Introduction
Folkman (1)
proposed that tumor growth was vascularization-dependent, as there
were numerous antitumor studies focused on anti-angiogenesis.
Angiogenesis is the process by which new capillaries are formed
from existing blood vessels to supply the tumor, and it is
hypothesized to be essential for the continued growth and
metastasis of solid tumors (2).
Therefore, preventing tumor angiogenesis may prevent tumor growth.
However, the results of anti-angiogenic therapies for treating
patients with cancer remain unsatisfactory (3,4).
An increasing number of studies have shown that the process of
angiogenesis is far more complicated than initially expected
(5). Aside from angiogenesis,
there are other cellular mechanisms contributing to the generation
of vasculature in tumors, including vasculogenic mimicry, bone
marrow-derived vasculogenesis and cancer stem-like cell-derived
vasculogenesis (6). Cancer
stem-like cells have been reported to differentiate into vascular
endothelial cells and contribute to the formation of a vascular
system in tumors (7,8). Therefore, cancer stem-like
cell-derived vasculogenesis should be considered in anti-angiogenic
therapies for cancer treatment.
Autophagy is a physiological process that captures
and degrades intracellular erroneous proteins and damaged
organelles to maintain intracellular metabolism (9). Numerous studies have shown that
autophagy serves an important role in cell differentiation. For
example, terminal differentiation of immune cells is highly
dependent on functional autophagy (10-12). Nuschke et al (13) demonstrated that undifferentiated
bone marrow mesenchymal stem cells do not employ autophagy despite
the accumulation of non-degraded autophagic vacuoles; however, when
cells are stimulated by osteogenic differentiation, the rate of
autophagy also increases. The essential role of autophagy in
differentiation has been demonstrated in hepatic stem/progenitor
cells (14), cardiac stem cells
(15) and neuronal cells
(16).
Bussolati et al (17) reported that breast tumor
stem/progenitor cells could differentiate into endothelial cells.
In the present study, breast cancer stem-like cells (BCSLCs),
characterized as CD44+CD24−/low, were
isolated from MCF-7 breast cancer cells and induced to
differentiate into endothelial cells using vascular endothelial
growth factor (VEGF), and the association between autophagy and
endothelial differentiation of BCSLCs was examined.
Materials and methods
Cells
MCF-7 cells were purchased from the China Center for
Type Culture Collection and cultured in DMEM/F12 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin
(Sangon Biotech Co., Ltd.) at 37°C in 5% CO2 atmosphere.
Human umbilical vein endothelial cells (HUVECs; cat. no.
ATCC® CRL-1730™; American Type Culture Collection)
supplemented with F-12K medium (cat. no. ATCC 30-2004™; American
Type Culture Collection) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.), 1% penicillin-streptomycin (Sangon
Biotech Co., Ltd.), 0.1 mg/ml heparin (cat. no. H3393;
Sigma-Aldrich; Merck KGaA) and 1% endothelial cell growth
supplement (cat. no. 354006; BD Biosciences) at 37°C in 5%
CO2. Pre-made lentiviral particles expressing a fusion
target of green fluorescent protein (GFP)-red fluorescent protein
(RFP)-LC3 (cat. no. JM-1314L204H-S) were purchased from Shanghai
Genomeditech Co. Ltd. and transfected into MCF-7 cells according to
the manufacturer's protocol. In brief, 1×104 MCF-7
cells/well were plated in a 6-well plate and cultured for 12 h to
ensure that the cell density reached 30-50% when the virus was
infected the following day. Health Gene transgene reagent (Health
Gene Technologies) and 3×108 TU/ml lentivirus were used
to transfect MCF-7 cells; 6 µg/ml polybrene was added to
promote the transfection. After culture for 24 h, the medium was
renewed and cultured for another 8 h, then 1.5 µg/ml
puromycin (Sigma-Aldrich; Merck KgA) was added to isolate resistant
cells. Then, monoclonal transfected cells were obtained and used
for subsequent experiments.
Isolation of BCSLCs
BCSLCs, defined as
CD44+CD24−/low MCF-7 cells, were isolated
using a Miltenyi Microbead kit (Miltenyi Biotec GmbH) according to
the manufacturer's instructions and cultured in stem cell medium,
comprising serum-free medium supplemented with 20 ng/ml epidermal
growth factor (EGF; Gibco; Thermo Fisher Scientific, Inc.), 20
ng/ml basic fibroblast growth factor (bFGF; Gibco; Thermo Fisher
Scientific, Inc.) and 10 ng/ml B27 (Gibco; Thermo Fisher
Scientific, Inc.). BCSLCs were grown to microsphere formation at
37°C with 5% CO2 and observed under an inverted
fluorescence microscope (magnification, ×20; Leica DM IL LED Fluo;
Leica Microsystems GmbH).
Flow cytometry
Sorted BCSLCs were cultured for 12 h in stem cell
medium supplemented with 10 ng/ml VEGF, and VEGF-induced BCSLC and
control BCSLCs were analyzed using flow cytometry. Cells were
collected and adjusted to a cell density of 5×105
cells/tube. Cells were centrifuged at 300 × g for 10 min at 4°C,
the supernatant was discarded, and cells were resuspended in 100
µl buffer plus the corresponding antibody (1:20), and
incubated at 4°C for 8 min in the dark. Subsequently, the cells
were washed with 1 ml buffer and centrifuged at 300 × g for 10 min
at 4°C. The supernatant was discarded, cells were resuspended in
500 µl buffer, stored in the dark on ice, and flow cytometry
was performed within 30 min. The following primary antibodies were
used: Fluorescein isothiocyanate (FITC)-conjugated anti-human CD44
(cat. no. 130-113-903; Miltenyi Biotec GmbH), phycoerythrin
(PE)-conjugated anti-human CD24 (cat. no. 130-098-861; Miltenyi
Biotec GmbH) PE-conjugated anti-human CD31 (cat. no. 130-110-807;
Miltenyi Biotec GmbH) and FITC-conjugated anti-human CD105 (cat.
no. 130-098-778; Miltenyi Biotec GmbH). Flow cytometry was
performed using a FACSCalibur flow cytometer (BD Biosciences). The
flow cytometry analysis software used was FlowJo™ v10.6.1 (BD
Biosciences).
Matrigel tube formation assay
The growth and differentiation status of BCSLCs
in vitro was evaluated by a Matrigel formation experiment.
Matrigel (BD Biosciences) was thawed, and the blot was added to a
96-well plate at 40 µl/well, with 3 replicate wells. The
96-well plate was then placed in a 37°C incubator for 45 min. After
the gel had coagulated, MCF-7 cells and BCSLCs were seeded onto
Matrigel at a density of 104 cells/well. The cells were
cultured for 12 h in complete or stem cell medium supplemented with
or without 10 ng/ml VEGF. Tube formation was observed under an
inverted microscope (magnification, ×10; Olympus Corporation).
Griess method for detecting nitric oxide
(NO) content
A total of 5×104 cells/well were plated
in a 6-well plate, and cultured in stem cell medium supplemented
with or without 10 ng/ml VEGF. After 3 days, the cell were ~80%
confluent and sub-cultured. After passaging, the cells were
considered second generation of differentiation. The
second-generation cell culture solution was used to detect the NO
content. The content of NO in the supernatant was measured using
the Griess assay protocol as previously described (18). The NO content in HUVEC culture
medium was used as the control.
Confocal microscopy of LC3
mRFP-GFP-LC3 lentivirus was purchased from Shanghai
Genomeditech Co. Ltd. MCF-7 cells were infected with lentivirus as
aforementioned and transfected cells were selected for with 1.5
µg/ml puromycin. mRFP-GFP-LC3-transformed BCSLCs were
isolated using a Miltenyi Microbead kit. The selected mRFP-GFP-LC3
double-labeled BCSLCs were seeded at a density of 1×104
cells/well in complete culture medium without VEGF, or stem cell
culture solution with or without 10 ng/ml VEGF. The localization of
RFP and GFP was observed using a confocal laser scanning microscope
(Olympus Corporation) at ×100 or ×600 magnification.
Reverse transcription-quantitative
(RT-q)PCR analysis
The expression of CD105 was detected via RT-qPCR.
Total RNA was extracted from cells using TRIzol extraction reagent
(Takara Bio, Inc.). For reverse transcription, a PrimeScript™
Reverse Transcription kit (Takara Bio, Inc.) was used according to
the manufacturer's instructions. qPCR was performed using SYBR
Premix Ex Taq with Tli RNaseH Plus (Takara Bio, Inc.) according to
the manufacturer's instructions. GAPDH was used as the internal
reference. The PCR reaction conditions consisted of
pre-denaturation at 95°C for 30 sec, followed by 40 cycles of
denaturation at 95°C for 5 sec and extension at 59°C for 30 sec.
The sequence of the qPCR primers were as follows: CD105, forward
5′-AGA GGA CAG GGG TGA CAA GGT-3′, reverse 5′-AAG TGT GGG CTG AGG
TAG AGG-3′; and GAPDH, forward 5′-AGA AGG CTG GGG CTC ATT TG-3′,
reverse 5′-AGG GGC CAT CCA CAG TCT TC-3′. Expression was measured
using the 2−∆∆Cq method (19).
For the evaluation of endothelial differentiation,
after sorting, cells were seeded at 2×105 cells/well in
6-well plates and divided into four groups: Control group; 10 ng/ml
VEGF, 10 ng/ml VEGF + 100 nM rapamycin (RAPA; Sigma-Aldrich; Merck
KGaA) or 10 ng/ml VEGF + 400 nM bafilomycin A1 (Baf A1; Abcam).
Cells were treated for 24 h, and cell morphology and CD105
expression was evaluated.
RNA interference
Short hairpin (sh)-autophagy related 5 (Atg5) was
inserted into a lentiviral vector (pGMLV-SC7 RNAi; Shanghai
Genomeditech Co. Ltd.). The sequences of the shRNA were: Forward
5′-GCA GAT GGA CAG TTG CAC ACA C-3′, reverse, 5′-AGG TGT TTC CAA
CAT TGG CTC A-3′. The transfection of sh-Atg5 lentivirus into MCF-7
was conducted according to the same protocol as the transfection of
GFP-RFP-LC3 lentivirus into MCF-7 cells described above. sh-Atg5
BCSLCs were isolated using a Miltenyi Microbead kit, seeded in
6-well plates at a density of 2×105 cells/well, and
cultured for 24 h in stem cell medium with or without 10 ng/ml
VEGF.
Western blot analysis
Western blot analysis was used to detect the
expression of autophagy-associated proteins. Cells were seeded at a
density of 2×105 cells/well in a 6-well plate and
treated according to the experimental design. After the end of the
experiment, total protein was extracted using a Whole Protein
Extraction kit (cat. no. BC3710; Beijing Solarbio Science &
Technology Co., Ltd.) and the protein concentration was determined
using a Bicinchoninic Acid Protein Assay kit (cat. no. PC0020;
Beijing Solarbio Science & Technology Co., Ltd.). Proteins (20
µg/lane) were separated via 12% SDS-PAGE and transferred to
a PVDF membrane. Membranes were blocked with 5% skimmed milk for 1
h at room temperature, and subsequently incubated with primary
antibodies (1:1,000) at 4°C overnight and incubated for 1 h at room
temperature the following day. After washing with TBS-0.05%
Tween-20 (TBST), the membrane was incubated with secondary antibody
(1:1,000) for 2 h at room temperature. The membrane was washed
thoroughly with TBST and signals were visualized using enhanced
chemiluminescence reagent (Beijing Solarbio Science &
Technology Co., Ltd.). Grayscale analysis was performed using
Gel-Pro Analyzer V.4.0 (Meyer Instruments, Inc.). The primary
antibodies used were β-actin (cat. no. 4970), LC3 (cat. no. 4108),
p62 (cat. no. 5114), Beclin1 (cat. no. 3495), Atg3 (cat. no. 3415),
Atg5 (cat. no. 12994), Atg7 (cat. no. 8558), Atg16L1 (cat. no.
8089), Unc-51-like autophagy activating kinase (ULK; cat. no.
8054), phosphorylated (p)-ULK (cat. no. 5869), AMP kinase (AMPK;
cat. no. 5832) and p-AMPK (cat. no. 2535); the secondary antibody
used was a horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G antibody (cat. no. 7074). All antibodies were
purchased from Cell Signaling Technology, Inc.
To monitor autophagy flux during endothelial
differentiation, after sorting, cells were seeded at
2×105 cells/well in 6-well plates and divided into three
groups: 0 h VEGF; 24 h VEGF (10 ng/ml); and 24 h VEGF + 1 h
chloroquine (CQ; 50 µM; Sigma-Aldrich; Merck KGaA).
Statistical analysis
Results are presented as the mean ± standard
deviation of at least three independent experiments. Data were
analyzed by t-test, or by one-way ANOVA followed by Tukey's test
post hoc analysis. Statistical analysis was performed using
Graph-Pad Prism 6.01 software (GraphPad Software, Inc.). P<0.05
was considered to indicate a statistically significant
difference.
Results
BCSLC sorting and microsphere
culture
Cancer stem cells (CSCs) are a type of tumor cell
that are capable of self-renewal and differentiation (20). A previous study showed that CSCs
can survive in serum-free medium containing bFGF, EGF, B27 and
other growth factors, and gradually form microspheres during
culture (21). Cells
characterized as CD44+CD24−/low are
considered to be breast cancer stem cells. First,
CD24−/low cells were isolated by sorting using
immunomagnetic beads, and the expression of CD24 was detected via
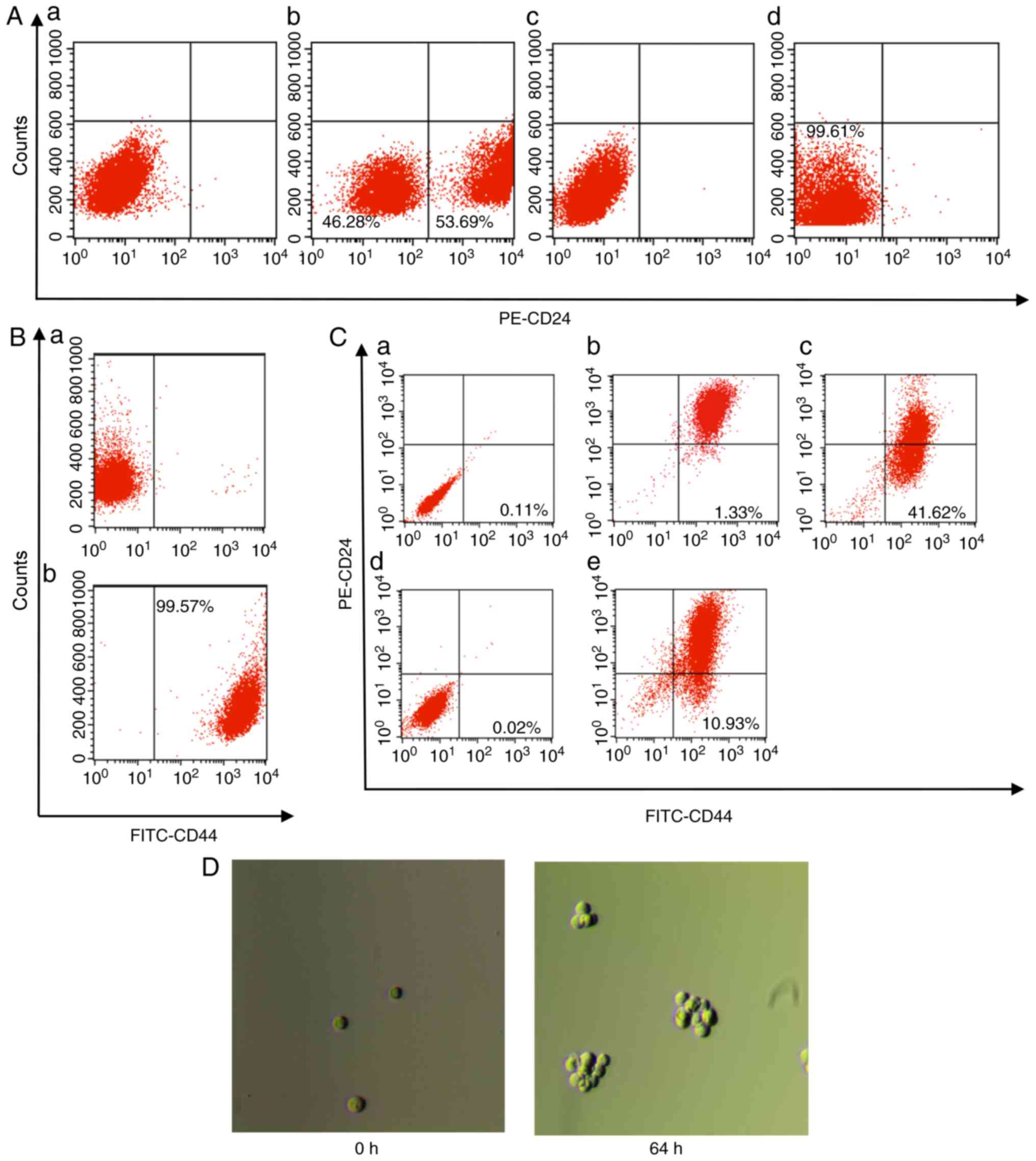
flow cytometry. As presented in Fig.
1A, the proportion of cells characterized as CD24+
in MCF-7 was 53.69 and 46.28% were cells characterized as
CD24−/low; the two cell populations had notable
partitions (Fig. 1Ab). The
proportion of CD24−/low cells obtained using
immunomagnetic beads was 99.61%, indicating that the purity of the
obtained CD24−/low type cell was high and could be used
for the following isolations (Fig.
1Ad). CD44+ cells were further sorted from the
isolated CD24−/low cells using immunomagnetic beads, and
the results of flow cytometry showed that the proportion of
CD44+ cells was 99.57% (Fig. 1B). The obtained
CD44+CD24−/low cells were considered BCSLCs,
and the obtained BCSLCs were identified using flow cytometry; the
results showed that the proportion of
CD44+CD24−/low cells in BCSLCs was 41.62%
(Fig. 1Cc), considerably higher
than the proportion of cells obtained from the MCF-7 cells (1.33%;
Fig. 1Cb). Therefore, the
CD44+CD24−/low cells were enriched using
immunomagnetic beads. The obtained BCSLCs were sub-cultured, and
the proportion of CD44+CD24−/low cells
decreased to 10.93% when cultured up to passage 8 (Fig. 1Ce).
Microspheres gradually formed when BCSLCs were
cultured in serum-free medium containing bFGF and EGF. The obtained
BCSLCs with CD44+CD24−/low specificity were
cultured in stem cell culture medium, and the results showed that
microspheres gradually formed when the cells were cultured for 64 h
or more (Fig. 1D), suggesting
that the obtained BCSLCs exhibited characteristics associated with
cancer stem cells.
Endothelial differentiation of BCSLCs in
vitro
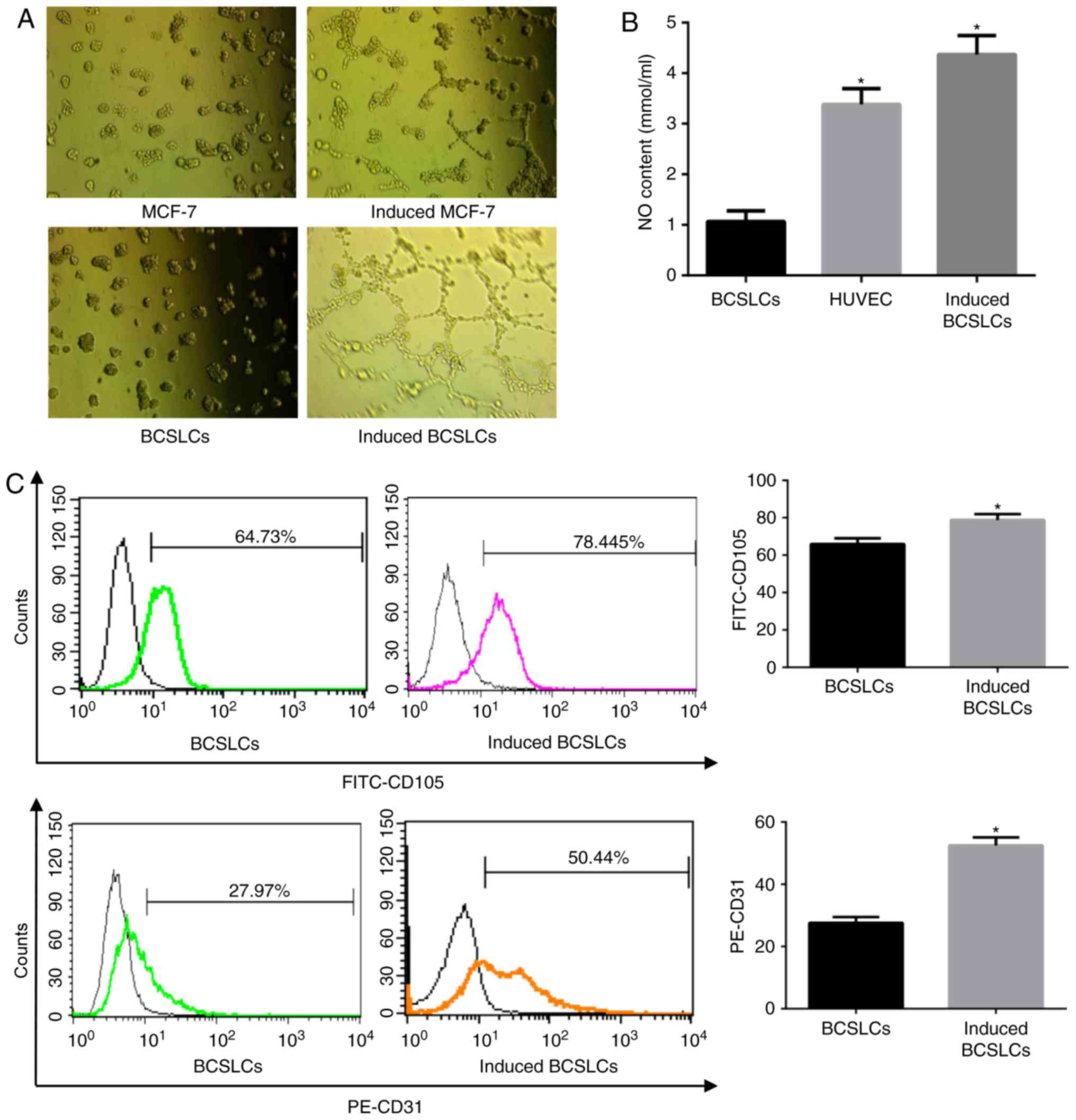
VEGF was used as an inducer of endothelial
differentiation. MCF-7 and BCSLCs were seeded on Matrigel and
cultured for 12 h in stem cell medium with or without VEGF to mimic
the growth and differentiation of cells in vivo. As
presented in Fig. 2A, after
incubation with VEGF-containing stem cell culture medium, BCSLCs
formed a tubular structure, whereas MCF-7 cells did not.
NO, which is an endothelium-dependent relaxation
factor, is secreted by vascular endothelial cells expressing active
NO synthase (22). The release of
NO can be used as an indicator of endothelial cell viability
(23). The results showed that
BCSLCs treated with VEGF released significantly higher quantities
of NO compared with cells not treated with VEGF (Fig. 2B). The quantity of NO released by
induced BCSLCs was higher compared with the levels released by
HUVECs. Thus, BCSLCs exhibited a property typically associated with
endothelial cells when induced by VEGF.
CD31 and CD105 are used as markers of endothelial
cells (24). The BCSLCs were
cultured with or without VEGF for 12 h, and the expression of CD105
and CD31 was assessed using flow cytometry. The results showed that
in induced cells, the proportion of cells expressing CD105 and CD31
was 78.45 and 50.44%, respectively, significantly higher compared
with cells not treated with VEGF (Fig. 2C). These results suggested that
after being induced by VEGF, BCSLCs expressed endothelial cell
markers.
Alterations in autophagy during
endothelial differentiation of BCSLCs
Autophagy is a dynamic physiological metabolic
process in the cell. Detection of autophagy flow can more
accurately reflect the changes of autophagy metabolism (25). Chloroquine (CQ) is used as an
autophagy inhibitor, as it can alter the pH of the lysosome to
abrogate lysosomal function, and inhibit binding of the
autophagosome to the lysosome, thus blocking autophagy and
resulting in an accumulation of autophagosomes (26). To monitor autophagy flux during
endothelial differentiation of BCSLCs, CQ was used to inhibit
autophagy, and the expression of Beclin1, p62 and LC3-II was
detected via western blotting. The results showed that the
expression levels of Beclin1, p62 and LC3-II were significantly
increased when the BCSLCs were induced by VEGF for 24 h (Fig. 3A), suggesting that autophagy was
increased. When the BCSLSs were treated with CQ for 1 h following
treatment with VEGF, the expression of LC3-II and p62 was increased
significantly, and the expression of Beclin1 was decreased
significantly compared with cells not treated with CQ (Fig. 3A). Treatment with CQ would result
in the accumulation of autophagosomes, which would reduce
expression of upstream Beclin1 expression via negative feedback
regulation (27). The
accumulation of LC3-II reflects the increase in autophagy flux when
induced by VEGF.
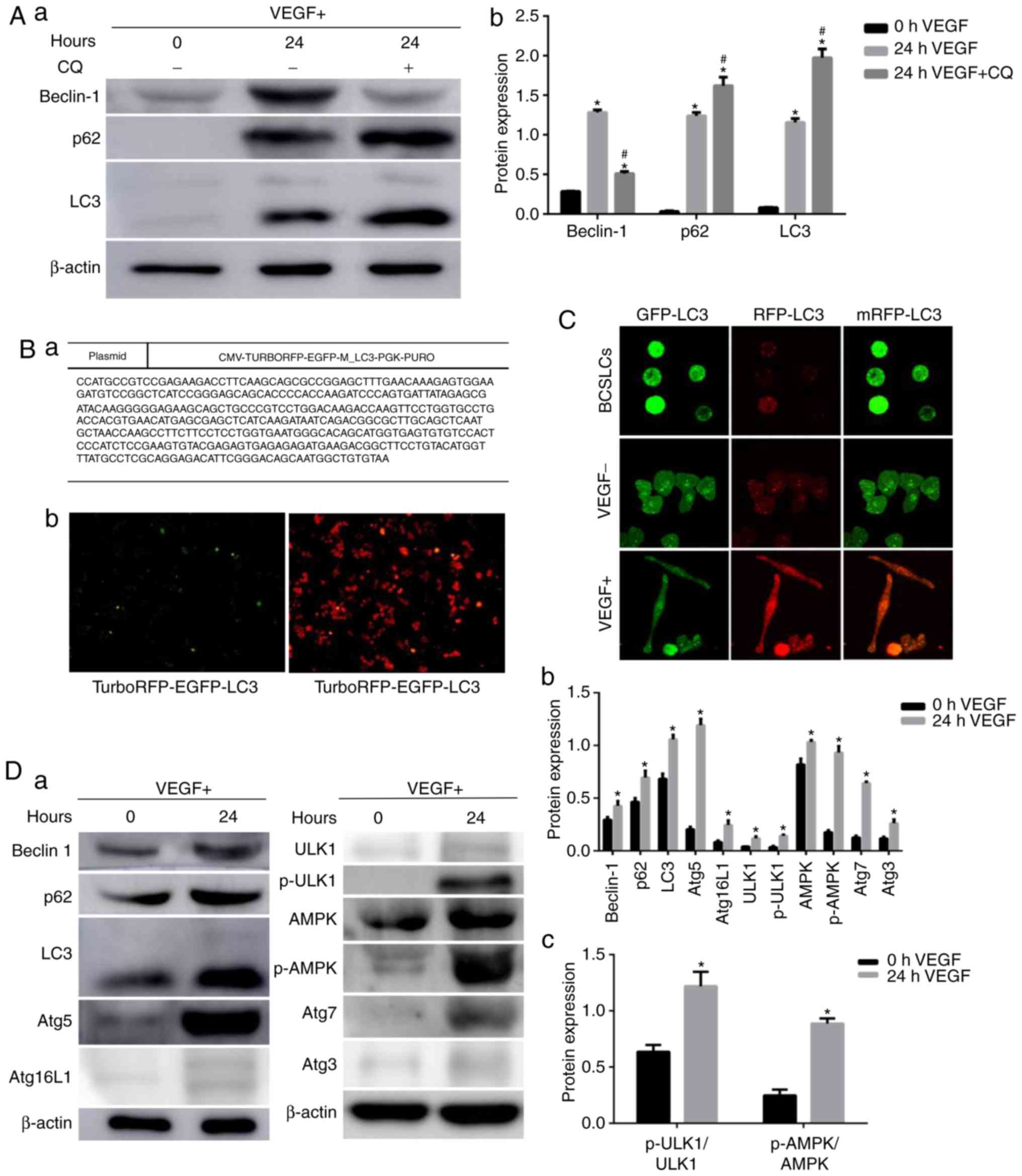 | Figure 3Alterations in autophagy activity
during endothelial differentiation of BCSLCs. (A) BCSLCs were
cultured in stem cell culture medium containing VEGF for 0 and 24
h, with or without CQ. Western blotting was used to detect the
expression of Beclin1, p62 and LC3-II. (a) Example western blot
figure, (b) grayscale analysis. Data were analyzed by one-way ANOVA
followed by Tukey's test post hoc analysis; *P<0.05
vs. 0 h VEGF group; #P<0.05 vs. 24 h VEGF group. (B)
MCF7 cells transfected with RFP-GFP-LC3. (Ba) Sequencing results of
the RFP-GFP-LC3 plasmid. (B-b) Fluorescent expression of GFP and
RFP in MCF7 cells following transfection. (C) BCSLCs were cultured
in complete medium or stem cell medium with or without VEGF for 24
h, and the RFP and GFP intensity were observed under a confocal
laser scanning microscope. (D) BCSLCs were cultured in stem cell
culture medium containing VEGF for 0 and 24 h, and the expression
of autophagy signaling pathway-associated proteins were detected
using western blotting. (a) Example western blot figure, (b and c)
grayscale analysis. Data were analyzed by t-test;
*P<0.05 vs. 0 h VEGF group. AMPK, AMP kinase; Atg,
autophagy related; BCSLC, breast cancer stem-like cell; CQ,
chloroquine; GFP, green fluorescent protein; p, phosphorylated;
RFP, red fluorescent protein; ULK, Unc-51-like autophagy activating
kinase; VEGF, vascular endothelial growth factor. |
MCF-7 cells stably expressing mRFP-GFP-LC3 were
created to further examine the association between autophagy and
endothelial differentiation of BCSLCs. Fig. 3B shows the sequencing results. The
sequence of the inserted fragment in the recombinant clone was
completely identical to the sequence of the target fragment, and
thus the construction of the overexpression vector was successful.
Fluorescent signals were detected after lentiviral infection, and
GFP and RFP were visible, demonstrating successful transfection of
mRFP-GFP-LC3 in MCF-7 cells (Fig.
3B). BCSLCs were isolated from the RFP-GFP-LC3-MCF-7 cells, and
cultured in complete medium, or stem cell medium with or without
VEGF for 24 h, and the cells were observed under a confocal laser
scanning microscope (Fig. 3C).
After 24 h of cell culture, the green fluorescence intensity of the
VEGF-containing group was lower compared with stem cell culture
cells. There was an increase in the number of red and yellow spots
in the cells cultured in the VEGF-containing stem cell culture
medium compared with cells cultured in the complete medium. LC-3
has two forms, LC3-І and LC3-II. LC3-І is diffusely distributed in
cells, and LC3-II accumulates on the inner and outer membranes of
autophagic vesicles, including autophagy precursors, and
autophagosomes, making these autophagic structures appear green in
the transfected cells. When autophagosomes fuse with lysosomes, the
green fluorescence should be quenched due to the low pH, but the
red fluorescence should remain stable (28). Therefore, RFP was used to track
LC3, and the weakening of green fluorescence was used to indicate
the fusion of autophagosomes and lysosomes. Autophagy flux of
BCSLCs in stem cell culture medium containing VEGF was markedly
higher compared with cells grown in stem cell culture medium or
complete medium. BCSLCs were induced into a spindle shape akin to
the typical shape of vascular endothelial cells when treated with
VEGF. These data suggested that the initiation of
transdifferentiation of BCSLCs was accompanied by an increase in
autophagic flux.
Proteins associated with autophagy signaling
pathways were detected by western blotting to further determine the
changes in autophagy levels during the endothelial differentiation
of BCSLCs. The results showed that treatment with VEGF
significantly increased the expression levels of ULK1, p-ULK1, AMPK
and p-AMPK, all of which are upstream of the autophagy signaling
pathway; the expression levels of key proteins involved in the
steps between autophagy formation to autophagic lysosome
degradation, such as Beclin1, Atg5, Atg16L1, Atg7, Atg3 and p62,
were also increased compared with BCSLCs (Fig. 3D). There results suggested that
autophagy levels were increased overall during endothelial
differentiation of BCSLCs induced by VEGF. The analysis of ULK1,
p-ULK1, AMPK, and p-AMPK showed that the ratios of p-AMPK/AMPK and
p-ULK1/ULK1 were also increased significantly (Fig. 3Dc), indicating that the AMPK
protein was activated, which in turn affects downstream ULK1
expression and phosphorylation, thus initiating autophagy (25).
Regulation of autophagy affects
endothelial differentiation of BCSLCs
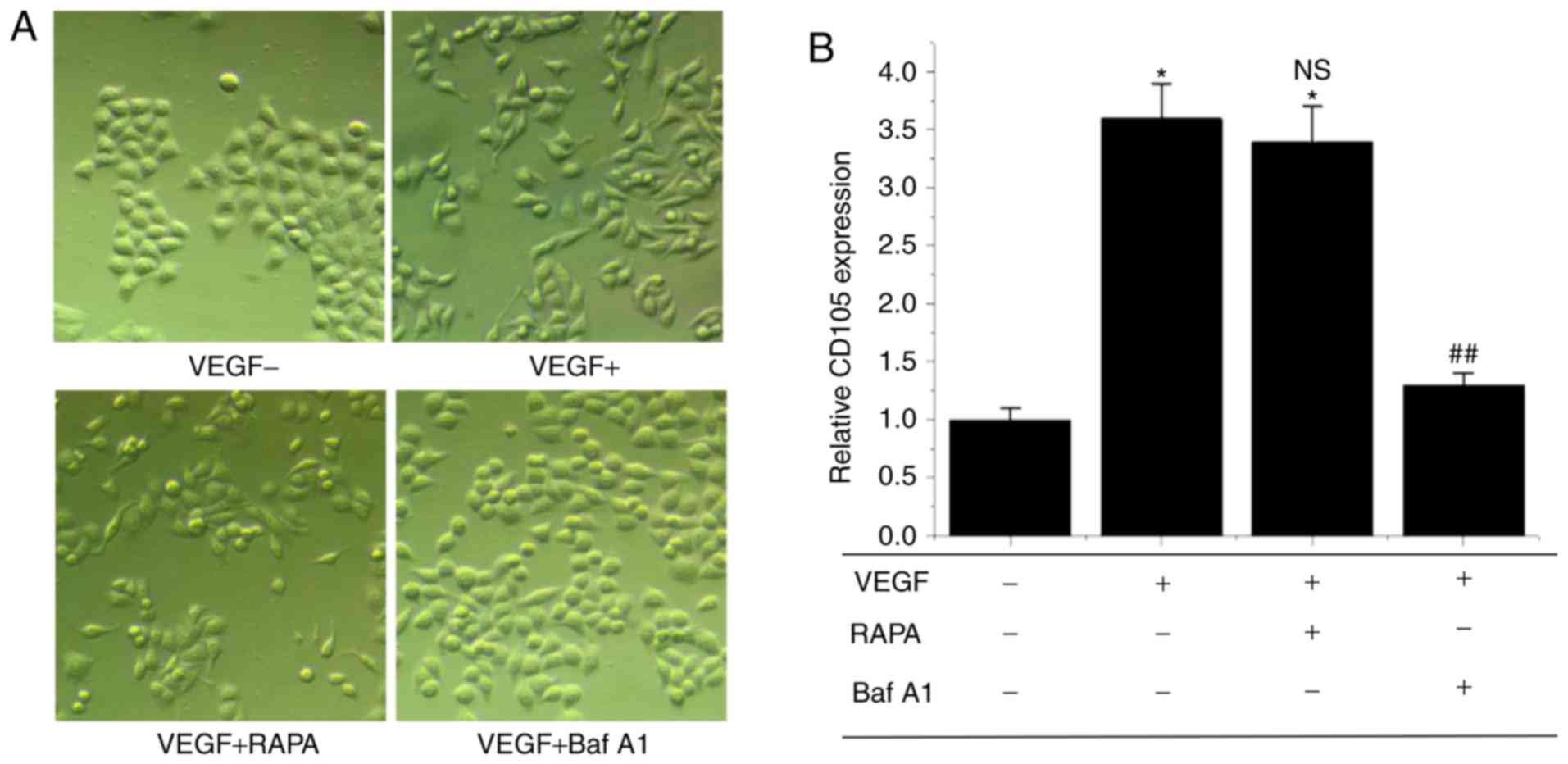
In order to investigate the association between
autophagy and endothelial differentiation of BCSLCs, the autophagy
activator RAPA and inhibitor Baf A1 were used to treat BCSLCs
separately. As shown in Fig. 4A,
cells initially showed an elongated spindle-like shape, similar to
endothelial cells when treated with VEGF. When the cells were
treated with VEGF and RAPA, the cellular morphology was also
elongated and spindle-like, but when the cells were treated with
VEGF and Baf A1, the cellular morphology was cobblestone-like
similar to that of BCSLCs without VEGF. The results suggest a
positive association between autophagy and endothelial
differentiation of BCSLCs. Expression of CD105 was assessed using
RT-qPCR, and the results showed that treatment with VEGF or VEGF +
RAPA increased expression of CD105 significantly compared with
BCSLCs not treated with VEGF, and there was no significant
difference in CD105 expression levels between these two groups.
However, the expression of CD105 was decreased significantly when
BCSLCs were treated with VEGF + Baf A1 compared with the VEGF group
(Fig. 4B). These results were in
agreement with the cellular morphology experiments, and a positive
association between autophagy and the endothelial differentiation
of BCSLCs was further suggested.
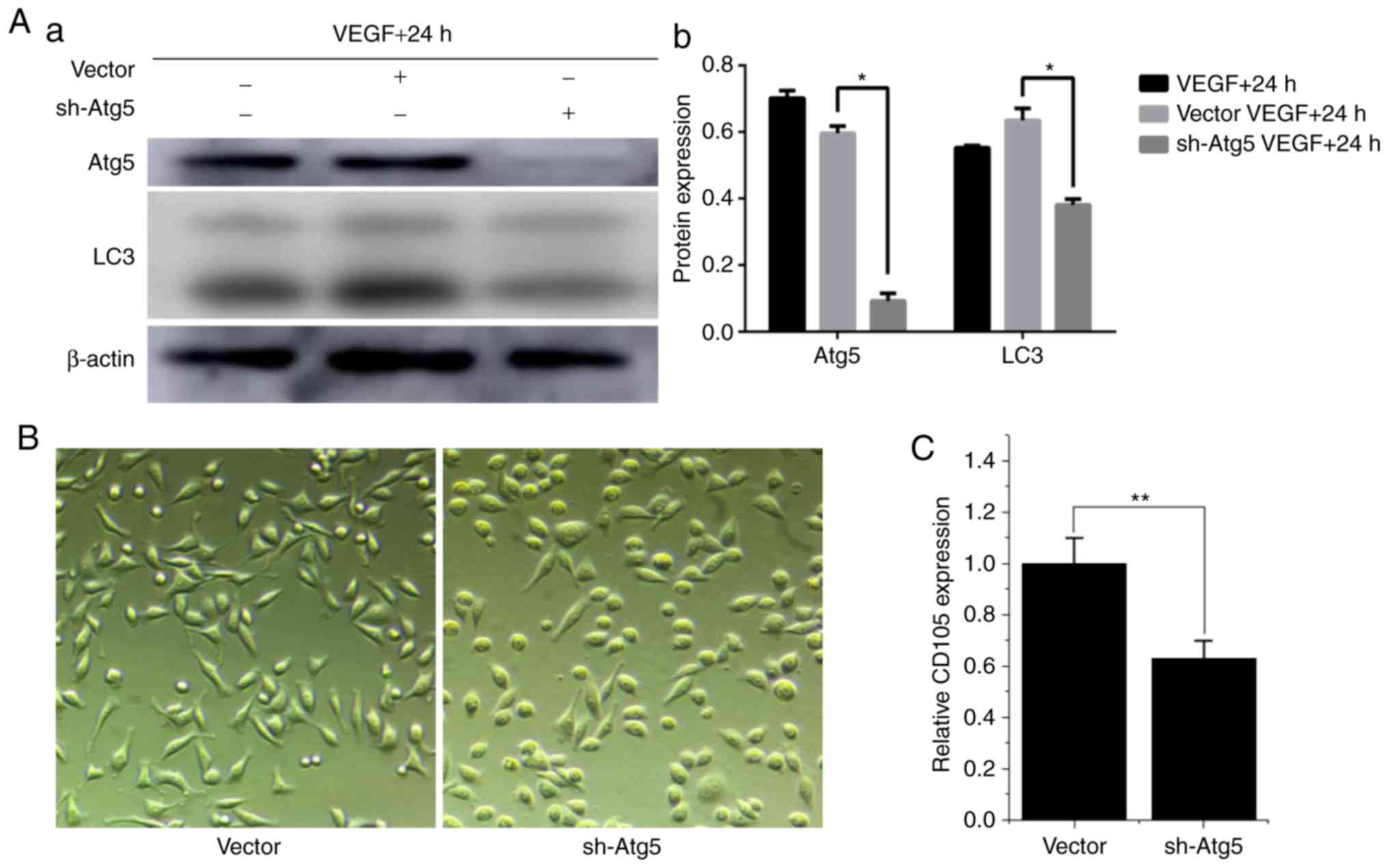
As Atg5 is one of the core proteins involved in
autophagosome formation, an Atg5-knockdown MCF-7 cell line was
established to further investigate if autophagy was critical for
endothelial differentiation of BCSLCs. sh-Atg5-BCSLCs were isolated
from sh-Atg5-MCF-7 and were induced with VEGF for 24 h, and the
expression of Atg5 and LC3-II was assessed via western blotting. As
shown in Fig. 5A, after being
induced with VEGF, the expression levels of Atg5 and LC3-II were
similar in the control and vector groups, but significantly
downregulated in the sh-Atg5 group. This indicated that autophagy
was inhibited in sh-Atg5-BCSLCs. When the sh-Atg5-BCSLCs and
vector-BCSLCs (negative control) were cultured with 1 ng/ml VEGF
for 24 h, the vector-BCSLCs exhibited an elongated spindle-like
morphology similar to endothelial cells; however, the majority of
the sh-Atg5-BCSLCs retained their cobblestone-like morphology
(Fig. 5B). CD105 mRNA expression
levels were measured and the results showed that the expression of
CD105 was significantly decreased in sh-Atg5-BCSLCs following
treatment with VEGF compared with the vector-BCSLCs (Fig. 5C). All the above results suggested
that autophagy is required for effective endothelial
differentiation of BCSLCs.
Discussion
Since Folkman (1)
suggested that blood vessels are necessary for the development and
growth of solid tumors in 1971, there have been a large amount of
data supporting this hypothesis (29). The notion that CSCs are cancer
cells that cause tumor growth and metastasis was first proposed in
leukemia and later in solid tumors, including breast cancer
(30). Studies have shown that
CSCs can differentiate into endothelial progenitor cells (31), endothelial cells (32,33) or vascular smooth muscle-like cells
(34), highlighting an alternate
means of tumor blood vessel formation, and provides a new target
for anti-angiogenic therapy in tumor.
The presence of CSCs has been demonstrated in solid
tumors such as the colon, liver, lung, and pancreas, and breast
cancer stem cells (BCSCs) were the first CSCs to be reported in a
solid tumor (35). Recent
findings have identified different types of BCSCs with various
markers (36). Among these,
CD44+CD24−/low was most frequently used to
characterize BCSCs since it was discovered by Al-Hajj et al
(30) in 2003. In the present
study, CD44+CD24−/low BCSLCs were isolated
from MCF-7 using the magnetic activated cell sorting method, and
the isolated BCSLCs exhibited stemness by forming microspheres in
the stem cell culture medium. VEGF was used as an endothelial
differentiation inducer, and the BCSLCs presented with a long
spindle-like morphology, similar to endothelial cells, and formed
tube-like structures. Additionally, the induced BCSLCs released
increased quantities of NO, and these results showed that the
induced BCSLCs behaved in a similar manner to endothelial cells.
Flow cytometry showed that the induced BCSLCs expressed increased
amounts of CD31 and CD105, both of which are endothelial
cell-specific markers. All the above results indicated that BCSLCs
can be induced to differentiate into endothelial cells by VEGF.
Previous studies have found that autophagy is
associated with stemness maintenance and the differentiation of
stem cells. For example, in hepatocellular carcinoma, it was found
that autophagy promoted cancer cell survival and resistance to
chemotherapeutic drugs, as well as stem cell-like properties
(37). Autophagy was reported to
decrease reactive oxygen species generation and maintain stemness
in mesenchymal stem cells (38).
Notably, Zhang et al (39)
found that increased autophagy in cardiac stem cells promoted
myocardial differentiation. Since then, there have been numerous
studies demonstrating the requirement of autophagy for
differentiation of stem/progenitor cells (20,40). However, whether autophagy
participated in the endothelial differentiation of BCSLCs was
unknown. In the present study, LC3-II, Beclin1 and p62 were used as
autophagy flux indicators, and it was shown that autophagy was
increased significantly when BCSLCs were induced by VEGF for 24 h,
suggesting that autophagy participated in endothelial
differentiation of BCSLCs. The RFP-GFP-LC3-BCSLCs were induced by
VEGF, and an increase in red and yellow puncta, and weakened green
fluorescence intensity were observed, indicative of enhanced
autophagy flux during endothelial differentiation of BCSLCs. In the
autophagy signaling pathway, the initiation of autophagy flux
starts from the phosphorylation of ULK1, and the phosphorylation of
AMPK can activate the phosphorylation of ULK1 (25,41,42). Western blot analysis showed that
after treatment with VEGF, the ratios of p-AMPK/AMPK and
p-ULK1/ULK1 increased significantly, indicating that the AMPK
protein was activated, which in turn affects downstream ULK1
expression and phosphorylation, initiating autophagy. Additionally,
the expression levels of key proteins involved in autophagosome
formation to autophagic lysosome degradation were increased
notably.
Autophagy flux was upregulated during the
endothelial differentiation of BCSLCs; however, whether autophagy
was essential for the endothelial differentiation of BCSLCs
remained to be demonstrated. RAPA and Baf A1 were used to activate
or inhibit autophagy, respectively. VEGF could not induce BCSLCs to
differentiate into endothelial cells if autophagy was inhibited by
Baf A1. This result was further validated using BCSLCs with
knockdown of Atg5. Atg5 is one of the key autophagy proteins, and
Atg5-knockdown is commonly used to investigate the role of
autophagy (43-46). In the present study,
Atg5-knockdown BCSLCs could not be induced to differentiate into
endothelial cells, indicating the important role of autophagy in
endothelial differentiation of BCSLCs.
In conclusion, BCSLCs could be induced to
differentiate into endothelial cells by VEGF in vitro, and
autophagy participates in the endothelial differentiation of
BCSLCs. There was a positive association between autophagy and the
endothelial differentiation of BCSLCs, and autophagy was required
for endothelial differentiation in BCSLCs. Therefore, autophagy may
be a potential target for the treatment of breast cancer, and the
present study provides a theoretical basis for the treatment of
breast cancer with anti-angiogenic therapy by targeting
autophagy.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant. nos. 81373429 and
81671243).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
ZYao and HW contributed to the conception and design
of the study, and revised the manuscript. FC, ZYan and YJ designed
and performed the experiments, and drafted the original manuscript.
CF, YW and RL analyzed the data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bergers G and Hanahan D: Modes of
resistance to anti-angio-genic therapy. Nat Rev Cancer. 8:592–603.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sennino B and McDonald DM: Controlling
escape from angio-genesis inhibitors. Nat Rev Cancer. 12:699–709.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jain RK and Carmeliet P: SnapShot: Tumor
Angiogenesis. Cell. 149:1408–1408.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ricci-Vitiani L, Pallini R, Biffoni M,
Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G,
Larocca LM and De Maria R: Tumour vascularization via endothelial
differentiation of glioblastoma stem-like cells. Nature.
468:824–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang R, Chadalavada K, Wilshire J, Kowalik
U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C and
Tabar V: Glioblastoma stem-like cells give rise to tumour
endothelium. Nature. 468:829–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
White E: The role for autophagy in cancer.
J Clin Invest. 125:42–46. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizushima N and Levine B: Autophagy in
mammalian development and differentiation. Nat Cell Biol.
12:823–830. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bronietzki AW, Schuster M and Schmitz I:
Autophagy in T-cell development, activation and differentiation.
Immunol Cell Biol. 93:25–34. 2015. View Article : Google Scholar
|
|
12
|
Zhang Y, Morgan MJ, Chen K, Choksi S and
Liu ZG: Induction of autophagy is essential for monocyte-macrophage
differentiation. Blood. 119:2895–2905. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nuschke A, Rodrigues M, Stolz DB, Chu CT,
Griffith L and Wells A: Human mesenchymal stem cells/multipotent
stromal cells consume accumulated autophagosomes early in
differentiation. Stem Cell Res Ther. 5:1402014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sugiyama M, Yoshizumi T, Yoshida Y, Bekki
Y, Matsumoto Y, Yoshiya S, Toshima T, Ikegami T, Itoh S, Harimoto
N, et al: p62 Promotes amino acid sensitivity of mTOR pathway and
hepatic differentiation in adult liver stem/progenitor cells. J
Cell Physiol. 232:2112–2124. 2017. View Article : Google Scholar
|
|
15
|
Shi X, Li W, Liu H, Yin D and Zhao J:
β-Cyclodextrin induces the differentiation of resident cardiac stem
cells to cardiomyocytes through autophagy. Biochim Biophys Acta Mol
Cell Res. 1864:1425–1434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vazquez P, Arroba AI, Cecconi F, de la
Rosa EJ, Boya P and de Pablo F: Atg5 and Ambra1 differentially
modulate neurogenesis in neural stem cells. Autophagy. 8:187–199.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bussolati B, Grange C, Sapino A and
Camussi G: Endothelial cell differentiation of human breast tumour
stem/progenitor cells. J Cell Mol Med. 13:309–319. 2009. View Article : Google Scholar
|
|
18
|
Ghasemi A and Zahediasl S: Preanalytical
and analytical considerations for meastutriing nitric oxide
metabolites in serum or plasma using the griess method. Clin Lab.
58:615–624. 2012.
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Ciurea ME, Georgescu AM, Purcaru SO,
Artene SA, Emami GH, Boldeanu MV, Tache DE and Dricu A: Cancer stem
cells: Biological functions and therapeutically targeting. Int J
Mol Sci. 15:8169–8185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kandel J, Bossy-Wetzel E, Radvanyi F,
Klagsbrun M, Folkman J and Hanahan D: Neovascularization is
associated with a switch to the export of bFGF in the multistep
development of fibrosarcoma. Cell. 66:1095–1104. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sturtzel C: Endothelial cells. Adv Exp Med
Biol. 1003:71–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parinandi NL, Sharma A, Eubank TD, Kaufman
BF, Kutala VK, Marsh CB, Ignarro LJ and Kuppusamy P: Nitroaspirin
(NCX-4016), an NO donor, is antiangiogenic through induction of
loss of redox-dependent viability and cytoskeletal reorganization
in endothelial cells. Antioxid Redox Signal. 9:1837–1849. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deliu IC, Neagoe CD, Bezna M,
Genunche-Dumitrescu AV, Toma SC, Ungureanu BS, Uscatu CD, Beznă MC,
Lungulescu CV, Pădureanu V, et al: Correlations between endothelial
cell markers CD31, CD34 and CD105 in colorectal carcinoma. Rom J
Morphol Embryol. 57:1025–1030. 2016.PubMed/NCBI
|
|
25
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, Adhihetty PJ, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy (3rd edition).
Autophagy. 12:1–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maycotte P, Aryal S, Cummings CT, Thorburn
J, Morgan MJ and Thorburn A: Chloroquine sensitizes breast cancer
cells to chemotherapy independent of autophagy. Autophagy.
8:200–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wirth M, Joachim J and Tooze SA:
Autophagosome formation-the role of ULK1 and Beclin1-PI3KC3
complexes in setting the stage. Semin Cancer Biol. 23:301–309.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanida I, Ueno T and Uchiyama Y: Use of
pHlurorin-mKate2-human LC3 to monitor autophagic responses. Methods
Enzymol. 587:87–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ao LY, Li WT, Zhou L, Yan YY, Ye AQ, Liang
BW, Shen WY, Zhu X and Li YM: Therapeutic effects of JLX-001 on
ischemic stroke by inducing autophagy via AMPK-ULK1 signaling
pathway in rats. Brain Res Bull. 153:162–170. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaur S and Bajwa P: A ‘tete-a tete'
between cancer stem cells and endothelial progenitor cells in tumor
angiogenesis. Clin Transl Oncol. 16:115–121. 2014. View Article : Google Scholar
|
|
32
|
Fazioli F, Colella G, Miceli R, Di
Salvatore MG, Gallo M, Boccella S, De Chiara A, Ruosi C and de
Nigris F: Post-surgery fluids promote transition of cancer stem
cell-to-endothelial and AKT/mTOR activity, contributing to relapse
of giant cell tumors of bone. Oncotarget. 8:85040–85053. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chroscinski D, Sampey D and Maherali N:
Reproducibility Project: Cancer Biology; Reproducibility Project
Cancer Biology: Registered report: Tumour vascularization via
endothelial differentiation of glioblastoma stem-like cells. Elife.
4:2015. View Article : Google Scholar
|
|
34
|
El Hallani S, Boisselier B, Peglion F,
Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas JL,
Eichmann A, et al: A new alternative mechanism in glioblastoma
vascularization: Tubular vasculogenic mimicry. Brain. 133:973–982.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pattabiraman DR and Weinberg RA: Tackling
the cancer stem cells-what challenges do they pose? Nat Rev Drug
Discov. 13:497–512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang F, Xu J, Tang L and Guan X: Breast
cancer stem cell: The roles and therapeutic implications. Cell Mol
Life Sci. 74:951–966. 2017. View Article : Google Scholar
|
|
37
|
Liu G, Fan X, Tang M, Chen R, Wang H, Jia
R, Zhou X, Jing W, Wang H, Yang Y, et al: Osteopontin induces
autophagy to promote chemo-resistance in human hepatocellular
carcinoma cells. Cancer Lett. 383:171–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hou J, Han ZP, Jing YY, Yang X, Zhang SS,
Sun K, Hao C, Meng Y, Yu FH, Liu XQ, et al: Autophagy prevents
irradiation injury and maintains stemness through decreasing ROS
generation in mesenchymal stem cells. Cell Death Dis. 4:e8442013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang J, Liu J, Huang Y, Chang JY, Liu L,
McKeehan WL, Martin JF and Wang F: FRS2α-mediated FGF signals
suppress premature differentiation of cardiac stem cells through
regulating autophagy activity. Circ Res. 110:E29–E39. 2012.
View Article : Google Scholar
|
|
40
|
Takao S, Ding Q and Matsubara S:
Pancreatic cancer stem cells: Regulatory networks in the tumor
microenvironment and targeted therapy. J Hepatobiliary Pancreat
Sci. 19:614–620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chun Y and Kim J: Autophagy: An essential
degradation program for cellular homeostasis and life. Cells.
7:E2782018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang S, Li T, Ji T, Yi W, Yang Z, Wang S,
Yang Y and Gu C: AMPK: Potential therapeutic target for ischemic
stroke. Theranostics. 8:4535–4551. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Q, Bu S, Xin D, Li B, Wang L and Lai
D: Autophagy is indispensable for the self-renewal and quiescence
of ovarian cancer spheroid cells with stem cell-like properties.
Oxid Med Cell Longev. 2018:70104722018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shroff A and Reddy KVR: Autophagy gene
ATG5 knockdown upregulates apoptotic cell death during Candida
albicans infection in human vaginal epithelial cells. Am J Reprod
Immunol. 80:e130562018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qian G, Liu D, Hou L, Hamid M, Chen X, Gan
F, Song S and Huang K: Ochratoxin A induces cytoprotective
autophagy via blocking AKT/mTOR signaling pathway in PK-15 cells.
Food Chem Toxicol. 122:120–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee SG and Joe YA: Autophagy mediates
enhancement of proangiogenic activity by hypoxia in mesenchymal
stromal/stem cells. Biochem Biophys Res Commun. 501:941–947. 2018.
View Article : Google Scholar : PubMed/NCBI
|