein samples were
mixed with 5X loading buffer. Denatured protein (80 µg/lane)
was separated using 10% SDS PAGE and transferred to PVDF membranes.
After blocking with non fat milk in TBST (10 mmol/l Tris-HCl, 0.5
ml/l Tween-20 and 0.15 mol/l NaCl, pH 7.2) for 1 h at the room
temperature, the membranes were incubated at 4°C overnight (about
16 h) with the following primary antibodies: Anti-CRF (1:500;
10944-1-AP, ProteinTech Group, Inc.), anti-CRF-R1 (1:1,000;
20967-1-AP, ProteinTech Group, Inc.), anti-CRF-R2 (1:1,000;
CSB-PA623797LA01HU, Cusabio Technology LLC), anti-ZO-1 (1:1,000;
21773-1-AP, ProteinTech Group, Inc.), anti-JAM-1 (1:500; BS-3651R,
BIOSS), anti-β-catenin (1:1,000; 51067-2-AP, ProteinTech Group,
Inc.), anti-E-cadherin (1:1,000; 20874-1-AP, ProteinTech Group,
Inc.) and anti-β-actin (1:10,000; GB12001, Wuhan Servicebio
Technology Co., Ltd.). After washing three times with TBST, the
membranes were incubated with the following secondary antibodies:
Goat anti-rabbit or goat anti-mouse IgG (1:10,000; 072-06-15-06 and
072-07-18-06, KPL, Inc.). The protein levels were quantified as
relative grey value using ImageJ software v1.51 (National
Institutes of Health).
ELISA
The duodenal tissues were homogenized and the
supernatants were collected. After quantifying the total protein
concentration using a BCA assay kit, the concentrations of
histamine were determined with a histamine ELISA kit (E-EL-0032c,
Elabscience Biotechnology Co., Ltd.) according to the
manufacturer's instructions.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from the duodenal tissue
using TRIzol® reagent (Thermo Fisher Scientific, Inc.),
and 5 µg total RNA was reverse-transcribed into cDNA. The
gene expression levels of tryptase and PAR-2 were determined using
the SYBR® Master Mix (Promega Corporation) and the CFX
96 Real-time PCR System (Bio-Rad Laboratories, Inc.), and
individual gene expression levels were normalized to those of
β-actin. The thermocycling conditions were as follows: An initial
step at 95°C for 2 min, 40 cycles of denaturation at 95°C for 15
sec, and annealing/extension at 60°C for 1 min. The primer
sequences used were: Tryptase forward, 5′-GCT TCC ATC CAG TAC
CGC-3′, and reverse, 5′-ACC TTT GCA GAT CAA AGG-3′; PAR-2 forward,
5′-TTG GCA GAC CTC CTC TCT GT-3′, and reverse, 5′-GAG GCA GGT CAT
GAA AAG GA-3′; β-actin forward, 5′-AGT TGC GTT ACA CCC TTT C-3′,
and reverse, 5′-CAC CTT CAC CGT TCC AGT-3′.
Immunohistochemistry (IHC)
Duodenal and spinal cord (T8-T10) sections were
fixed in 4% paraformaldehyde at room temperature, embedded in
paraffin, cut into sections (4-µm), and mounted on glass
slides. The sections were heated to 60°C for 20 min, deparaffinized
in xylene and dehydrated in alcohol. Antigen retrieval was
performed in a pressure cooker using sodium citrate buffer (pH
6.0). The sections were then soaked in 3%
H2O2/methanol solution to inhibit endogenous
peroxidase activity, and then blocked with 3% BSA. The sections
were incubated overnight at 4°C with anti-CRF (1:100; 10944-1-AP,
ProteinTech Group, Inc.), and anti-tryptase (1:100; ab2378, Abcam)
primary antibodies. After washing in PBS (pH 7.4), the sections
were incubated with the following secondary antibodies: Horseradish
peroxidase-labeled goat anti-rabbit or goat anti-mouse IgG (1:200;
GB2301 and GB23303, Wuhan Servicebio Technology Co., Ltd.). The
tissues were then incubated with 0.05% 3,3′-diaminobenzidine
tetrachloride for colorimetric development, and subsequently
counterstained with hematoxylin prior to dehydration with ethanol
and xylene. Images were obtained using a light microscope of
100-200 times multiplication (Carl Zeiss AG), and the
immunoreactivity of CRF and MC tryptase was determined using the
Image Pro Plus 6.0 image analysis software system (Media
Cybernetics, Inc.).
Statistical analysis
Statistical analyses were performed using SPSS 20.0
software (IBM Corp). Data are expressed as the mean ± SEM. One-way
analysis of variance (ANOVA) followed by the least significant
difference post hoc test was used to determine the statistical
significance among the groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
SNS reduces the expression levels of CRF
in the duodenum and central nervous system
The CRF signaling pathway is an important component
of the brain-gut interaction, coordinating visceral responses to
stress (17). We aimed to
determine whether a CRF-related pathway was involved in the
response to SNS treatment in FD. The central and peripheral CRF
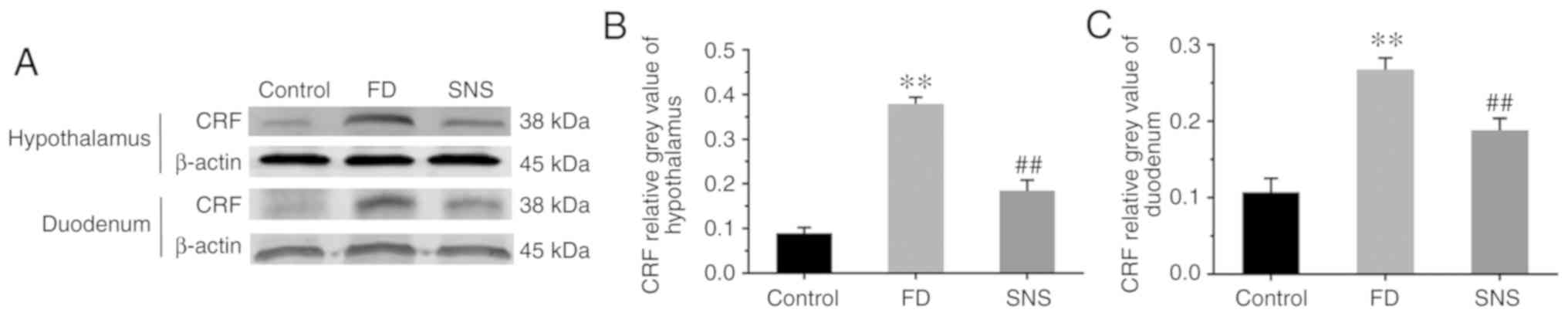
expression levels were determined using western blotting (Fig. 1A) and/or IHC (Fig. 2). The results showed that the
protein expression level of CRF was markedly increased in the
hypothalamus (Fig. 1B) and
duodenum (Fig. 1C) of FD rats,
compared with the control group (hypothalamus: 0.38±0.01 vs.
0.09±0.01; P<0.01) (duodenum: 0.27±0.02 vs. 0.11±0.02,
P<0.01). SNS treatment ameliorated the expression levels of CRF
in both the hypothalamus and the duodenum (hypothalamus: 0.18±0.02
vs. 0.38±0.01, P<0.01) (duodenum: 0.19±0.02 vs. 0.27±0.02,
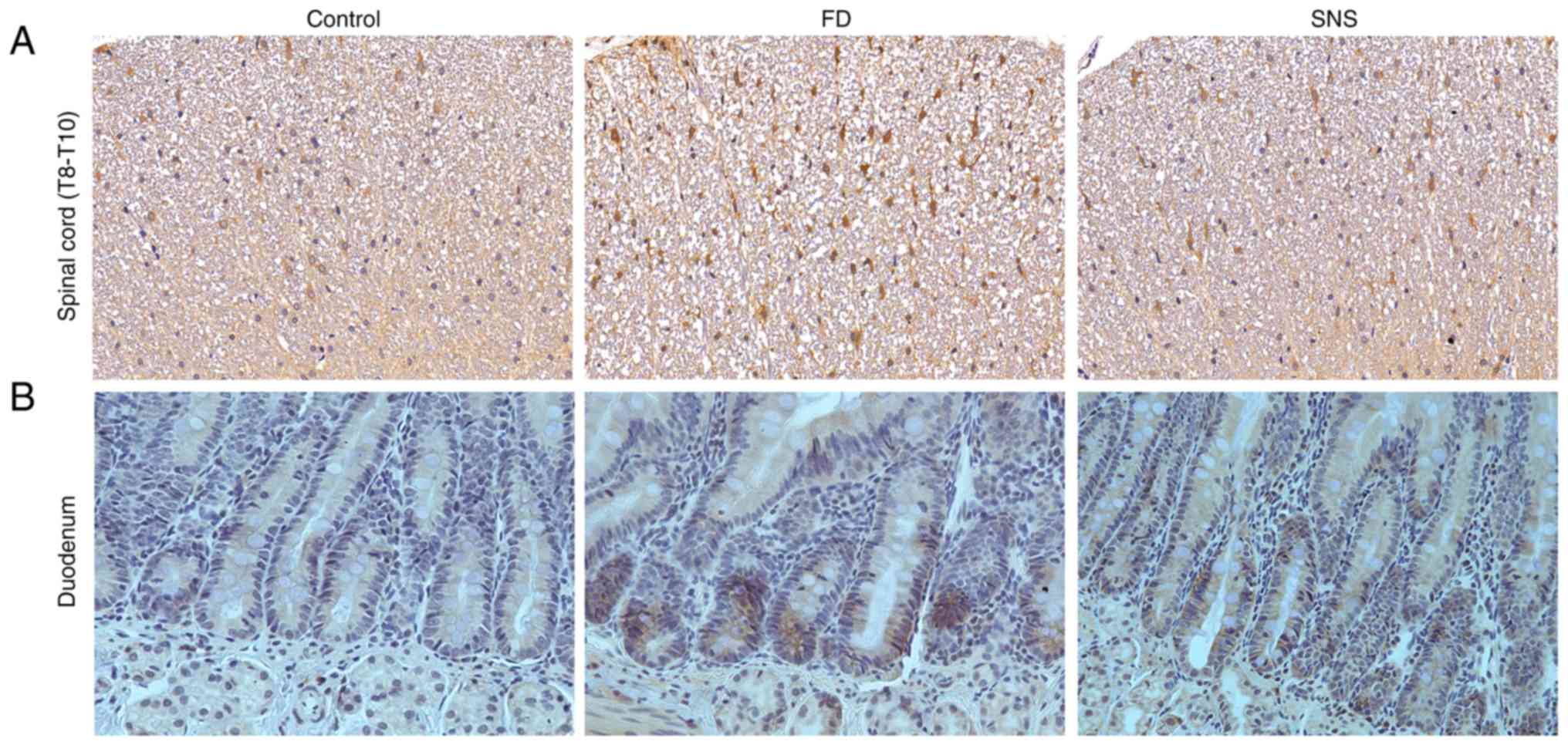
P<0.01). A similar expression trend was observed in spinal cord
tissues. The protein expression levels of CRF in spinal cord
(T8-T10) (Fig. 2A) and duodenal
tissues (Fig. 2B) were also
detected, using IHC. The average optical density value (IOD/area)
increased in spinal cord and duodenum of model group (both
P<0.01), but was decreased following SNS treatment (both
P<0.01).
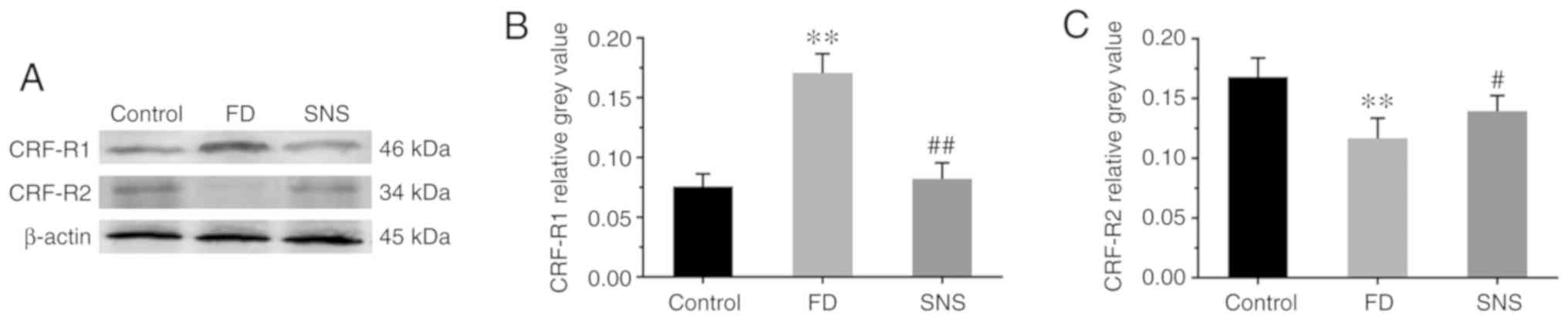
SNS regulates the expression of CRF-R1
and CRF-R2 in the duodenum
CRF activity is initiated via the activation of CRF
receptors, primarily CRF-R1 and CRF-R2, within the gut wall
(18). Therefore, the expression
of CRF-R1 and CRF-R2 was detected in duodenal tissues (Fig. 3A). The expression levels of CRF-R1
(Fig. 3B) increased markedly in
the FD group compared with the control group (0.17±0.02 vs.
0.08±0.01; P<0.01), but were reduced in the SNS group (0.08±0.01
vs. 0.17±0.02; P<0.01). However, the expression level of CRF-R2
(Fig. 3C) was the opposite of
that of CRF-R1 (FD group vs. control group, 0.12±0.02 vs. 0.17±0.02
P<0.01; SNS group vs. FD group, 0.14±0.01 vs. 0.12±0.02;
P<0.05). These findings suggested that SNS downregulated the
expression level of CRF in the duodenum and central nervous system,
and modulated the expression of both CRF-R1 and CRF-R2.
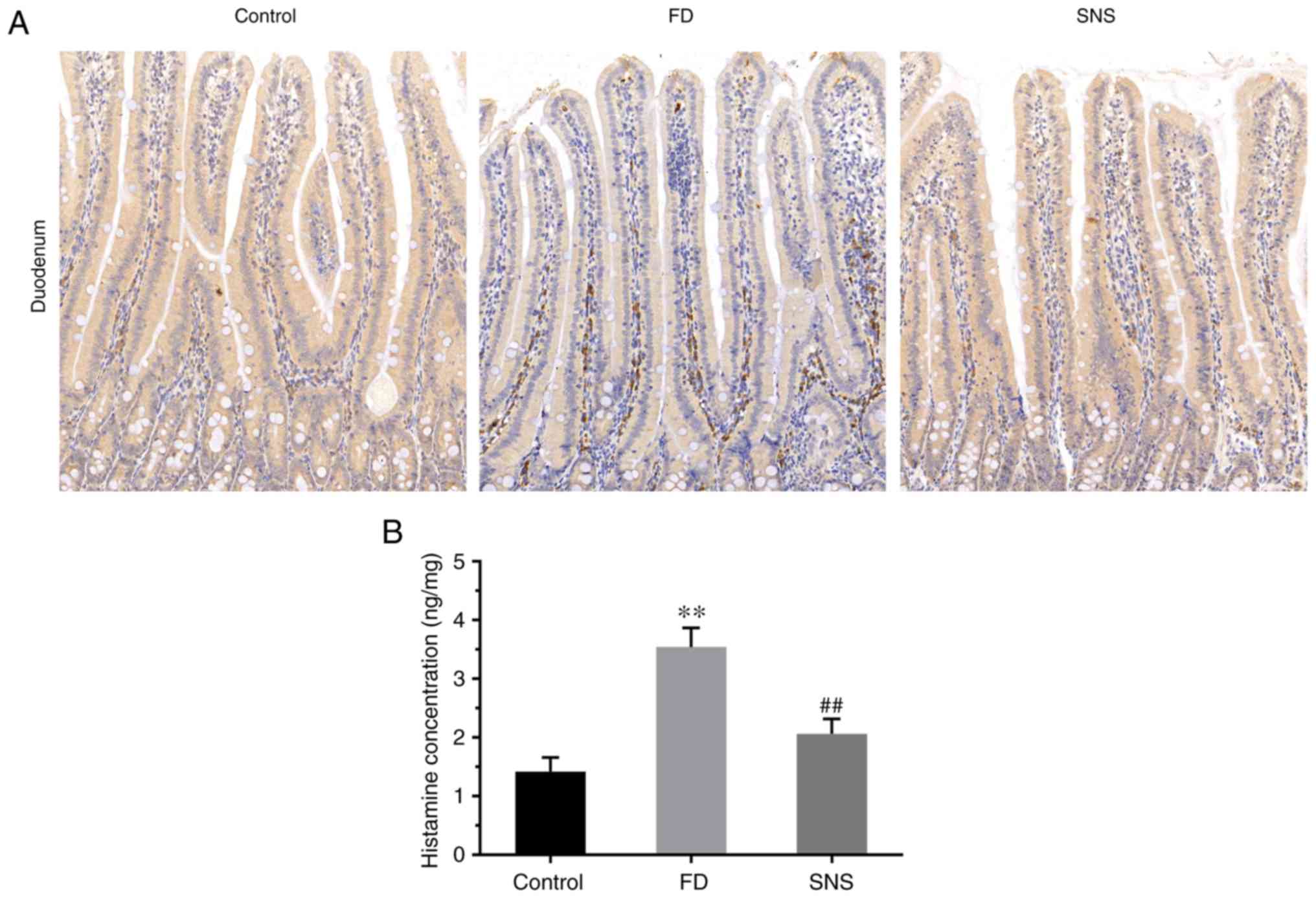
SNS modulates MC infiltration
MCs are an important contributor to the mucosal
micro-inflammation observed in FD (19), and the fact that MC activation
negatively impacts intestinal permeability is well known (4,20,21). Histamine and tryptase are
important indicators of MC activation, thus the influence of SNS on
MC infiltration was assessed by IHC staining of tryptase (Fig. 4A) and the histamine concentration
quantified using ELISA (Fig. 4B).
The results indicated a prominent increase in the average optical
density of tryptase-positive cells in FD rats, compared with that
of the control rats [(6.27±0.54) ×103 vs. (1.57±0.26)
×103; P<0.01]. Furthermore, the average optical
density of tryptase-positive cells was decreased following SNS
treatment [(2.30±0.32) ×103 vs. (6.27±0.54)
×103; P<0.01]. The ELISA results showed that the
concentration of histamine was significantly higher in the FD rats,
compared with the control group (3.54±0.33 vs. 1.42±0.24;
P<0.01), which was suppressed in response to SNS treatment
(2.06±0.25 vs. 3.54±0.33; P<0.01). These data demonstrate that
SNS suppressed low-grade inflammation by alleviating MC
infiltration.
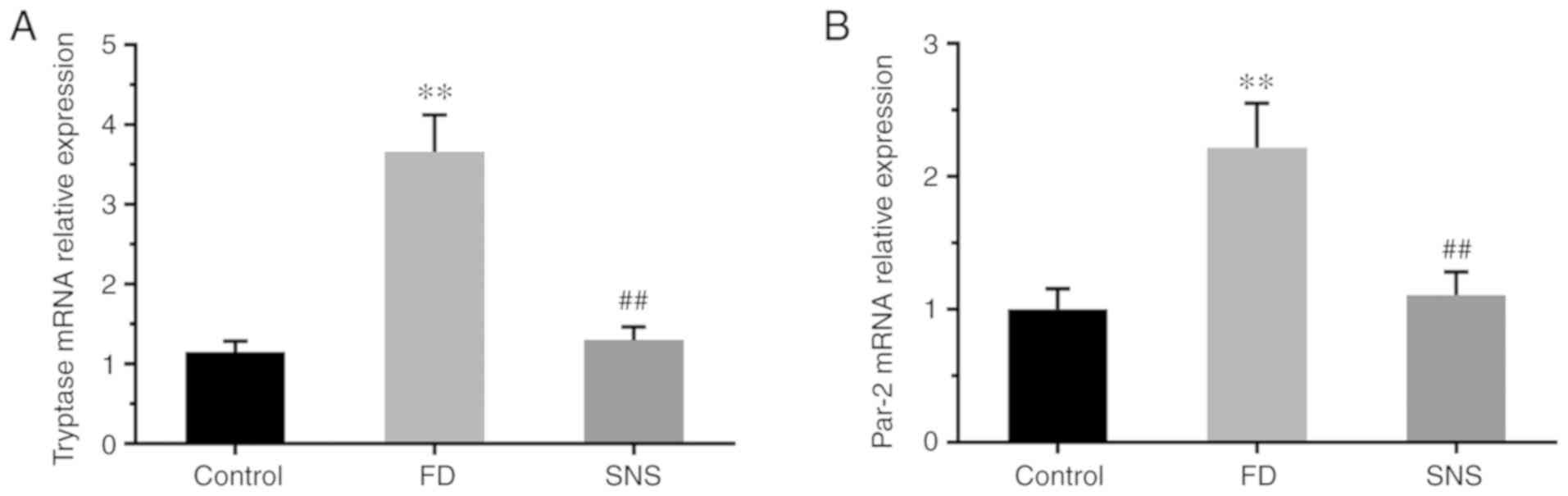
SNS decreases the mRNA expression level
of tryptase and PAR-2
PAR-2 regulates gastrointestinal motility and
epithelial barrier function (22), and is activated by proteases
(principally MC tryptase) in the intestine (23). The potential effects of SNS on
MC-associated tryptase/PAR-2 in the duodenum of FD rats were
therefore determined. Tryptase (Fig.
5A) and PAR-2 (Fig. 5B) mRNA
expression levels were significantly higher in the duodenum of FD
rats than in the control group (PAR-2: 2.22±0.36 vs. 1.00±0.15,
P<0.01; tryptase: 3.66±0.46 vs. 1.15±0.14, P<0.01). Following
SNS treatment in the FD group, the mRNA expression levels of PAR-2
(1.11±0.18 vs. 2.22±0.36; P<0.01) and tryptase (1.30±0.17 vs.
3.66±0.46; P<0.01) were significantly reduced. These findings
suggest that the MC tryptase/PAR-2 signaling pathway can be
regulated by SNS.
SNS restores duodenal mucosal barrier
function in FD rats
Impaired duodenal mucosal integrity is apparent in
patients with FD, and the symptoms of FD are attributable to this
phenomenon. Thus the present study also aimed to determine whether
SNS restores the duodenal mucosal barrier by regulating the
expression of tight junction (TJ) and adherens junction (AJ)
proteins (Fig. 6A and D). The
protein expression levels of ZO-1 (Fig. 6B; 0.22±0.02 vs. 0.51±0.02;
P<0.01), JAM-1 (Fig. 6C;
0.16±0.02 vs. 0.24±0.03; P<0.01) and β-catenin (Fig. 6E; 0.21±0.04 vs. 0.39±0.05;
P<0.01) were significantly reduced in the duodenal tissues of FD
rats, compared with those of the control group. Furthermore, SNS
treatment enhanced the expression levels of these proteins in FD
rats (ZO-1: 0.47±0.01 vs. 0.22±0.02, P<0.01; JAM-1: 0.19±0.03
vs. 0.16±0.02, P<0.05; and β-catenin: 0.37±0.04 vs. 0.21±0.04,
P<0.01). However, there were no marked abnormalities in the
protein expression levels of E-cadherin among the three groups
(Fig. 6F; control group vs. FD
group: P=0.868; FD group vs. SNS group: P=0.624). These results
indicated that SNS upregulated the expression of ZO-1, JAM-1 and
β-catenin, and that it may potentially be used to restore the
duodenal mucosal barrier.
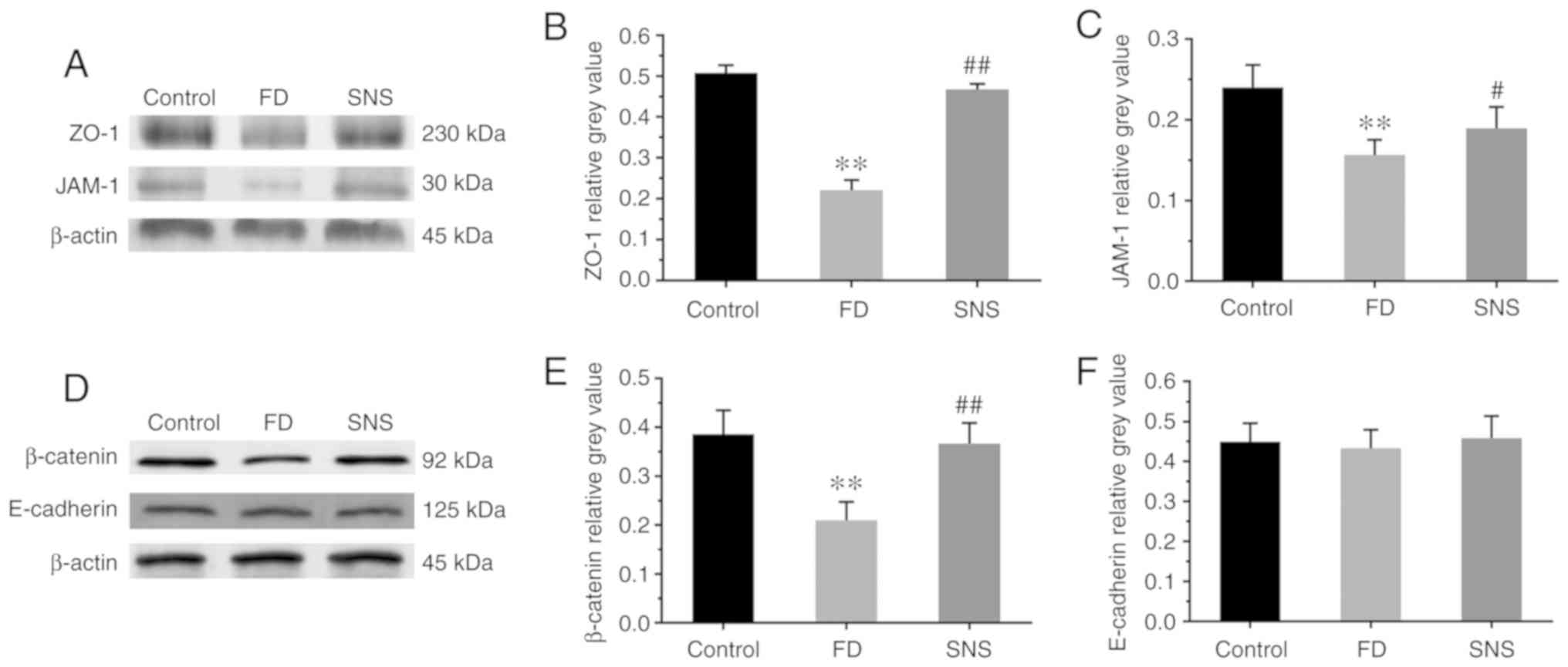 | Figure 6(A and D) Western blot analysis of
ZO-1, JAM-1, β-catenin and E-cadherin expression in duodenal
tissues. Relative grey values of ZO-1, JAM-1, β-catenin and
E-cadherin expression levels. (B, C, E and F) Compared with the
control group, the protein expression level of ZO-1, JAM-1 and
β-catenin was markedly reduced in the model rats, and SNS treatment
increased the expression levels of these three proteins. There was
no significant difference in the protein expression level of
E-cadherin between groups. N=6 for each group.
**P<0.01 compared with the control group;
#P<0.05 and ##P<0.01 compared with the
model group. ZO-1, zona occludens protein-1; JAM-1, junctional
adhesion molecule-1; SNS, Sini San; FD, functional dyspepsia. |
Discussion
The present study demonstrated that SNS treatment
restored duodenal mucosal barrier integrity and ameliorated
low-grade inflammation by modulating the gut-brain interaction in a
rat model of FD. The underlying therapeutic mechanisms of SNS are
the regulation of the CRF pathway and suppressed infiltration of
MCs in the duodenum, and a subsequent increase the expression
levels of ZO-1, JAM-1 and β-catenin.
The integrity of the duodenal mucosal barrier is of
great significance to keep the intestinal homeostasis (24). Changes in mucosal permeability and
tight junction protein expression have been reported in numerous
gastrointestinal diseases, including ulcerative colitis, Crohn's
disease and irritable bowel syndrome (25-27). Previous studies have found that
intestinal permeability is increased in patients with FD (4,20),
and that the intestinal epithelium between the enterocytes is
sealed by tight and adherens junctions, and desmosomes (28). As important components of the
epithelial mechanical barrier, TJ proteins protect the intestinal
mucosa from foreign antigens, toxins and invasion by environmental
microorganisms (29). ZO-1 is a
scaffolding protein that plays a key role in the formation of TJs
(30), while JAM-1 is localized
to the TJs of epithelial and endothelial cells and is closely
associated with the regulation of junctional integrity and
permeability (31). Research has
suggested that acute stress reduces the expression of TJ proteins
in the duodenal mucosa (32).
β-catenin and E-cadherin are important components of cell-to-cell
adhesion proteins, which maintain cell and tissue polarity and
integrity (33). Previous
research demonstrated that SNS reduced duodenum mucosal
permeability and restored the expression levels of claudin-1 and
occluding (14), but it remained
unclear whether SNS could regulate AJ proteins, or indeed, other TJ
proteins. In the present study, decreased expression levels of
ZO-1, JAM-1 and β-catenin were evident in FD rats, compared with
the control group, and SNS treatment restored these expression
levels to a significant degree. Another key finding of the present
study was that SNS treatment also restored duodenal epithelial
barrier integrity, which may be the underlying therapeutic
mechanism of SNS in the treatment of FD.
The importance of low-grade inflammation in FD has
been reported and confirmed in recent years (4). Systemic responses including an
increased number of circulating lymphocytes, elevated expression
levels of pro-inflammatory cytokines, and low-grade inflammation in
the duodenum may promote the symptoms of FD (19). MCs are one of the dominant factors
within the inflammatory infiltrate of the duodenum in FD, which has
been previously confirmed (34,35). In the present study, significant
increases in the number of tryptase-positive MCs and the
concentration of histamine were observed, suggesting that
MC-associated pathways are activated in FD model rats. Findings of
a previous study have suggested that there are interactive
relationships between the gut epithelial barrier and intestinal
inflammation (29). Degranulated
MCs release MC tryptase that activates PAR-2 in the intestine,
increasing paracellular permeability (10). The present study revealed that the
mRNA expression levels of tryptase and PAR-2 were significantly
increased in the FD group, indicating that the tryptase/PAR-2
pathway had been activated. Of note, it was also demonstrated for
the first time that SNS suppressed the infiltration of MCs and
inhibited the tryptase/PAR-2 pathway. Specifically, the influence
of SNS on MC infiltration was assessed by IHC staining of tryptase.
Results showed that the number of tryptase-positive MCs was
elevated in the model group, and reduced following SNS treatment.
The trend of histamine expression was the same, whereas tryptase
and PAR-2 mRNA expression levels were significantly reduced in the
SNS group. These findings suggest that the MC tryptase/PAR-2
signaling pathway can be regulated by SNS. However, it remains
unclear whether the central nervous system is involved in these
pathological alterations.
It is widely acknowledged that pathological
responses to gut-brain disorders are associated with FD (36). Anxiety and depression exist in a
considerable proportion of patients with FD, and these individuals
are prone to FD symptoms when exposed to stress (37). CRF is a key regulator of the
brain-gut axis in response to stress, and is widely expressed in
the central nervous system and the gastrointestinal tract. Various
studies have shown that CRF signaling pathways affect inflammation
and epithelial permeability in the intestine (9,38),
but as yet, it is not known whether CRF is an important regulatory
factor in low-grade inflammation and mucus barrier integrity in the
duodenum of FD patients. Another key finding of the present study
was the identification of differences in the expression levels of
CRF between the control and the FD groups, and that SNS
significantly reduced these expression levels in both the central
nervous system and the duodenum. It has also been reported that CRF
regulates mucosal permeability via MCs (39). CRF in the brain and its periphery
acts via its engagement with CRF-R1 and CRF-R2. Novel research
suggests that CRF-R1 and CRF-R2 signaling is dynamic, but reaches a
state of equilibrium in healthy individuals, thus can be utilized
to determine functional changes in the gastrointestinal tract
induced by stress (40). In the
acute responses to immunologic and psychologic stress, CRF-R1
expressed on MCs as positive, global modulators of MC degranulation
(41), while CRF-R2 plays a
negative role in modulating the degranulation of MCs (42). In the present study, SNS treatment
restored the balance of CRF1 and CRF2 signaling, suggesting that
SNS is involved in the gut-brain interaction by regulating the CRF
signaling pathway.
To conclude, the present study confirmed that
decreased expression levels of AJ and TJ proteins, increased
infiltration of MCs, and differential expression of CRF pathway
components are associated with FD. Moreover, it was elucidated for
the first time that the therapeutic effect of SNS on FD was
achieved by restoring mucosal barrier integrity and suppressing
low-grade inflammation in the duodenum, which was mediated via the
CRF signaling pathway.
Abbreviations:
|
FD
|
functional dyspepsia
|
|
SNS
|
Sini San
|
|
CRF
|
corticotropin-releasing factor
|
|
CRF-R
|
corticotropin-releasing factor
receptor
|
|
PAR-2
|
protease-activated receptor 2
|
|
ZO-1
|
zona occludens protein 1
|
|
JAM-1
|
junctional adhesion molecule 1
|
Acknowledgments
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81774215).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ and CZ contributed to the study concept and
design; CZ and JZ performed the experiments and statistical
analysis; CZ and LZ were involved in manuscript drafting and
revision. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Guide for the Care and Use of Laboratory Animals published by
the National Institutes of Health, and was approved by the Animal
Care and Use Committee of Beijing Institute of Traditional Chinese
Medicine (no. 2018030101).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Tack J, Talley NJ, Camilleri M, Holtmann
G, Hu P, Malagelada JR and Stanghellini V: Functional
gastroduodenal disorders. Gastroenterology. 130:1466–1479. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stanghellini V, Chan FK, Hasler WL,
Malagelada JR, Suzuki H, Tack J and Talley NJ: Gastroduodenal
disorders. Gastroenterology. 150:1380–1392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Konturek SJ, Konturek PC, Pawlik T,
Sliwowski Z, Ochmański W and Hahn EG: Duodenal mucosal protection
by bicarbonate secretion and its mechanism. J Physiol Pharmacol.
55(Suppl 2): S5–S17. 2014.
|
|
4
|
Vanheel H, Vicario M, Vanuytsel T, Van
Oudenhove L, Martinez C, Keita ÅV, Pardon N, Santos J, Söderholm
JD, Tack J and Farré R: Impaired duodenal mucosal integrity and
low-grade inflammation in functional dyspepsia. Gut. 63:262–271.
2014. View Article : Google Scholar
|
|
5
|
Ishigami H, Matsumura T, Kasamatsu S,
Hamanaka S, Taida T, Okimoto K, Saito K, Minemura S, Maruoka D,
Nakagawa T, et al: Endoscopy-guided evaluation of duodenal mucosal
permeability in functional dyspepsia. Clin Transl Gastroenterol.
8:e832017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koloski NA, Jones M, Kalantar J, Weltman
M, Zaguirre J and Talley NJ: The brain-gut pathway in functional
gastrointestinal disorders is bidirectional: A 12-year prospective
population-based study. Gut. 61:1284–1290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stengel A and Taché YF: Activation of
brain somatostatin signaling suppresses CRF receptor-mediated
stress response. Front Neurosci. 11:2312017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hagiwara SI, Kaushal E, Paruthiyil S,
Pasricha PJ, Hasdemir B and Bhargava A: Gastric
corticotropin-releasing factor influences mast cell infiltration in
a rat model of functional dyspepsia. PLoS One. 13:e2037042018.
View Article : Google Scholar
|
|
9
|
Wouters MM, Vicario M and Santos J: The
role of mast cells in functional GI disorders. Gut. 65:155–168.
2016. View Article : Google Scholar
|
|
10
|
Jacob C, Yang PC, Darmoul D, Amadesi S,
Saito T, Cottrell GS, Coelho AM, Singh P, Grady EF, Perdue M and
Bunnett NW: Mast cell tryptase controls paracellular permeability
of the intestine. Role of protease-activated receptor 2 and
beta-arrestins. J Biol Chem. 280:31936–31948. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng He and Qin Ling: Clinical efficacy of
Sini powder and western medicine in the treatment of mixed cold and
heat functional dyspepsia. Acta Chin Med Pharmacol. 42:148–150.
2014.
|
|
12
|
Han YY and Wang HB: Clinical observation
of modified sini powder in the treatment of 30 cases of functional
dyspepsia with depression. Beijing J Trad Chin Med. 30:457–458.
2011.
|
|
13
|
Li Y, Sun Y, Ma X, Xue X, Zhang W, Wu Z,
Ouyang Y, Chen J and Wang W, Guo S and Wang W: Effects of Sini san
used alone and in combination with fluoxetine on central and
peripheral 5-HT levels in a rat model of depression. J Tradit Chin
Med. 33:674–681. 2013. View Article : Google Scholar
|
|
14
|
Chang X, Zhao L, Wang J, Lu X and Zhang S:
Sinisan improves duodenal tight junction integrity in a rat model
of functional dyspepsia. BMC Complement Altern Med. 17:4322017.
View Article : Google Scholar
|
|
15
|
Chinese Pharmacopoeia Commission:
Pharmacopoeia of the People's Republic of China. 1. China Medical
Science Press; pp. 86–280. 2015
|
|
16
|
Wu ZY, Zhang SS, Li PC, Lu XF, Wang ZF,
Wang JJ and Li XL: Rat model of functional dyspepsia resulting from
iodoacet-amide-treated and tail-squeezed. Chin J Integrated
Traditional Western Med Digestion. 23:462–466. 2015.
|
|
17
|
Taché Y and Perdue MH: Role of peripheral
CRF signalling pathways in stress-related alterations of gut
motility and mucosal function. Neurogastroenterol Motil. 16(Suppl
1): S137–S142. 2004. View Article : Google Scholar
|
|
18
|
Taché Y, Martinez V, Million M and Rivier
J: Corticotropin-releasing factor and the brain-gut motor response
to stress. Can J Gastroenterol. 13(Suppl A): A18–A25. 1999.
View Article : Google Scholar
|
|
19
|
Walker MM and Talley NJ: The role of
duodenal inflammation in functional dyspepsia. J Clin
Gastroenterol. 51:12–18. 2017. View Article : Google Scholar
|
|
20
|
Neilan NA, Garg UC, Schurman JV and
Friesen CA: Intestinal permeability in children/adolescents with
functional dyspepsia. BMC Res Notes. 7:2752014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Larson D and Mitre E: Histamine release
and surface CD200R1 staining as sensitive methods for assessing
murine mast cell activation. J Immunol Methods. 379:15–22. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fernández-Blanco JA, Hollenberg MD,
Martinez V and Vergara P: PAR-2-mediated control of barrier
function and motility differs between early and late phases of
postinfectious gut dysfunction in the rat. Am J Physiol
Gastrointest Liver Physiol. 304:G390–G400. 2013. View Article : Google Scholar
|
|
23
|
Li S, Guan J, Ge M, Huang P, Lin Y and Gan
X: Intestinal mucosal injury induced by tryptase-activated
protease-activated receptor 2 requires β-arrestin-2 in vitro. Mol
Med Rep. 12:7181–7187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Camilleri M, Madsen K, Spiller R,
Greenwood-Van Meerveld B and Verne GN: Intestinal barrier function
in health and gastrointestinal disease. Neurogastroenterol Motil.
24:503–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stremmel W, Staffer S, Schneider MJ,
Gan-Schreier H, Wannhoff A, Stuhrmann N, Gauss A, Wolburg H,
Mahringer A, Swidsinski A and Efferth T: Genetic mouse models with
intestinal-specific tight junction deletion resemble an ulcerative
colitis phenotype. J Crohns Colitis. 11:1247–1257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Landy J, Ronde E, English N, Clark SK,
Hart AL, Knight SC, Ciclitira PJ and Al-Hassi HO: Tight junctions
in inflammatory bowel diseases and inflammatory bowel disease
associated colorectal cancer. World J Gastroenterol. 22:3117–3126.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martinez C, Vicario M, Ramos L, Lobo B,
Mosquera JL, Alonso C, Sánchez A, Guilarte M, Antolín M, de Torres
I, et al: The jejunum of diarrhea-predominant irritable bowel
syndrome shows molecular alterations in the tight junction
signaling pathway that are associated with mucosal pathobiology and
clinical manifestations. Am J Gastroenterol. 107:736–746. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boeckxstaens G, Camilleri M, Sifrim D,
Houghton LA, Elsenbruch S, Lindberg G, Azpiroz F and Parkman HP:
Fundamentals of neurogastroenterology:
Physiology/motility-sensation. Gastroenterology. Feb. 18–2016.Epub
ahead of print. View Article : Google Scholar
|
|
29
|
Pastorelli L, De Salvo C, Mercado JR,
Vecchi M and Pizarro TT: Central role of the gut epithelial barrier
in the pathogenesis of chronic intestinal inflammation: Lessons
learned from animal models and human genetics. Front Immunol.
4:2802013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hamada K, Shitara Y, Sekine S and Horie T:
Zonula occludens-1 alterations and enhanced intestinal permeability
in methotrexate-treated rats. Cancer Chemother Pharmacol.
66:1031–1038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Naik UP and Eckfeld K: Junctional adhesion
molecule 1 (JAM-1). J Biol Regul Homeost Agents. 17:341–347.
2003.
|
|
32
|
Lee HS, Kim DK, Kim YB and Lee KJ: Effect
of acute stress on immune cell counts and the expression of tight
junction proteins in the duodenal mucosa of rats. Gut Liver.
7:190–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian X, Liu Z, Niu B, Zhang J, Tan TK, Lee
SR, Zhao Y, Harris DC and Zheng G: E-cadherin/β-catenin complex and
the epithelial barrier. J Biomed Biotechnol. 2011:5673052011.
View Article : Google Scholar
|
|
34
|
Yuan HP, Li Z, Zhang Y, Li XP, Li FK and
Li YQ: Anxiety and depression are associated with increased counts
and degranulation of duodenal mast cells in functional dyspepsia.
Int J Clin Exp Med. 8:8010–8014. 2015.PubMed/NCBI
|
|
35
|
Vanheel H, Vicario M, Boesmans W,
Vanuytsel T, Salvo-Romero E, Tack J and Farré R: Activation of
eosinophils and mast cells in functional dyspepsia: An
ultrastructural evaluation. Sci Rep. 8:53832018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Powell N, Walker MM and Talley NJ: The
mucosal immune system: Master regulator of bidirectional gut-brain
communications. Nat Rev Gastroenterol Hepatol. 14:143–159. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
De la Roca-Chiapas JM, Solís-Ortiz S,
Fajardo-Araujo M, Sosa M, Córdova-Fraga T and Rosa-Zarate A: Stress
profile, coping style, anxiety, depression, and gastric emptying as
predictors of functional dyspepsia: A case-control study. J
Psychosom Res. 68:73–81. 2010. View Article : Google Scholar
|
|
38
|
Overman EL, Rivier JE and Moeser AJ: CRF
induces intestinal epithelial barrier injury via the release of
mast cell proteases and TNF-α. PLoS One. 7:e399352012. View Article : Google Scholar
|
|
39
|
Wallon C, Yang PC, Keita AV, Ericson AC,
McKay DM, Sherman PM, Perdue MH and Söderholm JD:
Corticotropin-releasing hormone (CRH) regulates macromolecular
permeability via mast cells in normal human colonic biopsies in
vitro. Gut. 57:50–58. 2008. View Article : Google Scholar
|
|
40
|
Nozu T and Okumura T:
Corticotropin-releasing factor receptor type 1 and type 2
interaction in irritable bowel syndrome. J Gastroenterol.
50:819–830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ayyadurai S, Gibson AJ, D'Costa S, Overman
EL, Sommerville LJ, Poopal AC, Mackey E, Li Y and Moeser AJ:
Frontline science: Corticotropin-releasing factor receptor subtype
1 is a critical modulator of mast cell degranulation and
stress-induced pathophysiology. J Leukoc Biol. 102:1299–1312. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
D'Costa S, Ayyadurai S, Gibson AJ, Mackey
E, Rajput M, Sommerville LJ, Wilson N, Li Y, Kubat E, Kumar A, et
al: Mast cell corticotropin-releasing factor subtype 2 suppresses
mast cell degranulation and limits the severity of anaphylaxis and
stress-induced intestinal permeability. J Allergy Clin Immunol.
143:1865–1877. 2019. View Article : Google Scholar
|