Introduction
Periodontitis, which has a high incidence rate, is a
chronic inflammatory disease that results in tooth loss (1). Periodontitis is characterized by the
loss of alveolar bone periodontal ligament and gingival tissue, and
is caused by numerous factors, including poor dental hygiene, the
deposition of bacterial plaque on the teeth, traumatic misalignment
of the teeth and imbalances of the inflammatory and immune response
(2,3). In addition, periodontitis has been
reported to be associated with a number of diseases, such as
hypertension, chronic kidney disease and diabetes (4-7).
Therefore, the treatment for periodontitis is vital for health. The
current treatments for periodontitis include flap curettage,
scaling and root planing procedures (8-10).
However, the effects of the aforementioned methods can only control
the development of periodontitis (10). At present, there is still no
effective treatment for periodontitis, at least to the best of our
knowledge.
As mesenchymal stem cells, periodontal ligament stem
cells (PDLSCs) have been the focus of research in the field of
restoration and regeneration (11,12). Regeneration therapy based on
mesenchymal stem cells has yielded promising results (13). PDLSCs have been reported to
possess a high proliferative, self-renewal and
multi-differentiation abilities (14-17). Following transplantation, PDLSCs
are expected to generate new tissues or reconstruct damaged
tissues. PDLSC damage is a critical factor behind tooth loss. In
addition, elevated inflammation levels promote the progression of
periodontitis (18,19). Therefore, it is vital to control
the inflammatory damage of PDLSCs for the restoration of tissue and
to inhibit the progression of periodontitis.
Lipopolysaccharide (LPS), a major stimulator of
inflammation, rapidly promotes the generation of pro-inflammatory
cytokines or inflammatory mediators. Therefore, LPS has been widely
used for the construction of experimental models of periodontitis
(20). Studies have indicated
that LPS levels are high in patients with periodontitis (21). LPS is a vital factor and
LPS-stimulated cell models can provide a reference for clinical
diagnosis and therapy. LPS-stimulated human periodontal ligament
cells were constructed as a cell model in the present study.
Chinese herbal monomers have exhibited improved
anti-inflammatory abilities in numerous diseases (22-25). Tetramethylpyrazine (TMP), which is
extracted from Ligusticum wallichii, has been used in
cardiovascular disorders in clinical practice (26). LPS has been confirmed to possess
numerous bioactivities, such as anti-inflammatory, anti-apoptotic
and antioxidant effects (26).
Furthermore, TMP has been reported to possess anti-inflammatory
bioactivity in spinal cord ischemia-reperfusion injury, human
umbilical vein endothelial cell inflammation injury and brain
injury (27-30). However, the effects of TMP on
periodontitis remain to be elucidated. The present study
investigated the effects of TMP in LPS-stimulated human PDLSCs
(hPDLSCs). The results revealed that TMP exerted anti-inflammatory
and anti-apoptotic effects against LPS-stimulated hPDLSCs via the
downregulation of microRNA (miR)-302b.
Materials and methods
Cells and cell culture
hPDLSCs (HUM-iCELL-m002; iCell Bioscience Inc.) were
cultured at a density of 1x104 cells/well in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS in 5%
CO2 at 37°C.
CCK-8 assay
To evaluate the effects of LPS on hPDLSCs, the cells
were divided into the control group and LPS group. Cells were
treated with 1 µg/ml LPS (Sigma-Aldrich; Merck KGaA).
Subsequently, the cells were incubated for 24, 48 and 72 h, at
37°C. To evaluate the effects of TMP on hPDLSCs, the cells were
divided into the control and experimental groups. Cells in the
experimental group were treated with various concentrations of TMP
(10, 20 and 30 µm) and incubated for 24, 48 or 72 h, at
37°C. To investigate the effects of TMP on LPS-stimulated hPDLSCs,
the cells were divided into the control and experimental groups.
The cells in the experimental groups were treated with various
concentrations (10, 20 and 30 µm) of TMP (purity ≥98%;
Beijing Solarbio Science and Technology Co., Ltd.) in the presence
of 1 µg/ml LPS. Following treatment, 10 µl CCK-8
solution was added to each well and the cells were incubated at
37°C for 1 h. Cell viability was determined by the reading the
absorbance at a wavelength of 450 nm using a Varioskan™ LUX
multimode microplate reader (Thermo Fisher Scientific, Inc.).
Assessment of inflammation levels
The inflammatory markers, tumor necrosis factor
(TNF)-α, interleukin (IL)-1β and IL-6, were evaluated. The cells
were treated with 1 µg/ml LPS and various concentrations of
TMP (10, 20 and 30 µm). The experimental groups were treated
with various concentrations of TMP in the presence of LPS. Cells
treated with 1 µg/ml LPS were considered as the LPS group.
Cells in the control, LPS and experimental groups were lysed with
0.25% trypsin and centrifuged at 16,000 x g 4°C for 15 min to
harvest the supernatant. The levels of TNF-α, IL-1β and IL-6 in the
supernatants were determined using the following ELISA kits: Human
TNF-α (cat. no. ab181421; Abcam), human IL-1β (cat. no. ab100562;
Abcam) and human IL-6 (cat. no. ab178013; Abcam).
Measurement of cell apoptosis
Following treatment, cells were digested with 0.25%
trypsin and harvested. Cells were then rinsed with PBS (0.01 mol/l)
and resuspended in binding buffer. A total of 1x106
cells were stained with Annexin V-FITC (5 µl) and propidium
iodide staining solution (10 µl). The cell apoptosis of the
different groups was analyzed using a flow cytometer (BD
Biosciences).
Western blot analysis
Cells in each experimental group were lysed in lysis
buffer (Beyotime). The protein concentration was measured using a
bicinchoninic acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Total protein was separated by 10% SDS-PAGE.
Separated proteins were then transferred to nitrocellulose
membranes. Following blocking with skim milk, the membranes were
incubated with primary antibodies against pro-caspase-3 (1:1,000;
cat. no. ab32499; Abcam), Bax (1:200; cat. no. ab53154; Abcam),
cleaved caspase-3 (1:1,000; cat. no. ab2302; Abcam), and Bcl-2
(1:500; cat. no. ab194583; Abcam) at 4°C overnight. Subsequently,
the membranes were incubated with horseradish peroxidase-labeled
secondary antibody (1:2,000; Beyotime Institute of Biotechnology)
for 1 h, at room temperature. Protein bands were visualized using
enhanced chemiluminescence reagent. Protein expression was
determined using an ImageQuant LAS500 chemiluminescence camera (GE
Healthcare Life Sciences) and ImageJ 1.52 k software (National
Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
miR-302b expression was measured using the RT-qPCR
detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Total RNA was extracted using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) RNA was reverse
transcribed into cDNA using a PrimeScript RT reagent kit (Takara
Biotechnology Co., Ltd.). RT-qPCR analysis was performed as
previously described (31).
Cell transfection
To verify the successful construction of the
miR-302b mimic group, cells were divided into the control,
miR-negative control (NC) and miR-302b mimic groups. The cells were
transfected with 50 nmol/l miR-NC and 50 nmol/l miR-302b mimic and
incubated for 5 min at 37°C. To investigate the effects of miR-302b
mimic on inflammation and apoptosis, the cells were divided into
the hPDLSC group, LPS group, LPS (1 µg/ml for 72 h) + TMP
(30 µm) group, LPS (1 µg/ml for 72 h) + TMP (30
µm) + miR-NC group and LPS (1 µg/ml for 72 h) + TMP
(30 µm) + miR-302b mimic group.
Statistical analysis
Data are presented as the means ± SD. Data analysis
was performed using SPSS software (version no. 11.0; IBM Corp.) and
GraphPad Prism (version no. 8.0; GraphPad Software, Inc.). One-way
and two-way ANOVA were performed followed by Tukey's post hoc test
for differential comparisons between groups.
Results
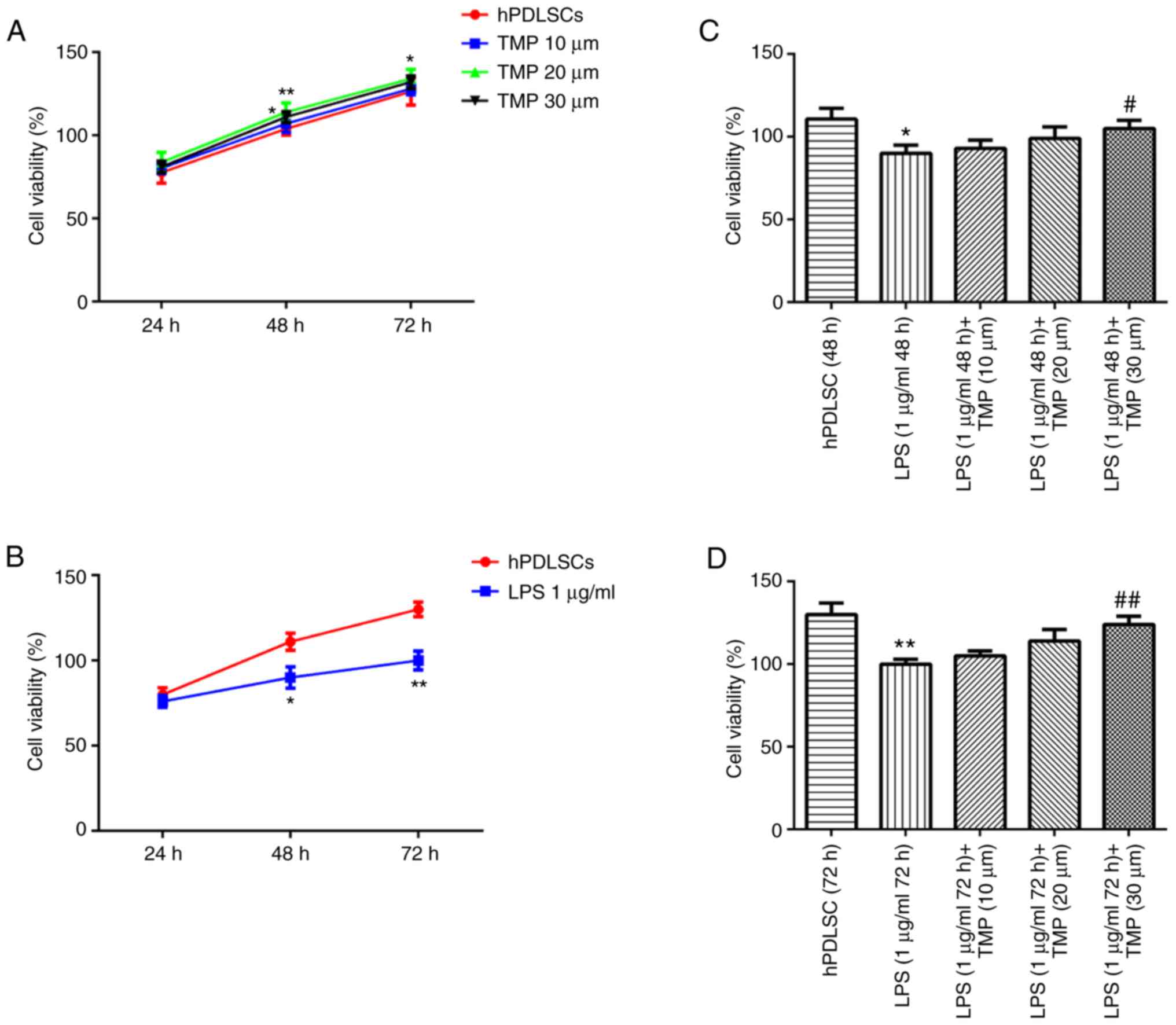
TMP alleviates the effects of LPS on cell
viability
The concentrations of 10, 20 and 30 µm TMP
exerted no effect on cell viability, confirming that TMP had no
toxic effect on the hPDLSCs (Fig.
1A). Following treatment with 1 µg/ml LPS for 48 and 72
h, the cell survival rate was significantly lower compared with
that of the control group, indicating that LPS exerted an
inhibitory effect on cell viability (Fig. 1B). TMP treatment increased the
survival rate of LPS-stimulated cells in a concentration-dependent
manner (Fig. 1C and D).
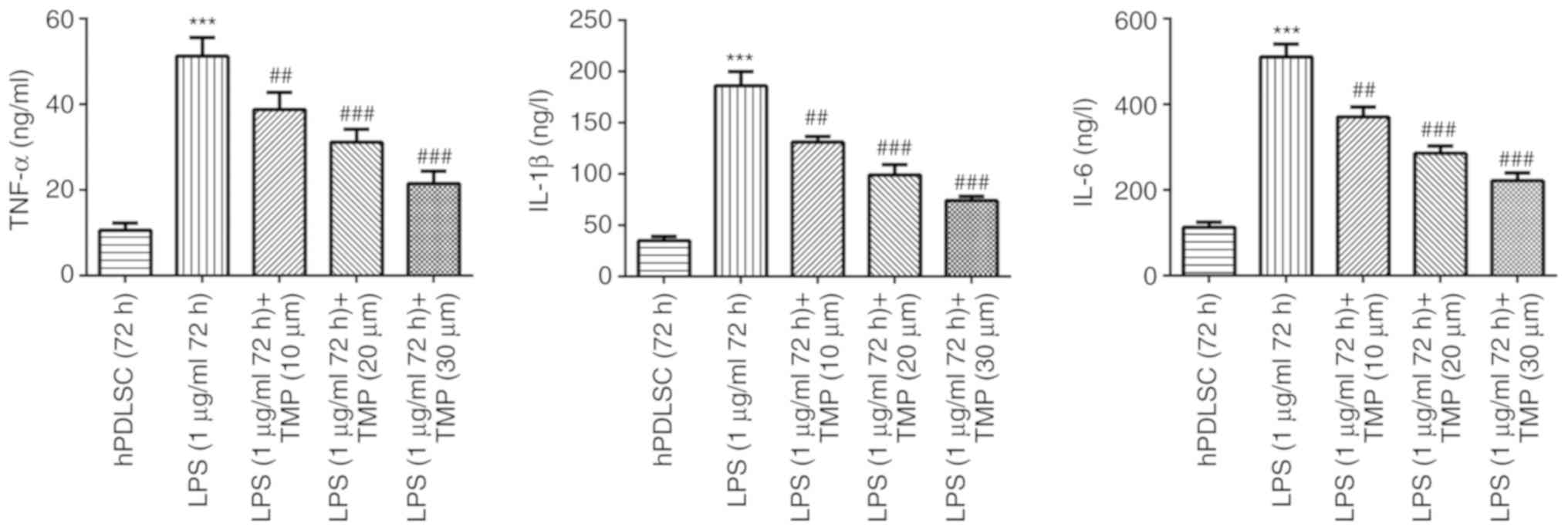
TMP reduces the inflammation levels in
LPS-stimulated hPDLSCs
Compared with the control group, TNF-α, IL-1β and
IL-6 levels were increased following LPS treatment, confirming that
the present cell model of periodontitis was successfully
established. The inflammation levels induced by LPS were reduced by
TMP treatment in a concentration-dependent manner (Fig. 2).
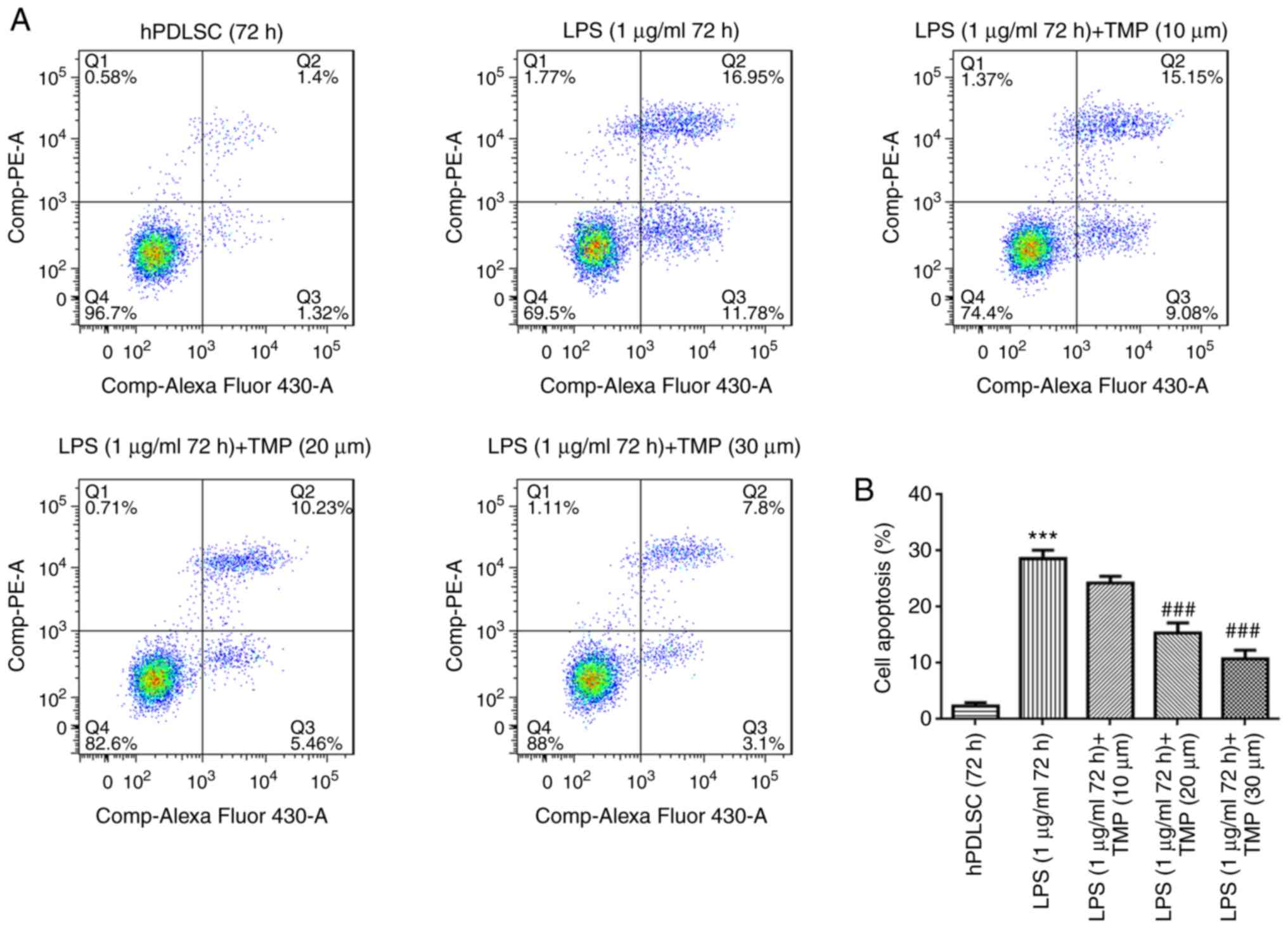
TMP reduces the cell apoptosis of
LPS-stimulated hPDLSCs
Cell apoptotic levels were evaluated by flow
cytometry. Cell apoptosis was elevated in the LPS group compared
with the control group (Fig. 3).
TMP alleviated the apoptosis of LPS-stimulated hPDLSCs in a
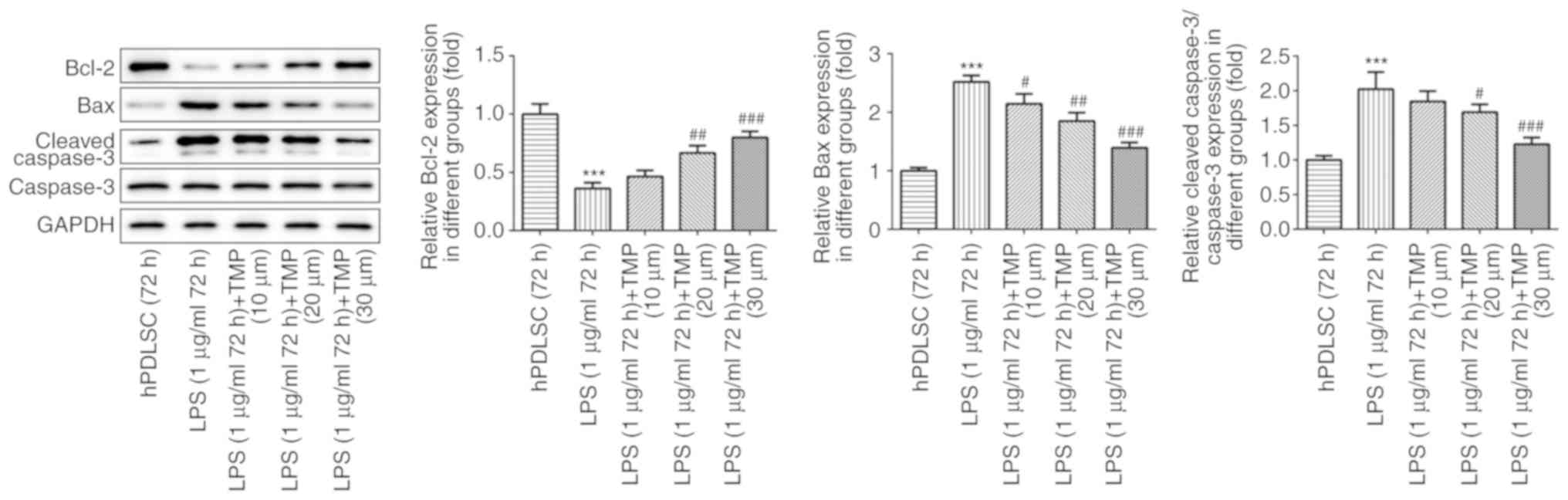
concentration-dependent manner. The expression of apoptosis-related
proteins was also evaluated. Compared with the control group, the
levels of the pro-apoptotic proteins, Bax and cleaved caspase-3,
were upregulated, while the levels of the anti-apoptotic protein,
Bcl-2, was decreased in the LPS-stimulated cells (Fig. 4). Following treatment with TMP,
the Bax and cleaved caspase-3 levels decreased, and the Bcl-2
levels increased in a concentration-dependent manner in the
LPS-stimulated cells. This indicated that TMP exerted protective
effects on cell apoptosis induced by LPS via the downregulation of
Bax and cleaved caspase-3, and the upregulation of Bcl-2.
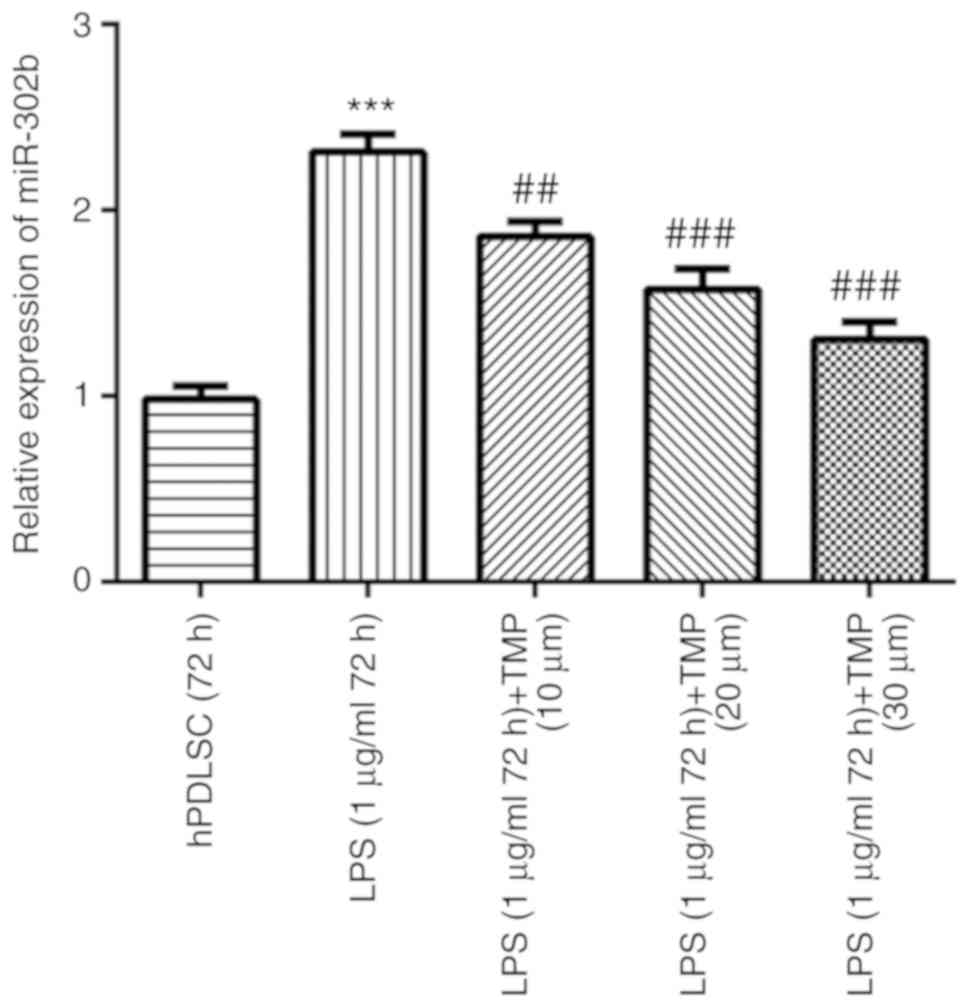
TMP downregulates the miR-302b levels in
LPS-stimulated cells
Compared with the control group, the miR-302b levels
were upregulated in the LPS group. The miR-302b levels were
downregulated by TMP in the LPS-stimulated cells in a
concentration-dependent manner, indicating that miR-302b may play a
role in LPS-induced injury and is a potential target of TMP
(Fig. 5).
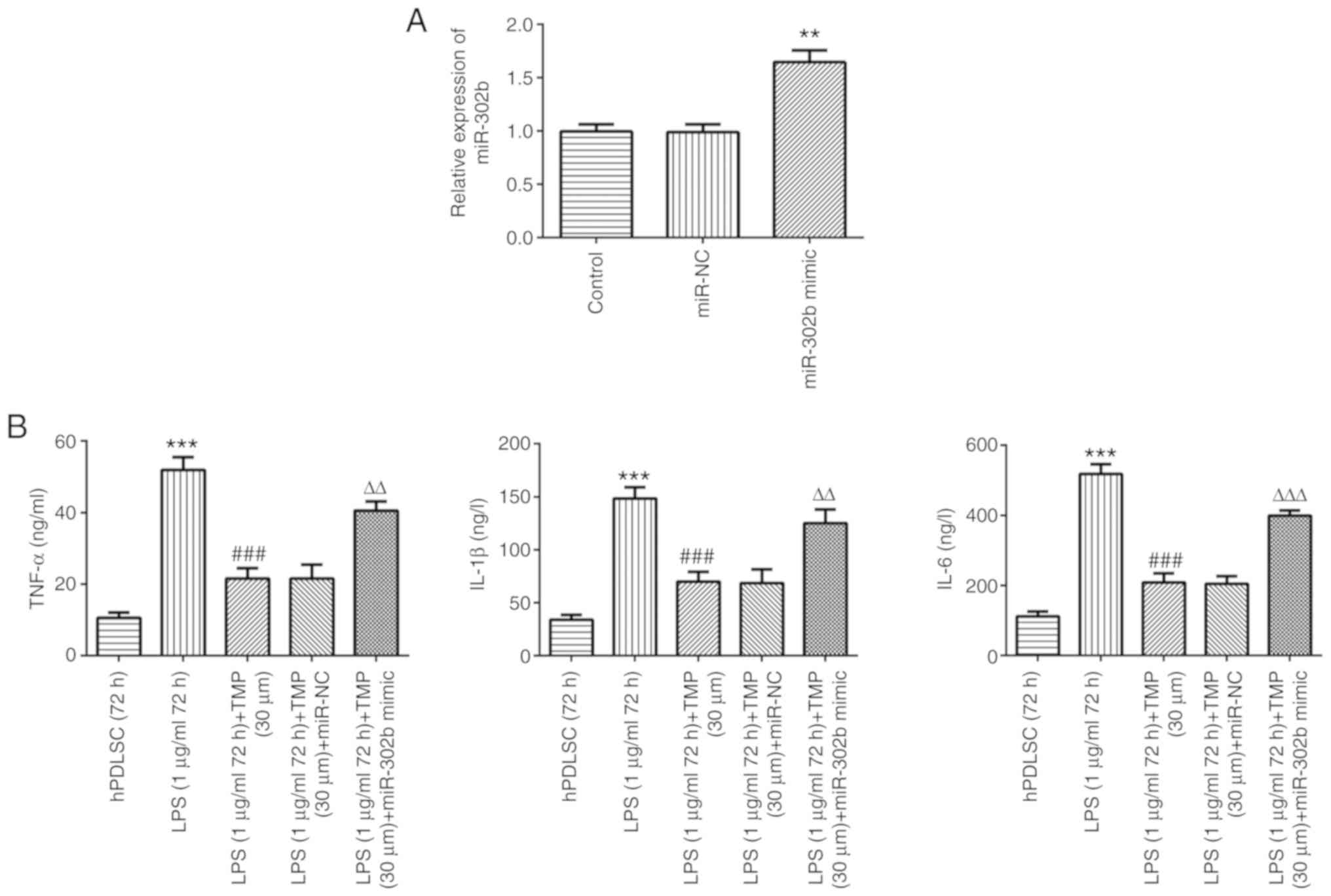
miR-302b mimic reverses the
anti-inflammatory effects of TMP in LPS-stimulated cells
As shown in Fig.
6A, the miR-302b levels were higher compared with those in the
control and miRNA-NC groups, indicating that miR-302b mimic was
successfully transfected. LPS stimulation increased the levels of
TNF-α, IL-1β and IL-6 compared with the controls, and this effect
was reversed by TMP in a concentration-dependent manner (Fig. 6B). Following transfection with
miR-302b mimic, inflammation levels increased in the LPS-stimulated
cells compared with the LPS (1 µg/ml 72 h) + TMP (30
µm) group, indicating that the anti-inflammatory effect of
TMP in LPS-stimulated cells was achieved via the downregulation of
miR-302b.
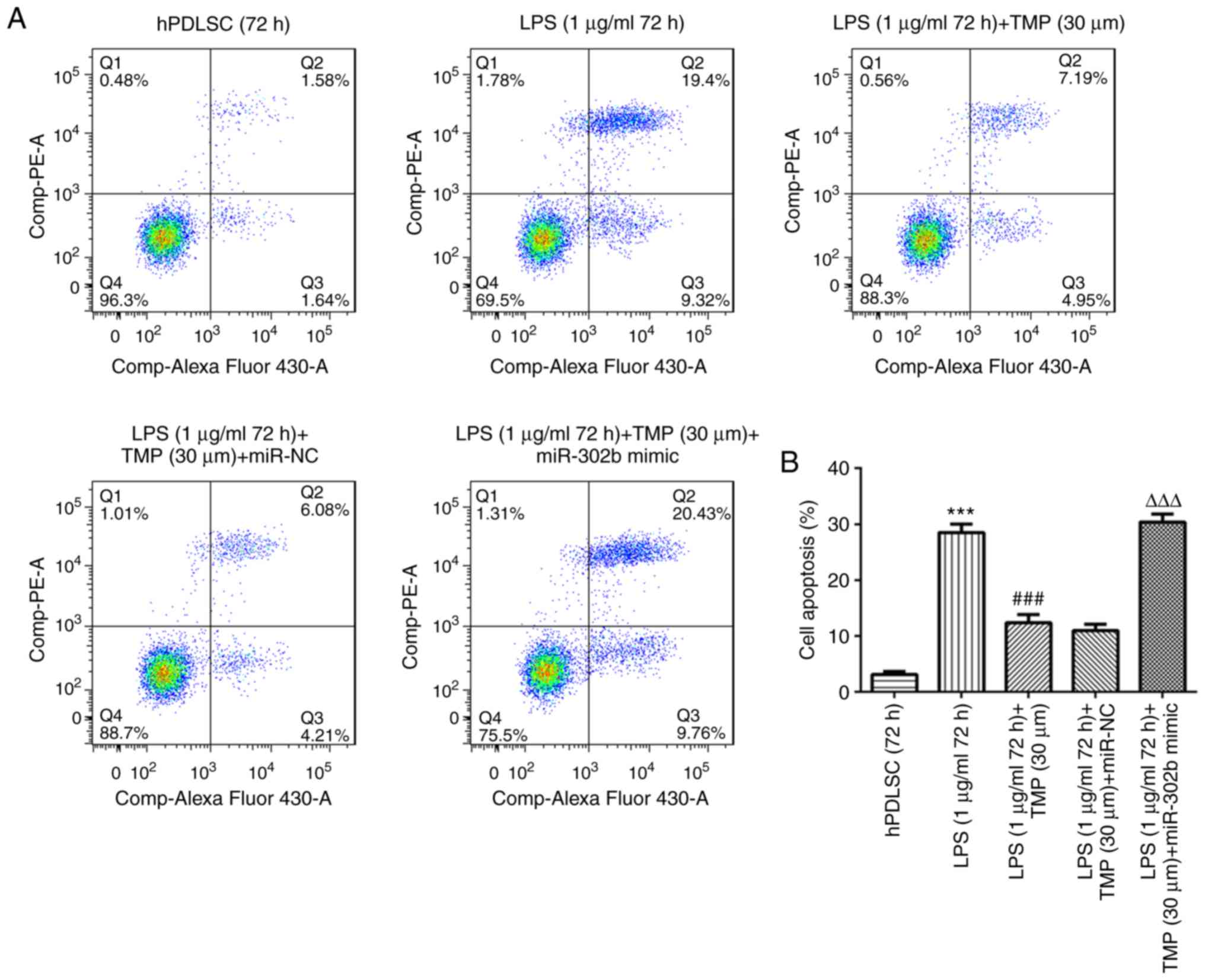
miR-302b mimic reverses the
anti-apoptotic effects of TMP in LPS-stimulated cells
Cell apoptotic levels were higher in the LPS group
compared with the control group (Fig.
7). LPS-induced cell apoptosis was reduced by TMP and this
effect was weakened by miR-302b mimic transfection, indicating that
the anti-apoptotic effect of TMP was achieved via the
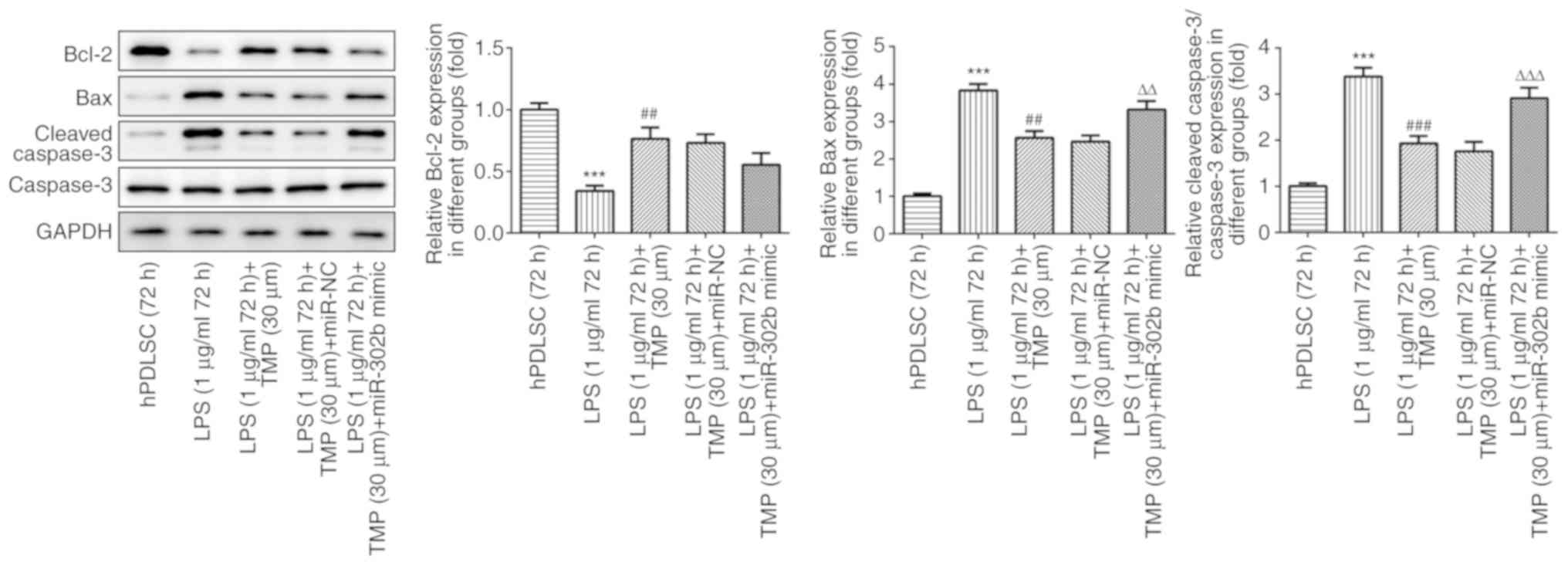
downregulation of miRNA-302b. The levels of the pro-apoptotic
proteins, Bax and cleaved caspase-3, were elevated by LPS
stimulation and the levels of the anti-apoptotic protein, Bcl-2,
were decreased by LPS stimulation (Fig. 8). The effect induced by LPS was
reversed by TMP. In addition, compared with the LPS (1
µg/ml, 72 h) + TMP (30 µm) group, the levels of
pro-apoptotic proteins increased, while those of Bcl-2 were
downregulated in the LPS (1 µg/ml, 72 h) + TMP (30
µm) + miR-302b mimic group, demonstrating that the
anti-apoptotic effect of TMP was reversed by miR-302b mimic. The
results revealed that the anti-apoptotic effect of TMP on the
LPS-stimulated cells was achieved via the down-regulation of
miR-302b mimic.
Discussion
Periodontitis is a chronic disease that results in
the loss of alveolar bone periodontal ligament and gingival tissue.
Considering the poor microorganism sessile growth and biofilm
penetration by biocides or antibiotics, current drug therapies,
such as biocides or antibiotics are not ideal (32). Based on the high-proliferation,
self-renewal and multi-differentiation abilities of PDLSCs, PDLSCs
are vital for tissue restoration induced by periodontitis (17). Therefore the discovery of a novel
a drug that can protect PDLSCs from apoptosis and
inflammation-related injury is vital. The present study used LPS as
the inflammation stimulator for the construction of the cell injury
model.
The results of the present study demonstrated that
the inflammation levels induced by LPS were higher, and this
finding is consistent with those of previous studies (33-35). Previous studies have demonstrated
that cell proliferation increases under inflammatory conditions
(36,37). In the present study, although the
inflammation level was increased, the cell proliferation was
decreased. This may be due to the different concentration and
LPS-induced inflammation exposure time used from the previous
study. The effects of inflammation on cell proliferation need to be
further elucidated in future research.
The results of the present study demonstrated that
TMP is a promising Chinese medicine monomer that protects PDLSCs
from apoptosis and inflammation injury. Firstly, the cytotoxicity
of TMP was evaluated. The present data indicated that treatment
with 30 µm TMP had no effect on cell survival. A previous
study indicated that treatment with 400 µm TMP exerted no
toxic effects on PC12 cells and exerted protective effects against
neurotoxicity (38).
Subsequently, the effects of LPS on PDLSCs were
investigated. The results revealed that stimulation with 1
µg/ml LPS for 24 h significantly inhibited cell viability.
Previous studies have demonstrated that TMP exerts
anti-inflammatory effects on hepatic stellate cells, a mouse model
of Parkinson's disease and myocardial cells (39-41). The present study demonstrated that
inflammation levels induced by LPS were significantly reduced by
TMP in a concentration-dependent manner.
Previous studies have demonstrated that TMP exerts
an inhibitory effect on cell apoptosis in numerous cells, such as
vascular endothelial cells, HepG2 cells, myocardial cells and bone
marrow-derived mesenchymal stem cells (42-45). In the present study, cell
apoptotic levels induced by LPS were markedly decreased by TMP in a
concentration-dependent manner. In addition, the expression levels
of apoptotic proteins were investigated as further evidence. The
expression of the anti-apoptotic protein, Bcl-2, was decreased, and
the levels of the pro-apoptotic proteins, Bax and cleaved
caspase-3, were elevated in the LPS-stimulated PDLSCs. The
aforementioned effects induced by LPS in the cells were all
reversed by TMP. The results demonstrated that TMP is a promising
agent for use in protecting PDLSCs from apoptosis and
inflammation-related injury.
The mechanisms of action of TMP were also
investigated in the present study. miRNAs have been confirmed as
vital biomarkers in a number of diseases and regulate protein
expression at the post-transcriptional level. miR-302b
overexpression was confirmed to enhance the effects of LPS on
apoptosis, inflammation and cell viability (46). In the present study, the miR-302b
levels were elevated in the LPS-stimulated PDLSCs and were
downregulated by TMP in a concentration-dependent manner. The
present study further investigated whether miR-302b overexpression
exerts effects on the LPS + TMP-treated cells. The results revealed
that the anti-inflammatory and anti-apoptotic effects of TMP in the
LPS-stimulated cells were inhibited by miR-302b overexpression. The
results demonstrated that the anti-inflammatory and anti-apoptotic
effects of TMP on LPS-induced cells was achieved via the
downregulation of miR-302b.
In addition, since inflammation exerts negative
effects on the multi-lineage differentiation potential and
self-renewal capability of PDLSCs, the inflammatory response needs
to be inhibited and the osteogenic differentiation of PDLSCs in the
inflammatory microenvironment needs to be improved when periodontal
tissue engineering is applied to repair alveolar bone defects.
Therefore, further experiments are required to confirm the effects
of TMP on multi-lineage differentiation.
To conclude, the present study found that TMP, with
its anti-inflammatory and anti-apoptotic effects, may be a
promising agent for use in protecting PDLSCs from
periodon-titis-induced injury. TMP is expected to be a novel agent
for use in the treatment of periodontitis, while further research
is still required to confirm the effects of TMP in vivo.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
The first author YD prepared the manuscript, and was
involved in all the experiments and analyzed the data. WA was
involved in the cell experiments, as well as in the literature
search and figure preparation. YW was involved in some sample
preparations and literature searches. JW was involved in the
communication with the journal during the manuscript submission and
peer review and also contributed to the conception and design of
the study. The present study was designed by YD and WA with the
guidance of JW. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Serrano C and Suarez E: Prevalence of
severe periodontitis in a colombian adult population. J Int Acad
Periodontol. 21:53–62. 2019.PubMed/NCBI
|
|
2
|
Zhang J, Zhang AM, Zhang ZM, Jia JL, Sui
XX, Yu LR and Liu HT: Efficacy of combined orthodontic-periodontic
treatment for patients with periodontitis and its effect on
inflammatory cytokines: A comparative study. Am J Orthod
Dentofacial Orthop. 152:494–500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahmad N, Ahmad FJ, Bedi S, Sharma S, Umar
S and Ansari MA: A novel nanoformulation development of eugenol and
their treatment in inflammation and periodontitis. Saudi Pharm J.
27:778–790. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Czesnikiewicz-Guzik M, Osmenda G,
Siedlinski M, Nosalski R, Pelka P, Nowakowski D, Wilk G,
Mikolajczyk TP, Schramm-Luc A, Furtak A, et al: Causal association
between periodontitis and hypertension: Evidence from mende-lian
randomization and a randomized controlled trial of non-surgical
periodontal therapy. Eur Heart J. 40:3459–3470. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wallace K, Shafique S and Piamjariyakul U:
The relationship between oral health and hemodialysis treatment
among adults with chronic kidney disease: A systematic review.
Nephrol Nurs J. 46:375–394. 2019.PubMed/NCBI
|
|
6
|
Zhou X, Zhang W, Liu X, Zhang W and Li Y:
Interrelationship between diabetes and periodontitis: Role of
hyperlipidemia. Arch Oral Biol. 60:667–674. 2015. View Article : Google Scholar
|
|
7
|
Franca LFC, Vasconcelos ACCG, da Silva
FRP, Alves EHP, Carvalho JS, Lenardo DD, de Souza LKM, Barbosa ALR,
Medeiros JR, de Oliveira JS and Vasconcelos DFP: Periodontitis
changes renal structures by oxidative stress and lipid
peroxidation. J Clin Periodontol. 44:568–576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trikka D and Vassilopoulos S: Periodontal
regeneration with enamel matrix derivative in the management of
generalized aggressive periodontitis: A case report with 11-year
follow-up and literature review. J Int Soc Prev Community Dent.
9:13–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bella P and Istvan G: The comprehensive
periodontal, resorative end prosthodontic therapy of chronic
periodontitis case presentation. Fogorv Sz. 109:125–135. 2016.In
English, Hungarian. PubMed/NCBI
|
|
10
|
Ashouri Moghaddam A, Radafshar G,
Jahandideh Y and Kakaei N: Clinical evaluation of effects of local
application of aloe vera gel as an adjunct to scaling and root
planning in patients with chronic periodontitis. J Dent (Shiraz).
18:165–172. 2017.
|
|
11
|
Portron S, Soueidan A, Marsden AC, Rakic
M, Verner C, Weiss P, Badran Z and Struillou X: Periodontal
regenerative medicine using mesenchymal stem cells and
biomaterials: A systematic review of pre-clinical studies. Dent
Mater J. 38:867–883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Son H, Jeon M, Choi HJ, Lee HS, Kim IH,
Kang CM and Song JS: Decellularized human periodontal ligament for
periodontium regeneration. PLoS One. 14:e02212362019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang
J, Xu GT, Liang A and Liu S: Concise reviews: Characteristics and
potential applications of human dental tissue-derived mesenchymal
stem cells. Stem Cells. 33:627–638. 2015. View Article : Google Scholar
|
|
14
|
Nagata M, Iwasaki K, Akazawa K, Komaki M,
Yokoyama N, Izumi Y and Morita I: Conditioned medium from
periodontal ligament stem cells enhances periodontal regeneration.
Tissue Eng Part A. 23:367–377. 2017. View Article : Google Scholar :
|
|
15
|
Zheng DH, Wang XX, Ma D, Zhang LN, Qiao QF
and Zhang J: Erythropoietin enhances osteogenic differentiation of
human periodontal ligament stem cells via Wnt/β-catenin signaling
pathway. Drug Des Devel Ther. 13:2543–2552. 2019. View Article : Google Scholar :
|
|
16
|
Gu K, Fu X, Tian H, Zhang Y, Li A, Wang Y,
Wen Y and Gu W: TAZ promotes the proliferation and osteogenic
differentiation of human periodontal ligament stem cells via the
p-SMAD3. J Cell Biochem. 121:1101–1113. 2020. View Article : Google Scholar
|
|
17
|
Menicanin D, Mrozik KM, Wada N, Marino V,
Shi S, Bartold PM and Gronthos S: Periodontal-ligament-derived stem
cells exhibit the capacity for long-term survival, self-renewal,
and regeneration of multiple tissue types in vivo. Stem Cells Dev.
23:1001–1011. 2014. View Article : Google Scholar :
|
|
18
|
Li J, Li Y, Pan S, Zhang L, He L and Niu
Y: Paeonol attenuates ligation-induced periodontitis in rats by
inhibiting osteoclasto-genesis via regulating Nrf2/NF-κB/NFATc1
signaling pathway. Biochimie. 156:129–137. 2019. View Article : Google Scholar
|
|
19
|
Zhou W, Su L, Duan X, Chen X, Hays A,
Upadhyayula S, Shivde J, Wang H, Li Y, Huang D and Liang S:
MicroRNA-21 down-regulates inflammation and inhibits periodontitis.
Mol Immunol. 101:608–614. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elburki MS, Rossa C Jr, Guimarães-Stabili
MR, Lee HM, Curylofo-Zotti FA, Johnson F and Golub LM: A chemically
modified curcumin (CMC 2.24) inhibits nuclear factor κB activation
and inflammatory bone loss in murine models of LPS-induced
experimental periodontitis and diabetes-associated natural
periodontitis. Inflammation. 40:1436–1449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li F and Xu HS: Effects of low level laser
combined with basic periodontal therapy on cytokines and LPS,
leptin in gingival crevicular fluid of diabetes mellitus
complicated with chronic periodontitis patients. Shanghai Kou Qiang
Yi Xue. 27:637–640. 2018.In Chinese.
|
|
22
|
Wei S, Chi J, Zhou M, Li R, Li Y, Luo J
and Kong L: Anti-inflammatory lindenane sesquiterpeniods and dimers
from Sarcandra glabra and its upregulating AKT/Nrf2/HO-1 signaling
mechanism. Industrial Crops and Products. 137:367–376. 2019.
View Article : Google Scholar
|
|
23
|
Zhou J, Xu G, Yan J, Li K, Bai Z, Cheng W
and Huang K: Rehmannia glutinosa (Gaertn.) DC. polysaccharide
ameliorates hyperglycemia, hyperlipemia and vascular inflammation
in streptozotocin-induced diabetic mice. J Ethnopharmacol.
164:229–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Yi L, Song S, Wang L, Liang Q,
Wang Y, Wu Y and Gao Q: Puerarin attenuates palmitate-induced
mitochondrial dysfunction, impaired mitophagy and inflammation in
L6 myotubes. Life Sci. 206:84–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong J, Liang W, Wang T, Sui J, Wang J,
Deng Z and Chen D: Saponins regulate intestinal inflammation in
colon cancer and IBD. Pharmacol Res. 144:66–72. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Michel HE and Menze ET:
Tetramethylpyrazine guards against cisplatin-induced nephrotoxicity
in rats through inhibiting HMGB1/TLR4/NF-κB and activating Nrf2 and
PPAR-γ signaling pathways. Eur J Pharmacol. 857:1724222019.
View Article : Google Scholar
|
|
27
|
Fan L, Wang K, Shi Z, Die J, Wang C and
Dang X: Tetramethylpyrazine protects spinal cord and reduces
inflammation in a rat model of spinal cord ischemia-reperfusion
injury. J Vasc Surg. 54:192–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen J, Wang H, Gao C, Liu D, Fan Y, Li W,
Chen Y and Pan S: Tetramethylpyrazine alleviates LPS-induced
inflammatory injury in HUVECs by inhibiting Rho/ROCK pathway.
Biochem Biophys Res Commun. 514:329–335. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kao TK, Chang CY, Ou YC, Chen WY, Kuan YH,
Pan HC, Liao SL, Li GZ and Chen CJ: Tetramethylpyrazine reduces
cellular inflammatory response following permanent focal cerebral
ischemia in rats. Exp Neurol. 247:188–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liao SL, Kao TK, Chen WY, Lin YS, Chen SY,
Raung SL, Wu CW, Lu HC and Chen CJ: Tetramethylpyrazine reduces
ischemic brain injury in rats. Neurosci Lett. 372:40–45. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ge T, Yin M, Yang M, Liu T and Lou G:
MicroRNA-302b suppresses human epithelial ovarian cancer cell
growth by targeting RUNX1. Cell Physiol Biochem. 34:2209–2220.
2014. View Article : Google Scholar
|
|
32
|
Lazar V, Saviuc CM and Chifiriuc MC:
Periodontitis and periodontal disease-innovative strategies for
reversing the chronic infectious and inflammatory condition by
natural products. Curr Pharm Des. 22:230–237. 2016. View Article : Google Scholar
|
|
33
|
Jia R, Yi Y, Liu J, Pei D, Hu B, Hao H, Wu
L, Wang Z, Luo X and Lu Y: Cyclic compression emerged dual effects
on the osteogenic and osteoclastic status of LPS-induced
inflammatory human periodontal ligament cells according to loading
force. BMC Oral Health. 20:72020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Duan Y, An W, Wu H and Wu Y: Salvianolic
acid C attenuates LPS-induced inflammation and apoptosis in human
periodontal ligament stem cells via toll-like receptors 4
(TLR4)/nuclear factor kappa B (NF-κB) pathway. Med Sci Monit.
25:9499–9508. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang L, Xia J, Liu Q and Jin Y: Effect of
lipopolysaccharide on proliferation and inflammatory factors
expression of human periodontal ligament stem cells. Hua Xi Kou
Qiang Yi Xue Za Zhi. 31:286–290. 2013.In Chinese. PubMed/NCBI
|
|
36
|
Yu Y, Bi CS, Wu RX, Yin Y, Zhang XY, Lan
PH and Chen FM: Effects of short-term inflammatory and/or hypoxic
pretreatments on periodontal ligament stem cells: In vitro and in
vivo studies. Cell Tissue Res. 366:311–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang HN, Xia Y, Yu Y, Wu RX, Gao LN and
Chen FM: Stem cells derived from 'inflamed' and healthy periodontal
ligament tissues and their sheet functionalities: A patient-matched
comparison. J Clin Periodontol. 43:72–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guan D, Su Y, Li Y, Wu C, Meng Y, Peng X
and Cui Y: Tetramethylpyrazine inhibits CoCl2-induced neurotoxicity
through enhancement of Nrf2/GCLc/GSH and suppression of
HIF1α/NOX2/ROS pathways. J Neurochem. 134:551–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu X, Zhang F, Xiong X, Lu C, Lian N, Lu Y
and Zheng S: Tetramethylpyrazine reduces inflammation in liver
fibrosis and inhibits inflammatory cytokine expression in hepatic
stellate cells by modulating NLRP3 inflammasome pathway. IUBMB
Life. 67:312–321. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao H, Xu ML, Zhang Q, Guo ZH, Peng Y, Qu
ZY and Li YN: Tetramethylpyrazine alleviated cytokine synthesis and
dopamine deficit and improved motor dysfunction in the mice model
of Parkinson's disease. Neurol Sci. 35:1963–1967. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo M, Liu Y and Shi D: Cardiovascular
actions and therapeutic potential of tetramethylpyrazine (Active
Component Isolated from Rhizoma Chuanxiong): Roles and mechanisms.
Biomed Res Int. 2016:24303292016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu JZ, Wang XK, Cao Y, Li DZ, Wu TD, Zhang
T, Xu DQ and Lu HB: Tetramethylpyrazine facilitates functional
recovery after spinal cord injury by inhibiting MMP2, MMP9, and
vascular endothelial cell apoptosis. Curr Neurovasc Res.
14:110–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bi L, Yan X, Chen W, Gao J, Qian L and Qiu
S: Antihepatocellular carcinoma potential of tetramethylpyrazine
induces cell cycle modulation and mitochondrial-dependent
apoptosis: Regulation of p53 signaling pathway in HepG2 cells in
vitro. Integr Cancer Ther. 15:226–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin KH, Kuo WW, Jiang AZ, Pai P, Lin JY,
Chen WK, Day CH, Shen CY, Padma VV and Huang CY:
Tetramethylpyrazine ameliorated hypoxia-induced myocardial cell
apoptosis via HIF-1α/JNK/p38 and IGFBP3/BNIP3 inhibition to
upregulate PI3K/Akt survival signaling. Cell Physiol Biochem.
36:334–344. 2015. View Article : Google Scholar
|
|
45
|
Fang Y, Chu L, Li L, Wang J, Yang Y, Gu J
and Zhang J: Tetramethylpyrazine protects bone marrow-derived
mesenchymal stem cells against hydrogen peroxide-induced apoptosis
through PI3K/Akt and ERK1/2 pathways. Biol Pharm Bull.
40:2146–2152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Yu T, Jin H, Zhao C and Wang Y:
Knockdown MiR-302b alleviates LPS-induced injury by targeting smad3
in C28/I2 chondrocytic cells. Cell Physiol Biochem. 45:733–743.
2018. View Article : Google Scholar : PubMed/NCBI
|