Introduction
Androgen-deprivation therapy (ADT) in combination
with radiation therapy (RT) is the standard of care for locally
advanced prostate cancer (1).
ADT, or chemical castration, is a highly effective therapeutic
option for patients with prostate cancer and has been shown to
prolong survival (2,3). The beneficial effect of ADT relates
to the dependence of prostate tumor cell growth on androgen
receptor signaling. Upon binding to testosterone, androgen
receptors homodimerize, leading to phosphorylation, subsequent
nuclear translocation and binding to the androgen response
elements, which eventually results in transcription of target genes
that facilitate tumor cell growth, thus extending survival
(2). RT uses ionizing radiation
to induce double-stranded DNA breaks in cancer cells and is
well-established as an effective treatment for local-ized prostate
cancer (4). Patients treated with
RT were less likely to further develop metastases and exhibited a
decreased rate of disease progression compared to an active
surveillance (control) group (5).
A number of large, randomized clinical trials in
previous years have demonstrated improved long-term survival using
combined ADT and RT compared to RT or ADT alone (6-11).
The synergistic effect of ADT and RT is likely related to the role
of ADT as a 'radiosensitizer' by downregulating DNA repair genes,
thus accelerating DNA damage induced by RT (12-14). Alternatively, an enhanced immune
response has been suggested as another possible mechanism of the
synergistic effect of ADT and RT (15,16).
Despite these benefits, treatment with ADT can
result in a multitude of iatrogenic conditions including increased
risk for diabetes, cardiovascular diseases, sexual health
dysfunction, depression, cognitive and mood dysfunction (11,17). These treatment-related conditions
can greatly reduce health-related quality of life (18). One of the most common and
burdensome symptoms experienced during RT with concomitant ADT is
fatigue (19,20). A total of ~40% of men experience
clinically-significant fatigue while taking ADT and 60% experience
clinically-significant fatigue during RT for non-metastatic
prostate cancer (19).
Various mechanisms have been hypothesized for the
etiology of treatment-related fatigue; however, the exact
underlying mechanism especially related to combined therapies
remains unknown (20,21). Previous studies suggest a possible
role of mitochondrial dysfunction in cancer-related fatigue
(22-24). Interestingly, oxidative stress
induced by RT as well as androgen deprivation can separately
influence mitochondrial function (25). Furthermore, anemia induced by
combined treatment of ADT and RT can lead to cerebral hypoxia and
neuronal mitochondrial dysfunction (26,27).
Given the prominent use of combined ADT and RT for
localized prostate cancer, there is an increasing need to better
understand adverse effects of the combined treatment. The goal of
the current study is to explore the influence of ADT on fatigue
progression and mitochondrial function during RT for localized
prostate cancer. To examine mitochondrial function in human blood
samples, a method previously published by the present group was
used (28). The effect of the ADT
and RT on brain mitochondria was examined using a previously
developed mouse model of radiation-induced fatigue (29).
Materials and methods
Participants
The present study (NCT00852111) was approved by the
Institutional Review Board of the National Institutes of Health
(NIH). All participants enrolled in this study were male, ≥18 years
of age, diagnosed with non-metastatic prostate cancer with or
without prior prostatectomy and scheduled to receive external beam
radiation therapy (EBRT). Patient characteristics are presented in
Table I. Participants were
excluded if they had known progressive diseases causing significant
fatigue including mitochondrial diseases, psychiatric disease
within the past five years, uncorrected hypothyroidism or anemia,
or a second malignancy. Individuals who used sedatives, steroids,
or non-steroidal anti-inflammatory agents were also excluded. All
participants recruited in the study completed EBRT which lasted
38-44 days with a total dosage of 68.4-75.6 Gray (Gy), depending on
the clinical stage of the disease. The majority of the enrolled
participants received neoadjuvant ADT 76 days before EBRT and
continued to receive ADT during and after EBRT. Participants
receiving ADT were on a daily dose of 50 mg Bicalutamide, an
androgen receptor antagonist, prior to receiving a
gonadotropin-releasing hormone agonist (GnRH) (30). Participants often receive an
injection of 22.5 mg leuprolide acetate, the GnRH, two weeks after
receiving the androgen receptor antagonist. They continue to
receive the leuprolide acetate every three months. This combination
stops the release of hormones and prevents any residual hormones
from functioning. The Bicalutamide continued throughout RT and
often stopped on the last day of radiation. Participants remained
on the leuprolide acetate up to 2-3 years after completing RT.
Participants were recruited between September 2009 and November
2015 at the Magnuson Clinical Research Center at the NIH. Signed
written informed consents were obtained prior to study
participation.
 | Table IDemographics and clinical
characteristics of sample population. |
Table I
Demographics and clinical
characteristics of sample population.
|
Characteristics | Total (n=64) | +ADT all time
points (n=27) | +ADT during EBRT
(n=20) | No ADT (n=17) |
|---|
| Age, years | 65.41±7.78 | 66.37±8.10 | 65.25±7.06 | 64.06±8.31 |
| BMI,
kg/m2 | 30.26±4.91 | 30.15±4.51 | 31.20±5.86 | 29.32±4.34 |
| Ethnicity, % | | | | |
| Asian | 6.25 | 7.41 | 0.00 | 11.76 |
| Black | 26.56 | 25.93 | 30.00 | 23.53 |
| Hispanic | 3.13 | 0.00 | 0.00 | 11.76 |
| White | 64.06 | 66.67 | 70.00 | 11.76 |
| Education, % | | | | |
| Did not complete
high school | 6.25 | 3.70 | 3.70 | 0.00 |
| High school
grad/GED | 10.94 | 11.11 | 10.00 | 11.76 |
| Associate
degree/some college | 7.81 | 11.11 | 5.00 | 5.88 |
| Bachelor's
degree | 46.88 | 48.15 | 40.00 | 52.94 |
| Advanced
degree | 26.56 | 22.22 | 30.00 | 29.41 |
| No answer | 1.56 | 3.70 | 0.00 | 0.00 |
| T stage, % | | | | |
| T1c | 29.69 | 18.52 | 45.00 | 29.41 |
| T2 | 1.56 | 0.00 | 0.00 | 5.88 |
| T2a | 29.69 | 29.63 | 35.00 | 23.53 |
| T2b | 6.25 | 7.41 | 0.00 | 11.76 |
| T2c | 10.94 | 11.11 | 5.00 | 17.65 |
| T3 | 21.88 | 33.33 | 15.00 | 11.76 |
| Gleason score,
% | | | | |
| 3+3=6 | 6.25 | 3.70 | 0.00 | 17.65 |
| 3+4=7 | 26.56 | 0.00 | 50.00 | 41.18 |
| 3+5=8 | 1.56 | 3.70 | 0.00 | 0.00 |
| 4+3=7 | 14.06 | 14.81 | 10.00 | 17.65 |
| 4+4=8 | 32.81 | 44.44 | 25.00 | 23.53 |
| 4+5=9 | 14.06 | 29.63 | 5.000 | 0.00 |
| 5+4=9 | 4.69 | 3.70 | 10.00 | 0.00 |
| CBC,
1,000/µl | | | | |
| WBC | 6.52±1.74 | 6.03±1.68 | 6.77±1.92 | 7.01±1.47 |
| RBC | 4.59±0.42 | 4.46±0.43 | 4.54±0.32 | 4.83±0.42 |
| Neutrophils
absolute | 3.86±1.42 | 3.54±1.49 | 3.96±1.36 | 4.27±1.32 |
| Lymphocytes
absolute | 1.91±0.63 | 1.79±0.60 | 2.06±0.79 | 1.92±0.45 |
| Monocytes
absolute | 0.54±0.23 | 0.46±0.13 | 0.61±0.34 | 0.57±0.16 |
| Eosinophils
absolute | 0.19±0.15 | 0.19±0.18 | 0.19±0.11 | 0.19±0.16 |
| Basophils
absolute | 0.03±0.02 | 0.03±0.02 | 0.04±0.02 | 0.03±0.01 |
| PSA baseline,
ng/ml | 5.99±13.59 | 4.32±6.64 | 9.77±22.76 | 4.19±3.83 |
| PSA completion of
EBRT, ng/ml | 0.41±0.92 | 0.07±0.10 | 0.07±0.07 | 1.35±1.43 |
Instruments
Clinical and demographic data were obtained from
chart review. Fatigue was measured using the frequently-used
13-item Functional Assessment of Cancer Therapy-Fatigue (FACT-F), a
validated, reliable, stand-alone measure of fatigue in cancer
therapy (questionnaire items and scoring method can be found at
www.facit.org) (31). FACT-F had good internal
consistency reliability with a Cronbach's α=0.81 when tested in the
present study participants. Each item response is rated on a 0-4
scale (0='not at all', 4='very much'). Total FACT-F scores
typically range from 16-53, with lower scores reflecting higher
fatigue intensity. A FACT-F score of 43 best divides fatigue scores
of cancer patients and the general population (32), and is used for cross-sectional
comparisons with the US general population (32). Subjects with a FACT-F score <43
were considered fatigued. Subjects were considered to have
significantly increased fatigue when there was at least a
three-point decrease in FACT-F score relative to the baseline. The
3-point change in FACT-F score satisfies the Clinically Minimally
Important Difference threshold, which has been shown to be
clinically meaningful (defined by effect size: Cohen's d >0.2)
(33,34).
Depressive symptoms were measured using the
validated, 24-item Hamilton Depression Rating Scale (HAM-D)
(35,36). Scores ranged from 0-54 with high
scores reflecting increasing severity of depressive symptoms: A
score of 0-7 indicated no depression, 8-16 indicated mild
depression and a score of ≥17 indicated moderate to severe
depression (37). HAM-D has good
internal consistency (standardized Cronbach α=0.67-0.80) and
test-retest reliability (Pearson correlation coefficient=0.88,
P<0.001) (38).
Blood cell counts were measured using standard
procedures adapted by the Department of Laboratory Medicine, NIH.
Anemia was defined as hemoglobin level <13 g/dl for men based on
the World Health Organization guidelines (39).
Mitochondrial function measurement
Mitochondrial function parameters were measured as
previously described (28).
Briefly, peripheral blood mononuclear cells (PBMCs) were isolated
from human peripheral blood samples using the BD Vacutainer CPT
Mononuclear Cell Preparation Tubes based on manufacturer's protocol
(BD Biosciences; Becton, Dickinson and Company). Each well in the
cell plate was coated with freshly prepared Cell-Tak cell and
tissue adhesive solution (Corning, Inc.). PBMCs were plated at
1.5×105 cells/well to reach 80-90% confluency.
Respiratory inhibitors were reconstituted immediately before the
experiment to reach the following working concentrations:
Oligomycin, 1 µM; carbonyl
cyanide-4-(trifluoromethoxy)phenylhydrazone, 1 µM; and
antimycin A/rotenone, 0.5 µM. After incubating in a
non-CO2 incubator for 45-60 min in the presence of pH
7.4 assay media [for details on assay media preparation, see
(28)], the cell plate was
inserted in to a Seahorse XFp extracellular flux instrument
(Agilent Technologies, Inc.). The respiratory inhibitors were
injected sequentially into the corresponding ports and oxygen
consumption rate (OCR) was measured. Live cells were stained with
the Cell Proliferation Assay (Thermo Fisher Scientific, Inc.) and
quantified using the Cytation 1 instrument (Biotek Instruments,
Inc.). OCR measurements from each well were normalized to the
number of live cells. ATP coupling efficiency is calculated as:
Coupling Efficiency=100% x (ATP Production-linked OCR)/(Basal
Respiration Rate). Basal respiration rate was measured as baseline
OCR before the injection of mitochondrial respiration inhibitors
and ATP production-linked OCR was measured as the difference
between basal respiration rate and the post-oligomycin OCR.
Mouse model of cancer-related
fatigue
Ethics
This study was approved by the National Heart Lung
and Blood Institute (NHLBI) Animal Care and Use Committee of the
NIH. All investigators working with animals were properly trained
by the NIH Office of Animal Care and Use and the NHLBI Murine
Phenotyping Core. All interactions with animals in this study were
in compliance with The Guide for the Care and Use of Laboratory
Animals (40).
Animals
A total of 60 male C57Bl/6 mice were ordered from
Charles River Laboratories and were 6-9 weeks old and 15-20 g at
the beginning of each study. Mice were given ad libitum
access to food and water and were individually housed on a 12-h
light-dark cycle at ~22.2°C and 50% humidity throughout all
studies. Tails were tattooed for identification and mice received
three days of gentle handling by experimenters before procedures
began. Mice received daily visual health inspection and were
removed from study if any health problems were apparent.
Flutamide implants
Mice were randomly split into two groups. Implant
surgery took place over a two-day period, with half the animals in
each group receiving implants on each of the two days. The 'ADT'
group had a flutamide pellet (SA-152 5 mg/pellet, 60 Day Release;
Innovative Research of America) surgically implanted subcutaneously
on their backs. Mice were anesthetized using isoflurane anesthesia
(3-5% isoflurane was used to induce anesthesia and 1-3% was used to
maintain anesthesia) and placed atop a heating pad. A stab incision
was made at the nape of the neck, the incision site was swabbed
with disinfectant and a pellet was inserted subcutaneously after
all disinfectant had completely dried. The wound was closed with
tissue glue and a skin staple and mice were returned to their
cages. The control (CTL) group underwent the same surgery as the
ADT group, but no pellet was implanted. Skin staples were removed
one week after surgery. When removing the staples, implanted
pellets could be felt when touching the animal's back and could
often be seen as a small bump underneath the skin; mice were
removed from the study if they were in the ADT group but a pellet
was not detected in this way.
Irradiation
Mice in each of the ADT and CTL groups were randomly
subdivided into irradiated (Irrad) or sham (Sham) groups, resulting
in four groups: Irrad-ADT, Irrad-CTL, Sham-ADT, and Sham-CTL. The
procedure is described in detail by Wolff et al (2017),
though in the present study a lower dose of radiation was used. In
brief, on each of the three days of irradiation, all mice were
anesthetized with an intraperitoneal injection of ketamine (100
mg/kg; VetOne/MWI Animal Health) and xylazine (10 mg/kg; Akorn
Animal Health). Mice were placed inside a lead shielding and
received 4 Gy of γ radiation at a dose rate of 1 Gy/min targeted to
a narrow pelvic region. Mice in the sham group were anesthetized
and placed inside the shielding but remained outside the
irradiator. All mice recovered from the anesthesia in their cages
above heating pads.
Voluntary wheel running activity
(VWRA)
Mice were housed in cages with a running wheel
(Lafayette Instrument Neuroscience), which recorded wheel rotation
in one-min intervals. After at least one week acclimating to the
animal facility in standard plastic home cages, mice were housed in
running wheel cages for at least 2 weeks before surgery, then for
~2 weeks prior to irradiation, then for 10-12 days after
irradiation and before euthanasia. Mice were removed from their
running wheel cages during two days of surgery and the three days
of irradiation. Mice that did not consistently use the running
wheels were removed from the study. Data were collected by the
Lafayette Running Wheel software (Lafayette Instrument
Neuroscience; version 11.16). The VWRA outcome measure was time
spent using the wheel.
Tissue harvest and western blotting
Mice were anesthetized with ketamine/xylazine
(120/20 mg/kg) and decapitated immediately after exsanguination.
The skull was opened and the brain was removed. The whole brain was
placed in a petri dish with ice-cold PBS. Cortical tissues were
extracted by removing the brain stem, cerebellum, midbrains and
were immediately placed into ceramic bead tubes on ice. Modified
radioimmunoprecipitation assay buffer (50 mm Tris-HCl pH 7.4, 1%
NP-40, 0.25% sodium deoxycholate and 150 mm NaCl) supplemented with
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA) was added
to the samples for cell lysis using a bead-mill homogenizer (Thermo
Fisher Scientific, Inc.). Lysates were centrifuged at 14,000 × g 15
min at 4°C. Supernatants were retained as the soluble lysate,
boiled at 100°C for 10 min in the presence of Laemmli Sample Buffer
(Bio-Rad Laboratories, Inc.) supplemented with dithiothreitol. All
protein samples (30 µg at 1 µg/µl) were
subjected to denaturing 10% SDS-polyacrylamide gel electrophoresis
followed by transfer to polyvinylidene difluoride membranes using
the Trans-Blot Turbo Transfer System (Bio-Rad Laboratories, Inc.).
The membranes were blocked at 4°C for 1 h with a 5% Non-Fat Dry
Milk Omniblock (AB10109-00100; AmericanBio, Inc.) or bovine serum
albumin (Sigma-Aldrich; Merck KGaA) solution in phosphate-buffered
saline with 0.1% Tween and incubated overnight at 4°C with a rabbit
polyclonal antibodies mitochondrial transcription factor A (TFAM;
1:1,000; cat. no: ab131607; Abcam) and glucose transporter 4
(GLUT4; 1:750; cat. no: ab654; Abcam). Membranes were re-probed
with a primary antibody against GAPDH (anti-rabbit, 1:1,000, cat.
no: ab9485; Abcam) as a loading control. After incubation overnight
at 4°C, the membranes were further probed with a goat anti-rabbit
IgG horseradish peroxidase antibody (1:1,000; cat. no: ab6721;
Abcam). Immunoreactive complexes were visualized using Super Signal
West Pico Chemiluminescent Substrate (Thermo Fisher Scientific),
imaged and quantified using the ChemiDoc MP Imaging Systems Image
Lab 6.0.1 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Descriptive analyses were used to describe
demographic characteristics of the sample. All data were expressed
as the mean ± standard error of mean. To assess changes in clinical
variables over time, a two-way repeated-measures analysis of
variance (ANOVA) was employed. The presence or absence of ADT was
defined as between-subject factors, while the within-subject factor
was defined by study time points. The sphericity assumption was
tested with Mauchly's test and fatigue differences at each time
point were determined by non-directional Student's t-test with
Bonferroni corrections for multiple comparisons. One-way ANOVA was
used to determine significant differences in comparisons involving
more than 2 groups. Post hoc non-directional Student's t-test with
Bonferroni correction was used for between group comparisons.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed with SPSS
statistics software version 23 (IBM Corps.). Behavioral data
analysis was conducted in python using the statsmodels library for
ANOVA and the scipy and pandas modules for all other tests. Two-way
ANOVAs were used to test for main effects, Shapiro-Wilk tests were
used for evaluating normality and post-hoc t-tests were used for
pairwise comparisons with Holm-Sidak corrections for multiple
comparisons. Pearson correlation coefficient analysis was performed
to analyze correlations between variables. All experiments were
performed with an n>8 and repeated three times. In all plots,
error bars represent the standard error of the mean. Threshold α
values were 0.05 for all tests.
Results
Clinical characteristics of
participants
The clinical sample was predominantly Caucasian
(62.50%) with an average age of 65.23±7.41 years and a body mass
index (BMI) of 30.3±4.96 (Table
I). The majority of the subjects had locally confined prostate
cancer with 28.07% at T stage T1, 47.37% at T2 and 15.79% at T3. Of
the 64 subjects enrolled in the study, 17 subjects received only
EBRT with no ADT at any study time point. A total of 27 subjects
received EBRT with concomitant ADT at all study time points
(baseline, midpoint, completion and one-year post-EBRT). ADT (both
Bicalutamide and leuprolide acetate) treatment was terminated upon
EBRT completion in 20 of the subjects. There were no significant
differences in clinical characteristics between the two groups
including BMI, Gleason scores and T stage (Table I). In addition, there was no
significant correlation between FACT-F scores and prostate-specific
antigen levels after treatment completion (r=0.162, P=0.255).
Fatigue worsened during EBRT and ADT
treatment
Subjects receiving ADT experienced significantly
worse fatigue at the midpoint (P=0.00005) as well as completion of
EBRT (P=0.0008), compared to subjects without ADT (Fig. 1A). Using the previously described
definition of fatigue, a FACT-F cutoff score of 43 (21), the present study found that 61% of
subjects in the ADT group reported fatigue compared to 13% in the
non-ADT group prior to EBRT initiation (Fig. 1B). The percentage of fatigued
subjects in the ADT group increased to 72% at midpoint and 68% at
EBRT completion. The effect of ADT on fatigue lessened one year
after EBRT with 32% of the ADT group experiencing fatigue (Fig. 1B). On the other hand, the
percentage of fatigued subjects in the non-ADT group remained
stable during EBRT (midpoint: 24%, completion: 21%) and at one-year
post-EBRT (28%) (Fig. 1B).
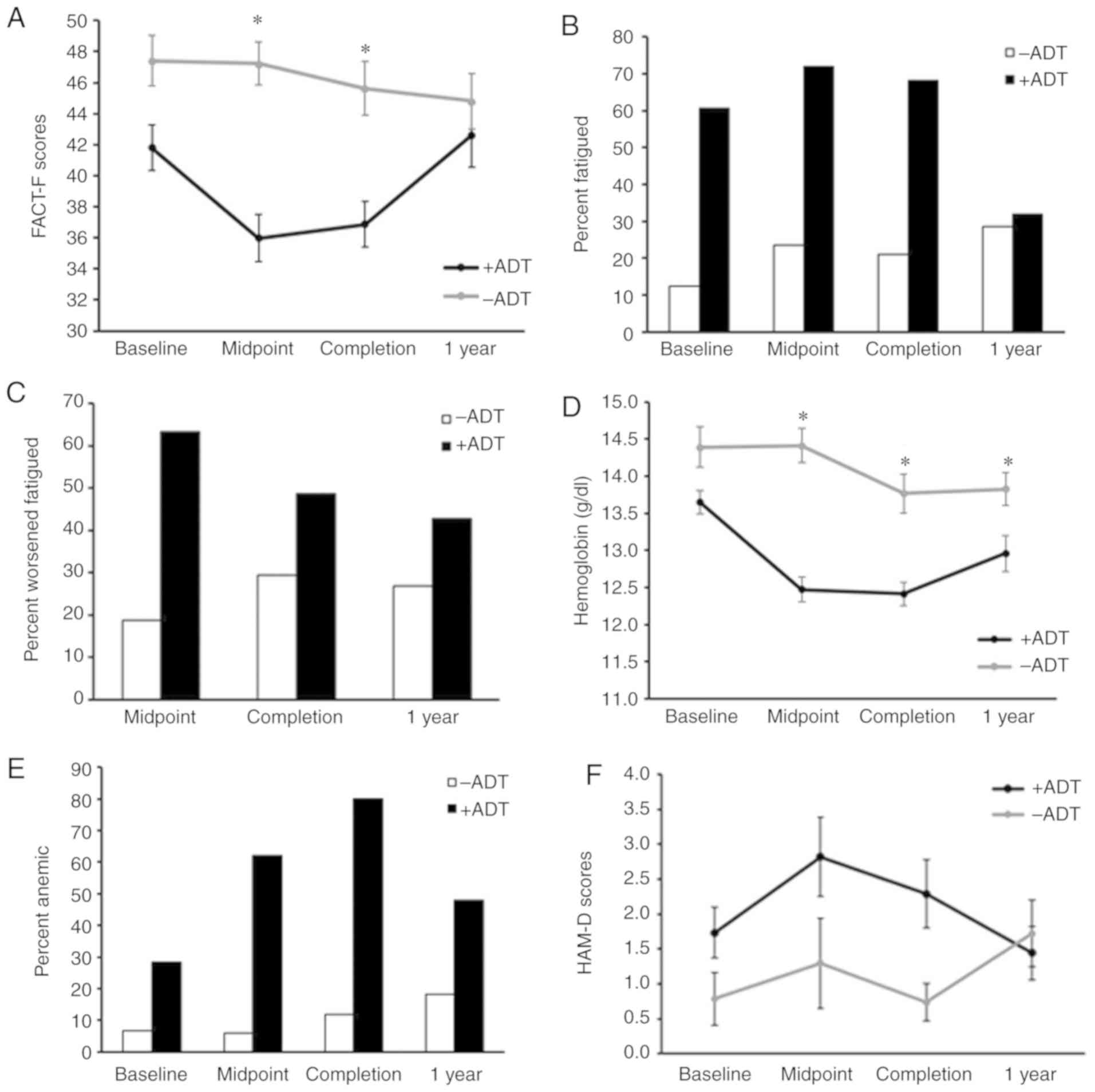 | Figure 1ADT contributes to anemia during RT
in men with non-metastatic prostate cancer. (A) ADT significantly
affected fatigue development over time
(F3,42=3.80, P=0.02) with the most significant
difference occurring at midpoint (P=0.00005) and completion of RT
(P=0.0008). (B)Percentage of subjects with fatigue, or a FACT-F
score less than 43. (C) Percentage of subjects with worsened
fatigue, or a decrease in FACT-F scores ≥3 from baseline. (D) ADT
treatment significantly affects changes in hemoglobin levels over
time (F3,37=6.12, P=0.002). (E) Percentage of
subjects with anemia at each time point. (F) Percentage of subjects
with anemia at each time point. The presence of absence of ADT did
not affect levels of depressive symptoms
(F3,40=0.34, P=0.80). * P<0.05 vs.
−ADT. ADT, androgen deprivation therapy; FACT-F, Functional
Assessment of Cancer Therapy-Fatigue; RT, radiation therapy. |
A 3-point longitudinal change in FACT-F score has
been found to represent clinically important worsening of fatigue
(33). The percentage of subjects
receiving ADT with worsened fatigue increased during EBRT (63% at
midpoint, 49% at completion of EBRT). Only 19% of non-ADT subjects
experienced significantly worsened fatigue during EBRT and 30%
after treatment completion (Fig.
1C).
Hemoglobin levels decreased significantly over time
in subjects treated with ADT (Fig.
1D; F3,37=6.12, P=0.002). Prior to EBRT
initiation (at baseline), 28% of subjects receiving ADT were anemic
with hemoglobin levels lower than 13 g/dl compared to 7% of
subjects without ADT (Fig. 1E).
The percentage of subjects with anemia increased in the ADT group
during EBRT-62% at the midpoint and 80% upon completion of EBRT,
compared to 6% at the midpoint and 12% at completion of EBRT in the
no ADT group (Fig. 1E). One year
after EBRT completion, ADT continued to affect hemoglobin levels,
though to a lesser degree, resulting in 48% of anemia in the ADT
group, compared to 18% in the no ADT group (Fig. 1E).
Despite the similarity of depressive symptoms with
fatigue in terms of the subjective experience and underlying
physiological mechanism (41),
the effect of ADT during EBRT appeared to be specific to fatigue,
as ADT did not affect HAM-D scores at any study time point
(Fig. 1F;
F3,40=0.34, P=0.80).
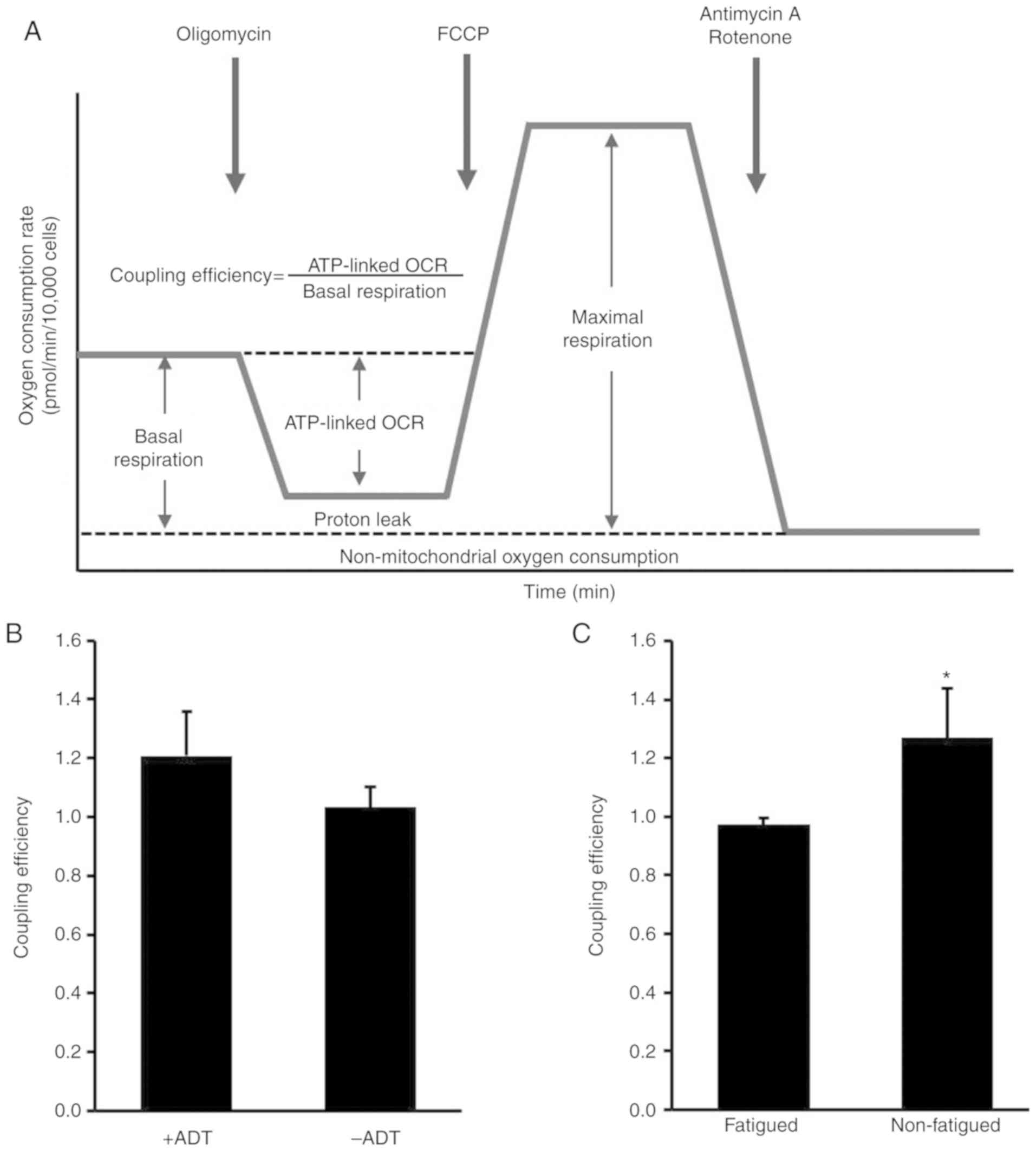
Fatigued subjects exhibit lower
mitochondrial coupling efficiency
A schematic illustration of the mitochondrial
function assay and coupling efficiency calculation is shown in
Fig. 2A. Although ADT treatment
during RT did not affect mitochondria coupling efficiency (+ADT vs.
-ADT P=0.633, Fig. 2B), fatigued
subjects during RT exhibited lower ATP coupling efficiency compared
to non-fatigued controls (fatigued vs. non-fatigued P=0.017;
Fig. 2C).
Mouse model of ADT/RT-induced
fatigue
Due to limitations of sample availability (assessing
brain mitochondrial function was not possible in human subjects),
the present study decided to use a previously published mouse model
of fatigue (29), to examine the
role of brain mitochondria in fatigue behavior. VWRA was used to
measure fatigue-like behavior in mice. To model ADT, flutamide
pellets were implanted that slowly released 5 mg flutamide over a
60-day period. Over the next two weeks, VWRA was monitored
(Fig. 3A) and found there was no
significant difference between these ADT mice and control (CTL)
mice (Fig. 3B,
t48=0.48, P=0.63).
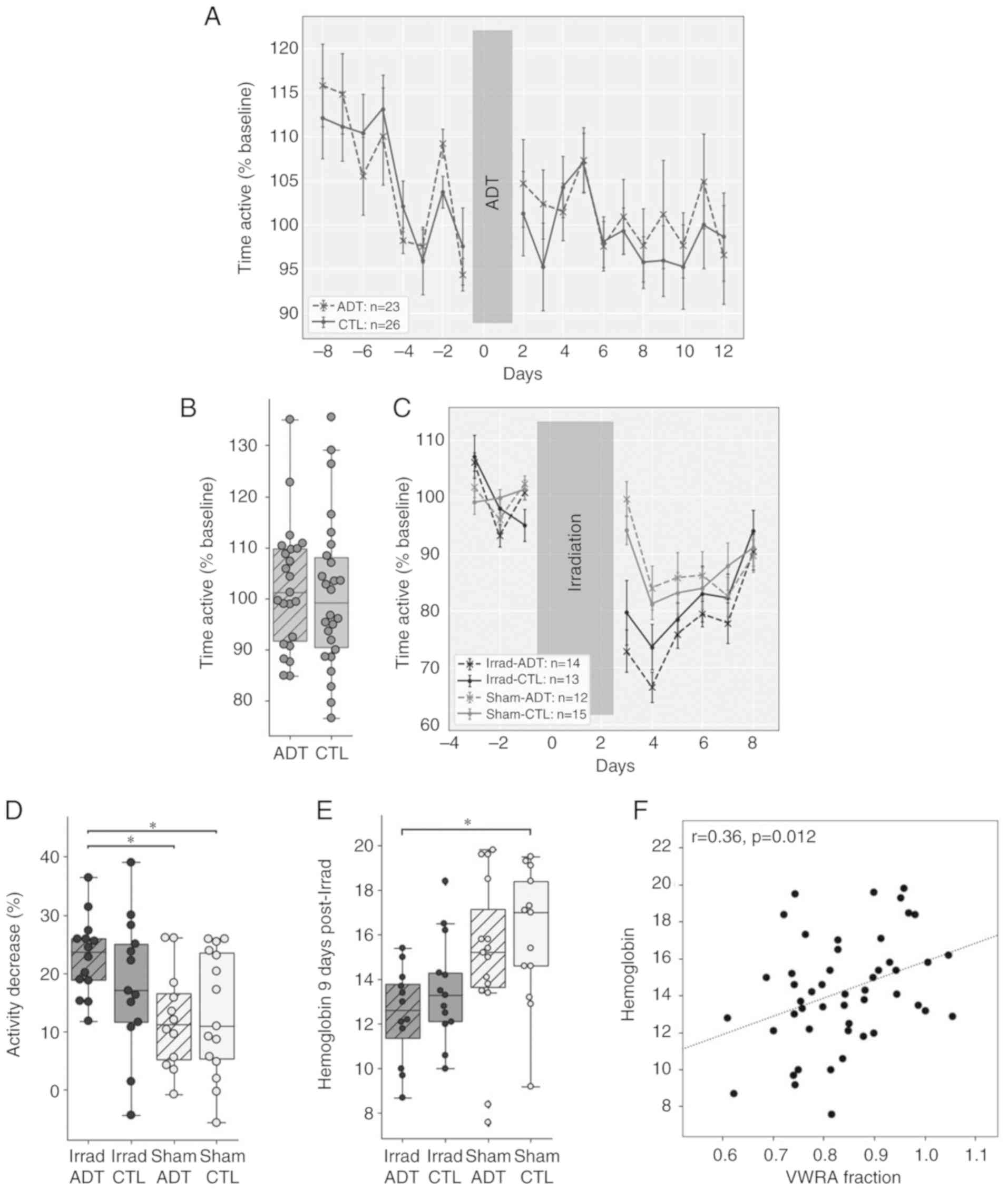 | Figure 3ADT/radiation therapy-induced fatigue
in a mouse model. (A) VWRA before and after implanting flutamide
pellets on days 0 and 1. Data are normalized to the mean daily VWRA
over the four days before surgery (n=12-15 mice per group). (B)
There was no difference between groups in total activity over the
12 days post-surgery (t48=0.48, P=0.63). (C) VWRA
before and after three days of irradiation on days 0-2. Data are
normalized to the mean VWRA over the four days before irradiation.
(D) There was a significant effect of irradiation
(F2,50=8.82, P=0.0046) but not ADT
(F2,50=0.47, P=0.50) on VWRA totals over the six
days after irradiation. Post-hoc comparisons showed a significant
difference only when comparing the Irrad-ADT group to Sham-ADT
(t25=3.62, P=0.0084) or Sham-CTL
(t28=2.91, P=0.036). (E) There was a significant
effect of irradiation (F2,44=4.62, P=0.037) but
not ADT (F2,44=0.66, P=0.42) on hemoglobin
levels. Post-hoc comparisons showed a significant effect only when
comparing Irrad-ADT to Sham-CTL (t16=3.36,
P=0.016). (F) Hemoglobin levels showed a significant correlation
with VWRA (r=0.33, P=0.022). *P<0.05. ADT,
androgen deprivation therapy; Irrad, irradiated; CTL, control;
VWRA, voluntary wheel-running activity. |
A total of two weeks after initiating ADT, mice
received three days of irradiation targeted to the pelvic region.
Previous studies indicate that lower-abdominal irradiation causes
fatigue-like behavior that lasts about six days (29,42), so the present study measured
average VWRA across six days post-irradiation (Fig. 3C). All mice, including Sham-CTL,
showed a reduction in wheel running, with mice in the Irrad-ADT
group showing the largest decrease. A two-way ANOVA reported a
significant effect of irradiation (F2,50=8.82,
P=0.0046) but not of ADT (F2,50=0.47, P=0.50) on
VWRA (Fig. 3D). Post-hoc pairwise
comparisons only reported a significant reduction in VWRA from the
combination of ADT and irradiation (t28=2.91,
P=0.036) compared to Sham-CTL.
Hemoglobin levels were measured 9 days after
irradiation to test whether the mice showed anemia-like conditions
(Fig. 3E). As with VWRA, a
two-way ANOVA showed a significant effect of irradiation
(F2,44=9.84, P=0.0029) but not ADT
(F2,44=1.68, P=0.20) on blood hemoglobin levels.
Again, similar to VWRA, post-hoc pairwise comparisons of hemoglobin
levels only showed a significant reduction in the group that
received both irradiation and ADT (t16=3.36,
P=0.016) compared to Sham-CTL. In addition, hemoglobin levels
across all mice were found to be significantly positively
correlated with VWRA totals (r=0.36, P=0.012; Fig. 3F).
Fatigue in the mouse model appears to be
related to mitochondrial function in the cerebral cortex
To investigate whether brain mitochondria are
affected by the ADT and peripheral irradiation procedures, mice
were euthanized 10-12 days after irradiation and whole brain
protein lysates were collected and immunoblotted for glucose
transporter GLUT4 and mitochondrial transcription factor TFAM.
Representative bands from each group are shown in Fig. 4A. Densitometry data were
quantified (n=8 mouse samples per group for a total of 32 samples)
and shown as bar graphs (Fig.
4B). Levels of GLUT4 were found to be lower in the brains of
irradiated mice relative to sham (Fig. 4B) and a two-way ANOVA showed a
significant effect of irradiation (F2,27=4.87,
P=0.036) but not ADT (F2,27=1.00, P=0.33). The
Sham-ADT group showed a lower mean level of GLUT4 than Sham-CTL,
which could suggest that floor effects were masking an effect of
ADT, but the interaction term of the ANOVA was just shy of
significance (F2,27=3.03, P=0.052). On brain
levels of TFAM (Fig. 4B), a
significant effect of irradiation was found
(F2,25=5.54, P=0.027) but not ADT
(F2,25=0.18, P=0.68) and no interaction
(F2,25=0.0010, P=0.97). Across all GLUT4 and TFAM
post-hoc pairwise comparisons, only one comparison was significant:
GLUT4, Irrad-CTL vs. Sham-CTL (t14=−3.31,
P=0.0342).
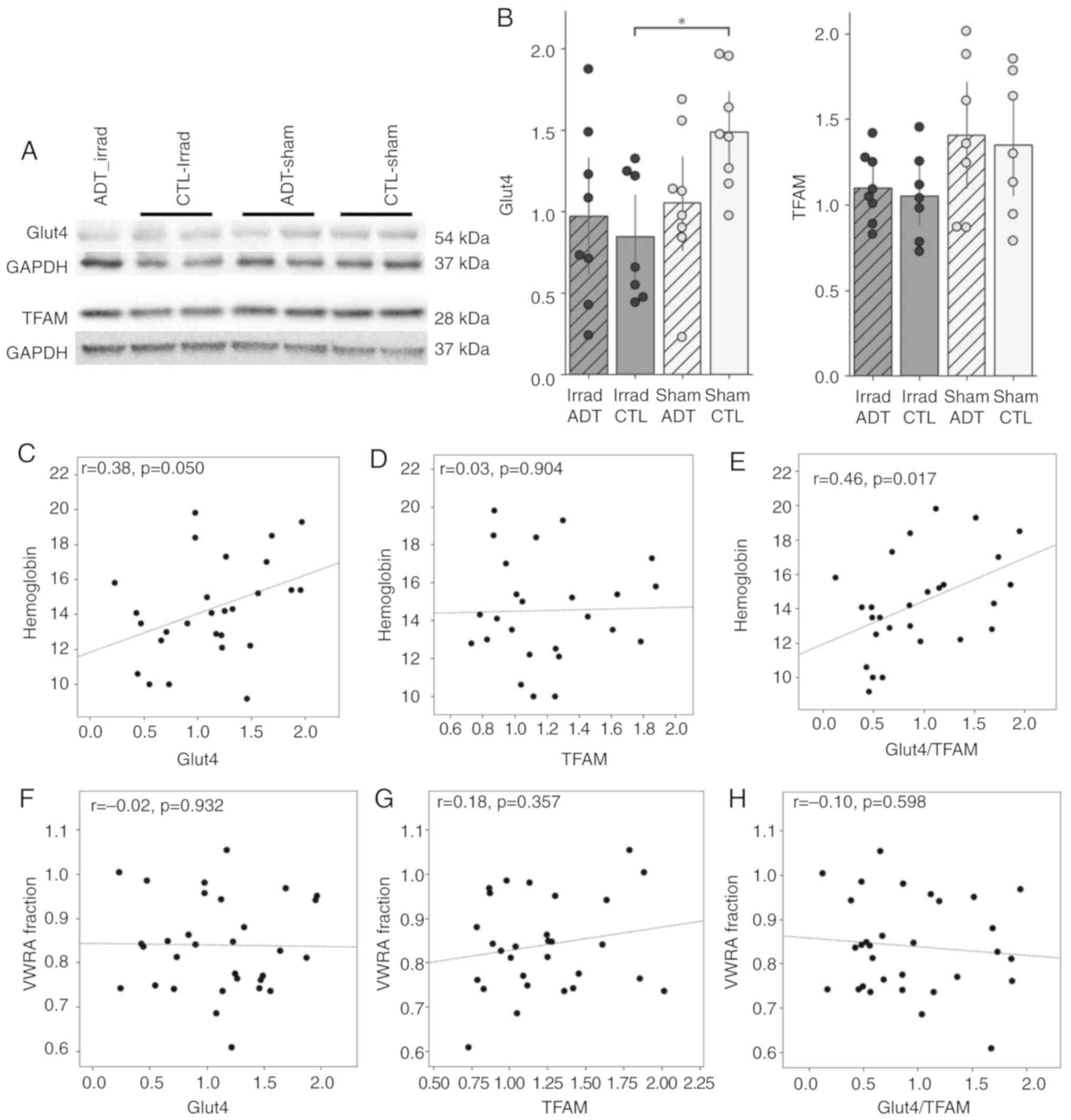 | Figure 4Brain mitochondrial effects in the
mouse model of ADT/radiation therapy-induced fatigue. (A) Western
blot analysis of GLUT4 and TFAM. Blots indicate representative
bands. (B) Densitometry data were quantified (n=8 mouse samples per
group for a total of 32 samples) and shown as bar graphs.
Irradiation had a significant effect on GLUT4 levels in the mouse
brain (F2,27=4.87, P=0.036) but ADT did not
(F2,27=1.00, P=0.33); the interaction of
irradiation and ADT was on the edge of significance
(F2,27=3.03, P=0.052). Irradiation had a
significant effect on TFAM levels in the mouse brain
(F2,25=5.54, P=0.027) but ADT did not (ADT:
F2,25= 0.18, P= 0.68). Hemoglobin levels of (C) GLUT4,
(D) TFAM and (E) GLUT4/TFAM showed a borderline significant
correlation with GLUT4 levels in the mouse brain (C: r=0.38,
P=0.050), no significant correlation with TFAM (D: r=0.03,
P=0.90) and a strong correlation with GLUT4/TFAM ratio
(r=−0.56, P=0.003). VWRA did not significantly correlate
with (F) GLUT4, (G) TFAM, nor (H) TFAM/GLUT4 ratio (P>0.35 for
all). *P<0.05. ADT, androgen deprivation therapy;
Irrad, irradiated; CTL, control; VWRA, voluntary wheel-running
activity; GLUT4, glucose transporter 4; TFAM, transcription factor
A mitochondrial. |
Whether these levels of brain mitochondrial proteins
correlated with either the anemia measure (hemoglobin) or the
fatigue measure (VWRA) was looked at next. The present study found
that hemoglobin levels were positively correlated with both GLUT4
(r=0.38, P=0.050; Fig. 4C)
and the GLUT4/TFAM ratio (r=0.46, P=0.017; Fig. 4E), but not with TFAM
(r=0.03, P=0.904; Fig.
4D). VWRA did not correlate with any of these measures (all
P>0.35; Fig. 4F-H).
Discussion
The current study describes a novel finding that RT
exacerbated the effects of ADT on fatigue in patients with
non-metastatic localized prostate cancer. The present study showed
that the combination of ADT and RT caused worsened fatigue that was
associated with anemia and mitochondrial dysfunction. This combined
effect of ADT and radiotherapy appeared to be specific to fatigue,
as depressive symptoms were unaffected. As anemia can lead to
hypoxia in brain tissues (43),
the mouse model was used to examine markers of brain bioenergetics,
TFAM and GLUT4 (44,45). The mouse model did not use
tumor-bearing mice but showed that the combination of ADT and RT
were sufficient to induce a fatigue-like behavior that mimicked the
clinical behaviors that were observed: ADT alone did not cause
fatigue; instead, ADT exacerbated the effect of RT on fatigue. The
present findings suggest a possible role of brain bioenergetics in
decreased wheel running activity and fatigue. It is possible that
ADT combined with RT not only results in anemia in patients, but
also mitochondrial dysfunction in the CNS leading to cognitive
fatigue.
Although it has been shown that ADT and RT may
independently cause anemia and fatigue, adverse effects of combined
therapy have been less explored (46). In the current study, ADT or RT
alone did not significantly affect anemia or fatigue. Instead, ADT
and RT acted synergistically to worsen anemia and fatigue during
EBRT, and the trajectory of hemoglobin level changes mimicked that
of fatigue. Interestingly, a subset of subjects who did not receive
ADT still experienced anemia one year after RT. This may be due to
the fact that aging contributes to anemia in elderly patients-for
example, both the Framingham cohort and the Third National Health
and Nutrition Examination Survey found that the prevalence of
anemia in men over 65 years of age is 6.1-11% (47), comparable to what was observed in
the non-ADT group. Depressive symptoms were included in the present
analysis due to the significant overlap between fatigue and
depression both in regard to the subjective experience and the
underlying mechanism (48,49).
No difference in HAM-D was observed at any study time point. This
suggests that the effect of combined ADT and radiotherapy appeared
to be specific to fatigue, as depressive symptoms did not follow
the same longitudinal trend of anemia or fatigue.
Mitochondria coupling efficiency represents the
proportion of O2 consumed to drive ATP synthesis and is
calculated as the fraction of basal mitochondrial respiration rate
used for ATP production (50).
The present study found that self-reported subjective fatigue
appeared to be associated with decreased ATP coupling efficiency,
indicative of less efficient mitochondrial ATP production. A
limitation with the clinical study is that it was only able to
obtain blood samples. Even though there is evidence in the
literature to support the validity of measuring mitochondrial
function in PBMCs as a proxy marker for systemic bioenergetics
(51), the similarity in
bioenergetics between PBMCs and neurons remains to be established.
In order to examine whether brain bioenergetics is influenced by
ADT/RT, a mouse model was developed using a low irradiation dosage
and subcutaneous flutamide implants, which was not sufficient to
induce fatigue on their own. Similar to the present clinical
findings, ADT and irradiation induced fatigue only when
administered together. Also supporting current clinical findings,
hemoglobin levels after irradiation correlated with changes in
wheel running.
The present study showed that irradiation in the
ADT/RT-induced fatigue mouse model resulted in the downregulation
of GLUT4, a glucose transporter that is preferentially expressed in
brain regions involved in the control of motor activity and is
essential for maintaining neuronal metabolic homeostasis (52,53). In addition to its role in
glycolysis, GLUT4 levels have also been shown to decrease in the
presence of mitochondrial dysfunction and serves as an indirect
marker of mitochondrial health (52,54). ADT with the sham irradiation
procedure also decreased GLUT4 expression, though the effect did
not reach statistical significance (P=0.052). It is possible that
irradiation caused floor effects masking any effect of ADT in the
downregulation of GLUT4, even at a dosage that did not cause a
behavioral change. Similarly, irradiation resulted in a
downregulation of TFAM, a mitochondrial transcription factor that
is important for maintaining normal cellular respiratory function
and serves as a marker for mitochondrial function (55). Interestingly, altered TFAM levels
has been shown to contribute to mitochondrial dysfunction in
various neurodegenerative diseases (54,56). The addition of ADT did not appear
to further contribute to GLUT4 or TFAM downregulation. This
suggests that the synergistic effect of ADT and irradiation in
behavior may extend beyond brain bioenergetics. Future studies will
examine the effects of different irradiation and ADT dosages, as
well as the contribution of mitochondrial dysfunction in skeletal
muscle tissues.
A limitation in the present study is the small
clinical sample size, particularly in the non-ADT group. Future
studies could validate findings from this study in a larger sample
size. The current study did not detect any significant difference
between fatigue scores and anemia status between subjects with or
without ADT at baseline, at which point subjects had already
received ADT but not RT. Future studies will examine the fatigue
status in subjects with metastatic cancer that receive ADT
monotherapy. PBMCs functional tests were used to assess the role of
mitochondrial function in cancer-related fatigue. Even though as a
heterogenous population, PBMCs serve as a useful tool for assessing
global mitochondrial dysfunction. Future studies will further
examine bioenergetics in each cell subpopulation. Additional
efforts will also be made to establish baseline mitochondrial
function test measurements in healthy individuals. In the current
clinical study, the sample type available was a limitation. Blood
collection is well tolerated by patients even at multiple time
points. Future studies will examine markers in cerebral-spinal
fluid to get a better picture of the CNS aspect of fatigue.
The ADT/RT mouse model mimicked clinical symptoms of
human subjects going through the combined therapy. However, fatigue
measured by VWRA lasted for days in mice, but lasts for months up
to years in human subjects. Continued efforts are made to develop a
mouse model with the more physiologically relevant 'persistent'
fatigue. One limitation in the ADT model is that patients received
ADT for on average 76 days prior to EBRT initiation, whereas mice
in the animal model of fatigue received 12 days of ADT prior to
irradiation. While it is difficult to determine what the
'equivalent' ADT treatment time would be for a mouse (57), the mouse model is consistent with
the clinical observation that ADT without radiation did not cause
fatigue. Furthermore, a small pilot study (data not shown) treating
with flutamide for 38 days was conducted and no difference in VWRA
in the flutamide-treated animals relative to controls was seen;
therefore on a shorter experiment design was decided upon.
Another limitation in the ADT model is that patients
received both an anti-androgen (Bicalutamide) and an LH-RH agonist,
whereas mice received only an anti-androgen (flutamide). The
flutamide-ADT mouse model was developed based on other studies
showing that flutamide treatment reduced tumor incidence compared
to a placebo in animal models (58-60), but future studies would benefit
from combining LH-RH agonists with an anti-androgen to model ADT
and fatigue-like behavior. Lastly, measurements of mitochondrial
function in the mouse model were limited to indirect markers
including TFAM and GLUT4. Due to the limited blood volume that can
be collected from a mouse (<0.5 ml), it was not possible to
extract enough PBMCs from a mouse to perform the same mitochondrial
function assay that was done using human PBMC samples. Future
studies will explore mitochondrial function assays that utilize
isolated mitochondria from brain tissues of the mouse model.
In conclusion, the present study demonstrated that
ADT exacerbated fatigue in subjects receiving RT. Anemia appeared
to be a significant contributor of fatigue during EBRT, but not
prior to treatment initiation, suggesting worsened hematologic
toxicity with a combination of ADT and RT. Furthermore,
self-reported fatigue was associated with mitochondrial dysfunction
suggesting a physiological basis for the subjective phenomenon.
Additionally, ADT/RT induced fatigue-like behaviors in a mouse
model and mimicked clinical observations of human subjects
receiving concomitant ADT and RT. Using the mouse model,
alterations in markers of mitochondrial dysfunction and brain
bioenergetics were found in mice receiving irradiation, suggesting
the contribution of non-mitochondrial factors in the synergistic
effect on fatigue exerted by combined ADT and RT. Results from this
study will inform patients and clinicians of mechanisms related to
the combined treatment and thus manage these symptoms more
effectively.
Abbreviations:
|
ADT
|
androgen deprivation therapy
|
|
RT
|
radiation therapy
|
|
NMPC
|
non-metastatic prostate cancer
|
|
PBMC
|
peripheral blood mononuclear cells
|
|
VWRA
|
voluntary wheel-running activity
|
|
GLUT4
|
glucose transporter 4
|
|
TFAM
|
transcription factor A
mitochondrial
|
|
CNS
|
central nervous system
|
|
EBRT
|
external beam radiation therapy
|
|
Gy
|
Gray
|
|
GnRH
|
gonadotropin-releasing hormone
agonist
|
|
FACT-F
|
Functional Assessment of Cancer
Therapy-Fatigue
|
|
HAM-D
|
Hamilton Depression Rating Scale
|
|
CTL
|
control
|
|
Irrad
|
irradiated
|
|
OCR
|
oxygen consumption rate
|
|
ANOVA
|
analysis of variance
|
Acknowledgments
The authors would like to thank Ms. Diane Cooper
(National Institutes of Health Library Writing Center, Bethesda,
MD, USA) for assistance with manuscript editing.
Funding
The present study is fully supported by the Division
of Intramural Research of the National Institute of Nursing
Research of the National Institutes of Health, Bethesda, MD, USA
(grant no. ZIA NR000020-06).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LRF, JMR, JL and LNS designed, collected, analyzed
and interpreted patient data regarding the clinical aspect and
mitochondrial function. BSW, SA and SR performed the mouse model
experiments and analyzed the data. All authors contributed to
writing the manuscript and approved the final version. All authors
were involved in drafting the manuscript. All authors have agreed
to be accountable for all aspects of the work.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board of the National Institutes of Health (approved protocol no.
NCT00852111). Written informed consent was obtained prior to study
participation. This study was approved by the National Heart Lung
and Blood Institute Animal Care and Use Committee of the NIH.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Spina CS: Androgen deprivation therapy and
radiation therapy for prostate cancer: The mechanism underlying
therapeutic synergy. Transl Cancer Res. 7(Suppl 6): S695–S703.
2018. View Article : Google Scholar
|
|
2
|
Harris WP, Mostaghel EA, Nelson PS and
Montgomery B: Androgen deprivation therapy: Progress in
understanding mechanisms of resistance and optimizing androgen
depletion. Nat Clin Pract Urol. 6:76–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duchesne GM, Woo HH, Bassett JK, Bowe SJ,
D'Este C, Frydenberg M, King M, Ledwich L, Loblaw A, Malone S, et
al: Timing of androgen-deprivation therapy in patients with
prostate cancer with a rising PSA [TROG 03.06 and VCOG PR 01-03
(TOAD)]: A randomised, multicentre, non-blinded, phase 3 trial.
Lancet Oncol. 17:727–737. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barker HE, Paget JT, Khan AA and
Harrington KJ: The tumour microenvironment after radiotherapy:
Mechanisms of resistance and recurrence. Nat Rev Cancer.
15:409–425. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamdy FC, Donovan JL, Lane JA, Mason M,
Metcalfe C, Holding P, Davis M, Peters TJ, Turner EL, Martin RM, et
al: 10-year outcomes after monitoring, surgery, or radiotherapy for
localized prostate cancer. N Engl J Med. 375:1415–1424. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rusthoven CG, Jones BL, Flaig TW, Crawford
ED, Koshy M, Sher DJ, Mahmood U, Chen RC, Chapin BF, Kavanagh BD
and Pugh TJ: Improved survival with prostate radiation in addition
to androgen deprivation therapy for men with newly diagnosed
metastatic prostate cancer. J Clin Oncol. 34:2835–2842. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horwitz EM, Bae K, Hanks GE, Porter A,
Grignon DJ, Brereton HD, Venkatesan V, Lawton CA, Rosenthal SA,
Sandler HM and Shipley WU: Ten-year follow-up of radiation therapy
oncology group protocol 92-02: A phase III trial of the duration of
elective androgen deprivation in locally advanced prostate cancer.
J Clin Oncol. 26:2497–2504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roach M III, Bae K, Speight J, Wolkov HB,
Rubin P, Lee RJ, Lawton C, Valicenti R, Grignon D and Pilepich MV:
Short-term neoadjuvant androgen deprivation therapy and
external-beam radiotherapy for locally advanced prostate cancer:
Long-term results of RTOG 8610. J Clin Oncol. 26:585–591. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
D'Amico AV, Chen MH, Renshaw AA, Loffredo
M and Kantoff PW: Androgen suppression and radiation vs. radiation
alone for prostate cancer: A randomized trial. JAMA. 299:289–295.
2008.PubMed/NCBI
|
|
10
|
Shipley WU, Seiferheld W, Lukka HR, Major
PP, Heney NM, Grignon DJ, Sartor O, Patel MP, Bahary JP, Zietman
AL, et al: Radiation with or without antiandrogen therapy in
recurrent prostate cancer. N Engl J Med. 376:417–428. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siddiqui ZA and Krauss DJ: Adjuvant
androgen deprivation therapy for prostate cancer treated with
radiation therapy. Transl Androl Urol. 7:378–389. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Milosevic M, Chung P, Parker C, Bristow R,
Toi A, Panzarella T, Warde P, Catton C, Menard C, Bayley A, et al:
Androgen withdrawal in patients reduces prostate cancer hypoxia:
Implications for disease progression and radiation response. Cancer
Res. 67:6022–6025. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao M, Rogers L, Suchowerska N, Choe D,
Al-Dabbas MA, Narula RS, Lyons JG, Sved P, Li Z and Dong Q:
Sensitization of prostate cancer to radiation therapy: Molecules
and pathways to target. Radiother Oncol. 128:283–300. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Polkinghorn WR, Parker JS, Lee MX, Kass
EM, Spratt DE, Iaquinta PJ, Arora VK, Yen WF, Cai L, Zheng D, et
al: Androgen receptor signaling regulates DNA repair in prostate
cancers. Cancer Discov. 3:1245–1253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kissick HT, Sanda MG, Dunn LK, Pellegrini
KL, On ST, Noel JK and Arredouani MS: Androgens alter T-cell
immunity by inhibiting T-helper 1 differentiation. Proc Natl Acad
Sci USA. 111:9887–9892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalina JL, Neilson DS, Comber AP, Rauw JM,
Alexander AS, Vergidis J and Lum JJ: Immune modulation by androgen
deprivation and radiation therapy: Implications for prostate cancer
immunotherapy. Cancers (Basel). 9. pii: E13. 2017, View Article : Google Scholar
|
|
17
|
Nead KT, Gaskin G, Chester C,
Swisher-McClure S, Dudley JT, Leeper NJ and Shah NH: Androgen
deprivation therapy and future Alzheimer's disease risk. J Clin
Oncol. 34:566–571. 2016. View Article : Google Scholar
|
|
18
|
Evans JR, Zhao S, Daignault S, Sanda MG,
Michalski J, Sandler HM, Kuban DA, Ciezki J, Kaplan ID, Zietman AL,
et al: Patient-reported quality of life after stereotactic body
radiotherapy (SBRT), intensity modulated radiotherapy (IMRT), and
brachytherapy. Radiother Oncol. 116:179–184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nelson AM, Gonzalez BD, Jim HS, Cessna JM,
Sutton SK, Small BJ, Fishman MN, Zachariah B and Jacobsen PB:
Characteristics and predictors of fatigue among men receiving
androgen deprivation therapy for prostate cancer: A controlled
comparison. Support Care Cancer. 24:4159–4166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holliday EB, Dieckmann NF, McDonald TL,
Hung AY, Thomas CR Jr and Wood LJ: Relationship between fatigue,
sleep quality and inflammatory cytokines during external beam
radiation therapy for prostate cancer: A prospective study.
Radiother Oncol. 118:105–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng LR, Dickinson K, Kline N and Saligan
LN: Different phenotyping approaches lead to dissimilar biologic
profiles in men with chronic fatigue following radiation therapy. J
Pain Symptom Manage. 52:832–840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vichaya EG, Molkentine JM, Vermeer DW,
Walker AK, Feng R, Holder G, Luu K, Mason RM, Saligan L, Heijnen
CJ, et al: Sickness behavior induced by cisplatin chemotherapy and
radiotherapy in a murine head and neck cancer model is associated
with altered mitochondrial gene expression. Behav Brain Res.
297:241–250. 2016. View Article : Google Scholar
|
|
23
|
Hsiao CP and Hoppel C: Analyzing
mitochondrial function in human peripheral blood mononuclear cells.
Anal Biochem. 549:12–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsiao CP, Daly B and Hoppel C:
Mitochondrial bioenergetics and cancer-related fatigue in patients
with prostate cancer undergoing localized radiation therapy. Oncol
Nurs Forum. 42:E2132015.
|
|
25
|
Toro-Urrego N, Garcia-Segura LM,
Echeverria V and Barreto GE: Testosterone protects mitochondrial
function and regulates neuroglobin expression in astrocytic cells
exposed to glucose deprivation. Front Aging Neurosci. 8:1522016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng LR, Chen MK, Lukkahatai N, Hsiao CP,
Kaushal A, Sechrest L and Saligan LN: Clinical predictors of
fatigue in men with non-metastatic prostate cancer receiving
external beam radiation therapy. Clin J Oncol Nurs. 19:744–750.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li M, Bertout JA, Ratcliffe SJ, Eckenhoff
MF, Simon MC and Floyd TF: Acute anemia elicits cognitive
dysfunction and evidence of cerebral cellular hypoxia in older rats
with systemic hypertension. Anesthesiology. 113:845–858. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng LR, Nguyen Q, Ross A and Saligan LN:
Evaluating the role of mitochondrial function in cancer-related
fatigue. J Vis Exp. 2018. View
Article : Google Scholar :
|
|
29
|
Wolff BS, Renner MA, Springer DA and
Saligan LN: A mouse model of fatigue induced by peripheral
irradiation. J Vis Exp. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Merseburger AS, Hammerer P, Rozet F,
Roumeguère T, Caffo O, da Silva FC and Alcaraz A: Androgen
deprivation therapy in castrate-resistant prostate cancer: How
important is GnRH agonist backbone therapy? World J Urol.
33:1079–1085. 2015. View Article : Google Scholar :
|
|
31
|
Yellen SB, Cella DF, Webster K, Blendowski
C and Kaplan E: Measuring fatigue and other anemia-related symptoms
with the functional assessment of cancer therapy (FACT) measurement
system. J Pain Symptom Manage. 13:63–74. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cella D, Eton DT, Lai JS, Peterman AH and
Merkel DE: Combining anchor and distribution-based methods to
derive minimal clinically important differences on the functional
assessment of cancer therapy (FACT) anemia and fatigue scales. J
Pain Symptom Manage. 24:547–561. 2002. View Article : Google Scholar
|
|
33
|
Yost KJ, Eton DT, Garcia SF and Cella D:
Minimally important differences were estimated for six
patient-reported outcomes measurement information system-cancer
scales in advanced-stage cancer patients. J Clin Epidemiol.
64:507–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cella D, Yount S, Sorensen M, Chartash E,
Sengupta N and Grober J: Validation of the functional assessment of
chronic illness therapy fatigue scale relative to other
instrumentation in patients with rheumatoid arthritis. J Rheumatol.
32:811–819. 2005.PubMed/NCBI
|
|
35
|
Lydiatt WM, Denman D, McNeilly DP, Puumula
SE and Burke WJ: A randomized, placebo-controlled trial of
citalopram for the prevention of major depression during treatment
for head and neck cancer. Arch Otolaryngol Head Neck Surg.
134:528–535. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Milam JE, Meeske K, Slaughter RI,
Sherman-Bien S, Ritt-Olson A, Kuperberg A, Freyer DR and Hamilton
AS: Cancer-related follow-up care among Hispanic and non-Hispanic
childhood cancer survivors: The project forward study. Cancer.
121:605–613. 2015. View Article : Google Scholar
|
|
37
|
Zimmerman M, Martinez JH, Young D,
Chelminski I and Dalrymple K: Severity classification on the
Hamilton depression rating scale. J Affect Disord. 150:384–388.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
González-Pinto A, Mosquera F, Reed C,
Novick D, Barbeito S, Vega P, Bertsch J, Alberich S and Haro JM:
Validity and reliability of the Hamilton depression rating scale (5
items) for manic and mixed bipolar disorders. J Nerv Ment Dis.
197:682–686. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ferrucci L, Maggio M, Bandinelli S,
Basaria S, Lauretani F, Ble A, Valenti G, Ershler WB, Guralnik JM
and Longo DL: Low testosterone levels and the risk of anemia in
older men and women. Arch Intern Med. 166:1380–1388. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
National Research Council (US): Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. Institute for Laboratory Animal Research (US) and National
Academies Press (US): Guide for the care and use of laboratory
animals. 8th edition. National Academies Press; Washington (DC):
2011
|
|
41
|
van Dam A: Subgroup analysis in burnout:
Relations between fatigue, anxiety, and depression. Front Psychol.
7:902016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wolff BS, Raheem SA and Saligan LN:
Comparing passive measures of fatigue-like behavior in mice. Sci
Rep. 8:142382018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kurtz P, Schmidt M, Claassen J, Carrera E,
Fernandez L, Badjatia N, Mayer S and Lee K: Anemia is associated
with brain tissue hypoxia and metabolic crisis after severe brain
injury. Crit Care. 13(Suppl 1): P922009. View Article : Google Scholar
|
|
44
|
Gan L, Ma D, Li M, Yang FC, Rogers RS,
Wheatley JL, Koch LG, Britton SL, Thyfault JP, Geiger PC and
Stanford JA: Region-specific differences in bioenergetic proteins
and protein response to acute high fat diet in brains of low and
high capacity runner rats. Neurosci Lett. 674:49–53. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ding F, Yao J, Rettberg JR, Chen S and
Brinton RD: Early decline in glucose transport and metabolism
precedes shift to ketogenic system in female aging and Alzheimer's
mouse brain: Implication for bioenergetic intervention. PLoS One.
8:e799772013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Price EA, Mehra R, Holmes TH and Schrier
SL: Anemia in older persons: Etiology and evaluation. Blood Cells
Mol Dis. 46:159–165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Andrès E, Serraj K, Federici L, Vogel T
and Kaltenbach G: Anemia in elderly patients: New insight into an
old disorder. Geriatr Gerontol Int. 13:519–527. 2013. View Article : Google Scholar
|
|
48
|
Bower JE, Ganz PA, Irwin MR, Kwan L, Breen
EC and Cole SW: Inflammation and behavioral symptoms after breast
cancer treatment: Do fatigue, depression, and sleep disturbance
share a common underlying mechanism. J Clin Oncol. 29:3517–3522.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dantzer R: Cytokine, sickness behavior,
and depression. Immunol Allergy Clin North Am. 29:247–264. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dott W, Mistry P, Wright J, Cain K and
Herbert KE: Modulation of mitochondrial bioenergetics in a skeletal
muscle cell line model of mitochondrial toxicity. Redox Biol.
2:224–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Karamercan MA, Weiss SL, Villarroel JP,
Guan Y, Werlin E, Figueredo R, Becker LB and Sims C: Can peripheral
blood mononuclear cells be used as a proxy for mitochondrial
dysfunction in vital organs during hemorrhagic shock and
resuscitation? Shock. 40:476–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ashrafi G, Wu Z, Farrell RJ and Ryan TA:
GLUT4 mobilization supports energetic demands of active synapses.
Neuron. 93:606–615. e32017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Alquier T, Leloup C, Lorsignol A and
Pénicaud L: Translocable glucose transporters in the brain.
Diabetes. 55(Suppl 2): S131–S138. 2006. View Article : Google Scholar
|
|
54
|
Kang I, Chu CT and Kaufman BA: The
mitochondrial transcription factor TFAM in neurodegeneration:
Emerging evidence and mechanisms. FEBS Lett. 592:793–811. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chandrasekaran K, Anjaneyulu M, Inoue T,
Choi J, Sagi AR, Chen C, Ide T and Russell JW: Mitochondrial
transcription factor A regulation of mitochondrial degeneration in
experimental diabetic neuropathy. Am J Physiol Endocrinol Metab.
309:E132–E141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Oka S, Leon J, Sakumi K, Ide T, Kang D,
LaFerla FM and Nakabeppu Y: Human mitochondrial transcriptional
factor A breaks the mitochondria-mediated vicious cycle in
Alzheimer's disease. Sci Rep. 6:378892016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dutta S and Sengupta P: Men and mice:
Relating their ages. Life Sci. 152:244–248. 2016. View Article : Google Scholar
|
|
58
|
Ragnow S, Hooshdaran MZ, Sanjay K and
Steiner MS: Toremifene prevents prostate cancer in the transgenic
adenocarcinoma of mouse prostate model. Cancer Res. 62:1370–1376.
2002.
|
|
59
|
Wu CT, Chen WC and Chen MF: The response
of prostate cancer to androgen deprivation and irradiation due to
immune modulation. Cancers (Basel). 11. pii: E20. 2018, View Article : Google Scholar
|
|
60
|
Lin TH, Lee SO, Niu Y, Xu D, Liang L, Li
L, Yeh SD, Fujimoto N, Yeh S and Chang C: Differential androgen
deprivation therapies with anti-androgens casodex/bicalutamide or
MDV3100/Enzalutamide versus anti-androgen receptor ASC-J9(R) lead
to promotion versus suppression of prostate cancer metastasis. J
Biol Chem. 288:19359–19369. 2013. View Article : Google Scholar : PubMed/NCBI
|