Introduction
Brain metastases are the most common intracerebral
tumors. In the majority of cases, brain metastases have a
devastating prognostic effect, as treatment options like
chemotherapy and radiotherapy have only limited efficacy (1). Median survival is <1 year in most
patients (2). The primary cancer
types that frequently metastasize to the brain are lung and breast
cancer, melanoma, renal and colorectal cancer (3). As the incidence is 2- to 10-fold
higher compared with primary brain malignancies like glioblastoma
(4), brain metastases have a
major clinical relevance. The average age at diagnosis in western
populations is increasing, and overall survival in a number of
primary tumors improved due to new targeted treatment strategies
and the increased availability of cranial magnetic resonance
imaging. As a consequence, the incidence of cerebral metastases is
also increasing (5). In a number
of cases, brain metastases occur following successful treatment of
the primary tumor including resection, chemotherapy and radiation
therapy. This is mainly due to a small percentage of tumor cells,
which hardly proliferate and are insensitive to chemotherapy,
remaining in the patient following treatment (6,7).
These dormant cells have the ability to be reactivated and
therefore can be the source of recurrence. Dormancy is understood
as a combination of three different mechanisms (8), including cellular dormancy (9-11)
and tumor dormancy (12-15). Cellular dormancy is described as a
state in which solitary disseminated cancer cells either circulate
in the blood or settle at secondary sites in a quiescent state.
Tumor dormancy is described when tumors, as an accumulation of
cells, do not expand in size over a long period of time.
Importantly, tumor dormancy crucially depends on the
microenvironment and tumor stroma, which both induce tumor cell
quiescence. Additionally, systemic factors, like the immune system,
hormonal control or blockage and insufficiency of tumor
angiogenesis may result in tumor dormancy (11,16-24). The majority of studies
investigating dormant cells have focused on the bone marrow niche
as a potential microenvironment.
Dormant cells are defined by their low proliferation
rate, which is associated with a decreased expression of
proliferation marker protein Ki-67, a protein that is exclusively
expressed in the G1-, S-, G2- and M-phases of the cell cycle
(25). Identification of dormant
cells in vivo is challenging, but there are several markers
that are known to be present on dormant cells. Induction of
dormancy has been closely associated with the effect of fibroblast
growth factor 2 (FGF-2) in breast cancer (26). Cells being stimulated with FGF-2
in the bone marrow niche turned into dormant cells, making FGF-2
one of the key regulators of dormancy (27). Other possible markers for dormant
cells in breast cancer are thrombos-pondin-1 (25) and cyclin-dependent kinase
inhibitor p27 (28). de Jong
et al (29) indicated
that, in invasive breast cancer, expression of platelet-derived
growth factor β (PDGFβ) was positively correlated with the
apoptotic index. Additionally, mice bearing microscopic dormant
liposarcomas exhibited a significant increase in
platelet-associated angiogenesis regulatory proteins including
basic fibroblast growth factor (bFGF) and PDGFβ. These proteins
have also been suggested to serve as potential biomarkers for
dormant cells (30). PDGFβ
serves, among other functions, an important role in the metastasis
process (26). Hypoxia is
considered to be an important inductor of dormancy, as upregulation
of dormancy genes is closely associated with genes like glucose
transporter, type 1 (GLUT1) and hypoxia-inducible factor 1-α
(HIF1-α) (31). This has been
described for disseminated tumor cells in the bone marrow for
breast cancer, but additionally in lung cancer, where induction of
dormancy is markedly associated with hypoxia (32). Under hypoxic conditions, which
frequently occur on a cellular level in lung cancer, HIF1-α is
upregulated and leads to a glutamate dehydrogenase-dependent
increase in glutamine uptake, glutamate to α-ketoglutarate flux and
generation of ATP, which serves an important role in survival and
drug-resistance in lung cancer cells (33) and breast cancer (31). In summary, fibroblast growth
factor 2 (FGF2), PDGFβ, and HIF1-α are dormancy markers that may be
used to identify dormant cells outside the central nervous system.
They are designated as 'peripheral' dormancy markers in the
following text.
Almog et al (19) performed a genome wide
transcriptional analysis of dormant breast cancer, glioblastoma,
osteosarcoma and liposarcoma tumors derived from human cell lines.
This led to, among the confirmation of known dormancy markers like
thrombospondin-1, angiomotin and tropomyosin, the identification of
novel dormancy specific biomarkers. Histone cluster 1 H2B family
member K (H2BK), Ephrin receptor A5 (EphA5) and insulin-like growth
factor-binding protein 5 (IGFBP5) were markedly upregulated in
dormant cells derived from glioblastoma, which is a highly
malignant primary brain tumor.
The Ephrin family of receptor tyrosine kinases and
their ligands are involved in embryonic and adult neurogenesis
(34,35). EphA5 itself is considered to be a
membrane receptor, but is also identified at increased levels of
dormant-tumor bearing mice. Levels decrease with increasing tumor
stage in glioma.
Histone H2BK is a core component of the nucleosome.
Whereas histone acetylation is well known to affect angiogenesis,
the role of histone H2BK in tumor progression remains unclear
(19).
The insulin-like growth factor (IGF) axis is known
to be an important pathway in carcinogenesis (36,37). IGFPBs control the binding of IGF
to its receptor and were demonstrated to serve a critical role in
the conversion of dormant tumors to fast-growing angiogenic tumors
(19).
Recently we were able to demonstrate that H2BK,
IGFBP5 and EphA5 are also expressed in human glioblastoma cell
lines in situ. Stimulation with the chemotherapeutic agent
temozolomide, which is frequently used in clinical therapy of
glioblastoma, leads to upregulation of IGFBP5 and EphA5 under
certain conditions (38).
In summary, H2BK, IFGBP5 and EphA5 are markers of
dormant cells in the central nervous system, and were termed
'central' dormancy markers in the present study.
The Natural Killer Group 2D (NKG2D) system is
crucial for recognition of malignant cells. Induced by challenging
environmental conditions, malignant cells in the brain express
several stress inducible molecules like the Natural Killer Group 2D
ligands (NKG2DL), which are usually recognized by the NKG2D
receptor (NKG2DR) (39,40). NKG2DL are not expressed under
healthy conditions, but are induced due to different types of
cellular stress, for example viral infection, genotoxic stress, or
malignant transformation (41).
To date, 8 different NKG2DL are known: MHC class I chain-related
protein A (MICA) and B (MICB) and 6 members of the UL16-binding
protein family (ULBP1-6) (42).
NKG2DR are located on effector cells of the immune system,
including NK cells, γδ T cells, CD8+ T cells and a minor
subset of immune regulatory CD4+ T cells (43). Binding of the ligand to the
receptor results in killing of the NKG2DL-carrying cell. To avoid
this, tumor cells can release NKG2DL by shedding them from the
surface or releasing them in exosomes (44). In primary brain tumors, such as
glioblastoma, the NKG2D system serves an important role in tumor
immunosurveillance (45). In
contrast to a number of other tumors, cells isolated from
glioblastoma rarely express NKG2DL on their surface (40). Notably, glioma stem-like cells
appear to carry certain subsets of NKG2DL on their surface, which
they tend to upregulate when treated with temozolomide (45). As therapy itself might be part of
the acquisition of dormancy (46), and chemotherapy has been suggested
to be involved in the regulation of immune-associated factors like
the NKG2D system, dormancy and immunosurveillance are closely
connected. At present, the molecular mechanisms behind the survival
of dormant cells in the neural niche and their invisibility to
effector cells of the immune system are mainly unknown.
The aim of the present study was to investigate the
NKG2DL expression pattern of dormant cells in cerebral metastases
of breast and lung cancer patients in situ, and to determine
whether there was a brain-specific 'central' dormancy phenotype,
represented by markers like EphA5, H2BK and IGFBP5, which has been
described primarily in glioblastoma, or a 'peripheral' dormancy
phenotype, represented by markers like PDGFβ, FGF2 and HIF1-α in
association with immunogenicity represented by the NKG2DL system.
As dormant cells serve a crucial role in tumor recurrence and
survival, deciphering the immunogenicity in cerebral metastases
might assist to identify future therapeutic targets.
Materials and methods
Tissue samples
Cerebral metastases of 6 patients with breast cancer
and 9 patients with lung cancer were surgically resected at the
Department of Neurosurgery, University Medical Center
Schleswig-Holstein. The present study was approved by the Ethics
Committee of the University of Kiel (approval no. D536/15) and was
in accordance with the Helsinki Declaration of 1964 and its later
amendments. Informed consent was obtained from all individual
patients included in the study. Patient information is provided in
Tables I and II. Tissue was frozen in liquid nitrogen
directly following resection. The median age of breast cancer
patients was 61 years. All patients with breast cancer were female;
brain metastases were located in the frontal lobe in 4 cases, and
occipital and cerebellum in 1 case. A total of 55.6% of patients
with lung cancer were female, and the median age was 57 years. The
metastases were located in the frontal lobe in 1 cases, the
temporal lobe in 2 cases, the cerebellum in 3 cases, the parietal
lobe in 3 cases and the occipital lobe in 2 cases. Certain patients
presented with >1 metastases or metastases on the border of two
lobes.
 | Table IPatients with diagnosis of cerebral
metastases of breast cancer. |
Table I
Patients with diagnosis of cerebral
metastases of breast cancer.
| Sample number | Sex | Age, years | Area of
localization | Histology | Receptor
status |
|---|
| Bcm1 | Female | 64 | Left frontal
lobe | Carcinoma
metastasis | ER 20%; PR
negative |
| Bcm2 | Female | 61 | Right
fronto-parietal lobe | Carcinoma
metastasis | ER >80%; PR
negative |
| Bcm3 | Female | 65 | Right occipital
lobe | Carcinoma
metastasis | ER 10-50%; PR
negative |
| Bcm4 | Female | 79 | Right cerebellar
hemisphere | Carcinoma
metastasis | ER positive; PR
negative |
| Bcm5 | Female | 57 | Left frontal
lobe | Carcinoma
metastasis | ER negative; PR
negative |
| Bcm6 | Female | 40 | Left frontal
lobe | Carcinoma
metastasis | ER <10%; PR
<10% |
 | Table IIPatients with diagnosis of cerebral
metastases of lung cancer. |
Table II
Patients with diagnosis of cerebral
metastases of lung cancer.
| Sample number | Gender | Age, years | Area of
localization | Histology |
|---|
| Lcm1 | Female | 64 | left temporal
lobe | Adenocarcinoma |
| Lcm2 | Male | 44 | Left cerebellar
hemisphere | Adenocarcinoma |
| Lcm3 | Male | 51 | Right
frontotemporal lobe | Adenocarcinoma |
| Lcm4 | Female | 54 | Right cerebellar
hemisphere | Adenocarcinoma |
| Lcm5 | Female | 65 | Left occipital
lobe | Adenocarcinoma |
| Lcm6 | Male | 72 | Right
parietooccipital lobe, left occipital lobe | Adenocarcinoma |
| Lcm7 | Female | 49 | Left frontoparietal
lobe, right frontal lobe | Adenocarcinoma |
| Lcm8 | Female | 46 | Right parietal
lobe | Adenocarcinoma |
| Lcm9 | Male | 68 | Left cerebellar
hemisphere | Adenocarcinoma |
A total of 4 of the 6 patients with breast cancer
had received chemotherapy according to clinical guidelines prior to
diagnosis of the cerebral metastasis. All patients with breast
cancer were treated with mastectomy or segment resection of the
primary tumor prior to surgical removal of the cerebral metastasis.
Only 1 of the patients was diagnosed with brain metastases prior to
the diagnosis of breast cancer. A total of 4 breast cancer
metastases samples were positive for estrogen receptors. None were
positive for progesterone receptors.
A total of 8 of 9 patients with lung cancer were
diagnosed with cerebral metastases prior to diagnosis of the
primary tumor. Histologically, adenocarcinoma was diagnosed in all
patients with lung cancer. All diagnoses were verified by a
pathologist.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was isolated using TRIzol® (Thermo
Fisher Scientific, Inc.). The method used was first described by
Chomczynski and Sacchi (47)
following modifications made in accordance to the manufacturer's
protocol. DNA was digested by RNase free DNase (Promega
Corporation). cDNA synthesis and RT-qPCR were performed using
TaqMan MasterMix and Primer Probes (Applied Biosystems; Thermo
Fisher Scientific, Inc.): Human (h)GAPDH [hGAPDH; (assay ID:
Hs99999905_m1)], hH2BK (assay ID: Hs00955067_g1), hIGFBP5 (assay
ID: Hs00181213_m1), hEphA5 (assay ID: Hs00300724_m1), hPDGFβ (assay
ID: Hs00966522_m1), hHIF1A (assay ID: Hs00153153_m1), hFGF2 (assay
ID: Hs00266645_m1), hMICA (assay ID: Hs00792195_m1), hMICB (assay
ID: Hs04187752_mH), hULBP1 (assay ID: Hs00360941_m1) and hULBP2
(assay ID: Hs00607609_mH) and hULBP3 (assay ID: Hs00225909_m1).
Cycle of threshold values (Cq) were measured by a cycler
(QuantStudio 5, Applied Biosystems by Thermo Fisher Scientific).
Gene expression levels were normalized to the housekeeping gene
GAPDH using the following formula: ΔCq=Cq (gene of interest)-Cq
(GAPDH). As ΔCq values correspond to the exponential course of the
RT-qPCR, ΔCq=3.33 corresponds to a 10-fold lower expression
compared to GAPDH and ΔCq=6.67 corresponds to a 100-fold lower
expression. The relative gene expression compared with GAPDH
(2−ΔCq) was used for statistical analysis (48). Cq of n=41, which was the maximum
cycle number, was defined as undetectable, as this would commonly
represent the detection limit if 40 amplification cycles are
run.
Immunohistochemistry
For in situ analysis of marker expression, 10
µm cryosections of 5 different patients with breast cancer
and 4 different patients with lung cancer were stained by
immunohistochemistry. Sections were fixated with an ice-cold
acetone-methanol mixture (1:1) for 10 min. Blockade for
autofluorescence was performed with sudan black (1% in 70% ethanol;
Carl Roth GmbH + Co., KG). Subsequently, blockage of unspecific
antibody binding was performed by adding 0.5% glycine/0.5% bovine
serum albumin. The slides were then incubated with primary
antibodies overnight at 4°C, followed by secondary antibodies at
37°C for 1 h. Nuclei were stained with DAPI (Sigma-Aldrich; Merck
KGaA; 1:30,000), which was applied at room temperature for 30 min.
All antibodies were diluted in TBS with 0.1% Tween (TBST). Sections
were embedded with Immumount (Thermo Fisher Scientific, Inc.).
Following each step, slides were washed with TBST. The primary
antibodies used were rabbit anti-FGF2 (1:1,250; Abcam), mouse
anti-H2BK (1:400, Biorbyt, Ltd), rabbit anti-IGFBP5 (1:400, Santa
Cruz Biotechnology, Inc.), rabbit anti-EphA5 (1:400; Santa Cruz
Biotechnology, Inc.), mouse anti-PDGFβ (1:20; Abcam), mouse
anti-MICA (1:150; Santa Cruz Biotechnology, Inc.), mouse anti-MICB
(1:500; R&D Systems, Inc.), rabbit anti-ULBP1 (1:200;
ProteinTech Group, Inc.), rabbit anti-ULBP2 (1:500; Abcam) and
mouse anti-ULBP3 (1:250; R&D Systems). When both primary
antibodies were derived from the same species, unspecific binding
was blocked by adding F(ab) fragments from the same species (donkey
anti-mouse and anti-rabbit F(ab) fragments; 1:1,000; Jackson
ImmunoResearch, Laboratories Inc.). Subsequently, secondary
antibodies were added. Isotype controls were performed by applying
mouse or rabbit IgG1 (both R&D Systems) in concentrations
adapted to the other primary antibodies used (1:1,000; Fig. 1). As secondary antibodies, donkey
anti-mouse or anti-rabbit IgGs labelled with Alexa Fluor 488 or
Alexa Fluor 555 (1:1,000; Invitrogen; Thermo Fisher Scientific,
Inc.) were used (Fig. 1). For
negative controls, the primary antibodies were omitted.
Microscopy
Images were obtained with a Zeiss Oberver. Z1
microscope at ×40 magnification (Carl Zeiss AG). A Plan-Apochromat
40x/1.4 Oil DIC (CN) VIS-IR M27 was used as a lens. The camera
AxioCam MR MR5 (Zeiss) with a focus depth of 0.89 µm for 555
nm, 0.80 µm for 488 nm, and 0.72 µm for DAPI was
used. For image processing, ZEN 2 (blue edition) software was used
(Carl Zeiss AG). Images had a size of 1,388×1,040 pixels
(223.82×167.70 µm) at an image bit depth of 12 bits.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). For testing significance of increase or decrease of
expression, Student's t-test was used. P<0.05 were considered to
indicate a statistically significant difference. A Pearson
correlation analysis was performed to analyze the associations
between marker expression levels. Microsoft Excel 2016 (Microsoft
Corporation) and SPSS Statistics (version 24.0; IBM Corp) were used
for all statistical analyses.
Results
Expression and cellular localization of
NKG2DL and dormancy markers in cerebral metastases of breast and
lung cancer in situ
The expression of NKG2DL and the different 'central'
and 'peripheral' dormancy markers mentioned above were examined at
mRNA and protein levels by RT-qPCR and double immunohistochemistry,
respectively. Samples were obtained directly from metastases
tissues of patients with breast and lung cancer, who underwent
surgery at the Department of Neurosurgery, University Hospital
Schleswig-Holstein, Campus Kiel.
mRNA expression of NGK2DL and different
dormancy markers
All NKG2L were identified to be expressed at
detectable mRNA levels in breast and lung cancer metastases, with
ULBP1 exhibiting the lowest expression level in the patients with
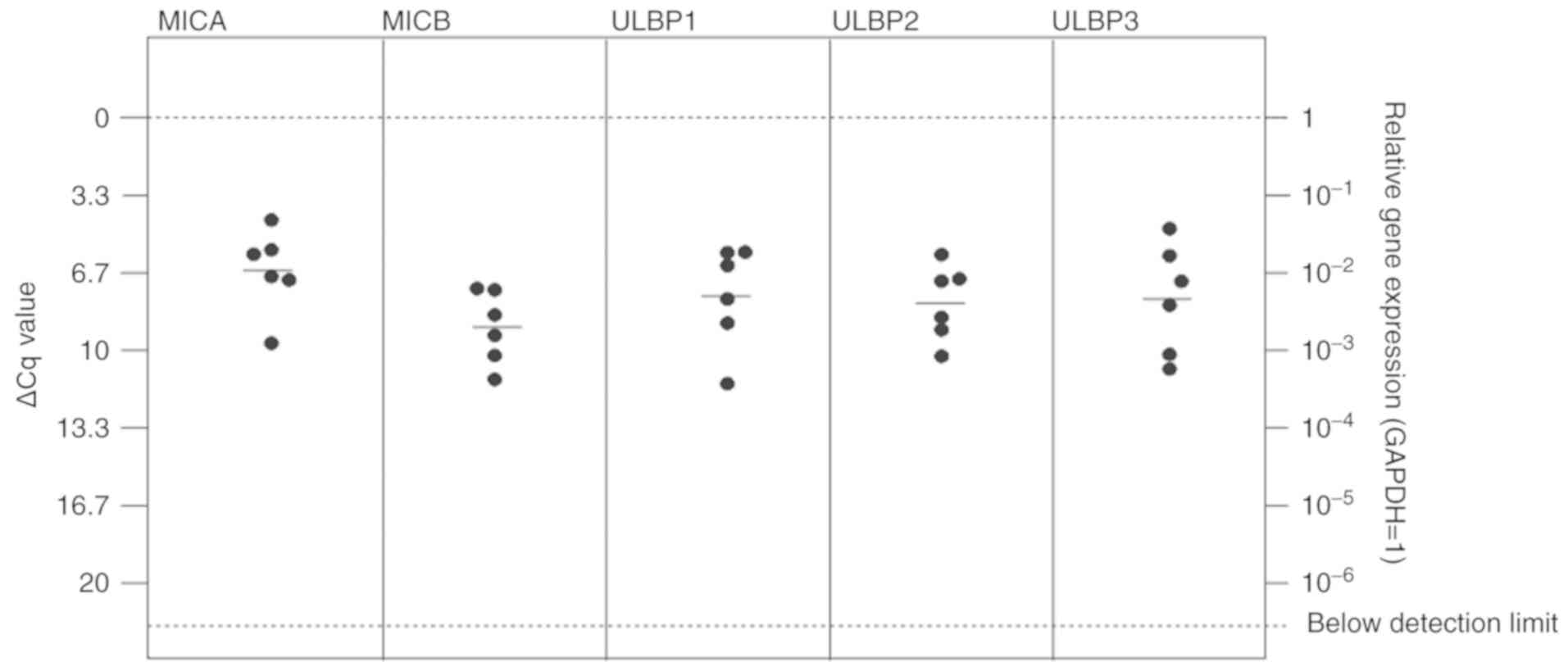
lung cancer [Figs. 2 and 3; median ΔCq values in breast cancer
tissues: MICA=6.57 (SD, 1.78); MICB=9.00 (SD, 1.56); ULBP1=7.66
(SD, 2.20); ULBP2=7.96 (SD, 1.62); ULBP3=7.79 (SD, 2.36); median
ΔCq values in lung cancer tissues: MICA=6.90 (SD, 1.28); MICB=5.83
(SD, 1.98); ULBP1=9.84 (SD, 3.12); ULBP2=9.76 (SD, 1.37); and
ULBP3=3.49 (SD, 1.51)].
In the breast cancer metastases tissues, no
significant differences between mRNA expression of the NKG2DL MICA,
MICB, ULBP1, 2 and 3 were observed (Fig. 2). In lung cancer, MICA and MICB
were highly expressed, with a significantly increased expression
level of MICA compared with ULPB2 and 3 (Fig. 3).
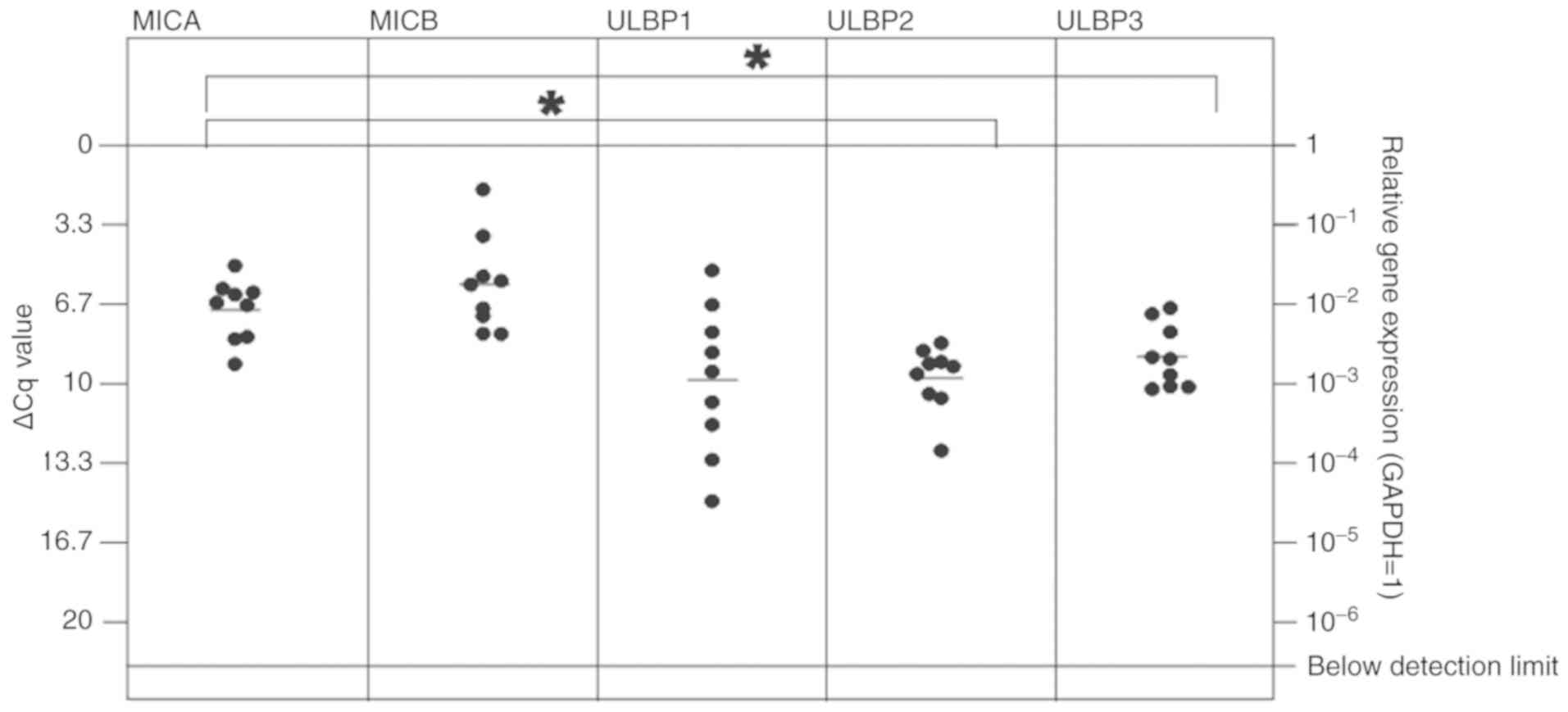
The majority of the other dormancy markers were also
expressed at detectable mRNA levels in breast and lung cancer
metastases [Figs. 4 and 5; median ΔCq values in breast cancer
tissues: H2BK=4.77 (SD, 2.56); IGFBP5=2.88 (2.50); EphA5=12.04 (SD,
2.36); PDGFβ=3.98 (SD, 2.85); FGF2=9.08 (SD, 2.71); HIF1-α=2.20
(SD, 1.85); median ΔCq values in lung cancer: H2BK=3.50 (SD, 1.51);
IGFBP5=3.50 (SD, 2.66); EphA5=9.60 (SD, 1.42); PDGFβ=4.58 (SD,
1.34); FGF2=7.96 (SD, 1.40); HIF1-α=1.00 (1.06)]. EphA5 was
expressed at the lowest levels in both cancer types. In breast
cancer metastases, the 'peripheral' dormancy markers PDGFβ and
HIF1-α were expressed at significantly increased levels compared
with the 'central' dormancy marker EphA5 (Fig. 4). Similar results were obtained in
lung cancer metastases tissues, where HIF1-α and PDGFβ also
exhibited significantly increased expression levels compared with
EphA5 (Fig. 5). In lung cancer
metastases tissues, HIF1-α also was expressed at a significantly
increased level compared with the 'central' dormancy marker H2BK.
Among the 'central' dormancy markers, H2BK and IGFBP5 exhibited the
highest expression levels, with significantly increased H2BK
expression levels compared with the 'peripheral' dormancy marker
FGF2 (Fig. 5).
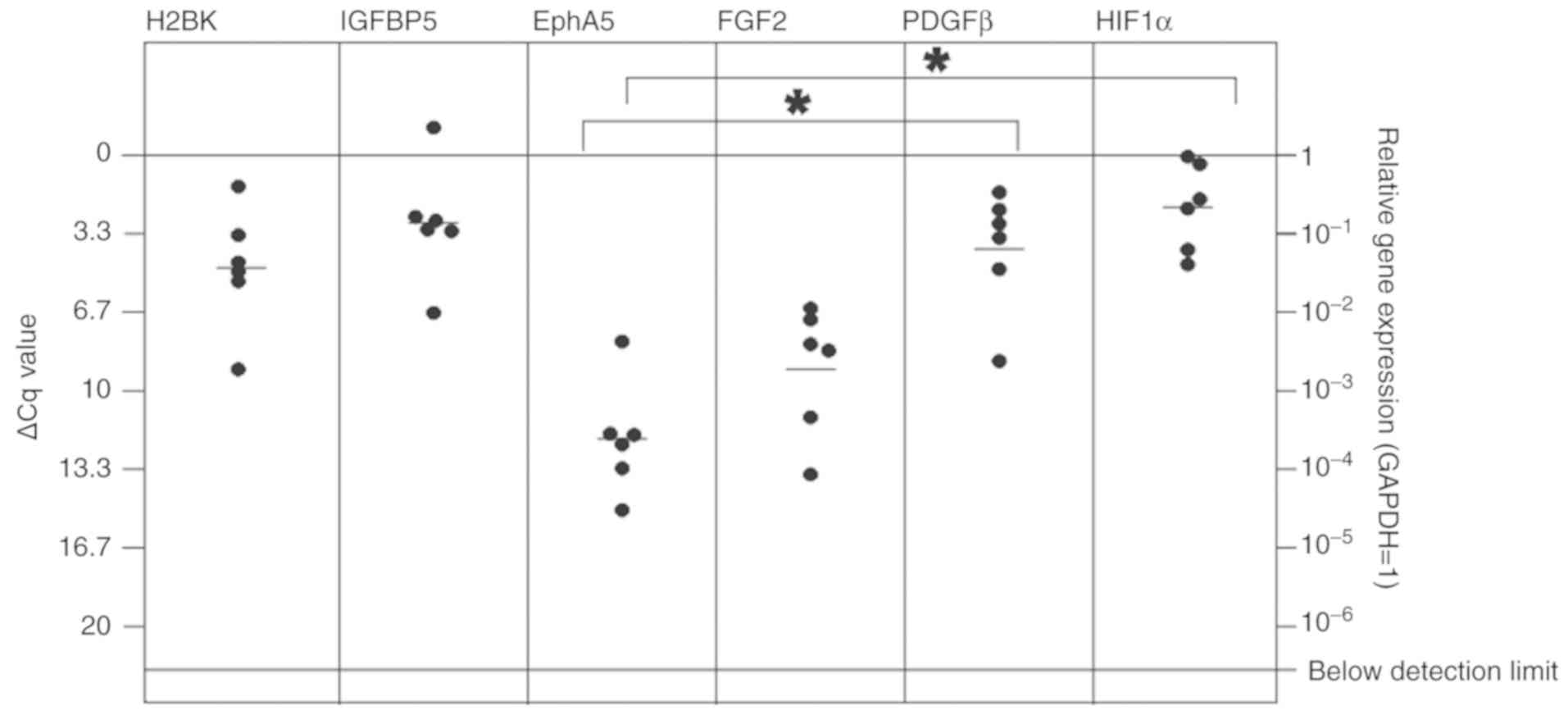 | Figure 4In vitro expression of central
(H2BK, IGFBP5, EphA5) and peripheral (FGF2, PDGFβ, HIF1-α) dormancy
markers in the cerebral metastasis tissues from patients with
breast cancer. mRNA levels were analyzed by reverse
transcription-quantitative polymerase chain reaction. Comparable
with measurements in the patients with lung cancer metastases, all
dormancy markers were expressed at a stable level. The 'peripheral'
dormancy markers HIF1-α and PDGFβ exhibited significantly increased
expression levels compared with the 'central' dormancy marker
EphA5. The expression level of EphA5 was decreased compared with
H2BK and IGFBP5, but this was not statistically significant.
*P≤0.05. H2BK, Histone cluster 1 H2B family member K;
IGFBP5, insulin-like growth factor-binding protein 5; EphA5, Ephrin
receptor A5; FGF2, fibroblast growth factor 2; PDGFβ,
platelet-derived growth factor β; HIF1-α, hypoxia-inducible factor
1-α. |
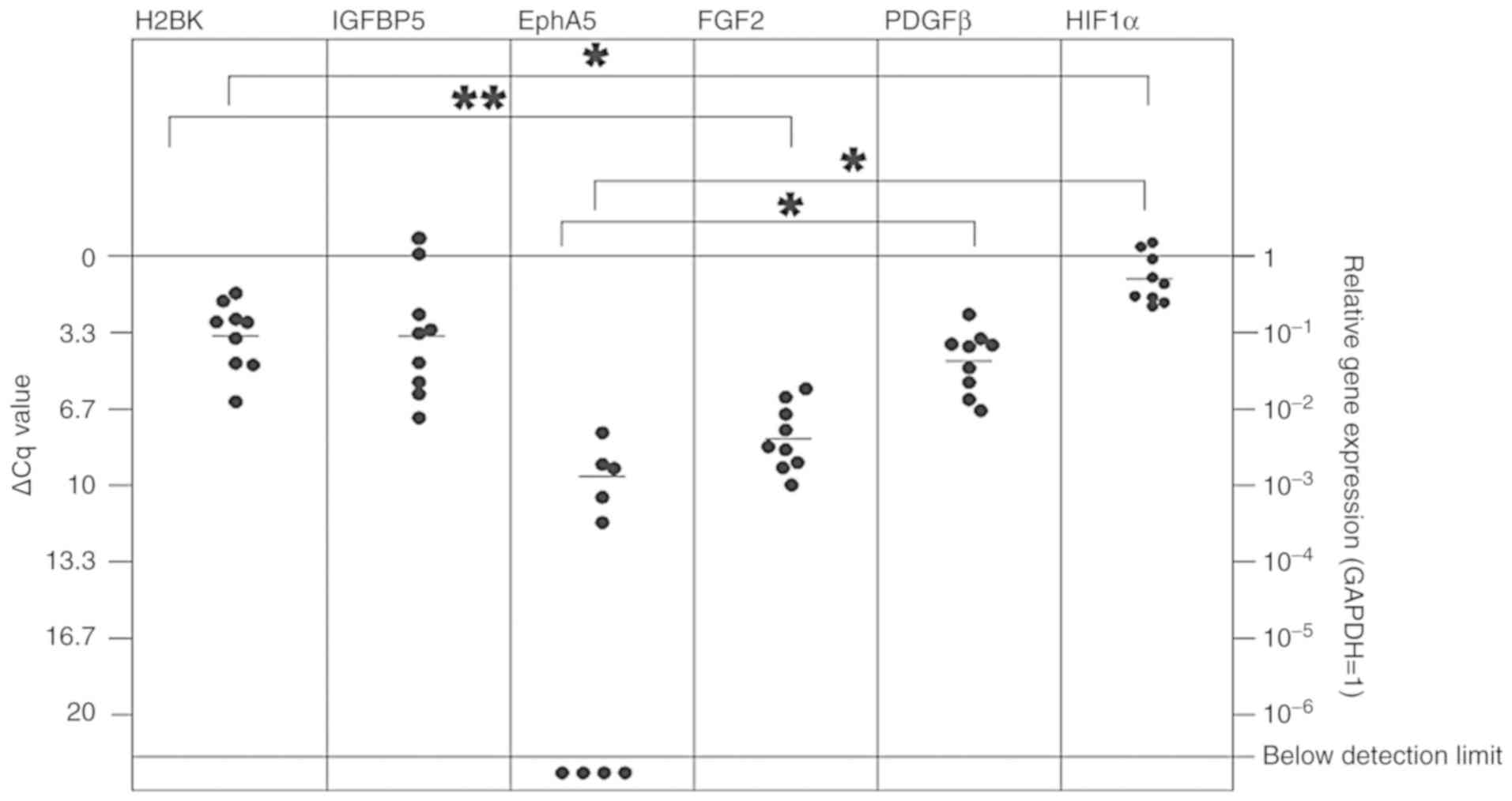 | Figure 5In vitro expression of
'central' (H2BK, IGFBP5, EphA5) and 'peripheral' (FGF2, PDGFβ,
HIF1-α) dormancy markers in the cerebral metastasis tissues from
patients with lung cancer. All dormancy markers were expressed at a
stable level. The 'peripheral' dormancy marker HIF1-α was expressed
at a significantly increased level compared with H2BK and EphA5,
which are both 'central' dormancy markers. The 'peripheral'
dormancy marker PDGFβ was expressed at a significantly increased
level compared with the 'central' dormancy marker EphA5. Among the
'central' dormancy makers, H2BK and IGFBP5 were expressed at the
highest levels. H2BK exhibited a significantly higher expression
level compared with FGF2, which was expressed at relatively low
levels. Overall, the expression of EphA5, one of the 'central'
dormancy markers, was the lowest and was not detectable in 4 of the
probes. *P≤0.05 and **P≤0.01. H2BK, Histone
cluster 1 H2B family member K; IGFBP5, insulin-like growth
factor-binding protein 5; EphA5, Ephrin receptor A5; FGF2,
fibroblast growth factor 2; PDGFβ, platelet-derived growth factor
β; HIF1-α, hypoxia-inducible factor 1-α. |
Bravais-Pearson correlation analysis
A Bravais-Pearson correlation analysis of the
RT-qPCR data, including all NKG2DL and dormancy markers, was
performed.
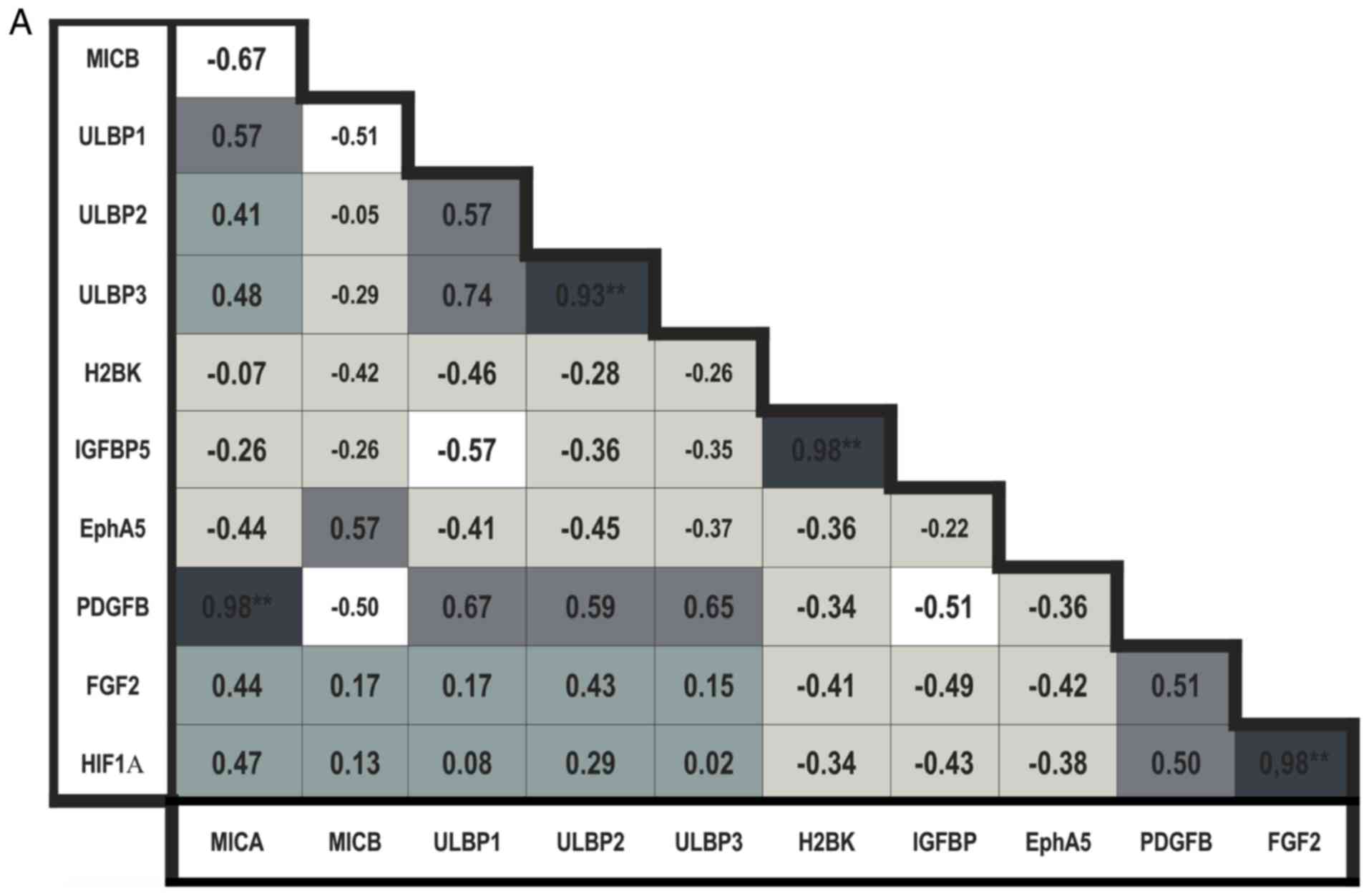
In the breast cancer metastases tissues, the
expression of MICB was negatively correlated with expression of all
other NKG2DL. The expression levels of MICA, ULBP1, 2 and 3 were
positively correlated with each other; the correlation between
ULBP2 and 3 was observed to be significant (P<0.01; Fig. 6A).
When analyzing the 'central' dormancy markers in the
breast cancer metastases tissues, the expression levels of H2BK and
IGFBP5 were identified to be positively correlated (P<0.01;
Fig. 6A).
The expression levels of the 'peripheral' dormancy
markers in breast cancer metastases tissues were positively
correlated with each other, but this result was not statistically
significant (Fig. 6A). There was
a significantly positive correlation between the expression of FGF2
and HIF1-α (P<0.01; Fig.
6A).
The expression of 'central' dormancy markers was
negatively correlated with expression of 'peripheral' dormancy
markers in breast cancer.
The expression levels of all NKG2DL were positively
correlated with the expression of nearly all 'peripheral' dormancy
markers, with exclusion of the association between MICB and PDGFβ;
the correlation between MICA and PDGFβ was observed to be
statistically significant (P<0.01; Fig. 6A) in breast cancer. The expression
levels of all NKG2DL were negatively correlated with the expression
of 'central' dormancy markers, with exception of MICB, whose
expression exhibited a slightly positive correlation with
expression of EphA5 (Fig.
6A).
When examining the cerebral metastases tissues from
the patients with lung cancer, the results of the correlation
analysis were more complex. Similar to the data from the metastases
tissues from the patients with breast cancer, the expression of
MICB was negatively correlated with expression of all other NKG2DL.
The expression of MICA, ULBP1 and 3 were positively correlated. A
significant correlation in lung cancer was observed in the
expression of ULBP1 and 3, which were positively correlated
(P<0.05; Fig. 6B).
When examining the associations between the
expression levels of the dormancy markers, mostly positive
correlations were observed between the expression levels of all
'peripheral' dormancy markers with all other 'central' dormancy
markers in lung cancer metastases. Only the expression of EphA5 was
negatively correlated with the expression of the other 'peripheral'
dormancy markers FGF2 and HIF1-α, but this was not significantly
different (Fig. 6B).
The correlation analysis concerning NKG2DL and
dormancy marker expression in lung cancer metastases was
heterogeneous. The MICA and ULBP3 expression levels were negatively
correlated with the expression of 'central' and 'peripheral'
dormancy markers, with the exception of FGF2, which exhibited a
moderate, non-significant positive correlation. Expression of MICB
was significantly positively correlated with the expression of the
'central' dormancy marker IGFBP5 and the 'peripheral' marker
HIF1-α. The expression levels of ULBP1 and 2 were significantly
positively correlated with the expression of the 'central' dormancy
marker EphA5. There was no significant correlation between MICB and
EphA5 observed.
Double immunostaining
To specify the in situ distribution and
localization of dormancy markers, cryosections of cerebral
metastases from patients with breast and lung cancer were stained.
The NKG2D ligands MICA, MICB, ULBP1 and ULBP2 were stained in
combination with the 'central' dormancy markers EphA5, H2BK and
IGFBP5 and the 'peripheral' dormancy markers PDGFβ, FGF2 and
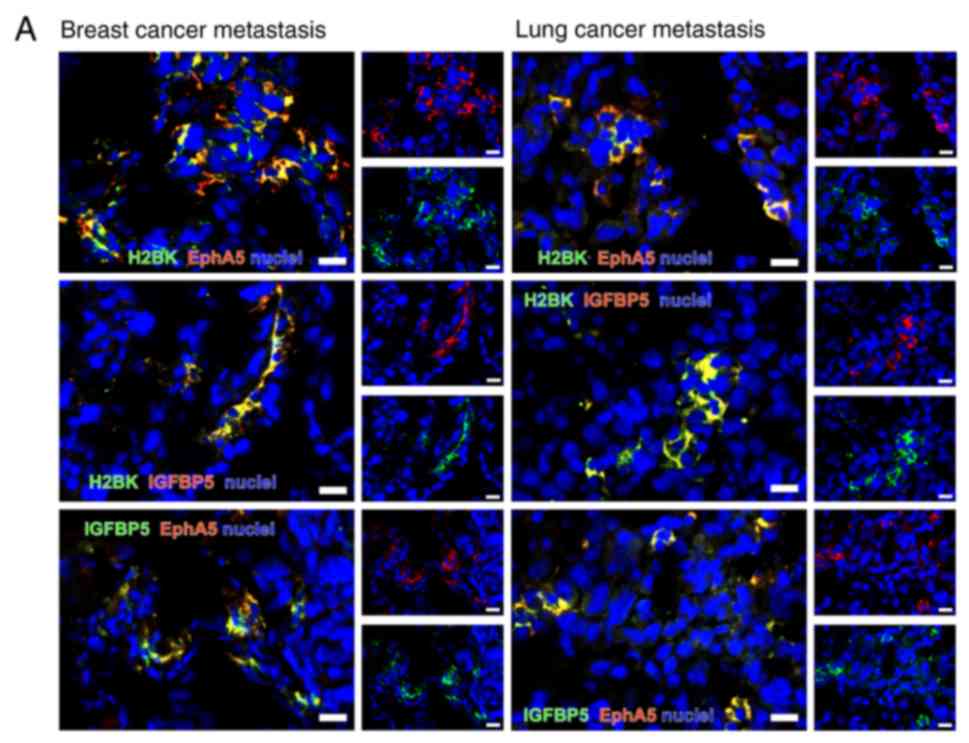
HIF1-α. The staining results are summarized in Fig. 7A and B. The numbers of stained
tumors is also presented in Fig. 7A
and B. A high degree of consistency concerning the staining
results between the different patients and between different areas
of the tumors was observed. Example images of the staining results
are presented in Figs. 8-11. In order to provide a
respresentative impression, only one image per combination of
markers and per tumor entity has been presented. In cases where
this one image does not represent the results obtained in all areas
the tumors, a second image was added in a white box from a second
area of a second tumor. If the image in the white box was taken
from another tumor of the same entity, it was marked with an
asterix.
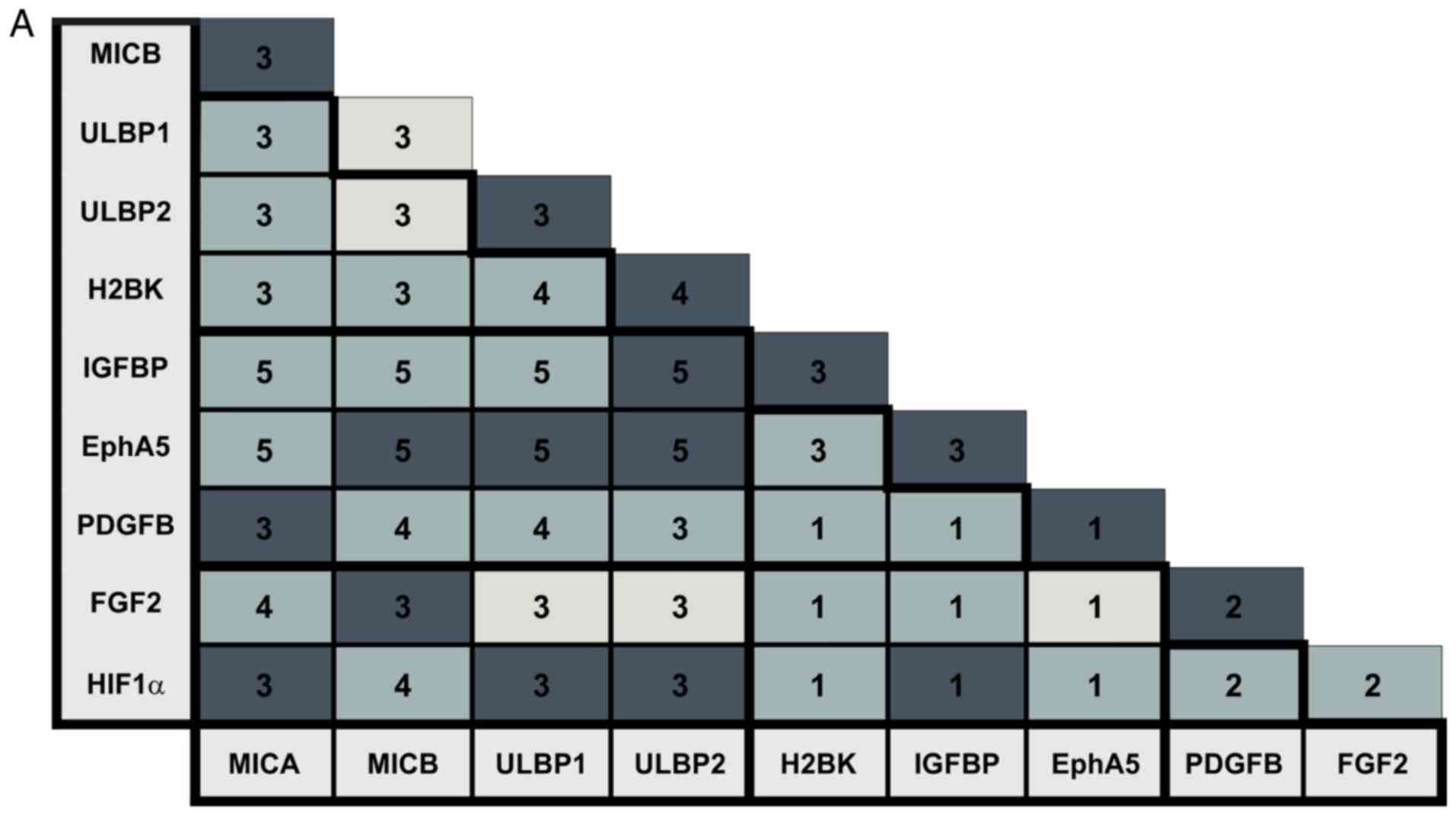 | Figure 7Staining patterns in the breast and
lung cancer patients. (A) Overview of staining pattern in breast
cancer metastases. (B) Overview of staining pattern in lung cancer
metastases. The numbers in the box represent the number of
different tumors that were examined. Light grey color represents no
co-staining, normal grey represents partial co-staining and dark
grey represents complete co-staining. MICA, MHC class I
chain-related protein A; MICB, MHC class I chain-related protein B;
ULBP, UL16-binding protein family; H2BK, Histone cluster 1 H2B
family member K; IGFBP5, insulin-like growth factor-binding protein
5; EphA5, Ephrin receptor A5; FGF2, fibroblast growth factor 2;
PDGFβ, platelet-derived growth factor β; HIF1-α, hypoxia-inducible
factor 1-α. |
Double immunostaining for dormancy
markers among each other
All dormancy markers were detectable using double
immunostaining (Fig. 8A-C). The
majority of the marker-positive cells were identified as single
cells or in small clusters.
When staining for 'central' dormancy markers
exclusively, a broad co-staining of IGFBP5 with H2BK and IGFBP5
with EphA5 was observed in breast cancer metastases. In the lung
cancer tissues, a broad co-staining of all 'central' dormancy
markers was identified. This was the case in different areas of one
tumor, but also when comparing tumors from different patients. The
distribution pattern was similar in breast and lung cancer
metastases tissues (Fig. 8A).
When considering the 'peripheral' dormancy markers,
several partial co-staining patterns were observed in breast
cancer: HIF1-α with FGF2; HIF1-α with PDGFβ; and PDGFβ with FGF2
(Fig. 8B), and a partial or
complete co-staining in lung cancer metastases (Fig. 8B).
When staining for 'peripheral' and 'central'
dormancy markers in breast cancer, a rare but partial co-staining
of most markers was observed. Representative images for H2BK
co-staining with 'peripheral' dormancy markers are presented in
Fig. 8C.
In the lung cancer metastases tissues, the majority
of the 'central' and 'peripheral' dormancy markers were partially
co-stained with each other in a small number of samples.
Representative sections are presented in Fig. 8C.
Clearly observable co-staining between the majority
of the 'central' dormancy markers and a lack of co-staining between
the 'central' and 'peripheral' dormancy markers in both tumor types
suggested that there may be a circumscribed population of dormant
cells in each tumor type, which is defined by the expression of
'central' dormancy markers. As only a partial co-staining between
the 'peripheral' dormancy markers was observed in both tumor types,
these appear mark non-overlapping populations of dormant cells.
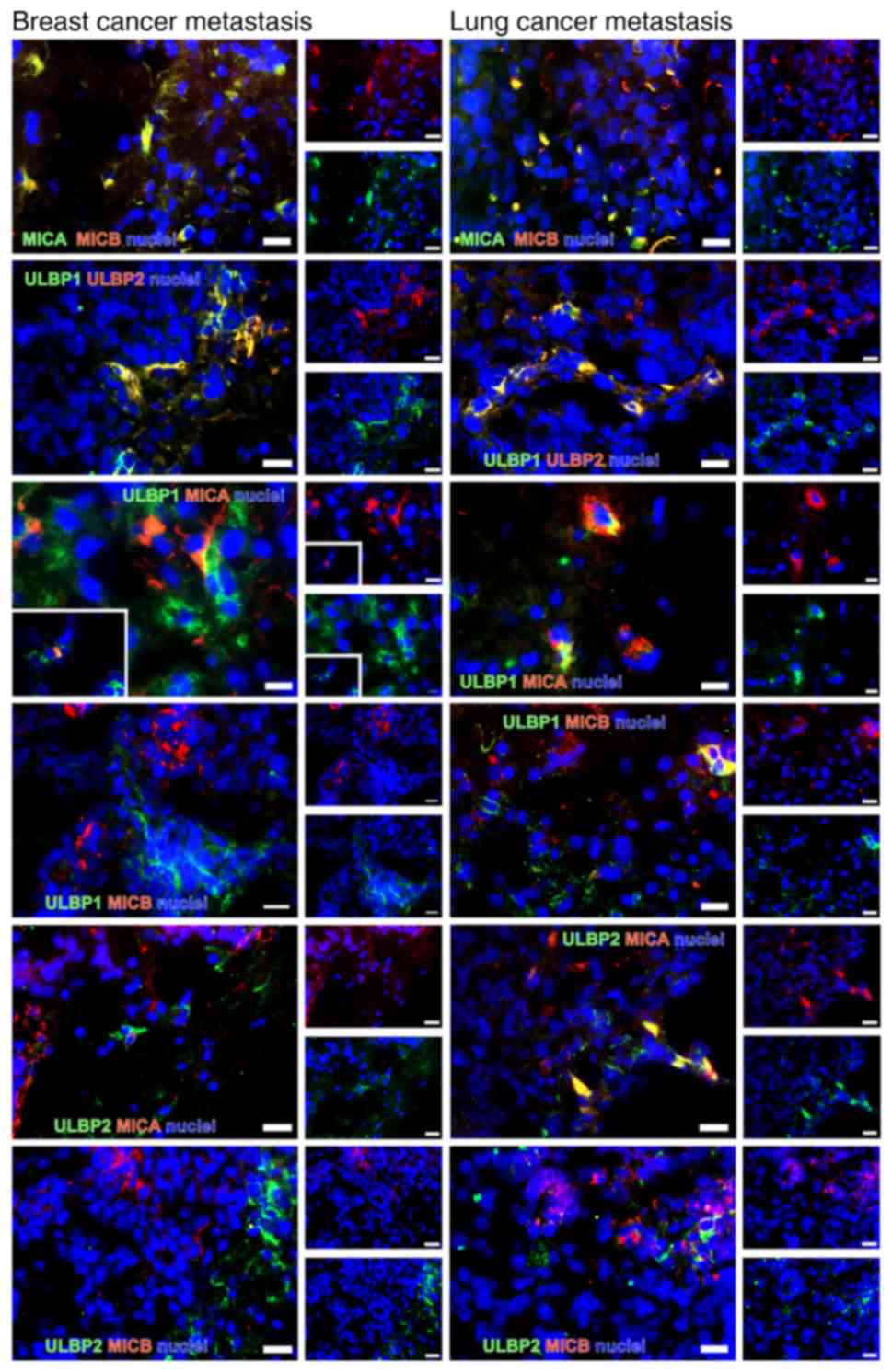
Double immunostaining of NKG2DL
The majority of the NKG2DL+ cells were
identified in small groups or as single cells scattered over the
tumor. Notably, most tumors exhibited a similar staining pattern,
irrespective of the tumor type. MICA and MICB were identified to be
co-stained. MICA was very rarely co-stained with ULBP1 and 2 in the
breast cancer tissues. In the lung cancer samples, MICA and ULBP2
were observed as co-stained. ULBP1 was co-stained with ULBP2 in
both tumor types. MICB was not co-stained with ULBP1 and 2 in
breast cancer and partially co-stained with ULBP1 in lung cancer
(Fig. 9). As observed previously
in glioma stem-like cells (45),
only a subset of NKG2DL was identified to be expressed
simultaneously in each cell.
Double immunostaining of dormancy markers
with NKG2DL
Staining pattern for 'central' and 'peripheral'
dormancy markers and NKG2DL was similar in breast and lung cancer
metastases tissues.
In breast cancer metastases, co-staining for MICA,
MICB and ULBP1 with 'central' dormancy markers H2BK and IGFBP5 was
observed in a small number of samples. EphA5 was frequently
co-stained with MICB, ULBP1 and 2. ULBP2 was identified to be
co-stained with 'central', but not 'peripheral' dormancy markers in
breast cancer tissues. Representative images are presented in
Fig. 10A.
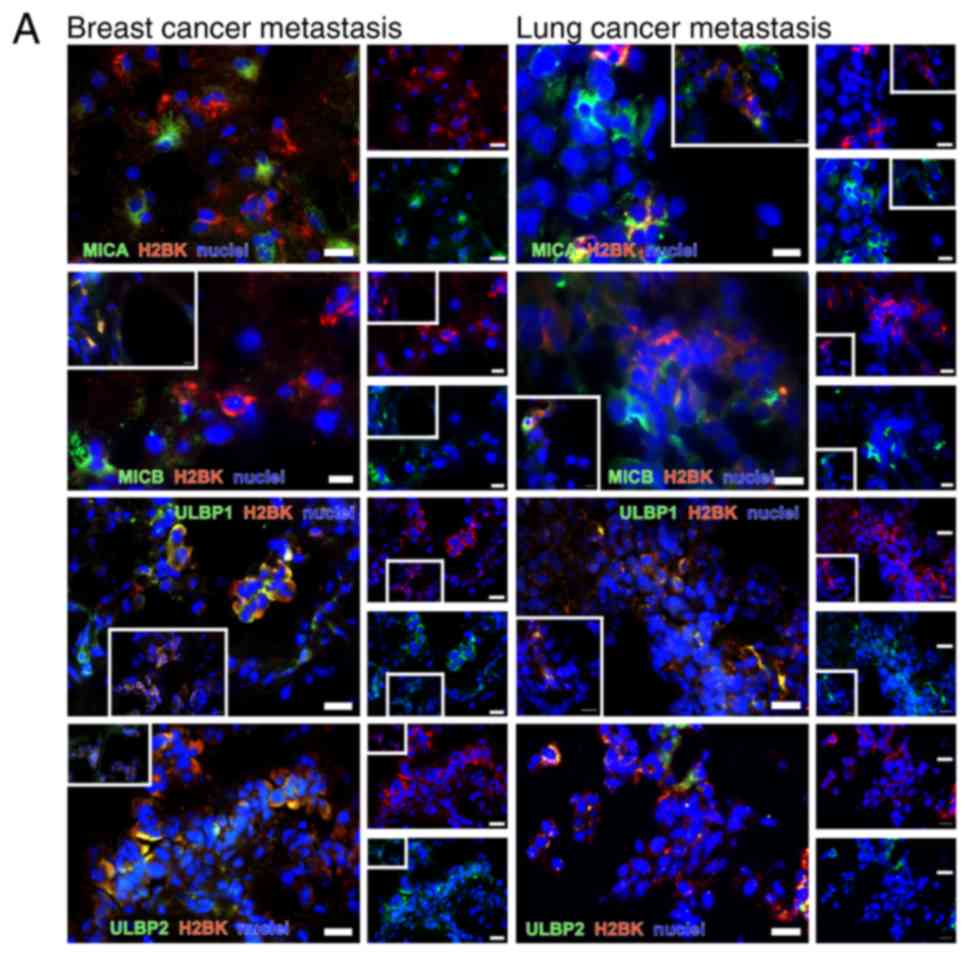 | Figure 10Double immunostaining of dormancy
markers with NKG2DL. (A) Double immunostaining of 'central'
dormancy markers with H2BK as a representative marker in
combination with NKG2DL MICA, MICB, ULBP1 and ULBP2 in cryosections
from cerebral metastases tissues from patients with breast (left
column) and lung cancer (right column). Staining analyses for EphA5
and IGFBP5 with all NKG2D ligands were also performed. (B) Double
immunostaining of 'peripheral' dormancy markers, here with PDGFβ as
an example, in combination with the NKG2DL MICA, MICB, ULBP1 and 2
in cryosections from cerebral metastases of breast (left column)
and lung cancer (right column). *Representative staining
of different tumor tissues compared with those in the larger
picture. Scale bar=20 µm. The images in the white boxes show
representative areas of the markers in the same tumor. If the image
in the white box was taken from another tumor, it was marked with
an asterix. NKG2DL, Natural Killer Group 2 member D ligands; MICA,
MHC class I chain-related protein A; MICB, MHC class I
chain-related protein B; ULBP, UL16-binding protein family; H2BK,
Histone cluster 1 H2B family member K; PDGFβ, platelet-derived
growth factor β. |
NKG2DL+ cells therefore appear to be
hallmarked by a spectrum of 'central' dormancy markers in breast
cancer metastases tumors.
In the lung cancer metastases tumors, co-staining
for MICB and ULBP1 with IGFBP5 was observed in a small number of
samples; representative images are presented in Fig. 10A. MICA and MICB were co-stained
with H2BK and EphA5 in some, but not all cells. ULBP1 was
co-stained with H2BK and EphA5 in examined all cells. Unlike in
breast cancer metastases, ULBP2 was co-stained with H2BK, IGFBP5
and EphA5 (Fig. 10A).
When analyzing the 'peripheral' dormancy markers,
similar staining patterns were observed when comparing breast and
lung cancer metastases tissues. Representative images are presented
in Fig. 10B. MICA was completely
co-stained with PDGFβ and HIF1-α. MICB was completely co-stained
with FGF2, but only partly with PDGF and HIF1-α. ULBP1 and 2 were
rarely co-stained with PDGFβ in both tumor types, completely
co-stained with HIF1-α in breast cancer, and partially co-stained
with HIF1-α in lung cancer. ULBP1 and 2 were not co-stained with
FGF2 (Fig. 10).
Although a positive NKG2DL and HIF1-α co-staining
result was considered to be representative of 'peripheral' dormancy
markers, the majority of the NKG2DL+ cells were
identified to express 'central' dormancy markers in lung cancer
metastases.
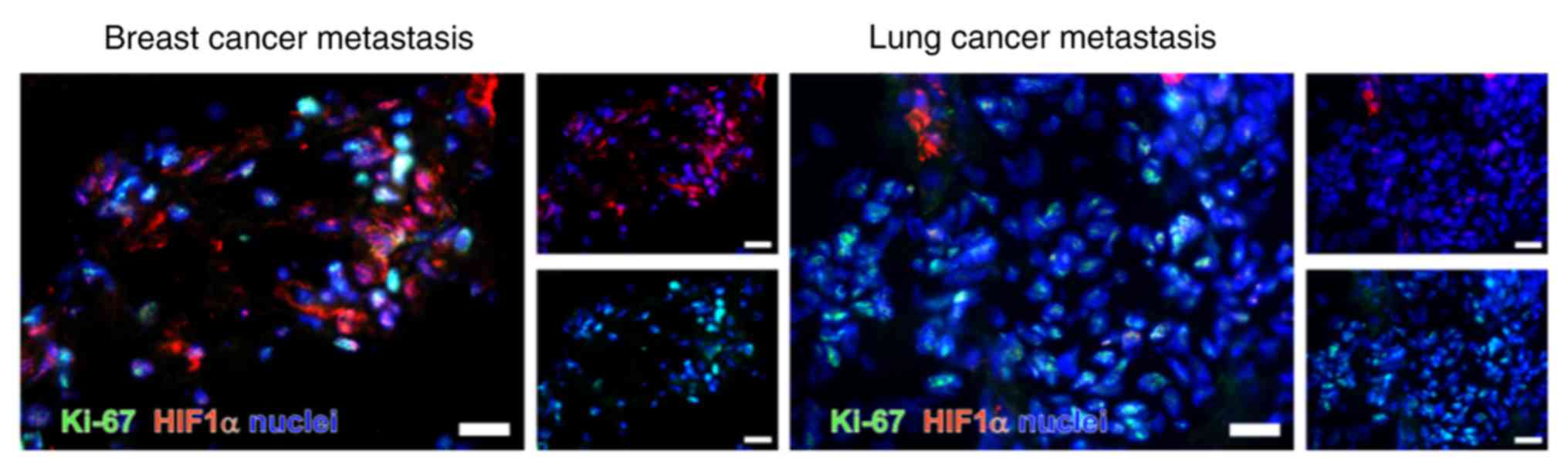
Double immunostaining of dormancy markers
with Ki-67
As previously described, Ki-67 is a protein
primarily expressed during the G1-, S-, G2- and M-phases of the
cell cycle (25). Therefore, is
not expected to be expressed in dormant cells. In the present
study, HIF1-α was co-stained with Ki-67 (Fig. 11). Ki-67 was observed to be
primarily identified in the nucleus. As indicated in Fig. 11, HIF1-α and Ki-67 were not
observed to be co-stained.
Discussion
Prognosis of patients with cerebral metastases is
poor (49,50). Mechanisms of tumor escape in the
periphery involve the NKG2D system and therapy resistance of
dormant cells. NKG2DL+ cells are usually detected by
NKG2DR+ cells and then killed by cytotoxic T-cells and
NK cells. In cells that proliferate normally, this mechanism is
avoided by the shedding of NKG2DL and its subsequent secretion in
exosomes. Dormancy, as a universal mechanism of chemo-resistance,
crucially depends on the microenvironment that surrounds the
disseminated tumor cells. The bone marrow and peri-vascular niches
in particular have been closely investigated as important sites for
the development of dormant tumor cells (46,51). To the best of our knowledge, the
role of the NKG2D system in dormant cells in the brain has not been
investigated fully.
EphA5, H2BK and IGFBP5 serve a role in
glioblastoma and cerebral metastases
RT-qPCR analysis and IHC on cerebral metastases
tissues from patients with breast and lung cancer indicated that
the 'central' dormancy markers EphA5, H2BK and IGFBP5 were
expressed at a stable level in both tumor entities.
Immunohistochemistry analysis revealed that these
'central' dormancy markers were co-stained with each other, but
only occasionally with 'peripheral' dormancy markers. 'Central'
dormancy markers therefore appeared to be expressed by a special
cell population in cerebral metastases, which may also be
identified in other types of tumors of the brain. EphA5, H2BK and
IGFBP5 as dormancy markers have been identified to be relevant in
glioblastoma (19,38). None of these 'central' dormancy
markers have been demonstrated to serve a role in the dormancy
processes occurring outside of the central nervous system.
Nevertheless, IGFBP5 has been identified in metastases of lung
cancer and low levels of IGFBP5 were observed to be associated with
improved survival in non-small cell lung cancer (52). In breast cancer, IGFBP5 serves a
role in peripheral tumor progression and is associated with tumor
cell differentiation, apoptosis and regulation of cellular growth
(53,54). EphA5 has been suggested to be
involved in anticancer processes in breast cancer, and a deletion
of EphA5 leads to a significantly increased susceptibility towards
chemotherapeutics (55). EphA5
has been demonstrated to be expressed in ~70% of all lung cancer
cells and is markedly associated with radio resistance of lung
cancer cells; the expression of EphA5 is markedly correlated with
an increased mortality rate (56). To the best of our knowledge, H2BK
expression has not been identified in the development of peripheral
cancer.
Notably, the microenvironment of the neural niche
appears to lead to the development of a central type of dormancy in
brain metastases, which is different from the type of dormancy
observed in cells in the periphery. This confirms the importance of
the microenvironment on the development of dormancy and may explain
the varying susceptibility of tumor cell populations in different
anatomical sites to chemo- and radiotherapy.
Dormancy marker expression of cerebral
metastases is tumor type-specific, but homogeneous when comparing
patients
All examined markers, in particular H2BK and IGFBP5,
were expressed at detectable levels in RT-qPCR analysis.
In the breast cancer tissues, a negative correlation
between the expression levels of the 'central' and peripheral'
dormancy markers was observed. Therefore, 'central' and
'peripheral' dormancy marker expression was used to distinguish
between the two populations of dormant cells. In the lung cancer
tumor tissues, a positive correlation between 'central' and
'peripheral' dormancy markers was observed, with exception of
EphA5, suggesting that the population of dormant cells cannot be
divided into two separate populations based on these markers.
Breast and lung cancer metastases differ concerning
the dormancy marker expression. This heterogeneity may, in part,
explain the differences observed in chemo-resistance patterns
(49), which is frequently
observed in numerous patients with brain metastases, particularly
in breast cancer (57).
Despite the clear tumor type-specific patterns
observed, the staining results were highly homogeneous among
different patients with the same tumor type. In contrast to
glioblastoma, which exhibited a very high level of inter- and
intratumoral heterogeneity, metastases clearly follow a distinct
evolutionary path, which depends on their parent tumor type
(58). The present study
demonstrated that the microenvironment of the brain affected the
dormancy marker and NKG2DL expression levels in a site-specific
manner. This is in concordance with the data from Kienast et
al (59), who demonstrated
that the unique microenvironmental architecture of the brain exerts
a selective drive on metastasized cells and leads to an induction
of vascular dormancy in the perivascular neural niche. Therefore,
it is not clear whether the distinct features of dormant metastatic
cells in the brain are induced by the surrounding cerebral
microenvironment, or whether they are selected from a minority of
cells already expressing 'central' dormancy markers.
NKG2DL are differentially expressed in
dormant cells
Similar to observations in glioblastoma and glioma
stem-like cells (43,45), the expression levels of NKG2DL in
dormant cells of cerebral metastases appear to differ on a cellular
level. The present study demonstrated that, in breast cancer
tissues, the populations of 'central' dormant cells contain a
subset of NKG2DL+ cells, which differs from the
'peripheral' dormant cells. In the breast cancer tissues, 'central'
dormancy marker-expressing cells also expressed MICB and
'peripheral' dormancy marker-expressing cells also expressed MICA,
ULBP1 and 2, as demonstrated by the RT-qPCR data. In the lung
cancer cases, MICB appeared to be expressed in cells that also
expressed 'central' dormancy markers, primarily IGFBP5, as
demonstrated by the RT-qPCR data. Notably, MICB expression was
correlated with HIF1-α expression in lung cancer metastases. This
may be due to the overall high expression level of HIF1-α in the
breast cancer metastases tissues, which was hypothesized to have
occurred due to the hypoxic cerebral microenvironment. HIF1-α is a
known key factor in the hypoxic response in cancer (60). Concerning the other NKG2DL, the
expression profile patterns of NKG2DL in the dormant cells was not
as able to distinguish between central and peripheral dormancy as
in breast cancer.
Malladi et al (61) indicated that the depletion of NK
cells in athymic mice resulted in permissive outgrowth of dormant
tumors cells of breast and lung cancer and an increased number of
bone metastases. They also demonstrated that dormant cells that
survived NK cell surveillance exhibited a downregulation of several
UL16 binding proteins (ULBPs). As the present study identified a
preserved expression of NKG2DL in cells expressing 'central'
dormancy markers, it was hypothesized that these cells may be
susceptible to therapeutic approaches targeting 'central' dormancy
markers. In addition, the mechanisms leading to downregulation of
ULBPs in dormant cells in the periphery may not occur in the
central nervous system.
Analysis of the expression of NKG2DL provides
potential new therapeutic approaches. As a mechanism of tumor
immune escape, tumor cells shed NKG2DL from their surface using a
disintegrin metalloproteinases (ADAMs) (62). There are several known inhibitors
of ADAMs (63). These may be
effective in targeting a subgroup of dormant cells in the central
nervous system.
The heterogeneity of NKG2DL expression in dormant
cells may be an important immunological escape mechanism and may
explain the heterogenous treatment responses and survival curves of
patients with cerebral metastases (49). Additionally, heterogeneity in
NKG2DL expression has been described in other tumor entities
including lymphoma (64),
melanoma (65), leukemia
(66), colorectal cancer
(67), ovarian carcinoma
(68) and glioblastoma (45). The data from the present study
demonstrates that this pattern of heterogeneity was also present in
cerebral metastases tissues from patients with breast and lung
cancer.
NKG2DL and dormancy marker RNA expression
levels differ from surface expression
In the breast cancer cases, the mRNA expression
levels of 'peripheral' dormancy markers were increased compared
with the expression levels of the 'central' dormancy markers. The
increased mRNA levels of the 'peripheral' dormancy markers were
associated with a high expression level of NKG2DL. At a protein
level, NKG2DL expression occurred primarily in cells carrying
'central' dormancy markers. In the lung cancer metastases tissues,
the absolute mRNA expression levels of the 'peripheral' dormancy
markers were also increased compared with the expression levels of
the 'central' dormancy markers; however, a positive correlation
between mRNA expression levels was observed. At the protein level,
NKG2DL+ cells were identified to express mostly
'central' dormancy markers.
Localization in-situ and protein expression
levels, which were examined using IHC, and mRNA expression, which
was examined using RT-qPCR, are not directly comparable.
Wolpert et al demonstrated that NKG2DL mRNA
and surface expression levels may differ, which is most likely due
to posttranslational and posttranscriptional modification (69). Other mechanisms, including the
induction of surface loss of MICB via the secretory pathway by
human cytomegalovirus, may also serve a role (70,71) in this process.
Study limitations
As a very limited number of patients were included
in the present study, the results are only preliminary. However,
the staining results were highly consistent between the different
patients. Nevertheless, due to the reproducibility of the results,
they may be considered as representative. Due to the limited
availability of frozen tissue and corresponding DNA,
immunocytochemistry was only performed in 5 of the 6 patients
breast cancer and in 4 of the 9 patients with lung cancer. A
quantitative analysis of protein expression would be valuable, but
would not be meaningful in the low number of patients analyzed in
the present study. The majority of the patients with breast cancer
metastases in the present cohort received chemotherapy prior to the
development and consequent surgical resection of brain metastases.
This was not the case in the patients with lung cancer; in these
patients, the brain metastases were the first detected
manifestation of the tumor in 8 of the 9 patients with lung cancer
who were included into the study. Chemotherapy is known to induce
dormancy, and therefore may have changed the expression patterns of
the markers observed in the patients with breast cancer.
Additionally, the cohort of lung cancer patients analyzed in the
present study only represented a subgroup of patients, as the
majority of cases of pulmonary adenocarcinoma are diagnosed prior
to manifestation of brain metastases. It therefore cannot be
excluded that the cohort of the present study is susceptible to
systematic bias. An additional important limitation of the present
study is that no healthy brain tissues were available to compare
the dormancy markers and NKG2DL expression profiles. This is due to
the fact that living cerebral cells and cryoprotected tissue from
healthy donors are difficult to obtain. Using tissue from patients
who underwent surgery for conditions such as epilepsy or brain
trauma would incur a significant risk of bias, as trauma, ischemia
and other pathologic stimuli are known to alter NKG2DL expression
(41). Using tissue from cadavers
is also not an acceptable alternative, as the hypoxia occurring
during death may also alter NKG2DL expression (72).
In conclusion, breast and lung cancer brain
metastases were demonstrated to express 'peripheral' dormancy
markers at increased levels compared with 'central' dormancy
markers at an mRNA level, which was predicted, due to the
peripheral origin of the tumors. Notably, EphA5, H2BK and IGFBP5,
which have been demonstrated to serve roles in glioblastoma, were
observed to be important factors in cerebral metastases. NKG2DL are
expressed at high levels, with tumor type-specific differences
observed between different members of the family. NKG2DL were
identified to be predominantly co-localized with 'central', not
'peripheral', dormancy markers in both tumor types examined. This
cell population, which was demonstrated to be positive for NKG2DL
and dormancy markers, may represent a highly attractive target for
therapy in the future.
Acknowledgments
The authors would like to thank the
medical-technical assistants Mrs Fereshteh Ebrahim and Mrs Brigitte
Rehmke (both from the Department of Neurosurgery, University
Hospital of Schleswig-Holstein, Campus Kiel, Germany) for their
expert technical support.
Funding
The present study was supported by a sponsorship
from the University Hospital of Schleswig-Holstein, Campus Kiel
(Forschungsförderung 2016, grant no. F356971; Juniorförderung 2018,
grant no. F358916) which was awarded to CF. Additionally, the study
was funded by the German Research Foundation as part as of the
Research Training Group 'Materials4Brain' (grant no. RTG2154;
P8).
Availability of data and materials
The datasets analyzed during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
CF performed the histological examination of the
cryosections, performed the statistical analysis, analyzed and
interpreted patient data, generated the figures and wrote the
manuscript. VM performed the reverse transcription-quantitative
polymerase chain reaction, immunohistochemical staining and
quantitative analysis of immunohistochemical staining. VA assisted
with the immunohistochemical staining procedure. MS and JF
supervised the project and edited the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the University of Kiel (approval no. D536/15) and was
in accordance with the Helsinki Declaration of 1964 and its later
amendments. Informed consent was obtained from all individual
patients included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Doron H, Pukrop T and Erez N: A Blazing
Landscape: Neuroinflammation shapes brain metastasis. Cancer Res.
79:423–436. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cagney DN, Martin AM, Catalano PJ, Redig
AJ, Lin NU, Lee EQ, Wen PY, Dunn IF, Bi WL, Weiss SE, et al:
Incidence and prognosis of patients with brain metastases at
diagnosis of systemic malignancy: A population-based study. Neuro
Oncol. 19:1511–1521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berghoff AS, Schur S, Füreder LM,
Gatterbauer B, Dieckmann K, Widhalm G, Hainfellner J, Zielinski CC,
Birner P, Bartsch R and Preusser M: Descriptive statistical
analysis of a real life cohort of 2419 patients with brain
metastases of solid cancers. ESMO Open. 1:e0000242016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ekici K, Temelli O, Dikilitas M, Halil
Dursun I, Bozdag Kaplan N and Kekilli E: Survival and prognostic
factors in patients with brain metastasis: Single center
experience. J BUON. 21:958–963. 2016.PubMed/NCBI
|
|
5
|
Takei H, Rouah E and Ishida Y: Brain
metastasis: Clinical characteristics, pathological findings and
molecular subtyping for therapeutic implications. Brain Tumor
Pathol. 33:1–12. 2016. View Article : Google Scholar
|
|
6
|
Luzzi KJ, MacDonald IC, Schmidt EE,
Kerkvliet N, Morris VL, Chambers AF and Groom AC: Multistep nature
of metastatic inefficiency: Dormancy of solitary cells after
successful extrava-sation and limited survival of early
micrometastases. Am J Pathol. 153:865–873. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gužvić M and Klein CA: Cancer dormancy:
Time to explore its clinical relevance. Breast Cancer Res.
15:3212013. View
Article : Google Scholar
|
|
8
|
Naumov GN, Bender E, Zurakowski D, Kang
SY, Sampson D, Flynn E, Watnick RS, Straume O, Akslen LA, Folkman J
and Almog N: A model of human tumor dormancy: An angiogenic switch
from the nonangiogenic phenotype. J Natl Cancer Inst. 98:316–325.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Townson JL and Chambers AF: Dormancy of
solitary metastatic cells. Cell Cycle. 5:1744–1750. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barkan D, El Touny LH, Michalowski AM,
Smith JA, Chu I, Davis AS, Webster JD, Hoover S, Simpson RM,
Gauldie J and Green JE: Metastatic growth from dormant cells
induced by a col-I-enriched fibrotic environment. Cancer Res.
70:5706–5716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wikman H, Vessella R and Pantel K: Cancer
micrometastasis and tumour dormancy. APMIS. 116:754–770. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Holmgren L, O'Reilly MS and Folkman J:
Dormancy of micro-metastases: Balanced proliferation and apoptosis
in the presence of angiogenesis suppression. Nat Med. 1:149–153.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Achilles EG, Fernandez A, Allred EN,
Kisker O, Udagawa T, Beecken WD, Flynn E and Folkman J:
Heterogeneity of angiogenic activity in a human liposarcoma: A
proposed mechanism for 'no take' of human tumors in mice. J Natl
Cancer Inst. 93:1075–1081. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Udagawa T, Fernandez A, Achilles EG,
Folkman J and D'Amato RJ: Persistence of microscopic human cancers
in mice: Alterations in the angiogenic balance accompanies loss of
tumor dormancy. FASEB J. 16:1361–1370. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bissell MJ and Hines WC: Why don't we get
more cancer? A proposed role of the microenvironment in restraining
cancer progression. Nat Med. 17:320–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gimbrone MA Jr, Leapman SB, Cotran RS and
Folkman J: Tumor dormancy in vivo by prevention of
neovascularization. J Exp Med. 136:261–276. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Folkman J, Watson K, Ingber D and Hanahan
D: Induction of angiogenesis during the transition from hyperplasia
to neoplasia. Nature. 339:58–61. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Almog N, Ma L, Raychowdhury R, Schwager C,
Erber R, Short S, Hlatky L, Vajkoczy P, Huber PE, Folkman J and
Abdollahi A: Transcriptional switch of dormant tumors to
fast-growing angiogenic phenotype. Cancer Res. 69:836–844. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quesnel B: Dormant tumor cells as a
therapeutic target? Cancer Lett. 267:10–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moserle L, Amadori A and Indraccolo S: The
angiogenic switch: Implications in the regulation of tumor
dormancy. Curr Mol Med. 9:935–941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koebel CM, Vermi W, Swann JB, Zerafa N,
Rodig SJ, Old LJ, Smyth MJ and Schreiber RD: Adaptive immunity
maintains occult cancer in an equilibrium state. Nature.
450:903–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kenny PA and Bissell MJ: Tumor reversion:
Correction of malignant behavior by microenvironmental cues. Int J
Cancer. 107:688–695. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barkan D, Green JE and Chambers AF:
Extracellular matrix: A gatekeeper in the transition from dormancy
to metastatic growth. Eur J Cancer. 46:1181–1188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gomis RR and Gawrzak S: Tumor cell
dormancy. Mol Oncol. 11:62–78. 2017. View Article : Google Scholar :
|
|
26
|
Walker ND, Patel J, Munoz JL, Hu M, Guiro
K, Sinha G and Rameshwar P: The bone marrow niche in support of
breast cancer dormancy. Cancer Lett. 380:263–271. 2016. View Article : Google Scholar
|
|
27
|
Tivari S, Korah R, Lindy M and Wieder R:
An in vitro dormancy model of estrogen-sensitive breast cancer in
the bone marrow: A tool for molecular mechanism studies and
hypothesis generation. J Vis Exp. e526722015.PubMed/NCBI
|
|
28
|
Patel P and Chen EI: Cancer stem cells,
tumor dormancy, and metastasis. Front Endocrinol (Lausanne).
3:1252012. View Article : Google Scholar
|
|
29
|
de Jong JS, van Diest PJ, van der Valk P
and Baak JP: Expression of growth factors, growth factor receptors
and apoptosis related proteins in invasive breast cancer: Relation
to apoptotic rate. Breast Cancer Res Treat. 66:201–208. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cervi D, Yip TT, Bhattacharya N, Podust
VN, Peterson J, Abou-Slaybi A, Naumov GN, Bender E, Almog N,
Italiano JE Jr, et al: Platelet-associated PF-4 as a biomarker of
early tumor growth. Blood. 111:1201–1207. 2008. View Article : Google Scholar
|
|
31
|
Fluegen G, Avivar-Valderas A, Wang Y,
Padgen MR, Williams JK, Nobre AR, Calvo V, Cheung JF, Bravo-Cordero
JJ, Entenberg D, et al: Phenotypic heterogeneity of disseminated
tumour cells is preset by primary tumour hypoxic microenvironments.
Nat Cell Biol. 19:120–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Endo H, Okami J, Okuyama H, Nishizawa Y,
Imamura F and Inoue M: The induction of MIG6 under hypoxic
conditions is critical for dormancy in primary cultured lung cancer
cells with activating EGFR mutations. Oncogene. 36:2824–2834. 2017.
View Article : Google Scholar
|
|
33
|
Jiang ZF, Wang M, Xu JL and Ning YJ:
Hypoxia promotes mitochondrial glutamine metabolism through
HIF1α-GDH pathway in human lung cancer cells. Biochem Biophys Res
Commun. 483:32–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abdollahi A, Hahnfeldt P, Maercker C,
Gröne HJ, Debus J, Ansorge W, Folkman J, Hlatky L and Huber PE:
Endostatin's antiangiogenic signaling network. Mol Cell.
13:649–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yancopoulos GD, Klagsbrun M and Folkman J:
Vasculogenesis, angiogenesis, and growth factors: Ephrins enter the
fray at the border. Cell. 93:661–664. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Foulstone E, Prince S, Zaccheo O, Burns
JL, Harper J, Jacobs C, Church D and Hassan AB: Insulin-like growth
factor ligands, receptors, and binding proteins in cancer. J
Pathol. 205:145–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lassalle P, Molet S, Janin A, Heyden JV,
Tavernier J, Fiers W, Devos R and Tonnel AB: ESM-1 is a novel human
endothelial cell-specific molecule expressed in lung and regulated
by cytokines. J Biol Chem. 271:20458–20464. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Adamski V, Hempelmann A, Flüh C, Lucius R,
Synowitz M, Hattermann K and Held-Feindt J: Dormant glioblastoma
cells acquire stem cell characteristics and are differentially
affected by Temozolomide and AT101 treatment. Oncotarget.
8:108064–108078. 2017. View Article : Google Scholar
|
|
39
|
Jung TY, Choi YD, Kim YH, Lee JJ, Kim HS,
Kim JS, Kim SK, Jung S and Cho D: Immunological characterization of
glioblastoma cells for immunotherapy. Anticancer Res. 33:2525–2533.
2013.PubMed/NCBI
|
|
40
|
Chitadze G, Flüh C, Quabius ES,
Freitag-Wolf S, Peters C, Lettau M, Bhat J, Wesch D, Oberg HH,
Luecke S, et al: In-depth immunophenotyping of patients with
glioblastoma multiforme: Impact of steroid treatment.
Oncoimmunology. 6:e13588392017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nausch N and Cerwenka A: NKG2D ligands in
tumor immunity. Oncogene. 27:5944–5958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Raulet DH, Gasser S, Gowen BG, Deng W and
Jung H: Regulation of ligands for the NKG2D activating receptor.
Annu Rev Immunol. 31:413–441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chitadze G, Lettau M, Luecke S, Wang T,
Janssen O, Fürst D, Mytilineos J, Wesch D, Oberg HH, Held-Feindt J
and Kabelitz D: NKG2D- and T-cell receptor-dependent lysis of
malignant glioma cell lines by human γδ T cells: Modulation by
temozolomide and A disintegrin and metalloproteases 10 and 17
inhibitors. Oncoimmunology. 5:e10932762015. View Article : Google Scholar
|
|
44
|
Chitadze G, Bhat J, Lettau M, Janssen O
and Kabelitz D: Generation of soluble NKG2D ligands: Proteolytic
cleavage, exosome secretion and functional implications. Scand J
Immunol. 78:120–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Flüh C, Chitadze G, Adamski V, Hattermann
K, Synowitz M, Kabelitz D and Held-Feindt J: NKG2D ligands in
glioma stem-like cells: Expression in situ and in vitro. Histochem
Cell Biol. 149:219–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Werner-Klein M and Klein CA: Therapy
resistance beyond cellular dormancy. Nat Cell Biol. 21:117–119.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–498. 2001.
View Article : Google Scholar
|
|
49
|
Achrol AS, Rennert RC, Anders C, Soffietti
R, Ahluwalia MS, Nayak L, Peters S, Arvold ND, Harsh GR, Steeg PS
and Chang SD: Brain metastases. Nat Rev Dis Primers. 5:52019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hall WA, Djalilian HR, Nussbaum ES and Cho
KH: Long-term survival with metastatic cancer to the brain. Med
Oncol. 17:279–286. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Carlson P, Dasgupta A, Grzelak CA, Kim J,
Barrett A, Coleman IM, Shor RE, Goddard ET, Dai J, Schweitzer EM,
et al: Targeting the perivascular niche sensitizes disseminated
tumour cells to chemotherapy. Nat Cell Biol. 21:238–250. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shersher DD, Vercillo MS, Fhied C, Basu S,
Rouhi O, Mahon B, Coon JS, Warren WH, Faber LP, Hong E, et al:
Biomarkers of the insulin-like growth factor pathway predict
progression and outcome in lung cancer. Ann Thorac Surg.
92:1805–1811; discussion 1811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Beattie J, Allan GJ, Lochrie JD and Flint
DJ: Insulin-like growth factor-binding protein-5 (IGFBP-5): A
critical member of the IGF axis. Biochem J. 395:1–19. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sureshbabu A, Okajima H, Yamanaka D,
Tonner E, Shastri S, Maycock J, Szymanowska M, Shand J, Takahashi
S, Beattie J, et al: IGFBP5 induces cell adhesion, increases cell
survival and inhibits cell migration in MCF-7 human breast cancer
cells. J Cell Sci. 125:1693–1705. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li Y, Chu J, Feng W, Yang M, Zhang Y,
Zhang Y, Qin Y, Xu J, Li J, Vasilatos SN, et al: EPHA5 mediates
trastuzumab resistance in HER2-positive breast cancers through
regulating cancer stem cell-like properties. FASEB J. 33:4851–4865.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Staquicini FI, Qian MD, Salameh A, Dobroff
AS, Edwards JK, Cimino DF, Moeller BJ, Kelly P, Nunez MI, Tang X,
et al: Receptor tyrosine kinase EphA5 is a functional molecular
target in human lung cancer. J Biol Chem. 290:7345–7359. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sperduto PW, Kased N, Roberge D, Chao ST,
Shanley R, Luo X, Sneed PK, Suh J, Weil RJ, Jensen AW, et al: The
effect of tumor subtype on the time from primary diagnosis to
development of brain metastases and survival in patients with
breast cancer. J Neurooncol. 112:467–472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Perus LJM and Walsh LA: Microenvironmental
heterogeneity in brain malignancies. Front Immunol. 10:22942019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kienast Y, von Baumgarten L, Fuhrmann M,
Klinkert WE, Goldbrunner R, Herms J and Winkler F: Real-time
imaging reveals the single steps of brain metastasis formation. Nat
Med. 16:116–122. 2010. View Article : Google Scholar
|
|
60
|
Keith B, Johnson RS and Simon MC: HIF1α
and HIF2α: Sibling rivalry in hypoxic tumour growth and
progression. Nat Rev Cancer. 12:9–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Malladi S, Macalinao DG, Jin X, He L,
Basnet H, Zou Y, de Stanchina E and Massagué J: Metastatic latency
and immune evasion through autocrine inhibition of WNT. Cell.
165:45–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chitadze G, Lettau M, Bhat J, Wesch D,
Steinle A, Fürst D, Mytilineos J, Kalthoff H, Janssen O, Oberg HH
and Kabelitz D: Shedding of endogenous MHC class I-related chain
molecules A and B from different human tumor entities:
Heterogeneous involvement of the 'a disintegrin and
metalloproteases' 10-17. Int J Cancer. 133:1557–1566. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Malemud CJ: Inhibition of MMPs and
ADAM/ADAMTS. Biochem Pharmacol. 165:33–40. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Pende D, Rivera P, Marcenaro S, Chang CC,
Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L and
Moretta A: Major histocompatibility complex class I-related chain A
and UL16-binding protein expression on tumor cell lines of
different histotypes: Analysis of tumor susceptibility to
NKG2D-dependent natural killer cell cytotoxicity. Cancer Res.
62:6178–6186. 2002.PubMed/NCBI
|
|
65
|
Vetter CS, Groh V, thor Straten P, Spies
T, Bröcker EB and Becker JC: Expression of stress-induced MHC class
I related chain molecules on human melanoma. J Invest Dermatol.
118:600–605. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Salih HR, Antropius H, Gieseke F, Lutz SZ,
Kanz L, Rammensee HG and Steinle A: Functional expression and
release of ligands for the activating immunoreceptor NKG2D in
leukemia. Blood. 102:1389–1396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Watson NF, Spendlove I, Madjd Z, McGilvray
R, Green AR, Ellis IO, Scholefield JH and Durrant LG: Expression of
the stress-related MHC class I chain-related protein MICA is an
indicator of good prognosis in colorectal cancer patients. Int J
Cancer. 118:1445–1452. 2006. View Article : Google Scholar
|
|
68
|
Castriconi R, Dondero A, Negri F, Bellora
F, Nozza P, Carnemolla B, Raso A, Moretta L, Moretta A and Bottino
C: Both CD133+ and CD133- medulloblastoma cell lines express
ligands for triggering NK receptors and are susceptible to
NK-mediated cytotoxicity. Eur J Immunol. 37:3190–3196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wolpert F, Tritschler I, Steinle A, Weller
M and Eisele G: A disintegrin and metalloproteinases 10 and 17
modulate the immunogenicity of glioblastoma-initiating cells. Neuro
Oncol. 16:382–391. 2014. View Article : Google Scholar :
|
|
70
|
Wu J, Chalupny NJ, Manley TJ, Riddell SR,
Cosman D and Spies T: Intracellular retention of the MHC class
I-related chain B ligand of NKG2D by the human cytomegalovirus UL16
glyco-protein. J Immunol. 170:4196–4200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Spreu J, Stehle T and Steinle A: Human
cytomegalovirus-encoded UL16 discriminates MIC molecules by their
alpha2 domains. J Immunol. 177:3143–3149. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang ZX, Wang S, Huang X, Min WP, Sun H,
Liu W, Garcia B and Jevnikar AM: NK cells induce apoptosis in
tubular epithelial cells and contribute to renal
ischemia-reperfusion injury. J Immunol. 181:7489–7498. 2008.
View Article : Google Scholar : PubMed/NCBI
|