Introduction
Insulin-like growth factor (IGF) mediates the
growth-promoting effects of growth hormone (GH), which is mainly
produced in the liver (1-4). IGF-1 regulates growth, glucose
uptake and protein metabolism, whereas the IGF-1-dependent effects
of GH induce insulin secretion and lipolysis (5). The GH-liver axis provides signals
related to growth and nutrient partitioning (6,7).
GH also serves an important role in somatic cell growth.
Signal transducers and activators of transcription
(STATs) are activated by various factors and cytokines and
subsequently activate Jak tyrosine kinases, leading to receptor
tyrosine phosphorylation (8).
STAT5 signaling depends on the ability of this protein to
translocate to the nucleus and bind to the nuclear response element
of a target gene (9). IGF1
expression is associated with STAT5, which binds to a region
(TTCNNNGAA) in this gene (10,11). As a result, GH is stimulated by
IGF1 expression. This process begins with the stimulation of
STAT5 via cell signaling, which increases IGF1 mRNA
expression by binding to the gene promoter (10-12).
Sulfur has long been used and collected from sulfur
stones or emulsified minerals by heating, melting and obtaining
liquid sulfur from the upper layer of the mineral. Pure sulfur is
odorless and either colorless or pale yellow, and is not
electrically conductive or water-soluble (13). Sulfur is a component of the
essential amino acids cysteine and methionine. This element has
been widely used. Historically, it was used in paints, gunpowder
and weapons. At present, it is used in industries ranging from
cosmetics to food supplements; ~85% of all sulfur is used to
produce fertilizer (14). A
number of studies have elucidated the functions of sulfur in the
human body, namely as a component of skin, bones, hair and
cartilage tissue (15,16). Sulfur is also essential for enzyme
and immune reactions (17,18).
In humans, sulfur must be consumed indirectly; it is
present in duck meat, as well as in garlic (Allium sativum),
onions (A. cepa) and hooker chives (A. hookeri)
(19,20). Direct administration of
sulfur-containing natural compounds is generally toxic to humans
and causes side effects, and thus sulfur-containing compounds are
not used directly unless their toxic components are removed
(21). The USA and other
countries use non-toxic forms of natural and dietary sulfur, e.g.,
methylsulfonylmethane (MSM) as growth enhancers, pain relievers and
drugs for rheumatoid arthritis, depression, skin hardening, cancer
and inflammatory disease (22-26). These applications indirectly
demonstrate the efficacy of MSM; however, MSM is associated with
high production costs and limited plant resources.
To utilize mineral sulfur, various substances
(boiled pine tree or black soybean extract) are mixed with
precipitated mineral sulfur, centrifuged and dehydrated to remove
harmful components (27). The
addition of non-toxic sulfur (NTS) to livestock feed has been
demonstrated to improve immunity and meat quality (28). In addition, repeated oral
administration of NTS did not induce toxicity in rats (29). However, to the best of our
knowledge, no studies have addressed the underlying mechanisms of
the effects of NTS on cell growth due to the inability to dissolve
NTS. The present study aimed to describe a method for verifying the
efficacy of NTS in vitro.
Materials and methods
Antibodies and cell culture reagents
DMEM was purchased from Gibco; Thermo Fisher
Scientific, Inc. Penicillin-streptomycin solution and fetal bovine
serum (FBS) were purchased from HyClone; GE Healthcare Life
Sciences. Trypsin-EDTA (0.05%) was obtained from Gibco; Thermo
Fisher Scientific, Inc. Antibodies specific for β-actin (cat. no.
sc-47778), GH receptor (GHR; cat. no. sc-57161), IGF-1 receptor β
(IGF-1Rβ; cat. no. sc-713), STAT5b (cat. no. sc-1656) and
horseradish peroxidase-conjugated goat anti-mouse (cat. no.
sc-516102) and anti-rabbit (cat. no. sc-2357) secondary antibodies
were obtained from Santa Cruz Biotechnology, Inc. An anti-IGF-1
antibody (cat. no. ab9572) was purchased from Abcam, and pIGF-1Rβ
(cat. no. 3021s), pJak2 (cat. no. 3776s), Jak2 (cat. no. 3230s) and
pSTAT5 (cat. no. 9314s) antibodies were purchased from Cell
Signaling Technology Inc. Recombinant growth hormone (cat. no.
100-40) was purchased from PeproTech Inc. NTS was purchased from
Nara Bio Co., Ltd.
Cell culture and treatment
Mouse muscle C2C12 cells (ATCC CRL-1772) were
cultured in DMEM supplemented with 10% FBS and 1% penicillin and
streptomycin at 37°C in 5% CO2. For each experiment,
cells at 70-80% confluence were gently washed twice with PBS. For
differentiation studies, cells at 85% confluence were transferred
in DMEM supplemented with 2% horse serum (differentiation medium)
and treated with NTS in fresh media. Unless otherwise specified,
cells were treated with 0.2 µg/ml NTS in 99.9% DMSO
(Sigma-Aldrich; Merck KGaA) for 24 h at 37°C.
Cell viability assay
Cell viability was assessed using an MTT assay
(Sigma-Aldrich; Merck KGaA). The day before treatment, cells were
re-suspended in DMEM at a density of 1×104 cells/well in 24-well culture plates.
The next day, the medium was replaced with fresh DMEM alone
(vehicle control) or with different concentrations of NTS (0.1-10
µg/ml), followed by a 24 h incubation at 37°C. Subsequently,
MTT (5 mg/ml) was added and the cells were incubated at 37°C for 4
h. The resulting formazan product was dissolved in DMSO, and the
absorbance was measured at 560 nm using an Ultra Multifunctional
Microplate Reader (Tecan US, Inc.). All measurements were performed
in triplicate, and experiments were repeated at least thrice.
Western blotting
Whole cell lysates were prepared from untreated or
NTS-treated mouse muscle cells in Radioimmunoprecipitation assay
buffer (EMD Millipore) containing phosphatase and protease
inhibitors on ice. Cells were disrupted by aspiration through a
23-gauge needle, and the lysates were centrifuged at 18,300 × g and
4°C for 10 min to remove cellular debris. Protein concentrations
were measured using the Bradford method (Thermo Fisher Scientific,
Inc.). Equal amounts of protein (100 µg/lane) were separated
by 10% SDS-PAGE and transferred onto nitrocellulose membranes. The
membranes were blocked for 1 h with 5% skimmed milk (BD
Biosciences) in TBS-T buffer (20 mM Tris-HCl, pH 7.6, 137 mM NaCl,
0.1X Tween-20). The membranes were probed overnight at 4°C with
relevant primary antibodies diluted in 5% bovine serum albumin (EMD
Millipore) or skimmed milk, washed with TBS-T and incubated for 1 h
at room temperature with horseradish peroxidase-conjugated
secondary antibodies. Visualization was performed using an Enhanced
Chemiluminescence Plus detection kit (Amersham; GE Healthcare) and
an ImageQuant LAS-4000 imaging device (Fujifilm) with LAS-4000
Control Software (Version 1.1; Fujifilm). The blots were stripped
using Restore Western Blot Stripping Buffer (Thermo Fisher
Scientific, Inc.).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from cells using the RNeasy
Mini kit (Qiagen GmbH) according to the manufacturer's protocol and
quantified spectrophotometrically at 260 nm. Subsequently, RT-PCR
analyses were performed to detect IGF-1 and GAPDH RNA expression.
Briefly, cDNA was synthesized from total RNA at 42°C for 1 h and
95°C for 5 min using a first-strand cDNA synthesis kit (cat. no.
K-2041; Bioneer Corporation) and oligo d(T) primers. A RT-PCR
Premix kit (cat. no. K-2016; Bioneer Corporation) was used to
amplify IGF-1 and GAPDH using specific primers (Bioneer
Corporation): IGF-1 forward, 5′-CTA CGC CAA TGT GGT GCT AT-3′ and
reverse, 5′-TCT GCC ATT TGC CTG AAG TT-3′ (409 bp); GAPDH forward,
5′-AAG GCC ATC ACC ATCT TCC A-3′ and reverse, 5′-ACG ATG CCA AAG
TGG TCA TG-3′ (320 bp). The PCR conditions were as follows: 95°C
for 10 min; 32 cycles at 95°C for 45 sec, 58°C for 60 sec and 72°C
for 60 sec; and 72°C for 10 min. The PCR products were resolved by
electrophoresis on a 2% agarose gel and visualized using ethidium
bromide (Sigma-Aldrich; Merck KGaA). Davinch-K Gel Imaging System
(Davinch-K Co., Ltd.) and Multi Gauge V3.1 (Fujifilm) were used for
densitometry analysis.
Transfection and STAT5b overexpression
analysis
C2C12 cells (2×105) were cultured in
6-well plates to 60% confluence. The cells were transfected with a
STAT5b-pMX vector (22) (kindly
provided by Dr Koichi Ikuta, Kyoto University, Japan) using the
DharmaFECT transfection reagent (GE Healthcare Dharmacon, Inc.) for
24 h at 37°C. Transfected cells were washed with ice-cold PBS and
treated for 24 h with media containing DMSO with or without NTS.
Western blotting was used to isolate and analyze target protein and
β-actin expression levels as described above.
Small interfering RNA (siRNA)
transfection and analysis
C2C12 cells (2×105) were cultured in
6-well plates to 60% confluence and subsequently transfected with
On-Target plus SMARTpool siRNA specific for STAT5b or non-targeting
siRNA (cat. no. L-010539-00-0005; GE Healthcare Dharmacon) with
FuGENE6 (Roche Diagnostics) according to the manufacturer's
instructions. After 24 h, NTS was added for another 24 h at 37°C.
Proteins were isolated and subjected to western blotting for
further analysis as described above.
Chromatin immunoprecipitation (ChIP)
assay
The ChIP assay was performed using the
Imprint® Chromatin Immunoprecipitation kit
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. Briefly, mouse muscle cells were fixed with 1%
formaldehyde for 10 min at room temperature and quenched with 1.25
M glycine. Following washing with PBS, the cells were suspended in
nuclear preparation buffer and shearing buffer and sonicated under
optimized conditions (amplitude, 25%; pulse, 30 sec on/30 sec off
for 20 min on ice). The sheared DNA was centrifuged at 21,000 × g
for 10 min at 4°C, and the cleared supernatant was subjected to
protein/DNA immunoprecipitation. The clarified supernatant was
diluted with the dilution buffer (ratio, 1:1), and 5 µl
aliquots of the diluted samples were used as internal controls. The
diluted supernatant was incubated with anti-STAT5b antibodies in
Parafilm pre-coated wells for 90 min. The controls were incubated
with normal goat IgG and anti-RNA polymerase II. Unbound DNA was
removed using immunoprecipitation wash buffer, and bound DNA was
collected using the cross-link reversal method with DNA release
buffer containing proteinase K. The released DNA and internal
control DNA were purified using the GenElute Binding Column G. DNA
was then quantified using conventional quantitative PCR. The primer
sequences were as follows: IGF-1 forward 5′-CCA CAC ACA CCT ATT CAC
CC-3′ and reverse, 5′-CCT GGA GCC ATA GGG TAT GA-3′. The qPCR
conditions were as follows: 95°C for 3 min; 40 cycles at 95°C for
30 sec, 60°C for 30 sec and 72°C for 40 sec; and 72°C for 5
min.
Growth hormone assay
Analysis of the growth hormone levels was conducted
using Human Growth Hormone SimpleStep® ELISA kit
(ab190811; Abcam). C2C12 cells (2×105) were cultured in
6-well plates to 60% confluence and treated with 99.9% DMSO and NTS
for 24 h. The samples were centrifuged at 2,000 × g for 10 min, and
the supernatants were collected. The samples were then added to
96-well plate along with equal amounts of antibody cocktail and
incubated for 1 h at room temperature on a plate shaker at 400 rpm.
The plates were washed with 1X wash buffer, and the development
solution was added and incubated for 10 min in the dark on a plate
shaker set to 400 rpm. Finally, the stop solution was added, and
optical density was recorded at 450 nm using an Ultra
Multifunctional Microplate Reader (Tecan US, Inc.). All
measurements were performed in triplicate, and experiments were
repeated at least thrice.
Statistical analysis
Data are expressed as the mean ± SEM of at least
three experiments. Statistical analysis was performed using
Student's t-test or one-way ANOVA with Tukey's post hoc test.
Analyses were performed using the SAS 9.3 program (SAS Institute,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
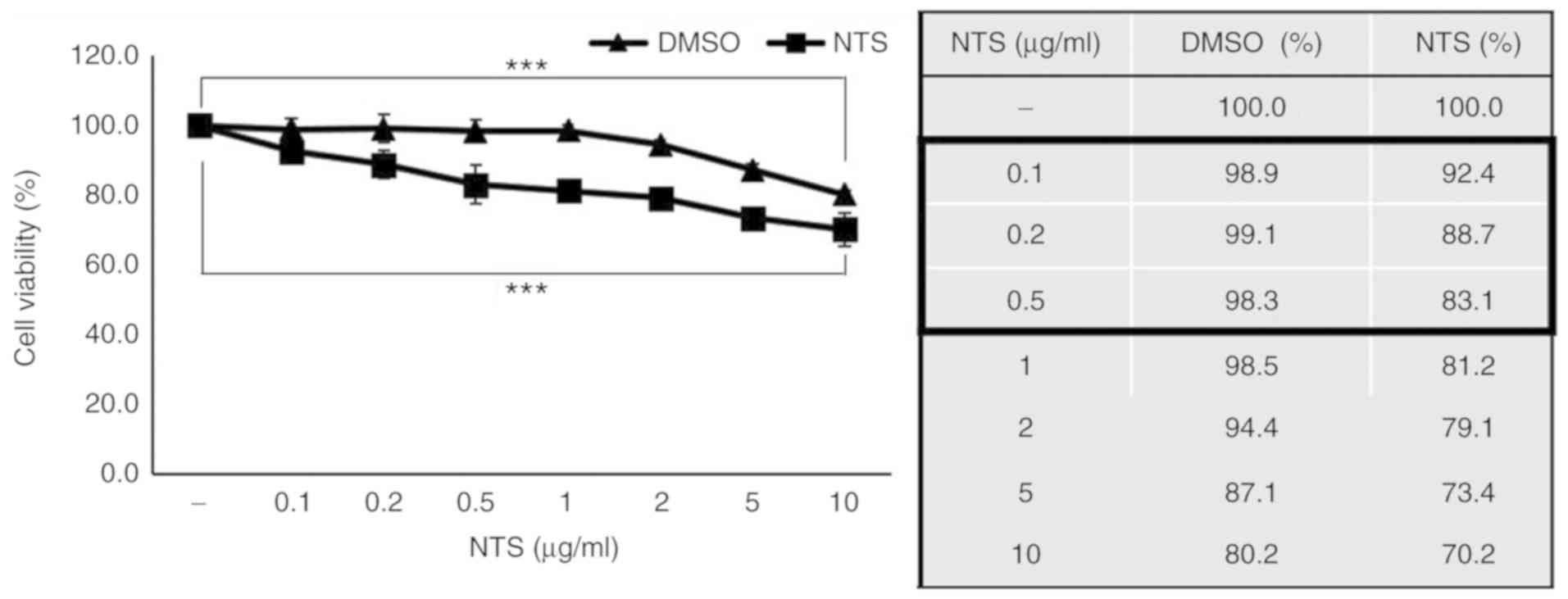
NTS induces C2C12 cell death
NTS is not easily dissolved in common solvents such
as water, ethanol or DMSO. Through a series of experiments
involving normal and heated water, DMSO and ethanol, NTS was
determined to dissolve completely in DMSO at a maximum
concentration of 1 mg/ml (Table
SI and Fig. S1). To evaluate
the effects of NTS on C2C12 cell proliferation, MTT assay was
conducted using 0.1-10 µg/ml NTS and DMSO alone (control).
NTS induced significantly more cell death compared with an equal
amount of DMSO (Fig. 1). For
further experiments, NTS concentrations <0.5 µg/ml were
selected, since they did not induce significantly greater cell
death compared with the controls.
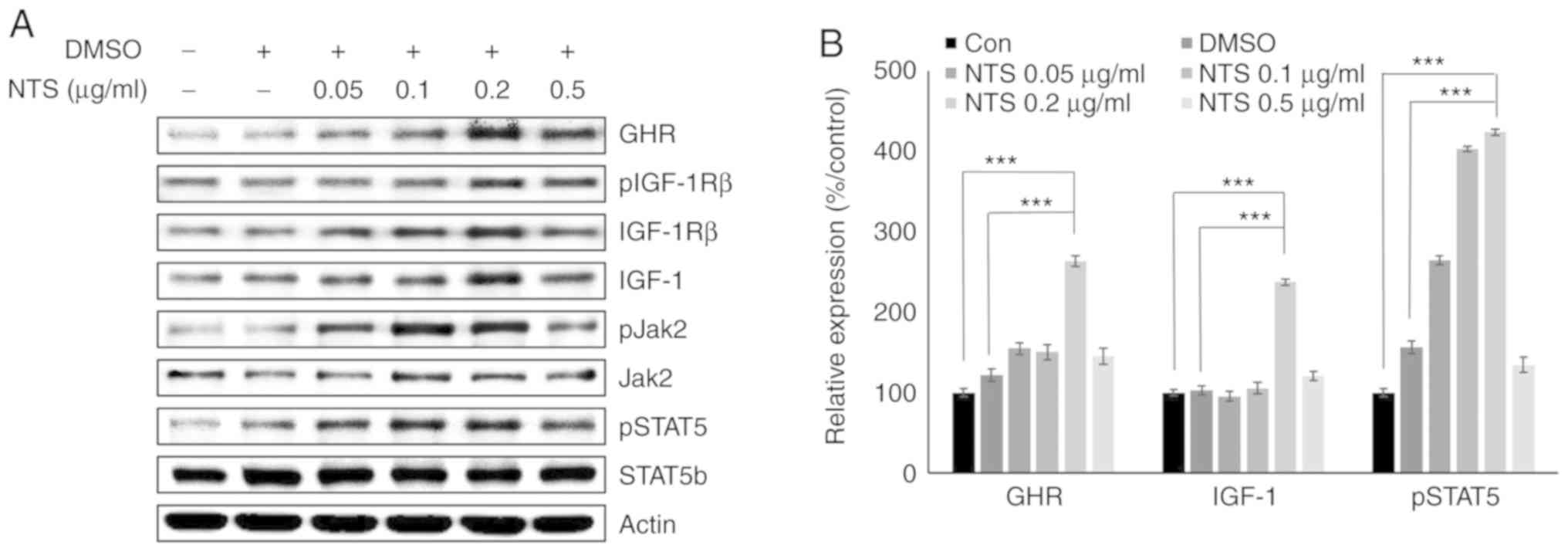
NTS increases the expression of GHR,
pSTAT5 and IGF-1 in C2C12 cells
In the MTT assay, NTS concentrations ≤0.5
µg/ml induced <18% mortality in C2C12 mouse muscle cells.
Western blotting was used to analyze translational expression of
GHR, pSTAT5 and IGF-1 in C2C12 cells treated with 0.05-0.5
µg/ml NTS. The results demonstrated that compared with lower
concentrations, 0.2 µg/ml NTS increased the expression of
GHR, pIGF-1Rβ, pJak2, pSTAT5 and IGF-1 without inducing notable
changes in total Jak2, STAT5b and IGF-1Rβ (Figs. 2A and S3). Slight decreases in the levels of
GHR, pSTAT5 and IGF-1 proteins were observed at 0.5 µg/ml
NTS, possibly due to increased cell death (Fig. 2B). To confirm the ability of NTS
to induce growth hormone signaling, a growth hormone assay was
performed in C2C12 cells in presence of NTS, and the results
revealed increased levels of growth hormone in NTS compared with
control cells (Fig. S2).
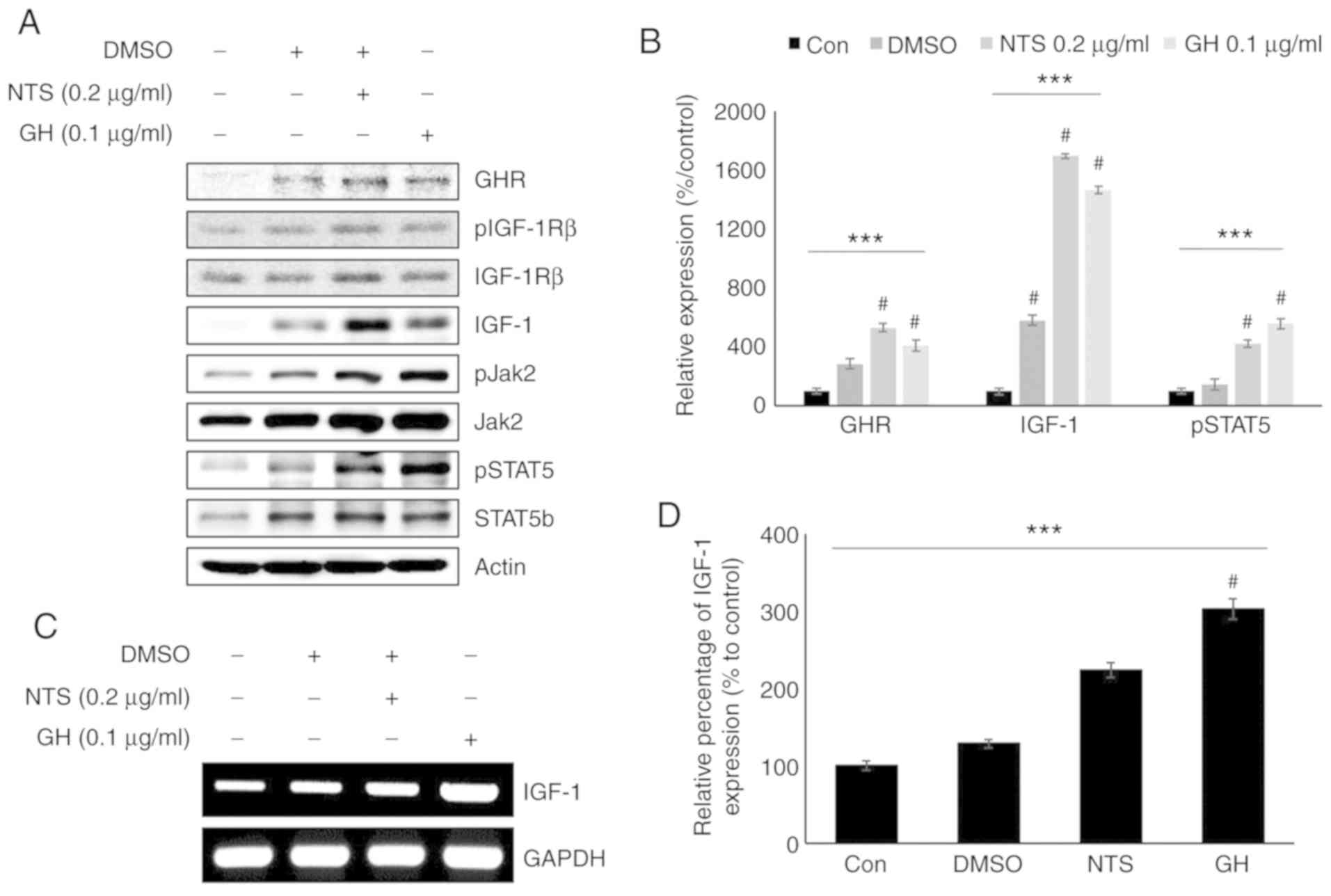
NTS increases the expression of pSTAT5
and IGF-1, similar to GH signaling
As NTS increased the levels of GHR, pSTAT5 and
IGF-1, it was hypothesized that it may exert similar effects to
those of GH signaling. Western blotting was used to analyze cells
treated with 0.1 µg/ml recombinant GH (Fig. 3A). The patterns of GHR, STAT5b,
pSTAT5, Jak2, pJak2 and IGF-1 expression were similar between cells
treated with NTS and GH (Figs. 3B
and S4). pIGF-1Rβ and IGF-1Rβ
expression did not exhibit any differences compared with the
control. Igf1 mRNA levels in the presence of 0.2
µg/ml NTS or 0.1 µg/ml GH were also evaluated
(Fig. 3C). Similar to the protein
levels, increased Igf1 mRNA expression was observed in
response to NTS and GH, suggesting that these factors induce
similar signaling pathways (Fig.
3D).
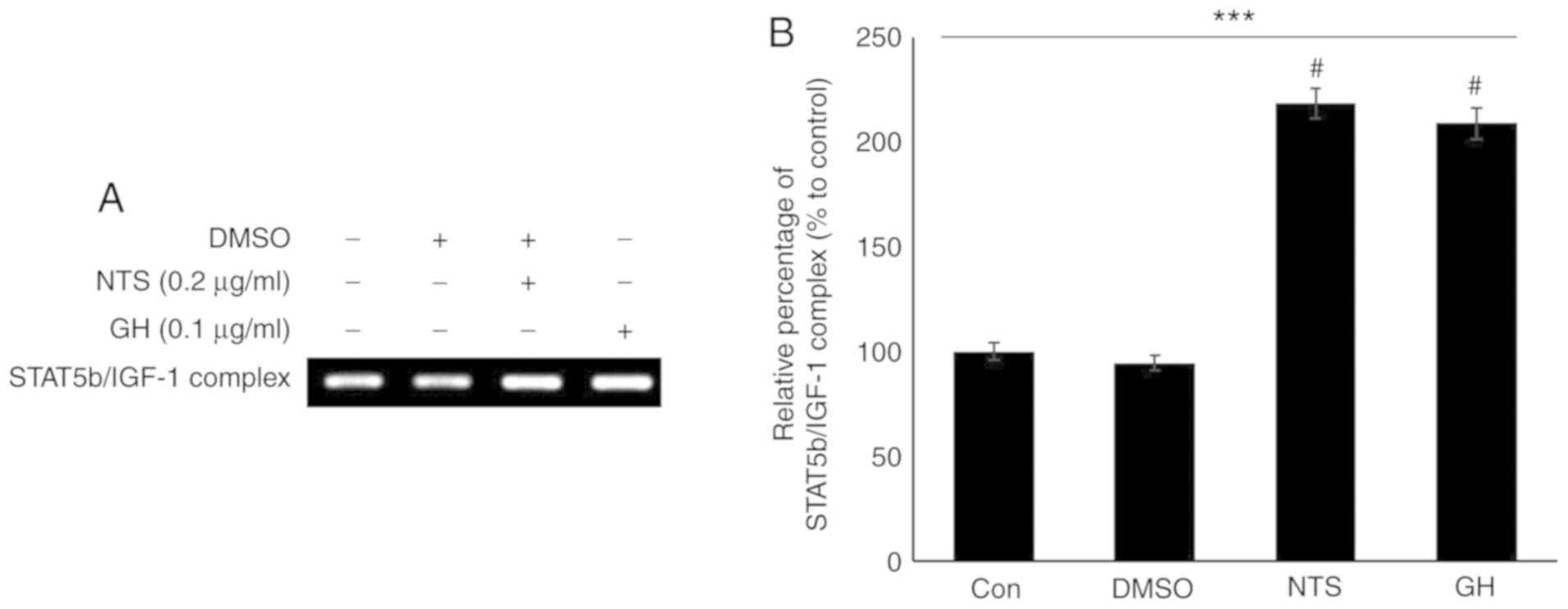
NTS and GH increase the binding of STAT5b
to the Igf1 promoter
As NTS and GH signaling exhibited similar effects,
the effects of NTS on the binding of STAT5b to the Igf1
promoter region were evaluated using 0.1 µg/ml recombinant
GH for comparison. The primer was designed to cover the Igf1
promoter region. The results of ChIP with a STAT5b antibody
demonstrated that 0.2 µg/ml NTS increased the binding
activity of STAT5b to the Igf1 promoter and thus may have
initiated transcription (Fig.
4A). The increased formation of the STAT5b/Igf1 complex
in response to GH signaling also suggested similarity with NTS
activity (Fig. 4B).
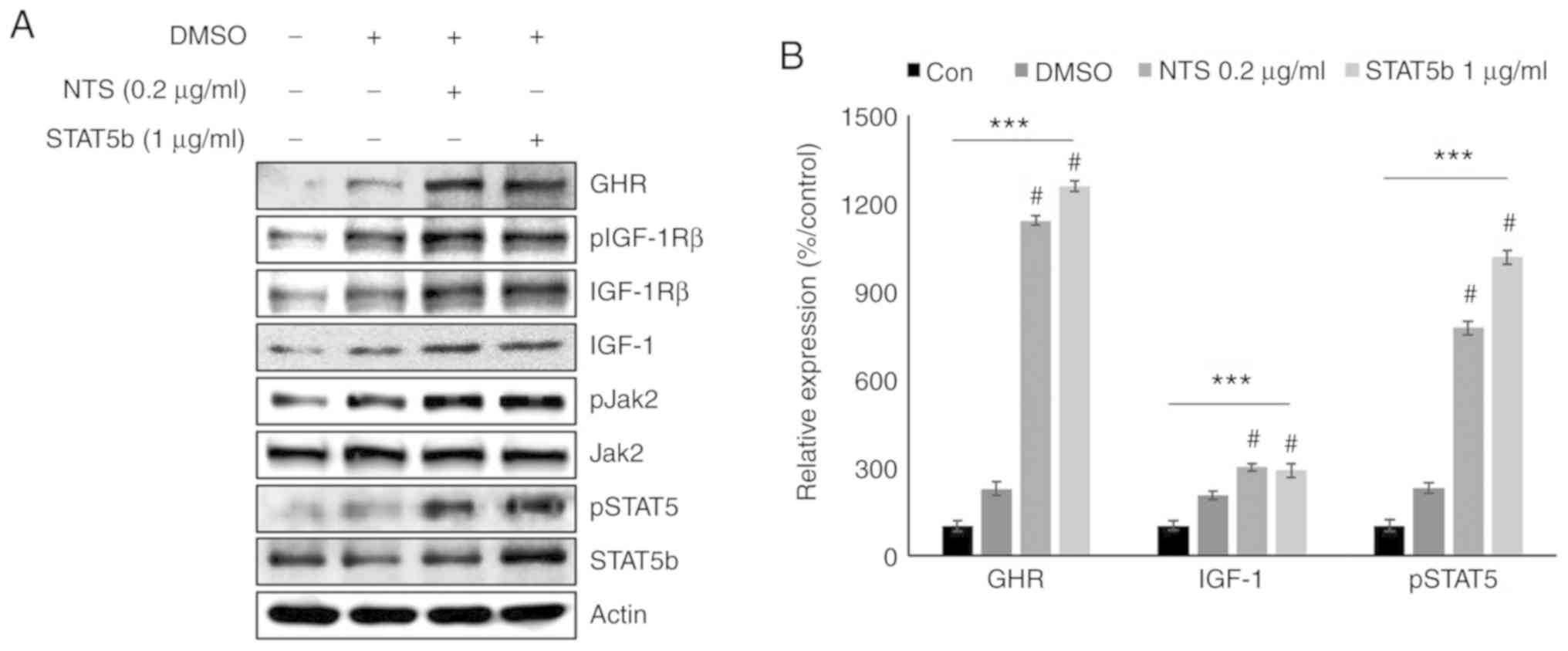
NTS increases the expression of GHR and
IGF-1 similar to STAT5b signaling
Considering the similarities between NTS activity
and GH signaling, the role of STAT5b was compared with NTS
treatment by overexpressing STAT5b. Western blotting analysis of
NTS-treated and STAT5b-overexpressing cells exhibited similar
increases in the levels of GHR, IGF-1 and pSTAT5 (Fig. 5A). NTS also upregulated pJak2,
which suggested that it may induce GH signaling by regulating
Jak2/STAT5b/IGF-1 signaling. Similar upregulation of GHR in
response to NTS treatment and STAT5b overexpression also suggested
an association between these signaling axes (Fig. 5B).
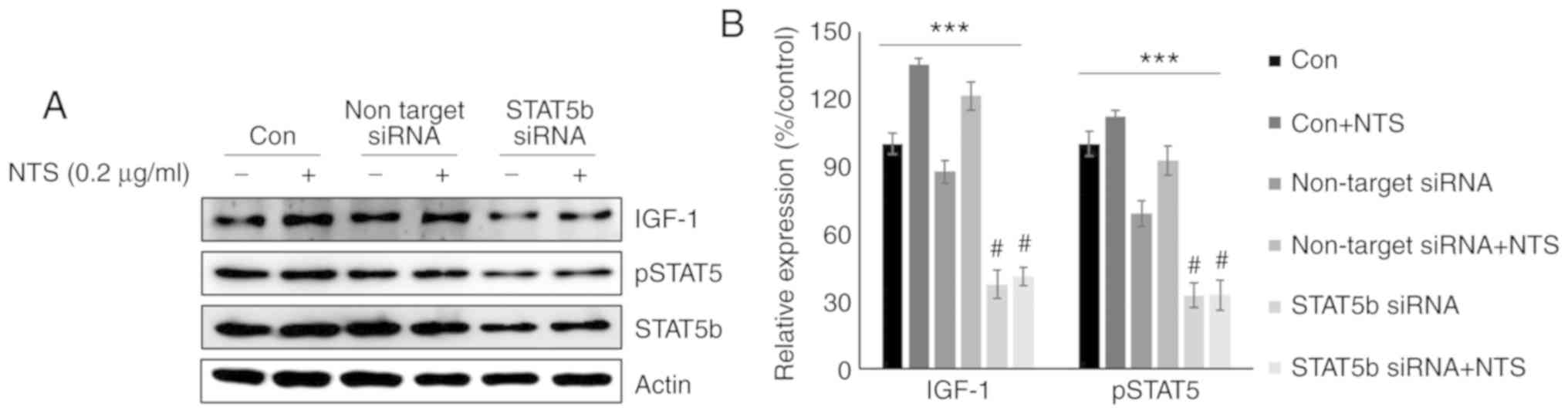
STAT5b regulates IGF-1 expression in
C2C12 cells following NTS treatment
To confirm the role of STAT5b in the NTS-mediated
regulation of IGF-1, Stat5b expression was silenced using
siRNA. Western blotting analysis of on-target STAT5b inhibition
revealed increases in the levels of pSTAT5 and IGF-1 following NTS
treatment (Fig. 6A). The relative
levels of pSTAT5 and IGF-1 suggested that STAT5b may be a key
mediator of the effects of NTS in C2C12 mouse muscle cells
(Fig. 6B). These results
suggested that NTS may act as a GH mimic to regulate STAT5b and
IGF-1 signaling.
Discussion
Sulfur is the third-most abundant mineral element
present in the human body after calcium and phosphorous, and it is
used in metabolism (30). As
direct sulfur administration is generally toxic, this element is
normally derived from dietary protein sources. NTS is important as
it enables non-toxic sulfur supplementation that can be delivered
via non-dietary methods. However, the research on NTS is
preliminary, and a suitable solvent for NTS is uncertain. In the
present study, NTS was successfully dissolved in DMSO ≤1 mg/ml.
Sulfur is essential for growth and development. The
sulfur-containing amino acids methionine and cysteine are required
for protein synthesis and optimal growth (31). Sulfur exerts antibacterial effects
against acne-causing bacteria (32) and facilitates the shedding of
skin, and thus could be used to treat certain skin conditions
(33). External sulfur therapy
may be an effective growth-promoting option in a system that cannot
produce sulfur-containing amino acids. The present study
hypothesized that NTS may act a as growth factor by mimicking GH
signaling. GH is an important promoter of stem cell activation,
cell growth and differentiation (34). GHR is a class 1 receptor to which
GH binds to promote cell growth (35). A previous study demonstrated that
MSM could enhance GH signaling by regulating the Jak2/STAT5b
pathway (36). Therefore, it was
hypothesized in the present study that NTS may enhance GH activity
in C2C12 mouse muscle cells. The results demonstrated an increase
of GH signaling by NTS in C2C12 cells, which indicated the ability
of NTS to enhance GH activity.
GH promotes cell growth by regulating Jak2 and
STAT5b activity and subsequently inducing IGF-1 expression
(37). STAT5b acts as a
transcription factor for IGF-1 (11). GH binds to GHR to promote cell
proliferation, whereas GHR knockdown prevents the phosphorylation
of STAT5 by Jak2, thus inhibiting growth (38). In the present study, NTS
upregulated the expression of GHR, pSTAT5 and IGF-1, suggesting a
role in growth enhancement similar to that of GH. Therefore, the
effects of NTS were compared with those of recombinant GH in C2C12
cells; the results demonstrated that both NTS and GH upregulated
the levels of GHR, pSTAT5, pJak2 and IGF-1. These results confirmed
that NTS may act similarly to GH or mimic GH signaling to promote
cell growth. NTS and GH also increased the formation of the
STAT5b/Igf1 complex, which further confirmed that enhanced
STAT5b signaling may be mediated by IGF-1.
STAT5b serves an important role in cell growth, as
GH promotes cell growth by regulating IGF-1 production via the
GHR/STAT5b cascade (39). IGF-1
production also depends on Jak2-dependent STAT5b phosphorylation
during cell growth and development (40). The present study evaluated the
role of STAT5b in NTS-mediated cell growth enhancement by comparing
the effects of NTS and STAT5b overexpression. Of note, cells
treated with NTS or overex-pressing STAT5b exhibited increased
levels of GHR, pJak2, pSTAT5 and IGF-1. These results confirmed
that NTS may enhance GH signaling by regulating the
Jak2/STAT5b/IGF-1 signaling cascade. STAT5b is a major molecule
associated with sulfur-containing compound-mediated growth
enhancement (41). Combined
STAT5b knockdown and NTS treatment increased the expression of
IGF-1, thus confirming the role of STAT5b in mediating the effect
of NTS on GH signaling.
In conclusion, NTS may act to enhance GH signaling
by upregulating the expression of GHR. Specifically, NTS enhanced
GH signaling by regulating the Jak2/STAT5b/IGF-1 signaling pathway
in C2C12 mouse muscle cells. STAT5b may also serve a vital role in
the ability of NTS to enhance GH signaling.
Supplementary Data
Funding
This study was supported by Nara Bio Co., Ltd.,
Republic of Korea in 2018 and the National Research Foundation of
Korea (NRF) grant funded by the Korea government (MSIT) (grant no.
2018R1C1B6006146).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DYK and NS conceived, designed and performed the
experiments and wrote the manuscript. YMY and K-JJ contributed in
designing the experiments and data analysis. ESJ, HDK, IHK and SWB
analyzed the data. All authors contributed to revising the
manuscript and approved the final version for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Hyoung Do Kim is affiliated with Nara Bio Co., Ltd.,
which provided funding for this study and supplied non-toxic
sulfur. The remaining authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Duan C: Specifying the cellular responses
to IGF signals: Roles of IGF-binding proteins. J Endocrinol.
175:41–54. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sjogren K, Liu JL, Blad K, Skrtic S, Vidal
O, Wallenius V, LeRoith D, Törnell J, Isaksson OG, Jansson JO and
Ohlsson C: Liver-derived insulin-like growth factor I (IGF-I) is
the principal source of IGF-I in blood but is not required for
postnatal body growth in mice. Proc Natl Acad Sci USA.
96:7088–7092. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin MB and Stoica A: Insulin-Like
growth factor-I and estrogen interactions in breast cancer. J Nutr.
132:3799S–3801S. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krajcik RA, Borofsky ND, Massardo S and
Orentreich N: Insulin-like growth factor I (IGF-I), IGF-binding
proteins, and breast cancer. Cancer Epidemiol Biomarkers Prev.
11:1566–1573. 2002.PubMed/NCBI
|
|
5
|
Dominici FP, Argentino DP, Munoz MC,
Miquet JG, Sotelo AI and Turyn D: Influence of the crosstalk
between growth hormone and insulin signalling on the modulation of
insulin sensitivity. Growth Horm IGF Res. 15:324–336. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mingarro M, Vega-Rubin de Celis S, Astola
A, Pendon C, Valdivia MM and Perez-Sanchez J: Endocrine mediators
of seasonal growth in gilthead sea bream (Sparus aurata): The
growth hormone and somatolactin paradigm. Gen Comp Endocrinol.
128:102–111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beckman BR and Dickhoff WW: Plasticity of
smolting in spring chinook salmon: Relation to growth and
insulin-like growth factor-I. J Fish Biol. 53:808–826. 1998.
View Article : Google Scholar
|
|
8
|
Joung YH, Lim EJ, Lee MY, Park JH, Ye SK,
Park EU, Kim SY, Zhang Z, Lee KJ, Park DK, et al: Hypoxia activates
the cyclin D1 promoter via the Jak2/STAT5b pathway in breast cancer
cells. Exp Mol Med. 37:353–364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Furth PA: STAT signaling in different
breast cancer sub-types. Mol Cell Endocrinol. 382:612–615. 2014.
View Article : Google Scholar
|
|
10
|
Wang Y and Jiang H: Identification of a
distal STAT5-binding DNA region that may mediate growth hormone
regulation of insulin-like growth factor-I gene expression. J Biol
Chem. 280:10955–10963. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joung YH, Lee MY, Lim EJ, Kim MS, Hwang
TS, Kim SY, Ye SK, Lee JD, Park T, Woo YS, et al: Hypoxia activates
the IGF-1 expression through STAT5b in human HepG2 cells. Biochem
Biophys Res Commun. 358:733–738. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalita A, Gupta S, Singh P, Surolia A and
Banerjee K: IGF-1 stimulated upregulation of cyclin D1 is mediated
via STAT5 signaling pathway in neuronal cells. IUBMB Life.
65:462–471. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meyer B: Elemental sulfur. Chem Rev.
76:367–388. 1976. View Article : Google Scholar
|
|
14
|
Mattiello EM, da Silva RC, Degryse F,
Baird R, Gupta VV and McLaughlin MJ: Sulfur and zinc availability
from co-granulated zn-enriched elemental sulfur fertilizers. J
Agric Food Chem. 65:1108–1115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bragulla HH and Homberger DG: Structure
and functions of keratin proteins in simple, stratified,
keratinized and cornified epithelia. J Anat. 214:516–559. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muizzuddin N and Benjamin R: Beneficial
effects of a sulfur-containing supplement on hair and nail
condition. Nat Med J. 11:2019.
|
|
17
|
Grimble RF: The effects of sulfur amino
acid intake on immune function in humans. J Nutr. 136:1660S–1665S.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grimble RF and Grimble GK:
Immunonutrition: Role of sulfur amino acids, related amino acids,
and polyamines. Nutrition. 14:605–610. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Block E: The organosulfur chemistry of the
Genus Allium-Implications for the organic chemistry of sulfur.
Angew Chem Int Ed Engl. 31:1135–1178. 1992. View Article : Google Scholar
|
|
20
|
Koh E and Surh J: Influence of sulfur
fertilization on the antioxidant activities of onion juices
prepared by thermal treatment. Prev Nutr Food Sci. 21:160–164.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim CH and Choi GH: Growth inhibition of
extract from sulfur fed duck carcass against various cancer cell
lines. Korean J Food Sci Ani Resour. 22:348–351. 2002.
|
|
22
|
Kang DY, Darvin P, Yoo YB, Joung YH, Sp N,
Byun HJ and Yang YM: Methylsulfonylmethane inhibits HER2 expression
through STAT5b in breast cancer cells. Int J Oncol. 48:836–842.
2016. View Article : Google Scholar
|
|
23
|
Lim EJ, Hong DY, Park JH, Joung YH, Darvin
P, Kim SY, Na YM, Hwang TS, Ye SK, Moon ES, et al:
Methylsulfonylmethane suppresses breast cancer growth by
down-regulating STAT3 and STAT5b pathways. PLoS One. 7:e333612012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Joung YH, Darvin P, Kang DY, Sp N, Byun
HJ, Lee CH, Lee HK and Yang YM: Methylsulfonylmethane inhibits
RANKL- induced osteoclastogenesis in BMMs by suppressing NF-κB and
STAT3 activities. PLoS One. 11:e01598912016. View Article : Google Scholar
|
|
25
|
Caron JM, Bannon M, Rosshirt L, Luis J,
Monteagudo L, Caron JM and Sternstein GM: Methyl sulfone induces
loss of metastatic properties and reemergence of normal phenotypes
in a metastatic cloudman S-91 (M3) murine melanoma cell line. PLoS
One. 5:e117882010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miller LE: Methylsulfonylmethane decreases
inflammatory response to tumor necrosis factor-α in cardiac cells.
Am J Cardiovasc Dis. 8:31–38. 2018.
|
|
27
|
Kwon MD: Method of manufacturing natural
edible sulfur. US Patent US20100203224A1. Filed April 8, 2010;
issued August 12, 2010.
|
|
28
|
Lim CI, Choe HS, Kang C, Lee BK and Ryu
KS: Effects of dietary organic sulfur on performance, egg quality
and cell-mediated immune response of laying hens. Korean J Poult
Sci. 45:97–107. 2018. View Article : Google Scholar
|
|
29
|
Lee JS, Kwon JK, Han SH, An IJ, Kim SJ,
Lee SH, Park YS, Park BK, Kim BS, Kim S, et al: Toxicity study of
detoxication sulphur at 3 months post-treatment in rats. J Fd Hyg
Safety. 25:263–268. 2010.
|
|
30
|
Nimni ME, Han B and Cordoba F: Are we
getting enough sulfur in our diet? Nutr Metab (Lond). 4:242007.
View Article : Google Scholar
|
|
31
|
Griffith OW: Mammalian sulfur amino acid
metabolism: An overview. Methods Enzymol. 143:366–376. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mills OH Jr and Kligman AM: Is sulphur
helpful or harmful in acne vulgaris? Br J Dermatol. 86:620–627.
1972. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu TY: Mechanism and treatment of sulfur
mustard-induced cutaneous injury. Chin J Traumatol. 17:345–350.
2014.PubMed/NCBI
|
|
34
|
Messias de Lima CF, Dos Santos Reis MD, da
Silva Ramos FW, Ayres-Martins S and Smaniotto S: Growth hormone
modulates in vitro endothelial cell migration and formation of
capillary-like structures. Cell Biol Int. 41:577–584. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang S, Wu J, Wang N, Zeng L and Wu Y: The
role of growth hormone receptor in beta cell function. Growth Horm
IGF Res. 36:30–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Joung YH, Lim EJ, Darvin P, Chung SC, Jang
JW, Do Park K, Lee HK, Kim HS, Park T and Yang YM: MSM enhances GH
signaling via the Jak2/STAT5b pathway in osteoblast-like cells and
osteoblast differentiation through the activation of STAT5b in
MSCs. PLoS One. 7:e474772012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Waters MJ and Brooks AJ: Growth hormone
and cell growth. Endocr Dev. 23:86–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rowland JE, Lichanska AM, Kerr LM, White
M, d'Aniello EM, Maher SL, Brown R, Teasdale RD, Noakes PG and
Waters MJ: In vivo analysis of growth hormone receptor signaling
domains and their associated transcripts. Mol Cell Biol. 25:66–77.
2005. View Article : Google Scholar :
|
|
39
|
Hwa V: STAT5B deficiency: Impacts on human
growth and immunity. Growth Horm IGF Res. 28:16–20. 2016.
View Article : Google Scholar :
|
|
40
|
Chaudhari A, Gupta R, Patel S, Velingkaar
N and Kondratov R: Cryptochromes regulate IGF-1 production and
signaling through control of JAK2-dependent STAT5B phosphorylation.
Mol Biol Cell. 28:834–842. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Preetha NS, Kang DY, Darvin P, Kim DN,
Joung YH, Kim SY, Cho KH, Do CH, Park KD, Lee JH, et al: Induction
of in vitro ketosis condition and suppression using
methylsulfonylmethane by altering ANGPTL3 expression through STAT5b
signaling mechanism. Anim Cells Syst. 19:30–38. 2015. View Article : Google Scholar
|