Introduction
Pterygium is a common disease of the ocular surface,
characterized by invasion of triangular inflammatory fibrovascular
lesions into the cornea, which can cause inflammation, irregular
corneal astigmatism and stromal opacity (1). Currently, the underlying mechanisms
of pterygium have not been well elucidated and surgical excision is
the standard treatment; however, pterygium recurrence is common
following surgery (2). In order
to devise strategies for the non-surgical treatment of pterygium or
the prevention of recurrence after resection, it is important to
understand the underlying molecular mechanisms of the pathogenesis
of pterygium.
Pterygium is more common in tropical and subtropical
zones, and epidemiological evidence indicates that the pathogenesis
of pterygium is strongly associated with high exposure to
ultraviolet (UV) radiation (3).
UV radiation induces expression of several proinflammatory
cytokines, including transforming growth factor-β, interleukin
(IL)-1, IL-6, and IL-8, and growth factors, including fibroblast
growth factor and vascular endothelial growth factor, which promote
the progression of pterygium (3-6);
however, the exact mechanism by which UV leads to the onset of
pterygium remains unclear.
Pterygium exhibits tumor-like features, including
propensity to invade normal tissue, recurrence after excision and
coexistence with premalignant lesions (4,7,8).
Previous studies have demonstrated that pterygium epithelial cells
(PECs) are highly proliferative, have features of
epithelial-mesenchymal transition (EMT) and overexpress
anti-apoptotic proteins (9-13).
Livin is a member of the inhibitors of apoptosis protein (IAP)
family (14), which function in
tumor initiation, progression and resistance to chemotherapy
(15). Livin has been reported to
serve a prominent and specific role in certain malignancies and is
associated with diverse cellular behaviors, including
proliferation, invasiveness and motility (14). However, the role of Livin in
ocular surface diseases has not been previously reported, and to
the best of our knowledge, there is no research investigating the
expression and molecular mechanisms of Livin in the development of
pterygium.
In the present study, the expression levels and
distribution of Livin in pterygium was investigated and the
association between UVB radiation and Livin expression levels was
explored. Knockdown of Livin expression was used to investigate the
function of Livin in the cellular behavior of pterygium epithelium
and the potential underlying molecular mechanisms.
Materials and methods
Patients and samples
The present study was in compliance with The
Declaration of Helsinki and was approved by the Institutional
Review Board of Hangzhou Red-Cross Hospital (Hangzhou, China). All
patients provided written informed consent. A total of 19 male and
16 female patients with primary pterygium (age range, 52-79 years;
mean age, 60.3±7.7 years), treated at Hangzhou Red-Cross Hospital
between November 2018 and March 2019, were included in the present
study. Exclusion criteria included a history of any other ocular
surface disorders, previous ocular surgery, ocular trauma, systemic
autoimmune disease or inflammation and use of any topical steroids
or non-steroidal anti-inflammatory drugs. Normal conjunctival
tissue segments from the nasal bulbar conjunctiva near the limbus
were obtained from 10 individuals (6 men and 4 women; age range,
44-69 years; mean age, 56.6±8.9 years) during retinal surgeries as
control group samples.
Pterygia were classified into two stages based on
the invasion of the pterygium, the number of new blood vessels and
the transparency of the tissue (16). A total of 17 samples were
classified as of quiescent stage, characterized by mild invasion,
hyperemia and moderate transparency, whereas 18 samples were
classified as of advanced stage, characterized by aggression, dense
neovascularization and opacity of the pterygium. Excised tissues
were divided laterally into two symmetrical pieces of identical
size. One piece (for measurement of Livin mRNA) was frozen and
stored at −80°C and the other piece was fixed in 10% buffered
formaldehyde solution and embedded in paraffin before subsequent
experiments (<3 months).
Reverse transcription-quantitative
(RT-q)PCR
Normal conjunctival tissue and pterygium were cut
and ground, then 1 ml TRIzol® (EZBioscience) was used to
extract total cellular RNA according to the manufacturer's
protocol. Subsequently, RNA purity and concentration were measured
using a Nanodrop 2000 (Thermo Fisher Scientific, Inc.), then RNA
was reverse transcribed to cDNA using a Revert Aid First Strand
cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). qPCR was
performed using SsoAdvance Universal SYBR-Green Supermix (Bio-Rad
Laboratories, Inc.). PCR was performed at 95°C for 3 min, then for
40 cycles at 95°C for 10 sec, 60°C for 30 sec and 72°C for 30 sec.
The primer sequences of the target genes were as follows: Livin
forward, 5′-ACA GAG GAG GAA GAG GAG GAG G-3′ and reverse 5′-GCA GTC
AGC GGC CAG T CA T-3′; and β-actin forward, 5′-GAA CCC TAA GGC TAA
CAG AGA AA-3′ and reverse, 5′-CCA CTA GCA TAA AGG GAG AG A AC-3′.
β-actin was used as an internal control. The experimental results
were automatically calculated using a CFX96TouchTM
Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.). The
relative expression of Livin gene was expressed as a fold-increase
of β-actin calculated using the 2−ΔΔCq method (17).
Immunohistochemistry analysis
The tissues were fixed in a 10% formalin solution
and embedded in paraffin. Samples were cut into 4-µm
sections, mounted on glass and dried overnight at 37°C. All
sections were then deparaffinized in xylene, rehydrated with
alcohol (100% ethanol for 2×3 min; 95% ethanol for 3 min; 70%
ethanol for 3 min; 50% ethanol for 3 min) and washed in PBS.
Sections were then inactivated using 3% H2O2
for 10 min. Sections were heated to 100°C in EDTA (pH 9.0) for 10
min and then naturally cooled and soaked in PBS three times for 3
min each. The sections were blocked in a 37°C oven for 45 min using
5% BSA (Sigma-Aldrich; Merck KGaA). The immunohistochemistry
experiment was conducted using the streptavidin-biotin-peroxidase
method (18). Anti-Livin primary
antibody (cat no. ab93750; 1:50; Abcam) was applied to the sections
and incubated overnight at 4°C. 3,3′-Diaminobenzidine was used as a
chromogen, followed by rinsing with water for 5 min and
counterstaining with hematoxylin for 2 min at room temperature.
The numbers of epithelial cells and Livin-positive
cells of the pterygium or normal conjunctiva were counted in three
independent fields using a high-power field under a light
microscope (magnification, ×40). Cells positively stained for the
anti-Livin antibody were scored according to the percentage extent
of staining and the measurements were averaged. Scores were as
follows: 0, no positive staining; +, 1-10%; ++, 11-50%; and +++,
>50% positive cells. Scores of +, ++ and +++ were considered to
represent positive immunostaining, whereas a score of 0 indicated
negative immunostaining. All evaluations of the staining were
conducted by an observer blinded to the origins of the tissue
samples.
Western blot analysis
Total protein was extracted from patient tissues
using RIPA lysis buffer (Beyotime Institute of Biotechnology) and
20 µg protein/lane was loaded onto a 12% gel, resolved using
SDS-PAGE and subsequently transferred to PVDF membranes (EMD
Millipore). PVDF membranes were immersed in a blocking solution
containing 5% skimmed milk powder in PBS or 5% BSA and blocked at
room temperature for 2 h. Subsequently, the membranes were
incubated with primary antibodies at 4°C overnight, followed by
secondary antibodies at room temperature for 2 h. The proteins were
visualized using a Clarity™ Western ECL Substrate (Bio-Rad
Laboratories, Inc.) and detected using a chemiluminescence
detection system (ChemiDoc MP; Bio-Rad Laboratories, Inc.) and the
bands were analyzed using ImageJ software (v1.46; National
Institutes of Health). Anti-GAPDH antibodies were used as an
internal control (cat. no. cst5174; 1:1,000; Cell Signaling
Technology, Inc.). The primary antibodies included anti-Livin (cat.
no. NB100-56548; 1:500-1:2,000; Novus Biologicals, Ltd.),
anti-caspase-7 (cat. no. ab32522, 1:1,000; Abcam), anti-caspase-3
(cat. no. cst9662; 1:1,000; Cell Signaling Technology, Inc.),
anti-E-cadherin (cat. no. 610405; 1:1,000; BD Biosciences),
anti-Snail (cat. no. cst3879; 1:1,000; Cell Signaling Technology,
Inc.) and anti-poly ADP-ribose polymerase (cat. no. ab74290; PARP;
1:4,000, Abcam). The secondary antibodies included goat
anti-rabbitIgG (cat. no. BL003A; 1:5,000; Biosharp Life Sciences)
and goat anti-mouse IgG (cat. no. BL001A; 1:5,000; Biosharp Life
Sciences).
Culture of PECs
PECs were isolated and cultured using previously
established methods (10,16). Fresh primary pterygium samples at
the advanced stage were washed with PBS with 1% penicillin (100
units/ml) and streptomycin (100 µg/ml) (Gibco; Thermo Fisher
Scientific, Inc.). After the fibrovascular tissue was removed as
much as possible, the remaining epithelial tissues were cut into
small pieces of 1×1 mm and then cultured in DMEM supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 mg/ml streptomycin at 37°C with 5%
CO2 in a humidified incubator. The solution was changed
once every 2-3 days, and after the cells covered the bottom of the
bottle, the cells were digested with 0.25% trypsin (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C for 2 min. Under a phase-contrast
microscope, once cytoplasmic retraction and enhanced refractive
index had been observed, 2 ml medium with 10% FBS was added to
terminate digestion. The cell suspension was prepared, counted and
inoculated. Finally, PECs were seeded in serum-free DMEM and
cultured at 37°C, 5% CO2, in a saturated humidity
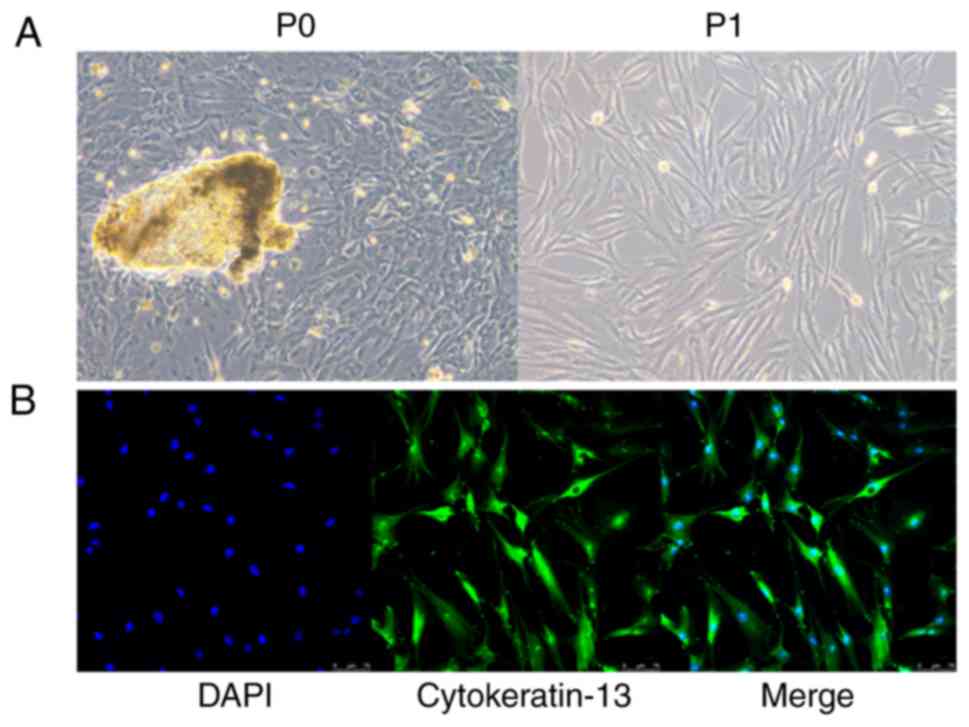
incubator. The cells from primary culture using tissue were defined
as primary passage cells (P0) and the cells from the first passage
after the primary culture were defined as first passage PECs (P1).
The morphology of cells was relatively uniform, showing a
fibrous/fusiform shape. Immunofluorescence results confirmed that
almost all cells (P2) were positively stained with anti-cytokeratin
13 (cat. no. ab32522; 1:200; Abcam) at 4°C overnight, so the cell
purity was considered to be very high, close to 100% (Fig. 1).
UVB irradiation of PECs
The cultured PECs were incubated in a 96-well
culture plate with 4×103 cells/well for 24 h. Culture
medium was removed and replaced with PBS. The PECs were irradiated
with UVB at a dose of 0, 0.5, 1 and 2.0 J/cm2 using an
UV irradiation chamber BS-02 (Opsytec Dr. Gröbel GmbH). PECs were
collected for analysis of Livin expression, and analysis of cell
viability and invasion ability following UVB irradiation.
CCK-8 assay for cell viability
Following UVB irradiation, PECs were incubated in
96-well plates at 100 µl/well with 5 replicate wells for
each group for 24 h. Subsequently, 10 µl CCK8 was added to
each well for analysis of cell viability according to the
manufacturer's protocol (Shanghai Yeasen Biotech Co., Ltd.). Blank
control wells were set and incubated at 37°C in the dark. The
optical density (OD) value was read at a wavelength of 450 nm using
a microplate reader Plus384 (Molecular Devices LLC) and the cell
survival rate was calculated using the following formula: Cell
viability (%)=(experimental group OD450/normal group
OD450) ×100. The experiment was repeated three
times.
PEC transfection
Small interfering RNA (siRNA) was used to knockdown
endogenous Livin gene expression in PECs. Cells were transfected
with Livin-specific siRNA (GE Healthcare Dharmacon, Inc.) and
negative control scrambled siRNA using Lipofectamine®
2000 (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The PECs were seeded at a density of
1×105 cells/well in 6-well plates and transfected with
30 nM Livin-siRNA using Lipofectamine 2000. After 48 h of
incubation at 37°C, the cells were harvested and subjected to cell
viability, migration and invasion assays, and flow cytometry
analysis.
Cell apoptosis assay
Apoptosis was determined using an Annexin
V-FITC/7-AAD assay. After transfection with Livin-siRNA, PECs were
incubated at 37°C for 48 h. A total of 5×104 cells were
collected by centrifugation at 500 × g for 5 min at room
temperature, washed twice with PBS and centrifuged again at 500 × g
for 10 min at 37°C. A total of 100 µl binding buffer was
used to re-suspend the cells. After adding 5 µl Annexin
V-FITC and 5 µl 7-AAD (both Beijing Jiamay Biotech), the
cells were incubated for 15 min at room temperature in the dark.
After adding 400 µl binding buffer, cell apoptosis was
detected using a NovoCyte flow cytometer (ACEA Biosciences, Inc.).
The cells that were FITC-positive and 7-AAD-negative were indicated
as apoptotic cells.
Cell migration assay
A total of 1×105 cultured PECs/well were
seeded in a 6-well culture plate. After incubation for 24 h at
37°C, a straight line was scratched across the cells using a P200
pipette tip to create a wound. The plates were then rinsed with PBS
to remove the cells in suspension. Identical sites were observed at
×40 magnification at 0 and 24 h post-scratching using a light
microscope. The migration of cells toward the wounds was expressed
as percentage of wound closure: % of wound closure=[(At= 0 h-At=∆
h)/At= 0 h] ×100%. At=0 h is the area of wound measured immediately
after scratching; At=Δ h is the area of wound measured 24 h after
scratching.
Cell invasion assay
Cell invasion was measured in a Transwell cell
culture chamber. Cultured PECs were seeded in the upper chambers
with 25 µg Matrigel (8-12 mg/ml). A total of 100 µl
cell suspension using serum-free medium was added to the upper
chamber (9×103 cells/chamber) and 600 µl DMEM
containing 10% serum as a chemokine was added to the lower chamber.
Incubation was performed at 37°C with 5% CO2 for 48 h.
The chamber was then removed, washed three times with PBS, fixed
with immobilization solution for 20 min and washed three times with
PBS. After staining with crystal violet for 20 min at room
temperature, the cells were counted under a light microscope
(magnification, ×200). Results of three individual experiments were
averaged.
Statistical analysis
The data are expressed as the mean ± standard
deviation of at least three independent experiments. All datasets
were tested for normality with the Kolmogorov-Smirnov test. When
data were normally distributed, one-way ANOVA with Tukey's post hoc
test was performed for multiple comparisons, and Student's t-test
was used for comparison between two groups. A χ2 test
was used for compare Livin staining scores. P<0.05 was
considered to indicate a statistically significant difference.
Results
Livin expression levels are upregulated
in pterygium compared with those in normal conjunctiva
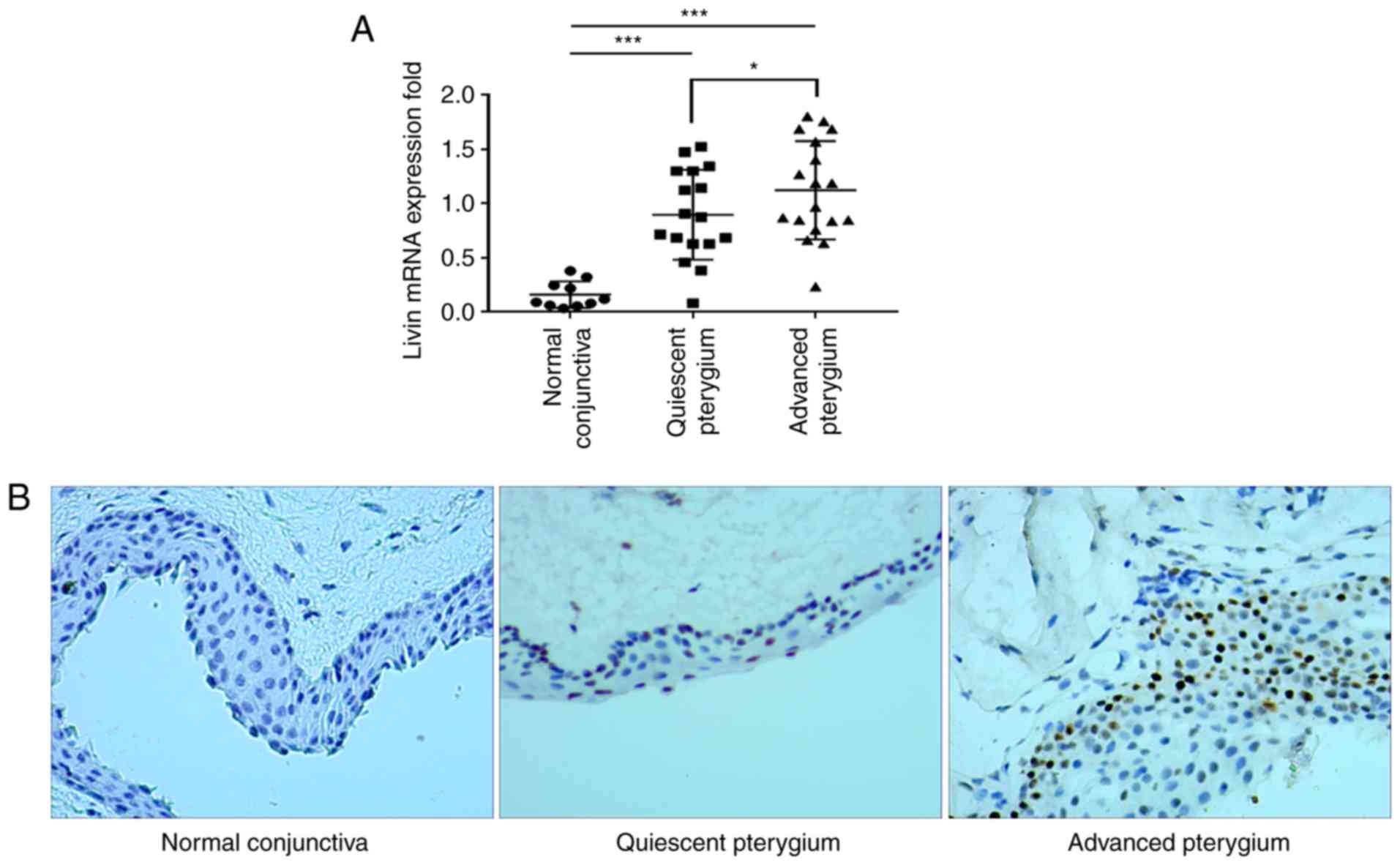
The Livin expression levels were analyzed in normal
human conjunctiva and pterygium tissues using RT-qPCR. Livin mRNA
expression levels were increased 5-fold in quiescent stage
pterygium and 5.3-fold in the pterygium at the advanced stage
compared with that in the normal conjunctiva (Fig. 2A). Advanced stage pterygium
tissues exhibited significantly higher Livin mRNA expression levels
compared with quiescent stage tissues (Fig. 2A). Immunohistochemistry
demonstrated that Livin protein was expressed in pterygium tissues
primarily located in the nuclei of epithelial cells, whereas it was
seldom expressed in the normal conjunctiva (Fig. 2B).
In the pterygium group, 28/35 samples (80.0%)
stained positively for Livin, whereas in the normal conjunctiva
group only 1/10 samples (10.0%) demonstrated Livin-positive
staining. The difference between the pterygium and normal
conjunctiva groups was significant (80.0 vs. 10%; P<0.001).
Staining scores were higher in advanced-stage compared with
quiescent-stage samples (Table
I).
 | Table ILivin expression in pterygium and
normal conjunctiva tissues. |
Table I
Livin expression in pterygium and
normal conjunctiva tissues.
| Livin expression
level | Normal conjunctiva,
n (%) | Quiescent
pterygium, n (%) | Advanced pterygium,
n (%) |
|---|
| 0 | 9 (90.0) | 4 (23.5) | 3 (16.7) |
| + | 1 (10.0) | 5 (29.4) | 3 (16.7) |
| ++ | 0 (0.0) | 6 (35.3) | 7 (38.9) |
| +++ | 0 (0.0) | 2 (11.8) | 5 (27.8) |
| Total positive | 1 (10) | 13 (76.5) | 15 (83.3) |
Knockdown of Livin expression impairs
cell migration, invasion and viability, and induces apoptosis in
PECs
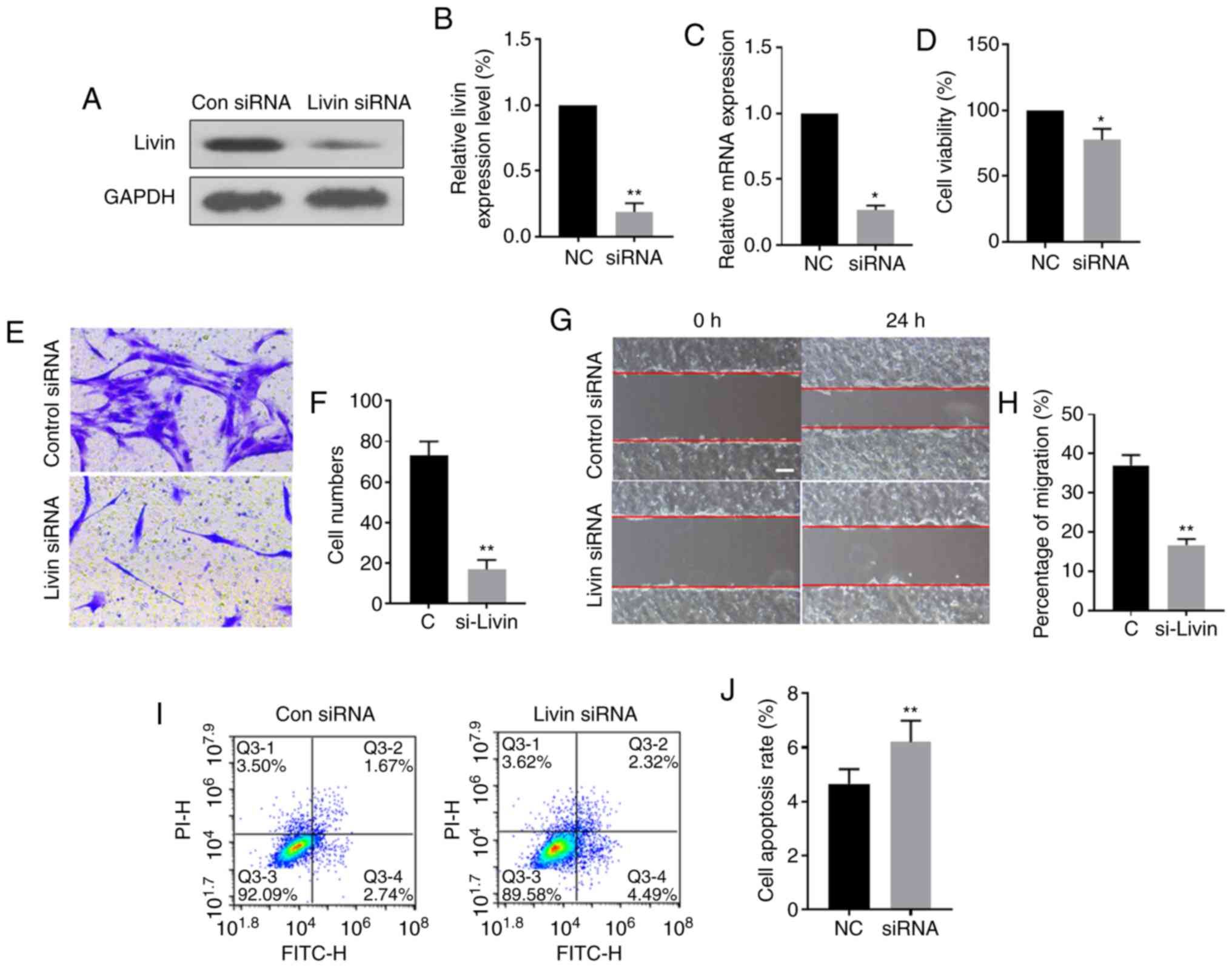
To further evaluate the role of Livin in pterygium
development and progression, ex vivo cultured PECs were
transfected with Livin-siRNA or control siRNA. Western blot
analysis showed that the expression levels of Livin protein and
mRNA were significantly downregulated in the
Livin-siRNA-transfected group compared with those in the control
group, suggesting successful knockdown of Livin in
Livin-siRNA-transfected PECs (Fig.
3A-C). Knockdown of Livin expression significantly reduced
invasion, migration and viability of PECs (Fig. 3D-H) and the proportion of
apoptotic cells was increased in Livin-siRNA-transfected PECs
compared with that in negative control siRNA-transfected PECs
(Fig. 3I and J).
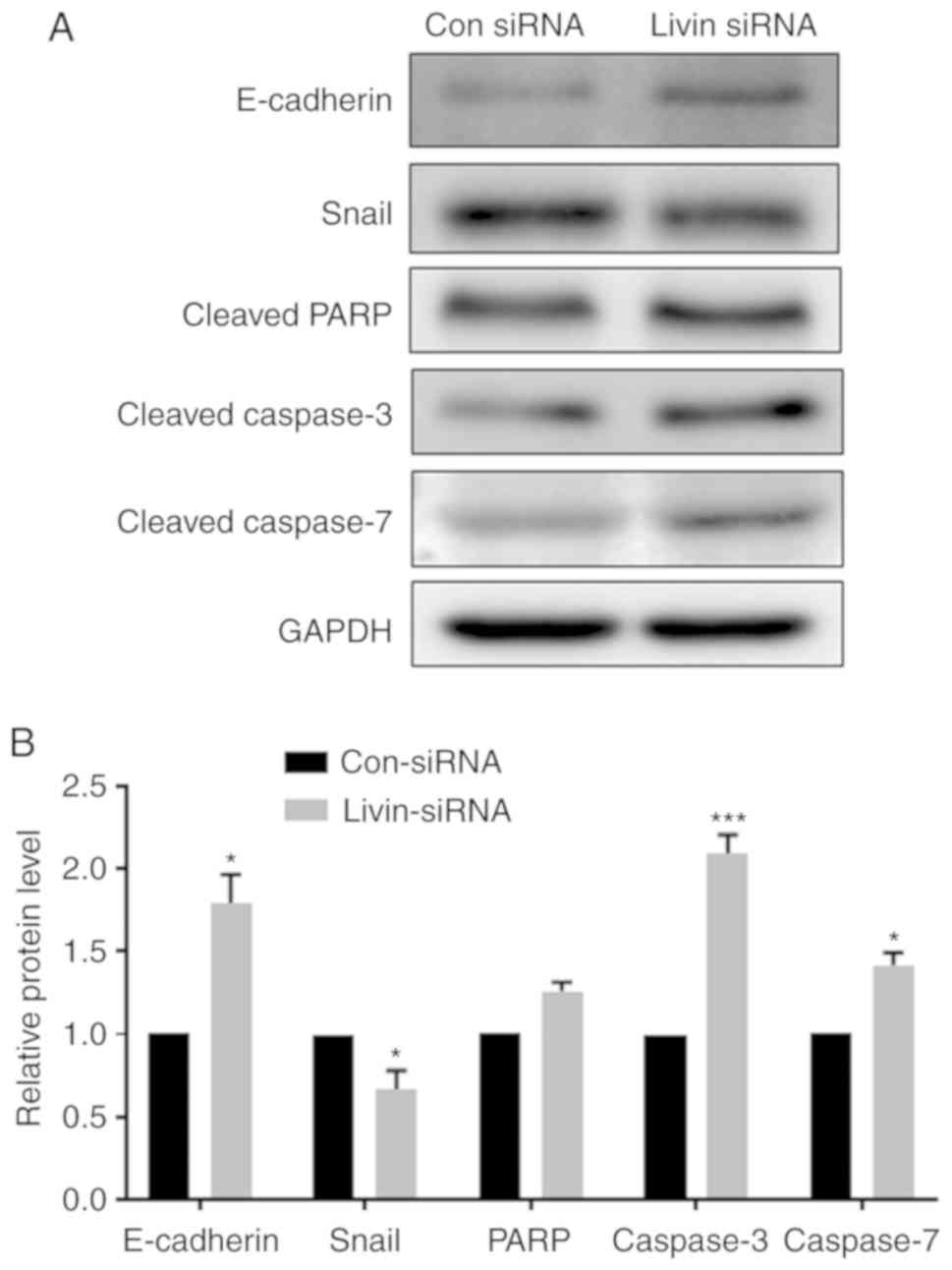
Livin regulates EMT and apoptosis via
E-cadherin, Snail, caspases and PARP
Previous studies have demonstrated that EMT is an
initial step required for cell metastasis and that it has a role in
apoptotic resistance (19).
Therefore, the role of Livin in regulating the expression of
EMT-associated proteins was also investigated. The data indicate
that knockdown of Livin expression significantly increased
E-cadherin and reduced Snail expression levels (Fig. 4A and B), indicating Livin is
involved in the regulation of EMT. The protein expression levels of
apoptosis-associated enzymes, caspase-3 and 7, were significantly
increased in the Livin-knockdown PECs. PARP protein expression
levels were also increased in Livin-knockdown PECs, but this
difference was not significant (Fig.
4B).
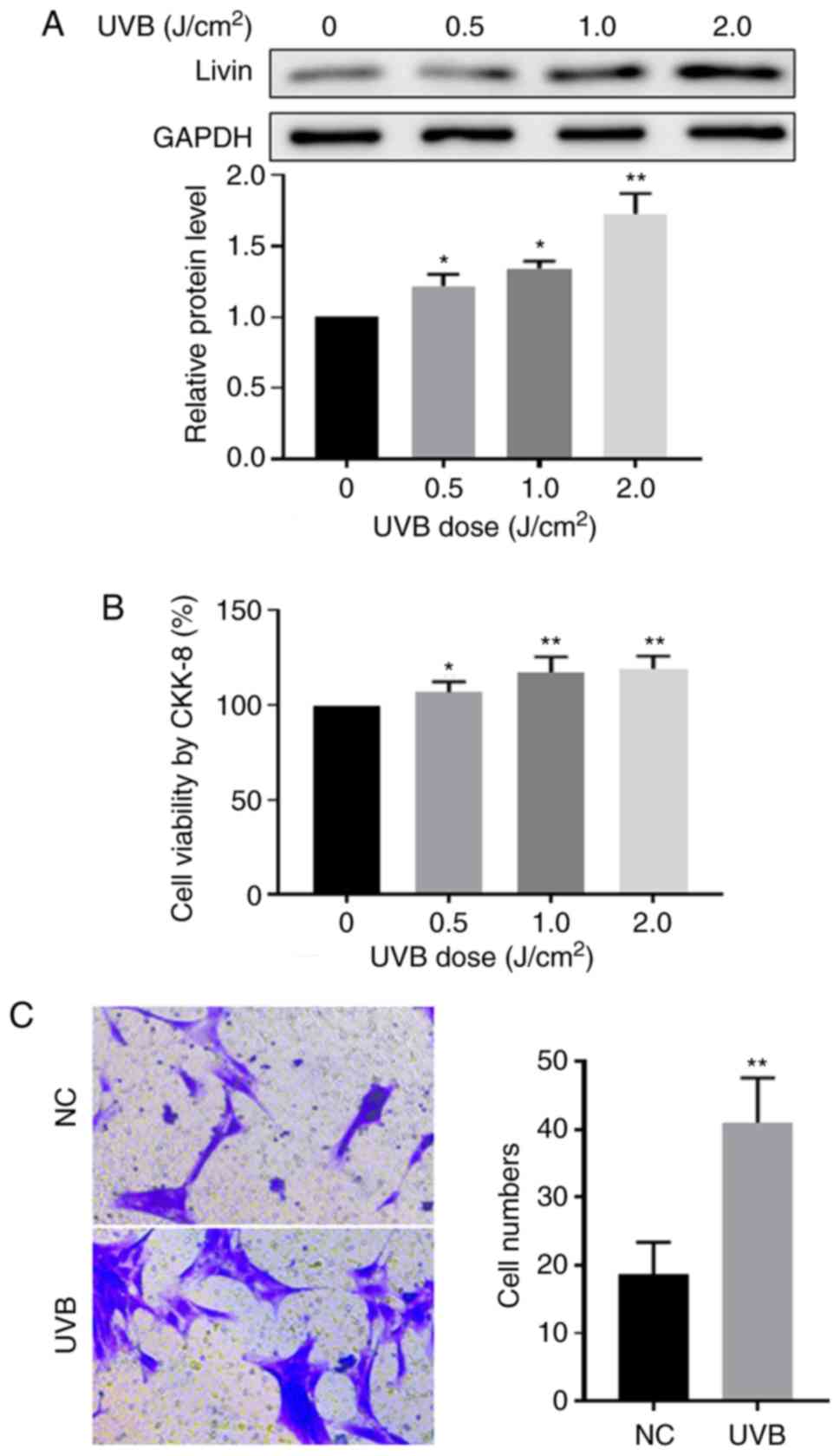
Effect of UVB irradiation on Livin
expression, cell viability and invasion ability in PECs
UVB is a major risk factor for pterygium development
(3). To further investigate the
association between Livin expression levels and cell viability and
invasion following UVB irradiation, ex vivo PECs in culture
were treated with UVB radiation. Compared with the non-radiation
treatment group, Livin protein expression levels were significantly
increased in UVB irradiation-treated PECs (Fig. 5A) and PEC viability was
significantly increased with increasing doses of UVB radiation
(Fig. 5B). In addition, cell
invasion was promoted by UVB irradiation (Fig. 5C).
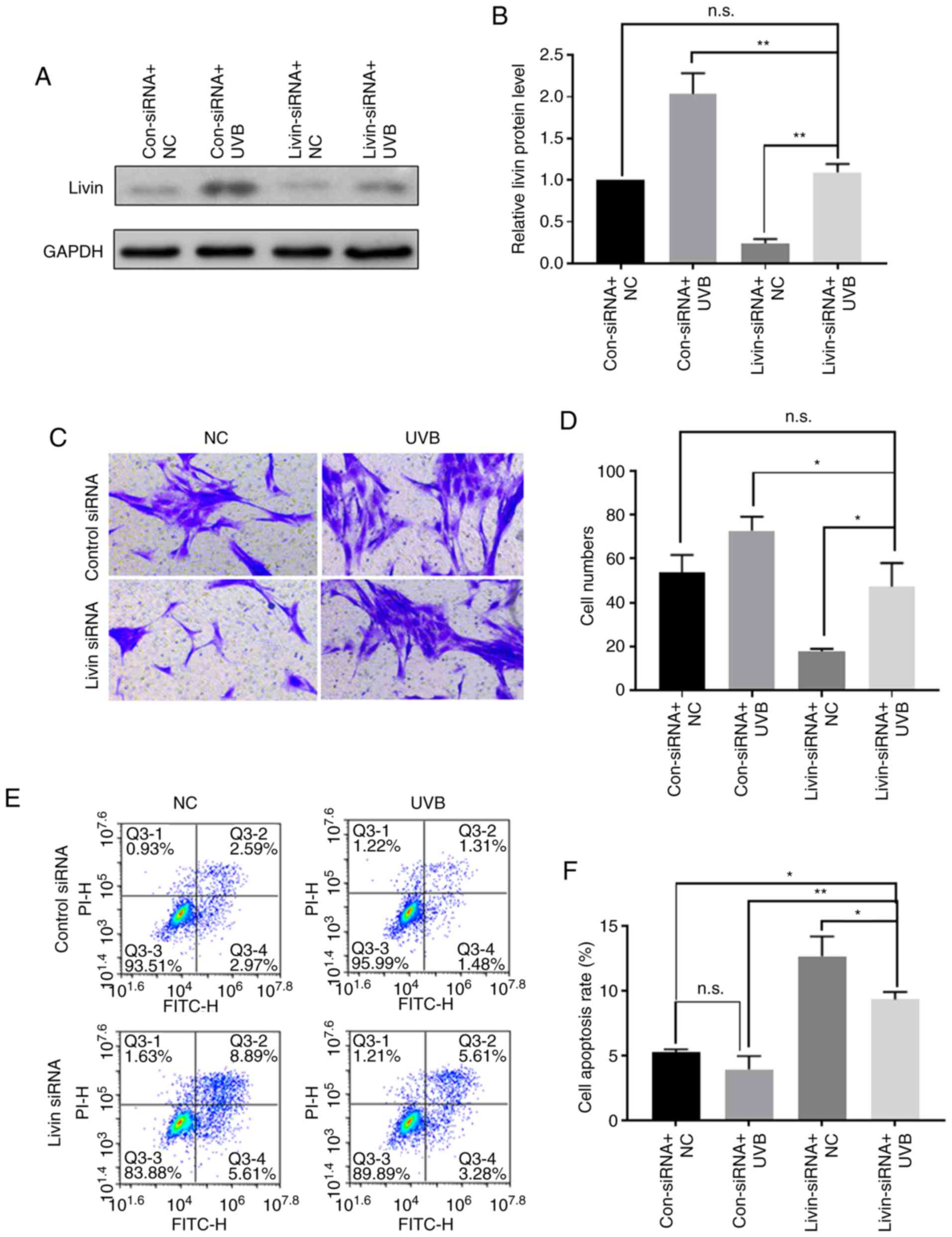
UVB irradiation may promote the
development and progression of pterygium by inducing the expression
of Livin
Western blot analysis was used to evaluate Livin
protein expression following UVB irradiation with or without
Livin-knockdown. Livin-knockdown followed by UVB irradiation
resulted in partial inhibition of UVB-induced Livin protein
expression (Fig. 6A and B). In
the invasion experiments, UVB induced an increase in cell invasion
ability, which was attenuated by Livin silencing (Fig. 6C and D). Cell apoptosis analysis
revealed that UVB irradiation reduced apoptosis of PECs, and
transfection with Livin-siRNA prior to UVB irradiation increased
the apoptosis of PECs compared with the control siRNA group
(Fig. 6E and F).
Discussion
Pterygium is a fibrovascular neoformation composed
of epithelium and highly vascular loose subepithelial connective
tissue (20). Previous studies
have demonstrated that apoptotic and oncogenic proteins,
microsatellite instability, inflammatory mediators, extracellular
matrix modulators, EMT and other factors are involved in the
development of pterygium (4,9-11,13). However, to the best of our
knowledge, the precise molecular mechanisms of pterygium
pathogenesis have not been resolved. It is hypothesized that
epithelial cells function in the pathogenesis of pterygium, with
EMT and secretion of matrix metalloproteinases from epithelial
cells associated with the invasive and recurrent behavior of
pterygium (21-23).
Previous studies have demonstrated that a number of
apoptosis-associated genes and proteins are differentially
expressed in pterygium compared with normal conjunctiva tissues,
including p53, Survivin and Bcl-2, indicating the role of
apop-tosis in the pathogenesis of pterygium (12,16,24). Pterygium may be associated with
disrupted control of cellular apoptosis rather than an increase in
proliferative capacity, therefore, the regulation of PEC apoptosis
may have a role in the pathogenesis of pterygium (25). As a member of the IAP family,
Livin participates in apoptosis, proliferation and the cell cycle
by binding to caspases and regulation of EMT (14,26). Livin is not expressed in the
majority of normal adult tissues; however, it is present in
developmental tissues and highly expressed in most tumor tissues
(27). In the present study, the
molecular mechanism underlying the function of Livin in the
pathogenesis of pterygium was investigated. Livin mRNA and protein
expression levels were significantly upregulated in pterygium
tissues compared with those in the normal conjunctiva. Moreover,
Livin expression levels were higher in advanced pterygium compared
with those in quiescent pterygium tissues. This difference may be
due to the higher cell proliferation pattern in advanced pterygium
compared with that in quiescent pterygium. Therefore, upregulated
Livin may be involved in the occurrence and development of
pterygium, which requires further research. In the present study,
cultured PECs were also used for further investigation of the
association between Livin and the development of pterygium. Livin
expression in advanced pterygium may have a role in promoting
epithelial proliferation, resulting in progression of the
pterygium.
To explore whether Livin expression is involved in
the occurrence and development of pterygium, PECs were transfected
with Livin-siRNA in the present study. Knockdown of Livin
expression levels increased apoptosis of PECs and decreased their
proliferation, migration and invasion abilities, which is
consistent with the results of a previous study investigating other
IAPs (16). It is hypothesized
that EMT occurs during the pathogenesis of pterygium (13). Snail is a key transcription factor
involved in EMT regulation (28).
E-cadherin is downregulated in during EMT, associated with the
detachment and invasion of cells from their primary site, and
results in metastasis and progression (13,28,29). In the present study,
Livin-knockdown reduced Snail expression and increased E-cadherin
expression, consistent with the reduced proliferation, migration
and invasion of PECs following Livin-knockdown, indicating that
Livin may be associated with EMT. Therefore, Livin may be involved
in the pathogenesis of pterygium via a function in EMT.
IAPs suppress apoptosis induced by a range of
stimuli, primarily via direct binding of the BIR domain to specific
intracellular proteases, including caspase-3, -7 and -9, inhibiting
their activity (24). PARP is an
important downstream substrate of caspase-3 (30). In the present study,
Livin-knockdown increased the expression of caspase-3, -7 and PARP,
consistent with results of a previous study investigating the
function of Livin in oral squamous cells (27,31). Therefore, the anti-apoptotic
function of Livin may be mediated by suppressing the activity of
caspases in PECs.
A number of studies have demonstrated that UV
exposure is a factor precipitating the development of pterygium
(3,21,32,33). Histological examination has
confirmed that elastic connective tissue lesions and severely
damaged areas of the Bowman membrane indicate direct damage caused
by solar radiation or indirect damage due to excessive proteolytic
activity (3,21). The present study showed that Livin
expression levels were increased by UVB irradiation and contributed
to the increased cell viability and invasion ability in PECs.
Meanwhile, knockdown of Livin expression attenuated the effects of
UVB-induced Livin expression level on cell viability and cell
invasion in PECs. This decreased cell viability and invasion
ability in Livin-knockdown PECs following UVB exposure suggests
that Livin may have a role in inducing pterygium formation by
increasing cell viability and invasion. PEC apoptosis was reduced
by low-dose UVB irradiation, and knockdown of Livin prior to UVB
irradiation increased the apoptosis of PECs. Therefore, the
increase in cell viability and invasion induced by UVB irradiation
may be due to upregulated Livin expression, and silencing of Livin
expression may protect against pterygium development.
Overall, the present study suggests that increased
Livin expression levels may be involved in the pathogenesis and
development of pterygium. Knockdown of Livin reduced the
proliferation, migration and invasion of PECs, and induced
apoptosis by regulating EMT and apoptosis-associated proteins. UVB
radiation increased the expression of Livin and cell invasion;
therefore, UVB is a risk factor for pterygium. The present study
provides novel insight into the pathogenesis of pterygium and may
contribute to the development of novel therapeutic agents for
treatment. However, the interaction between Livin and other
cytokines and growth factors in pterygium development still
requires further investigation.
Funding
This study was supported by The Natural Science
Foundation of Zhejiang Province (grant no. LY17H120009) and The
Zhejiang Traditional Chinese Medicine Science and Technology
Project (grant no. 2016ZA151).
Availability of data and materials
The datasets during the current study are available
from the corresponding author on reasonable request.
Authors' contributions
SQW, QBX, WYS and LWZ designed the study. SQW, QBX
and LWZ drafted the original manuscript, collected the data and
reviewed the literature. QBX, WYS and LYS interpreted the data and
critically reviewed the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Institutional
Review Board at Hangzhou Red-Cross Hospital (Hangzhou, China) and
written informed consent was provided by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Liu T, Liu Y, Xie L, He X and Bai J:
Progress in the pathogenesis of pterygium. Curr Eye Res.
38:1191–1197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nuzzi R and Tridico F: How to minimize
pterygium recurrence rates: Clinical perspectives. Clin Ophthalmol.
12:2347–2362. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou WP, Zhu YF, Zhang B, Qiu WY and Yao
YF: The role of ultraviolet radiation in the pathogenesis of
pterygia (Review). Mol Med Rep. 14:3–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cardenas-Cantu E, Zavala J, Valenzuela J
and Valdez-Garcia JE: Molecular basis of pterygium development.
Semin Ophthalmol. 31:567–583. 2016.
|
|
5
|
Di Girolamo N, Chui J, Coroneo MT and
Wakefield D: Pathogenesis of pterygia: Role of cytokines, growth
factors, and matrix metalloproteinases. Prog Retin Eye Res.
23:195–228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maurizi E, Schiroli D, Atkinson SD, Mairs
L, Courtney DG, O'Hagan B, McGilligan VE, Pagnamenta AT, Taylor JC,
Vasquez JJD, et al: A novel role for CRIM1 in the corneal response
to UV and pterygium development. Exp Eye Res. 179:75–92. 2019.
View Article : Google Scholar
|
|
7
|
Segev F, Mimouni M, Tessler G, Hilely A,
Ofir S, Kidron D and Bahar I: A 10-year survey: Prevalence of
ocular surface squamous neoplasia in clinically benign pterygium
specimens. Curr Eye Res. 40:1284–1287. 2015. View Article : Google Scholar
|
|
8
|
Oellers P, Karp CL, Sheth A, Kao AA,
Abdelaziz A, Matthews JL, Dubovy SR and Galor A: Prevalence,
treatment, and outcomes of coexistent ocular surface squamous
neoplasia and pterygium. Ophthalmology. 120:445–450. 2013.
View Article : Google Scholar
|
|
9
|
Qin YJ, Chu WK, Huang L, Ng CHY, Chan TCY,
Cao D, Yang C, Zhang L, Huang SP, Li J, et al: Induction of
apoptosis in pterygium cells by antagonists of growth
hormone-releasing hormone receptors. Invest Ophthalmol Vis Sci.
59:5060–5066. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui YH, Li HY, Gao ZX, Liang N, Ma SS,
Meng FJ, Li ZJ and Pan HW: Regulation of apoptosis by miR-122 in
pterygium via targeting Bcl-w. Invest Ophthalmol Vis Sci.
57:3723–3730. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang K, Jiang Z, Ding BQ, Cheng P, Huang
DK and Tao LM: Expression of cell proliferation and apoptosis
biomarkers in pterygia and normal conjunctiva. Mol Vis.
17:1687–1693. 2011.PubMed/NCBI
|
|
12
|
Tan DT, Tang WY, Liu YP, Goh HS and Smith
DR: Apoptosis and apoptosis related gene expression in normal
conjunctiva and pterygium. Br J Ophthalmol. 84:212–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meshkani SE, Kooshan N, Moghadam AB,
Falanji F, Adli A, Baghbani-Arani F, Arian AG and Rad A: Signaling
roadmap to epithelial-mesenchymal transition in pterygium, TWIST1
centralized. J Cell Physiol. 234:18146–18155. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kasof GM and Gomes BC: Livin, a novel
inhibitor of apoptosis protein family member. J Biol Chem.
276:3238–3246. 2001. View Article : Google Scholar
|
|
15
|
Wang Z, Liu S, Ding K, Ding S, Li C, Gao
D, Zhang T and Bi D: Silencing Livin induces apoptotic and
autophagic cell death, increasing chemotherapeutic sensitivity to
cisplatin of renal carcinoma cells. Tumour Biol. 37:15133–15143.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu YX, Zhang LY, Zou DL, Liu ZS, Shang XM,
Wu HP, Zhou Y, He H and Liu ZG: Differential expression and
function of survivin during the progress of pterygium. Invest
Ophthalmol Vis Sci. 55:8480–8487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Di Girolamo N, McCluskey P, Lloyd A,
Coroneo MT and Wakefield D: Expression of MMPs and TIMPs in human
pterygia and cultured pterygium epithelial cells. Invest Ophthalmol
Vis Sci. 41:671–679. 2000.PubMed/NCBI
|
|
19
|
Pearlman RL, Montes de Oca MK, Pal HC and
Afaq F: Potential therapeutic targets of epithelial-mesenchymal
transition in melanoma. Cancer Lett. 391:125–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Safi H, Kheirkhah A, Mahbod M, Molaei S,
Hashemi H and Jabbarvand M: Correlations between histopathologic
changes and clinical features in pterygia. J Ophthalmic Vis Res.
11:153–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Di Girolamo N, Wakefield D and Coroneo MT:
UVB-mediated induction of cytokines and growth factors in pterygium
epithelial cells involves cell surface receptors and intracellular
signaling. Invest Ophthalmol Vis Sci. 47:2430–2437. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng J, Sha XY, Liu Y, Yang RM and Wen Y:
Pterygium epithelium abnormal differentiation related to activation
of extracellular signal-regulated kinase signaling pathway in
vitro. Int J Ophthalmol. 8:1118–1125. 2015.PubMed/NCBI
|
|
23
|
Tsai YY, Chiang CC, Yeh KT, Lee H and
Cheng YW: Effect of TIMP-1 and MMP in pterygium invasion. Invest
Ophthalmol Vis Sci. 51:3462–3467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weinstein O, Rosenthal G, Zirkin H, Monos
T, Lifshitz T and Argov S: Overexpression of p53 tumor suppressor
gene in pterygia. Eye (Lond). 16:619–621. 2002. View Article : Google Scholar
|
|
25
|
Maxia C, Perra MT, Demurtas P, Minerba L,
Murtas D, Piras F, Corbu A, Gotuzzo DC, Cabrera RG, Ribatti D and
Sirigu P: Expression of survivin protein in pterygium and
relationship with oxidative DNA damage. J Cell Mol Med.
12:2372–2380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han Y, Zhang L, Wang W, Li J and Song M:
Livin promotes the progression and metastasis of breast cancer
through the regulation of epithelialmesenchymal transition via the
p38/GSK3beta pathway. Oncol Rep. 38:3574–3582. 2017.PubMed/NCBI
|
|
27
|
Yan B: Research progress on Livin protein:
An inhibitor of apoptosis. Mol Cell Biochem. 357:39–45. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kato N, Shimmura S, Kawakita T, Miyashita
H, Ogawa Y, Yoshida S, Higa K, Okano H and Tsubota K: Beta-catenin
activation and epithelial-mesenchymal transition in the
pathogenesis of pterygium. Invest Ophthalmol Vis Sci. 48:1511–1517.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Slee EA, Adrain C and Martin SJ:
Executioner caspase-3, -6, and -7 perform distinct, non-redundant
roles during the demolition phase of apoptosis. J Biol Chem.
276:7320–7326. 2001. View Article : Google Scholar
|
|
31
|
Lee HS, Lee JH and Yang JW: Effect of
porcine chondrocyte-derived extracellular matrix on the pterygium
in mouse model. Graefes Arch Clin Exp Ophthalmol. 252:609–618.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aletras AJ, Trilivas I, Christopoulou ME,
Drakouli S, Georgakopoulos CD and Pharmakakis N: UVB-mediated
down-regulation of proteasome in cultured human primary pterygium
fibroblasts. BMC Ophthalmol. 18:3282018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Di Girolamo N, Kumar RK, Coroneo MT and
Wakefield D: UVB-mediated induction of interleukin-6 and -8 in
pterygia and cultured human pterygium epithelial cells. Invest
Ophthalmol Vis Sci. 43:3430–3437. 2002.PubMed/NCBI
|