Introduction
In the US, there are ~64,000 new cases of renal cell
carcinoma (RCC) and ~14,000 RCC-related deaths each year (1). In the past decade, systemic therapy
for metastatic RCC has notably improved, moving from the use of
immunotherapeutic interferon α to a variety of targeted
therapeutics, which include anti-angiogenic drugs targeting
vascular endothelial growth factor and its receptors, mTOR
inhibitors, receptor tyrosine kinase inhibitors and immune
checkpoint inhibitors (2).
Despite the progress in treatment options, metastatic RCC remains
incurable with median progression-free survival ranging between 8
and 11 months for patients treated with sunitinib and pazopanib
(3-5). Therefore, more effective drugs are
needed to improve treatment outcomes for patients with metastatic
RCC.
Glycogen synthase kinase-3 (GSK-3) is a
multifunctional kinase involved in a broad range of pathological
processes, including neurodegenerative diseases and cancer
(6). GSK-3 is a serine/threonine
protein kinase that phosphorylates and inactivates glycogen
synthase (GS) (7). GSK-3 has two
isoforms, GSK-3α and GSK-3β (6).
Despite their homology, GSK-3α and GSK-3β are encoded by different
genes, serve independent functions, and the loss of one is not
compensated by the other (8).
GSK-3β has been considered as a potential tumor suppressor as it
phosphorylates and targets pro-oncogenic molecules including c-Jun
(9), c-Myc (10), cyclin D1 (11) and β-catenin (12) for ubiquitin-dependent proteasomal
degradation. However, over the past decade, GSK-3β has emerged as a
therapeutic target in several different types of cancer (13), including renal cancer (14). The GSK-3β inhibitor 9-ING-41 has
entered clinical trials in patients with advanced cancer (clinical
trial no. NCT03678883). 9-ING-41 is a maleimide-based
ATP-competitive small molecule GSK-3β inhibitor with high
selectivity and low toxicity (15,16). The antitumor activity of 9-ING-41
has been demonstrated in models of glioblastoma (17), neuroblastoma (18), breast (19), ovarian (15), pancreatic (16) and renal (20) cancer. The present study aimed to
determine whether 9-ING-41 may potentiate the antitumor effects of
chemotherapeutic drugs and targeted therapeutics and increase the
cytotoxic effects of human immune cells in RCC cell lines.
Materials and methods
Cell culture and reagents
RCC cell lines ACHN and KRCY were obtained from the
American Type Culture Collection. Caki-1 was obtained from Japanese
Collection of Research Bioresources Cell Bank. KU19-20 was kindly
provided by Dr Mototsugu Oya (Department of Urology, School of
Medicine, Keio University, Tokyo, Japan). The cells were cultured
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
1% MEM Non-Essential Amino Acids (Gibco; Thermo Fisher Scientific,
Inc.), 1% MEM sodium pyruvate solution 100 mM (Gibco; Thermo Fisher
Scientific, Inc.) and 90 µg/ml kanamycin in a 37°C incubator
containing 5% CO2. 9-ING-41 was provided by Actuate
Therapeutics, Inc. and used at 0.5-50 µM. Sorafenib was
obtained from ChemScence, LLC and used at 3-8 µM. Sunitinib
was obtained from Sigma-Aldrich; Merck KGaA and used at 1-4
µM. Cabozantinib was obtained from LC Laboratories and used
at 0.5-6 µM. Pazopanib, chloroquine and bafilomycin were
obtained from Cayman Chemical Company and used at 0.5-1, 8-30 and
1-10 µM, respectively. Control cells were treated with an
equal amount of DMSO. Mycoplasma testing was performed for all cell
lines; if mycoplasma was positive, the cells were treated with
MC-210 (DS Pharma Promo Co., Ltd.) at 0.5 µg/ml for two
weeks, and the reagent was washed out for another one week at 37°C
in an incubator.
Cell viability and proliferation
assays
Cell viability was detected with a colorimetric
CellTiter 96® AQueous One Solution Cell Proliferation
assay (Promega Corporation), using a tetrazolium compound according
to the manufacturer's instructions using ACHN, Caki-1, KRCY and
KU19-20 cell lines. The cells were treated with 0.5-5 µM
9-ING-41 during the assay, and cell viability was measured at 0,
24, 48, 72 and 96 h in 9-ING-41 monotherapy. In the combination
treatment, 9-ING-41 was used at 0.5-4 µM during the assay,
and cell viability was measured at 0 and 72 h. For the estimation
of cell proliferation, a 5-bromo-2-deoxyuridine (BrdU) Cell
Proliferation Assay kit (EMD Millipore) was used according to the
manufacturer's instructions using ACHN, Caki-1, KRCY and KU19-20
cell lines. The cells were treated with 0-25 µM 9-ING-41.
Both experiments were performed in three or four replicates using a
flat-bottom 96-well plate (Corning, Inc.) and an iMark™ 96-well
microplate reader (Bio-Rad Laboratories, Inc.). Absorbance was
measured at 490 nm in the cell viability assay and at 450-595 nm in
the cell proliferation assay. GI50, a concentration of
the drug that inhibits the proliferation of cancer cells by 50%,
was calculated using GraphPad Prism 7 (GraphPad Software,
Inc.).
Analysis of cell cycle and apoptosis
ACHN and KRCY cells were fixed in cold 70% ethanol
for 30 min. Propidium iodide (PI) staining of fixed cells was
performed for cell cycle analysis and quantification of apoptosis
(sub-G1 population) using FxCycle™ PI/RNase Staining
Solution (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Stained cells were analyzed using BD
Accuri™ C6 software and a BD Accuri™ C6 Flow Cytometer (BD
Biosciences).
Western blotting
Subconfluent cell cultures were washed with cold PBS
and lysed in lysis buffer (150 mM sodium chloride, 5 mM EDTA, 1%
Triton X-100, 100 mM Tris-HCl and a protease inhibitor). Following
clarification of the lysates by centrifugation at 15,000 × g for 30
min at 4°C, protein concentration was detected by the Bradford
method, and 30 µg of each protein was electrophoretically
separated on a 10% SDS-polyacrylamide gel and transblotted to a
PVDF membrane. Immunoblots were blocked with 10% skimmed milk in
TBS followed by incubation with primary antibodies. Horseradish
peroxidase-labeled ECL™ Anti-mouse IgG (1:2,000-1:5,000) and ECL™
Anti-rabbit IgG (1:5,000-1:20,000) from GE Healthcare were used as
secondary antibodies and detected using Clarity Max Western ECL
Substrate from Bio-Rad Laboratories, Inc. according to the
manufacturer's instructions. Expression of β-actin was used as a
loading control. The images were analyzed using Ez-Capture MG (Atto
Corporation). The following antibodies were used: Anti-cyclin D1
(cat. no. 2922), anti-cyclin B1 (cat. no. 4135S), anti-E2F
transcription factor 1 (E2F-1; cat. no. 3742),
anti-cyclin-dependent kinase 1 (CDK1; cat. no. 77055S), anti-GS
(cat. no. 3893), anti-phospho-GS (Ser641) (cat. no. 3891),
anti-poly (ADP-ribose) polymerase (PARP; cat. no. 9542),
anti-GSK-3β (cat. no. 12456), anti-β-actin (cat. no. 12262) from
Cell Signaling Technology, Inc.; anti-X-linked inhibitor of
apoptosis (XIAP; cat. no. 610716) and anti-Bcl-2 (cat. no. 610538)
from BD Biosciences. The dilution ratios of the primary antibodies
were 1:250-1:1,000.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
ACHN, Caki-1, KRCY and KU19-20 cell lines were
treated with 0, 25 and 50 µM 9-ING-41. Total cellular RNA
was extracted using the SV Total RNA Isolation System (Promega
Corporation) and the first-strand DNA was synthesized using a cDNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) following the manufacturer's instructions. qPCR
was performed in a 7300 Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with 40 cycles of denaturation
(95°C for 15 sec), annealing and elongation (60°C for 1 min).
Predesigned TaqMan® Gene Expression assays (Applied
Biosystems; Thermo Fisher Scientific, Inc.) targeting human Bcl-2
(assay ID, Hs00236808_s1), E2F1 (assay ID, Hs00153451_m1) mRNA were
used, and GAPDH (assay ID, Hs02758991_g1) was used as an endogenous
control. Each experiment was performed in triplicate wells for each
sample in a final reaction volume of 20 µl using a
TaqMan® Universal PCR Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The expression of the target mRNA was quantified relative
to that of the GAPDH mRNA using the 2-ΔΔCq method as
previously described (21) and
untreated controls were used as a reference.
Cytotoxicity assay
Human peripheral blood mononuclear cells (PBMC) were
separated from human blood obtained from healthy volunteers using
Lymphocyte Separation Solution (Nacalai Tesque, Inc.). For
activation, PBMCs were suspended at a concentration of
2×106 cells/ml in RPMI-1640 medium containing 10% FBS,
and recombinant human interleukin 2 (IL-2; cat. no. 0617AFC12;
Peprotech, Inc.) was added at a concentration of 2,000 IU/ml. PBMCs
were cultured for 3 days at 37°C in a 5% CO2 atmosphere.
CytoTox96® Non-radioactive Cytotoxic Assay (Promega
Corporation) was used according to the manufacturer's instructions.
The CytoTox96® colorimetric assay measures lactate
dehydrogenase (LDH), a stable cytosolic enzyme that is released
upon cell lysis. Briefly, RCC cells ACHN and Caki-1 and activated
PBMCs were added to a round-bottom 96-well plate (Corning, NY) and
mixed at the lymphocyte to cancer cell ratios between 1:2.5 and
1:80. Following 4-h incubation at 37°C, 50 µl of the
supernatants were transferred to a fresh 96-well flat-bottom plate
(Corning, Inc.), and the absorbance signal was measured at 490 nm
using an iMark™ Microplate Reader. Experiments were performed in
triplicate.
Statistical analysis
Continuous variables are presented as the mean ± SD.
All continuous variables in this study met the criteria for a
normal distribution and were assumed to be parametric. Data were
analyzed using one-way ANOVA with Dunnett's test for multiple
comparisons. Statistical analysis was performed using GraphPad
Prism 7 software (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Treatment with 9-ING-41 inhibits the
proliferation of renal cancer cells
Immunoblotting was used to determine GSK-3β
expression in ACHN, Caki-1, KRCY and KU19-20 RCC cell lines
(Fig. 1A). Treatment with
9-ING-41 resulted in reduced GSK-3β activity in all RCC cell lines,
indicated by the decreased expression of phospho-GS, a downstream
target of GSK-3β, compared with control cells treated with DMSO
(Fig. 1A). The results of the MTS
assay demonstrated that treatment with 9-ING-41 decreased the
proliferation of RCC cells at low micromolar concentrations in a
dose-dependent manner with a GI50 range of 0.5-1.7
µM (Fig. 1B). The results
of the BrdU incorporation assay confirmed that treatment with
9-ING-41 inhibited the proliferation of RCC cells compared with the
respective control groups (Fig.
1C).
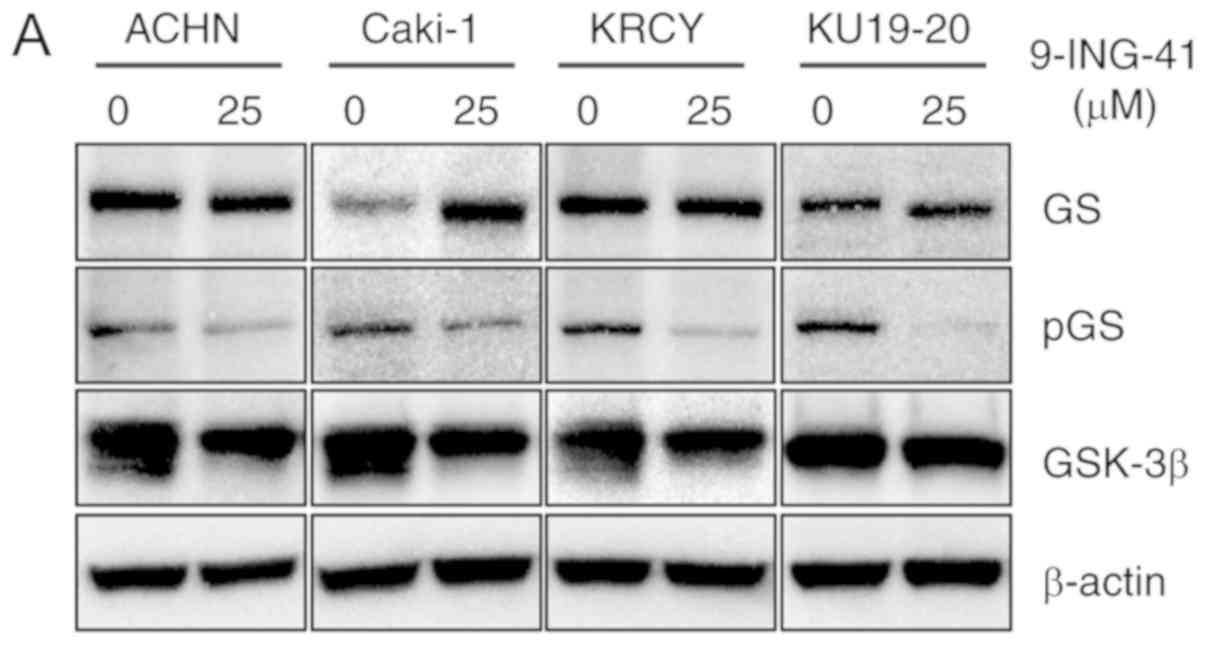 | Figure 1Treatment with 9-ING-41 inhibits the
proliferation and survival of RCC cells. (A) RCC cells were treated
with the indicated concentrations of 9-ING-41 for 96 h, and protein
expression was analyzed by western blotting. (B) Relative cell
proliferation was measured by MTS assay in RCC cells treated with
the indicated doses of 9-ING-41 for 24, 48, 72 and 96 h.
Differences were analyzed by one-way ANOVA. (C) BrdU colorimetric
assay was performed in RCC cells treated with diluent (DMSO) or
9-ING-41 at indicated concentrations for 48 h.
*P<0.05, **P<0.01 and
***P<0.001. RCC, renal cell carcinoma; GS, glycogen
synthase; p, phosphorylated; GSK-3β, glycogen synthase kinase-3β;
GI50, concentration that inhibits cell proliferation by
50%; BrdU, 5-bromo-2-deoxyuridine; OD, optical density. |
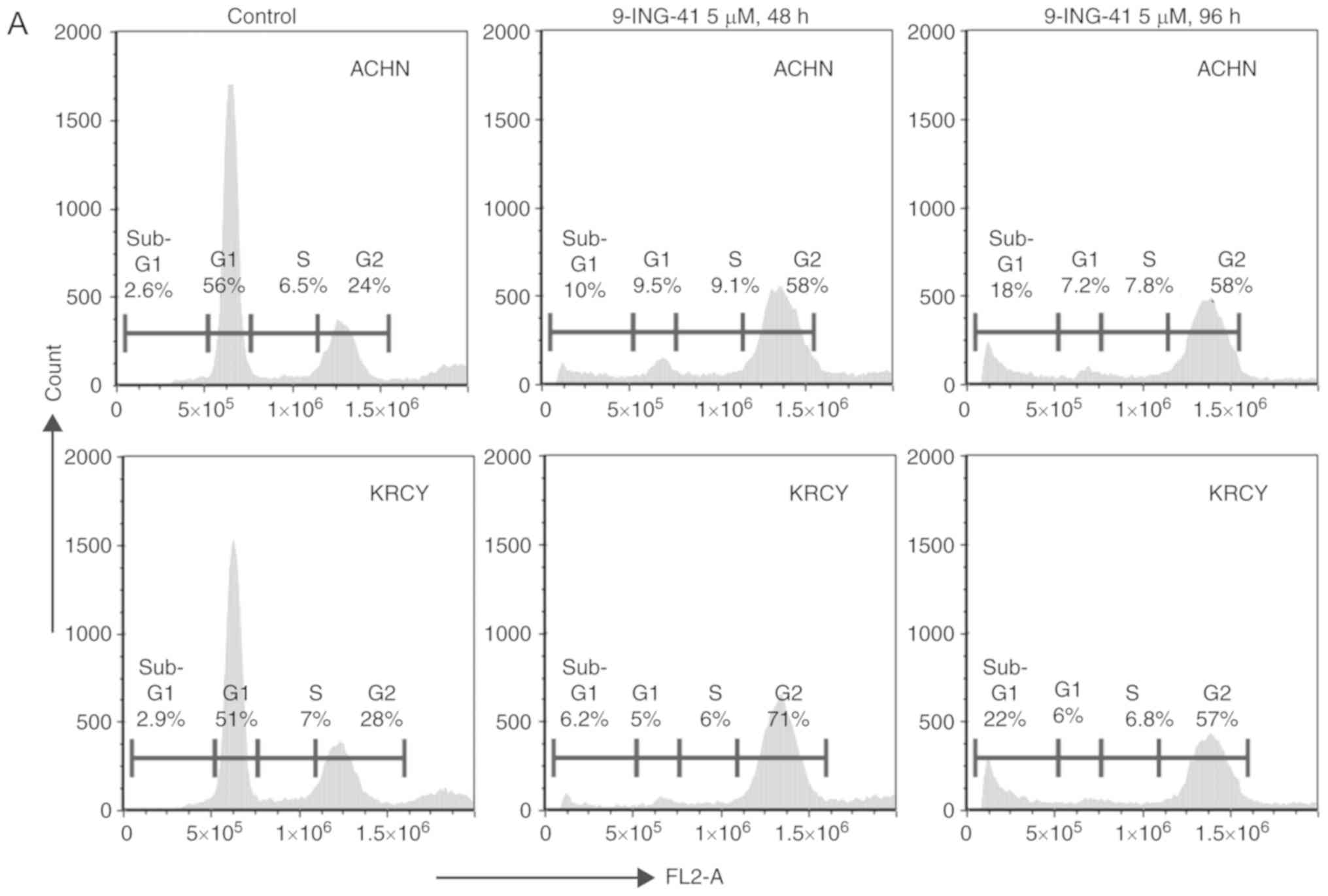
Treatment with 9-ING-41 induces cell
cycle arrest and apoptosis in renal cancer cells
PI-fluorescence-activated cell sorting revealed that
treatment with 9-ING-41 for 48 h induced cell cycle arrest at the
G2 phase, and treatment for 96 h induced cell cycle
arrest with an increased sub-G1 cell population, which
is an indicator of apoptosis, in ACHN and KRCY cells compared with
the control groups (Fig. 2A and
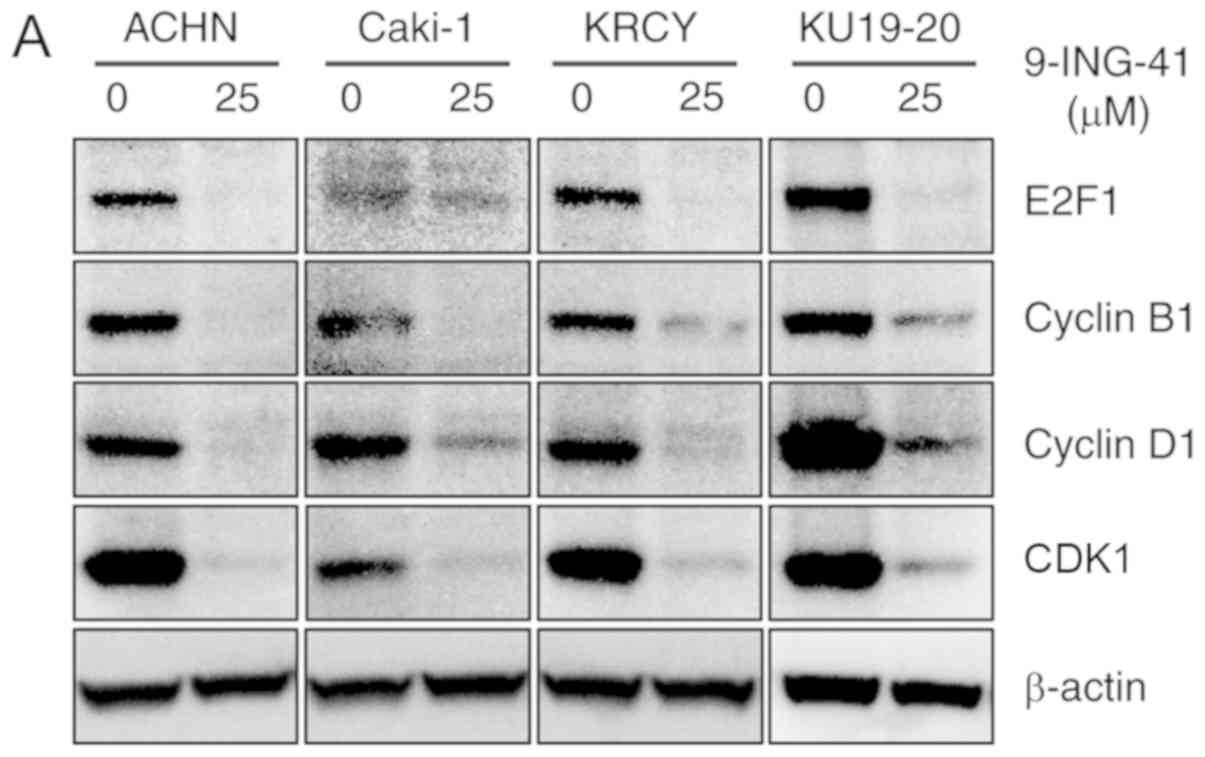
B). Mechanistically, the expression of Cyclin B1 and CDK1
proteins, which serve an important role in the transition from
G2 to M phase, was decreased following treatment with
9-ING-41 (Fig. 3A). In addition,
the expression of E2F-1 and cyclin D1 proteins, which serve a
crucial role in the cell cycle, was decreased following treatment
with 9-ING-41 (Fig. 3A).
Treatment with 9-ING-41 decreased the expression of antiapoptotic
proteins, Bcl-2 and XIAP, leading to an increase in apoptosis
indicated by PARP cleavage, which is a marker of apoptosis
(Fig. 3B). However, the
immunoblotting results also indicated that Bcl-2 was scarcely
expressed in KU19-20. RT-qPCR results demonstrated decreased Bcl-2
and E2F1 mRNA expression in RCC cells treated with 9-ING-41
compared with the controls, with the exception of Bcl-2 in KU19-20
(Fig. 3C).
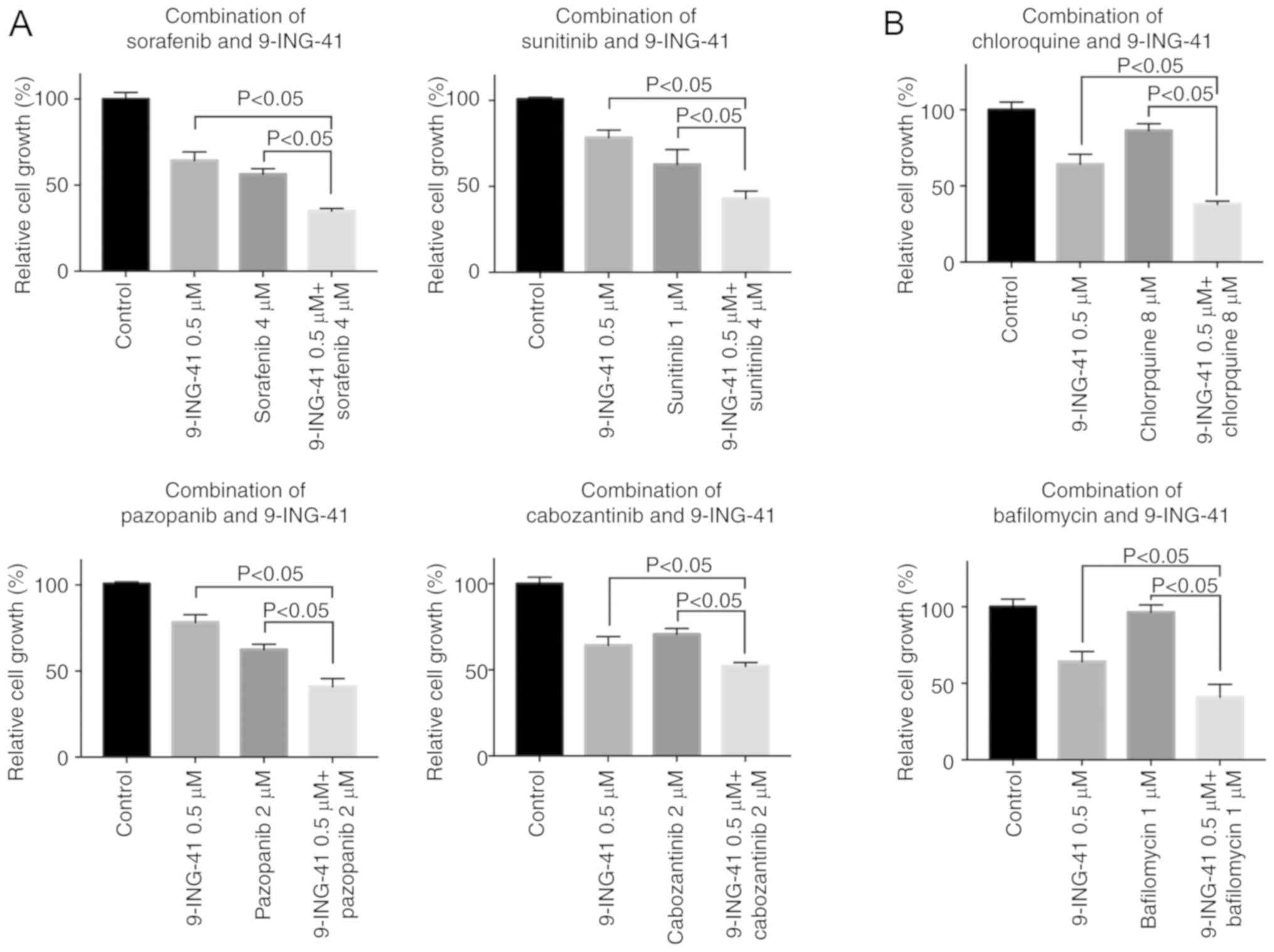
9-ING-41 potentiates the antitumor
effects of targeted therapeutics and autophagy inhibitors in RCC
cells
Using RCC cell lines, the effects of 9-ING-41 in
combination with first- and second-line RCC targeted therapeutics
sunitinib, pazopanib, sorafenib and cabozantinib were investigated
(Figs. 4A, C and S1). For combination experiments, a
minimally effective and clinically relevant concentration of 0.5-2
µM 9-ING-41 was used for ACHN (GI50=0.8
µM), Caki-1 (GI50=1.7 µM), KRCY
(GI50=1 µM) and KU19-20 (GI50=0.5
µM) cells. The results of the MTS assay demonstrated that
9-ING-41 potentiated the antitumor effects of sorafenib
(P<0.05), cabozantinib (P<0.05), sunitinib (P<0.05) and
pazopanib (P<0.05) in RCC cells (Figs. 4A, C and S1).
To test the hypothesis that an autophagy inhibitor
may potentiate the antitumor effects of 9-ING-41, RCC cells were
treated with a combination of 9-ING-41 (0.5-2 µM) and the
autophagy inhibitors chloroquine (8-30 µM) and bafilomycin
(1-10 µM). The results of the MTS assay revealed that the
antitumor effect of 9-ING-41 was significantly increased when
9-ING-41 was combined with chloroquine (P<0.05) or bafilomycin
(P<0.05) compared with single treatments in RCC cells (Figs. 4B, D and S1).
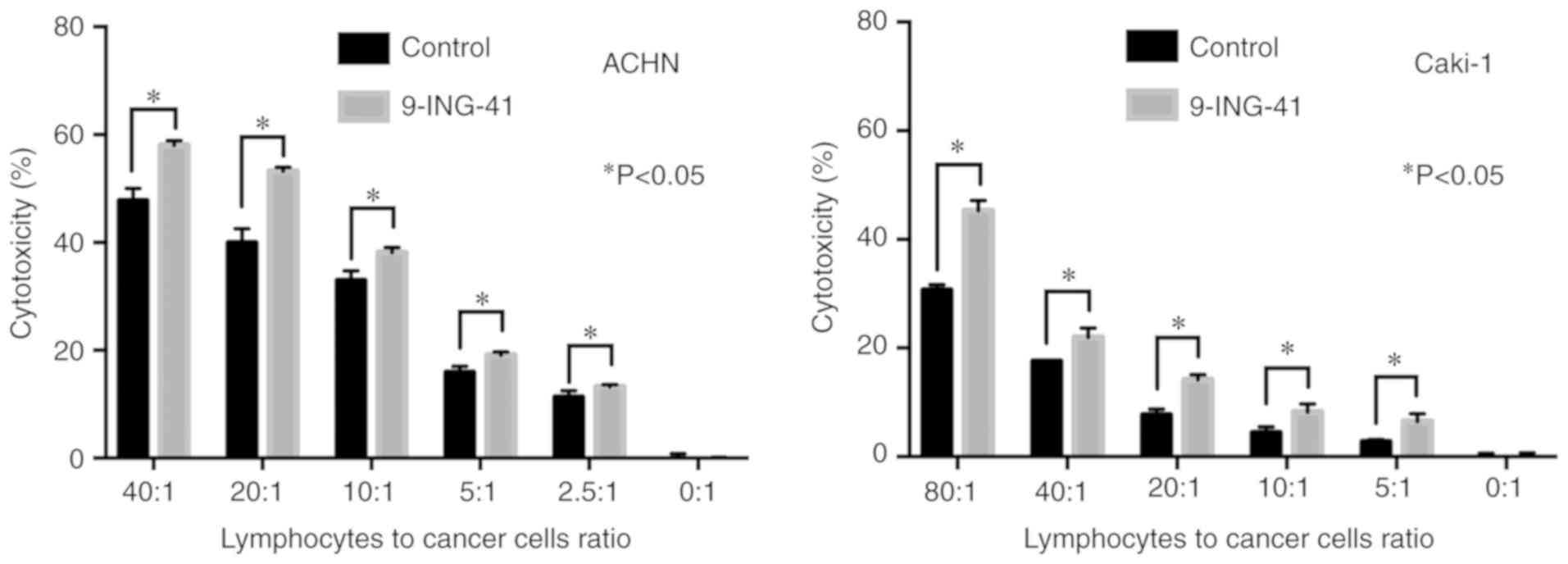
Treatment with 9-ING-41 potentiates the
antitumor effects of immune cells in RCC
To investigate whether GSK-3 inhibition affects the
antitumor effects of human immune cells in RCC cell lines, ACHN and
Caki-1 renal cancer cells were treated with 9-ING-41 for 72 h, and
then mixed with activated PBMCs at various ratios. LDH activity of
the supernatant was measured to evaluate the cytotoxic effects
(Fig. 5). The results
demonstrated that treatment with 9-ING-41 significantly increased
the cytotoxic effects of activated PBMCs in representative RCC cell
lines ACHN and Caki-1 compared with control cells treated with DMSO
(Fig. 5).
Discussion
GSK-3β has been identified as a potential
therapeutic target in human RCC (14,20). The results of our previous study
identified GSK-3 as a positive regulator of RCC cell survival,
proliferation and chemoresistance (14). Since the two isoforms of GSK-3, α
and β, are 98% homologous in the kinase domain, the majority of
known competitive inhibitors of GSK-3 inhibit both isoforms, and
should thus be referred to as GSK-3 inhibitors rather than GSK-3β
inhibitors (22). Previous
studies have demonstrated that treatment with 9-ING-41, a clinical
stage GSK-3β inhibitor, at clinically relevant concentrations of
0.5-2 µM suppresses the viability of neuroblastoma, ovarian,
pancreatic and breast cancer cells in vitro (15,16,18,19). Pharmacokinetic studies have
demonstrated that 9-ING-41 (20 mg/kg at 30 min after intravenous
administration) could reach mouse plasma and brain concentration of
~7 µM and 44 µM, respectively (17). The results of the present study
demonstrated that treatment with 0.5-2 µM 9-ING-41
suppressed the viability of renal cancer cells. These results were
supported by another study, which demonstrated that treatment with
ARA-014418, a toolkit GSK-3 inhibitor, resulted in a significant
decrease of antiapoptotic proteins Bcl-2 and XIAP and induction of
apoptosis in RCC cells (14).
Consistent with the previous report by Pal et al (20), the results of the present study
indicated that treatment with 9-ING-41 induced cell cycle arrest in
RCC cells.
Resistance to the current standard treatments in
metastatic RCC has led to a poor prognosis for patients with this
disease (2). NF-κB-mediated drug
resistance results in RCC progression and recurrence (23). Inhibition of GSK-3β, a positive
regulator of NF-κB-mediated survival in cancer cells (24,25), may be an effective therapeutic
approach to overcome RCC resistance to antitumor drugs. Recently,
treatment with the GSK-3 inhibitor 9-ING-41 has been demonstrated
to overcome the resistance to chemotherapeutic drugs in models of
breast cancer (19), glioblastoma
(17) and neuroblastoma (18). Another study has reported that
ARA-014418 enhances the antitumor effect of sorafenib in RCC cells
(26). The results of the present
study demonstrated that 9-ING-41 potentiated the antitumor effects
of the first- and second-line RCC targeted therapeutics sunitinib,
pazopanib, sorafenib and cabozantinib. These results provided a
rationale for the combination of 9-ING-41 with targeted
therapeutics for effective treatment of RCC.
Although the role of autophagy in cancer is complex
and context-dependent, autophagy has been suggested as a potential
mechanism of evading apoptosis in cancer cells (27). A number of antitumor therapies
have been identified to induce autophagy in human cancer cells
(28-30). Whether autophagy induced by
antitumor therapy contributes to apoptosis of cancer cells or
represents a mechanism of resistance to therapy-mediated apoptosis
remains unclear. An increase of intracellular glucose storage and
induction of autophagy have been demonstrated in 9-ING-41-treated
renal cancer cells (20).
Inhibition of GSK-3 triggers an autophagic response in prostate
(31), pancreatic (32) and renal (20) cancer. Pal et al (20) have demonstrated that GSK-3
inhibition by 9-ING-41 affects energy homeostasis and triggers a
pro-survival autophagic response in renal cancer cells. In the
present study, 9-ING-41 increased the antitumor effects of the
autophagy inhibitors chloroquine and bafilomycin in RCC cells.
These results are in agreement with previously published work
demonstrating that the inhibition of autophagy by bafilomycin
sensitized pancreatic cancer cells to GSK-3 inhibition-induced
apoptosis (32). The results of
the present study support the hypothesis that autophagy-mediated
resistance to 9-ING-41 therapy may be overcome by combining
9-ING-41 with autophagy inhibitors in human RCC.
Historically, the treatment of metastatic RCC
included immune-modulating therapies, such as interferon α and IL-2
(33). However, these therapies
exhibit significant toxicity and low efficacy (33). Since the introduction of targeted
therapy, targeted tyrosine kinase inhibitors and vascular
endothelial growth factor have become the standard treatments for
advanced RCC (2). The development
of immune checkpoint inhibitors for RCC treatment has further
improved treatment outcomes for patients with advanced RCC
(2). Results from clinical trials
have demonstrated that immune checkpoint inhibitors improved
overall survival in treatment-naïve or previously treated
metastatic RCC (34,35). In the present study, treatment
with 9-ING-41 significantly increased the cytotoxic effect of human
immune cells added to RCC cell lines. These results suggested
9-ING-41-treatment increased RCC cells vulnerability to activated
human immune cells. Further experiments using autologous models are
being performed in our laboratory to explore the molecular
mechanisms of this phenomenon.
The results of the present study support the
hypothesis that treatment with the specific small molecule GSK-3β
inhibitor 9-ING-41 may potentiate the antitumor immune response in
patients with RCC. 9-ING-41 has exhibited significant clinical
activity in patients with advanced cancer (clinical trial no.
NCT03678883). These results provide a compelling rationale for the
inclusion of patients with advanced renal cancer in studies of
9-ING-41, both as a single agent and in combination with current
standard therapies.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by research grant
from the Department of Urology, Niigata University (Niigata City,
Japan).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TA, VB, AU and YT conceived and designed the study.
TA, HK, AK, VB, MT, AU and YT developed the methodology. TA, HK and
AK performed the experiments. TA, VB, AU and YT analyzed and
interpreted the data. TA, HK, AK, VB, MT, DS, AM, FG, AU and YT
wrote, reviewed and/or revised the manuscript. DS, AM, FG and YT
provided administrative, technical, or material support. YT, TA and
VB supervised the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by Niigata University
Ethical Committee (approval no. 2620), and informed consent was
obtained from healthy volunteers.
Patient consent for publication
Not applicable.
Competing interests
9-ING-41 has been licensed to Actuate Therapeutics,
Inc. Andrew Mazar, Andrey Ugolkov, Daniel Schmitt and Francis Giles
hold an equity interest in Actuate Therapeutics, Inc.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodriguez-Vida A, Hutson TE, Bellmunt J
and Strijbos MH: New treatment options for metastatic renal cell
carcinoma. ESMO Open. 2:e0001852017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Motzer RJ, Hutson TE, Cella D, Reeves J,
Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, et
al: Pazopanib versus sunitinib in metastatic renal-cell carcinoma.
N Engl J Med. 369:722–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatic renal
cell carcinoma: Results of a randomized phase III trial. J Clin
Oncol. 28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eldar-Finkelman H: Glycogen synthase
kinase 3: An emerging therapeutic target. Trends Mol Med.
8:126–132. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schlender KK, Beebe SJ, Willey JC, Lutz SA
and Reimann EM: Isolation and characterization of cyclic
AMP-independent glycogen synthase kinase from rat skeletal muscle.
Biochim Biophys Acta. 615:324–340. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagini S, Sophia J and Mishra R: Glycogen
synthase kinases: Moonlighting proteins with theranostic potential
in cancer. Semin Cancer Biol. 56:25–36. 2019. View Article : Google Scholar
|
|
9
|
de Groot RP, Auwerx J, Bourouis M and
Sassone-Corsi P: Negative regulation of Jun/AP-1: Conserved
function of glycogen synthase kinase 3 and the drosophila kinase
shaggy. Oncogene. 8:841–847. 1993.PubMed/NCBI
|
|
10
|
Sears R, Nuckolls F, Haura E, Taya Y,
Tamai K and Nevins JR: Multiple Ras-dependent phosphorylation
pathways regulate Myc protein stability. Genes Dev. 14:2501–2514.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Diehl JA, Cheng M, Roussel MF and Sherr
CJ: Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis
and subcellular localization. Genes Dev. 12:3499–3511. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rubinfeld B, Albert I, Porfiri E, Fiol C,
Munemitsu S and Polakis P: Binding of GSK3beta to the
APC-beta-catenin complex and regulation of complex assembly.
Science. 272:1023–1026. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walz A, Ugolkov A, Chandra S, Kozikowski
A, Carneiro BA, O'Halloran TV, Giles FJ, Billadeau DD and Mazar AP:
Molecular pathways: Revisiting glycogen synthase kinase-3β as a
target for the treatment of cancer. Clin Cancer Res. 23:1891–1897.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bilim V, Ougolkov A, Yuuki K, Naito S,
Kawazoe H, Muto A, Oya M, Billadeau D, Motoyama T and Tomita Y:
Glycogen synthase kinase-3: A new therapeutic target in renal cell
carcinoma. Br J Cancer. 101:2005–2014. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hilliard TS, Gaisina IN, Muehlbauer AG,
Gaisin AM, Gallier F and Burdette JE: Glycogen synthase kinase 3β
inhibitors induce apoptosis in ovarian cancer cells and inhibit
in-vivo tumor growth. Anticancer Drugs. 22:978–985. 2011.PubMed/NCBI
|
|
16
|
Gaisina IN, Gallier F, Ougolkov AV, Kim
KH, Kurome T, Guo S, Holzle D, Luchini DN, Blond SY, Billadeau DD
and Kozikowski AP: From a natural product lead to the
identification of potent and selective
benzofuran-3-yl-(indol-3-yl)maleimides as glycogen synthase kinase
3beta inhibitors that suppress proliferation and survival of
pancreatic cancer cells. J Med Chem. 52:1853–1863. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ugolkov A, Qiang W, Bondarenko G, Procissi
D, Gaisina I, James CD, Chandler J, Kozikowski A, Gunosewoyo H,
O'Halloran T, et al: Combination treatment with the GSK-3 inhibitor
9-ING-41 and CCNU cures orthotopic chemoresistant glioblastoma in
patient-derived xenograft models. Transl Oncol. 10:669–678. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ugolkov AV, Bondarenko GI, Dubrovskyi O,
Berbegall AP, Navarro S, Noguera R, O'Halloran TV, Hendrix MJ,
Giles FJ and Mazar AP: 9-ING-41, a small-molecule glycogen synthase
kinase-3 inhibitor, is active in neuroblastoma. Anticancer Drugs.
29:717–724. 2018.PubMed/NCBI
|
|
19
|
Ugolkov A, Gaisina I, Zhang JS, Billadeau
DD, White K, Kozikowski A, Jain S, Cristofanilli M, Giles F,
O'Halloran T, et al: GSK-3 inhibition overcomes chemoresistance in
human breast cancer. Cancer Lett. 380:384–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pal K, Cao Y, Gaisina IN, Bhattacharya S,
Dutta SK, Wang E, Gunosewoyo H, Kozikowski AP, Billadeau DD and
Mukhopadhyay D: Inhibition of GSK-3 induces differentiation and
impaired glucose metabolism in renal cancer. Mol Cancer Ther.
13:285–296. 2014. View Article : Google Scholar :
|
|
21
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cormier KW and Woodgett JR: Recent
advances in understanding the cellular roles of GSK-3. F1000Res.
6:2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morais C, Gobe G, Johnson DW and Healy H:
The emerging role of nuclear factor kappa B in renal cell
carcinoma. Int J Biochem Cell Biol. 43:1537–1549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ougolkov AV, Bone ND, Fernandez-Zapico ME,
Kay NE and Billadeau DD: Inhibition of glycogen synthase kinase-3
activity leads to epigenetic silencing of nuclear factor kappaB
target genes and induction of apoptosis in chronic lymphocytic
leukemia B cells. Blood. 110:735–742. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ougolkov AV, Fernandez-Zapico ME, Savoy
DN, Urrutia RA and Billadeau DD: Glycogen synthase kinase-3beta
participates in nuclear factor kappaB-mediated gene transcription
and cell survival in pancreatic cancer cells. Cancer Res.
65:2076–2081. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawazoe H, Bilim VN, Ugolkov AV, Yuuki K,
Naito S, Nagaoka A, Kato T and Tomita Y: GSK-3 inhibition in vitro
and in vivo enhances antitumor effect of sorafenib in renal cell
carcinoma (RCC). Biochem Biophys Res Commun. 423:490–495. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye
WC, Zhang DM and Chen ZS: Autophagy and multidrug resistance in
cancer. Chin J Cancer. 36:522017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abdel-Mohsen MA, Ahmed OA and El-Kerm YM:
BRCA1 gene mutations and influence of chemotherapy on autophagy and
apoptotic mechanisms in Egyptian breast cancer patients. Asian Pac
J Cancer Prev. 17:1285–1292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park JM, Huang S, Wu TT, Foster NR and
Sinicrope FA: Prognostic impact of Beclin 1, p62/sequestosome 1 and
LC3 protein expression in colon carcinomas from patients receiving
5-fluorouracil as adjuvant chemotherapy. Cancer Biol Ther.
14:100–107. 2013. View Article : Google Scholar :
|
|
30
|
Gao S, Yang XJ, Zhang WG, Ji YW and Pan Q:
Mechanism of thalidomide to enhance cytotoxicity of temozolomide in
U251-MG glioma cells in vitro. Chin Med J (Engl). 122:1260–1266.
2009.
|
|
31
|
Sun A, Li C, Chen R, Huang Y, Chen Q, Cui
X, Liu H, Thrasher JB and Li B: GSK-3β controls autophagy by
modulating LKB1-AMPK pathway in prostate cancer cells. Prostate.
76:172–183. 2016. View Article : Google Scholar
|
|
32
|
Marchand B, Arsenault D, Raymond-Fleury A,
Boisvert FM and Boucher MJ: Glycogen synthase kinase-3 (GSK3)
inhibition induces prosurvival autophagic signals in human
pancreatic cancer cells. J Biol Chem. 290:5592–5605. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3:170092017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Motzer RJ, Tannir NM, McDermott DF, Arén
Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P,
Porta C, George S, et al: Nivolumab plus ipilimumab versus
sunitinib in advanced renal-cell carcinoma. N Engl J Med.
378:1277–1290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|