Introduction
The term pesticide is generic, including a wide and
miscellaneous category of chemicals conceived to prevent and defeat
weeds or pests, with different targets, chemical structures and
biological effects. Although their efficacy has contributed to
their wide use, occupational and environmental exposure is a threat
to the public health (1). Low
levels persist in the environment; however, certain categories of
workers (e.g., greenhouses workers) may be exposed to high
concentrations of pesticides with potential health consequences.
Even though ‘pesticide exposure’ may appear to be a very unspecific
statement, epidemiological studies are often biased by the lack of
a proper assessment of exposure and cannot consider exposure to
specific pesticides, since they are almost invariably used as
mixtures (2).
The symptoms of acute exposure are easily
identified; however, chronic exposure can contribute to the
development of chronic pesticide-related illness (3-12).
Chronic exposure to pesticide has been associated with both genetic
and epigenetic alterations underlying the development of different
diseases (13). Some classes of
pesticides are recognized as causative factors of gene mutations
and polymorphisms affecting key factors involved in the regulation
of toxic agents and xenobiotic metabolisms (14). In addition, it has been
demonstrated that pesticides are able to modulate the expression
levels of microRNAs (miRNAs or miRs) (15,16), known to be involved in the
development of different chronic degenerative diseases (17-19).
Recent studies have suggested oxidative stress as
one of the mechanisms for the adverse health effects of pesticides;
moreover, pesticides can act as endocrine disruptors, particularly
if used in mixtures (20).
Oxidative stress results from the inability of the cell to
neutralize an excess of oxidative species, altering the
physiological balance between the production and the elimination of
oxidant chemical species by antioxidant enzymes. The production of
free radicals can disrupt all the components of the cell, including
proteins, lipids and DNA (21,22). Furthermore, oxidative stress
enhances the inflammatory response, and thus contributes to the
pathophysiology of several illnesses (23,24). Also the diet can alter the
oxidative status because of the daily intake of substances with
antioxidant properties, such as polyphenols (25,26). These mechanisms form several
molecules which could represent useful biomarkers for the
evaluation of oxidative stress in workers exposed to pesticides,
such as advanced oxidation protein products (AOPP), advanced
glycation end-products (AGE) and reactive oxygen metabolites
(ROMs). An increase in the levels of these biomarkers is usually
inversely associated with the depletion of the biological
antioxidant potential (BAP) (27). The main challenge in occupational
exposure assessment originates from the variability in individual
response and genetic susceptibility, revealing diverse sensitivity
to xenobiotics. In fact, certain individuals seem to be at a higher
risk of pesticide-induced oxidative stress due to the presence of
genetic polymorphisms which influence the metabolism of such
xenobiotics (28,29). Some of these genetic polymorphisms
have been widely studied. Glutathione S-transferases (GSTs) are
enzymes involved in phase II metabolization participating in the
detoxification of xenobiotics or endogenous compounds. GSTs play a
key role in the interaction between glutathione (GSH) and the
substrate. Genetic differences in the expression and activity of
these enzymes are due to polymorphic alleles. These polymorphisms
alter GST activity and consequently, the susceptibility to toxic
compounds (30). Paraoxonase
(PON) family genes are located in region 21.3-22.1 (7q21.3-22.1) of
chromosome 7. The family includes 3 proteins (PON1, PON2 and PON3)
exhibiting different activities. PON1 plays a defensive role
against atherosclerosis due to its esterase and lipoprotein
antioxidant activity. The functions of PON2 and PON3 are less clear
than those of PON1. It has been demonstrated that they exhibit
antioxidant and anti-atherosclerotic properties, similar to those
of PON1 (31).
The genotypic characterization of these genes may be
used as predictor for susceptibility to pesticides. The present
study aimed to assess the contribution of genetic polymorphisms of
some pesticide-metabolizing enzymes on the serum levels of AOPP,
AGE, ROMs and BAP as biomarkers of oxidative stress in a cluster of
occupationally exposed farmers.
Materials and methods
Subjects
In total, 62 subjects, working as farmers in Eastern
Sicily, Italy, were enrolled in this study. All farmers were
Caucasian, with a mean age of 46.48±12.72 years and had been
employed for 21.97±12.44 years. The subjects, included in a
compulsory medical surveillance program, provided written informed
consent for this survey. Workers included in the study were those
who accepted voluntary enrollment between 120 subjects and are
representative of a population occupationally exposed to
pesticides. A questionnaire was used to obtain information
regarding the sociodemographic characteristics and lifestyle of the
subjects.
Genotyping
Briefly, 3 ml peripheral blood samples were
collected from each participant in vacuum tubes containing
K3-EDTA. Genomic DNA was then isolated from peripheral
blood using the Gentra PureGene DNA Purification system (Qiagen).
Following qualitative analysis, DNA was quantified
spectrophotometrically. The genotyping of polymorphisms was
performed using real-time polymerase chain reaction (PCR) allelic
discrimination technique on a 7500 Real-time PCR instrument
(Applied Biosystems), using Pre-Designed TaqMan SNP Genotyping
assay 5 (Applied Biosystems). The homozygous wild-type genotype was
recognized on the basis of a VIC fluorescent signal, heterozygous
genotype on the basis of a VIC/FAM fluorescent signal, and
homozygous mutated genotype on the basis of a FAM fluorescent
signal.
Quantification of AGE
AGE levels were determined as previously described
(27). The fluorescence intensity
with λexc=350 nm and λem=440 nm was measured using a Sinergy HT
microplate absorbance reader (Biotek Instruments, Inc.) and
expressed as AU/ml.
Quantification of AOPP
The serum concentrations of AOPP were determined as
previously described by Costa et al (27). The absorbance was measured using a
Sinergy HT microplate absorbance reader, with a calibration curve
of 0-128 µM chloramine T.
Quantification of ROMs and BAPs
In order to assess reactive oxygen metabolite levels
and biological antioxidant potential in serum, the d-ROMS test and
BAP test were used (Diacron International). The absorbance at 505
nm was recorded and measurements were expressed as Carr Units (U
CARR) and as nmol/ml respectively (32,33).
Statistical analysis
The normality of the variables was assessed using
the D’Agostino and Pearson test. Mann-Whitney test was used to
evaluate a possible influence of sex in AOPP, AGE and BAP while
Student’s t-test was used for ROMs. The stratification of workers
in 3 risk classes, male vs. female, was performed using the
Chi-square contingency test. The Kruskal-Wallis test followed by
Dunn’s multiple comparison test was used to assess differences
between the AOPP, AGE and BAP levels between subjects with
different polymorphic alleles and between risk classes, while for
the for ROM levels, one-way ANOVA followed by Tukey’s multiple
comparison test was used. Grouped analysis of biomarker levels
related to sex and risk class was performed using two-way analysis
of variance. All analyzes were performed using Prism version 6.01
(GraphPad software). A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of the study
population
The sociodemographic and lifestyle characteristics
of the study population are presented in Table I. The majority were smokers
(61.3%), and only 9.7% subjects consumed >2 glasses of wine or
beer or one serving of liquor/day. All subjects included used
personal protective equipment provided by the employer. Other
information collected concerned occupational features and the
absence of known disorders or diseases in the 3 months preceding
the survey. Data regarding the demographic and lifestyle
characteristics of the exposed subjects are presented in Table I. The subjects were exposed to a
mixture of pesticides (including imidacloprid, cypermethrin,
pirimetanil, dimetomorf and carbendazim), with the prevalent use of
chlorpyrifos, which is an organophosphate (OP). No cases of
exposure to pesticides from non-occupational sources were
registered. No infectious or inflammatory diseases and no drug use
was reported in the subjects in the 3 months preceding the survey.
The frequency of polymorphisms and the levels of AGE, AOPP, ROMs
and BAP are presented in Table
II.
 | Table ISociodemographic characteristics and
lifestyle of subjects. |
Table I
Sociodemographic characteristics and
lifestyle of subjects.
| Characteristic | No. (%) |
|---|
| Number of
subjects | 62 (100) |
| Age (years) | 46.48±12.72 |
| Sex | |
| Male | 43 (69.3) |
| Female | 18 (29.0) |
| Missing data | 1 (1.7) |
| Occupational
seniority (years) | 21.97±12.44 |
| Ethnicity | Caucasian 62
(100) |
| Smoking
statusa | |
| Smoker | 38 (61.3) |
| Non smoker | 24 (38.7) |
| Alcohol
consumptionb | |
| Yes | 6 (9.7) |
| No | 56 (90.3) |
| Personal protective
equipment | |
| Yes | 62 (100) |
| No | 0 (0) |
 | Table IIFrequency and effect of genotype on
oxidative stress biomarkers levels in farmers exposed to
pesticides. |
Table II
Frequency and effect of genotype on
oxidative stress biomarkers levels in farmers exposed to
pesticides.
| Gene | Polymorphism | Genotype | N (%) | AGE (AU/ml) | AOPP (nmol/ml) | ROMs (U CARR) | BAP nmol/ml |
|---|
| PON1 | Q192R | Wild-type (QQ) | 31 (50.0) | 147.20±38.82 | 1.60±0.98 | 314.90±96.13 |
2,101.16±649.55 |
| Heterozygote
(QR) | 27 (43.5) | 150.05±26.02 | 1.69±1.27 | 311.55±99.01 |
1,944.63±631.53 |
| Mutant homozygote
(RR) | 1 (1.7) | 189.20 | 0.73 | 346.00 | 1,867.00 |
| Missing data | 3 (4.8) | | | | |
| PON2 | S331C | Wild-type (CC) | 37 (59.6) | 153.49±35.80 | 1.29±0.76 | 279.65±82.15 |
2,276.11±563.21 |
| Heterozygote
(CG) | 17 (27.4) | 140.41±29.44 | 2.14±1.48 | 350.76±99.94 |
1,700.88±628.05 |
| Mutant homozygote
(GG) | 7 (11.3) | 146.60±20.08 | 1.96±1.06 | 398.57±62.37 |
1,672.71±477.26 |
| Missing data | 1 (1.7) | | | | |
| A148G | Wild-type (CC) | 26 (41.9) | 152.75±38.95 | 1.42±0.84 | 297.88±84.48 |
2,119.58±631.99 |
| Heterozygote
(CG) | 7 (11.3) | 156.68±20.54 | 1.92±0.66 | 336.00±60.83 |
1,753.00±475.63 |
| Mutant homozygote
(GG) | 6 (9.7) | 121.17±26.98 | 1.29±1.28 | 289.17±125.96 |
2,280.67±731.40 |
| Missing data | 23 (37.1) | | | | |
| GSTP1 | lle105Val | Wild-type | 33 (53.2) | 154.11±35.14 | 1.60±0.98 | 323.15±88.44 |
1,998.00±628.86 |
| Heterozygote | 21 (33.8) | 146.88±29.61 | 1.59±1.29 | 291.38±101.80 |
2,116.67±648.94 |
| Mutant
homozygote | 7 (11.3) | 132.23±26.39 | 1.59±1.17 | 317.57±109.58 |
2,036.28±632.87 |
| Missing data | 1 (1.7) | | | | |
| Ala114Val | Wild-type | 57 (91.9) | 148.98±33.88 | 1.63±1.13 | 314.03±96.66 |
2,039.37±651.59 |
| Heterozygote | 2 (3.2) | 155.90±13.70 | 0.73±0.30 | 184.00±30.00 |
2,469.50±235.50 |
| Mutant
homozygote | 1 (1.7) | 169.80 | 1.78 | 374.00 | 1,534.00 |
| Missing data | 2 (3.2) | | | | |
| GSTM1 | | Deleted | 28 (45.1) | 148.94±32.89 | 1.59±1.10 | 324.11±103.35 |
1,902.32±662.06 |
| Non-deleted | 24 (38.7) | 153.67±35.77 | 1.48±0.47 | 307.96±90.45 |
2,098.04±588.16 |
| Missing data | 10 (16.2) | | | | |
| GSTT1 | | Deleted | 34 (54.8) | 147.59±37.08 | 1.58±1.30 | 311.50±101.45 |
2,066.65±634.68 |
| Non-deleted | 18 (29.0) | 158.82±26.19 | 1.79±0.82 | 326.39±90.14 |
1,852.89±616.30 |
| Missing data | 10 (16.2) | | | | |
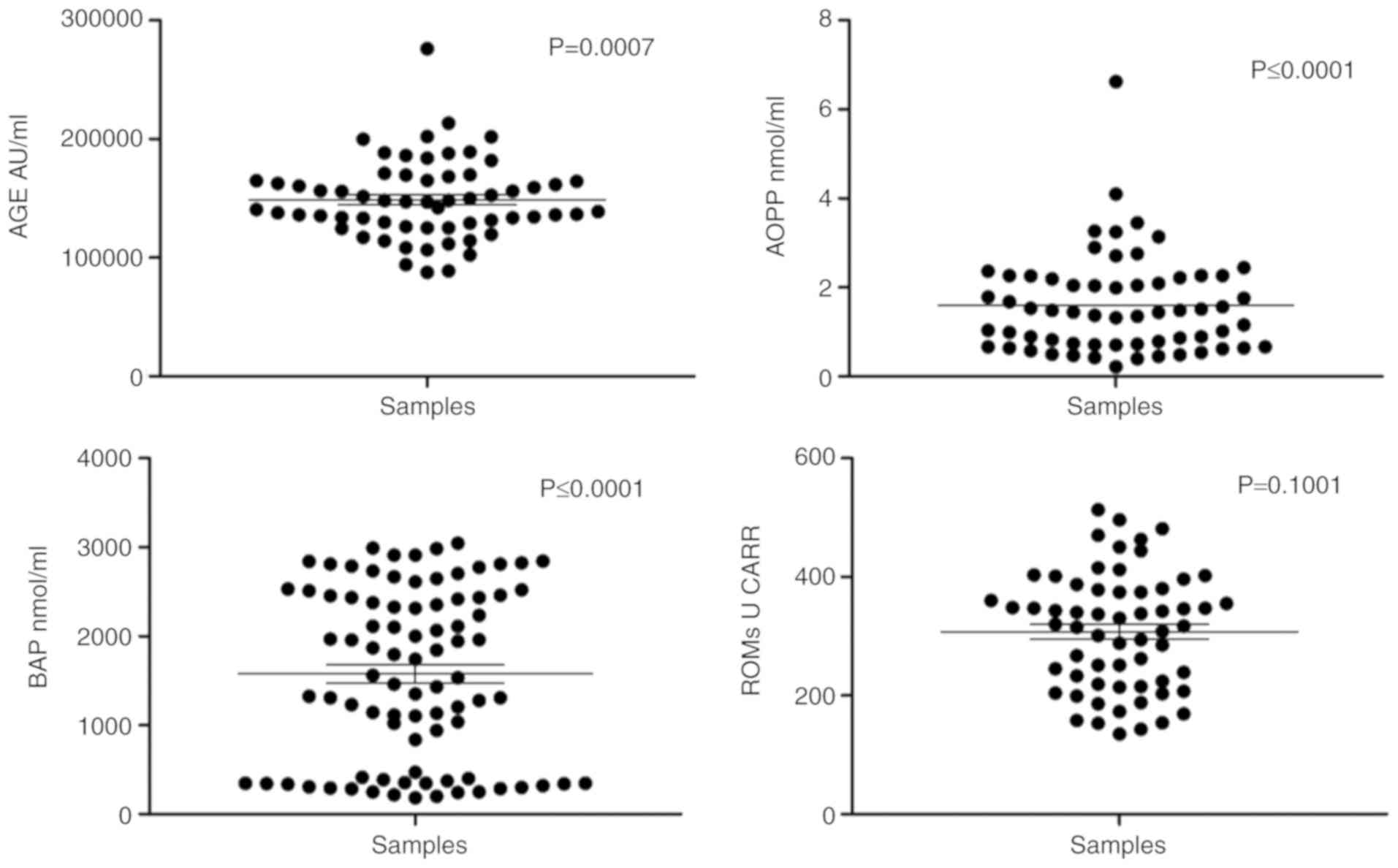
The D’Agostino and Pearson normality test revealed
that the AGE, AOPP and BAP levels did not follow a normal
distribution. Therefore, non-parametric tests were performed on
these biomarkers. Conversely, the ROM serum levels seemed to follow
a normal distribution; thus, parametric tests were used for this
variable (Fig. 1).
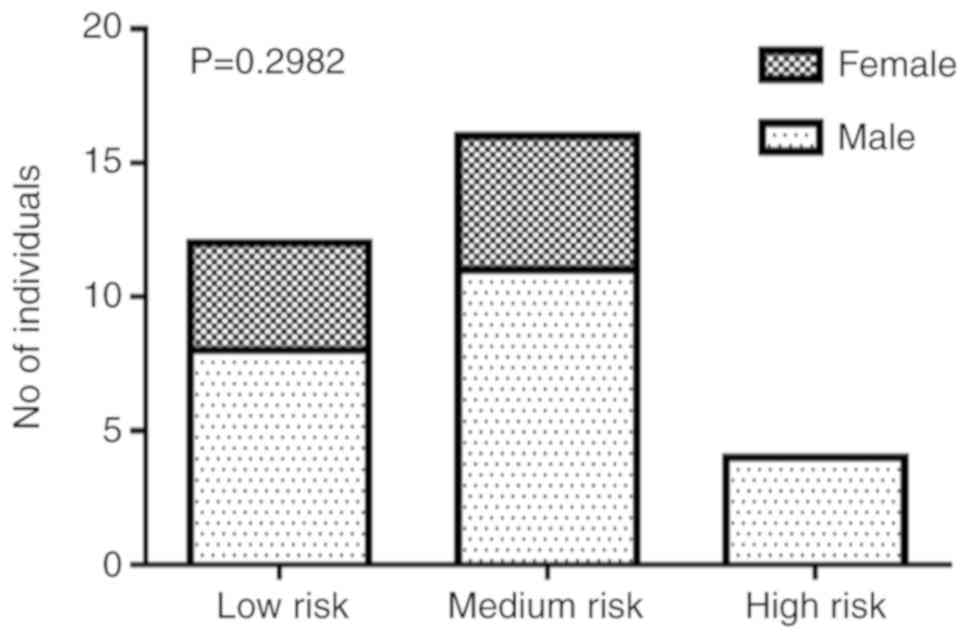
Since some data regarding the polymorphic profile of
the studied genes were missing, all the subjects whose genetic
profile was not complete were excluded to proceed with a grouping
according to different hypothetical risk classes, in order to avoid
statistical bias. The 32 resulting subjects were subsequently
grouped into 3 risk classes, assigning the score 0 to the wild‑type
profile, 1 to the heterozygote profile and 2 to the homozygous
profile for the polymorphisms of PON1, PON2 and glutathione
S-transferase Pi 1 (GSTP1); mutations of glutathione S-transferase
mu 1 (GSTM1) and glutathione S-transferase theta 1 (GSTT1) were
assigned scores 0 where there was no deletion, and 1 where the
deletion was present. The sum of the scores resulting from
polymorphic alterations ranged from 0 to 7. The 3 risk classes were
thus constituted: Class 1 (score 0-2, low risk); Class 2 (score
3-4, medium risk); and Class 3 (score 5-7, high risk). The
Chi-square test performed on these datasets was statistically not
significant. The data contingency analysis revealed that no female
individual belonged to the high-risk group (Fig. 2).
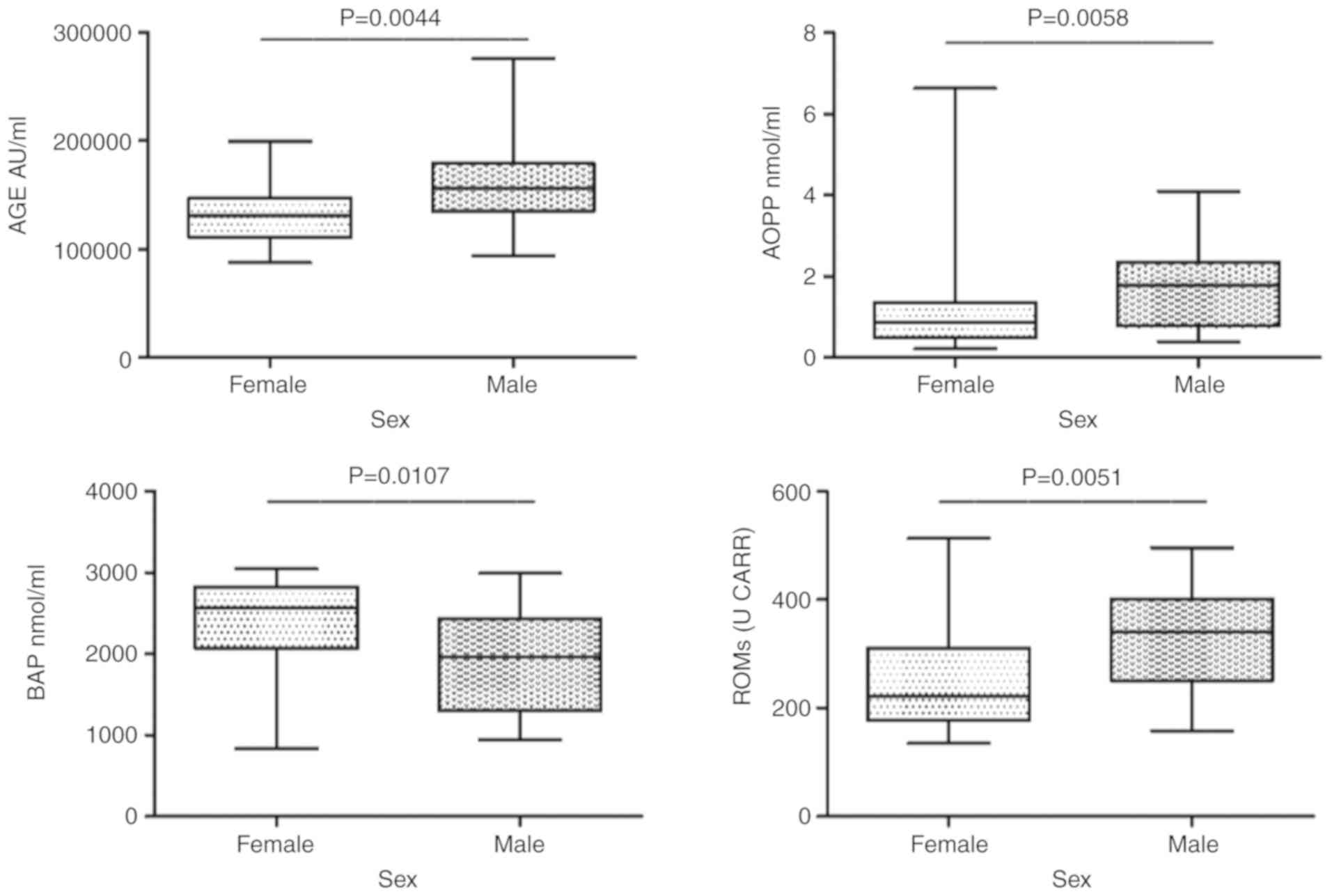
Oxidative stress biomarkers
There was a statistically significant difference in
the sex-dependent variation of the AGE, BAP, AOPP and ROMs levels.
Statistical significance was calculated using the Mann-Whitney
non-parametric test, apart from the ROM data, where the Student’s
t-test was used (Fig. 3).
Conversely, the same analysis revealed that alcohol and smoking do
not influence oxidative stress biomarkers (data not shown).
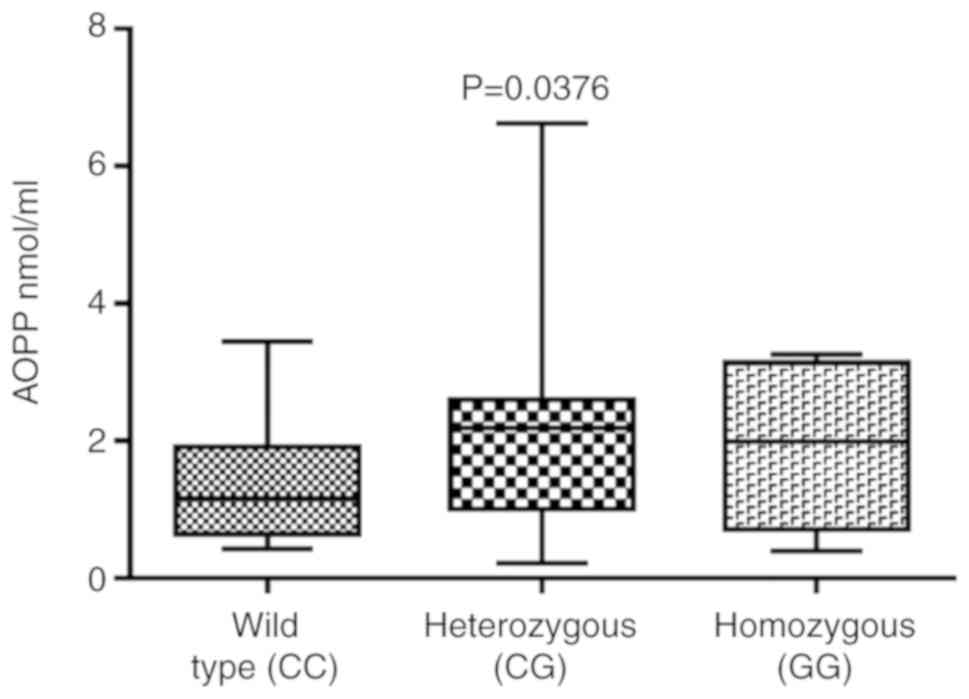
Statistically significant differences in the AOPP
and ROM levels in subjects with the PON2 S331C polymorphism were
highlighted by Kruskal-Wallis and ANOVA tests, respectively;
analyzing the individual groups by Mann-Whitney and t-test (data
not shown), respectively, for AOPP (Fig. 4), although the Kruskal-Wallis test
revealed an overall statistical difference among the three groups
(P=0.0376), Dunn’s multiple post-hoc test comparisons test did not
reveal any statistical differences between individual groups
(Fig. 4). The only statistically
significant difference found was between the wild-type subjects
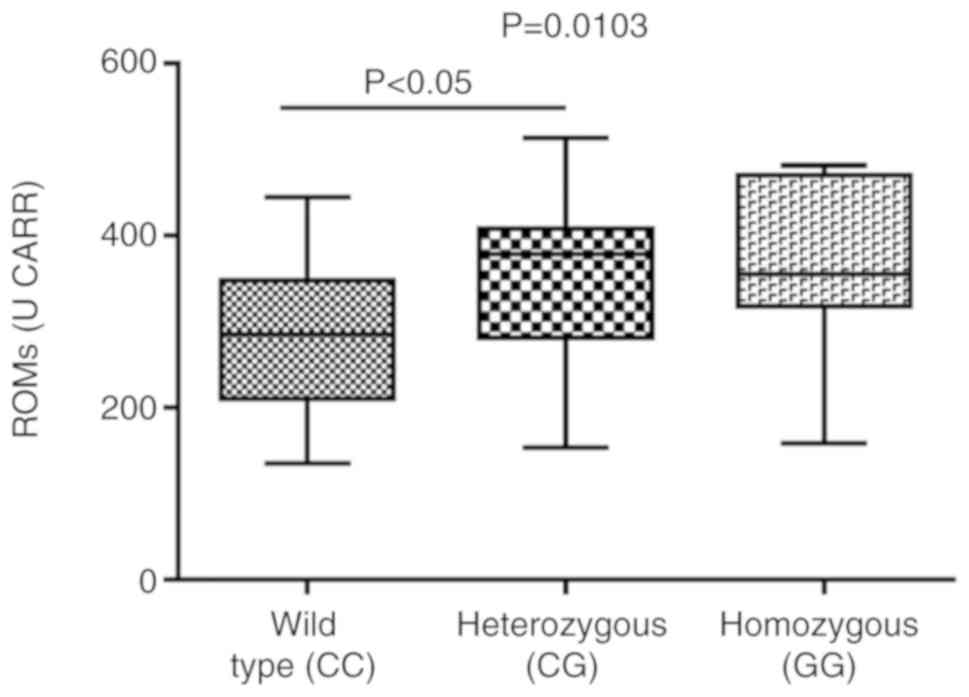
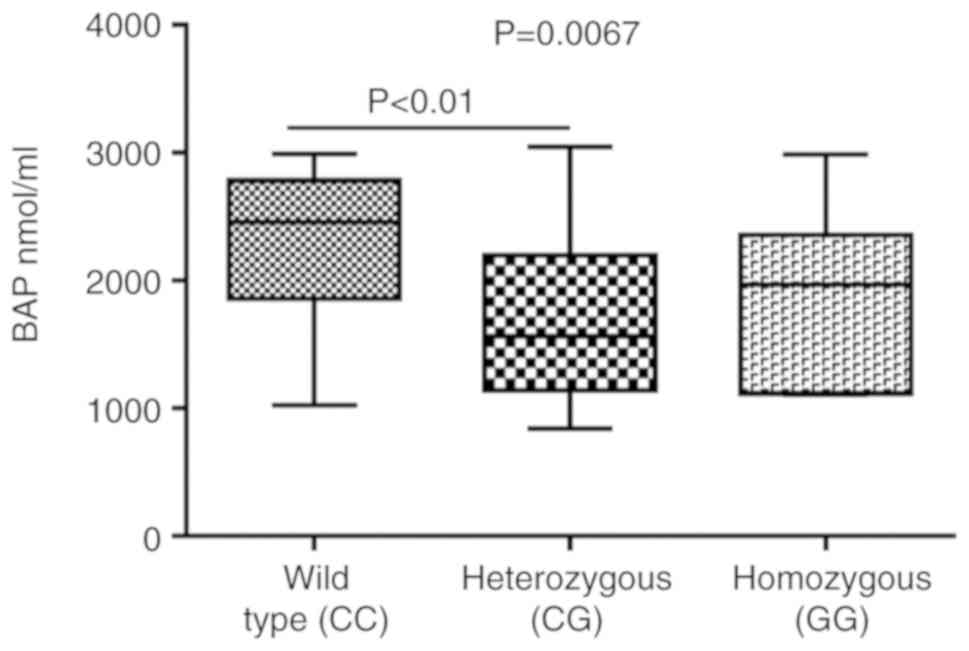
compared to the heterozygous subjects for ROMs (Fig. 5). A similar trend was observed for
the BAP levels (significant difference between wild‑type vs.
heterozygous), but with an inverse association (BAP levels were
higher in wild-type compared to both homo- and heterozygous groups)
(Fig. 6).
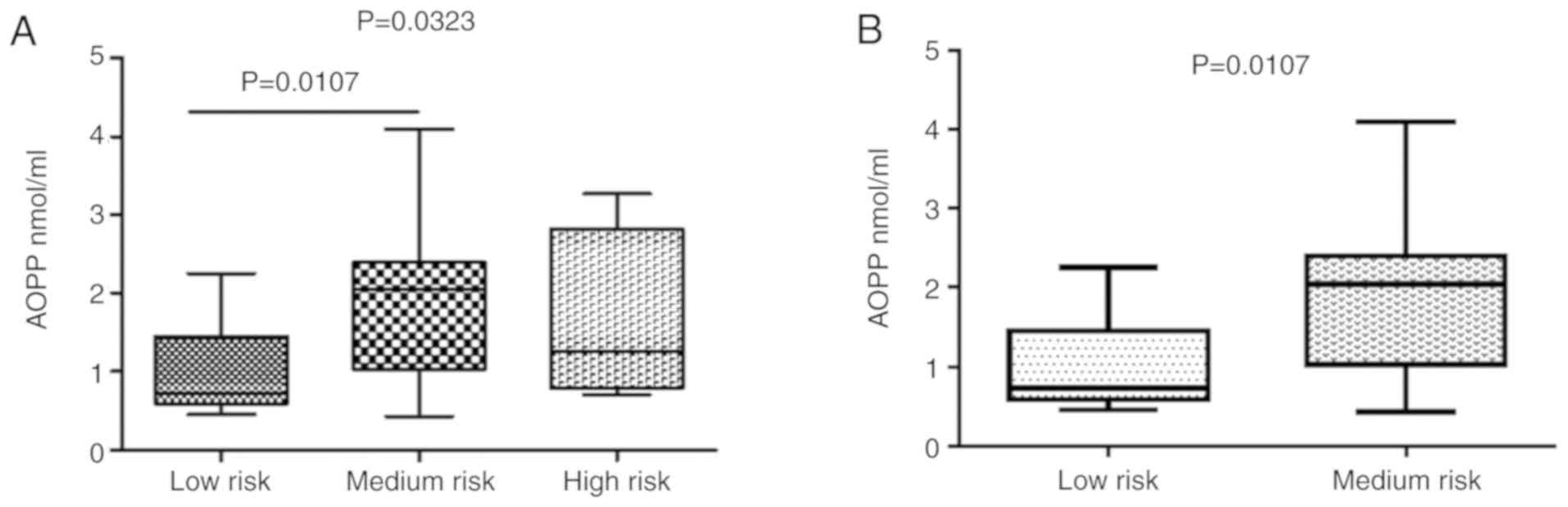
Following the stratification of the subjects exposed
to pesticides into 3 filtered classes, statistically significant
differences in AOPP levels were observed (Fig. 7A) in the subjects who were at
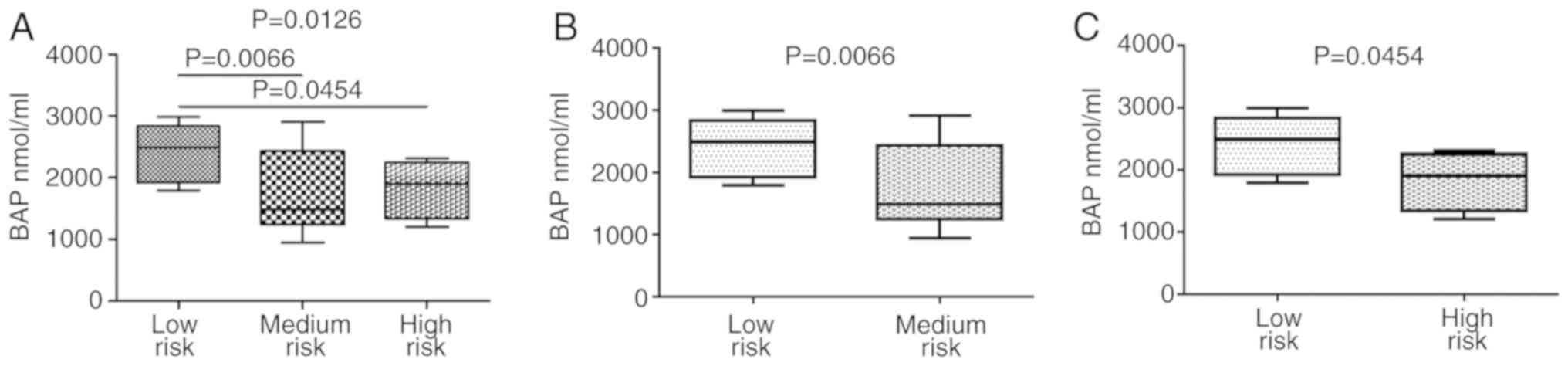
low-risk compared to those at medium-risk (Fig. 7B). A similar trend was observed
with an inverse association, for the BAP levels. The Kruskal-Wallis
test followed by Dunn’s comparison test revealed significant
differences between the low-risk and medium-risk individuals and
between the low-risk and high-risk individuals (Fig. 8).
No statistically significant differences were
observed with regards to AGE and ROMs protein levels taking into
consideration the filtered risk classes of subjects exposed to
pesticides. By performing the t‑test there was no statistically
significant difference between the individual groups.
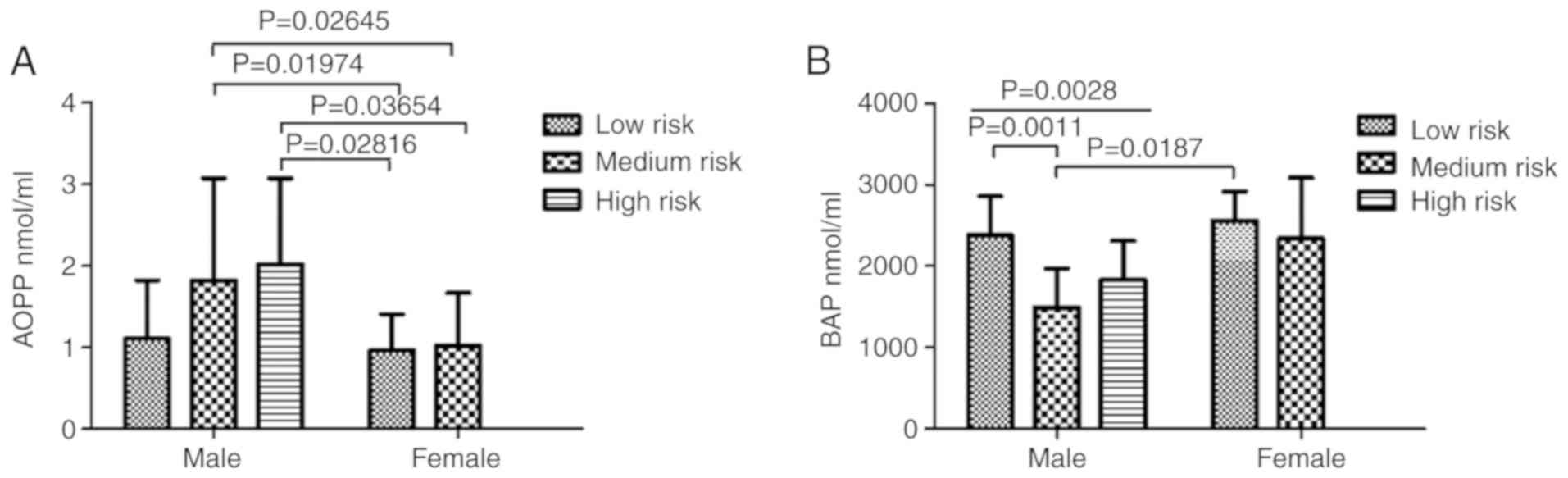
By performing a grouped analysis using two-way
ANOVA, statistically significant differences were found for AOPP
and BAP between medium-risk male and female workers (Fig. 9). In particular, for AOPP,
statistically significant differences were identified between the
male medium risk vs. the female medium risk groups (P=0.02645), the
male medium risk vs. female low risk groups (P=0.01974), the male
high risk vs. female low risk groups (P=0.02816) and between the
male high risk vs. female medium risk groups (P=0.03654) (Fig. 9A). For BAP, significant
differences were observed between the male the low risk vs. male
medium risk groups (P=0.011), the male low risk vs. male high risk
groups (P=0.0028) and between the male medium risk vs. female low
risk groups (P=0.0187).
Discussion
The results of this study associating the PON2 S331C
polymorphism with altered levels of serum oxidative stress
biomarkers associated with a low antioxidant potential of the
organism leads to the hypothesis of a possible role of this PON
family in the metabolism of pesticides. In fact, the AOPP and ROMs
levels were higher in individuals who had the heterozygous profile
of the polymorphism with respect to the wild‑type profile, and
coherently, the levels of BAP followed an inverse trend with
respect to the first two markers. These oxidative biomarkers have
been linked to the toxicity of pesticides due to their pathogenetic
mechanisms (34). Thus, compared
with AGE, the levels of AOPP and ROMs provided a more sensitive
biomarker, with an improved association with the PON2 genotype.
The findings of this study are in contrast to the
results of a previously study conducted by the authors (27), in which no significant
associations were found regarding the polymorphism of the PON1
(Q192R) gene investigated. This divergence could be justified by
differences concerning the characteristics of the population and
above all, the class of pesticides handled by the workers examined.
In the previous study, the workers were exposed to organophosphates
and this would explain the positive association with the PON1
polymorphism, with PON1 being a key enzyme in the metabolism of
these xenobiotics; by contrast, this study population was not only
exposed to organophosphorus, but to a mixture of pesticides which
also contained other classes of compounds. This may provide an
explanation for the contrasting data derived from the two studies.
The second explanation could be the different composition of the
study population. In the previous study, the sample consisted of
only male subjects, while the sample under examination in this
study was almost 30% female individuals. A difference was also
observed between male and female individuals, having the latter
lower values of AGE, AOPP and ROMs and consistently a higher BAP
value. This suggests that women have a better response to oxidative
damage from exposure to pesticides.
The allele frequency is coherent with most studies
conducted on populations from different geographical areas
(35). There are a number of
studies in the literature concerning the role of PON1 and the
metabolism of pesticides; however, the role of PON2 has not yet
been fully clarified. A recent study demonstrated the increased
expression of PON1 and PON2 related to exposure to toxicants
(36). Another study suggested
PON2 polymorphisms to play a role in male infertility (37). PON2 has also been suggested as an
alternative potential treatment for Parkinson’s disease,
functioning as an endogenous antioxidant system (38), or playing a role in the etiology
of noise-induced hearing loss (39). The antioxidant properties of PONs
seem to be useful in the prevention and treatment of cardiovascular
diseases and related disorders, as PON2 reduces oxidative stress in
vascular cells protecting against cell-mediated oxidation of LDL
(40). Others also have
investigated the role of PON2, although with weak results (41). Two polymorphic sites of PON2,
S331C and G148A, have been investigated in several disorders, both
cardiovascular and neurological, such as Alzheimer’s disease,
diabetes and coronary artery disease (42-48).
The whole population is exposed on daily basis to
pesticide residues through food, water and other commercial
products. These substances are found at very low concentrations;
however, a long-term exposure to these residues can have negative
repercussions on health. The main aim of standard toxicological
trials performed by regulatory agencies is to assess safe exposure
parameters. These studies usually adopt dosage schemes that are too
high for real-life risk simulation and explore a specific type of
toxicity by evaluating a linear dose-effect response. In order to
better evaluate the real risk, study protocols have been proposed,
taking into consideration the realistic setting of long-term
low-dose exposure to mixtures containing pesticides, either alone
or in combination with common chemicals in commercial products, in
order to establish a standardized method to evaluate the daily
exposures of the general population. This could lead to a new
cumulative risk assessment and no longer to the current single risk
(49,50). Clearly, it is improbable to test
thousands of substances; however, if the hypothesis of an increase
in the cumulative risk or even of a different identification of the
risk is demonstrated at doses around the regulatory limits, then a
new step supporting the effort to pass to the era of low-dose
cumulative risk assessment is necessary.
The PON family is one of the key enzymes involved in
organophosphate compound metabolism and is believed to be
associated in oxidative stress-related pathology. Genetic studies
on PON aim to associate the most common polymorphisms with disease
occurrence. This study revealed an association between the PON2
S331C polymorphism and the serum levels of some oxidative stress
biomarkers, although the direct and indirect effects on health
status have not yet been well established. According to these
results, the above-mentioned genotypes could be considered for the
health surveillance of individuals occupationally exposed to
pesticides, in order to define a cluster of susceptible workers so
as to guarantee greater protection.
Evidence on the equivalence of human versus
experimental pesticide exposure is not easy to achieve, as
pesticide levels in environment are influenced by climate and a
variety of other factors, e.g., the design of the present study did
not allow the evaluation of possible exposure to minor amounts of
pesticides from food and water.
Simulating a representative scenario of long-term
exposure to doses lower than regulatory limits of mixtures
containing different pesticides, could help the researchers to
collect more information from a single chronic toxicity study,
adopting a more effective approach and taking a step forward in
risk assessment switching from the single to the cumulative risk
assessment (51,52).
In conclusion, the results of this study indicated
that a chronic OP pesticide exposure may result in enduring
oxidative stress, and polymorphic genes encoding PON can enhance
pesticide toxicity. The gene-environment interaction and pesticide
exposure seem to be important in the development of several chronic
and degenerative disease. The early identification of these
chemical biomarkers is warranted in order to implement health
prevention programs for workers who are more susceptible to the
adverse effects of pesticide exposure.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors’ contributions
CC, DC, CF, GB and MT made substantial contributions
to the conception and design of the study. DC, CC, FG and RC were
involved in data acquisition, analysis and interpretation. GB and
MT drafted the article or critically revised it for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The subjects included in this study were healthy
workers included in a compulsory medical surveillance program and
participation to the study did not expose them to any additional
danger. They were neither administered any drug or other compound,
nor were submitted to any invasive procedure. Biological samples
used for the study were not primarily collected for the study
itself, but for mandatory biological monitoring and medical
surveillance of occupational hazards. Workers included in the study
were those who accepted voluntary enrollment and provided written
informed consent for this survey, formulated according to the
International Declaration of Helsinki. Consequently, as safety and
well-being of subjects could not be affected, approval from ethics
committee was not requested.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martenies SE and Perry MJ: Environmental
and occupational pesticide exposure and human sperm parameters: A
systematic review. Toxicology. 307:66–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sultana Shaik A, Shaik AP, Jamil K and
Alsaeed AH: Evaluation of cytotoxicity and genotoxicity of
pesticide mixtures on lymphocytes. Toxicol Mech Methods.
26:588–594. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gangemi S, Gofita E, Costa C, Teodoro M,
Briguglio G, Nikitovic D, Tzanakakis G, Tsatsakis AM, Wilks MF,
Spandidos DA and Fenga C: Occupational and environmental exposure
to pesticides and cytokine pathways in chronic diseases (Review).
Int J Mol Med. 38:1012–1020. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mamane A, Baldi I, Tessier JF, Raherison C
and Bouvier G: Occupational exposure to pesticides and respiratory
health. Eur Respir Rev. 24:306–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parrón T, Requena M, Hernández AF and
Alarcón R: Environmental exposure to pesticides and cancer risk in
multiple human organ systems. Toxicol Lett. 230:157–165. 2014.
View Article : Google Scholar
|
|
6
|
Yu Y, Yang A, Zhang J and Hu S: Maternal
exposure to the mixture of organophosphorus pesticides induces
reproductive dysfunction in the offspring. Environ Toxicol.
28:507–515. 2013. View Article : Google Scholar
|
|
7
|
Fenga C, Gangemi S, Teodoro M, Rapisarda
V, Golokhvast K, Docea AO, Tsatsakis AM and Costa C:
8-Hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in
workers exposed to low-dose benzene. Toxicol Rep. 4:291–295. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Costa C, Miozzi E, Teodoro M, Briguglio G,
Rapisarda V and Fenga C: New insights on ‘old’ toxicants in
occupational toxicology (Review). Mol Med Rep. 15:3317–3322. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gangemi S, Miozzi E, Teodoro M, Briguglio
G, De Luca A, Alibrando C, Polito I and Libra M: Occupational
exposure to pesticides as a possible risk factor for the
development of chronic diseases in humans (Review). Mol Med Rep.
14:4475–4488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suratman S, Edwards JW and Babina K:
Organophosphate pesticides exposure among farmworkers: Pathways and
risk of adverse health effects. Rev Environ Health. 30:65–79.
2015.PubMed/NCBI
|
|
11
|
Falzone L, Marconi A, Loreto C, Franco S,
Spandidos DA and Libra M: Occupational exposure to carcinogens:
Benzene, pesticides and fibers (Review). Mol Med Rep. 14:pp.
4467–4474. 2016, View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Polo A, Crispo A, Cerino P, Falzone L,
Candido S, Giudice A, De Petro G, Ciliberto G, Montella M, Budillon
A and Costantini S: Environment and bladder cancer: Molecular
analysis by interaction networks. Oncotarget. 8:65240–65252. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collotta M, Bertazzi PA and Bollati V:
Epigenetics and pesticides. Toxicology. 307:35–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koutros S, Andreotti G, Berndt SI, Hughes
Barry K, Lubin JH, Hoppin JA, Kamel F, Sandler DP, Burdette LA,
Yuenger J, et al: Xenobiotic-metabolizing gene variants, pesticide
use, and the risk of prostate cancer. Pharmacogenet Genomics.
21:615–623. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weldon BA, Shubin SP, Smith MN, Workman T,
Artemenko A, Griffith WC, Thompson B and Faustman EM: Urinary
microRNAs as potential biomarkers of pesticide exposure. Toxicol
Appl Pharmacol. 312:19–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan H, Yuan M, Tang Y, Wang B and Zhan X:
MicroRNA expression profiling in human acute organophosphorus
poisoning and functional analysis of dysregulated miRNAs. Afr
Health Sci. 18:333–342. 2018. View Article : Google Scholar
|
|
17
|
Falzone L, Romano GL, Salemi R, Bucolo C,
Tomasello B, Lupo G, Anfuso CD, Spandidos DA, Libra M and Candido
S: Prognostic significance of deregulated microRNAs in uveal
melanomas. Mol Med Rep. 19:2599–2610. 2019.PubMed/NCBI
|
|
18
|
Falzone L, Lupo G, La Rosa GRM, Crimi S,
Anfuso CD, Salemi R, Rapisarda E, Libra M and Candido S:
Identification of novel MicroRNAs and their diagnostic and
prognostic significance in oral cancer. Cancers (Basel). 11:2019.
View Article : Google Scholar
|
|
19
|
Candido S, Lupo G, Pennisi M, Basile MS,
Anfuso CD, Petralia MC, Gattuso G, Vivarelli S, Spandidos DA, Libra
M and Falzone L: The analysis of miRNA expression profiling
datasets reveals inverse microRNA patterns in glioblastoma and
Alzheimer’s disease. Oncol Rep. 42:911–922. 2019.PubMed/NCBI
|
|
20
|
Petrakis D, Vassilopoulou L, Mamoulakis C,
Psycharakis C, Anifantaki A, Sifakis S, Docea AO, Tsiaoussis J,
Makrigiannakis A and Tsatsakis AM: Endocrine disruptors leading to
obesity and related diseases. Int J Environ Res Public Health.
14:2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Surajudeen YA, Sheu RK, Ayokulehin KM and
Olatunbosun AG: Oxidative stress indices in Nigerian pesticide
applicators and farmers occupationally exposed to organophosphate
pesticides. Int J Appl basic Med Res. 4:S37–S40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wafa T, Nadia K, Amel N, Ikbal C, Insaf T,
Asma K, Hedi MA and Mohamed H: Oxidative stress, hematological and
biochemical alterations in farmers exposed to pesticides. J Environ
Sci Health B. 48:1058–1069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aouey B, Derbali M, Chtourou Y, Bouchard
M, Khabir A and Fetoui H: Pyrethroid insecticide lambda-cyhalothrin
and its metabolites induce liver injury through the activation of
oxidative stress and proinflammatory gene expression in rats
following acute and subchronic exposure. Environ Sci Pollut Res
Int. 24:5841–5856. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fenga C, Gangemi S, Di Salvatore V,
Falzone L and Libra M: Immunological effects of occupational
exposure to lead (Review). Mol Med Rep. 15:3355–3360. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Costa C, Tsatsakis A, Mamoulakis C,
Teodoro M, Briguglio G, Caruso E, Tsoukalas D, Margina D, Dardiotis
E, Kouretas D and Fenga C: Current evidence on the effect of
dietary polyphenols intake on chronic diseases. Food Chem Toxicol.
110:286–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Costa C, Ozcagli E, Gangemi S, Schembri F,
Giambò F, Androutsopoulos V, Tsatsakis A and Fenga C: Molecular
biomarkers of oxidative stress and role of dietary factors in
gasoline station attendants. Food Chem Toxicol. 90:30–35. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Costa C, Gangemi S, Giambò F, Rapisarda V,
Caccamo D and Fenga C: Oxidative stress biomarkers and paraoxonase
1 polymorphism frequency in farmers occupationally exposed to
pesticides. Mol Med Rep. 12:6353–6357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Teodoro M, Briguglio G, Fenga C and Costa
C: Genetic polymorphisms as determinants of pesticide toxicity:
Recent advances. Toxicol Rep. 6:564–570. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Costa C, Miozzi E, Teodoro M and Fenga C:
Influence of genetic polymorphism on pesticide-induced oxidative
stress. Curr Opin Toxicol. 13:1–7. 2019. View Article : Google Scholar
|
|
30
|
Ginsberg G, Smolenski S, Hattis D, Guyton
KZ, Johns DO and Sonawane B: Genetic polymorphism in glutathione
transferases (GST): Population distribution of GSTM1, T1, and P1
conjugating activity. J Toxicol Environ Health B. 12:389–439. 2009.
View Article : Google Scholar
|
|
31
|
Ginsberg G, Neafsey PJ, Hattis D, Guyton
KZ, Johns DO and Sonawane B: Genetic polymorphism in paraoxonase 1
(PON1): Population distribution of PON1 activity. J Toxicol Environ
Health B. 12:473–507. 2009. View Article : Google Scholar
|
|
32
|
Hirose H, Kawabe H, Komiya N and Saito I:
Relations between serum reactive oxygen metabolites (ROMs) and
various inflammatory and metabolic parameters in a Japanese
population. J Atheroscler Thromb. 16:77–82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kakita H, Hussein MH, Daoud GA, Kato T,
Murai H, Sugiura T, Mizuno K, Yamada Y, Ito T, Fukuda S, et al:
Total hydroperoxide and biological antioxidant potentials in a
neonatal sepsis model. Pediatr Res. 60:675–679. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hernández AF, Lacasaña M, Gil F,
Rodríguez-Barranco M, Pla A and López-Guarnido O: Evaluation of
pesticide-induced oxidative stress from a gene-environment
interaction perspective. Toxicology. 307:95–102. 2013. View Article : Google Scholar
|
|
35
|
Shin BS: Paraoxonase gene polymorphism in
south-western Korean population. J Korean Med Sci. 24:561–566.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bordoni L, Nasuti C, Fedeli D, Galeazzi R,
Laudadio E, Massaccesi L, López-Rodas G and Gabbianelli R: Early
impairment of epigenetic pattern in neurodegeneration: Additional
mechanisms behind pyrethroid toxicity. Exp Gerontol.
124:1106292019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Volk M, Jaklič H, Zorn B and Peterlin B:
Association between male infertility and genetic variability at the
PON1/2 and GSTM1/T1 gene loci. Reprod Biomed Online. 23:105–110.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aguirre-Vidal Y, Monroy-Noyola A,
Anaya-Ramos L, Arteaga-Silva M, Mendez-Armenta M, Ostoa-Saloma P,
Díaz-Zaragoza M, Morales-Montor J, Ríos C and Montes S:
β-estradiol-3-benzoate confers neuroprotection in Parkinson
MPP+ rat model through inhibition of lipid peroxidation.
Steroids. 126:7–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li X, Cao J, Wang J, Song H, Ji G, Dong Q,
Wei C, Cao Y, Wang B, Zhu B and Xiao H: PON2 and ATP2B2 gene
polymorphisms with noise-induced hearing loss. J Thorac Dis.
8:430–438. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li YR, Zhu H, Kauffman M, Danelisen I,
Misra HP, Ke Y and Jia Z: Paraoxonases function as unique
protectors against cardiovascular diseases and diabetes: Updated
experimental and clinical data. Exp Biol Med (Maywood).
239:899–906. 2014. View Article : Google Scholar
|
|
41
|
Liu YJ, Huang PL, Chang YF, Chen YH, Chiou
YH, Xu ZL and Wong RH: GSTP1 genetic polymorphism is associated
with a higher risk of DNA damage in pesticide-exposed fruit
growers. Cancer Epidemiol Biomarkers Prev. 15:659–666. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shi J, Zhang S, Tang M, Liu X, Li T, Han
H, Wang Y, Guo Y, Zhao J, Li H and Ma C: Possible association
between Cys311Ser polymorphism of paraoxonase 2 gene and late-onset
Alzheimer’s disease in Chinese. Brain Res Mol Brain Res.
120:201–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ren H, Tan SL, Liu MZ, Banh HL and Luo JQ:
Association of PON2 gene polymorphisms (Ser311Cys and Ala148Gly)
with the risk of developing type 2 diabetes mellitus in the Chinese
population. Front Endocrinol (Lausanne). 9:4952018. View Article : Google Scholar
|
|
44
|
González-Castro TB, Tovilla-Zárate CA,
Juárez-Rojop IE, Hernández-Díaz Y, López-Narváez ML,
Rodríguez-Pérez C, González-Hernández YK and Ramos-Méndez MÁ: PON2
and PPARG polymorphisms as biomarkers of risk for coronary heart
disease. Biomark Med. 12:287–297. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen ML, Zhao H, Liao N and Xie ZF:
Association between paraoxonase 2 Ser311Cys polymorphism and
coronary heart disease risk: A meta-analysis. Med Sci Monit.
22:3196–3201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Andalib S, Vaseghi G, Motavallian A,
Sadeghi HM, Eshraghi A, Amini M and Majlesi AR: Association of
polymorphism of ser311cys paraoxonase-2 gene with type 2 diabetes
mellitus in Iran. Int J Prev Med. 4:517–522. 2013.PubMed/NCBI
|
|
47
|
Teranishi M, Uchida Y, Nishio N, Kato K,
Otake H, Yoshida T, Suzuki H, Sone M, Sugiura S, Ando F, et al:
Polymorphisms in genes involved in oxidative stress response in
patients with sudden sensorineural hearing loss and Ménière’s
disease in a Japanese population. DNA Cell Biol. 31:1555–1562.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li BH, Zhang LL, Yin YW, Pi Y, Yang QW,
Gao CY, Fang CQ, Wang JZ and Li JC: Association between paraoxonase
2 Ser311Cys polymorphism and ischemic stroke risk: A meta-analysis
involving 5,008 subjects. Mol Biol Rep. 39:5623–5630. 2012.
View Article : Google Scholar
|
|
49
|
Tsatsakis A, Kouretas D, Tzatzarakis M,
Stivaktakis P, Tsarouhas K, Golokhvast KS, Rakitskii VN, Tutelyan
VA, Hernandez AF, Rezaee R, et al: Simulating real-life exposures
to uncover possible risks to human health: A proposed consensus for
a novel methodological approach. Hum Exp Toxicol. 36:554–564. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Docea AO, Calina D, Goumenou M, Neagu M,
Gofita E and Tsatsakis AM: Study design for the determination of
toxicity from long-term-low-dose exposure to complex mixtures of
pesticides, food additives and lifestyle products. Toxicol Lett.
258:S1792016. View Article : Google Scholar
|
|
51
|
Tsatsakis AM, Docea AO and Tsitsimpikou C:
New challenges in risk assessment of chemicals when simulating real
exposure scenarios; simultaneous multi-chemicals’ low dose
exposure. Food Chem Toxicol. 96:174–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Docea AO, Gofita E, Goumenou M, Calina D,
Rogoveanu O, Varut M, Olaru C, Kerasioti E, Fountoucidou P,
Taitzoglou I, et al: Six months exposure to a real life mixture of
13 chemicals’ below individual NOAELs induced non monotonic
sex-dependent biochemical and redox status changes in rats. Food
Chem Toxicol. 115:470–481. 2018. View Article : Google Scholar : PubMed/NCBI
|