Introduction
Placentation is a dynamic and complicated process.
After implantation, trophoblasts differentiate into cyto- and
syncytiotrophoblast cells, and proliferate much more rapidly than
embryo cells, creating an organ in just a few weeks that properly
fulfils its function and supports foetus development (1). Any abnormalities in formation and
function of the placenta may have serious consequences in foetal
life, like intrauterine growth restriction (IUGR) (2), preeclampsia (3), miscarriage (4), as well as long-term complications
like cardiovascular (5) and
chronic diseases in adulthood (6). Placentation requires the production
of growth factors, adhesion proteins, hormones and transcription
factors. Numerous factors have been identified in placenta cells
and their role has been established in implantation and subsequent
development of the placenta (1,7).
For example, insulin-like growth factors 1 (IGF1) and IGF2
stimulate cytotrophoblast proliferation and decreased cell
apoptosis (8), while leptin
promotes trophoblast cell proliferation, survival and invasion
(9-11). Moreover, adiponectin has
antiproliferative effect in placenta cell lines JEG-3 and BeWo
(12).
Apelin is another newly discovered adipokine. In
addition to adipose tissue, it is expressed in other tissues like
lung, kidney, ovary, heart, brain and blood vessels (13,14). Produced as a 77-amino acid
prepropeptide, it is enzymatically cleaved into smaller fragments
of 12, 17 and 36 amino acids in length and a 13-amino acid
pyroglutamylated peptide that has the strongest physiological
effect (15). Of the numerous
processes in which apelin is involved, it primarily regulates fluid
homeostasis (16), energy
metabolism (17), blood pressure
(18) and angiogenesis (19). Its actions are mediated by the APJ
receptor, a transmembrane receptor associated with a G protein
(20). Previous data identified
the apelinergic (apelin/apelin receptor APJ) system in
cytotrophoblast, syncytiotrophoblast and foetal endothelial cells
(3), where it was demonstrated
that the dominant form in the placental villi is pyr-apelin-13 and
apelin-13 (21). In mice, an APJ
defect leads to embryo lethality due to significant growth
retardation and vascular deformations (22). Studies on rat models showed that
in normal pregnancies, the level of apelin in the maternal serum is
elevated in the early stages of pregnancy, after which it drops by
50% in late pregnancy. These changes are caused by the increased
clearance of apelin by the gain of activity of the Ang-converting
enzyme-related carboxypeptidase-2 (ACE2), which hydrolyses apelin
(23). Moreover, during the
increase of apelin in the maternal serum, the placenta releases
significant amounts of ACE2 (24). Nevertheless, higher foetal apelin
concentrations suggest a potential role in foetal growth and ex
utero angiogenesis (25).
Numerous studies focus on the role of the apelin in the
pathophysiology of preeclampsia and in IUGR (6,21,26); however, the action of apelin on
trophoblastic cell function, such as proliferation and cell cycle,
is still unknown.
Published data led the present study to hypothesise
that apelin and APJ can regulate the placenta formation process by
action on placental cell proliferation. To verify this hypothesis,
two placental cell lines reflecting both syncytiotrophoblast (BeWo)
and cytotrophoblast (JEG-3) cells were used. First, the mRNA and
protein expression as well as immunolocalisation of the apelinergic
system in both cell lines were measured. Moreover, human placenta
slides were used to confirm apelin and APJ positive
immunolocalisation. Next, the effect of human recombinant apelin-13
on the placental cell proliferation, cell cycle and cyclins D, E, A
and B protein expression were analysed. As for the molecular
mechanism by which apelin regulates proliferation, the activation
of different kinases such as extracellular signal-regulated kinases
1/2 (ERK1/2), phosphatidylinositol 3′-kinase/protein kinase B
(Akt), 5′-monophosphate-activated protein kinase α (AMPKα) and
signal transducer and activator of transcription 3 (Stat3) was
studied. Kinases PI3K/Akt, ERK1/2, AMPKα and JAK/Stat3 are
signalling molecules involved in most types of cell growth,
proliferation, survival and apoptosis (27-29) and in the major molecular mechanism
of apelin action in other cell types (30-32).
Materials and methods
Reagents
Phosphate buffered saline (PBS), DMEM/F12 medium and
trypsin were purchased from Gibco; Thermo Fisher Scientific, Inc.
Insulin, glycerol, EDTA, dithiothreitol, 3,3′-diaminobenzidine
(DAB), bromophenol blue, sodium deoxycholate, Nonidet P-40 (NP-40),
Tween-20, PD098059, AG490 and apelin-13 (cat. no. A6469) were
obtained from Sigma-Aldrich; Merck KGaA. Foetal bovine serum (FBS;
heat inactivated) was purchased from Biowest. Tris base, SDS and
bovine serum albumin (BSA) were purchased from Bioshop (Canada,
Inc.). ML221, LY294002 and Compound C were obtained from Tocris
Bioscience, Cell Signaling Technology, Inc. and Merck KGaA,
respectively. The WesternBright™ Sirius kit was purchased from
Advansta, Inc. Bradford protein assay kit, 4-20% gels (cat. no.
456-1093) and membranes (cat. no. 1704156) were obtained from
Bio-Rad Laboratories, Inc.
Cell culture and treatment
Syncytiotrophoblast BeWo (cat. no. CCL-98) and
cytotrophoblast JEG-3 (cat. no. HTB-36) cell lines were obtained
from the American Type Culture Collection. BeWo cells were cultured
in DMEM/F12 medium without phenol red, supplemented with 0.01 mg/ml
insulin and 10% FBS, while JEG-3 cells were cultured in DMEM/F12
medium without phenol red, supplemented only with 10% FBS. Cell
lines were grown in 75-cm2 tissue culture flasks in a
37°C incubator with a humidified mixture of 5% CO2 and
95% air.
Treatment 1
The aim of this experiment was to analyse mRNA and
protein expression of apelin and APJ, as well as
immunolocalisation. JEG-3 or BeWo cells (1×104
cells/96-well) were cultured in DMEM/F12 with 5% FBS for 24 h and
then cells were carefully rinsed with PBS and stored in −70°C for
mRNA expression analysis, or lysed in ice-cold lysis buffer
including 50 mM Tris-HCl (pH 7.5) containing 100 mM NaCl, 0.5%
sodium deoxycholate, 0.5% NP-40, 0.5% SDS and protease inhibitors
and stored at −20°C for protein expression analysis.
Immunofluorescence labelling was performed on JEG-3 or BeWo cells,
seeding at 2×104 cells/4-well labtech (BD Biosciences;
Becton, Dickinson and Company) cultured in DMEM/F12 with 5% FBS for
24 h. Then cells were rinsed with PBS and fixed using absolute
methanol for 40 min at 20°C.
Treatment 2
In this experiment the effect of human recombinant
apelin-13 on cell proliferation and cyclins D, E, A and B protein
expression was focused on examine. JEG-3 or BeWo cells
(4×103 cells/per well of 96-well plate) were cultured in
DMEM/F12 with 10% FBS for 24 h. Next, media were replaced by
DMEM/F12 with 1% FBS and cells were treated for 24, 48 and 72 h
with apelin at doses 0.02, 0.2, 2.0, 20 and 200 ng/ml. The
concentrations of apelin were chosen based on the authors'
preliminary research and previous study (33). The alamarBlue stock solution or
Cell Counting Kit-8 (CCK-8) reagent in amounts equal to 10% of the
incubation volume was added, according to the manufacturers'
protocol. BeWo cells (4×103 cells/per well of 96-well
plate) were cultured with apelin at doses 2 and 20 ng/ml for 24, 48
and 72 h, then cells were lysed in ice-cold lysis buffer and stored
at −20°C for cyclin protein expression analysis.
Treatment 3
The aim of this experiment was to examine the effect
of human recombinant apelin-13 on the cell cycle. JEG-3 or BeWo
cells (2.5×104 cells/per 24-well plate) were cultured in
DMEM/F12 with 10% FBS for 24 h. Then, media were replaced by
DMEM/F12 with 1% FBS and cells were incubated for 24, 48 and 72 h
with 2 ng/ml of apelin. Both trophoblastic cells were harvested by
trypsinisation and washed in PBS. Then, cells were fixed with 70%
cold ethanol at 4°C for 60 min and stored at −20°C. These cells
were used for measuring the percentage of the total cell population
in each phase of the cell cycle.
Treatment 4
The aim of this experiment was to examine the
molecular mechanism of the apelin action on BeWo cell
proliferation. After 24 h, media were replaced by DMEM/F12 with 1%
FBS and cells were treated with apelin at 2 ng/ml for 1, 5, 15, 30,
45, 60 and 90 min. Then, cells were lysed in ice-cold lysis buffer
and stored at −20°C for protein expression of kinases
phosphorylated p-ERK1/2, total ERK, p-Akt, total Akt, p-Stat3,
total-Stat3, p-AMPKα and total AMPKα study. To test the activation
of kinases in different signalling pathways, cells were pre-treated
with selective inhibitors of ERK1/2, Akt, Stat3 and AMPKα kinases
(PD98059 at 10 µM, LY294002 at 10 µM, AG490 at 50
µM and Compound C at 10 µM, respectively), as well as
with the inhibitor of APJ (ML221 at 10 µM) for 1 h, and then
apelin at 2 ng/ml was added. The concentrations of inhibitors were
chosen based on the authors' preliminary research and previous
study (33). The alamarBlue stock
solution was added to the wells in amounts equal to 10% of the
incubation volume to measurement cell proliferation after 72 h of
culture.
Real time PCR
Total RNA isolation and cDNA synthesis (for 1 h at
37°C) were carried out on cells at the baseline using the TaqMan
Gene Expression Cells-to-CT kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.), following the manufacturer's protocol. The
resulting preamplified cDNA preparations were analysed by
quantitative PCR using the StepOnePlus Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and TaqMan
Gene Expression Assays for apelin (cat. no. Hs00936329_m1; RefSeq
NM_017413.4) and APJ (cat. no. Hs00270873_s1; RefSeq NM_005161.4)
(Applied Biosystems; Thermo Fisher Scientific, Inc.), in
combination with TaqMan Gene Expression Master Mix containing ROX
reference dye (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The thermal cycling conditions were as follows: 50°C for 2 min,
95°C for 10 min and then 40 cycles of 95°C for 15 sec and 60°C for
60 sec. Duplicate control samples lacking cDNA were prepared for
each gene and showed no DNA contamination. The gene expression was
normalized using the geometric mean of three housekeeping genes:
GAPDH (cat. no. 4310884E; RefSeq NM_002046.3), TBP (cat. no.
Hs00920495_m1; RefSeq NM_001172085.1), YWHAZ (cat. no.
Hs01122445_g1; RefSeq NM_001135699.1) the expression of which was
stable under these conditions (data not shown). 3T3L1 adipose
tissue cells [culture of cells was described (34)] and ovarian follicles cells
[isolation and culture of cells were described previously (33)] were used as a positive control to
study apelin/APJ expression in the placenta. The normalized values
for relative expression (R) were calculated according to the
following equation: R=(EGene−Cq
Gene)/(geometric mean (EGAPDH −Cq
GAPDH; E TBP−Cq TBP;
EYWHAZ−Cq YWHAZ), where Cq was the cycle
threshold and E was the PCR efficiency for each primer pair
(35).
Western blotting
The western blotting procedure used to determine
apelin/APJ, cyclins and kinases proteins expression was described
previously (33). Briefly, the
proteins (80 µg) were separated by 4-20% Mini-Protean TGX
System Precast Protein Gels and transferred to Trans-Blot Turbo
Mini PVDF Transfer Packs (Bio-Rad Laboratories, Inc.). The
membranes were blocked for 1 h in 0.02 M Tris-buffered saline
containing 5% BSA and 0.1% Tween-20 at room temperature (RT), then
incubated overnight at 4°C with appropriate primary antibodies
(Table I). The membranes were
then washed in Tris-buffered saline containing 0.1% Tween-20 and
incubated for 1 h at RT with a horseradish peroxidase-conjugated
secondary antibody (Table I).
β-actin was used as a loading control. Signals were detected by
chemiluminescence using a WesternBright™Sirius (cat. no.
K-12043-D20; Advansta, Inc.) and visualised using the Chemidoc™
XRS+ System (Bio-Rad Laboratories, Inc.). All bands were quantified
using a densitometer and ImageJ software (version 1.51; US National
Institutes of Health).
 | Table IAntibodies used for western blotting
and immunofluorescence. |
Table I
Antibodies used for western blotting
and immunofluorescence.
| Antibody | Host species | Vendor/cat.
no. | Dilution |
|---|
| Apelin | Mouse | Santa Cruz
Biotechnology, Inc./sc-293441 | 1:50 (IHC), 1:200
(WB) |
| APJ | Rabbit | Santa Cruz
Biotechnology, Inc./sc-33823 | 1:100 (IHC), 1:200
(WB) |
| Cyclin D | Rabbit | CST/2978 | 1:1,000 (WB) |
| Cyclin E | Mouse | Abcam/ab3927 | 1:500 (WB) |
| Cyclin A | Mouse | CST/4656 | 1:2,000 (WB) |
| Cyclin B | Rabbit | CST/4138 | 1:1,000 (WB) |
| Phospho-ERK1/2 | Rabbit | CST/9101 | 1:1,000 (WB) |
| Total-ERK1/2 | Rabbit | CST/9102 | 1:1,000 (WB) |
| Phospho-Akt | Rabbit | CST/9271 | 1:1,000 (WB) |
| Total-Akt | Rabbit | CST/9272 | 1:1,000 (WB) |
| Phospho-Stat3 | Rabbit | CST/9131 | 1:1,000 (WB) |
| Total-Stat3 | Rabbit | CST/4904 | 1:2,000 (WB) |
| Phospho-AMPKα | Rabbit | Thermo Fisher
Scientific, Inc./PA5-17831 | 1:1,000 (WB) |
| Total-AMPKα | Rabbit | Thermo Fisher
Scientific, Inc.//PA5-17398 | 1:1,000 (WB) |
| β-actin | Mouse | Sigma-Aldrich;
Merck KGaA/A5316 | 1:3,000 (WB) |
| Secondary | Anti-rabbit | CST/7074 | 1:500 (IHC),
1:1,000 (WB) |
| Secondary | Anti-mouse | CST/7076 | 1:500 (IHC),
1:1,000 (WB) |
Immunocytochemistry
After fixation, placenta cells were incubated for 5
min with 0.2% Triton in PBS. Next, nonspecific binding sites were
blocked with 3% BSA for 3 h at RT. Thereafter, cells were incubated
overnight at 4°C in a humidified chamber in the presence of primary
antibodies against apelin/APJ (Table
I). On the next day, goat anti-mouse antibodies for apelin
(cat. no. A11001; Thermo Fisher Scientific, Inc) or goat
anti-rabbit antibodies for APJ (cat. no. A10520, Thermo Fisher
Scientific, Inc) were applied overnight at 4°C. After each step,
sections were carefully rinsed with PBS. The antibodies were
diluted to 1:200 in 3% BSA. Immunofluorescent staining was
protected from light and coverslips were mounted with Fluoroshield
mounting medium (Sigma-Aldrich; Merck KGaA) with
4′,6-diamidino-2-phenylindole (DAPI) and examined with
epifluorescence microscope Leica DMR. To quantitatively evaluate
the intensity of immunofluorescent reaction, digital colour images
were obtained using a Nikon Coolpix 4500 Video Camera (Nikon
Corporation) connected to a video capture card (PV-BT878P1; Prolink
Computer, Inc.).
Immunohistochemistry
To optimize immunohistochemical staining, paraffin
sections of human placenta tissue slides (GTX24360; GeneTex Inc.)
were immersed in 10 mM citrate buffer (pH 6.0) and heated in a
microwave oven (2×5 min, 700 W). Thereafter, sections were immersed
sequentially in 3% H2O2 for 10 min at RT and
normal 5% goat serum (Sigma-Aldrich; Merck KGaA) for 30 min that
were used as blocking solutions. Afterwards, sections were
incubated overnight at 4°C with primary antibodies against
apelin/APJ (Table I). Next,
anti-rabbit biotinylated antibody and avidinbiotinylated
horseradish peroxidase complex (ABC/HRP; 1:100; Dako; Agilent
Technologies, Inc.) were applied at RT in succession. Bound
antibody was visualized with 0.05% DAB as a chromogenic substrate.
Control sections included omission of primary antibody and/or
substitution by irrelevant IgG. Thereafter, sections were washed
and were counterstained with Mayer's hematoxylin at RT for 10 sec
and mounted using DPX mounting media (Sigma-Aldrich, Merck
KGaA).
AlamarBlue assay
The alamarBlue® assay (cat. no. DAL1100;
Invitrogen; Thermo Fisher Scientific, Inc.) is based on
quantitation of the cells' metabolic activity and is designed to
measure the proliferation of different cell types. Cellular
metabolism induces a chemical reduction of the alamarBlue medium,
i.e. this assay is based on the quantitative metabolic conversion
of the blue, non-fluorescent resazurin to pink, fluorescent
resorufin by living cells. AlamarBlue stock solution was
aseptically added to the wells in amounts equal to 10% of the
incubation volume as described in a previous study (33). The resazurin reduction was
determined after 3 h incubation with alamarBlue by measuring the
absorbance at 570 and 600 nm wavelengths using a FLUORO reader
(BioTek Instruments, Inc.).
Cell Counting Kit-8
Cell Counting Kit-8 (CCK-8; cat. no. 96992; Sigma
Aldrich; Merck KGaA) allows sensitive colorimetric assays for the
determination of cell proliferation. Highly water-soluble
tetrazolium salt, CCK-8, is reduced by dehydrogenase activities in
cells to give a yellow-coloured formazan dye, which is also soluble
in the culture media. The amount of the formazan dye is directly
proportional to the number of living cells. After treating cells
with apelin, 10 µl CCK-8 solution was added and the plate
was further incubated for 4 h. Then, the absorbance at 450 nm
wavelength was measured using an ELISA reader (BioTek Instruments,
Inc.).
Flow cytometry
Before staining with propidium iodide (PI), the
cells were washed twice in 1 ml of PBS, then the cell pellet was
resuspended in 300 µl of the PI/RNase staining buffer (BD
Biosciences; Becton, Dickinson and Company) and incubated for 30
min in the dark at RT. The red fluorescence of PI was measured by
FACSCalibur flow cytometer (Becton, Dickinson and Company). A total
of 10,000 cells were examined per sample. The percentage of cell
population in each cell cycle phase (G0/G1, S and G2/M) was
calculated from DNA content histograms using the WinMDI 2.8
Software (The Scripps Research Institute).
Statistical analysis
All experimental data were expressed as the mean ±
standard error of the mean of three independent experiments (n=3).
Distribution of normality was checked by Shapiro-Wilk test. A
one-way or two-way analysis of variance, followed by Tukey's test
(GraphPad Prism 5 Software, Inc.), was used to determine any
difference between treatments with apelin and control. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of apelin/APJ in human
trophoblastic cells
The expression (mRNA and protein) of apelin and its
receptor, APJ, were studied in cytotrophoblast JEG-3 and
syncytiotrophoblast BeWo cells to compare their expression in both
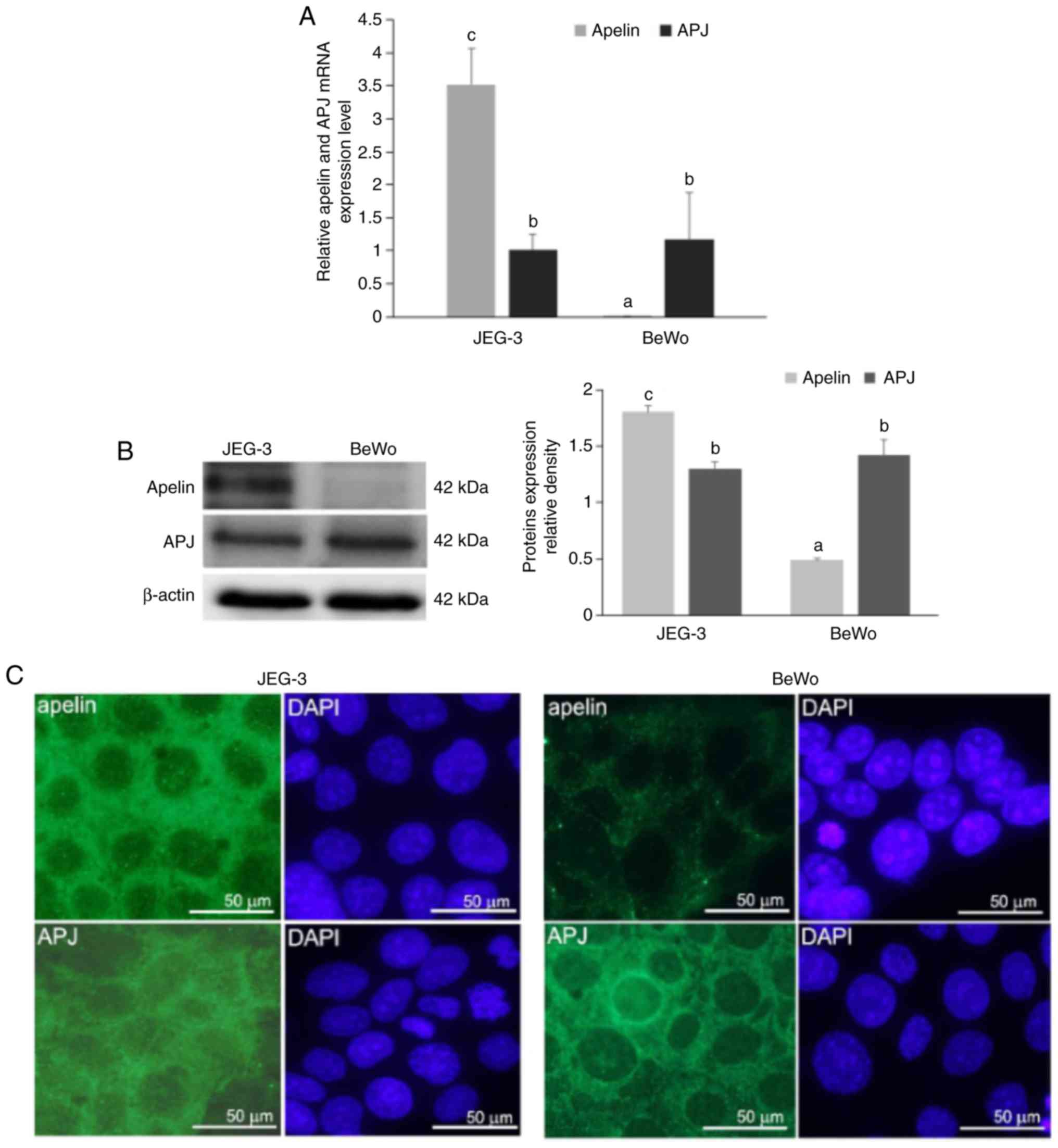
cell lines. Using quantitative PCR, it was demonstrated that mRNA
expression of apelin was 5-fold higher in JEG-3 compared with BeWo
cells (5.518 vs. 0.002, respectively), while mRNA expression of APJ
was at the same level in both placental cells (P<0.05; Fig. 1A). Additionally, the present study
observed that in JEG-3 cells, apelin expression was increased
compared with APJ, while in BeWo cells, APJ expression was higher
than apelin. Immunoblotting results confirmed these data
(P<0.05; Fig. 1B).
Immunocytochemical analyses revealed a strong cytoplasmic signal
for apelin in JEG-3 cells. On the other hand, BeWo cells exhibited
a weak punctuate signal for apelin in the cell cytoplasm. A
moderate immunosignal for APJ was revealed in the cell cytoplasm of
both placental cell lines. Occasionally in BeWo cells, a strong
signal was located in the perinuclear area (Fig. 1C).
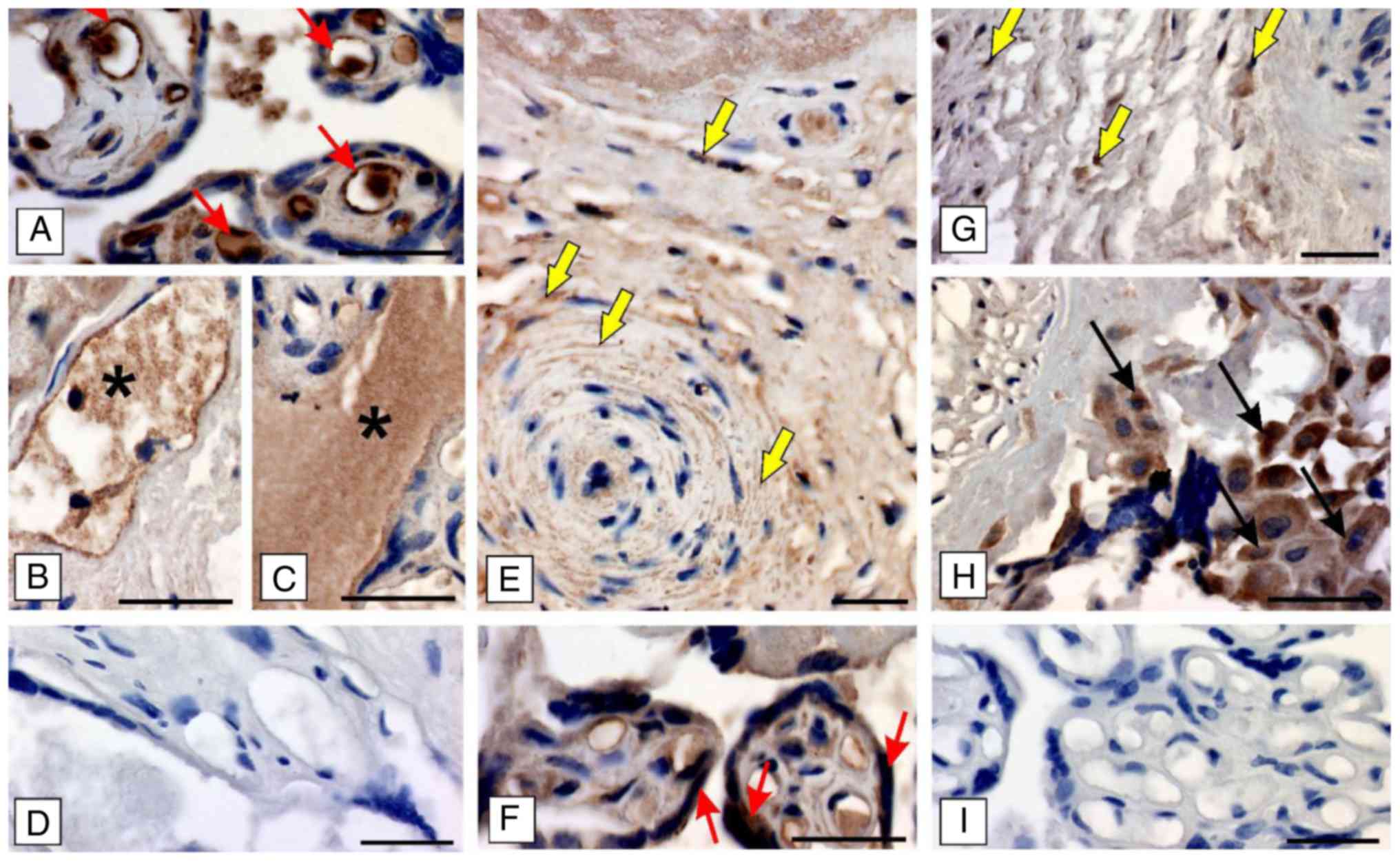
To confirm the results from immunocytochemical
studies in the cell lines apelin/APJ expression was analysed in the
human placenta slides. It was observed that both apelin and the
presence of APJ was revealed, however the signal intensity was
dependent on the type of placental cell/structure. In detail, a
strong signal for apelin was visible in the cytoplasm of
endothelial lining of blood capillaries (Fig. 2A) and in the maternal blood
(Fig. 2B and C), while cells of
the placental artery exhibited a moderate intensity of the apelin
signal (Fig. 2E). A moderate to
weak signal intensity for APJ was demonstrated in the cytoplasm of
epithelial cells of vesicles and mesenchymal cells of the Whartons
jelly (Fig. 2F), but only a few
cells of the placental artery exhibited signal (Fig. 2G). A strong signal for APJ was
seen in cells of the syncytiotrophoblast (Fig. 2H). No positive signals were found
when tissues were incubated with omission of primary antibodies
(Fig. 2D and I).
Dose- and time-dependent effect of apelin
on trophoblastic cell proliferation
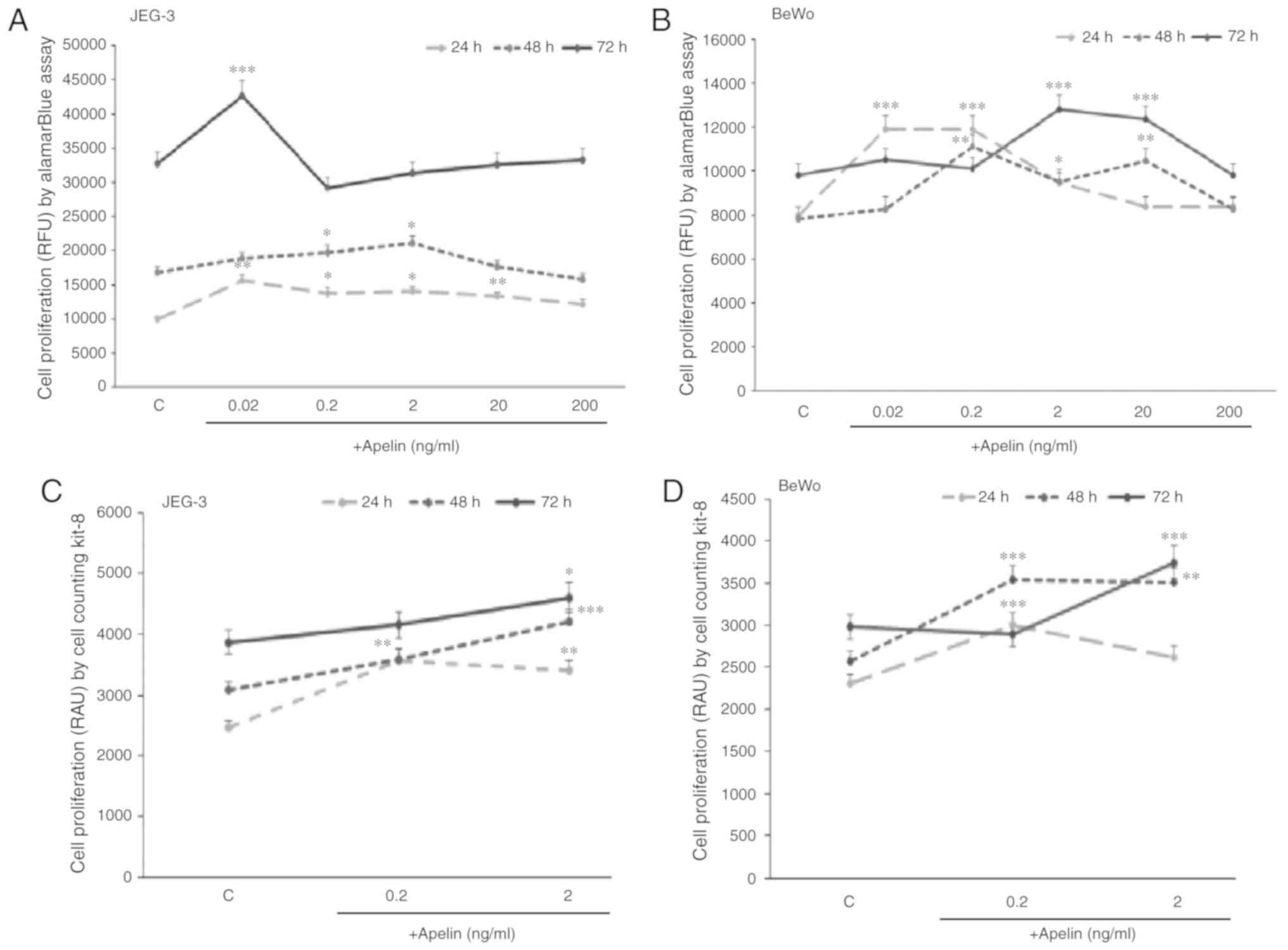
Based on the results showing higher expression of
apelin in the cytotrophoblast, which represent the proliferative
population inside placental villi, it was decided to study the
direct role of apelin on human placenta cells proliferation. The
present study showed that in JEG-3 cells, apelin significantly
increased cell proliferation after 24 h at 0.02, 0.2, 2.0 and 20
ng/ml doses, after 48 h at 0.2 and 2.0 ng/ml doses and after 72 h
at 0.02 ng/ml dose (P<0.05, P<0.01 and P<0.001,
respectively; Fig. 3A). In BeWo
cells, it was also observed that apelin increased cell
proliferation after 24 h at 0.02, 0.2 and 2.0 ng/ml doses, after 48
h at 0.2, 2.0 and 20 ng/ml doses and after 72 h at 2.0 and 20 ng/ml
doses (P<0.05, P<0.05 and P<0.001, respectively; Fig. 3B).
To confirm the current findings, a CCK-8 assay was
used as a second method to measure cell proliferation. The present
study showed that in JEG-3 during 24 and 48 h incubation, apelin at
0.2 and 2.0 ng/ml and during 72 h at 2 ng/ml had a significant
stimulatory effect on cell proliferation (P<0.05; Fig. 3C), while in BeWo, after 24 and 48
h at 0.2 ng/ml and after 48 and 72 h at 2.0 ng/ml of apelin, cell
proliferation was increased (P<0.01; Fig. 3D).
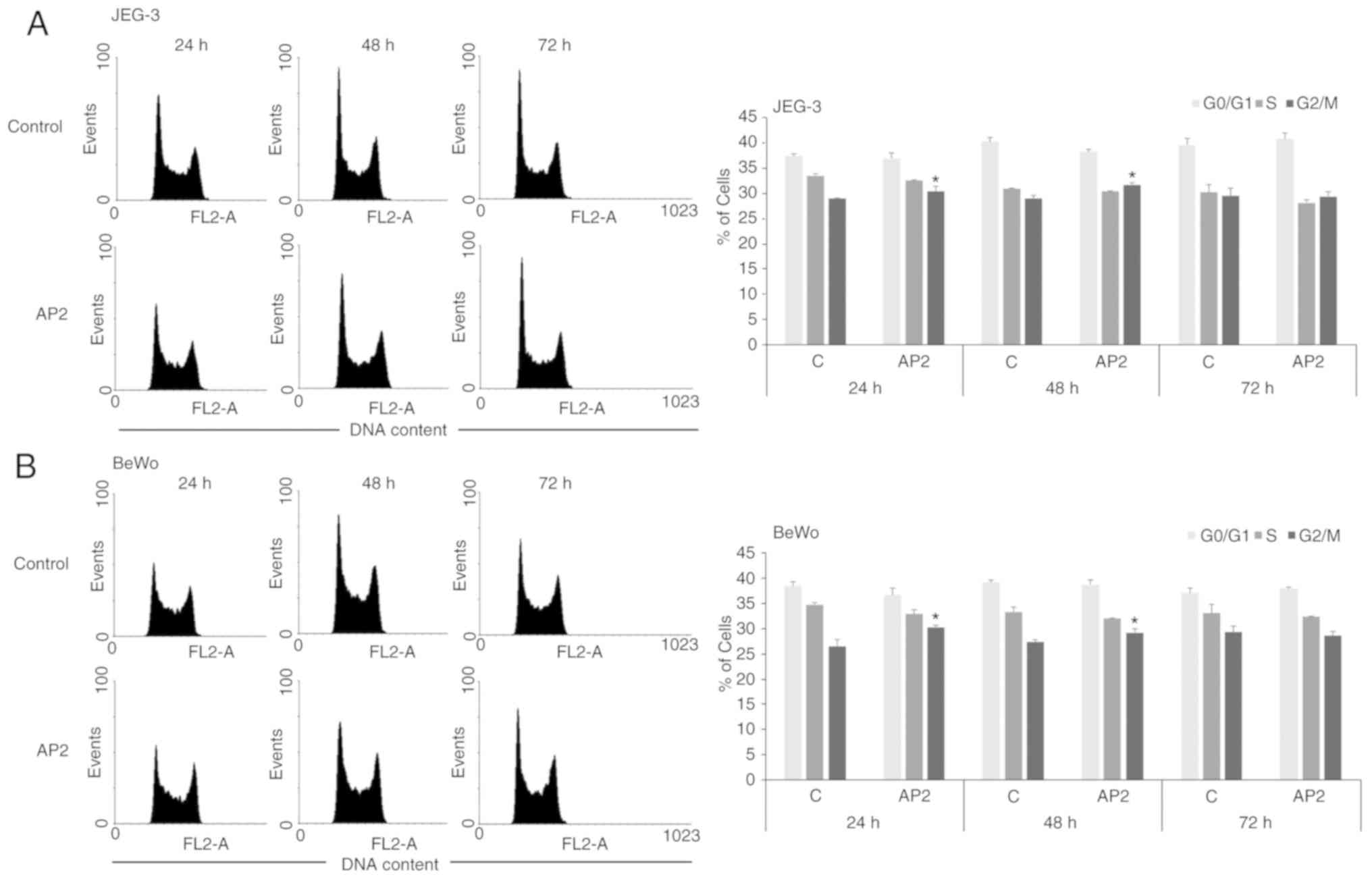
Effect of apelin on the cell cycle in
human trophoblastic cells
To further confirm the influence of apelin on
placental cell proliferation, the action of apelin on cell cycle
distribution was investigated. In both trophoblastic JEG-3 and BeWo
cells it was observed that apelin at dose 2.0 ng/ml significantly
increased the percentage of cells in G2/M phase of cell cycle after
24 and 48 h of incubation (P<0.05; Fig. 4).
Effect of apelin on protein expression of
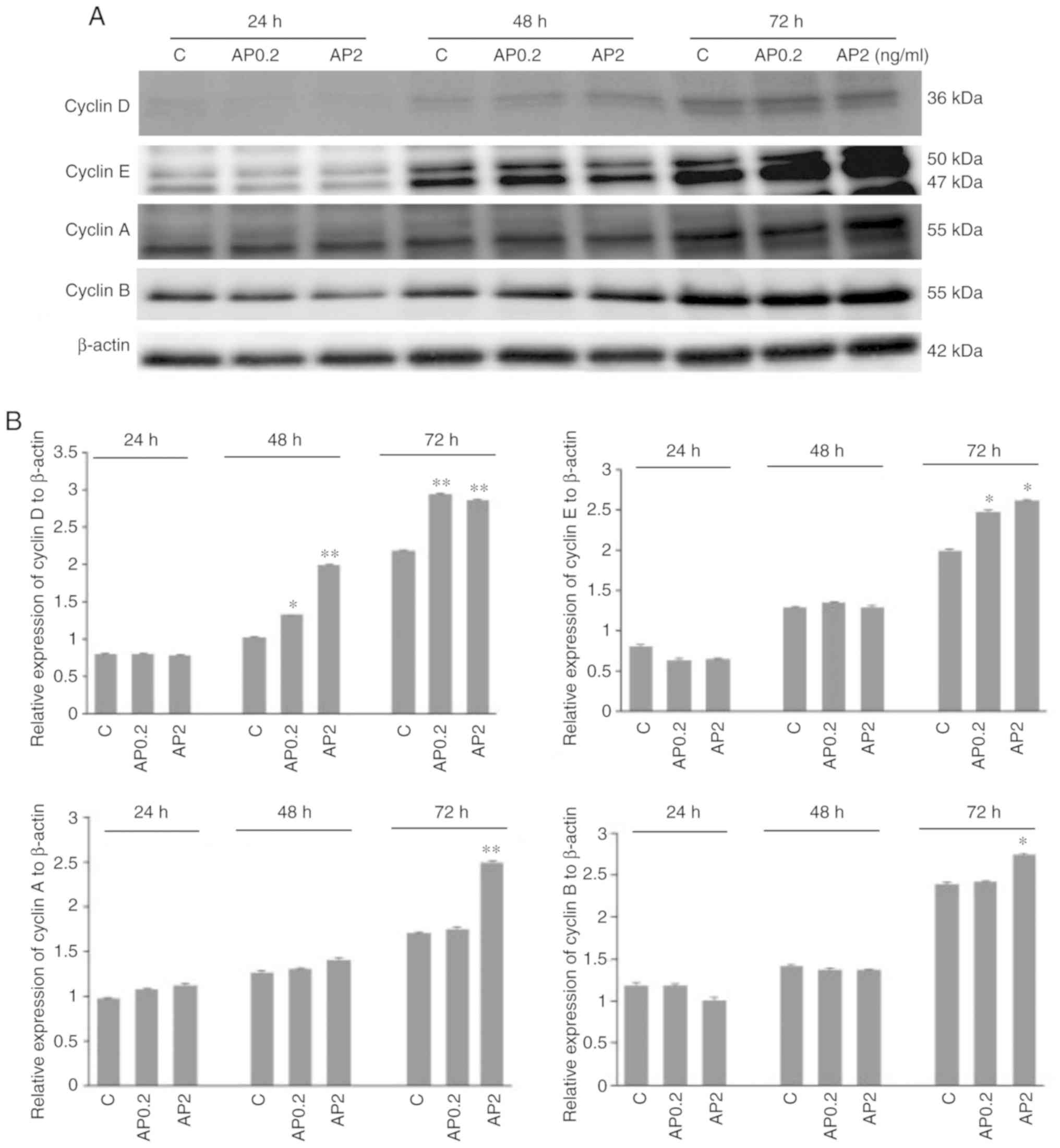
cyclins in human trophoblastic BeWo cells
It is well established that cyclins function as
regulators of proliferation-related cell cycle progression.
Therefore, in the present study, the effect of apelin on protein
expression of cyclins D, E, A and B in human trophoblastic BeWo
cells was studied. Apelin at both doses, 0.2 and 2.0 ng/ml
significantly increased cyclin D protein expression after 48 and 72
h incubation (*P<0.05 and **P<0.01;
Fig. 5A and B). It was also
observed that apelin increased cyclins E, A and B protein
expression after 72 h of cell incubation at 0.2 and 2.0 ng/ml for
cyclin E, and 2.0 ng/ml for cyclins A and B (*P<0.05
and **P<0.01; Fig. 5A
and B). Analysis of cyclins blots showed that cyclin E can be
detected as a doublet of 50 kDa and a single band of 42 kDa, as
described in datasheet of producer antibodies (Abcam). Moreover, in
cyclin B a molecular weight shift was observed among bands,
suggesting the possibility of posttranslational modification in the
proteins.
Molecular mechanism of apelin action on
human trophoblastic BeWo cell proliferation
Apelin is known to activate various signalling
pathways in different cell types (31-33). Therefore, the effect of apelin
(2.0 ng/ml) on ERK1/2, PI3K/Akt, JAK/Stat3 and AMPKα was
investigated over different incubation periods. These pathways were
focused on as they have previously been shown to be involved in
regulation of cell proliferation (28-30). Apelin has a stimulatory effect on
the phosphorylation of kinase p42 of ERK1/2 after 30 min, Akt after
1 min and Stat3 after 1, 5 and 45 min of incubation (P<0.05;
Fig. 6A and B). Moreover, AMPKα
activation was increased from 1 to 45 min and 90 min of incubation
with apelin. Kinase inhibitors PD098059, AG490 and Compound C as
well as ML221 resulted in a reversal of apelin-stimulated cell
proliferation to control levels, except inhibitor LY294002
(Fig. 6C).
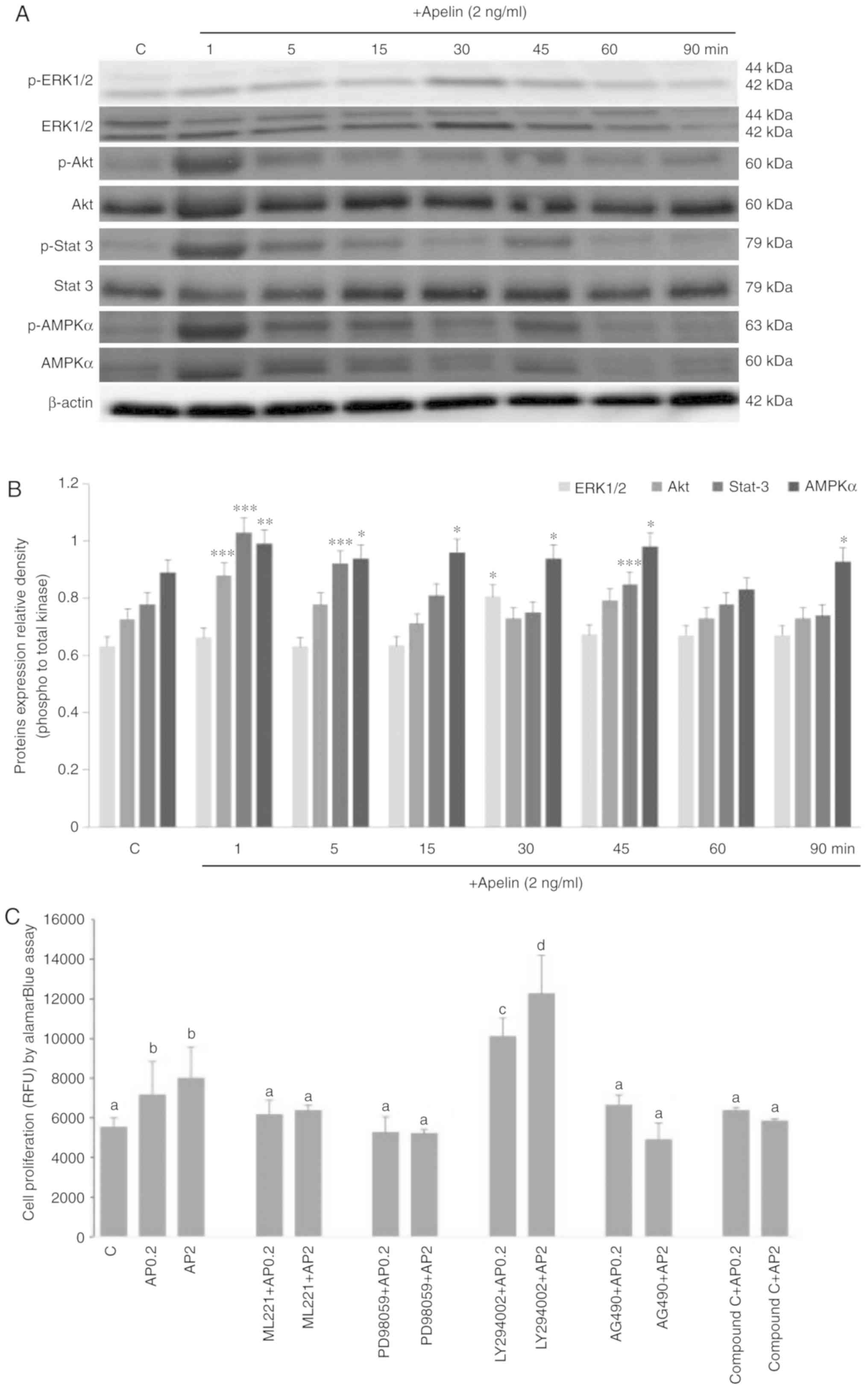 | Figure 6Molecular mechanism of mitogenic
action of apelin-13. Kinases p-ERK1/2, total ERK, p-Akt, total Akt,
p-Stat3, total-Stat3, p-AMPKα and total AMPKα were detected by (A)
western blotting and (B) densitometry analysis. Cells were
pre-treated for 1 h with selective inhibitors of ERK 1/2, Akt,
Stat3 and AMPKα kinases (PD98059, LY294002 and AG490 Compound C
respectively) and APJ inhibitor (ML221), after which apelin (2
ng/ml) was added for 72 h and cell proliferation was studied (C).
The results are expressed as the mean ± standard error of the mean
of three independent cultures. Statistical analysis was carried out
using one-way analysis of variance *P<0.05,
**P<0.01, ***P<0.001 vs. the control or
two-way analysis of variance, followed by Tukey's test. Different
letters indicate significant differences at P<0.05. RFU,
relative fluorescent unit; p, phosphorylated; ERK, extracellular
signal regulated kinase; Akt, protein kinase B; Stat3, signal
transducer and activator of transcription 3; AMPKα,
5′-monophosphate-activated protein kinase α. |
Discussion
Apelin is a pleiotropic peptide, but its major
action relates to energy metabolism, cardiovascular function, body
fluid homeostasis and the last study also documented female
reproduction. Previously published studies indicate that apelin is
involved in pregnancy by i) regulation of blood pressure and
angiogenesis in the preeclampsia pathophysiology; ii) effects on
plasma volume expansion in foetal growth; and iii) regulation of
glucose metabolism in the gestational diabetes pathophysiology
(21,23,25). However, there are no data
regarding the direct effects of apelin in trophoblast cell
proliferation or the cell cycle. In the present study, the
expression and immunolocalization of the apelinergic system was
first checked in both human trophoblastic cell lines
syncytiotrophoblast BeWo and cytotrophoblast JEG-3 as well as in
human placenta slides. Both these cells lines maintained numerous
characteristics of human trophoblastic cells and have been widely
used to study cellular and molecular signalling in the placenta
(36-38). Additionally, the molecular
mechanism involved in the human recombinant apelin-13 action on
cell proliferation was investigated.
The results of the present study clearly
demonstrated that apelin expression was higher in cytotrophoblast
JEG-3 cells than syncytiotrophoblast BeWo cells, while APJ was at
the same level in both placental cell lines. Moreover, in JEG-3
cells, apelin expression was increased compared with APJ, while in
BeWo cells, higher expression of APJ than apelin was noted. These
results agree with previous data by Cobellis et al (3), showing that the level of apelin
expression in the human placenta was much higher in cytotrophoblast
cells compared to syncytiotrophoblast. Cytotrophoblast are
mononucleated cells that represent the proliferative population
inside placental villi; this particular localization suggests the
direct role of apelin/APJ on human placenta cells proliferation.
Additionally, high cytoplasmic and/or membrane apelin localisation
was also described in JEG-3 cells, while BeWo cells exhibited
weaker apelin signal in the cell cytoplasm. Immunolocalization of
APJ revealed a moderate signal in the cell cytoplasm in both
placental cells, while a weaker signal was observed within the
nuclei. Additionally, immunohistochemistry on human placental
slides showed a strong signal for apelin in the cytoplasm of the
endothelial lining of blood capillaries and in maternal blood,
while cells of the placental artery exhibited a moderate intensity
of the apelin signal. A moderate to weak signal intensity for APJ
was located in the cytoplasm of epithelial cells of vesicles and
mesenchymal cells of the Whartons jelly and a strong signal for APJ
was observed in cells of the syncytiotrophoblast. The results of
the present study demonstrating expression of apelin/APJ in
endothelial and epithelial cells of vesicles were confirmed by a
previous study documenting apelin expression in the vascular
endothelial cells lining blood vessels of the human heart, rat
blood vessels and a high level in endothelial cells of other
vessels (39). In the light of
these observations Cobellis et al (3) presented the hypothesis that apelin
expressed by endothelial cells, acting on vascular smooth muscle in
a paracrine fashion has a vasoconstrictor potential and can be a
biochemical marker in preeclampsia, which is characterized by
endothelial dysfunction. The current results partly confirmed this
hypothesis. To summarize the first part of the present study, the
expression of both components (ligand and receptor) of the apelin
signalling system provides the opportunity for analysis of the
direct role of apelin in human placenta cells.
Since the cytotrophoblast cells represent the
proliferative population inside placental villi, this particular
localisation led the present study to investigate a possible role
for apelin in the regulation of proliferation, as previously
demonstrated on other models like gastric epithelial cells
(40) or umbilical endothelial
cells (41). The present study
observed that apelin increased placental cell proliferation. This
effect was observed with human recombinant apelin-13 concentrations
ranging from 0.02 to 20 ng/ml, whereas the literature data
documented that apelin is significantly decreased in pregnant women
(4.45-8.7 ng/ml vs. 5.0-9.3 ng/ml in control) (42). In the present study, apelin-13 was
used because data presented by Yamaleyeva et al (21) documented that apelin-13,
(Pyr1)-apelin and possibly oxidised apelin-12 were the major forms
in both normal and preeclamptic chorionic villi. In fact, apelin
has been shown to exhibit mitogenic action in numerous cell types,
including cardiomyoblast cells (43), ovarian cells (33), vascular smooth muscle cells (VSMC)
(44) and endothelial cells
(45).
Cell cycle analysis was performed to determine the
mechanisms underlying the proliferative effects of apelin on
placenta cells. The present data indicate the important role of
apelin in placenta cell cycle progression. Apelin stimulates the
transition to G2/M phase that is required for cell division and, as
shown by further analysis, cyclins were involved in the observed
apelin effect. Therefore, modulation of the cell cycle and
expression of cyclins by apelin is strictly connected with its
biological activity. It is well known that progression through the
G2/M is connected with the expression of cyclin B, among other
proteins. An enhancement of cyclins D, E, A and B in response to
human recombinant apelin-13 was found. Cyclins and cyclin-dependent
kinases are subunits of cell cycle-dependent protein kinases that
regulate key events during the progression of the cell cycle
(46). Moreover, the level of
cyclin proteins is selectively expressed in different phases of the
cell cycle, for example the rise in cyclin D is followed later
during G1 by the appearance of cyclin E, which rapidly disappears
towards the beginning of DNA synthesis (S phase) (47). Mitogenic signals (e.g., growth
factors and serum compounds) that stimulate cell progress through
G1 coincide with an increased expression of cyclin D. The present
study found enhancement of cyclin D expression in response to
apelin at the 48 time point. Therefore, earlier modulation of
cyclin D by apelin facilitated cell cycle progression. It is known
that after progression through the cyclin D-dependent portion of
the cell cycle, cyclin E becomes activated (48) and further facilitates cell cycle
progression through S towards G2/M phase. Current results strongly
indicate that apelin modulates cell cycle progression by affecting
the activation of cyclins, especially cyclin D and E. Cyclin A
begins to appear towards the end of G1, continues to rise during
the S phase and then through G2 phase rapidly declines as the cell
progresses, while level of cyclin B begins to appear during the S
phase. This rise continues into the G2 phase before rapidly falling
during the mitotic phase (47).
Therefore, apelin can enhance the cell cycle by promoting the
switch of cells from S phase to G2/M phase. Additionally, it is a
well-known fact that the increase in expression of the cell cycle
machinery is a key event in regulating cell proliferation. Further
studies are needed to understand how apelin cooperates with precise
signalling pathways to regulate these cyclins. The present results
agree with data of Li et al (44) indicating that apelin increased
cell cycle progress by stimulating the expression of cyclins D1 and
E in VSMC cells.
The last part of the current study focused on
description of the molecular mechanism of apelin action in placenta
cells by studying the effect of apelin on signalling pathways and
next the role of APJ receptor and activation of different kinases
was investigated. The present study showed that apelin at 2 ng/ml
stimulated phosphorylation of kinases ERK1/2, Akt, Stat3 and AMPKα,
but cell proliferation was reduced to control levels in the culture
with pharmacological inhibitors of APJ receptor and kinases ERK1/2,
Stat3 and AMPKα but not Akt. In the authors' preliminary studies
all inhibitors of kinases ERK1/2, Akt, Stat3 and AMPKα (PD98059 at
10 µM, LY294002 at 10 µM, AG490 at 50 µM and
Compound C at 10 µM, respectively), as well as inhibitor of
APJ (ML221 at 10 µM) were used alone to check that their
concentration and no effect on cell proliferation was observed.
Previous data showed that apelin induced cell proliferation by
activation of different signalling pathways through PI3/Akt
activation in rat granulosa cells (49), endothelial progenitor cells
(50) and retinal pigment
epithelial cells (30); or via
ERK1/2 in human breast cancer cells (MCF-7) (51) and lung adenocarcinoma (52). These observations suggest that the
molecular mechanism of the mitogenic action of apelin is cell
specific. Thus, a potentially new mechanism of apelin action can be
described as follows: Apelin-induced stimulation of trophoblastic
cell proliferation regulates early placental development. As a
result, the condition of the placenta directly indicates the
well-being of the developing foetus.
In conclusion, the results of the present study
noted that apelin is produced by trophoblastic cells and can be
involved in the human placenta physiology. It was observed that
this adipokine by stimulation of placenta cell proliferation via
APJ activation and kinases ERK1/2, Stat3 and AMPKα pathways can be
new regulator of early placental development. The limitations of
the study must also be acknowledged. All in vitro results
were obtained using human placenta cell lines, so the structure and
local microenvironment in the placenta tissue did not exist. To
complete these results, future studies on placenta explants or
in vivo animals models will be necessary for understanding
the role of apelin on placenta cells physiology. For example, Van
Mieghem et al (23)
conducting the apelin studies on rats documented that maternal
plasma apelin levels decreased in the last week of gestation due to
increased elimination by the fetoplacental unit, however role of
apelin on animal placenta models are still unknown.
Funding
The present study was supported by the Jagiellonian
University (grant no. K/ZDS/008063). The cost of Open Access
publication was covered by the Society for Biology of Reproduction
in Poland.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
EM and AR designed the study. EM, PK, ED, MOC, WT
and MKB performed the experiements. EM, MOC and MKB analysed the
data. EM and AR prepared the manuscript. All authors have seen and
approved the final published version of this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Maltepe E, Bakardjiev AI and Fisher SJ:
The placenta: Transcriptional, epigenetic, and physiological
integration during development. J Clin Invest. 120:1016–1025. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scifres CM and Nelson DM: Intrauterine
growth restriction, human placental development and trophoblast
cell death. J Physiol. 587:3453–3458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cobellis L, De Falco M, Mastrogiacomo A,
Giraldi D, Dattilo D, Scaffa C, Colacurci N and De Luca A:
Modulation of apelin and APJ receptor in normal and
preeclampsia-complicated placentas. Histol Histopathol. 22:1–8.
2007.
|
|
4
|
Reus AD, El-Harbachi H, Rousian M,
Willemsen SP, Steegers-Theunissen RP, Steegers EA and Exalto N:
Early first-trimester trophoblast volume in pregnancies that result
in live birth or miscarriage. Ultrasound Obstet Gynecol.
42:577–584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murphy VE, Smith R, Giles WB and Clifton
VL: Endocrine regulation of human fetal growth: The role of the
mother, placenta, and fetus. Endocr Rev. 27:141–169. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Briana DD and Malamitsi-Puchner A:
Intrauterine growth restriction and adult disease: The role of
adipocytokines. Eur J Endocrinol. 160:337–347. 2009. View Article : Google Scholar
|
|
7
|
Knöfler M and Pollheimer J: IFPA Award in
Placentology lecture: Molecular regulation of human trophoblast
invasion. Placenta. 33(Suppl): S55–S62. 2012. View Article : Google Scholar :
|
|
8
|
Forbes K, Westwood M, Baker PN and Aplin
JD: Insulin-like growth factor I and II regulate the life cycle of
trophoblast in the developing human placenta. Am J Physiol Cell
Physiol. 294:C1313–C1322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Caüzac M, Czuba D, Girard J and Hauguel-de
Mouzon S: Transduction of leptin growth signals in placental cells
is independent of JAK-STAT activation. Placenta. 24:378–384. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schulz LC and Widmaier EP: The effect of
leptin on mouse trophoblast cell invasion. Biol Reprod.
71:1963–1967. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Magariños MP, Sánchez-Margalet V, Kotler
M, Calvo JC and Varone CL: Leptin promotes cell proliferation and
survival of trophoblastic cells. Biol Reprod. 76:203–210. 2007.
View Article : Google Scholar
|
|
12
|
Benaitreau D, Dieudonné MN, Dos Santos E,
Leneveu MC, Mazancourt Pd and Pecquery R: Antiproliferative effects
of adiponectin on human trophoblastic cell lines JEG-3 and BeWo.
Biol Reprod. 80:1107–1114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Falco M, De Luca L, Onori N, Cavallotti
I, Artigiano F, Esposito V, De Luca B, Laforgia V, Groeger AM and
De Luca A: Apelin expression in normal human tissues. In Vivo.
16:333–336. 2002.PubMed/NCBI
|
|
14
|
Kurowska P, Barbe A, Różycka M,
Chmielińska J, Dupont J and Rak A: Apelin in reproductive
physiology and pathology of different species: A critical review.
Int J Endocrinol. 2018:91704802018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boucher J, Masri B, Daviaud D, Gesta S,
Guigné C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B,
Carpéné C, et al: Apelin, a newly identified adipokine up-regulated
by insulin and obesity. Endocrinology. 146:1764–1771. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee DK, Cheng R, Nguyen T, Fan T,
Kariyawasam AP, Liu Y, Osmond DH, George SR and O'Dowd BF:
Characterization of apelin, the ligand for the APJ receptor. J
Neurochem. 74:34–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bertrand C, Valet P and Castan-Laurell I:
Apelin and energy metabolism. Front Physiol. 6:1152015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tatemoto K, Takayama K, Zou MX, Kumaki I,
Zhang W, Kumano K and Fujimiya M: The novel peptide apelin lowers
blood pressure via a nitric oxide-dependent mechanism. Regul Pept.
99:87–92. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kidoya H and Takakura N: Biology of the
apelin-APJ axis in vascular formation. J Biochem. 152:125–131.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tatemoto K, Hosoya M, Habata Y, Fujii R,
Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et
al: Isolation and characterization of a novel endogenous peptide
ligand for the human APJ receptor. Biochem Biophys Res Commun.
251:471–476. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamaleyeva LM, Chappell MC, Brosnihan KB,
Anton L, Caudell DL, Shi S, McGee C, Pirro N, Gallagher PE, Taylor
RN, et al: Downregulation of apelin in the human placental
chorionic villi from preeclamptic pregnancies. Am J Physiol
Endocrinol Metab. 309:E852–E860. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Charo DN, Ho M, Fajardo G, Kawana M, Kundu
RK, Sheikh AY, Finsterbach TP, Leeper NJ, Ernst KV, Chen MM, et al:
Endogenous regulation of cardiovascular function by apelin-APJ. Am
J Physiol Heart Circ Physiol. 297:H1904–H1913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Van Mieghem T, van Bree R, Van Herck E,
Pijnenbor R, Deprest J and Verhaeghe J: Maternal apelin physiology
during rat pregnancy: The role of the placenta. Placenta.
31:725–730. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hanssens A, Marx-Deseure S, Lecoutre L,
Butruille A, Fournel C, Knauf C, Besengez C, Breton L, Storme P,
Deruelle, et al: Maternal obesity alters the apelinergic system at
the feto-maternal interface. Placenta. 39:41–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malamitsi-Puchner A, Gourgiotis D,
Boutsikou M, Baka S, Hassiakos D and Briana DD: Circulating apelin
concentrations in mother/infant pairs at term. Acta Paediatr.
96:1751–1754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van Mieghem T, Doherty A, Baczyk D, Drewlo
S, Baud D, Carvalho J and Kingdom J: Apelin in normal pregnancy and
pregnancies complicated by placental insufficiency. Reprod Sci.
23:1037–1043. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng JQ, Lindsley CW, Cheng GZ, Yang H
and Nicosia SV: The Akt/PKB pathway: Molecular target for cancer
drug discovery. Oncogene. 24:7482–7492. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thompson N and Lyons J: Recent progress in
targeting the Raf/MEK/ERK pathway with inhibitors in cancer drug
discovery. Curr Opin Pharmacol. 5:350–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pereira de Sousa FL, Chaiwangyen W,
Morales-Prieto DM, Ospina-Prieto S, Weber M, Photini SM, Sass N,
Daher S, Schleussner E and Markert UR: Involvement of STAT1 in
proliferation and invasiveness of trophoblastic cells. Reprod Biol.
17:218–224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qin D, Zheng XX and Jiang YR: Apelin-13
induces proliferation, migration, and collagen I mRNA expression in
human RPE cells via PI3K/Akt and MEK/Erk signaling pathways. Mol
Vis. 19:2227–2236. 2013.PubMed/NCBI
|
|
31
|
Li Y, Bai YJ, Jiang YR, Yu WZ, Shi X, Chen
L, Feng J and Sun GB: Apelin-13 is an early promoter of
cytoskeleton and tight junction in diabetic macular edema via
PI-3K/Akt and MAPK/Erk and MAPK/Erk signaling pathways. Biomed Res
Int. 2018:32425742018.
|
|
32
|
Różycka M, Kurowska P, Grzesiak M,
Kotula-Balak M, Tworzydło W, Rame C, Gregoraszczuk E, Dupont J and
Rak A: Apelin and apelin receptor at different stages of corpus
luteum development and effect of apelin on progesterone secretion
and 3β-hydroxysteroid dehydrogenase (3β-HSD) in pigs. Anim Reprod
Sci. 92:251–260. 2018. View Article : Google Scholar
|
|
33
|
Rak A, Drwal E, Rame C, Knapczyk-Stwora K,
Słomczyńska M, Dupont J and Gregoraszczuk EL: Expression of apelin
and apelin receptor (APJ) in porcine ovarian follicles and in vitro
effect of apelin on steroidogenesis and proliferation through APJ
activation and different signaling pathway. Theriogenology.
96:126–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jasaszwili M, Wojciechowicz T, Billert M,
Strowski MZ, Nowak KW and Skrzypski M: Effects of adropin on
proliferation and differentiation of 3T3-L1 cells and rat primary
preadipocytes. Mol Cell Endocrinol. 496:1105322019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
36
|
Rebut-Bonneton C, Segond N, Demignon J,
Porquet D and Evain-Brion D: Effects of calcitonin on human
trophoblastic cells in culture: absence of autocrine control. Mol
Cell Endocrinol. 85:65–71. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zygmunt M, Hahn D, Munstedt K, Bischof P
and Lang U: Invasion of cytotrophoblastic JEG-3 cells is stimulated
by hCG in vitro. Placenta. 19:587–593. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Standley PR and Standley CA:
Identification of a functional Na+/Mg2+ exchanger in human
trophoblast cells. Am J Hypertens. 15:565–570. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wysocka MB, Pietraszek-Gremplewicz K and
Nowak D: The role of apelin in cardiovascular diseases, obesity and
cancer. Front Physiol. 9:5572018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang G, Anini Y, Wei W, Qi X, OCarroll AM,
Mochizuki T, Wang HQ, Hellmich MR, Englander EW and Greeley GH Jr:
Apelin, a new enteric peptide, localization in the gastrointestinal
tract, ontogeny, stimulation of gastric cell proliferation and of
cholecystokinin secretion. Endocrinology. 145:1342–1348. 2004.
View Article : Google Scholar
|
|
41
|
Masri B, Knibiehler B and Audigier Y:
Apelin signaling: A promising pathway from cloning to pharmacology.
Cell Signal. 17:415–426. 2005. View Article : Google Scholar
|
|
42
|
Kourtis A, Gkiomisi A, Mouzaki M, Makedou
K, Anastasilakis AD, Toulis KA, Gerou S, Gavana E and Agorastos T:
Apelin levels in normal pregnancy. Clin Endocrinol (Oxf).
75:367–371. 2011. View Article : Google Scholar
|
|
43
|
Yin L, Zhang P, Li C, Si J, Wang Y, Zhang
X, Zhang D, Zhang H and Lin C: Apelin-13 promotes cell
proliferation in the H9c2 cardiomyoblast cell line by triggering
extracellular signal-regulated kinase 1/2 and protein kinase B
phosphorylation. Mol Med Rep. 17:447–451. 2018.
|
|
44
|
Li F, Li L, Qin X, Pan W, Feng F, Chen F,
Zhu B, Liao D, Tanowitz H, Albanese C and Chen L: Apelin-induced
vascular smooth muscle cell proliferation: The regulation of cyclin
D1. Front Biosci. 13:3786–3792. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sorli SC, van den Berghe L, Masri B,
Knibiehler B and Audigier Y: Therapeutic potential of interfering
with apelin signalling. Drug Discov Today. 11:1100–1106. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sánchez I and Dynlacht BD: New insights
into cyclins, CDKs, and cell cycle control. Semin Cell Dev Biol.
16:311–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bertoli C, Skotheim JM and de Bruin RA:
Control of cell cycle transcription during G1 and S phases. Nat Rev
Mol Cell Biol. 14:518–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Foster DA, Yellen P, Xu L and Saqcena M:
Regulation of G1 cell cycle progression: Distinguishing the
restriction point from a nutrient-sensing cell growth
checkpoint(s). Genes Cancer. 11:1124–1131. 2010. View Article : Google Scholar
|
|
49
|
Shuang L, Jidong W, Hongjuan P and Zhenwei
Y: Effects of apelin on proliferation and apoptosis in rat ovarian
granulosa cells. Clin Exp Obstet Gynecol. 43:409–413.
2016.PubMed/NCBI
|
|
50
|
Zhang J, Liu Q, Hu X, Fang Z, Huang F,
Tang L and Zhou S: Apelin/APJ signaling promotes hypoxia-induced
proliferation of endothelial progenitor cells via
phosphoinositide-3 kinase/Akt signaling. Mol Med Rep. 12:3829–3834.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Peng X, Li F, Wang P, Jia S, Sun L and Huo
H: Apelin-13 induces MCF-7 cell proliferation and invasion via
phosphorylation of ERK1/2. Int J Mol Med. 36:733–738. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang L, Su T, Lv D, Xie F, Liu W, Cao J,
Sheikh IA, Qin X, Li L and Chen L: ERK1/2 mediates lung
adenocarcinoma cell proliferation and autophagy induced by
apelin-13. Acta Biochim Biophys Sin (Shanghai). 6:100–111. 2014.
View Article : Google Scholar
|