Introduction
According to the World Health Organization, coronary
artery disease is the most common cause of clinical mortality
worldwide (1,2). Acute myocardial infarction (AMI)
mainly occurs upon coronary occlusion, which is caused by
detachment and rupture of unstable atherosclerotic plaques
(3,4). At present, percutaneous coronary
intervention (5), coronary artery
bypass grafting (6,7) or thrombolytic therapy (8) are effective treatments against AMI,
which work by restoring blood perfusion and oxygen supply. However,
coronary reperfusion can cause secondary damage to ischemic
tissues, which is known as myocardial ischemia reperfusion injury
(MIRI). MIRI usually leads to irreversible damage to
ultrastructure, cell death and increased infarct size; therefore,
MIRI has become a major obstacle in clinical treatment (9,10).
It is widely accepted that the mechanism underlying
MIRI is mainly associated with a sudden increase in the generation
of reactive oxygen species (ROS), neutrophil infiltration, nitric
oxide aggregation, inflammatory reaction, Ca2+ overload,
endoplasmic reticulum stress, disordered energy metabolism,
cardiomyocyte apoptosis, autophagy, necrosis and necroptosis
induced by prolonged IRI (11,12). Cardiomyocyte apoptosis, which is a
type of programmed cell death, is a rare event in healthy
myocardium; however, it is the earliest and predominant form of
cell death in infarcted myocardium and has been associated with
MIRI (13). Following MIRI,
although the blood supply is restored, oxygen free radicals and
overload of Ca2+ produced during ischemia initiate
cardiomyocyte apoptosis. Previous studies have reported that the
structural and functional damage to myocardial cells may primarily
result from apoptosis during MIRI. Preventing the occurrence of
cardiomyocyte apoptosis has become a target for certain drugs that
interfere with MIRI (14,15). It has been reported that curcumin
is a potent cardioprotective compound, which may have beneficial
effects on MIRI by reducing oxidative stress, preventing
inflammation and inhibiting cardiomyocyte apoptosis (16). Numerous studies have indicated
that traditional Chinese medicine (TCM) exerts a potent
cardioprotective effect on MIRI and may be used as a therapeutic
approach for its treatment (17,18).
As a drug used in TCM, ginseng is a perennial herb
belonging to the Araliaceae family and is a species within the
genus Panax. Ginseng has been used in Asian and Western
countries since ancient times. Among the 17 different species
assigned to this genus, the major commercial ginseng varieties,
namely Panax ginseng, Panax notoginseng and Panax
quinquefolium L., are most commonly used as medicines (19). The primary constituents of ginseng
are ginsenosides, triterpenoids and saponins, which have been
reported to exert strong pharmacological activities, including
anti-diabetic, anti-fatigue, anti-depressant and anti-cancer
properties (20,21). Among the 200 types of ginsenosides
and saponins, Rb1, Rb2, Rg1, Rg3, Rd, Re, Rh1 and Rh2 have been
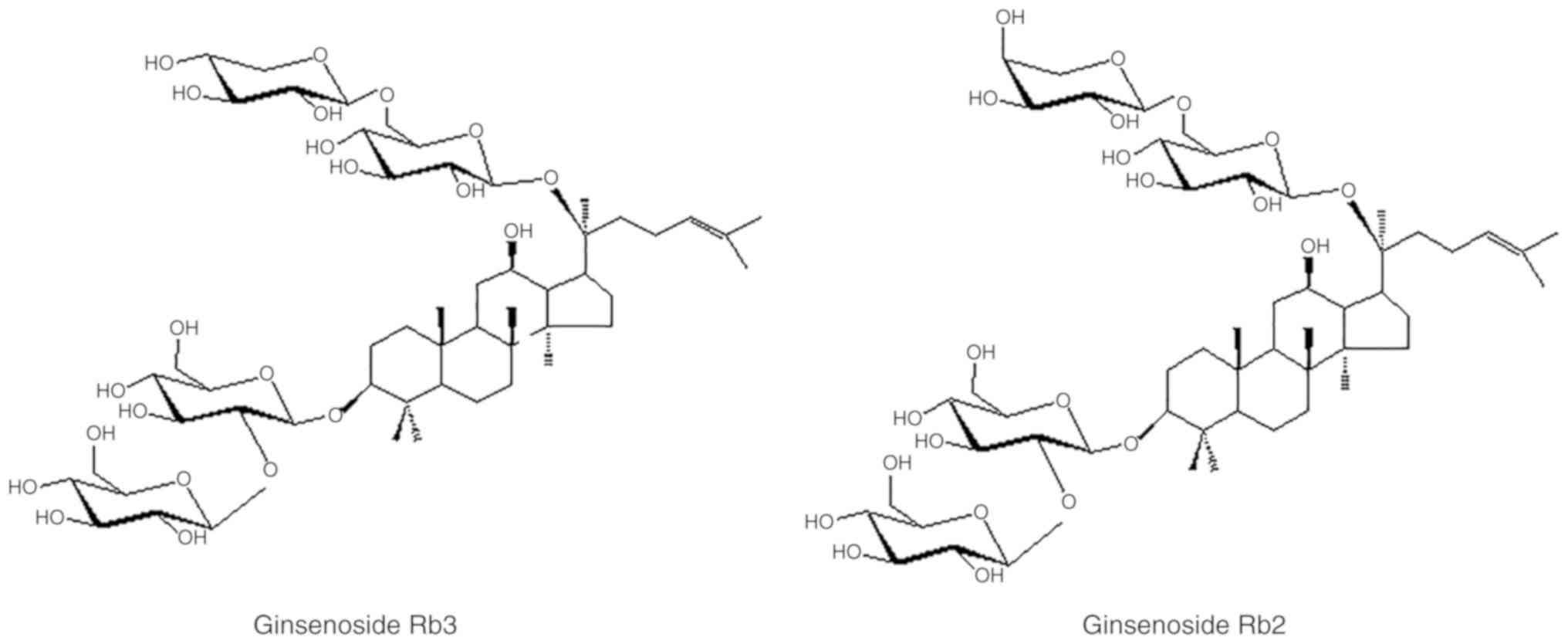
studied most extensively. Ginsenoside Rb2 (G-Rb2) and G-Rb3 share
the same backbone structure of four-ring dammarane, but they differ
in their carbohydrate moieties at C20; G-Rb2 possesses
α-L-arabinose (pyranose), whereas G-Rb3 possesses β-D-xylose
(Fig. 1) (22).
It is difficult to separate G-Rb2 from its isomer
G-Rb3. The process of G-Rb2 removal is complex, which may markedly
increase development costs, making it unsuitable for large-scale
production. Previous studies have demonstrated that G-Rb3 and G-Rb2
exert cardioprotective effects when used alone (23,24). Therefore, the combined use of
G-Rb3 and G-Rb2 (G-Rb3/Rb2) may simplify the isolation process,
reduce production costs and even exert synergistic effects. A
Chinese national patent (CN201210474548.0) has been applied for the
method of extraction of G-Rb3/Rb2, and the content of G-Rb3 and
G-Rb2 in the obtained extract was 95-80 and 5-20%, respectively, as
determined by high-performance liquid chromatography (HPLC)
(25). The present study aimed to
compare the protective effect of G-Rb3/Rb2 and G-Rb3 on MIRI in
rats.
Diltiazem (DLZ) is a representative
non-dihydropyridine calcium antagonist, which possesses mild
peripheral vasodilatation properties, and can increase coronary and
renal blood flow. DLZ has been widely used in the treatment of
ischemic heart disease and hypertension (26,27). Previous studies have reported that
DLZ can significantly reduce myocardial cell injury, apoptosis
induced by ischemia reperfusion and myocardial infarct size
(28,29). Therefore, DLZ was selected as a
positive control in the present study.
Materials and methods
Chemical reagents
Nitrotetrazolium blue chloride (NBT; cat. no. N6876)
was purchased from Sigma-Aldrich; Merck KGaA. Aspartate
aminotransferase (AST; cat. no. 2401157), lactate dehydrogenase
(LDH; cat. no. 2401131) and creatine kinase MB (CK-MB; cat. no.
20162400887) isoenzyme biochemical kits were obtained from Biosino
Bio-technology & Science, Inc. Malondialdehyde (MDA; cat. no.
A003-1-2), superoxide dismutase (SOD; cat. no. A001-1-2),
glutathione peroxidase (GSH-Px; cat. no. A005-1-2) and catalase
(CAT; A007-2-1) biochemical assay kits were purchased from Nanjing
Jiancheng Bioengineering Institute. Tumor necrosis factor-α (TNF-α;
cat. no. KYRD-0011) and interleukin-6 (IL-6; cat. no. KYRD-0017)
radioimmunoassay kits were obtained from Beijing KangyuanRuide
Biotech. Co., Ltd. Terminal deoxy-nucleotidyl-transferase-mediated
dUTP nick end labeling (TUNEL) apoptosis detection kit (cat. no.
11684817910) was purchased from Sigma-Aldrich; Merck KGaA.
Antibodies against B-cell lymphoma 2 (Bcl-2; cat. no. 15071),
Bcl-2-associated X protein (Bax; cat. no. 14796) and caspase-3
(cat. no. 9661) were obtained from Cell Signaling Technology, Inc.
for immunohistochemistry (IHC). PerfectStart™ Green qPCR SuperMix
kit (cat. no. AQ601-01) was obtained from TransGen Biotech Co.,
Ltd. All other chemicals were of analytical grade.
Drug preparation
G-Rb3/Rb2 was extracted and identified by Professor
Yanping Chen (Department of Natural Medicinal Chemistry, Jilin
University, Changchun, China), as follows: Total ginsenosides were
extracted from the caudexes or leaves of Panax quinquefolium
L., as previously described (30), and were then dissolved in ethanol
(1:10-50). Subsequently, an equal volume of 0.5% NaOH ethanol
solution was added to obtain panaxadiol saponins, which were
subjected to silica gel column chromatography and eluted with
chloroform:methanol:ethyl acetate:H2O (15:22:40:10, the
upper layer). Upon elution, the eluent solvent was recovered and
the sample was dried to a constant weight to obtain G-Rb3/Rb2. The
content of G-Rb3 and G-Rb2 in the obtained extract was 95-80 and
5-20%, respectively, as determined by HPLC.
G-Rb3 was prepared by Professor Yanping
Chen as described previously (30)
All specimens were retained at the herbarium of
Jilin University. As the positive control, DLZ was purchased from
Tianjin Tanabe Seiyaku Co., Ltd.
Animals
The present study was approved by the Ethics
Committee of Jilin University and was conducted in accordance with
the Guide for the Care and Use of Laboratory Animals published by
the National Institutes of Health (31). A total of 84 healthy male
Sprague-Dawley rats (weight, 220-240 g; age, 7-8 weeks) were
obtained from the Experimental Animal Center of Jilin University.
All rats were housed in an environment at a constant temperature of
22-24°C and relative humidity of 45-55% under a 12-h light/dark
cycle with free access to food and water. Prior to the start of the
experiment, the rats were acclimated to their environment for 1
week.
Experimental protocols
All 84 healthy rats were randomly divided into five
groups: i) Sham surgery [sham surgery and treatment with double
distilled (dd)H2O]; ii) MIRI (MIRI surgery and treatment
with ddH2O); iii) G-Rb3/Rb2 (20 mg/kg); iv) G-Rb3 (20
mg/kg); and v) DLZ (20 mg/kg) as a positive control. G-Rb3/Rb2,
G-Rb3 and DLZ were dissolved in ddH2O prior to
administration. The drugs and ddH2O were orally
administered to the rats once a day for 3 consecutive days.
A MIRI rat model was established by trained
personnel as follows: Briefly, 30 min after the final dose of drug
administration, rats were anesthetized with 3% isoflurane in a
closed chamber. During surgery, anesthesia was maintained with
isoflurane (2-2.5% in oxygen) using a precision vaporizer. The
parameters evaluated to ensure the animals were fully anesthetized
prior to heart exposure were righting reflex and paw withdrawal
reflex. Subsequently, a left parasternal incision was made between
the third and fourth intercostal muscles to expose the rat heart,
and the left anterior descending coronary artery was ligated with a
6-0 operative silk thread. After 30 min of ischemia, reperfusion
was performed for 120 min by releasing the thread. The rats in the
sham group underwent the same procedures, with the exception that
the left anterior descending coronary artery was not ligated.
During the 30 min of ischemia, ventricular fibrillation and
ventricular tachycardia may occur in rats; therefore chest
compressions were administered at the early stage (32). However, when this occurred more
than three times and the rats could not be resuscitated by first
aid, it was considered that the rats had reached humane endpoints
and were euthanized using CO2 inhalation; the flow rate
of CO2 displaced 25% of the chamber volume per
minute.
Determination of cardiac function
After 120 min of reperfusion, the rats were
anesthetized with an intraperitoneal injection of sodium
pentobarbital (40 mg/kg; Sigma-Aldrich; Merck KGaA); the bilateral
common carotid arteries were isolated and cannulated into the left
ventricle from the right common carotid artery using a 2-F
polyethylene catheter. Subsequently, heart rate (HR), systolic
blood pressure (SBP), diastolic blood pressure (DBP), left
ventricular systolic pressure (LVSP), left ventricular end
diastolic pressure (LVEDP), maximal rate of the increase of left
ventricular pressure and maximal rate of the decrease of left
ventricular pressure (±dp/dtmax) were measured using a
multi-channel physiological recording system (RM-6000; Nihon Kohden
Corporation).
Determination of AST, CK-MB and LDH
activities in the serum
According to a previously described protocol
(33), serum was prepared as
follows: Following the determination of cardiac functional
parameters, while rats were still under anesthesia, 5 ml blood
samples were collected from the abdominal aorta of each rat. The
blood samples were completely coagulated by incubation at room
temperature for 1 h, and were then centrifuged at 1,000 × g for 10
min at 4°C. The supernatant was collected for analysis. Commercial
biochemical assay kits and an automatic biochemical analyzer
(COBAS-FARA; Roche Diagnostics) were used to measure the activities
of AST, LDH and CK-MB, according to the manufacturer's
protocols.
Measurement of infarct size
After blood sample collection, the heart of each rat
was removed, in order to harvest the left ventricular myocardium,
which was weighed and cut into four horizontal slices from the apex
to the base. Subsequently, the slices were incubated with 0.5 mg/ml
NBT at 37°C for 10 min to achieve complete staining, which was
shown as a purple-black color in normal myocardial tissues and
colorless in ischemic myocardial tissues. The ischemic myocardial
zone was identified, separated from normal myocardial tissues and
weighed. The myocardial infarct size was calculated as the weight
of ischemic myocardium zone/the weight of left ventricular
myocardium.
Measurement of MDA expression, and SOD,
GSH-Px and CAT activities in the left ventricle
The homogenate of the left ventricle was prepared as
follows: In total, 1 g left ventricle tissues were homogenized by
adding nine volumes of ice-cold saline and then centrifuged for 15
min at 1,500 × g and 4°C. The supernatant was collected to analyze
MDA content, and SOD, GSH-Px and CAT activities using biochemical
kits and a spectrophotometer [7202B; Unico (Shanghai) Instrument
Co., Ltd.], according to the manufacturer's protocols.
Determination of TNF-α and IL-6 levels in
the serum
In order to determine alterations in the levels of
inflammatory factors in the serum, TNF-α and IL-6 contents were
measured by radioimmunoassay kits (Beijing KangyuanRuide Biotech.
Co., Ltd.) and a gamma-scintillation counter (DFM-96; Beijing
Zongcheng Electromechanical Technology Development Co., Ltd.),
according to the manufacturers' protocols.
Histopathological examination via
histology, TUNEL assay and IHC
Myocardial tissue samples were fixed in a 4%
paraformaldehyde solution for 24 h at room temperature, dehydrated
in a graded series of ethanol (50, 75, 85, 95 and 100%), and then
embedded in paraffin. According to the experimental protocol,
specimens were cut into 4-µm sections and were then stained
with hematoxylin for 5 min, followed by 1% hydrochloric acid
alcohol differentiation for 3 sec and eosin staining for 3 min at
room temperature. According to the manufacturer's protocol,
myocardial cell apoptosis was detected using the TUNEL apoptosis
detection kit (Roche Diagnostics), prior to being examined by a
pathologist blinded to the experimental design, under a light
microscope (Nikon E100; Nikon Corporation) at ×200 magnification.
The five fields of view were automatically selected and the
percentage of apoptosis-positive cells was calculated for each
field of view. The mean was calculated to obtain the percentage of
apoptotic cells and was expressed as apoptotic index. Apoptotic
index (%)=(apoptotic nuclei count/total nucleus count) ×100%.
The protein expression of Bax, Bcl-2 and caspase-3
in myocardial tissues was detected by IHC. The samples were
prepared as aforementioned and sliced into 4-µm sections.
Following dehydration and paraffin embedding, the sections were
blocked in 3% methanol-H2O2 for 10 min and
incubated with primary antibodies (1:500) against Bcl-2, Bax and
caspase-3 for immunostaining at room temperature for 2 h. After
washing, polyclonal goat anti-mouse and goat anti-rabbit
immunoglobulin G secondary antibody (1:1,500; cat. no. IB-0021 and
IB-0061; Beijing Dingguo Changsheng Biotech Co., Ltd.) was added
for 1 h at room temperature. Subsequently, sections were washed
prior to development with avidin-biotin-streptavidin complex
(OriGene Technologies, Inc.) at 30°C for 20 min and
diami-nobenzidine chromogen for 15 min at room temperature. The
images of the stained sections were captured using the
aforementioned light microscope at ×200 magnification. Brown
staining was recognized as positive expression. Five areas of each
sample from all experimental groups were randomly captured.
For examination of myocardial ultrastructure, heart
samples were initially fixed in a 2.5% phosphate-buffered
glutaraldehyde solution at 4°C for 4 h and then further fixed in a
1% phosphate-buffered osmium tetroxide solution at 4°C for 2 h.
After dehydration with graded ethanol, the samples were embedded in
resin and polymerized at 72°C for 48 h. Ultrathin sections (50-70
nm) were then stained with 10% uranyl acetate for 30 min and 0.1%
lead acetate for 15 min at room temperature. The myocardial
ultrastructure was observed under a transmission electron
microscope (JEM-1200 EX; JEOL, Ltd.) at ×7,500 magnification.
Detection of caspase-3, Bcl-2 and Bax
mRNA expression
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was used to determine the mRNA expression levels
of Bax, Bcl-2 and caspase-3. According to a previously described
protocol (34), total RNA (1
µg) was extracted from the left ventricular myocardial
tissues using TRIzol® reagent (cat. no. 15596018;
Invitrogen; Thermo Fisher Scientific, Inc.), and then subjected to
RT using a riboSCRIPTTM Reverse Transcription kit
(Guangzhou RiboBio Co., Ltd.). The RT temperature protocol used was
42°C for 60 min and 72°C for 10 min. RT-qPCR was performed using a
TransStart Green qPCR Super Mix kit (Beijing Transgen Biotech Co.,
Ltd.) and a qPCR system (Mx3000P; Agilent Technologies, Inc.) with
β-actin as the housekeeping gene. The thermocycling conditions were
as follows: Initial denaturation at 94°C for 30 sec, followed by 40
cycles at 94°C for 5 sec, 60°C for 30 sec and 72°C for 30 sec, and
a final extension step at 72°C for 5 min. The results were analyzed
using the 2−ΔΔCq method (35). The following primer sequences were
used: Caspase-3, forward 5′-TCC TCG TTT CCG TGT TTG-3′, reverse
5′-CAG GGC ATC TCC ACT TTG-3′; Bcl-2, forward 5′-TTG CTT GGC TGG
TTC TAC-3′, reverse 5′-TCT ATG CCC TAC CTA TGA G-3′; Bax, forward
5′-TAG CCA CAG TGT TGT AAG C-3′, reverse 5′-TCT GAT CCG TCT CAA TAG
TC-3′; and β-actin, forward 5′-TTG TGC CTT GAT AGT TCG C-3′ and
reverse 5′-GGA GTC CTT CTG ACC CAT AC-3′.
Statistical analysis
All data are presented as the mean ± standard
deviation of at least three independent replicates. One-way
analysis of variance followed by the Tukey-Kramer post hoc test was
carried out using GraphPad Prism 5 software (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of G-Rb3/Rb2 and G-Rb3 on cardiac
function parameters
After 30 min of ischemia and 120 min of reperfusion,
cardiac function parameters were measured to determinate the extent
of MIRI. As shown in Fig. 2,
compared with the sham rats, HR, SBP, DBP, LVSP and ±dp/dtmax were
reduced, whereas LVEDP was significantly increased, in MIRI rats
(P<0.05). In addition, HR, SBP, DBP, LVSP and ±dp/dtmax were
increased (P<0.05) following the administration of G-Rb3/Rb2 (20
mg/kg), G-Rb3 (20 mg/kg) and DLZ (20 mg/kg), whereas LVEDP was
significantly reduced (P<0.05) following the administration of
G-Rb3/Rb2 (20 mg/kg) and DLZ (20 mg/kg).
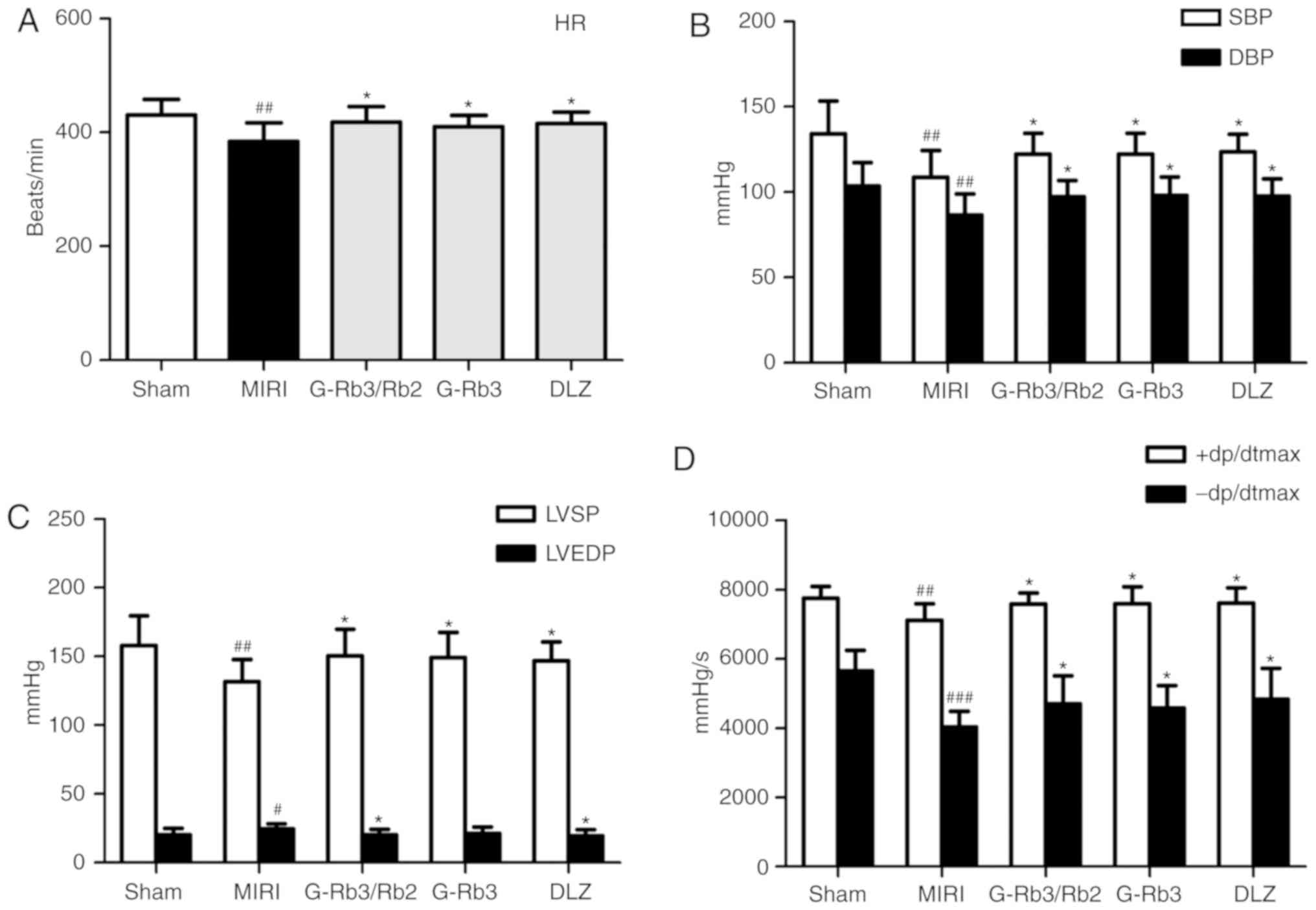 | Figure 2Effects of G-Rb3/Rb2 and G-Rb3 on
cardiac function parameters in MIRI rats. (A) HR, (B) SBP and DBP,
(C) LVSP and LVEDP, and (D) +dp/dtmax and -dp/dtmax were measured
after 30 min of ischemia and 120 min of reperfusion (n=10 rats).
Data are expressed as the mean ± standard deviation.
#P<0.05, ##P<0.01 and ###P<0.001
compared with the sham group; *P<0.05 compared with
the MIRI group. +dp/dtmax, maximal rate of the increase of left
ventricular pressure; -dp/dtmax, maximal rate of the decrease of
left ventricular pressure; DBP, diastolic blood pressure; DLZ,
diltiazem; G, ginsenoside; HR, heart rate; LVEDP, left ventricular
end diastolic pressure; LVSP, left ventricular systolic pressure;
MIRI, myocardial ischemia and reperfusion injury; SBP, systolic
blood pressure. |
Effects of G-Rb3/Rb2 and G-Rb3 on infarct
size, and AST, LDH and CK-MB activities
MIRI resulted in augmented myocardial infarct size,
and increased AST, LDH and CK-MB activities (P<0.05). G-Rb3/Rb2
(20 mg/kg), G-Rb3 (20 mg/kg) and DLZ (20 mg/kg) significantly
inhibited the MIRI-induced increase in myocardial infarct size, and
AST, LDH and CK-MB activities (P<0.05; Fig. 3). There was no statistically
significant difference among the three treatment groups.
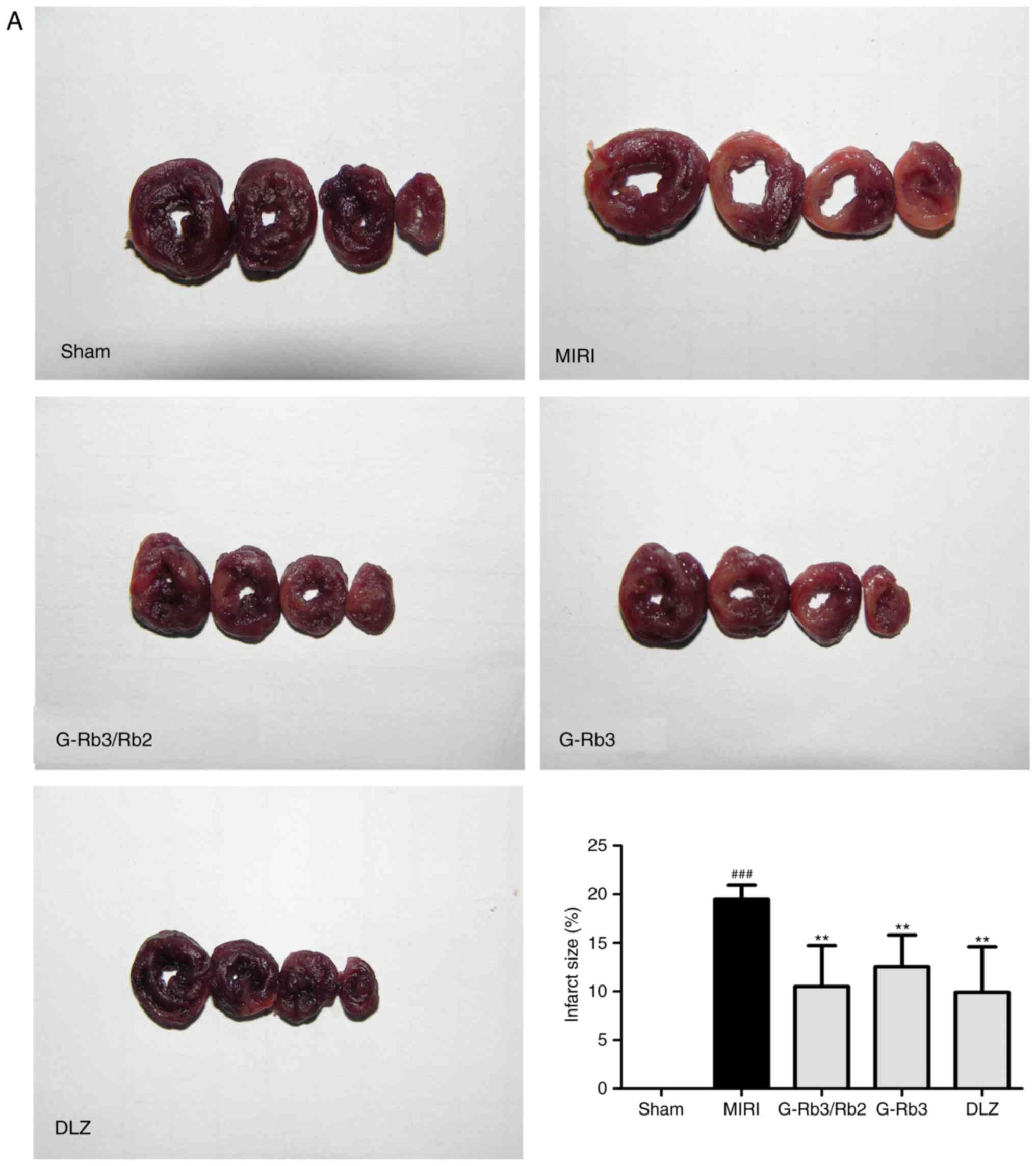 | Figure 3Effects of G-Rb3/Rb2 and G-Rb3 on
myocardial infarct size, and the activities of AST, LDH and CK-MB
in MIRI rats. (A) Representative myocardial cross-sections of
nitrotetrazolium blue chloride-stained heart tissues from each
group. Colorless sections indicate infarcted myocardium, whereas
normal myocardium is stained purple-black (n=4 rats). (B) AST, (C)
CK-MB and (D) LDH activities in the serum (n=10 rats). Data are
expressed as the mean ± standard deviation. ##P<0.01
and ###P<0.001, compared with the sham group;
*P<0.05 and **P<0.01, compared with the
MIRI group. AST, aspartate aminotransferase; CK-MB, creatine kinase
MB; DLZ, diltiazem; G, ginsenoside; LDH, lactate dehydrogenase;
MIRI, myocardial ischemia and reperfusion injury. |
Effects of G-Rb3/Rb2 and G-Rb3 on MDA
expression, and SOD, GSH-Px and CAT activities
Compared with in the sham group, a higher level of
oxidative stress was detected in MIRI rats, as manifested by
significantly increased MDA expression, and reduced SOD, GSH-Px and
CAT activities (P<0.05). The level of MDA was reduced, whereas
the activities of SOD, GSH-Px and CAT were elevated, following
treatment with G-Rb3/Rb2 (20 mg/kg), G-Rb3 (20 mg/kg) or DLZ (20
mg/kg) (P<0.05; Fig. 4A-D).
All three treatment groups exhibited similar results.
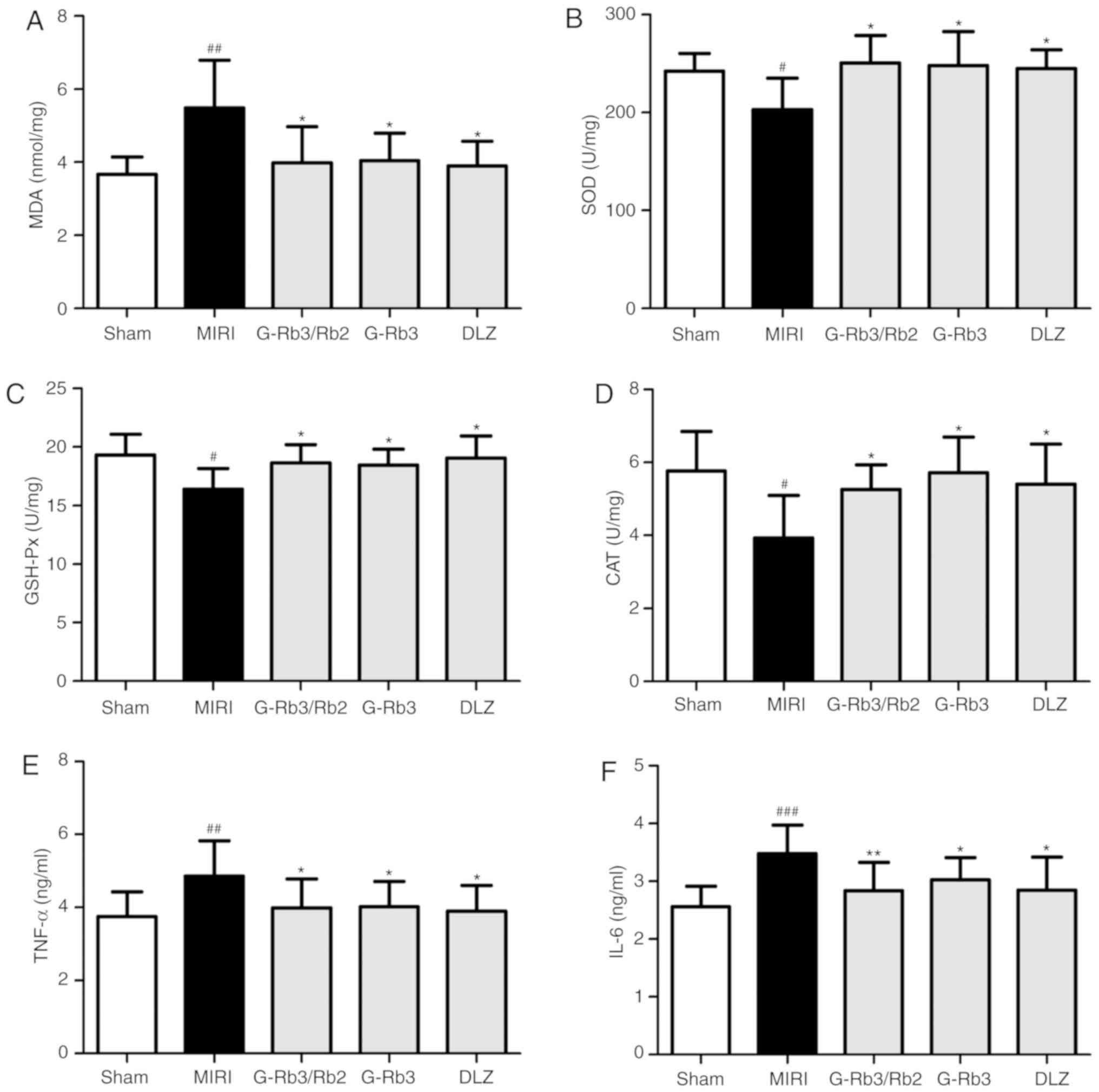 | Figure 4Effects of G-Rb3/Rb2 and G-Rb3 on
oxidative stress and inflammatory factors. Levels of (A) MDA, and
the activities of (B) SOD, (C) GSH-Px and (D) CAT in the left
ventricular tissues were analyzed using biochemical assay kits (n=6
rats). Levels of (E) TNF-α and (F) IL-6 in the serum were detected
by radioimmunoassay kits (n=10 rats). Data are expressed as the
mean ± standard deviation. #P<0.05,
##P<0.01 and ###P<0.001 compared with
the sham group; *P<0.05 and **P<0.01
compared with the MIRI group. CAT, catalase; DLZ, diltiazem; G,
ginsenoside; GSH-Px, glutathione peroxidase; IL-5, interleukin-6;
MDA, malondialdehyde; MIRI, myocardial ischemia and reperfusion
injury; SOD, superoxide dismutase; TNF-α, tumor necrosis
factor-α. |
Effects of G-Rb3/Rb2 and G-Rb3 on TNF-α
and IL-6 levels
The serum levels of TNF-α and IL-6 were determined
using radioimmunoassay kits. The levels of TNF-α and IL-6 in the
MIRI group were significantly increased compared with in the sham
group (P<0.05). Treatment with G-Rb3/Rb2 (20 mg/kg), G-Rb3 (20
mg/kg) and DLZ (20 mg/kg) all attenuated the MIRI-induced increase
in TNF-α and IL-6 levels (P<0.05; Fig. 4E and F). The effects of the three
treatment groups were similar.
Effects of G-Rb3/Rb2 and G-Rb3 on
histology, ultrastructural alterations, apoptosis and
expression
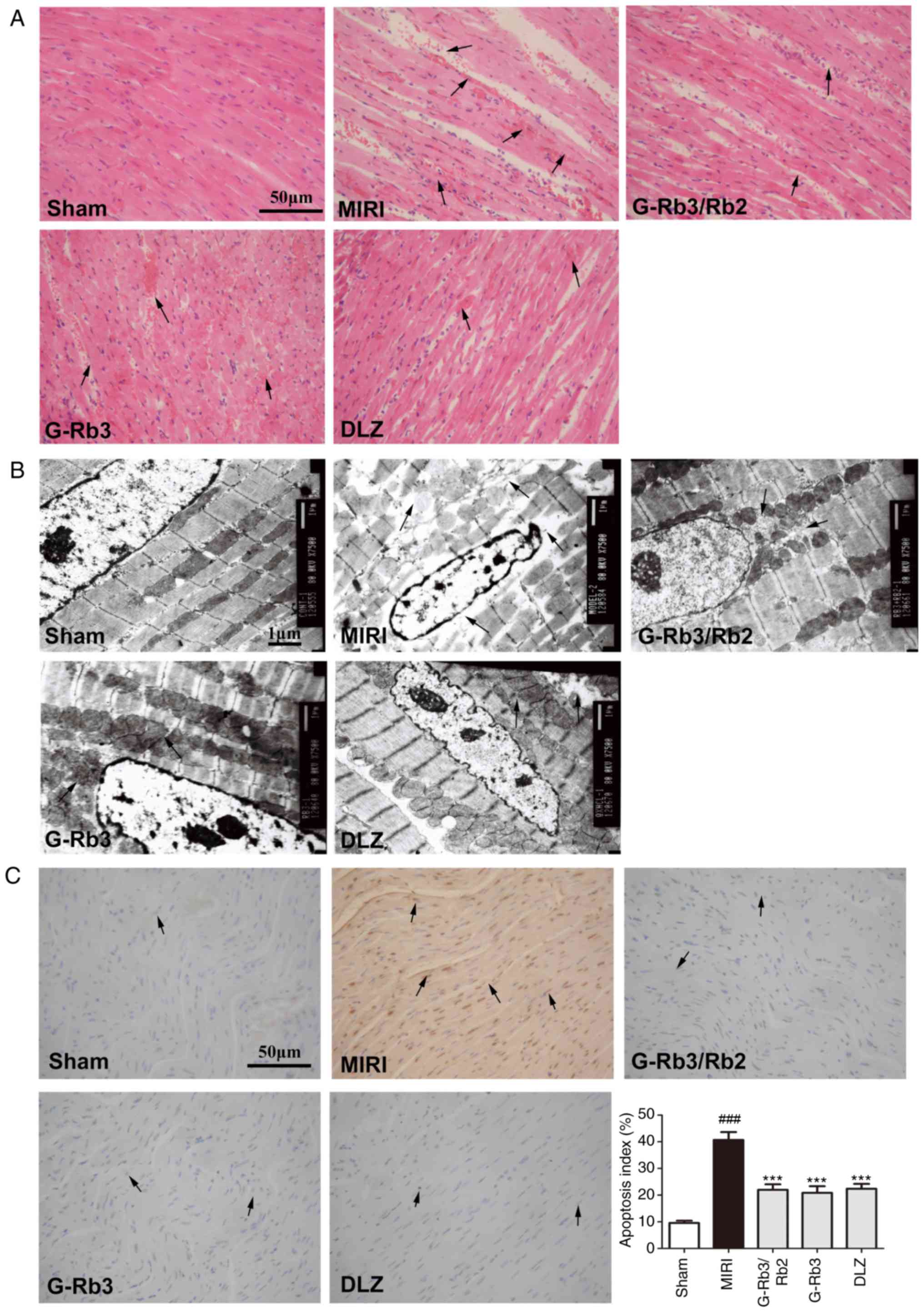
No focal separation of myocardial fibers was
detected in the myocardium of sham rats and these rats exhibited an
intact myocardial cell membrane. However, widespread myocardial
structure disorder and myocyte necrosis were observed in the
myocardial tissue samples of the MIRI group. The other three groups
exhibited mild necrosis and neutrophil granulocyte infiltration.
These morphological alterations were not obviously alleviated
compared with the MIRI group. The condition of myocardial injury
was similar in these three groups (Fig. 5A).
According to the myocardial micrographs obtained by
transmission electron microscopy (Fig. 5B), the myocardial fibers in the
sham group were arranged in a regular manner, with a clear and
ordered mitochondrial structure. The mitochondria in the MIRI group
were swollen with distorted cristae, vacuolar degeneration and
large areas of cytoplasmic vacuolization caused by irregularity of
myofilaments. The pathological alterations were alleviated
following treatment with G-Rb3/Rb2 (20 mg/kg), G-Rb3 (20 mg/kg) or
DLZ (20 mg/kg); however, these groups still exhibited slightly
damaged cristae.
As shown in Fig.
5C, a TUNEL assay was used to detect apoptotic cells in
myocardial tissues. The results revealed that the extent of DNA
damage was significantly increased in the MIRI group but was
significantly reduced in the G-Rb3/Rb2 (20 mg/kg), G-Rb3 (20 mg/kg)
and DLZ (20 mg/kg) groups (P<0.05).
In addition, the IHC results indicated that Bcl-2
expression was significantly reduced, whereas Bax and caspase-3
expression were increased, in the MIRI group. Administration of
G-Rb3/Rb2 (20 mg/kg), G-Rb3 (20 mg/kg) and DLZ (20 mg/kg) increased
Bcl-2 expression, and reduced Bax and caspase-3 expression
(P<0.05; Fig. 6A).
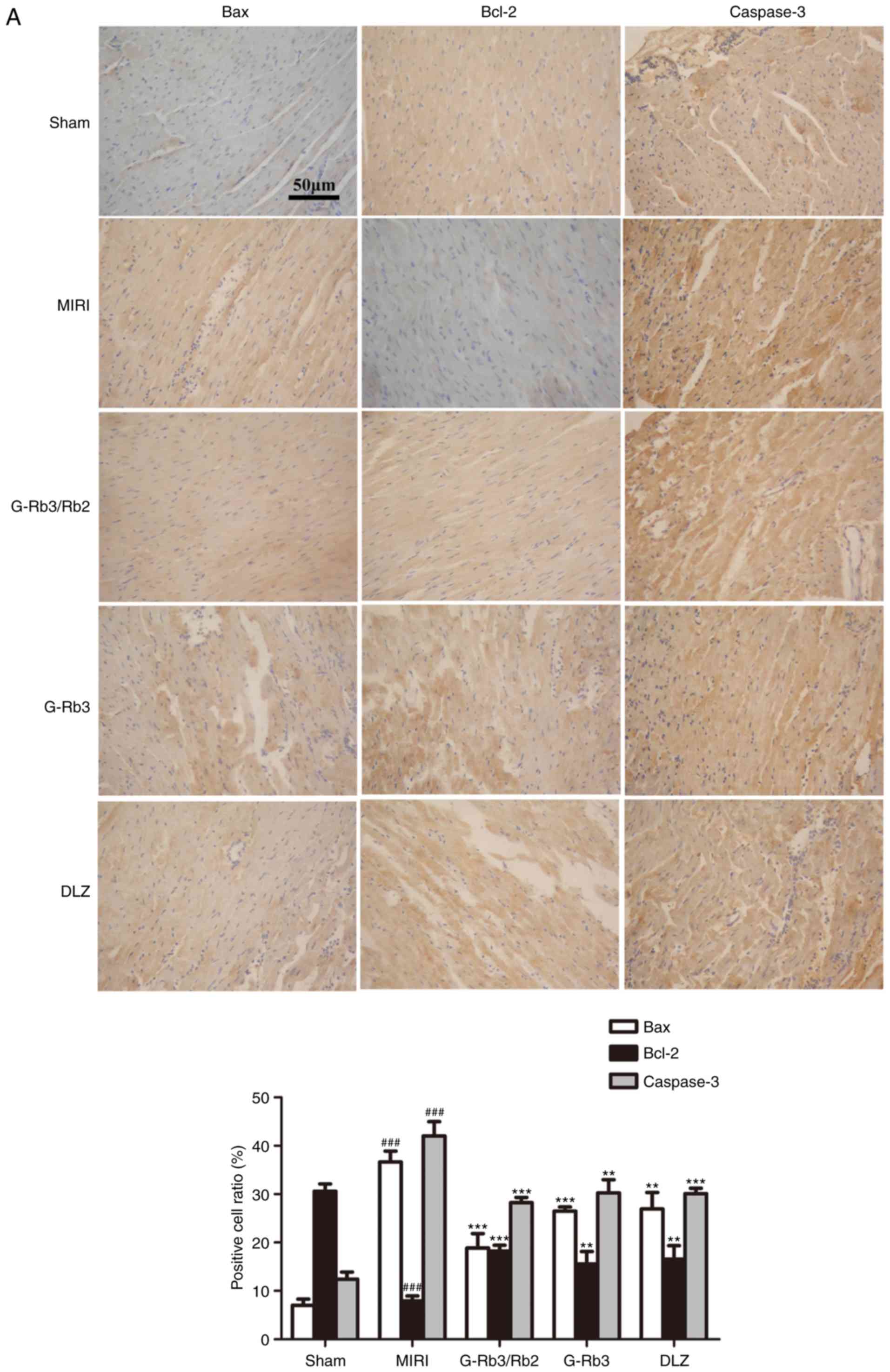 | Figure 6Effects of G-Rb3/Rb2 and G-Rb3 on
caspase-3, Bcl-2 and Bax expression. (A) Representative IHC
photomicrographs of myocardial tissues examined under a light
microscope at ×200 magnification (n=4 rats). The results were
expressed as positive cell ratio (%). Effects of G-Rb3/Rb2 and
G-Rb3 on caspase-3, Bcl-2 and Bax expression. (B-E) mRNA expression
levels of (B) Bax, (C) Bcl-2 and (E) caspases-3 were detected by
reverse transcription-quantitative polymerase chain reaction. (D)
Ratio of Bax/Bcl-2 was calculated to assess the degree of
myocardial tissue apoptosis (n=4 rats). Data are expressed as the
mean ± standard deviation. ###P<0.001 compared with
the sham group; *P<0.05, **P<0.01 and
***P<0.001, compared with the MIRI group. Bax,
Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; DLZ,
diltiazem; G, ginsenoside; IHC, immunohisto-chemistry; MIRI,
myocardial ischemia and reperfusion injury. |
Effects of G-Rb3/Rb2 and G-Rb3 on the
mRNA expression levels of Bax, Bcl-2 and caspase-3
RT-qPCR was used to detect the effects of G-Rb3/Rb2
on the mRNA expression levels of Bax, Bcl-2 and caspase-3. Compared
with in the sham group, rats in the MIRI group exhibited a
significant reduction in Bcl-2 mRNA expression, whereas Bax and
caspase-3 mRNA expression was increased, as was the ratio of
Bax/Bcl-2 expression (P<0.05). As shown in Fig. 6B-E, the mRNA expression levels of
Bcl-2 were elevated, whereas the mRNA expression levels of Bax and
caspase-3 were significantly reduced, along with a reduced ratio of
Bax/Bcl-2 expression, following treatment with G-Rb3/Rb2 (20
mg/kg), G-Rb3 (20 mg/kg) and DLZ (20 mg/kg) (P<0.05). No
significant difference was observed among the three treatment
groups.
Discussion
A rat model of MIRI was established by 30 min of
ligation of the left anterior descending coronary artery followed
by 120 min of reperfusion; this method has been widely used to
research the underlying mechanisms of cardiomyocyte apoptosis
(36). Several studies have
reported that rats are prone to death after ligation of the left
anterior descending coronary artery due to arrhythmia; the
mortality rate of this model can be as high as 60% (32,37). In the present study, 27 rats died
and the mortality rate was ~30%; five rats died during the
operation due to excess blood loss, 18 rats were euthanized due to
reaching humane endpoints and four rats were deeply anesthetized
and died of respiratory depression. It has been reported that the
safety range of sodium pentobarbital is too narrow, which causes
poor control of anesthesia depth, and some rats may die of
respiratory depression even at the recommended anesthetic dose
(38,39). This rat model of MIRI was used to
evaluate the cardioprotective effect of G-Rb3/Rb2 compared with
that of G-Rb3. The most important indicators of cardiac function
are hemodynamic parameters, such as HR, SBP, DBP, LVSP, LVEDP and
±dp/dtmax. It was previously reported that MIRI can lead to
hemodynamic disorders and inhibition of heart function
characterized by reduced HR, increased LVEDP, and decreased HR,
SBP, DBP, LVSP and ±dp/dtmax (40). In the present study, hemodynamic
parameters were incorporated into the experimental design to
evaluate the effects of G-Rb3/Rb2 on cardiac function. Consistent
with the results from previous studies, the MIRI rats exhibited
significant cardiac dysfunction, whereas the administration of
G-Rb3/Rb2 (20 mg/kg) and DLZ (20 mg/kg) significantly blocked these
adverse changes. These results indicated that the concomitant use
of G-Rb3 and G-Rb2 may ameliorate MIRI-induced impairment of
cardiac function. No significant difference was observed between
the two groups.
Infarct size is an important parameter for
evaluating left ventricular function and the effectiveness of
cardiovascular drugs. It can also be used to assess the condition
and prognosis of coronary artery diseases. G-Rb3/Rb2 (20 mg/kg),
G-Rb3 (20 mg/kg) and DLZ (20 mg/kg) significantly inhibited the
MIRI-induced increase in myocardial infarct size. The integrity of
the lipid membrane is important for maintaining cell structure and
functionality, and increased levels of oxygen free radicals can
lead to disordered cellular structures (41). As myocardial marker enzymes, AST,
LDH and CK-MB can be released into the bloodstream when the cell
membrane is ruptured in MIRI; therefore, changes in membrane
integrity and the degree of myocardial injury can be reflected by
the serum activities of AST, LDH and CK-MB (42). The results of the present study
revealed a significant elevation in the activities of AST, LDH and
CK-MB in MIRI rats. However, treatment with G-Rb3/Rb2 (20 mg/kg),
G-Rb3 (20 mg/kg) and DLZ (20 mg/kg) significantly inhibited the
MIRI-induced increase in AST, LDH and CK-MB activities. Taken
together, these results demonstrated that the protective effects of
these three drugs against MIRI may be mediated by increasing
cardiomyocyte membrane stability, while decreasing the release of
enzymes and myocardial infarct size. In addition, the three
treatment groups exhibited similar effects.
Oxygen free radicals are a key factor in MIRI. A
sudden increase in potent free radicals can be evoked within the
first few minutes of reestablished blood flow when the onset of
reperfusion reintroduces abundant oxygen. The release of free
radicals in the early phase of reperfusion, in combination with the
ischemia and reperfusion-induced decrease in antioxidant activity,
renders the myocardium vulnerable to damage. During MIRI, an
excessive amount of ROS is produced in the blood or in myocardial
tissues to induce cardiomyocyte apoptosis (43,44). Oxygen free radicals can also
damage the myocardial cell membrane by peroxidizing the
polyunsaturated fatty acids located in the cell membrane. As an end
product of peroxidization of polyunsaturated fatty acids in the
membrane, MDA is attacked by oxygen free radicals; therefore, the
content of MDA can reflect the degree of lipid peroxidization. As
free radical-scavenging enzymes, SOD, GSH-Px and CAT act as the
first line of defense against oxidative stress by eliminating
reactive oxygen radicals (45).
In the present study, treatment with G-Rb3/Rb2 (20 mg/kg), G-Rb3
(20 mg/kg) and DLZ (20 mg/kg) reduced the MIRI-induced elevation in
MDA content, and increased the activities of SOD, GSH-Px and CAT.
These findings indicated that G-Rb3/Rb2, G-Rb3 and DLZ may protect
against oxidative damage by enhancing the activity of endogenous
antioxidant enzymes. Excessive inflammatory responses, including
leukocyte exudation, edema, tissue necrosis and increased release
of inflammatory cytokines (IL-6 and TNF-α), are hallmarks of MIRI,
which can activate the cytokine cascade, and promote the production
of oxygen free radicals to activate neutrophils and induce the
apoptosis of cardiomyocytes. IL-6 exerts a multifaceted effect on
immune response, acute phase response, hematopoiesis and the
nervous system (46). It has been
reported that ischemia and reperfusion can significantly induce the
production of IL-6 (47). TNF-α
can inhibit myocardial contractile function, stimulate endothelial
cells and neutrophils to produce adhesion molecules, and accelerate
the adhesion between cells and intravascular coagulation, thus
leading to necrosis and apoptosis of cardiomyocytes, which
aggravate the degree of MIRI (48). Therefore, detection of TNF-α and
IL-6 concentration around the infarct area can indirectly reflect
the degree of myocardial inflammatory infiltration and myocardial
damage. According to the present data, G-Rb3/Rb2, G-Rb3 and DLZ
treatment reduced the levels of IL-6 and TNF-α in the blood, thus
suggesting that the cardioprotective effects of these drugs may be
mediated through their anti-inflammatory properties, and at least
in part through the regulation of cardiomyocyte apoptosis via
TNF-α-mediated activation of the death receptor pathway.
In the present study, the myocardium underwent
morphological alterations after 30 min of ischemia and 120 min of
reperfusion. H&E staining revealed swollen myocardial cells
with unclear boundaries, an enlarged intercellular space and
irregular granularity in the cytoplasm, degenerated and swollen
myocardial fibers, and marked infiltration of red blood cells and
neutrophils. These morphological alterations were not obviously
alleviated by G-Rb3/Rb2, G-Rb3 and DLZ treatment, which may be
attributed to the duration of myocardial ischemia and reperfusion
used in the present study and/or the experimental conditions of
H&E staining. In addition, serious damage was detected in the
nuclei and mitochondria of cardiomyocytes under transmission
electron microscopy, which is considered a 'gold standard' for
determining apoptosis. G-Rb3/Rb2, G-Rb3 and DLZ treatment markedly
alleviated myocardial ultrastructural damage; however, no marked
alterations were observed under light microscopy after H&E
staining. These findings indicated that apoptosis occurred in the
early stage of MIRI (after 120 min of reperfusion), consistent with
previous studies (49,50). It has been reported that MIRI and
apoptosis appear in reperfused myocardium within ≤4 h (51). Serious damage to tissues was also
observed by H&E staining and transmission electron microscopy
after 30 min of ischemia and 120 min of reperfusion.
The TUNEL assay is capable of staining intact and
single apoptotic nuclei or apoptotic body in situ, in order
to accurately reflect the biochemical characteristics of apoptosis.
The results of TUNEL staining indicated that G-Rb3/Rb2, G-Rb3 and
DLZ could ameliorate apoptosis during ischemia reperfusion to
reduce myocardial damage. A major pathological variant of MIRI is
apoptosis. The Bcl-2 gene family regulates apoptosis via the
mitochondrial pathway. Bax promotes apoptosis by promoting
cytochrome c release, activating caspases and forming a
dimer with Bcl-2 to inhibit Bcl-2 activity (52). The anti-apoptotic effect of Bcl-2
is caused by its ability to inhibit activation of mitochondrial
cytochrome c and caspase-3; notably, the ratio of Bax/Bcl-2
can further reflect the regulation of apoptosis mediated by the
Bcl-2 gene family (53). In the
present study, the protein expression levels of caspase-3, Bcl-2
and Bax were detected in myocardial tissues by IHC, which can
directly and accurately locate the protein of interest in cells or
tissues with high sensitivity in a qualitative manner. The IHC
results indicated that Bcl-2 expression was significantly reduced,
whereas Bax and caspase-3 expression were increased, in the MIRI
group. Administration of G-Rb3/Rb2, G-Rb3 and DLZ could increase
Bcl-2 expression, and reduce Bax and caspase-3 expression. In
addition, the mRNA expression levels of Bax, Bcl-2 and caspase-3
were detected by RT-qPCR. The mRNA expression levels of Bax, Bcl-2
and caspase-3 were similar to the protein expression levels
detected by IHC. The ratio of Bax/Bcl-2 expression was increased
after MIRI, and reduced following treatment with G-Rb3/Rb2, G-Rb3
and DLZ. The results indicated that G-Rb3/Rb2, G-Rb3 and DLZ may
exert myocardial protective effects by affecting genes responsible
for regulating apoptosis.
In conclusion, the protective effects of G-Rb3/Rb2
on MIRI were similar to those of G-Rb3. The underlying mechanisms
may be attributed to regulation of oxidative stress and
inflammatory factors, as well as inhibition of cardiomyocyte
apoptosis, at least in part. Therefore, G-Rb3/Rb2 may be used as a
concomitant treatment for MIRI.
Acknowledgments
The authors would like to thank Professor Yanping
Chen (Department of Natural Medicinal Chemistry, Jilin University,
Changchun, China) for providing G-Rb3/Rb2 and G-Rb3.
Funding
The present study received financial support from
the National Natural Science Foundation of China (grant no.
81473378) and the Natural Science Foundation of Jilin Province
(grant no. 20170101002JC).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DS and XL conceived and designed the study. YJ, WF
and XY performed the experiments. XL wrote the paper. YJ and WF
analyzed data, and reviewed and edited the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jilin University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karimi M, Zare H, Bakhshian Nik A, Yazdani
N, Hamrang M, Mohamed E, Sahandi Zangabad P, Moosavi Basri SM,
Bakhtiari L and Hamblin MR: Nanotechnology in diagnosis and
treatment of coronary artery disease. Nanomedicine (Lond).
11:513–530. 2016. View
Article : Google Scholar
|
|
2
|
Krokhaleva Y and Vaseghi M: Update on
prevention and treatment of sudden cardiac arrest. Trends
Cardiovasc Med. 29:394–400. 2019. View Article : Google Scholar
|
|
3
|
Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ and
Chen Y: Protective role of melatonin in cardiac
ischemia-reperfusion injury: From pathogenesis to targeted therapy.
J Pineal Res. 64:2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rude RE, Muller JE and Braunwald E:
Efforts to limit the size of myocardial infarcts. Ann Intern Med.
95:736–761. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nesher N, Zisman E, Wolf T, Sharony R,
Bolotin G, David M, Uretzky G and Pizov R: Strict thermoregulation
attenuates myocardial injury during coronary artery bypass graft
surgery as reflected by reduced levels of cardiac-specific troponin
I. Anesth Analg. 96:328–335, table of contents. 2003.PubMed/NCBI
|
|
7
|
Jo MS, Lee J, Kim SY, Kwon HJ, Lee HK,
Park DJ and Kim Y: Comparison between creatine kinase MB,
heart-type fatty acid-binding protein, and cardiac troponin T for
detecting myocardial ischemic injury after cardiac surgery. Clin
Chim Acta. 488:174–178. 2019. View Article : Google Scholar
|
|
8
|
Virmani R, Forman MB and Kolodgie FD:
Myocardial reperfusion injury. Histopathological effects of
perfluorochemical. Circulation. 81(3 Suppl): IV57–IV68.
1990.PubMed/NCBI
|
|
9
|
Hou H, Wang Y, Li Q, Li Z, Teng Y, Li J,
Wang X, Chen J and Huang N: The role of RIP3 in cardiomyocyte
necrosis induced by mitochondrial damage of myocardial
ischemia-reperfusion. Acta Biochim Biophys Sin (Shanghai).
50:1131–1140. 2018.
|
|
10
|
Ibáñez B, Heusch G, Ovize M and Van de
Werf F: Evolving therapies for myocardial ischemia/reperfusion
injury. J Am Coll Cardiol. 65:1454–1471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu L, Zhang W, Huang C, Liang Q, Bao H,
Gong Z, Xu M, Wang Z, Wen M and Cheng X: FoxO4 promotes myocardial
ischemia-reperfusion injury: The role of oxidative stress-induced
apoptosis. Am J Transl Res. 10:2890–2900. 2018.PubMed/NCBI
|
|
12
|
Davidson SM, Ferdinandy P, Andreadou I,
Bøtker HE, Heusch G, Ibáñez B, Ovize M, Schulz R, Yellon DM,
Hausenloy DJ, et al: Multitarget strategies to reduce myocardial
ischemia/reperfusion injury: JACC review topic of the week. J Am
Coll Cardiol. 73:89–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gottlieb RA, Burleson KO, Kloner RA,
Babior BM and Engler RL: Reperfusion injury induces apoptosis in
rabbit cardiomyocytes. J Clin Invest. 94:1621–1628. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu S, Ai Q, Feng K, Li Y and Liu X: The
cardioprotective effect of dihydromyricetin prevents
ischemia-reperfusion-induced apoptosis in vivo and in vitro via the
PI3K/Akt and HIF-1α signaling pathways. Apoptosis. 21:1366–1385.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shu Z, Yang Y, Yang L, Jiang H, Yu X and
Wang Y: Cardioprotective effects of dihydroquercetin against
ischemia reperfusion injury by inhibiting oxidative stress and
endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt
pathway. Food Funct. 10:203–215. 2019. View Article : Google Scholar
|
|
16
|
Mokhtari-Zaer A, Marefati N, Atkin SL,
Butler AE and Sahebkar A: The protective role of curcumin in
myocardial ischemia-reperfusion injury. J Cell Physiol.
234:214–222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun J, Yu X, Huangpu H and Yao F:
Ginsenoside Rb3 protects cardiomyocytes against
hypoxia/reoxygenation injury via activating the antioxidation
signaling pathway of PERK/Nrf2/HMOX1. Biomed Pharmacother.
109:254–261. 2019. View Article : Google Scholar
|
|
18
|
Liu K, Chen H, You QS, Ye Q, Wang F, Wang
S, Zhang SL, Yu KJ and Lu Q: Curcumin attenuates myocardial
ischemia-reperfusion injury. Oncotarget. 8:112051–112059. 2017.
View Article : Google Scholar
|
|
19
|
Wang CZ, Wu JA, McEntee E and Yuan CS:
Saponins composition in American ginseng leaf and berry assayed by
high-performance liquid chromatography. J Agric Food Chem.
54:2261–2266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JH: Pharmacological and medical
applications of Panax ginseng and ginsenosides: A review for use in
cardiovascular diseases. J Ginseng Res. 42:264–269. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai L, Gao J, Wei F, Zhao J, Wang D and
Wei J: Therapeutic potential of ginsenosides as an adjuvant
treatment for diabetes. Front Pharmacol. 9:4232018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wan JB, Yang FQ, Li SP, Wang YT and Cui
XM: Chemical characteristics for different parts of Panax
notoginseng using pressurized liquid extraction and HPLC-ELSD. J
Pharm Biomed Anal. 41:1596–1601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Jiang Y, Yu X, Fu W, Zhang H and
Sui D: Ginsenoside-Rb3 protects the myocardium from
ischemia-reperfusion injury via the inhibition of apoptosis in
rats. Exp Ther Med. 8:1751–1756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang Y, Zhong GG, Chen L and Ma XY:
Influences of ginsenosides Rb1, Rb2, and Rb3 on electric and
contractile activities of normal and damaged cultured
myocardiocytes. Zhongguo Yao Li Xue Bao. 13:403–406.
1992.PubMed/NCBI
|
|
25
|
Sui DY, Chen YP, Yu XF, Wang ZC, Qu SC and
Ma XY: Application of composition containing ginsenoside-Rb3 and
ginsenoside-Rb2 to treatment of heart and cerebral vascular
diseases. CN2012104745480. filed. November 21–2012.
|
|
26
|
Moukarbel GV, Ayoub CM and Abchee AB:
Pharmacological therapy for myocardial reperfusion injury. Curr
Opin Pharmacol. 4:147–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pizzetti G, Mailhac A, Li Volsi L, Di
Marco F, Lu C, Margonato A and Chierchia SL: Beneficial effects of
diltiazem during myocardial reperfusion: A randomized trial in
acute myocardial infarction. Ital Heart J. 2:757–765.
2001.PubMed/NCBI
|
|
28
|
Herzog WR, Vogel RA, Schlossberg ML,
Edenbaum LR, Scott HJ and Serebruany VL: Short-term low dose
intracoronary diltiazem administered at the onset of reperfusion
reduces myocardial infarct size. Int J Cardiol. 59:21–27. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen C, Chen W, Nong Z, Ma Y, Qiu S and Wu
G: Cardioprotective effects of combined therapy with hyperbaric
oxygen and diltiazem pretreatment on myocardial
ischemia-reperfusion injury in rats. Cell Physiol Biochem.
38:2015–2029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi Y, Han B, Yu X, Qu S and Sui D:
Ginsenoside Rb3 ameliorates myocardial ischemia-reperfusion injury
in rats. Pharm Biol. 49:900–906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press; Washington, DC: 2011
|
|
32
|
Cao Y, Li YK, Chen MQ and Yao KW: Research
progress on preparation of rat models of acute myocardial
infarction. Chin J Compat Med. 27:96–100. 2017.
|
|
33
|
Zhang H, Xu H, Xie H, Li F, Yu X and Sui
D: Cardiovascular protective effects of IL-1 ra-Fc-IL-18BP on
experimental myocardial infarction by inhibiting oxidative stress
and inflammation in a rat model. Pharmazie. 69:769–774. 2014.
|
|
34
|
Dai S, Hong Y, Xu J, Lin Y, Si Q and Gu X:
Ginsenoside Rb2 promotes glucose metabolism and attenuates fat
accumulation via AKT-dependent mechanisms. Biomed Pharmacother.
100:93–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
36
|
Maulik N, Yoshida T, Engelman RM, Deaton
D, Flack JE III, Rousou JA and Das DK: Ischemic preconditioning
attenuates apoptotic cell death associated with
ischemia/reperfusion. Mol Cell Biochem. 186:139–145. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Animal Models of Human Diseases. EQ L:
People's Medical Publishing House; China: pp. p1132008
|
|
38
|
Zeller A, Arras M, Jurd R and Rudolph U:
Identification of a molecular target mediating the general
anesthetic actions of pentobarbital. Mol Pharmacol. 71:852–859.
2007. View Article : Google Scholar
|
|
39
|
Archer DP, Samanani N and Roth SH:
Small-dose pentobarbital enhances synaptic transmission in rat
hippocampus. Anesth Analg. 93:1521–1525, table of contents. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nassiri AA, Hakemi MS, Asadzadeh R, Faizei
AM, Alatab S, Miri R and Yaseri M: Differences in cardiovascular
disease risk factors associated with maximum and mean carotid
intima-media thickness among hemodialysis patients. Iran J Kidney
Dis. 6:203–208. 2012.PubMed/NCBI
|
|
41
|
Peng S, Wang Y, Zhou Y, Ma T, Wang Y, Li
J, Huang F, Kou J, Qi L, Liu B and Liu K: Rare ginsenosides
ameliorate lipid overload-induced myocardial insulin resistance via
modulating metabolic flexibility. Phytomedicine. 58:1527452019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luo Y, Pan YZ, Zeng C, Li GL, Lei XM, Liu
Z and Zhou SF: Altered serum creatine kinase level and cardiac
function in ischemia-reperfusion injury during percutaneous
coronary intervention. Med Sci Monit. 17:CR474–CR479. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ryter SW, Kim HP, Hoetzel A, Park JW,
Nakahira K, Wang X and Choi AM: Mechanisms of cell death in
oxidative stress. Antioxid Redox Signal. 9:49–89. 2007. View Article : Google Scholar
|
|
44
|
Han SY, Li HX, Ma X, Zhang K, Ma ZZ and Tu
PF: Protective effects of purified safflower extract on myocardial
ischemia in vivo and in vitro. Phytomedicine. 16:694–702. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhai KF, Duan H, Khan GJ, Xu H, Han FK,
Cao WG, Gao GZ, Shan LL and Wei ZJ: Salicin from Alangium Chinense
ameliorates rheumatoid arthritis by modulating the Nrf2-HO-1-ROS
pathways. J Agric Food Chem. 66:6073–6082. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lyu M, Cui Y, Zhao T, Ning Z, Ren J, Jin
X, Fan G and Zhu Y: Tnfrsf12a-mediated atherosclerosis signaling
and inflammatory response as a common protection mechanism of
shuxuening injection against both myocardial and cerebral
ischemia-reperfusion injuries. Front Pharmacol. 9:3122018.
View Article : Google Scholar :
|
|
47
|
Zhai KF, Duan H, Luo L, Cao WG, Han FK,
Shan LL and Fang XM: Protective effects of paeonol on inflammatory
response in IL-1β-induced human fibroblast-like synoviocytes and
rheumatoid arthritis progression via modulating NF-κB pathway.
Inflammopharmacology. Aug 10–2017.Epub ahead of print. View Article : Google Scholar
|
|
48
|
Sun N, Wang H and Wang L: Protective
effects of ghrelin against oxidative stress, inducible nitric oxide
synthase and inflammation in a mouse model of myocardial
ischemia/reperfusion injury via the HMGB1 and TLR4/NF-κB pathway.
Mol Med Rep. 14:2764–2770. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Saraste A, Pulkki K, Kallajoki M,
Henriksen K, Parvinen M and Voipio-Pulkki LM: Apoptosis in human
acute myocardial infarction. Circulation. 95:320–323. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fliss H and Gattinger D: Apoptosis in
ischemic and reperfused rat myocardium. Circ Res. 79:949–956. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Haunstetter A and Izumo S: Apoptosis:
Basic mechanisms and implications for cardiovascular disease. Circ
Res. 11:1111–1129. 1998. View Article : Google Scholar
|
|
52
|
Guo M, Chen K, Lv Z, Shao Y, Zhang W, Zhao
X and Li C: Bcl-2 mediates coelomocytes apoptosis by suppressing
cytochrome c release in Vibrio splendidus challenged Apostichopus
japonicus. Dev Comp Immunol. 103:1035332019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhai KF, Duan H, Chen Y, Khan GJ, Cao WG,
Gao GZ, Shan LL and Wei ZJ: Apoptosis effects of imperatorin on
synoviocytes in rheumatoid arthritis through
mitochondrial/caspase-mediated pathways. Food Funct. 9:2070–2079.
2018. View Article : Google Scholar : PubMed/NCBI
|