Introduction
The human umbilical cord is often considered to be a
substitute source of stem or progenitor cells (1). Acquisition of Human umbilical cord
blood-derived mesenchymal stem cells (hUCB-MSCs) is more efficient
compared with bone marrow-derived MSCs as these cells are acquired
by non-invasive methods (2).
Transplantation of hUCB-MSCs is a well-established alternative
treatment for a wide spectrum of diseases, including visual
impairment, glioma, ischemic brain damage, liver disease and
cartilage degeneration (3-7).
Alopecia is the hereditary thinning of hair induced
by androgens in men and women (8). In alopecia, the size of the hair
follicles is reduced, and the hair shaft thins and ultimately
disappears (9). The anagen or
growth phase gradually shortens, revealing smaller follicular
tissue and irregularly shaped hair shafts covering the scalp
(10). Based on morphological
changes observed during the degeneration of the hair follicle, the
regression of this mini-organ is predominantly caused by
coordinated keratinocyte apoptosis (9).
Glucocorticoid (GC) levels are regulated by the
adrenocorticotropic hormone, which is regulated by the
hypothalamic-pituitary-adrenal (HPA) axis (11). A previous study has suggested that
in keratinocytes, the glucocorticoid receptor (GR) is associated
with the expression of a variety of genes, including those involved
in apoptosis, cell adhesion, lipid metabolism and formation of the
stratum corneum (12). The skin
and hair are targets of HPA axis activity and also produce GCs
through their own neuroendocrine systems; this nominal 'peripheral'
HPA axis is associated with hair loss (13,14). Previous studies demonstrated that
corticotropin-releasing hormone significantly inhibited hair shaft
elongation in vitro, induced hair regression, inhibited hair
matrix keratinocyte proliferation and upregulated apoptosis of root
sheath cells ex vivo (15,16).
The present study aimed to demonstrate the
preventive effects exerted by hUCB-MSCs on hair loss and the
mechanisms that underlie alopecia prevention by investigating the
effect of hUCB-MSCs on dexamethasone (Dex)-induced hair loss in
mouse catagen induction models. The effects of hUCB-MSCs on human
dermal papilla cells (hDPCs) and HaCaT cells under Dex-induced
stress were also investigated to elucidate the molecular mechanisms
underlying hair follicle protection.
Materials and methods
Animals
All animal procedures were approved by the
Institutional Animal Care and Use Committee (IACUC) of Chung-Ang
University (2018-03-20) and performed in accordance with the Guide
for the Care and Use of Laboratory Animals published by the US
National Institutes of Health (17). Female C57/BL6 mice (6 weeks old)
were obtained from Saeron Bio, Inc. and allowed to adapt for one
week with ad libitum feeding. The animals were randomly
divided into 3 groups (n=6) as follows: i) Normal control group
(NoC), saline injection; ii) 0.1% Dex only treatment group (Dex),
0.1% Dex + saline injection; and iii) hUCB-MSC treatment group (Dex
+ hUCB-MSCs), 0.1% Dex + hUCB-MSCs (8 sites/head, 100
µl/site). The mice were anesthetized with Zoletil 50 (50
mg/kg) and xylazine (10 mg/kg) by IP injection as described
previously (18,19). Hair cycle synchronization of mouse
models was induced by taping of the dorsal skin during the telogen
phase of the hair cycle, as described previously (20). On the final day of depilation, the
percentage of dorsal skin color change on the back of each mouse
was evaluated by comparing the change in skin color progression,
and a hair cycle score (HCS) was calculated using the formula
described by Müller-Röver et al (21).
Histological analysis
Histology was analyzed by hematoxylin and eosin
(H&E) staining. Immunohistochemistry (IHC) and
immunofluorescence (IF) were performed on 4% paraformaldehyde-fixed
and paraffin-embedded sections. The tissues were blocked 3% BSA and
5% normal goat in Tris-buffered saline with 0.1% Tween 20 (TBS-T,
pH 7.4) for 2 h at room temperature. For IHC, antibodies against
β-catenin (cat. no. 610154; 1:500; BD Biosciences) and Dickkopf WNT
signaling pathway inhibitor 1 (DKK-1; cat. no. ab109416; 1:500;
Abcam) were used. The sections were washed with Tris-buffered
saline containing 0.1% Tween 20 for 10 min three times, and the
primary antibodies were detected with biotinylated Goat Anti-Mouse
IgG Antibody (cat. no. BP-9200; 1:1,000; Vector Laboratories) and
biotinylated Goat Anti-Rabbit IgG Antibody (cat. no. BP-9500;
1:1,000; Vector Laboratories) for 4 h at room temperature plus a
streptavidin-peroxidase complex (Vector Laboratories, Inc.) and
brown FAST DAB (Thermo Fisher Scientific, Inc.) staining. Slides
were observed by light microscopy (DM750; Leica Microsystems GmbH)
in five consecutive fields at ×100 magnification. Antibodies
against microtubule-associated protein 1 light chain 3 β (LC3BI/II;
cat. no. PM036; 1:500; MBL International Co.) and p62 (cat. no.
PM045; 1:500, MBL International Co.) were used for IF. Samples were
mounted on slides, and images were acquired using a confocal
microscope (LSM700; Zeiss AG) in five consecutive fields at
magnification, ×200.
Preparation of hUCB-MSCs
This study was approved by the Institutional Review
Board of MEDIPOST Co., Ltd. Collection of hUCB and isolation and
culture of hUCB-MSCs were performed as previously described
(22). Mononuclear cells were
isolated from hUCBs by centrifugation on a Ficoll-Hypaque gradient
(density, 1.077 g/cm3; Sigma-Aldrich; Merck KGaA). Cells
were then seeded in culture flasks at 5×105
cells/cm2. Following the formation of spindle-shaped
cell colonies, cells were reseeded for expansion. hUCB-MSCs were
cultured in αMEM medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and gentamycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified atmosphere containing 5% CO2 and 3%
O2. Cells were passaged when they reached 80% confluency
and used either for experiments or redistribution to new culture
plates. In all experiments, hUCB-MSCs were used at passage 6.
Measurement of apoptosis by TUNEL
assay
A TUNEL assay was performed on mouse dorsal skin
tissue using the DeadEnd fluorometric in situ cell death
detection kit (Promega Corporation) according to the manufacturer's
protocol in order to analyze DNA fragmentation, which is indicative
of apoptosis. DAPI was used to visualize the nuclei. Images were
acquired using a confocal microscope (LSM700; Zeiss AG).
Cell viability assay
hDPCs were obtained from CEFO Co., Ltd. (cat. no.
CB-HDP-001) and cultured for 6 passages prior to use in
experiments. The experiments were conducted using short incubation
periods as long in vitro culture resulted in hDPCs losing
their original characteristics and functions. HaCaT cells, which
are immortalized human keratinocytes, were obtained from Addexbio
Technologies (cat. no. T0020001). The two cell lines were cultured
in Dulbecco's Modified Eagle's Medium (DMEM; Welgene, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% Penicillin-Streptomycin (Gibco; Thermo Fisher Scientific,
Inc.). During the culture, 5% CO2 was continuously
supplied while maintaining the temperature at 37°C. Transwell
plates (0.4 µm pore; Corning, Inc.) were used for
co-culture, with hDPCs or HaCaT cells seeded on the 6-well plate
and hUCB-MSCs seeded in the 6-well inserts. Following 48 h
co-culture, the inserts were removed in accordance with the
experimental schedule to isolate hUCB-MSCs. To determine cell
viability, hDPCs or HaCaT cells alone seeded in 6-well plates at a
density of 2×105 cells/ml were observed. Culture media
were discarded, and cells were counted using the Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.) according to
the manufacturer's instructions. Absorbance was measured at 450 nm
using a SpectraMax i3x Multi-Mode Detection Platform (Molecular
Devices, LLC).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from hDPCs and HaCaT cells
using FavorPrep™ Tri-RNA reagent (Favorgen Biotech Corp.).
First-strand cDNA synthesis was performed on the total RNA template
using PrimeScript™ RT master mix (Takara Bio, Inc.). The resulting
cDNA was subjected to qPCR using TOPreal™ qPCR 2X PreMIX containing
SYBR® (Enzynomics, Inc.) with a CFX-96 Touch™ Real-Time
PCR detection system (Bio-Rad Laboratories, Inc.). The
thermocycling conditions were as follows: 10 min at 95°C, followed
by 40 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 30
sec. Expression data were calculated from the cycle threshold (Cq)
value using the 2−ΔΔCq method (23). Oligonucleotides used for qPCR were
as follows: Human autophagy-related protein (ATG) 6 forward,
5′-AACCTCAGCCGAAGACTGAA-3′ and reverse, 5′-CCTCTAGTGCCAGCTCCTTT-3′;
human ATG8 forward, 5′-CGCACCTTCGAACAAAGAGT-3′ and reverse,
5′-GACCATGCTGTGTCCGTTC-3′; human cathepsin D (CTSD) forward,
5′-CAAGTTCGATGGCATCCTGG-3′ and reverse, 5′-CGGGTGACATTCAGGTAGGA-3′;
human lysosomal-associated membrane protein 1 (LAMP1)
forward, 5′-CTTTCAAGGTGGAAGGTGGC-3′ and reverse,
5′-GATAGTCTGGTAGCCTGCGT-3′; human peroxi-some
proliferator-activated receptor gamma coactivator 1α
(PGC-1α) forward, 5′-AGCGCCGTGTGATTTATGTC-3′ and reverse,
5′-TGCGTCCACAAAAGTACAGC-3′; human DKK-1 forward,
5′-TTCCGAGGAGAAATTGAGGA-3′ and reverse, 5′-CCTGAGGCACAGTCTGATGA-3′;
human alkaline phosphatase liver/bone/kidney isozyme (ALPL)
forward, 5′-CCTCCTCGGAAGACACTCTG-3′ and reverse,
5′-AGACTGCGCCTGGTAGTTGT-3′; human β-catenin (CTNNB1)
forward, 5′-GCCGGCTATTGTAGAAGCTG-3′ and reverse,
5′-GAGTCCCAAGGAGACCTTCC-3′.
Vascular endothelial growth factor (VEGF)
concentration
hDPC culture media was collected following each
experiment, and the VEGF levels in the supernatant were quantified
using a Human VEGF Quantikine ELISA Kit (cat. no. DVE00; R&D
Systems, Inc.) according to the manufacturer's protocol. Absorbance
was measured at 450 nm using a SpectraMax i3x Multi-Mode Detection
Platform.
Propidium iodide (PI) fluorescence
images
Following stimulation under each condition, HaCaT
cells were washed with PBS and stained with 10 µg/ml PI for
5 min at 37°C, and fluorescent images were observed under a DP70
fluorescence microscope with DP Controller software (DP-BSV ver.
03.03; Olympus Optical Co., Ltd.).
Western blot analysis
Mouse skin tissues or treated cells were harvested
and washed twice with PBS at 4°C. Protein was collected by lysing
the cells in 200 µl ice-cold PRO-PREP™ buffer (iNtRON
Biotechnology, Inc.). The protein concentration was evaluated using
a Bicinchoninic Acid Protein Assay kit (Thermo Fisher Scientific,
Inc.). Aliquots of protein (30 µg/lane) were separated by
10-15% SDS-PAGE and transferred onto PVDF membranes. The membranes
were blocked 5% non-fat milk in TBS-T for 2 h at room temperature
and incubated with primary antibodies against poly (ADP-ribose)
polymerase (PARP; cat. no. 9532S; 1:1,000; Cell Signaling
Technology, Inc.), Bcl-2 (cat. no. SC-492; 1:1,000; Santa Cruz
Biotechnology, Inc.), Bax (cat. no. SC-526; 1:1,000; Santa Cruz
Biotechnology, Inc.), pro-caspase-3 (cat. no. ab32499; 1:1,000;
Abcam), cleaved-caspase-3 (cat. no. ab2302; 1:1,000; Abcam),
pro-caspase-9 (cat. no. ab138412; 1:1,000, Abcam),
cleaved-caspase-9 (cat. no. ab2302; 1:1,000; Abcam), β-catenin
(cat. no. ab16051; 1:1,000; Abcam), DKK-1 (cat. no. ab61034;
1:1,000; Abcam), LC3BI/II (cat. no. PM036; 1:1,000; MBL
International Co.), p62 (cat. no. PM045; 1:1,000; MBL International
Co.), Beclin1 (cat. no. PD017; 1:1,000; MBL International Co.),
LAMP1 (cat. no. sc-20011; 1:1,000; Santa Cruz Biotechnology, Inc.),
transcription factor C/EBP homologous protein (CHOP; cat. no.
sc-7351; 1:1,000; Santa Cruz Biotechnology, Inc.), protein kinase R
(PKR)-like endoplasmic reticulum kinase (PERK; cat. no. sc-377400;
1:1,000; Santa Cruz Biotechnology, Inc.) and β-actin (cat. no.
sc-47778; 1:1,000, Santa Cruz Biotechnology, Inc.) at 4°C
overnight. Goat anti-rabbit (cat. no. BA-1000; Vector Laboratories,
Inc.) and anti-mouse (cat. no. WB-2000; Vector Laboratories, Inc.)
were used as secondary antibodies (1:10,000) and incubated for 2 h
at room temperature. The blots were analyzed by densitometry using
ImageJ 1.44 software (National Institutes of Health).
Determination of mitochondrial mass and
mitochondrial membrane potential (MMP)
Mitochondrial mass (MitoTracker Green FM; cat. no.
M7514; 100 nM; excitation/emission, 490/525 nm; Invitrogen; Thermo
Fisher Scientific, Inc.) and MMP (Tetramethylrhodamine, Ethyl
Ester, Perchlorate; cat. no. T669; 200 nM excitation/emission,
549/582 nm; Invitrogen Thermo Fisher Scientific, Inc.) were
measured in HaCaT cells based on the fluorescence levels following
staining with selective dyes at 37°C for 30 min. HaCaT cells were
washed once with PBS and promptly evaluated using a SpectraMax i3x
Multi-Mode Detection Platform.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD) of three independent experiments. Statistical analyses were
performed using SPSS 17.0 (SPSS, Inc.). Differences between two
groups were evaluated using a paired t-test. For multiple
comparisons, one-way ANOVA followed by Tukey's multiple comparisons
test was used. P<0.05 was considered to indicate a statistically
significant difference.
Results
hUCB-MSCs prevent hair regression induced
by topical treatment with Dex
The schedule of the animal experiments are presented
Fig. 1A. To determine whether
hUCB-MSCs extended the anagen phase of the hair cycle, the growth
of hair shafts in all groups was visually observed on day 16 after
depilation (Fig. 1B, upper row).
Hair cycle stage was determined by measuring the skin color status,
and the results revealed diffuse darkening of the dorsal skin 16
days after removing hair (Fig.
1B, lower row). However, no significant differences were
observed among the Con, Dex and Dex + hUCB-MSCs groups (Fig. 1B and C).
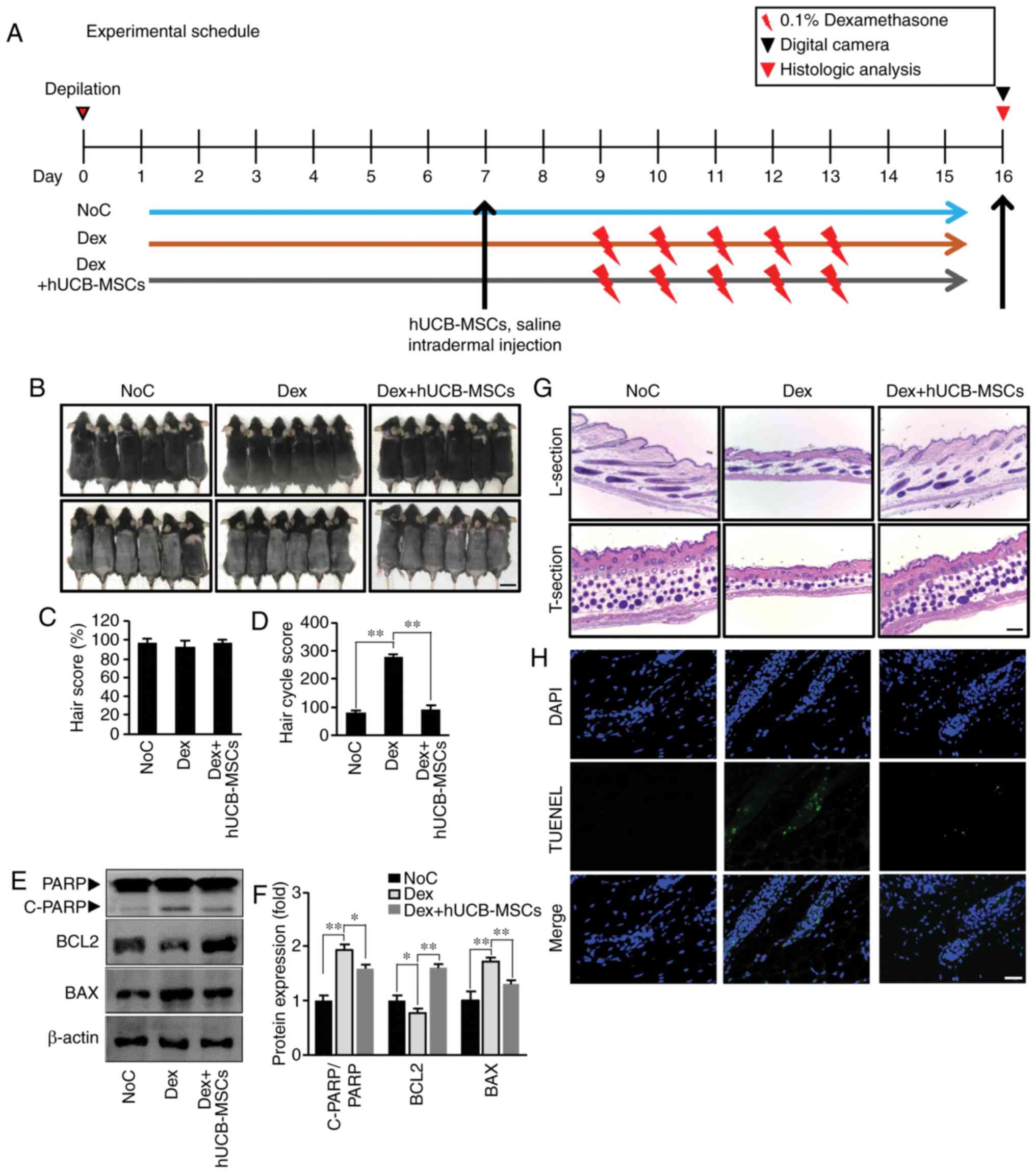 | Figure 1hUCB-MSCs prevent the catagen phase
in C57BL/6 mice. (A) Experimental schedule. On day 7 of tape
depilation, the NoC group was treated with 800 µl saline;
1.25×105 cells/ml of hUCB-MSCs were injected into the
intradermal site in the Dex + hUCB-MSCs group, and the Dex group
was not treated. From day 9 to 13, 1 ml dexamethasone (0.1%) was
topically administered to the Dex and Dex + hUCB-MSCs groups. On
day 16, all animals were sacrificed. (B) The dorsal skin was
photographed on day 16 (upper panel, pre-depilation; lower panel,
post-depilation). Scale bar, 2.5 cm. (C) Quantification of the hair
score. Data are expressed as the mean ± SD. (D) HCS on day 16
post-depilation. Higher HCS indicates the progression of catagen.
(E) Western blot analysis of PARP, Bcl-2 and Bax levels in dorsal
tissue from the three groups. (F) Densitometry analysis if protein
expression. (G) Sections of the dorsal skin. L-section,
longitudinal section; T-section, transverse section. Scale bar, 100
µm. (H) TUNEL staining. Representative images of TUNEL
staining (green fluorescence, middle panel). DAPI was used as a
counterstain (upper panel), and merged pictures are presented in
the bottom panel. Scale bar, 25 µm. *P<0.05,
**P<0.01 vs. Dex. NoC, normal control; Dex,
dexamethasone; HCS, hair cycle score; hUCB-MSCs, human umbilical
cord blood-derived mesenchymal stem cells; PARP, poly (ADP-ribose)
polymerase. |
Dorsal skin was harvested for histological analysis
to determine whether hUCB-MSCs prolonged anagen (Fig. 1G, longitudinal section). Hair
follicles treated with hUCB-MSCs maintained a longer anagen phase
compared with the Dex group. The Dex + hUCB-MSCs group also
maintained a higher hair follicle number compared with the Dex
group (Fig. 1G, transverse
section). In addition, the HCS in the Dex + hUCB-MSC group was
lower compared with that in the Dex group, indicating that
hUCB-MSCs prevented Dex-modulated transition to catagen (Fig. 1D).
The present study also investigated histological
changes following hair regression to confirm the protective effect
of hUCB-MSCs. Mice treated with Dex exhibited severe
histopathological degeneration, which was determined by TUNEL
staining, whereas injection with hUCB-MSCs ameliorated these
changes (Fig. 1H). This suggested
that Dex-induced apoptosis in hair follicles may be responsible for
hair regression. In addition, the present study examined whether
hUCB-MSCs regulated PARP-dependent cell death. Dex induced an
increase in the expression of the pro-apoptotic protein Bax and
cleavage of PARP, as well as a decrease in the expression of the
anti-apoptotic protein Bcl-2. By contrast, hUCB-MSCs counteracted
PARP-dependent apoptosis compared with the Dex group (Fig. 1E and F). These results indicated
that hUCB-MSC transplantation ameliorated follicular cell death by
reducing hair follicle apoptosis.
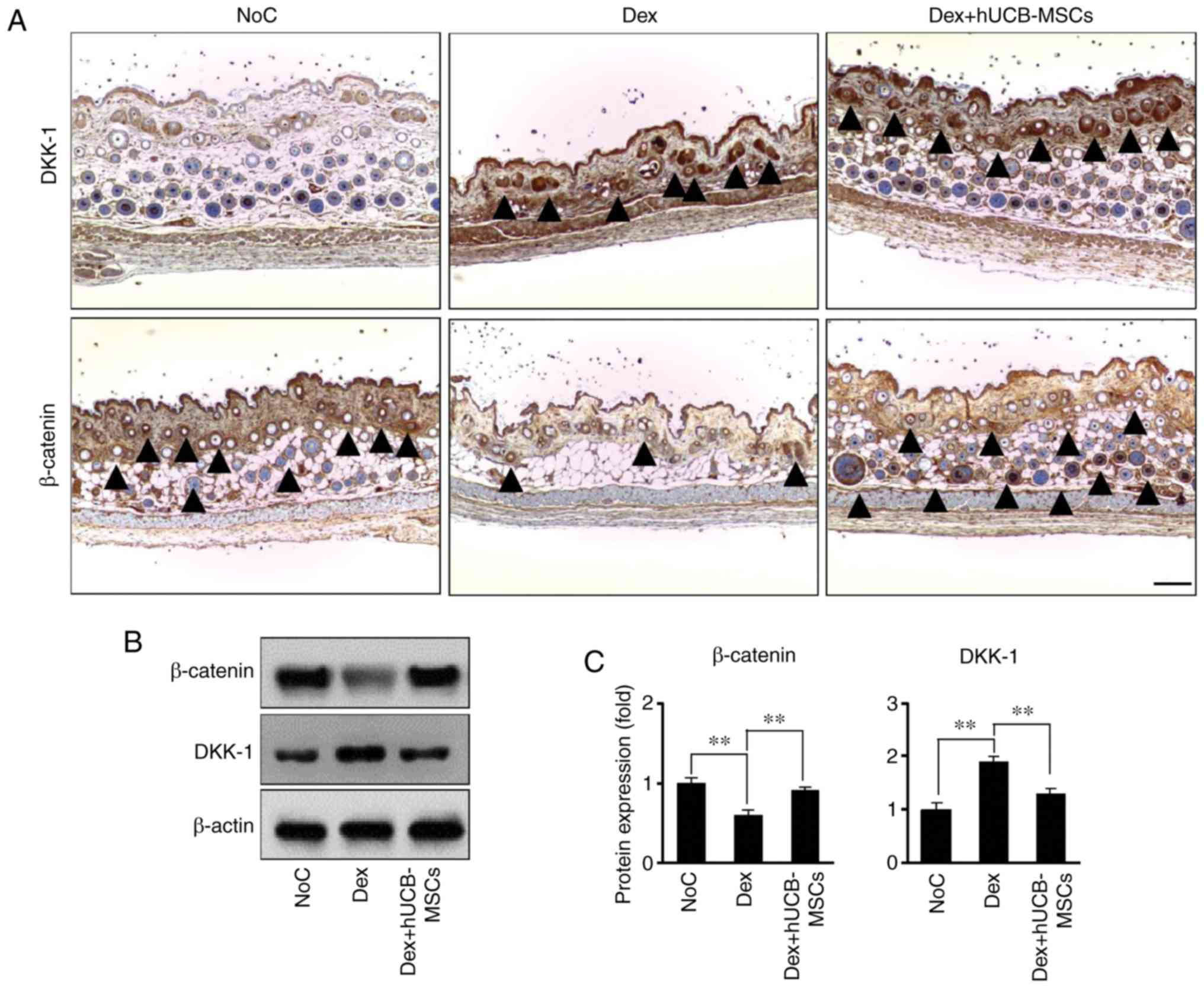
hUCB-MSCs prevent DKK-1 increase and
β-catenin decrease in hair follicles
Mouse dorsal skin tissue samples were used to
investigate whether Dex-stimulated hair regression was associated
with DKK-1 and β-catenin expression. Compared with the NoC group,
DKK-1 expression increased in the Dex group (Fig. 2A, upper panel). However, hUCB-MSCs
significantly attenuated this upregulated DKK-1 expression in hair
follicles. In addition, hUCB-MSC treatment significantly changed
the expression of β-catenin, which is a positive regulator of hair
growth, compared with the Dex group (Fig. 2A, lower panel). The levels of
DKK-1 and β-catenin were significantly reversed in mouse skin
tissues; in the Dex group, DKK-1 expression was increase and
β-catenin expression was decreased compared with the control group,
whereas in the Dex + hUCB-MSCs group, DKK-1 expression was decrease
and β-catenin expression was increased compared with the Dex group
(Fig. 2B and C).
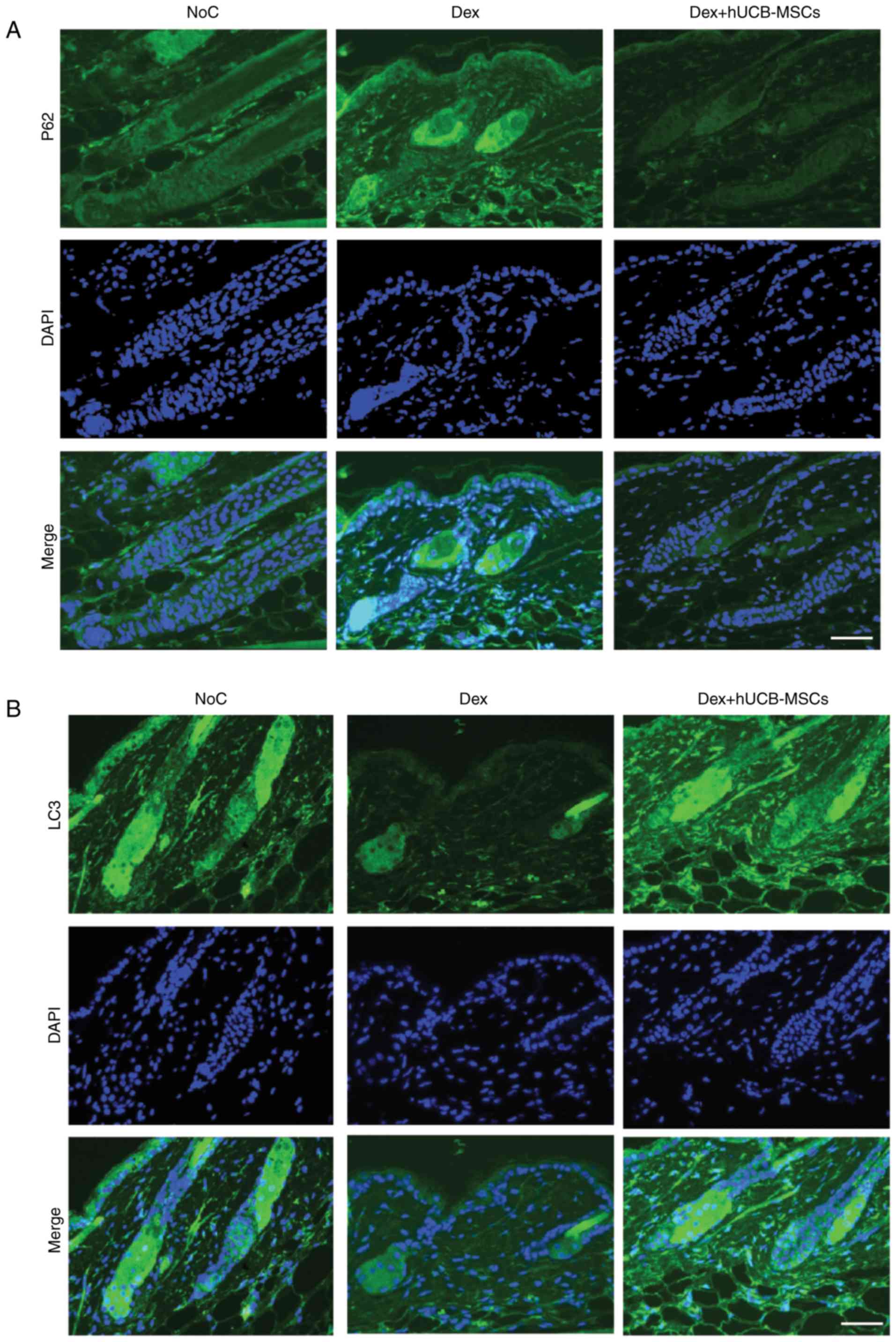
hUCB-MSCs reduce the deposition of p62
and increase LC3 expression in hair follicles
To investigate the role of autophagy in hUCB-MSC
intra-dermal transplantation and hair regression in mouse skin
tissue, the expression levels of the negative regulator p62 and the
positive regulator LC3, which are key markers of autophagy
regulation (24,25), were determined. The results
demonstrated that Dex-induced hair regression increased the
deposition of p62 in mouse hair follicles in the Dex group
(Fig. 3A). However, the
accumulation of p62 was diminished in the Dex + hUCB-MSCs group in
a manner similar to that of the NoC group.
In the present study, LC3 expression in the Dex +
hUCB-MSCs group significantly recovered compared with the Dex
group, and the recovery level was similar to that of the NoC group
(Fig. 3B). Of note, LC3II was
significantly increased in the Dex + hUCB-MSCs group compared with
the Dex group; by contrast, p62 was increased in the Dex group but
significantly decreased in the Dex + hUCB-MSCs group (Fig. 3C and D). These results
demonstrated that Dex-induced hair follicle damage was likely due
to malfunction of the autophagic process.
hUCB-MSCs reduce the inhibitory effects
of Dex on cell viability and decrease DKK-1 expression in
hDPCs
Analysis of the viability of hDPCs stimulated by Dex
(25, 50 and 100 µM) for 48 h revealed that cell viability
decreased in a concentration-dependent manner compared with the
control group (Fig. 4B). To
confirm the effects of hUCB-MSCs on the inhibition of hDPC
viability by Dex, hUCB-MSCs were co-cultured with hDPCs using a
Transwell plate. Co-culture with hUCB-MSC not only increased the
viability of hDPCs, but also partially restored the reduction of
hDPC viability induced by Dex (Fig.
4C).
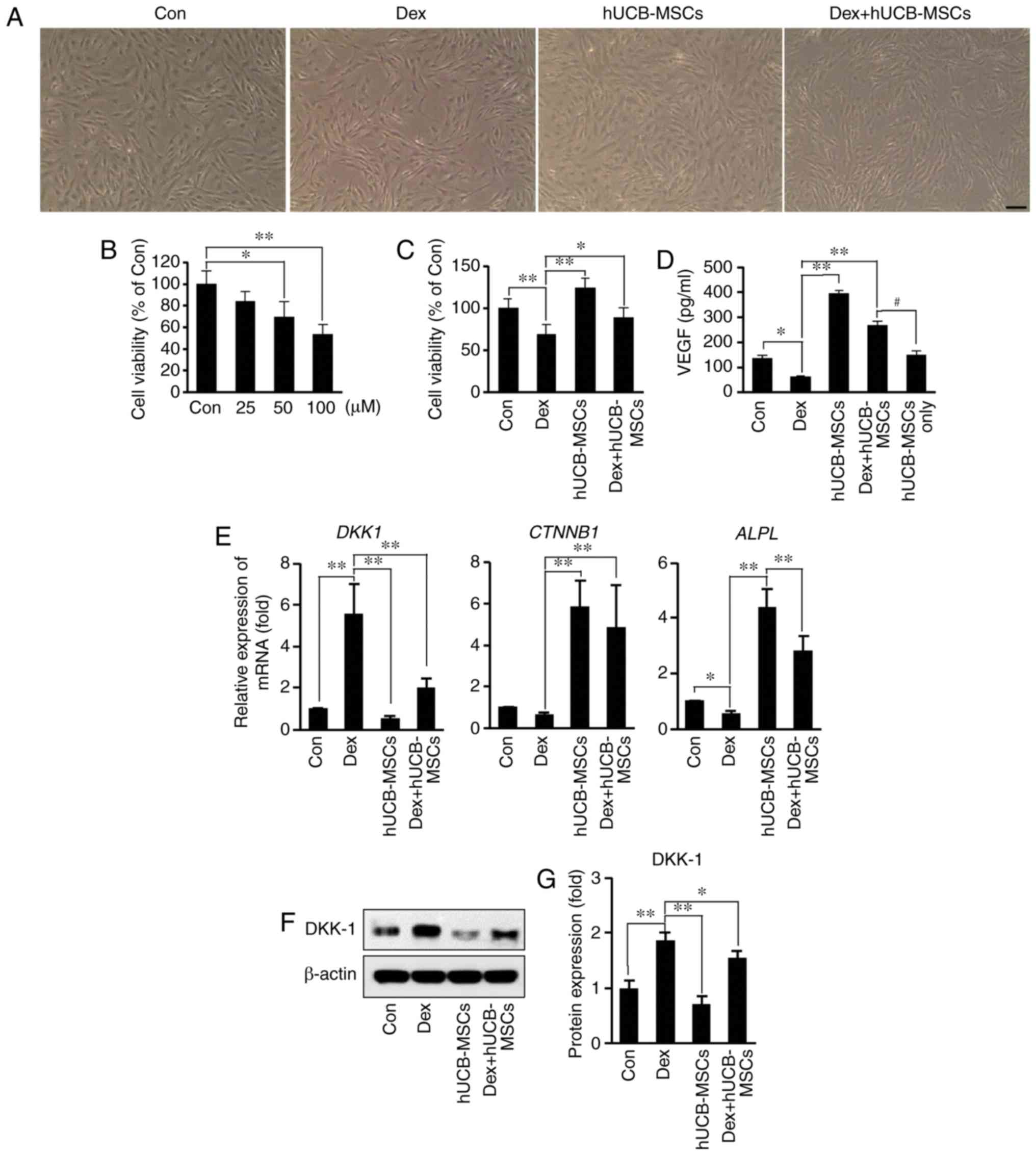 | Figure 4Effects of hUCB-MSC co-culture on
viability, VEGF secretion, mRNA and protein expression in hDPCs
following Dex stimulation. (A) Representative images of hDPCs. Cell
density was decreased by Dex stimulation; hUCB-MSCs counteracted
the reduction. Scale bar, 50 µm. (B) hDPCs were treated with
25-100 µM Dex for 48 h. Cell viability was determined via
MTT assay. *P<0.05, **P<0.01 vs. Con.
(C) Effects of hUCB-MSCs on the viability of hDPCs stimulated with
100 µM Dex for 48 h in the presence or absence of hUCB-MSCs.
Cell viability was determined via MTT assay. *P<0.05,
**P<0.01 vs. Dex. (D) Secretory concentration of
VEGF. hDPCs were stimulated with 100 µM Dex for 48 h in the
presence or absence of hUCB-MSCs, VEGF concentration in the culture
supernatant was evaluated using ELISA. *P<0.05,
**P<0.01 vs. Dex,; #P<0.05 vs. Dex +
hUCB-MSCs. (E) Expression of DKK-1, CTNNB1 and
ALPL mRNA was determined by reverse
transcription-quantitative PCR in hDPCs stimulated with 100
µM Dex for 24 h in the presence or absence of hUCB-MSCs.
*P<0.05, **P<0.01 vs. Dex. (F) Western
blot analysis of DKK-1 levels in hDPCs stimulated with 100
µM Dex for 24 h in the presence or absence of hUCB-MSCs. (G)
Bar diagram of densitometry analysis. *P<0.05,
**P<0.01 vs. Dex. hDPCs, human dermal papilla cells;
Con, control; Dex, dexamethasone; hUCB-MSC, human umbilical cord
blood-derived mesenchymal stem cell; VEGF, vascular endothelial
growth factor; DKK-1, Dickkopf WNT signaling pathway inhibitor 1;
CTNNB1, β-catenin; ALPL, human alkaline phosphatase
liver/bone/kidney isozyme. |
VEGF has previously been identified as a key
mediator of hair generation and growth (26). Therefore, growth factor secretion
in the presence or absence of Dex and hUCB-MSCs was compared in the
present study using ELISA. VEGF secretion following treatment with
100 µM Dex was significantly decreased compared with the
control group (Fig. 4D). However,
co-treatment with Dex and hUCB-MSCs demonstrated that hUCB-MSCs
counteracted the inhibitory effect of Dex on VEGF secretion. To
evaluate the amount of VEGF secreted from hUCB-MSCs, secretion of
VEGF in hUCB-MSCs alone was measured in the culture medium; the
concentration of VEGF obtained from hUCB-MSCs was 152.73±12.7
pg/ml. The results indicated that the amount of VEGF released from
hDPCs in the Dex + hUCB-MSCs group (~117 pg/ml) was higher compared
with that in the Dex group, even when the amount of VEGF secreted
by hUCB-MSCs was excluded.
To investigate whether hUCB-MSCs modulated the
expression of genes associated with hair growth in hDPCs, the
expression levels of DKK-1, CTNNB1 and ALPL
mRNA, as well as DKK-1 protein (27), were analyzed in the presence or
absence of Dex. DKK-1 expression was decreased at the mRNA and
protein levels in the Dex + hUCB-MSCs group compared with the Dex
group (Fig. 4E-G). Additionally,
CTNNB1 and ALPL mRNA expression increased in the
hUCB-MSC co-culture groups in the presence and absence of Dex.
These results suggested that hUCB-MSCs may protect against Dex
stimulation-induced cell damage in hDPCs.
hUCB-MSCs protect HaCaT cells against
apoptosis Dex-induced
To determine whether Dex induced keratinocyte cell
death directly, HaCaT cells were treated with different
concentrations of Dex for 48 h, and the cell viability was
measured. Cell viability was decreased compared with the Con group
by treatment with 25, 50 and 100 µM Dex (Fig. 5A). To confirm the effect of
hUCB-MSCs on the inhibitory effects of Dex on HaCaT proliferation,
hUCB-MSCs were co-cultured with HaCaT using a Transwell plate. Of
note, co-culture with hUCB-MSCs increased HaCaT cell viability and
rescued the Dex-reduced viability during co-culture of hUCB-MSCs in
the presence of Dex (Fig. 5B). To
investigate whether the inhibitory effects of Dex on HaCaT
viability were apoptosis-dependent, cells were stained with PI and
the expression of Bcl-2, Bax, caspase-9, caspase-3 and PARP
proteins was analyzed. PI-positive expression was increased in the
Dex group, which was counteracted by hUCB-MSCs in the Dex +
hUCB-MSCs group (Fig. 5C). To
verify apoptosis activation, PARP-dependent apoptosis upon Dex
treatment was analyzed using western blotting. The cleaved form of
caspase-9, caspase-3, PARP and the Bax/Bcl-2 ratio were increased
in the Dex group, whereas hUCB-MSC co-culture counteracted the
upregulation of apoptosis (Fig. 5D
and E).
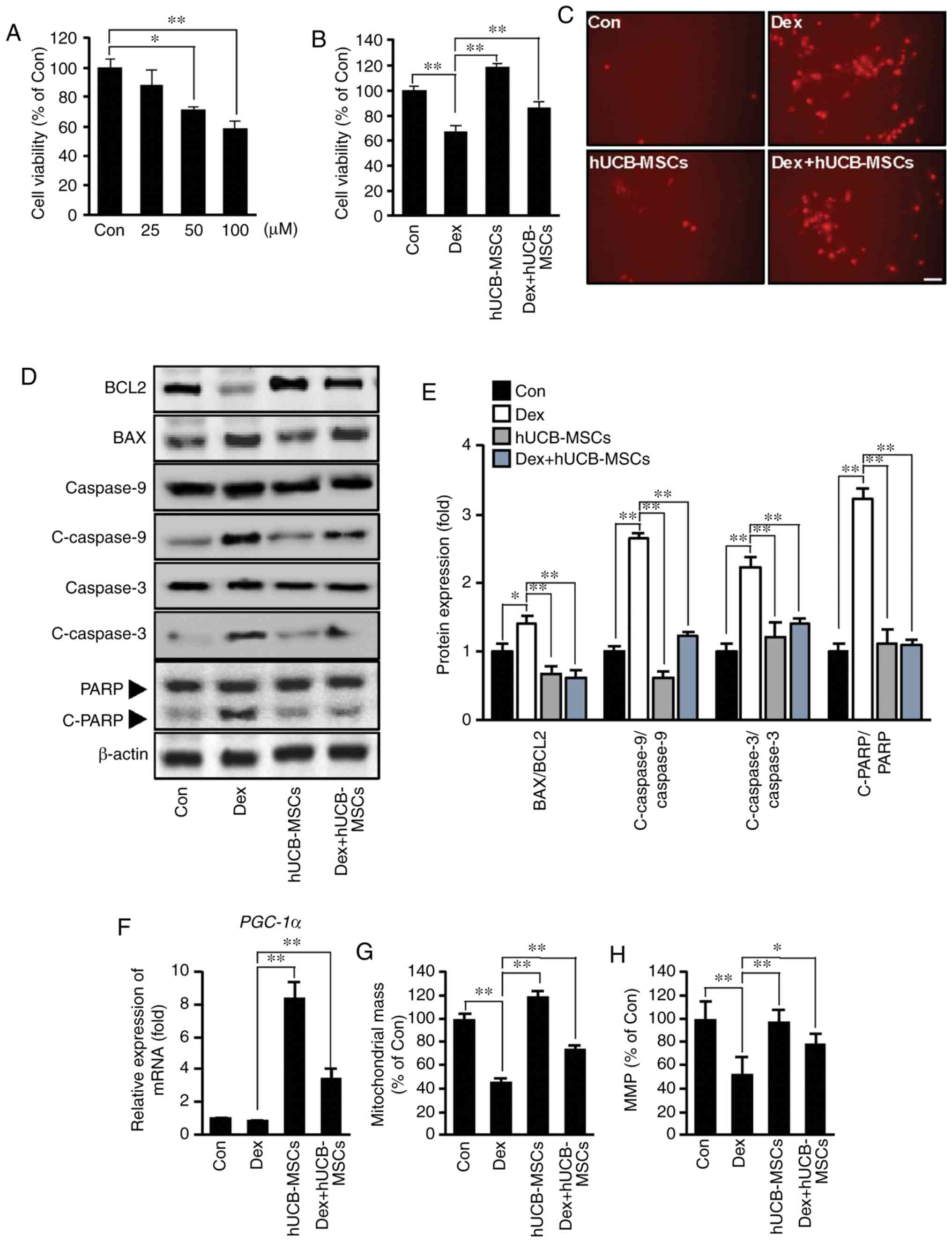 | Figure 5Effects of hUCB-MSC co-culture on
apoptosis and mitochondria biogenesis after Dex stimulation in
HaCaT cells. (A) HaCaT cells were treated with 25-100 µM Dex
for 48 h. Cell viability was determined using MTT assay.
*P<0.05, **P<0.01 vs. Con. (B) Effects
of hUCB-MSCs on the viability of HaCaT cells stimulated with 100
µM Dex for 48 h in the presence or absence of hUCB-MSCs.
Cell viability was determined via MTT assay. **P<0.01
vs. Dex. (C) Representative images of HaCaT cells stained with PI.
Positive reactions were increased by Dex stimulation, whereas
hUCB-MSCs counteracted apoptotic reactions. Scale bar, 50
µm. (D) Western blot analysis of Bcl-2, Bax, caspase-9,
C-caspase-9, caspase-3, C-caspase-3 and PARP levels in HaCaT cells
stimulated with 100 µM Dex for 48 h in the presence or
absence of hUCB-MSCs. (E) Bar diagram of densitometry analysis.
**P<0.01 vs. Dex. (F) PGC-1α mRNA expression
was determined using reverse transcription-quantitative PCR in
HaCaT cells stimulated with 100 µM Dex for 24 h in the
presence or absence of hUCB-MSCs. **P<0.01 vs. Dex.
(G) Mitochondrial mass was measured using MitoTracker fluorescence
signals. **P<0.01 vs. Dex. (H) MMP was measured under
the indicated conditions using the MMP-sensitive probe.
*P<0.05, **P<0.01 vs. Dex. Con,
control; Dex, dexamethasone; hUCB-MSC, human umbilical cord
blood-derived mesenchymal stem cell; PI, propidium iodide; C,
cleaved; PARP, poly (ADP-ribose) polymerase; MMP, mitochondrial
membrane potential; PGC-1α, peroxisome
proliferator-activated receptor gamma coactivator 1α. |
Mitochondrial biogenesis markers, mitochondrial mass
and MMP were evaluated to elucidate the protective effect of
hUCB-MSCs on Dex-induced cell death. PGC-1α transcription
was upregulated by hUCB-MSCs (Fig.
5F). Decreased mitochondrial mass and MMP were counteracted in
HaCaT cells by the presence of hUCB-MSCs (Fig. 5G and H). These results suggested
that co-culture with hUCB-MSCs may protect the quantity and
function of mitochondria. In addition, Dex stimulation may induce
keratinocyte death directly via apoptosis, and hUCB-MSCs may exert
anti-apoptotic effects on Dex stimulation.
hUCB-MSCs regulate autophagy and decrease
unfolding protein response (UPR) in HaCaT cells
To investigate whether autophagy regulation and UPR
were influenced by Dex stimulation and to determine the effects of
hUCB-MSCs in this context, changes in autophagy-related genes and
ER stress markers at the protein and mRNA levels were quantified in
HaCaT cells. Co-culture with hUCB-MSCs increased the conversion of
LC3-I to LC3-II compared with that in the Dex group (Fig. 6A and B). In addition, Beclin1 and
p62 expression levels indicated that autophagy regulation increased
in the presence of hUCB-MSCs. The expression of LAMP-1, which
degrades macromolecules from the cytoplasm through autophagy
(28), was significantly
increased in cells co-cultured with hUCB-MSCs compared with the Dex
group. Similarly, the transcription levels of autophagy-related
genes ATG6, ATG8, p62, CTSD and LAMP1
were increased in the hUCB-MSC co-culture groups compared with the
control and Dex groups (Fig. 6C).
Transcription of p62 was increased by hUCB-MSCs, whereas the p62
protein level was decreased in cells treated with hUCB-MSCs
(Fig. 6A and B). To understand
the association between UPR, cell damage and autophagy, the
expression levels of PERK (an ER membrane sensor) and CHOP (a
command executor in ER stress), which are ER stress markers, we
observed. PERK and CHOP protein expression levels were increased by
Dex treatment, indicating that unfolding proteins accumulated in
HaCaT cells, resulting in ER stress. However, hUCB-MSC co-culture
decreased the ER stress markers in the presence or absence of Dex
treatment. These results suggested that hUCB-MSC treatment enhanced
the autophagic flux and reduced the accumulation of unfolding
proteins in keratinocytes.
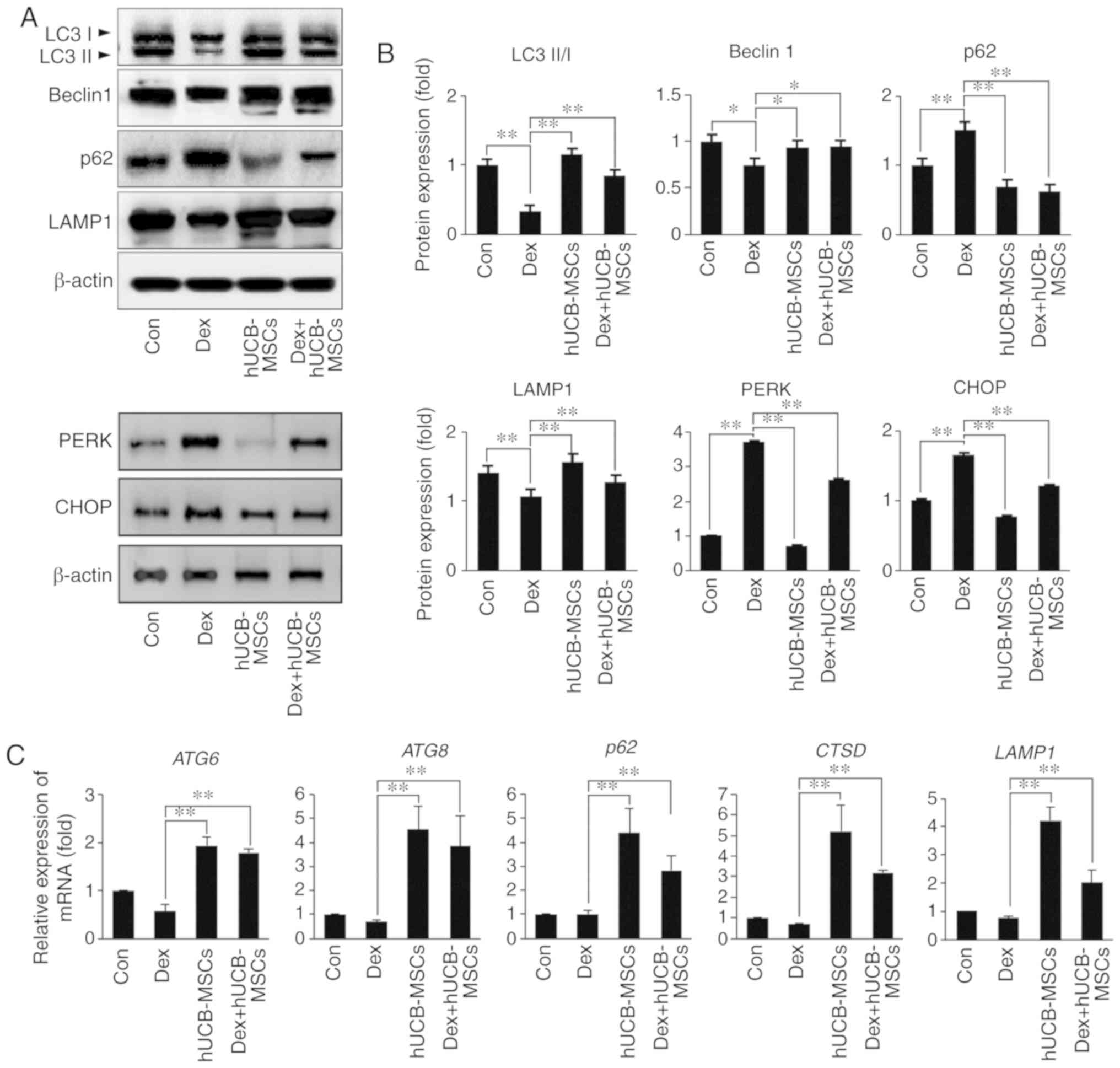 | Figure 6Effects of hUCB-MSC co-culture on
autophagy and ER stress in HaCaT cells following Dex stimulation.
(A) HaCaT cell lysates were immunoblotted with antibodies against
LC3I/II, Beclin1, p62, LAMP1, PERK, CHOP or β-actin. (B)
Densitometry analysis results. **P<0.01 vs. Dex
group. (C) Transcript levels of autophagy-related genes were
determined by reverse transcription-quantitative PCR.
**P<0.01 vs. Dex group. LC3, microtubule-associated
protein 1 light chain 3 β Con, control; Dex, dexamethasone;
hUCB-MSC, human umbilical cord blood-derived mesenchymal stem cell;
LAMP1, human lysosomal-associated membrane protein 1; CHOP,
transcription factor C/EBP homologous protein; PERK, protein kinase
R (PKR)-like endoplasmic reticulum kinase; ATG, autophagy-related
protein. |
Discussion
Dermal papilla cells are small, elongated, rain
droplet-shaped cells that extend from the epidermis into the dermis
(29). Dermal cells with
mesoderm-derived stem cell characteristics serve a pivotal role in
the formation of hair follicle tissues, and their function is
important in hair loss (30).
Therefore, the possibility of treating hair loss using
human-derived MSCs has been suggested in previous studies. Yoo
et al (31) have
demonstrated that dermal papilla-like tissues produced by
mesenchymal stem cells exhibited novel hair follicle-inducing
activity upon transplantation in athymic mice, suggesting that
human stem cells from the bone marrow or the umbilical cord may be
novel and usable sources of stem cells. Our previous study
demonstrated that conditioned media from hUCB-MSCs contain various
beneficial proteins such as growth factors and cytokines (32). In addition, as a precedent, the
application of adipose tissue-derived stem cell-conditioned media
containing paracrine factors has exhibited potential as a
therapeutic alternative in female patients with alopecia (33). Paracrine factors from MSCs
actively participate in anti-apoptotic mechanisms, as well as
stimulation of proliferation and removal of toxic proteins
(34,35). These findings suggest that
human-derived stem cells or their paracrine factors have a
potential for hair loss treatment.
Topical administration of Dex causes hair follicle
degeneration, thus indicating the clinical phenotype of the catagen
phase (36). In addition, Dex
application increased DKK-1 protein expression levels in human
dermal papilla cells, which was followed by hair follicle apoptosis
(37). DKK-1 acts as an
antagonist of the Wnt/β-catenin pathway, and secretory DKK-1
induces apoptosis of follicular keratinocytes (38). The Wnt/β-catenin pathway acts as
an inductive signal for hair growth by maintaining dermal papilla
cells in the anagen phase (39).
In the case of Wnt stimulation in dermal papilla cells, β-catenin
(encoded by the CTNNB1 gene) expression increases, and
β-catenin accumulates in the nuclei of dermal papilla during
anagen, providing hair growth signals (39). These findings suggest that the
β-catenin pathway may be a target for the development of drugs that
activate hair growth by inducing anagen phase genes (40). In the present study, intra-dermal
injection of hUCB-MSCs not only decreased DKK-1 expression in mouse
skin, but also normalized β-catenin expression compared with that
in the control group. Expression of ALPL, a prominent dermal
papilla marker, is dependent on the hair cycle and is most highly
expressed during the anagen phase; thus, it has been identified as
a critical marker for hair growth promotion (41). The expression of the ALPL
gene was also increased in hUCB-MSC co-culture, indicating that
hUCB-MSCs affected anagen maintenance via β-catenin signaling, as
reported previously (42). Of
note, apoptosis was decreased in the Dex + hUCB-MSC group compared
with the Dex group in vivo. Furthermore, in vitro,
hUCB-MSCs protected hDPCs against Dex-induced cell damage and
decreased DKK-1 mRNA and protein expression levels. These results
suggested that hUCB-MSCs may prevent hair catagen by suppressing
DKK-1 expression. Although a previous study indicated that Dex
increased DKK-1 secretion from hDPCs followed by outer root sheath
apoptosis, this did not confirm that Dex directly influences
keratinocyte cell death (37).
The results of the present study demonstrated that Dex inhibited
cell proliferation, followed by the induction of keratinocyte
apoptosis. GC mainly binds GRs and influences cell proliferation or
cell damage (43); although a
number of GRs are located in skin keratinocytes (44), previous studies have identified
the positive effects of Dex in atopic dermatitis and wound healing
(45,46). However, another study has reported
that an elevated concentration of Dex decreased cell proliferation
and induced apoptosis and/or necrosis in endothelial cells
(47). In the present study, Dex
reduced cell proliferation and induced apoptosis by increasing the
Bax/Bcl-2 ratio, cleaved-caspase-3, cleaved-caspase-9 and cleaved
PARP in keratinocytes.
Llambiand and Green (48) have reported that
mitochondria-mediated apoptosis is mainly associated with the Bcl-2
family. Therefore, in the apoptosis pathway, the ratio of Bax/Bcl-2
and Bax/Bcl-extra large serve an important role, as do the
expression levels of Bcl-2 family members (49). In the present study, hUCB-MSCs not
only decreased the Bax/Bcl-2 ratio, but also increased the mRNA
levels of PGC-1α, a master regulator of mitochondrial
biogenesis (50). In addition, in
the presence of hUCB-MSCs, mitochondrial mass and MMP were
increased compared with the Dex group in HaCaT cells. These results
suggested that hUCB-MSCs and their paracrine factors protected
HaCaT cells against apoptosis and that there may be an underlying
mechanism associated with mitochondrial function and
biogenesis.
The role of autophagy as a degradative pathway is
crucial in regenerative medicine. Induction of autophagy under
pathological conditions or cell stress may serve as an adaptive and
protective mechanism for cell survival (51,52). In a previous study, genetic
inhibition of follicular autophagic flux induced and enhanced
premature hair keratinocyte apoptosis, suggesting that autophagic
flux in anagen phase keratinocytes is important for the maintenance
of the hair growth phase (53).
In the present study, hUCB-MSCs transplanted in mice were involved
in LC3 lipidation, which was suppressed by Dex. Additionally,
hUCB-MSCs protected HaCaT cells against Dex-induced apoptosis and
increased autophagy-related mRNA and protein expression. In
particular, the expression levels of lysosomal markers were
significantly increased in the presence of hUCB-MSCs in HaCaT
cells. The autophagosome cargo protein p62 gathers intracellular
constructs that have broken structures or expired functions
(54). If the autophagy process
has not matured or is interrupted by specific stimuli, the
autophagosomes cannot fuse with the lysosome to generate the
autophagolysosome; thus, unnecessary cellular components are not
lysed, resulting in p62 accumulation (55). In the present study, hUCB-MSC
transplantation effectively decreased p62 accumulation in mouse
hair follicles. In addition, an increase in the lysosomal markers
and a decrease in the ER stress markers was confirmed in the
detached environment in vitro. These results suggested that
hUCB-MSCs and their paracrine factors restored autophagy processes
disrupted by GCs.
To the best of our knowledge, the current study is
the first to demonstrate that hUCB-MSCs may prevent hair regression
caused by Dex-induced hair follicular damage. Although other
mechanisms underlying these protective effects remain to be
determined, maintaining autophagic flux was identified as a crucial
mechanism of anagen phase expansion during hair loss.
Glossary
Abbreviations
Abbreviations:
|
hDPCs
|
human dermal papilla cells
|
|
hUCB-MSCs
|
human umbilical cord blood-derived
mesenchymal stem cells
|
|
GC
|
glucocorticoid
|
|
HPA
|
hypothalamic-pituitary-adrenal
|
|
GR
|
glucocorticoid receptor
|
|
H&E
|
hematoxylin and eosin
|
|
HCS
|
hair cycle score
|
|
VEGF
|
vascular endothelial growth factor
|
|
IHC
|
immunohistochemistry
|
|
IF
|
immunofluorescence
|
|
MMP
|
mitochondrial membrane potential
|
|
PARP
|
poly (ADP-ribose) polymerase
|
|
UPR
|
unfolded protein response
|
Acknowledgments
Not applicable.
Funding
The present was co-supported by the Global High-tech
Biomedicine Technology Development Program of the National Research
Foundation & Korea Health Industry Development Institute funded
by the Korean government (grant nos. NRF-2 015M3D6A1065114 and
NRF-2015M3D6A1065363).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
DHB and EL designed the study. BCL, MJC, TRK, JHK,
ESJ, WO, SKM and BCP performed the experiments and analyzed the
data. JN and BJK contributed to drafting the manuscript. All
authors have read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy and integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All animal procedures were approved by the
Institutional Animal Care and Use Committee of Chung-Ang University
(approval no. 2018-00032) and conformed to all applicable National
Institutes of Health guidelines. Neonatal hUCB-MSCs were collected
from umbilical veins after neonatal delivery with informed consent
of the pregnant mothers. This study was approved by the
Institutional Review Board of MEDIPOST Co., Ltd.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ding DC, Chang YH, Shyu WC and Lin SZ:
Human umbilical cord mesenchymal stem cells: A new era for stem
cell therapy. Cell Transplant. 24:339–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oh W, Kim DS, Yang YS and Lee JK:
Immunological properties of umbilical cord blood-derived
mesenchymal stromal cells. Cell Immunol. 251:116–123. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen M, Xiang Z and Cai J: The
anti-apoptotic and neuro-protective effects of human umbilical cord
blood mesenchymal stem cells (hUCB-MSCs) on acute optic nerve
injury is transient. Brain Res. 1532:63–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang SG, Jeun SS, Lim JY, Kim SM, Yang YS,
Oh WI, Huh PW and Park CK: Cytotoxicity of human umbilical cord
blood-derived mesenchymal stem cells against human malignant glioma
cells. Child's Nerv Syst. 24:293–302. 2008. View Article : Google Scholar
|
|
5
|
Lim JY, Jeong CH, Jun JA, Kim SM, Ryu CH,
Hou Y, Oh W, Chang JW and Jeun SS: Therapeutic effects of human
umbilical cord blood-derived mesenchymal stem cells after
intrathecal administration by lumbar puncture in a rat model of
cerebral ischemia. Stem Cell Res Ther. 2:382011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JY, Jeon HB, Yang YS, Oh W and Chang
JW: Application of human umbilical cord blood-derived mesenchymal
stem cells in disease models. World J Stem Cells. 2:34–38. 2010.
View Article : Google Scholar
|
|
7
|
Chung JY, Song M, Ha CW, Kim JA, Lee CH
and Park YB: Comparison of articular cartilage repair with
different hydrogel-human umbilical cord blood-derived mesenchymal
stem cell composites in a rat model. Stem Cell Res Ther. 5:392014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hillmer AM, Hanneken S, Ritzmann S, Becker
T, Freudenberg J, Brockschmidt FF, Flaquer A, Freudenberg-Hua Y,
Jamra RA, Metzen C, et al: Genetic variation in the human androgen
receptor gene is the major determinant of common early-onset
androgenetic alopecia. Am J Hum Genet. 77:140–148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lindner G, Botchkarev VA, Botchkareva NV,
Ling G, van der Veen C and Paus R: Analysis of apoptosis during
hair follicle regression (catagen). Am J Pathol.
151:16011997.PubMed/NCBI
|
|
10
|
Courtois M, Loussouarn G, Hourseau C and
Grollier JF: Hair cycle and alopecia. Skin Pharmacol. 7:84–89.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Silverman MN and Sternberg EM:
Glucocorticoid regulation of inflammation and its functional
correlates: From HPA axis to glucocorticoid receptor dysfunction.
Ann N Y Acad Sci. 1261:55–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pérez P: Glucocorticoid receptors,
epidermal homeostasis and hair follicle differentiation.
Dermatoendocrinol. 3:166–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Slominski A, Wortsman J, Tuckey RC and
Paus R: Differential expression of HPA axis homolog in the skin.
Mol Cell Endocrinol. 265:143–149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Yu M, Yu W, Weinberg J, Shapiro J
and McElwee KJ: Development of alopecia areata is associated with
higher central and peripheral hypothalamic-pituitary-adrenal tone
in the skin graft induced C3H/HeJ mouse model. J Invest Dermatol.
129:1527–1538. 2009. View Article : Google Scholar
|
|
15
|
Ito N, Ito T, Kromminga A, Bettermann A,
Takigawa M, Kees F, Straub RH and Paus R: Human hair follicles
display a functional equivalent of the
hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB
J. 19:1332–1334. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arck PC, Handjiski B, Hagen E, Joachim R,
Klapp BF and Paus R: Indications for a 'brain-hair follicle axis
(BHA)': Inhibition of keratinocyte proliferation and up-regulation
of keratinocyte apoptosis in telogen hair follicles by stress and
substance P. FASEB J. 15:2536–2538. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Albus U: Guide for the Care and Use of
Laboratory Animals. 8th edition. SAGE Publications; Sage UK,
London: 2012
|
|
18
|
Kwon TR, Kim JH, Hong JY, Seok J, Kim JM,
Bak DH, Choi MJ, Mun SK, Kim CW and Kim BJ: Irradiation with 310 nm
and 340 nm ultraviolet light-emitting-diodes can improve atopic
dermatitis-like skin lesions in NC/Nga mice. Photochem Photobiol
Sci. 17:1127–1135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crema A, Ledda M, Fioretti D, Lolli MG,
Sanchez M, Carico E, Marchese R, Rinaldi M and Lisi A: Combination
of cord blood-derived human hepatic progenitors and hepatogenic
factors strongly improves recovery after acute liver injury in mice
through modulation of the Wnt/β-catenin signaling. J Tissue Eng
Regen Med. 13:1031–1043. 2019.PubMed/NCBI
|
|
20
|
Park S, Erdogan S, Hwang D, Hwang S, Han
EH and Lim YH: Bee venom promotes hair growth in association with
inhibiting 5α-reductase expression. Biol Pharm Bull. 39:1060–1068.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Müller-Röver S, Handjiski B, van der Veen
C, Eichmüller S, Foitzik K, McKay IA, Stenn KS and Paus R: A
comprehensive guide for the accurate classification of murine hair
follicles in distinct hair cycle stages. J Invest Dermatol.
117:3–15. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi W, Kwon SJ, Jin HJ, Jeong SY, Choi
SJ, Oh W, Yang YS, Jeon HB and Jeon ES: Optimization of culture
conditions for rapid clinical-scale expansion of human umbilical
cord blood-derived mesenchymal stem cells. Clin Transl Med.
6:382017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Bjørkøy G, Lamark T, Brech A, Outzen H,
Perander M, Overvatn A, Stenmark H and Johansen T: P62/SQSTM1 forms
protein aggregates degraded by autophagy and has a protective
effect on huntingtin-induced cell death. J Cell Biol. 171:603–614.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lippai M and Lőw P: The role of the
selective adaptor p62 and ubiquitin-like proteins in autophagy.
BioMed Res Int. 2014:8327042014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lachgar S, Charveron M, Gall Y and Bonafe
J: Minoxidil upregulates the expression of vascular endothelial
growth factor in human hair dermal papilla cells. Br J Dermatol.
138:407–411. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ito M, Yang Z, Andl T, Cui C, Kim N,
Millar SE and Cotsarelis G: Wnt-dependent de novo hair follicle
regeneration in adult mouse skin after wounding. Nature.
447:316–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y and Yu L: Autophagic lysosome
reformation. Exp Cell Res. 319:142–146. 2013. View Article : Google Scholar
|
|
29
|
Young TH, Lee CY, Chiu HC, Hsu CJ and Lin
SJ: Self-assembly of dermal papilla cells into inductive spheroidal
microtissues on poly (ethylene-co-vinyl alcohol) membranes for hair
follicle regeneration. Biomaterials. 29:3521–3530. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sennett R and Rendl M:
Mesenchymal-epithelial interactions during hair follicle
morphogenesis and cycling. Semi Cell Dev Biol. 23:917–927. 2012.
View Article : Google Scholar
|
|
31
|
Yoo BY, Shin YH, Yoon HH, Seo YK, Song KY
and Park JK: Application of mesenchymal stem cells derived from
bone marrow and umbilical cord in human hair multiplication. J
Dermatolol Sci. 60:74–83. 2010. View Article : Google Scholar
|
|
32
|
Kim Y, Jin HJ, Heo J, Ju H, Lee HY, Kim S,
Lee S, Lim J, Jeong SY, Kwon J, et al: Small hypoxia-primed
mesenchymal stem cells attenuate graft-versus-host disease.
Leukemia. 32:26722018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shin H, Ryu HH, Kwon O, Park BS and Jo SJ:
Clinical use of conditioned media of adipose tissue-derived stem
cells in female pattern hair loss: A retrospective case series
study. Int J Dermatol. 54:730–735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hocking AM and Gibran NS: Mesenchymal stem
cells: Paracrine signaling and differentiation during cutaneous
wound repair. Exp Cell Res. 316:2213–2219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lai RC, Arslan F, Lee MM, Sze NS, Choo A,
Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, et al:
Exosome secreted by MSC reduces myocardial ischemia/reper-fusion
injury. Stem Cell Res. 4:214–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paus R, Handjiski B, Czarnetzki BM and
Eichmüller S: A murine model for inducing and manipulating hair
follicle regression (catagen): Effects of dexamethasone and
cyclosporin A. J Invest Dermatol. 103:143–147. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kwack MH, Lee JH, Seo CH, Kim JC, Kim MK
and Sung YK: Dickkopf-1 is involved in dexamethasone-mediated hair
follicle regression. Exp Dermatol. 26:952–954. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Premanand A and Rajkumari BR: Androgen
modulation of Wnt/β-catenin signaling in androgenetic alopecia.
Arch Dermatol Res. 310:1–9. 2018. View Article : Google Scholar
|
|
39
|
Kishimoto J, Burgeson RE and Morgan BA:
Wnt signaling maintains the hair-inducing activity of the dermal
papilla. Genes Dev. 14:1181–1185. 2000.PubMed/NCBI
|
|
40
|
Huelsken J, Vogel R, Erdmann B, Cotsarelis
G and Birchmeier W: β-Catenin controls hair follicle morphogenesis
and stem cell differentiation in the skin. Cell. 105:533–545. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Iida M, Ihara S and Matsuzaki T: Hair
cycle-dependent changes of alkaline phosphatase activity in the
mesenchyme and epithelium in mouse vibrissal follicles. Dev Growth
Differ. 49:185–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou L, Xu M, Yang Y, Yang K, Wickett RR,
Andl T, Millar SE and Zhang Y: Activation of β-catenin signaling in
CD133-positive dermal papilla cells drives postnatal hair growth.
Plos One. 11:e01604252016. View Article : Google Scholar
|
|
43
|
He J, Zhou J, Yang W, Zhou Q, Liang X,
Pang X, Li J, Pan F and Liang H: Dexamethasone affects cell
growth/apoptosis/chemo-sensitivity of colon cancer via
glucocorticoid receptor α/NF-κB. Oncotarget. 8:67670–67683.
2017.PubMed/NCBI
|
|
44
|
Boix J, Bigas J, Sevilla LM, Iacobone M,
Citton M, Torresan F, Caroccia B, Rossi GP and Pérez P: Primary
aldosteronism patients show skin alterations and abnormal
activation of glucocorticoid receptor in keratinocytes. Sci Rep.
7:158062017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Deckers J, Bougarne N, Mylka V, Desmet S,
Luypaert A, Devos M, Tanghe G, Van Moorleghem J, Vanheerswynghels
M, De Cauwer L, et al: Co-Activation of glucocorticoid receptor and
peroxisome proliferator-activated receptor-γ in murine skin
prevents worsening of atopic march. J Invest Dermatol.
138:1360–1370. 2018. View Article : Google Scholar
|
|
46
|
Gauthier A, Fisch A, Seuwen K, Baumgarten
B, Ruffner H, Aebi A, Rausch M, Kiessling F, Bartneck M,
Weiskirchen R, et al: Glucocorticoid-loaded liposomes induce a
pro-resolution phenotype in human primary macrophages to support
chronic wound healing. Biomaterials. 178:481–495. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vogt CJ and Schmid-Schönbein GW:
Microvascular endothelial cell death and rarefaction in the
glucocorticoid-induced hypertensive rat. Microcirculation.
8:129–139. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Llambi F and Green DR: Apoptosis and
oncogenesis: Give and take in the BCL-2 family. Curr Opin Genet
Dev. 21:12–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:492014. View Article : Google Scholar
|
|
50
|
Palikaras K, Lionaki E and Tavernarakis N:
Balancing mitochondrial biogenesis and mitophagy to maintain energy
metabolism homeostasis. Cell Death Differ. 22:1399–1401. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Crotzer VL and Blum JS: Autophagy and
adaptive immunity. Immunology. 131:9–17. 2010.PubMed/NCBI
|
|
52
|
Levine B and Deretic V: Unveiling the
roles of autophagy in innate and adaptive immunity. Nat Rev
Immunol. 7:767–777. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Parodi C, Hardman JA, Allavena G, Marotta
R, Catelani T, Bertolini M, Paus R and Grimaldi B: Autophagy is
essential for maintaining the growth of a human (mini-) organ:
Evidence from scalp hair follicle organ culture. Plos Biol.
16:e20028642018. View Article : Google Scholar
|
|
54
|
Jiang P and Mizushima N: LC3-and p62-based
biochemical methods for the analysis of autophagy progression in
mammalian cells. Methods. 75:13–18. 2015. View Article : Google Scholar
|
|
55
|
Wurzer B, Zaffagnini G, Fracchiolla D,
Turco E, Abert C, Romanov J and Martens S: Oligomerization of p62
allows for selection of ubiquitinated cargo and isolation membrane
during selective autophagy. Elife. 4:e089412015. View Article : Google Scholar : PubMed/NCBI
|