Introduction
Osteoarthritis (OA) is a joint disease commonly
occurring in middle-aged and elderly individuals (1,2),
in which the bones, synovium, joint capsule and other structures
develop chronic aseptic inflammation due to degenerative changes in
the joint. OA can affect multiple joints of the body, particularly
the joints of the lower limb, among which knee OA (KOA) has the
highest incidence rate, with ~250 million patients with KOA
reported in 2010, accounting for 3.6% of the total world population
(1). Among individuals aged
>65 years, this percentage may be as high as 60%. The main
symptoms of KOA are joint pain, loss of function and stiffness,
which can cause inconvenience or even disability, resulting in the
loss of the ability to work and a significant socioeconomic burden.
Multiple causes of KOA have been identified, including joint
injuries, abnormal joint development and genetic factors. Previous
studies have demonstrated that obesity, high joint pressure or mild
inflammation in the joints are risk factors for the development of
KOA (3). The molecular mechanism
of KOA is complicated, involving a variety of cell types and
signaling pathways. Thus, the mechanism underlying the occurrence
and development of KOA requires further investigation (4).
High mobility group box protein 1 (HMGB1) is a
non-histone in the nucleus, the biological activity of which varies
with its location and post-translational modifications.
Accumulating evidence suggests that the mRNA expression levels of
HMGB1 and receptor for advanced glycation end products (RAGE) are
increased in patients with KOA. Cartilage is mainly composed of
chondrocytes and various extracellular matrix proteins, including
highly sulfated polymeric protein polysaccharides, small
proteoglycans and collagen (3).
During the development of OA, the synthetic catabolic balance of
the cartilage matrix is dysregulated. Matrix metalloproteinases
(MMPs) and a disintegrin and metalloproteinase with thrombospondin
motifs (ADAMTS), which are secreted by chondrocytes, are the major
extracellular matrix-degrading enzymes. In rheumatoid arthritis and
synovial fibroblasts from patients with KOA, HMGB1 forms a complex
with lipopolysaccharide, interleukin (IL)-1α or IL-1β, promoting
the production of pro-inflammatory cytokines and MMPs (5). Another study has demonstrated that
HMGB1 increases the expression level of MMP3 and MMP13 in wild-type
mouse chondrocytes, whereas this effect was not observed in the
knee joint chondrocytes of toll-like receptor
(TLR)2/TLR4−/− mice (6). In addition, HMGB1 promotes
chondrocyte apoptosis (7). These
results suggest that HMGB1 may promote the production of MMPs in
chondrocytes through the TLR2/TLR4 receptor pathway and that HMGB1
may promote the apoptosis of chondrocytes and the deterioration of
KOA. However, the specific underlying molecular mechanism remains
unclear.
Numerous signaling pathways are involved in the
process of OA, including bone morphogenetic proteins (8), Indian hedgehog (9), hypoxia-induced and Wnt signaling
pathways (10,11). As an important component of the
Wnt signaling pathway, β-catenin is activated by Wnt family
proteins and participates in the pathological process of KOA
(12). Previous studies have also
demonstrated that the Wnt signaling pathway is involved in the
homeostasis and degradation of the cartilage matrix by regulating
the expression of anabolic or catabolic genes, such as increased
expression of MMP-2, MMP-3, MMP-9, MMP-13, ADAMTS4 and ADAMTS5,
which promote the degradation of the extracellular matrix and
trigger KOA (13). Thus, the
Wnt/β-catenin signaling pathway may enhance the degradation of
articular cartilage matrix and promote the occurrence and
development of KOA by increasing the expression of MMPs and
ADAMTs.
Recent studies have revealed that, during
epithelial-to-mesenchymal transition (EMT), overexpression of HMGB1
promotes AKT phosphorylation and triggers the inactivation of
glycogen synthase kinase (GSK)-3β, leading to the intracellular
aggregation of GSK-3β and the transfer of β-catenin into the
nucleus; HMGB1 also affects the expression of the corresponding EMT
marker gene β-catenin, and RAGE is involved in this process,
suggest that HMGB1 may activate the Wnt signaling pathway by
binding to RAGE to activate intracellular phosphoinositide 3-kinase
(PI3K)/AKT, thus inducing EMT (14).
HMGB1 has been previously studied in OA; it is
highly expressed in the articular cartilage and synovium and plays
an important role in the process of KOA (15,16). However, the specific mechanism
underlying the activity of HMGB1 in KOA has not been fully
elucidated. The aim of the present study was to determine how HMGB1
regulates the PI3K/AKT/GSK-3β/ β-catenin signaling pathway.
Materials and methods
Cell line and manipulation
The ATDC5 mouse pre-chondral cell line is a
universal cell model for extracellular study of cartilage derived
from mouse teratoma cells. The ATDC5 cell line was obtained from
ATCC and was cultured according to the ATCC protocol. Cells were
seeded into a 96-well plate at 7×103 cells/well and
cultured in a humidified incubator with 5% CO2 at 37°C
overnight in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.). Subsequently, ATDC5 cells were cultured in ITS
(containing 3×10−8 mol/l sodium selenite, 5.5
µM/ml human transferrin and 10 µM/ml bovine insulin)
for 14 days and allowed to differentiate into chondrocytes. To
study the effects of HMGB1 on chondrocyte apoptosis and catabolism,
the chondrocytes were divided into two groups and treated with
HMGB1 [0, 10, 50, 100 or 300 ng/ml recombinant HMGB1 protein, with
purity >95% (SDS-PAGE), purchased from Abcam (cat. no.
ab167718)] or IL-1β (5 ng/ml) and PBS. To study the mechanism of
KOA, the chondrocytes were divided into four groups: i)
Chondrocytes + HMGB1; ii) chondrocytes + HMGB1 + LY294002 [10
µM (17); PI3K inhibitor];
iii) chondrocytes + HMGB1 + MK-2206 [1 µM (18); AKT inhibitor]; iv) chondrocytes +
SB415286 [10 µM (19);
GSK-3β inhibitor]. The concentration of HMGB1 used was 100 ng/ml.
Cells were harvested at 48 h for further analysis. The experiment
was performed in triplicate.
Hoechst 33342 staining assay
Hoechst 33342 staining was used to study the
apoptosis of ATDC5 cells. Cells were seeded into a 96-well plate at
a density of 7×103 cells/well, cultured in 100 µl
medium and treated with HMGB1 (0, 10, 50, 100 and 300 ng/ml) or
IL-1β (5 ng/ml) in a humidified incubator with 5% CO2 at
37°C overnight. Subsequently, the medium was removed, and the cells
were fixed at 4°C in 4% paraformaldehyde for 1 h and permeabilized
in saponin (0.1% v/v in PBS-BSA). Hoechst 33342 (1 µg/ml;
Promega Corporation) was added to each well, and the cells were
further incubated in the dark for 30 min at 37°C. To assess
specific apoptosis, apoptotic and living cells with condensed and
fragmented nuclei were visualized using a blue filter of a Nikon
TE2000-U inverted fluorescence microscope (Nikon Corporation) at
×200 magnification. The experiments were repeated three times.
Flow cytometry
ATDC5 cells were seeded into a 96-well plate at a
density of 7×103 cells/well, and subjected to different
treatments. Subsequently, the cells were digested and harvested,
then fixed with ethanol overnight at 4°C. Apoptosis was examined
using the Annexin V-FITC/propidium iodide (PI) kit (Multisciences
Lianke Biotech) according to the manufacturer's protocol. Cell
apoptosis rate was measured by a FACSCalibur flow cytometer (BD
Biosciences) with Cell Quest software version 5.1 (BD Biosciences).
The experiments were repeated three times.
siRNA and cell transfection
ATDC5 cells were seeded in 6-well plates and
transfected with 100 pmol negative control (NC) or siRNA fragments
against HMGB1 (RiboBio) by using Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The siRNA sequences for NC and siHMGB1
were as follows: siHMGB1-#1, 5′-GGA ATA ACA CTG CTG CAG A-3′;
siHMGB1-#2, 5′-CTG CGA AGC TGA AGG AAA A-3′; NC, 5′-GCC AGA TTT CTC
AGG TGA TAA-3′. The experiments were repeated three times.
Alcian blue staining
Alcian blue staining was performed on ATDC5 cells.
Briefly, cells were fixed with 100% methanol and stained with 0.1%
Alcian blue 8GS (Sigma-Aldrich; Merck KGaA) in 0.1 N HCl for 4 h at
room temperature and images were captured. The dye was quantified
by solubilizing the sample in 6M guanidine HCL overnight. The
absorbance at 620 nm was measured by a spectrophotometer. The
experiments were repeated three times.
Western blotting
Western blotting was performed according to the
standard procedure using polyclonal antibodies specific for MMPs,
ADAMTs, pAKT, GSK-3β, pGSK-3β, β-catenin, estrogen sulfotransferase
(EST-1) and Runt-related transcription factor 2 (Runx2).
Immunoblotting and protein extraction were performed as previously
described (20,21). Total protein was extracted from
ATDC5 cells using a radioimmuno-precipitation assay buffer
(Sigma-Aldrich; Merck KGaA) supplemented with protease and
phosphatase inhibitors. Protein concentration was quantified by the
BCA assay. A total of 30 µg proteins were loaded per lane
and separated by 10% SDS-PAGE and a transferred to a PVDF membrane
(EMD Millipore). The membrane was blocked with 5% non-fat milk and
incubated with primary antibodies (all from Cell Signaling
Technology, Inc., except where otherwise indicated) against MMPs
(1:1,000, MMP-2 cat. no. 40994, MMP-3 cat. no. 14351, MMP-9 cat.
no. 13667 and MMP-13 cat. no. 69926), ADAMTs (Abcam, 1:1,000,
ADAMT-4 cat. no. ab185722 and ADAMT-5 cat. no. ab41037), GSK-3β
(1:2,000, cat. no. 12456), pGSK-3β (1:1,000, cat. no. 5558),
β-catenin (1:1,000, cat. no. 9582), EST-1 (Abcam, 1:1,000, cat. no.
ab63877) and Runx2 (1:1,000, cat. no. 8486) in TBS buffer at 4°C
overnight. Tubulin was used as a loading control (1:3,000; cat. no.
60008-1-Ig; Proteintech Group, Inc.). The membranes were
subsequently incubated with the secondary antibodies of horseradish
peroxidase-conjugated goat anti-rabbit IgG and goat anti-mouse IgG
(1:3,000; cat. no. 7076S; EarthOx, Life Sciences). SuperSignal™
West Femto Chemiluminescent Substrate (Thermo Fisher Scientific,
Inc.) and Gel Doc™ XR+ System (Bio-Rad Laboratories, Inc.) were
used to develop the blots. The intensity of the bands was analyzed
using Quantity One software (Bio-Rad Laboratories, Inc.) according
to the manufacturer's instructions. The results represented at
least three independent experiments.
Statistical analysis
GraphPad Prism 5 software (GraphPad Software, Inc.)
was used to analyze and plot the data. The results are presented as
the mean ± standard error of the mean. To determine the
significance of the differences between the control and treatment
groups, Student's t-test and ANOVA followed by Bonferroni's
post-hoc test were used. P<0.05 was considered to indicate a
statistically significant difference.
Results
HMGB1 promotes chondrocyte apoptosis and
cartilage matrix degradation
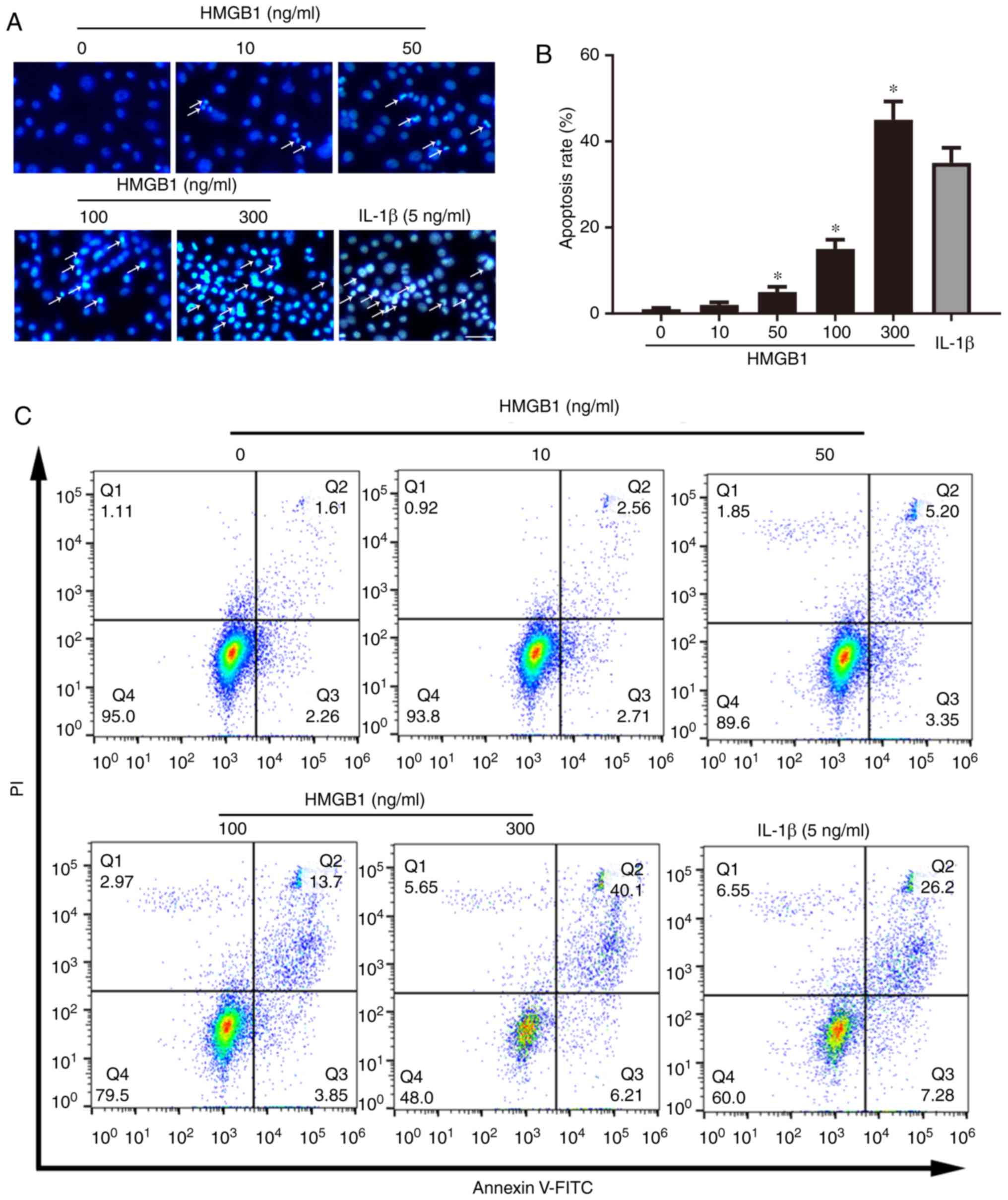
To investigate whether HMGB1 promoted apoptosis and
participates in the development of KOA, ATDC5 cells were cultured
and treated with recombinant HMGB1 t different concentrations.
IL-1β was used as inflammatory control. Hoechst 33342 staining was
performed to detect apoptosis (Fig.
1A and B). Representative images of Hoechst 33342 staining
demonstrated that the number of apoptotic ATDC5 cells (white
arrows) increased with increasing dose of HMGB1. Apoptotic ATDC5
cells displayed a round and shrunken cell body with condensed or
fragile nuclei. To further confirm the extent of apoptosis, cells
were subjected to flow cytometry detection. The results also
demonstrated that HMGB1 induced apoptosis in a
concentration-dependent manner (Fig.
1C and D). The positive control IL-1β induced apoptosis of
ATDC5 cells. In order to lower the toxicity, the concentration of
100 ng/ml HMGB1 was selected for the following experiments. The
major pathological change in KOA is cartilage degeneration and
necrosis, and cartilage proteoglycan is the main component of
normal cartilage tissue. Therefore, the changes in proteoglycanase
and its cleavage products associated with cartilage proteoglycan
metabolism during the progression of KOA were investigated. To
evaluate the effects of HMGB1 on the endochondral ossification of
ATDC5 cells, the deposition of sulfated glycosaminoglycans was
determined using Alcian blue staining. ATDC5 cells treated with
HMGB1 and IL-1β exhibited decreased Alcian Blue staining (Fig. 1E and F). These results suggested
that HMGB1 induces chondrocyte apoptosis and promotes cartilage
matrix degradation.
HMGB1 promotes the expression of
cartilage matrix degradation genes
To further determine whether HMGB1 promotes
cartilage matrix degradation, the expressions of cartilage matrix
degradation genes, including MMP-2, MMP-3, MMP-9, MMP-13, ADAMTS4
and ADAMTS5, were examined by western blotting. The results
revealed that, compared with the control group, the expression
levels of cartilage matrix degradation genes were significantly
upregulated in ATDC5 cells treated with HMGB1 (Fig. 2A and B). In the positive control
group treated with IL-1β, the expression levels of these genes were
also upregulated. Moreover, siRNA fragments against endogenous
HMGB1 were synthesized. The transfection significantly decreased
the expression levels of HMGB1 along with the downregulated
expression of MMPs and ADAMTS (Fig.
2C and D). These data further demonstrate that HMGB1 exposure
promotes cartilage matrix degradation and apoptosis via the
regulation of matrix degradation genes.
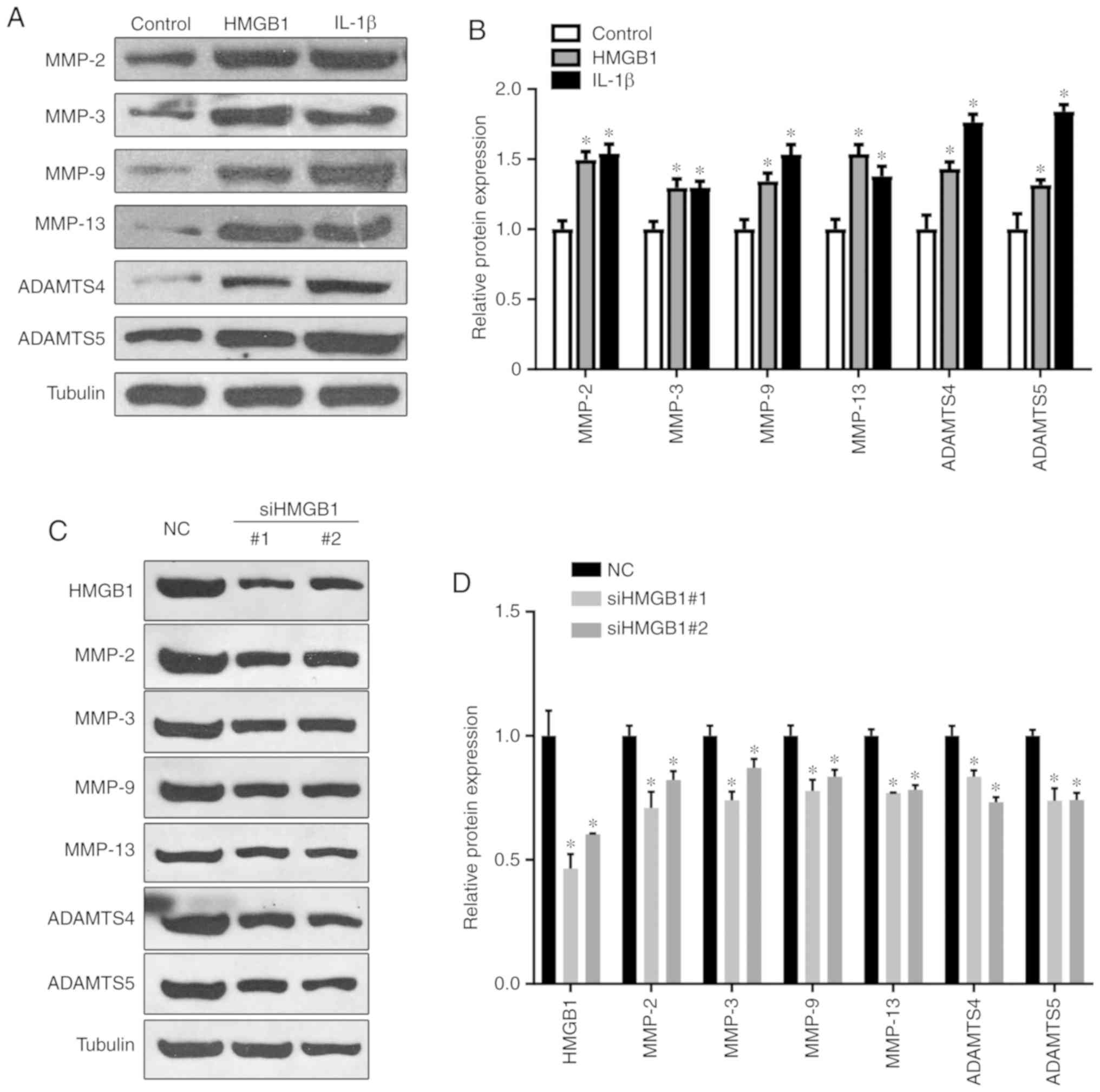 | Figure 2HMGB1 regulates the expression of
cartilage matrix degradation genes. (A and B) Cells were treated
with HMGB1 (100 ng/ml) or IL-1β (5 ng/ml) and western blotting
demonstrated the expression levels of MMP-2, MMP-3, MMP-9, MMP-13,
ADAMTS4 and ADAMTS5 protein expression in ATDC5 cells.
*P<0.05 compared with control group. (C and D) Cells
were transfected with siRNA fragments against HMGB1, and the
expression levels of the indicated proteins were detected by
western blotting; the relative expression levels are shown in (D).
*P<0.05 compared with the NC group. HMGB1, high
mobility group box 1 protein; IL-1β, interleukin 1β; MMP, matrix
metalloproteinase; ADAMTS, a disintegrin and metalloproteinase with
thrombospondin motifs; NC, negative control. |
HMGB1 is involved in the regulation of
the GSK-3β/β-catenin pathway
β-Catenin is an important component of the Wnt
signaling pathway that can be activated by Wnt family proteins and
participates in the pathological process of KOA. However, whether
the GSK-3β/β-catenin pathway is also involved in the pathological
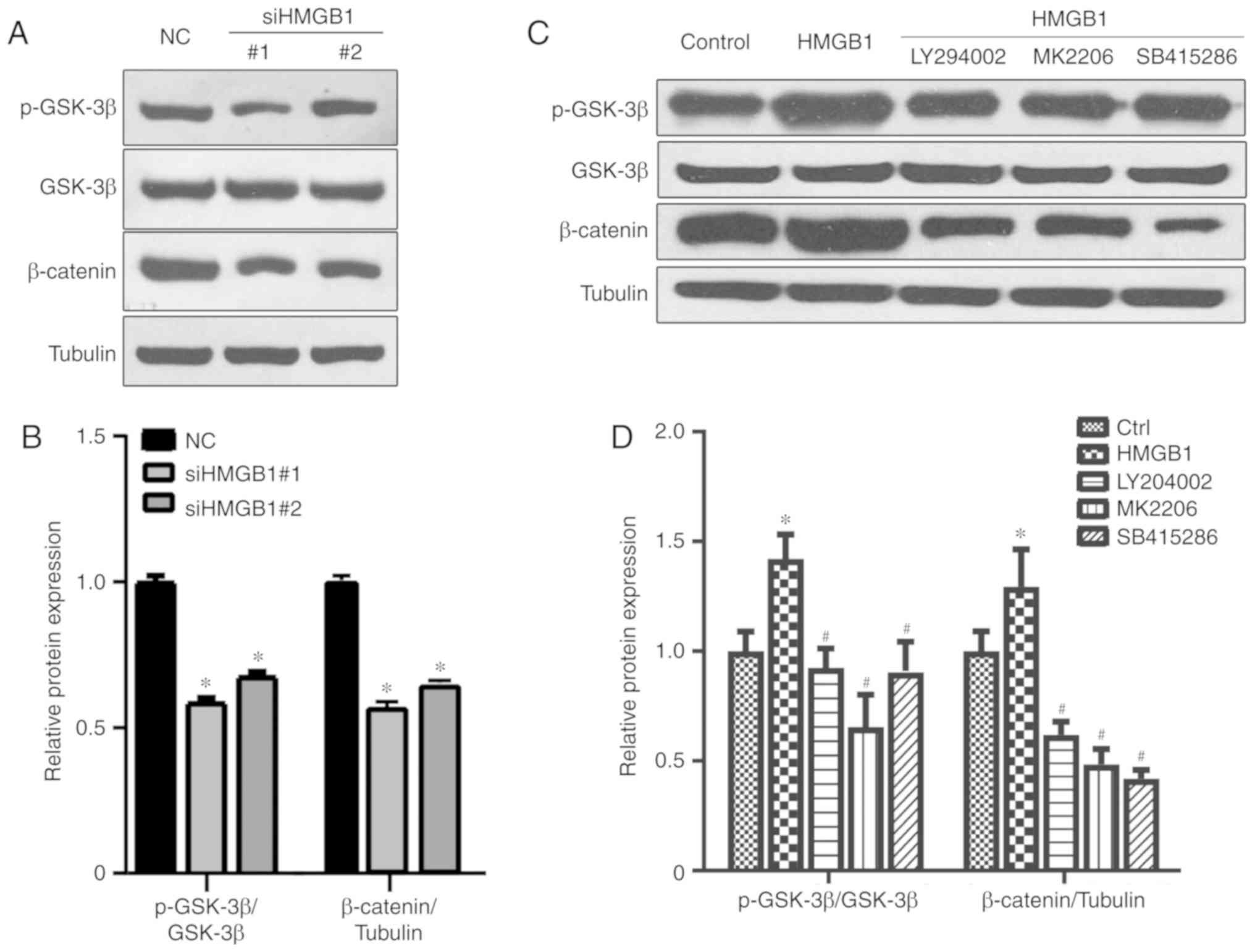
process of HMGB1-induced KOA remains elusive. As shown in Fig. 3A and B, cells with HMGB1 knockdown
exhibited a decrease in the expression levels of phosphorylated
GSK-3β (p-GSK-3β) and β-catenin. Following HMGB1 treatment,
significant upregulation of pGSK-3β and β-catenin expression levels
was observed, which was reversed by the addition of the GSK-3
upstream kinase PI3K inhibitor LY294002, the AKT inhibitor MK-2206
and the GSK-3β inhibitor SB415286 (Fig. 3C and D). These results suggest
that HMGB1 may induce the activation of the GSK-3β/β-catenin
pathway, which may subsequently lead to the abnormal regulation of
chondrocytes.
Regulation of the GSK-3β/β-catenin
pathway contributes to HMGB1-induced expression of cartilage matrix
degradation genes and apoptosis
Increased GSK-3 phosphorylation (inactivation) leads
to the cytoplasmic accumulation and translocation of β-catenin into
the nucleus, leading to the expressions of downstream target genes
(22). To further explore the
mechanisms underlying HMGB1-induced cartilage matrix degradation,
the expression levels of cartilage matrix degradation genes
targeted by β-catenin accumulation were determined by western
blotting. As shown in Fig. 4A and
B, compared with the control group, the expression levels of
MMP-2, MMP-3, MMP-9, MMP-13, ADAMTS4 and ADAMTS5 were significantly
increased following treatment with HMGB1, an effect that was
partially reversed by blocking the PI3K/AKT/GSK-3β/β-catenin
pathway with SB415286, LY294002 and MK-2206. To explore the
potential involvement of the GSK-3β/β-catenin pathway in
HMGB1-induced chondrocyte apoptosis, Hoechst 33342 staining was
performed. Compared with the control group, the number of apoptotic
ATDC5 cells (white arrows) was significantly increased following
treatment with HMGB1. However, this effect was reversed by the
application of LY294002, MK-2206 and SB415286 (Fig. 4C and D). In addition, the
cytometry assay revealed the same trend (Fig. 4E and F). These results indicated
that the activation of the GSK-3β/β-catenin pathway may contribute
to HMGB1-induced expression of cartilage matrix degradation genes
and chondrocyte apoptosis.
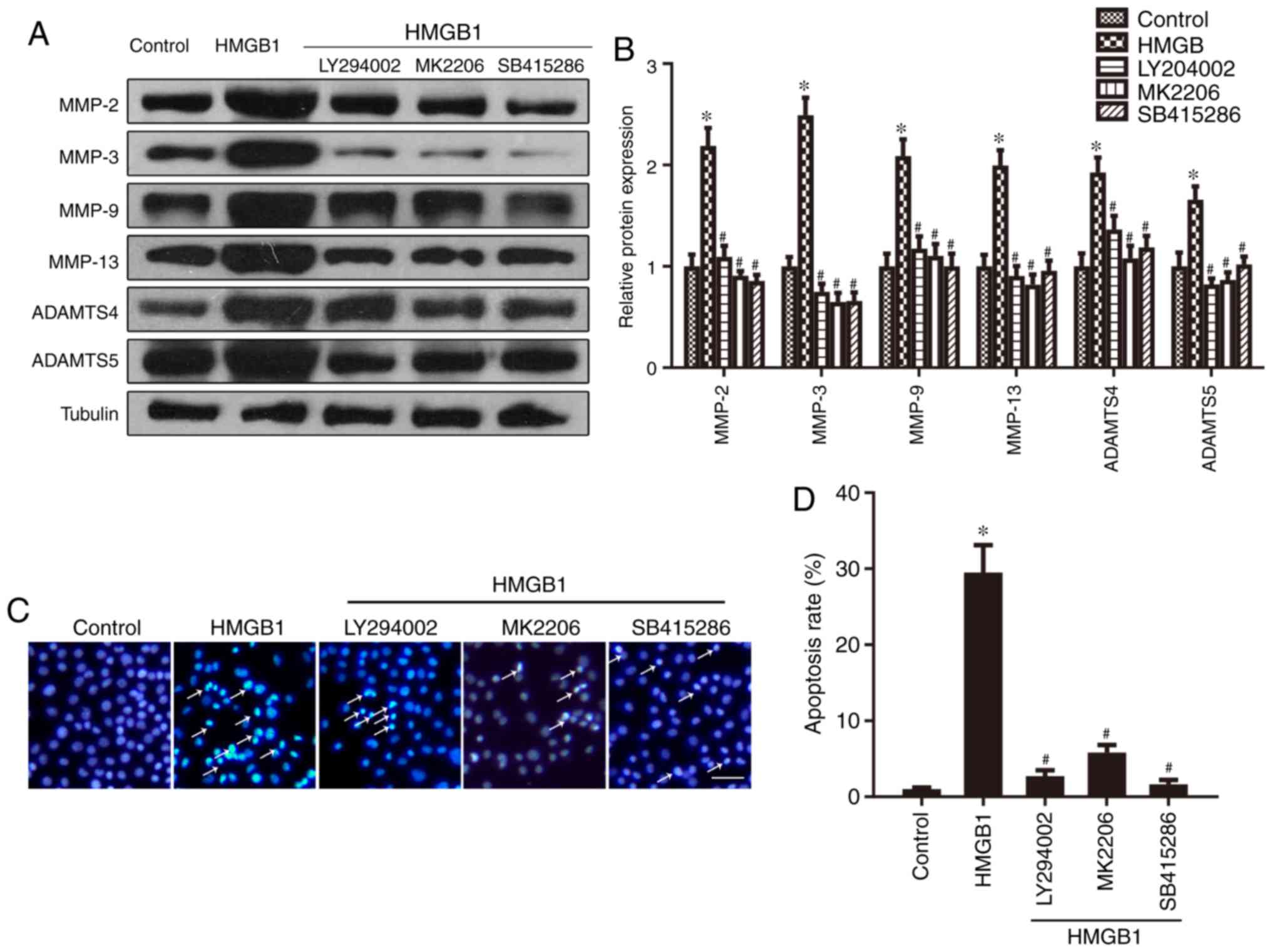 | Figure 4The GSK-3β/β-catenin pathway
contributes to the HMGB1-induced expression of cartilage matrix
degradation genes and cell apoptosis. (A and B) Western blotting
revealed increased protein expression of MMP-2, MMP-3, MMP-9,
MMP-13, ADAMTS4 and ADAMTS5 in ATDC5 cells following HMGB1
treatment, which was reversed by LY294002, MK-2206 and SB415286. (C
and D) Hoechst 33342 staining was performed on ATDC5 cells and the
stained cells were quantified. The number of apoptotic ATDC5C cells
(arrows) increased in cells treated with HMGB1, which was reversed
by LY294002, MK-2206 and SB415286. Scale bar, 20 µm. (E and
F) Cells subjected to the indicated treatments were subjected to
flow cytometry. *P<0.05 compared with the control
group. #P<0.05 compared with the HMGB1 group.
HMGB1, high mobility group box 1 protein; MMP, matrix
metalloproteinase; ADAMTS, a disintegrin and metalloproteinase with
thrombospondin motifs; GSK, glycogen synthase kinase. |
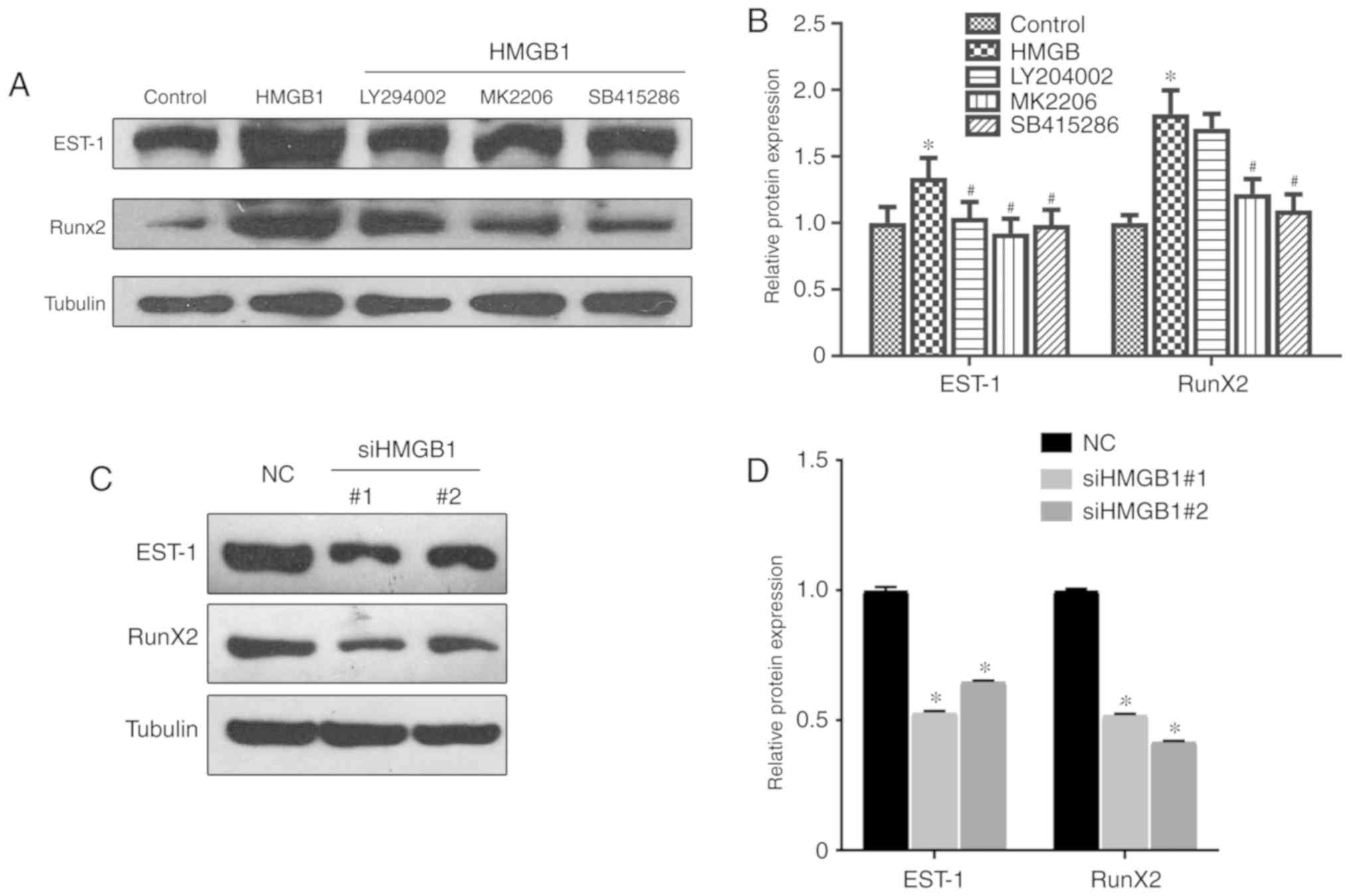
HMGB1 promotes chondrocyte dysfunction
through the upregulation of EST-1 and Runx2
EST-1 and Runx2 regulate the transcription of
multiple genes and play key roles in the differentiation and
maturation of chondrocytes. Recently, Runx2 was found to be
regulated by β-catenin accumulation (23,24). To determine whether HMGB1 may
regulate chondrocyte differentiation and maturation through EST-1
and Runx2, the expression levels of EST-1 and Runx2 were analyzed
by western blotting. As shown in Fig.
5A and B, HMGB1 significantly increased the protein expression
levels of EST-1 and Runx2, which was partially reversed following
treatment with LY294002, MK-2206 and SB415286 (Fig. 5A and B). Furthermore, genetic
knockdown of HMGB1 markedly downregulated the expression levels of
EST-1 and Runx2 (Fig. 5C and D).
These results suggest that HMGB1 may promote chondrocyte
differentiation via the regulation of EST-1 and Runx2 in OA.
Discussion
KOA is a common joint disease with a high incidence
rate among middle-aged and elderly individuals; however, the
underlying molecular mechanisms have yet to be fully elucidated.
The results of the present study demonstrated that HMGB1 may
promote chondrocyte apoptosis and the expression of cartilage
matrix degradation genes, such as MMP-2, MMP-3, MMP-9, MMP-13,
ADAMTS4 and ADAMTS5, which may induce the occurrence and
progression of KOA. In addition, HMGB1 upregulated the expression
levels of p-GSK-3β and β-catenin; when the GSK-3β/β-catenin pathway
was inhibited, the number of apoptotic cells and the expression of
MMPs and ADAMTs were decreased, which indicated that HMGB1 may
induce the occurrence and development of KOA by activating the
GSK-3β/β-catenin pathway. The results also demonstrated that HMGB1
may promote chondrocyte dysfunction by upregulating EST-1 and
Runx2.
HMGB1 was firstly identified as a nuclear protein
regulating DNA replication and chromatin function (25). Despite its role in the
pathogenesis of inflammatory diseases (26) and rheumatoid arthritis (27), accumulating evidence suggests that
HMGB1 also plays an important role in OA. HMGB1 is mainly
distributed in the cytoplasm and matrix of chondrocytes in OA
cartilage (28). The expression
levels of HMGB1 have been associated with the degree of synovitis,
pain and daily activity in patients with KOA (29). Inhibition of HMGB1 suppressed the
inflammatory symptoms (30). In a
rat model or collagen-induced arthritis, treatment with HMGB1
antibody alleviated the arthritis symptoms (14). Recently, HMGB1 was reported to be
secreted in the extracellular environment and act as a cytokine
(31). HMGB1 can be released by
osteoarthritic synoviocytes by IL-1β stimuli (32,33). However, the detailed mechanisms of
action of secreted HMGB1 in OA remain to be fully elucidated. In
the present study, recombinant HMGB1 was applied to treat the
cultured chondrogenic cells, and the effect was compared with that
of IL-1β. The results demonstrated that HMGB1 induced cell
apoptosis and the expression of various cartilage matrix
degradation-related genes, including MMPs and ADAMTS. Although the
pro-inflammatory effects were not revealed by observing the
expression of inflammatory factors, the data suggested that HMGB1
acted as a ligase to affect the surrounding cells, mediating
inflammation and cell death during OA.
The pathogenesis of OA is complex, and the major
cause of OA is cartilage degradation (34). The extracellular matrix
components, such as aggrecan and collagen II, are mainly secreted
by chondrocytes (35). In normal
cartilage tissue, the extracellular matrix synthesis and catabolic
processes are balanced, and the thickness and properties of the
cartilage matrix tend to be stable (36,37). During the process of OA, the
synthetic catabolic balance of the cartilage matrix is disturbed,
the cartilage matrix synthesis is insufficient, catabolism
increases, and the cartilage matrix is degraded, which results in
the thinning of the cartilage layer and degeneration. It is well
known that proinflammatory factors, such as IL-1β, promote
cartilage degradation by stimulating the production of MMPs
(38). MMPs degrade matrix
components, including collagen and other basement membrane
proteins, resulting in cartilage matrix degradation (39). It has been reported that HMGB1
treatment of OA chondrocytes results in the phosphorylation of
nuclear factor-κB (NF-κB) and MMP expression (40). The results of the present study
demonstrated that HMGB1 promoted chondrocyte apoptosis and
upregulated the expression levels of the cartilage matrix
degradation genes MMP-2, MMP-3, MMP-9, MMP-13, ADAMTS4 and ADAMTS5.
Although changes in the mRNA levels were not detected, the data are
consistent with those of previous studies.
Wnt signaling regulation of β-catenin plays critical
role in variety of physiological processes (41). Previous studies have demonstrated
that the expression level of β-catenin was upregulated in
degenerative chondrocytes, resulting in loss of cartilage (42,43). In addition, conditional activation
of β-catenin led to the abnormal differentiation of adult mouse
chondrocytes and induced an OA-associated phenotype in mice
(44). Takamatsu et al
(29) demonstrated that verapamil
inhibited the Wnt signaling pathway and the expression of the Wnt
response gene MMP3 by increasing the expression of the antagonistic
protein secreted frizzled-related protein 3, thus inhibiting the
progression of KOA. The results of the present study revealed that
HMGB1 upregulated the expression levels of p-GSK-3β and β-catenin;
blocking the PI3K/AKT/GSK-3 signaling pathway decreased the cell
apoptosis rate and the expression levels of MMPs and ADAMTs,
indicating that HMGB1 may induce the occurrence and development of
KOA by activating the GSK-3β/β-catenin pathway. EST-1 and Runx2 are
osteogenic differentiation-specific transcription factors that
regulate the transcription of multiple genes (45,46) that play important roles in the
formation and differentiation of osteoblasts, differentiation and
maturation of chondrocytes, formation and absorption of osteoclasts
and production of bone matrix proteins (47,48). In addition, in rheumatoid
arthritis, a number of other signaling pathways that may regulate
MMPs have also been identified, such as Nrf2/HO-1 signaling
(49), NF-κB inflammatory
signaling (50,51), mitochondrial/caspase-mediated
pathways (52) or
mitogen-activated protein kinase signaling pathway (53); however, its role in HMGB1-induced
chondrocyte apoptosis requires further investigation. The results
of the present study also indicated that HMGB1 may promote
chondrocyte dysfunction by upregulating EST-1 and Runx2.
In conclusion, the results of the present study
preliminarily demonstrated the effects of HMGB1 on KOA, including
changes in the GSK-3β/β-catenin pathway, and explored the possible
mechanism underlying the role of HMGB1 in KOA, providing a
theoretical basis and new evidence for the in-depth study of the
molecular mechanism of KOA pathogenesis. The findings of the
present study may aid in identifying molecular targets for the
development of new drugs and the establishment of novel treatment
options for KOA. However, the precise molecular biological
mechanisms remain to be fully elucidated.
Funding
The present was supported by the Zhuhai People's
Hospital Scientific Research Development and Research Fund (grant
no. 201711).
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
ZS, XM, TT, PZ and LZ performed and experiments
analyzed the results. XM and LZ revised and analyzed the data. TT
and PZ contributed to the revised experiment. ZS, XM and YJ wrote
the manuscript and supervised the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent to publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ashford S and Williard J: Osteoarthritis:
A review. Nurse Pract. 39:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ke X, Jin G, Yang Y, Cao X, Fang R, Feng X
and Lei B: Synovial fluid HMGB-1 levels are associated with
osteoarthritis severity. Clin Lab. 61:809–818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X, Guo Y, Wang C and Yu H, Yu X and
Yu H: MicroRNA-142-3p Inhibits chondrocyte apoptosis and
inflammation in osteoarthritis by targeting HMGB1. Inflammation.
39:1718–1728. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Snelling SJ, Davidson RK, Swingler TE, Le
LT, Barter MJ, Culley KL, Price A, Carr AJ and Clark IM: Dickkopf-3
is upregulated in osteoarthritis and has a chondroprotective role.
Osteoarthritis Cartilage. 24:883–891. 2016. View Article : Google Scholar :
|
|
6
|
Ley C, Svala E, Nilton A, Lindahl A,
Eloranta ML, Ekman S and Skiöldebrand E: Effects of high mobility
group box protein-1, interleukin-1β, and interleukin-6 on cartilage
matrix metabolism in three-dimensional equine chondrocyte cultures.
Connective Tissue Res. 52:290–300. 2011. View Article : Google Scholar
|
|
7
|
Puzovic V, Brcic I, Ranogajec I and
Jakic-Razumovic J: Prognostic values of ETS-1, MMP-2 and MMP-9
expression and co-expression in breast cancer patients. Neoplasma.
61:439–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin Y and Wang Y: Association of BMP-14
rs143383 ploymorphism with its susceptibility to osteoarthritis: A
meta-analysis and systematic review according to PRISMA guideline.
Medicine (Baltimore). 96:e74472017. View Article : Google Scholar
|
|
9
|
Zhang RK, Li GW, Zhang DW, Yu B and Feng
SY: Research of the expression of subchondral bone of Indian
hedgehog with early experimental osteoarthritis induced by
mechanical stress. Zhonghua yi xue za zhi (Chinese). 97:53–56.
2017.
|
|
10
|
Xu W, Gao P, Zhang Y, Piao L and Dong D:
microRNA-138 Induces Cell Survival and Reduces WNT/β-catenin
signaling of osteoarthritis chondrocytes through NEK2. IUBMB Life.
71:1355–1366. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okura T, Ohkawara B, Takegami Y, Ito M,
Masuda A, Seki T, Ishiguro N and Ohno K: Mianserin suppresses
R-spondin 2-induced activation of Wnt/β-catenin signaling in
chondrocytes and prevents cartilage degradation in a rat model of
osteoarthritis. Sci Rep. 9:28082019. View Article : Google Scholar
|
|
12
|
Fernandez-Torres J, Zamudio-Cuevas Y,
Lopez-Reyes A, Garrido-Rodríguez D, Martínez-Flores K, Lozada CA,
Muñóz-Valle JF, Oregon-Romero E and Martínez-Nava GA: Gene-gene
interactions of the Wnt/β-catenin signaling pathway in knee
osteoarthritis. Mol Biol Rep. 45:1089–1098. 2018. View Article : Google Scholar
|
|
13
|
Sanchez-Adams J, Leddy HA, McNulty AL,
O'Conor CJ and Guilak F: The mechanobiology of articular cartilage:
Bearing the burden of osteoarthritis. Curr Rheumatol Rep.
16:4512014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YC, Statt S, Wu R, Chang HT, Liao JW,
Wang CN, Shyu WC and Lee CC: High mobility group box 1-induced
epithelial mesenchymal transition in human airway epithelial cells.
Sci Rep. 6:188152016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang Y, Zhu L, Zhang T, Lu H, Wang C, Xue
B, Xu X, Liu Y, Cai Z, Sang W, et al: BRD4 has dual effects on the
HMGB1 and NF-κB signalling pathways and is a potential therapeutic
target for osteoarthritis. Biochim Biophys Acta Mol Basis Dis.
1863:3001–3015. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang C, Yu W, Huang C, Ding Q, Liang C,
Wang L, Hou Z and Zhang Z: Chrysin protects human osteoarthritis
chondrocytes by inhibiting inflammatory mediator expression via
HMGB1 suppression. Mol Med Rep. 19:1222–1229. 2019.
|
|
17
|
Flemming A, Brummer T, Reth M and Jumaa H:
The adaptor protein SLP-65 acts as a tumor suppressor that limits
pre-B cell expansion. Nat Immunol. 4:38–43. 2003. View Article : Google Scholar
|
|
18
|
Shen C, Cai GQ, Peng JP and Chen XD:
Autophagy protects chondrocytes from glucocorticoids-induced
apoptosis via ROS/Akt/FOXO3 signaling. Osteoarthritis Cartilage.
23:2279–2287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hui W, Litherland GJ, Jefferson M, Barter
MJ, Elias MS, Cawston TE, Rowan AD and Young DA: Lithium protects
cartilage from cytokine-mediated degradation by reducing
collagen-degrading MMP production via inhibition of the P38
mitogen-activated protein kinase pathway. Rheumatology (Oxford).
49:2043–2053. 2010. View Article : Google Scholar
|
|
20
|
Li Y, Wang XY, Zhang ZL, Cheng X, Li XD,
Chuai M, Lee KK, Kurihara H and Yang X: Excess ROS induced by AAPH
causes myocardial hypertrophy in the developing chick embryo. Int J
Cardiol. 176:62–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He YQ, Li Y, Wang XY, He XD, Jun L, Chuai
M, Lee KK, Wang J, Wang LJ, Yang X, et al: Dimethyl phenyl
piperazine iodide (DMPP) induces glioma regression by inhibiting
angio-genesis. Exp Cell Res. 320:354–364. 2014. View Article : Google Scholar
|
|
22
|
Yavropoulou MP and Yovos JG: The role of
the Wnt signaling pathway in osteoblast commitment and
differentiation. Hormones (Athens). 6:279–294. 2007. View Article : Google Scholar
|
|
23
|
Iwata M, Aikawa T, Hakozaki T, Arai K,
Ochi H, Haro H, Tagawa M, Asou Y and Hara Y: Enhancement of Runx2
expression is potentially linked to beta-catenin accumulation in
canine intervertebral disc degeneration. J Cell Physiol.
230:180–190. 2015. View Article : Google Scholar
|
|
24
|
Vega OA, Lucero CMJ, Araya HF, Jerez S,
Tapia JC, Antonelli M, Salazar-Onfray F, Las Heras F, Thaler R,
Riester SM, et al: Wnt/β-catenin signaling activates expression of
the bone-related transcription factor RUNX2 in select human
osteosarcoma cell types. J Cell Biochem. 118:3662–3674. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andersson U and Tracey KJ: HMGB1 is a
therapeutic target for sterile inflammation and infection. Annu Rev
Immunol. 29:139–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nalesso G, Sherwood J, Bertrand J, Pap T,
Ramachandran M, De Bari C, Pitzalis C and Dell'accio F: WNT-3A
modulates articular chondrocyte phenotype by activating both
canonical and noncanonical pathways. J Cell Biol. 193:551–564.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park SY, Lee SW, Kim HY, Lee WS, Hong KW
and Kim CD: HMGB1 induces angiogenesis in rheumatoid arthritis via
HIF-1α activation. Eur J Immunol. 45:1216–1227. 2015. View Article : Google Scholar
|
|
28
|
Heinola T, Kouri VP, Clarijs P, Ciferska
H, Sukura A, Salo J and Konttinen YT: High mobility group box-1
(HMGB-1) in osteoarthritic cartilage. Clin Exp Rheumatol.
28:511–518. 2010.PubMed/NCBI
|
|
29
|
Takamatsu A, Ohkawara B, Ito M, Masuda A,
Sakai T, Ishiguro N and Ohno K: Verapamil protects against
cartilage degradation in osteoarthritis by inhibiting Wnt/β-catenin
signaling. PLoS One. 9:e926992014. View Article : Google Scholar
|
|
30
|
Yuan Z, Luo G, Li X, Chen J, Wu J and Peng
Y: PPARgamma inhibits HMGB1 expression through upregulation of
miR-142-3p in vitro and in vivo. Cell Signal. 28:158–164. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gardella S, Andrei C, Ferrera D, Lotti LV,
Torrisi MR, Bianchi ME and Rubartelli A: The nuclear protein HMGB1
is secreted by monocytes via a non-classical, vesicle-mediated
secretory pathway. Embo Rep. 3:995–1001. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garcia-Arnandis I, Guillen MI, Gomar F,
Pelletier JP, Martel-Pelletier J and Alcaraz MJ: High mobility
group box 1 potentiates the pro-inflammatory effects of
interleukin-1β in osteoarthritic synoviocytes. Arthrit Res Ther.
12:R1652010. View
Article : Google Scholar
|
|
33
|
Garcia-Arnandis I, Guillen MI, Castejon
MA, Gomar F and Alcaraz MJ: Haem oxygenase-1 down-regulates high
mobility group box 1 and matrix metalloproteinases in
osteoarthritic synoviocytes. Rheumatology (Oxford). 49:854–861.
2010. View Article : Google Scholar
|
|
34
|
Ghosh S, Basu M and Roy SS: ETS-1 protein
regulates vascular endothelial growth factor-induced matrix
metalloproteinase-9 and matrix metalloproteinase-13 expression in
human ovarian carcinoma cell line SKOV-3. J Biol Chem.
287:15001–15015. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ji Q, Xu X, Xu Y, Fan Z, Kang L, Li L,
Liang Y, Guo J, Hong T, Li Z, et al: miR-105/Runx2 axis mediates
FGF2-induced ADAMTS expression in osteoarthritis cartilage. J Mol
Med (Berl). 94:681–694. 2016. View Article : Google Scholar
|
|
36
|
Mekala LP, Mohammed M, Chintalapati S and
Chintalapati VR: Stable isotope-assisted metabolic profiling
reveals growth mode dependent differential metabolism and multiple
catabolic pathways of l-phenylalanine in rubrivivax benzoatilyticus
JA2. J Proteome Res. 17:189–202. 2018. View Article : Google Scholar
|
|
37
|
Dell'Isola A, Allan R, Smith SL, Marreiros
SS and Steultjens M: Identification of clinical phenotypes in knee
osteoarthritis: A systematic review of the literature. BMC
Musculoskelet Disord. 17:4252016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arend WP and Dayer JM: Inhibition of the
production and effects of interleukin-1 and tumor necrosis factor
alpha in rheumatoid arthritis. Arthritis Rheum. 38:151–160. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Loeser RF, Yammani RR, Carlson CS, Chen H,
Cole A, Im HJ, Bursch LS and Yan SD: Articular chondrocytes express
the receptor for advanced glycation end products: Potential role in
osteoarthritis. Arthritis Rheum. 52:2376–2385. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schulte G and Bryja V: WNT signalling:
Mechanisms and therapeutic opportunities. Br J Pharmacol.
174:4543–4546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xia C, Wang P, Fang L, Ge Q, Zou Z, Dong
R, Zhang P, Shi Z, Xu R, Zhang L, et al: Activation of β-catenin in
Col2-expressing chondrocytes leads to osteoarthritis-like defects
in hip joint. J Cell Physiol. 234:18535–18543. 2019.PubMed/NCBI
|
|
43
|
Nishimura R, Hata K and Kida J: Regulation
of osteoblasts and chondrocytes by Wnt signaling. Clin Calcium
(Japanese). 29:299–307. 2019.
|
|
44
|
Ma L, Liu Y, Zhao X, Li P and Jin Q:
Rapamycin attenuates articular cartilage degeneration by inhibiting
β-catenin in a murine model of osteoarthritis. Connect Tissue Res.
60:452–462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen Y, Hu Y, Yang L, Zhou J, Tang Y,
Zheng L and Qin P: Runx2 alleviates high glucose-suppressed
osteogenic differentiation via PI3K/AKT/GSK3 β/β-catenin pathway.
Cell Biol Int. 41:822–832. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Deng L, Hu G, Jin L, Wang C and Niu H:
Involvement of microRNA-23b in TNF-α-reduced BMSC osteogenic
differentiation via targeting runx2. J Bone Miner Metab.
36:648–660. 2018. View Article : Google Scholar
|
|
47
|
Cai T, Sun D, Duan Y, Wen P, Dai C, Yang J
and He W: WNT/β-catenin signaling promotes VSMCs to osteogenic
trans-differentiation and calcification through directly modulating
Runx2 gene expression. Exp Cell Res. 345:206–217. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu YL, Wang S, Ding DG, Xu L and Zhu HT:
miR217 inhibits osteogenic differentiation of rat bone
marrowderived mesenchymal stem cells by binding to Runx2. Mol Med
Rep. 15:3271–3277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhai KF, Duan H, Khan GJ, Xu H, Han FK,
Cao WG, Gao GZ, Shan LL and Wei ZJ: Salicin from alangium chinense
ameliorates rheumatoid arthritis by modulating the Nrf2-HO-1-ROS
pathways. J Agric Food Chem. 66:6073–6082. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhai KF, Duan H, Cao WG, Gao GZ, Shan LL,
Fang XM and Zhao L: Protective effect of Rabdosia amethystoides
(Benth) Hara extract on acute liver injury induced by Concanavalin
A in mice through inhibition of TLR4-NF-κB signaling pathway. J
Pharmacol Sci. 130:94–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhai KF, Duan H, Luo L, Cao WG, Han FK,
Shan LL and Fang XM: Protective effects of paeonol on inflammatory
response in IL-β-induced human fibroblast-like synoviocytes and
rheumatoid arthritis progression via modulating NF-kappaB pathway.
Inflammopharmacology. Aug 10–2017.Epub ahead of print. View Article : Google Scholar
|
|
52
|
Zhai KF, Duan H, Chen Y, Khan GJ, Cao WG,
Gao GZ, Shan LL and Wei ZJ: Apoptosis effects of imperatorin on
synoviocytes in rheumatoid arthritis through
mitochondrial/caspase-mediated pathways. Food Funct. 9:2070–2079.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhai KF, Duan H, Cui CY, Cao YY, Si JL,
Yang HJ, Wang YC, Cao WG, Gao GZ and Wei ZJ: Liquiritin from
Glycyrrhiza uralensis attenuating rheumatoid arthritis via reducing
inflammation, suppressing angiogenesis, and inhibiting MAPK
signaling pathway. J Agric Food Chem. 67:2856–2864. 2019.
View Article : Google Scholar : PubMed/NCBI
|