Introduction
An inflammatory response is the result of a complex
and interconnected series of events of the immune system aimed at
destroying foreign agents or altered cells, but inevitably leading
to healthy tissue damage at the site of injury (1). In particular, activated neutrophils,
which are key effectors of the innate immune responses, generate
reactive oxygen species (ROS) and reactive nitrogen species (RNS),
proteolytic enzymes and neutrophil extracellular traps to eliminate
pathogens and dying cells (2).
However, these molecules can also interact with nucleic acids,
proteins and lipids of the surrounding healthy tissues, causing
changes in their structure and function with detrimental
consequences for cellular homeostasis (3). The neutrophil-mediated activation of
the immune response, as a causative or secondary factor, occurs in
a broad range of pathologies, including post-ischemic reperfusion
damage, neurodegeneration, obstructive pulmonary disease, obesity,
diabetes and cancer (4). The
excessive activated neutrophil recruitment also takes place in a
variety of intestinal dysfunctions and inflammatory conditions,
including intestinal ischemia and inflammatory bowel diseases
(IBD), including Crohn’s disease and ulcerative colitis (5,6).
Therefore, there is a growing effort to understand the mechanisms
associated with the action of neutrophils in inflammatory sites,
and to identify novel factors that may modulate neutrophil behavior
in order to develop therapeutic strategies to limit or neutralize
their destructive action.
Serotonin, histamine, the putrescine diamine, the
spermine and spermidine polyamines exert immunomodulatory,
neuroactive and proliferative functions (7). Consequently, during the last few
decades copper-containing amine oxidases (Cu-AOs) have been
proposed as efficient anti-inflammatory and anti-allergic agents
(8), due to their ability to
metabolize the primary amine groups of these biogenic amines as
follows (9):
R-CH2-NH2+O2+H2O →
RCHO+NH3+H2O2. The beneficial
effects of Cu-AOs from vegetal sources have been documented both in
in vivo and ex vivo animal models of inflammatory or
allergic conditions, including asthma-like reaction (10) and myocardial (11) or intestinal (12) ischemia-reperfusion injuries, in
which the treatment with vegetal Cu-AO was demonstrated to promote
the alleviation of these dysfunctions and the decrease of tissue
histamine level, neutrophil infiltration and cellular oxidative
damage. Also, the removal of histamine, which is an efficient
substrate of vegetal Cu-AO (13)
(for this reason the enzyme is also called histaminase, as well as
diamine oxidase (DAO) for its ability to metabolize diamines), was
associated with the anti-inflammatory function of this enzyme and
its anti-allergic properties. Histamine, which is a major mast
cell-derived mediator, is not only an efficient modulator of
endothelial cell contraction and vascular permeability, but also a
pro-inflammatory amine able to induce the expression of endothelial
cell adhesion molecules, thus promoting the rolling and
extravasation of leukocytes (14).
Cu-AO purified from bovine serum (BSAO) has been
reported to exert a cardioprotective effect in isolated rat hearts
exposed to electrolysis-generated ROS, and to have a scavenging
capacity against ROS and their by-products generated by
electrolysis in Krebs-Henseleit buffer (superoxide, hydroxyl
radical, singlet oxygen, hydrogen peroxide and hypochlorous acid)
(15), despite the fact that
Cu-AOs generate hydrogen peroxide. It has been hypothesized that
the beneficial effects of plant Cu-AO are associated with its
scavenging capacity of ROS and RNS that are generated by activated
neutrophils and the consequent reduction of tissue oxidative damage
(8). However, direct evidence of
the scavenging activity of vegetal Cu-AO is lacking.
With the aim to clarify the antioxidant and
anti-inflammatory properties of vegetal Cu-AO and further define
its potential use as a novel antiallergic and anti-inflammatory
agent, the present study analyzed the scavenging properties of
Cu-AO purified from Lathyrus sativus (LSAO) seedlings
against ROS and RNS generated chemically in cell-free systems.
Moreover, vascular adhesion protein 1 (VAP-1) is a peculiar Cu-AO
of the plasma membrane of vascular endothelial and smooth muscle
cells, and it is able to interact with specific determinants on the
membrane of circulating leukocytes, promoting their extravasation
to the site of the inflammation (16). Therefore, the ability of LSAO to
directly affect neutrophil functions was studied in vitro by
investigating the effects of the enzyme on some functional
responses of isolated human neutrophils activated by
formyl-methionyl-leucyl-phenylalanine peptide (fMLP). Fabaceae
contain a high concentration of Cu-AO in the cellular periplasm
(17). In particular, crude
homogenates of Lathyrus sativus seedlings are a rich source
of the enzyme (18), justifying
the use of this plant.
Materials and methods
Materials
Aminoantipyrine (AAP), bovine heart cytochrome
c, bovine milk xanthine oxidase, bovine serum albumin (BSA),
4-bromo-calcium ionophore A23187, cytochalasin B,
3,5-dichloro-2-hydroxybenzensulfonic acid (DCHBS), fMLP, glucose,
horseradish peroxidase (HRP), hydrogen peroxide
(H2O2),
N-methoxysuccinyl-Ala-Ala-Pro-Val-7-amido-4-methylcoumarin
(MeO-Suc-Ala-Ala-Pro-Val-AMC), porcine pancreas elastase,
sulphanilamide, sodium diethyldithiocarbamate (DDC), sodium
nitroprusside, sodium nitrite, thapsigargin (TG), trypan blue and
xanthine were purchased from Merck KGaA. Fura-2-acetoxymethyl ester
(Fura-2AM) was purchased from Thermo Fisher Scientific, Inc.,
Polymorphprep™ solution from Sentinel Diagnostics and Superdex D200
FPLC column from GE Healthcare. Ultrafiltration devices with a
cut-off of 30 kDa were obtained from Sartorious AG. QCM™ Chemotaxis
3 μm 96-well cell migration assay system was from Merck KGaA. Other
chemicals were analytical grade and were used without further
purification.
LSAO purification and
characterization
LSAO was prepared from Lathyrus sativus
seedlings, according to a large-scale preparation method (19). Following purification, samples
were dialyzed at 4°C overnight against 50 mM phosphate buffer
(1:1,000; pH 7.4), concentrated on ultrafiltration devices and
further purified by size exclusion chromatography on a Superdex
D200 FPLC column, equilibrated and eluted with 50 mM phosphate
buffer (pH 7.4) at a flow rate of 0.5 ml/min using a FPLC System
(GE Healthcare). Aliquots with the highest AO-specific activity
were pulled together, concentrated using ultrafiltration and stored
at −20°C until use. The purified protein (10 μg/lane) moved as a
single band on 7.5% SDS-PAGE. LSAO preparations were characterized
for protein content and enzymatic activity was measured in terms of
H2O2 generation from the oxidation of 3 mM
putrescine, as previously reported (20). LSAO preparations with a specific
activity of 40±5 U/mg protein were used. 1 U enzyme oxidizes 1 μmol
substrate/min.
Catalytically inactive LSAO samples were prepared
upon the depletion of copper from the active site by DDC treatment
(21). The residual copper
content was measured as previously described (22).
Neutrophil isolation
Neutrophils were purified from heparinized human
blood, as previously reported (23), freshly drawn from repeat healthy
blood donors at the Immunohematology and Transfusion Medicine Unit
at Policlinico Umberto I, Sapienza University of Rome (Rome, Italy)
(~7 ml blood/donor). All repeat blood donors (23 women and 47 men;
age 18–65 years) gave written informed consent approved by the
Ethic Committee of Policlinico Umberto I, Sapienza University of
Rome. Following separation in Polymorphprep™ density gradient
medium by centrifugation at 500 x g for 30 min at 20°C, the cells
were collected according to the manufacturer’s protocol, suspended
in PBS (pH 7.4), containing 140 mM NaCl, 2.7 mM KCl, 12 mM
Na2HPO4, 1.5 mM KH2PO4,
5 mM glucose and 0.5 mM MgCl2 and stored on ice. Each
preparation contained 90–98% neutrophils and was free of
contaminating erythrocytes as detected using an inverted Axiovert
40 C microscope at a magnification of x100 (Carl Zeiss AG). The
neutrophils had a viability of >90% when kept in ice for up to 6
h after purification, as determined using the trypan blue exclusion
test (24).
Effect of LSAO on superoxide generated
from the oxidation of xanthine by xanthine oxidase and from
fMLP-activated human neutrophils
The effect of LSAO on superoxide, which was produced
from the oxidation of xanthine by xanthine oxidase as previously
reported (25), was measured for
20 min by adding 10 μM LSAO enzyme to 50 mM phosphate buffer (pH
7.8) containing 10 μM bovine heart cytochrome c, 0.1 mM
EDTA, 50 μM xanthine and bovine milk xanthine oxidase (sufficient
to reduce cytochrome c at a rate of 0.025 absorbance unit
per min in the absence of LSAO) in a spectrophotometric cuvette at
25°C.
To examine the effect of LSAO on the generation of
superoxide by human neutrophils, a suspension of cells
(2x106 cells/ml) was incubated at 37°C for 5 min in a
spectrophotometric cuvette in PBS containing 60 μM bovine heart
cytochrome c, 1 mM CaCl2 and 1 μg/ml cytochalasin
B in the presence of 10 μM LSAO. For neutrophil activation, 1 μM
fMLP was added to induce superoxide production (26).
In both cases, superoxide formation was evaluated by
recording absorbance at 550 nm in a UV-1700 Shimadzu
spectrophotometer (Shimadzu Corporation) to assess the change of
absorbance associated with the superoxide-induced cytochrome
c reduction. The concentration of reduced cytochrome
c was calculated assuming an extinction coefficient of
27,600 M−1 cm−1 (27).
Scavenging effect of LSAO on
H2O2 and nitric oxide (NO)
The effect of 10 μM LSAO on
H2O2 was examined by incubating the enzyme at
25°C for 195 min in 50 mM phosphate buffer pH 7.0 containing 30 μM
hydrogen peroxide. The concentration of H2O2
was estimated by adapting the previously reported method (20) to a microplate procedure, in which
the presence of H2O2 is detected by the
formation of a pink adduct generated in the presence of AAP, DCHBS
and HRP. At time intervals of 60 min, 110 μl aliquots were
withdrawn and added to 90 μl of a solution containing 25 U/ml HRP,
4.5 mM AAP and 9 mM DCHBS on a 96 well-microplate. The microplate
was incubated at room temperature for 15 min, and the optical
density was then read at 515 nm using an Appliskan microplate
reader (Thermo Fisher Scientific, Inc.). The
H2O2 concentrations were calculated using a
standard curve obtained with stock solutions of
H2O2, whose concentrations were estimated
spectrophometrically assuming a molar extinction coefficient of
26,000 M−1 cm−1 (20).
The scavenging activity of LSAO on NO was evaluated
in terms of the ability of the enzyme to decrease the rate of
nitrite formation from NO generated in sodium nitroprusside
solution, as previously reported (28). Briefly, sodium nitroprusside at a
concentration of 50 mM was dissolved in 50 mM phosphate buffer (pH
7.4) continuously purged by N2. Aliquots were then
diluted ten times in 50 mM phosphate buffer (pH 7.4) containing 10
μM LSAO and incubated at 25°C for 60, 150, 230 and 290 min.
Aliquots were then withdrawn, added to an equal volume of Griess
reagent (2% w/v sulphanilamide, 4% w/v H3PO4,
0.2% w/v naphthylethylenediamide) and the absorbance was read at
546 nm. NO concentration was expressed as nitrite using a standard
curve prepared with sodium nitrite solutions at concentrations of
2.5, 5, 10, 25 and 50 μM.
Effect of LSAO on azurophilic
degranulation in fMLP-activated human neutrophils
Human neutrophils were incubated for 5 min at a
concentration of 2x106 cells/ml at 37°C in PBS
containing 1 mM CaCl2, 1 μg/ml cytochalasin B, different
concentrations of LSAO (0.5, 1, 2.5, 5 and 10 μM) and 40 μM
MeO-Suc-Ala-Ala-Pro-Val-AMC, a fluorogenic substrate of elastase.
Cells were then exposed to 1 μM fMLP and the concentration of
released elastase was evaluated fluorimetrically on a Jasco-750
spectrofluorometer (Jasco Inc.) in terms of its catalytic activity
by recording the fluorescence developed from the proteolysis of its
substrate MeO-Suc-Ala-Ala-Pro-Val-AMC, as previously reported
(29). To verify that LSAO did
not inhibit the elastase catalytic activity, LSAO was incubated at
37°C with purified porcine elastase in PBS containing 1 mM
CaCl2 and 1 μg/ml cytochalasin B. The elastase activity
was then recorded following the addition of 40 μM
MeO-Suc-Ala-Ala-Pro-Val-AMC as aforementioned.
Effect of LSAO on cellular migration in
fMLP-activated human neutrophils
The effect of LSAO on neutrophil migration was
assessed using the QCM™ Chemotaxis 3-μm 96-well cell migration kit.
Neutrophil suspension (100 μl; 1x106 cells/ml) in PBS
containing 1 mM CaCl2 were added to the upper migration
chamber of the provided microplate device; 150 μl PBS aliquots were
placed in the lower microplate chamber, and 10 nM fMLP and/or LSAO
(1 and 10 μM) were added. To avoid uncontrolled cell adhesion to
the plates, PBS was supplemented with BSA at a final concentration
of 1 mg/ml. The microplate was then kept in an incubator in a
humidified atmosphere of 5% CO2 for 1 h at 37°C to allow
the migration of neutrophils from the upper to the lower chamber.
Following incubation, the upper migration chamber was removed, the
wells of the lower chamber were treated for 15 min at room
temperature with the lysis buffer and the dye solution according to
the manufacturer’s protocol, and the fluorescence of each sample,
due to the binding of the dye to DNA, was read at a 535 nm emission
upon excitation at 485 nm using an Appliskan microplate reader
(Thermo Fisher Scientific, Inc.). The number of migrated cells was
estimated using a standard curve obtained by adding from
0.2x105 to 1.0x105 total cells per well.
Effect of LSAO on calcium flux in
fMLP-activated human neutrophils
Human neutrophils at a final concentration of
107 cells/ml were incubated with 5 μM Fura-2AM, a
specific calcium probe, for 30 min at 37°C in PBS. Cells were then
washed, suspended in PBS at a final concentration of
2x107 cells/ml and kept on ice to be used within the
next 2–4 h. Neutrophil suspensions were diluted ten times in PBS
buffer in a quartz cuvette, incubated at 37°C for 5 min and then
exposed to different agents (1 mM CaCl2; 0.2 or 2 mM
EGTA; 1 μM fMLP ; 10 μM LSAO or BSA; 8 nM TG or 1.6 μM 4-Bromo
A23187). Fluorescence emission was measured at 485 nm upon
excitation at 340 nm with a Jasco-750 spectrofluorometer (Jasco
Inc.). The cytosolic free calcium concentration was calculated as
previously described (30).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed using one-way ANOVA with
Tuckey’s post hoc test for comparisons involving three or more
groups. GraphPad Prism® version 4.00 for Windows
(GraphPad Software, Inc.) was used to perform statistical analysis.
P≤0.05 was considered to indicate a statistically significant
difference.
Results
Effect of LSAO on superoxide,
H2O2 and nitric oxide (NO)
Cu-AO prepared from Lathyrus sativus
seedlings, at concentrations of 10 μM, did not affect the rate of
bovine heart cytochrome c reduction by superoxide generated
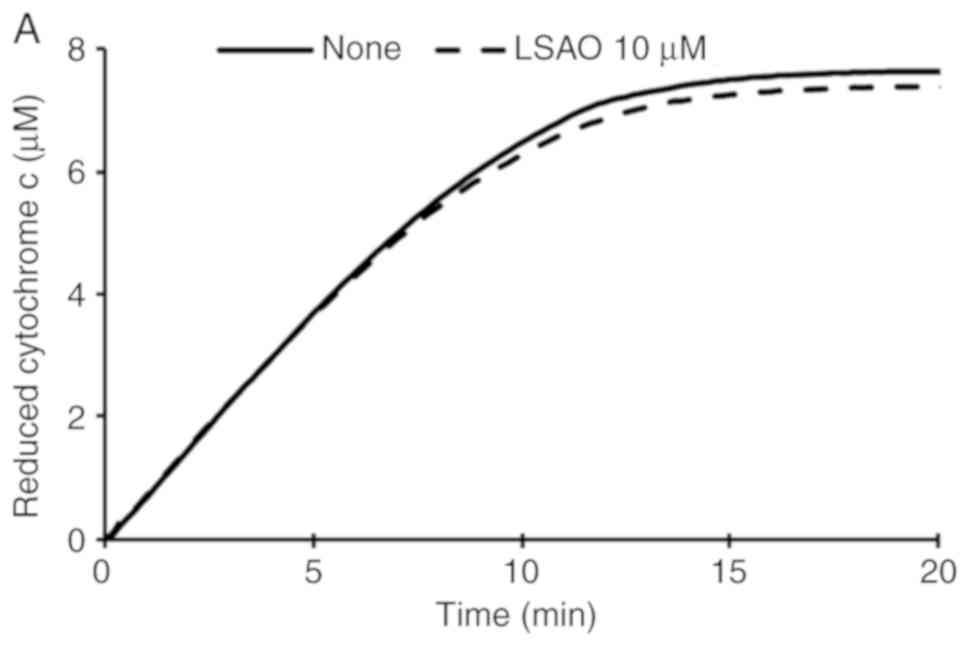
from the oxidation of xanthine by xanthine oxidase (Fig. 1A). However, it slowed down the
rate of cytochrome c reduction added to human neutrophils
exposed to 1 μM fMLP (Fig. 1B),
indicating that the protein is not a scavenger of superoxide, but
it is able to interfere with the fMLP-activated cellular mechanisms
of superoxide generation.
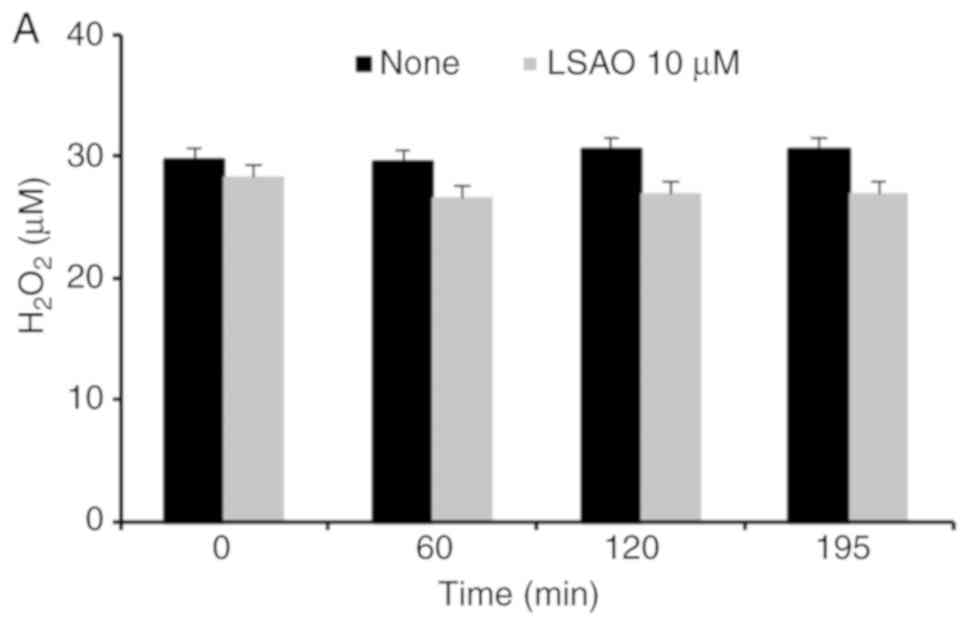
LSAO did not interact with
H2O2 either, with no decrease of hydrogen
peroxide concentration in solutions incubated with LSAO for 60, 120
and 195 min (Fig. 2A). Moreover,
similar amounts of nitrite were generated from solutions of 5 mM
nitroprusside in the absence or presence of 10 μM LSAO, indicating
that the enzyme did not scavenge NO (Fig. 2B).
Effect of LSAO on fMLP-activated human
neutrophil functional responses
Azurophilic degranulation
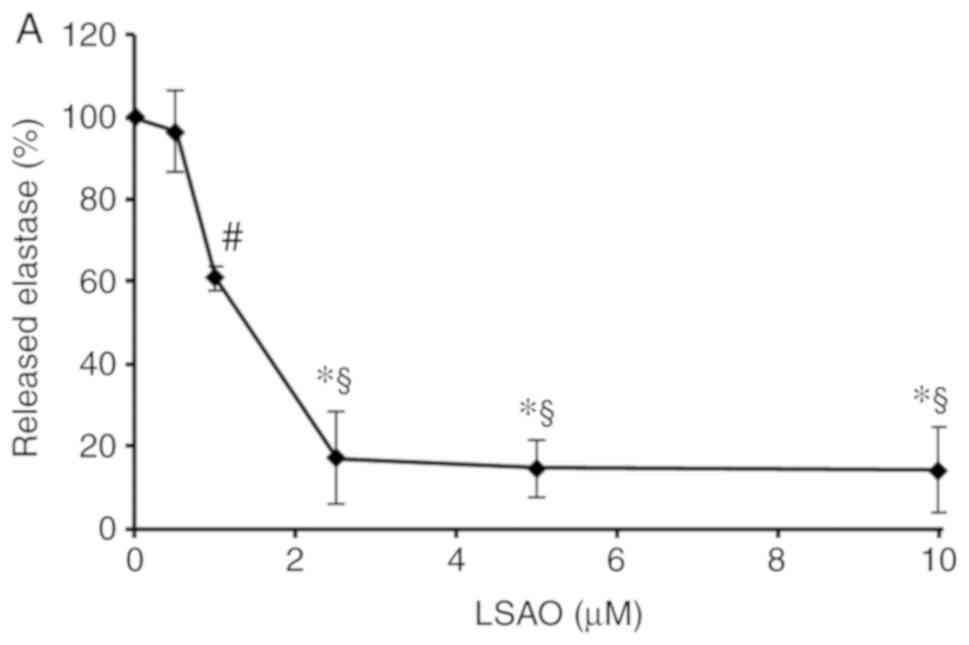
LSAO was indicated to markedly reduce the
fMLP-induced azurophilic degranulation in human neutrophils as
evaluated by elastase release (Fig.
3A). The activity of elastase released in neutrophil
suspensions exposed to fMLP was lower in the presence of LSAO than
in its absence, whereas in cell-free control experiments LSAO
incubated with purified porcine elastase did not affect its
proteolytic activity (data not shown). The effect of LSAO on the
elastase released from neutrophils activated by fMLP was
concentration-dependent with an IC50 of 1.2±0.2 μM
(Fig. 3A). Under the same
experimental conditions, degranulation was not affected by 10 μM
BSA (data not shown), suggesting that the inhibition was
LSAO-specific. The effect of the LSAO was not dependent on its
catalytic activity. LSAO, inactivated by treatment with DDC, which
removes copper from the active site of the enzyme, exhibited a
similar effect as that of the catalytically active form (data not
shown). In DDC-treated LSAO, the residual copper was <5% of the
original content, and the residual LSAO enzymatic activity was
<3%.
Cellular migration
Cu-AO from Lathyrus sativus was also
indicated to interfere in a concentration-dependent manner with
neutrophil migration. In the absence of fMLP, LSAO promoted a
slight, but not statistically significant, migration of neutrophils
when added at concentrations of 1 and 10 μM. However, when
neutrophils were activated by 10 nM fMLP, 10 μM LSAO inhibited cell
migration (Fig. 3B). Similar
effects were observed with DDC-inhibited LSAO (data not shown).
Furthermore, LSAO was not toxic under these experimental
conditions. In fact, human neutrophils had a >90% viability when
incubated for 1 h in PBS containing 1 mM CaCl2 with or
without 1 mg/ml BSA, either in the absence or in the presence of 10
μM LSAO.
Calcium flux
Exposure of human neutrophils to LSAO altered the
calcium cellular homeostasis. In the presence of 10 μM LSAO, the
entry of calcium following the addition of 1 mM CaCl2
was lower compared with the absence of the enzyme (Fig. 4A), indicating that LSAO can
interact with the neutrophil membrane systems responsible for
calcium influx from the extracellular space. In addition, the
enzyme inhibited ~50% of the increase in the intracellular calcium
level promoted by the exposure to 1 μM fMLP, demonstrating that
LSAO also interfered with the fMLP-dependent activation of calcium
mobility. BSA (10 μM) was not observed to affect cellular calcium
mobility (Fig. 4A).
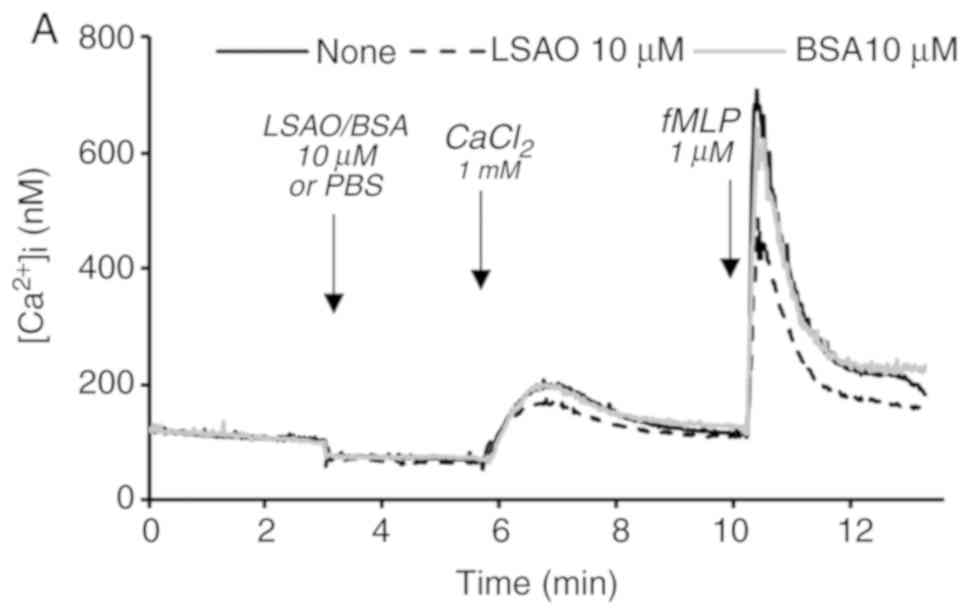 | Figure 4Effect of LSAO on: (A) Extracellular
calcium entry into Ca2+-depleted neutrophils and
transient increase of [Ca2+]i induced by fMLP
addition. One experiment representative of n=3. (B) Intracellular
calcium release from internal stores triggered by fMLP. One
experiment representative of n=3. (C) Store-operated calcium entry
promoted by thapsigargin. One experiment representative of n=3.
Different agents were added, as indicated by the arrows. Cells in
the absence (continuous black line) or presence of 10 μM LSAO
(dashed black line) or 10 μM BSA (continous grey line). LSAO, Cu-AO
purified from Lathyrus sativus; fMLP,
formyl-methionyl-leucyl-phenylalanine peptide; BSA, bovine serum
albumin; CaCl2, calcium chloride; EGTA, ethylene
glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid; PBS,
phosphate-buffered saline; TG, thapsigargin. |
In neutrophils treated with 1 mM CaCl2
(to load the intracellular calcium reserves) and then exposed to 2
mM EGTA (to chelate the extracellular calcium, and detect the
calcium efflux from the internal stores to the cytosol), the
addition of 10 μM LSAO caused a small, transient increase of the
cytosolic calcium level (Fig.
4B). This behavior showed that LSAO may serve a role in the
cell systems, promoting calcium mobility from the internal stores.
Moreover, LSAO treatment affected the cytosolic calcium level
increase when the cells were subsequently activated by 1 μM fMLP
(Fig. 4B). This indicated that
LSAO also interfered with the fMLP-mediated release of calcium from
the intracellular stores. In line with this evidence, following the
addition of 1.6 μM 4-Bromo A23187 (a Ca2+ ionophore used
in the presence of EGTA to evaluate the concentration of calcium in
the internal stores), the internal stores were indicated to be
almost completely empty with a slightly higher residual calcium
level in samples treated with LSAO (Fig. 4B). This confirmed that, in the
presence of LSAO the fMLP-dependent release of calcium from
internal stores was impaired. The exposure of neutrophils to 10 μM
BSA did not affect cellular calcium mobility (Fig. 4B), suggesting again that the
effect of LSAO was protein-specific.
The effect of LSAO on calcium influx in the absence
of fMLP was confirmed in neutrophils treated with 8 nM TG (Fig. 4C), which inhibits the endoplasmic
reticulum Ca2+-ATPase with consequent emptying of the
intracellular calcium reserves and activation of the store-operated
calcium entry (SOCE) (31). The
SOCE is the major mechanism of calcium influx into neutrophils,
through which the calcium-filling level in the endoplasmic
reticulum regulates the entry of calcium from the extracellular
space to the cytosol (32). In
human neutrophils treated with TG, the presence of 10 μM LSAO
inhibited the increase of the intracellular calcium level by ~40%
when 1 mM CaCl2 was added (Fig. 4C). This suggests a possible
interaction of LSAO with the plasma membrane systems responsible
for calcium entry, such as the calcium-release activated channels
(CRAC) promoted by SOCE (33).
Discussion
Over the last few decades, the administration of
vegetal Cu-AO has been proposed as a potential antioxidant,
anti-allergic and anti-inflammatory strategy, due to its beneficial
effects in different animal model systems (8). This approach was also supported by
its well-known ability to metabolize histamine (13) and by the capability of BSAO to
scavenge electrolysis-induced ROS in Krebs-Henseleit buffer
(15).
The current study into the properties of vegetal
Cu-AO was extended to include an exploration of the ROS and RNS
scavenging properties of the enzyme purified from Lathyrus
sativus seedlings. Moreover, the effect of LSAO on neutrophil
functions was analyzed. An altered activation of these cells is
involved in the pathogenesis of different autoimmune and
inflammatory conditions (i.e., Crohn’s disease, ulcerative colitis,
rheumatoid arthritis and systemic lupus erythematosus) (4). Therefore, the regulation of these
functions is of therapeutic interest.
The obtained data indicated that Cu-AO purified from
Lathyrus sativus seedlings does not have intrinsic
scavenging properties on superoxide and hydrogen peroxide (Figs. 1A and 2A). This suggests that the previously
reported scavenging capability of BSAO against ROS and their
by-products, generated by electrolysis in Krebs-Henseleit buffer
(15) might be due to the
interaction of this protein with reactive species other than
superoxide and hydrogen peroxide, including hydroxyl radical,
hypochlorous acid or singlet oxygen. Furthermore, the different
behaviors of LSAO and BSAO might be due to different structural
features. The primary structure of LSAO is not known; however, a
sequence homology of ~23% has been reported between Cu-AO purified
from pea and lentil seedlings with BSAO (34). In addition, LSAO is not a
scavenger of NO (Fig. 2B). This
is consistent with previous data showing that, in isolated hearts
from guinea pigs during anaphylaxis, pea-seedling Cu-AO promoted
the release of NO, which would not be observed if the enzyme was a
scavenger of NO (35).
Despite the lack of ROS and RNS scavenging activity,
LSAO exerted an antioxidant effect by inhibiting the generation of
superoxide in human neutrophils stimulated by fMLP (Fig. 1B). This effect might be due to a
possible competition of the enzyme with fMLP interfering with its
binding to FPR1, a G protein-coupled receptor constitutively
expressed on the surface of neutrophils (36). Consequently, the activation of the
cellular signaling pathways responsible for the assembly of the
NADPH-oxidase complex may be reduced. However, a direct interaction
of LSAO with the subunits gp91-phox and p22 of the NADPH oxidase
complex located on the cellular membrane cannot be excluded,
leading to an impaired complex assembly.
The binding of fMLP to FPR1 also regulates the
exocytosis of the intracellular granules and vesicles, chemotactic
migration and calcium release from endoplasmic reticulum (37,38). The exposure of neutrophils to fMLP
in the presence of LSAO results in the inhibition of elastase
release, cell migration (Fig. 3A and
B) and calcium mobility (Fig. 4A
and B), confirming that the vegetal enzyme can affect the
interaction of fMLP with its receptor.
However, the ability of LSAO to affect cellular
migration (Fig. 3B) and calcium
mobility (Fig. 4B and C), in the
absence of fMLP, suggests that in addition to FPR1, the vegetal
enzyme can also directly interact with other components of the
plasma membrane, contributing to the regulation of the neutrophil
functions. In particular, the inhibition of calcium entry by LSAO
in neutrophils treated with TG (Fig.
4C) suggests that vegetal DAO can directly interact with
components of the CRAC channels responsible of calcium transport
(i.e., stromal interaction molecule, calcium release-activated
calcium modulator 1 and transient receptor potential channels)
(33). Since fMLP-dependent
signaling regulates neutrophil generation of superoxide, elastase
release and chemotactic migration via cytosolic calcium increase
(36), the capacity of LSAO to
interfere with the CRAC-dependent calcium entry could also explain
the inhibitory effect of the enzyme on the activation of
neutrophils by fMLP.
The ability of LSAO to interact with neutrophil
proteins is consistent with the capacity of free BSAO to interact
with human hemoglobin (39) or
that of gold-complexed BSAO to bind protein clusters on the surface
of cultured human hepatocytes (40). In the latter case, the binding of
the enzyme to the cellular membrane proteins was partially
inhibited by N-acetyl-galactosamine, N-acetyl-glucosamine and
mannose, suggesting that the BSAO carbohydrate chains are also
important for protein-protein interaction. Although the
carbohydrate moiety of LSAO has not been characterized, it is well
known that pea and lentil Cu-AO are glycoproteins (41); therefore, it is possible that LSAO
can associate with the proteins of the human neutrophil membrane
via its glycosidic moiety.
In addition, BSAO has been documented to generate
H2O2 when incubated with polylysine, lysozyme
or ribonuclease A (42),
suggesting its ability to modify target proteins by the oxidative
deamination of their lysines to the corresponding aldehydes. In
addition, the vascular adhesion protein, VAP-1, of the endothelial
cells, has been reported to hook granulocytes, monocytes and
lymphocytes and contribute to their extravasation through the
formation of a covalent, but transient, Schiff base between its
2,4,5-trihydroxyphenylalanine cofactor and lysines of Siglec-9 and
Siglec-10 proteins present on the surface of these white cells
(43). However, under our
experimental conditions, a modification of the neutrophil membrane
proteins upon deamination of their amine groups by the LSAO
enzymatic activity may be ruled out since the catalytically
inactive LSAO was able to inhibit neutrophil functions, such as the
release of elastase and cell migration.
In conclusion, the results of the current study
indicated that LSAO up to 10 μM concentration, reported to protect
the human intestinal Caco-2 cell line from histamine-induced damage
(20), is able to interfere with
the activation of human neutrophils. This property, together with
the ability to catabolyze histamine, confirms the anti-inflammatory
effect of this enzyme and supports the use of vegetal Cu-AO for the
treatment of inflammatory conditions; in particular enteric
dysfunctions, including Crohn’s disease and ulcerative colitis,
where the transmigration of neutrophils into the intestinal lumen
triggers drastic mucosal injury (44). In recent years, there has been
increasing interest in the oral administration of vegetal Cu-AO as
a novel complementary therapeutic strategy for the alleviation of
food allergies and IBD symptoms (8). Vegetal Cu-AO has already been
formulated for oral administration as monolithic tablets with
carboxymethyl-starch (45). This
is a pH-responsive excipient, which is protonated in gastric
acidity, forming an external compact layer, and is then swollen and
dissoluted in neutral intestinal media where it favors the delivery
of catalytically active enzymes. Furthermore, the effect of Cu-AO
from Lathyrus sativus, which is rectally administered, in an
in vivo murine intestinal inflammation model is currently
under investigation (manuscript in preparation).
Acknowledgements
The authors would like to thank Professor G.
Girelli, Dr E. Panzini (Immunohaematology and Transfusion Medicine
Unit at the Policlinico Umberto I, Sapienza University of Rome) for
providing blood samples.
Abbreviations:
|
AAP
|
aminoantipyrine
|
|
BSA
|
bovine serum albumin
|
|
BSAO
|
bovine serum Cu-AO
|
|
CRAC
|
calcium-release activated channels
|
|
Cu-AO
|
copper-containing amine oxidase
|
|
DAO
|
diamine oxidase
|
|
DCHBS
|
3,5-dichloro-2-hydroxybenzensulfonic
acid
|
|
EGTA
|
ethylene glycol-bis(β-aminoethyl
ether)-N,N,N′,N′-tetraacetic acid
|
|
fMLP
|
formyl-methionyl-leucyl-phenylalanine
peptide
|
|
HRP
|
horseradish peroxidase
|
|
IBD
|
inflammatory bowel disease
|
|
LSAO
|
Cu-AO purified from Lathyrus
sativus
|
|
PBS
|
phosphate-buffered saline
|
|
NO
|
nitric oxide
|
|
ROS
|
reactive oxygen species
|
|
RNS
|
reactive nitrogen species
|
|
SOCE
|
store-operated calcium entry
|
|
TG
|
thapsigargin
|
|
VAP-1
|
vascular adhesion protein 1
|
Funding
The study was supported from the Regione Lazio of
Italy (grant no. FILAS-RU-2014-1020), MAECI (Italy)-MRIF (Québec,
Canada) Joint Project (2017–2019) and Fondation Courtois
(Canada).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
PP purified, modified and characterized the
catalytic activity of LSAO, as well as its scavenging properties on
ROS and RNS. PP also contributed to the isolation of neutrophils
from whole human blood and to the analysis of calcium mobility,
performed the assay of cellular migration, collected and analyzed
the data and contributed to manuscript editing. EC isolated
neutrophils from whole human blood, designed and performed the
study on calcium mobility and elastase release, analyzed the data
and contributed to manuscript editing. MAM contributed to the
concept of the intestinal administration of Cu-AO, the acquisition
of funding and manuscript editing. LM purified, modified LSAO and
characterized its catalytic activity, as well as its scavenging
properties on ROS and RNS. LM also contributed to the analysis of
calcium mobility, performed the assay of cellular migration,
conceived and designed the current study, contributed to the
acquisition of funding, collected, analyzed the data and wrote the
original draft of the manuscript. All authors read and approved the
manuscript.
Ethics approval and consent to
partecipate
Healthy blood donors gave written informed consent
approved by the Ethics Committee of Policlinico Umberto I, Sapienza
University of Rome (Rome, Italy).
Patient consent for publication
Healthy blood donors gave written informed consent
for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kipanyula MJ, Seke Etet PF, Vecchio L,
Farahna M, Nukenine EN and Nwabo Kamdje AH: Signaling pathways
bridging microbial-triggered inflammation and cancer. Cell Signal.
25:403–416. 2013. View Article : Google Scholar
|
|
2
|
Witko-Sarsat V, Rieu P, Descamps-Latscha
B, Lesavre P and Halbwachs-Mecarelli L: Neutrophils: Molecules,
functions and pathophysiological aspects. Lab Invest. 80:617–653.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liew PX and Kubes P: The neutrophil’s role
during health and disease. Physiol Rev. 99:1223–1248. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tintinger GR, Anderson R and Feldman C:
Pharmacological approaches to regulate neutrophil activity. Semin
Immunopathol. 35:395–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dinallo V, Marafini I, Di Fusco D, Laudisi
F, Franzè E, Di Grazia A, Figliuzzi MM, Caprioli F, Stolfi C,
Monteleone I and Monteleone G: Neutrophil extracellular traps
sustain inflammatory signals in ulcerative colitis. J Crohns
Colitis. 27:772–784. 2019. View Article : Google Scholar
|
|
6
|
Mumy KL and McCormick BA: The role of
neutrophils in the event of intestinal inflammation. Curr Opin
Pharmacol. 9:697–701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Medina MA, Urdiales JL, Rodríguez-Caso C,
Ramírez FJ and Sánchez-Jiménez F: Biogenic amines and polyamines:
Similar biochemistry for different physiological missions and
biomedical applications. Crit Rev Biochem Mol Biol. 38:23–59. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mondovì B, Fogel WA, Federico R, Calinescu
C, Mateescu MA, Rosa AC and Masini E: Effects of amine oxidases in
allergic and histamine-mediated conditions. Recent Pat Inflamm
Allergy Drug Discov. 7:20–34. 2013. View Article : Google Scholar
|
|
9
|
McIntire WS and Hartmann C: Copper
containing amine oxidases. Principles and Applications of
Quinoproteins. Davidson VL: Marcel Decker; New York, NY: pp.
97–171. 1992
|
|
10
|
Masini E, Vannacci A, Giannini L, Befani
O, Nistri S, Mateescu MA, Mannaioni PF, Mondovì B and Federico R:
Effect of a plant histaminase on asthmalike reaction induced by
inhaled antigen in sensitized guinea pig. Eur J Pharmacol.
502:253–264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masini E, Pierpaoli S, Marzocca C,
Mannaioni PF, Pietrangeli P, Mateescu MA, Zelli M, Federico R and
Mondovì B: Protective effects of a plant histaminase in myocardial
ischaemia and reperfusion injury in vivo. Biochem Biophys Res
Commun. 309:432–439. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Masini E, Cuzzocrea S, Bani D, Mazzon E,
Muja C, Mastroianni R, Fabrizi F, Pietrangeli P, Marcocci L,
Mondovì B, et al: Beneficial effect of a plant histaminase in a rat
model of splanchnic artery occlusion and reperfusion. Shock.
27:409–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pietrangeli P, Federico R, Mondovì B and
Morpurgo L: Substrate specificity of copper-containing plant amine
oxidases. J Inorg Biochem. 101:997–1004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamaki K, Thorlacius H, Xie X, Lindbom L,
Hedqvist P and Raud J: Characteristics of histamine-induced
leukocyte rolling in the undisturbed microcirculation of the rat
mesentery. Br J Pharmacol. 123:390–399. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mateescu MA, Dumoulin MJ, Wang XT, Nadeau
R and Mondovì B: A new physiological role of copper amine oxidases:
Cardioprotection against reactive oxygen intermediates. J Physiol
Pharmacol. 48(Suppl): S110–S121. 1997.
|
|
16
|
Jalkanen S and Salmi M: VAP-1 and CD73,
endothelial cell surface enzymes in leukocyte extravasation.
Arterioscler Thromb Vasc Biol. 28:18–26. 2008. View Article : Google Scholar
|
|
17
|
Angelini R, Rea G, Federico R and D’Ovidio
R: Spatial distribution and temporal accumulation of mRNA encoding
diamine oxidase during lentil (Lens culinaris Medicus) seedling
development. Plant Sci. 119:103–113. 1996. View Article : Google Scholar
|
|
18
|
Padiglia A, Cogoni A and Floris G:
Characterization of amine oxidases from Pisum, Lens, Lathyrus and
Cicer. Phytochemistry. 30:3895–3897. 1991. View Article : Google Scholar
|
|
19
|
Pietrangeli P, Nocera S, Federico R,
Mondovì B and Morpurgo L: Inactivation of copper-containing amine
oxidases by turnover products. Eur J Biochem. 71:146–152. 2004.
View Article : Google Scholar
|
|
20
|
Jumarie C, Séïde M, Marcocci L,
Pietrangeli P and Mateescu MA: Diamine oxidase from white pea
(Lathyrus sativus) combined with catalase protects the human
intestinal Caco-2 cell line from histamine damage. Appl Biochem
Biotechnol. 182:1171–1181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morpurgo L, Agostinelli E, Befani O and
Mondovì B: Reactions of bovine serum amine oxidase with
NN-diethyldithiocarbamate. Selective removal of one copper ion.
Biochem J. 248:865–870. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brumby PE and Massey V: Determination of
nonheme iron, total iron, and copper. Methods Enzymol. 10:473–474.
1967.
|
|
23
|
Ferrante A and Thong YH: Optimal
conditions for simultaneous purification of mononuclear and
polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll
method. J Immunol Methods. 36:109–117. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tennant JR: Evaluation of the Trypan Blu
technique for determination of cell viability. Transplantation.
2:685–694. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McCord JM and Fridovich I: Superoxide
Dismutase: An enzymic function for erythrocuprein (hemocuprein). J
Biol Chem. 24:6049–6055. 1969.
|
|
26
|
Cross AR, Parkinson JF and Jones OT: The
superoxide-generating oxidase of leucocytes. NADPH-dependent
reduction of flavin and cytochrome b in solubilized preparations.
Biochem J. 223:337–344. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Margoliash E and Frohwirt N: Spectrum of
horse heart cytochrome c. Biochem J. 71:570–572. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marcocci L, Packer L, Droy-Lefaix MT,
Sekaki A and Gardès-Albert M: Antioxidant action of Ginkgo biloba
extract EGb 761. Methods Enzymol. 234:462–475. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Capuozzo E, Pecci L, Giovannitti F,
Baseggio Conrado A and Fontana M: Oxidative and nitrative
modifications of enkephalins by human neutrophils: effect of
nitroenkephalin on leukocyte functional responses. Amino Acids.
43:875–884. 2012. View Article : Google Scholar
|
|
30
|
Salerno C and Capuozzo E: Effects of the
semisynthetic bis-indole derivative KAR-2 on store-operated calcium
entry in human neutrophils. Arch Biochem Biophys. 537:133–137.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thastrup O, Cullen PJ, Drøbak BK, Hanley
MR and Dawson AP: Thapsigargin, a tumor promoter, discharges
intracellular Ca2+ stores by specific inhibition of the endoplasmic
reticulum Ca2(+)-ATPase. Proc Natl Acad Sci USA. 87:2466–2470.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clemens RA and Lowell CA: Store-operated
calcium signaling in neutrophils. J Leukoc Biol. 98:497–502. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Clemens RA and Lowell CA: CRAC channel
regulation of innate immune cells in health and disease. Cell
Calcium. 78:56–65. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Salminen TA, Smith DJ, Jalkanen S and
Johnson MS: Structural model of the catalytic domain of an enzyme
with cell adhesion activity: Human vascular adhesion protein-1
(HVAP-1) D4 domain is an amine oxidase. Protein Eng. 11:1195–1204.
1998. View Article : Google Scholar
|
|
35
|
Masini E, Vannacci A, Marzocca C,
Mannaioni PF, Befani O, Federico R, Toma A and Mondovì B: A plant
histaminase modulates cardiac anaphylactic response in guinea pig.
Biochem Biophys Res Commun. 296:840–846. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dorward DA, Lucas CD, Chapman GB, Haslett
C, Dhaliwal K and Rossi AG: The role of formylated peptides
receptor 1 in governing neutrophil function during acute
inflammation. Am J Pathol. 185:1172–1184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Futosi K, Fodor S and Mócsai A: Neutrophil
cell surface receptors and their intracellular signal transduction
pathways. Int Immunopharmacol. 17:638–650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang X, Jin H, Cai X, Li S and Shen Y:
Structural and mechanistic insights into the activation of stromal
interaction molecule 1 (STIM1). Proc Natl Acad Sci USA.
109:5657–5662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marcocci L, Pietrangeli P, Befani O,
Mavelli I and Mondovì B: Inhibition of bovine serum amine oxidase
activity by hemoglobin. Life Chem Rep. 9:171–177. 1991.
|
|
40
|
Dini L, Agostinelli E and Mondoví B:
Cultured hepatocytes bind and internalize bovine serum amine
oxidase-gold complexes. Biochem Biophys Res Commun. 179:1169–1174.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Franc V, Řehulka P, Medda R, Padiglia A,
Floris G and Šebela M: Analysis of the glycosylation pattern of
plant copper amine oxidases by MALDITOF/TOF-MS coupled manual
chromatographic separation of glycans and glycopeptides.
Electrophoresis. 34:2357–2367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang X, Pietrangeli P, Mateescu MA and
Mondovì B: Extended substrate specificity of serum amine oxidase:
Possible involvement in protein posttranslational modification.
Biochem Biophys Res Commun. 223:91–97. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kivi E, Elima K, Aalto K, Nymalm Y,
Auvinen K, Koivunen E, Otto DM, Crocker PR, Salminen TA, Salmi M
and Jalkanen S: Human Siglec-10 can bind to vascular adhesion
protein-1 and serves as its substrate. Blood. 114:5385–5392. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fournier BM and Parkos CA: The role of
neutrophils during intestinal inflammation. Mucosal Immunol.
5:354–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Calinescu C, Mondovi B, Federico R,
Ispas-Szabo P and Mateescu MA: Carboxymethyl starch: Chitosan
monolithic matrices containing diamine oxidase and catalase for
intestinal delivery. Int J P harm. 428:48–56. 2012. View Article : Google Scholar
|