Introduction
Cordyceps sinensis, the caterpillar fungus,
is an ascomycete species that is among the most important in
traditional Chinese medicine (1).
This species has abundant nucleosides, polysaccharides, organic
acids, amino acids, peptides, steroids, trace elements and other
chemical components (1). C.
sinensis has numerous therapeutic effects, in that it regulates
immune function, intrinsic renal cell proliferation and synthesis
of the extracellular matrix and cytokines. Practitioners of
traditional Chinese medicine extensively use C. sinensis to
treat diabetic nephropathy, chronic renal diseases and similar
conditions, because of its stimulatory effect on the immune system
and its antioxidative activity (2,3).
The authors' previous study found that C.
sinensis attenuates the disease progression of renal fibrosis
by suppressing Bcl-2-associated athanogene 3 induction in a rat
model (4). However, no studies
have yet addressed the potential protective effect of C.
sinensis on hepatitis B virus-associated glomerulonephritis
(HBV-GN). HBV-GN is a severe health issue in China, the primary
cause of secondary renal damage in Chinese children (5-9).
HBx is one of the HBV proteins with numerous functions, including
activation of the signaling pathways in multiple cell types,
regulation of cell proliferation and induction of apoptosis
(10-12). The authors' previously
demonstrated that HBx-induced apoptosis of renal tubular epithelial
cells is one of the major renal injuries from HBV infection
(9,13,14).
The integration of multiple pro-apoptotic and
anti-apoptotic signals determines whether cells undergo apoptosis
(15). Wang et al
(16) showed that HBx activates
the phosphatidylino-sitol-3-kinase (PI3K)/protein kinase B (Akt)
signaling pathway in HepG-2 cells. The PI3Ks are a family of
enzymes that transduce intracellular signaling to regulate diverse
cellular functions, including proliferation, growth,
differentiation, survival, motility and intracellular trafficking
(17-21).
Previous studies demonstrated that C.
sinensis attenuates the apoptosis of renal tubular epithelial
cells following induction by angiotensin II and ischemia (22-24). However, there is no research on
the protective effect of C. sinensis on HBx-induced renal
tubular cell apoptosis. In this study, a cell line stably
expressing HBx was first established, then the role of the PI3K/Akt
signaling pathway on HBx-induced renal tubular cell apoptosis was
examined. The efficacy and mechanism of C. sinensis
attenuation of HBx-induced apoptosis in renal tubular cells was
subsequently investigated.
Materials and methods
Reagents
Rabbit polyclonal anti-PI3K, rabbit polyclonal
anti-phospho-(p-)p85 PI3K, rabbit polyclonal anti-Akt and rabbit
polyclonal anti-p-Akt (ser473) were obtained from Cell Signaling
Technology, Inc. Mouse monoclonal anti-HBx antibody was obtained
from Chemicon International; Thermo Fisher Scientific, Inc. Rabbit
polyclonal anti-Bcl-2 and rabbit polyclonal anti-Bax were obtained
from Santa Cruz Biotechnology, Inc. Cell Counting Kit-8 (CCK-8) was
obtained from Nanjing KGI Biological Technology Development Co.,
Ltd. Caspase-3 and -9 activity assay kit and LY294002 were obtained
from Sigma-Aldrich; Merck KGaA. An Annexin V-Fluorescein
Isothiocyanate (FITC) and propidium iodide (PI) double staining kit
were obtained from Nanjing KeyGen Biotech Co., Ltd. Artificially
cultured C. sinensis extract [trade name Corbrin capsule
(Bailing Jiaonang)] was provided by Hangzhou Zhongmei Huadong
Pharmaceutical Co., Ltd. and was dissolved in sterile distilled
water. The final working concentration was 40 mg/l according to
0.5% (v/v) dilution to the medium (24,25).
Cell culture
A human renal proximal tubular epithelial cell line
(HK-2) was obtained from the China Center for Type Culture
Collections. HK-2 cells were cultured in Dulbecco's modified
Eagle's medium (Sigma-Aldrich; Merck KGaA) containing 4.5 mM
glucose, 100 µg/ml streptomycin, 100 U/ml penicillin and 10%
fetal bovine serum in a humidified atmosphere of 5% CO2
at 37°C. Cells were detached using 0.25% trypsin and 0.02%
ethylenediaminetetraacetic acid. Before treatment, 80-85% of
confluent cells were cultured in serum-free media for 12 h to
arrest and synchronize the cell cycle. This method can block the
cell cycle in the G0/G1 phase and subsequent treatments and cell
cycle change analysis based on this method are more convincing
(26). In the present study, six
repetitions were conducted in each group in every experiment. All
experiments were repeated three times. In the C. sinensis
treatment group, cells were incubated with C. sinensis (40
mg/l) for 24 h. In the LY294002 (PI3K inhibitor) treatment
experiments, cells received 40 µmol/l LY294002 treatment for
60 min before C. sinensis induction (17).
Stable transfection
HK-2 cells were transfected with pCMV-HBx or
pCMV-tag2A (empty vector) with Lipofectamine™ LTX and PLUS™
transfection reagents (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. At 24 h after transfection, the cells
were diluted to 1:10 and cultured in growth medium containing G418
(600 µg/ml) for 3 weeks. Stable transfected clones were
chosen and maintained in a medium containing 300 µg/ml of
G418 for further studies.
Cell proliferation assay
Cell viability was assayed by a colorimetric
procedure using CCK-8 (Sigma-Aldrich; Merck KGaA) according to the
manufacturer's protocol. The absorbance at 450 nm was determined by
a microplate reader. The cell suspension was inoculated into
96-well plates (100 µl/well) and incubated overnight. CCK-8
(10 µl) was added to each well and the cells were
continuously cultured at 37°C for 2 h. The optical density value
(OD) at 450 nm was determined by a microplate reader. The cellular
survival rate was calculated according to the following formulas:
Survival rate (%)=(OD value of the test group/OD value of the
control group) ×100. Inhibition of proliferation rate
(%)=(1−survival rate) ×100.
Measurement of caspase-3 and -9
activity
HK-2 cells were harvested and centrifuged at 250 × g
at 4°C for 5 min. Cells were washed twice with PBS (pH 7.4) at 4°C,
then re-suspended in 50 µl cell lysis buffer at 4°C. All
steps were performed on ice. The protein concentration was measured
using a micro bicin-choninic acid (BCA) kit (Thermo Fisher
Scientific, Inc.). Each 50 µl cell extract (containing 100
µg protein) was combined with equal volumes of 2X reaction
buffer in a microplate and 5 µl peptide substrates of
caspase-3 and -9. After incubating overnight in the dark at 37°C,
the samples were examined using a microplate reader at 405 nm. The
activity of caspase-3 and -9 was calculated as the absorbance ratio
of treated/control samples.
Western blot analysis
Total cell proteins were extracted with
radioimmunoprecipitation assay lysate (Sigma-Aldrich; Merck KGaA)
containing phenylmethane sulfonyl fluoride. Protein concentration
was determined using the BCA kit according to the manufacturer's
protocol. Immunoblotting was performed with 50 µg protein,
which was separated using 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and transferred onto polyvinylidene fluoride
membranes (EMD Millipore). The membranes were blocked with 5%
fat-free milk or 5% bovine serum albumin (Sigma-Aldrich; Merck
KGaA) in TBST [Tris-buffered saline (TBS; pH 7.4) containing 0.21%
Tween-20] for 2 h at room temperature. The primary antibodies: HBx
(1:1,000; cat. no. MA1-081), t-PI3K (1:1,000; cat. no. 3011T),
p-p85 PI3K (1:1,000; cat. no. 4228T), t-Akt (1:1,000; cat. no.
4691S), p-Akt (ser473) (1:1,000; cat. no. 4060S), Bcl-2 (1:500;
cat. no. PRS3335), or Bax (1:500; cat. no. SAB4502546) were
respectively added and incubated at 4°C overnight. Next, the
secondary antibodies, anti-rabbit IgG (1:5,000; HRP-linked
antibody; cat. no. 7074S; Cell Signaling Technology, Inc.) or
anti-mouse IgG (1:5,000; HRP-linked antibody; cat. no. 7076S; Cell
Signaling Technology, Inc.), were added and incubated at room
temperature for 2 h. Bound proteins were visualized using
electrochemiluminescence (ECL kit; Pierce; Thermo Fisher
Scientific, Inc.) and detected using a DNR BioImaging system 3.2
(DNR Bio-Imaging Systems, Ltd.). β-actin (1:1,000; cat. no. 4970;
Cell Signaling Technology, Inc.) was used as an internal
control.
Hoechst 33342 staining
Morphological variations in the nuclei of apoptotic
cells were observed by staining with HO33342 (Sigma-Aldrich; Merck
KGaA). The cell slides were taken out, washed with PBS for three
times, fixed with 4% paraformaldehyde at 4°C for 20 min and washed
with PBS again for three times. After HO33342 fluorochrome (5 mg/l)
was added, the cell slides were incubated for 8 min at 37°C
(protected from light) and rewashed with PBS three times.
Observations and imaging were immediately conducted under a
fluorescence microscope. A total of 3 sections were selected in
each group and 20 fields of vision were randomly selected for each
section. The number of apoptotic cells and the total cells in each
random field were counted by two independent researchers. Then the
statistical analysis was performed. Finally, the equation
(apoptotic cells/total cells ×100%) was used to indicate the
apoptotic rate.
Flow cytometry analysis
The percentage of apoptotic cells was determined
using an Annexin V-FITC Apoptosis Detection kit (Nanjing KeyGen
Biotech Co., Ltd.), quantified by flow cytometry and analyzed with
BD CellQuest™ Pro (version S7; BD Biosciences). Cells were stained
at 4°C for 30 min according to the manufacturer's protocol and flow
cytometry was conducted using a FACScan flow cytometer
(Becton-Dickinson, and Company). Cells were divided into four
quadrants: Left lower quadrant (Annexin V-FITC− and
PI−, representing normal live cells), left upper
quadrant (Annexin V-FITC− and PI+,
representing cells with mechanical damages), right lower quadrant
(Annexin V-FITC+ and PI−, representing early
apoptotic cells), and right upper quadrant (Annexin
V-FITC+ and PI+, representing late apoptotic
cells). The results show the sums of early and late apoptosis.
Statistical analysis
All results are expressed as means ± standard
deviations from three independent experiments, and data were
analyzed with SPSS version 21.0 (IBM, Corp.). Results were compared
using analysis of variance (ANOVA). When the ANOVA indicated a
statistically significant difference, multiple comparisons were
performed using Tukey's. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of C. sinensis in HK-2 cells
stably expressing HBx
HK-2 cells stably expressing HBx were first
established by transfection with pCMV-HBx. In the present study,
the empty vector pCMV-tag2A was utilized as a negative control
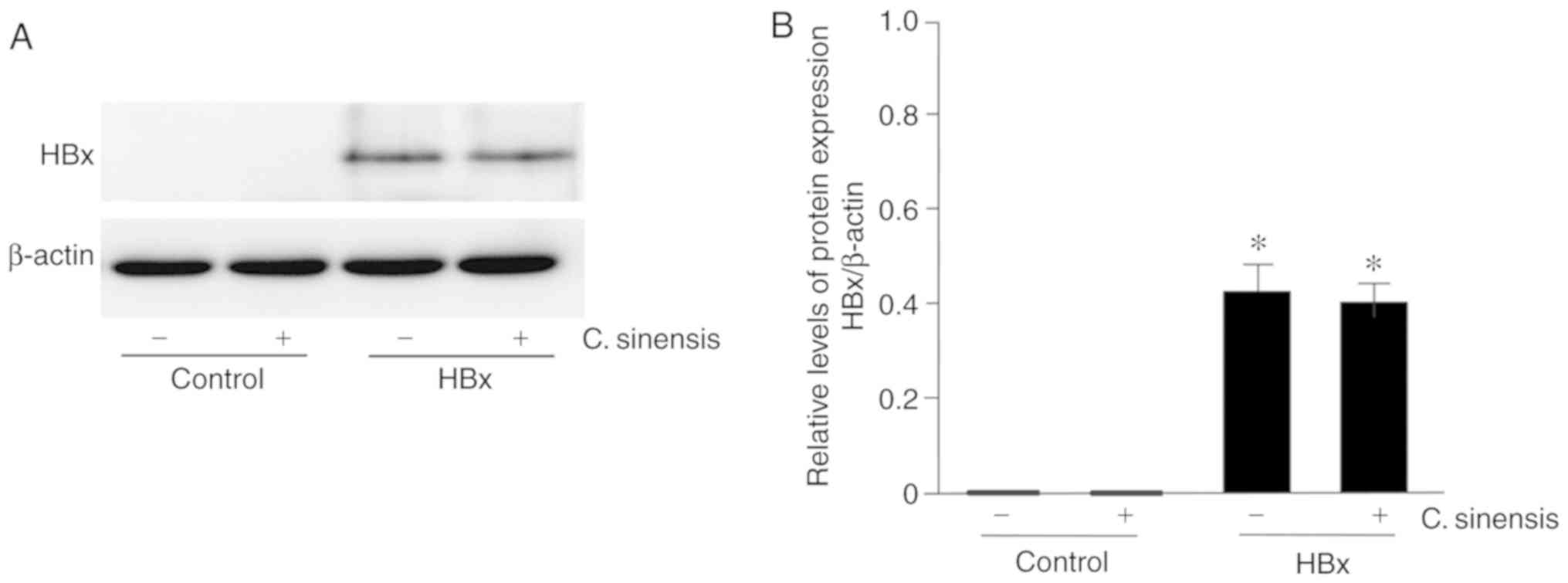
(Fig. 1). Western blotting
confirmed no expression of HBx in control cells; on the other hand,
a prominent protein band at 17 kDa, corresponding to the HBx
protein, was detected in lysates from cells stably expressing HBx.
Treatment with C. sinensis had no effect on the expression
of HBx (Fig. 1A and B).
C. sinensis attenuates HBx-induced
apoptosis
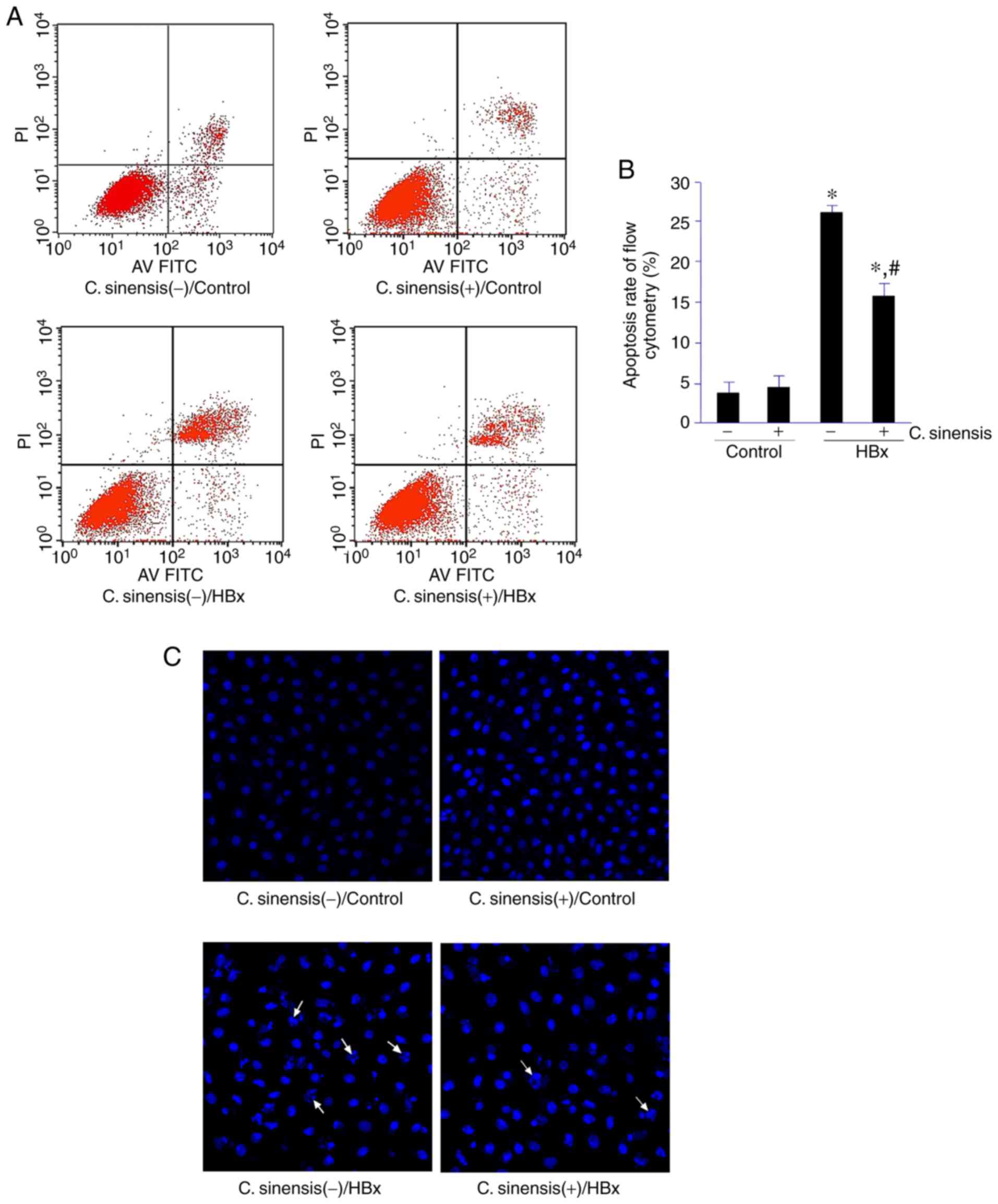
Cell apoptosis analyzed by flow cytometry indicated
that C. sinensis treatment had no effect on the apoptosis of
control cells (3.53±1.28 vs. 4.21±1.34%; Fig. 2A and C). By contrast, C.
sinensis treatment significantly inhibited the apoptosis of
cells in the HBx group (26.11±4.01 vs. 16.42±3.73%;
P<0.05). In the control cells, the nuclei of the HK-2 cells
showed clear outlines after HO33342 staining. In the HBx groups,
nuclear fragmentations were observed and dissolutions and chromatin
margination occurred in some nuclei. C. sinensis treatment
significantly improved the damage described above (Fig. 2B and D).
Upregulated caspase-3 activity (17.72±2.93%) and
caspase-9 activity (16.04±2.11%) were found in HBx-overexpressing
cells. In addition, C. sinensis treatment decreased the
activity of caspase-3 by 7.71±1.81% (P<0.05) and caspase-9 by
8.47±1.36% (P<0.05; Fig.
2E).
C. sinensis attenuates HBx-inhibited cell
proliferation
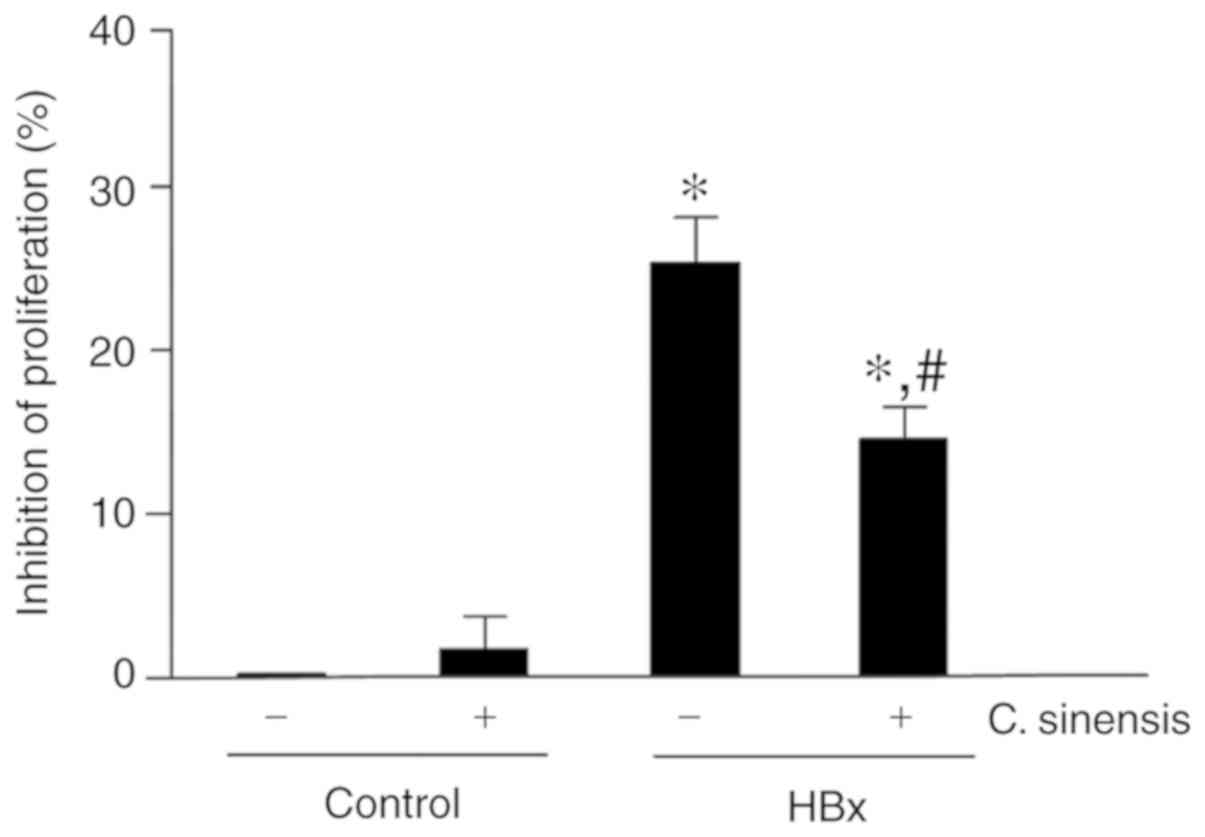
The effect of C. sinensis on proliferation
was examined in HBx-overexpressing cells. The results showed that
HBx over-expression inhibited cell proliferation by 25.27±2.14% and
C. sinensis treatment significantly attenuated this
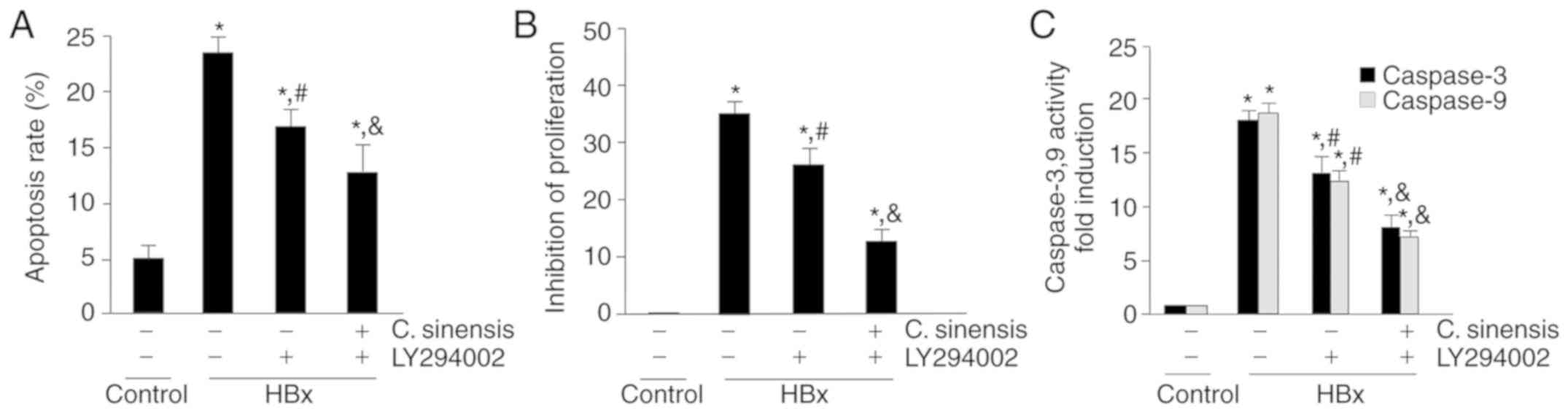
inhibitory effect (inhibition of 15.02±2.96%, P<0.05; Fig. 3).
C. sinensis treatment attenuates
HBx-induced activation of the PI3K/Akt pathway
The phosphorylation level of proteins involving in
the PI3K/Akt pathway was examined by western blotting analysis
(Fig. 4A and B). The results show
that HBx-overexpressing cells had significantly higher levels of
the phosphorylated forms of these proteins (P<0.05 for all
comparisons) and C. sinensis treatment significantly
attenuated this effect.
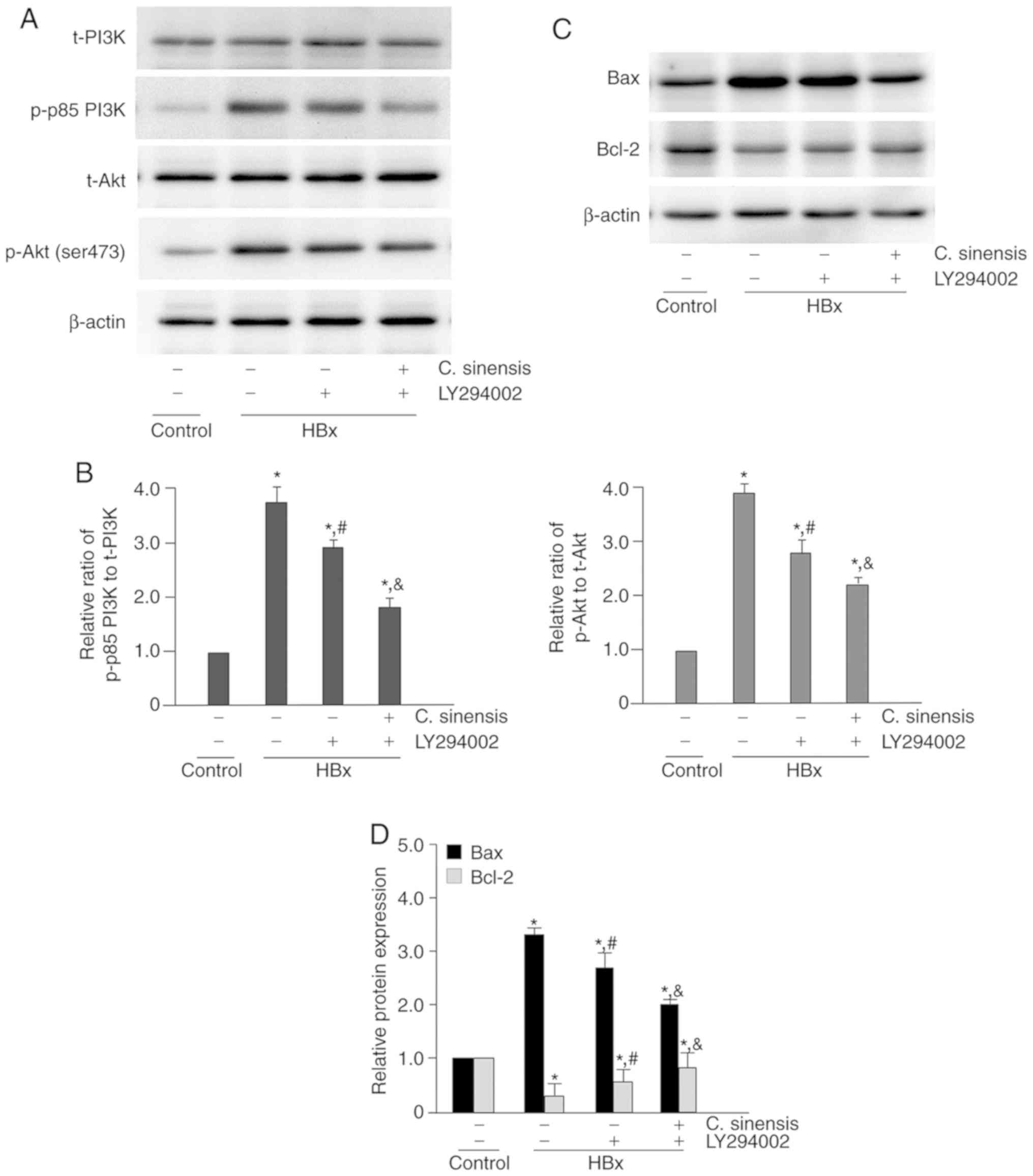
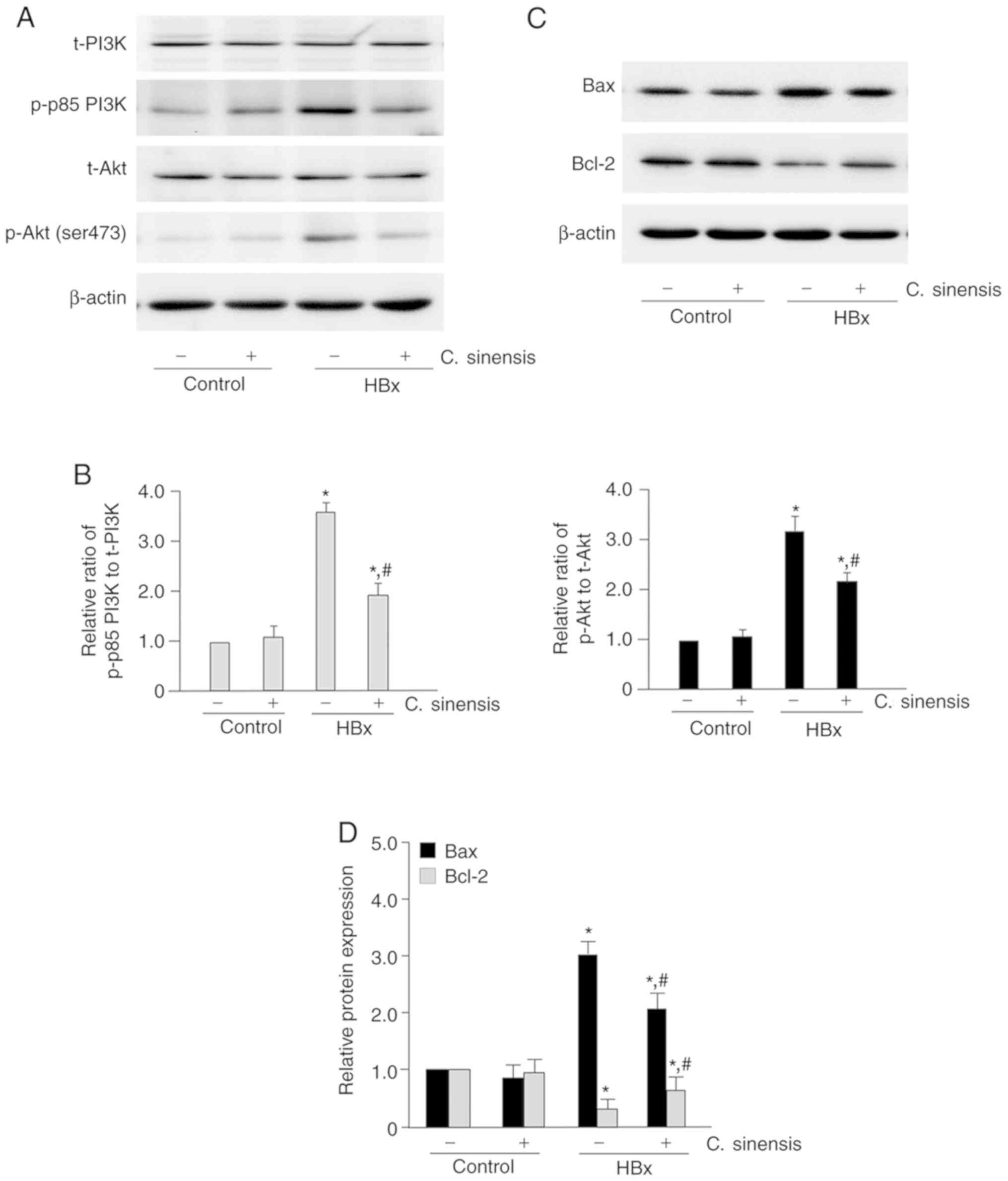 | Figure 4Effect of Cordyceps sinensis
treatment on the PI3K/Akt signaling pathway and expression of Bax
and Bcl-2. (A) Representative western blotting results of p-p85
PI3K, t-PI3K, p-Akt (ser473) and t-Akt in the control group, the
control/C. sinensis group, the HBx group, and the HBx/C.
sinensis group. In the C. sinensis treatment groups,
cells were incubated with C. sinensis (40 mg/l) for 24 h.
After treatment, total cell lysates were analyzed for the amount of
protein of p-p85 PI3K, t-PI3K, p-Akt (ser473) and t-Akt by western
blotting. (B) Quantitative results of western blotting. The ratios
of p-p85 PI3K to t-PI3K and p-Akt to t-Akt in the control group
were set as 1. The relative ratio of p-p85 PI3K to t-PI3K and p-Akt
to t-Akt in the other groups was compared with that of the control
group. (C) Representative western blotting of Bax and Bcl-2 in the
control group, the control/C. sinensis group, the HBx group,
and the HBx/C. sinensis group. (D) Quantitative results of
western blotting. The protein expression level of Bax and Bcl-2 in
the control group were set as 1. The relative expression level of
Bax and Bcl-2 in the other groups was compared with that of the
control group. *P<0.05 vs. the control group;
#P<0.05 vs. the HBx group. PI3K,
phosphatidylinositol-3-kinase; Akt, protein kinase B; p,
phosphorylated; t, total. |
Next, western blotting analysis was performed to
examine the expression level of Bcl-2 and Bax (apoptosis
regulators) in HK-2 cells (Fig. 4C
and D). The results indicated that HBx-overexpressing cells had
higher levels of Bax and lower levels of Bcl-2 (P<0.05 for both
comparisons). However, C. sinensis treatment significantly
attenuated these effects (P<0.05 for both comparisons).
The effect of the PI3K inhibitor LY294002 on
HBx-induced activation of the PI3K/Akt pathway was further
investigated (Fig. 5). The
results showed that LY294002 treatment alone significantly
attenuated the HBx-increased phosphorylated protein level in this
pathway (P<0.05 for all comparisons). In addition, treatment
with C. sinensis and LY294002 together led to further
attenuation (Fig. 5A and B).
LY294002 had similar effects on the expression of Bax and Bcl-2,
and C. sinensis treatment also increased this effect
(Fig. 5C and D).
Next, the role of the PI3K/Akt signaling pathway in
regulating the apoptosis of HBx-overexpressing cells was examined.
Consistent with above results, LY294002 attenuated HBx-induced
apoptosis and inhibited proliferation, and C. sinensis
treatment increased this effect (Fig.
6A and B). Similarly, treatment with LY294002 attenuated the
HBx-induced changes in the activity of caspase-3 and -9, and C.
sinensis treatment increased this effect (Fig. 6C). Taken together, the present
data indicate that C. sinensis treatment attenuates
HBx-induced apoptosis and inhibits proliferation by inhibiting the
PI3K/Akt pathway in HK-2 cells.
Discussion
Previous studies demonstrated that HBx is involved
in multiple cellular processes, including gene transcription,
repair of DNA damage, cell cycle, proliferation and apoptosis
(27,28). Zhang et al (29) reported that HBx inhibited
apoptosis. However, other studies reported that HBx enhanced
apoptosis by modulating other signaling molecules, such as c-FLIP
(30) and Hsp60 (31). These results suggest that HBx
promotes or inhibits cell apoptosis, depending on the cell type and
the experimental conditions. In this study, it was found that
overexpression of HBx significantly increased apoptosis in HK-2
cells. These results are consistent with those of the authors'
previous study (13).
C. sinensis is a Chinese herbal medicine
commonly used to improve kidney function and to treat renal
dysfunction/failure. Specifically, the efficacy of co-treatment
with C. sinensis in patients with chronic allograft
nephropathy is superior to that of immunosuppressive drugs alone
and C. sinensis has been used to treat chronic allograft
nephropathy and in long-term therapy in China (4,32,33). The present study showed that C.
sinensis treatment attenuated HBx-induced apoptosis. It is well
known that PI3K/Akt signaling can regulate cell apoptosis in
numerous types of cells (16,34). Therefore, western blotting
analysis was used to measure the activity of the PI3K/Akt signaling
pathway, such as the phosphorylation of PI3K and Akt in
HBx-transfected HK-2 cells. The results showed that HBx
transfection activated the PI3K/Akt signaling pathway but C.
sinensis treatment attenuated the HBx-induced PI3K/Akt
signaling. In addition, C. sinensis treatment attenuated
HBx-induced upregulation of Bax and Bcl-2, both of which play
important roles in apoptosis. These data indicate that C.
sinensis attenuates HBx-enhanced apoptosis in HK-2 cells
through suppressing the PI3K/Akt/Bcl-2 pathway.
The P13K/Akt signaling pathway is involved in the
regulation of cell proliferation, differentiation, apoptosis and
glucose transport. The regulation of apoptosis by the P13K/Akt
signaling pathway is very complex. In different cells and
environments, its effect can be either anti-apoptotic or
pro-apoptotic. To confirm that C. sinensis decreases
apoptosis by suppressing the PI3K/Akt pathway, a PI3K inhibitor,
LY294002, was utilized to suppress PI3K activity in HBx-over
expressing cells. LY294002 is mostly known to inactivate Akt,
consequently inhibiting cell proliferation and inducing apoptosis.
This study indeed observed that LY294002 ameliorated HBx-induced
apoptosis by inhibiting the activation of PI3K/Akt. It was found,
in several related studies, that under different conditions or in
different cell types, LY294002 treatment can reduce cell apoptosis
by inhibiting the activation of PI3K/Akt signaling (35,36). This result suggests that the
double-sided character of LY294002 in promoting or inhibiting cell
apoptosis depends on the experimental cell type and its
environmental conditions. In this context, the present results
indicate that LY294002 and C. sinensis act synergistically
to attenuate HBx-enhanced apoptosis. This is the first study to the
best of our knowledge to show that C. sinensis attenuates
PI3K/Akt signaling in stable HBx-overexpressing HK-2 cells.
Cysteine proteases play a critical role in apoptosis
regulation. Previous studies indicated that caspase-8, -9 and -10
couple cell death stimuli to the downstream effector caspases,
including caspase-3, -6, and -7, to initiate the apoptosis process
(37). Once apoptosis is
triggered, cellular proteins are cleaved at specific aspartate
residues by the effector caspases (38,39). Other studies have reported that
HBx over-expression promotes the activation of caspase-3 and -9
(40,41). Thus, activation of caspase-3 and
-9 was examined in stable HBx-overexpressing HK -2 cells treated
with LY294002 and C. sinensis. The current results showed
that LY294002 and C. sinensis attenuated caspase-3 and -9
activity in these HBx-overexpressing cells in an additive manner.
Thus, caspase-3 and -9 activation is required for PI3K/Akt-induced
apoptosis in HBx-overexpressing HK-2 cells.
In conclusion, the present results demonstrated that
HBx induces apoptosis in stable HBx-overexpressing HK-2 cells
through PI3K/Akt-Bcl-2 signaling cascades. Treatment with C.
sinensis significantly attenuated HBx-enhanced apoptosis, at
least partly by suppressing the PI3K/Akt-Bcl-2 pathway. Therefore,
the current study provides a molecular basis for C. sinensis
as a treatment option for HBV-GN. Further studies should focus on
this regulatory circuit in other in vitro and in vivo
models. In the present study, the relationship between inflammation
and HBx-induced apoptosis in HK-2 cells was not investigated.
Future studies should seek to characterize the role of inflammation
governing this process. In addition, the current team is also
working on another cell line, the rat renal tubular epithelial cell
NRK52E. Preliminary results showed that C. sinensis could
also improve the apoptosis induced in these cells by HBx by
inhibiting PI3K/Akt signaling. The final data are still being
assembled for statistical analysis. It is anticipated that the
results will be consistent with those of this study.
Funding
The present study was supported by the 345 Talent
Project of Shengjing Hospital of China Medical University (grant
no. 201940B), the Liaoning Province Key Research and Development
Guidance Project (grant no. 2018225008), the Dr Start Fund of
Liaoning Province of China (grant no. 201501005), and the Education
Commission of Liaoning Province of China (grant no. L2013295).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PH and JL were responsible for the cell culture and
treatments; CCK-8 experiments and analyses; and for figure
preparation. JNM performed the Hoechst 33342 staining and analyses.
DW performed the flow cytometry analysis. PH performed the
statistical analysis of the data. PH, DW, JNM and JL prepared the
images and revised the manuscript. PH and CW performed the western
blot experiments. JL prepared the Cordyceps sinensis for the
experiments. PH and CW performed transfection and cell selection.
PH conceived the study. PH and JL wrote the manuscript. All authors
reviewed and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Li SP, Zhao KJ, Ji ZN, Song ZH, Dong TT,
Lo CK, Cheung JK, Zhu SQ and Tsim KW: A polysaccharide isolated
from Cordyceps sinensis, a traditional Chinese medicine, protects
PC12 cells against hydrogen peroxide-induced injury. Life Sci.
73:2503–2513. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jordan JL, Sullivan AM and Lee TD: Immune
activation by a sterile aqueous extract of Cordyceps sinensis:
Mechanism of action. Immunopharmacol Immunotoxicol. 30:53–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li SP, Li P, Dong TT and Tsim KW:
Anti-oxidation activity of different types of natural Cordyceps
sinensis and cultured Cordyceps mycelia. Phytomedicine. 8:207–212.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du F, Li S, Wang T, Zhang HY, Zong ZH, Du
ZX, Li DT, Wang HQ, Liu B, Miao JN and Bian XH: Cordyceps sinensis
attenuates renal fibrosis and suppresses BAG3 induction in
obstructed rat kidney. Am J Transl Res. 7:932–940. 2015.PubMed/NCBI
|
|
5
|
Xu H, Sun L, Zhou LJ, Fang LJ, Sheng FY
and Guo YQ: The effect of hepatitis B vaccination on the incidence
of childhood HBV-associated nephritis. Pediatr Nephrol.
18:1216–1219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Venkataseshan VS, Lieberman K, Kim DU,
Thung SN, Dikman S, D'Agati V, Susin M, Valderrama E, Gauthier B,
Prakash A, et al: Hepatitis-B-associated glomerulonephritis:
Pathology, pathogenesis, and clinical course. Medicine. 69:200–216.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He XY, Fang LJ, Zhang YE, Sheng FY, Zhang
XR and Guo MY: In situ hybridization of hepatitis B DNA in
hepatitis B-associated glomerulonephritis. Pediatr Nephrol.
12:117–120. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lai KN, Ho RT, Tam JS and Lai FM:
Detection of hepatitis B virus DNA and RNA in kidneys of HBV
related glomerulonephritis. Kidney Int. 50:1965–1977. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He P, Zhou G, Qu D, Zhang B, Wang Y and Li
D: HBx inhibits proliferation and induces apoptosis via Fas/FasL
upregulation in rat renal tubular epithelial cells. J Nephrol.
26:1033–1041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bouchard MJ and Schneider RJ: The
enigmatic X gene of hepatitis B virus. J Virol. 78:12725–12734.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arbuthnot P, Capovilla A and Kew M:
Putative role of hepatitis B virus X protein in
hepatocarcinogenesis: Effects on apoptosis, DNA repair,
mitogen-activated protein kinase and JAK/STAT pathways. J
Gastroenterol Hepatol. 15:357–368. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee YI, Kang-Park S, Do SI and Lee YI: The
hepatitis B virus-X protein activates a phosphatidylinositol
3-kinase-dependent survival signaling cascade. J Biol Chem.
276:16969–16977. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He P, Zhang D, Li H, Yang X, Li D, Zhai Y,
Ma L and Feng G: Hepatitis B virus X protein modulates apoptosis in
human renal proximal tubular epithelial cells by activating the
JAK2/STAT3 signaling pathway. Int J Mol Med. 31:1017–1029. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He P, Zhang B, Liu D, Bian X, Li D, Wang
Y, Sun G and Zhou G: Hepatitis B virus x protein modulates
apoptosis in NRK-52E cells and activates fas/fasl through the
MLK3-MKK7-JNK3 signaling pathway. Cell Physiol Biochem.
39:1433–1443. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Szliszka E, Zydowicz G, Janoszka B, Dobosz
C, Kowalczyk-Ziomek G and Krol W: Ethanolic extract of Brazilian
green propolis sensitizes prostate cancer cells to TRAIL-induced
apoptosis. Int J Oncol. 38:941–953. 2011.PubMed/NCBI
|
|
16
|
Wang P, Guo QS, Wang ZW and Qian HX: HBx
induces HepG-2 cells autophagy through PI3K/Akt-mTOR pathway. Mol
Cell Biochem. 372:161–168. 2013. View Article : Google Scholar
|
|
17
|
Qiu XM, Bai X, Jiang HF, He P and Wang JH:
20-(s)-ginsenoside Rg3 induces apoptotic cell death in human
leukemic U937 and HL-60 cells through PI3K/Akt pathways.
Anti-Cancer Drugs. 25:1072–1080. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cantley LC and Neel BG: New insights into
tumor suppression: PTEN suppresses tumor formation by restraining
the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA.
96:4240–4245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang X, Fraser M, Moll UM, Basak A and
Tsang BK: Akt-mediated cisplatin resistance in ovarian cancer:
Modulation of p53 action on caspase-dependent mitochondrial death
pathway. Cancer Res. 66:3126–3136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Altomare DA, Wang HQ, Skele KL, De Rienzo
A, Klein-Szanto AJ, Godwin AK and Testa JR: AKT and mTOR
phosphorylation is frequently detected in ovarian cancer and can be
targeted to disrupt ovarian tumor cell growth. Oncogene.
23:5853–5857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou Q and Hu S: Effect of Cordyceps
Cinensis extractant on apoptosis and expression of Toll-like
receptor 4 mRNA in the ischemia-reperfusion injured NRK-52E cells.
Zhong Nan Da Xue Xue Bao Yi Xue Ban (Chinese). 35:77–84. 2010.
|
|
23
|
Tang R, Zhou Q, Shu J, Tang T, Ao X, Peng
W and Zhang Y: Effect of cordyceps sinensis extract on Klotho
expression and apoptosis in renal tubular epithelial cells induced
by angiotensin II. Zhong Nan Da Xue Xue Bao Yi Xue Ban (Chinese).
34:300–307. 2009.
|
|
24
|
Tu S, Zhou Q, Tang R, Tang T, Hu S and Ao
X: Proapoptotic effect of angiotensin II on renal tubular
epithelial cells and protective effect of Cordyceps sinensis. Zhong
Nan Da Xue Xue Bao Yi Xue Ban (Chinese). 37:67–72. 2012.
|
|
25
|
Yang CH, Kao YH, Huang KS, Wang CY and Lin
LW: Cordyceps militaris and mycelial fermentation induced apoptosis
and autophagy of human glioblastoma cells. Cell Death Dis.
3:e4312012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goissis MD, Caetano HV, Marques MG, de
Barros FR, Feitosa WB, Milazzotto MP, Binelli M, Assumpcao ME and
Visintin JA: Effects of serum deprivation and cycloheximide on cell
cycle of low and high passage porcine fetal fibroblasts. Reprod
Domes Anim. 42:660–663. 2007. View Article : Google Scholar
|
|
27
|
Chung TW, Lee YC and Kim CH: Hepatitis B
viral HBx induces matrix metalloproteinase-9 gene expression
through activation of ERK and PI-3K/AKT pathways: Involvement of
invasive potential. FASEB J. 18:1123–1125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang FZ, Fei HR, Lian LH, Wang JM and Qiu
YY: Hepatitis B x-interacting protein induces HepG2 cell
proliferation through activation of the phosphatidylinositol
3-kinase/Akt pathway. Exp Biol Med (Maywood). 236:62–69. 2011.
View Article : Google Scholar
|
|
29
|
Zhang X, Dong N, Yin L, Cai N, Ma H, You
J, Zhang H, Wang H, He R and Ye L: Hepatitis B virus X protein
upregulates survivin expression in hepatoma tissues. J Med Virol.
77:374–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim KH and Seong BL: Pro-apoptotic
function of HBV X protein is mediated by interaction with c-FLIP
and enhancement of death-inducing signal. EMBO J. 22:2104–2116.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Satoh T, Enokido Y, Aoshima H, Uchiyama Y
and Hatanaka H: Changes in mitochondrial membrane potential during
oxidative stress-induced apoptosis in PC12 cells. J Neurosci Res.
50:413–420. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Z, Wang X, Zhang Y and Ye G: Effect
of Cordyceps sinensis on renal function of patients with chronic
allograft nephropathy. Urol Int. 86:298–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ding C, Tian PX, Xue W, Ding X, Yan H, Pan
X, Feng X, Xiang H, Hou J and Tian X: Efficacy of Cordyceps
sinensis in long term treatment of renal transplant patients. Front
Biosci (Elite Ed). 3:301–307. 2011.
|
|
34
|
Wang W, Shi Y, Bai G, Tang Y, Yuan Y,
Zhang T and Li C: HBxAg suppresses apoptosis of human placental
trophoblastic cell lines via activation of the PI3K/Akt pathway.
Cell Biol Int. 40:708–715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou M, Shen S, Zhao X and Gong X:
Luteoloside induces G0/G1 arrest and pro-death autophagy through
the ROS-mediated AKT/mTOR/p70S6K signalling pathway in human
non-small cell lung cancer cell lines. Biochem Biophys Res Commun.
494:263–269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Liu Y, Shi F, Cheng L and She J:
Knockdown of rap1b enhances apoptosis and autophagy in gastric
cancer cells via the PI3K/Akt/mTOR pathway. Oncol Res. 24:287–293.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim MN, Lee KE, Hong JY, Heo WI, Kim KW,
Kim KE and Sohn MH: Involvement of the MAPK and PI3K pathways in
chitinase 3-like 1-regulated hyperoxia-induced airway epithelial
cell death. Biochem Biophys Res Commun. 421:790–796. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Degterev A and Yuan J: Expansion and
evolution of cell death programmes. Nat Rev Mol Cell Biol.
9:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Walsh JG, Cullen SP, Sheridan C, Luthi AU,
Gerner C and Martin SJ: Executioner caspase-3 and caspase-7 are
functionally distinct proteases. Proc Natl Acad Sci USA.
105:12815–12819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choi JH, Jeong H and Jang KL: Hepatitis B
virus X protein suppresses all-trans retinoic acid-induced
apoptosis in human hepatocytes by repressing p14 expression via DNA
methylation. J Gen Virol. 98:2786–2798. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fei H, Zhou Y, Li R, Yang M, Ma J and Wang
F: HBXIP, a binding protein of HBx, regulates maintenance of the
G2/M phase checkpoint induced by DNA damage and enhances
sensitivity to doxorubicin-induced cytotoxicity. Cell Cycle.
16:468–476. 2017. View Article : Google Scholar : PubMed/NCBI
|