Introduction
Nitric oxide (NO) synthase, endothelial
(NOS3)-derived NO is an important signaling molecule in the
vascular system (1-3). The bioavailability of
tetrahydrobiopterin (BH4) is critical for the catalytic activity of
NOS3. The decreased effectiveness of BH4 leads to the decoupling of
NOS3 and the production of reactive oxygen species (ROS) in
endothelial cells (4,5). Decreased bioavailability of NO is an
important factor in myocardial ischemia-reperfusion injury. The
mechanism of NO reduction is associated with the lack of NOS
substrate L-arginine and the cofactor BH4 leading to NOS decoupling
(6).
Ischemia time-dependently decreases cardiac BH4
content and NOS3 activity, and increases NOS-derived superoxide
anion (O2·-) production, which is considered
to contribute to post-ischemic endothelial dysfunction and
myocardial injury (6). The
decrease in BH4 bioavailability is associated with the uncoupling
of NOS3 activity and the production of NOS3-dependent
O2·-. Increased resistance of the heart to
ischemia is associated with the combination of heat shock protein
90 (HSP90) and NOS3 and subsequent increases in NO production
(7). BH4 supplementation may
restore endothelium-dependent coronary blood flow and decrease
ischemia/reperfusion (I/R) injury in rat hearts (8,9),
while inhibition of BH4 synthesis may increase I/R injury (10). These results suggest that
myocardial BH4 bioavailability is an important factor in
cardioprotection and supports the hypothesis that BH4 may be a
novel therapeutic target for the treatment of I/R injury.
Increasing evidence indicates that NOS3-derived NO
is a critical mediator of anesthetic preconditioning (APC)
(10-13). Under hyperglycemic conditions, the
cardioprotective effects of volatile anesthetics by BH4 and
HSP90-regulated NOS3 activity was abrogated (14). However, the role of BH4
availability and the association of HSP90 with NOS3 in APC-mediated
cardioprotection against I/R injury remain to be elucidated. The
aims of the present study were to determine: i) Whether BH4 levels
were different in I/R rat hearts with or without APC, and whether
increasedBH4 levels contributed to resistance to I/R injury by APC;
ii) whether BH4 supplementation with BH4 precursorsepiapterin (SP)
and GTP cyclohydrolase I (GCH-1) inhibitor,
2,4-diamino-6-hydroxypyrimidine (DAHP) differentially modulated
resistance to I/R injury in the control (I/R alone) and APC rat
hearts and iii) whether BH4 supplementation alteredthe
BH4:BH2ratio, consequently affecting NOS3 activity and the
association of HSP90 with NOS3 in APC-induced cardioprotection.
Materials and methods
Animals
The present study was approved by the Institutional
Animal Care and Use Committee of Nanjing University. Male
Sprague-Dawley rats (250±50 g), aged 8-10 weeks, were purchased
from the Animal Center of Suzhou University and were housed at 25°C
with 60% humidity in a 12:12 h light: Dark cycle. All rats were
housed in each cage and were allowed ad libitum access to
food and water. All procedures performed on the rats used in the
present study were in accordance with the National Institute Health
Guide for the care and Use of Laboratory Animals.
Isolated heart preparation
The isolated heart preparation was performed as
previously described (8,13,15). Briefly, rats were anesthetized
with sodium pentobarbital (50 mg/kg i.p.) and decapitated when
unresponsive to noxious stimulation (pinching the paw). The hearts
were excised and perfused in the Langendorff mode at a perfusion
pressure equivalent to 80 mmHg. Perfusate and bath temperatures
were maintained at 37.2±0.1°C using a thermostatically controlled
water circulator (Lauda E100; LAUDA). Left ventricular pressure
(LVP) was measured isovolumetrically with a transducer connected to
a thin saline-filled latex balloon inserted into the left ventricle
through the mitral valve from an incision in the left atrium. The
hearts were immersed in gassed physiological buffer solution at
37.2°C, and subjected to 30 min global ischemia followed by 120 min
reperfusion. At the time points prior to ischemia or at the end of
the experiments, the hearts were freeze-clamped and stored at −80°C
until use in the BH4 and BH2 analyses by high performance liquid
chromatography (HPLC) or western blot analysis.
Experimental protocols
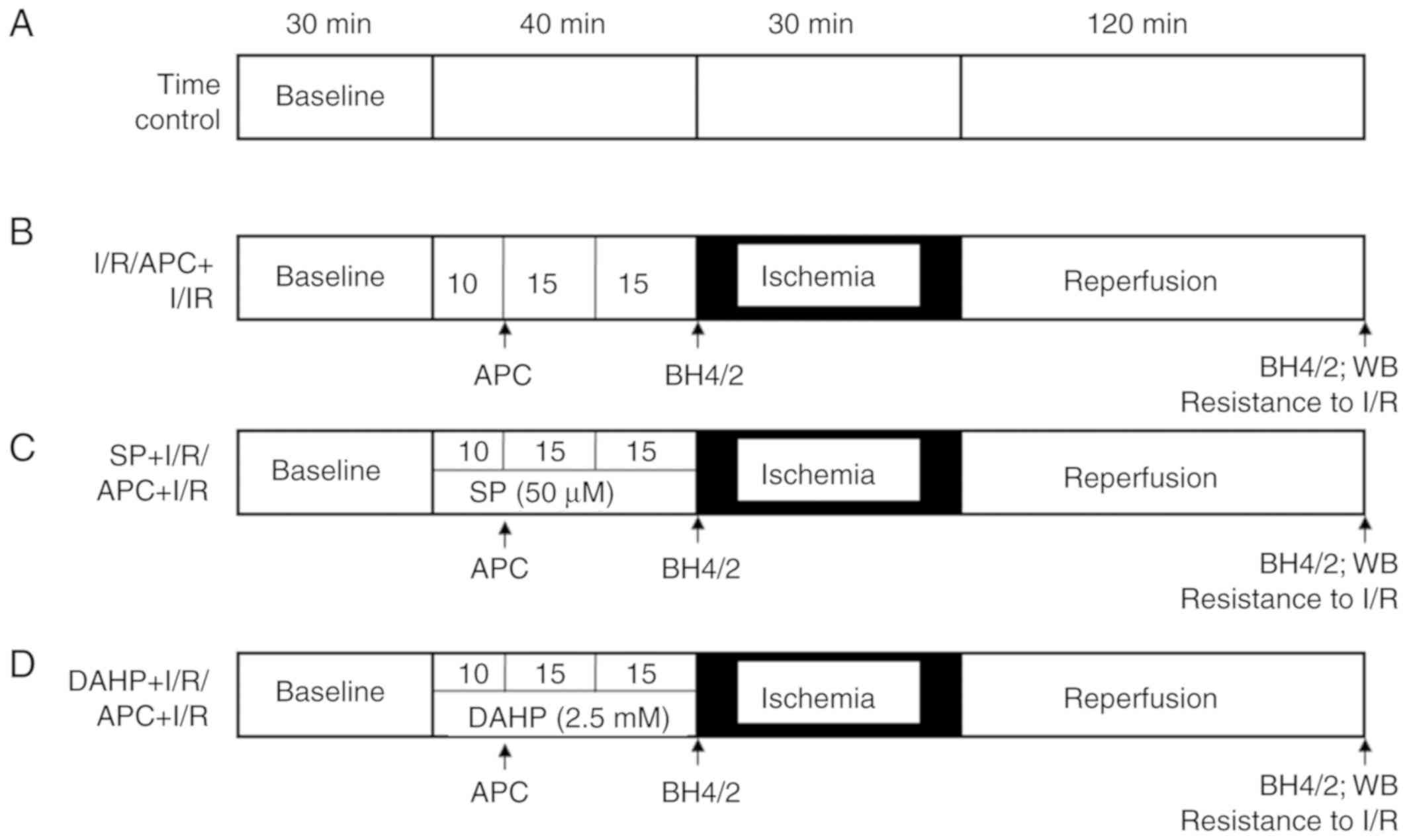
Each experimental treatment phase lasted for 220
min. A total of 70 rats were used. The functional parameters were
stabilized for 30 min; after that the hearts were randomly divided
into 7 groups with 10 hearts in each group (Fig. 1). Untreated time controls (TC;
without I/R) were perfused for 220 min without drugs or ischemia
(timeline A). After 40 min of vehicle perfusion, the I/R group was
subjected to 30 min of global ischemia and 120 min of reperfusion;
the APC group was exposed to sevoflurane (3.5%; Abbott
Pharmaceutical Co., Ltd.) for 15 min followed by a 15 min washout
prior to the onset of ischemia (timeline B). In the SP + I/R or SP
+ APC groups, SP (50 µM; cat. no. 11.225; Schircks
Laboratories) was administered for 40 min prior to the onset of
ischemia (timeline C). In the DAHP + I/R or DAHP + APC groups, DAHP
(2.5 mM; cat. no. D19206; Sigma-Aldrich, Merck KGaA) was
administered for 40 min prior to the onset of ischemia (timeline
D).
Measurement of BH4 and BH2
The contents of BH4 and BH2 in all cardiac
homogenates were determined viaHPLC as previously described
(8). BH4 and BH2 were quantified
via HPLC with an electrochemical detector (ESA Biosciences
CoulArray® system Model 542) using a Synergi Polar-RP
column (Phenomenex) eluted with argon degassed 50 mM phosphate
buffer (pH 2.6). Multi-channel colorimetric detection was set
between 0 and 600 mV. A separate channel was set at -250 mV in
order to verify the reversibility of BH4 oxidative peak detection.
The peak areas were collected at 0 and 150 mV for BH4, and 280 and
365 mV for BH2, and the data were combined to obtain a calibration
curve. Intracellular concentrations of BH4 and BH2 were calculated
using authentic external BH4 and BH2 standards as previously
described (8). Cellular BH4 and
BH2 levels were then normalized to cell protein concentrations.
Western blot analysis
The western blot analysiswas performed as previously
described (8). The protein
content of the samples was determined using a BCA assay kit (P0010;
Biyuntian Biotechnology Company) and was adjusted to the same
concentration. After denaturation, 20 µg of each sample was
dissolved in Laemmli sample buffer (cat. no. S3401; Sigma-Aldrich,
Merck KGaA) and separated via SDS-PAGE (12% gel). Following
transfer to nitrocellulose membranes, membranes were blocked with
5% non-fat milk in PBS and probed with primary antibodies at 4°C
overnight and were incubated with HRP-Conjugated goat anti-rabbit
secondary antibodies (1:10,000; cat. no. ab6721; Abcam) or
HRP-conjugated goat anti-mouse secondary antibody (1:10,000; cat.
no. sc-2031; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. The protein bands were visualized with a SuperSignal
West Pico kit (cat. no. 34577; Pierce; Thermo Fisher Scientific,
Inc.) and band densities were quantified using UN-SCAN-IT software
(v.7.0; Silk Scientific, Inc.). Antibodies specific for GCH-1 (cat.
no. ab26439, 1:2,000) and GAPDH (cat. no. ab9485, 1:2,500) used in
the study were purchased from Abcam. The NOS3 (cat. no. PA3-031A;
1:1,000) and HSP90 (cat. no. MA1-10372; 1:1,000) antibodies were
purchased from Invitrogen; Thermo Fisher Scientific, Inc.
Immunoprecipitation assay
Immunoprecipitation was performed as previously
described (8). Hearts were
homogenized in ice-cold lysis buffer and subjected to SDS-PAGE (12%
gel). Proteins were detected using enhanced chemiluminescence
detection reagents (cat. no. 34577; Pierce; Thermo Fisher
Scientific, Inc.) for densitometric analysis with UN-SCAN-IT
software.
Reactive oxygen species (ROS)
detection
ROS (O2·-) production was
detected using lucigenin-enhanced chemiluminescence as previously
described (8). During the first
minute of reperfusion, coronary effluent (1 ml) was collected and
500 µl was immediately transferred to a 1.5 ml vial, in
which 5 µl of 500 µM lucigenin (cat. no. M8010;
Sigma-Aldrich; Merck KGaA) was added. The final concentration was 5
µM. The vial was placed in a luminometer (Turner BioSystems,
Inc.) in order to measure chemiluminescence for 5 min in the dark.
A vial containing lucigenin was used to measure background
luminescence, and this background value was subtracted from each
sample value.The data of O2·- production was
expressed as relative light units.
NO detection
In order to assess the effects of BH4 on NOS3
coupling, at 1 min of reperfusion, 1 ml coronary effluent was
collected and NO concentrations were immediately determined using a
NO electrode (World Precision Instruments). The values were
normalized by heart wet weight and coronary flow rate as previously
described (8). The NO electrodes
were calibrated using NaNO2 (cat. no. 72586;
Sigma-Aldrich; Merck KGaA) and KI (cat. no. 746428)
+H2SO4 (cat. no. 339741) (both Sigma-Aldrich;
Merck KGaA) following the manufacturer's protocol, to quantify the
amount of NO produced.
Statistics analysis
The data are presented as the means ± standard error
of the mean. In order to examine the overall differences between
the groups, a two-way analysis of variance was used. The
Student-Newman-Keuls multiple comparison post hoc test was used to
differentiate within the groups. SPSS 19.0 (IBM Corp.) was used to
perform the statistical analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
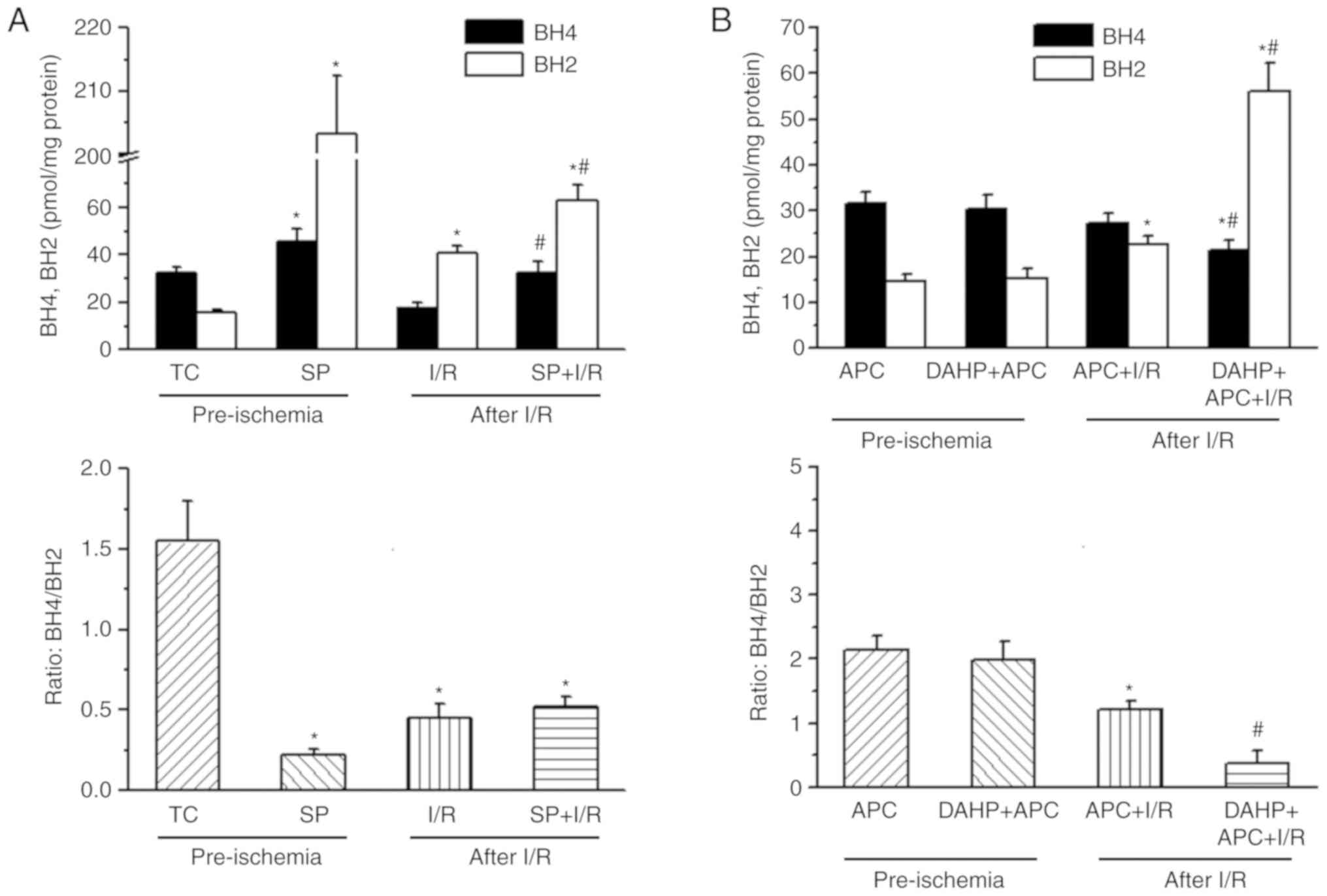
BH4 and BH2 levels in APC and I/R rat
hearts
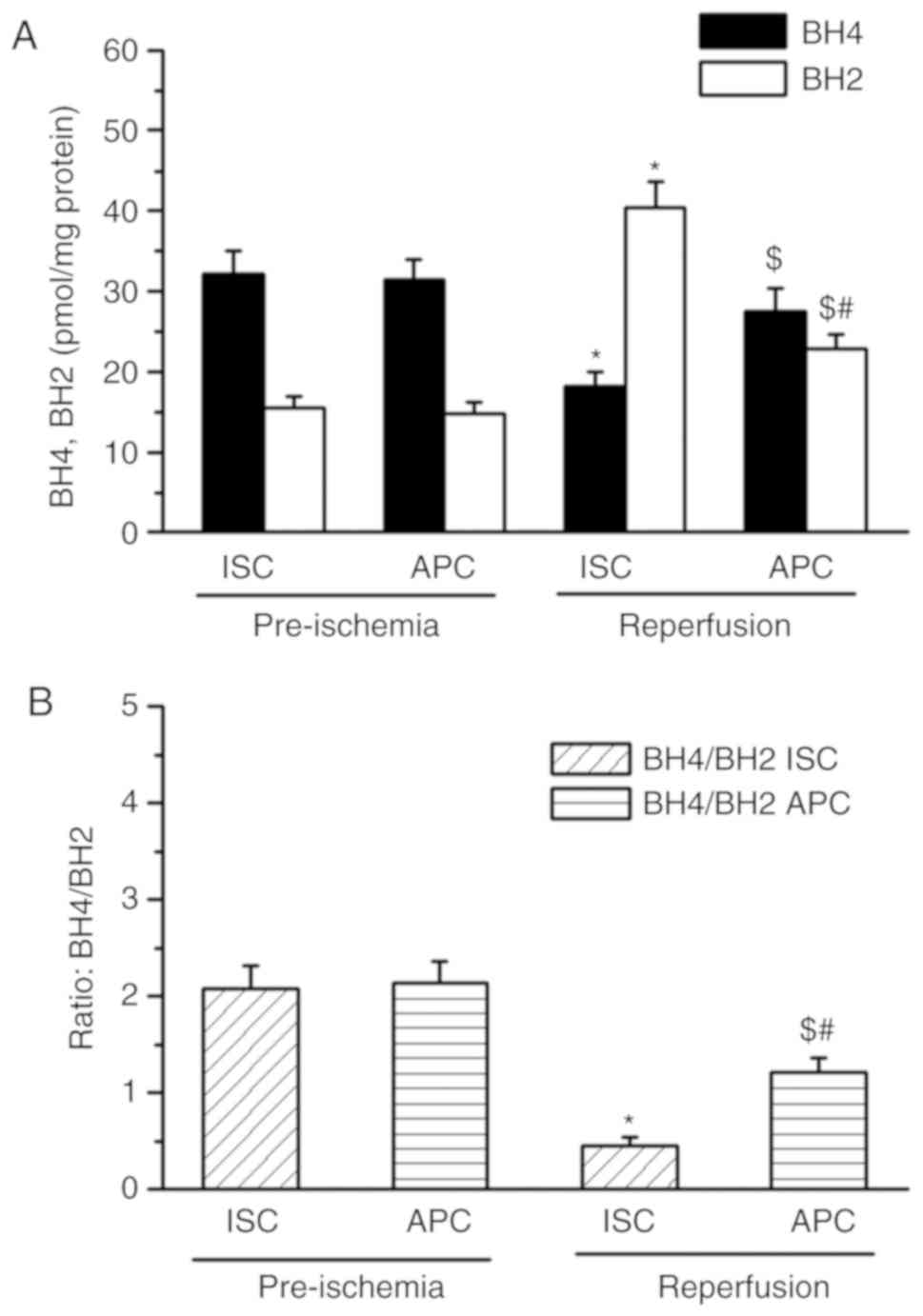
As presented in Fig.
2, prior to ischemia, BH4 and BH2 levels were similar in both
the I/R and APC + I/R groups (Fig.
2A). The BH2 level was significantly increased and the BH4
level decreased significantly in I/R hearts when compared with the
pre-ischemia baseline values. Following I/R, the BH4 levels
(27.4±1.9 vs. 18.1±1.9 pmols/mg protein) were increased and BH2
levels (22.7±1.8 vs. 40.3±3.2 pmols/mg protein) were decreased in
the APC + I/R hearts when compared with the I/R hearts (P<0.05;
n=10/group). The BH4:BH2 ratio in APC + I/R rat hearts was
increased 2-fold compared with the I/R hearts (1.2±0.15 vs.
0.45±0.09).
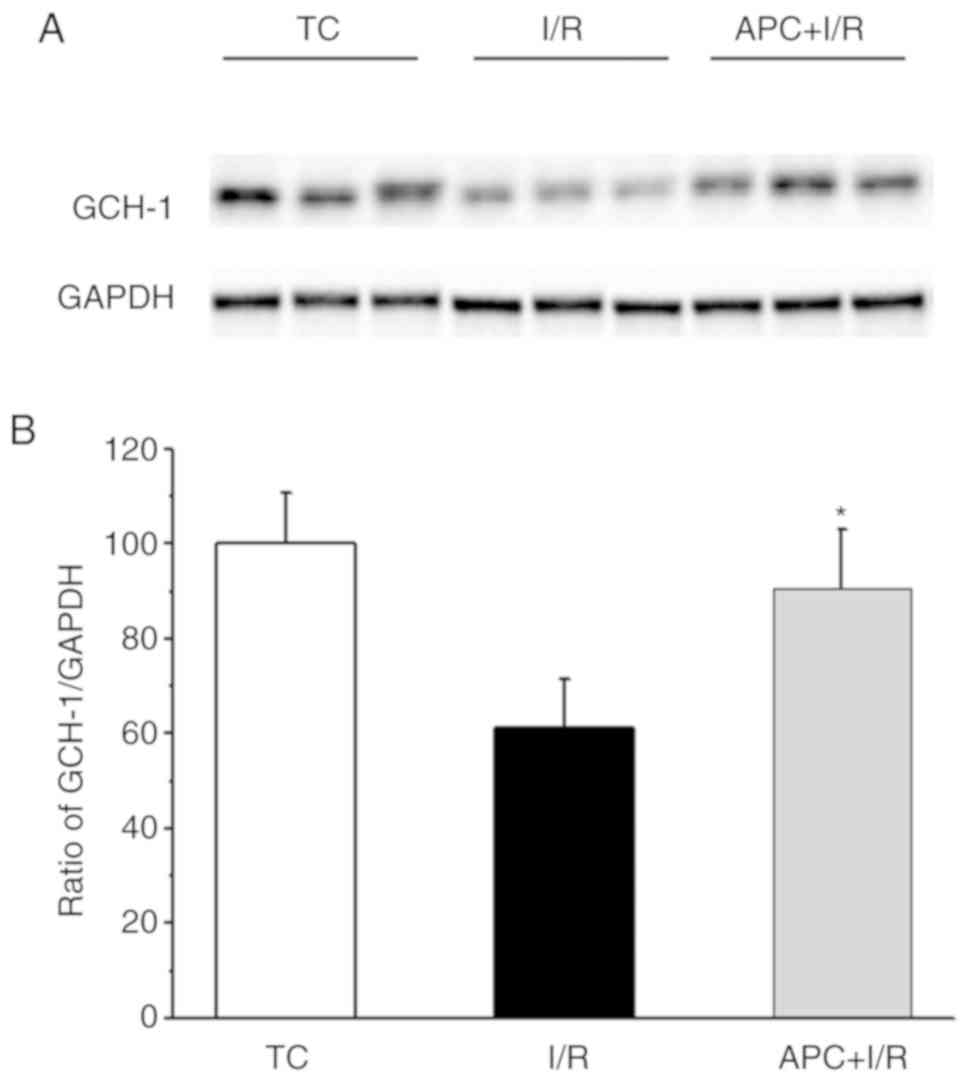
GCH-1 protein levels
Fig. 3 presents
the GCH-1 contents in TC, I/R and APC + I/R heart tissues. The
GCH-1 expression level in APC + I/R hearts was increased by almost
30% compared with that in I/R hearts after I/R (n=3/group).
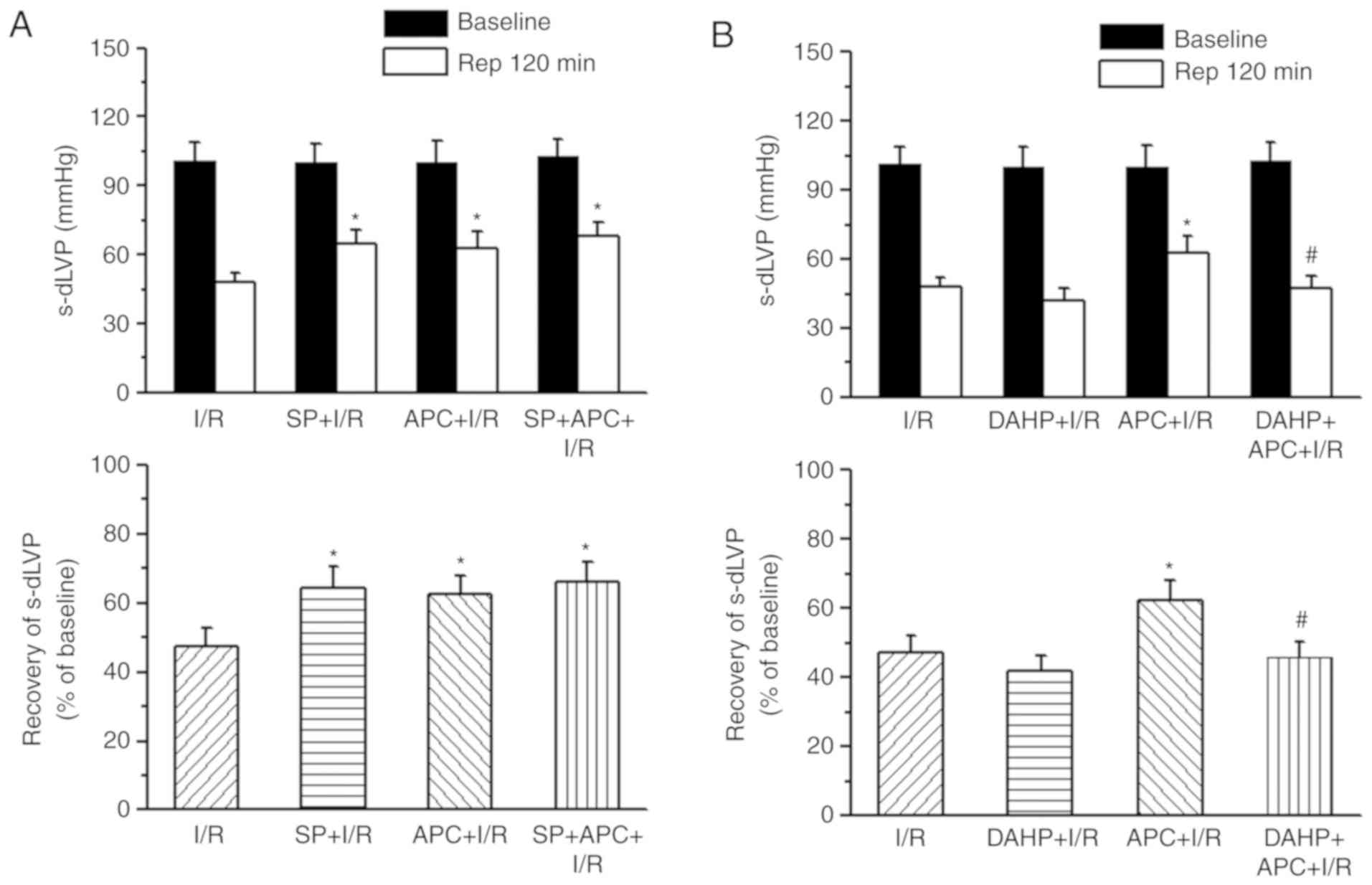
Supplementation of SP or DAHP on cardiac
function
In order to determine whether BH4 contributed to the
increased resistance to I/R injury in APC + I/R hearts compared
with I/R hearts, the present study perfused isolated hearts with
either a GCH-1 inhibitor (DAHP, 2.5 mM) or a BH4 donor (SP, 50
µM) for 40 min prior to ischemia with or without APC
treatment. Left ventricular developed pressure (LVDP) was measured
after 120 min reperfusion, using the percentage of pre-drug and
pre-ischemic values. The recovery rate of LVDP following I/R in the
APC + I/R hearts (62.7±7.6 mmHg and 62.6±5.2%) was significantly
increased compared with that in the I/R group (48.0±4.0 mmHg and
47.4±5.1%) (P<0.01). SP treatment significantly improved the
LVDP in I/R hearts (64.5±6.7 mmHg and 64.3±6.2%), but did not
increase the recovery of LVDP in APC + I/R hearts (68±6.1 mmHg and
66±5.9%; Fig. 4A). DAHP treatment
decreased the recovery of LVDP in APC hearts (41.7±5.1 mmHg and
45.7±4.6%) to the values observed in the I/R group hearts (Fig. 4B).
Supplementation of SP or DAHP on cardiac
BH4 and BH2 levels
In order to observe the changes in BH4 and BH2
levels following SP or DAHP treatment in I/R or APC + I/R hearts,
BH4 and BH2 levels were measured at 40 min after perfusion with SP
or DAHP (pre-ischemia) or at 120 min reperfusion. Following SP
treatment and prior to ischemia in the SP + I/R hearts, the levels
of BH4 (45.4±5.4 vs. 32.1±2.9 pmols/mg protein; P<0.05) and BH2
(203±19 vs. 15.5±1.4 pmol/mg protein) were significantly increased
when compared with the I/R hearts (P<0.001; Fig. 5A). At 120 min reperfusion, SP
treatment increased BH4 levels significantly in the SP+IR group
compared with the I/R group (32.4±4.5 vs. 18.1±1.9 pmol/mg protein;
P<0.05). In the APC + I/R rat hearts, DAHP treatment prior to
ischemia did not affect BH4 or BH2 levels (Fig. 5B). Following I/R, DAHP treatment
significantly decreased BH4 levels (21.4±2.2 vs. 27.4±2.2 pmol/mg
protein) and increased BH2 levels (56.1±6.2 vs. 22.7±1.8 pmol/mg
protein) in the DAHP+APC hearts compared with the APC + I/R hearts
(P<0.01). After 120 min reperfusion, the BH4:BH2 ratio was
decreased in DAHP+APC + I/R hearts compared with the APC + I/R
hearts (P<0.01).
Supplementation of SP or DAHP on the
association of HSP90 and NOS3
In order to observe the effects of SP or APC on the
interaction between HSP90 and NOS3 in I/R rat hearts, NOS3 was
immunoprecipitated from the homogenates of I/R hearts following
APC, SP or DAHP treatment and probed for NOS3 and HSP90 proteins
(Fig. 6). The results
demonstrated an increased association between HSP90 and NOS3
(>30%) in the SP+IR (IB hsp90-SR+I/R row) and APC + I/R hearts
(IB hsp90-APC + I/R row) compared with the I/R group. However, this
increase in association betweenHSP90 and NOS3 in the APC + I/R
hearts was inhibited by treatment with DAHP (IB hsp90-DAHP+APC +
I/R row).
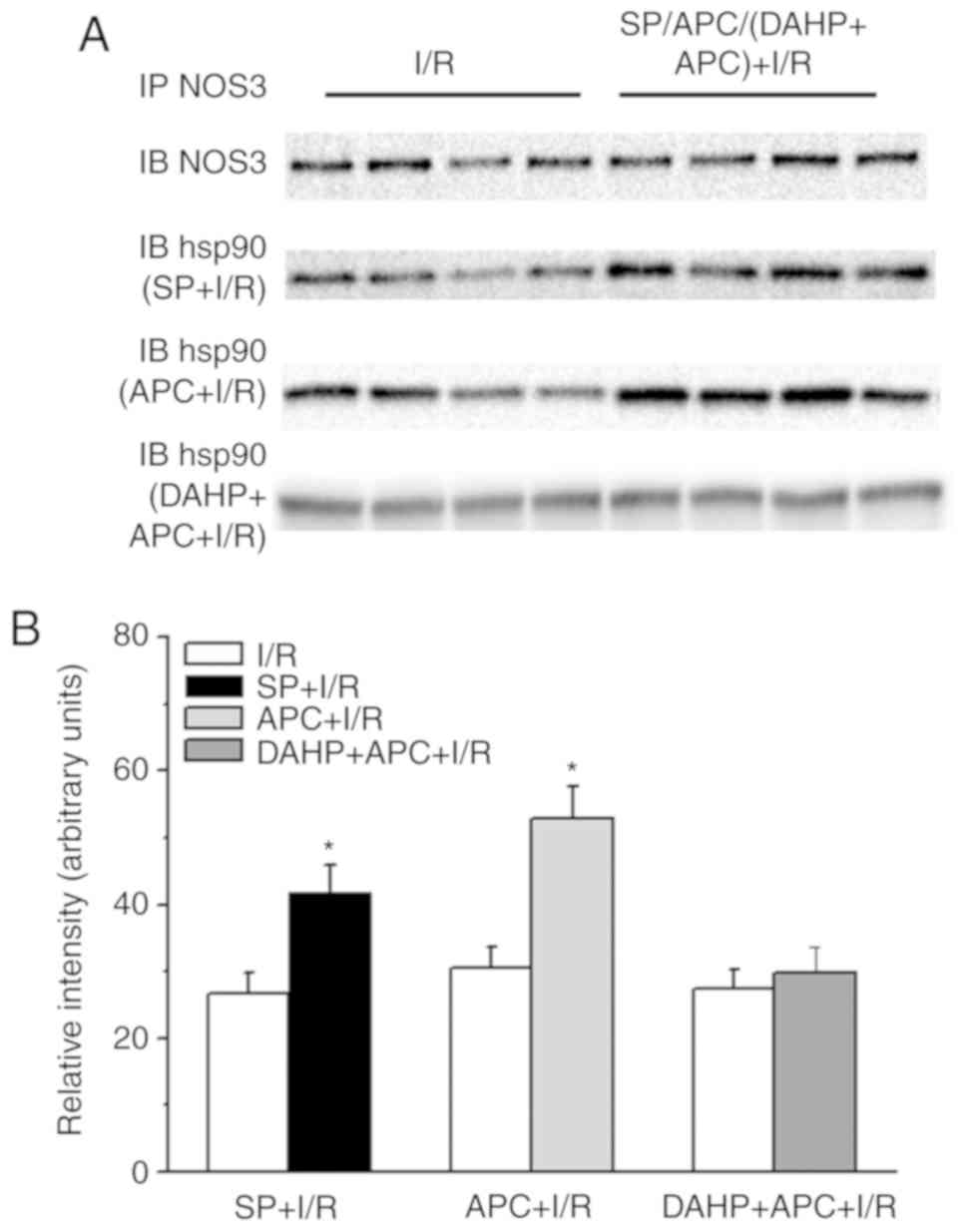 | Figure 6Western blot analysis of the
association between NOS3 and HSP90 in heart homogenates from rat
hearts. (A) The IB NOS3 row represents the I/R and APC + I/R heart
tissues. The IB hsp90 (SP + I/R) row represents the I/R and SP + IR
heart tissues. The IB hsp90 (APC + I/R) row represents the I/R and
APC + IR heart tissues. The IB hsp90 (DAHP + APC + I/R) row
represents the I/R and DAHP + APC + I/R heart tissues. The effects
of SP (50 µM), APC (sevoflurane at 3.5%) or DAHP (2.5 mM)
treatment on HSP90 association with NOS3. (B) The densitometric
analyses of the blot gel in part A. n=3/group. IP,
immunoprecipitation; IB, immunoblot. *P<0.05 vs. I/R
group. NOS3, nitric oxide synthase, endothelial; HSP90, heat shock
protein 90; I/R, ischemia/reperfusion; APC, anesthetic
preconditioning; SP, sepiapterin; DAHP,
2,4-diamino-6-hydroxypyrimidine. |
NO and O2·- levels
following I/R
The present study also measured •NO and
O2·- production in I/R hearts following SP
treatment or APC + I/R hearts following DAHP treatment (Fig. 7). The data revealed that the
lucigenin chemiluminescence signal intensity, which represents
O2·-(ROS) production, was significantly
decreased in the SP + I/R and APC + I/R rat hearts, but increased
in DAHP + APC + I/R rat hearts at the end of 120 min reperfusion.
Following treatment with SP, the NO levels in the SP + I/R group
was significantly increased compared with those in the I/R group,
whereas DAHP treatment significantly decreased the NO levels in
DAHP + APC + I/R hearts. APC treatment also significantly increased
NO production in APC + I/R hearts compared with the I/R group.
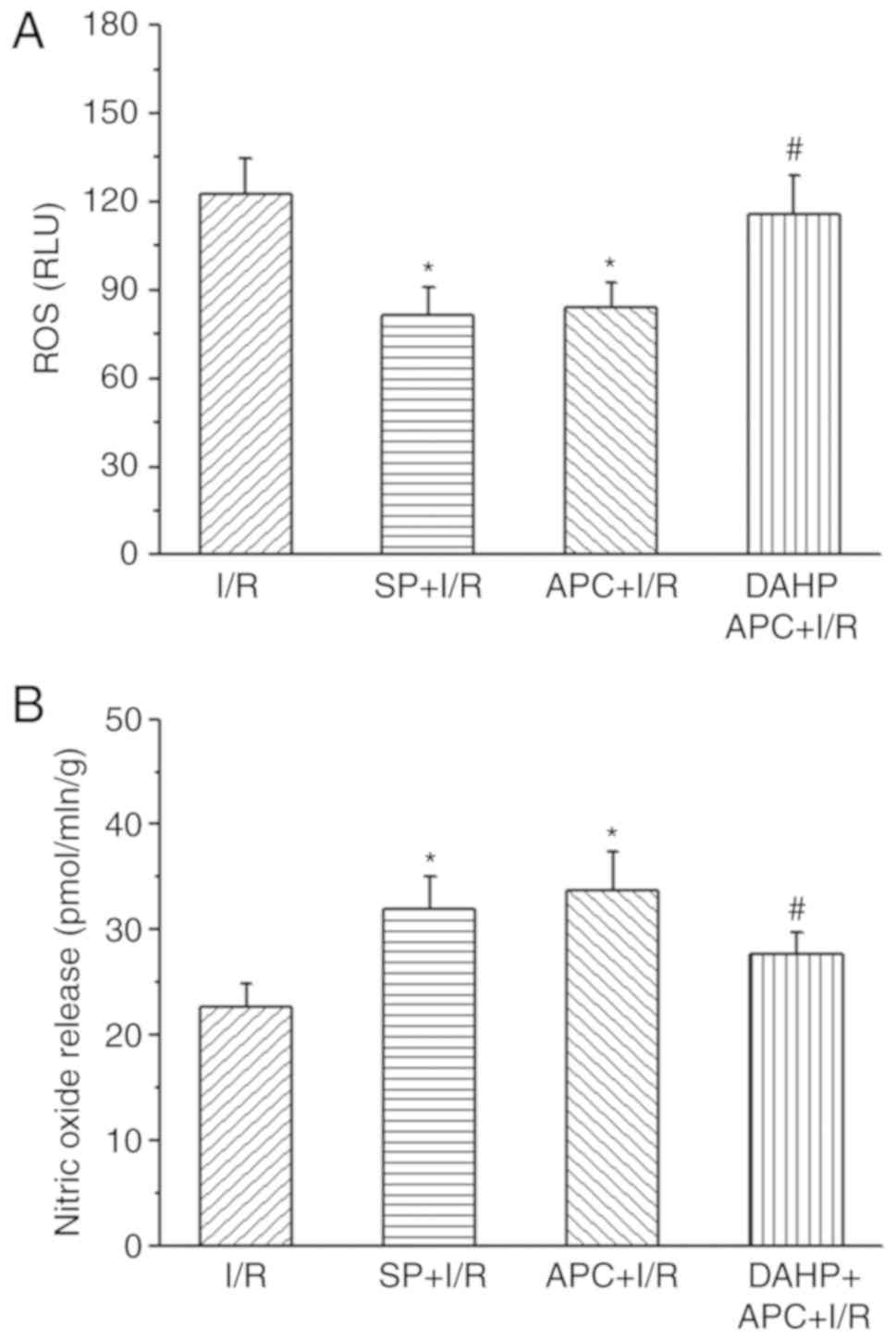 | Figure 7Lucigenin chemiluminescence signal
intensity. Superoxide and NO production from hearts of I/R rats
with/without SP (50 µM) treatment and APC + I/R with/without
DAHP (2.5 mM) treatment at 1 min reperfusion. (A) ROS production in
I/R, SP + I/R, APC + I/R and DAHP + APC + I/R groups. (B) NO levels
in the I/R, SP + I/R, APC + I/R and DAHP + APC + I/R groups. The
values are presented as mean ± standard error of the mean.
n=6/group. *P<0.05 vs. I/R group.
#P<0.05 vs. I/R + APC group. NO, nitric oxide; I/R,
ischemia/reperfusion; APC, anesthetic preconditioning; SP,
sepiapterin; DAHP, 2,4-diamino-6-hydroxypyrimidine. |
Discussion
The maintenance of physiological levels of NO
produced by NOS3 represents a key element for vascular function
(16,17). As a pteridine cofactor, BH4 is an
important regulator of NOS3 function, as BH4 is required to
maintain enzymatic coupling of L-arginine oxidation, to produce NO
(18,19). The present study assessed the role
of BH4 and the BH4 biosynthetic precursor, SP, in the mechanisms
underlying resistance to I/R injury in I/R hearts with or without
APC. The results revealed that: i) BH4 levels were significantly
increased and BH2 levels were significantly decreased in APC + I/R
hearts compared with the I/R group hearts. The BH4:BH2 ratio in the
APC-treated hearts was increased 2-fold compared with that in the
I/R group hearts. The recovery of cardiac function in the
APC-treated hearts was consistent with the alterations of BH4 and
BH2 levels, and the BH4:BH2 ratio; ii) SP supplementation increased
the resistance to I/R injury in the I/R group, and GCH-1 inhibition
decreased APC-induced cardiac function recovery; iii) SP
supplementation increased the association between HSP90 and NOS3;
while GCH-1 inhibition by DAHP decreased this association; iv) ROS
(O2·-) production decreased and NO levels
were increased significantly following SP treatment in the I/R
group, and ROS production increased and NO levels decreased
following DAHP treatment in APC + I/R hearts at the initial time of
reperfusion. The results of the present study indicate that BH4 and
the ongoing synthesis of BH4 participated in the susceptibility to
I/R injury in the APC + I/R and I/R group hearts. APC-induced
resistance to I/R injury was mediated by the NOS3 cofactor BH4 and
the elevation of HSP90-NOS3 association.
In I/R-injured hearts, lowering BH4 levels may be an
important cellular defect involving endothelial and cardiomyocyte
dysfunction (6,20). The role of BH4 in NOS activity is
important in cardioprotection. The decrease in BH4 levels not only
prevents NOS3 from generating NO, but also causes NOS3
'uncoupling', resulting in NOS3 generating
O2·- instead of NO (21). BH4 deficiency due to decreased BH4
biosynthesis or BH4 oxidation to BH2 (less catalytic activity) is
known to be one of the causes of I/R injury (8). The results of the present study are
consistent with these data, in that BH4 level and the BH4:BH2 ratio
in APC-treated hearts were significantly increased compared with
that in the I/R group, confirming that BH4 serves a crucial role in
I/R injury. The importance of the BH4:BH2 ratio in determining
whether BH4 and BH2 bind to NOS3 has been revealed in recent
studies. As the affinity of BH4 and BH2 to NOS3 is equal, BH4 in
NOS3 can be rapidly and efficiently replaced by BH2, resulting in
the uncoupling of NOS3 activity, and decreased NO synthesis
(2,22). APC treatment demonstrated
increased BH4 and decreased BH2 levels following I/R. Therefore, in
addition to decreased BH4 levels, the BH4:BH2 ratio was
significantly decreased in I/R group hearts compared with that in
the APC + I/R hearts, which may also lead to NOS3 uncoupling, an
increase in O2·- production and decreased
cardiac function recovery.
Increasing the availability of BH4 by modulating
GCH-1 has been recognized as a novel strategy for protecting the
heart during post-infarction remodeling, dilated myopathic
remodeling and cardiac hypertrophy (23). Certain studies have demonstrated
that the treatment of exogenous BH4 or BH4 precursor SP may promote
NO production and NOS-dependent coronary flow recovery following
isch-emia (6,24). SP supplementation enhanced the
resistance to I/R injury in the I/R group. Notably, the results of
the present study demonstrated that SP did not alter the
APC-induced cardioprotection observed, suggesting that the cardiac
protection induced by APC had reached its maximum effect.
Resistance to I/R injury by APC was abrogated to levels that were
not significantly different from the I/R group when GCH-1 was
inhibited with DAHP. In addition, the GCH-1 inhibitor DAHP did not
affect the functional recovery of the I/R group, but SP
supplementation increased the functional recovery to a level
comparable with that of APC-treated hearts. This association
provides evidence that BH4 levels are associated with APC-induced
cardioprotection.
The present study hypothesized that BH4 serves a
crucial role in NOS3 activity, NO generation, potentially with
O2·- formation as suggested by the lucigenin
chemiluminescence assay, and vascular reactivity during I/R. The
increased lucigenin chemiluminescence signal intensity,
representing the level of O2·- released, or
net emission, from I/R rat hearts was significantly attenuated by
SP and APC, but increased in the DAHP +APC +I/R group. Furthermore,
there was a greater lucigenin chemiluminescence signal intensity
from I/R alone hearts. NO release was increased in I/R alone hearts
through SP supplementation, while DAHP significantly decreased NO
production in APC + I/R hearts. These data confirmed that BH4
levels were associated with NOS-dependent NO production, and
decreased O2·- generation.
A previous study has demonstrated that APC enhanced
GTPCH-1 and NOS3 expression levels, and promoted the production of
NO in the myocardium following reperfusion (11). In C57BL/6 mice hearts, the rate of
LV pressure rise, BH4 level, Ca2+ handling proteins and
SR Ca2+ release were decreased following I/R, which was
associated with GCH1 degradation (25). In the coronary arteries of rats
with heart failure, both NOS3 and GCH1 protein expression levels
increased concomitantly with the decrease in ROS generation,
increase of NO bioavailability and NOS3 coupling (26). A high level of GCH-1 expression in
APC + I/R rats was also observed in the present study, indicating
that increased GCH-1 expression is involved in volatile
anesthetic-induced cardio-protection.
Previous studies have demonstrated that an increased
association between HSP90 and NOS3 contributed to cardio-protection
against I/R by increasing NO generation and decreasing
O2·- production (7,27).
The results of the present study also demonstrated that SP
supplementation increased the association between HSP90 and NOS3,
while DAHP supplementation decreased this association in APC + I/R
hearts, consistent with the results of a previous study (8). These data demonstrated that BH4
improved NOS3 activity and function, which is an important
mechanism underlying APC-induced cardioprotection.
In summary, the present study demonstrated that BH4
and GCH-1 differentially modulated APC-induced cardioprotection
against I/R injury. BH4 biosynthesis, regulated by GCH-1, served a
key role in the enhancement of NO generation during APC, and NO, in
turn, conferred a cardioprotective effect. Furthermore, the
differences in the BH4:BH2 ratio, which were associated with NOS3
coupling state and the increase in heart BH4 oxidation, conferred a
cardioprotective effect in APC-treated hearts. These results
indicated that APC may mediate resistance to I/R injury by
enhancement of BH4 level and the association between HSP90 and
NOS3. In addition, the combination of BH4 and HSP90 that
contributes to the resistance to I/R injury may be dependent on
increasing NOS3-coupled activity and function. Overall, the
pharmacological targeting of the BH4-HSP90-NOS3 axis may represent
a novel approach to alleviating myocardial I/R injury.
Funding
The present study was supported by the Suzhou
Science and Technology Development Plan (grant nos. SS201756 and
SS201613); the Suzhou New District Science and Technology Project
(grant no. 2017Z004); and the National Science and Technology
Development Plan (grant no. NSFC 81703501).
Availability of data and materials
All data generated or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW, JA and AC were involved in the experimental
design, data collection, data analysis and manuscript writing. SQ,
LH, JS and TC performed the experiments. CW, JA and AC provided
critical comments throughout the process of the present study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Nanjing University
Patient consent for publication
Not applicable.
Competing interests
The authors have declared that they have no
competing interests.
Acknowledgments
Not applicable.
References
|
1
|
Bredt DS and Snyder SH: Nitric oxide: A
physiologic messenger molecule. Annu Rev Biochem. 63:175–195. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ziolo MT, Kohr MJ and Wang H: Nitric oxide
signaling and the regulation of myocardial function. J Mol Cell
Cardiol. 45:625–632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gaynullina DK, Schubert R and Tarasova OS:
Changes in endothelial nitric oxide production in systemic vessels
during early ontogenesis-A key mechanism for the perinatal
adaptation of the circulatory system. Int J Mol Sci. 20:pii: E1421.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuyun MF, Ng LL and Ng GA: Endothelial
dysfunction, endothelial nitric oxide bioavailability,
tetrahydrobiopterin, and 5-methyltetrahydrofolate in cardiovascular
disease. Where are we with therapy? Microvasc Res. 119:7–12.
2018.
|
|
5
|
Xie L, Hu D, Qin H, Zhang W, Zhang S, Feng
Y, Yao H, Xiao Y, Yao K and Huang X: In vivo gum arabic-coated
tetrahydrobi-opterin protects against myocardial ischemia
reperfusion injury by preserving eNOS coupling. Life Sci.
219:294–302. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dumitrescu C, Biondi R, Xia Y, Cardounel
AJ, Druhan LJ, Ambrosio G and Zweier JL: Myocardial ischemia
results in tetrahydrobiopterin (BH4) oxidation with impaired
endothelial function ameliorated by BH4. Proc Natl Acad Sci USA.
104:15081–15086. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi Y, Hutchins W, Ogawa H, Chang CC,
Pritchard KA Jr, Zhang C, Khampang P, Lazar J, Jacob HJ, Rafiee P
and Baker JE: Increased resistance to myocardial ischemia in the
Brown Norway vs Dahl S rat: Role of nitric oxide synthase and
Hsp90. J Mol Cell Cardiol. 38:625–635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
An J, Du J, Wei N, Xu H, Pritchard KA Jr
and Shi Y: Role of tetrahydrobiopterin in resistance to myocardial
ischemia in Brown Norway and Dahl S rats. Am J Physiol Heart Circ
Physiol. 297:H1783–H1791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie L, Talukder MA, Sun J, Varadharaj S
and Zweier JL: Liposomal tetrahydrobiopterin preserves eNOS
coupling in the post-ischemic heart conferring in vivo
cardioprotection. J Mol Cell Cardiol. 86:14–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawahara K, Takase M and Yamauchi Y:
Increased vulnerability to ischemia/reperfusion-induced ventricular
tachyarrhythmias by pre-ischemic inhibition of nitric oxide
synthase in isolated rat hearts. Cardiovasc Pathol. 12:49–56. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baotic I, Weihrauch D, Procknow J,
Vasquez-Vivar J, Ge ZD, Sudhakaran S, Warltier DC and Kersten JR:
Isoflurane favorably modulates guanosine triphosphate
cyclohydrolase-1 and endo-thelial nitric oxide synthase during
myocardial ischemia and reperfusion injury in rats. Anesthesiology.
123:582–589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guerrero-Orriach JL, Escalona Belmonte JJ,
Ramirez Fernandez A, Ramirez Aliaga M, Rubio Navarro M and Cruz
Manas J: Cardioprotection with halogenated gases: How does it
occur? Drug Des Devel Ther. 11:837–849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Novalija E, Varadarajan SG, Camara AK, An
J, Chen Q, Riess ML, Hogg N and Stowe DF: Anesthetic
preconditioning: Triggering role of reactive oxygen and nitrogen
species in isolated hearts. Am J Physiol Heart Circ Physiol.
283:H44–H52. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amour J, Le Manach YL, Borel M, Lenfant F,
Nicolas-Robin A, Carillion A, Ripart J, Riou B and Langeron O:
Comparison of single-use and reusable metal laryngoscope blades for
orotracheal intubation during rapid sequence induction of
anesthesia: A multicenter cluster randomized study. Anesthesiology.
112:325–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stowe DF, Gadicherla AK, Zhou Y, Aldakkak
M, Cheng Q, Kwok WM, Jiang MT, Heisner JS, Yang M and Camara AK:
Protection against cardiac injury by small Ca(2+)-sensitive K(+)
channels identified in guinea pig cardiac inner mitochondrial
membrane. Biochim Biophys Acta. 1828:427–442. 2013. View Article : Google Scholar
|
|
16
|
Chatterjee A, Black SM and Catravas JD:
Endothelial nitric oxide (NO) and its pathophysiologic regulation.
Vascul Pharmacol. 49:134–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomas SR, Chen K and Keaney JF Jr:
Oxidative stress and endothelial nitric oxide bioactivity. Antioxid
Redox Signal. 5:181–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vasquez-Vivar J, Kalyanaraman B, Martasek
P, Hogg N, Masters BS, Karoui H, Tordo P and Pritchard KA Jr:
Superoxide generation by endothelial nitric oxide synthase: The
influence of cofactors. Proc Natl Acad Sci USA. 95:9220–9225. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hemmens B and Mayer B: Enzymology of
nitric oxide synthases. Methods Mol Biol. 100:1–32. 1998.
|
|
20
|
Leucker TM, Ge ZD, Procknow J, Liu Y, Shi
Y, Bienengraeber M, Warltier DC and Kersten JR: Impairment of
endothelial-myocardial interaction increases the susceptibility of
cardiomyocytes to ischemia/reperfusion injury. PLoS One.
8:e700882013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vasquez-Vivar J, Kalyanaraman B and
Martasek P: The role of tetrahydrobiopterin in superoxide
generation from eNOS: Enzymology and physiological implications.
Free Radic Res. 37:121–127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gorren AC, List BM, Schrammel A, Pitters
E, Hemmens B, Werner ER, Schmidt K and Mayer B:
Tetrahydrobiopterin-free neuronal nitric oxide synthase: Evidence
for two identical highly anticooperative pteridine binding sites.
Biochemistry. 35:16735–16745. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moens AL and Kass DA: Tetrahydrobiopterin
and cardiovascular disease. Arterioscler Thromb Vasc Biol.
26:2439–2444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tiefenbacher CP, Lee CH, Kapitza J, Dietz
V and Niroomand F: Sepiapterin reduces postischemic injury in the
rat heart. Pflugers Arch. 447:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Baumgardt SL, Fang J, Shi Y, Qiao
S, Bosnjak ZJ, Vasquez-Vivar J, Xia Z, Warltier DC, Kersten JR and
Ge ZD: Transgenic overexpression of GTP cyclohydrolase 1 in
cardio-myocytes ameliorates post-infarction cardiac remodeling. Sci
Rep. 7:30932017. View Article : Google Scholar
|
|
26
|
Couto GK, Paula SM, Gomes-Santos IL,
Negrao CE and Rossoni LV: Exercise training induces eNOS coupling
and restores relaxation in coronary arteries of heart failure rats.
Am J Physiol Heart Circ Physiol. 314:H878–H887. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vladic N, Ge ZD, Leucker T, Brzezinska AK,
Du JH, Shi Y, Warltier DC, Pratt PF Jr and Kersten JR: Decreased
tetrahy-drobiopterin and disrupted association of Hsp90 with eNOS
by hyperglycemia impair myocardial ischemic preconditioning. Am J
Physiol Heart Circ Physiol. 301:H2130–H2139. 2011. View Article : Google Scholar : PubMed/NCBI
|