Introduction
Wound healing is one of the most complex biological
processes and affects the whole organism, in addition to the site
of injury (1). The cutaneous
wound healing process can be divided into six phases: Hemostasis;
inflammation; proliferation and migration; angiogenesis;
re-epithelialization; and synthesis or remodeling (2-4);
the three broad stages of the healing process are inflammation,
proliferation and remodeling. The inflammation phase is
characterized by the presence of tumor necrosis factor-α and
interleukin (IL)-6, which are released by keratinocytes; this
release leads to the infiltration of macrophages and neutrophils to
the wound site (5). This
infiltration ensures the regulation of wound healing in the phases
to follow via proinflammatory cytokines such as IL-1β and IL-6, as
well as growth factors such as epidermal growth factor,
transforming growth factor β-1 (TGF-β1) and platelet-derived growth
factor, which are released by macrophages (6). In the next phase, known as the
proliferation phase, the release of critical cytokines and growth
factors mediate key events such as angiogenesis,
re-epithelialization and endothelial proliferation (7). Polarization and migration of skin
keratinocytes then lead to stratification and differentiation,
resulting in wound contraction (8). Fibroblasts and keratinocytes also
play a vital role in this migration process (9). The migration of fibroblasts and
keratinocytes is initiated by intracellular polarization induced
via critical proteins such as Rac1, cell division cycle 42 (Cdc42)
and α-p21-activated kinase (α-PAK) (10-12). In the final stage of remodeling,
TGF-β1 mediates the degradation of granular tissue, and a scar
containing substantial quantities of type I collagen and immature
blood vessels is formed (13,14).
Sirtuins (Sirts) are class III histone deacetylases
that regulate numerous biological processes and diseases through
the deacetylation of histone and non-histone targets, including
aging, inflammation and cancer (15). Among the seven members of the Sirt
family of proteins, Sirt1 is the most studied and characterized;
Sirt1 plays critical roles in various important diseases and
conditions, such as inflammation (16), oxidative damage (17), apoptosis (18), diabetes (19) and aging (20). Due to its diverse range of targets
and functions, certain studies have also elucidated the possible
role of Sirt1 in wound healing and cell migration (21-25).
Our previous studies focused on identifying novel
Sirt1 activators and evaluating their potential for skin wound
healing and regeneration (26,27). In the present study, a novel
synthetic activator of Sirt1,
(E)-3-(2,4-dichlorophenyl)-N-phenylacrylamide
(NED416; Fig. S1), was
investigated for its effects on Sirt1 regulation and the process of
cutaneous wound healing.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs; cat.
no. CRL-1730™) and normal human dermal fibroblasts (NHDFs; cat. no.
PCS-201-012™) were purchased from the American Type Culture
Collection, while HaCaT epidermal keratinocytes were provided by
Professor Tae Yoon Kim from the College of Medicine of The Catholic
University of Korea. All the cell lines used in this study were
tested for mycoplasma contamination and used in experiments between
passage numbers 5 and 10. HaCaT cells were cultured in high-glucose
DMEM (Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum
(FBS) and 1% penicillin-streptomycin. HUVECs were cultured in M200
medium (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented
with 20% FBS and 1% low-serum growth supplement (Invitrogen; Thermo
Fisher Scientific, Inc.). NHDFs were cultured in fibroblast medium
(ScienCell Research Laboratories, Inc.). Cultures were at all times
incubated in a humidified atmosphere at 37°C and 5% CO2
unless stated otherwise.
Preparation of NED416
A piper amide derivative, NED416, was synthesized
according to a previously reported method (28), purified by silica gel column
chromatography and characterized. The spectral data obtained were
consistent with previously reported values (29). 1H-NMR (300 MHz,
CDCl3): δ 8.17 (br s, 1H), 8.00 (d, J=15.6
Hz, 1H), 7.65 (d, J=7.8 Hz, 2H), 7.48 (d, J=8.4 Hz,
1H), 7.38 (d, J=1.8 Hz, 1H), 7.29 (t, J=7.8 Hz, 2H),
7.15 (dd, J=8.0, 8.6 Hz, 1H), 7.08 (t, J=7.2 Hz, 1H),
6.67 (d, J=15.6 Hz, 1H). 13C-NMR (100 MHz,
CD3OD): δ 165.7, 139.8, 137.1, 137.0, 136.4,
133.1, 130.9, 129.9 (2C), 129.8, 128.9, 125.8, 125.5, 121.2 (2C;
Fig. S2). HRMS (fast atom
bombardment): Calculated for
C15H11Cl2NO [(M)+] 292.0296, found
292.0299.
Cell treatment
Cells at 80% confluence were rinsed and treated with
1, 5, or 10 µM NED416 solutions prepared and diluted in
dimethyl sulfoxide (DMSO; Thermo Fisher Scientific, Inc.) and
serum-free medium, respectively, after washing the cells twice with
PBS (PAA Laboratories GmbH; GE Healthcare).
Sirt1 activity measurement
The Sirt1 activity assay was performed in triplicate
by using a SIRT1 Direct Fluorescent Screening Assay kit (Cayman
Chemical Company). Briefly, 25 µl of assay buffer was added
to each well of a 96-well plate followed by 5 µl of Sirt1, 5
µl of NED416 or resveratrol solution, and 15 µl of
Sirt1 substrate solution. Background wells were treated with
solvent but not with Sirt1.
Western blotting
Treated cells were harvested and lysed with lysis
buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS
and 50 mM Tris-Cl). The concentration of proteins in the
supernatants of lysates was determined using a Bio-Rad Protein
Assay (Bio-Rad Laboratories, Inc.), with bovine serum albumin
(SERVA Electrophoresis GmbH) as the standard. Protein samples (30
µg) were subjected to SDS-PAGE on 8-15% polyacrylamide gels
and transferred to nitrocellulose membranes. Membranes were blocked
for 1 h at room temperature with a 5% (w/v) non-fat milk solution
in TBS-0.05% Tween-20. Membranes were then subjected to overnight
incubation at 4°C with the solutions of primary antibodies against
Sirt1 (1:1,000; cat. no. sc-15404; Santa Cruz Biotechnology, Inc.),
p53 and acetylated p53 (1:1,000; cat. nos. 9282 and 2570; Cell
Signaling Technologies, Inc.), α-tubulin (1:1,000; cat. no.
SAB3501072; Sigma-Aldrich; Merck KGaA), Rac1 and phosphorylated
(p)-Rac1 (1:1,000; cat. nos. 2465 and 2461; Cell Signaling
Technologies, Inc.), Cdc42 and p-Cdc42 (1:1,000; cat. nos. 2462 and
2461; Cell Signaling Technologies, Inc.), α-PAK (1:1,000; cat. no.
sc-166887; Santa Cruz Biotechnology, Inc.), ERK and p-ERK (1:1,000;
cat. nos. sc-271291 and sc-136521; Santa Cruz Biotechnology, Inc.),
JNK and p-JNK (1:1,000; cat. nos. sc-1648 and sc-6254; Santa Cruz
Biotechnology, Inc.), and p38 and p-p38 (1:1,000; cat. nos.
sc-136210 and sc-166182; Santa Cruz Biotechnology, Inc.), followed
by incubation at room temperature for 2 h with horseradish
peroxidase conjugated anti-rabbit and anti-mouse secondary
antibodies (1:500; cat. nos. sc-2357 and sc-516102; Santa Cruz
Biotechnology, Inc.). Bands were visualized using a solution
comprised of luminal (cat. no. 42586.A.01) and hydrogen peroxide
(cat. no. 42585.B.01; both SERVA Electrophoresis GmbH) in a 1:1
mixture. The membrane was incubated with this solution for 1 min,
then bands were visual-ized using a chemiluminescence detection
system (Bio-Rad Laboratories, Inc.); protein expression was
quantified using Alpha View SA Version 3.4.0.0 software
(ProteinSimple, Inc.).
MTT assays
To determine cell viability, 1×105 HaCaT
cells, which were seeded in 6-well plates or 40-mm culture dishes,
were treated with 1, 5 or 10 µM solutions of NED416 prepared
in serum-free medium. The medium was removed after 24 h, and 2 ml
of 500 µg/ml MTT solution was added. The cells were then
incubated at 37°C for 1 h. Then, MTT was replaced with 1 ml of
DMSO, and the plates were incubated on a shaker for 30 min at room
temperature. The absorbance at 570 nm was determined using a
microplate reader (Molecular Devices LLC).
Small interfering RNA (siRNA)
knockdown
HaCaT cells seeded in 96-well plates (~60%
confluence) were transfected with 100 nM (final concentration) of
Sirt1 siRNA or scramble siRNA (Bioneer Corporation) dissolved in
serum-free media with an equal volume of a 1.4-1.5% solution of
Lipofectamine® (Invitrogen; Thermo Fisher Scientific,
Inc.) prepared in serum-free medium. Cells were then incubated for
6 h before washing with PBS and then subjected to the cell
migration assay. The independent sequences used for silencing Sirt1
were as follows: Sequence 1, sense, 5′-ACU UUG CUG UAA CCC UGU
A(dTdT)-3′ and antisense, 5′-UAC AGG GUU ACA GCA AAG U(dTdT)-3′;
sequence 2, sense, 5′-AGA GUU GCC ACC CAC ACC U(dTdT)-3′ and
antisense, 5′-AGG UGU GGG UGG CAA CUC U(dTdT)-3′. The sequence for
the scramble siRNA was as follows: Sense, 5′-AGA GUU CAA AAG CCC
UUC A(dTdT)-3′ and antisense, 5′-UGA AGG GCU UUU GAA CUC
U(dTdT)-3′.
Cell migration assays
HaCaT cells were seeded in 96-well plates 1 day
before the assay. A wound was created in the cell layer using a
Wound Maker™ tool (Essen BioScience, Ltd.). Wounded cells were
incubated at 37°C for 24 h in the presence of NED416 or serum-free
medium. In the experiments demonstrating the effect of the ERK
pathway on cell migration, the wounded cells were pretreated with
10 µM of ERK inhibitors SP600125, SB203580 and U0126 (cat.
nos. S5567, S8307 and U120; all Sigma-Aldrich; Merck KGaA) for 1 h
prior to treatment with NED416. Images of the cells were captured
every 4 h using IncuCyte ZOOM® (magnification, ×40;
Essen BioScience, Ltd.) to monitor the effects of NED416 on cell
migration.
Endothelial cell tube formation
assay
HUVECs were cultured in M200 medium (Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 20% FBS and 1%
low-serum growth supplement (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C and 5% CO2. Each well of the 96-well
plates was coated with 50 µl of Matrigel matrix and
incubated at 37°C for 30 min. HUVECs were suspended in serum-free
medium containing 1X LGS, and 1×106 HUVECs were seeded
in each well of the 96-well plates pretreated with Matrigel matrix
with the vehicle (M200) or NED416 for 18 h. Tube formation was
captured using an inverted microscope (magnification, ×40; Nikon
Corporation).
Ethical approval
All procedures for animal experiments were reviewed
and approved by the Animal Care Committee of the Center of Animal
Care and Use at the Lee Gil Ya, Cancer and Diabetes Institute,
Gachon University (permit no. LCDI-2013-0022).
Full-thickness wounds and quantification
of healing
SKH-1 hairless mice (5 weeks old, male, 25-30 g)
were purchased from Shizuoka Laboratory Animal Center and housed in
a temperature-controlled room (23°C) with 65% humidity and a 12-h
light/dark cycle. A total of 40 mice were randomly divided into
four groups: Vehicle (n=10); 0.05% NED416 (n=10); 0.1% NED416
(n=10); and 0.5% NED416 (n=10). Two identical wounds were created
on the posterior dorsal region of each mouse using a 6-mm biopsy
punch. NED416 solutions (0.05, 0.1 and 0.5%) dissolved in a mixture
of distilled water, ethanol and propanediol (5:3:2, respectively)
were applied topically to the wound every day for 10 days. The
vehicle group was treated with solvent only.
Histological analysis
Skin samples containing the central part of the
wound were collected on days 3, 5, 7 and 10 after wound induction.
Specimens were fixed in 10% formalin for 18 h at room temperature.
Fixed tissues were subjected to H&E and Masson's Trichrome
staining as described by Wahedi et al (27). Images were captured using an
inverted microscope (magnification, ×10). For epidermal
regeneration and granulation, a semi-quantitative score system was
used as described in Table I
(30).
 | Table ICriteria to evaluate the histological
score of wound healing. |
Table I
Criteria to evaluate the histological
score of wound healing.
| Score | Epidermal and
dermal regeneration | Granulation tissue
thickness |
|---|
| ±1 | Little epidermal
and dermal organization | Thin granulation
layer |
| ±2 | Moderate epidermal
and dermal organization | Moderate
granulation layer |
| ±3 | Complete remodeling
of dermis and epidermis | Thick granulation
layer |
| ±4 | | Very thick
granulation layer |
Statistical analysis
Differences between groups were determined using
one-way ANOVA with Dunnett's test or Tukey's test as a post hoc
test. P<0.05 was considered to indicate a statistically
significant difference. The results are presented as the mean ±
standard error of the mean from three independent experiments.
Statistical analysis was performed using GraphPad Prism version
6.01 (GraphPad Software, Inc.).
Results
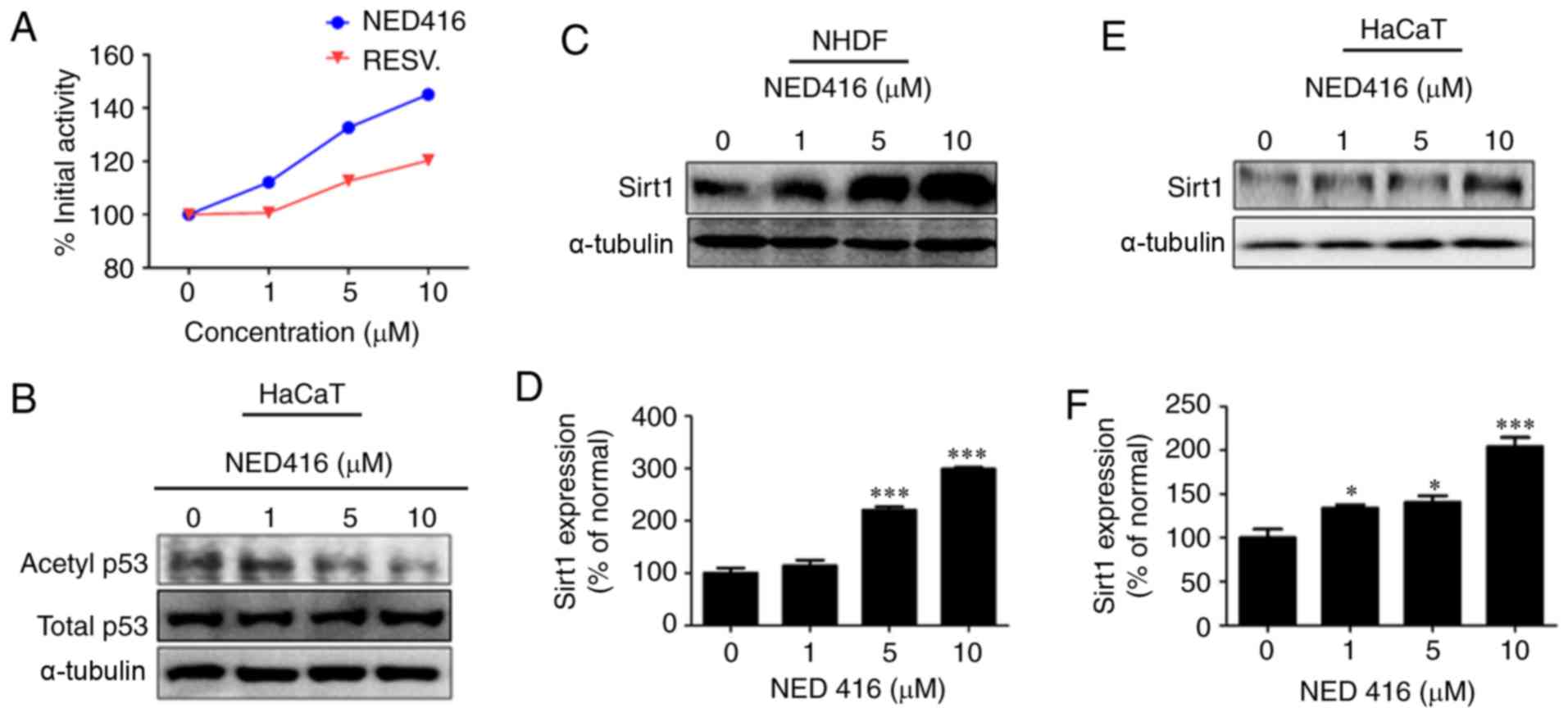
NED416 increases the activity and protein
expression of Sirt1
The effect of NED416 on Sirt1 activity was analyzed
using an enzymatic activity assay. It was shown that NED416
enhanced the enzymatic activity of Sirt1 in a
concentration-dependent manner. This effect of NED416 on Sirt1
activity was greater than that of resveratrol, a well-known Sirt1
activator (Fig. 1A). Western blot
analysis showed that NED416 treatment resulted in a
concentration-dependent increase in the deacetylation of p53,
validating the assay results (Fig.
1B).
NED416 treatment increased Sirt1 expression in NHDFs
in a concentration-dependent manner (Fig. 1C and D). A similar
concentration-dependent effect of NED416 was observed on Sirt1
expression in HaCaT cells (Fig. 1E
and F). An MTT assay showed that NED416 treatment did not
significantly decrease NHDF or HaCaT cell viability (Fig. S3).
NED416 promotes skin cell migration
To investigate the role of Sirt1 in the migration of
HaCaT cells, Sirt1 knockdown was performed, followed by a migration
assay in HaCaT cells. Cells treated with Sirt1 siRNA exhibited a
slower rate of migration than the scramble siRNA-transfected and
untreated cells (Fig. 2A and B).
These differences in the rates of migration were significant at 4,
8 and 12 h after treatment (Fig.
2C).
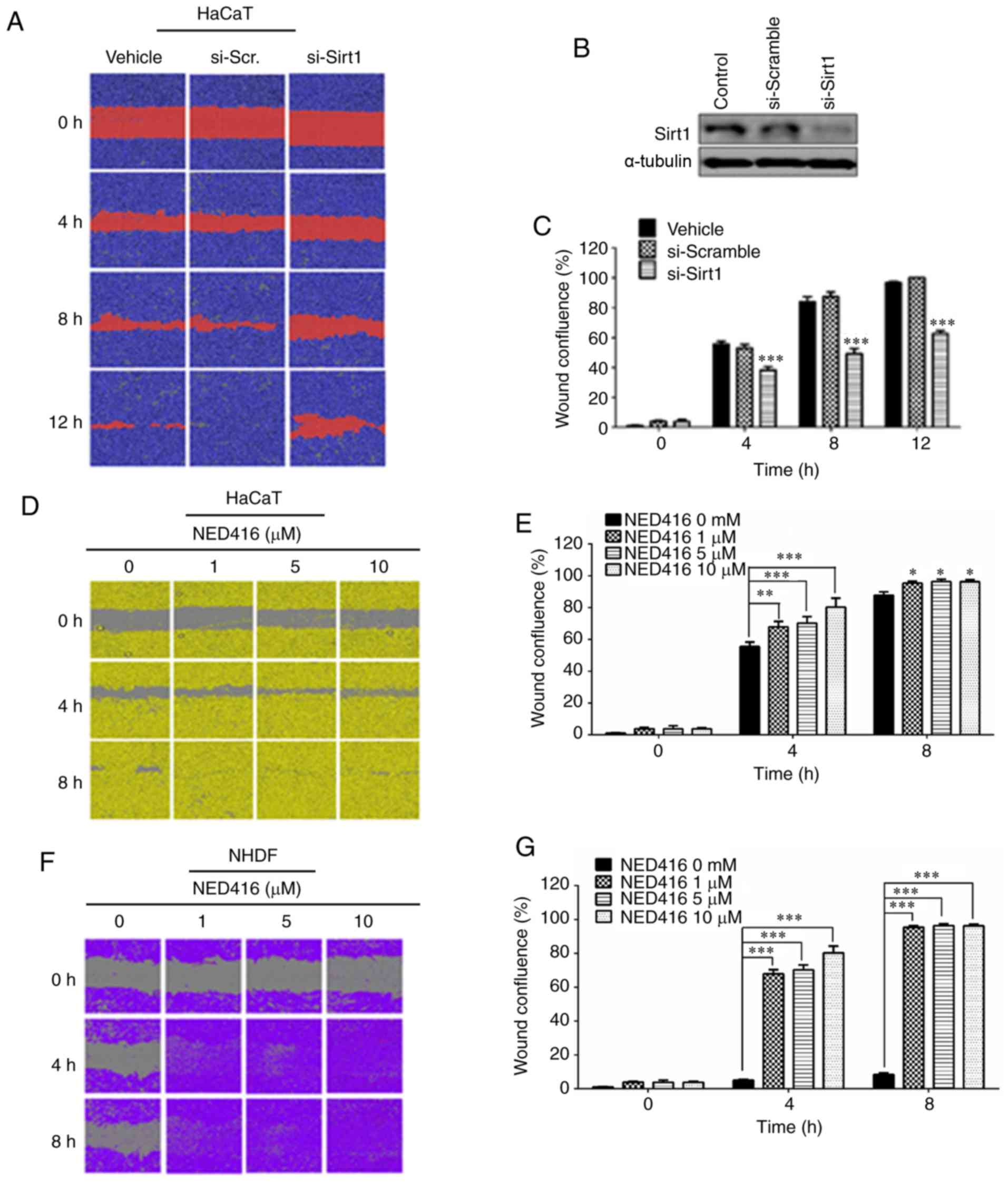 | Figure 2NED416 promotes skin cell migration.
(A) Healing of wounded HaCaT cell monolayers following Sirt1
siRNA-mediated knockdown resulted in a slower rate of migration
than control or scramble siRNA-treated cells. (B) Western blot
showing siRNA knockdown of Sirt1. (C) Quantification of the average
wound confluence in control, scramble and Sirt1 siRNA-treated HaCaT
cells. ***P<0.001 vs. control. (D) HaCaT cells
cultured in the presence of medium only or NED416 after wounding
showed that NED416 treatment increases cell migration in a
concentration-dependent manner. (E) Quantification of wound
confluence in control and NED416-treated HaCaT cells. (F) NHDF
cells cultured in the presence of medium only or NED416 after
wounding revealed that 5 µM NED416 induced the most
pronounced wound healing, followed by 1 and 10 µM NED416.
(G) Quantification of wound confluence in control and
NED416-treated NHDF cells. Data are presented as the mean ± SEM.
Magnification, ×40 of images. *P<0.05,
**P<0.01, ***P<0.001 vs. 0 mM. NED416,
(E)-3-(2,4-dichlorophenyl)-N-phenylacrylamide; Sirt1,
sirtuin 1; NHDF, normal human dermal fibroblasts; si(RNA), small
interfering (RNA). |
Moreover, cells grown in the presence of NED416
exhibited faster growth rates than the cells grown in the absence
of NED416 (Fig. 2D). Treatment
with NED416 resulted in faster growth and migration rates, which
were concentration-dependent, than those of untreated cells. This
difference in the rate of migration was significant at 4 and 8 h
(Fig. 2E). NHDF cells grown in
the presence of NED416 also exhibited faster growth rates than the
cells grown in the absence of NED416, as observed in HaCaT cells
(Fig. 2F). These differences in
the rates of migration were significant at 4 and 8 h (Fig. 2G).
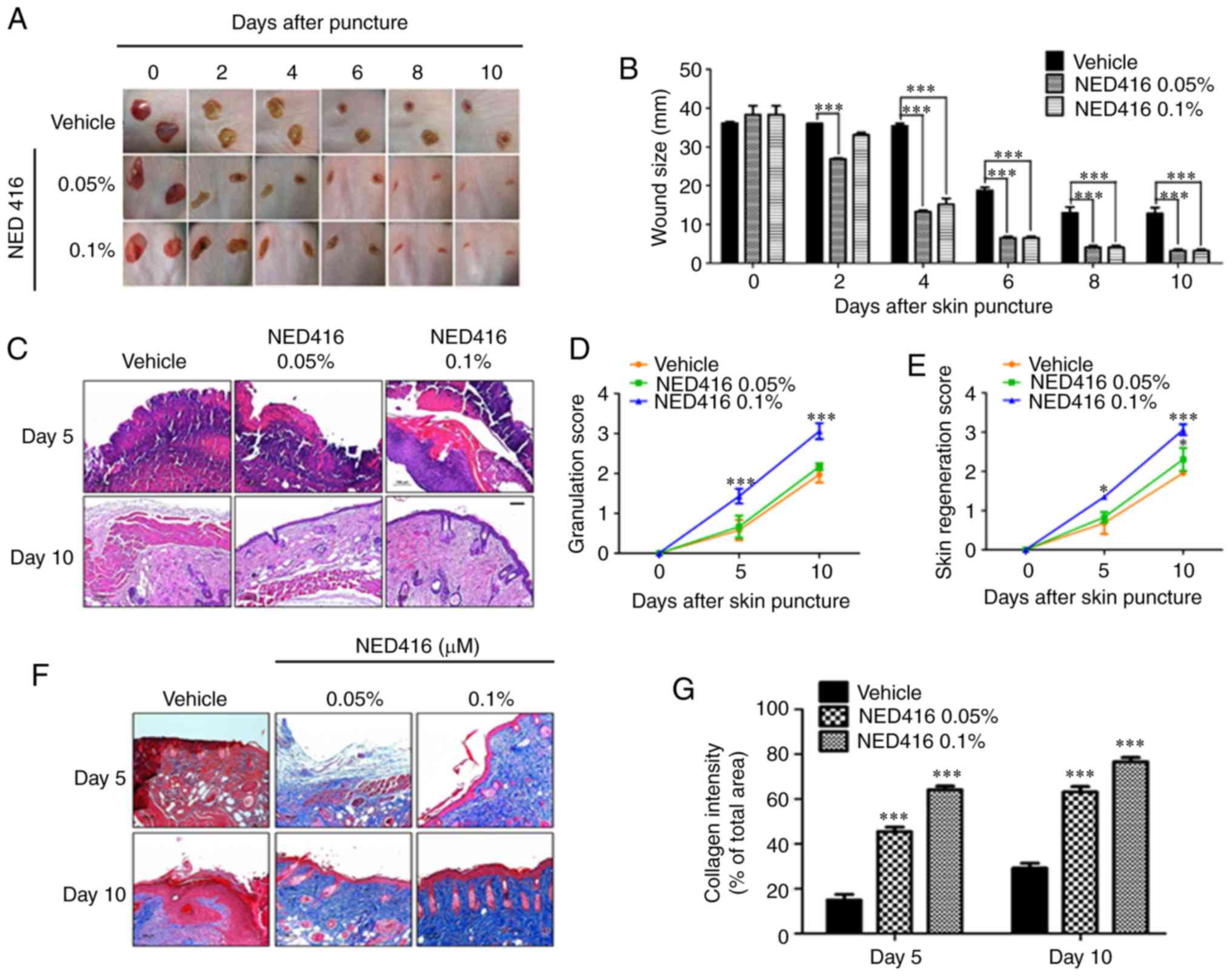
NED416 promotes wound healing and tissue
regeneration in mouse skin
NED416 was investigated for wound healing potential
in a hairless mouse model. According to digital photographs,
hairless mice treated with different concentrations of NED416
showed faster wound closure and dermal regeneration than
vehicle-treated mice (Fig. 3A).
Up to day 2 of injury, wound size was not significantly different
between the vehicle and 0.1% NED groups; however, from day 3
onward, the difference was significant for both concentrations of
NED416 (Fig. 3B). NED416-treated
mice showed complete wound healing after 8 days, while the wounds
in vehicle-treated mice were not healed completely even after 10
days.
Skin tissues were stained with H&E to monitor
tissue development. The skin tissue of NED416-treated mice showed
faster and more complete development of granulation tissue and
neoepithelium when compared with the skin tissue of untreated mice
(Fig. 3C). Based on the criteria
presented in Table I, improved
dermal and epidermal regeneration was found in NED416-treated mice,
along with improved granulation tissue formation, compared with in
vehicle-treated mice (Fig. 3D and
E). Moreover, the deposition and organization of collagen
improved in the skin tissue from the mice treated with NED416 than
in those from the mice treated with vehicle only (Fig. 3F and G).
NED416 activates migration-related
proteins and angiogenesis
To confirm the finding that NED416 positively
affects skin cell migration, the expression and phosphorylation of
cell migration-related marker proteins (Rac1, Cdc42 and α-Pak) were
measured after NED416 treatment of HaCaT cells (Fig. 4A). Western blot analysis showed
that expression of Rac1 was not altered by NED416 (Fig. 4B). However, the GTP-bound active
form of Cdc42 was upregulated by NED416 treatment (Fig. 4C), indicating the activation of
Cdc42. The expression of α-Pak was also not altered by NED416
treatment (Fig. 4D).
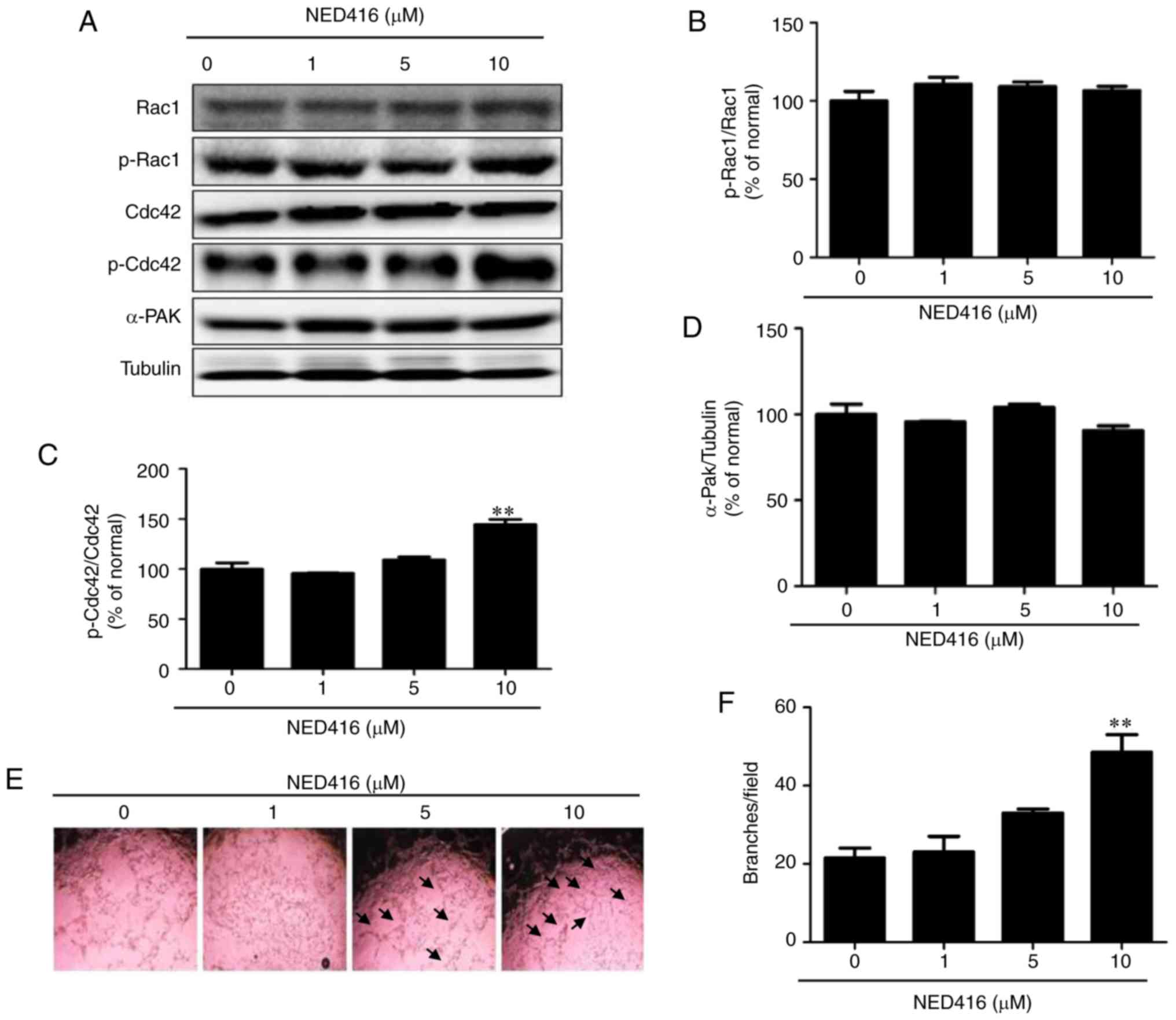 | Figure 4NED416 activates migration-related
proteins in HaCaT cells and promotes angiogenesis. (A) Cellular
levels of migration-related proteins, including Rac1, Cdc42 and
their phosphorylated forms, as well as α-Pak, upon treatment with
NED416 in HaCaT cells. Quantification of the expression profiles of
(B) p-Rac1, (C) p-Cdc42 and (D) α-Pak. (E) Microscopic images of
HUVECs indicating tube formation after treatment with NED416 and
medium only. Magnification, ×40. (F) Quantification of the number
of branches in HUVECs after 18 h of NED416 treatment. Data are
presented as the mean ± SEM. **P<0.01 vs. 0 mM.
NED416,
(E)-3-(2,4-dichlorophenyl)-N-phenylacrylamide; p,
phosphorylated; Cdc42, cell division cycle 42; α-PAK,
α-p21-activated kinase; HUVEC, human umbilical vein endothelial
cell. |
To measure the effect of NED416 on angiogenesis,
HUVECs cultured with NED416 and medium only were photographed to
monitor the progress of tube formation. Microscopic images captured
16 h post-treatment showed that cells treated with NED416 exhibited
notably enhanced angiogenesis, in that the number of branches in
these cells was greater than in untreated cells (Fig. 4E). Although the effect of NED416
was only significant at 10 µM, a notable trend towards
increased angiogenesis was also observed in 5 µM-treated
cells (Fig. 4F).
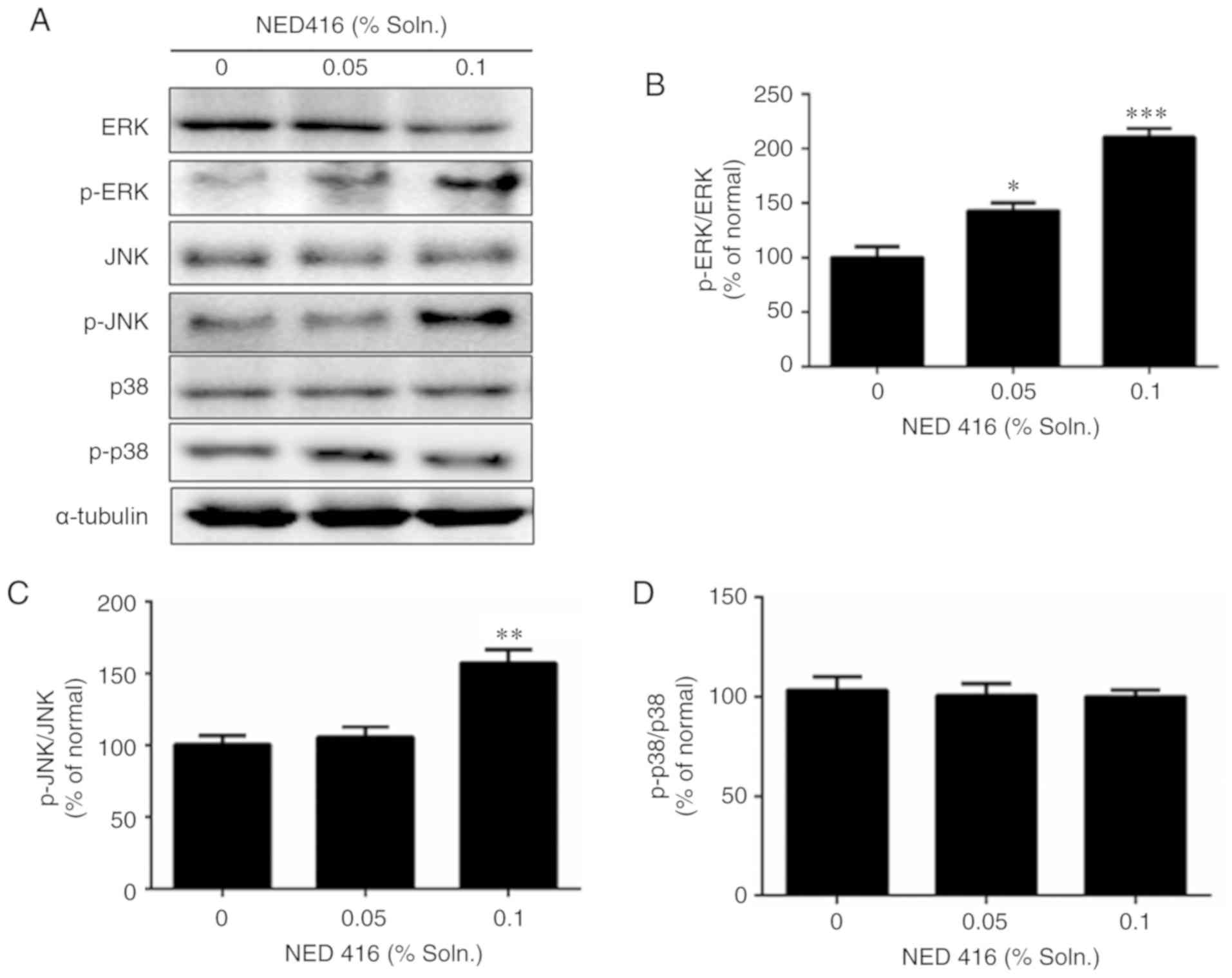
NED416 activates mitogen-activated
protein kinase (MAPK) signaling proteins
The expression of MAPKs was evaluated, as they are
intracellular signaling molecules that may interact with Rho family
proteins to mediate cell migration (31). As determined via western blot
analysis, skin samples treated with NED416 exhibited increases in
ERK and JNK activation (Fig. 5A),
with significantly increased relative levels of p-ERK1/2 (Fig. 5B) and p-JNK compared with the
untreated tissues (Fig. 5C).
However, NED416 did not affect the expression of p-p38 (Fig. 5D). To confirm the role of MAPK
signaling in increased cell migration, ERK, JNK and p38 were
blocked by using their specific inhibitors in the presence of
NED416 in cultured HaCaT cells. Each of the inhibitors reduced
NED416-mediated skin cell migration (Fig. S4).
Discussion
The present study aimed to reveal the effects of
NED416 on Sirt1 activation and thus on the process of cutaneous
wound healing based on the fact that Sirt1 and its regulators have
been shown to play a role in cell migration and tissue healing
(24,25). The ability of NED416 to increase
Sirt1 activity, as well as Sirt1 expression, in NHDF and HaCaT
cells indicated that it is a Sirt1 activator. NED416 promoted the
cellular levels of Sirt1 and enhanced its enzymatic activity. At
present, isoflavones and resveratrol are the most widely known
natural compounds that can increase both the protein expression and
activity of Sirt1 (32); most
synthetic regulators of Sirt1 only alter the activity of Sirt1
(33,34). NED416 not only enhanced Sirt1
activity over that of control cells but was more effective than
resveratrol, which is an established Sirt1 activator (35). The MTT assay for NED416 also
showed that NED416 was not toxic at effective concentrations in
NHDF or HaCaT cells. The ability of NED416 to increase Sirt1
activity and expression over that of resveratrol suggests that its
mode of action differs from that of resveratrol and may provide a
diverse approach to targeting Sirt1.
Knocking down Sirt1 expression in skin keratinocytes
resulted in decreased migration, suggesting that Sirt1 may play a
role in cell migration during wound healing. Treatment with NED416
promoted the rate of migration in HaCaT cells. Differences in
migration rates were significant at all time points between
NED416-treated and untreated cells. Similar results were found when
the same experiment was performed using NHDFs. Both skin
fibroblasts and keratinocytes were included in the study as they
are involved in rejuvenation and wound healing (9,36).
Migration assays using NHDFs and HaCaT cells revealed increased
migration rates in the presence of NED416, proving it an effective
Sirt1 activator for its effects on skin cell migration and pointing
toward the involvement of Sirt1 in cell migration during wound
healing. These findings are consistent with the results of previous
studies of the involvement of Sirt1 and its activators in tissue
healing and cell migration (24,25,27).
To confirm the in vitro effects of NED416 in
an animal model, its effects on cutaneous wound healing were tested
in hairless mice. As hypothesized, the differences in wound healing
were significant at all time points from day 2 onward for animals
treated with NED416 compared with vehicle. Skin samples from the
NED416-treated mice exhibited faster neoepithelium, dermal and
epidermal regeneration when subjected to H&E staining. These
results not only complemented the results found in cultured
keratinocyte and fibroblast monolayers, but also offered insight
into epithelial migration facilitated by NED416 in injured skin
tissue. Furthermore, these findings demonstrated the wound healing
potential of NED416 in vivo as re-epithelialization is one
of the most critical and complex steps of wound healing (1). Thus, in addition to later stages
such as migration and tissue remodeling, NED416 positively
regulated the early stages of wound closure and inflammation.
Resveratrol and juglone are Sirt1 activators from plant sources
that showed wound healing potential in previous studies (27,37,38). Sirt1 activators, including NED416,
regulate wound healing mainly in the later phases. However,
extracts from Aloe vera and Vitis vinifera regulate
the overall process of wound healing (38,39).
NED416 increased the quantity of collagen present in
skin tissue samples that were collected 5 and 10 days after injury.
The amounts of collagen deposited were also significantly higher in
the NED416-treated groups than in the vehicle-treated group. This
finding indicated that NED416 promoted the generation and
deposition of collagen, a marker of the remodeling phase, and is
consistent with the results of previous studies in which collagen
fibers and elastin indicate the replacement and rearrangement of
old and disorganized collagen (7,40).
To confirm the wound healing effects of NED416, the
expression and phosphorylation levels of migration-related proteins
(Rac1, Cdc42 and α-Pak) were monitored, as these proteins play
important roles in cell migration and thus the promotion of wound
healing (10-12). Protein expression of Cdc42 induced
by NED416 treatment was significantly increased. The expression of
Rac1 was not different between its phosphorylated and
unphosphorylated forms upon treatment with NED416. However, the
relative expression of p-Cdc42 compared with its unphosphorylated
form was increased following treatment with NED416. The expression
of α-Pak was also not markedly increased. These findings showed
that NED416 promoted wound healing by enhancing skin cell migration
via the Rac1/Cdc42 pathway. The activation of Cdc42 is significant
in the sense that activation of Rac1 depends on Cdc42, and α-Pak is
a downstream target of the Cdc42/Rac1 complex (41).
Cdc42 is involved in controlling cell migration
through filopodia formation in actin filaments, whereas Rac1 and
α-Pak control lamellipodia and stress fiber formation, respectively
(42-47). Thus, Cdcd42 activation is the most
significant and vital event in cell migration induced by NED416
treatment. Neoangiogenesis plays an essential role in the
development and nourishment of nascent tissue, and the removal of
waste from the wound area (48).
The ability of NED416 to promote angiogenesis in cultured HUVECs
also supported the wound healing potential of NED416.
Compared with vehicle treatment, NED416 treatment
activated MAPK signaling, as western blot analysis revealed
increased levels of ERK and JNK in their phosphorylated forms. Of
note, NED416 increased p-ERK and p-JNK expression in a
dose-dependent manner. However, phosphorylation of p38 was not
increased. These findings regarding MAPK signaling pathway
activation by NED416 are of note as cell migration has been shown
to involve the ERK and JNK signaling pathways to promote the
process of wound healing (49,50).
In conclusion, NED416 is a novel synthetic Sirt1
activator that can increase Sirt1 activity more than resveratrol,
and ameliorates the overall process of wound healing in cultured
skin cells and a mouse wound model, mainly in the later processes
of cell migration, angiogenesis and tissue remod-eling. NED416
promotes these processes by regulating the Rac1/Cdc42 and MAPK
signaling pathways. Future studies with NED416 and other Sirt1
activators not only have the potential for developing new
therapeutic strategies for wound healing, but may also lead to a
novel paradigm of research to reveal the role of Sirt1 in cutaneous
wound healing, which is one of the most complex and essential
repair and regeneration processes.
Supplementary Data
Funding
This study was supported by a grant from the Korean
Health Technology R&D Project, Ministry of Health &
Welfare, Republic of Korea (grant no. HN10C0017).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HMW performed the experiments, analyzed the data and
prepared the manuscript. JKC, LS and MCK performed experiments. HC
and SK performed the compound synthesis. SYK conceived, designed
and supervised the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures for animal experiments were reviewed
and approved by the Animal Care Committee of the Center of Animal
Care and Use at the Lee Gil Ya, Cancer and Diabetes Institute,
Gachon University (permit no. LCDI-2013-0022).
Patient consent for participation
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
We acknowledge Professor Tae Yoon Kim of the College
of Medicine of The Catholic University of Korea for providing the
HaCaT cell line.
References
|
1
|
Gurtner GC, Werner S, Barrandon Y and
Longaker MT: Wound repair and regeneration. Nature. 453:314–321.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Chen J and Kirsner R:
Pathophysiology of acute wound healing. Clin Dermatol. 25:9–18.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kondo T and Ishida Y: Molecular pathology
of wound healing. Forensic Sci Int. 203:93–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reinke JM and Sorg H: Wound repair and
regeneration. Eur Surg Res. 49:35–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Werner S and Grose R: Regulation of wound
healing by growth factors and cytokines. Physiol Rev. 83:835–870.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hantash BM, Zhao L, Knowles JA and Lorenz
HP: Adult and fetal wound healing. Front Biosci. 13:51–61. 2008.
View Article : Google Scholar
|
|
8
|
Raja Sivamani K, Garcia MS and Isseroff
RR: Wound re-epithelialization: Modulating keratinocyte migration
in wound healing. Front Biosci. 12:2849–2868. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wojtowicz AM, Oliveira S, Carlson MW,
Zawadzka A, Rousseau CF and Baksh D: The importance of both
fibroblasts and keratinocytes in a bilayered living cellular
construct used in wound healing. Wound Repair Regen. 22:246–255.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Braun A, Dang K, Buslig F, Baird MA,
Davidson MW, Waterman CM and Myers KA: Rac1 and Aurora A regulate
MCAK to polarize microtubule growth in migrating endothelial cells.
J Cell Biol. 206:97–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baek SH, Cho HW, Kwon YC, Lee JH, Kim MJ,
Lee H and Choe KM: Requirement for Pak3 in Rac1-induced
organization of actin and myosin during Drosophila larval wound
healing. FEBS Lett. 586:772–777. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Funasaka K, Ito S, Hasegawa H, Goldberg
GS, Hirooka Y, Goto H, Hamaguchi M and Senga T: Cas utilizes Nck2
to activate Cdc42 and regulate cell polarization during cell
migration in response to wound healing. FEBS J. 277:3502–3513.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: Growth factors and cytokines in wound
healing. Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar
|
|
14
|
Welch MP, Odland GF and Clark RA: Temporal
relationships of F-actin bundle formation, collagen and fibronectin
matrix assembly, and fibronectin receptor expression to wound
contraction. J Cell Biol. 110:133–145. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Michan S and Sinclair D: Sirtuins in
mammals: Insights into their biological function. Biochem J.
404:1–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Orecchia A, Scarponi C, Di Felice F,
Cesarini E, Avitabile S, Mai A, Mauro ML, Sirri V, Zambruno G,
Albanesi C, et al: Sirtinol treatment reduces inflammation in human
dermal micro-vascular endothelial cells. PLoS One. 6:e243072011.
View Article : Google Scholar
|
|
17
|
Liu Y, He XQ, Huang X, Ding L, Xu L, Shen
YT, Zhang F, Zhu MB, Xu BH, Qi ZQ and Wang HL: Resveratrol protects
mouse oocytes from methylglyoxal-induced oxidative damage. PLoS
One. 8:e779602013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci
USA. 105:13421–13426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JH, Song MY, Song EK, Kim EK, Moon WS,
Han MK, Park JW, Kwon KB and Park BH: Overexpression of SIRT1
protects pancreatic beta-cells against cytokine toxicity by
suppressing the nuclear factor-kappaB signaling pathway. Diabetes.
58:344–351. 2009. View Article : Google Scholar :
|
|
20
|
Satoh A, Brace CS, Rensing N, Cliften P,
Wozniak DF, Herzog ED, Yamada KA and Imai S: Sirt1 extends life
span and delays aging in mice through the regulation of Nk2
homeobox 1 in the DMH and LH. Cell Metabol. 18:416–430. 2013.
View Article : Google Scholar
|
|
21
|
Valente S, Mellini P, Spallotta F, Carafa
V, Nebbioso A, Polletta L, Carnevale I, Saladini S, Trisciuoglio D,
Gabellini C, et al: 1,4-Dihydropyridines active on the SIRT1/AMPK
pathway ameliorate skin repair and mitochondrial function and
exhibit inhibition of proliferation in cancer cells. J Med Chem.
59:1471–1491. 2016. View Article : Google Scholar
|
|
22
|
Wang Y, Zhao X, Shi D, Chen P, Yu Y, Yang
L and Xie L: Overexpression of SIRT1 promotes high
glucose-attenuated corneal epithelial wound healing via p53
regulation of the IGFBP3/IGF-1R/AKT pathway. Invest Ophthalmol Vis
Sci. 54:3806–3814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hunt ND, Li GD, Zhu M, Miller M, Levette
A, Chachich ME, Spangler EL, Allard JS, Hyun DH, Ingram DK and de
Cabo R: Effect of calorie restriction and refeeding on skin wound
healing in the rat. Age (Dordr). 34:1453–1458. 2012. View Article : Google Scholar
|
|
24
|
Zeytin K, Ciloğlu NS, Ateş F, Vardar Aker
F and Ercan F: The effects of resveratrol on tendon healing of
diabetic rats. Acta Orthop Traumatol Turc. 48:355–362. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Casarin RC, Casati MZ, Pimentel SP, Cirano
FR, Algayer M, Pires PR, Ghiraldini B, Duarte PM and Ribeiro FV:
Resveratrol improves bone repair by modulation of bone
morphogenetic proteins and osteopontin gene expression in rats. Int
J Oral Maxillofac Surg. 43:900–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wahedi HM, Lee TH, Moon EY and Kim SY:
Juglone up-regulates sirt1 in skin cells under normal and UVB
irradiated conditions. J Dermatol Sci. 81:210–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wahedi HM, Park YU, Moon EY and Kim SY:
Juglone ameliorates skin wound healing by promoting skin cell
migration through Rac1/Cdc42/PAK pathway. Wound Repair Regen.
24:786–794. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim S, Lim C, Lee S, Lee S, Cho H, Lee JY,
Shim DS, Park HD and Kim S: Column chromatography-free
solution-phase synthesis of a natural piper-amide-like compound
library. ACS Comb Sci. 15:208–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiu J and Zhang R: Direct transformation
of arylpropynes to acrylamides via a three-step tandem reaction.
Org Biomol Chem. 12:1556–1560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galeano M, Deodato B, Altavilla D,
Cucinotta D, Arsic N, Marini H, Torre V, Giacca M and Squadrito F:
Adeno-associated viral vector-mediated human vascular endothelial
growth factor gene transfer stimulates angiogenesis and wound
healing in the genetically diabetic mouse. Diabetologia.
46:546–555. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng C, Kong X, Wang H, Gan H, Hao Y, Zou
W, Wu J, Chi Y, Yang J, Hong Y, et al: Trihydrophobin 1 interacts
with PAK1 and regulates ERK/MAPK activation and cell migration. J
Biol Chem. 284:8786–8796. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dai H, Sinclair DA, Ellis JL and Steegborn
C: Sirtuin activators and inhibitors: Promises, achievements, and
challenges. Pharmacol Ther. 188:140–154. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumar A and Chauhan S: How much successful
are the medicinal chemists in modulation of SIRT1: A critical
review. Eur J Med Chem. 119:45–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hubbard BP and Sinclair DA: Small molecule
SIRT1 activators for the treatment of aging and age-related
diseases. Trends Pharmacol Sci. 35:146–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alcain FJ and Villalba JM: Sirtuin
activators. Expert Opin Ther Pat. 19:403–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee TH, Lee GW, Park KH, Mohamed MA, Bang
MH, Baek YS, Son Y, Chung DK, Baek NI and Kim J: The stimulatory
effects of Stewartia koreana extract on the proliferation and
migration of fibroblasts and the wound healing activity of the
extract in mice. Int J Mol Med. 34:145–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Amanat S, Taymouri S, Varshosaz J,
Minaiyan M and Talebi A: Carboxymethyl cellulose-based wafer
enriched with resvera-trol-loaded nanoparticles for enhanced wound
healing. Drug Deliv Transl Res. Jan 24–2020.Epub ahead of print.
View Article : Google Scholar
|
|
38
|
Lin LX, Wang P, Wang YT, Huang Y, Jiang L
and Wang XM: Aloe vera and Vitis vinifera improve wound healing in
an in vivo rat burn wound model. Mol Med Rep. 13:1070–1076. 2016.
View Article : Google Scholar
|
|
39
|
Lodhi S, Jain AP, Rai G and Yadav AK:
Preliminary investigation for wound healing and anti-inflammatory
effects of Bambusa vulgaris leaves in rats. J Ayurveda Integr Med.
7:14–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hornstra IK, Birge S, Starcher B, Bailey
AJ, Mecham RP and Shapiro SD: Lysyl oxidase is required for
vascular and diaphragmatic development in mice. J Biol Chem.
278:14387–14393. 2003. View Article : Google Scholar
|
|
41
|
Manser E, Leung T, Salihuddin H, Zhao ZS
and Lim L: A brain serine/threonine protein kinase activated by
Cdc42 and Rac1. Nature. 367:40–46. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wakayama Y, Fukuhara S, Ando K, Matsuda M
and Mochizuki N: Cdc42 mediates Bmp-induced sprouting angiogenesis
through Fmnl3-driven assembly of endothelial filopodia in
zebrafish. Dev Cell. 32:109–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Reddy PN, Radu M, Xu K, Wood J, Harris CE,
Chernoff J and Williams DA: p21-activated kinase 2 regulates HSPC
cytoskeleton, migration, and homing via CDC42 activation and
interaction with beta-Pix. Blood. 127:1967–1975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abraham S, Scarcia M, Bagshaw RD, McMahon
K, Grant G, Harvey T, Yeo M, Esteves FOG, Thygesen HH, Jones PF, et
al: A Rac/Cdc42 exchange factor complex promotes formation of
lateral filopodia and blood vessel lumen morphogenesis. Nat Commun.
6:72862015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
King SJ, Asokan SB, Haynes EM, Zimmerman
SP, Rotty JD, Alb JG Jr, Tagliatela A, Blake DR, Lebedeva IP,
Marston D, et al: Lamellipodia are crucial for haptotactic sensing
and response. J Cell Sci. 129:2329–2342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gujdar A, Sipeki S, Bander E, Buday L and
Faragó A: Phorbol ester-induced migration of HepG2 cells is
accompanied by intensive stress fibre formation, enhanced integrin
expression and transient down-regulation of p21-activated kinase 1.
Cell Signal. 15:307–318. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kundumani-Sridharan V, Singh NK, Kumar S,
Gadepalli R and Rao GN: Nuclear factor of activated T cells c1
mediates p21-activated kinase 1 activation in the modulation of
chemo-kine-induced human aortic smooth muscle cell F-actin stress
fiber formation, migration, and proliferation and injury-induced
vascular wall remodeling. J Biol Chem. 288:22150–22162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Carmeliet P: Angiogenesis in health and
disease. Nat Med. 9:653–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang M, Sun L, Wang X, Chen S, Kong Y,
Liu N, Chen Y, Jia Q and Zhang L and Zhang L: Activin B promotes
BMSC-mediated cutaneous wound healing by regulating cell migration
via the JNK-ERK signaling pathway. Cell Transplant. 23:1061–1073.
2014. View Article : Google Scholar
|
|
50
|
Zhang L, Deng M, Parthasarathy R, Wang L,
Mongan M, Molkentin JD, Zheng Y and Xia Y: MEKK1 transduces activin
signals in keratinocytes to induce actin stress fiber formation and
migration. Mol Cell Biol. 25:60–65. 2005. View Article : Google Scholar :
|