Introduction
As a non-invasive and pre-cancerous growth of the
endometrium, endometrial hyperplasia (EH) is featured by an
increased amount of endometrial tissues, exhibiting an increased
endometrial gland to stroma ratio of over 1:1 and a modified
glandular architecture (1). At
present, approximately 200,000 new cases of EH are diagnosed
annually in developed countries (2). The majority of EH cases are caused
by long-term exposure to elevated levels of estrogen (3). In fact, the production of excessive
estrogen by fat cells has been demonstrated to increase the risk of
endometrial cancer (EC) and EH in obese patients (4).
As a primary contributor to fibrosis in the kidney,
colon and lung, transforming growth factor-β1 (TGF-β1) can bind to
type I and type II ALK5 receptors located on the plasma membrane,
subsequently triggering the phosphorylation of Smad2/3, which in
turn binds to Smad4 located in the nucleus, eventually activating
the transcription and expression of TGF-β1 (5,6).
TGF-β1 can also increase the risk of fibrosis by activating the
non-Smad-dependent signaling pathways. β-catenin, integrin-linked
kinase, protein kinase B (AKT), Ras homolog gene family member A
and p38 mitogen-activated protein kinase have all been identified
as downstream effectors of TGF-β1 signaling in different types of
cells, such as keratinocytes and mammary epithelial cells (7). In addition, TGF-β1 signaling has
been implicated in regulating the proliferation and hyperplasia of
uterine epithelial cells (8).
As a type of RNA molecules without protein-coding
functions, non-coding RNAs (ncRNAs) include several sub-classes,
such as long non-coding RNAs (lncRNAs), microRNAs (miRNAs), small
nuclear RNAs, small nucleolar RNAs, ribosomal RNAs and transfer
RNAs. Among the different types of ncRNAs, lncRNAs refer to ncRNAs
that contain at least 200 nucleotides (9). Growing evidence has indicated that
lncRNAs are closely involved in the regulation of gene expression
at the epigenetic, post-transcriptional and transcriptional levels
(10). By contrast, miRNAs are
short ncRNAs with a length of <25 nucleotides. Nevertheless,
miRNAs can also regulate the expression of their target genes by
binding to the 3′-untranslated region of their target mRNAs
(11). Extensive studies have
revealed that miRNAs are involved in the regulation of numerous
essential biological processes, whereas the abnormal expression of
miRNAs was observed in a number of diseases, including cancer
(12).
It has previously been reported that the
deregulation of the TGF-β1 signaling pathway was involved in the
pathogenesis of EH (13). In
addition, the lncRNA urothelial cancer associated 1 (UCA1) was
demonstrated to deregulate the TGF-β1 signaling pathway (13). Furthermore, miR-144-3p acts as a
competing endogenous RNA for UCA1, while TGF-β1 acts as a direct
target gene of miR-144-3p (14).
Notably, it has been reported that metformin (Met) modulated the
expression of the lncRNA UCA1 (15); thus, it is hypothesized that Met
may exert its therapeutic effect via targeting the
UCA1/miR-144-3p/TGF-β1 signaling pathway. In the present study, an
EH mouse model was established by treating mice with increased
concentrations of tamoxifen, and subsequently the effect of
tamoxifen on the expression levels of UCA1, miR-144 and other
factors along the TGF-β1/AKT signaling pathway was investigated.
Furthermore, the therapeutic role of Met in the treatment of EH was
studied by measuring the effect of Met on the expression levels of
UCA1, miR-144 and other factors along the TGF-β1/AKT signaling
pathway.
Materials and methods
Animals and treatments
In the present study, 60 female mice (8-weeks-old)
weighing between 20 and 31 g were obtained from the Animal Center
of Baoji Maternal and Child Health Hospital and kept in a
specific-pathogen-free animal facility with a relative humidity of
50-60% and a temperature of 20-25°C. The light/dark cycle of the
animal facility was set to 12/12 h, and the mice had free access to
water and food. After one week of adaptation, all mice were
anesthetized using intra-peritoneal injections of xylazine
hydrochloride (16-20 mg/kg) and ketamine hydrochloride (75-150
mg/kg). Subsequently, the mid-lumbar dorsal region of each mouse
was exposed for the surgical procedure. During the surgery, a
square incision was created at the dorsal terminal of the ribcage
through the muscle, thus exposing the fat pad region around the
ovary. In the next step, the oviduct of the mouse was ligated and
subsequently cauterized using heated forceps to prevent bleeding
during the excision of the ovary. Following ovary excision, the
remaining ovarian tissues were placed back into the peritoneal
cavity prior to repeating the aforementioned procedure at the other
side. Finally, the muscle openings were sealed with a 5-0 suture in
conjunction with skin clips. Subsequent to the surgery, all mice
were allowed to rest for 1 week prior to conducting subsequent
experimental steps to ensure the successful establishment of a
post-menopausal model.
Following the successful ovariectomy, the mice were
divided into four groups (each, n=15), as follows: Sham group that
included untreated control mice, and three tamoxifen-treated
groups, in which mice received a daily dose of 1.0, 2.0 and 3.5
µg tamoxifen for 7 consecutive days, respectively. For
tamoxifen treatment, tamoxifen tablets (Yangtze River
Pharmaceutical Co., Ltd.) were crushed into a thin powder,
suspended in ethanol and then diluted using canola oil. The
administration of tamoxifen in mice was performed orally using a
gavage needle. In the sham group, the mice received only the same
volume of canola oil instead of the tamoxifen suspension.
Subsequent to the treatment with tamoxifen and the successful
induction of EH (as determined via hematoxylin and eosin staining),
the 3.5 µg tamoxifen treated-mice were again divided into
five groups (each, n=3) to receive Met treatment, as follows: Sham
group, treated with phosphate-buffered saline (PBS); Met group,
treated with a daily dose of 5 mg Met (Tocris Bioscience); EH
group, treated with a daily dose of 3.5 µg tamoxifen; EH +
Met group, treated with a daily dose of 5 mg Met in conjunction
with a daily dose of 3.5 µg tamoxifen; and EH + AKT
inhibitor group, treated with the AKT inhibitor MK-2206 (Lifespan
Biosciences, Inc.) dissolved in 30% Captisol at a dose of 120 mg/kg
and administered by oral gavage in conjunction with a daily dose of
3.5 µg tamoxifen. All treatments were administered once a
day for 7 consecutive days. The successful establishment of the
animal models was confirmed by hematoxylin and eosin (H&E)
staining among these groups. All experiments were performed
according to the protocol approved by the Ethical Committee of
Baoji Maternal and Child Health Hospital (Baoji, China).
Immunohistochemistry
Tissue samples were harvested, fixed in 4%
paraformaldehyde for 48 h, dehydrated, embedded in paraffin and
sliced into 5 µm sections. Gradient ethanol was used to
dewax and hydrate the samples, followed by 2 min of antigen
retrieval in 10 mM citrate (pH 6.0) under a 720 W heating condition
in a microwave. Subsequently, the sections were cooled-down at room
temperature for 30 min, blocked in 3% hydrogen peroxide for 15 min
and incubated at room temperature for 120 min with primary
anti-TGF-β1 (cat. no. ab92468, 1:5,000; Abcam) antibodies.
Subsequent to sample washing with Tris-buffered saline/Tween-20,
HRP-linked IgG secondary antibodies (cat no. ab6721, 1:1,500;
Sigma-Aldrich; Merck KGaA) were used to treat the samples for 2 h
at room temperature. A DAB substrate kit (Vector Laboratories,
Burlingame, CA, USA) was then used to visualize the bound
antibodies, while hematoxylin was used to stain the cell
nuclei.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
A miRNeasy Mini kit (Qiagen GmbH, Düsseldorf,
Germany) was used to extract total RNA from endometrial tissue
samples of each group after 7 days of treatment. A NanoDrop 1000
spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) was used to assess the RNA purity and concentration. Next, a
MiScript Reverse Transcription kit (Qiagen GmbH) was used to
synthesize the cDNA of UCA1, miR-144 and TGF-β1 using the following
reaction conditions: 37°C for 60 min and 95°C for 5 min. An ABI
7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and a miScript SYBR-Green PCR kit (Qiagen GmbH,
Hilden, Germany) were then used to analyze the expression levels of
UCA1, miR-144 and TGF-β1 mRNA in a One-Step Real-Time PCR 96-well
optical plate. The sequences of primers used in qPCR were the
following: UCA1 forward, 5′-GCC CCT TGG ACC ATC ACA-3′, and
reverse, 5′-GAC GGC AGT TGG TGT GCT AT-3′; miR-144 forward, 5′-CGG
TAC AGT ATA GAT GAT GTA CT-3′, and reverse, 5′-CAG TGC GTG TCG TGG
AGT-3′; TGF-β1 mRNA forward, 5′-AAC TGC TTC CTG TAT GGG GTC-3′, and
reverse, 5′-AAG GCG TCG TCA ATG GAC TC-3′; U6 forward, 5′-CTC GCT
TCG GCA GCA CA-3′, and reverse, 5′-AAC GCT TCA CGA ATT TGC GT-3′;
GAPDH forward, 5′-CGG AGT CAA CGG ATT TGG TCG TAT-3′ and reverse,
5′-AGC CTT CTC CAT GGT GGT GAA GAC-3′. The reaction was performed
using the following conditions: Initial activation at 95°C for 15
min and denaturation at 94°C for 15 sec, followed by annealing at
55°C for 30 sec and a final extension at 72°C for 60 sec. U6 and
GAPDH served as the internal control to normalize the expression
levels of UCA1, miR-144 and TGF-β1 mRNA, which were calculated
using the 2-ΔΔCq method (16). Each reaction was repeated at least
three times.
Cell culture and treatment
Two human endometrial cancer cell lines, AN3CA and
Ishikawa, were obtained from the Cell Bank of the Chinese Academy
of Sciences (Shanghai, China) and used to establish cell models in
the current study. The two cell lines were cultured in a Dulbecco's
modified Eagle's medium (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (Invitrogen; Thermo
Fisher Scientific, Inc.), 100 mg/ml streptomycin sulfate and 100
U/ml penicillin. The cells were maintained in an incubator at 37°C
and 5% CO2. Subsequently, the cells were treated for 48
h with 10 and 20 µM Met and compared with untreated cells,
which served as the control group, to study the effect of Met on
the expression of multiple target genes. Three independent
experiments were performed.
Cell proliferation (MTT) assay
When AN3CA and Ishikawa cells reached 80%
confluence, they were made into single cell suspensions. The cells
were then seeded into a 96-well plate at a density of
6×103 cells/well in 0.2 ml culture medium. Each well was
treated with a 10% MTT solution at 24, 48 and 72 h after cell
culture. Following further culturing for 4 h, the supernatant was
discarded and 100 µl dimethyl sulfoxide (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was added into each well, followed
by 10 min of incubation on a shaker to fully dissolve the formazan
crystals produced by the living cells. The optical density (OD)
value of each well was measured at 490 nm on a microplate reader. A
cell viability curve was generated using time as the x-axis and the
OD value as the y-axis to evaluate the viability of AN3CA and
Ishikawa cells subjected to different treatments.
Western blot analysis
To analyze the protein expression levels of TGF-β1,
total AKT and p-AKT in tissue and cell samples, the samples were
treated with ice-cold lysis buffer (containing 1% NP-40, 0.1%
sodium dodecyl sulfate, 50 mM Tris-HCl, pH 7.4, and 150 mM NaCl)
supplemented with protease inhibitors (Roche, Indianapolis, IN,
USA). A bicinchoninic acid Protein Assay kit (Bio-Rad Laboratories,
Inc.) was used to determine the concentration of protein according
to the manufacturer's protocol. Next, 12% w/v sodium dodecyl
sulfate-polyacrylamide gel electrophoresis was used to resolve the
isolated protein, which was then electro transferred to a
nitrocellulose membrane (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) for 2 h at 90 V. PBS containing 5% nonfat dry milk was then
used to block the membrane for 60 min to eliminate non-specific
binding. Subsequently, the membrane was incubated at 4°C overnight
with polyclonal primary antibodies against TGF-β1 (cat. no.
ab92468, 1:5,000; Abcam), total AKT (cat. no. ab81283, 1:5,000;
Abcam), p-AKT (cat. no. ab131443, 1:5,000; Abcam), total Caspase-3
(cat. no. ab13847, 1:5,000; Abcam), active Caspase-3 (cat no.
ab2302, 1:5,000; Abcam) or β-actin (cat. no. ab8226, 1:5,000;
Abcam) as the internal control. After the membrane was washed twice
by PBS, it was further incubated at room temperature for 2 h with
horseradish peroxidase (HRP)-conjugated secondary antibodies (cat.
no. ab6721, 1:12,000; Abcam). An enhanced chemiluminescent kit
(Pierce, Waltham, MA) was used to visualize antigen-antibody
complexes, which were then analyzed by Quantity One software
(Bio-Rad Laboratories, Inc.) to quantify the protein levels of
TGF-β1, total AKT, p-AKT, total Caspase-3 and active Caspase-3. All
experiments were run in triplicate.
Apoptosis analysis
Cells were harvested, washed twice with PBS and
resuspended in 1X binding buffer to a final density of
4×105 cells per well. A total of 5 µl PE Annexin
V and 5 µl 7-AAD were added to 100 µl cell
suspension. Samples were then incubated in darkness for 15 min. The
status of cell apoptosis was measured using an Annexin V-FITC
apoptosis detection kit (Thermo Fisher Scientific, Inc.) and flow
cytometry (BD Biosciences) following the manufacturer's
protocols.
Statistical analysis
All data are presented as the mean ± standard
deviation, and SPSS software (version 11.5; SPSS, Inc., Chicago,
IL, USA) was used to perform all statistical analyses. Analysis of
variance with a Holms-Sidak post-hoc test was used to perform the
statistical comparisons between different groups. A P-value of
<0.05 was considered to denote a statistically significant
difference.
Results
Differential expression of UCA1, miR-144
and TGF-β1 among various groups
Different doses of tamoxifen (1.0, 2.0 and 3.5
µg) were used to establish an animal model of EH, and the
expression levels of UCA1, miR-144 and TGF-β were then detected
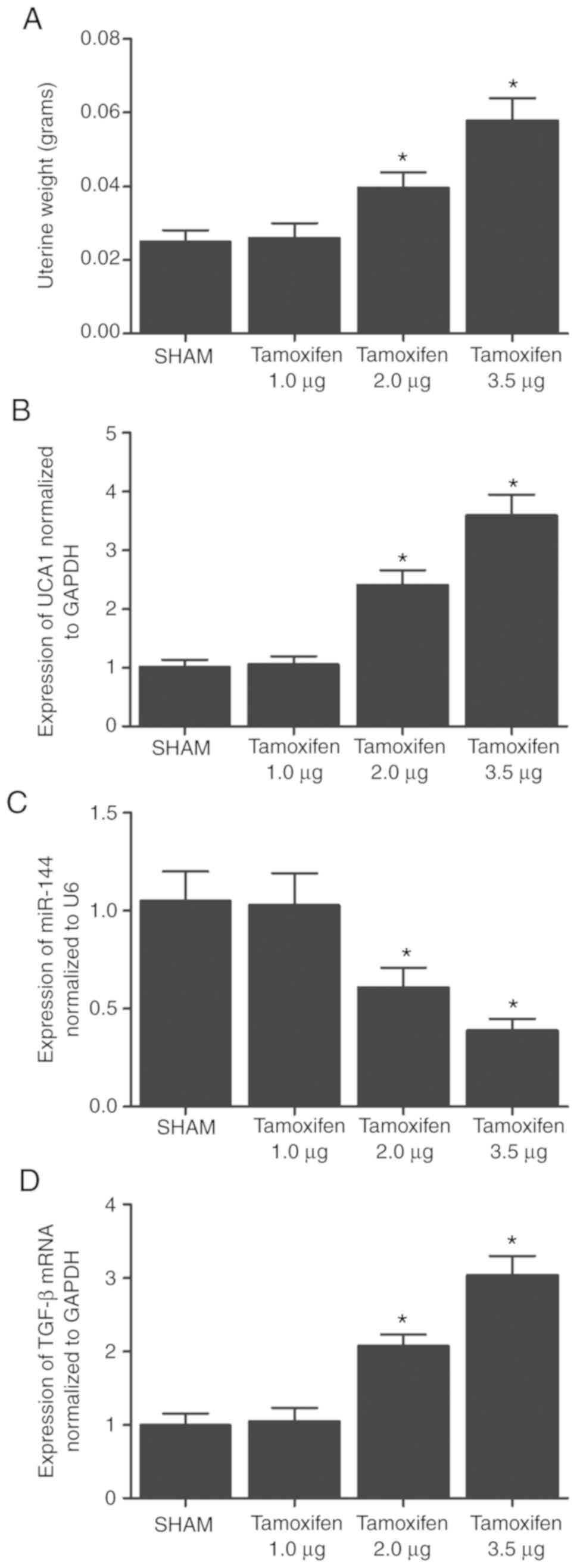
using RT-qPCR. As shown in Fig.
1, the uterine weight (Fig.
1A), as well as the expression levels of UCA1 (Fig. 1B), miR-144 (Fig. 1C) and TGF-β (Fig. 1D), exhibited no significant
differences between the sham and 1.0 µg tamoxifen groups.
However, when the concentration of tamoxifen was increased from 1
to 3.5 µg, the uterine weight (Fig. 1A) and the expression levels of
UCA1 (Fig. 1B) and TGF-β
(Fig. 1D) were also markedly
increased in a dose-dependent manner, while the expression of
miR-144 was significantly decreased by the tamoxifen treatment.
Furthermore, western blot analysis was performed to
compare the protein expression levels of TGF-β, total AKT, p-AKT,
total Caspase-3 and active Caspase-3 among the four groups. As
shown in Fig. 2, the protein
levels of TGF-β and p-AKT and in the sham group were comparable
with those in the 1.0 µg tamoxifen group. However, when the
concentration of tamoxifen was increased from 1 to 3.5 µg,
the protein levels of TGF-β and p-AKT became significantly higher.
By contrast, the protein levels of total AKT and total Caspase-3
were comparable among the sham and tamoxifen (1.0, 2.0 and 3.5
µg) groups. In addition, when the concentration of tamoxifen
increased from 1 to 3.5 µg, the protein levels of active
Caspase-3 were significantly decreased.
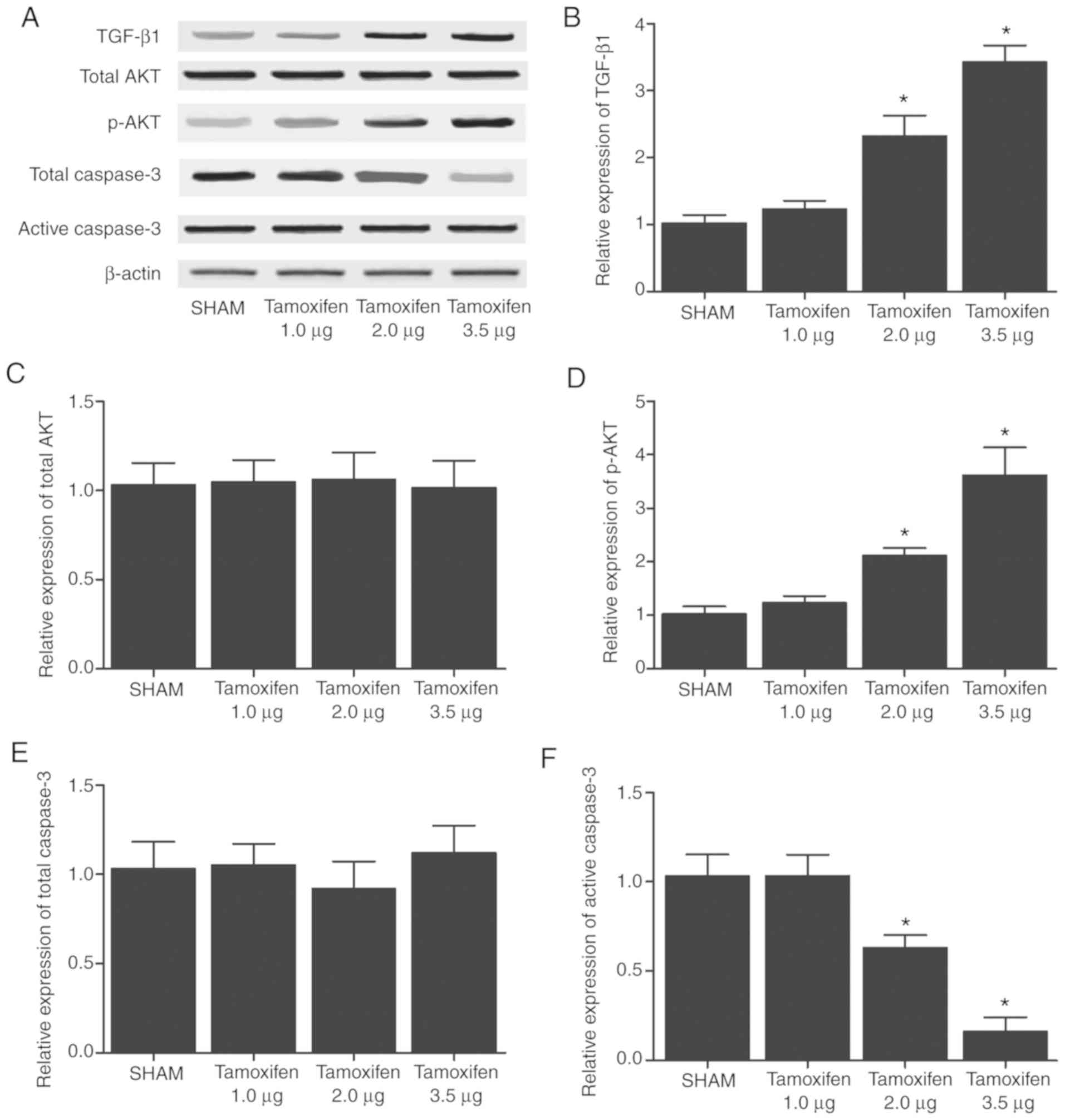 | Figure 2Expression levels of TGF-β, total
AKT, p-AKT, total Caspase-3 and active Caspase-3 in the various
treatment groups. (A) Western blots of TGF-β, total AKT, p-AKT,
total Caspase-3 and active Caspase-3 in mice treated with tamoxifen
(1.0, 2.0 and 3.5 µg). (B) TGF-β1, (C) total AKT, (D) p-AKT,
(E) total Caspase-3 and (F) active Caspase-3 expression levels are
shown. Tamoxifen (2.0 and 3.5 µg) dose-dependently
upregulated the protein expression levels of TGF-β and p-AKT, while
it dose-dependently downregulated the protein expression of active
Caspase-3. Tamoxifen exerted no effect on the protein expression
levels of total AKT and total Caspase-3. *P<0.05 vs.
sham group (N=3). UCA1, urothelial cancer associated 1; miR-144,
microRNA-144; TGF-β1, transforming growth factor-β1. |
Met regulated the expression of key EH
markers
Met was used to treat mice with EH induced by 3.5
µg tamoxifen, and then the expression levels of UCA1,
miR-144 and TGF-β were compared among the sham, EH, Met, EH + Met
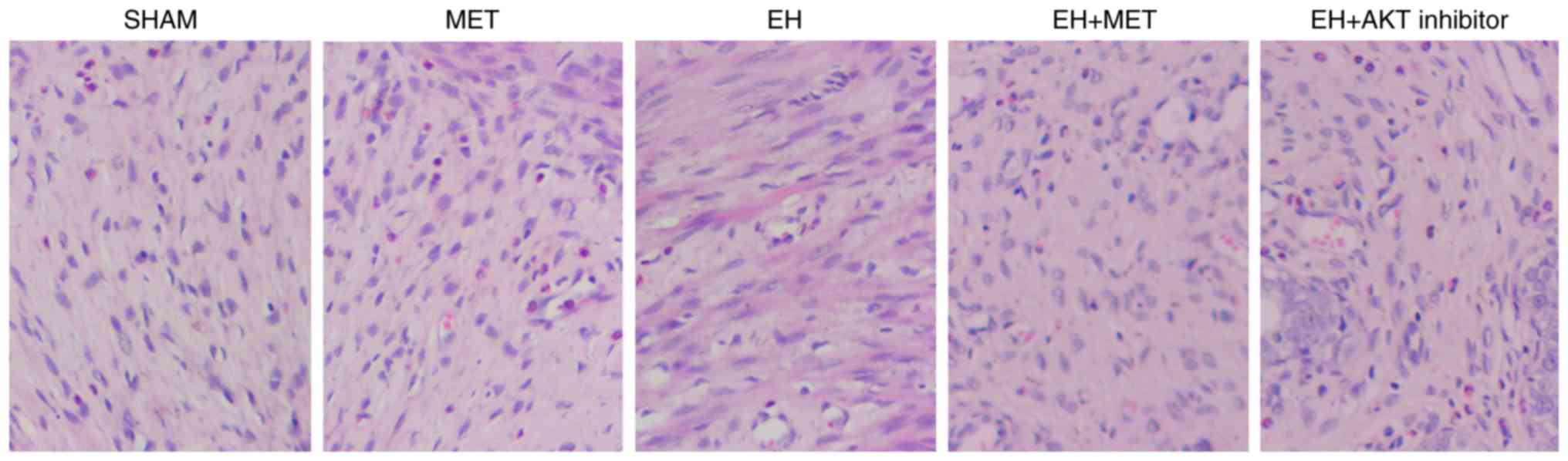
and EH + AKT inhibitor groups using RT-qPCR. The successful
establishment of the animal model was validated via H&E
staining, as shown in Fig. 3. As
shown in Fig. 4, the
administration of Met exerted no marked effect on the uterine
weight (Fig. 4A), or on the
expression levels of UCA1 (Fig.
4B), miR-144 (Fig. 4C) and
TGF-β (Fig. 4D) compared with
those in the sham group. On the contrary, the uterine weight
(Fig. 4A), and the expression
levels of UCA1 (Fig. 4B) and
TGF-β (Fig. 4D) were
significantly increased in the EH group, along with a significantly
decreased level of miR-144 (Fig.
4C). Notably, the treatment with Met in EH mice partially
restored the abnormal expression levels of UCA1, TGF-β and miR-144,
while treatment with AKT inhibitor in EH mice exerted similar
effects.
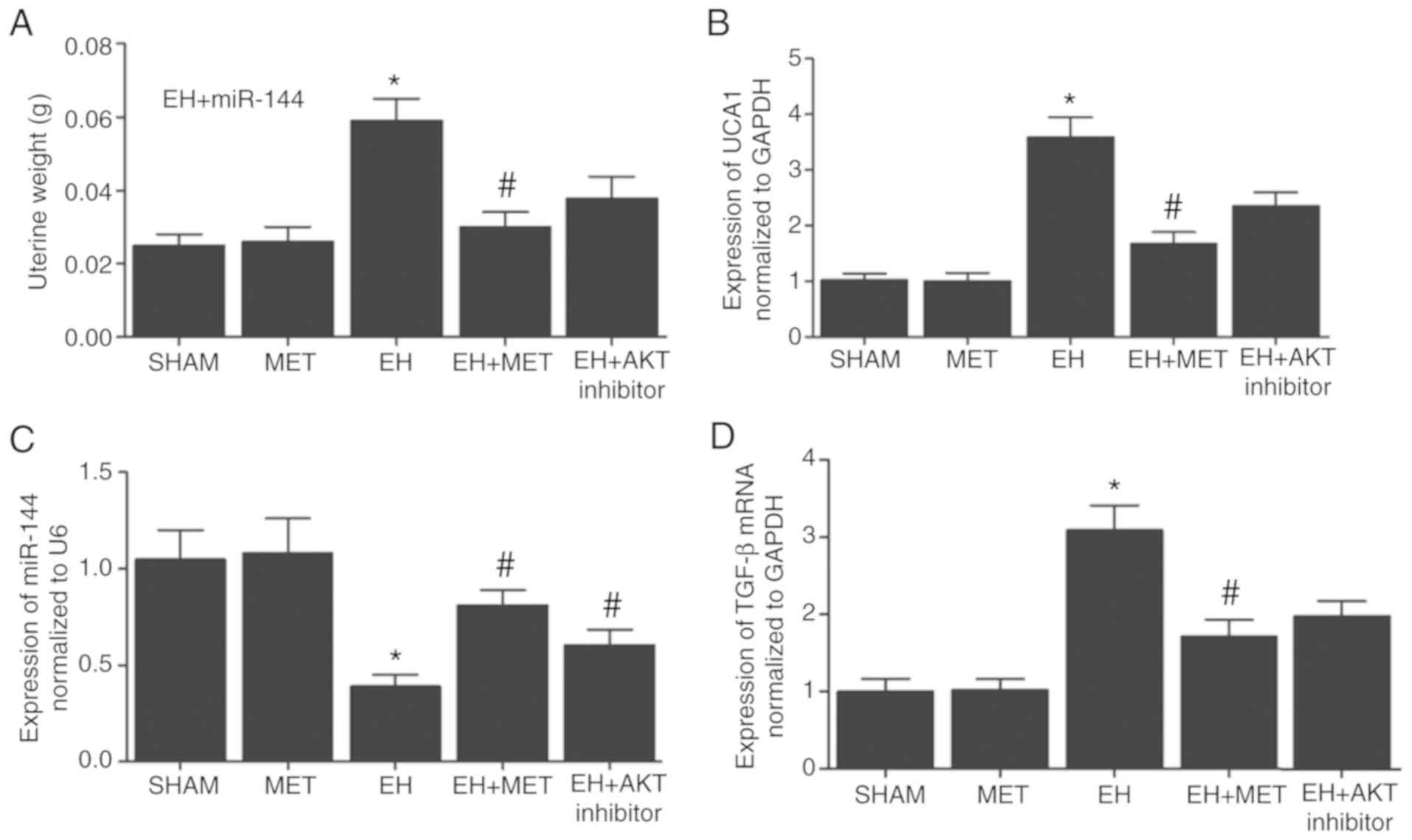 | Figure 4Differential expression levels of
UCA1, miR-144 and TGF-β following Met treatment. Effect of Met on
(A) uterine weight, and on (B) UCA1, (C) miR-144 and (D) TGF-β
expression levels. Met partially restored the normal uterine weight
that was increased by tamoxifen treatment, as well as Met partially
restored the normal mRNA expression levels of UCA1, miR-144 and
TGF-β in the EH group. *P<0.05 vs. sham group;
#P<0.05 vs. EH group (N=3). Met, metformin; EH,
endometrial hyperplasia; UCA1, urothelial cancer associated 1;
miR-144, microRNA-144; TGF-β, transforming growth factor-β. |
Furthermore, western blot analysis was performed to
compare the protein expression levels of TGF-β, total AKT, p-AKT,
total Caspase-3 and active Caspase-3 and p-AKT among the sham, EH,
Met and EH + Met groups. As shown in Fig. 5, the protein levels of TGF-β
(Fig. 5A and B) and p-AKT
(Fig. 5A and D) in the sham group
were comparable with those in the Met group, while the EH group
exhibited much higher levels of TGF-β and p-AKT. However, the
treatment of EH mice with Met partially restored the normal protein
expression of TGF-β and p-AKT. Furthermore, the protein levels of
total AKT (Fig. 5A and C) and
total Caspase-3 (Fig. 5A and E)
were similar among the sham, EH, Met, EH + Met and EH + AKT
inhibitor groups. The protein levels of active Caspase-3 (Fig. 5A and F) were similar in the sham
and Met groups, whereas treatment with 3.5 µg tamoxifen
significantly reduced the level of active Caspase-3 (EH group). In
addition, the inhibitory effect of tamoxifen on the expression of
active Caspase-3 (Fig. 5F) was
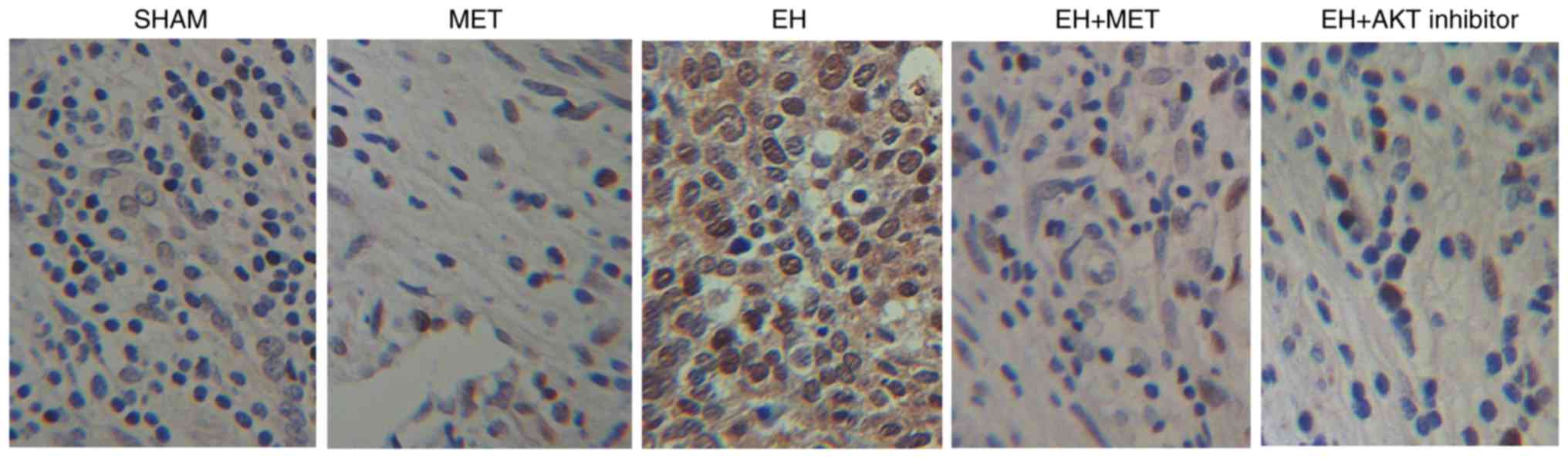
partially offset by the administration of Met or AKT inhibitor. IHC
assays also produced similar results to those of the western blot
analysis (Fig. 6).
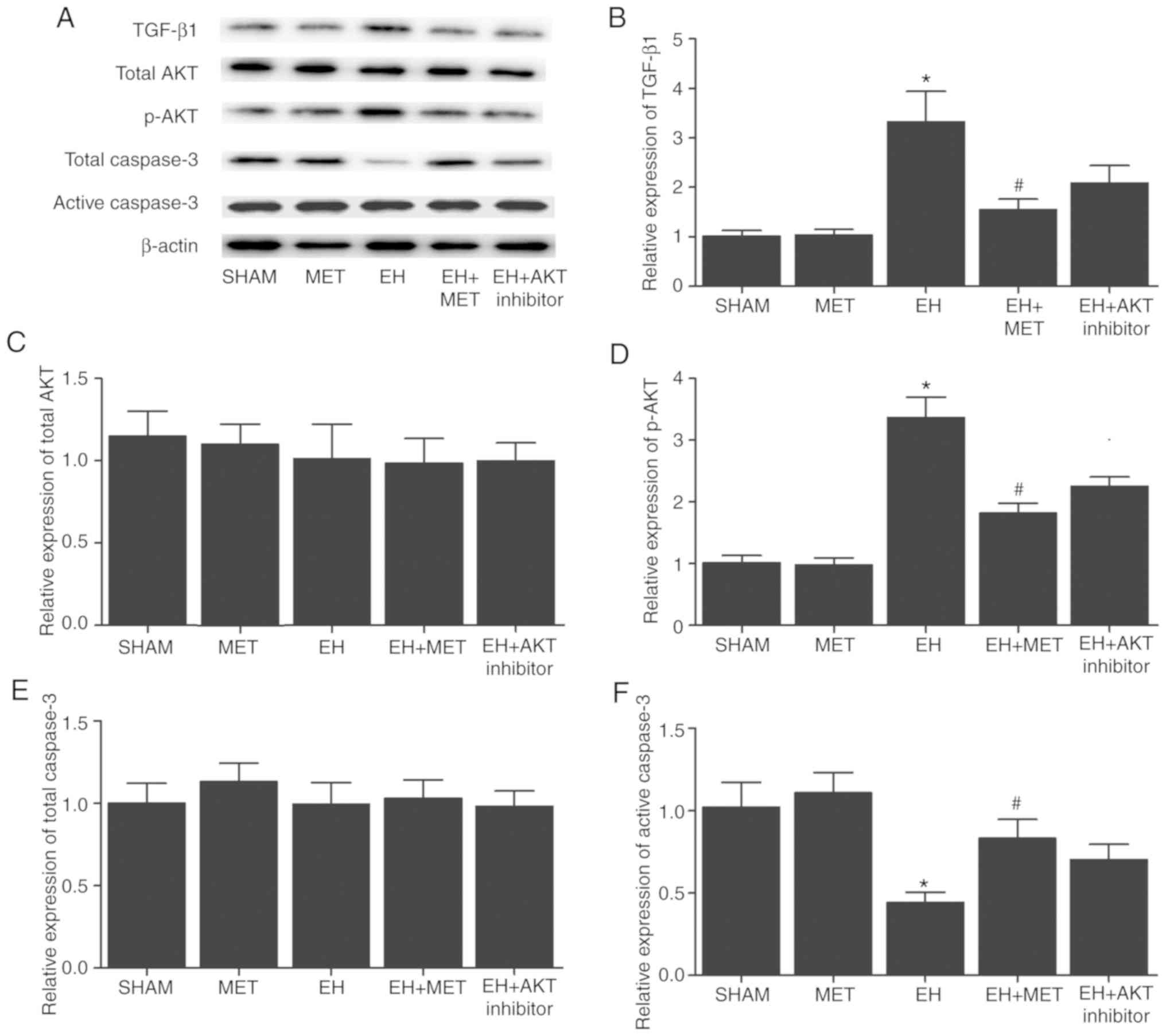 | Figure 5Expression levels of TGF-β, total
AKT, p-AKT and active Caspase-3 following Met treatment. (A)
Western blots of TGF-β, total AKT, p-AKT, total Caspase-3 and
active Caspase-3 in the different groups. (B) TGF-β1, (C) total
AKT, (D) p-AKT, (E) total Caspase-3 and (F) active Caspase-3
protein expression levels are shown. The protein levels of TGF-β
and p-AKT in the EH group were significantly higher compared with
those in the sham group, while Met treatment in the EH group
reduced these levels to a certain extent. By contrast, the protein
level of active Caspase-3 was significantly reduced in the EH group
compared with the sham group, while Met treatment in the EH group
increased this protein level to a certain extent. Met treatment
exerted no effect on the protein expression levels of total AKT and
total Caspase-3. *P<0.05 vs. sham group;
#P<0.05 vs. EH group (N=3). Met, metformin; EH,
endometrial hyperplasia; UCA1, urothelial cancer associated 1;
miR-144, microRNA-144; TGF-β1, transforming growth factor-β1. |
Met affected cell survival and the in
vitro expression of key EH markers
Various doses of Met (10 and 20 µM) were used
to treat AN3CA and Ishikawa cells, and subsequently the effect of
Met on cell proliferation and apoptosis was detected by MTT and
flow cytometry assays, respectively. Furthermore, RT-qPCR and
western blot analysis were conducted to compare the levels of UCA1,
miR-144, TGF-β, total AKT, p-AKT, total Caspase-3 and active
Caspase-3 between the control and Met (10 and 20 µM) groups.
As shown in Fig. 7, Met inhibited
the proliferation (Fig. 7A) and
promoted the apoptosis (Fig. 7B)
of AN3CA cells in a dose-dependent manner. In addition, in AN3CA
cells, treatment with Met reduced the expression of UCA1 mRNA
(Fig. 7C), TGF-β mRNA (Fig. 7E), TGF-β protein (Fig. 7F and G) and p-AKT protein
(Fig. 7F and I), while increasing
the levels of miR-144 (Fig. 7D)
and the expression of active Caspase-3 (Fig. 7F and K) in a dose-dependent
manner. However, Met exerted no effect on the protein expression
levels of total AKT (Fig. 7F and
H) and total Caspase-3 (Fig.
7F and 7J). Similar results
were also obtained from Ishikawa cells (Fig. 8).
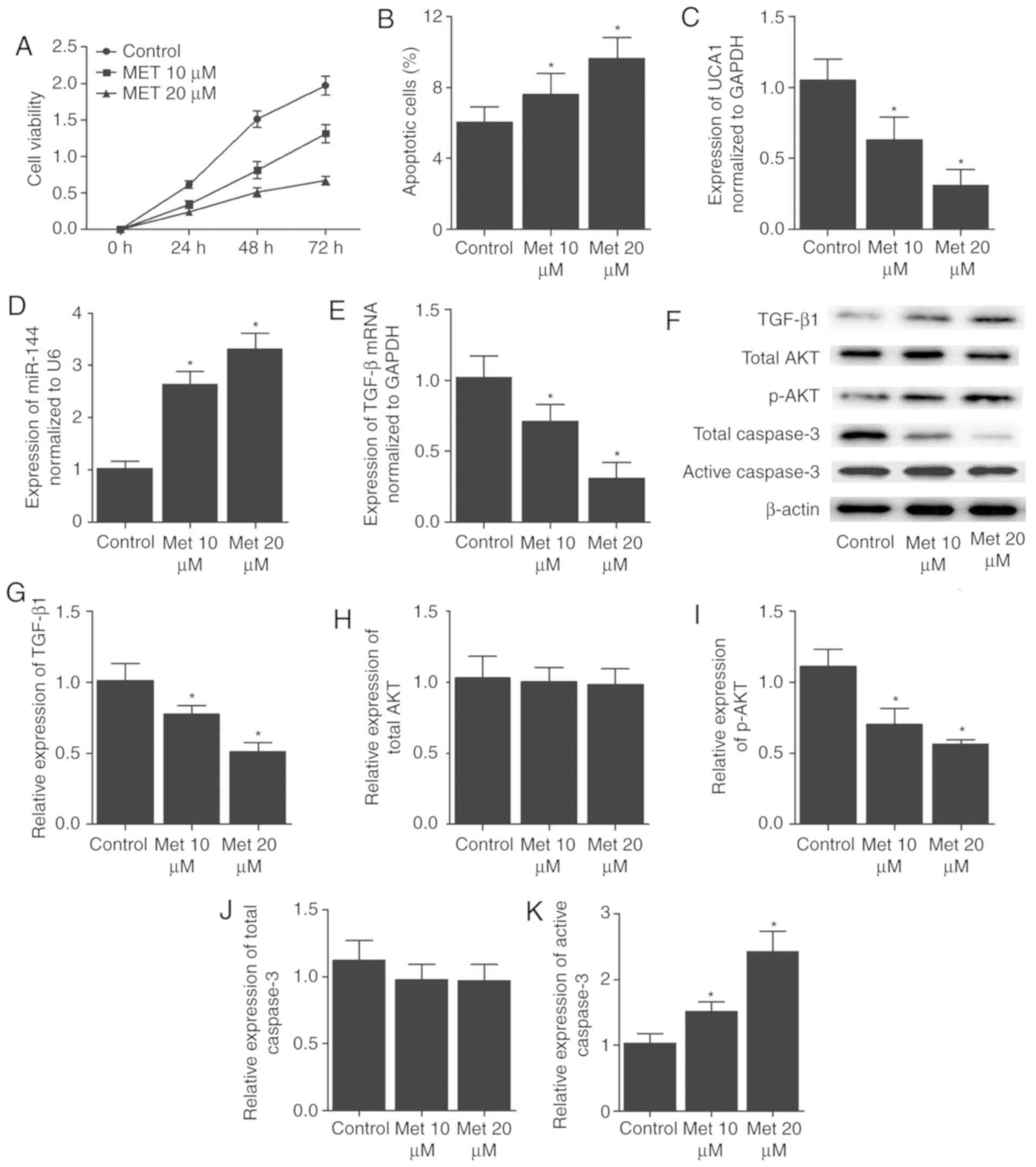 | Figure 7Met affected cell survival and the
expression levels of key EH markers in AN3CA cells. (A) Viability
was reduced and (B) apoptosis was increased dose-dependently in
AN3CA cells following the administration of Met. (C) UCA1 mRNA, (D)
miR-144 and (E) TGF-β mRNA expression levels in AN3CA cells. Met
treatment dose-dependently reduced the mRNA levels of UCA1 and
TGF-β, and enhanced miR-144 expression. (F) Western blot analysis,
and protein levels of (G) TGF-β1, (H) total AKT, (I) p-AKT, (J)
total Caspase-3 and (K) active Caspase-3. Met treatment
dose-dependently reduced the protein levels of TGF-β and p-AKT,
while increasing the protein expression of active Caspase-3. By
contrast, Met treatment exerted no effect on the protein level of
total AKT and total Caspase-3 in AN3CA cells. *P<0.05
vs. control group (N=3). Met, metformin; UCA1, urothelial cancer
associated 1; miR-144, microRNA-144; TGF-β, transforming growth
factor-β. |
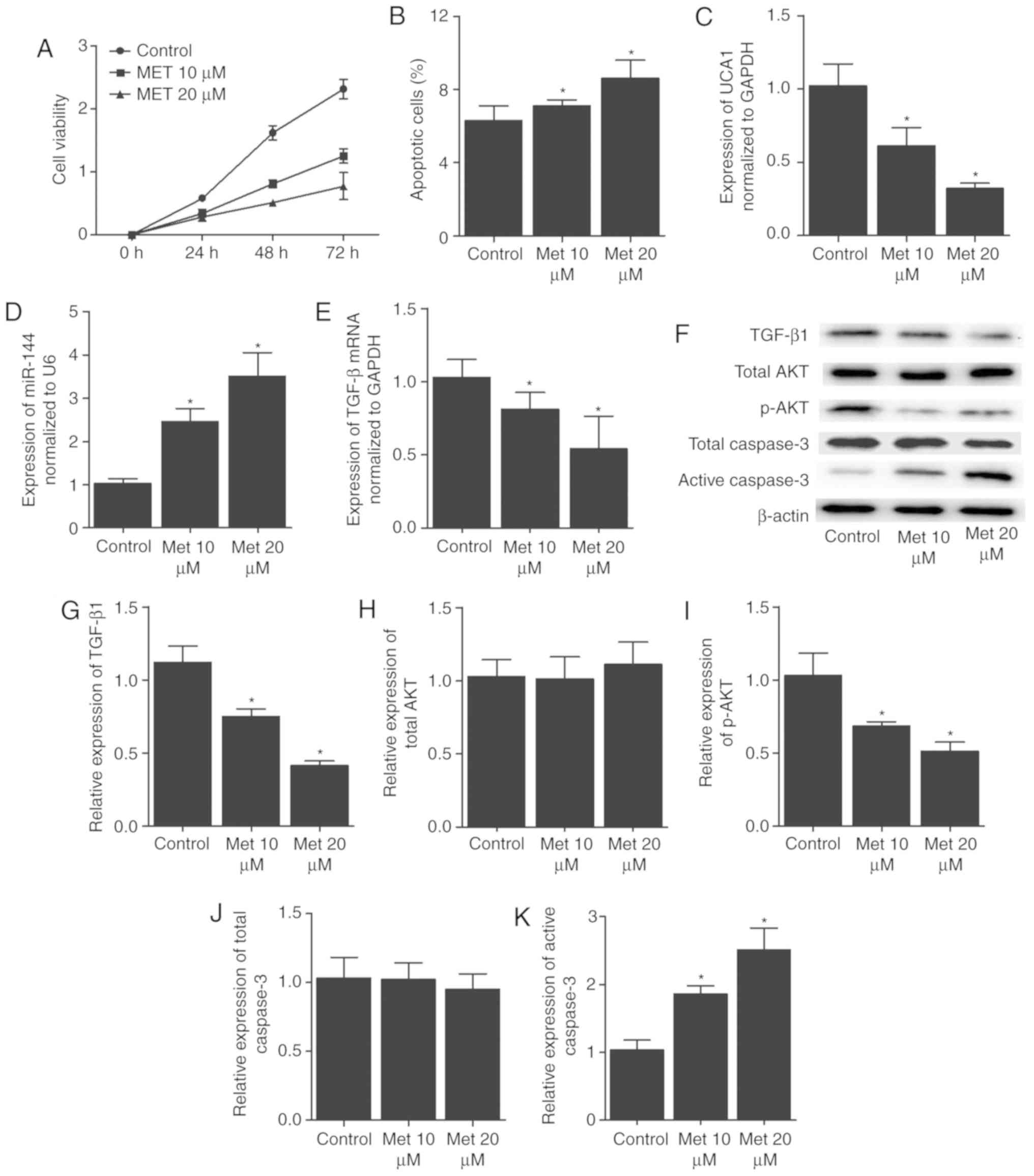 | Figure 8Met affected cell survival and the
expression levels of key EH markers in Ishikawa cells. (A)
Viability was reduced and (B) apoptosis was increased
dose-dependently in Ishikawa cells following the administration of
Met. (C) UCA1 mRNA, (D) miR-144 and (E) TGF-β mRNA expression
levels in Ishikawa cells. Met treatment in Ishikawa cells
dose-dependently reduced the mRNA levels of UCA1 and TGF-β, and
enhanced miR-144 expression. (F) Western blot analysis, and protein
levels of (G) TGF-β1, (H) total AKT, (I) p-AKT, (J) total Caspase-3
and (K) active Caspase-3. Met treatment in Ishikawa cells
dose-dependently decreased the protein expression levels of TGF-β
and p-AKT, while increasing the protein expression of active
Caspase-3. However, Met treatment exerted no effect on the protein
level of total AKT and total Caspase-3 in Ishikawa cells.
*P<0.05 vs. control group (N=3). Met, metformin;
UCA1, urothelial cancer associated 1; miR-144, microRNA-144; TGF-β,
transforming growth factor-β. |
Discussion
EH, considered as a precursor of EC, is caused by
long-term exposure to estrogen and subsequent stimulation of
endome-trial growth. In fact, the presence of EH has been reported
to increase the risk of EC (17).
As a type of biguanide, Met (also known as N,N-dimethylbiguanide)
is frequently used in the treatment of polycystic ovarian syndrome
(PCOS) and type 2 diabetes mellitus, particularly in patients
presenting insulin resistance or obesity (18-20). Given that EH is implicated in the
development of insulin resistance, while Met has been found to
exert an anti-metastatic, anti-invasive and anti-proliferative
effect in several types of cancer, Met may also be used in EH
treatment (20,21). In vitro, Met has been
demonstrated to induce the expression of PR in EC cells, thus
enhancing the efficiency of progestin therapy and reducing the
severity of progestin resistance that is often observed in
long-term progestin treatment (22). In the present study, an animal of
tamoxifen-induced EH was established and the EH mice were
subsequently treated with Met. The results revealed that tamoxifen
increased the uterine weight and the expression levels of UCA1,
TGF-β and p-AKT, while decreasing the expression levels of miR-144
and active Caspase-3. However, treatment with MT partially restored
the normal expression of miR-144 and active Caspase-3.
A previous clinical trial revealed that the effect
of Met is comparable with that of megestrol for the treatment of
simple EH (23). Another study
demonstrated that Met may directly reverse impaired glycolysis and
normalize mitochondrial function in PCOS patients with EH (24). Met was used to successfully treat
several cases of atypical EH that was not responding to progestin
treatment (25). In vitro
studies also reported that Met was able to reduce the proliferation
of prostate, ovarian, endometrial and breast cancer cells (26-29). A previous study on EC cell line
also demonstrated a dose-dependent effect of Met treatment, while a
meta-analysis confirmed that Met was able to reduce the incidence
of pancreatic, hepatic, colorectal and breast cancer (25,30). In a retrospective study of ~1,000
EC patients who were followed up for over 3 years, the patients
treated with Met exhibited a longer overall survival in comparison
with the patients not treated with Met (31). In another study on PCOS patients,
an increasing dose of Met more significantly reduced the risk of EC
(32). Since the risk of EC is
increased by >4-fold in PCOS patients, the protective effect of
Met against EC is significant in PCOS patients (30). In addition, since >30% of PCOS
patients eventually develop progestin resistance, novel treatments
are required to overcome progestin resistance and to reduce the
proliferation of endometrial cells (30).
It has been previously demonstrated that lncRNA UCA1
can bind to miR-144 expressed in A549 cells (14). In the current study, the effect of
Met on cell proliferation and apoptosis, as well as on the
expression levels of UCA1, miR-144, TGF-β, total AKT, p-AKT, total
Caspase-3 and active Caspase-3, was investigated in AN3CA and
Ishikawa cells. The results demonstrated that Met reduced the cell
viability and promoted cell apoptosis. In addition, Met treatment
dose-dependently reduced the expression levels of UCA1, TGF-β and
p-AKT, while increasing the expression of miR-144 and active
Caspase-3. However, Met treatment exerted no effect on the
expression of total AKT and total Caspase-3.
As a major signaling pathway of TGF-β1, the
phosphatidylinositol 3-kinase (PI3K) signaling serves an essential
role in a wide range of cellular processes (33). For instance, by phosphorylating
and converting PIP2 to PIP3 on the cell membrane, PI3K facilitates
the interaction between PIP3 and GTP-binding proteins, such as AKT,
PKC, and Rac. As a major effector of PI3K, AKT is activated by a
wide range of extracellular signals and growth factors to control
numerous basic cellular processes, including cell proliferation,
apoptosis, division and survival (33). Other intracellular signaling
pathways, including the extracellular signal-regulated kinase 1/2
pathway, have been implicated in carcinogenesis (34). Notably, the inactivation of the
TGF-β pathway has been reported to occur prior to the onset of EH.
Thus, the identification of TGF-β targets in the development of EH
and EC may provide a novel way to prevent tumor escape from immune
responses (35). Furthermore, if
the level of TGF-β receptor expression can be increased during the
treatment of EH, a novel therapeutic modality may be developed to
restore impaired TGF-β signaling in EH and EC (31). It has also been demonstrated that
K-1 inactivated the Wnt/β-catenin and Wnt7a/FZD6 signaling pathways
in EH cells. K-1 can also reduce PI3K/AKT phosphorylation, thus
promoting the apoptosis of primary EH cells (36).
Nevertheless, there are limitations in the current
study. Although the effect of Met in the prognosis and treatment of
EH was demonstrated, only animal and cellular models were utilized.
In addition, while treatment of the EH animal model with miR-144
was attempted, it was observed that the concentration in the target
organ, the uterus, was not significantly elevated, as expected. To
further confirm the study findings, further clinical data will be
needed and corresponding clinical trials are necessary.
In conclusion, in the current study, tamoxifen was
used to establish a mouse model of EH, which exhibited increased
levels of UCA1, TGF-β and p-AKT, along with decreased levels of
miR-144 and active Caspase-3. Notably, Met treatment partially
restored the normal expression levels of UCA1, TGF-β, p-AKT,
miR-144 and active Caspase-3 in a dose-dependent manner.
Furthermore, Met treatment inhibited cell proliferation and
promoted cell apoptosis.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MG designed the current study. MG, JZ and WH
collectted and analyzed the data. JZ collected the literature. WH
and MG composed the manuscript.
Ethics approval and consent to
participate
All experiments were performed according to the
protocol approved by the Ethical Committee of Baoji Maternal and
Child Health Hospital (Baoji, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Horn LC, Schnurrbusch U, Bilek K,
Hentschel B and Einenkel J: Risk of progression in complex and
atypical endometrial hyper-plasia: Clinicopathologic analysis in
cases with and without progestogen treatment. Int J Gynecol Cancer.
14:348–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ozdegirmenci O, Kayikcioglu F, Bozkurt U,
Akgul MA and Haberal A: Comparison of the efficacy of three
progestins in the treatment of simple endometrial hyperplasia
without atypia. Gynecol Obstet Invest. 72:10–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Daud S, Jalil SS, Griffin M and Ewies AA:
Endometrial hyperplasia-the dilemma of management remains: A
retrospective observational study of 280 women. Eur J Obstet
Gynecol Reprod Biol. 159:172–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nieman KM, Romero IL, Van Houten B and
Lengyel E: Adipose tissue and adipocytes support tumorigenesis and
metastasis. Biochim Biophys Acta. 1831:1533–1541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sheppard D: Transforming growth factor
beta: A central modulator of pulmonary and airway inflammation and
fibrosis. Proc Am Thorac Soc. 3:413–417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
ten Dijke P and Hill CS: New insights into
TGF-beta-Smad signalling. Trends Biochem Sci. 29:265–273. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bakin AV, Rinehart C, Tomlinson AK and
Arteaga CL: p38 mitogen-activated protein kinase is required for
TGFbeta-mediated fibroblastic transdifferentiation and cell
migration. J Cell Sci. 115:3193–3206. 2002.PubMed/NCBI
|
|
8
|
Gao Y, Li S and Li Q: Uterine epithelial
cell proliferation and endometrial hyperplasia: Evidence from a
mouse model. Mol Hum Reprod. 20:776–786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eulalio A, Huntzinger E and Izaurralde E:
Getting to the root of miRNA-mediated gene silencing. Cell.
132:9–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang ZS, Wang J, Zhu BQ and Ge L: Long
noncoding RNA UCA1 promotes multiple myeloma cell growth by
targeting TGF-β. Eur Rev Med Pharmacol Sci. 22:1374–1379.
2018.PubMed/NCBI
|
|
14
|
Li D, Li H, Yang Y and Kang L: Long
noncoding RNA urothelial carcinoma-associated 1 promotes the
proliferation and metastasis of human lung tumor cells by
regulating MicroRNA-144. Oncol Res. 26:537–546. 2018. View Article : Google Scholar
|
|
15
|
Li T, Sun X and Jiang X: UCA1 involved in
the metformin-regulated bladder cancer cell proliferation and
glycolysis. Tumour Biol. 39:1010428317710823. 2017. View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Matias-Guiu X, Catasus L, Bussaglia E,
Lagarda H, Garcia A, Pons C, Muñoz J, Argüelles R, Machin P and
Prat J: Molecular pathology of endometrial hyperplasia and
carcinoma. Hum Pathol. 32:569–577. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pernicova I and Korbonits M:
Metformin-mode of action and clinical implications for diabetes and
cancer. Nat Rev Endocrinol. 10:143–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nestler JE: Metformin for the treatment of
the polycystic ovary syndrome. N Engl J Med. 358:47–54. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shao R, Li X, Feng Y, Lin JF and Billig H:
Direct effects of metformin in the endometrium: A hypothetical
mechanism for the treatment of women with PCOS and endometrial
carcinoma. J Exp Clin Cancer Res. 33:412014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen ZQ, Zhu HT and Lin JF: Reverse of
progestin-resistant atypical endometrial hyperplasia by metformin
and oral contraceptives. Obstet Gynecol. 112:465–467. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie Y, Wang YL, Yu L, Hu Q, Ji L, Zhang Y
and Liao QP: Metformin promotes progesterone receptor expression
via inhibition of mammalian target of rapamycin (mTOR) in
endometrial cancer cells. J Steroid Biochem Mol Biol. 126:113–120.
2011. View Article : Google Scholar
|
|
23
|
Sharifzadeh F, Aminimoghaddam S, Kashanian
M, Fazaeli M and Sheikhansari N: A comparison between the effects
of metformin and megestrol on simple endometrial hyperplasia.
Gynecol Endocrinol. 33:152–155. 2017. View Article : Google Scholar
|
|
24
|
Wang T, Zhang J, Hu M, Zhang Y, Cui P, Li
X, Li J, Vestin E, Brännström M, Shao LR and Billig H: Differential
expression patterns of glycolytic enzymes and
mitochondria-dependent apoptosis in PCOS patients with endometrial
hyperplasia, an early hallmark of endometrial cancer, in vivo and
the impact of metformin in vitro. Int J Biol Sci. 15:714–725. 2019.
View Article : Google Scholar :
|
|
25
|
Shan W, Wang C, Zhang Z, Gu C, Ning C, Luo
X, Zhou Q and Chen X: Conservative therapy with metformin plus
megestrol acetate for endometrial atypical hyperplasia. J Gynecol
Oncol. 25:214–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sivalingam VN, Kitson S, McVey R, Roberts
C, Pemberton P, Gilmour K, Ali S, Renehan AG, Kitchener HC and
Crosbie EJ: Measuring the biological effect of presurgical
metformin treatment in endometrial cancer. Br J Cancer.
114:281–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ben Sahra I, Tanti JF and Bost F: The
combination of metformin and 2-deoxyglucose inhibits autophagy and
induces AMPK-dependent apoptosis in prostate cancer cells.
Autophagy. 6:670–671. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiralerspong S, Palla SL, Giordano SH,
Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi
GN and Gonzalez-Angulo AM: Metformin and pathologic complete
responses to neoadjuvant chemotherapy in diabetic patients with
breast cancer. J Clin Oncol. 27:3297–3302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rattan R, Graham RP, Maguire JL, Giri S
and Shridhar V: Metformin suppresses ovarian cancer growth and
metastasis with enhancement of cisplatin cytotoxicity in vivo.
Neoplasia. 13:483–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Guo YR, Lin JF, Feng Y, Billig H and
Shao R: Combination of diane-35 and metformin to treat early
endometrial carcinoma in PCOS women with insulin resistance. J
Cancer. 5:173–181. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee TY, Martinez-Outschoorn UE, Schilder
RJ, Kim CH, Richard SD, Rosenblum NG and Johnson JM: Metformin as a
therapeutic target in endometrial cancers. Front Oncol. 8:3412018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tabrizi AD, Melli MS, Foroughi M,
Ghojazadeh M and Bidadi S: Antiproliferative effect of metformin on
the endometrium-a clinical trial. Asian Pac J Cancer Prev.
15:10067–10070. 2014. View Article : Google Scholar
|
|
33
|
Cantrell DA: Phosphoinositide 3-kinase
signalling pathways. J Cell Sci. 114:1439–1445. 2001.PubMed/NCBI
|
|
34
|
Santarpia L, Lippman SM and El-Naggar AK:
Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy.
Expert Opin Ther Targets. 16:103–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parekh TV, Gama P, Wen X, Demopoulos R,
Munger JS, Carcangiu ML, Reiss M and Gold LI: Transforming growth
factor beta signaling is disabled early in human endometrial
carcinogenesis concomitant with loss of growth inhibition. Cancer
Res. 62:2778–2790. 2002.PubMed/NCBI
|
|
36
|
Chandra V, Fatima I, Manohar M, Popli P,
Sirohi VK, Hussain MK, Hajela K, Sankhwar P and Dwivedi A:
Inhibitory effect of 2-(piper
idinoethoxyphenyl)-3-(4-hydroxyphenyl)-2H-benzo(b)pyran (K-1) on
human primary endometrial hyperplasial cells mediated via combined
suppression of Wnt/β-catenin signaling and PI3K/Akt survival
pathway. Cell Death Dis. 5:e13802014. View Article : Google Scholar
|