Introduction
Cistanche Hoffmg. Et Link is a perennial
parasite herb of a genus of the Orobanchaceae family. Herba
Cistanche, the stem of Cistanche deserticola Y. C. MA
and Cistanche tubulosa (Schenk) R. Weight, have been used as
a tonic agent to treat kidney deficiency, impotence, morbid
leukorrhea and senile constipation (1), which may be due to its androgen-like
or sex hormone regulatory effect (2). Echinacoside (ECH;
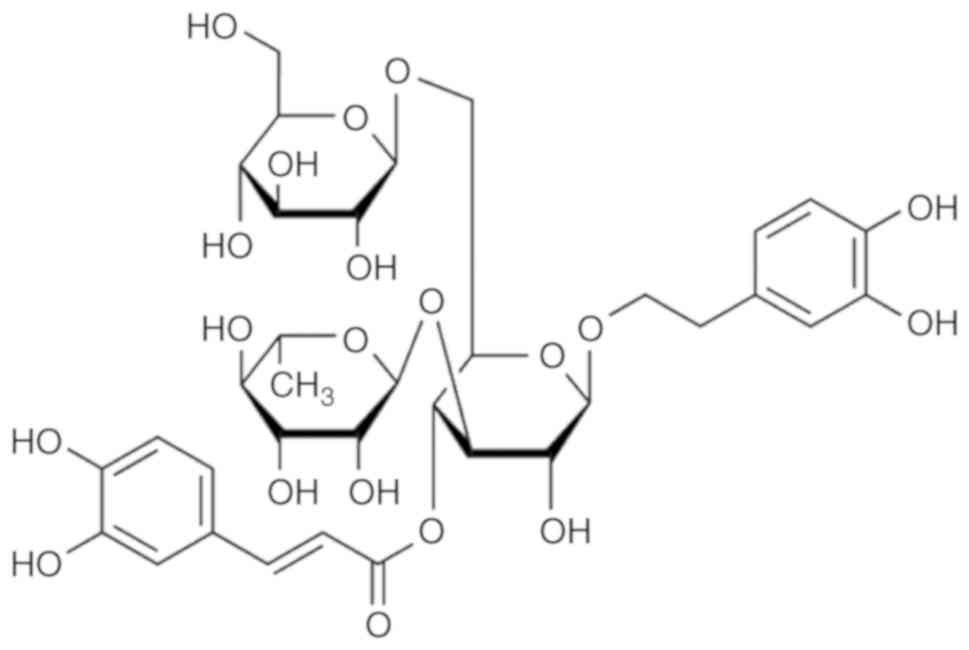
C35H46O20; molecular weight,
786.73; Fig. 1), is one of the
phenylethanoid glycosides (PhGs) isolated from the stems of Herba
Cistanche, and exhibits multiple biological properties,
including antioxidant, antiaging, anti-inflammatory,
hepatoprotective and neuroprotective effects (3). Modern pharmacology investigations
have demonstrated that various constituents of Herba
Cistanche, including ECH, acteoside, kankanose, kankanoside
F and cistanoside F, exhibit vasorelaxant activity (4).
The endothelium is a critical regulator of the
vasculature, and nitric oxide (NO) is an important relaxing factor
released by endothelial cells. By diffusing into smooth muscle
cells, NO activates guanylate cyclase, increases the levels of
cyclic guanosine monophosphate (cGMP), and then activates
cGMP-dependent protein kinases (PKGs) to promote smooth muscle
relaxation (5). It was previously
demonstrated that ECH at 350-400 µM directly acts on
vascular smooth muscle and inhibits hypoxia-induced proliferation
in rat pulmonary artery smooth muscle cells (6), whereas 30-300 µM ECH caused
acute vasorelaxation in endothelium-intact rings in a
concentration-dependent manner, and enhanced cGMP production in the
corpus cavernosum smooth muscle of aortic rings contracted by
phenylephrine (7). By opening the
NO-cGMP-PKG-BKCa channels in smooth muscle cells, 100 or
300 µM ECH suppressed noradrenaline-induced contraction in
the pulmonary arteries of rats, particularly in endothelium-denuded
rings (8). Endothelial cells are
key regulators of cardiovascular function and, in several cases, it
has been found that the endothelial-dependent relaxation was due to
a transferrable substance, such as NO, released from the
endothelium (9). However, the
upstream molecular mechanism of ECH-induced NO production in
vascular endothelial cells requires further investigation.
In the vascular endothelium, NO generation is
primarily mediated by endothelial NO synthase (eNOS). Several
groups have demonstrated that the phosphatidylinositol 3-kinase
(PI3K) pathway activates the serine threonine protein kinase B
(Akt), which causes direct eNOS phosphorylation at serine 1177
(Ser1177) (10). The androgen
receptor (AR) is a member of the nuclear receptor subfamily s3,
which canonically modifies gene expression. AR localization to the
caveolae in the cell membrane is involved in the non-genomic
regulation of endothelial cell function or gene expression by
triggering the c-Src/PI3K/Akt cascade, which ultimately results in
eNOS phosphorylation and NO production (11). In human aortic endothelial cells,
testosterone has been reported to activate PI3K/Akt signaling and
rapidly induce NO production due to the direct interaction of AR
and the p85α subunit of PI3K on the cardiovascular system (12). It was reported that ECH aggravates
hormone deficiency-related symptoms and exerts its androgen-like
effects due to competitive binding to the AR instead of
testosterone (2). Therefore, the
present study hypothesized that the PI3K/Akt pathway may be
involved in NO production through AR-dependent eNOS phosphorylation
induced by ECH. The aim of the present study was to evaluate the
following effects of ECH: i) Induction of NO production and eNOS
phosphorylation; ii) involvement of AR in eNOS phosphorylation; and
iii) activation of the PI3K/Akt pathway in human umbilical vein
endothelial cells (HUVECs), a well-known experimental model for
studying the regulation of endothelial cell functions and
angiogenesis (13).
Materials and methods
Chemicals and reagents
ECH (purity, 92.5%) was obtained from the National
Drug Reference Standards in National Institute for the Food and
Drug Control. Antibodies against p-Akt (Ser473; cat. no. ab8805),
Akt (cat. no. ab81283), p-eNOS (Ser1177; cat. no. ab184154) and
eNOS (cat. no. ab76198) were purchased from Abcam. The inhibitors
of nilutamide and ICI 182780 were obtained from Sigma-Aldrich;
Merck KGaA. L-NAME was purchased from Adamas-Beta, Ltd., and
wortmannin was purchased from Pribolab.
Cell culture and drug treatment
HUVECs were obtained from ScienCell Research
Laboratories Inc. and cultured in endothelial cell medium (ECM;
ScienCell Research Laboratories) with 5% (v/v) fetal bovine serum
(FBS; Gibco, Thermo Fisher Scientific, Inc.) and 1% endothelial
cell growth supplement (ECGS) at 37°C (5% CO2 and 95%
humidity) (14). Upon reaching
confluence, the cells were digested with trypsin and plated in ECM
with 1% FBS and 1% ECGS. For all experiments, HUVECs were plated at
a concentration of 1×104/ml and grown until reaching
confluence. Prior to treatment with ECH or other stimulators, the
cells were incubated in phenol red-free ECM without FBS and ECGS
for 6 h to induce growth arrest. In the inhibitory experiments,
HUVECs were pre-incubated with various antagonists or inhibitors,
including 10 µM nilutamide, 10 µM ICI 182780, 0.5 mM
L-NAME or 5 µM wortmannin, for 30 min, with or without ECH,
at 37°C. In all groups, including the control, DMSO was used as a
solvent at equal concentrations of 0.001%.
Measurement of intracellular NO
production
Relative changes in cytosolic NO concentration in
HUVECs were monitored using the fluorescent NO probe DAF-FM (Cayman
Chemical Company), as previously reported (12). Briefly, the cells were loaded with
5 µM DAF-FM diacetate for 20 min at 37°C in a black
microtiter plate and rinsed several times with PBS (pH 7.4). The
fluorescence was determined at excitation and emission wavelengths
of 495 and 515 nm, respectively, using a fluorescent microplate
reader (Biotek Synergy H4; BioTek Instruments, Inc.) and a compact
inverted microscope (Nikon eclipse Ts2R; Nikon Corporation).
Western blot analysis
As previously reported (15), confluent monolayers of cells were
washed twice in ice-cold PBS and lysed with RIPA buffer (P0013D;
Beyotime Institute of Biotechnology). The protein concentration in
the supernatant was measured using the bicinchoninic acid assay
method (16). Subsequently, 30
µg protein were loaded per lane, separated using 10%
polyacrylamide gels and transferred onto polyvi-nylidene difluoride
membranes. The membranes were blocked with 5% skimmed milk for 1 h
at 25°C. After incubation with monoclonal antibodies against Akt
(1:1,000 dilution), p-Akt (1:500 dilution), eNOS (1:2,000
dilution), or p-eNOS (1:1,000 dilution) at 4°C overnight, the
membranes were washed with TBST (containing 0.1% Tween-20) 4 times
for ~15 min per wash at 25°C. Subsequently, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:5,000 dilution; anti-mouse antibody, cat. no. A0216,
or anti-rabbit antibody, cat. no. A0208, Beyotime Institute of
biotechnology) for 1 h at 25°C, and detected using an enhanced
chemiluminescence kit (cat. no. 32209, Thermo Fisher Scientific,
Inc.). Band intensities were quantified using Image J gel analysis
software, version 1.8.0_112 (National Institutes of Health).
Small interfering (si) RNA preparation
and transfection
The long double-stranded RNAs were synthesized by
the target mRNA of the AR and estrogen receptor (ER) with sequences
shown in Table I. The conditions
for siRNA knockdown involved transfecting HUVECs at 70% confluence
maintained in non-antimicrobial culture medium in 60 mm
collagen-coated culture dishes. Transfection of 5 nM AR-siRNA or 10
nM ERα-siRNA with Lipofectamine 3000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was performed separately, according to the
manufacturer's protocols. Transfection efficiency was evaluated by
reverse transcription quantitative-PCR analysis (RT-qPCR), as
previously reported (17). The
thermo-cycling conditions were as follows: 10 min at 95°C; 40
cycles of 95°C for 5 sec and 60°C for 1 min, and a melting curve at
95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec; three
independent biological replicates were performed for each sample.
The subsequent experiments were conducted 48 h after transfection.
Primer pairs were designed using Primer Premier v5.0 software
(PREMIER Biosoft) with the following sequences: AR forward, 5′-GGT
TAC ACC AAA GGG CTA GAA-3′ and reverse, 5′-GAC TTG TAG AGA GAC AGG
GTA GA-3′; ERα forward, 5′-CCA GTA CCA ATG ACA AGG GAA G-3′ and
reverse, 5′-TCA CAG GAC CAG ACT CCA TAA-3′; and GAPDH forward,
5′-CAG GGC TGC TTT TAA CTC TGG TAA-3′ and reverse, 5′-GGG TGG AAT
CAT ATT GGA ACA TGT-3′.
 | Table IThe sequences of the sense RNA strand
targeting AR and ER in siRNA experiments. |
Table I
The sequences of the sense RNA strand
targeting AR and ER in siRNA experiments.
| Target gene | Name | Sequences
(5′-3′) |
|---|
| AR | siRNA-1 |
AAACAGGUACUUCUGUUUCCC |
| siRNA-2 |
AAUGCAAAGGUUCUCUGCUAG |
| siRNA-3 |
AAGGUCUUCUUCAAAAGAGCC |
| ERα | siRNA-1 |
GAUCAAACGCUCUAAGAAG |
| siRNA-2 |
GAAUGUGCCUGGCUAGAGA |
| siRNA-3 |
GAUGAAAGGUGGGAUACGA |
| ERβ | siRNA-1 |
GGAAAUGCGUAGAAGGAAU |
| siRNA-2 |
UUCAAGGUUUCGAGAGUUA |
| siRNA-3 |
GCACGGCUCCAUAUACAUA |
| Control | siRNA-random |
AAGUGCGAUCUAACUGACCUA |
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical comparisons between groups were performed
using the Kruskal-Wallis test or two-way ANOVA with Tukey's post
hoc test for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
ECH induces NO production and eNOS
phosphorylation
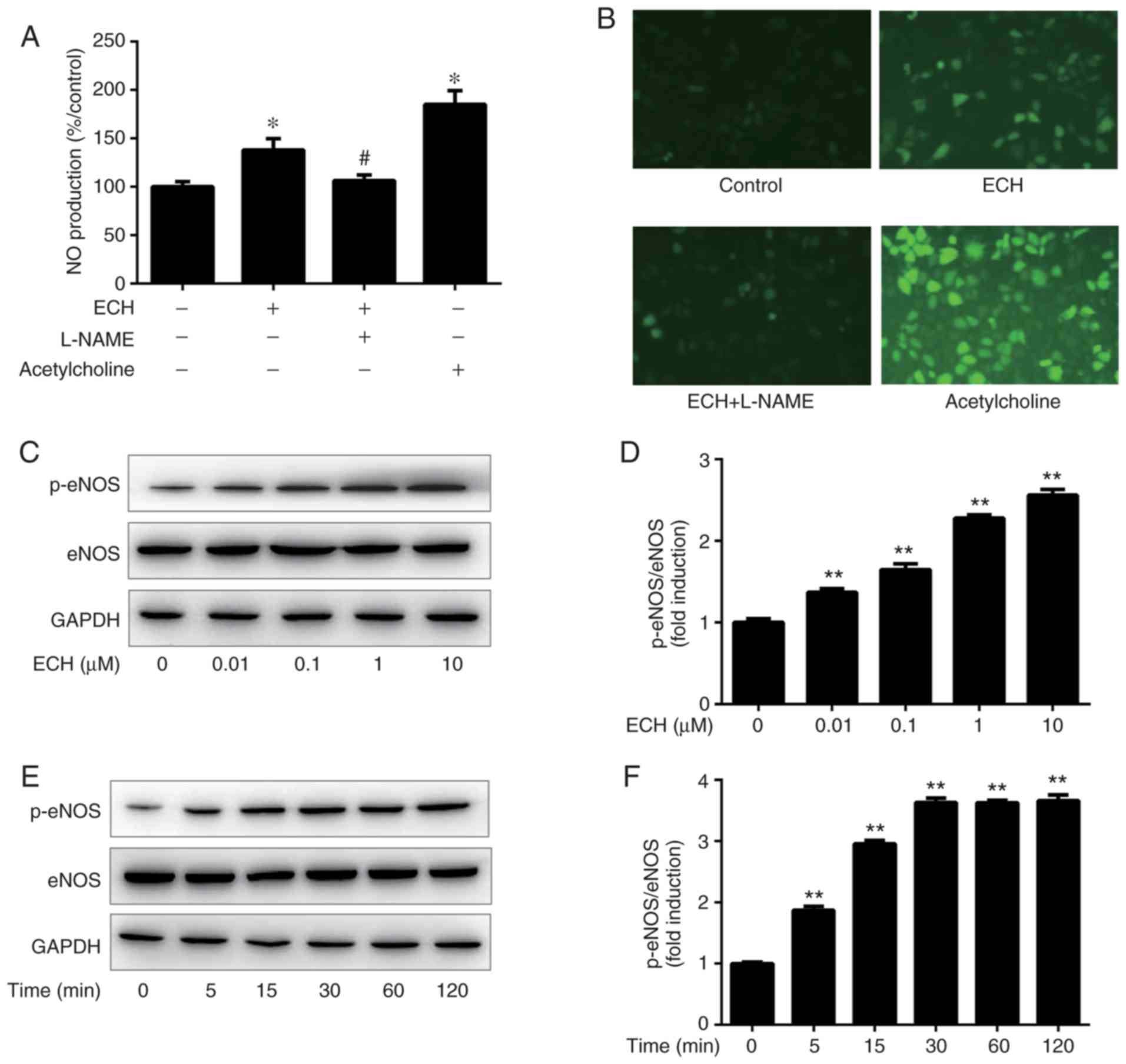
As shown in Fig. 2A
and B, 1 µM ECH significantly increased intracellular NO
production in HUVECs compared with the negative control cells,
while the stimulatory effect of ECH was attenuated to the control
level by pretreatment with L-NAME. To obtain the optimal
concentration, eNOS phosphorylation was tested at 60 min after
treatment with ECH at concentrations of 0, 0.01, 0.1, 1 and 10
µM. The results revealed that ECH at concentrations of
0.01-10 µM may significantly induced eNOS phosphorylation at
Ser 1177 in a concentration-dependent manner, with the maximal eNOS
phosphorylation observed at 10 µM ECH induction (Fig. 2C and D). Furthermore, eNOS
phosphorylation at Ser1177 was examined by western blot analysis at
0, 5, 15, 30, 60 and 120 min after incubation with 1 µM ECH.
It was observed that eNOS phosphorylation was rapidly triggered by
ECH at 5 min, and continued to increase until 30 min of incubation
(Fig. 2E and F). Subsequently,
despite ECH treatment, the relative magnitude of eNOS
phosphorylation remained stable from 30 min onwards; therefore, ECH
did not affect total eNOS expression (Fig. 2C and E).
AR mediates ECH-induced NO production and
eNOS phosphorylation
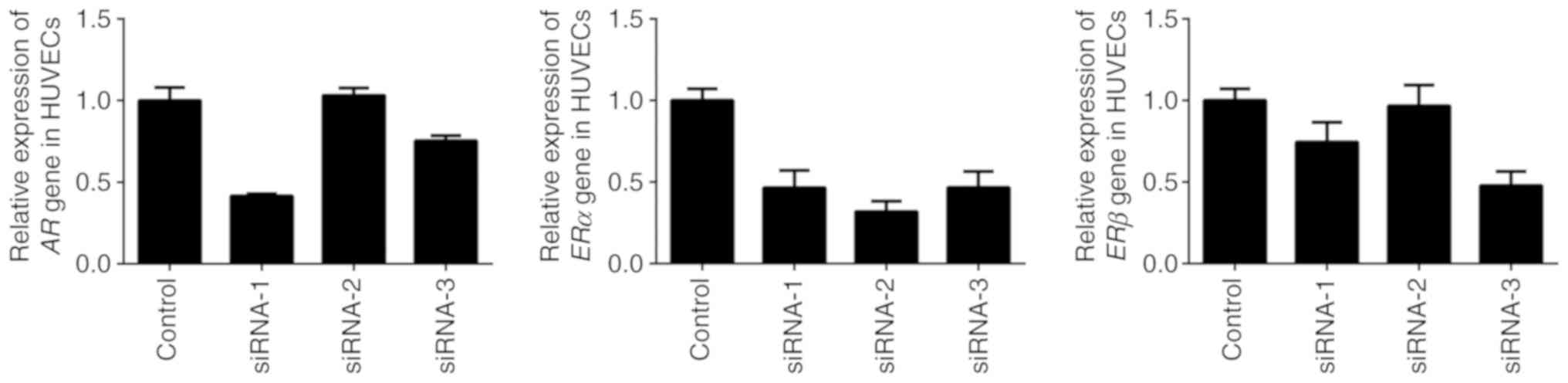
RT-qPCR assay demonstrated that the interfering
effects of AR-siRNA-1 and ERα-siRNA-2 were the most successful in
inhibiting the expression of AR and ER in HUVECs in the present
study (Fig. 3). Subsequently, an
antagonist for AR inhibition-of-function analysis and siRNA for AR
loss-of-function analysis were applied to evaluate the involvement
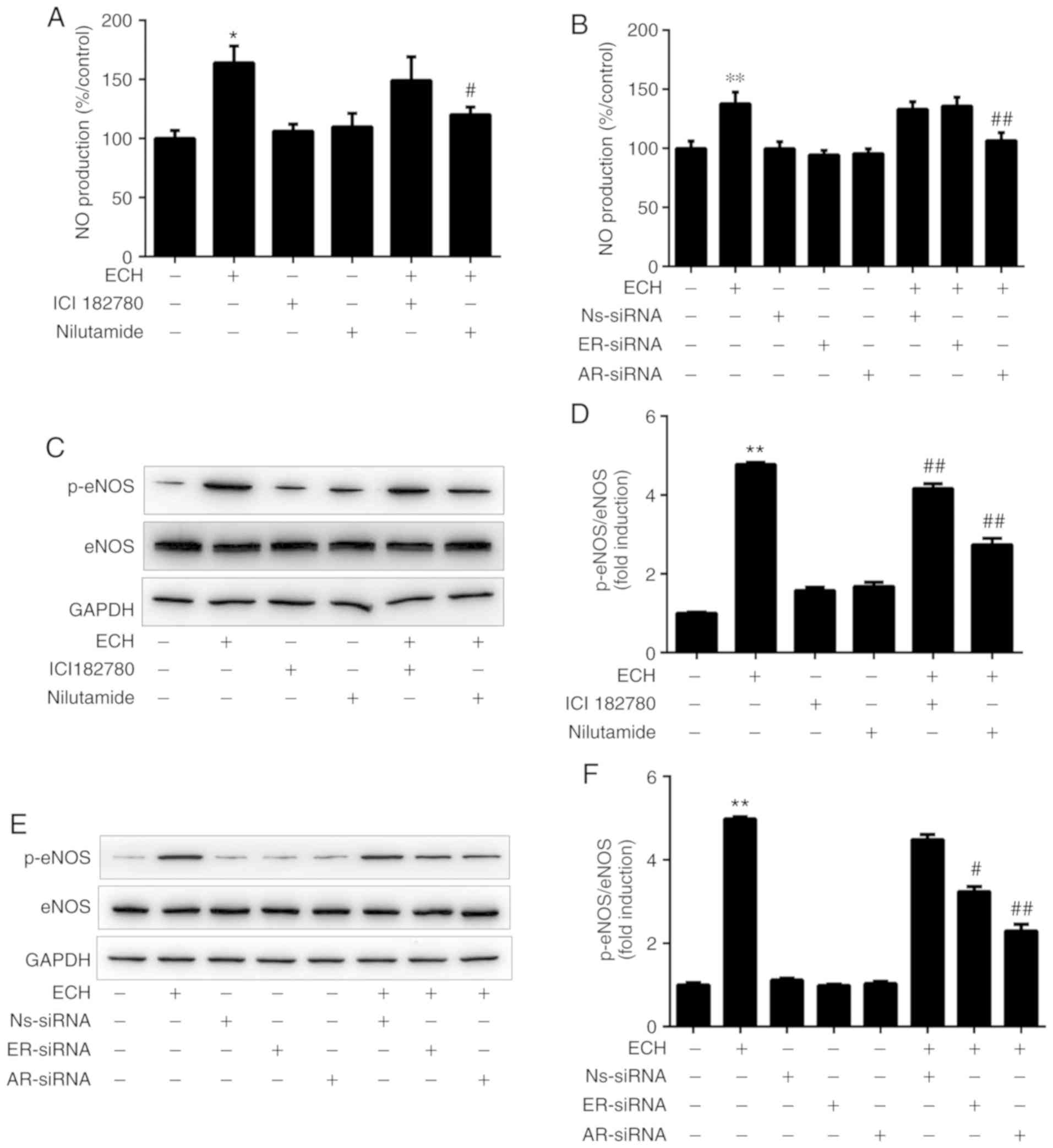
of AR in ECH-induced eNOS activation and NO production. As shown in
Fig. 4A, 1 µM ECH significantly increased
NO production in HUVECs (P<0.05). Pretreatment with the AR
antagonist nilutamide (10 µM) abolished ECH-induced NO
production, whereas ICI182789 (an ER antagonist) did not exert the
same effects. Furthermore, the effects of siRNA-mediated AR
knockdown on NO production were examined in cultured cells,
indicating that NO production was diminished by transfection with
AR siRNAs; however, it was not affected by ER siRNAs or control
random siRNAs (Fig. 4B).
Representative western blots and semi-quantitative analysis
(Fig. 4C and D) revealed that the
phosphorylation of eNOS induced by ECH was inhibited by nilutamide
and ICI182789, and that the inhibitory effect of nilutamide on
ECH-induced eNOS activation was significantly higher compared with
that of ICI182789, which indicated that inhibition of AR function
had a greater impact than ER on eNOS phosphorylation induced by
ECH. Similarly, the phosphorylation of eNOS induced by ECH was
reduced significantly in cells transfected with AR-siRNA and
ER-siRNA compared with the control random siRNA; the inhibitory
effect of AR-siRNA on ECH-induced eNOS activation was significantly
stronger compared with that of ER-siRNA (Fig. 4E and F). Therefore, the
aforementioned results suggested that ECH may cause AR-dependent
activation of eNOS to induce NO production in HUVECs.
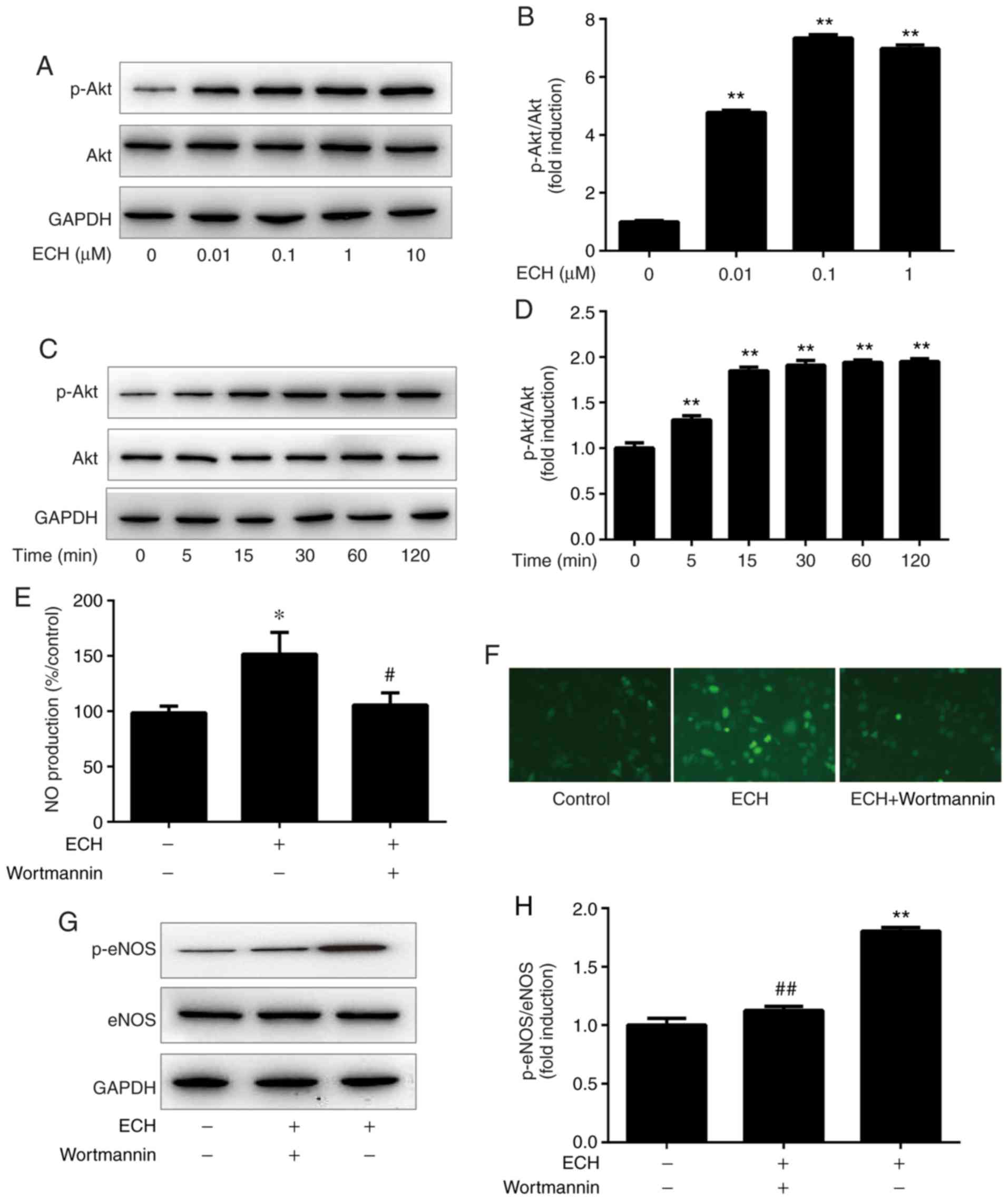
ECH activates the PI3K/Akt pathway
In the present study, Akt phosphorylation at Ser473
was tested 60 min after ECH incubation at concentrations of 0,
0.01, 0.1, 1 and 10 µM. The results indicated that ECH
(0.01-10 µM) may significantly induce Akt phosphorylation.
The relative expression levels of p-Akt peaked at 1 and 10
µM of ECH treatment (Fig. 5A
and B). Furthermore, Akt phosphorylation was examined by
western blot analysis at 0, 5, 15, 30, 60 and 120 min after the
addition of 1 µM ECH to HUVEC cultures. As shown in Fig. 5C and D, ECH rapidly increased Akt
phosphorylation after 5 min of incubation, and the maximum protein
level of p-Akt was observed at 60 min. By contrast, ECH did not
affect the total Akt expression (Fig.
5A and C). Moreover, to investigate the potential effect of
PI3K pathway on eNOS phosphorylation, HUVECs were pre-treated with
the PI3K inhibitor wortmannin prior to ECH application. Wortmannin
was found to reduce ECH-induced NO production to baseline levels
(Fig. 5E). A similar phenomenon
was observed using fluorescence microscopy when examining
ECH-induced DAF-FM fluorescence (Fig.
5F). Furthermore, wortmannin may have the ability to abolish
rapid eNOS phosphorylation in HUVECs (Fig. 5G and H). The aforementioned
results suggest that ECH may activate eNOS phosphorylation and NO
production via the PI3K/Akt pathway.
Discussion
ECH is a natural compound isolated from Herba
Cistanche, with various pharmacological properties. It was
previously demonstrated that ECH exerted an endothelium-dependent
vascular relaxation effect by opening NO-cGMP-PKG-BKCa
channels in blood vessel smooth muscle cells (7,8).
The current findings provide evidence that ECH exerted AR-dependent
activation of eNOS to induce NO production with the involvement of
the PI3K/Akt signaling pathway in vascular endothelial cells.
As the innermost layer of the vessel wall, the
endothelium can quickly sense and respond to changes in blood flow,
in turn resulting in signal transmission to the underlying smooth
muscle cells in order to regulate vascular tone (18). It has been widely reported that
multiple endothelial-derived vasodilatory compounds exist, with the
most prototypical substance being NO, formed from the endothelial
isoform of eNOS, which results in phosphorylation (19). Under normal conditions, eNOS
remains inactive when bound to caveolin, and is activated with the
following succession of events in endothelial cells: i) eNOS
dissociates from caveolin-1 and associates with
Ca2+/CaM; ii) heat shock protein (HSP)90 promotes eNOS
dimerization and favors a steric formation to recruit Akt; and iii)
calcineurin dephosphorylates Thr495 to reinforce the activation
state (20). In the present
study, NO production was significantly increased in HUVECs treated
with 1 µM ECH. This phenomenon was similar to previous
results, where ECH increased NO release and stimulated the
synthesis of cGMP in rat thoracic aortic rings (7). Furthermore, two phosphorylation
sites, Ser1177 and Thr495, appear to be particularly important for
regulating eNOS activity (20),
and the present study revealed that ECH induced acute eNOS
phosphorylation at Ser1177 in a concentration-dependent manner. In
addition, the stimuli associated with the effects on eNOS protein
levels mainly determine eNOS mRNA stability, and if the stimulus is
maintained for a longer period of time, eNOS mRNA transcription
occurs via mitogen-activated protein kinase and nuclear factor κB
(20). Taken together, these
observations indicate that the activation of eNOS at Ser1177 is
responsible for NO synthesis induced by ECH.
Furthermore, an increasing number of studies have
demonstrated that AR is expressed in endothelial cells in a number
of human tissues, which suggests a potential role for androgens and
their analogues, that act through AR-mediated processes, in the
modulation of human endothelial cell homeostasis (21). In the non-classical PI3K/Akt
pathway, AR may activate PI3K by directly interacting with PI3K
regulatory subunit p85α (22).
The present study demonstrated that an AR antagonist or AR siRNA
diminished the NO production and eNOS phosphorylation induced by
ECH in HUVECs. It has been previously reported that estrogens
induce eNOS phosphorylation and stimulate NO production via classic
ER activation in endothelial cells (23), and the administration of ECH
significantly enhances the expression of ER in the uterus (24). However, in the present study, the
effect of AR was more prominent compared with that of ERα on
ECH-induced eNOS activation and NO production. In addition, it was
observed that ECH caused acute NO production within minutes via
AR-involved eNOS activation in HUVECs, which is consistent with the
non-genomic nature of the response in endothelial cells. The AR is
associated with scaffolding proteins, including HSP90, HSP70 and
kinase Src, in the cytoplasm, and it can be transported to the
membrane from the AR complex within 5 min of testosterone treatment
(23). Based on the 'target
fishing' strategy, HSP90 was identified as the PhGs-coupled target,
which indicated that ECH may facilitate the dissociation of AR from
the scaffolding proteins (25).
Another study suggested that, in the hypothalamus, ECH may combine
with the AR pocket at amino acids Met-894 and Val-713 and inhibit
the transport of cytoplasmic AR to the nucleus (26). However, the underlying mechanism
through which ECH mediates cytoplasmic AR translocation to the
membrane requires further investigation. Therefore, further
research is required to elucidate the mechanism through which ECH
achieves AR-dependent eNOS activation and how it may be associated
with binding to HSP90 in vascular endothelial cells.
The PI3K/Akt pathway is one of the most important
signaling cascades, the activation of which is induced by producing
phosphatidylinositol-3,4,5-trisphosphate to bind the N-terminal
pleckstrin homology domain of the Ser/Thr kinase Akt. This
facilitates Akt recruitment to the plasma membrane (27). The PI3K/Akt pathway may play an
important role in the control of NO-dependent relaxation induced by
ECH. The present study revealed that ECH-induced NO production was
significantly reduced when the cells were incubated with the PI3K
inhibitor wortmannin. A previous report demonstrated that 15 mg/kg
ECH activated the PI3K/Akt signaling pathway in
5-fluorouracil-suppressed bone marrow cells (28). In the present study, PI3K
inhibitors significantly decreased ECH-induced eNOS phosphorylation
at Ser1177. Moreover, Akt activity was predominantly regulated by
upstream regulatory pathways, particularly PI3K-dependent
phosphorylation at Ser473 (20).
In the present study, ECH induced Akt phosphorylation at Ser473 in
a dose-dependent manner; similarly, a previous study demonstrated
that 5, 10 or 20 µM ECH exerted a cardioprotective effect
against anoxia/reperfusion treatment in a dose-dependent manner by
potentially upregulating p-Akt and SLC8A3 (29). The transcriptional regulation of
the Akt gene remains largely unknown (30); therefore, the present study
focused on the post-transcriptional regulatory effects of ECH on
Akt. Taking the aforementioned findings into consideration, it was
inferred that the application of ECH to HUVECs may lead to the
activation of the PI3K/Akt pathway, which phosphorylates eNOS and,
subsequently, increases NO production.
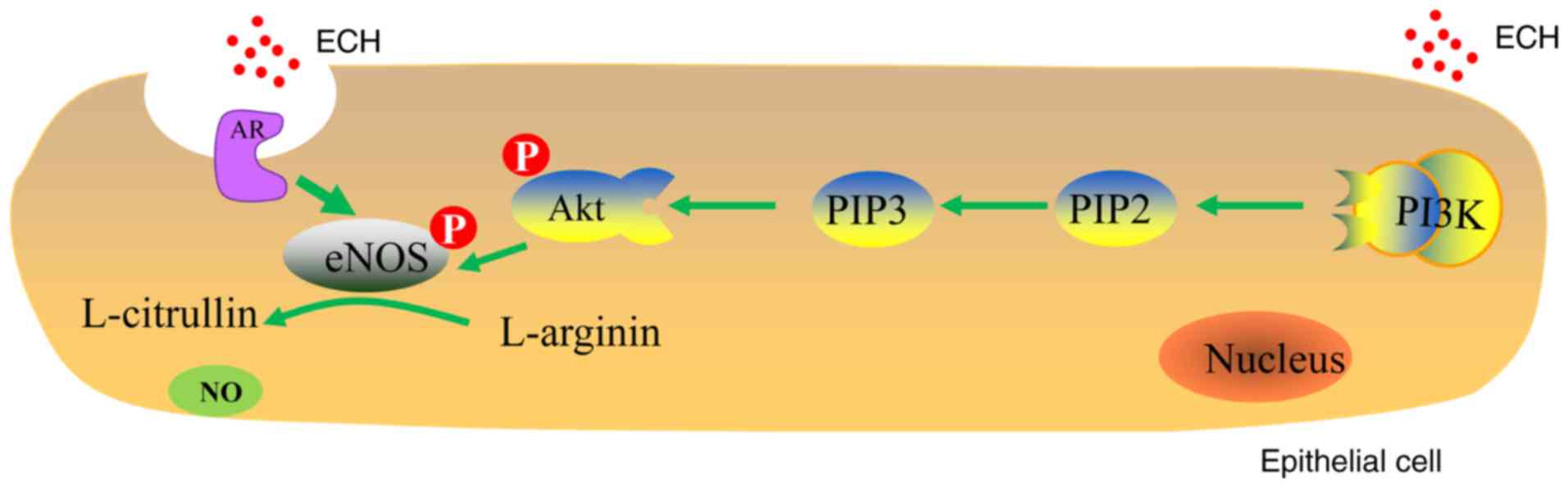
In conclusion, ECH is a natural product that is
mainly isolated from Herba Cistanche. The potential
mechanism underlying ECH-induced NO production in endothelial cells
may include the following (Fig.
6): i) ECH acts as a functional ligand of AR that is localized
to the caveolae in the cell membrane; ii) PI3K binds to the
hydrophobic domain of Akt at Ser473 and facilitates Akt recruitment
to the cell membrane; iii) the recruitment of the PI3K/Akt cascades
triggers AR-dependent eNOS phosphorylation; and iv) the generation
of NO is mediated by eNOS in endothelial cells. The observation
that ECH induces NO production through the AR-dependent
phosphorylation of eNOS with the involvement of the PI3K/Akt
pathway may contribute to further understanding of the vasorelaxant
effects of ECH. Furthermore, the ECH targeting the
endothelial-derived NO pathways may be due to non-genomic effects.
Therefore, the present study may help elucidate the mechanisms
through which ECH exerts its pharmacological effects to prevent
cardiovascular disease.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81503333).
Availability of data and materials
The data sets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
LG and XL conceived and designed the experiments. DL
and YZ performed the experiments. WZ and JG analyzed the data. LG
drafted the manuscript. XL reviewed and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
NO
|
nitric oxide
|
|
ECH
|
echinacoside
|
|
AR
|
androgen receptor
|
|
ER
|
estrogen receptor
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
Akt
|
serine/threonine-protein Akt kinase
(protein kinase B)
|
|
eNOS
|
endothelial isoform of nitric oxide
synthase
|
|
siRNA
|
small interfering RNA
|
|
cGMP
|
cyclic guanosine monophosphate
|
|
PKG
|
cGMP-dependent protein kinase
|
|
HUVEC
|
human umbilical vein endothelial
cell
|
|
ECM
|
endothelial cell medium
|
|
FBS
|
fetal bovine serum
|
|
ECGS
|
endothelial cell growth supplement
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
DMSO
|
dimethylsulfoxide
|
Acknowledgments
Not applicable.
References
|
1
|
Chinese Pharmacopoeia Commission:
Cistanche Herba. Pharmacopoeia of the People's Repulic of China 1.
Chin. Med. Sci. Press; Beijing: pp. 1352015
|
|
2
|
Jiang Z, Wang J, Li X and Zhang X:
Echinacoside and Cistanche tubulosa (Schenk) R. wight ameliorate
bisphenol A-induced testicular and sperm damage in rats through
gonad axis regulated steroidogenic enzymes. J Ethnopharmacol.
193:321–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu J, Yang L, Dong Y, Zhang B and Ma X:
Echinacoside, an inestimable natural product in treatment of
neurological and other disorders. Molecules. 23:pp. piiE12132018,
View Article : Google Scholar
|
|
4
|
Yoshikawa M, Matsuda H, Morikawa T, Xie H,
Nakamura S and Muraoka O: Phenylethanoid oligoglycosides and
acylated oligosugars with vasorelaxant activity from Cistanche
tubulosa. Bioorg Med Chem. 14:7468–7475. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arnold WP, Mittal CK, Katsuki S and Murad
F: Nitric oxide activates guanylate cyclase and increases guanosine
3′:5′-cyclic monophosphate levels in various tissue preparations.
Proc Natl Acad Sci USA. 74:3203–3207. 1977. View Article : Google Scholar
|
|
6
|
Gai XY, Tang F, Ma J, Zeng KW, Wang SL,
Wang YP, Wuren TN, Lu DX, Zhou Y and Ge RL: Antiproliferative
effect of echinaco-side on rat pulmonary artery smooth muscle cells
under hypoxia. J Pharmacol Sci. 126:155–163. 2014. View Article : Google Scholar
|
|
7
|
He WJ, Fang TH, Ma X, Zhang K, Ma ZZ and
Tu PF: Echinacoside elicits endothelium-dependent relaxation in rat
aortic rings via an NO-cGMP pathway. Planta Med. 75:1400–1404.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gai XY, Wei YH, Zhang W, Wuren TN, Wang
YP, Li ZQ, Liu S, Ma L, Lu DX, Zhou Y and Ge RL: Echinacoside
induces rat pulmonary artery vasorelaxation by opening the
NO-cGMP-PKG-BKCa channels and reducing intracellular
Ca2+ levels. Acta Pharmacol Sin. 36:587–596. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maruhashi T, Kihara Y and Higashi Y:
Assessment of endothelium-independent vasodilation: From
methodology to clinical perspectives. J Hypertens. 36:1460–1467.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmad KA, Ze H, Chen J, Khan FU, Chen X,
Xu J and Ding Q: The protective effects of a novel synthetic
β-elemene derivative on human umbilical vein endothelial cells
against oxidative stress-induced injury: Involvement of
antioxidation and PI3k/Akt/eNOS/NO signaling pathways. Biomed
Pharmacother. 106:1734–1741. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu J, Akishita M, Eto M, Koizumi H,
Hashimoto R, Ogawa S, Tanaka K, Ouchi Y and Okabe T: Src
kinase-mediates androgen receptor-dependent non-genomic activation
of signaling cascade leading to endothelial nitric oxide synthase.
Biochem Bioph Res Commun. 424:538–543. 2012. View Article : Google Scholar
|
|
12
|
Yu J, Akishita M, Eto M, Ogawa S, Son B,
Kato S, Ouchi Y and Okabe T: Androgen receptor-dependent activation
of endothelial nitric oxide synthase in vascular endothelial cells:
Role of phosphatidylinositol 3-kinase/Akt pathway. Endocrinology.
151:1822–1828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pittarella P, Squarzanti DF, Molinari C,
Invernizzi M, Uberti F and Reno F: NO-dependent proliferation and
migration induced by vitamin D in HUVEC. J Steroid Biochem.
149:35–42. 2015. View Article : Google Scholar
|
|
14
|
He Y, Luan Z, Fu X and Xu X:
Overexpression of uncoupling protein 2 inhibits the high
glucose-induced apoptosis of human umbilical vein endothelial
cells. Int J Mol Med. 37:631–638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao-Hong D, Chang-Qin X, Jian-Hua H,
Wen-Jiang Z and Bing S: Icariin delays homocysteine-induced
endothelial cellular senescence involving activation of the
PI3K/AKT-eNOS signaling pathway. Pharm Biol. 51:433–440. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith PK, Krohn RI, Hermanson GT, Mallia
AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ and
Klenk DC: Measurement of protein using bicinchoninic acid. Anal
Biochem. 150:76–85. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu L, Zhong X, Lian D, Zheng Y, Wang H and
Liu X: Triterpenoid biosynthesis and the transcriptional response
elicited by nitric oxide in submerged fermenting Ganoderma lucidum.
Process Biochem. 60:19–26. 2017. View Article : Google Scholar
|
|
18
|
Ellinsworth DC, Sandow SL, Shukla N, Liu
Y, Jeremy JY and Gutterman DD: Endothelium-derived
hyperpolarization and coronary vasodilation: Diverse and integrated
roles of epoxyeicosatrienoic acids, hydrogen peroxide, and gap
junctions. Microcirculation. 23:15–32. 2016. View Article : Google Scholar :
|
|
19
|
Freed JK and Gutterman DD: Communication
is key: Mechanisms of intercellular signaling in vasodilation. J
Cardiovasc Pharm. 69:264–272. 2017. View Article : Google Scholar
|
|
20
|
Quillon A, Fromy B and Debret R:
Endothelium microenvironment sensing leading to nitric oxide
mediated vasodilation: A review of nervous and biomechanical
signals. Nitric Oxide. 45:20–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Torres-Estay V, Carreno DV, Francisco IF,
Sotomayor P, Godoy AS and Smith GJ: Androgen receptor in human
endothelial cells. J Endocrinol. 224:131–137. 2015. View Article : Google Scholar
|
|
22
|
Deng Q, Zhang Z, Wu Y, Yu WY, Zhang J,
Jiang ZM, Zhang Y, Liang H and Gui YT: Non-genomic action of
androgens is mediated by rapid phosphorylation and regulation of
androgen receptor trafficking. Cell Physiol Biochem. 43:223–236.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Oliveira TS, de Oliveira LM, de
Oliveira LP, Costa RMD, Tostes RC, Georg RC, Costa EA, Lobato NS,
Filgueira FP and Ghedini PC: Activation of PI3K/Akt pathway
mediated by estrogen receptors accounts for estrone-induced
vascular activation of cGMP signaling. Vascul Pharmacol. 110:42–48.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li F, Yang X, Yang Y, Guo C, Zhang C, Yang
Z and Li P: Antiosteoporotic activity of echinacoside in
ovariectomized rats. Phytomedicine. 20:549–557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng KW, Liao LX, Wan YJ, Jiang Y and Tu
PF: Pharmacological targets identification and efficacy analysis of
phenylethanoid glycosides from Cistanches Herba based on 'target
fishing' strategy. Chin Tradit Herbal Drugs. 49:173–178. 2018.
|
|
26
|
Jiang Z, Zhou B, Li X, Kirby GM and Zhang
X: Echinacoside increases sperm quantity in rats by targeting the
hypothalamic androgen receptor. Sci Rep. 8:3839–3850. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coffer PJ, Jin J and Woodgett JR: Protein
kinase B (c-Akt): A multifunctional mediator of
phosphatidylinositol 3-kinase activation. Biochem J. 335:1–13.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang S, Zheng G, Tian S, Zhang Y, Shen L,
Pak Y, Shen Y and Qian J: Echinacoside improves hematopoietic
function in 5-FU-induced myelosuppression mice. Life Sci.
123:86–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen M, Wang X, Hu B, Zhou J, Wang X, Wei
W and Zhou H: Protective effects of echinacoside against
anoxia/reperfusion injury in H9c2 cells via up-regulating p-AKT and
SLC8A3. Biomed Pharmacother. 104:52–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abeyrathna P and Su Y: The critical role
of Akt in cardiovascular function. Vascul Pharmacol. 74:38–48.
2015. View Article : Google Scholar : PubMed/NCBI
|