Introduction
Sepsis is a systemic inflammatory response syndrome
caused by infection (1). The
common clinical features of this disease are fever, tachycardia,
shortness of breath and increased peripheral blood leukocytes
(1). Sepsis involves various
pathophysiological mechanisms, such as cell injury, apoptosis,
oxidative stress and mitochondrial dysfunction, which are
associated with the occurrence of renal injury (2). The probability of acute renal injury
in patients with severe sepsis is 60% (3). The clinical diagnosis of acute
kidney injury (AKI) can be determined by the increase of blood urea
nitrogen and serum creatinine (4). However, the clinical outcome of this
disease remains unsatisfactory as recent data has revealed that the
mortality rate of patients with AKI is ≤22% (5). Therefore, it is necessary to develop
an effective therapeutic strategy for acute kidney damage caused by
sepsis.
Long non-coding RNA (lncRNA) is a class of
non-protein coding RNAs that regulate the expression of genes at
the transcriptional or post-transcriptional level (6). Recent studies have demonstrated that
lncRNAs serve important roles in cell biology, including cell
proliferation, differentiation, development, invasion, migration
and apoptosis (7). An increasing
number of studies have demonstrated that lncRNAs, such as H19
(8), MALAT1 (9), SNHG16 (10) and NEAT1 (9), are involved in sepsis-induced
AKI.
The lncRNA cancer susceptibility candidate 2
(CASC2), located on chromosome 10q26, was originally reported to be
downregulated in the endometrium (11). Subsequent studies have shown that
CASC2 is involved in several human diseases (12). Notably, CASC2 can act as a tumor
suppressor gene, which is able to inhibit cell proliferation,
invasion and metastasis, and promote cell apoptosis in multiple
human types of cancer, such as pancreatic carcinoma, papillary
thyroid carcinoma and gastric cancer (13). Certain studies have recommended
CASC2 as a good prognostic biomarker for tumors, such as
hepatocellular carcinoma and pancreatic cancer (11,14–16). Another study has revealed that
CASC2 expression is significantly reduced in renal cell carcinoma
(17). However, to the best of
our knowledge, whether CASC2 is involved in sepsis-induced AKI has
not been studied. The aim of the present study was to investigate
the effect of CASC2 on sepsis-induced AKI, as well as the
underlying molecular mechanism.
Materials and methods
Sample collection
Blood samples were obtained from 20 healthy
volunteers and 69 patients with sepsis and AKI after clinical
diagnosis following the standards of the American College of Chest
Physicians/Society of Critical Care Medicine consensus conference.
All patients with sepsis-induced AKI and healthy volunteers from
the Huai’an no. 1 People’s Hospital of Nanjing Medical University
were enrolled in this study between May 2015 and June 2018. The
patient group included 30 males and 39 females, with a mean age of
43.4 (range, 23–72) years. Healthy volunteers included 8 males and
12 females, with a mean age of 45 (range, 25–58) years. All
participants provided written informed consent, and ethical
approval was granted by the Ethics Committee of Nanjing Medical
University (Nanjing, China).
Cell culture
A human renal tubular epithelial HK-2 cell line was
purchased from the American Type Culture Collection, and the 293
cell line was obtained from the Cell Bank of Chinese Academy of
Sciences. HK-2 cells were cultured in keratinocyte serum
immunization medium containing L-glutamine (Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin
(HyClone; GE Healthcare Life Sciences), and 293 cells were cultured
in DMEM (high glucose; Thermo Fisher Scientific, Inc.) supplemented
with 10% FBS (HyClone; GE Healthcare Life Sciences). To establish
an in vitro lipopolysaccharide (LPS)-induced sepsis model,
HK-2 cells were treated with 1 μg/ml LPS (Sigma-Aldrich; Merck
KGaA) for 24 h.
Animals and grouping
A total of 20 male BALB/c mice (6–8 weeks old; 22–25
g) were purchased from Beijing HFK Biotechnology. Mice were
maintained in a SPF-grade room at 18–22°C and 40–60% humidity under
a light/dark cycle of 12/12 h with free access to food and water.
The mice were randomly divided into two groups after one week of
feeding (sham surgery group, n=10; and model group, n=10). The
sepsis mouse model was established as previously described
(18). Briefly, mice were
anesthetized with ketamine-xylazine (20–25 mg/kg body weight) and
injected intraperitoneally with 15 mg/kg LPS. The control group was
injected with the same amount of saline. All animal experiments
were approved by the Animal Ethics Committee of the Nanjing Medical
University and performed in accordance with the Guide for the Care
and Use of Laboratory Animals.
Blood biochemical index
The blood samples of the mice were collected and
centrifuged at 3,000 x g for 10 min at 4°C to obtain the serum.
Then, the blood biochemical indicators were detected using a
diagnostic kit and a biochemical analyzer.
Cell transfection and nuclear factor κ-B
(NF-κB) inhibitor treatment
The CASC2 overexpression plasmid was constructed
using the pcNDA3 expression vector (pc-CASC2) (Guangzhou Ribobio
Co. Ltd). The mircRNA-155 (miR-155) mimic and inhibitor, as well as
the corresponding negative control (NC), were purchased from
Guangzhou Ribobio Co., Ltd., and the sequences were as follows:
miR-155 forward, 5′-ACGCTCAGTTAATGCTAATCGTGATA-3′ and reverse,
5′-ATTCCATGTTGTCCACTGTCTCTG-3′. The cells were transfected with
miR-155 (1 μg/well) or negative control (1 μg/well) using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Following 48-h transfection, cells were
subjected to subsequent experiments. The NF-κB inhibitor QNZ
(EVP4593) was purchased from MedChem Express, and the final
concentration was 10 μM.
MTT assay
HK-2 cells (1x104) were seeded in 96-well
plates. After 12 h of culture, cells were treated with or without
LPS for 24 h and then washed three times in PBS. Subsequently, 500
μg/ml MTT (Merck KGaA) was added to each well. A total of three h
later, 200 μl dimethyl sulfoxide solution (Merck KGaA) was added.
Finally, the samples were measured at 570 nm using a microplate
reader (Bio-Rad Laboratories, Inc.).
Cell apoptosis analysis
Cell apoptosis was measured using the Annexin
V-FITC/7-AAD kit (BD Biosciences; Becton, Dickinson and Company)
according to the manufacturer’s protocol. Samples were analyzed
using a FACSCanto flow cytometer (BD Bioscience) Data analysis was
performed using the FlowJo10.0 software (TreeStar, Inc.).
ELISA
Mouse peripheral blood (1 ml) was collected by
enucleating eyeballs prior to euthanasia and centrifuged at 10,000
x g for 10 min at 4°C. Subsequently, the serum was used to detect
the concentration of tumor necrosis factor (TNF)-α (cat. no.
BMS607-3), interleukin (IL)-6 (cat. no. BMS603-2), IL-8 (cat. no.
BMS6001) and IL-1β (cat. no. BMS6002) using ELISA kits (Thermo
Fisher Scientific, Inc.).
Superoxide measurement
HK-2 cells treated with or without LPS were
incubated for 1 h and then were collected. Following centrifuged at
300 x g for 5 min at 4°C, the pellet was resuspended in 900 μl
Krebs buffer containing 5 mmol KCl, 130 mmol NaCl, 1 mmol
MgCl2, 1 mmol K2HPO4, 5 mmol
CaCl2 and 20 mmol HEPES (pH 7.4), supplemented with 1
mg/ml bovine serum albumin (Sigma-Aldrich; Merck KGaA).
Subsequently, the samples were transferred into a measuring
chamber. Finally, the suspension was mixed with 100 μl lucigenin
(final concentration 4x104 mmol/l) and evaluated using a
chemiluminescence analyzer.
Detection of nitrite
The nitrite content in HK-2 medium was measured
using the Measure-iTTM High-Sensitivity Nitrite Assay kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer’s
protocol.
RT-qPCR
Total RNA was isolated from the cells using the
TRIzol reagent (Thermo Fisher Scientific, Inc.). For the detection
of CASC2, cDNA was synthesized from 1 μg RNA using the PrimeScript
RT reagent kit (Takara Bio, Inc.) under the following conditions:
42°C for 2 min, 37°C for 15 min and 85°C for 5 sec. QPCR was
performed using a SYBR Premix Ex Taq kit (Takara Bio, Inc.) with a
three-step PCR protocol. The thermocycling conditions were as
follows: 95°C for 2 min, followed by 38 cycles of 95°C for 5 sec,
60°C for 30 sec and 72°C for 30 sec. GAPDH served as an internal
reference gene. The primers used were as follows: CASC2 forward,
5′-TACAGGACATCAGTGGTGGT-3′ and reverse,
5′-ACATCTAGCTTAGGAATGTGGC-3′; GAPDH forward,
5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse,
5′-TGTAGACCATGTAGTTGAGGTCA-3′. For the detection of miR-155, cDNA
was generated using the TaqMan MicroRNA Reverse Transcription kit
(Thermo Fisher Scientific, Inc.) at 42°C for 60 min and 95°C for 3
min. Subsequently, qPCR was performed using a TaqMan Universal
Master Mix II kit (Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 94°C for 3 min, followed
by 30 cycles of 95°C for 30 sec, 55°C for 30 sec and 72°C for 1 min
and a final extension at 72°C for 3 min. U6 snRNA served as an
internal reference gene. The following primers were used: miR-155
forward, 5′-TTAATGCTAATCGTGATAGGGG-3′ and reverse,
5′-TCATGCCGTTAGGTAGCGTA-3′; U6 snRNA forward,
5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse,
5′-GGAACGCTTCACGAATTTG-3′. The relative expression of lncRNA or
miRNA were normalized to GAPDH or U6 using a 2−ΔΔCq
method as previously described (19).
Western blotting
This assay was performed as previously described
(20). Primary antibodies against
human p-IκBαSer32 (14D4; 1:1,000; cat. no. 2859S; Cell
Signaling Technology, Inc.), IκBα (ab32518, Abcam), p50 (E381;
1:1,000; cat. no. ab32360), p65 (E379; 1:1,000; cat. no. ab32536)
and GAPDH (6C5; 1:1,000; cat. no. ab8245; Abcam) were used.
Histological examination
The kidney was fixed in 4% paraformaldehyde (4°C, 24
h) and embedded in paraffin. Sections (4-μm thick) were cut and
processed, and then stained with hematoxylin for 5 min at room
temperature, differentiated by 0.6% hydrochloric alcohol for 30
sec, stained with eosin (1%) at room temperature for 1 min,
sterilized with 80% ethanol for 2 min, 95% ethanol for 2 min, 100%
ethanol for 2 min, 100% ethanol for 2 min. Finally, samples were
visualization by light microscopy (magnification, x400; Olympus
Corporation).
Electrophoretic mobility shift assay
(EMSA)
The NF-κB activity was measured by EMSA as
previously described (21).
Briefly, HK-2 cells were pretreated with AP (1 mg/ml) and then
treated with LPS (10 mg/ml) for 30 min. Subsequently, nuclear
extracts isolated from these cells were mixed with binding buffer
[20 mM HEPES-NaOH (pH 7.9), 100 mM NaCl, 10% glycerol, 2 mM EDTA
and 0.2% NP-40], Poly(dI-dC) and 32P-labelled NF-κB oligonucleotide
probes (Promega Corporation) for 30 min at room temperature.
Finally, the DNA-nuclear protein complexes were separated using 10%
PAGE, followed by DNA binding detection by autoradiography.
Luciferase reporter assay
Luciferase reporter assay was performed on 293 cells
using dual-luciferase reporter assay kit (Promega Corporation).
Cells were transfected with Luc reporter plasmid containing
wild-type or mutant CASC2 (synthesized by Shanghai Sangon Biotech
Co., Ltd.) using Lipofectamine® 3000 (Thermo Fisher
Scientific, Inc.), according to the manufacturer’s protocol.
Luciferase activities were measured at 48 h post-transfection.
Renilla luciferase was used as an internal reference.
Bioinformatics
The prediction of the interaction between CASC2 and
miR-155 was performed using Starbase 3.0 (http://starbase.sysu.edu.cn).
Statistical analysis
All data analyses were carried out using SPSS19.0
software (IBM Corp.) and all graphs were made using Graph Prism 6.0
software (GraphPad Software, Inc.). Student’s t-test was used to
compare the differences between two groups and one-way analysis of
variance followed by Tukey-Kramer post hoc test was used to compare
the differences among multiple groups. All experiments were
performed three times and the obtained data are expressed as the
mean ± standard error of mean. *P<0.05 was considered
to indicate a statistically significant difference.
Results
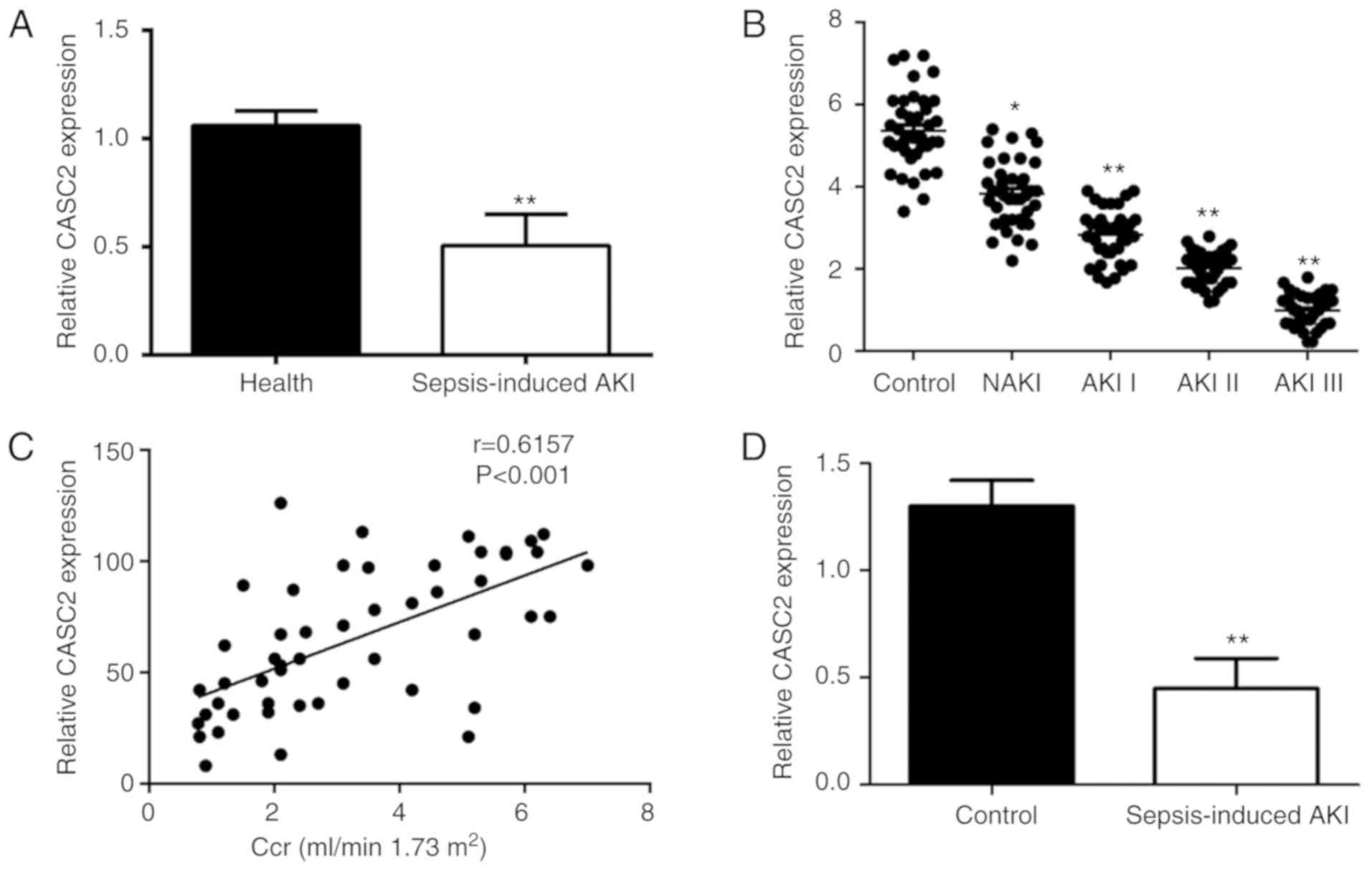
CASC2 expression is significantly
decreased in sepsis-induced AKI
To investigate the role of the lncRNA CASC2 in
sepsis, the expression of CASC2 mRNA was first measured in serum
obtained from sepsis patients and control groups by RT-qPCR
analysis. It was found that, compared with the control group, the
expression level of CASC2 was significantly decreased in sepsis
patients (Fig. 1A).
Interestingly, the present study observed that the expression level
of CASC2 decreased with the severity of AKI (Fig. 1B) and was inversely associated
with the level of creatinine clearance rate (Fig. 1C). Next, a sepsis-induced AKI
mouse model was established and confirmed by the pathological
changes observed in the kidneys and certain biochemical indicators
of blood (Fig. S1). Consistently,
the expression of CASC2 is significantly downregulated in mice with
AKI caused by sepsis (Fig. 1D).
The above data suggest that CASC2 may play a protective role in
sepsis-induced AKI.
CASC2 promotes cell viability and
inhibits inflammatory factor secretion, apoptosis and oxidative
stress in LPS-stimulated HK-2 cells
To assess the extract function of CASC2 in
sepsis-induced AKI, LPS was used to induce sepsis in a cell model.
Then, the CASC2 overexpression plasmid was transfected into HK-2
cells (Fig. 2A). The effect of
CASC2 upregulation on cell viability, apoptosis, inflammatory
factor expression and oxidative stress in LPS-stimulated HK-2 was
examined. It was found that the overexpression of CASC2 increased
cell viability (Fig. 2B) and
reduced the percentage of apoptotic cells in LPS-treated HK-2 cells
(Fig. 2C). The results of the
ELISA showed that the concentration of TNF-α, IL-6, IL-8 and IL-1β
in the cell suspension was significantly increased following LPS
stimulation, while the overexpression of CASC2 significantly
decreased the secretion of these inflammatory factors (Fig. 2D). In addition, the present study
observed that CASC2 overexpression significantly suppressed the
production of superoxide and nitrite in HK-2 cells after LPS
stimulation (Fig. 2E and F).
However, CASC2 overexpression did not have a significant effect on
normal HK-2 cells (Fig. 2).
Altogether, these results demonstrate that the upregulation of
CASC2 may contribute to the inhibition of the inflammatory
response, apoptosis and oxidative stress in sepsis-induced AKI.
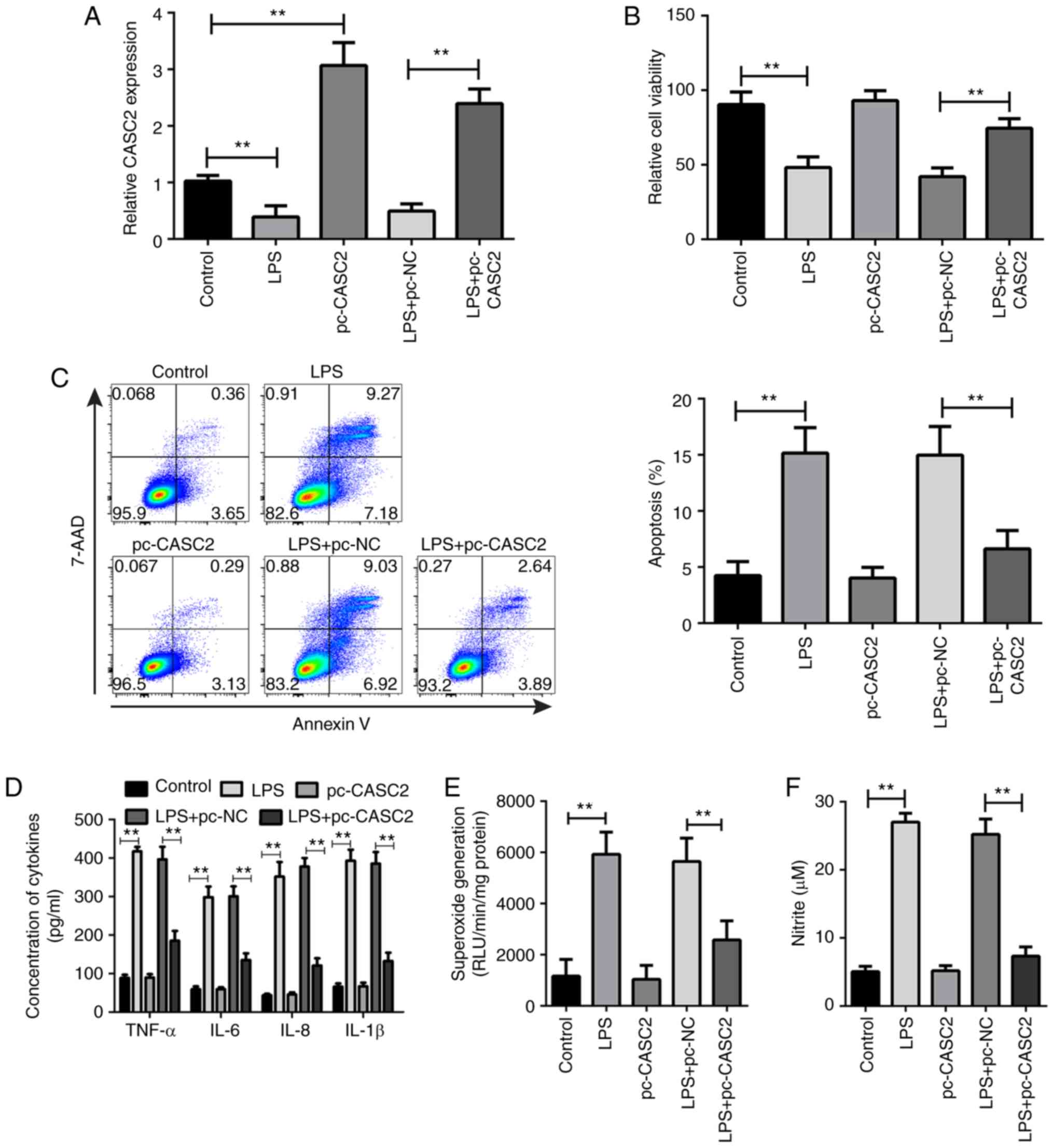 | Figure 2CASC2 promotes cell viability and
inhibits inflammatory factors secretion, apoptosis and oxidative
stress in LPS-stimulated HK-2 cells. (A) The expression of CASC2 in
HK-2 cells from the control, LPS, pc-CASC2, LPS+pc-NC and
LPS+pc-CASC2 groups. (B) The relative viability of HK-2 cells in
control, LPS, pc-CASC2, LPS+pc-NC and LPS+pc-CASC2 groups. (C) Flow
cytometric analysis of the apoptosis of HK-2 cells in control, LPS,
pc-CASC2, LPS+pc-NC and LPS+pc-CASC2 groups. (D) ELISA analysis of
the expression level of TNF-α, IL-6, IL-8 and IL-1β in control,
LPS, pc-CASC2, LPS+pc-NC and LPS+pc-CASC2 groups. The production of
(E) superoxide and (F) nitrite in HK-2 in the control, LPS,
pc-CASC2, LPS+pc-NC and LPS+pc-CASC2 groups.
**P<0.01. CASC2, cancer susceptibility candidate 2;
LPS, lipopolysaccharide; IL, interleukin; NC, negative control;
TNF, tumor necrosis factor. |
CASC2 negatively regulates the expression
of miR-155 in sepsis-induced AKI
Next, the current study set out to explore the
underlying molecular mechanism of CASC2 downregulation in the
pathogenesis of sepsis-induced AKI. Considering that lncRNA can act
as an endogenous RNA to competitively bind to miRNA, the expression
of several miRNAs associated with sepsis was measured in HK-2 cells
after CASC2 overexpression (8,10,20,22–25). Intriguingly, the present study
found that only miR-155 was significantly increased in
LPS-stimulated HK-2 cells when CASC2 was overexpressed (Fig. 3A). In addition, bioinformatics
predictions showed that CASC2 can directly bind to miR-155, as
demonstrated by the luciferase reporter assay (Fig. 3B). In accordance with these
findings, it was noticed that there was a negative regulation of
CASC2 and miR-155 in the serum of patients with sepsis (Fig. 3C). To further verify whether the
increased expression of miR-155 is responsible for sepsis-induced
AKI, the miR-155 inhibitor was then transfected into HK-2 cells
after LPS stimulation. As expected, the inhibition of miR-155
partially reversed cell viability, apoptosis, cytokine secretion
and oxidative stress (Fig. 3D–H).
On the contrary, the transfection of the miR-155 mimic into
LPS-stimulated HK-2 cells markedly weakened the protective role of
CASC2 (Fig. 3D–H). Altogether,
these data indicate that CASC2 overexpression can prevent HK-2
cells from LPS-induced injury at least partly by inhibiting miR-155
expression.
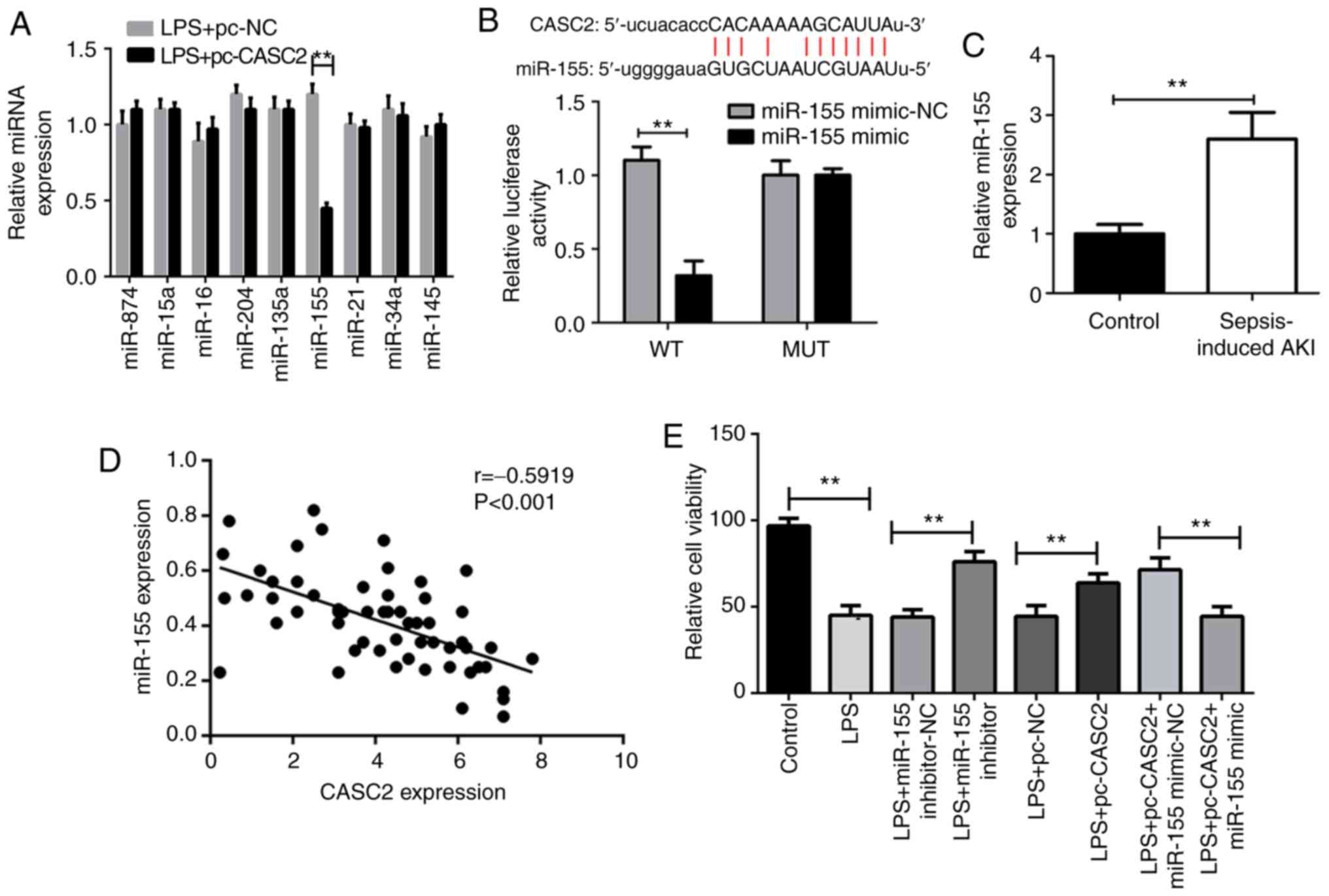 | Figure 3CASC2 negatively regulates the
expression of miR-155 in sepsis-induced AKI. (A) RT-qPCR analysis
of the expressions of miR-874, miR-15a, miR-16, miR-204, miR-135a,
miR-155, miR-21, miR-34a and miR-145 in HK-2 cells from LPS+pc-NC
and LPS+pc-CASC2 groups. (B) Relative luciferase activity in 293
cells after co-transfected with wt or mut CASC2 and miR-155 mimic
or control miRNA (miR-155 mimic-NC), determined by the luciferase
reporter assay. (C) Reverse transcription-quantitative PCR analysis
of the expression of miR-155 in the kidney from the control and
sepsis-induced AKI mice. (D) Pearson correlation analysis showing
the negative relationship between the expression of CASC2 and
miR-155 in the serum of 69 sepsis patients and 20 healthy controls.
The (E) cell viability, (F) cytokines concentrations, (G)
apoptosis, (H) superoxide generation and (I) nitrite production in
HK-2 cells from control, LPS, LPS+miR-155 inhibitor-NC, LPS+miR-155
inhibitor, LPS+pc-NC, LPS+pc-CASC2, LPS+pc-NC+miR-155 mimic-NC,
LPS+pc-NC+miR-155 mimic groups. *P<0.05 and
**P<0.01. CASC2, cancer susceptibility candidate 2;
LPS, lipopolysaccharide; miR, microRNA; NC, negative control; AKI,
acute kidney injury; wt, wild-type; mut, mutant; IL, interleukin;
TNF, tumor necrosis factor. |
CASC2 attenuates sepsis-induced AKI via
the NF-κB signaling pathway
It has been reported that the NF-κB signaling
pathway plays an important role in sepsis-induced AKI (20). Therefore, IκBα, p50 and p65 were
used as surrogate markers of NF-κB activation. As shown in Fig. 4A and B, LPS significantly
activated the NF-κB signaling pathway in LPS-treated HK-2 cells.
Importantly, the overexpression of CASC2 distinctly decreased the
expression levels of p50 and p65 in the cytoplasm and nucleus.
Consistent with these data, EMSA results confirmed that the
upregulation of CASC2 significantly inhibited the activation of the
NF-κB pathway (Fig. 4C). However,
the inhibition of NF-κB activity did not alter miR-155 expression
(Fig. 4D) and the suppression of
miR-155 did not affect the activity of NF-κB in LPS-stimulated HK-2
cells (Fig. S2A–C). Collectively,
these findings demonstrate that CASC2 can also inhibit the NF-κB
signaling pathway to protect sepsis-induced AKI, which is
independent of miR-155.
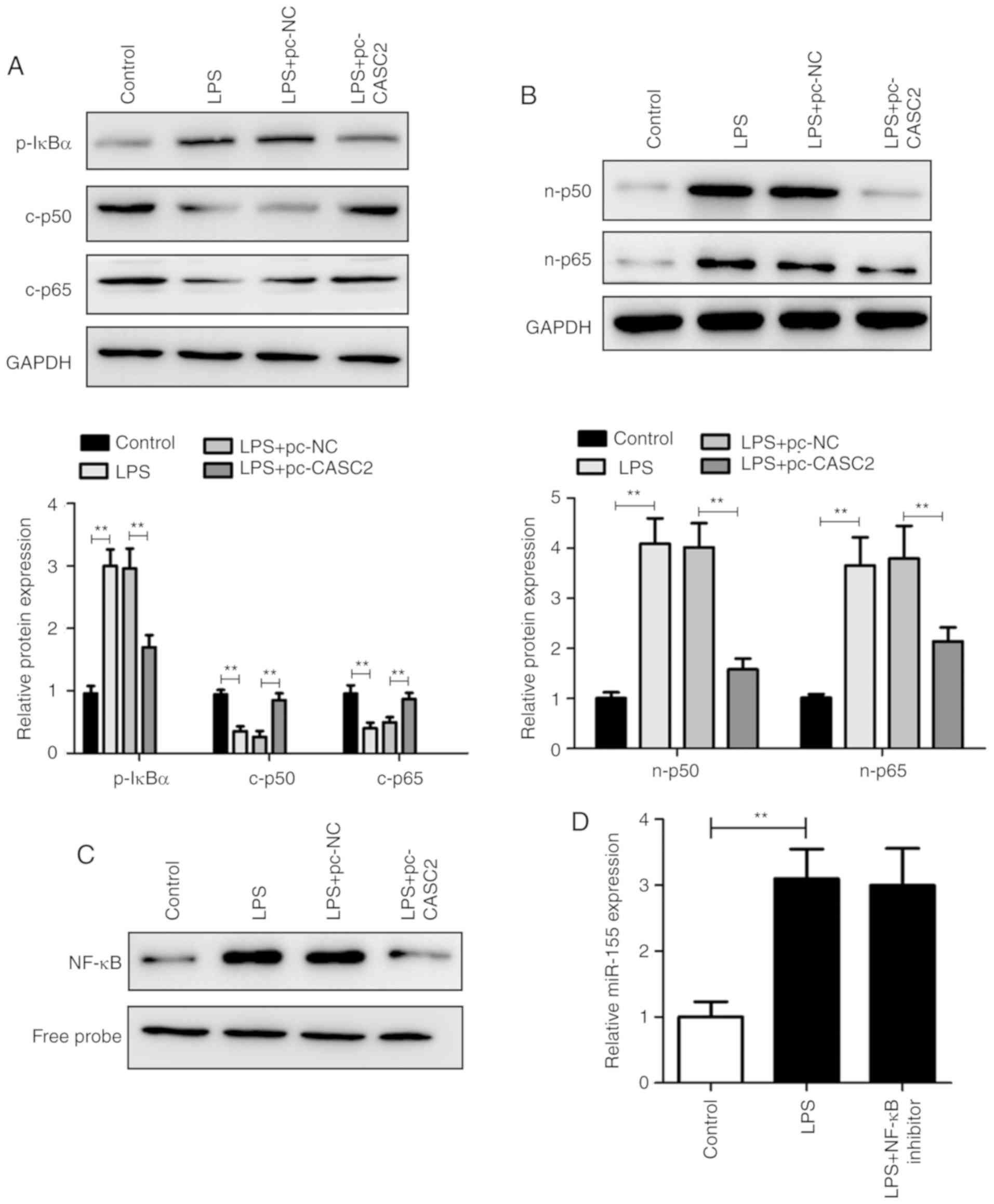 | Figure 4CASC2 attenuates sepsis-induced AKI
via the NF-κB signaling pathway. (A) Western blot analysis of the
expression of p-IκBα, t-IκBα, c-p50 and c-p65 in the HK-2 cells
from the control, LPS, LPS+pc-NC and LPS+pc-CASC2 groups. The
expression of p-IκBα was normalized to t-IκBα, and the expression
of c-p50 and c-p65 was normalized to GAPDH. (B) Western blot
analysis of the expression of n-p50 and n-p65 in the HK-2 cells
from the control, LPS, LPS+pc-NC and LPS+pc-CASC2 groups. (C) EMSA
analysis of the activity of the NF-κB pathway in HK-2 cells from
control, LPS, LPS+pc-NC and LPS+pc-CASC2 groups. (D) Reverse
transcription-quantitative PCR analysis of miR-155 expression in
HK-2 cells from control, LPS and LPS+NF-κB inhibitor groups.
**P<0.01. CASC2, cancer susceptibility candidate 2;
LPS, lipopolysaccharide; miR, microRNA; NC, negative control; AKI,
acute kidney injury; NF, nuclear factor; p−, phosphorylated; t−,
total; c-p50, cytosolic p50; n-p50, nuclear p50. |
Discussion
Sepsis can cause multiple-organ dysfunction and even
death, thus posing a major threat to human life (26). The most common complication of
sepsis is AKI, which is closely associated with its high mortality
(27). Therefore, it is important
to determine its pathogenesis and find an effective method for
treating sepsis-induced kidney injury. In this study, an
LPS-induced sepsis cell model was established to investigate the
role and underlying mechanism of the lncRNA CASC2 in sepsis-induced
AKI.
In recent years, an increasing number of studies
have focused on the biological function of lncRNAs; it has been
reported that lncRNAs have a different expression pattern in the
renal innate cells under disease conditions (28,29). Indeed, numerous lncRNAs serve
important roles in several kidney diseases, including diabetic
nephropathy, renal inflammation and fibrosis, renal transplant
rejection, renal cell carcinoma and kidney injury (30). However, the role of the majority
of lncRNAs in sepsis-induced AKI has not been explored. The present
study showed for the first time to the best of our knowledge that
the lncRNA CASC2 can ameliorate sepsis-induced AKI by targeting the
miR-155 and NF-κB pathway.
Previous studies have shown that CASC2 plays an
important role in multiple physiological and pathological
processes, such as cell differentiation, osteoarthritis, pulmonary
hypertension and tumor progression (15,31,32), while its expression and function
in kidney disease, particularly sepsis-induced AKI, remains
unclear. In the present study, it was found that the CASC2
expression was evidently reduced in patients with sepsis-induced
AKI, compared with healthy controls. In addition, the CASC2 level
was negatively associated with the severity of AKI. Furthermore,
the decrease in CASC2 expression was verified in mice and a cell
model of sepsis. These data indicate that the downregulation of
CASC2 may be harmful to sepsis-induced AKI. Thus, CASC2 may also
function as a diagnostic marker for sepsis-induced AKI.
Apoptosis is cell suicide or programmed cell death,
which is also involved in sepsis-induced AKI (33). The present study found that CASC2
overexpression downregulates LPS-induced apoptosis in HK-2 cells.
This led the current study to hypothesize that CASC2 can prevent
sepsis-induced AKI by inhibiting the apoptotic pathway. The
inflammatory response is recognized as a major cause of
sepsis-induced AKI, due to the release of a large number of
pro-inflammatory factors in the organism during sepsis (34). An increase in the levels of the 4
most common pro-inflammatory cytokines (TNF-α, IL-6, IL-8 and
IL-1β) was observed in a septic cell model. Specifically, it was
found that the overexpression of CASC2 inhibits the LPS-induced
expression of inflammatory factors in HK-2 cells. On the other
hand, the increase of free radicals has been observed in multiple
organs during sepsis. Therefore, reducing oxidative stress is one
of the ways to interfere with sepsis (35,36). The present results showed that the
production of superoxide and NO in HK-2 cells was increased under
LPS stimulation, which was reversed following CASC2 overexpression.
However, the present data showed that the overexpression of CASC2
has no significant effect on normal HK-2 cells. These data suggest
that CASC2 may function mainly in disease conditions, such as
AKI.
A large number of studies have identified the
interaction between lncRNA and miRNA (37). In the current study, it was
confirmed that CASC2 can directly bind to miR-155 to inhibit its
expression. Further investigation revealed that CASC2
overexpression can ameliorate LPS-induced injury in HK-2 cells,
while inhibiting miR-155 partially reversed these effects,
suggesting that CASC2 can interact with miR-155 to affect the
progression of sepsis-induced AKI. Also, these findings hint that
there may be other mechanisms. NF-κB plays a key role in
inflammatory diseases (38) and
studies have confirmed that the activation of NF-κB can increase
the secretion of cytokines, resulting in multiple-organ injury in
sepsis (39). In particular, the
present study noticed that CASC2 can inhibit the activation of the
NF-κB signaling pathway in sepsis-induced AKI, which also
contributes to alleviating disease progression. Although studies
have reported that the NF-κB pathway can regulate the expression of
miR-155 (40–42), current data found that the
inhibition of NF-κB activity did not alter miR-155 expression and
the suppression miR-155 did not affect the activity of NF-κB in the
present research model. Therefore, the mechanism through which
CASC2 inhibits the NF-κB pathway requires further research.
In summary, these results demonstrated that CASC2
expression was significantly decreased in sepsis-induced AKI. In
addition, overexpression of CASC2 was confirmed to attenuate
LPS-induced damage in human renal tubular epithelial HK-2 cells by
targeting the miR-155 and NF-κB pathway. Therefore, CASC2 may be a
potential therapeutic target for sepsis induced AKI.
Supplementary Information
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
MW designed the study, performed experiments and
wrote the manuscript. JW and FS performed the clinical study. KZ
and TJ contributed to the animal experiments and data analysis. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki. This study was approved by the Ethics
Committee of the Huai’an no. 1 People’s Hospital of Nanjing Medical
University. According to the approval that was received, informed
consent was not required. All animal experiments were approved by
the Animal Ethics Committee of the Nanjing Medical University and
performed in accordance with the Guide for the Care and Use of
Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boomer JS, Green JM and Hotchkiss RS: The
changing immune system in sepsis: Is individualized
immuno-modulatory therapy the answer? Virulence. 5:45–56. 2014.
View Article : Google Scholar :
|
|
3
|
Zafrani L, Ergin B, Kapucu A and Ince C:
Blood transfusion improves renal oxygenation and renal function in
sepsis-induced acute kidney injury in rats. Crit Care. 20:4062016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manoeuvrier G, Bach-Ngohou K, Batard E,
Masson D and Trewick D: Diagnostic performance of serum blood urea
nitrogen to creatinine ratio for distinguishing prerenal from
intrinsic acute kidney injury in the emergency department. BMC
Nephrol. 18:1732017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singbartl K and Kellum JA: AKI in the ICU:
Definition, epidemiology, risk stratification, and outcomes. Kidney
Int. 81:819–825. 2012. View Article : Google Scholar
|
|
6
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei S, Du M, Jiang Z, Hausman GJ, Zhang L
and Dodson MV: Long noncoding RNAs in regulating adipogenesis: New
RNAs shed lights on obesity. Cell Mol Life Sci. 73:2079–2087. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang Y, Hu J, Wang Z, Zong H, Zhang L,
Zhang R and Sun L: lncRNA H19 functions as an Aquaporin 1
competitive endogenous RNA to regulate microRNA-874 expression in
LPS sepsis. Biomed Pharmacother. 105:1183–1191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen H, Wang X, Yan X, Cheng X, He X and
Zheng W: lncRNA MALAT1 regulates sepsis-induced cardiac
inflammation and dysfunction via interaction with miR-125b and p38
MAPK/NFKB. Int Immunopharmacol. 55:69–76. 2018. View Article : Google Scholar
|
|
10
|
Wang W, Lou C, Gao J, Zhang X and Du Y:
lncRNA SNHG16 reverses the effects of miR-15a/16 on LPS-induced
inflammatory pathway. Biomed Pharmacother. 106:1661–1667. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Liu Z, Yao B, Li Q, Wang L, Wang
C, Dou C, Xu M, Liu Q and Tu K: Long non-coding RNA CASC2
suppresses epithelial-mesenchymal transition of hepatocellular
carcinoma cells through CASC2/miR-367/FBXW7 axis. Mol Cancer.
16:1232017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang F, Zhang Q, Chen W, Zhang H, Lu G,
Chen J and Qiu C: Long noncoding RNA cancer susceptibility
candidate 2 suppresses papillary thyroid carcinoma growth by
inactivating the AKT/ERK1/2 signaling pathway. J Cell Biochem.
120:10380–10390. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Feng X, Zhang M, Liu A, Tian L,
Bo W, Wang H and Hu Y: Long non-coding RNA CASC2 upregulates PTEN
to suppress pancreatic carcinoma cell metastasis by downregulating
miR-21. Cancer Cell Int. 19:182019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun J, Liu L, Zou H and Yu W: The long
non-coding RNA CASC2 suppresses cell viability, migration, and
invasion in hepatocellular carcinoma cells by directly
downregulating miR-183. Yonsei Med J. 60:905–913. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu Y, Liang S, Zhou Y, Li S, Li Y and Liao
W: HNF1A/CASC2 regulates pancreatic cancer cell proliferation
through PTEN/Akt signaling. J Cell Biochem. 120:2816–2827. 2019.
View Article : Google Scholar
|
|
16
|
Ba Z, Gu L, Hao S, Wang X, Cheng Z and Nie
G: Downregulation of lncRNA CASC2 facilitates osteosarcoma growth
and invasion through miR-181a. Cell Prolif. 51:102018. View Article : Google Scholar
|
|
17
|
Cao Y, Xu R, Xu X, Zhou Y, Cui L and He X:
Downregulation of lncRNA CASC2 by microRNA-21 increases the
proliferation and migration of renal cell carcinoma cells. Mol Med
Rep. 14:1019–1025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsoyi K, Lee TY, Lee YS, Kim HJ, Seo HG,
Lee JH and Chang KC: Heme-oxygenase-1 induction and carbon
monoxide-releasing molecule inhibit lipopolysaccharide
(LPS)-induced high-mobility group box 1 release in vitro and
improve survival of mice in LPS-and cecal ligation and
puncture-induced sepsis model in vivo. Mol Pharmacol. 76:173–182.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Yuan F, Zhang X, Chen W, Tang X and
Lu L: Elevated MIR100HG promotes colorectal cancer metastasis and
is associated with poor prognosis. Oncol Lett. 18:6483–6490.
2019.PubMed/NCBI
|
|
20
|
Chen Y, Qiu J, Chen B, Lin Y, Chen Y, Xie
G, Qiu J, Tong H and Jiang D: Long non-coding RNA NEAT1 plays an
important role in sepsis-induced acute kidney injury by targeting
miR-204 and modulating the NF-κB pathway. Int Immunopharmacol.
59:252–260. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang D, Mi M, Jiang F, Sun Y, Li Y, Yang
L, Fan L, Li Q, Meng J, Yue Z, et al: Apple polysaccharide reduces
NF-Kb mediated colitis-associated colon carcinogenesis. Nutr
Cancer. 67:177–190. 2015. View Article : Google Scholar
|
|
22
|
Zheng G, Pan M, Jin W, Jin G and Huang Y:
MicroRNA-135a is up-regulated and aggravates myocardial depression
in sepsis via regulating p38 MAPK/NF-κB pathway. Int
Immunopharmacol. 45:6–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao X, Zhang C, Zhang X, Chen Y and Zhang
H: miR-145 negatively regulates TGFBR2 signaling responsible for
sepsis-induced acute lung injury. Biomed Pharmacother. 111:852–858.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang ZJ, Zhang MY, Fan ZW, Sun WL and
Tang Y: Influence of lncRNA HOTAIR on acute kidney injury in sepsis
rats through regulating miR-34a/Bcl-2 pathway. Eur Rev Med
Pharmacol Sci. 23:3512–3519. 2019.PubMed/NCBI
|
|
25
|
Lin Z, Liu Z, Wang X, Qiu C and Zheng S:
miR-21-3p plays a crucial role in metabolism alteration of renal
tubular epithelial cells during sepsis associated acute kidney
injury via AKT/CDK2-FOXO1 pathway. Biomed Res Int. 2019:2821731.
2019. View Article : Google Scholar
|
|
26
|
Huang S, Qian K, Zhu Y, Huang Z, Luo Q and
Qing C: Diagnostic value of the lncRNA NEAT1 in peripheral blood
mononuclear cells of patients with sepsis. Dis Markers.
2017:7962836. 2017. View Article : Google Scholar
|
|
27
|
Bellomo R, Kellum JA, Ronco C, Wald R,
Martensson J, Maiden M, Bagshaw SM, Glassford NJ, Lankadeva Y,
Vaara ST and Schneider A: Acute kidney injury in sepsis. Intensive
Care Med. 43:816–828. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou P, Chen Z, Zou Y and Wan X: Roles of
non-coding RNAs in acute kidney injury. Kidney Blood Press Res.
41:757–769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng X, Ye C, Zhao J, Bian P, Zhang Y and
Jia Z: Alterations and clinical signifecance of exosome-containing
innate immunity related lncRNAs in patients of hemorrhagic fever
with renal syndrome. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
32:1522–1526. 2016.(In Chinese). PubMed/NCBI
|
|
30
|
Li S, Zhou J, Wang Z, Wang P, Gao X and
Wang Y: Long noncoding RNA GAS5 suppresses triple negative breast
cancer progression through inhibition of proliferation and invasion
by competitively binding miR-196a-5p. Biomed Pharmacother.
104:451–457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pan L, Chen H, Bai Y, Wang Q and Chen L:
Long non-coding RNA CASC2 serves as a ceRNA of microRNA-21 to
promote PDCD4 expression in oral squamous cell carcinoma. Onco
Targets Ther. 12:3377–3385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang H, Kan QE, Su Y and Man H: Long
non-coding RNA CASC2 improves diabetic nephropathy by inhibiting
JNK pathway. Exp Clin Endocrinol Diabetes. 127:533–537. 2019.
View Article : Google Scholar
|
|
33
|
Kockara A and Kayatas M: Renal cell
apoptosis and new treatment options in sepsis-induced acute kidney
injury. Ren Fail. 35:291–294. 2013. View Article : Google Scholar
|
|
34
|
Venkatachalam MA and Weinberg JM: The
tubule pathology of septic acute kidney injury: A neglected area of
research comes of age. Kidney Int. 81:338–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goode HF and Webster NR: Free radicals and
antioxidants in sepsis. Crit Care Med. 21:1770–1776. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Knotek M, Esson M, Gengaro P, Edelstein CL
and Schrier RW: Desensitization of soluble guanylate cyclase in
renal cortex during endotoxemia in mice. J Am Soc Nephrol.
11:2133–2137. 2000.PubMed/NCBI
|
|
37
|
Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB,
Yin DD, Kong R, Xia R, Lu KH, Li JH, et al: lnc RNA HOTAIR
functions as a competing endogenous RNA to regulate HER2 expression
by sponging miR-331–3p in gastric cancer. Mol Cancer. 13:922014.
View Article : Google Scholar
|
|
38
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schrier RW and Wang W: Acute renal failure
and sepsis. N Engl J Med. 351:159–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cremer TJ, Fatehchand K, Shah P, Gillette
D, Patel H, Marsh RL, Besecker BY, Rajaram MV, Cormet-Boyaka E,
Kanneganti TD, et al: miR-155 induction by microbes/microbial
ligands requires NF-κB-dependent de novo protein synthesis. Front
Cell Infect Microbiol. 2:732012. View Article : Google Scholar
|
|
41
|
Chen C, Luo F, Yang Q, Wang D, Yang P, Xue
J, Dai X, Liu X, Xu H, Lu J, et al: NF-κB-regulated miR-155, via
repression of QKI, contributes to the acquisition of CSC-like
phenotype during the neoplastic transformation of hepatic cells
induced by arsenite. Mol Carcinog. 57:483–493. 2018. View Article : Google Scholar
|
|
42
|
Wang M, Yang F, Qiu R, Zhu M, Zhang H, Xu
W, Shen B and Zhu W: The role of mmu-miR-155-5p-NF-κB signaling in
the education of bone marrow-derived mesenchymal stem cells by
gastric cancer cells. Cancer Med. 7:856–868. 2018. View Article : Google Scholar : PubMed/NCBI
|