Introduction
Rheumatoid arthritis (RA) is a chronic,
inflammatory, autoimmune disease that primarily affects the joints.
In a recent study, it was reported that approximately 0.5-1% of the
world population suffers from RA and the prevalence of this disease
is on the increase (1). Although
the etiology of RA is not clear, it is believed to be caused by a
combination of genetic and environmental factors (2). RA is characterized by chronic joint
inflammation, persistent synovial inflammation, progressive
cartilage destruction, and bone erosion, leading to painful, stiff,
and deformed joints, and joint instability (3). Chronic immune activation results in
systemic inflammation as well as in local inflammation within the
inflamed joints (3). These
systemic inflammatory responses affect extra-articular organs,
including those of the gastrointestinal, cardiovascular, and
respiratory systems. These non-articular complications of RA are
associated with early mortality (4).
Although the mechanism underlying the pathogenesis
of RA is not fully understood, both humoral and cellular immunity,
including the production of autoantibodies and infiltration of
inflammatory cells in the synovium are believed to be involved
(5). Autoantibodies produced by
plasma cells are involved in the pathogenesis of RA via the
formation of immune complexes in the joint (6). Autoantibodies and their immune
complexes promote the production of pro-inflammatory cytokines via
the activation of Fc receptor on macrophages and stimulate the
formation of neutrophil extracellular traps leading to inflammatory
response in the synovial tissue (7). Furthermore, autoantibodies enhance
the differentiation and activation of osteoclasts by directly
binding to the osteoclast surface or by indirect induction of
pro-inflammatory cytokines, resulting in bone erosion in RA
(8). Infiltrating immune cells
and synovial fibroblasts produce pro-inflammatory cytokines, such
as interleukin (IL)-6, IL-1, tumor necrosis factor-α (TNF-α), and
inflammatory enzymes, including metalloproteinases (MMPs),
cyclooxygenase (COX)-2, and inducible nitric oxide (NO) synthase
(iNOS). These inflammatory mediators activate genes related to the
inflammation response, which is followed by tissue destruction
(9,10). In particular, macrophages
constitute the major proportion of the cell population in the
inflamed synovium and are critically involved in the pathogenesis
of RA (11). Macrophages are the
major source of pro-inflammatory cytokines and release
tissue-degrading enzymes that contribute to inflammation and
articular destruction in RA. Increased infiltration of synovial
macrophages correlates with the destruction of cartilage and bone
(12). Among the pro-inflammatory
cytokines, IL-6, which is a pleiotropic cytokine, is produced by
various cell types, and regulates the inflammatory response and
bone homeostasis in RA (13). The
IL-6/IL-6 receptor and gp130 complex can activate the downstream
Janus kinase/signal transducer and activator of transcription 3
(JAK/STAT3) signaling pathway (14), and activate osteoclast formation
by stimulating the expression of osteoclastogenic genes (15). Abnormal activation of osteoclasts
in RA eventually leads to bone resorption and articular bone
destruction, one of the major symptoms in RA (16).
Humulus japonicus (HJ), known as 'Japanese
hop,' in the family Cannabaceae is an annual vine that originated
in countries of East Asia, including China and Korea, and was
introduced to North America. The pollen of HJ is a major cause of
allergic rhinitis (17). It is
cultivated for use in Asian herbal medicine and has been used to
treat pulmonary disease and skin diseases, such as dermatitis,
pruritus, and atopic diseases in Korea. Additionally, the
anti-oxidative and anti-microbial effects of this plant have been
validated (18,19). In a previous study, it was
reported that HJ exerts anti-atherosclerotic effects by inhibiting
pro-inflammatory mediators, including NO, prostaglandin E2 (PGE2)
and cytokines, such as IL-1β, IL-6, and TNF-α (20).
Notwithstanding decades of study, safe and specific
medicine for RA has not yet been established. Therefore, there is a
need for development of additional new therapeutic agents and
discovery of natural plant extracts for the treatment of RA that
can suppress joint inflammation and cartilage and bone destruction
without adverse effects. These would help in the development of new
drugs. Collagen-induced arthritis (CIA) in mice is the most
commonly used animal model for RA (21). Generation of self-reactive T cells
and antibody-mediated autoimmune reactivity against joint-specific
antigen, type II collagen, play an important role in the
pathogenesis of CIA (22). CIA
mice share histological and immunological features with
RA-afflicted humans. The chief shared features include
proliferative synovitis with infiltration of immune cells, pannus
formation, and erosion of cartilage and bone (23). This model is usually used to
assess the therapeutic effects of novel compounds and to study the
mechanisms involved in the pathogenesis of RA (21).
In the present study, we examined the anti-arthritic
effects of HJ using CIA mice and a murine macrophage cell line.
Materials and methods
Animal studies
Eight-week-old male DBA/1 mice (Orient Bio Inc.)
were acclimatized to a 12-h light/dark cycle at 22±2°C for 2 weeks
with unlimited food and water in a specific pathogen-free facility.
The mice were randomly divided into two groups: i) vehicle group
(n=12) treated with 0.5% carboxymethyl cellulose; ii) HJ group
(n=12) treated with 300 mg/kg of HJ. Starting 3 days before second
immunization, HJ was administered daily by oral gavage for 18 days
and changes in body weight were measured each day (Fig. 1A). The humane endpoint for these
experiments was set when the mice showed the following clinical
signs: Severe paw swelling, severe lameness caused by pain, loss of
≥20% of body weight, or blistering and ulceration at the injection
site associated with immunization. There was no animal lost to any
of these causes in the present experiments. All the mice were
humanely euthanized by CO2 asphyxiation for at least one
minute until death confirmed by absence of heart rate, no
breathing, and no reflexes. Animal experiments were approved by the
Institutional Animal Care and Use Committee of the Korea Research
Institute of Bioscience and Biotechnology (KRIBB-AEC-19142) and
were performed in accordance with the Guide for the Care and Use of
Laboratory Animals published by the US National Institutes of
Health (Bethesda).
Preparation of HJ extract
HJ was purchased from Gangwon Herbs, Gangwon,
Republic of Korea, on July, 2014. Professor W.K. Oh identified the
voucher specimen (SNU-2014-0004), which was then deposited at the
College of Pharmacy, Seoul National University, Korea. The HJ
extract was prepared and supplied by the Korea Bioactive Natural
Material Bank (Seoul). Briefly, the dried aerial parts of HJ were
soaked in 70% ethanol in an extraction container for 2 days at room
temperature.
Cell culture
Murine macrophage RAW 264.7 cells were purchased
from the American Type Cell Culture (ATCC; Manassas). The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM; Hyclone;
GE Healthcare Life Sciences) containing 10% fetal bovine serum
(FBS; Gibco, Thermo Fisher Scientific, Inc.), 100 U/ml penicillin,
and 100 µg/ml streptomycin, in a humidified environment (5%
CO2/95% air) at 37°C. The cells were pre-treated with
different concentrations of HJ (50, 100, 200 µg/ml) for 1 h
and were subsequently stimulated with lipopolysaccharide (LPS, 0.4
µg/ml; Sigma) or vehicle for 24 h.
Induction and clinical assessment of
CIA
For induction of arthritis, bovine type II collagen
(Chondrex) was dissolved at 2 mg/ml in phosphate-buffered saline
containing 0.1 M acetic acid, and was emulsified in an equal volume
of 2 mg/ml complete Freund's adjuvant (Chondrex). Mice were
immunized intradermally at the base of the tail with 100 µl
emulsion containing 100 µg bovine type II collagen. After 21
days, a booster dose was administered intradermally in the same way
as described above. Seven days after the second immunization, the
animals were boosted with an intraperitoneal injection of 40
µg LPS. The mice were examined for paw swelling and a
clinical score was determined. Paw swelling was assessed through
measuring the mean thickness of all the paws with a micrometer
caliper. The clinical score was assessed using the following
system: 0, normal paw; 1, one toe inflamed and swollen; 2, >1
toe, but not the entire paw, inflamed and swollen, or mild swelling
of the entire paw; 3, entire paw inflamed and swollen; 4, very
inflamed and swollen or ankylosed paw (24). Each limb was graded, giving a
maximum possible score of 16 per animal.
Histopathological analysis
The rear paws from each mouse were collected on day
35 of first immunization. The paws were fixed, decalcified,
paraffin-embedded, sectioned (5 µm), and stained with
hematoxylin and eosin (H&E), safranin O, or toluidine blue. The
H&E sections were analyzed microscopically for the degree of
inflammation and for cartilage and bone destruction, using the
following scale: 0, normal synovium; 1, synovial membrane
hypertrophy and cell infiltrates; 2, pannus and cartilage erosion;
3, major erosion of cartilage and subchondral bone; 4, loss of
joint integrity and ankyloses (25). Synovitis was evaluated by H&E
staining and was scored according to the following scale: 0, no
inflammation; 1, slight thickening of the lining layer or some
infiltrating cells in the underlying layer; 2, slight thickening of
the lining layer plus some infiltrating cells in the underlying
layer; 3, thickening of the lining layer, an influx of cells in the
underlying layer, and the presence of cells in the synovial space;
4, highly infiltrated synovium, with many inflammatory cells
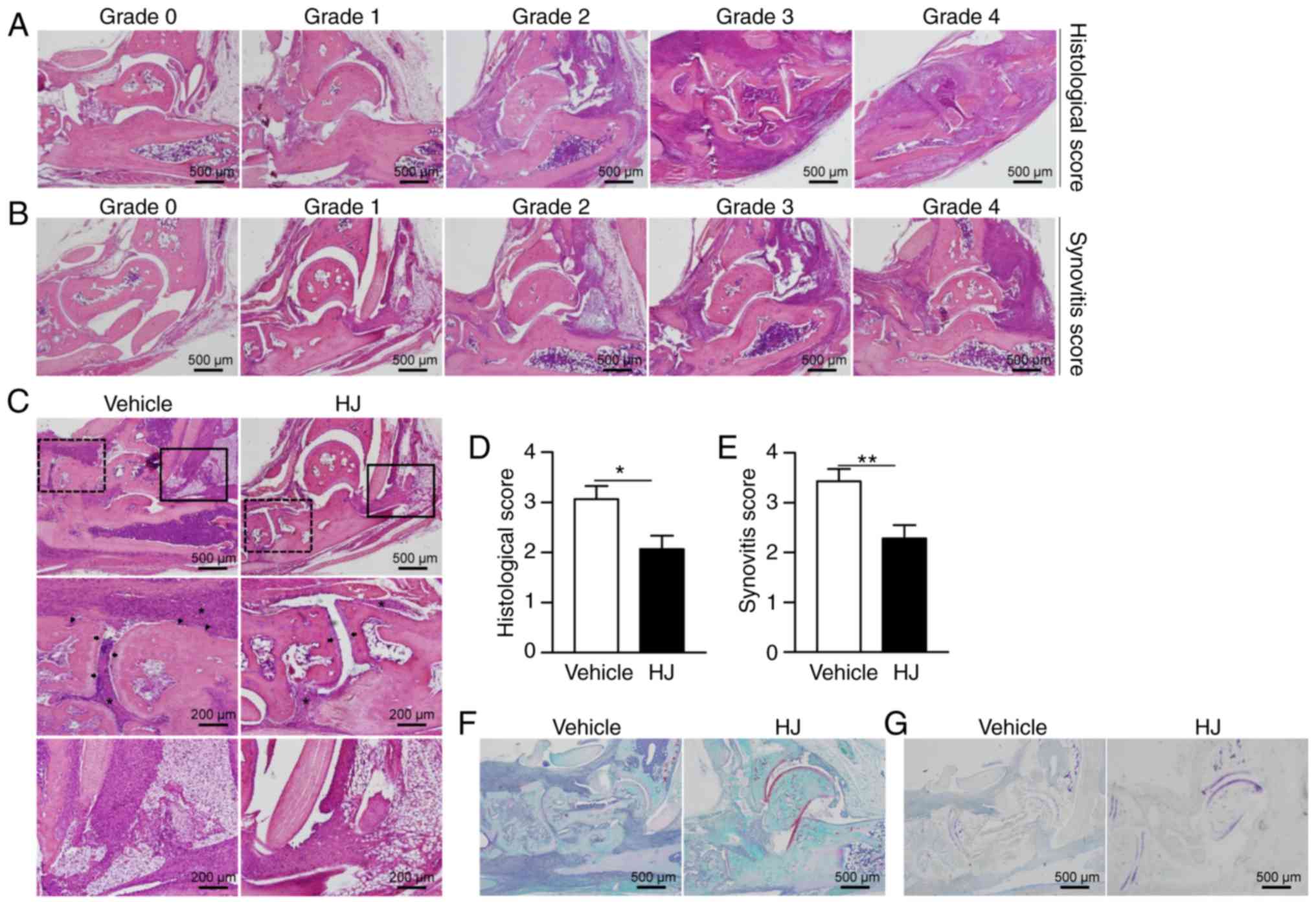
(26). Representative images of
the histopathological grading system are shown in Fig. 2A and B.
Reverse transcription
quantitative-polymerase chain reaction
Total RNA was isolated from the fore paw using
TRIzol reagent (Invitrogen), and reverse transcribed using the
iScript™ cDNA Synthesis kit (Bio-Rad) with a primer annealing step
at 25°C for 5 min, followed by reverse transcription at 46°C for 20
min, inactivation at 95°C for 1 min, and storage at 4°C. The
resulting cDNA was subjected to qPCR using the StepOnePlus™
Real-Time PCR System (Applied Biosystems) with
AccuPower® 2X Greenstar qPCR Master Mix (Bioneer),
according to the manufacturers' protocol. The cycling conditions
were 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec, and
60°C for 1 min. To detect and remove possible primer-dimer
artifacts, a dissocia tion curve was generated for the following
cycling conditions: 95°C for 15 sec, 60°C for 1 min, and 95°C for
15 sec. Relative gene expression levels were analyzed using the
2−ΔΔCq method (27)
and normalized against the expression of 18S rRNA. The primer
sequences used in the experiments are listed in Table I.
 | Table IPCR primer sequences used in this
study. |
Table I
PCR primer sequences used in this
study.
| Gene | Gene bank accession
no. | Primer
sequence |
|---|
| Mmp3 | NM_010809.2 | Forward
5′-GCCATCTCTTCCATCCAACA-3′ |
| Reverse
5′-CCAGGGTGTGAATGCTTTTA-3′ |
| Mmp13 | NM_008607.2 | Forward
5′-GGAGCCACAGATGAGCACAGA-3′ |
| Reverse
5′-TGAACGCTCGCAGTGAAAAG-3′ |
| Cox-2 | NM_011198.4 | Forward
5′-GGGTGTCCCTTCACTTCTTTCA-3′ |
| Reverse
5′-GAGTGGGAGGCACTTGCATT-3′ |
| iNOS | NM_001313921.1 | Forward
5′-GTTCTCAGCCCAACAATACAAGA-3′ |
| Reverse
5′-GTGGACGGGTCGATGTCAC-3′ |
| Cd68 | NM_001291058.1 | Forward
5′-TCACAGTTCACACCAGCTCC-3′ |
| Reverse
5′-CTTGGACCTTGGACTAGGCG-3′ |
| Cd11c | NM_001363984.1 | Forward
5′-CTGGATAGCCTTTCTTCTGCTG-3′ |
| Reverse
5′-GCACACTGTGTCCGAACTCA-3′ |
| Cd80 | NM_001359898.1 | Forward
5′-ACCCCCAACATAACTGAGTCT-3′ |
| Reverse
5′-TTCCAACCAAGAGAAGCGAGG-3′ |
| Cd86 | NM_019388.3 | Forward
5′-TCTTCCTCTGTTCCTTGGGC-3′ |
| Reverse
5′-TGCGGCTCCCTGTGTGT-3′ |
| Cd163 | NM_001170395.1 | Forward
5′-GGTGGACACAGAATGGTTCTT-3′ |
| Reverse
5′-CCAGGAGCGTTAGTGACAGC-3′ |
| Arginase
1 | NM_007482.3 | Forward
5′-ACATTGGCTTGCGAGACGTA-3′ |
| Reverse
5′-ATCACCTTGCCAATCCCCAG-3′ |
|
Il-12rβ1 | NM_001311141.1 | Forward
5′-CTGCACCCACTCACATTAAC-3′ |
| Reverse
5′-CAGTTGGCTTTGCCCTGTGG-3′ |
| Ccr2 | NM_009915.2 | Forward
5′-GGGCTGTGAGGCTCATCTTT-3′ |
| Reverse
5′-TGCATGGCCTGGTCTAAGTG-3′ |
| Ccr5 | NM_009917.5 | Forward
5′-CGAAAACACATGGTCAAACG-3′ |
| Reverse
5′-GTTCTCCTGTGGATCGGGTA-3′ |
| Ccr3 | NM_009914.4 | Forward
5′-TGCTGAGATGTCCCAATA-3′ |
| Reverse
5′-GCCAGGTCCAGATGTTTA-3′ |
| Ccr4 | NM_009916.2 | Forward
5′-GGAAGGTATCAAGGCATTTGGG-3′ |
| Reverse
5′-GTACACGTCCGTCATGGACTT-3′ |
| Il-2 | NM_008366.3 | Forward
5′-CTGGAGCAGCTGTTGATGGA-3′ |
| Reverse
5′-GCCTGCTTGGGCAAGTAAAA-3′ |
| Il-13 | NM_008355.3 | Forward
5′-ATTGCAATGCCATCTACAGG-3′ |
| Reverse
5′-TTGCTTTGTGTAGCTGAGCA-3′ |
| Il-6 | NM_031168.2 | Forward
5′-TTCCATCCAGTTGCCTTCTTG-3′ |
| Reverse
5′-GGGAGTGGTATCCTCTGTGAAGTC-3′ |
| Rank | NM_009399.3 | Forward
5′-AGAGGGGAGCCTCAGGGTCC-3′ |
| Reverse
5′-AAGTTCATCACCTGCCCGCTAGA-3′ |
| Nfatc1 | NM_001164111.1 | Forward
5′-GCCTCGAACCCTATCGAGTG-3′ |
| Reverse
5′-AGTTATGGCCAGACAGCACC-3′ |
| CtsK | NM_007802.4 | Forward
5′-TACCCATATGTGGGCCAGGA-3′ |
| Reverse
5′-TTCAGGGCTTTCTCGTTCCC-3′ |
| Trap | NM_001102405.1 | Forward
5′-GGAACTTCCCCAGCCCTTAC-3′ |
| Reverse
5′-AGGTCTCGAGGCATTTTGGG-3′ |
| Oscar | NM_001290377.1 | Forward
5′-GTAACGGATCAGCTCCCCAG-3′ |
| Reverse
5′-TGCAAAACTCATGCCCGGTA-3′ |
| Calr | NM_007588.2 | Forward
5′-TAGTTAGTGCTCCTCGGGCT-3′ |
| Reverse
5′-AGTACTCTCCTCGCCTTCGT-3′ |
| 18s
rRNA | NR_003278.3 | Forward
5′-GACACGGACAGGATTGACAGATTGATAG-3′ |
| Reverse
5′-GTTAGCATGCCAGAGTCTCGTTCGTT-3′ |
Measurement of serum anti-type II
collagen antibody IgG, IgG1, and IgG2a by ELISA
Plasma samples were collected at the end of the
experiment (day 35) for the determination of IgG, IgG1, and IgG2a
antibody levels with three commercially available test kits, mouse
anti-mouse Type II collagen IgG TMB (2036T, Chondrex), IgG1 TMB
(20361T, Chondrex), and IgG2a (20362T, Chondrex) antibody subtype
assay kit TMB, according to the manufacturer's instructions.
Antibody levels were quantified using seven standard serum samples
(0.16-10 ng/ml).
Measurement of nitrite, PGE2 production,
and IL-6 secretion in the LPS-stimulated RAW264.7 cells
The level of nitrite was measured in the culture
supernatant from the LPS-stimulated RAW 264.7 cells using a NO
estimation kit, according to the manufacturer's instructions
(Intron). The NO estimation kit is based on the principle of
diazotization (Griess method) technique. The levels of PGE2 and
IL-6 in the culture supernatant were measured via competitive PGE2
ELISA kit (ENZO Life Sciences) and BD OptEIA™ Set (BD Biosciences),
respectively, according to the manufacturers' instructions.
Western blot analysis
The paws from the DBA/1 mice were collected at the
end of the experiment (day 35). Paws and RAW264.7 cells were
prepared by homogenization in a RIPA lysis buffer containing 1%
NP-40, 0.25% sodium deoxycholate, 50 mmol/l Tris-HCl pH 7.4, 1
mmol/l EDTA and 120 mmol/l NaCl added with the protease and
phosphatase inhibitors. Centrifugation was carried out three times
at 12,000 × g for 10 min at 4°C and the protein concentration in
the supernatant was measured using the Bradford method. Protein
samples were separated by electrophoresis on 10% sodium dodecyl
sulfate-polyacrylamide gel and transferred onto a polyvinylidene
fluoride membrane (Millipore). The membranes were blocked with 5%
skimmed milk in Tris-buffered saline-Tween 0.1% for 30 min at room
temperature. The membranes were incubated with the primary
antibodies specific to COX-2 (1:100 dilution; ab15191; Abcam), iNOS
(1:100 dilution; ab49999; Abcam), p-STAT3 Ser727 (1:100 dilution;
CST9134; Cell Signaling), and STAT3 (1:100 dilution; CST9139; Cell
Signaling) at 4°C overnight prior to application of HRP-conjugated
secondary antibodies (1:1,000 dilution) for 1 h at room
temperature. After washing with Tris-buffered saline and Tween-20,
bands were detected using EzWestLumi plus (ATTO). TINA software,
2.09 (Raytest Isotopenmessgeräte) was used for measuring density of
western blot bands. The ratio was determined in arbitrary
units.
Statistical analysis
Numerical data are presented as means ± SEM.
Comparisons between two groups were performed using a two-tailed
Student's t-test. Comparisons among multiple groups were performed
using Tukey-Kramer HSD test after one-way ANOVA or Wilcoxon test
after two-way ANOVA. The threshold of significance was set at
P<0.05.
Results
HJ treatment ameliorates CIA in mice
DBA/1 mice were immunized on days 0 and 21 with
bovine type II collagen and were treated with HJ orally starting 3
days prior to the second immunization, as detailed in the methods
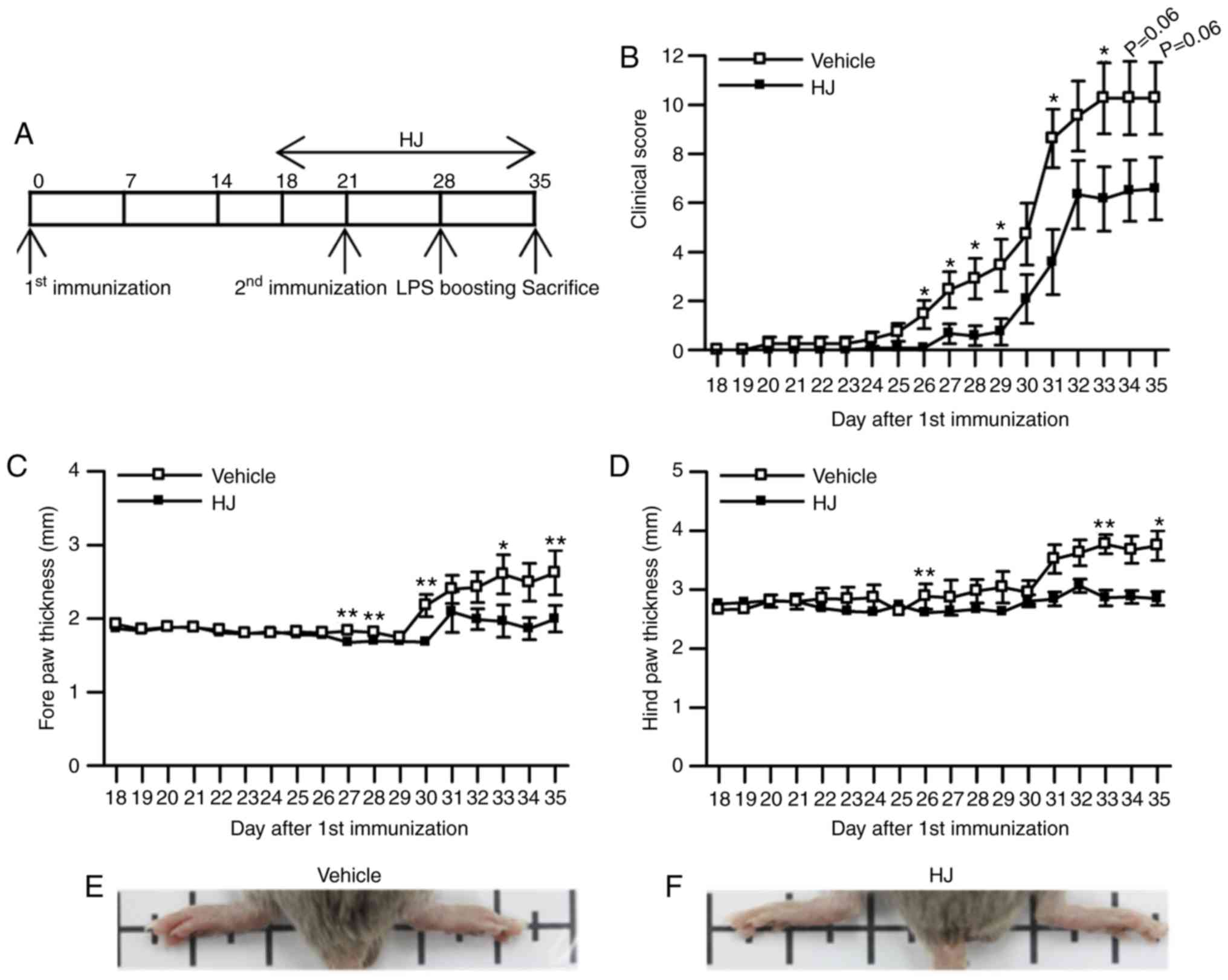
section (Fig. 1A). The gross
score of paw arthritis was significantly reduced from day 26 to day
29, and showed a trend toward a decrease from day 30 to the last
day of the experiment following the first immunization in the HJ
group compared to that in the vehicle group (Fig. 1B). Paw edema was evaluated by
measuring the paw thickness from the first day of treatment with
vehicle or HJ until the end of the experiment. Mice in the HJ group
showed a significant reduction in the size of hind paw (3.29±0.18
vs. 2.70±0.14 mm for vehicle versus HJ group) as well as of forepaw
(2.36±0.16 vs. 1.75±0.14 mm for vehicle versus HJ group) at the end
of the experiment (Fig. 1C and
D). In agreement with paw diameter, development of swelling or
redness of paw was diminished in the hind paws in HJ group
(Fig. 1E and F). These results
demonstrated that HJ has ameliorative effects on CIA.
HJ reduces articular inflammation and
injury in CIA mice
The severity of arthritis in CIA mice was also
evaluated by H&E staining of histological sections of mouse
hind paw. The mice in the vehicle group showed histopathological
changes typical of RA, including synovial hypertrophy with massive
infiltration of inflammatory cells and erosion of bone and
cartilage (Fig. 2C). By contrast,
mice in the HJ group showed a marked reduction in the infiltration
of inflammatory cells and less erosion of bone and cartilage
(Fig. 2C). In agreement with the
results of H&E staining, histological and synovitis scores were
significantly diminished in HJ-treated mice compared to those in
vehicle-treated mice (Fig. 2D and
E). The reduction in cartilage damage was further confirmed by
safranin O and toluidine blue staining in the HJ group (Fig. 2F and G). Based on these results,
it could be suggested that HJ improves CIA via the regulation of
synovial inflammation, cartilage damage, and bone erosion.
HJ inhibits the expression of
pro-inflammatory mediators in the paw of CIA mice and
LPS-stimulated RAW264.7 cells
We conducted RT-qPCR to determine whether HJ
inhibits the expression of inflammation-related proteases and
enzymes in the paw of CIA mice. The expression levels of
Mmp3, Mmp13, Cox-2, and iNOS were
significantly reduced in the paw of HJ-treated mice compared to the
levels in vehicle-treated mice (Fig.
3A-D). As macrophages play a crucial role in RA by expressing
inflammatory mediators, such as iNOS, COX-2, and MMPs (9,28),
we investigated whether HJ suppresses the expression of
inflammatory mediators in LPS-stimulated RAW 264.7 cells. The mRNA
expression of Mmp3, Mmp13, Cox-2, and iNOS
were markedly decreased after HJ treatment in a dose-dependent
manner (Fig. 3E-H). In addition
to the reduced expression of these genes, western blot analysis
revealed that the protein levels of Cox-2 and iNOS were also
decreased in a dose-dependent manner after HJ treatment compared
with their levels in cells treated with LPS only (Fig. 3I). Furthermore, the secretion
levels of PGE2 and NO produced by Cox-2 and iNOS, respectively,
were significantly decreased in HJ-treated cells compared to the
levels in cells treated only with LPS in a dose-dependent manner
(Fig. 3J and K). These results
suggest that HJ downregulates the induction of inflammatory
mediators under both in vitro and in vivo inflamed
situations.
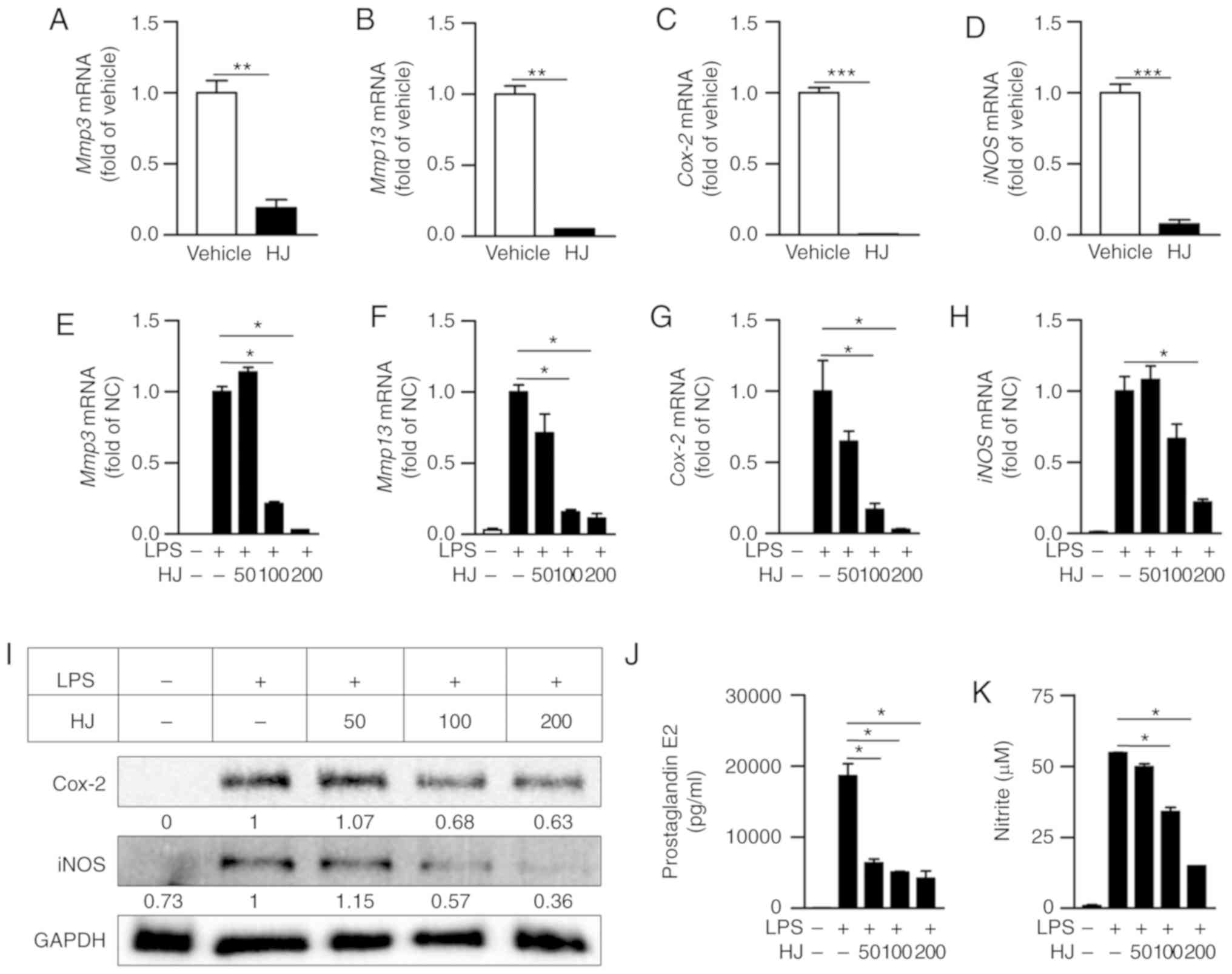 | Figure 3Regulatory effect of Humulus
japonicus on pro-inflammatory enzymes in collagen-induced
arthritis (CIA) mice and lipopolysaccharide (LPS)-stimulated RAW
264.7 cells. (A-D) Gene expression levels of Mmp3 (A),
Mmp13 (B), Cox-2 (C), and iNOS (D) were
analyzed by RT-qPCR in the paw of CIA mice on the last day of the
experiment. The vehicle group was set to a value of 1, and average
fold-change is shown. (E-K) RAW 264.7 cells were pre-treated with
different concentrations of HJ (0-200 µg/ml) for 1 h and
were stimulated with 0.4 µg/ml LPS or vehicle for 24 h. Gene
expression levels of (E) Mmp3, (F) Mmp13, (G)
Cox-2 and (H) iNOS in the RAW264.7 cell lysate were
analyzed by RT-qPCR. The LPS-only treated group (normal control)
was set to a value of 1, and average fold-change is shown. (I)
Protein levels of COX-2 and iNOS in the RAW264.7 cell lysate were
identified by western blot analysis. Band intensities were
quantified and normalized relative to the quantity of their
respective GAPDH bands, and expressed as fold changes of the values
in the LPS-only treated group. (J and K) Levels of (J)
prostaglandin E2 (PGE2) and (K) nitric oxide (NO) were evaluated in
the RAW264.7 cell culture supernatant by ELISA and Griess test,
respectively. Representative data from at least three independent
experiments are shown. Grouped quantitative data are presented as
means ± SEM (vehicle group; n=8, HJ group; n=6). Significance was
measured using (A-D) two-tailed Student's t-test or (E-K) the
Tukey-Kramer HSD test following one-way ANOVA.
*P<0.05, **P<0.01,
***P<0.001. |
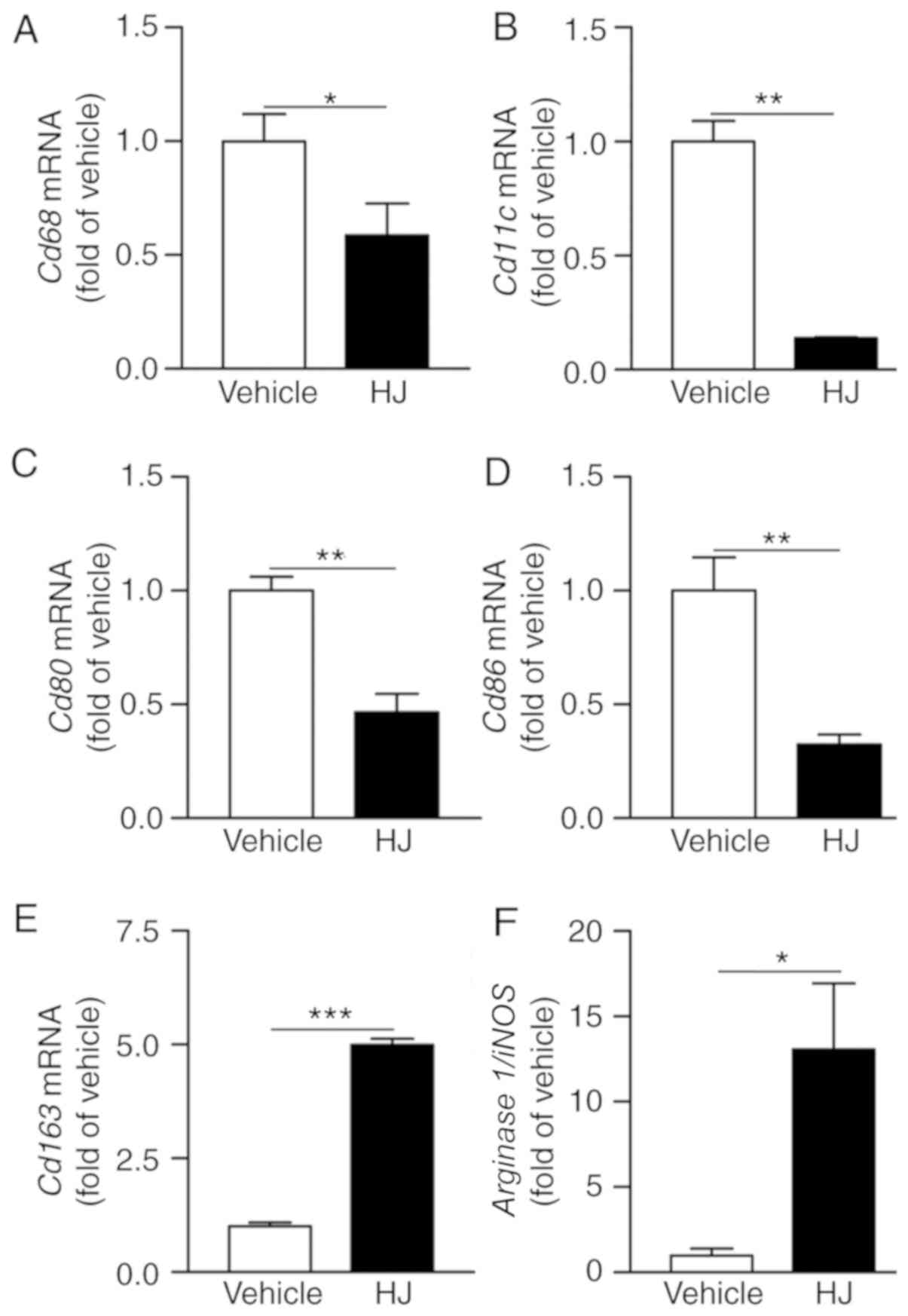
HJ reduces articular macrophage
infiltration in CIA
The degree of synovial macrophage infiltration
correlates with the severity of RA (29). Monocytes can differentiate into
classically activated pro-inflammatory M1 macrophages to aggravate
the RA symptom and alternatively into anti-inflammatory M2
macrophages to improve the RA phenotype (30). Therefore, we determined whether HJ
influences the infiltration of articular macrophages and the M1/M2
subsets in CIA mice. The gene expression levels of Cd68 as
pan-macrophage marker, and Cd11c, Cd80, and
Cd86 as M1 macrophage markers were significantly reduced in
paws of the HJ group compared to the levels in the vehicle group
(Fig. 4A-D). By contrast, the
expression level of M2 macrophage marker, Cd163 and the
arginase 1/iNOS ratio were significantly increased in the
paws of mice in the HJ group suggesting skewing from classically to
alternatively activated macrophages (Fig. 4E and F) (31). These results suggest that HJ
reduces the infiltration of macrophages and affects the M1/M2
subsets in the paw of CIA mice.
HJ inhibits autoantibody production in
CIA mice
We further investigated as to how HJ improves
inflammatory arthritis in CIA mice. Anti-collagen autoantibodies
have pathogenic properties in the RA and IgG2a has a critical role
in the development of RA (32).
Therefore, we investigated whether HJ inhibits the production of
collagen-specific antibody and affects humoral immunity in CIA
mice. To confirm this, we measured the level of type II
collagen-specific total IgG, IgG1, and IgG2a in the plasma of CIA
mice by ELISA. The levels of total IgG specific to type II collagen
were not significantly different between the vehicle and the HJ
group (Fig. 5A). The production
of collagen-specific IgG2a was significantly reduced in the HJ
group compared to that in the vehicle group and a trend toward
reduced IgG1 was also observed in the HJ group (Fig. 5B and C). The production of IgG2a
is induced by T-helper type 1 (Th1) cell-derived cytokines, such as
IFN-γ and IL-2, and production of IgG1 is associated with Th2
cell-dependent cytokines, such as IL-4 and IL-13 (33). We next investigated whether HJ
could regulate the Th1 and Th2 cells in the paw of CIA mice. The
expression levels of IL-12rβ2, Ccr2, and Ccr5
as Th1 cell-associated surface markers and Ccr3 and
Ccr4 as Th2 cell-related surface markers were significantly
reduced in the paw of mice in the HJ group compared to those in the
vehicle group mice (Fig. 5D-H).
Furthermore, the expression levels of Th1 cytokine, IL-2,
and Th2 cytokine, IL-13, were markedly decreased in the paw
of HJ-treated mice (Fig. 5I and
J). From these results, it is clear that HJ improves CIA via
downregulation of the Th1- and Th2-mediated autoantibody
production.
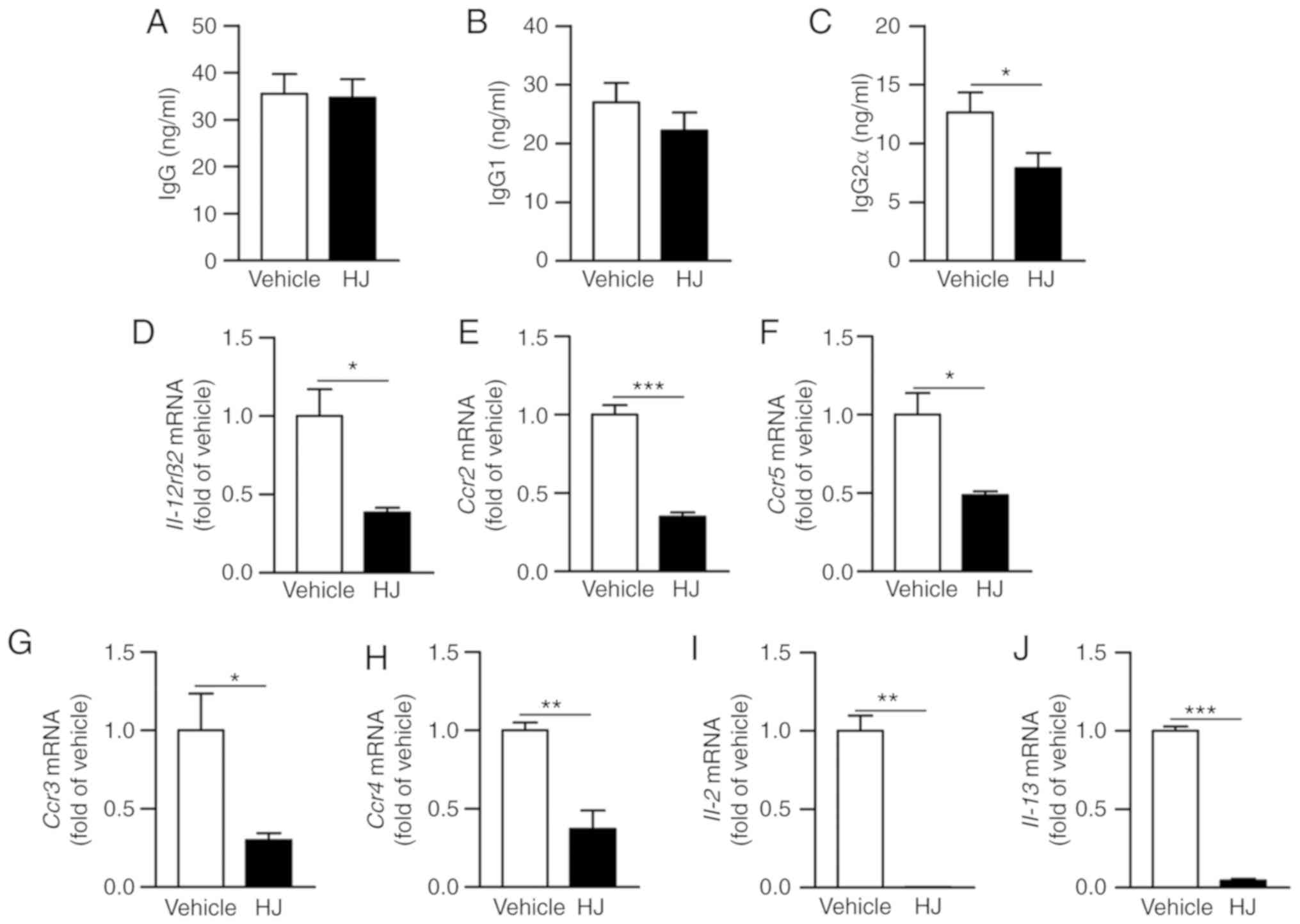 | Figure 5Effect of Humulus japonicas
(HJ) on the production of anti-type II collagen antibody in plasma.
Levels of (A) anti-type II collagen total IgG and its subtypes (B)
IgG1, and (C) IgG2a were measured by ELISA in plasma obtained on
day 35 from each mice group. Gene expression levels of (D)
Il-12rβ2, (E) Ccr2, (F) Ccr5, (G) Ccr3,
(H) Ccr4, (I) IL-2 and (J) IL-13 were analyzed
by RT-qPCR in the paw of CIA mice on day 35 following the first
immunization. The vehicle group was set to a value of 1, and
average fold-change is shown. Grouped quantitative data are
presented as means ± SEM (vehicle group; n=8, HJ group; n=6).
Significance was measured using two-tailed Student's t-test.
*P<0.05, **P<0.01,
***P<0.001. |
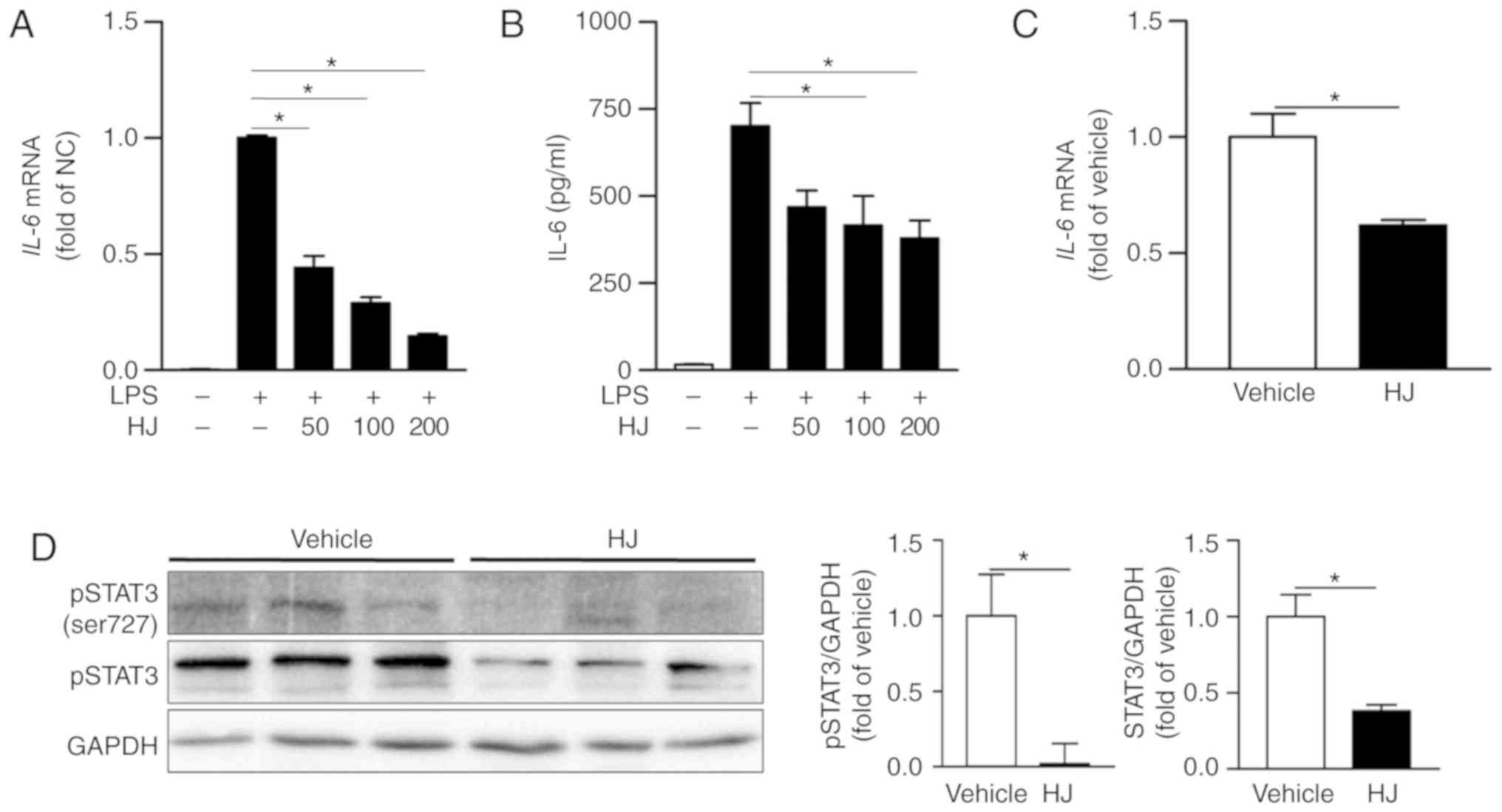
HJ suppresses the expression of IL-6 and
inhibits STAT3 signaling pathways in CIA mice
IL-6 is one of the most abundantly expressed
cytokines in the rheumatoid synovium and has critical roles in the
inflammatory process and osteoclast-mediated bone resorption in RA
(34). The expression level of
IL-6 was significantly reduced in a dose-dependent manner in
LPS-stimulated RAW 264. 7 cells treated with HJ compared to that in
cells treated only with LPS (Fig.
6A). The secretion level of IL-6 was also significantly reduced
in the supernatant of HJ-treated cells compared to that in cells
treated only with LPS in a dose-dependent manner, although the
differences among HJ concentration were not significant (Fig. 6B). Moreover, the expression of
IL-6 was markedly decreased in the paw mice in the HJ group
compared to that in mice of the vehicle group (Fig. 6C). IL-6 transduces signals via the
phosphorylation of STAT3, and STAT3 stimulates joint inflammation
and erosion in RA (35).
Treatment with HJ significantly reduced the phosphorylation and
expression of STAT3 in paw of CIA mice (Fig. 6D). Taken together, these results
suggest that HJ downregulates the expression of IL-6 and STAT3
signaling pathway in the paw of CIA mice.
HJ ameliorates CIA through inhibition of
osteoclast-specific genes and expression of transcription
factors
Considering that periarticular bone erosions and
generalized bone loss are hallmarks of RA and that osteoclasts play
a critical role in bone erosion (36), we analyzed
osteoclastogenesis-related gene expression in the paw of CIA mice.
In the HJ group mice, the expression levels of Rank and an
osteoclastogenic transcription factor, Nfatc1, were
decreased compared to that in the vehicle group (Fig. 7A and B). HJ treatment markedly
reduced the expression of osteoclast-specific genes, including
Ctsk, Trap, Calr, and Oscar, in the paw
of CIA mice, albeit the reduction in the expression of the latter
two genes was not statistically significant (Fig. 7C-F). These results suggest that HJ
can ameliorate CIA via inhibition of the induction of
osteoclast-related genes.
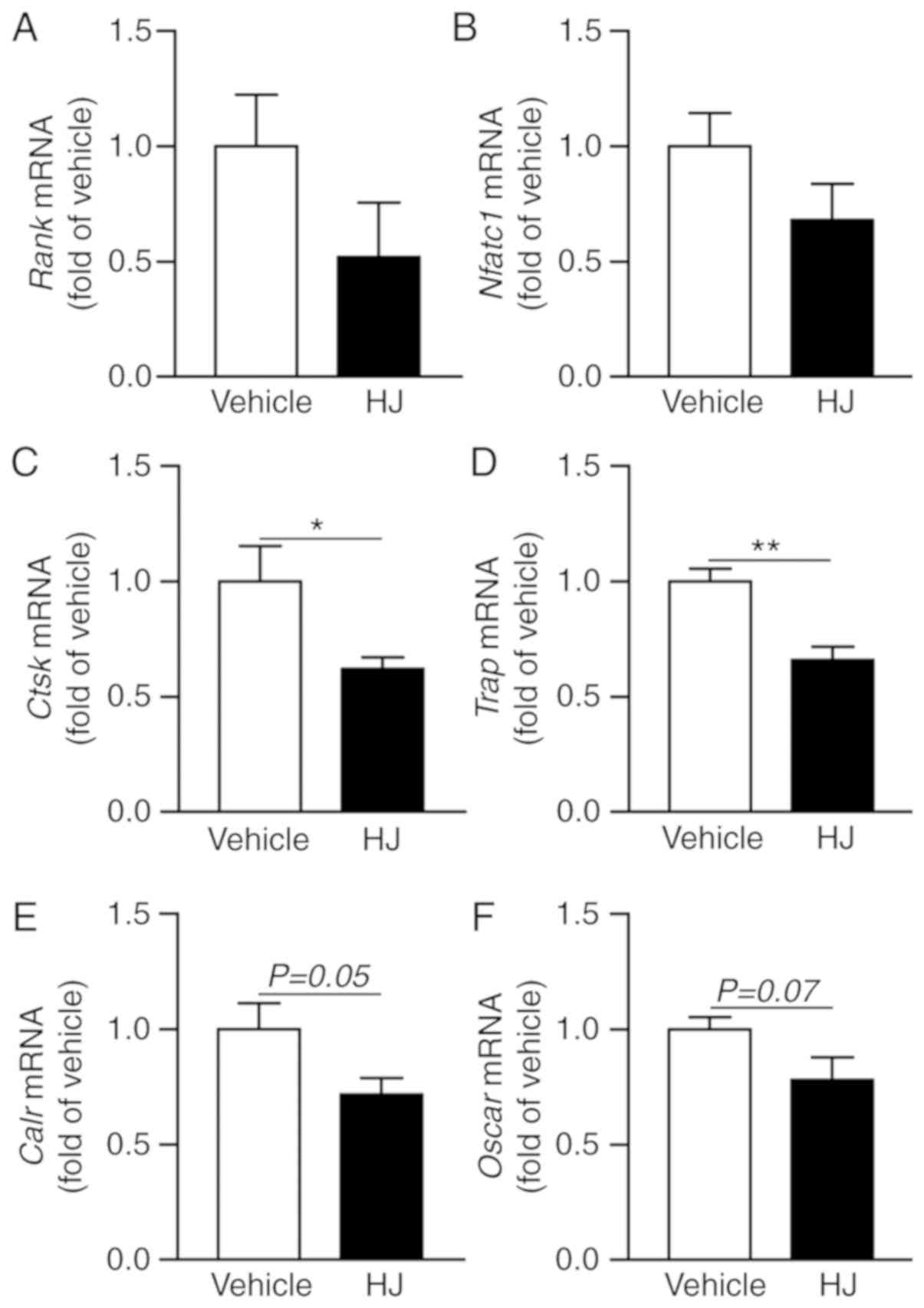 | Figure 7Effects of Humulus japonicas
(HJ) treatment on gene expression of osteoclast activity markers in
collagen-induced arthritis (CIA) mice. On day 35, following the
first immunization, paws were obtained from all CIA mice treated
either with vehicle or HJ. Gene expression levels of (A)
Rank (osteoclast surface receptor), (B) Nfatc1
(master transcription factor for osteoclastogenesis), and
osteoclast-specific markers, including (C) Ctsk, (D)
Trap, (E) Calr, and (F) Oscar, were analyzed
by RT-qPCR. Vehicle group was set to a value of 1, and the average
fold-change is shown. Grouped quantitative data are presented as
means ± SEM (vehicle group; n=8, HJ group; n=6). Two-tailed
Student's t-test was used to compare vehicle group with HJ group.
*P<0.05, **P<0.01. |
Discussion
Previous findings have shown that HJ has
anti-atherosclerotic and anti-inflammatory effects by inhibiting
pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, and
inflammatory mediators, including COX-2 and iNOS, in the
LPS-stimulated RAW264.7 macrophage cells (20). In addition, HJ exerts inhibitory
effects on LPS-induced phosphorylation of the inhibitor κB-α
(37). However, there are no
reports on the therapeutic potential of HJ against RA. In the
present study, we demonstrated the anti-arthritic effect of HJ,
in vitro and in vivo. HJ effectively ameliorated the
rheumatic symptoms by inhibiting inflammation and articular
destruction as revealed by diminished gross and histological
arthritic score.
RA is a systemic inflammatory disorder and is
characterized by increased levels of pro-inflammatory cytokines,
such as TNFα and IL-6, in the plasma, and of pro-inflammatory
enzymes, including MMPs, COX-2, and iNOS, in the synovium, which
contribute to articular inflammation, bone erosion, and cartilage
destruction (9,10). Moreover, MMPs secreted by synovial
fibroblasts, chondrocytes, and macrophages contribute to
degradation of the extracellular matrix in the articular cartilage
(38). Among them, MMP3 and MMP13
are considered important pathological mediators of RA (38). MMP3 can degrade a different
extracellular matrix such as aggrecan and fibronectin in joint, and
also activate other MMPs such as pro-MMP1 and pro-MMP13 (39). In addition, MMP13 plays a crucial
role in cleaving type II collagen, which is a major component of
the cartilage and also cleaves other cartilage collagen types, such
as types IX and X, and other extracellular matrix components
including fibronectin and aggrecan (40). Moreover, in RA, COX-2 and iNOS
induced by activated macrophage are responsible for the production
of PGE2 and NO, respectively. The overproduction of PGE2 and NO
play key roles in RA and are involved in pain, inflammation, and
tissue destruction (41). In the
present study, HJ significantly inhibited the expression of
pro-inflammatory cytokine, IL-6, and pro-inflammatory
enzymes, such as Mmp3, Mmp13, Cox-2, and iNOS, in a
dose-dependent manner after HJ treatment in LPS-stimulated RAW264.7
cells and in the paw of CIA mice. However, a limitation of this
study was that we could not measure the levels of MMP13, COX-2, and
iNOS by ELISA in the plasma of CIA mice. Instead, we measured
alternatively the levels of PGE2 and NO produced by COX-2 and iNOS,
respectively, in the HJ-treated Raw264.7 cell culture supernatant
after LPS stimulation. In accordance with the gene expression
levels of Cox-2 and iNOS, HJ reduced the protein
levels of COX-2 and iNOS and also reduced the production levels of
PGE2 and NO in a dose-dependent manner. These results suggest that
HJ can ameliorate CIA by suppressing pro-inflammatory cytokine and
enzymes.
In addition to the regulation of the expression of
inflammatory mediators in macrophages, HJ also markedly reduced the
expression of the pan-macrophage marker and M1 macrophage marker,
whereas the expression of M2 macrophage marker and the arginase
1/iNOS ratio were significantly increased in the paw of HJ-treated
CIA mice. The degree of macrophage infiltration and activation
correlates, not only with joint pain and inflammation, but also
with joint erosion in RA. A number of macrophages are present in
the inflamed synovium and play important roles in the pathogenesis
of RA by releasing various cytokines, chemokines, and MMP leading
to the erosion of cartilage and bone (11). Monocytes can differentiate into
classically activated pro-inflammatory M1 macrophage or
alternatively activated anti-inflammatory M2 macrophage. M1
macrophages, activated by LPS- or Th1-related cytokines, such as
interferon-γ, produce various pro-inflammatory cytokines resulting
in tissue damage in the development of RA (42). On the other hand, M2 macrophages
activated by Th2-related cytokines, including IL-4 and IL-13, are
involved in tissue remodeling by producing anti-inflammatory
cytokines (28). In other words,
an imbalance in the M1/M2 ratio is associated with the development
of RA. These findings suggest that the reduction of macrophage
infiltration and improvement of M1/M2 ratio in the joints of CIA
mice may attenuate CIA by decreasing the inflammatory mediators in
the paw of HJ-treated mice. However, further studies are needed to
understand the manner in which HJ regulates macrophage migration
and M1/M2 differentiation in the paw of CIA mice.
RA is an autoimmune disease characterized by
increased production of autoantibodies (7). Cytokines derived from T cells
stimulate the differentiation and proliferation of B cells into
plasma cells and enhance the production of autoantibody in RA
(43). Th1 cytokines, IFN-γ and
IL-2, induce class switching to IgG2a and are associated with
cellular immunity, whereas Th2 cytokines, IL-4 and IL-13, regulate
the switch from IgM/D to IgG1 and IgE and are associated with
humoral immunity (44). Thus, the
balance between Th1 and Th2 cells regulates the antibody-mediated
immune response, and RA is described as a Th1-dominant chronic
autoimmune disease (45). In the
present study, we found that HJ significantly suppressed the
production of anti-type II collagen-specific IgG2a, and tended to
reduce the production of anti-type II collagen-specific IgG1. Even
though we did not analyze T-cell subsets via flow cytometric
analysis in the paw of CIA mice, we additionally measured gene
expression levels of Il-12rβ2, Ccr2 and Ccr5
as Th1 cell-associated markers, and Ccr3 and Ccr4 as
Th2 cell-associated markers in the paw of HJ-treated CIA mice. HJ
markedly decreased the expression levels of Th1 and Th2 cell
surface markers in the paws of CIA mice. In accordance with these
changes, the gene expression levels of Th1 and Th2 cytokines,
IL-2 and IL-13, were also markedly reduced in the
paws of CIA mice. These results demonstrate that the HJ inhibits
the production of autoantibodies, especially Th1-mediated IgG2a, by
downregulating both Th1 and Th2 cells and cytokines in the CIA
mice. However, further investigation is required to elaborate the
regulatory effect of HJ on Th1 and Th2 cell differentiation and B
cell-derived autoantibody production in collagen-specific humoral
immunity.
Among the cytokines, IL-6, derived from synovial
fibroblasts and activated macrophages are critical in the
pathogenesis of RA. IL-6 contributes to synovial inflammation by
recruiting inflammatory cells and can also promote the production
of autoantibody acting on plasma blasts, which leads to destruction
of the cartilage (46). This
cytokine is also the major STAT3-activating factor in RA and
IL-6-mediated STAT3 is involved in the destruction of joints
through stimulation of the expression of RANKL in osteoblasts and
the induction of osteoclast differentiation (47). Osteoclasts are terminally
differentiated cells of the monocyte/macrophage lineage that resorb
the bone matrix. Periarticular bone erosion adjacent to the
inflamed joint and systemic bone loss are characteristic features
of RA (48). The destruction of
bones in RA is mainly attributable to the abnormal activation of
osteoclasts (49). The production
of autoantibody and pro-inflammatory cytokine-driven infiltrating
immune cells in the synovium stimulate the differentiation of bone
resorbing osteoclasts, thereby contributing to bone erosion
(50). Pro-inflammatory cytokines
promote osteoclastogenesis via the expression of RANKL on the
surface of osteoblasts (51).
Some researchers have shown that pro-inflammatory cytokines,
including TNF-α, IL-1β, and IL-6, are capable of inducing
osteoclast differentiation independently of RANKL (52). In the present study, HJ
significantly reduced the expression and secretion levels of IL-6
in LPS-simulated RAW264.7 macrophage cells and in the paw of CIA
mice. Furthermore, phosphorylated STAT3 and total STAT3 were
significantly reduced in the HJ group compared to that in the
vehicle group. Additionally, HJ reduced the expression of
osteoclast-specific markers, including Ctsk, Trap,
Calr, and Oscar, as well as of the transcription
factor, NFATc1. Therefore, these results suggest that HJ
modulates the activation of osteoclasts via regulation of the
expression of IL-6 and STAT3 signaling pathways in the paws of CIA
mice. Although STAT3 is the major downstream signaling pathway of
IL-6, further investigation is needed to determine whether HJ
modulates the IL-6 expression in a STAT3 signaling
pathway-dependent manner in CIA.
In conclusion, to the best of our knowledge, this is
the first study to suggest that HJ has protective effects on the
inflammation and destruction of cartilage and bones during the
development of RA in CIA mice by inhibiting the secretion of
pro-inflammatory mediators and osteoclast formation. The results of
the present study provide novel insights into the possibility of
using HJ extract as therapeutics for preventing RA.
Abbreviations:
|
CIA
|
collagen-induced arthritis
|
|
COX
|
cyclooxygenase
|
|
HJ
|
Humulus japonicas
|
|
iNOS
|
inducible nitric oxide synthase
|
|
IL
|
interleukin
|
|
MMPs
|
metalloproteinases
|
|
PGE2
|
prostaglandin E2
|
|
RA
|
rheumatoid arthritis
|
|
TNF-α
|
tumor necrosis factor-α
|
Acknowledgments
The authors would like to thank Mrs. Y.J. Seo and
Mrs. J.H. Choi (Laboratory Animal Resource Center, Korea Research
Institute of Bioscience and Biotechnology, Daejeon, Republic of
Korea) for their technical assistance.
Funding
This study was supported by grants from the KRIBB
Research Initiative Program, a grant from the National Research
Foundation of Korea (NRF) and the Korean government (MSIP)
(NRF-2019R1C1C1005319), and the Korea Bioactive Natural Material
Bank (KBNMB, NRF-2017M3A9B8069409) through the National Research
Foundation of Korea, funded by the Ministry of Science, ICT and
Planning.
Availability of data and materials
The datasets generated during the present study are
not currently available to the public but will be available from
the corresponding author on reasonable request.
Authors' contributions
EJK, HJK, YHK and CHL designed the experiments and
the study. EJK, HJK, JHC, JRN, JHK, IBL, YKC, DHC and JA collected
data and conducted experiments for the study. EJK, HJK, YHK and CHL
analyzed all the data. WKO and CHL contributed to critical
revisions of the text. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of the Korea Research
Institute of Bioscience and Biotechnology (KRIBB-AEC-19142) and
were performed in accordance with the Guide for the Care and Use of
Laboratory Animals published by the US National Institutes of
Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing of interest.
References
|
1
|
Myasoedova E, Crowson CS, Kremers HM,
Therneau TM and Gabriel SE: Is the incidence of rheumatoid
arthritis rising?: Results from Olmsted County, Minnesota
1955-2007. Arthritis Rheum. 62:1576–1582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Silman AJ and Pearson JE: Epidemiology and
genetics of rheumatoid arthritis. Arthritis Res. 4(Suppl 3):
S265–S272. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heidari B: Rheumatoid Arthritis: Early
diagnosis and treatment outcomes. Caspian J Intern Med. 2:161–170.
2011.PubMed/NCBI
|
|
4
|
Cojocaru M, Cojocaru IM, Silosi I, Vrabie
CD and Tanasescu R: Extra-articular manifestations in rheumatoid
arthritis. Maedica (Buchar). 5:286–291. 2010.
|
|
5
|
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ
and Xu J: Rheumatoid arthritis: Pathological mechanisms and modern
pharmacologic therapies. Bone Res. 6:152018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suurmond J, Zou YR, Kim SJ and Diamond B:
Therapeutics to block autoantibody initiation and propagation in
systemic lupus erythematosus and rheumatoid arthritis. Sci Transl
Med. 7:280ps2852015. View Article : Google Scholar
|
|
7
|
Derksen VFAM, Huizinga TWJ and van der
Woude D: The role of autoantibodies in the pathophysiology of
rheumatoid arthritis. Semin Immunopathol. 39:437–446. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bugatti S, Manzo A, Montecucco C and
Caporali R: The clinical value of autoantibodies in rheumatoid
arthritis. Front Med (Lausanne). 5:3392018. View Article : Google Scholar
|
|
9
|
Bingham CO III: The pathogenesis of
rheumatoid arthritis: Pivotal cytokines involved in bone
degradation and inflammation. J Rheumatol Suppl. 65:3–9.
2002.PubMed/NCBI
|
|
10
|
Choy E: Understanding the dynamics:
Pathways involved in the pathogenesis of rheumatoid arthritis.
Rheumatology (Oxford). 51(Suppl 5): pp. v3–v11. 2012, View Article : Google Scholar
|
|
11
|
Kinne RW, Stuhlmüller B and Burmester GR:
Cells of the synovium in rheumatoid arthritis. Macrophages
Arthritis Res Ther. 9:2242007. View
Article : Google Scholar
|
|
12
|
Ma Y and Pope RM: The role of macrophages
in rheumatoid arthritis. Curr Pharm Des. 11:569–580. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pablos Álvarez JL: Interleukin 6 in the
physiopathology of rheumatoid arthritis. Reumatol Clin. 5:34–39.
2009.In Spanish. View Article : Google Scholar
|
|
14
|
Blanchard F, Duplomb L, Baud'huin M and
Brounais B: The dual role of IL-6-type cytokines on bone remodeling
and bone tumors. Cytokine Growth Factor Rev. 20:19–28. 2009.
View Article : Google Scholar
|
|
15
|
Kwan Tat S, Padrines M, Théoleyre S,
Heymann D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: Interrelations
in bone resorption pathophysiology. Cytokine Growth Factor Rev.
15:49–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weitzmann MN: The role of inflammatory
cytokines, the RANKL/OPG axis, and the immunoskeletal interface in
physiological bone turnover and osteoporosis. Scientifica (Cairo).
2013:1257052013.
|
|
17
|
Park JW, Ko SH, Kim CW, Jeoung BJ and Hong
CS: Identification and characterization of the major allergen of
the Humulus japonicus pollen. Clin Exp Allergy. 29:1080–1086. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sung B, Chung JW, Bae HR, Choi JS, Kim CM
and Kim ND: Humulus japonicus extract exhibits antioxidative and
anti-aging effects via modulation of the AMPK-SIRT1 pathway. Exp
Ther Med. 9:1819–1826. 2015.In Korean. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park SW, Woo CJ, Chung SK and Chung KT:
Antimicrobial and antioxidative activities of solvent fraction from
Humulus japonicas. Korean J Food Sci Technol. 26:464–470. 1994.
|
|
20
|
Lim H, Noh JR, Kim YH, Hwang JH, Kim KS,
Choi DH, Go MJ, Han SS, Oh WK and Lee CH: Anti-atherogenic effect
of Humulus japonicus in apolipoprotein E-deficient mice. Int J Mol
Med. 38:1101–1110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brand DD, Latham KA and Rosloniec EF:
Collagen-induced arthritis. Nat Protoc. 2:1269–1275. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nandakumar KS, Bäcklund J, Vestberg M and
Holmdahl R: Collagen type II (CII)-specific antibodies induce
arthritis in the absence of T or B cells but the arthritis
progression is enhanced by CII-reactive T cells. Arthritis Res
Ther. 6:R544–R550. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brand DD, Kang AH and Rosloniec EF: The
mouse model of collagen-induced arthritis. Methods Mol Med.
102:295–312. 2004.PubMed/NCBI
|
|
24
|
Lee CH, Bae SJ and Kim M:
Mucosa-associated lymphoid tissue lymphoma translocation 1 as a
novel therapeutic target for rheumatoid arthritis. Sci Rep.
7:118892017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun J, Jia Y, Li R, Guo J, Sun X, Liu Y,
Li Y, Yao H, Liu X, Zhao J and Li Z: Altered influenza virus
haemagglutinin (HA)-derived peptide is potent therapy for CIA by
inducing Th1 to Th2 shift. Cell Mol Immunol. 8:348–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jhun JY, Yoon BY, Park MK, Oh HJ, Byun JK,
Lee SY, Min JK, Park SH, Kim HY and Cho ML: Obesity aggravates the
joint inflammation in a collagen-induced arthritis model through
deviation to Th17 differentiation. Exp Mol Med. 44:424–431. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Laria A, Lurati A, Marrazza M, Mazzocchi
D, Re KA and Scarpellini M: The macrophages in rheumatic diseases.
J Inflamm Res. 9:1–11. 2016.PubMed/NCBI
|
|
29
|
Kinne RW, Bräuer R, Stuhlmüller B,
Palombo-Kinne E and Burmester GR: Macrophages in rheumatoid
arthritis. Arthritis Res. 2:189–202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fukui S, Iwamoto N, Takatani A, Igawa T,
Shimizu T, Umeda M, Nishino A, Horai Y, Hirai Y, Koga T, et al: M1
and M2 monocytes in rheumatoid arthritis: A contribution of
imbalance of M1/M2 monocytes to osteoclastogenesis. Front Immunol.
8:19582018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Munder M, Eichmann K and Modolell M:
Alternative metabolic states in murine macrophages reflected by the
nitric oxide synthase/arginase balance: Competitive regulation by
CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol.
160:5347–5354. 1998.PubMed/NCBI
|
|
32
|
Mukherjee P, Wu B, Mayton L, Kim SH,
Robbins PD and Wooley PH: TNF receptor gene therapy results in
suppression of IgG2a anticollagen antibody in collagen induced
arthritis. Ann Rheum Dis. 62:707–714. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Corry DB and Kheradmand F: Induction and
regulation of the IgE response. Nature. 402:B18–B23. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Srirangan S and Choy EH: The role of
interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther
Adv Musculoskelet Dis. 2:247–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oike T, Sato Y, Kobayashi T, Miyamoto K,
Nakamura S, Kaneko Y, Kobayashi S, Harato K, Saya H, Matsumoto M,
et al: Stat3 as a potential therapeutic target for rheumatoid
arthritis. Sci Rep. 7:109652017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rehman Q and Lane NE: Bone loss.
Therapeutic approaches for preventing bone loss in inflammatory
arthritis. Arthritis Res. 3:221–227. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hwang SY, Jo MJ, Kim SC and Jee SY:
Anti-inflammaory effects of the MeOH extract of Humulus japonicus
in vivo. J Korean Orient Med Ophthalmol Otolaryngol Dermatol.
22:92–103. 2009.
|
|
38
|
Burrage PS, Mix KS and Brinckerhoff CE:
Matrix metalloproteinases: Role in arthritis. Front Biosci.
11:529–543. 2006. View
Article : Google Scholar
|
|
39
|
Lerner A, Neidhöfer S, Reuter S and
Matthias T: MMP3 is a reliable marker for disease activity,
radiological monitoring, disease outcome predictability, and
therapeutic response in rheumatoid arthritis. Best Pract Res Clin
Rheumatol. 32:550–562. 2018. View Article : Google Scholar
|
|
40
|
Rose BJ and Kooyman DL: A tale of two
joints: The role of matrix metalloproteases in cartilage biology.
Dis Markers. 2016:48950502016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Amin AR, Attur M and Abramson SB: Nitric
oxide synthase and cyclooxygenases: Distribution, regulation, and
intervention in arthritis. Curr Opin Rheumatol. 11:202–209. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Y, Han CC, Cui D, Li Y, Ma Y and Wei
W: Is macrophage polarization important in rheumatoid arthritis?
Int Immunopharmacol. 50:345–352. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cope AP, Schulze-Koops H and Aringer M:
The central role of T cells in rheumatoid arthritis. Clin Exp
Rheumatol. 25:S4–S11. 2007.PubMed/NCBI
|
|
44
|
Kaplan C, Valdez JC, Chandrasekaran R,
Eibel H, Mikecz K, Glant TT and Finnegan A: Th1 and Th2 cytokines
regulate proteoglycan-specific autoantibody isotypes and arthritis.
Arthritis Res. 4:54–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Aarvak T, Chabaud M, Thoen J, Miossec P
and Natvig JB: Changes in the Th1 or Th2 cytokine dominance in the
synovium of rheumatoid arthritis (RA): A kinetic study of the Th
subsets in one unusual RA patient. Rheumatology (Oxford).
39:513–522. 2000. View Article : Google Scholar
|
|
46
|
Yoshida Y and Tanaka T: Interleukin 6 and
rheumatoid arthritis. Biomed Res Int. 2014:6983132014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yoshitake F, Itoh S, Narita H, Ishihara K
and Ebisu S: Interleukin-6 directly inhibits osteoclast
differentiation by suppressing receptor activator of NF-kappaB
signaling pathways. J Biol Chem. 283:11535–11540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Panagopoulos PK and Lambrou GI: Bone
erosions in rheumatoid arthritis: Recent developments in
pathogenesis and therapeutic implications. J Musculoskelet Neuronal
Interact. 18:304–319. 2018.PubMed/NCBI
|
|
49
|
Sato K and Takayanagi H: Osteoclasts,
rheumatoid arthritis, and osteoimmunology. Curr Opin Rheumatol.
18:419–426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schett G and Gravallese E: Bone erosion in
rheumatoid arthritis: Mechanisms, diagnosis and treatment. Nat Rev
Rheumatol. 8:656–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jung SM, Kim KW, Yang CW, Park SH and Ju
JH: Cytokine-mediated bone destruction in rheumatoid arthritis. J
Immunol Res. 2014:2636252014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lieben L: Bone: The concept of
RANKL-independent osteoclastogenesis refuted. Nat Rev Rheumatol.
12:6232016. View Article : Google Scholar : PubMed/NCBI
|