Introduction
With the aging of the global population and rapid
increases in the size of the elderly population in China, there is
an urgent need to develop interventions aimed at preventing
neurocognitive disorders. Aging leads to neurotoxicity, resulting
in the manifestation of cognitive dysfunction, which can affect
abstract thinking, skilled movement, emotions, cognition and memory
(1). Studies have found a strong
link between inflammatory responses in certain regions of the
central nervous system, a process termed 'neuroinflammation' and
neurodegenerative diseases (2,3).
Primary neuronal injury may be the result of local
neuroinflammation caused by the release of inflammatory mediators
(4). The stereotaxic injection of
lipopolysaccharide (LPS) is widely used to induce intracranial
nerve inflammation as a model of neuroinflammation (5). LPS is a component of the cell wall
of gram-negative bacteria, which prompts a number of different
immune responses that result in brain inflammation to induce this
model (6).
Traditional nonsteroidal anti-inflammatory drugs can
have adverse effects as a result of their mechanism of action.
Ligustrazine (TMP) is an amide alkaloid isolated from
Ligusticum tubers and has been shown to dilate
cerebrovascular vessels, decrease vascular resistance, increase
blood flow to the brain and limbs, and improve microcirculation.
TMP reduces platelet surface activity, inhibits platelet
aggregation, and prevents thrombosis. Furthermore, TMP has
anti-inflammatory and antioxidative effects (7). However, studies on the effects of
TMP on neurocognitive impairments are lacking.
Certain neurodegenerative diseases are characterized
by autophagy, a lysosome-mediated cell content-dependent
degradation pathway. Autophagy is often involved in the
pathological mechanism underlying certain diseases (8). For example, patients with
neurodegenerative diseases such as Alzheimer's disease and
Parkinson's disease exhibit accumulation of autophagosomes in
neurons. Autophagy is known to affect aging and age-related
diseases primarily through the regulation of metabolic pathways and
food consumption. Autophagy and longevity are regulated by the
insulin/insulin-like growth factor-1 signaling pathway, the
mammalian target of rapamycin (mTOR) pathway, dietary restriction,
and hunger (9). In numerous
physiological and pathological settings, autophagy protects cells
by promoting the degradation of excess or damaged cell components
and promotes the circulation of amino acids, lipids, nutrients, and
metabolites. Autophagy is the main pathway for the degradation of
cytoplasmic proteins and organelles (10). Therefore, abnormal autophagy
processes directly affect protein misfolding and accumulation, and
the regulation of autophagy may be a key mechanism in the treatment
of neurodegenerative diseases.
The authors' previous experiments demonstrated that
LPS induces mild neurocognitive impairments in rats. The present
study aimed to extend the previous findings by elucidating
potential methods for the screening of neurodegenerative diseases
to identify the pathway through which TMP improves LPS-induced
neurocognitive impairments. The study also explored
autophagy-related molecular mechanisms as well as the mechanisms
underlying the prevention of neurodegenerative diseases.
Materials and methods
Animals
A total of 36 specific pathogen free (SPF)
Sprague-Dawley (SD) rats (half male and female, ~4-8 weeks old,
120-180 g) were used in this study. The animals were provided by
the Jinan Pengyue Experimental Animal Center (animal qualification
certificate no. 3700920006246). The animals were raised in SPF
conditions at the experimental animal center of Jinan University
and were housed at a temperature of 22±2°C and a relative humidity
of 50±5% with a 12-h light and dark cycle throughout the study.
Food and water were provided ad libitum. Experiments were conducted
following a 10-day quarantine period to allow for environmental
habituation. Additionally, all experiments were conducted in
accordance with the requirements of the experimental animal ethics
committee of Jinan University and approved by them.
Behavioral training and water maze
test
Place navigation experiments were used to assess
learning and memory ability. Experiments were conducted over 6 days
and the rats were subjected to the Morris water maze with a water
depth of ~26 cm (11). The water
temperature during the experiments was 24±1°C and the maze platform
was hidden beneath the water. The water maze was divided into four
quadrants. On the first day, the rats were placed individually in
the water in the N, E, S and W (N: North, E: East, S: South, W:
West) quad-rants in order, with their nose facing the wall. The
time taken to find the hidden platform was recorded as the latency.
The total search time was limited to 90 sec. If a rat failed to
find the platform within the specified time, it was guided to swim
to the platform and kept on the platform for 10 sec, and the
latency was recorded as 90 sec. On the second day, the middle point
of the E quadrant was used as the first entry point and the rats
were placed individually in the pool in the N, E, S and W quadrants
in order. This was repeated on days 3 to 6 and the latencies were
recorded daily. Following the completion of water maze training, 36
SD rats were randomly divided into 6 groups (n=6 for each group)
based on the average escape time in the water maze on day 6 using a
random number table.
Establishment of model groups by
intraperitoneal injections of ligustrazine for assessing the
preventative and treatment effects of ligustrazine on
neurocognitive impairment
TMP hydrochloride for injection was obtained from
Chengdu Beite Biotechnology (http://www.btyy.com/#/). The animals were randomly
divided into six groups (n=6 for each group) based on the water
maze training results. The doses of TMP were based on preliminary
results and previously published literature (12,13), and 3 doses were used: 50, 100 and
200 mg/kg. Injections were administered intraperitoneally daily for
5 days starting at 3 pm. Animals in groups 4, 5 and 6 were injected
intraperitoneally with 50, 100, and 200 mg/kg TMP (1 ml/100 g body
weight), respectively, whereas animals in groups 2 and 3 were
treated with 0.9% normal saline (1 ml/100 g body weight) in a
similar manner. Rats in group 1 were used as the blank control.
Establishment of LPS-induced
neurocognitive impairments
Except for the control group, all rats underwent
lateral ventricle intubation and fixation using brain stereotaxic
locator according to the manufacturer's protocol (RWD Life
Science). For intracerebroventricular injections, 3% pentobarbital
sodium was injected intraperitoneally (45 mg/kg) as an anesthetic.
Once the rats were adequately anesthetized, they were placed in a
stereotaxic instrument. A razor was used to remove the fur. Iodine
was applied as a disinfectant. An incision was made in the scalp
along the midline using a blade. Based on a reference map, the
following coordinates were used as the injection point: 1.0 mm from
the anterior fontanelle, 1.8 mm (mediolateral) from the sagittal
suture and 3.8 mm deep (dorsoventral). After identifying the
injection point, a hole was drilled in the skull. Once the
cerebrospinal fluid was visible, drilling was stopped and a cannula
was inserted into the lateral ventricle and fixed with dental
cement. After 3 days of recovery, lateral ventricle injections were
performed. During drug administration, the rats were immobilized
and a microinjection needle was vertically inserted into the
cannula to a depth of 3.5-4.0 mm from the cerebral epidermis. For
the sham group, 5 µl artificial cerebrospinal fluid
(Shanghai Canspec Scientific Instruments Co., Ltd.) was
administered. The 150 µl/5 µl LPS was injected into
the lateral ventricle in the model group, 50 mg/kg TMP group, 100
mg/kg TMP group and 200 mg/kg TMP group. Injection was paused for 2
min after the infusion of every 2 µl. The needle was left in
place for 10 min at the end of the injection and then withdrawn
slowly. Water maze testing commenced the following day.
Morris water maze and behavioral
testing
Place navigation was performed as described above.
For the spatial probe test, the rats were evaluated for their
ability to remember the spatial location of the platform after
locating the hidden platform. The time spent in the target quadrant
and the number of times the animals crossed the target quadrant
were measured. After the escape trial, the hidden platform was
removed on the seventh day of the experiment. The quadrant diagonal
to the platform quadrant was selected as the entry point. The
number of times the animals crossed the platform area within 90 sec
and the time spent in the platform quadrant were recorded. However,
an animal exposed to 200 mg/kg TMP plus LPS was found dead on day 3
of Morris water maze of behavioral testing. In addition, the
remaining animals in 200 mg/kg TMP plus LPS group exhibited a worse
mental state, which was characterized by drowsiness, apathy, a
curled position in the home cage and disinterest in activities
during the study.
Cytokine immunoassays
After 24 h of the completion of the behavioral
tests, the remaining rats (n=35) were sacrificed after anesthesia
with pentobarbital sodium (45 mg/kg) by intraperitoneal injection
and then the brain tissues were immediately collected using
cervical decapitation. The cortex and hippocampus were dissected
for further neurochemical analyses. The protein concentrations of
interleukin (IL)-1β and tumor necrosis factor (TNF)-α in the brain
were detected using rat-specific ELISA kits [IL-1β, cat. no.
SEA563Ra; TNF-α, cat. no. SEA133Ra; Wuhan USCN Business Co., Ltd.].
Experimental procedures were performed in accordance with the
manufacturer's protocols. Briefly, the brain tissues were rinsed in
ice-cold PBS and the homogenates were centrifuged for 30 min at
5,000 × g at 4°C. Then, 100 µl resultant supernatant was
added to a 96-well plate coated with the appropriate purified
antigen and incubated for 1 h at 37°C. Absorption was measured at
450 nm using a microplate reader (Bio-Rad Laboratories, Inc.). The
mean and standard error of triplicate measurements were calculated
for each tested sample.
Western blot analysis
Brain tissues were treated with differing
concentrations of compounds as previously indicated. The tissues
were centrifuged once with PBS at 300 × g for 5 min at 4°C.
Subsequently, the tissue samples were suspended in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) containing 1% phenylmethanesulfonyl fluoride
(Beyotime Institute of Biotechnology). The tissues were
mechanically disrupted using steel balls and homogenized at 15 × g
for 5 min in a homogenizer. The tissue homogenates were centrifuged
at 4°C and 8,000 × g for 15 min and the protein concentration was
determined using a BCA kit (Beyotime Institute of Biotechnology),
in which 60 µg protein per sample was mixed with sample
loading buffer and boiled for 5 min. Samples were separated on an
8-15% SDS-PAGE gel (Beyotime Institute of Biotechnology) at 80 V
for 30 min followed by 120 V for 90 min in 1X running buffer and
transferred to a polyvinylidene difluoride (PVDF) membrane (EMD
Millipore). Each PVDF membrane was blocked in Tris-buffered
saline/Tween (TBST) with 5% nonfat milk or bovine serum albumin
(BSA; Beyotime Institute of Biotechnology) for 60 min with gentle
shaking at 25°C and then incubated with the designated primary
antibodies (1:1,000) overnight at 4°C. The primary antibodies were
purchased from Cell Signaling Technology, Inc., [anti-AKT (cat. no.
4685), anti-PI3K (cat. no. 4257), anti-P62 (cat. no. 397949s),
anti-Beclin1 (cat. no. 3495s), anti-NF-κB (cat. no. 8242p),
anti-LC3 (cat. no. 2775s), anti-p-AKT (cat. no. 13038p),
anti-p-mTOR (cat. no. 5536), anti-mTOR (cat. no. 2983)]. Each PVDF
membrane was washed three times in TBST for 10 min per wash and
then incubated with the 1:5,000 goat anti-rabbit IgG (Beijing Ding
guo Changsheng Biotechnology Co., Ltd.; cat. no. IA-0071) in TBST
with 5% nonfat milk or BSA for 1 h at 25°C. The membranes were
washed three times in TBST for 10 min per wash and then visualized
by horseradish peroxi-dase (EMD Millipore; cat. no. 69078). The
band intensities were quantified using Image-Pro Plus 6.0.
Statistical analysis
All experiments were repeated in triplicate. The
data are presented as the means ± standard errors of the mean.
Statistical analyses were performed using SPSS 19.0 (IBM, Corps.).
The data were statistically analyzed using repeated-measures ANOVA
or one-way ANOVA followed by the Student-Newman-Keuls test or
Bonferroni's T2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Water maze and behavioral training
results in SD rats
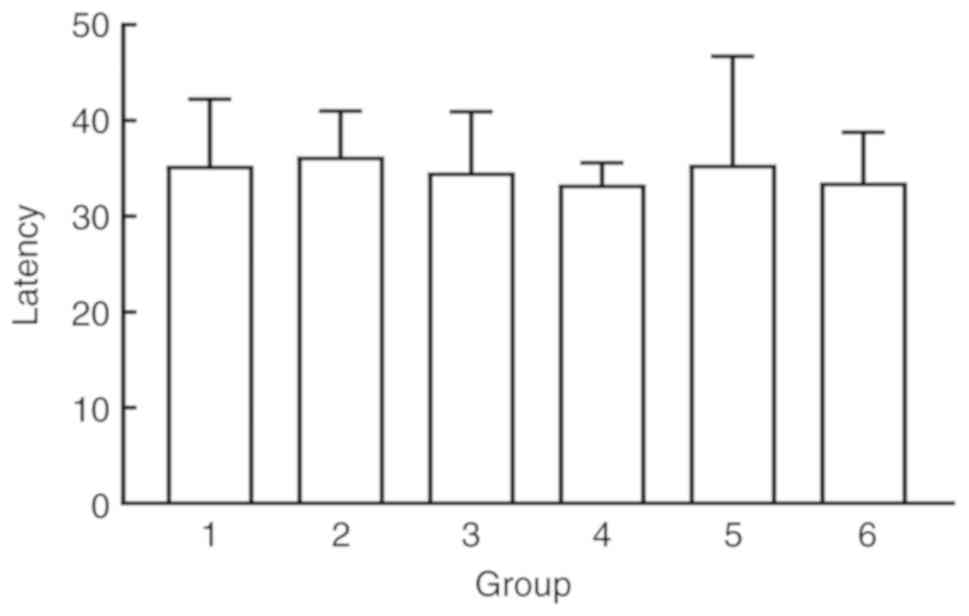
To rule out the effects of individual differences
and genetic mutations on the swimming ability of SD rats, the rats
were first trained to swim and remember the context of the water
maze. Learning and memory were evaluated according to escape
latency. The rats were trained for 6 days and calculated the
average escape latency from the four quadrants over the last two
days as the final escape latency. SD rats were randomly divided
into six groups using a random number table (n=6 for each group).
No significant differences in escape latency were observed between
the groups (P>0.05; Fig.
1).
Effects of TMP on spatial learning and
memory in rats with LPS-induced neurocognitive impairments
The results of the Morris water maze navigation
experiment on days 0 (escape trial after training), 1, 3, 5 and 6
revealed that compared with the blank control and sham operation
groups, the model group exhibited a significantly prolonged escape
latency (P<0.05). Compared with that of the model group, the
escape latency of the 50 mg/kg TMP group was significantly shorter
(P<0.05; Fig. 2A). The number
of platform crossings and time spent in the target quadrant were
significantly increased in the 50 mg/kg TMP group compared with the
model group (P<0.05), suggesting that LPS-induced neurocognitive
impairments were partly rescued in rats treated with TMP (Fig. 2B and C). On day 6, representative
navigation paths revealed that the navigation strategy of the rats
in the model group was poorer compared with that of the rats in the
control group. The navigation strategy of the rats in the 50 mg/kg
TMP group was improved compared with that of the rats in the model
group (Fig. 2D). The results of
the exploration experiment on day 7 revealed that the number of
platform crossings and time spent in the target quadrant were
reduced in the model group compared with the sham operation group,
indicating that rats in the model group exhibited LPS-induced
neurocognitive impairments.
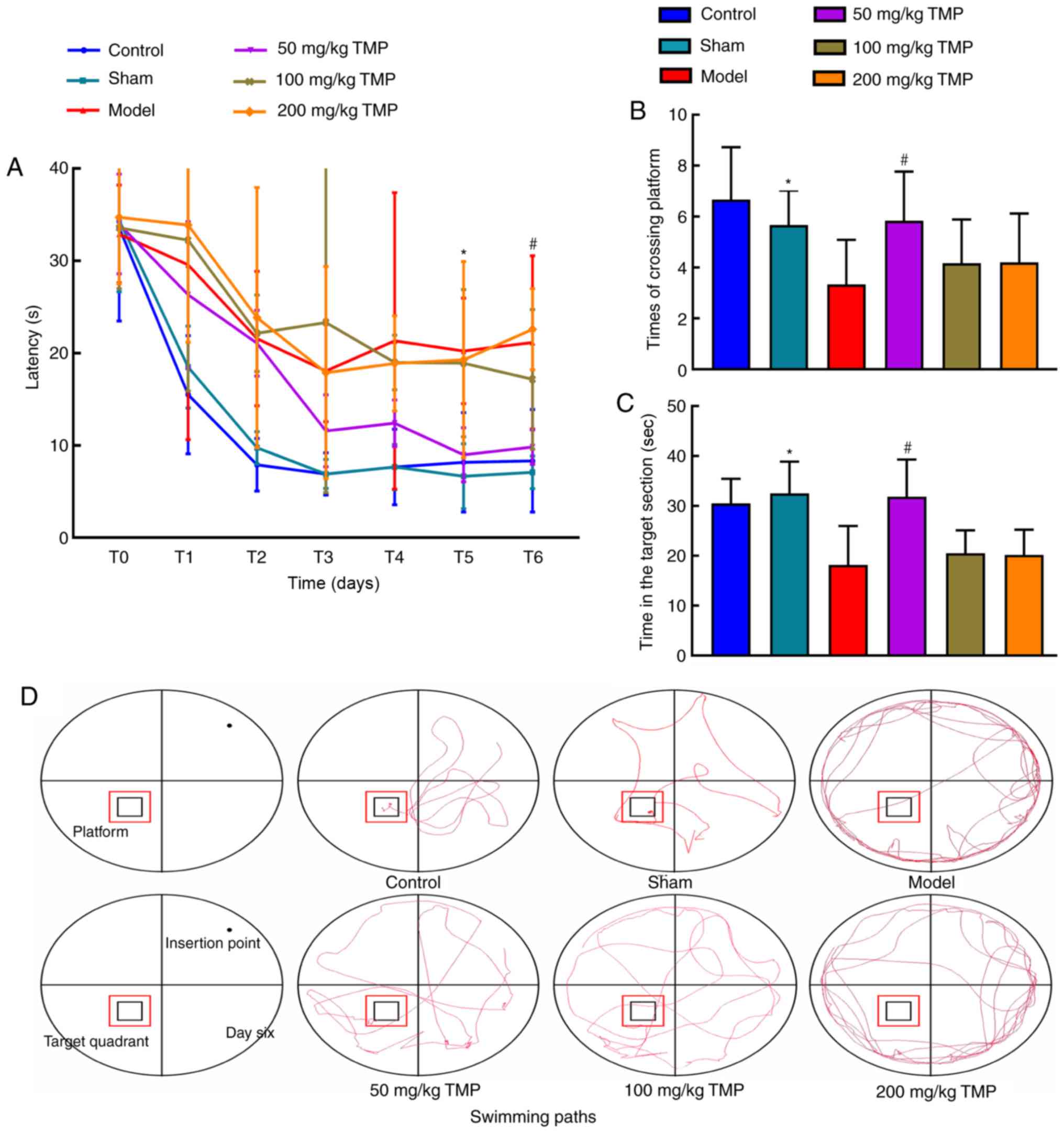 | Figure 2Ligustrazine hydrochloride improves
the spatial learning and memory of SD rats after LPS treatment. (A)
The water maze escape latencies of SD rats
intracerebroventricularly injected with artificial cerebrospinal
fluid or LPS; latencies on the fifth and sixth days, representing
spatial learning and recognition, are depicted. The spatial probe
test was used to measure of the (B) number of crossings of and the
(C) time spent in the target platform location after treatment with
ligustrazine hydrochloride. On the final day, a 90-sec probe trial
was conducted to detect the number of times the animals entered the
small target zone and the time they spent in the target quadrant.
(D) Representative swimming paths during exploration on day 6 of
training. The data are presented as the mean ± SEM, groups 1 to 5:
n=6, group 6: n=5. #P<0.05, the 50 mg/kg TMP group
vs. the model group; *P<0.01, the model group vs. the
sham group. SD rats, Sprague-Dawley rats; LPS, lipopolysaccharide;
TMP, ligustrazine. |
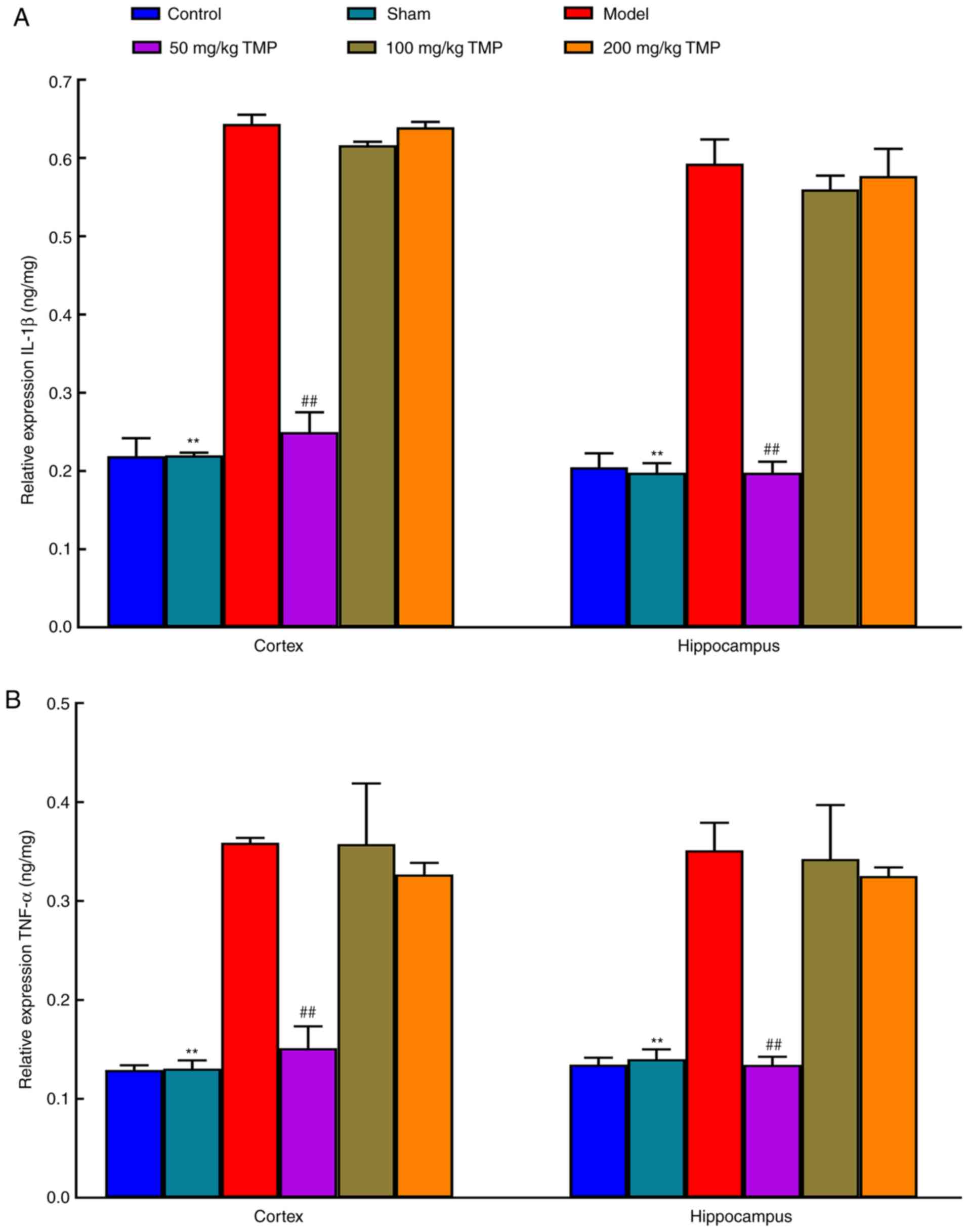
Expression of inflammatory factors
underlying LPS-induced neurocognitive impairments
Compared with those in the sham operation group, the
cortical and hippocampal IL-1β and TNF-α levels in the LPS
treatment group were elevated (P<0.05), suggesting that LPS
induced neuroinflammation in rats that resulted in neurocognitive
impairments. Compared with those in the model group, the levels of
IL-1β and TNF-α in the cortex and hippocampus of the 50 mg/kg TMP
group were decreased (P<0.05). There were no significant
differences between the 100 mg/kg TMP and 200 mg/kg TMP groups
(P>0.05), suggesting that low-dose TMP ameliorated LPS-induced
neuroinflammatory responses and improved neurocognitive impairments
(Fig. 3).
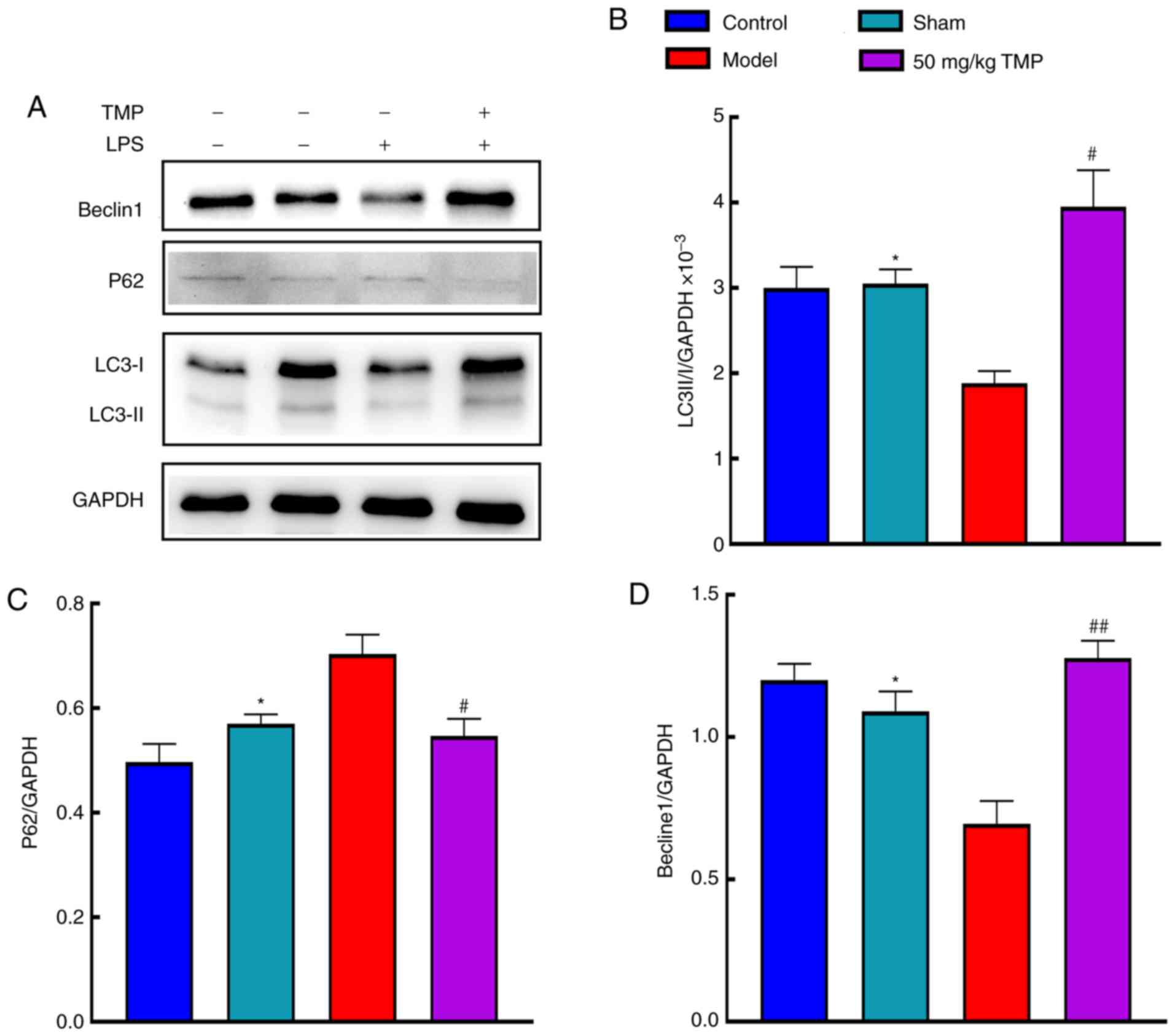
Effects of TMP on LPS-induced cognitive
impairments involve autophagy-related protein expression
Autophagy-related protein expression was analyzed by
western blotting (Fig. 4A).
Compared with the sham group, the model group exhibited
significantly decreased LC3 II/I expression (P<0.05). Compared
with the model group, the 50 mg/kg TMP group showed significantly
increased LC3 II/I expression (P<0.05; Fig. 4B). Compared with that in the sham
group, p62 expression in the model group was significantly
increased (P<0.05). Compared with that in the model group, p62
expression in the 50 mg/kg TMP group was reduced (P<0.05;
Fig. 4C). Beclin1 expression in
the model group was significantly decreased compared with that in
the sham group (P<0.05), and Beclin1 expression in the 50 mg/kg
TMP group was significantly increased (P<0.05; Fig. 4D).
TMP improves LPS-induced neurocognitive
impairments by activating autophagy through the PI3K/AKT/mTOR
signaling pathway
The expression levels of p-mTOR, mTOR, p-AKT, AKT,
PI3K and NF-κB were analyzed in the blank control group, sham
operation group, model group, and 50 mg/kg TMP group (Fig. 5A). Compared with that in the sham
group, p-mTOR/mTOR expression in the model group was significantly
increased (P<0.05). Compared with the model group, the 50 mg/kg
TMP group showed significantly reduced p-mTOR/mTOR expression
(P<0.05). p-AKT/AKT expression was significantly increased in
the model group compared with the sham group (P<0.05; Fig. 5B). There was a significant
difference in the reduction in p-AKT/AKT expression in the 50 mg/kg
TMP group compared with the model group (P<0.05; Fig. 5C). There was a significant
increase in PI3K levels in the model group compared with the sham
group (P<0.05) and a significant decrease in the low-dose group
compared with the model group (P<0.05; Fig. 5D). Additionally, there were
significant increases in NF-κB levels in the model group compared
to the sham group (P<0.05). However, there were no significant
differences in NF-κB levels between the model and 50 mg/kg TMP
groups (P>0.05; Fig. 5E).
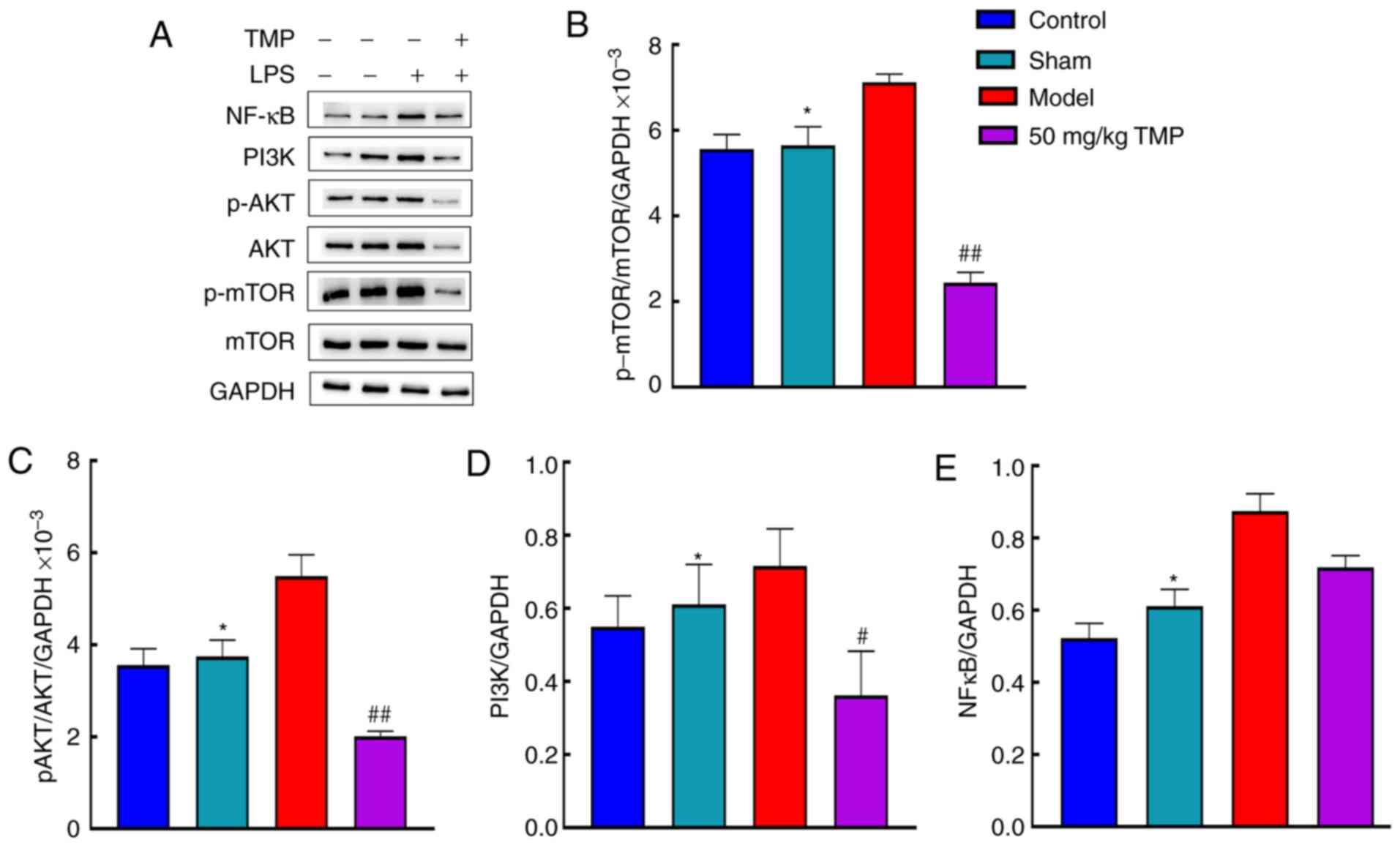 | Figure 5Ligustrazine hydrochloride improves
the protein expression levels of NFκB, PI3K, p-AKT, AKT, p-mTOR and
mTOR in the brain of SD rats after LPS treatment. (A) The protein
expression levels of NFκB, PI3K, p-AKT, AKT, p-mTOR and mTOR were
determined by western blot analysis. (B) Ligustrazine hydrochloride
improved the protein expression level of p-mTOR/mTOR in the brain
of SD rats after LPS treatment. (C) Ligustrazine hydrochloride
improved the protein expression level of (C) p-AKT/AKT, (D) PI3K
and (E) NFκB in the brain of SD rats after LPS treatment. The data
are presented as the mean ± SEM, n=3. #P<0.05 and
##P<0.01 for 50 mg/kg TMP vs. model group;
*P<0.05 for model group vs. sham. NF-κB, nuclear
transcription factor-κB; PI3K, phosphati-dylinositol 3 kinase;
p-AKT, protein kinase B; SD, Sprague-Dawley; LPS,
lipopolysaccharide; mTOR, mammalian target of rapamycin; TMP,
ligustrazine. |
Discussion
More research on the pharmacological effects of
traditional Chinese medicine may result in the discovery of novel
drugs and molecular mechanisms that may provide new opportunities
for the prevention and treatment of neurocognitive disorders. The
behavioral tests used in this study revealed that 50 mg/kg TMP
improved learning and memory in LPS-treated rats. Similar to the
results described by Bai et al (14), it was observed that TMP reduced
neuroinflammation caused by cerebral ischemia/reperfusion injury
and improved overall outcomes following brain injury. Additionally,
this study showed that TMP was protective against neurocognitive
impairments.
Recovery from neurocognitive impairments may be
attributed to the inhibition of neuroinflammation in the brain, the
relief of neuronal edema and increased central blood circulation.
In this study, IL-1β and TNF-α levels in the cortex and hippocampus
of the rats in the model group were increased, indicating the
occurrence of neuroinflammation in this group. In rats treated with
50 mg/kg TMP, IL-1β and TNF-α levels in the cortex and hippocampus
were reduced, suggesting that 50 mg/kg TMP inhibited
neuroinflammatory processes in the brain, thereby improving
neurocognitive impairments. The present study found that IL-1β and
TNF-α were not significantly reduced in the 100 mg/kg TMP and 200
mg/kg TMP groups following 5 days of ligustrazine pretreatment
compared with the blank control group. Additionally, when compared
with that of the model group, the escape latency of the 100 mg/kg
TMP and 200 mg/kg TMP groups was not shorter. These findings
indicate that neurocognitive impairments in the 100 mg/kg TMP and
200 mg/kg TMP rats were not significantly improved after
ligustrazine pretreatment; therefore, 100 mg/kg TMP and 200 mg/kg
TMP were considered to have had no effect.
LC3 attaches to the membrane surface of both
preautophagic and autophagic vesicles and is involved in the
formation of autophagosomes. LC3 expression is regarded as a marker
of the degree of autophagy activation and for the diagnosis of
altered autophagy (15). LC3 II
is a derivative of LC3 I formed by ubiquitin-proteasome proteolysis
after processing during autophagy and the formation of autophagic
bodies. Compared with the model group, the 50 mg/kg TMP group
exhibited increased LC3 II/I levels, suggesting that TMP activated
autophagy. Changes in autophagy activity can be measured by
observing changes in the expression of the autophagy-specific
degradation substrate p62 since p62 plays an important role as a
selective adaptor protein in autophagy. The PB1 domain of p62
promotes the packaging of ubiquitination substrates through
oligomerization and transports packaged substrates to participate
in the formation of autophagosomes (16). The p62 complex is formed through
the interaction between the LIR domain of p62 and LC3, and is
degraded as an autophagy-specific substrate in autophagic lysosomes
(17). This study found that in
the 50 mg/kg TMP group, which showed increased LC3 II/I levels, the
accumulation of p62 was reduced, suggesting the activation of
autophagy in the brain. Beclin1 is a key molecule in autophagy that
mediates the recruitment of autophagy-related proteins to
autophagic vesicles and promotes the formation and maturation of
autophagosomes by binding various proteins. Beclin1 regulates
autophagy alongside various factors that regulate mammalian class
III PI3K (Vps34). The regulation of the synthesis of the Beclin1
Vps34-Vps15 core complex can alter autophagy activity (18). The results of this study showed
that compared with the model group, the 50 mg/kg TMP group
exhibited increased Beclin1 synthesis following the activation of
autophagy. These results demonstrated that TMP can activate
autophagy and improve neurocognitive impairment. Interestingly, the
current study found that compared with the animals in the 50 mg/kg
TMP group, the animals in the 200 mg/kg TMP group exhibited a worse
mental state char-acterized by drowsiness, apathy, a curled
position in the home cage and disinterest in activities following
the injection of ligustrazine. In addition, an animal was found
dead with a stiff and cold body in the 200 mg/kg TMP group. No
abnormal changes were observed by the anatomy analysis. Combined
with the poor state of animals exposed to 200 mg/kg TMP plus LPS,
it is considered that the synergistic effect of 200 mg/kg TMP and
LPS may be the reason. However, the exact cause is unclear and will
be investigated further. Taken together, these findings, along with
the subsequent behavioral evaluations and detection of inflammatory
markers, support the hypothesis that 50 mg/kg doses of ligustrazine
can induce a significant pharmacological effect with respect to
improving neurocognitive functions.
Although the specific mechanisms of neuronal
autophagy are not fully understood, the PI3K/AKT/mTOR pathway is a
classical upstream activation pathway of autophagy (19). It is generally believed that
receptor tyrosine kinases, such as transmembrane glycoproteins, can
react with relevant extra-cellular ligands. The activation of
tyrosine residues of receptor tyrosine kinase recruits the p85
regulatory subunit to PI3K to activate the p110 catalytic subunit.
Phosphatidylinositol 4-phosphate and phosphatidylinositol
3,4-bisphosphate (PIP2) have a hydroxyl group on the inositol ring,
which is activated by PI3K phosphorylation; this produces PIP2 and
3,4,5-inositol (PIP3) independently. PIP3 is a second messenger and
an important molecule involved in PI3K signal transmission, as it
recruits signaling molecules of the PH domain to the plasma
membrane for activation (20).
The core structure of AKT is composed of three
parts: The regulatory active region at the C terminus, the
catalytic active region in the middle and the PH domain at the N
terminus. The PH domain of AKT is phosphorylated by activated PI3K.
AKT has catalytic activity at Thr308 and regulatory activity at
Ser473. When phosphoinositol-dependent kinases (PDK)1 and PDK2
participate in the reaction, AKT is phosphorylated at Ser473 and
Thr308, thereby activating and phosphorylating AKT to produce
p-AKT, which results in various physiological effects. Activated
p-AKT phosphorylates mTOR directly (21). Mammalian mTOR is a component of
mechanistic target of rapamycin C1 (mTORC1). When it is sensitive
to rapamycin, it regulates intracellular nutrition, growth factors,
pressure and other signals and activates PRAS40, a protein that
binds to mTORC1, causing PRAS40 to separate from mTORC1 (22). When it is not sensitive to
rapamycin, it controls cell proliferation and survival. When energy
supplies are impaired or the oxygen level is insufficient, Raptor,
a component of mTORC1, is phosphorylated. Phosphorylated Raptor
inhibits the activity of mTORC1, while phosphorylation activates
the downstream unc-5 autophagy activated kinase (ULK) complex
(including ulk1/2 and Atg13), increasing the activation of ulk1/2
and the phosphorylation level of Atg13. Once ULK1 is activated, it
forms a complex with Beclin1, leading to the activation and binding
of Beclin1 with the autophagy-related protein Atg1-Atg13 complex.
This promotes Atg1 activation and induces autophagy initiation or
upregulation to maintain cell homeostasis (23,24). The results of this study showed
that compared with the sham operation group, the model group showed
increased PI3K, p-AKT/AKT and p-mTOR/mTOR, suggesting that the
inhibition of autophagy in the model group may have been mediated
by the PI3K/AKT/mTOR signaling pathway. Compared with the levels in
the model group, the PI3K, p-AKT/AKT and p-mTOR/mTOR levels were
decreased in the 50 mg/kg TMP group, indicating that autophagy was
activated in the 50 mg/kg TMP group, possibly through the
PI3K/AKT/mTOR signaling pathway. Studies have shown that various
external factors can activate the NF-κB signaling pathway,
including cellular stress, ionizing radiation, LPS, cellular
membrane proteins and virus-related membrane proteins (25,26). The NF-κB signaling pathway is an
important pathway that regulates inflammatory responses. Studies
have shown that icariin activates autophagy by inhibiting the
expression of NF-κB signaling during chondrocyte apoptosis
(27,28). However, the present study found
that the expression levels of IL-1β, TNF-α and NF-κB were increased
in the model group compared with the sham group, suggesting that
LPS stimulation promoted the expression of NF-κB and induced
inflammation. However, compared with the model group, the 50 mg/kg
TMP group showed no significant difference in the protein
expression level of NF-κB, suggesting that the activation of
autophagy in the 50 mg/kg TMP group did not affect the NF-κB
signaling pathway. Autophagy activation in the 50 mg/kg TMP group
may have improved neurocognitive impairments through the
PI3K/AKT/mTOR signaling pathway rather than by reducing
inflammatory responses and inhibiting the expression of the NF-κB
protein. In this study, it was found that TMP activated autophagy,
not through the NF-κB pathway, but by inhibiting the PI3K/AKT/mTOR
pathway, which led to reduced neuroinflammation and an improvement
in neuro-cognition. However, the specific mechanisms underlying
these effects remain unclear and should be clarified in future
studies.
In conclusion, TMP improves LPS-induced
neurocognitive impairments in rats. TMP may activate autophagy
through the PI3K/AKT/mTOR signaling pathway, thereby improving the
inflammatory responses of neurons, increasing learning and memory,
and improving neurocognitive impairments in rats.
Abbreviations:
|
LC3
|
light chain 3
|
|
PI3K
|
phosphatidylinositol 3 kinase
|
|
AKT
|
protein kinase B
|
|
NF-κB
|
nuclear transcription factor-κB
|
|
mTOR
|
mammalian target of rapamycin
|
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81974185
and 81471235), the Guangdong Provincial Natural Science Foundation
of China (grant no. 2019A1515012024), the Program of Introducing
Talents of Discipline to Universities (grant no. B14036), the
Science and Technology Foundation of Guangdong (grant no.
2010B030700016), the Cultivation and Innovation Fund of Jinan
University (grant no. 21617460), and the Medical Group Fund of
Jinan University (grant no. 88016013039).
Availability of data and materials
The data are available from the corresponding author
upon reasonable request.
Authors' contributions
GL and JD designed the study and wrote the initial
draft of the manuscript. SL contributed to the analysis and
interpretation of data and assisted in the preparation of the
manuscript. HW contributed to data collection, and interpretation
and critically reviewed the manuscript. YW participated in and
performed the experiments in this study. YF supervised the methods
of all the experiments and interpreted of results. HT provided help
for analyzing the data. XY directed the study implementation,
including quality assurance and control of experiments. RP and FJ
supervised the present study and revised the manuscript. GL and JD
revised the manuscript. All authors approved the final version of
the manuscript and agreed to be accountable for all aspects of the
work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
All procedures performed in studies involving
animals were in accordance with the ethical standards of the
institution or practice at which the studies were conducted
(Experimental Animal Ethics Committee of Jinan University; Approval
no. I ACUC-20180604-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests. The founding sponsors had no role in the design of the
study; in the collection, analyses, or interpretation of the data;
in the writing of the manuscript; or in the decision to publish the
results.
References
|
1
|
Kanchi PK and Dasmahapatra AK: Polyproline
chains destabilize the Alzheimer's amyloid-beta protofibrils: A
molecular dynamics simulation study. J Mol Graph Model.
93:1074562019. View Article : Google Scholar
|
|
2
|
Leitner GR, Wenzel TJ, Marshall N, Gates
EJ and Klegeris A: Targeting toll-like receptor 4 to modulate
neuroinflammation in central nervous system disorders. Expert Opin
Ther Targets. 23:865–882. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ransohoff RM: How neuroinflammation
contributes to neurode-generation. Science. 353:777–783. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Augusto-Oliveira M, Arrifano GP,
Lopes-Araújo A, Santos-Sacramento L, Takeda PY, Anthony DC, Malva
JO and Crespo-Lopez ME: What do microglia really do in healthy
adult brain? Cells. 8:pii: E1293. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Batista CRA, Gomes GF, Candelario-Jalil E,
Fiebich BL and de Oliveira ACP: Lipopolysaccharide-induced
neuroinflammation as a bridge to understand neurodegeneration. Int
J Mol Sci. 20:pii: E2293. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li DW, Zhou FZ, Sun XC, Li SC, Yang JB,
Sun HH and Wang AH: Ginsenoside Rb1 protects dopaminergic neurons
from inflammatory injury induced by intranigral lipopolysaccharide
injection. Neural Regen Res. 14:1814–1822. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gong X, Ivanov VN, Davidson MM and Hei TK:
Tetramethylpyrazine (TMP) protects against sodium arsenite-induced
nephrotoxicity by suppressing ROS production, mitochondrial
dysfunction, pro-inflammatory signaling pathways and programed cell
death. Arch Toxicol. 89:1057–1070. 2015. View Article : Google Scholar :
|
|
8
|
Yang L, Wang H, Liu L and Xie A: The role
of insulin/IGF-1/PI3K/Akt/GSK3β signaling in parkinson's disease
dementia. Front Neurosci. 12:732018. View Article : Google Scholar
|
|
9
|
Suzuki H, Osawa T, Fujioka Y and Noda NN:
Structural biology of the core autophagy machinery. Curr Opin
Struct Biol. 43:10–17. 2017. View Article : Google Scholar
|
|
10
|
Fujikake N, Shin M and Shimizu S:
Association between autophagy and neurodegenerative diseases. Front
Neurosci. 12:2552018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu S, Li G, Tang H, Pan R, Wang H, Jin F,
Yan X, Xing Y, Chen G, Fu Y and Dong J: Madecassoside ameliorates
lipo-polysaccharide-induced neurotoxicity in rats by activating the
Nrf2-HO-1 pathway. Neurosci Lett. 709:1343862019. View Article : Google Scholar
|
|
12
|
Fu S, Wang J, Hao C, Dang H and Jiang S:
Tetramethylpyrazine ameliorates depression by inhibiting TLR4-NLRP3
inflammasome signal pathway in mice. Psychopharmacology (Berl).
236:2173–2185. 2019. View Article : Google Scholar
|
|
13
|
Ye Q, Zhang Y, Fu J, Zou Y, Zhao W, Chen C
and Liu K: Effect of ligustrazine on endometrium injury of thin
endometrium rats. Evid Based Complement Alternat Med.
2019:71619062019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bai XY, Wang XF, Zhang LS, Du PC, Cao Z
and Hou Y: Tetramethylpyrazine ameliorates experimental autoimmune
encephalomyelitis by modulating the inflammatory response. Biochem
Biophys Res Commun. 503:1968–1972. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lystad AH and Simonsen A: Mechanisms and
pathophysiological roles of the ATG8 conjugation machinery. Cells.
8:pii: E973. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kraft LJ, Dowler J, Manral P and Kenworthy
AK: Size, organization, and dynamics of soluble SQSTM1 and LC3
SQSTM1 complexes in living cells. Autophagy. 12:1660–1674. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamada T, Dawson TM, Yanagawa T, Iijima M
and Sesaki H: SQSTM1/p62 promotes mitochondrial ubiquitination
independently of PINK1 and PRKN/parkin in mitophagy. Autophagy.
15:2012–2018. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stjepanovic G, Baskaran S, Lin MG and
Hurley JH: Vps34 kinase domain dynamics regulate the autophagic pi
3-kinase complex. Mol Cell. 67:528–534. e5232017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li XT, Liang Z, Wang TT, Yang JW, Ma W,
Deng SK, Wang XB, Dai YF, Guo JH and Li LY: Brain-derived
neurotrophic factor promotes growth of neurons and neural stem
cells possibly by triggering the phosphoinositide
3-kinase/akt/glycogen synthase kinase 3β/β-catenin pathway. CNS
Neurol Disord Drug Targets. 16:828–836. 2017. View Article : Google Scholar
|
|
20
|
Ishii A, Furusho M, Macklin W and Bansal
R: Independent and cooperative roles of the Mek/ERK1/2-MAPK and
PI3K/Akt/mTOR pathways during developmental myelination and in
adulthood. Glia. 67:1277–1295. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gallardo-Vera F, Tapia-Rodriguez M, Diaz
D, Fortoul van der Goes T, Montaño LF and Rendón-Huerta EP:
Vanadium pentoxide increased PTEN and decreased SHP1 expression in
NK-92MI cells, affecting PI3K-AKT-mTOR and Ras-MAPK pathways. J
Immunotoxicol. 15:1–11. 2018. View Article : Google Scholar
|
|
22
|
Wang H, Liu Y, Wang D, Xu Y, Dong R, Yang
Y, Lv Q, Chen X and Zhang Z: The upstream pathway of mtor-mediated
autophagy in liver diseases. Cells. 8:pii: E1597. 2019. View Article : Google Scholar
|
|
23
|
Fusco C, Mandriani B, Di Rienzo M, Micale
L, Malerba N, Cocciadiferro D, Sjøttem E, Augello B, Squeo GM,
Pellico MT, et al: TRIM50 regulates Beclin 1 proautophagic
activity. Biochim Biophys Acta Mol Cell Res. 1865:908–919. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim BW, Jin Y, Kim J, Kim JH, Jung J, Kang
S, Kim IY, Kim J, Cheong H and Song HK: The C-terminal region of
ATG101 bridges ULK1 and PtdIns3K complex in autophagy initiation.
Autophagy. 14:2104–2116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caviedes A, Lafourcade C, Soto C and
Wyneken U: BDNF/NF-κB signaling in the neurobiology of depression.
Curr Pharm Des. 23:3154–3163. 2017. View Article : Google Scholar
|
|
26
|
Xie XK, Xu ZK, Xu K and Xiao YX: DUSP19
mediates spinal cord injury-induced apoptosis and inflammation in
mouse primary microglia cells via the NF-kB signaling pathway.
Neurol Res. 42:31–38. 2020. View Article : Google Scholar
|
|
27
|
Jiang LB, Meng DH, Lee SM, Liu SH, Xu QT,
Wang Y and Zhang J: Dihydroartemisinin inhibits catabolism in rat
chondrocytes by activating autophagy via inhibition of the NF-κB
pathway. Sci Rep. 6:389792016. View Article : Google Scholar
|
|
28
|
Mi B, Wang J, Liu Y, Liu J, Hu L, Panayi
AC, Liu G and Zhou W: Icariin activates autophagy via
down-regulation of the NF-κB signaling-mediated apoptosis in
chondrocytes. Front Pharmacol. 9:6052018. View Article : Google Scholar
|