Introduction
Infant hemangioma (IH) is a common benign vascular
lesion in childhood and consists of hyperactive endothelial cells
(1). IH usually grows slowly at 2
weeks or 4 weeks following birth and proliferates rapidly within
6-10 months. The growth rate of the tumor slows down at 12-14
months, and the tumor eventually degrades slowly following 5-10
years (1). IH is most commonly
located in the head, face and neck, followed by the limbs and trunk
(2). Approximately 20% of complex
cases are accompanied by a series of complications, including
deformity, visual impairment, bleeding, congestive heart failure
and even mortality (3). In
previous years, there have been numerous treatment developments,
including surgical hemangioma tissue removal and propranolol
injection. However, surgical treatment may result in postoperative
scarring, and the exact mechanism of propranolol in IH is not yet
understood (4). Therefore, it is
necessary to identify safer and more effective treatment strategies
and their associated mechanisms for the treatment of IH.
At present, it is known that the primary candidates
for the cellular sources of IH are hemangioma stem cells (HemSCs)
(5). One previous study confirmed
that insulin-like growth factor 1 (IGF-1) may facilitate HemSCs
proliferation, in addition to HemSCs differentiation into
adipocytes, and enhance lipogenesis (6). IGF-1 binds specifically to IGF-1
receptor (IGF-1R) and affects the expression of peroxisome
proliferator-activated receptor-γ2 (PPAR-γ2) through the
phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway
(7). PPAR-γ2 is a key factor that
directs adipocyte differentiation by controlling the expression of
specific differentiation-associated adipocyte genes (8,9).
MicroRNAs (miRs) are non-coding, endogenous, single-stranded, short
RNAs with lengths of 20-23 nucleotides. miRs may regulate the
expression of genes through binding to their complementary
sequences, which are in the target mRNA 3′-untranslated regions
(3′-UTRs) (10-12). miRs have been recognized for their
involvement in tumor development and their potential as prognostic
biomarkers (13). For instance,
miR-424 inhibits the basic fibroblast growth factor/fibroblast
growth factor receptor 1 pathway, thereby suppressing
proliferation, tube formation capability and migration in HemSCs
(14). In addition, miR-138
inhibits the differentiation of human adipose tissue-derived
mesenchymal stem cells into adipocytes (15). One previous study also confirmed
that miR-139-5p overexpression may inhibit non-small cell lung
cancer cell proliferation, migration and invasion by
down-regulating IGF-1R expression (16). Based on prior results, miR-139-5p
may influence HemSCs adipogenesis, migration and proliferation, and
IGF-1R may be its target.
The present study examined the effect, expression
and mechanism of miR-139-5p in IH. The aim was to provide novel
insight into how miR-139-5p may affect HemSCs proliferation and
adipogenesis through the IGF-1/IGF-1R pathway. This information may
be utilized to identify novel treatments for patients with IH.
Materials and methods
Tissue specimen collection
A total of 20 samples of IH were collected between
January 2018 and July 2019 at the Institute of Plastic Surgery of
Second Affiliated Hospital of Anhui Medical University (Anhui,
China). Among the 20 cases, male and female infantile skin
hemangioma accounted for 8 cases and 12 cases, respectively.
Inclusion criteria were as follows: i) Age ≤12 months; ii) specimen
confirmed as hemangioma by clinicians and pathologists; iii)
hemangioma specimens in the proliferative phase (determined
according to the WHO standards); iv) the patient did not receive
any other treatment prior to surgery; v) written informed consent
was obtained from the legal guardians of the patients. The study
was approved by the Ethics Committee of the Second Hospital of
Anhui Medical University (approval no. PJ-bb2017-026). Each
hemangioma tissue was acquired on a different day, and HemSCs were
rapidly isolated and cultured separately until the third generation
for subsequent experiments.
Isolation and identification of
HemSCs
Proliferating IH tissues removed from the patients
were immediately immersed in growth medium [10% fetal bovine serum
(FBS; GE Healthcare Life Sciences), 1% penicillin-streptomycin (PS;
Gibco; Thermo Fisher Scientific, Inc.) and Dulbecco's modified
Eagle's medium (DMEM; Beyotime Institute of Biotechnology)] at 4°C.
The following steps were performed on a clean cell bench. First,
the fat and skin tissues were removed from the hemangiomas, and the
samples were rinsed three times with phosphate-buffered saline
(PBS; BasalMedia). The tissues were then fully chopped and digested
with 0.2% collagenase (SERVA Electrophoresis GmbH) in a constant
temperature bath at 37°C for 2 h and shaken a number of times every
half hour until the samples were chylous. The samples were then
filtered through a 100-micron cell strainer. The cells expressing
CD133 (17) were selected using a
magnetic bead technique (Miltenyi Biotec, Inc.). Finally, the cells
were cultivated on fibronectin-coated plates in an appropriate
amount of the endothelial cell medium (ECM; ScienCell Research
Laboratories, Inc.) with 20% FBS and 1% PS in a wet incubator with
5% carbon dioxide at 37°C.
Cell culture and transfection
A miR-139-5p inhibitor and mimics were created by
Nanjing KeyGen Biotech Co., Ltd. The sequences were as follows:
miR-139-5p mimics sense, UCUACAGUGCACGUGUCUCCAGU and antisense,
ACUGGAGACACGUGCACUGUAGA; miR-139-5p inhibitor,
ACUGGAGACACGUGCACUGUAGA; miR-139-5p mimics negative control
(FAM-dN-CTL) sense, UUUGUACUACACAAAAGUACUG and antisense,
CAGUACUUUUGUGUAGUACAAA; and miR-139-5p inhibitor negative control
(FAM-N-CTL), CAGUACUUUUGUGUAGUACAA. HemSCs were seeded in 6-well
plates (Corning Inc.) at a density of 1×106 cells/ml per
well, and the cell density reached 50-60% confluence the following
day. Prior to transfection, the cells were rinsed twice with PBS
prior to transfection, and 2 ml ECM containing 10% FBS was added to
each well. Subsequently, 110 pmol miR-139-5p mimics or inhibitor
was diluted in 200 µl jetPRIME buffer (Polyplus-transfection
SA) and mixed. Then, 4 µl jetPRIME reagent
(Polyplus-transfection SA) was added and vortexed briefly for 10
sec and cultured for 10-50 min at room temperature. Finally,
miR-139-5p mimics or inhibitor were added to the appropriate wells.
At 24 h, the medium was replaced with fresh ECM, and a number of
groups were treated with 100 ng/ml IGF-1 (Peprotech, Inc.). The
next experiments were performed 48 h following transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was utilized to extract whole cellular RNA. cDNA
was synthesized through the reverse transcription of 2 µg
whole RNA utilizing the PrimeScriptTM RT reagent (Takara
Biotechnology Co., Ltd.). Reverse transcription was conducted at
37°C for 15 min, followed by 85°C for 5 sec and 4°C for 10 min for
heat inactivation. U6snRNA (Sangon Biotech Co., Ltd.) was utilized
as an endogenous control for the quantitative detection of
miR-139-5p expression. GAPDH (Sangon Biotech Co., Ltd.) was used as
an endogenous control for the quantitative detection of IGF-1R,
PPAR-γ, CCAAT-enhancer-binding protein (C/EBP)α and C/EBPβ
expression. RT-qPCR was performed using a CFX Connect Real-Time PCR
Detection System (Bio-Rad Laboratories, Inc.) according to the
manufacturer's protocol. The thermocycling conditions were as
follows: Preheating at 95°C for 10 min, followed by 40 cycles of
95°C for 15 sec and 60°C for 1 min, extension at 72°C for 35 sec,
and 4°C for preservation. The mRNA relative expression levels were
measured using the 2−ΔΔCq method in triplicate (18). The 20-µl reactions
consisted of 1 µl sense primer, 1 µl cDNA, 10
µl SYBR® Premix Ex Taq II (Takara Biotechnology
Co., Ltd.), 7 µl DNase/RNase-free water (Beijing Solarbio
Science & Technology Co., Ltd.) and 1 µl anti-sense
primer. The qPCR primer sequences are displayed in Table I.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5′→3′) | Reverse
transcription primer sequence (5′→3′) |
|---|
| U6 | F:
AGAGAAGATTAGCATGGCCCCTG |
GTCGTATCCAGTGCAGGGTCCGAGGTAT |
| R:
ATCCAGTGCAGGGTCCGAGG |
TCGCACTGGATACGACAAAATA |
| hsa-miR-139-5p | F:
CGCGTCTACAGTGCACGTGTC |
GTCGTATCCAGTGCAGGGTCCGAGGTA |
| R:
AGTGCAGGGTCCGAGGTATT |
TTCGCACTGGATACGACACTGGA |
| GAPDH | F:
GGCACCGTCAAGGCTGAGAAC | |
| R:
GGTGGCAGTGATGGCATGGAC | |
| PPAR-γ | F:
GGCAATTGAATGTCGTGTCTGTGG | |
| R:
CCGCCAACAGCTTCTCCTTCTC | |
| C/EBPα | F:
GCGAGGAGGATGAAGCCAAGC | |
| R:
TTGCTGTTCTTGTCCACCGACTTC | |
| C/EBPβ | F:
TACTACGAGGCGGACTGCTTGG | |
| R:
CGGAGAAGAGGTCGGAGAGGAAG | |
Western blot analysis
Ice-cold protease inhibitor cocktail (TargetMol) and
RIPA buffer (Beyotime Institute of Biotechnology) were mixed at a
ratio of 1:100. Then, 110 µl mixture was added to each well
of 6-well plates for total cellular protein extraction. A Bradford
Protein Assay kit (Beyotime Institute of Biotechnology) was
utilized to determine the protein concentrations. Cell protein
extracts (10 µl/lane) were subjected to SDS-PAGE (Wuhan
Servicebio Technology Co., Ltd.) on 10% polyacrylamide gels at 80 V
for 30 min and then 120 V for 1 h. The samples were transferred to
Immobilon-P transfer membranes (Merck KGaA) at a constant current
of 200 mA. Then, the samples were blocked with Tris-buffered saline
with 0.24% Tween-20 (TBST) containing 5% non-fat milk powder for 90
min. The membranes were washed several times with TBST and
incubated with primary antibodies overnight at 4°C. The primary
antibodies used were anti-β-actin (1:1,000; cat. no. AF7018;
Affinity Biosciences), anti-IGF-1R (1:500; cat. no. AF6123;
Affinity Biosciences), anti-PPAR γ (1:500; cat. no. AF6284;
Affinity Biosciences), anti-C/EBPβ (1:500; cat. no. AF7747;
Affinity Biosciences) and anti-C/EBPα (1:500; cat. no. AF6333;
Affinity Biosciences). The next day, peroxidase-conjugated goat
anti-rabbit immunoglobulin G (cat. no. ZB2301; OriGene
Technologies, Inc.) was mixed with TBST at a ratio of 1:10,000 and
incubated with the membranes for 1 h at 37°C with slow shaking and
then washed several times. Subsequently, the immune blots were
visualized with a western blotting detection kit (Advansta Inc.),
and the signal was detected using a Protein Imager (Find-dox6;
Tanon Science and Technology Co., Ltd.).
Dual luciferase reporter assay
Target gene prediction analysis using miRWalk
(http://mirwalk.umm.uni-heidelberg.de)
revealed that in the 3′-UTR IGF-1R mRNA sequence, there were
potential miR-139-5p binding sites. To determine the binding of
miR-139-5p to IGF-1R mRNA, the present study performed a luciferase
reporter assay. First, the fragments containing miR-139-5p binding
sites were amplified using the following primers: IGF-1R forward,
GCCTCGAGCTGGGATAGAAATGTTTAGGAG and reverse,
CGGCGGCCGCGCAACACAAAACAGGACATC (General Biosystems, Inc., Durham,
NC, USA). Thus, the wild-type (WT) 3′UTR of IGF-1R was obtained.
Then, the wild-type 3′UTR of IGF-1R was used as a template to
amplify the mutant-type (Mut) 3′UTR of IGF-1R with a point
mutation. The IGF-1R-Mut sequences were as follows: IGF-1R-Mut
forward, TTTCAATCACCATAGAAAAGCCCCATTATGAATT and reverse,
GGGGCTTTTCTATGGTGATTGAAACTGGTAATTT. Next, the segments (IGF-1R-WT
and IGF-1R-Mut) were cut by double enzyme digestion and inserted
into the psiCHECK™-2 vector (Shanghai GenePharma Co., Ltd.) at an
XhoI site upstream and NotI site downstream with
firefly luciferase to construct the corresponding plasmids. Cells
were inoculated in 24-well plates (Corning Incorporated) at a
density of 1×105 cells/well and then incubated with 5%
carbon dioxide at 37°C for 24 h. The plasmids (500
µg/µl; mutant and wild-type) were transfected into
HemSCs along with miR-139-5p mimics using jetPRIME buffer and
jetPRIME reagent (Polyplus-transfection, SA). After 24 h, the
luciferase activities were measured utilizing the Dual-Luciferase
Reporter Assay System (Promega Corporation) as well as an Infinite
M1000 PRO (Tecan Group Ltd.). Renilla luciferase activity
was used as the internal control.
Cell Counting Kit-8 (CCK-8) proliferation
assay
Logarithmic growth phase-transfected HemSCs were
digested and inoculated into 96-well plates (Corning Incorporated)
at a density of 1×104 cells/ml. Subsequent to 1, 3, 5
and 7 days of cultivation, the cells were treated with CCK-8
reagent (Dojindo Molecular Technologies, Inc.) and cultured at 37°C
for another 4 h. The absorbance was measured at a wavelength of 490
nm using a microplate reader (BioTek ELx 800; BioTek Instruments,
Inc.) and growth curves were constructed based on the optical
density values.
Transwell migration assay
Migration assays were performed using 24-well
Transwell chambers (Corning, Inc.). In brief, 600 µl ECM
containing 30% FBS was added to each lower chamber. A total of 200
µl serum-free DMEM containing 2×104 HemSCs that
were transfected and treated with IGF-1 were added to the
corresponding upper chamber. The cells on the bottom of the chamber
were fixed with 4% paraformaldehyde (Beyotime Institute of
Biotechnology) for 30 min at room temperature subsequent to 24 h of
incubation and stained with a 0.1% crystal violet (Beijing Solarbio
Science & Technology Co., Ltd.) for 25 min at room temperature.
Subsequent to washing with sterile water three times, the cells
were counted under a light microscope (Olympus IX71; Olympus
Corporation) at a magnification of ×100 using ImageJ software
version 1.8.0 (National Institutes of Health). The entire assay was
performed three times.
Oil red o-staining
HemSCs were incubated in 6-well plates at a density
of 1×106 cells/ml per well. The different groups were
transfected with miR-139-5p mimics or inhibitors, and some of the
groups were treated with IGF-1 (100 ng/ml) on the following day.
When cells were over-saturated, the original medium was replaced
with adipogenic differentiation media (Cyagen US Inc.), and the
cells were incubated at 37°C for 10 days. Then the cells were
stained with Oil Red O for 30 min at 37°C. An Eclipse E800 (Nikon
Corporation) microscope was used to photograph the cells.
Statistical analysis
All data are presented as the mean ± standard
deviation (n=3 for each experiment). Statistical analyses were
performed using SPSS 19.0 (IMB Corp.). For multiple groups, one-way
analysis of variance followed by Tukey's multiple comparisons test
was used to assess the mean values. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-139-5p regulates the expression of
IGF-1R in HemSCs
To investigate whether miR-139-5p was able to alter
IGF-1R expression in HemSCs, a pre-experiment was performed. HemSCs
transfected with miR-139-5p mimics as well as inhibitors were used
as experimental groups, and HemSCs transfected with miR-139-5p
mimics FAM-dN-CTL or miR-139-5p inhibitor FAM-N-CTL were utilized
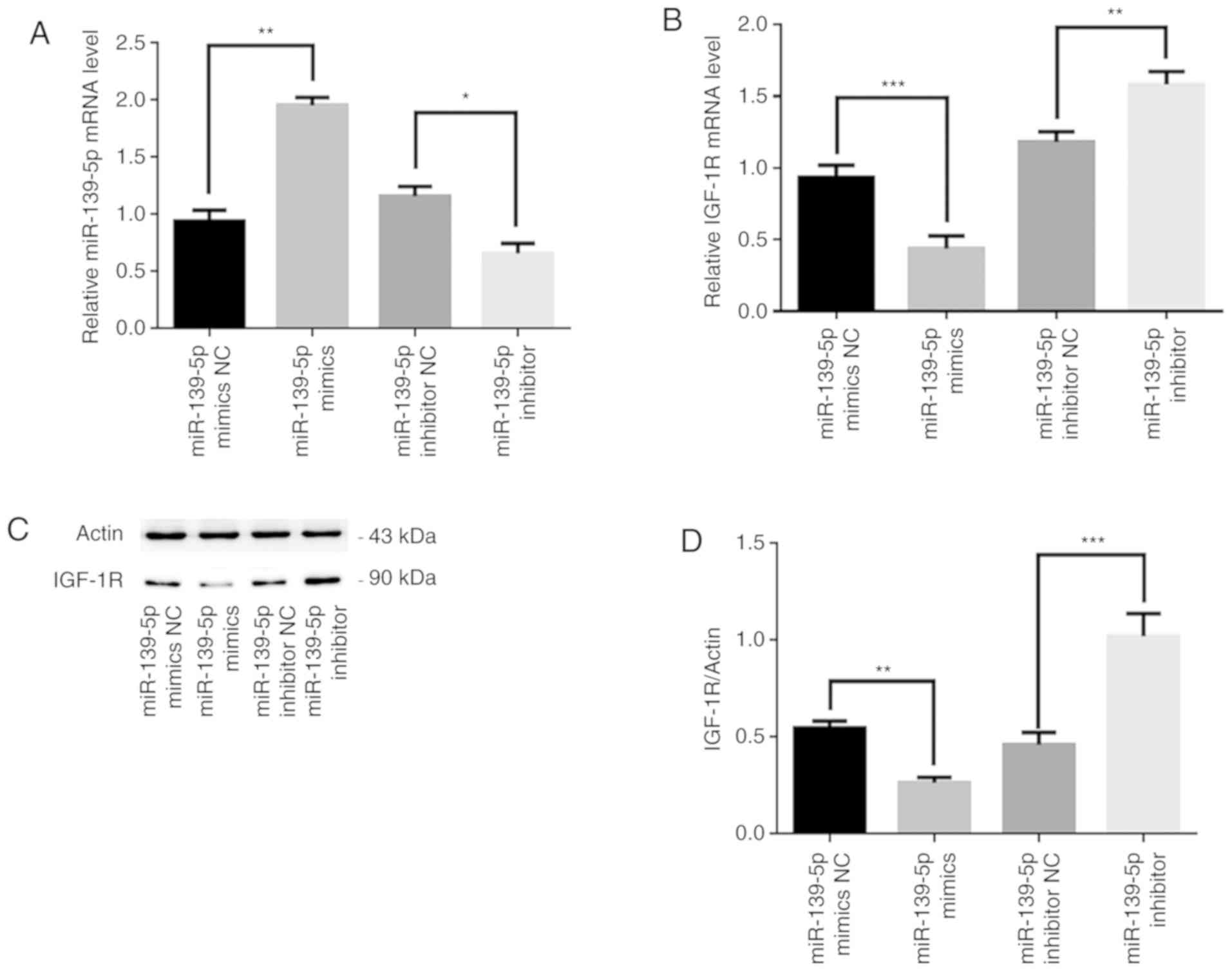
as negative controls. RT-qPCR revealed that miR-139-5p mRNA
expression levels following transfection with miR-139-5p mimics or
inhibitor were significantly higher and lower, respectively,
compared with that in their respective control groups (P<0.05;
Fig. 1A). Additionally, IGF-1R
mRNA expression levels were significantly lower in miR-139-5p
mimics-transfected HemSCs compared with in miR-139-5p mimics
FAM-dN-CTL-transfected HemSCs (P<0.001; Fig. 1B). In contrast, the IGF-1R mRNA
levels, which were altered using a miR-139-5p inhibitor, were
significantly higher compared with that in the miR-139-5p inhibitor
FAM-N-CTL group (P<0.01; Fig.
1B). Furthermore, western blot analysis was used to detect
IGF-1R protein expression in HemSCs in different groups following
transfection, and the same results were revealed. The protein
expression levels of IGF-1R were significantly lower in miR-139-5p
mimics-transfected HemSCs compared with in miR-139-5p mimics
FAM-dN-CTL cells (P<0.01), whereas the protein expression levels
of IGF-1R were significantly higher in miR-139-5p
inhibitor-transfected HemSCs compared with in miR-139-5p inhibitor
FAM-N-CTL cells (P<0.001; Fig. 1C
and D). Therefore, the pre-experiment results revealed that the
expression of miR-139-5p may be negatively associated with IGF-1R
in HemSCs.
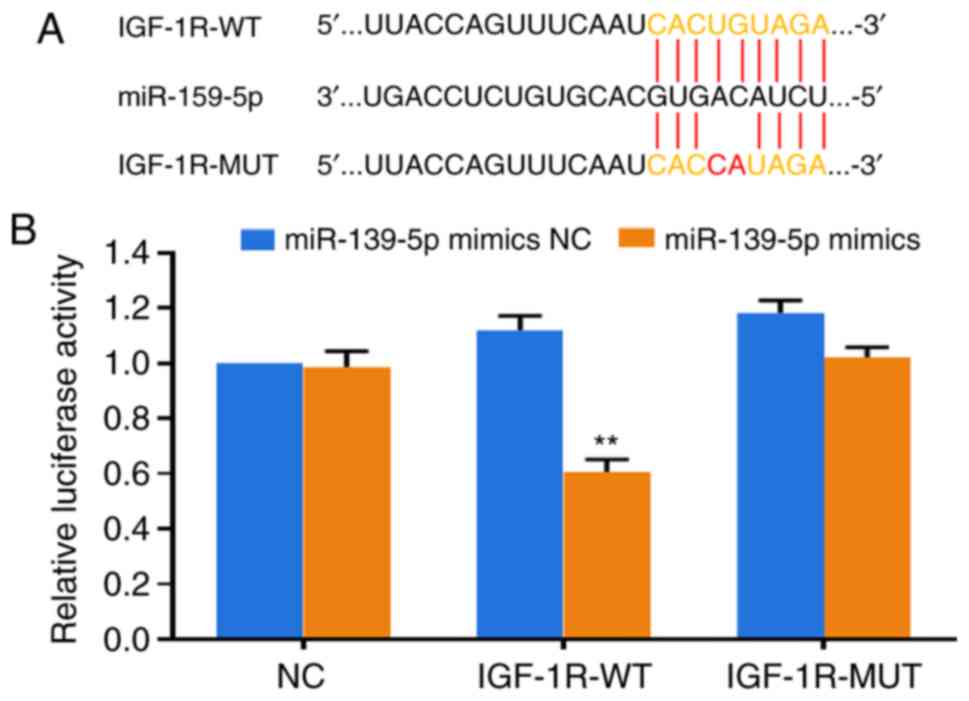
IGF-1R is a target of miR-139-5p
To further determine the molecular mechanism by
which miR-139-5p affected IGF-1R, the target gene software miRWalk
was used to predict the potential target genes of miR-139-5p. It
was revealed that IGF-1R was a potential target of miR-139-5p on
the basis of the presumed target sequences at the 2486-2493
location of the IGF-1R 3′-UTR. To test this prediction, luciferase
reporter constructs containing an IGF-1R gene mutant (Mut) or
wild-type 3′-UTR were constructed (Fig. 2A). The dual luciferase reporter
assay revealed that subsequent to miR-139-5p mimics transfection,
luciferase expression was the significantly inhibited in the
IGF-1R-WT group compared with the NC group due to IGF-1R binding to
miR-139-5p (P<0.01). The negative control group and the
IGF-1R-Mut group did not exhibit altered luciferase expression as
they did not bind to miR-139-5p (Fig.
2B). The results indicated that IGF-1R was indeed a miR-139-5p
target gene.
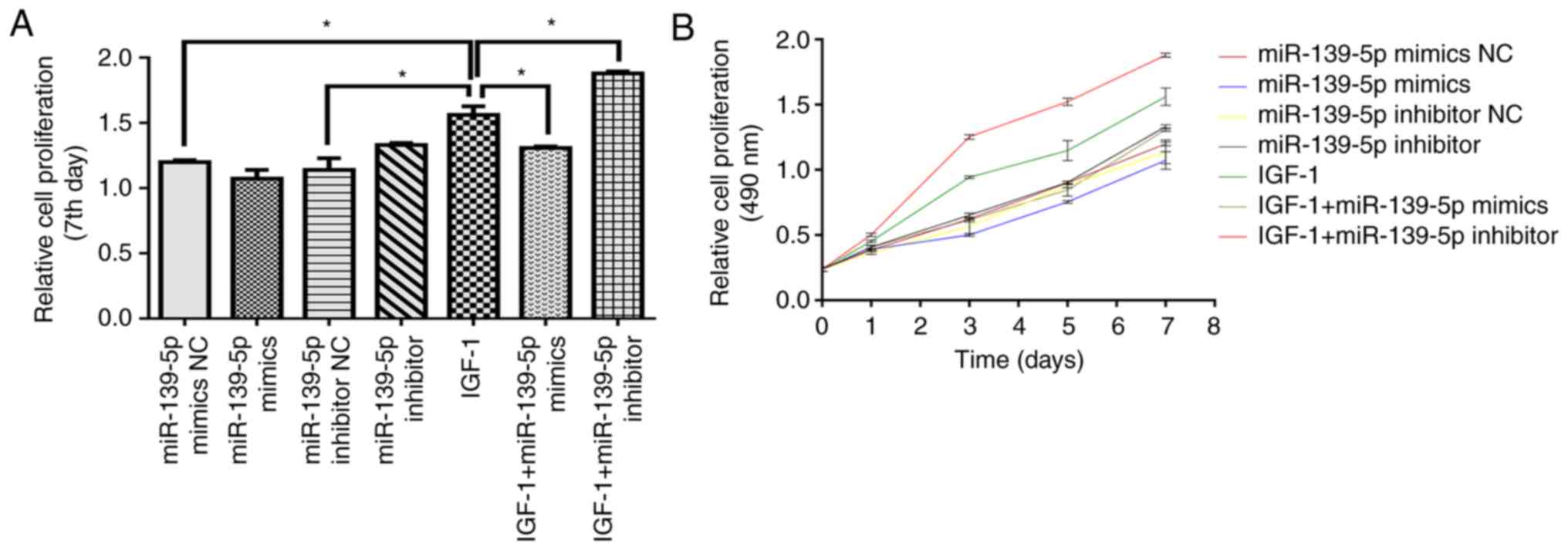
miR-139-5p modulates HemSCs proliferation
through IGF-1/IGF-1R
To elucidate whether miR-139-5p may influence HemSCs
proliferation in vitro, CCK-8 assays were used to determine
the alteration in the proliferation of cells transfected with
miR-139-5p inhibitor or mimics. The cell growth curve revealed that
IGF-1 significantly facilitated the proliferation of HemSCs
compared with the control conditions (P<0.05). Additionally,
miR-139-5p overexpression significantly suppressed HemSCs
proliferation compared with IGF-1 alone (P<0.05), while
miR-139-5p inhibition significantly increased HemSCs proliferation
(P<0.05; Fig. 3A and B). These
results suggested that miR-139-5p modulated IGF-1 binding to IGF-1R
by regulating IGF-1R expression and ultimately affected HemSCs
proliferation.
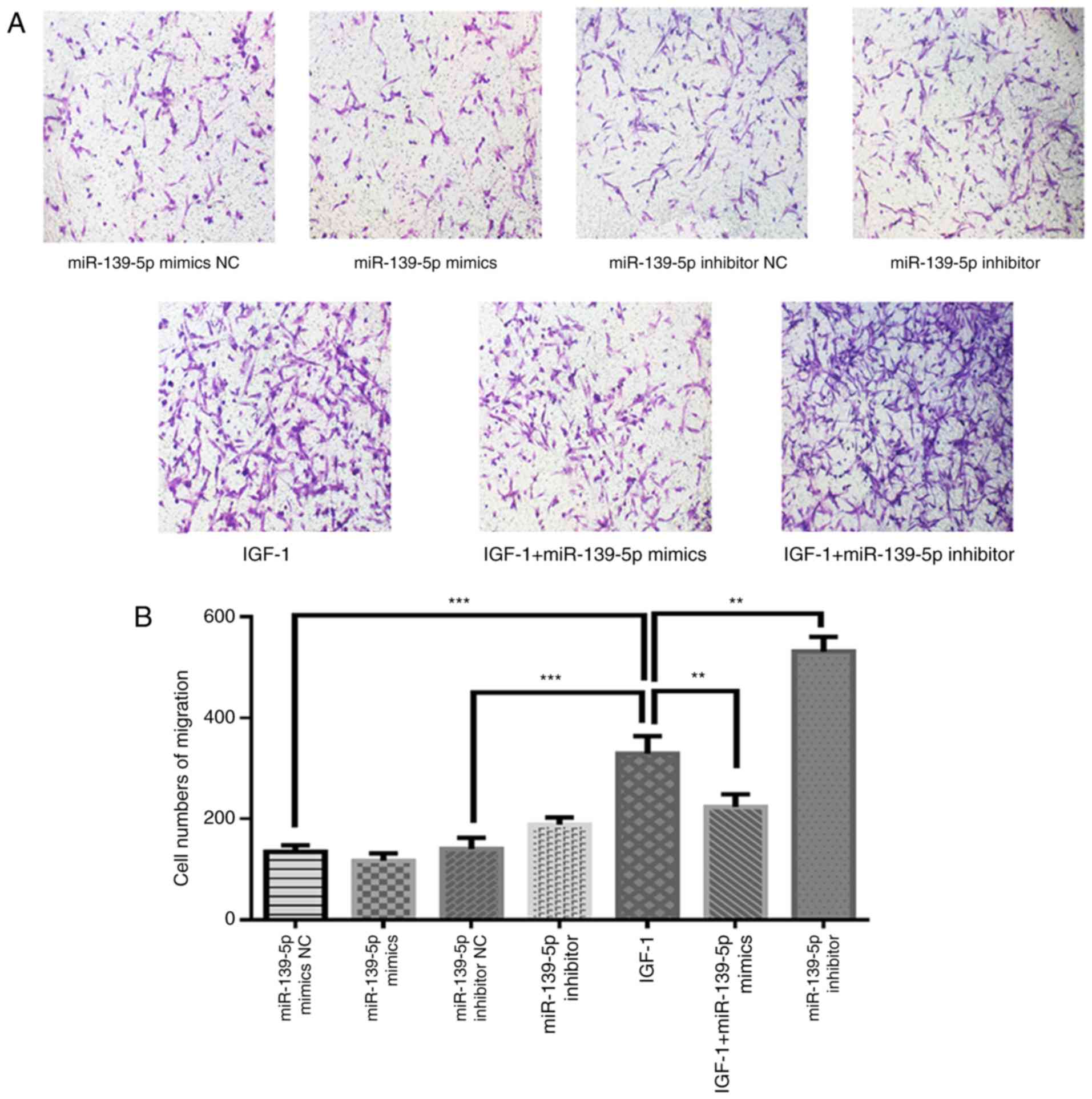
miR-139-5p modulates HemSCs migration
potentially through the IGF-1/IGF-1R pathway
Transwell migration assays were utilized to verify
whether miR-139-5p may affect HemSCs migration. Based on the
experimental results, it was revealed that IGF-1 treatment
significantly increased the HemSCs migration ability when compared
with the control conditions (P<0.001). In addition, miR-139-5p
overexpression significantly suppressed IGF-1-induced cell
migration (P<0.01) and miR-139-5p inhibition significantly
increased IGF-1-induced cell migration (P<0.01; Fig. 4A and B). Based on these
experimental results, it was hypothesized that miR-139-5p may
regulate the expression of IGF-1R, thereby affecting the binding of
IGF-1 and IGF-1R and further affecting the migration of HemSCs.
miR-139-5p modulates HemSCs
differentiation into adipocytes through IGF-1/IGF-1R
In order to further analyze the function of
miR-139-5p in the IGF-1/IGF-1R signaling pathway, RT-qPCR, oil red
o staining and western blot analysis were performed to examine the
effect of miR-139-5p on the adipogenic differentiation of HemSCs.
First, it was revealed that IGF-1 treatment stimulated adipogenesis
and lipid accumulation in HemSCs, as determined by oil red o
staining. Additionally, miR-139-5p overexpression significantly
suppressed IGF-1-induced lipid accumulation (P<0.01), whereas
miR-139-5p inhibition significantly strengthened IGF-1-induced
lipid accumulation (P<0.05; Fig.
5A and B). These results were further verified by western blot
analysis in addition to RT-qPCR. These data revealed that IGF-1
treatment significantly facilitated PPARγ, C/EBPα and C/EBPβ
expression in HemSCs compared with the controls (P<0.05), and
miR-139-5p overexpression significantly reduced the expression of
transcription regulators compared with IGF-1 treatment alone
(P<0.01). In contrast, miR-139-5p inhibition significantly
increased the expression of these transcription regulators
(P<0.01; Fig. 5C-I).
Therefore, the present results indicated that miR-139-5p may
modulate the differentiation of HemSCs into adipocytes by affecting
the IGF-1/IGF-1R signaling pathway.
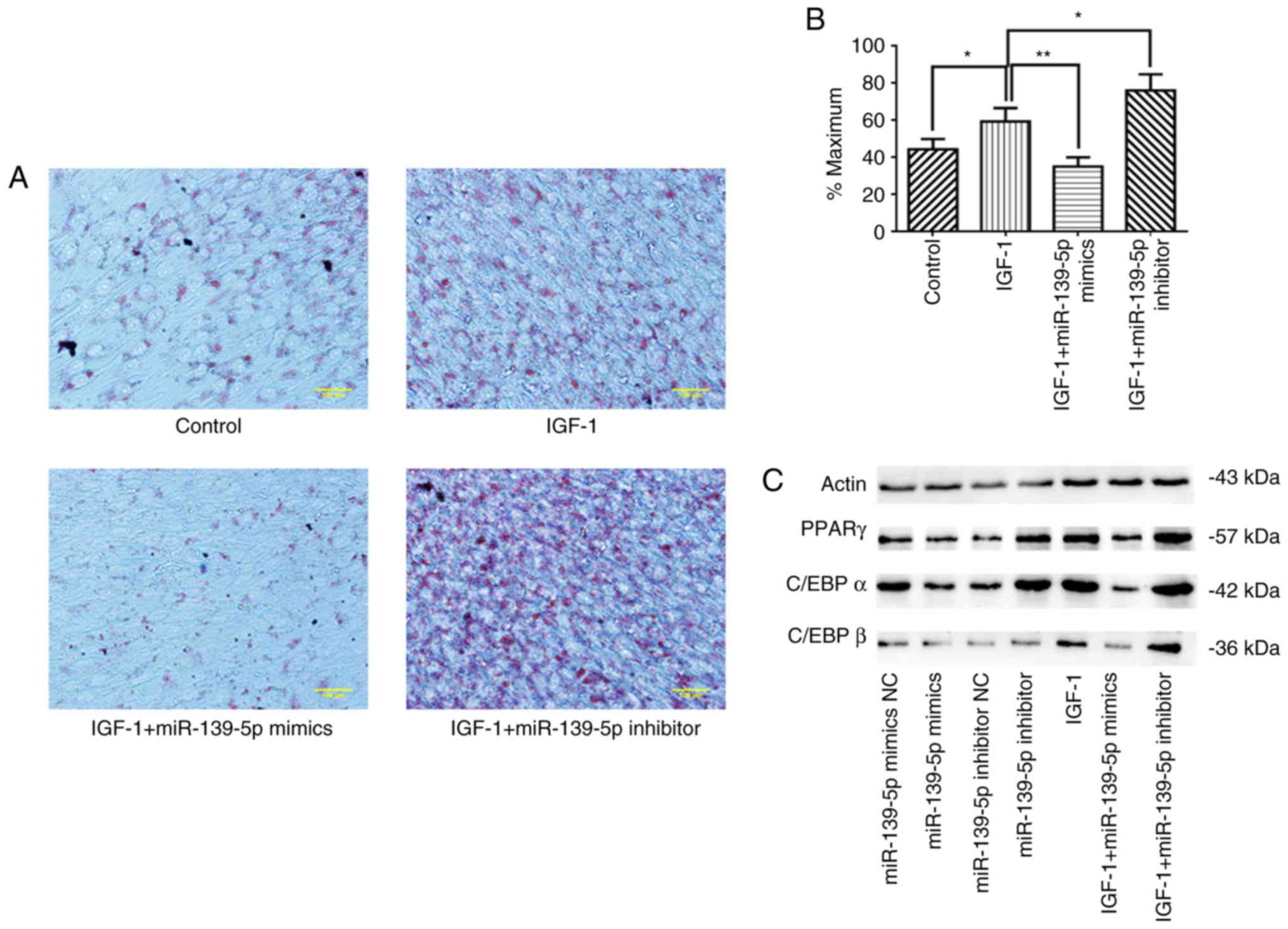 | Figure 5miR-139-5p modulated HemSCs
differentiation into adipocytes through IGF-1/IGF-1R. (A)
Adipogenic differentiation of HemSCs was determined by oil red o
staining to visualize intracellular lipid droplet accumulation. (B)
Oil red o-stained cells were quantified using ImageJ software. (C)
Western blot analysis demonstrated the expression levels of (D)
PPAR-γ, (E) C/EBPα and (F) C/EBPβ in HemSCs transfected with
miR-139-5p mimics or inhibitor and treated with or without 100
ng/ml IGF-1. (G-I) Expression of (G) PPAR-γ, (H) C/EBPα and (I)
C/EBPβ mRNA in HemSCs transfected with miR-139-5p mimics or
inhibitor and treated with or without 100 ng/ml IGF-1 were assessed
using reverse transcription-quantitative PCR.
*P<0.05, **P<0.01 and
***P<0.001. miR, microRNA; IGF-1, insulin-like growth
factor 1; IGF-1R, insulin-like growth factor 1 receptor; NC,
negative control; HemSCs, hemangioma stem cells; PPAR-γ, peroxisome
proliferator-activated receptor-γ; C/EBP, CCAAT-enhancer-binding
protein. |
Discussion
The cellular elements of IH change constantly
throughout the life cycle of growth and regression. The endothelial
cells that predominate in the proliferation period progressively
decrease during the involution phase. Conversely, adipocytes
increase and then ultimately dominate in the involution period
(19). However, the mechanisms
that are conducive to adipogenesis throughout spontaneous
regression are unclear.
A previous study confirmed that IGF-1 is able to
promote HemSCs proliferation in addition to adipogenesis (6). The biological effects of IGF-1 were
mediated through IGF-1R, a member of the growth factor receptor
tyrosine family (20,21). IGF-1R may induce differentiation
in certain types of cells, including adipocytes (22), osteoblasts (23) and central nervous system cells
(24-26). In previous years, numerous studies
have revealed that miRs may serve a crucial function in regulating
IGF-1R. For instance, miR-335 induces migration by targeting IGF-1R
(27), whereas miR-505 suppresses
hepatocellular carcinoma cell growth by targeting IGF-1R (28). On the basis of these reports,
miR-139-5p was revealed to affect the development of various tumor
types through IGF-1R. For instance, Cui et al (29) proved that miR-139 suppresses the
generation and proliferation of β-casein by targeting IGF-1R in
bovine mammary epithelial cells. Nam et al (30) confirmed that the overexpression of
miR-139 may inhibit IGF-1R and ultimately inhibit the proliferation
and migration of prostate cancer cells through the downstream
effects of the PI3K/AKT pathway. However, to the best of our
knowledge, there is no existing research on how miR-139-5p may
affect the proliferation, differentiation and migration of HemSCs.
The present study initially revealed that the expression of
miR-139-5p may be negatively associated with IGF-1R in HemSCs via a
pre-experiment. This may suggest that miR-139-5p is involved in the
migration and proliferation of HemSCs and that IGF-1R may be its
target. Therefore, the present study was conducted to further
investigate the function and specific mechanism of miR-139-5p in
HemSCs. First, the present study validated that IGF-1R was a
miR-139-5p target using a dual luciferase reporter assay. Next,
CCK-8 and Transwell assays revealed that miR-139-5p was able to
affect the migration and proliferation of HemSCs by modulating the
IGF-1/IGF-1R pathway.
In addition, one previous study has revealed that
the transcription regulator PPARγ and C/EBP family members serve
important functions in the development of adipose cells (8). Yuan et al (31) confirmed that PPAR-γ 2 gene
overexpression may upregulate adipogenic-associated genes and may
strengthen and speed up the differentiation of HemSCs into
adipocytes. Another study confirmed that IGF-1 is able to
participate in the regulation of PPARγ and C/EBP, so IGF-1 serves a
crucial function in preadipocyte growth in addition to
differentiation (32).
Furthermore, Maoa et al (33) revealed that the deletion of
miR-139-5p promoted the occurrence and development of colute
carrier family 25 member 20 through the PI3K/AKT and Wnt pathway
mediated by IGF-1R. Based on these and the present experimental
results, the present study examined whether miR-139-5p may affect
the adipogenesis of HemSCs through the IGF-1/IGF-1R pathway. It was
confirmed that IGF-1 may stimulate adipogenesis and lipid
accumulation in HemSCs via oil red o staining. miR-139-5p affected
the binding of IGF-1R to IGF-1 by regulating the expression level
of IGF-1R, which affected the differentiation of HemSCs into
adipocytes. Furthermore, the present study detected PPARγ, C/EBPα
and C/EBPβ expression through western blot analysis in addition to
RT-qPCR, further verifying the results of previous experiments. It
should also be noted that PI3K/AKT is a crucial signaling cascade
for the mediation of the IGF-1R signal (34). Numerous studies have revealed the
crucial function of the PI3K/AKT signaling cascade during
adipogenesis (35-39). For example, the PI3K/AKT signaling
pathway mediated by insulin may result in excessive lipids in
adipose tissue, so it serves a crucial function in the adipose
cells of patients with obesity (40). A previous study revealed that
IGF-1 upregulated the phosphorylation of AKT through the
IGF-1R-PI3K signaling pathway, which ultimately induced the
differentiation of HemSCs into adipocytes (6). In addition, a study performed by Mi
et al (41) proved that
miR-139-5p inhibited the differentiation of 3T3-L1 preadipocytes by
regulating the insulin receptor substrate 1/PI3K/AKT signaling
pathways. Based on the results of previous studies and the present
experimental results, it was hypothesized that miR-139-5p may
affect the adipogenesis of HemSCs through the IGF-1R-PI3K signaling
pathway. This hypothesis was not verified, but this will be the
direction of future research. In fact, the ideal treatment for IH
is to inhibit angiogenesis and promote adipogenesis. If the present
study is able to further verify whether miR-139-5p may affect IH
angiogenesis, it will better verify that miR-139-5p serves an
important function in the treatment mechanism of IH.
To the best of our knowledge, this is the first
study on the function of miR-139-5p and its potential mechanism in
HemSCs. Although the present study had certain limitations, there
are no relevant studies on the effect of miR-139-5p on the PI3K/AKT
signaling pathway. However, the present in vitro experiments
indicated that miR-139-5p may affect the migration, proliferation
and adipogenesis of HemSCs through the IGF-1/IGF-1R pathway by
regulating IGF-1R expression. These data lay the foundation for the
following experiments in vivo. The current treatments for IH
are limited, and miR-139-5p may be a novel potential therapeutic
target for IH.
Acknowledgments
Not applicable.
Funding
The present study was funded by the Natural Science
and Technology Fund Project of Anhui Province (grant no.
1808085MH282).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DC and YL designed the experiments. YW performed the
experiments and wrote the manuscript. HL, JX and FW analyzed the
experimental data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was ethically approved by the Ethics
Committee of the Second Hospital of Anhui Medical University
(approval no. PJ-bb2017-026). Written informed consent was obtained
from the guardians of the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boye E, Jinnin M and Olsen BR: Infantile
hemangioma: Challenges, new insights, and therapeutic promise. J
Craniofac Surg. 20(Suppl 1): S678–S684. 2009. View Article : Google Scholar
|
|
2
|
Haggstrom AN, Drolet BA, Baselga E,
Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW,
Newell B, et al: Prospective study of infantile hemangiomas:
Clinical characteristics predicting complications and treatment.
Pediatrics. 118:882–887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao F, Yang X, Xu G, Bi J, Lv R and Huo
R: Propranolol suppresses HUVEC viability, migration, VEGF
expression, and promotes apoptosis by downregulation of miR-4295. J
Cell Biochem. 120:6614–6623. 2019. View Article : Google Scholar
|
|
4
|
Zhang K, Wang F, Huang J, Lou Y, Xie J, Li
H, Cao D and Huang X: Insulin-like growth factor 2 promotes the
adipogenesis of hemangioma-derived stem cells. Exp Ther Med.
17:1663–1669. 2019.PubMed/NCBI
|
|
5
|
Khan ZA, Boscolo E, Picard A, Psutka S,
Melero-Martin JM, Bartch TC, Mulliken JB and Bischoff J:
Multipotential stem cells recapitulate human infantile hemangioma
in immunodeficient mice. J Clin Invest. 118:2592–2599.
2008.PubMed/NCBI
|
|
6
|
Wang F, Li H, Lou Y, Xie J, Cao D and
Huang X: Insulinlike growth factor I promotes adipogenesis in
hemangioma stem cells from infantile hemangiomas. Mol Med Rep.
19:2825–2830. 2019.PubMed/NCBI
|
|
7
|
Chen Q, Shou P, Zheng C, Jiang M, Cao G,
Yang Q, Cao J, Xie N, Velletri T, Zhang X, et al: Fate decision of
mesenchymal stem cells: Adipocytes or osteoblasts. Cell Death
Differ. 23:1128–1139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tontonoz P, Hu E and Spiegelman BM:
Regulation of adipocyte gene expression and differentiation by
peroxisome proliferator activated receptor gamma. Curr Opin Genet
Dev. 5:571–576. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao P, Deng Y, Gu P, Wang Y, Zhou H, Hu
Y, Chen P and Fan X: Insulin-like growth factor 1 promotes the
proliferation and adipogenesis of orbital adipose-derived stromal
cells in thyroid-associated ophthalmopathy. Exp Eye Res. 107:65–73.
2013. View Article : Google Scholar
|
|
10
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kasinski AL and Slack FJ: Epigenetics and
genetics MicroRNAs en route to the clinic: Progress in validating
and targeting microRNAs for cancer therapy. Nat Rev Cancer.
11:849–864. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Treiber T, Treiber N and Meister G:
Regulation of microRNA biogenesis and function. Thromb Haemost.
107:605–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye Y, Song Y, Zhuang J, Wang G, Ni J,
Zhang S and Xia W: MicroRNA-302a-3p suppresses hepatocellular
carcinoma progression by inhibiting proliferation and invasion.
Onco Targets Ther. 11:8175–8184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Dai J, Li F, Cheng H, Yan D and
Ruan Q: The expression and function of miR-424 in infantile skin
hemangioma and its mechanism. Sci Rep. 7:118462017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Z, Bian C, Zhou H, Huang S, Wang S,
Liao L and Zhao RC: MicroRNA hsa-miR-138 inhibits adipogenic
differentiation of human adipose tissue-derived mesenchymal stem
cells through adenovirus EID-1. Stem Cells Dev. 20:259–267. 2011.
View Article : Google Scholar
|
|
16
|
Xu W, Hang M, Yuan C, Wu F, Chen S and Xue
K: MicroRNA-139-5p inhibits cell proliferation and invasion by
targeting insulin-like growth factor 1 receptor in human non-small
cell lung cancer. Int J Clin Exp Pathol. 8:3864–3870.
2015.PubMed/NCBI
|
|
17
|
Yuan SM, Guo Y, Zhou XJ, Shen WM and Chen
HN: PDGFR-β (+) perivascular cells from infantile hemangioma
display the features of mesenchymal stem cells and show stronger
adipogenic potential in vitro and in vivo. Int J Clin Exp Patho.
7:2861–2870. 2014.
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta DeltaC(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Yu Y, Fuhr J, Boye E, Gyorffy S, Soker S,
Atala A, Mulliken JB and Bischoff J: Mesenchymal stem cells and
adipogenesis in hemangioma involution. Stem Cells. 24:1605–1612.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boney CM, Gruppuso PA, Faris RA and
Frackelton AR Jr: The critical role of Shc in insulin-like growth
factor-I-mediated mitogenesis and differentiation in 3T3-L1
preadipocytes. Mol Endocrinol. 14:805–813. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boney CM, Smith RM and Gruppuso PA:
Modulation of insulin-like growth factor I mitogenic signaling in
3T3-L1 preadipocyte differentiation. Endocrinology. 139:1638–1644.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smith PJ, Wise LS, Berkowitz R, Wan C and
Rubin CS: Insulin-like growth factor-I is an essential regulator of
the differentiation of 3T3-L1 adipocytes. J Biol Chem.
263:9402–9408. 1988.PubMed/NCBI
|
|
23
|
Schmid C, Steiner T and Froesch ER:
Insulin-like growth factor I supports differentiation of cultured
osteoblast-like cells. FEBS Lett. 173:48–52. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mcmorris FA, Smith TM, Desalvo S and
Furlanetto RW: Insulin-like growth factor I/somatomedin C: A potent
inducer of oligodendrocyte development. Proc Natl Acad Sci USA.
83:822–826. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mill JF, Chao MV and Ishii DN: Insulin,
insulin-like growth factor II, and nerve growth factor effects on
tubulin mRNA levels and neurite formation. Proc Natl Acad Sci USA.
82:7126–7130. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Recio-Pinto E, Lang FF and Ishii DN:
Insulin and insulin-like growth factor II permit nerve growth
factor binding and the neurite formation response in cultured human
neuroblastoma cells. Brain Res. 81:2562–2566. 1984.
|
|
27
|
Qi J, Shi LY, Wu Y, Shen XJ, Yuan J, Jin
CJ, Cong H and Ju SQ: Epigenetic silencing of miR-335 induces
migration by targeting insulin-like growth factor-1 receptor in
multiple myeloma. Leuk Lymphoma. 1–11. Jun 13–2019.Epub ahead of
print.
|
|
28
|
Ren L, Yao Y, Wang Y and Wang S: MiR-505
suppressed the growth of hepatocellular carcinoma cells via
targeting IGF-1R. Biosci Rep. 39:pii: BSR201824422019. View Article : Google Scholar
|
|
29
|
Cui Y, Sun X, Jin L, Yu G, Li Q, Gao X, Ao
J and Wang C: MiR-139 suppresses β-casein synthesis and
proliferation in bovine mammary epithelial cells by targeting the
GHR and IGF1R signaling pathways. BMC Vet Res. 13:3502017.
View Article : Google Scholar
|
|
30
|
Nam RK, Benatar T, Wallis CJD, Kobylecky
E, Amemiya Y, Sherman C and Seth A: MicroRNA-139 is a predictor of
prostate cancer recurrence and inhibits growth and migration of
prostate cancer cells through cell cycle arrest and targeting IGF1R
and AXL. Prostate. 79:1422–1438. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan SM, Guo Y, Wang Q, Xu Y, Wang M, Chen
HN and Shen WM: Overexpression of PPAR-γ2 gene enhances the
adipogenic differentiation of hemangioma-derived mesenchymal stem
cells in vitro and in vivo. Oncotarget. 8:115817–115828. 2017.
View Article : Google Scholar
|
|
32
|
Holly J, Sabin M, Perks C and Shield J:
Adipogenesis and IGF-1. Metab Syndr Relat Disord. 4:43–50. 2006.
View Article : Google Scholar
|
|
33
|
Maoa R, Zou F, Yang L, Lin S, Li Y, Ma M,
Yin P, Liang X and Liu J: The loss of MiR-139-5p promotes
colitis-associated tumorigenesis by mediating PI3K/AKT/Wnt
signaling. Int J Biochem Cell Biol. 69:153–161. 2015. View Article : Google Scholar
|
|
34
|
Xu J and Liao K: Protein kinase B/AKT 1
plays a pivotal role in insulin-like growth factor-1 receptor
signaling induced 3T3-L1 adipocyte differentiation. J Biol Chem.
279:35914–35922. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gagnon A, Chen CS and Sorisky A:
Activation of protein kinase B and induction of adipogenesis by
insulin in 3T3-L1 preadipocytes: Contribution of
phosphoinositide-3,4,5-trisphosphate versus
phosphoinositide-3,4-bisphosphate. Diabetes. 48:691–698. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kohn AD, Summers SA, Birnbaum MJ and Roth
RA: Expression of a constitutively active Akt Ser/Thr kinase in
3T3-L1 adipocytes stimulates glucose uptake and glucose transporter
4 translocation. J Biol Chem. 271:31372–31378. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Magun R, Burgering BM, Coffer PJ,
Pardasani D, Lin Y, Chabot J and Sorisky A: Expression of a
constitutively activated form of protein kinase B (c-Akt) in 3T3-L1
preadipose cells causes spontaneous differentiation. Endocrinology.
137:3590–3593. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peng XD, Xu PZ, Chen ML, Hahn-Windgassen
A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman
KG and Hay N: Dwarfism, impaired skin development, skeletal muscle
atrophy, delayed bone development, and impeded adipogenesis in mice
lacking Akt1 and Akt2. Gene Dev. 17:1352–1365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tomiyama K, Nakata H, Sasa H, Arimura S,
Nishio E and Watanabe Y: Wortmannin, a specific
phosphatidylinositol 3-kinase inhibitor, inhibits adipocytic
differentiation of 3T3-L1 cells. Biochem Bioph Res Commun.
212:263–269. 1995. View Article : Google Scholar
|
|
40
|
Cai R, Tang G, Zhang Q, Yong W, Zhang W,
Xiao J, Wei C, He C, Yang G and Pang W: A novel lnc-RNA, named
lnc-ORA, is identified by RNA-seq analysis, and its knockdown
inhibits adipogenesis by regulating the PI3K/AKT/mTOR signaling
pathway. Cells. 8:pii: E4772019. View Article : Google Scholar
|
|
41
|
Mi L, Chen Y, Zheng X, Li Y, Zhang Q, Mo D
and Yang G: MicroRNA-139-5p suppresses 3T3-L1 preadipocyte
differentiation through Notch and IRS1/PI3K/Akt insulin signaling
pathways. J Cell Biochem. 116:1195–1204. 2015. View Article : Google Scholar
|