Introduction
Multiple myeloma (MM) is a hematological malignancy
of terminally differentiated plasma cells. Myeloma bone disease
(MBD) is the most common complication in patients with MM (1). Adipose cytokines including visfatin,
leptin and adiponectin have been implicated in the stimulation or
inhibition of tumor cell growth (2-5).
For example, in a prospective study of 174 patients with MM and 348
controls (6), adiponectin was
revealed to be associated with an increased MM risk. In contrast,
Medina et al (7)
demonstrated that adiponectin had an anti-proliferative effect on
MM cells that was mediated by the protein kinase A/adenosine
monophosphate-activated protein kinase (AMPK) signaling pathway.
Adiponectin also was revealed to prevent MBD in a mouse myeloma
model (8).
Little is known about the impact of adiponectin on
bone disease induced by MM. To study this question, the present
study aimed to determine the concentrations of visfatin, leptin and
adiponectin in the serum and bone marrow and elucidate whether
correlations exist between these concentrations and bone disease in
patients with MM.
Osteoclasts are large multinucleated cells (9,10)
that are derived from tartrate-resistant acid phosphatase
(TRAP)-positive monocyte-osteoclast precursor cells [mostly cluster
of differentiation 14 (CD14)+ mononuclear cells
(11)] through the action of
receptor activator of nuclear factor-κB ligand (RANKL) and
macrophage colony-stimulating factor (M-CSF). Osteoclast activation
is associated with the development of MBD (12). For this reason, the present study
investigated the effects of adiponectin on the differentiation and
maturation of osteoclasts in MM.
Adiponectin exerts its functions by binding to
adiponectin receptor (AdipoR)1 and AdipoR2. The magnitude of the
effects of adiponectin on physiological functions in tissues is
directly associated with receptor expression levels (13). AdipoR1 is expressed significantly
higher in osteoclasts compared with AdipoR2, suggesting that
AdipoR1 has a higher affinity for this receptor isoform (14). Cell growth and metabolism also are
regulated by mechanistic target of rapamycin kinase (mTOR), which
integrates nutrient, energy and oxygen level information. Previous
studies have revealed that the mTOR pathway may be involved in the
generation of osteoclasts and affect their bone resorption function
(15) Walker et al
(16) reported that adiponectin
absence coincided with active AMPK/mTOR signaling in adiponectin
knockout hepatocellular carcinoma cells, which indicates that mTOR
lies downstream of adiponectin. However, it remains unclear how
AdipoR1, mTOR and its downstream effector molecule eukaryotic
translation initiation factor 4E-binding protein (4EBP1) are
involved in the effect of adiponectin on the differentiation and
maturation of osteoclasts in patients with MM. To study this
question, flow cytometry was used to detect the expression of
AdipoR1 on the surface of osteoclast precursor cells and the
phosphorylation of mTOR and 4EBP1.
Materials and methods
Study subjects
Subjects were recruited from the Hematology
Department of Tianjin Medical University General Hospital (Tianjin,
China). The present study was ethically approved by the Ethics
Committee of the Tianjin Medical University. Written informed
consent was obtained from all patients for the publication of this
report and any accompanying images. Bone marrow and peripheral
blood were collected from 39 newly diagnosed patients with MM
(including 24 men and 15 women; median age, 56 years; range, 46-72
years), according to the International Myeloma Working Group
(17). Peripheral blood from
normal controls were collected from 20 age-matched healthy
volunteers. Bone marrow mononuclear cells (BMMNCs) were extracted
from 32 newly diagnosed patients with MM. The diagnoses of patients
with MM were made according to the International Myeloma Workgroup
criteria. X-ray images were used to stage disease prior to
treatment. Bone disease was graded as follows: Stage A, no
osteolytic lesions or osteoporosis alone; stage B, one to three
osteolytic lesions; and stage C, more than three osteolytic lesions
and/or a pathological fracture (18). Patients with MM were classified
using the International Staging System (ISS). ISS stage I was
defined as serum β2-microglobulin levels <3.5 mg/l and serum
albumin levels >3.5 g/dl. ISS stage III was defined as serum
β2-microglobulin level >5.5 mg/l, independent from serum albumin
level. ISS stage II included patients who did not fulfill the
criteria for stages I and II (19).
Enzyme-linked immunosorbent assay
(ELISA)
Serum and bone marrow were collected prior to
treatment. Sera were centrifuged at 1,000 x g for 10 min at room
temperature and then stored at −80°C until use. Concentrations of
visfatin, leptin and adiponectin were measured using commercially
available ELISA kits (adiponectin (cat. no. SEA605Hu), visfatin
(cat. no. SEA638Hu) and leptin (cat. no. SEA084Hu) (all Uscn Life
Sciences, Inc., Wuhan, China) according to the manufacturer's
protocol.
Electrochemiluminescence immunoassay
The serum levels of carboxyterminal cross-linking
telopeptide of type I collagen (CTX), osteocalcin (OCN) and
procollagen I amino-terminal pro-peptide (PINP) were analyzed using
kits (cat. no. COBASE601) from Roche Diagnostics GmbH (Mannheim,
Germany) according to the manufacturer's protocol.
Differentiation and identification of
osteoclasts
In brief, BMMNCs were isolated from patients with MM
and cultured in α-Minimum Essential Medium containing 10% (v/v)
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 50 ng/ml M-CSF (Miltenyi Biotec, Inc., Cambridge, MA,
USA) and 100 ng/ml RANKL (Miltenyi Biotec, Inc.). Recombinant human
full-length adiponectin (PeproTech, Inc., Rocky Hill, NJ, USA) was
dissolved in water at a concentration of 1.0 mg/ml and then diluted
with 0.1% bovine serum albumin to a final concentration of 0.1
mg/ml. It then was stored at -20 to -80°C until use. BMMNCs were
cultured for 14 days in the presence of 10 µg/ml
adiponectin, following which the osteoclasts were identified using
TRAP staining at 37°C for 5 min using a commercial TRAP staining
kit (Sigma-Aldrich;). The number of TRAP-positive multinuclear
(>3 nuclei) cells in each well were counted.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Tiangen Biotech Co., Ltd., Beijing, China). The quality of RNA
samples was assessed spectrophotometrically (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Finally, the extracted RNA was dissolved
in 20 µl diethylpyrocarbonate-treated water and cDNA was
synthesized using a TIANScript RT kit (Tiangen Biotech Co., Ltd.),
according to the manufacturer's protocol: DNA removal reaction at
42°C for 3 min, RT reaction at 42°C for 15 min, then 95°C for 3
min. RNA levels were quantified by RT-qPCR using a Bio-Rad iQ5
Real-time system (Bio-Rad Laboratories, Inc.) and the thermocycling
conditions were as follows: 95°C for 30 sec, 95°C for 5 sec, then
annealing temperature for 30 sec, with a total of 45 cycles. The
levels of RNA were determined using the 2-ΔΔCq method
using β-actin as a control (20).
SYBR Green (Tiangen Biotech Co., Ltd.) was used as a double-strand
DNA-specific dye. Primers sequences are presented in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Target | Sense and antisense
sequences | Annealing
temperature, °C |
|---|
| RANKL | | |
| F |
5'-ATATCGTTGGATCACAGCACATCAGAG-3' | 58.7 |
| R |
5'-TGTCGGTGGCATTAATAGTGAGATGAG-3' | |
| OSCAR | | |
| F |
5'-GTTACCGCTGCTGCTACCGAAG-3' | 59.2 |
| R |
5'-GCGCAGGCTCACGTTGGC-3' | |
| TRAP | | |
| F |
5'-ATGACCACCTTGGCAATGTCTCTG-3' | 60.4 |
| R |
5'-AGGCTGCTGGCTGAGGAAGTC-3' | |
| Cathepsin K | | |
| F |
5'-CCATCCATAACCTTGAGGCTTCTCTTG-3' | 61.3 |
| R |
5'-CCAGTCATCTTCTGAACCACCTCTTC-3' | |
| GAPDH | | |
| F |
5'-CAGGAGGCATTGCTGATGAT-3' | 59.8 |
| R |
5'-GAAGGCTGGGGCTCATTT-3' | |
Flow cytometric analysis
Flow cytometric analysis was conducted using a
CytoFlex flow cytometer and CytExpert Pro analysis software 2.0
(Beckman Coulter, Inc., Brea, CA, USA). The side scatter area
represent the relative measures of complexity (21). According to a previous study by
Sorensen et al (22), CD14
is the specific marker of the osteoclasts precursor derived from
CD14+ monocytes cultured with M-CSF and RANKL. Thus,
CD14-positive cells are osteoclasts precursor cells. To detect
AdipoR1 expression, osteoclast precursor cells were labeled with
anti-CD14 (BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA;
cat. no. 562691) and anti-AdipoR1 (Abcam; cat. no. ab126611) for 15
min at room temperature. Phosphorylation of mTOR and 4EBP1 was
determined as follows: Anti-CD14 antibodies (BD Pharmingen; BD
Biosciences; cat. no. 562691) were incubated with cells at room
temperature for 15 min. Cells were washed with phosphate buffered
saline, then fixed at room temperature for 5 min and permeabilized
for 30 min using the Fixation/Permeabilization Solution kit (BD
Biosciences) according to the manufacturer's protocol. mTOR
signaling pathway-specific agonist (MHY1485) was added (10
µM; 4 h) at 37°C after 14 days in the presence of 10
µg/ml adiponectin to further determine whether the effects
of adiponectin may be reversed. Anti-mTOR (BD Phosflow; BD
Biosciences; cat. no. 563489) or anti-4EBP1 antibody (BD Phosflow;
BD Biosciences; cat. no. 560285) then were added for 20 min at room
temperature.
Statistical analysis
SPSS 21.0 software (IBM Corp., Armonk, NY, USA) was
used for statistical analysis. All results are expressed as the
mean ± SD, median and quartile range. An unpaired Student's t-test
and one-way analysis of variance with the LSD post hoc test were
used to analyze the significance of differences between groups. A
non-parametric test (Mann-Whitney U test) was used if the data were
not normally distributed. The correlation between visfatin, leptin
and adiponectin and CTX was performed using a Pearson's test. A
Spearman's test was used to determine the correlation between
visfatin, leptin or adiponectin, and OCN or PINP. P<0.05 was
considered to indicate a statistically significant difference.
Results
Levels of adiponectin are decreased in
newly diagnosed patients with MM
Clinical characteristics of the patients are
presented in Table II.
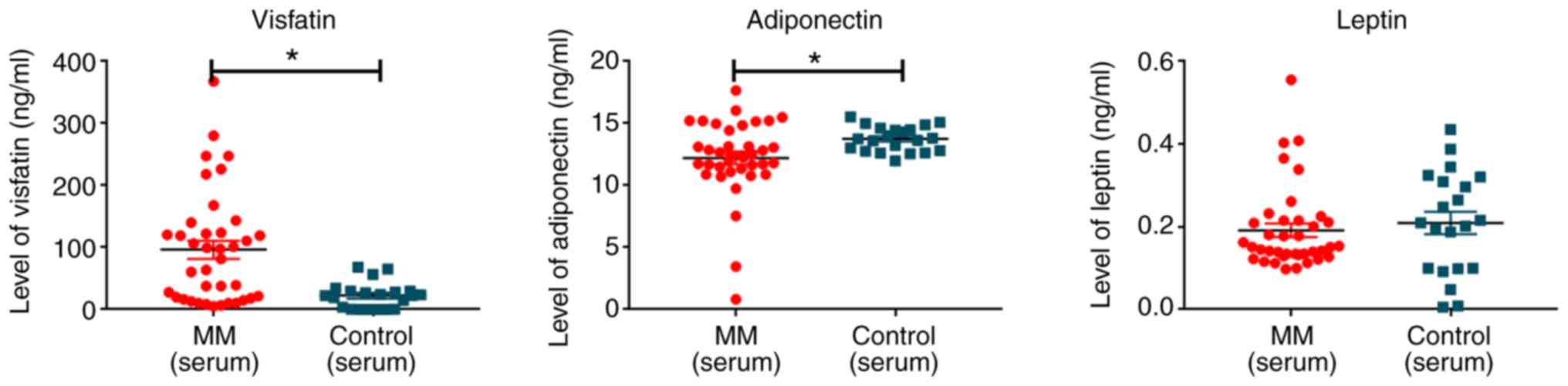
Adiponectin levels in the serum from patients with MM were
significantly lower compared with in normal controls (12.37±3.13
ng/ml vs. 13.80±0.95 ng/ml; P=0.045). Visfatin levels were
significantly higher in the serum from patients with MM compared
with the control (102.76±90.41 ng/ml vs. 22.55±21.41 ng/ml;
P<0.001). There was no significant difference between the level
of leptin in patients with MM and the normal controls (Table II; Fig. 1).
 | Table IIProfiles of patients with MM enrolled
in the present study. |
Table II
Profiles of patients with MM enrolled
in the present study.
| Group | Patient (n) | Sex
| Age (years), median
(range) | ISS
| Type of MM
| Visfatin (ng/ml,
x¯±s) | Leptin (ng/ml,
x¯±s) | Adiponectin (ng/ml,
x¯±s) |
|---|
| Male | Female | I/II | III | IgG | IgA | κ | λ |
|---|
| MM (serum) | 39 | 24 | 15 | 63 (47-83) | 8 | 31 | 22 | 8 | 8 | 1 |
102.76±90.41a | 0.21±0.11 | 12.37±3.13a |
| Control | 20 | 11 | 9 | 67 (57-75) | | | | | | | 22.55±21.41 | 0.21±0.13 | 13.80±0.95 |
Level of adiponectin is associated with
ISS and bone disease stage in MM
The present study compared the levels of adiponectin
according to the stage of ISS and bone disease. The serum levels of
adiponectin in patients with stage I/II MM was significantly higher
compared with in patients with stage III MM (P<0.05; Table III). However, the serum and bone
marrow levels of adiponectin in MBD stage A were significantly
higher compared with those in stages B or C (P<0.05; Table IV). No significant differences in
visfatin or leptin levels were correlated with stage of ISS or bone
disease (Tables III and
IV).
 | Table IIILevels of visfatin, leptin and
adiponectin in patients with different ISS phase multiple
myeloma. |
Table III
Levels of visfatin, leptin and
adiponectin in patients with different ISS phase multiple
myeloma.
| ISS | Patient (n) | Visfatin
(ng/ml) | Leptin (ng/ml) | Adiponectin
(ng/ml) |
|---|
| I/II | 8 | 60.66±54.68 | 0.20±0.05 | 14.47±1.29a |
| III | 31 | 113.62±95.19 | 0.21±0.12 | 11.83±3.24 |
 | Table IVLevels of visfatin, leptin and
adiponectin in different multiple myeloma bone disease stages. |
Table IV
Levels of visfatin, leptin and
adiponectin in different multiple myeloma bone disease stages.
| Bone disease | Patient (n) | Visfatin
(ng/ml) | Leptin (ng/ml) | Adiponectin
(ng/ml) |
|---|
| Stage A | 8 | 55.38±49.57 | 0.17±0.05 | 14.36±2.32a |
| Stage B/C | 26 | 114.98±95.01 | 0.22±0.12 | 11.86±3.13 |
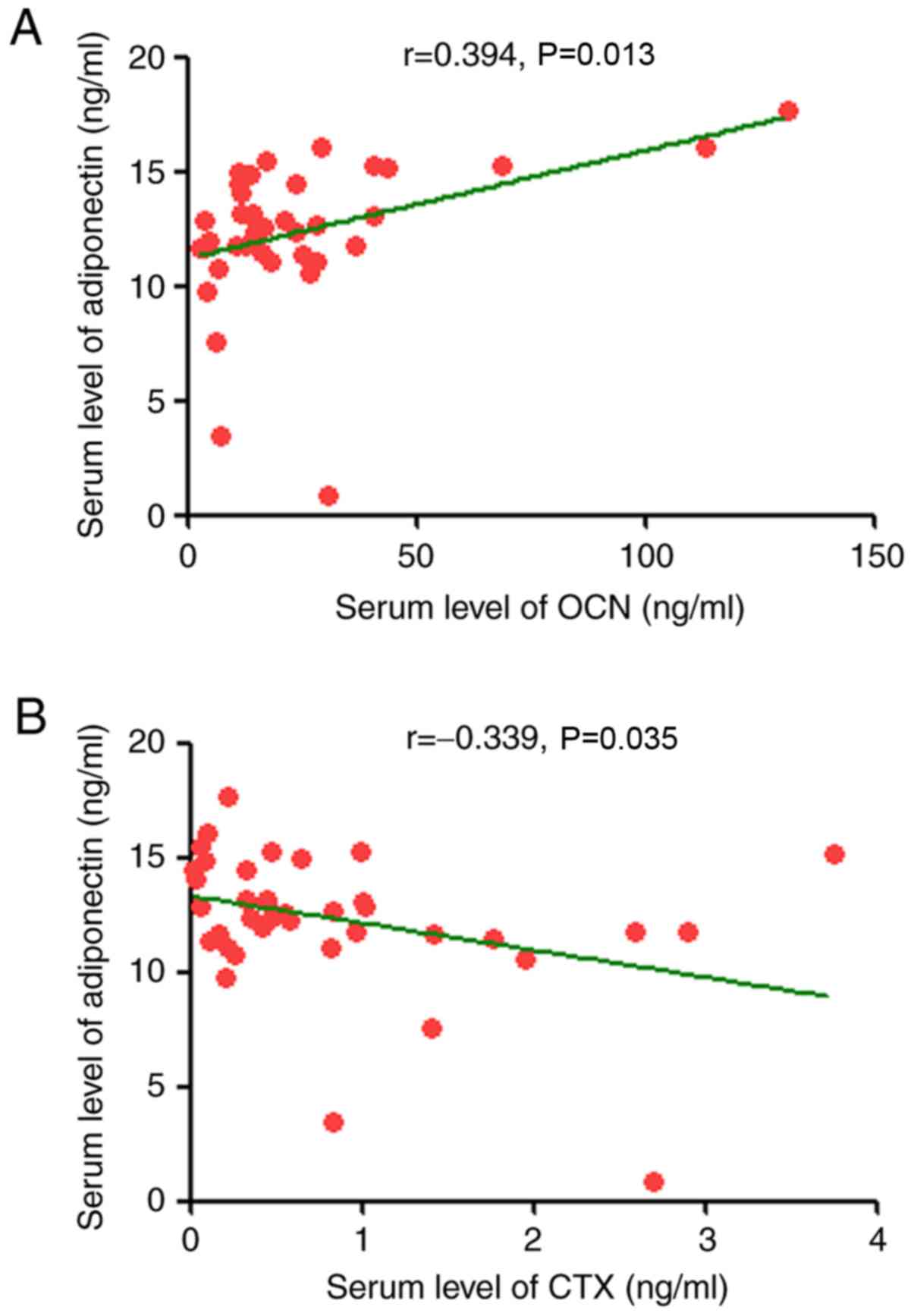
OCN is positively correlated and CTX is
negatively correlated with the level of adiponectin in patients
with MM
The present study determined the correlation between
serum levels of visfatin, leptin and adiponectin and measures of MM
load (β2-microglobulin, plasma cell percentage in bone
marrow, serum creatinine and LDH) or serum markers of bone disease
(OCN, CTX and PINP). Adiponectin levels significantly correlated
negatively with CTX (r=−0.339, P<0.05). Positive significant
correlations were identified for OCN (r=0.394, P<0.05; Table V and Fig. 2). The levels of visfatin and
leptin were not significantly correlated with MM load or serum
markers.
 | Table VCorrelations of visfatin, leptin and
adiponectin levels with indices of bone disease (OCN, CTX and
PINP). |
Table V
Correlations of visfatin, leptin and
adiponectin levels with indices of bone disease (OCN, CTX and
PINP).
| Visfatin | Leptin | Adiponectin |
|---|
| OCN | | | |
| r-value | −0.314 | 0.122 | 0.394 |
| P-value | 0.071 | 0.491 | 0.013 |
| CTX | | | |
| r-value | −0.045 | 0.011 | -0.339 |
| P-value | 0.799 | 0.949 | 0.035 |
| PINP | | | |
| r-value | 0.221 | −0.322 | −0.189 |
| P-value | 0.208 | 0.063 | 0.285 |
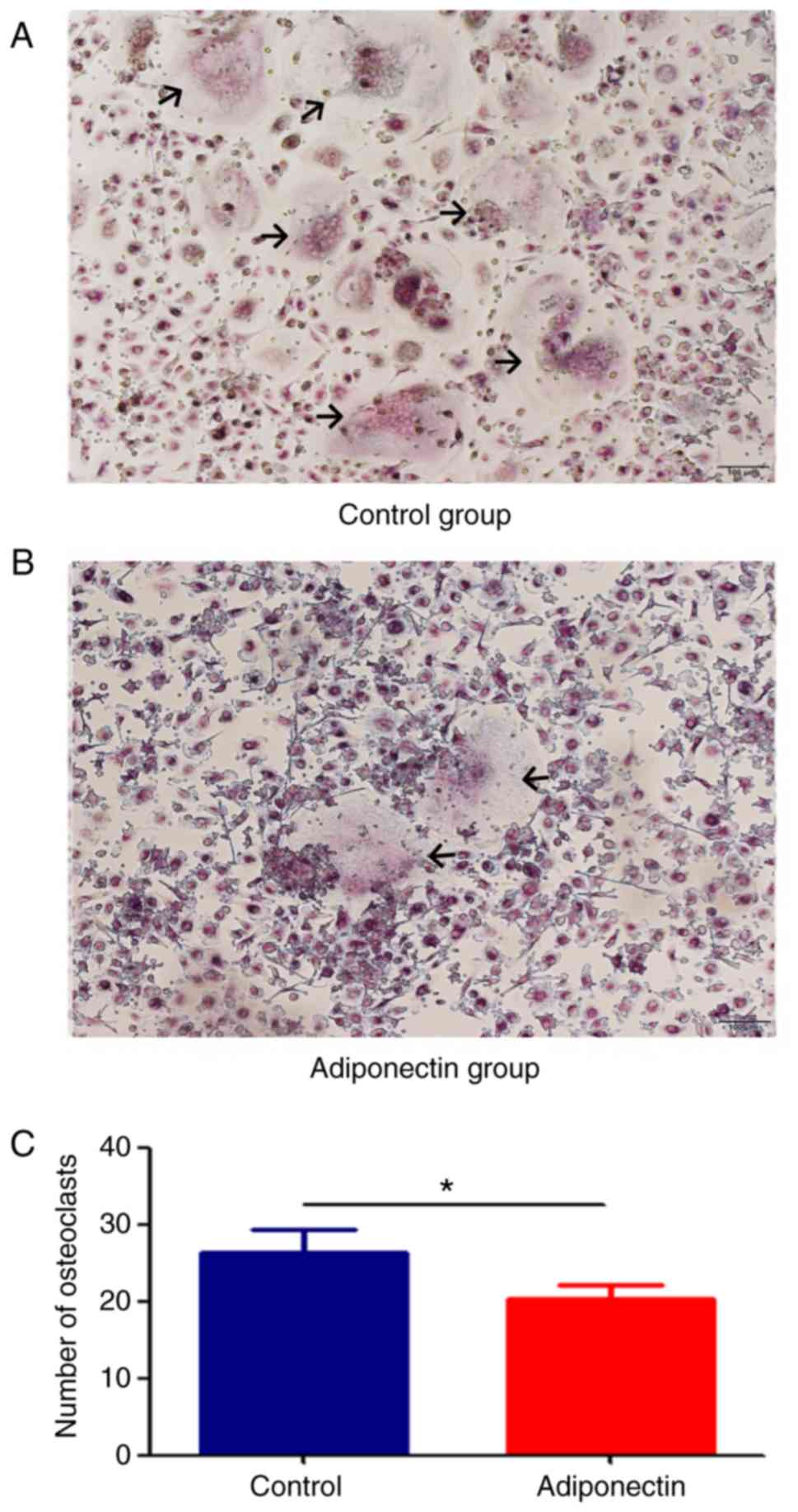
Adiponectin inhibits osteoclast
differentiation and maturation
BMMCs were cultured with RANKL and M-CSF for 14
days, following which TRAP staining was performed. Osteoclasts were
observed in each of the groups, but the number of osteoclasts in
the adiponectin group were significantly lower compared with that
in the control group (P<0.05; Fig.
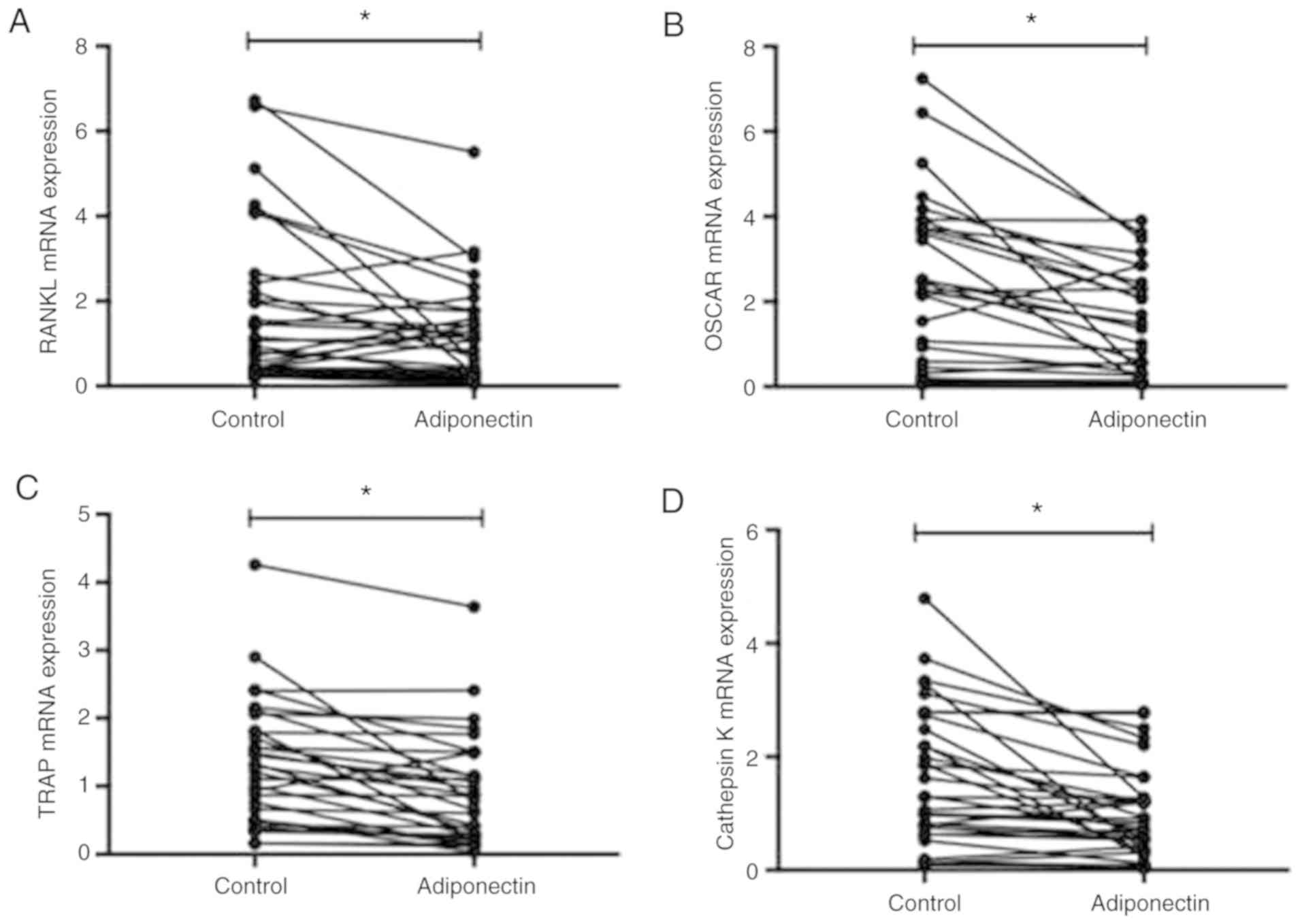
3). RT-qPCR was also performed to determine the mRNA expression
levels of the osteoclast specific factors RANKL, OSCAR, TRAP and
Cathepsin K. Expression levels for all factors were significantly
lower in the adiponectin group compared with in the control group
(P<0.05; Fig. 4).
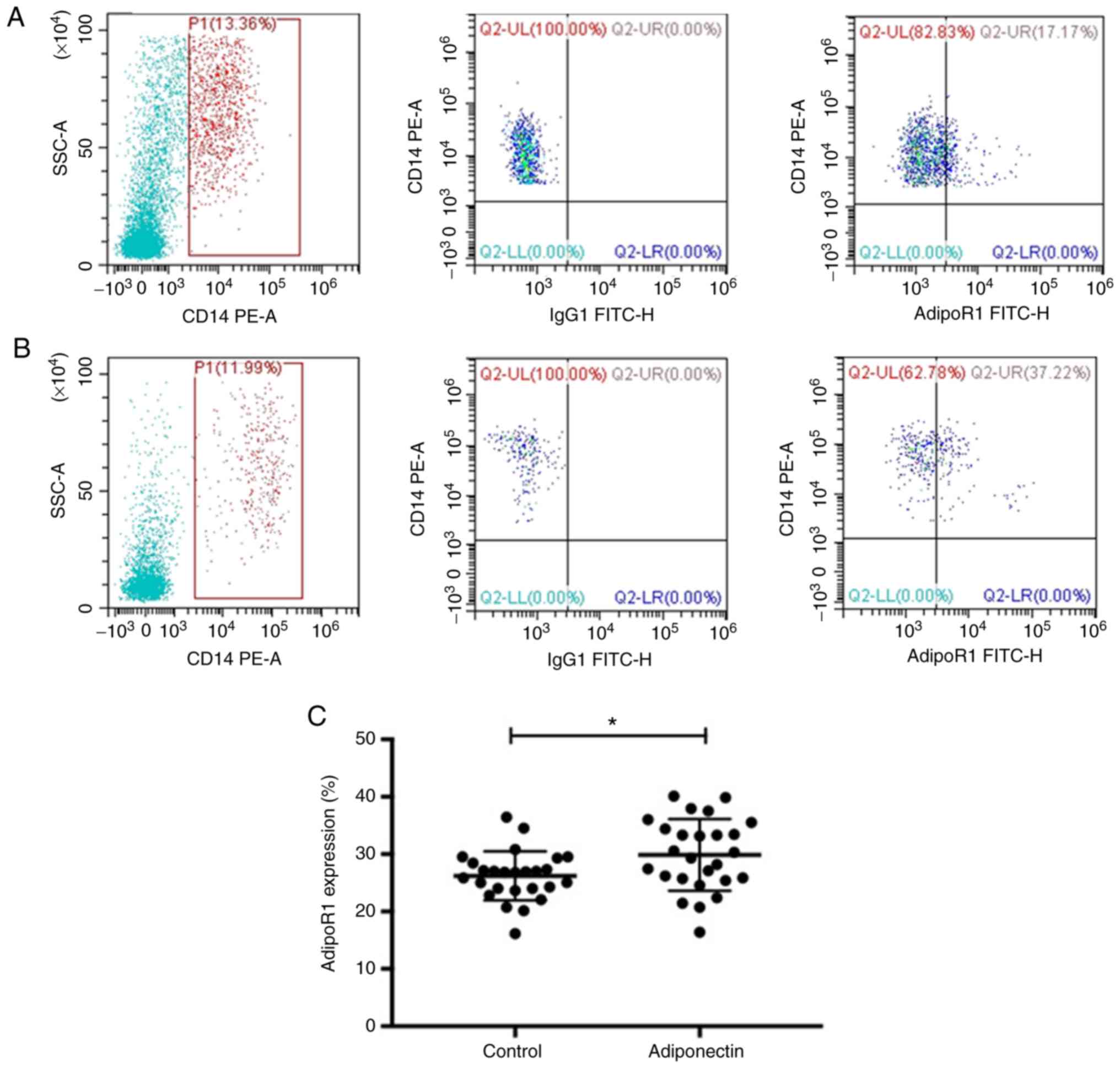
Adiponectin upregulates AdipoR1
expression on osteoclast precursors
To further investigate the mechanism of action of
adiponectin on the differentiation and maturation of MM
osteoclasts, the levels of the cell surface expression of AdipoR1
on osteoclast precursor cells (CD14+ cells) was
determined using flow cytometry. The levels of AdipoR1 in the
adiponectin group were significantly higher compared with in the
control group (P<0.05; Fig.
5).
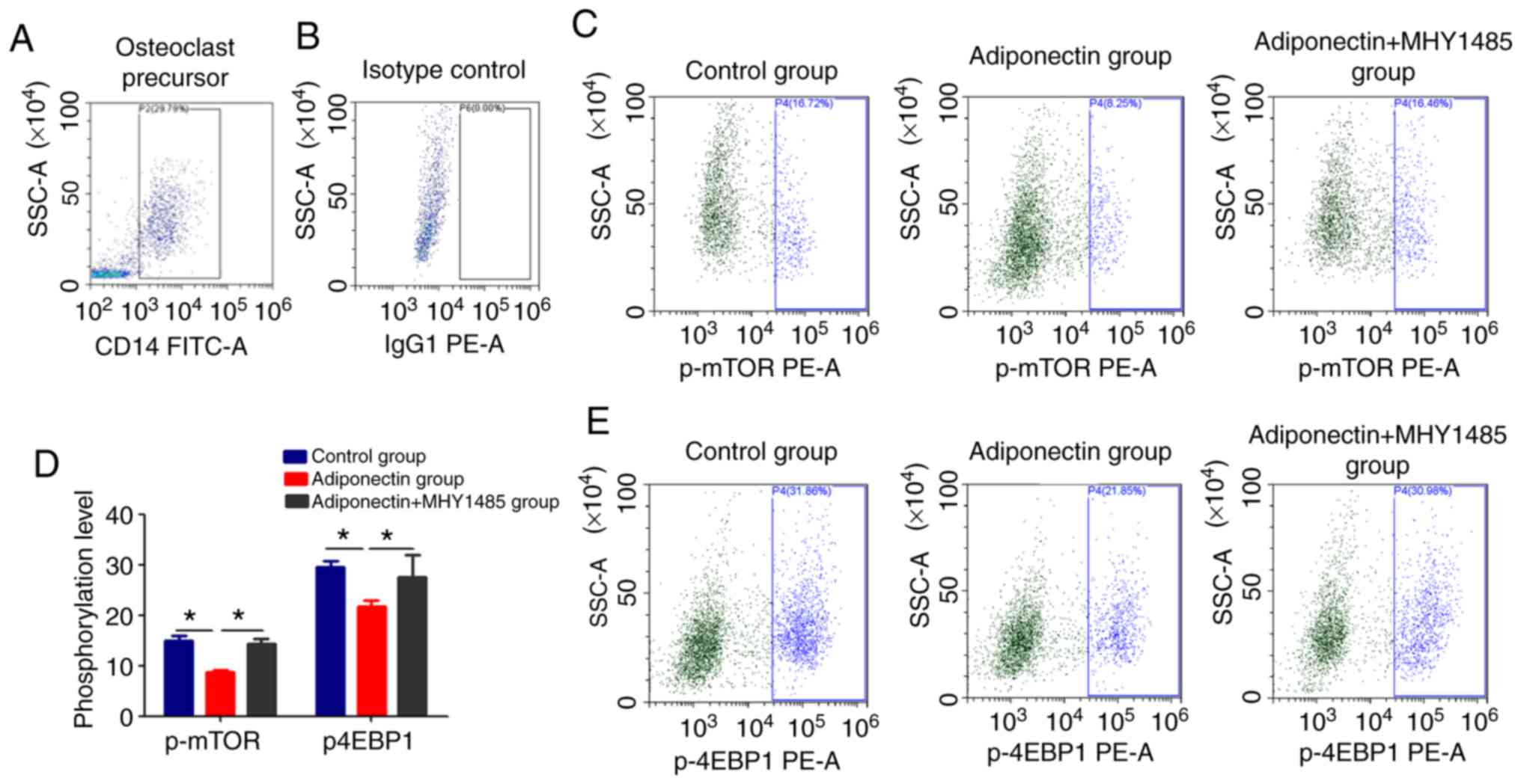
Adiponectin downregulates the
phosphorylation of mTOR and 4EBP1
The combination of RANK and RANKL have been revealed
to activate the mTOR pathway, which serves an important function in
the differentiation and maturation of osteoclasts (23). To determine the effect of
adiponectin on this pathway, the phosphorylation of mTOR and its
downstream signaling molecule 4EBP1 were measured. The
phosphorylation levels of mTOR and 4EBP1 were significantly lower
in the adiponectin group compared with in the control group
(P<0.05; Fig. 6).
Additionally, a mTOR signaling pathway-specific agonist (MHY1485)
was used to further determine whether the effects of adiponectin
may be reversed. The results revealed that the levels of p-mTOR and
p-4EBP1 in the adiponectin + MHY1485 group were significantly
higher compared with that in the adiponectin alone group
(P<0.05; Fig. 6). It revealed
that adiponectin may inhibit osteoclast differentiation and
maturation via the mTOR pathway.
Discussion
Previous studies have revealed that obesity is a
risk factor for MM (24) and MBD
due to the associated increase in the release of adipocytokines
(25). The present study studied
the association between markers of MBD and the adipocytokines
visfatin, leptin and adiponectin in patients with MM. Visfatin is
known to facilitate the proliferation of pre-B cells and digestive
system neoplasms (5,18). Another form of visfatin,
nicotinamide phosphoribosyl transferase, is indispensable for
myeloma cell growth and osteoclast activity, though its effect on
MM cells requires further investigation (25). The present study revealed that the
levels of visfatin were significantly higher in sera from patients
with MM compared with sera from normal controls (P<0.05) but
that there was no correlation between visfatin levels and the
severity of MBD.
Reseland et al (26) demonstrated that plasma
concentration of leptin was significantly higher in newly diagnosed
patients compared with a healthy control group. While the present
study demonstrated that serum leptin levels in 39 patients with MM
were not significantly different compared with those in normal
controls, and no correlation existed between leptin levels and MM
load or MBD. Further investigation is required to determine if the
levels of leptin may affect MM cells.
Adiponectin is secreted not only by adipocytes but
also by mesenchymal stem cells, osteoclasts and adipose cells in
the bone marrow (27).
Downregulation of adiponectin may increase the proliferation of MM
cells in mouse models of disease (8). Dalamaga et al (28) demonstrated that lower levels of
adiponectin predicted a higher risk of MM. In the present study,
adiponectin levels were significantly lower in newly diagnosed
patients with MM compared with in controls (P<0.05). Stage III
patients had lower levels compared with stage I/II patients. In
patients with MBD, adiponectin levels of stage B/C patients were
lower compared with those of stage A patients. These results are
consistent with previous studies (26,28). The present study observed a trend
toward lower adiponectin levels in normal controls compared with
patients with phase I/II MM or patients with stage A MM. Although
these differences did not reach significance, the present study
verified that the levels of adiponectin were negatively correlated
with β2-microglobulin, the percentage of plasma cells in
the bone marrow and serum creatinine. A previous study demonstrated
that the bone metabolic markers CTX, OC and PINP were useful for
the diagnosis and monitoring of MBD (29). CTX is a marker of bone resorption,
whereas OCN and PINP mark bone formation (30,31). The present study determined the
correlation among the levels of visfatin, leptin and adiponectin,
and those of OCN, CTX and PINP. Adiponectin levels were negatively
correlated with CTX and positively correlated with OCN. These
results suggest that adiponectin is a protective factor for MBD.
This is consistent with the observation that adiponectin may
increase osteoblast proliferation and differentiation while
inhibiting osteoclastogenesis in vitro (32). The present clinical results concur
with the results of a previous study (32). The pathway through which these
effects are mediated remains unclear, although previous studies
have revealed that the adiponectin receptor AdipoR1 is present on
osteoblasts and osteoclasts (24,27). Further investigation will be
required to elucidate the underlying mechanism.
Adiponectin has positive effects on insulin
sensitivity, inflammation, oxidative stress and tumor growth
(33,34). In the present study, osteoclasts
were successfully induced by RANKL and M-CSF subsequent to
extracting mononuclear cells from patients with MM. TRAP staining
revealed that the number of osteoclasts in the adiponectin group
was lower compared with that in the control group. In addition,
adiponectin decreased the mRNA levels of RANKL, OSCAR, TRAP and
Cathepsin K, indicating that adiponectin may inhibit osteoclast
differentiation and maturation in MM. The limitation of the present
study is that only RT-qPCR was performed to assess the expression
of osteoclast-specific factors RANKL, OSCAR, TRAP and Cathepsin K.
Therefore, it is unclear whether the protein levels of these
factors change in a similar manner, as western blot analysis was
not performed due to the objective difficulty that the samples from
the patients with MM were limited.
Adiponectin activates intracellular signaling
through its receptors, AdipoR1 and AdipoR2, and the expression
levels of these receptors correlate directly with the magnitude of
the ensuing signaling (13).
AdipoR1 is expressed at significantly higher levels in osteoclasts
compared with AdipoR2, suggesting that AdipoR1 has a higher
affinity for this receptor isoform (14). Thus, the present study did not
measure the expression of AdipoR2. To examine how adiponectin
affects osteoclast differentiation, the present study examined the
effect of adiponectin on the cell surface expression of AdipoR1 on
osteoclast precursors. The results revealed that the expression of
AdipoR1 increased following adiponectin treatment, suggesting that
adiponectin affects the differentiation and maturation of
osteoclasts by increasing the expression of AdipoR1.
The molecular pathways downstream of AdipoR1 have
not yet been fully elucidated. However, a number of studies have
revealed that the mTOR signaling pathway, which is also associated
with cell growth, is involved (35,36). mTOR serves an important function
in regulating cell proliferation, growth, differentiation,
migration and survival (35-37). Phosphorylated AMPK inhibits mTOR
signaling (29,32). 4EBP1 is a downstream molecule of
mTOR and may be activated by phosphorylated mTOR. The
phos-phoinositide-3-kinase/protein kinase B/mTOR pathway serves an
important function in regulating bone remodeling, which negatively
regulates bone mineralization (35-38) in vitro and promotes
osteoclastogenesis (39-41). Previous studies have revealed that
the inhibition of mTOR signaling may reduce bone loss in patients
with rheumatoid arthritis, multiple myeloma or neurofibromatosis
(42-44). Adiponectin also may inhibit the
growth of colorectal cancer cells by inhibiting the mTOR cell
pathway (45). In the present
study, it was revealed that adiponectin upregulates the expression
of AdipoR1 on osteoclast precursor cells and inhibits the
phosphorylation of mTOR and 4EBP1. Additionally, a mTOR signaling
pathway-specific agonist (MHY1485) was used to further determine
that the effects of adiponectin may be reversed. It revealed that
adiponectin may inhibit osteoclast differentiation and maturation
via the mTOR pathway. Therefore, adiponectin inhibits the
differentiation of osteoclasts in MM and this effect may be
mediated by the inhibition of mTOR signal transduction following
the binding of adiponectin by its receptor. However, the specific
molecular mechanism requires further investigation.
Adiponectin may serve a crucial function in MBD by
inhibiting the differentiation and maturation of osteoclasts in
patients with MM. This effect may be mediated by increasing the
expression of AdipoR1 on the surface of osteoclast precursor cells
through mTOR signaling.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81570106, 81400088,
81400085 and 81900131), the Tianjin Municipal Natural Science
Foundation (grant nos. 14JCYBJC25400, 15JCYBJC24300, 15KG150,
16ZXMJSY00180 and 18JCQNJC80400), the Tianjin Education Commission
Research Project (grant nos. 2018KJ045 and 2018KJ043) and The Youth
Incubation Fund of Tianjin Medical University General Hospital
(grant no. ZYYFY 2016006).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZL, HL and RF designed this research. ZL and YL
performed the majority of the experiments, analyzed the data, drew
the figures and drafted this manuscript. YW, RX and FM helped with
cell culture, reverse transcription-quantitative PCR and the flow
cytometry. CX performed the cell culture. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was ethically approved by the
Ethics Committee of the Tianjin Medical University (Tianjin,
China). Written informed consent was obtained from the
patients.
Patient consent for publication
Written informed consent was obtained from the
patients for the publication of this report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
CTX
|
carboxy-terminal cross-linking
telopeptide of type I collagen
|
|
ISS
|
International Staging System
|
|
MBD
|
multiple myeloma bone disease
|
|
MM
|
multiple myeloma
|
|
OCN
|
osteocalcin
|
|
PINP
|
procollagen I amino-terminal
propeptide
|
|
TRAP
|
tartrate-resistant acid
phosphatase
|
Acknowledgments
Not applicable.
References
|
1
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aparicio T, Kotelevets L, Tsocas A,
Laigneau JP, Sobhani I, Chastre E and Lehy T: Leptin stimulates the
proliferation of human colon cancer cells in vitro but does not
promote the growth of colon cancer xenografts in nude mice or
intestinal tumorigenesis in Apc(Min/+) mice. Gut. 54:1136–1145.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jardé T, Caldefie-Chézet F, Damez M,
Mishellany F, Perrone D, Penault-Llorca F, Guillot J and Vasson MP:
Adiponectin and leptin expression in primary ductal breast cancer
and in adjacent healthy epithelial and myoepithelial tissue.
Histopathology. 53:484–487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fenton JI, Birmingham JM, Hursting SD and
Hord NG: Adiponectin blocks multiple signaling cascades associated
with leptin-induced cell proliferation in Apc Min/+ colon
epithelial cells. Int J Cancer. 122:2437–2445. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohammadi M, Zarghami N, Hedayati M,
Ghaemmaghami S, Yamchi RM and Mohaddes M: Visfatin effects on
telomerase gene expression in AGS gastric cancer cell line. Indian
J Cancer. 52:32–35. 2015. View Article : Google Scholar
|
|
6
|
Hofmann JN, Liao LM, Pollak MN, Wang Y,
Pfeiffer RM, Baris D, Andreotti G, Lan Q, Landgren O, Rothman N and
Purdue MP: A prospective study of circulating adipokine levels and
risk of multiple myeloma. Blood. 120:4418–4420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Medina EA, Oberheu K, Polusani SR, Ortega
V, Velagaleti GV and Oyajobi BO: PKA/AMPK signaling in relation to
adiponectin's antiproliferative effect on multiple myeloma cells.
Leukemia. 28:2080–2089. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fowler JA, Lwin ST, Drake MT, Edwards JR,
Kyle RA, Mundy GR and Edwards CM: Host-derived adiponectin is
tumor-suppressive and a novel therapeutic target for multiple
myeloma and the associated bone disease. Blood. 118:5872–5882.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teitelbaum SL and Ross FP: Genetic
regulation of osteoclast development and function. Nat Rev Genet.
4:638–649. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Massey HM and Flanagan AM: Human
osteoclasts derive from CD14-positive monocytes. Br J Haematol.
106:167–170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim MS, Day CJ and Morrison NA: MCP-1 is
induced by receptor activator of nuclear factor-{kappa}B ligand,
promotes human osteoclast fusion, and rescues granulocyte
macrophage colony-stimulating factor suppression of osteoclast
formation. J Biol Chem. 280:16163–16169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamauchi T, Kamon J, Ito Y, Tsuchida A,
Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et
al: Cloning of adiponectin receptors that mediate antidiabetic
metabolic effects. Nature. 423:762–769. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pacheco-Pantoja EL, Waring VJ, Wilson PJ,
Fraser WD and Gallagher JA: Adiponectin receptors are present in
RANK-L-induced multinucleated osteoclast-like cells. J Recept
Signal Transduct Res. 33:291–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gan ZY, Fitter S, Vandyke K, To LB,
Zannettino AC and Martin SK: The effect of the dual PI3K and mTOR
inhibitor BEZ235 on tumour growth and osteolytic bone disease in
multiple myeloma. Eur J Haematol. 94:343–354. 2015. View Article : Google Scholar
|
|
16
|
Walker S, Wankell M, Ho V, White R, Deo N,
Devine C, Dewdney B, Bhathal P, Govaere O, Roskams T, et al:
Targeting mTOR and Src restricts hepatocellular carcinoma growth in
a novel murine liver cancer model. PLoS One. 14:e02128602019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rajkumar SV, Harousseau JL, Durie B,
Anderson KC, Dimopoulos M, Kyle R, Blade J, Richardson P, Orlowski
R, Siegel D, et al: Consensus recommendations for the uniform
reporting of clinical trials: Report of the international myeloma
workshop consensus panel 1. Blood. 117:4691–4695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Terpos E, de la Fuente J, Szydlo R,
Hatjiharissi E, Viniou N, Meletis J, Yataganas X, Goldman JM and
Rahemtulla A: Tartrate-resistant acid phosphatase isoform 5b: A
novel serum marker for monitoring bone disease in multiple myeloma.
Int J Cancer. 3:455–457. 2003. View Article : Google Scholar
|
|
19
|
Greipp PR, San Miguel J, Durie BG, Crowley
JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H,
Kyle RA, et al: International staging system for multiple myeloma.
J Clin Oncol. 23:3412–3420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Warhurst G, Dunn G, Chadwick P, Blackwood
B, McAuley D, Perkins GD, McMullan R, Gates S, Bentley A, Young D,
et al: Rapid detection of health-care-associated bloodstream
infection in critical care using multipathogen real-time polymerase
chain reaction technology: A diagnostic accuracy study and
systematic review. Health Technol Assess. 19:1–142. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sonzogni-Desautels K, Di Lenardo TZ,
Renteria AE, Gascon MA, Geary TG and Ndao M: A protocol to count
crypto-sporidium oocysts by flow cytometry without antibody
staining. PLoS Negl Trop Dis. 13:e00072592019. View Article : Google Scholar
|
|
22
|
Sorensen MG, Henriksen K, Schaller S,
Henriksen DB, Nielsen FC, Dziegiel MH and Karsdal MA:
Characterization of osteoclasts derived from CD14+ monocytes
isolated from peripheral blood. J Bone Miner Metab. 25:36–45. 2007.
View Article : Google Scholar
|
|
23
|
Tong X, Gu J, Song R, Wang D, Sun Z, Sui
C, Zhang C, Liu X, Bian J and Liu Z: Osteoprotegerin inhibit
osteoclast differentiation and bone resorption by enhancing
autophagy via AMPK/mTOR/p70S6K signaling pathway in vitro. J Cell
Biochem. 6:274682018.
|
|
24
|
Birmann BM, Giovannucci E, Rosner B,
Anderson KC and Colditz GA: Body mass index, physical activity, and
risk of multiple myeloma. Cancer Epidemiol Biomarkers Prev.
16:1474–1478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morris EV and Edwards CM: Adipokines,
adiposity, and bone marrow adipocytes: Dangerous accomplices in
multiple myeloma. J Cell Physiol. 233:9159–9166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reseland JE, Reppe S, Olstad OK,
Hjorth-Hansen H, Brenne AT, Syversen U, Waage A and Iversen PO:
Abnormal adipokine levels and leptin-induced changes in gene
expression profiles in multiple myeloma. Eur J Haematol.
83:460–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Berner HS, Lyngstadaas SP, Spahr A, Monjo
M, Thommesen L, Drevon CA, Syversen U and Reseland JE: Adiponectin
and its receptors are expressed in bone-forming cells. Bone.
35:842–849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dalamaga M, Karmaniolas K, Panagiotou A,
Hsi A, Chamberland J, Dimas C, Lekka A and Mantzoros CS: Low
circulating adiponectin and resistin, but not leptin, levels are
associated with multiple myeloma risk: A case-control study. Cancer
Causes Control. 20:193–199. 2009. View Article : Google Scholar
|
|
29
|
Peng F, Fu R, Liu H, Wang Y, Ding K, Ding
S, Liu Z, Ruan E, Qu W, Wang H, et al: Clinical significance of
serum bone metabolic markers in diagnosis and monitoring of myeloma
bone disease. Zhonghua Yi Xue Za Zhi. 95:3436–3439. 2015.In
Chinese.
|
|
30
|
Vasikaran S, Cooper C, Eastell R,
Griesmacher A, Morris HA, Trenti T and Kanis JA: International
osteoporosis foundation and international federation of clinical
chemistry and laboratory medicine position on bone marker standards
in osteoporosis. Clin Chem Lab Med. 49:1271–1274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Terpos E, Dimopoulos MA, Sezer O, Roodman
D, Abildgaard N, Vescio R, Tosi P, Garcia-Sanz R, Davies F,
Chanan-Khan A, et al: The use of biochemical markers of bone
remodeling in multiple myeloma: A report of the international
myeloma working group. Leukemia. 24:1700–1712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oshima K, Nampei A, Matsuda M, Iwaki M,
Fukuhara A, Hashimoto J, Yoshikawa H and Shimomura I: Adiponectin
increases bone mass by suppressing osteoclast and activating
osteoblast. Biochem Biophys Res Commun. 331:520–526. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kadowaki T, Yamauchi T, Kubota N, Hara K,
Ueki K and Tobe K: Adiponectin and adiponectin receptors in insulin
resistance, diabetes, and the metabolic syndrome. J Clin Invest.
116:1784–1792. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakayama S, Miyoshi Y, Ishihara H and
Noguchi S: Growth-Inhibitory effect of adiponectin via adiponectin
receptor 1 on human breast cancer cells through inhibition of
S-phase entry without inducing apoptosis. Breast Cancer Res Treat.
112:405–410. 2008. View Article : Google Scholar
|
|
35
|
Ogawa T, Tokuda M, Tomizawa K, Matsui H,
Itano T, Konishi R, Nagahata S and Hatase O: Osteoblastic
differentiation is enhanced by rapamycin in rat osteoblast-like
osteosarcoma (ROS 17/2.8) cells. Biochem Biophys Res Commun.
249:226–230. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Viñals F, López-Rovira T, Rosa JL and
Ventura F: Inhibition of PI3K/p70 S6K and p38 MAPK cascades
increases osteoblastic differentiation induced by BMP-2. FEBS Lett.
510:99–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oldham S and Hafen E: Insulin/IGF and
target of rapamycin signaling: A TOR de force in growth control.
Trends Cell Biol. 13:79–85. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee KW, Yook JY, Son MY, Kim MJ, Koo DB,
Han YM and Cho YS: Rapamycin promotes the osteoblastic
differentiation of human embryonic stem cells by blocking the mTOR
pathway and stimulating the BMP/Smad pathway. Stem Cells Dev.
19:557–568. 2010. View Article : Google Scholar
|
|
39
|
Lee SE, Woo KM, Kim SY, Kim HM, Kwack K,
Lee ZH and Kim HH: The phosphatidylinositol 3-kinase, p38, and
extracellular signal-regulated kinase pathways are involved in
osteoclast differentiation. Bone. 30:71–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sugatani T and Hruska KA: Akt1/Akt2 and
mammalian target of rapamycin/Bim play critical roles in osteoclast
differentiation and survival, respectively, whereas Akt is
dispensable for cell survival in isolated osteoclast precursors. J
Biol Chem. 280:3583–3589. 2005. View Article : Google Scholar
|
|
41
|
Glantschnig H, Fisher JE, Wesolowski G,
Rodan GA and Reszka AA: M-CSF, TNFalpha and RANK ligand promote
osteoclast survival by signaling through mTOR/S6 kinase. Cell Death
Differ. 10:1165–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kloos B, Chakraborty S, Lindner SG, Noack
K, Harre U, Schett G, Krämer OH and Kubatzky KF: Pasteurella
multocida toxin-induced osteoclastogenesis requires mTOR
activation. Cell Commun Signal. 13:402015. View Article : Google Scholar
|
|
43
|
Bertoldo F, Silvestris F, Ibrahim T,
Cognetti F, Generali D, Ripamonti CI, Amadori D, Colleoni MA, Conte
P, Del Mastro L, et al: Targeting bone metastatic cancer: Role of
the mTOR pathway. Biochim Biophys Acta. 1845:248–254.
2014.PubMed/NCBI
|
|
44
|
Hadji P, Coleman R and Gnant M: Bone
effects of mammalian target of rapamycin (mTOR) inhibition with
everolimus. Crit Rev Oncol Hematol. 87:101–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sugiyama M, Takahashi H, Hosono K, Endo H,
Kato S, Yoneda K, Nozaki Y, Fujita K, Yoneda M, Wada K, et al:
Adiponectin inhibits colorectal cancer cell growth through the
AMPK/mTOR pathway. Int J Oncol. 34:339–344. 2009.PubMed/NCBI
|