Introduction
Myocardial infarction (MI) is a leading cause of
death in the world; annually, 27% of people who experience an MI
die in the US (1). Current
pharmaceutical agents or medical devices can rescue the dying
cardiomyocytes to some extent; however, there are no effective
therapeutic means that can be used to directly repair the necrotic
myocardium (2). Increasing
evidence suggests that adult stem cells can be applied to
accelerate myocardial regeneration and improve cardiac function,
including skeletal myoblasts (3)
and bone marrow stem cells (4),
but the difficulties in acquisition and low numbers of stem cells
limit their clinical application. In 2001, Zuk et al
(5) reported that a
fibroblast-like population of cells from the human adipose tissue
differentiated in vitro into multilineage cells in the
presence of lineage-specific induction factors, and hypothesized
that human adipose tissue may represent an alternative stem cell
source to bone marrow-derived mesenchymal stem cells. Subsequent
studies have confirmed that adipose-derived stem cells (ASCs) exist
and have the capacity of self-renewal and differentiation into
multiple types of cells (6,7).
The population of ASCs represents a promising cell source for
tissue regeneration due to their easy accessibility.
Previous studies have demonstrated that ASCs improve
ventricular thickness and cardiac function of infracted rat hearts.
For example, Wang et al (8) and Schenke-Layland et al
(9) have demonstrated the
therapeutic efficacy of ASCs on infarcted rat heart by ligation of
the left anterior descending artery. In another study, using a
mouse model of myocardial infarct, Yang et al (10) revealed that both human ASCs and
ASC-conditioned medium significantly reduced the myocardial infarct
size and improved cardiac function. One recent clinical study
reported that autologous ASCs were feasible and scalable for the
treatment of ischemic cardiomyopathy (11). Increasing preclinical and clinical
evidence indicates that ASC-mediated myocardial regeneration may be
due to the paracrine effects of ASCs, such as anti-apoptosis,
anti-cardiac remodeling and promotion of angiogenesis.
ASCs can secrete cytokines such as vascular
endothelial growth factor (VEGF), transforming growth factor (TGF),
hepatocyte growth factor (HGF), basic fibroblast growth factor
(bFGF), placenta growth factor (PGF), granulocyte macrophage colony
stimulating factor (GM-CSF), insulin like growth factor-1 (IGF-1)
and angiopoietin (Ang) (12,13). Suga et al (14) have studied the paracrine mechanism
of ASCs by isolation and culturing of ASCs from transgenic mice
that express luciferase and green fluorescent protein. By injecting
these cells into the inguinal fat pads of wild-type mice to observe
the fate of transplanted ASCs, the effects of ASC transplantation
and cellular events after transplantation, they reported that ASCs
exerted an angiogenic effect by activating endothelial cells
through secreted cytokines HGF and VEGF in both in vitro
cultured cells and in vivo mice hearts (14). As an important angiogenic factor,
VEGF can promote cell migration during the process of angiogenesis
(15). IGF-1 can promote
proliferation and prevent apoptosis (16), and bFGF serves a significant role
in promoting cell proliferation and differentiation (17). The roles of VEGF, IGF-1 and bFGF
have been extensively studied in stem cell transplantation. For
instance, ASC treatment in aged rats can improve aging-related
erectile dysfunction partially through the secretion of IGF-1, bFGF
and VEGF (18).
The physiological O2 content of human
organs is estimated to range between 1 and 11% (19). However, in the majority of the
in vitro studies concerning the paracrine effects of
cultured ASCs, the O2 level was artificially set as 21%
(irrespective of 5% CO2 supplementation), corresponding
to the air oxygen content rather than the unique tissue normoxia,
which was referred to as ‘physioxia’ by Carreau et al
(19). It is estimated that the
oxygen content of venous blood and ventricular myocytes in the
human body is ~5% (19). ASCs
reside in anatomical sites that are relatively oxygen deficient,
and hypoxia may provide signals conducive to the maintenance of
definitive ASC properties (20).
Since oxygen is a crucial component of the stem cell niches that
influence cell proliferation and cell-fate commitment (21), elucidating the mechanisms on the
cardioprotective effects of ASCs under physioxic (5% O2)
conditions rather than the artificial normoxia (21% O2)
is essential, as it may more accurately reflect the bona fide
molecular and cellular changes in the body.
Therefore, the aim of the present study was to
compare the protective effects of rat ASCs on neonatal rat
ventricular myocytes (NRVMs) under normoxia and physioxia. In
addition, several secreted soluble factors involved in the
paracrine effects of ASCs were evaluated to determine the
cardioprotective effects of ASCs.
Materials and methods
Animals
A total of 10 adult Sprague-Dawley (SD) rats
(100–120 g, male) (22) and 12
neonatal SD rats (1–3 days old) were obtained from the Animal
Experiment Department at Shanxi Medical University (Jinzhong,
China). The rats were housed in a controlled environment (20–22°C,
12 h light/dark cycle and 50% relative humidity) and had ad
libitum access to food and water. All experiments involving the
use of animals were approved by the Animal Experimentation Ethics
Committee of Shanxi Medical University.
Isolation and culture of rat ASCs and
NRVMs
The isolation and culture of ASCs were performed as
previously described (23).
Briefly, adult SD rats were anesthetized with sodium pentobarbital
(30 mg/kg) by intraperitoneal injection, and their inguinal fat
pads were removed, followed by cervical dislocation. Fat pads were
rinsed several times with D-Hanks’ solution. Blood vessels and
fibrous tissues were excised under a dissecting microscope and
discarded. The remaining adipose tissues were minced into ~1
mm3 sections, digested with 0.2% type II collagenase
(cat. no. 17101-015; Gibco; Thermo Fisher Scientific, Inc.) for 10
min and agitated for 1 h at 37°C. Following centrifugation at 168 x
g for 10 min at 4°C, the cell pellet was resuspended in the ASC
culture medium (Dulbecco’s Modified Eagle’s Medium supplemented
with 15% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml
streptomycin; all from Thermo Fisher Scientific, Inc.). The ASCs
were transferred into culture flasks and incubated with 5%
CO2 at 37°C. The medium was changed 24 h after plating
and replaced every 2 days. ASCs at passage 3 were used in the
following experiments.
The isolation and culture of cardiomyocytes were
conducted as previously described (24). Cardiac tissue was derived from the
hearts of neonatal SD rats aged 1–3 days. Animals were sacrificed
with carbon dioxide (10–30% volume displacement/min) followed by
cervical dislocation. The hearts were removed and washed in
ice-cold PBS. The left ventricles of the rats were collected and
digested with type II collagenase. The NRVMs were plated at a
density of 1x105 cells/ml in cell culture medium
(Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal
bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin)
containing 0.1 mM bromodeoxyuridine (cat. no. B9285; Sigma-Aldrich;
Merck KGaA) to inhibit the proliferation of non-cardiomyocytes. The
NRVMs exhibited spontaneous contraction and synchronous beat
frequency was examined under microscope. The purity of
cardiomyocytes was examined under a microscope by
immunocytochemical identification of specific sarcomeric α-actin.
NRVMs in the primary culture were used in the following
experiments, and the cells were cultured in the incubator with 5%
CO2 and 21% O2 or 5% CO2 and 5%
O2 at 37°C.
Co-culture of NRVMs and ASCs
NRVMs were inoculated into 10 cm culture dishes and
cultured for 48 h under normoxic (21% O2, normoxia
group) or physioxic (5% O2, physioxia group) conditions.
NRVMs were treated with 0, 50, 100, 200, 400 and 800 μM
H2O2 for 3 h at 37°C (25). For co-culture, NRVMs were
transferred into 6-well plates, and ASCs were inoculated into the
chambers nested in the 6-well plates. ASCs and NRVMs were
co-cultured at a ratio of 1:5 in ASC culture medium. NRVMs in the
normoxia group were co-cultured with ASCs overnight under normoxia
and further cultured under normoxia for 24 h. To facilitate cell
adherence, NRVMs in the physioxia group were first co-cultured with
ASCs overnight under normoxia, and subsequently cultured under
physioxia for 24 h.
Immunofluorescence staining
NRVMs were cultured on glass slides, fixed with 4%
paraformaldehyde for 10 min at 37°C and washed with PBS. The cells
were incubated with an anti-sarcomeric α-actinin antibody (1:200
cat. no. ab137346; Abcam) in PBS supplemented with 1% bovine serum
albumin (cat. no. 0332; Amresco, Inc.) overnight at 4°C. Following
washing with PBS, the cells were incubated with a FITC-conjugated
goat anti-rabbit IgG secondary antibody (1:1,000; cat. no. ab6717;
Abcam) for 30 min at 37°C. Finally, the cells were stained with 10
μg/ml DAPI for 10 min at room temperature and observed under a
Nikon Ni-U fluorescence microscope (magnification, x200) (Nikon
Corporation, Japan), and three fields were analyzed per sample.
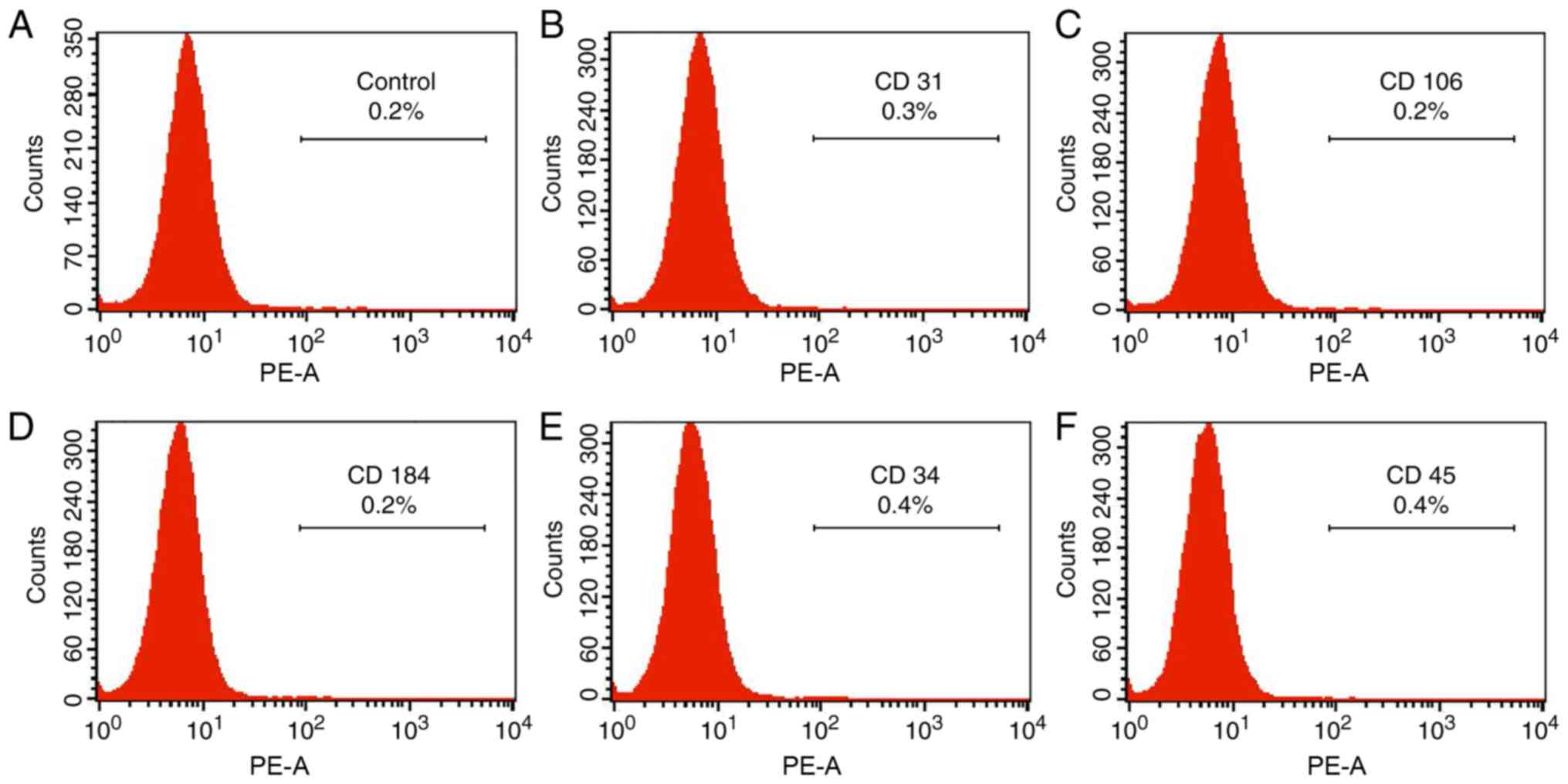
Flow cytometry
The phenotypic markers of ASCs (CD29 and CD44)
(26) and the negative markers of
ASCs (CD31, CD106, CD184, CD34 and CD45) were detected by flow
cytometry to identify rat ASCs. Following washing with PBS
containing 3% FBS, ASCs (200 μl; 3x106 cells/ml) were
incubated with 2 μl phycoerythrin (PE)-labeled anti-rat CD29 (cat.
no. sc-9970; Santa Cruz Biotechnology, Inc.) or PE-labeled anti-rat
CD44 (cat. no. sc-53069; Santa Cruz Biotechnology, Inc.) at 4°C for
1 h away from light. After washing with PBS supplemented with 3%
FBS twice, ASCs were centrifuged at 168 x g for 5 min at 4°C and
resuspended in PBS. A corresponding isotype control antibody (cat.
no. sc-2855; Santa Cruz Biotechnology, Inc.) was used to set up the
staining background. Cells were analyzed using a FACSCalibur flow
cytometer (BD Biosciences), and the results were analyzed with the
CellQuest acquisition software (BD Biosciences).
The apoptotic rate of NRVMs was assessed by the
Annexin V-FITC Apoptosis Detection kit (Beyotime Institute of
Biotechnology) according to the manufacturer’s protocol (25). Briefly, NRVMs were washed with
ice-cold PBS and resuspended at a density of 1x106
cells/ml in 100 μl binding buffer. The cells were incubated with 5
μl Annexin V-FITC solution in the dark for 15 min at room
temperature. Following washing with PBS, the cells were resuspended
in 100 μl PBS, mixed with 5 μl propidium iodide solution and
analyzed by flow cytometry as aforementioned.
ELISA
To estimate the concentrations of secreted VEGF,
IGF-1 and bFGF, the conditioned medium of untreated and
H2O2-treated NRVMs cultured alone or
co-cultured with ASCs under normoxia or physioxia was collected and
subjected to ELISA. ELISA was performed using the VEGF Rat ELISA
kit (Beijing Jiamay Biotech Co., Ltd.), Mouse/Rat IGF-I Quantikine
ELISA kit (R&D Systems, Inc.) and Mouse/Rat FGF basic
Quantikine ELISA kit (R&D Systems, Inc.) according to the
manufacturers’ protocols.
Myocardial glutathione (GSH)
measurement
Following co-culture, NRVMs were minced and broken
up with PBS. After centrifugation at 2,054 x g for 10 min at 4°C,
the supernatant was collected to measure the levels of total GSH
and oxidized glutathione (GSSG) using the total
Glutathione/Oxidized Glutathione Assay kit (cat. no. A061-1;
Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer’s instructions. The GSH/GSSG ratio was calculated to
determine the levels of oxidative stress in NRVMs.
Western blotting
NRVMs were collected, washed with PBS, lysed in 1%
Cell Lysis Buffer (Abcam) and centrifuged for 10 min at 24,149 x g.
The protein concentration was determined using a BCA Protein Assay
kit (Thermo Fisher Scientific, Inc.). A total of 30 μg protein/lane
was loaded onto 12% SDS-PAGE and transferred to a PVDF membrane.
Following blocking with 5% non-fat dry milk, the membrane was
incubated with a rat anti-Bcl-2 monoclonal antibody (1:500; cat.
no. sc-7382; Santa Cruz Biotechnology, Inc.), mouse anti-Bax
monoclonal antibody (1:500; cat. no. sc-493; Santa Cruz
Biotechnology, Inc.) or rabbit anti-rat β-actin polyclonal antibody
(1:1,000; cat. no. ab8227; Abcam) overnight at 4°C. The membranes
were then washed with TBS + 0.5% Tween-20 and incubated with
horseradish peroxidase-conjugated secondary antibodies (goat
anti-rat/mouse/rabbit; 1:4,000; cat. no. A0208; Beyotime Institute
of Biotechnology). The ECL kit (Thermo Fisher Scientific, Inc.) was
used to visualize the bands. The intensities of the protein bands
were quantified using a Gel Doc XR+ Photo-Image System
with Quantity One software version 4.6 (Bio-Rad Laboratories,
Inc.). The western blot experiments were repeated three times.
Statistical analysis
SPSS 17.0 (SPSS, Inc.) was used for statistical
analysis. Data are presented as the mean ± standard deviation.
Comparisons between two groups were performed using Student’s
t-test, whereas one-way ANOVA with Bonferroni post hoc test was
used for comparisons among multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
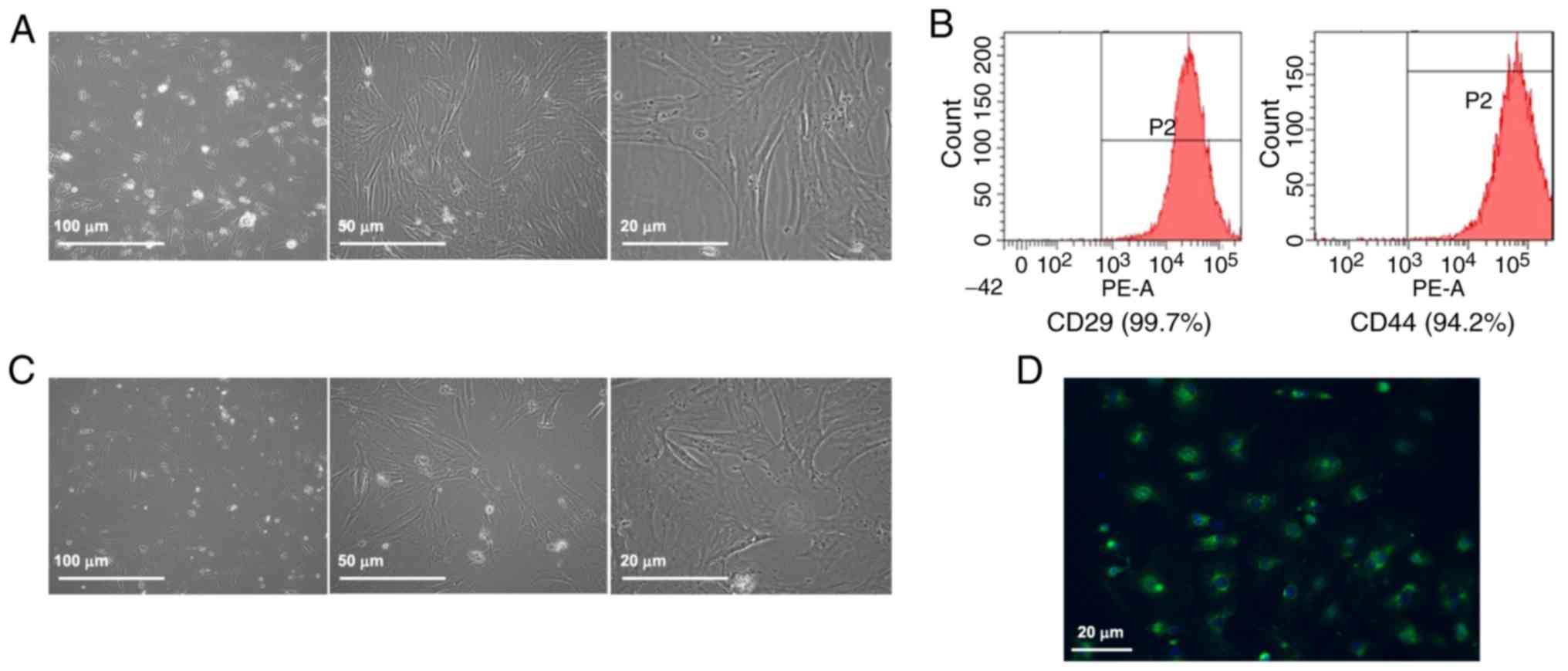
Characterization of rat ASCs
Rat ASCs were round with floating lipid vesicles in
the culture medium when initially inoculated into the culture
dishes (Fig. 1A). The cells
gradually extended and exhibited a short fusiform shape after 3
days. On day 7–8 after inoculation, the ASCs began to proliferate
and exhibited a fibroblast-like appearance. After the primary ASCs
were cultured for 3–5 passages, they proliferated with adherence
and formed a monolayer during further culture for 3–4 days. Then,
ASCs displayed uniform spindle shape and ‘whirlpool-like’ growth at
different magnification after four days (Fig. 1A). Flow cytometry analysis
revealed that the ASCs were positive for the surface expression of
CD29 and CD44 (Fig. 1B) and
negative for that of CD31, CD106, CD184, CD34 and CD45 (Fig. 2).
Characterization of NRVMs
The isolated NRVMs were validated by characterizing
their morphology, unique property of spontaneous contraction and
muscle tissue-specific expression of sarcomeric-α actinin. The
NRVMs were adherent at 24 h post-plating (Fig. 1C). The NRVMs exhibited spontaneous
contraction with synchronous beat frequency of 80–150 beats per
minute. The cells were long rod-, polygonal or spindle-shaped with
concentric or radial growth. Immunofluorescence staining of
sarcomeric α-actinin confirmed that the purity of the cultured
NRVMs was >90% (Fig. 1D).
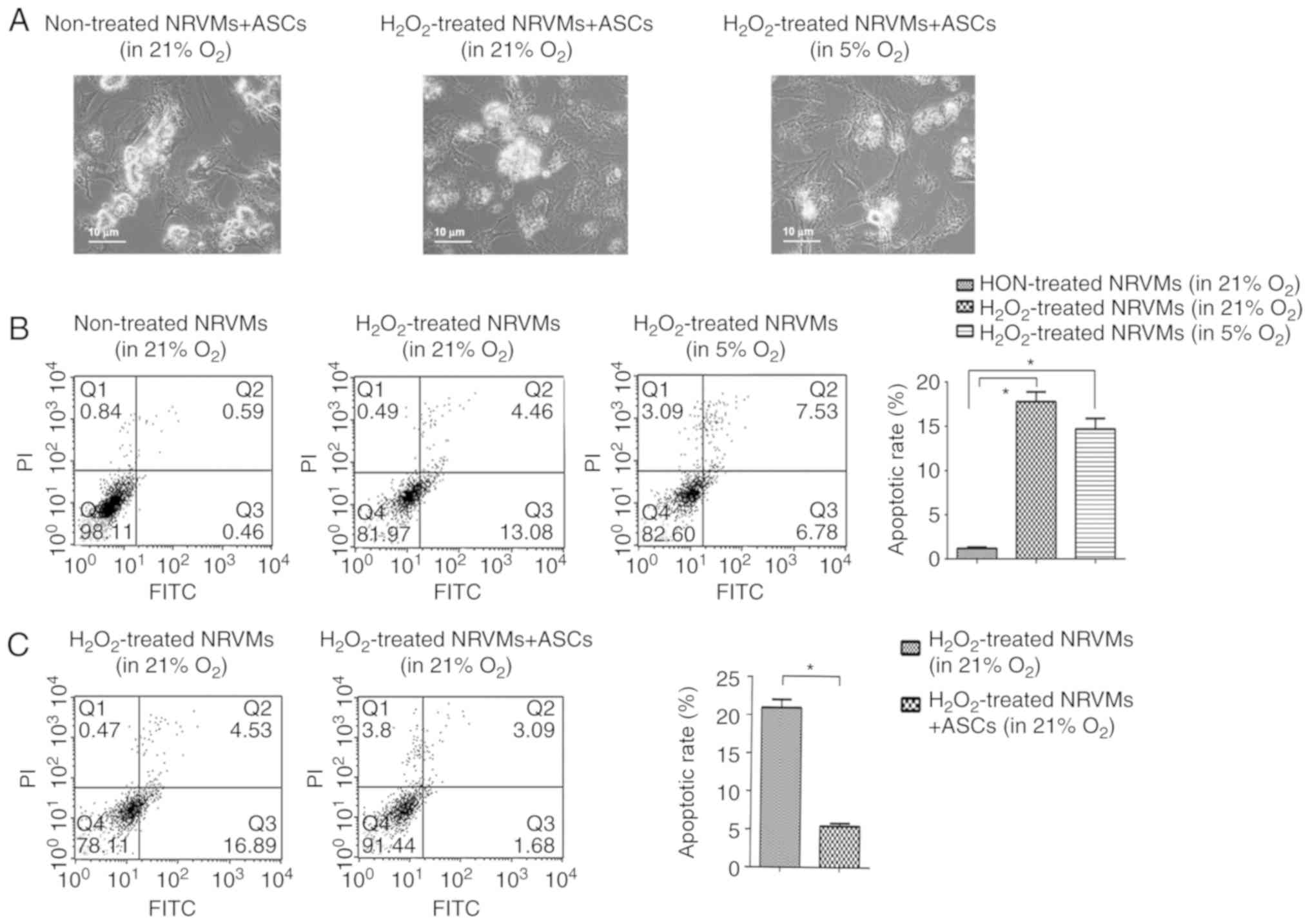
Co-culture with ASCs protects
H2O2-treated NRVMs from apoptosis under
normoxia or physioxia
In order to determine whether ASCs may exert the
protective effects on damaged NRVMs, treatment with
H2O2 was used to induce injury in NRVMs.
First, the toxicity of H2O2 was tested at
various concentrations (50, 100, 200, 400 and 800 μM). The results
demonstrated that treatment with >100 μM
H2O2 for 4 h resulted in the death of the
majority of NRVMs (data not shown). Therefore, 100 μM
H2O2 was selected to induce damage in NRVMs
in subsequent experiments.
A NRVM/ASC co-culturing system was established to
evaluate the protective roles of ASCs on 100 μM
H2O2-injured NRVMs. The morphology analysis
of the co-cultured NRVMs revealed that co-culture with ASCs
facilitated the recovery of damaged NRVMs; the viability of NRVMs
was increased in the co-culture with ASCs group compared with that
of the H2O2-injured NRVMs. This effect was
more evident under physioxia compared with normoxia (Fig. 3A).
The apoptotic rate of NRVMs following co-culture was
detected by flow cytometry. The apoptotic rate of NRVM following
100 μM H2O2 treatment was significantly
increased under normoxia (17.54%) and physioxia (14.31%) compared
with that of the untreated control (1.05%) (P<0.05; Fig. 3B). The extent to which NRVMs were
damaged by H2O2 (as reflected by the
apoptotic rate) did not differ significantly under normoxia or
physioxia conditions. When co-cultured with ASCs under normoxia,
the apoptotic rate of NRVMs was significantly decreased compared
with that of NRVMs cultured alone (4.77% vs. 21.42%, respectively;
P<0.05; Fig. 3C), suggesting
that ASCs exerted a cardioprotective effect in normoxia. The
apoptotic rate of NRVMs was decreased when NRVMs were co-cultured
with ASCs under physioxia (data not shown).
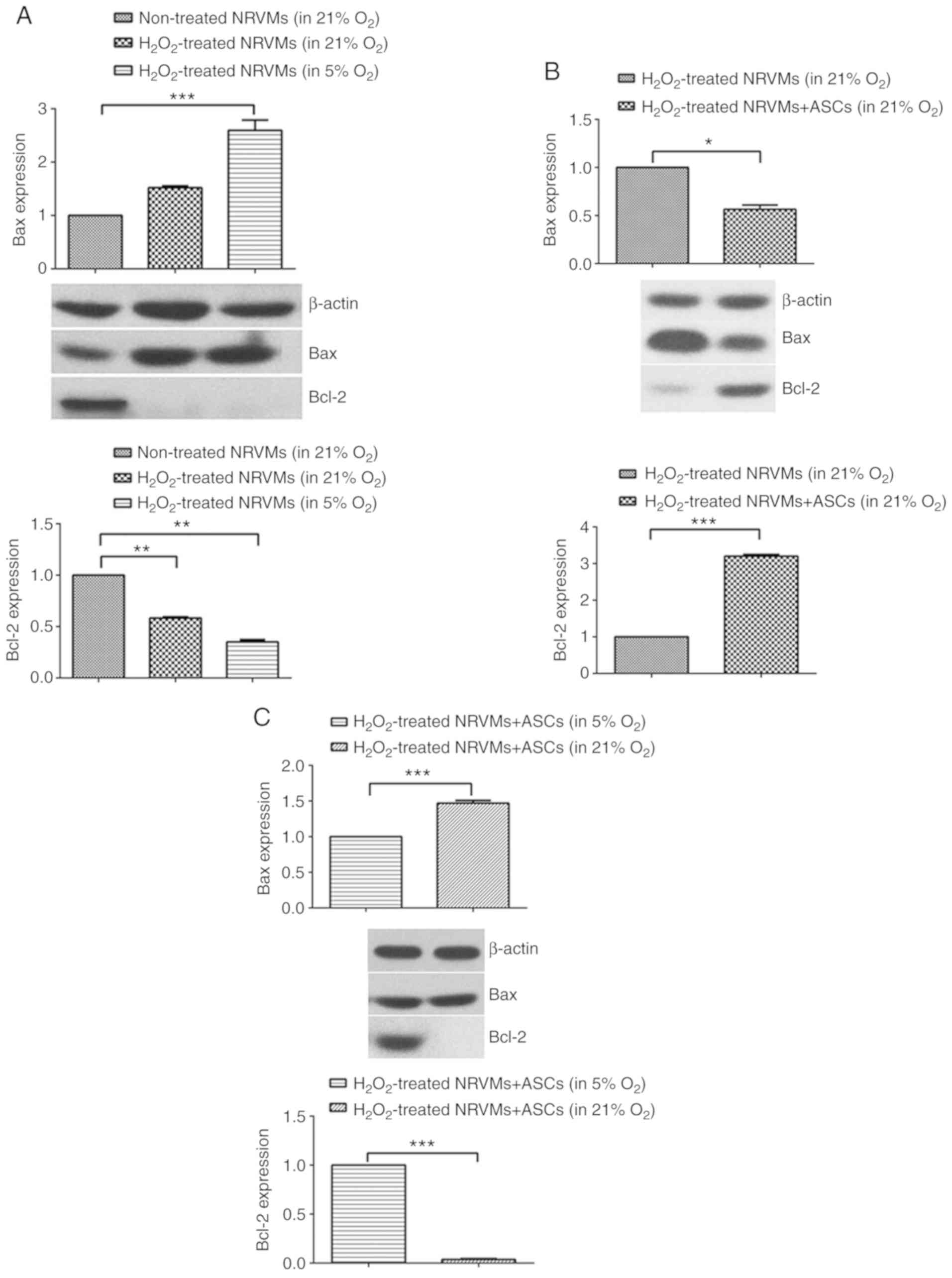
The protein levels of the pro-apoptotic protein Bax
and the anti-apoptotic protein Bcl-2 were determined by western
blotting to evaluate the apoptotic status of NRVMs co-cultured with
ASCs. In H2O2-injured NRVMs, the level of
Bcl-2 protein was significantly decreased, but the level of Bax
protein exhibited no significant difference in normoxia compared
with untreated cells (Fig. 4A).
Under physioxia, the level of Bax protein was further increased,
whereas the level of Bcl-2 protein was further decreased in
H2O2-injured NRVMs compared with untreated
cells under normoxia (Fig. 4A).
In the H2O2-injured NRVMs co-cultured with
ASCs, the protein expression of Bax was downregulated, and the
protein expression of Bcl-2 was upregulated in normoxia compared
with H2O2-injured NRVMs cultured alone
(Fig. 4B). Of note, in
H2O2-injured NRVMs co-cultured ASCs, the
apoptotic rate of NRVMs appeared to be more inhibited under
physioxia compared with normoxia, as evidenced by the reduced
expression of Bax protein and increased expression of Bcl-2 protein
in NRVMs co-cultured with ASCs in 5% O2 (Fig. 4C).
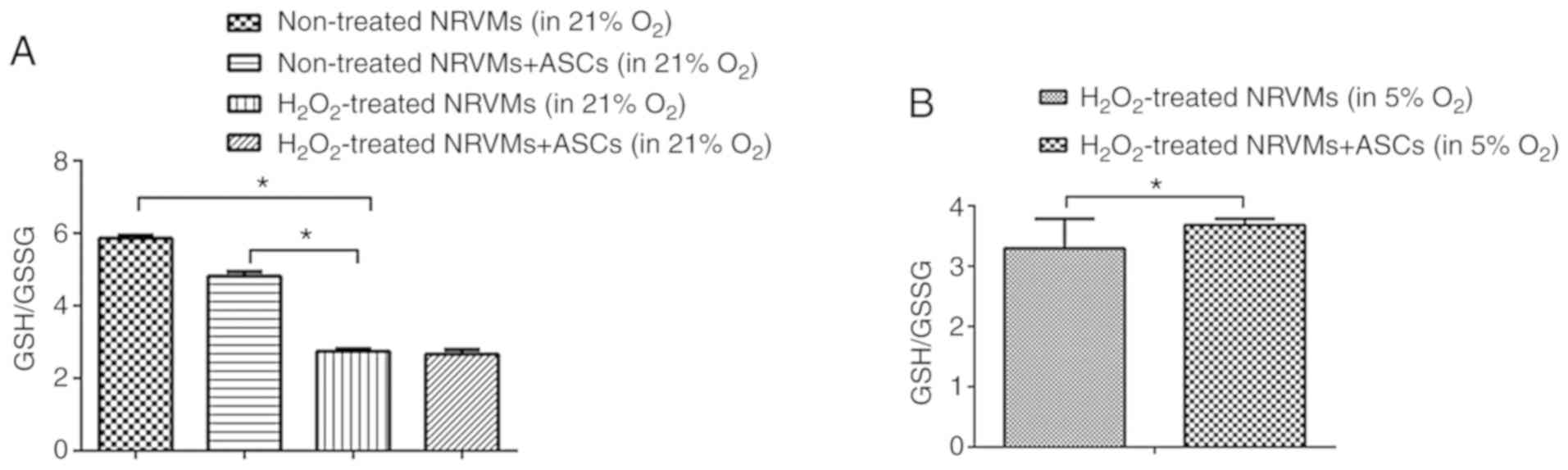
Co-culture with ASCs under physioxia
significantly alleviates the oxidative stress in
H2O2-injured NRVMs
It has been observed that oxidative stress increases
in the injured myocardium (27).
The GSH/GSSG ratio, a measure of overall cellular oxidative stress
(28), was used in the present
study to evaluate the degree of myocardial injury in NRVMs cultured
under various conditions. As presented in Fig. 5A, exposure to 100 μM
H2O2 significantly increased the levels of
oxidative stress in NRVMs cultured alone or co-cultured with ASCs.
Under normoxia, the GSH/GSSG ratio was lower in
H2O2 stimulated NRVMs compared with that in
untreated NRVMs (P<0.05); compared with the
H2O2-injured NRVMs cultured alone, the
GSH/GSSG ratio was not increased in the NRVMs co-cultured with ASCs
(Fig. 5A). However, under
physioxia, the GSH/GSSG ratio was significantly increased in
H2O2-treated NRVMs co-cultured with ASCs
compared with those cultured alone (P<0.05; Fig. 5B). Taken together, these results
suggested that co-culture with ASCs in physioxia, but not normoxia,
alleviated the oxidative stress in damaged NRVMs.
Co-culture of
H2O2-injured NRVMs with ASCs increases the
secretion of VEGF, IGF-1 and bFGF under normoxia or physioxia
To explore whether the paracrine mechanism of ASCs
contributed to their cardioprotective effect, the levels of soluble
factors VEGF, IGF-1 and bFGF in the co-culture medium were
measured. Under normoxia, the level of VEGF in the culture
supernatant was decreased after the NRVMs (cultured alone or
co-cultured with ASCs) were treated with H2O2
compared with untreated cells. Compared with the NRVMs cultured
alone, untreated or H2O2-injured NRVMs
co-cultured with ASCs exhibited significantly increased levels of
VEGF in the supernatant (P<0.001; Fig. 6A). Under physioxia, when the
H2O2-injured NRVMs were co-cultured with
ASCs, the VEGF concentration in the medium was significantly
increased compared with that in the medium of NRVMs cultured alone
(Fig. 6A). Co-culture with ASCs
slightly increased the secretion of IGF-1 in
H2O2-injured NRVMs under normoxia compared
with NRVMs cultured alone, although the difference was not
significant (Fig. 6B). However,
under physioxia, co-culture of injured
H2O2-NRVMs with ASCs significantly increased
IGF-1 secretion into the culture medium compared with that in NRVMs
cultured alone (P<0.05; Fig.
6B). Similarly, under normoxia, the exposure of NRVMs to
H2O2 reduced the concentration of secreted
bFGF to an undetectable level, whereas co-culture with ASCs
significantly increased the level of soluble bFGF compared with
NRVMs cultured alone (P<0.05; Fig.
6C). Consistently, when cells were cultured in 5%
O2, co-culture with ASCs increased the bFGF content in
the culture supernatant of the H2O2-injured
NRVMs compared with NRVMs cultured alone (P<0.01; Fig. 6C). Collectively, these results
indicated that the elevated levels of soluble factors VEGF, IGF-1
and bFGF in the co-culture condition may contribute to the
cardioprotective effect of ASCs.
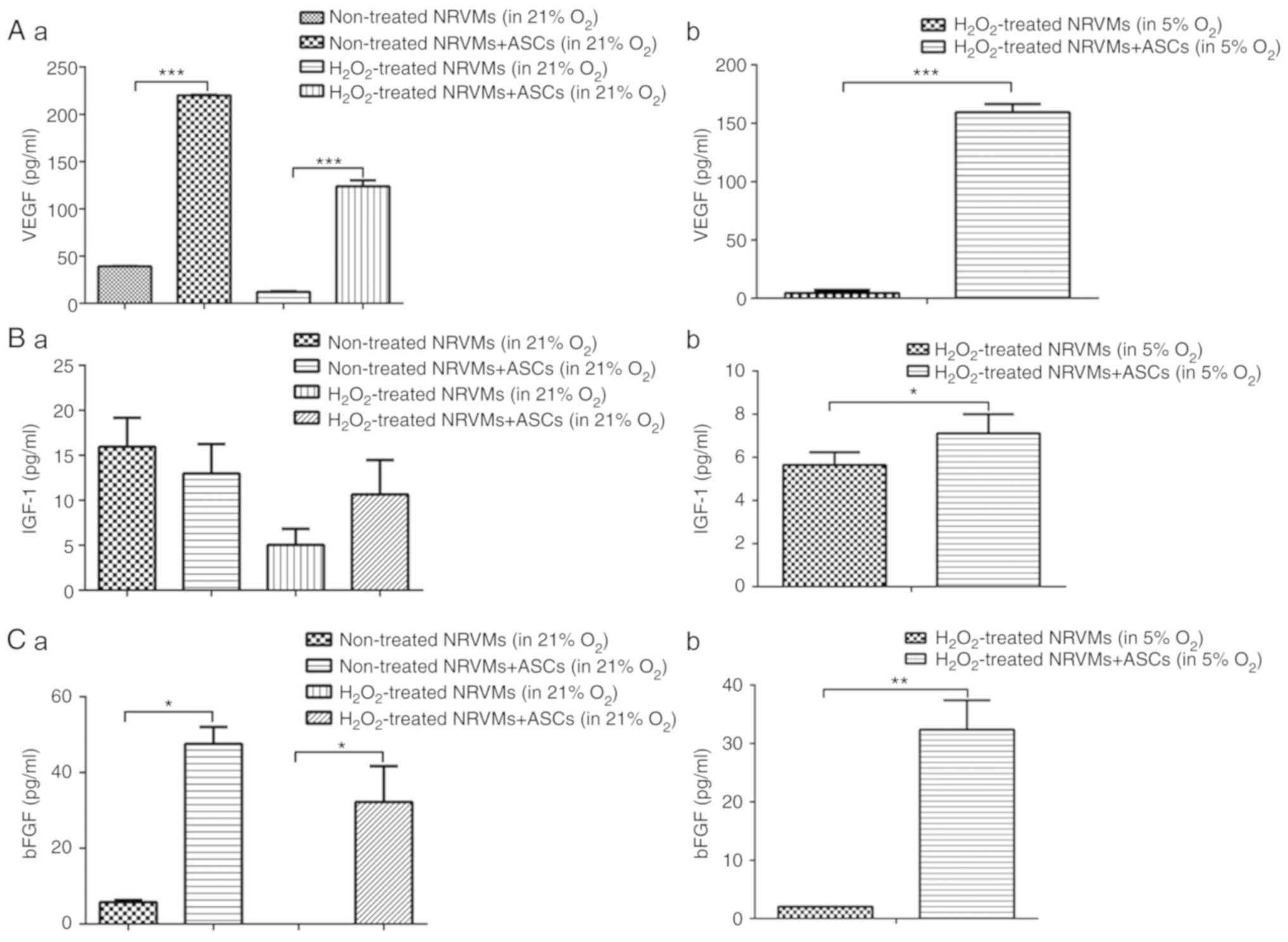 | Figure 6Co-culture of
H2O2-injured NRVMs with ASCs increases the
secretion of VEGF, IGF-1 and bFGF under normoxia or physioxia. (A)
The levels of VEGF in the culture supernatant of untreated and
H2O2-treated NRVMs under normoxia (21%
O2) and physioxia (5% O2). (A-a) The levels
of secreted VEGF in different groups under normoxia. (A-b) Under
physioxia, ASCs co-cultured with injured NRVMs increased the
secretion of VEGF. (B) The levels of IGF-1 in the culture
supernatant of untreated and H2O2-treated
NRVMs under normoxia and physioxia. (B-a) The levels of secreted
IGF-1 in different groups under normoxia. (B-b) Under physioxia,
ASCs co-cultured with injured NRVMs increased the secretion of
IGF-1. (C) The levels of bFGF in culture supernatant of untreated
and H2O2-treated NRVMs under normoxia and
physioxia. (C-a) The levels of bFGF in different groups under
normoxia. (C-b) Under physioxia, ASCs co-cultured with injured
NRVMs increased the secretion of bFGF. *P<0.05,
**P<0.01 and ***P<0.001. ASCs, adipose
tissue-derived stem cells; NRVMs, neonatal rat ventricular
myocytes; VEGF, vascular endothelial growth factor; IGF-1,
insulin-like growth factor; bFGF, basic fibroblast growth
factor. |
Discussion
Nearly half of patients with cardiovascular disease
die from ischemic heart failure, and most of them suffer from
myocardial infarction (29). The
necrotic myocardium cannot be repaired or reversed, and the current
treatment is limited to the approach of recovering with blood
reperfusion, which does not consistently result in a favorable
outcome (29). Transplantation of
stem cells for myocardial repair therapy is a promising treatment
method that has attracted wide attention (29). ASCs have been demonstrated to
possess the ability of multiple lineage differentiation (30) with the potential to treat
myocardial infarction. However, the cardioprotective effects of
ASCs under physioxia, which represents the oxygen content of venous
blood and ventricular myocytes, have not been investigated. The
present study established an in vitro NRVM/ASC co-culture
model to compare the protective effects of ASCs on
H2O2-injured NRVMS under 21 and 5%
O2 conditions. The results demonstrated that ASCs in 5%
O2 exerted a stronger protective effect against
H2O2-induced damage on NRVMs compared with
ASCs in 21% O2 in terms of apoptosis inhibition in NRVMs
and paracrine secretion of soluble factors such as VEGF, IGF-1 and
bFGF.
As ASCs are derived from the fat tissue and their
acquisition induces less pain compared with bone marrow-derived
mesenchymal stem cells, the clinical and translational application
of ASCs has become a research hotspot, especially in the field of
treating myocardial infarction (31,32). A previous study has demonstrated
that ASC transplantation promotes angiogenesis and improves
myocardial remodeling after myocardial infarction to improve
cardiac function (33). In the
majority of the studies concerning the beneficial effects of in
vitro cultured ASCs, the oxygen condition was either
artificially set as normoxia or hypoxia (<5% O2). For
example, Sadat et al (34)
reported that in an in vitro neonatal rat cardiomyocyte and
ASC co-culture system, the anti-apoptotic effect of ASCs was
observed under hypoxic conditions (a humidified atmosphere with 95%
N2 and 5% CO2 gas mixture). Consistent with
the results of the present study, a previous study demonstrated
that the ASCs markedly suppressed oxygen deprivation-induced
cardiomyocyte cell death, and the oxygen content in their cell
culture condition was also set as 5% O2 (35). To the best of our knowledge, the
present study is the first report directly comparing the beneficial
effects of ASCs under normoxia and physioxia on ventricular
myocytes following oxidative stress. The results of the present
study suggested that oxygen deprivation may influence the molecular
and cellular behavior of ASCs, particularly their paracrine
effects.
ASCs can differentiate into mature adipocytes,
myocardium and vascular endothelium under certain conditions
(36). However, further
understanding obtained in recent years poses a challenge to this
view; a number of studies have suggested that mesenchymal stem
cells cannot differentiate into cardiomyocytes and vascular cells,
and their therapeutic functions may be dependent on their paracrine
effects (37,38). This implies that the paracrine
mechanism in the therapeutic effect of ASCs serves an important
role. To further clarify the paracrine mechanism of ASCs, a model
of H2O2-induced NRVMs injury was established
in the present study to evaluate the effects of ASCs on NRVM
apoptosis. The results demonstrated that ASCs significantly reduced
H2O2-induced NRVM apoptosis in normoxia.
Similarly to other stem cells, ASCs secrete a variety of soluble
factors such as VEGF, HGF and IGF-1, which may participate in the
cardiac repair process, including the reduction of myocardial
apoptosis, the promotion of angiogenesis, increasing the survival
rate of transplanted cells, reducing inflammation and mobilizing
endogenous cardiac stem cells (39,40).
In the present study, notably increased secretion of
VEGF, IGF-1 and bFGF was observed after co-culture of NRVMs and
ASCs compared with that in NRVMs cultured alone, which may help
explain the protective role of ASCs by paracrine secretion of VEGF,
IGF-1 and bFGF. A previous study has demonstrated that ASCs promote
the secretion of VEGF and HGF in a hypoxic environment, and a
moderate or low amount of active oxygen can improve ASC expansion,
migration and regeneration (41).
The present study confirmed the protective effects of ASCs on
cardiomyocytes under physioxia, for which 5% oxygen was chosen.
However, whether VEGF, IGF-1 and bFGF also contributed to the
activation of ASCs themselves in the co-culture system under the 5%
O2 condition needs to be further studied. The main
limitation of the present study was that an ASC culture group was
not used as a control; additional experimental groups and soluble
factors are required to verify the cardioprotective role of ASCs by
paracrine secretion. In addition, although the clinical application
of ASCs appears promising, the main mechanisms of how ASCs improve
cardiac function have not been fully elucidated. Further in
vivo experiments with multiple animal models are warranted to
provide insights on the translational use of ASCs in the
clinic.
In conclusion, using an in vitro rat ASC/NRVM
co-culture system, the cardioprotective effects of ASCs were
evaluated under 21 and 5% O2 conditions. The results
demonstrated that oxygen concentrations influenced the
cardioprotective effects of ASCs. VEGF, IGF-1 and bFGF may serve a
role in the myocardial regeneration mediated by transplanted ASCs.
The present study highlights the crucial roles of the paracrine
effects of ASCs in treating myocardial diseases such as myocardial
infarction.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the
International Science and Technology Cooperation program of Shanxi
Province (grant no. 2014081050-2).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
YPG and YYL performed the experiments, collected the
data and drafted the manuscript. XWL and FY performed the
statistical analysis and participated in the study design. CHF, DJH
and WJG contributed analysis tools, analyzed the data and
participated in the drafting of the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All experiments involving the use of animals were
approved by the Animal Experimentation Ethics Committee of Shanxi
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boateng S and Sanborn T: Acute myocardial
infarction. Dis Mon. 59:83–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alagarsamy KN, Yan W, Srivastava A,
Desiderio V and Dhingra S: Application of injectable hydrogels for
cardiac stem cell therapy and tissue engineering. Rev Cardiovasc
Med. 20:221–230. 2019. View Article : Google Scholar
|
|
3
|
Povsic TJ, O’Connor CM, Henry T, Taussig
A, Kereiakes DJ, Fortuin FD, Niederman A, Schatz R, Spencer R 4th,
Owens D, et al: A double-blind, randomized, controlled, multicenter
study to assess the safety and cardiovascular effects of skeletal
myoblast implantation by catheter delivery in patients with chronic
heart failure after myocardial infarction. Am Heart J.
162:654–662.e1. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu B, Duan CY, Luo CF, Ou CW, Sun K, Wu
ZY, Huang H, Cheng CF, Li YP and Chen MS: Effectiveness and safety
of selected bone marrow stem cells on left ventricular function in
patients with acute myocardial infarction: A meta-analysis of
randomized controlled trials. Int J Cardiol. 177:764–770. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poglio S, De Toni-Costes F, Arnaud E,
Laharrague P, Espinosa E, Casteilla L and Cousin B: Adipose tissue
as a dedicated reservoir of functional mast cell progenitors. Stem
Cells. 28:2065–2072. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gimble JM and Guilak F: Differentiation
potential of adipose derived adult stem (ADAS) cells. Curr Top Dev
Biol. 58:137–160. 2003. View Article : Google Scholar
|
|
8
|
Wang L, Deng J, Tian W, Xiang B, Yang T,
Li G, Wang J, Gruwel M, Kashour T, Rendell J, et al:
Adipose-derived stem cells are an effective cell candidate for
treatment of heart failure: An MR imaging study of rat hearts. Am J
Physiol Heart Circ Physiol. 297:H1020–H1031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schenke-Layland K, Strem BM, Jordan MC,
Deemedio MT, Hedrick MH, Roos KP, Fraser JK and Maclellan WR:
Adipose tissue-derived cells improve cardiac function following
myocardial infarction. J Surg Res. 153:217–223. 2009. View Article : Google Scholar :
|
|
10
|
Yang D, Wang W, Li L, Peng Y, Chen P,
Huang H, Guo Y, Xia X, Wang Y, Wang H, et al: The relative
contribution of paracine effect versus direct differentiation on
adipose-derived stem cell transplantation mediated cardiac repair.
PLoS One. 8:e590202013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Henry TD, Pepine CJ, Lambert CR, Traverse
JH, Schatz R, Costa M, Povsic TJ, David Anderson R, Willerson JT,
Kesten S and Perin EC: The athena trials: Autologous
adipose-derived regenerative cells for refractory chronic
myocardial ischemia with left ventricular dysfunction. Catheter
Cardiovasc Interv. 89:169–177. 2017. View Article : Google Scholar
|
|
12
|
Hsiao ST, Lokmic Z, Peshavariya H,
Abberton KM, Dusting GJ, Lim SY and Dilley RJ: Hypoxic conditioning
enhances the angiogenic paracrine activity of human adipose-derived
stem cells. Stem Cells Dev. 22:1614–1623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rehman J, Traktuev D, Li J, Merfeld-Clauss
S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV
and March KL: Secretion of angiogenic and antiapoptotic factors by
human adipose stromal cells. Circulation. 109:1292–1298. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suga H, Glotzbach JP, Sorkin M, Longaker
MT and Gurtner GC: Paracrine mechanism of angiogenesis in
adipose-derived stem cell transplantation. Ann Plast Surg.
72:234–241. 2014. View Article : Google Scholar
|
|
15
|
Hoeben A, Landuyt B, Highley MS, Wildiers
H, Van Oosterom AT and De Bruijn EA: Vascular endothelial growth
factor and angiogenesis. Pharmacol Rev. 56:549–580. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Youssef A, Aboalola D and Han VK: The
roles of insulin-like growth factors in mesenchymal stem cell
niche. Stem Cells Int. 2017:9453108. 2017.
|
|
17
|
Kim JH, Lee MC, Seong SC, Park KH and Lee
S: Enhanced proliferation and chondrogenic differentiation of human
synovium-derived stem cells expanded with basic fibroblast growth
factor. Tissue Eng Part A. 17:991–1002. 2011. View Article : Google Scholar
|
|
18
|
Yang J, Zhang Y, Zang G, Wang T, Yu Z,
Wang S, Tang Z and Liu J: Adipose-derived stem cells improve
erectile function partially through the secretion of IGF-1, bFGF,
and VEGF in aged rats. Andrology. 6:498–509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carreau A, El Hafny-Rahbi B, Matejuk A,
Grillon C and Kieda C: Why is the partial oxygen pressure of human
tissues a crucial parameter? Small molecules and hypoxia. J Cell
Mol Med. 15:1239–1253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JH, Park SH, Park SG, Choi JS, Xia Y
and Sung JH: The pivotal role of reactive oxygen species generation
in the hypoxia-induced stimulation of adipose-derived stem cells.
Stem Cells Dev. 20:1753–1761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mohyeldin A, Garzón-Muvdi T and
Quiñones-Hinojosa A: Oxygen in stem cell biology: A critical
component of the stem cell niche. Cell Stem Cell. 7:150–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pushpan CK, V S, G S, Rathnam P, A J and A
H: Attenuation of atherosclerotic complications by modulating
inflammatory responses in hypercholesterolemic rats with dietary
Njavara rice bran oil. Biomed Pharmacother. 83:1387–1397. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tchkonia T, Tchoukalova YD, Giorgadze N,
Pirtskhalava T, Karagiannides I, Forse RA, Koo A, Stevenson M,
Chinnappan D, Cartwright A, et al: Abundance of two human
preadipocyte subtypes with distinct capacities for replication,
adipogenesis, and apoptosis varies among fat depots. Am J Physiol
Endocrinol Metab. 288:E267–E277. 2005. View Article : Google Scholar
|
|
24
|
Rutering J, Ilmer M, Recio A, Coleman M,
Vykoukal J and Alt E: Improved method for isolation of neonatal rat
cardiomyocytes with increased yield of C-Kit+ cardiac progenitor
cells. J Stem Cell Res Ther. 5:1–8. 2015.PubMed/NCBI
|
|
25
|
Chen A, Li G, Chen L, Guo J and Liu Y:
Downregulation of microRNA-100 protects
H2O2-induced apoptosis in neonatal
cardiomyocytes. Int J Clin Exp Pathol. 8:5491–5496. 2015.
|
|
26
|
Xu Y, Liu Z, Liu L, Zhao C, Xiong F, Zhou
C, Li Y, Shan Y, Peng F and Zhang C: Neurospheres from rat
adipose-derived stem cells could be induced into functional Schwann
cell-like cells in vitro. BMC Neurosci. 9:212008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Waddingham MT, Sonobe T, Tsuchimochi H,
Edgley AJ, Sukumaran V, Chen YC, Hansra SS, Schwenke DO, Umetani K,
Aoyama K, et al: Diastolic dysfunction is initiated by
cardiomyocyte impairment ahead of endothelial dysfunction due to
increased oxidative stress and inflammation in an experimental
prediabetes model. J Mol Cell Cardiol. 137:119–131. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Owen JB and Butterfiel DA: Measurement of
oxidized/reduced glutathione ratio. Methods Mol Biol. 648:269–277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fang Z, Yin X, Wang J, Tian N, Ao Q, Gu Y
and Liu Y: Functional characterization of human umbilical
cord-derived mesenchymal stem cells for treatment of systolic heart
failure. Exp Ther Med. 12:3328–3332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cawthorn WP, Scheller EL and MacDougald
OA: Adipose tissue stem cells meet preadipocyte commitment: Going
back to the future. J Lipid Res. 53:227–246. 2012. View Article : Google Scholar :
|
|
31
|
Zhu Y, Liu T, Song K, Fan X, Ma X and Cui
Z: Adipose-derived stem cell: A better stem cell than BMSC. Cell
Biochem Funct. 26:664–675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li TS, Cheng K, Malliaras K, Smith RR,
Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H,
et al: Direct comparison of different stem cell types and
subpopulations reveals superior paracrine potency and myocardial
repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol.
59:942–953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mazo M, Hernández S, Gavira J, Abizanda G,
Araña M, López-Martínez T, Moreno C, Merino J, Martino-Rodríguez A,
Uixeira A, et al: Treatment of reperfused ischemia with
adipose-derived stem cells in a preclinical Swine model of
myocardial infarction. Cell Transplant. 21:2723–2733. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sadat S, Gehmert S, Song YH, Yen Y, Bai X,
Gaiser S, Klein H and Alt E: The cardioprotective effect of
mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem
Biophys Res Commun. 363:674–679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tachida Y, Suda K, Nagase H, Shimada K,
Isono F and Kobayashi H: Secreted factors from adipose
tissue-derived mesenchymal stem cells suppress oxygen/glucose
deprivation-induced cardiomyocyte cell death via furin/PCSK-like
enzyme activity. Biochem Biophys Rep. 7:266–272. 2016.PubMed/NCBI
|
|
36
|
Panina YA, Yakimov AS, Komleva YK, Morgun
AV, Lopatina OL, Malinovskaya NA, Shuvaev AN, Salmin VV,
Taranushenko TE and Salmina AB: Plasticity of adipose
tissue-derived stem cells and regulation of angiogenesis. Front
Physiol. 9:16562018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grinnemo KH, Månsson-Broberg A, Leblanc K,
Corbascio M, Wärdell E, Siddiqui AJ, Hao X, Sylvén C and Dellgren
G: Human mesenchymal stem cells do not differentiate into
cardiomyocytes in a cardiac ischemic xenomodel. Ann Med.
38:144–153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Atsma DE, Fibbe WE and Rabelink TJ:
Opportunities and challenges for mesenchymal stem cell-mediated
heart repair. Curr Opin Lipidol. 18:645–649. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang JM, Wang JN, Zhang L, Zheng F, Yang
JY, Kong X, Guo LY, Chen L, Huang YZ, Wan Y and Chen SY: VEGF/SDF-1
promotes cardiac stem cell mobilization and myocardial repair in
the infarcted heart. Cardiovasc Res. 91:402–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deuse T, Peter C, Fedak PW, Doyle T,
Reichenspurner H, Zimmermann WH, Eschenhagen T, Stein W, Wu JC,
Robbins RC and Schrepfer S: Hepatocyte growth factor or vascular
endothelial growth factor gene transfer maximizes mesenchymal stem
cell-based myocardial salvage after acute myocardial infarction.
Circulation. 120(11 Suppl): S247–S254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park SG, Kim JH, Xia Y and Sung JH:
Generation of reactive oxygen species in adipose-derived stem
cells: Friend or foe? Expert Opin Ther Targets. 15:1297–1306. 2011.
View Article : Google Scholar : PubMed/NCBI
|