Introduction
Diabetic retinopathy (DR) is a disease secondary to
impaired glucose tolerance and retinal damage, which causes
decreased vision that can even lead to macular edema, retinal
detachment and vitreous hemorrhage, eventually resulting in
blindness, and is one of the most common complications of diabetes
mellitus (DM) (1). Data from the
World Health Organization indicate that DR has become a leading
cause of blindness among the working-age population in the United
States (2), and is the 5th leading cause of blindness worldwide,
while the number of patients with DR worldwide have been estimated
to increase to 191 million by the year 2030 (3). Previous studies have found that
sustained high glucose (HG) levels cause damage to retinal pigment
epithelial (RPE) cells in diabetic patients, resulting in
structural and secretory dysfunction, which proves that RPE cell
damage plays an important role in the early development of DR
(4).
RPE cells are derived from embryonic optic vesicles
and play a vital role in the growth, development and visual
function of the eye, and have also been found to exert antioxidant
functions, maintain secretory growth factors and participate in
physiological functions, such as circulating metabolism (5,6).
Moreover, RPE cells have been found to exhibit high levels of
apoptosis in in vitro models, resulting from causes, such as
oxidative stress (7) and blue
light damage (8). Therefore, the
apoptosis of RPE cells plays an important role in the pathogenesis
of retinal degenerative diseases; the protection of RPE cells and
the effective control of the apoptosis of RPE cells may delay the
development of retinal degeneration (9).
Astragaloside-IV (AIV) is one of the main active
ingredients extracted from Astragalus, and has been proven
to exert anti-inflammatory, antioxidant, energy metabolism,
neuroprotection and anticancer effects. AIV has also been used in
the treatment of cardiovascular and cerebrovascular diseases,
kidney diseases, respiratory diseases, diabetes and
diabetes-related complications (10,11). In addition, a previous study by
the authors found that miR-128 attenuates the apoptosis of RPE
cells induced by HG levels in vitro (12). miR-128 has also been found to be
associated with insulin resistance (13,14) and neuropathic susceptibility
(15) in diabetic patients.
Although AIV has been found to exert protective effects on diabetic
mouse retinopathy (16), its
specific molecular mechanisms of action remain unclear, and it is
unknown whether it is related to the inhibition of RPE cell
apoptosis and if its effects involve miR-128. In the present study,
the role and mechanisms of action of AIV in DR in rats with DM were
investigated. A rat model of DM was established by an
intra-peritoneal injection of streptozotocin (STZ). It was found
that AIV protected RPE cells from rats with DM from apoptosis by
upregulating miR-128 expression, which in turn attenuated
retinopathy in rats with DM.
Materials and methods
Experimental animal and grouping
The animal experiments performed in the present
study were approved and supervised by the Animal Care and Use
Committee of Weihai Municipal Hospital, and conformed with
guidelines of the National Institution of Health. A total of 38
Sprague-Dawley (SD) rats (SPF) were used in the present study, and
were kept under the following conditions: Temperature, 20-24°C,
humidity, 50-65% humidity, free access to food and water and 12-h
light/dark cycle. A rat model of DM was established by injecting
rats with an intraperitoneal injection of 100 mg/kg STZ (V900890;
Sigma-Aldrich; Merck KGaA) using SD rats (male:female ratio, 1:1; 6
weeks old; weighing 180-200 g). The day of the injection of STZ in
rats was defined as the first day. Blood glucose levels from the
tail vein were measured on the third day after the STZ injection.
Rats with a tail vein blood glucose level of >16.7 mmol/l were
defined as rats with DM. The rats in the Sham group were
intraperitoneally injected with the same dose of a solvent
(stroke-physiological saline solution) on the first day. The rats
with DM were randomly divided into 3 groups, namely the DM group,
the DM + L-AIV group, the DM + M-AIV group and the DM + H-AIV
group, where L, M and H represent low, medium, and high doses of
AIV, respectively. The rats in the DM + L-AIV, DM + M-AIV and DM +
H-AIV groups were intragastrically administered 20, 40 and 60 mg/kg
AIV (Xi'an Sobeo Pharmaceutical Technology Co., Ltd.) daily,
respectively. The other rats were administered the same volume of
solvent. Rat weight and blood glucose levels were measured at the
0, 4, 8 and 12th week of AIV administration and retinal function
was evaluated using an electroretinogram (ERG) at the 12th
week.
RPE cell separation and experiments
Rats were anesthetized by intraperitoneal injection
of sodium pentobarbital at 80 mg/kg, and the anesthesia status of
the rats was determined by observing righting reflex, eye reflex,
swallow reflex, pedal reflex and tail reflex. Subsequently, the
deeply anesthetized rats were sacrificed by cervical dislocation,
and the death of the rats was confirmed by observing breathing and
heartbeat. After the SD rats were euthanized, their eyeballs were
removed under aseptic conditions. The eyeballs were immersed in
physiological saline containing 400 U/ml gentamicin (E003632;
Sigma-Aldrich; Merck KGaA) for 30 min on ice. The eyeballs were
then placed in 50 U/ml hyaluronidase (H1115000; Sigma-Aldrich;
Merck KGaA) and 105 U/ml collagenase (1148089; Sigma-Aldrich; Merck
KGaA) mixture buffered at 37°C for 45 min. After washing 3 times
with D-Hanks buffer, the eyeball was placed in 0.1% Trypsin
(15090046, Thermo Fisher Scientific, Inc.) at 37°C for 30 min. The
eyeballs were dissected under an optical microscope (CX23, Olympus
Corporation) to harvest RPE-choroid-sclera tissue, and were
digested using 0.25% trypsin for 5 min at room temperature.
Finally, the cell suspension was harvested by washing the tissue
using the medium. The cell suspensions were incubated with 0.25%
trypsin for 5 min at room temperature after removing the medium
through centrifugation (500 x g, room temperature, 5 min). A
portion of the RPE cells were used directly to extract RNA for
reverse transcription-quantitative PCR (RT-qPCR) analysis and
protein for western blot analysis, while the other RPE cells were
cultured at 37°C with 5% CO2 in DMEM (12491-15, Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(10100-147, Thermo Fisher Scientific, Inc.). As described in a
previous study by the authors (12), 50 µmol miR-128-inhibitor
(5′-AGU GUC ACU UGG CCA GAG AAA -3′; Sangon Biotech) was first
transfected into the RPE cells using Lipofectamine™ 2000
transfection reagent (11668019, Invitrogen; Thermo Fisher
Scientific, Inc.). After 72 h, the cells were transferred to 4.5
g/l HG medium. The normal cell medium contained 1.0 g/l
glucose.
MTT assay
A total of 2×103 cells were seeded into a
96-well cell culture plate and cultured under normal conditions for
72 h. The cell culture medium was then removed and 4.5 g/l HG
medium was added for 6, 12, 24 or 48 h. Cell viability was then
measured using a Cell Proliferation Assay kit (C0009, Beyotime
Institute of Biotechnology).
TUNEL staining
The rat retinal tissues were fixed using
paraformaldehyde and were made into paraffin-embedded sections, and
TUNEL staining was used to detect the apoptosis of RPE cells in
each group. All reagents and procedures used for TUNEL assay were
according to the instructions provided by the manufacturer of the
TUNEL Cell Apoptosis Detection kit (TA201-02, TransGen Biotech Co.,
Ltd.).
Western blot analysis
RIPA lyssis buffer (R0010, Solarbio, Inc.) was used
to extract total protein from the RPE cells of the rats and the
protein concentration was detected using a BCA kit (P0011, Beyotime
Institute of Biotechnology), and 40 µg of total protein were
separated using 10% SDS-PAGE under a constant voltage of 90 V. The
proteins were then transferred from the SDS-PAGE gel onto a PVDF
membrane. After the samples were blocked using 5% skimmed milk at
room temperature for 1 h, the membrane was incubated with the
primary antibodies overnight at 4°C. The secondary antibody was
used for incubation at room temperature for 2 h. After being washed
3 times using phosphate-buffered saline/Tween-20, ECL solution
(WBKLS0100, Beijing Xinjingke Biotechnologies Co., Ltd.) was added
for detection, followed by densitometric analysis using ImageJ 3.0
software (IBM, Inc.) and β-actin was used as a loading control.
Information pertaining to the antibodies used in the present study
is presented in Table I.
 | Table IAntibody information. |
Table I
Antibody information.
| Antibody | Dilution | Cat. no. | Manufacturer |
|---|
| Bcl-2 | 1:2,000 | 15071 | Cell Signaling
Technology, Inc. |
| Bax | 1:2,000 | 5023 | Cell Signaling
Technology, Inc. |
| Fas | 1:1,000 | 4233 | Cell Signaling
Technology, Inc. |
| FasL | 1:1,500 | 68405 | Cell Signaling
Technology, Inc. |
| Active
caspase-3 | 1:500 | 9661 | Cell Signaling
Technology, Inc. |
| Active
caspase-8 | 1:1,000 | 9748 | Cell Signaling
Technology, Inc. |
| Active
caspase-9 | 1:1,000 | 9505 | Cell Signaling
Technology, Inc. |
| HOXB3 | 1:2,000 | ab83404 | Abcam |
| PI3K | 1:1,500 | ab180967 | Abcam |
| p-PI3K | 1:1,000 | 17366 | Cell Signaling
Technology, Inc. |
| AKT | 1:2,000 | ab18785 | Abcam |
| p-AKT | 1:1,000 | ab38449 | Abcam |
| p70s6k1 | 1:1,500 | ab32359 | Abcam |
| p-p70s6k1 | 1:500 | ab59208 | Abcam |
| β-actin | 1:5,000 | HC201 | TransGen Biotech
Co., Ltd. |
| Goat
anti-rabbit | 1:3,000 | ab6721 | Abcam |
| Goat
anti-mouse | 1:3,000 | ab205719 | Abcam |
RT-qPCR
An RNA extract kit (9767, Takara, Inc.) was used to
extract total RNA from the RPE cells of rats, and cDNA was
constructed using a one-step cDNA reverse transcription kit (D0401,
HaiGene). Finally, 20 µl of the qPCR reaction solution were
configured according to the instructions of the GoTaq qPCR Master
Mix (A6006A, Promega Corp.), and the relative expression level of
miR-128 against U6 RNA (internal control) was calculated using the
2−ΔΔCq method (17).
The following PCR primers were used: miR-128-F, 5′-CAA AGA CTA CTG
TGT AAC TGC GA-3′ and miR-128-R, 5′-TGG ACT GTA CTT GAC AAT GTT
GG-3′; β-actin-F, 5′-GGC TGT ATT CCC CTC CAT CG-3′; U6-F, 5′-CTC
GCT TCG GCA GCA CA-3′ and U6-R, 5′-AAC GCT TCA CGA ATT TGC
GT-3′.
Flow cytometric analysis for
apoptosis
RPE cells subjected to the different treatments were
harvested through centrifugation (500 × g, 5 min, room
temperature), and were washed 2 times with pre-cooled PBS on ice.
An Annexin V-FITC/PI Apoptosis Detection kit was used to stain the
cells and apoptotic cells were analyzed using a flow cytometer
(Flow-Count, Beckman Coulter, Inc.). All procedures were performed
according to the instructions of the manufacturers, and the
measurements were repeated 2 times for each sample.
Statistical analysis
GraphPad prism v8.3.0 software was used to record
and analyze the data collected. Data are expressed as the means ±
SD of 3 separate experiments, and one-way ANOVA with Tukey's test
as a post hoc test was used to compare differences between multiple
groups. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
AIV attenuates retinal damage in diabetic
rats
Through the intragastric administration of AIV
daily, 1 week after the intra-peritoneal injection of STZ, the body
weight of the rats and blood glucose levels were measured at the 4,
8 and 12th week. As shown in Fig.
1A, the body weight of the normal rats in the Sham group
increased during the study period; however, the body weight of the
rats with DM decreased in the 4th week and then slowly increased,
while the body weight of the rats with DM treated with AIV was
significantly higher than that of the rats with DM. Moreover, the
body weight of the rats with DM treated with a high dose of AIV was
higher than those administered a medium dose of AIV, while the body
weight of the rats with DM administered a low dose of AIV treatment
was the lowest. It was also found that (Fig. 1B) the blood glucose levels of rats
with DM were maintained at a high level; however, AIV treatment
decrease the blood glucose levels of rats with DM in a
dose-dependent manner, as the body weight of the rats changed.
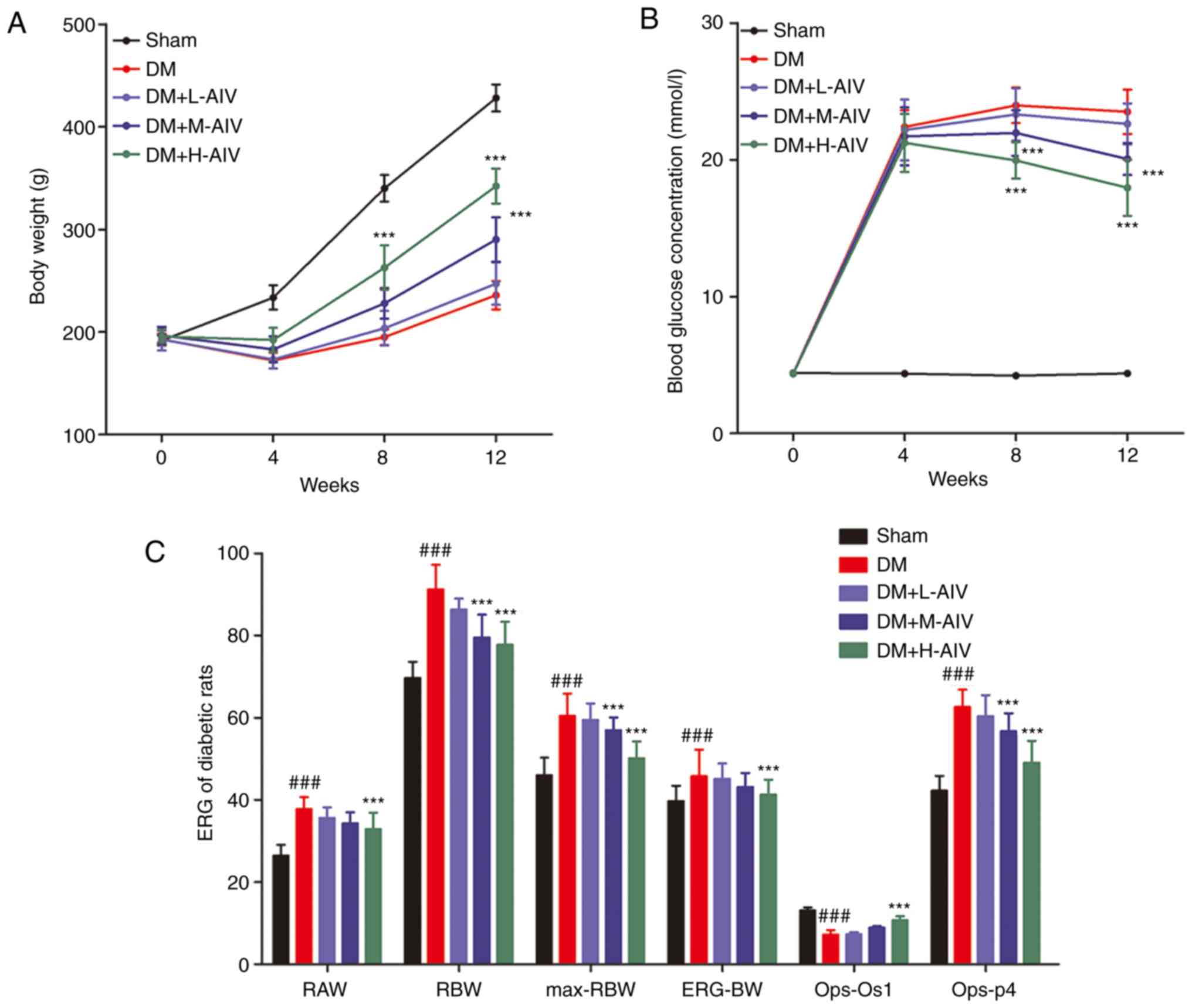 | Figure 1Effect of AIV on the ERG of DM rats.
(A) AIV significantly increased the body weight of rats with DM in
a dose-dependent manner; (B) AIV significantly induced blood
glucose levels of rats with DM in a dose-dependent manner; (C) AIV
significantly promoted the ERG of rats with DM at 12 weeks; 6 rats
were in the Sham group, and 8 rats were in the other groups;
***P<0.001 vs. DM group, and ###P<0.001
vs. Sham group. Data are expressed as the means ± SD of 3
individual experiments. AIV, astragaloside-IV; DM, diabetes
mellitus; ERG, electroretinogram; RAW, rod cell response a wave;
RBW, rod cell b wave; max-RBW, maximum response b wave; ERG-BW,
phot-ERG b wave; Ops, oscillatory potentials. |
Furthermore, retinal function was evaluated using an
ERG, and it was found that (Fig.
1C) compared with the rats in the Sham group, the rat rod cell
response a wave, b wave, maximum response b wave, photopic-ERG b
wave and oscillatory potential (OP) p4 wave latency increased
significantly, and the amplitude of OP os1 wave decreased
significantly in rats with DM. However, following 11 weeks of 60
mg/kg AIV (H-AIV) treatment, the rat rod cell response a wave, b
wave, maximum response b wave, phot-ERG b wave, OP p4 wave latency
decreased significantly and the amplitude of OP Os1 wave increased
significantly in DM rats.
AIV attenuates the apoptosis of REP cells
from diabetic rats
At the 12th week, the rats were euthanized to obtain
retinal tissues and RPE cells isolated. First, TUNEL staining was
performed to measure the level of apoptosis of RPE cells from DM
rats. It was found that (Fig. 2A)
RPE cells from normal rats did not undergo apoptosis; however, the
apoptosis of RPE cells from rats with DM was detected and the
proportion of apoptotic RPE cells decreased significantly following
treatment with AIV. Subsequently, apoptosis-related protein
expression was detected by western blot analysis. It was and found
that (Fig. 2B and C) the protein
expression levels of Bcl-2, Bcl-2/Bax and FasL decreased
significantly in the RPE cells from rats with DM, whereas AIV
treatment significantly increased these expression levels. The
protein expression levels of Bax, Fas, Fas/FasL, active caspase-3,
active caspase-8 and active caspase-9 significantly increased in
the RPE cells from rats with DM; however, AIV treatment
significantly decreased these expression levels.
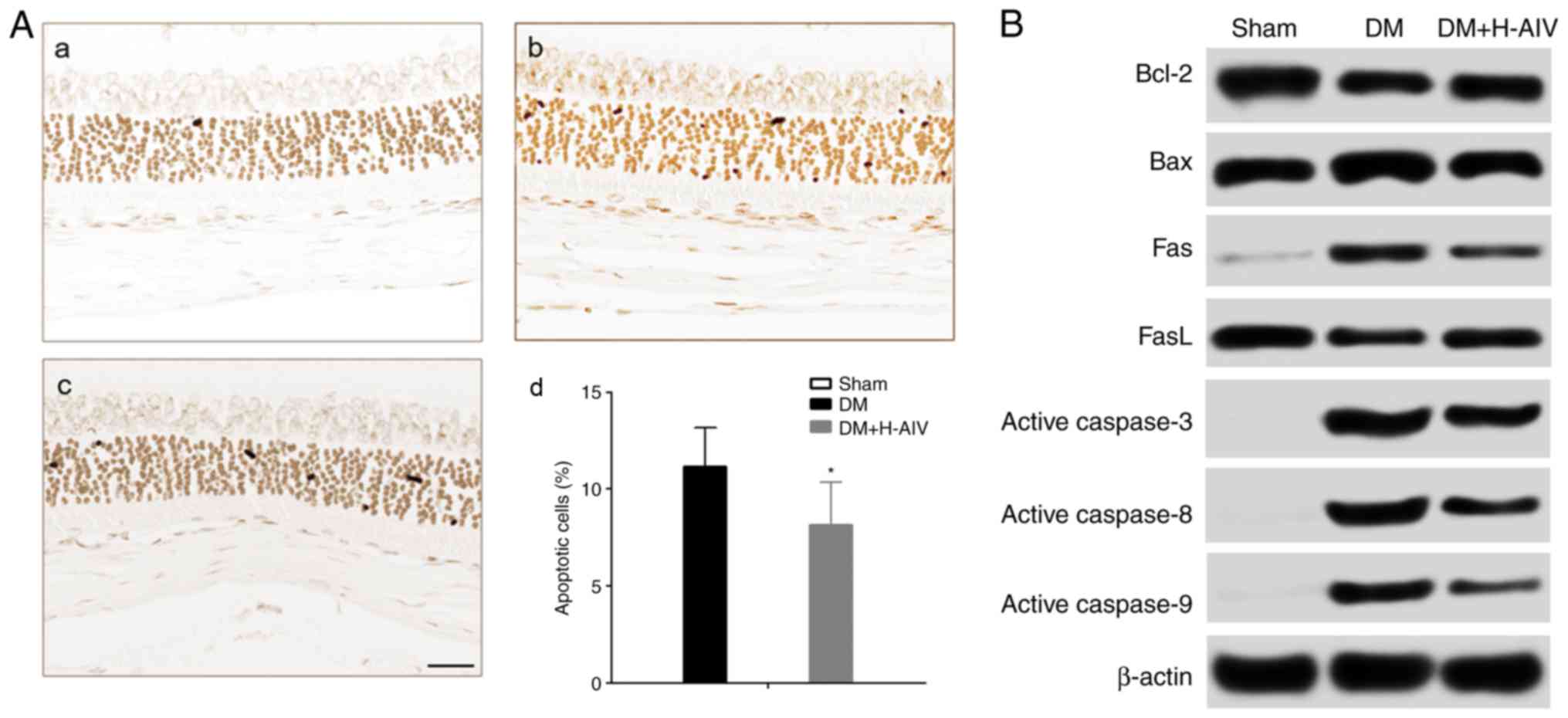 | Figure 2Effect of AIV on the apoptosis of RPE
cells from rats with DM. (A) AIV significantly decreased the
apoptosis of RPE cells from rats with DM as shown by TUNEL assay,
(a) Sham, (b) DM, (c) DM+H-AIV, (d) percentage apoptotic cells;
scale bar, 100 µm; (B) representative apoptosis-related
protein expression bands in RPE cells from rats with DM; (C)
statistical analysis of gray value of apoptosis-related protein
bands, (a) Bcl-2, (b) Bax, (c) Bcl-2/Bax, (d) Fas, (e) FasL, (f)
Fas/FasL, (g) active caspase-3, (h) active caspase-8, (i) active
caspase-9. *P<0.05 and ***P<0.001 vs.
DM group, and ###P<0.001 vs. Sham group. Data are
expressed as the means ± SD of 3 individual experiments. AIV,
astragaloside-IV; DM, diabetes mellitus; RPE cells, retinal pigment
epithelial cells. |
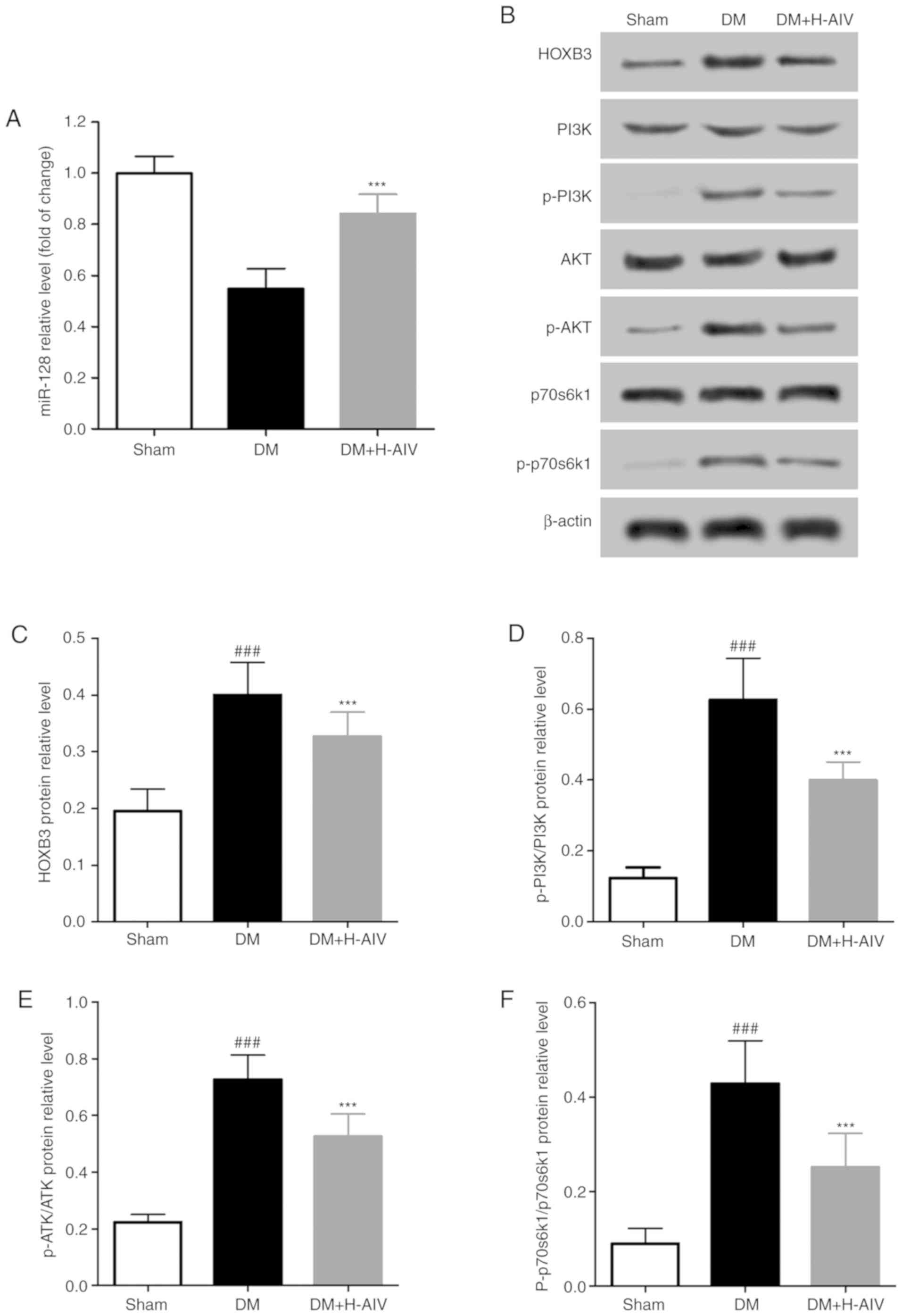
AIV increases miR-128 expression in REP
cells from diabetic rats
A previous study by the authors demonstrated that
miR-128 protects human RPE cells (ARPE-19) under HG conditions
through the homeobox B3 (HOXB3)/phosphoinositide 3-kinase
(PI3K)/extracellular regulated kinase (ERK)/mammalian target of
rapamycin (mTOR) pathway (12).
Therefore, the present study also detected the expression of
miR-128 in RPE cells of rats. It was found that (Fig. 3A) miR-128 expression in RPE cells
of DM rats was significantly lower than that in RPE cells from
normal rats; however, miR-128 expression in RPE cells from DM rats
treated with AIV (60 mg/kg) for 11 weeks increased significantly,
compared with that in RPE cells from rats with DM. In addition, the
expression of HOXB3, a target gene of miR-128, and the PI3K/AKT
pathway was detected in RPE cells from the rats. As shown in
Fig. 3B-F, the protein expression
levels of HOXB3, p-PI3K/PI3K, p-AKT/AKT and p-p70S6K1/p70S6K1 in
RPE cells from rats with without treatment were significantly
higher than those in RPE cells from normal rats. However, after 11
weeks of AIV treatment, the elevated protein expression levels of
HOXB3, p-PI3K/PI3K, p-AKT/AKT and p-p70S6K1/p70S6K1 decreased
significantly.
AIV reduces the HG-induced apoptosis of
REP cells in vitro
RPE cells were isolated from normal rats and treated
with HG, AIV or an miR-128-inhibitor. First, it was found that the
viability of RPE cells gradually decreased along with the
prolongation of HG treatment (Fig.
4A). The time point of 24 h was selected as the duration for
RPE cells to be exposed to HG levels in the following experiments.
Second, as shown in Fig. 4B,
compared with the control group, the expression of miR-128
significantly decreased following exposure to HG; however, AIV
significantly increased its expression. Subsequently, flow
cytometry was used to detect the level of apoptosis of rat RPE
cells. It was found that (Fig. 4C and
D) HG treatment induced the apoptosis of RPE cells in
vitro, whereas the ratio of apoptotic RPE cells decreased
significantly with the administration of AIV treatment. More
importantly, compared with the normal RPE cells, the ratio of
apoptotic RPE cells increased significantly following the knockdown
of miR-128 expression as a result of transfection with
miR-128-inhibitor.
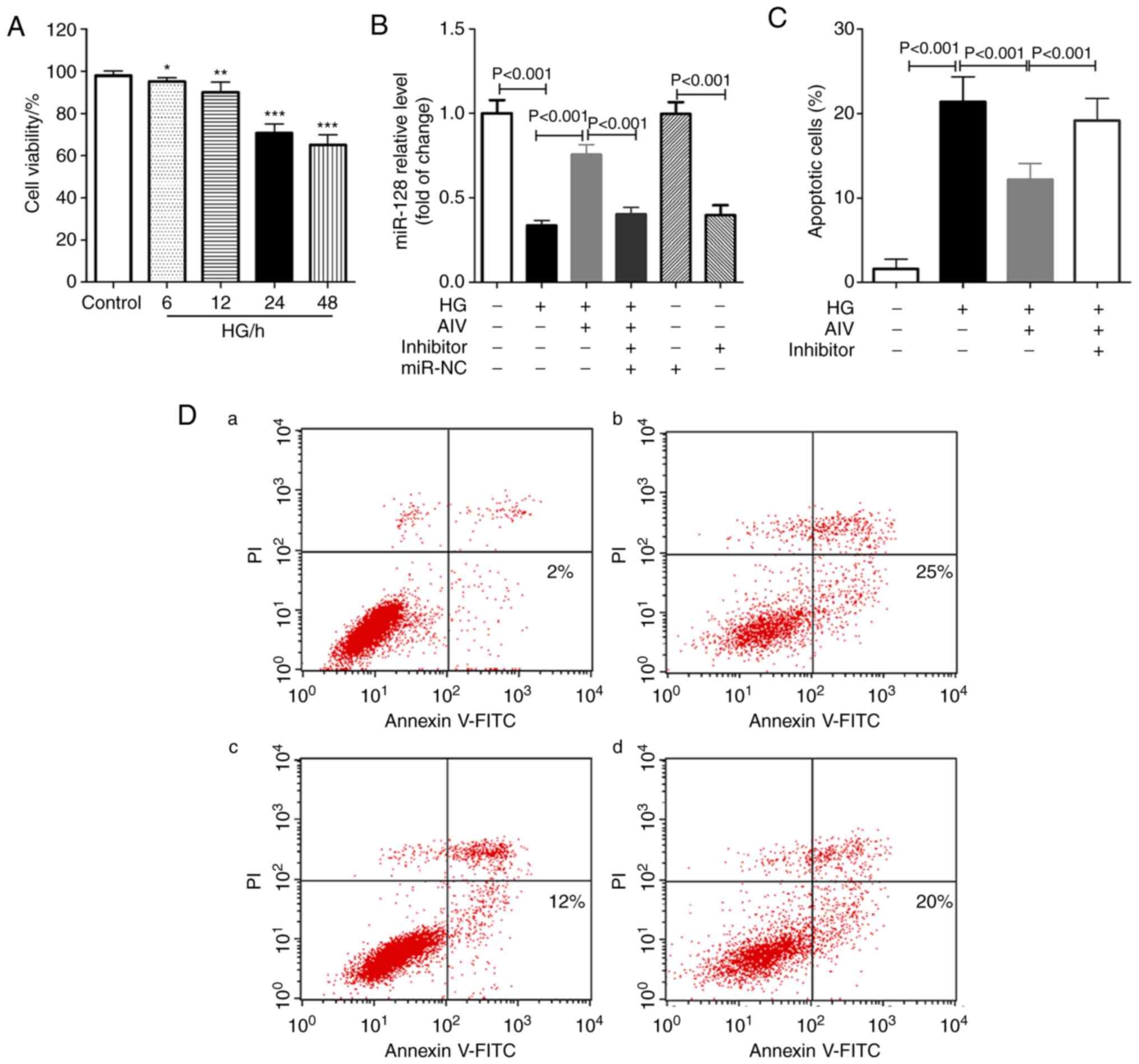 | Figure 4Effect of AIV on apoptosis of rat RPE
cells induced by high glucose in vitro. (A) MTT assay was
used to assess the viability of rat RPE cells after induction with
high glucose at different time points; (B) AIV significantly
increased miR-128 expression in rat RPE cells after induction with
high glucose at 24 h; (C) AIV protected rat RPE cells after
induction with high glucose at 24 h via miR-128; (D) flow cytometry
was used to detect the apoptosis of rat RPE cells following
treating with (a) nothing, (b) HG, (c) HG + AIV and (d) HG + AIV +
inhibitor; 3 independent experiments per test. Data are expressed
as the means ± SD of 3 individual experiments.
*P<0.05, **P<0.01,
***P<0.001, compared with control group. AIV,
astragaloside-IV; DM, diabetes mellitus; RPE cells, retinal pigment
epithelial cells; HG, high glucose; miR-NC, miR-128-inhibitor
negative control; inhibitor, miR-128-inhibitor. |
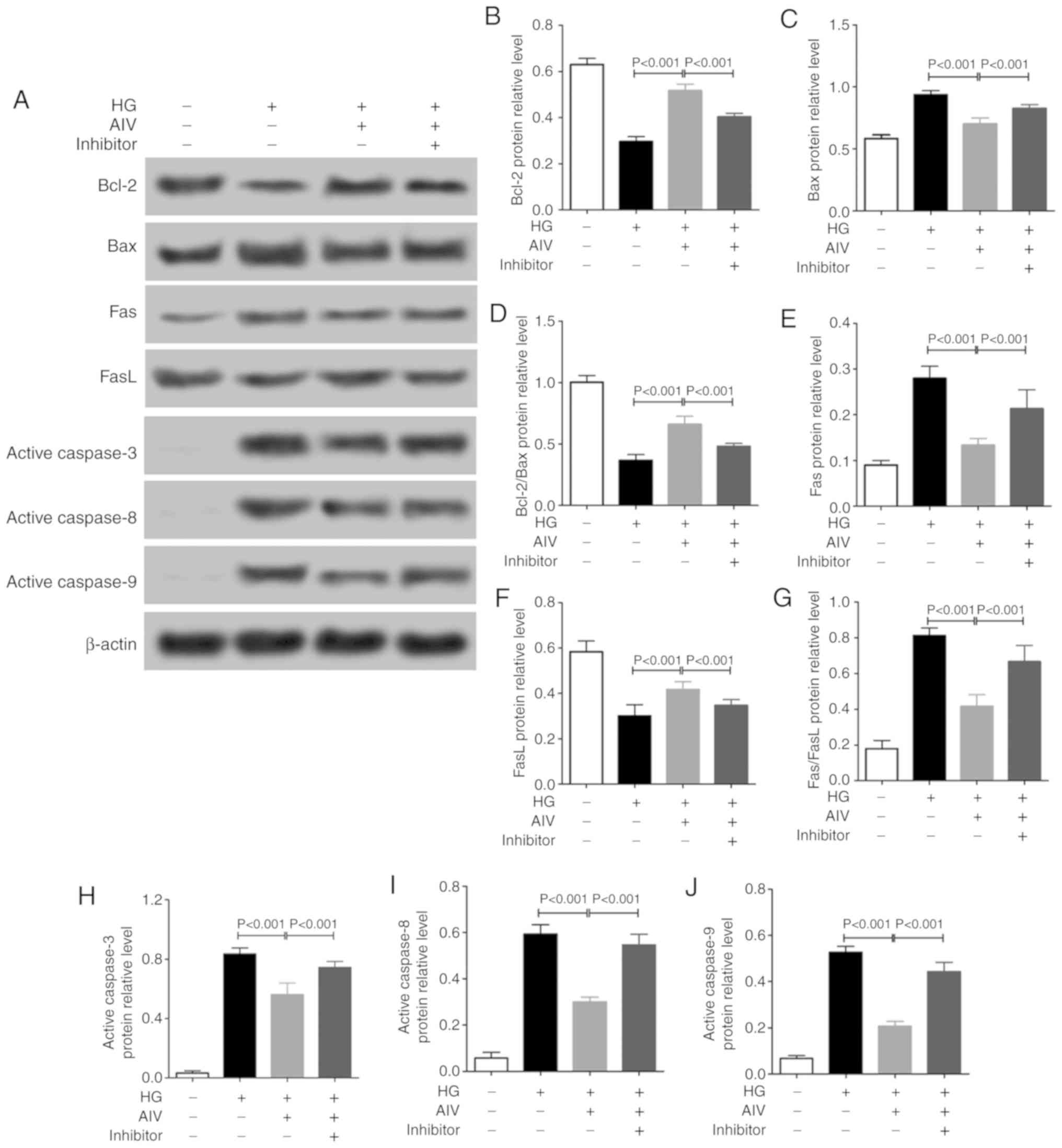
As regards the underlying the molecular mechanisms,
as shown in Fig. 5, AIV treatment
significantly increased the Bcl-2, Bcl2/Bax and FasL protein
expression levels, which were downregulated by HG in the RPE cells
in vitro, and significantly decreased the Bax, Fas,
Fas/FasL, active caspase-3, active caspase-8 and active caspase-9
protein expression levels, which were upregulated by HG in the RPE
cells. Moreover, it was found that (Fig. 6) AIV treatment significantly
decreased the p-PI3K/PI3K, p-AKT/AKT and p-p70S6K1/p70S6K1 protein
expression levels, which were upregulated by HG in RPE cells in
vitro. Similarly, compared with the normal RPE cells, the
effects of AIV treatment on changes in the expression levles of
apoptosis-related proteins and the PI3K/AKT pathway induced by HG
were weakened in the RPE cells following miR-128 knockdown.
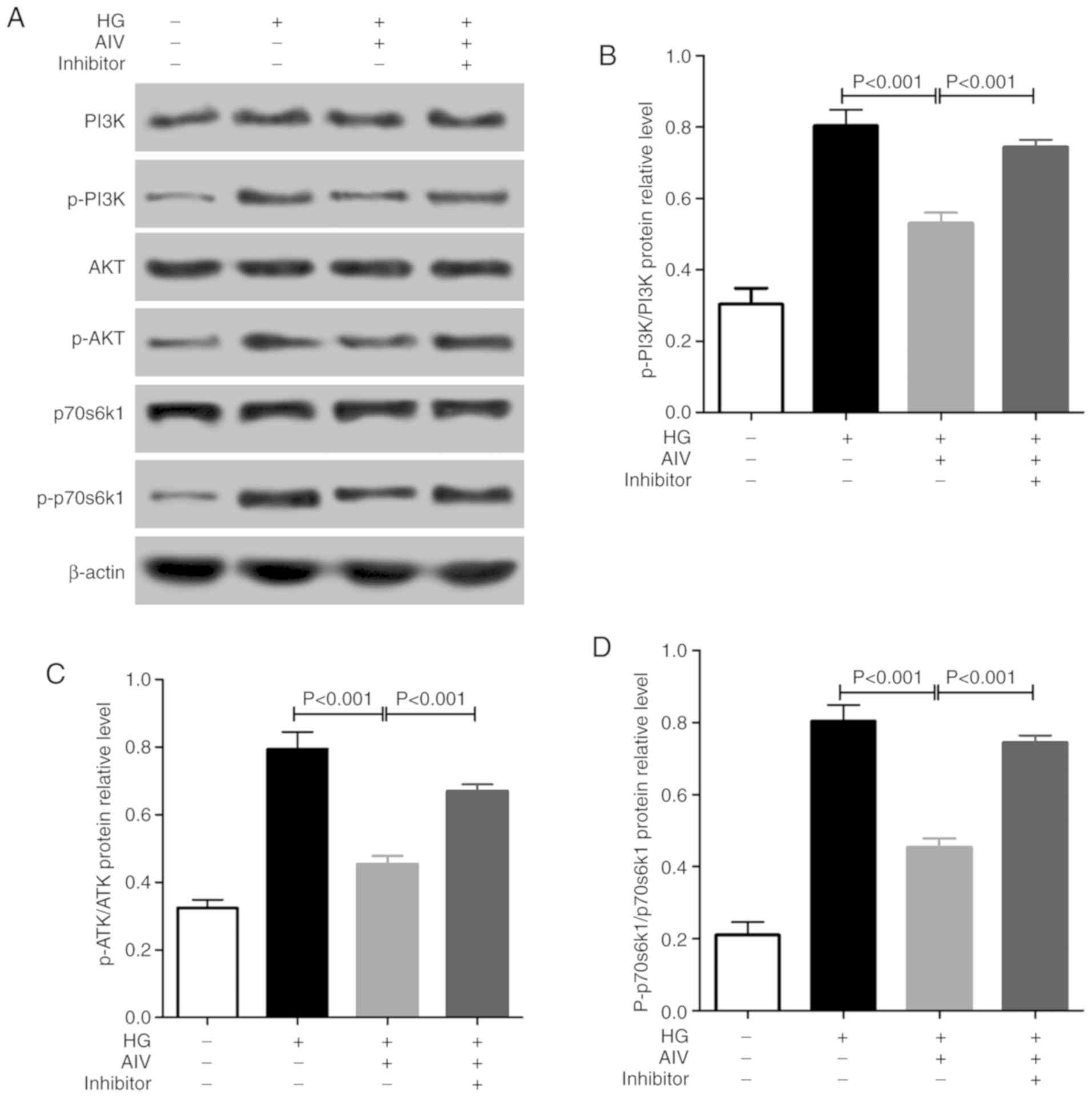 | Figure 6Effect of AIV on PI3K/AKT pathway in
rat RPE cells in vitro. (A-D) Western blot analysis was used
to detect the expression of p-PI3K, PI3K, p-AKT, AKT, p-p70s6k1 and
p70s6k1 protein; 3 independent experiments per test. Data are
expressed as the means ± SD of 3 individual experiments. AIV,
astragaloside-IV; DM, diabetes mellitus; RPE cells, retinal pigment
epithelial cells; HG, high glucose; miR-NC, miR-128-inhibitor
negative control; inhibitor, miR-128-inhibitor. |
Discussion
In the present study, it was first found that AIV
not only decreased the blood glucose levels, but also protected the
visual function of rats with DM. Previous studies have found that
AIV plays a role in lowering blood glucose levels in vivo
(16,18). Ding et al found that AIV
downregulated aldose reductase activity, prevented ERK1/2
phosphorylation and decreased the activity of cytokines, such as
nuclear factor (NF)-κB, and significantly decreased retinal
ganglion cell apoptosis in db/db mice with DR, relieved retinal
ganglion cell dysfunction, which proved that AS-IV exerted a
certain preventive effect against DR (16). However, the protective effects of
AIV against retinopathy have not been extensively studied and the
underlying molecular mechanisms remain unclear.
Under normal physiological conditions, RPE cells are
essential for maintaining normal retinal functions, such as the
absorption of scattered light, retinal pigmentation and synthesis,
the renewal and metabolism of photoreceptors, and the formation of
blood-retinal barriers. Therefore, they play an irreplaceable role
in maintaining visual function. The loss of any type of function of
RPE cells can lead to the degradation of the retina, which may
result in vision loss and even blindness. Chen et al
(19) found that the HG levels
induced a decrease in the permeability of ARPE-19 cells in
vitro, which induced mitochondrial dysfunction in ARPE-19 cells
by decreasing mitochondrial membrane potential and inhibiting Bcl-2
protein levels, which induced apoptosis by promoting the S0CS1 and
Fas/FasL signaling pathways. Crider et al (20) found that HG levels affected water
transport from the retina to the choroid by inducing a loss of
Na+-K+-ATPase function in bovine RPE cells.
In the present study, it ws found that AIV significantly attenuated
the apoptosis of REP cells from rats with DM. This indicated that
the protective effects of AIV on retinopathy in rats with DM may be
related to the inhibition of RPE cell apoptosis.
Although to the best of our knowledge, no previous
studies to date have demonstrated that AIV can protect RPE cells in
rats with DM, previous studies have found that AIV can inhibit
inflammatory and oxidative stress-induced apoptosis. Yin et
al (21) found that AIV
attenuated myocardial ischemia/reperfusion injury in rats via the
inhibition of calcium-sensing receptor-mediated apoptotic signaling
pathways, while Xiong et al (22) found that AIV inhibited guinea pig
cochlear impulse noise-induced apoptosis by inhibiting reactive
oxygen species (ROS) production. Apoptosis is a complex process
involving a number of different mechanisms, such as the
mitochondrial pathway, the Fas death receptor pathway and the
endoplasmic reticulum stress pathway (23). In the mitochondrial programmed
death pathway, cytochrome c released from the mitochondria
binds to the Apaf-1 protein in the cytoplasm to increase the
affinity of Apaf-1 to dATP/ATP, and then dATP/ATP binds to the
cytC/Apaf-1 complex to promote the oligomerization of the
cytC/Apaf-1 complex, thereby forming apoptosomes, which are able to
cause the auto-activation of caspase-3, caspase-8 and caspase-9,
resulting in chromosomal condensation, nuclear DNA fragmentation
and nuclear membrane rupture, which ultimately lead to cell death
(24,25). In the present study, it was found
that AIV significantly decreased the protein expression levels of
Bax/Bcl2, active caspase-3, active caspase-8 and active caspase-9
in RPE cells from rats with DM. Furthermore, it was found that AIV
significantly decreased the protein expression of Fas, and
significantly increased the expression of FasL and Fas/FasL. In the
Fas death receptor pathway, apoptotic signals induce the increased
expression of Fas, and apoptotic bodies are formed by FasL to
induce apoptosis (26,27). Therefore, these results suggested
that the apoptosis of RPE cells from rats with DM was regulated by
multiple pathways, including at least the mitochondrial apoptosis
pathway and the Fas death receptor pathway.
A previous study by the authors found that miR-128
attenuated the apoptosis induced by HG in RPE cells in vitro
(12). It was also found that
miR-128 expression was downregulated, whereas AIV treatment
significantly increased the expression of miR-128 and decreased the
expression levels of p-PI3K/PI3K, p-AKT/AKT and p-p70S6K1/p70S6K1
in RPE cells from rats with DM. This suggests that miR-128 may also
protect RPE cells in a HG environment in vivo and AIV
treatment may protect RPE cells by increasing the expression of
miR-128. The PI3K/Akt pathway is an important signaling pathway
that regulates cell survival and apoptosis (28), and has been found to be closely
related to mitochondria-mediated apoptosis pathway (29) and the Fas death receptor pathway
(19). In the PI3K/Akt pathway,
phosphatidylinositol-dependent kinase 1 (PDK1) binds to Akt to
phosphorylate Akt, which in turn activates p70S6K1, which
ultimately results in the inhibition of cell apoptosis (30,31). Therefore, AIV may be able to
protect RPE cells by increasing miR-128 expression via the PI3K/AKT
pathway in rats with DM.
In order to investigate whether miR-128 was the
target of AIV in RPE cells of DM rats, RPE cells were isolated from
healthy rats and miR-128 expression was knocked down by
transfection with an miR-128-inhibitor into RPE cells. It was found
that compared with normal RPE cells, the ratio of HG-induced
apoptotic RPE cells was significantly higher following the
knockdown of miR-128 expression by transfection with a
miR-128-inhibitor.
A limitation of the present study was that since no
miR-128 or PI3K/AKT knockout rats were used, the results obtained
from in vitro experiments need to be validated in
vivo.
In conclusion, the present study demonstrates that
treatment of rats with 60 mg/kg AIV may be able to inhibit the
apoptosis of RPE cells by upregulating the expression of miR-128 in
DM rats, and its specific molecular mechanisms may be related to
the PI3K/AKT-mediated mitochondrial apoptosis pathway and the Fas
death receptor pathway. Therefore, our study identified 60 mg/kg
AIV as a potential drug for the prevention and treatment of
diabetic retinopathy. However, the limitations of the present study
should be noted and further studies on miR-128 knockout in rats may
aid in the identification of the specific molecular mechanism of
action of AIV.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CS was involved in the conception and design of the
study. TW and ZZ analyzed the experimental data. LS, XS, QQ and JL
performed the experiments. TW, ZZ and CS were the major
contributors in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments performed in the present
study were approved and supervised by the Animal Care and Use
Committee of Weihai Municipal Hospital, and conformed with
guidelines of the National Institution of Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Frank RN: Diabetic retinopathy. N Engl J
Med. 350:48–58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roglic G: WHO Global report on diabetes: A
summary. Int J Noncommun Dis. 1:3–8. 2016. View Article : Google Scholar
|
|
3
|
Zheng Y, He M and Congdon N: The worldwide
epidemic of diabetic retinopathy. Indian J Ophthalmol. 60:428–431.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simó R, Villarroel M, Corraliza L,
Hernández C and Garcia-Ramírez M: The retinal pigment epithelium:
Something more than a constituent of the blood-retinal
barrier-implications for the pathogenesis of diabetic retinopathy.
J Biomed Biotechnol. 2010:1907242010. View Article : Google Scholar
|
|
5
|
Chiba C: The retinal pigment epithelium:
An important player of retinal disorders and regeneration. Exp Eye
Res. 123:107–114. 2014. View Article : Google Scholar
|
|
6
|
Spekker-Bosker K, Ufermann CM, Oldenburg
M, Däubener W and Eller SK: Interplay between IDO1 and iNOS in
human retinal pigment epithelial cells. Med Microbiol Immunol.
208:811–824. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsao YP, Ho TC, Chen SL and Cheng HC:
Pigment epithelium-derived factor inhibits oxidative stress-induced
cell death by activation of extracellular signal-regulated kinases
in cultured retinal pigment epithelial cells. Life Sci. 79:545–550.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seagle BL, Gasyna EM, Mieler WF and Norris
JR Jr: Photoprotection of human retinal pigment epithelium cells
against blue light-induced apoptosis by melanin free radicals from
Sepia officinalis. Proc Natl Acad Sci USA. 103:16644–16648. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steindl K and Binder S: Retinal
degeneration processes and transplantation of retinal pigment
epithelial cells: Past, present and future trends. Spektrum der
Augenheilkunde. 22:357–361. 2008. View Article : Google Scholar
|
|
10
|
Ren S, Zhang H, Mu Y, Sun M and Liu P:
Pharmacological effects of Astragaloside IV: A literature review. J
Tradit Chin Med. 33:413–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lia L, Houb X, Xub R, Liua C and Tub M:
Research review on the pharmacological effects of astragaloside IV.
Fundam Clin Pharmacol. 31:17–36. 2017. View Article : Google Scholar
|
|
12
|
Zhang Z, Sun J, Hu Y, Song C and Wu X:
miR-128 protects retinal pigment epithelium in high glucose through
HOXB3/PI3K/ERK-mTOR pathway. Int J Clin Exp Med. 9:1684–1691.
2016.
|
|
13
|
Chakraborty C, George Priya Doss C and
Bandyopadhyay S: miRNAs in insulin resistance and
diabetes-associated pancreatic cancer: The 'minute and miracle'
molecule moving as a monitor in the 'genomic galaxy'. Curr Drug
Targets. 14:1110–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chakraborty C, Doss CG, Bandyopadhyay S
and Agoramoorthy G: Influence of miRNA in insulin signaling pathway
and insulin resistance: Micro-molecules with a major role in type-2
diabetes. Wiley Interdiscip Rev RNA. 5:697–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ciccacci C, Morganti R, Di Fusco D,
D'Amato C, Cacciotti L, Greco C, Rufini S, Novelli G, Sangiuolo F,
Marfia GA, et al: Common polymorphisms in MIR146a, MIR128a and
MIR27a genes contribute to neuropathy susceptibility in type 2
diabetes. Acta Diabetol. 51:663–671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding Y, Yuan S, Liu X, Mao P, Zhao C,
Huang Q, Zhang R, Fang Y, Song Q, Yuan D, et al: Protective effects
of astragalo-side IV on db/db mice with diabetic retinopathy. PLoS
One. 9:e1122072014. View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Gui D, Huang J, Guo Y, Chen J, Chen Y,
Xiao W, Liu X and Wang N: Astragaloside IV ameliorates renal injury
in streptozotocin-induced diabetic rats through inhibiting
NF-κB-mediated inflammatory genes expression. Cytokine. 61:970–977.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen M, Wang W, Ma J, Ye P and Wang K:
High glucose induces mitochondrial dysfunction and apoptosis in
human retinal pigment epithelium cells via promoting SOCS1 and
Fas/FasL signaling. Cytokine. 78:94–102. 2016. View Article : Google Scholar
|
|
20
|
Crider JY, Yorio T, Sharif NA and Griffin
BW: The effects of elevated glucose on Na+/K(+)-ATPase of cultured
bovine retinal pigment epithelial cells measured by a new
nonradioactive rubidium uptake assay. J Ocul Pharmacol Ther.
13:337–352. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yin B, Hou XW and Lu ML: Astragaloside IV
attenuates myocardial ischemia/reperfusion injury in rats via
inhibition of calcium-sensing receptor-mediated apoptotic signaling
pathways. Acta Pharmacol Sin. 40:599–607. 2019. View Article : Google Scholar :
|
|
22
|
Xiong M, He Q, Lai H and Wang J:
Astragaloside IV inhibits apoptotic cell death in the guinea pig
cochlea exposed to impulse noise. Acta Otolaryngol. 132:467–474.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ugarte-Uribe B and García-Sáez AJ:
Apoptotic foci at mitochondria: In and around Bax pores. Philos
Trans R Soc Lond B Biol Sci. 372:pii: 20160217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Olson M and Kornbluth S: Mitochondria in
apoptosis and human disease. Curr Mol Med. 1:91–122. 2001.
View Article : Google Scholar
|
|
26
|
Stel AJ, Ten Cate B, Jacobs S, Kok JW,
Spierings DC, Dondorff M, Helfrich W, Kluin-Nelemans HC, de Leij
LF, Withoff S and Kroesen BJ: Fas receptor clustering and
involvement of the death receptor pathway in rituximab-mediated
apoptosis with concomitant sensitization of lymphoma B cells to
fas-induced apoptosis. J Immunol. 178:2287–2295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagata S and Golstein P: The Fas death
factor. Science. 267:1449–1456. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: Size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsuruta F, Masuyama N and Gotoh Y: The
phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax
translocation to mitochondria. J Biol Chem. 277:14040–14047. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao P, Meng Q, Liu LZ, You YP, Liu N and
Jiang BH: Regulation of survivin by PI3K/Akt/p70S6K1 pathway.
Biochem Biophys Res Commun. 395:219–224. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chai X, Sun D, Han Q, Yi L, Wu Y and Liu
X: Hypoxia induces pulmonary arterial fibroblast proliferation,
migration, differentiation and vascular remodeling via the
PI3K/Akt/p70S6K signaling pathway. Int J Mol Med. 41:2461–2472.
2018.PubMed/NCBI
|