Introduction
Inflammatory bowel disease (IBD) is a chronic
non-specific inflammatory disease that occurs in the
gastrointestinal tract, which includes ulcerative colitis (UC) and
Crohn's disease (1). The
pathogenesis of IBD has not yet been fully elucidated, however, it
is currently understood that intestinal mucosal immune overreaction
and dysfunction are the main causes (2). Current strategies for treating IBD
include anti-inflammatory drugs and immunomodulators (3). Hartnett and Egan (4) has shown that patients with IBD have
a lifetime risk of colorectal cancer that is two to three times
higher than the general population. Molodecky et al
(5) reported an increasing
incidence and prevalence of the inflammatory bowel diseases with
age. Ramos and Papadakis (6)
found that inflammatory bowel diseases were associated with
microbial dysbiosis. The number of methylated genes in
non-neoplastic colonic mucosa could predict colorectal cancer (CRC)
with good accuracy for both non-inflammatory and
inflammatory-related CRC (7). DNA
methylation is an epigenetic change that occurs on the cytosine of
genomic CpG dinucleotides and plays an important role in the
regulation of IBD gene expression (8). Covalent methylation of DNA CpG
islands is catalyzed by methyltransferases, which methylate C-5 of
cytosine nucleotides (9). The
global cytosine methylation pattern in the mammalian genome appears
to be established by the complex interaction of at least three
independently encoded DNA methyltransferases (DNMT): DNMT1, DNMT3A
and DNMT3B (1). The expression
levels of DNMT1 and DNMT3A are significantly increased in
UC-related carcinogenesis compared with non-inflammatory colorectal
carcinogenesis (10).
The DNMT inhibitor decitabine
(5-aza-2′-deoxycytidine) is a 5-azacytidine deoxyribose analog and
is currently used to treat hematological malignancies, including
myelodysplastic syndrome, acute myeloid leukemia and chronic
myelomonocytic leukemia (10).
Decitabine promotes the reduction of DNA methylation and induces
gene expression and differentiation. Decitabine exhibits
immunomodulatory potential both in vitro and in vivo
and induces demethylation of the forkhead box P3 (Foxp3) gene
(11).
To mimic human IBD, the present study established an
experimental colitis model by administering drinking water with 3%
dextran sulfate sodium (DSS) to BALB/c mice for 7 consecutive days.
The effect of the methyltransferase inhibitor decitabine on the
intestinal barrier function of mice with IBD and its potential
mechanism was investigated, and a theoretical basis for clinical
induction of immune tolerance in the treatment of IBD was
provided.
Materials and methods
Animal modeling
A total of 24 six-week-old male BALB/c mice were
purchased from Shanghai Jiesijie Experimental Animal Co., Ltd. Mice
were raised with a constant temperature of 23±2̊C, 50% humidity, a
12/12 h light/dark cycle and ad libitum access to food and
water. The animal protocol in this work was in accordance with
guidelines for the care and use of laboratory animals authorized by
the Medical Ethics Committee of Minhang Hospital, Shanghai, China.
The animal research was approved by the Ethics Committee of Minhang
Hospital, Shanghai, China [Medical Ethics Committee (2018) Approval
no. 2]. A total of 24 mice were randomly divided into four groups
(n=6 per group). Three groups of mice were used as experimental
colitis models and one group of mice served as normal controls. An
experimental colitis model was established by supplementing BALB/c
mice with 3% (w/v) DSS (Sigma-Aldrich; Merck KGaA) in drinking
water once a day for 7 days. On the 8th day, the decitabine,
sulfasalazine (SASP) positive control and model groups were
intraperitoneally administered with decitabine (Merck KGaA; cat.
no. 2353-33-5; 0.5 mg/kg), SASP (Merck KGaA; cat. no. 599-79-1; 100
mg/kg) and 1% DMSO (1 μl/20 g), respectively, for 7
consecutive days. Mice were injected twice a day at an interval of
12 h. During the experiment, no mice died unexpectedly. From the
day of treating with DSS changes in body weight, external
characteristics and clinical symptoms of the mice were monitored
every day. The combination of these indicators can reflect the pain
and suffering experienced by the animals during the experiment
(12). Mice were euthanized with
an intraperitoneal injection of 180 mg/kg sodium pentobarbital, and
then the heartbeat of the mice was examined. The duration of animal
experiments was 2 weeks from the first day of treatment with DSS to
euthanasia.
Mouse disease activity index score
Mice of each group were examined daily for body
mass, diarrhea and blood in the stool. Disease activity index (DAI)
was calculated as follows: DAI = (weight loss score + fecal trait
score + fecal occult blood score)/3) (13).
Gross morphological examination of colon
tissue
After the animals were sacrificed on the 8th day,
the entire colonic intestine from the anus to the end of the cecum
was isolated. Following washing, the extent of inflammation and
ulceration was assessed and the length of the colon was recorded.
Colon gross morphological damage index (CMDI) score was calculated
as reported previously (1).
Hematoxylin and eosin (H&E)
staining
Distal colon tissue (1 cm) was fixed in 4%
paraformaldehyde for 24 h at room temperature, embedded in
paraffin, sectioned to a thickness of 8-μm, and stained with
H&E for 1 min at room temperature. Histopathological changes
were observed under a light microscope (magnification, ×20 or ×40)
and scored according to the criteria described by Andújar et
al (14). The colon
histopathology index (CHPI) was determined as previously described
(15).
ELISA
Since the effect of DSS is more pronounced at the
distal end compared with the proximal colon (16), small sections (~1 cm) of excised
distal colonic tissue were collected for ELISA and western blot
assays. Subsequently, ~10 mg colon tissue of each group was
collected and the tissue was homogenized with 1 ml PBS (pH, 6.0;
containing 1 μg aprotinin (Shanghai Qcbio Science &
Technologies Co. Ltd.; cat. no. 20105ES08) and 1 μg
leupeptin pepstatin A (Maokangbio Co. Ltd.; cat. no. 103476-89-7).
After homogenate liquid was centrifuged at 16,099 × g for 20 min at
4°C, 500 μl supernatant was collected for ELISA. ELISA kits
for IL-17 (Boster Biological Technology; cat. no. EK0431), TGF-β
(Beijing Solarbio Science & Technology Co., Ltd.; cat. no.
SEKM-0035) and IL-10 (Boster Biological Technology; cat. no.
EK0417) were used to measure the cytokine levels, according to the
manufacturer's instructions.
Flow cytometry analysis
The spleens of the mice were cut, filtered through a
200-μm nylon mesh, centrifuged at 251.55 × g for 5 min at
4°C, and then the supernatant was removed. NH4Cl (2 ml;
0.83%) was added to lyse the red blood cells. After 2 min, 15 ml
PBS was added to terminate the lysis. The cell density was adjusted
to 1×106 cells/ml in PBS and the cells were blocked with
2.5% BSA at room temperature for 1 h. Subsequently, the cells were
incubated with FITC-rat anti-mouse CD4 (cat. no RM4-5; eBioscience;
Thermo Fisher Scientific, Inc.) in 2.5% BSA for 1 h at room
temperature in the dark and washed twice with PBS. Cells were then
fixed with fixation reagent (cat. no. 00-5523-00; eBioscience;
Thermo Fisher Scientific, Inc.) for 15 min at room temperature and
permeabilized for intracellular staining. Subsequently, 1 ml of a
3:1 permeabilization reagent (cat. no. 00-5523-00; eBioscience;
Thermo Fisher Scientific, Inc.) was added, and the cells were
incubated at 4°C for 1 h in the dark, followed by the addition of
cell permeabilization buffer (2 ml; cat. no. 39487s; Cell Signaling
Technology, Inc.), centrifugation twice, and the resuspension of
cells to a volume of 100 μl. PE-rat anti-mouse Foxp3 (cat.
no. 72-5775-40; eBioscience; Thermo Fisher Scientific, Inc.) in
2.5% BSA was added and incubated for 1 h at 4°C in the dark. Cell
phenotypes were detected using a flow cytometer and the data were
analyzed using FlowJo software (FlowJo LLC; version 7.6.1).
Immunofluorescence
Sections were blocked in 5% BSA for 1 h at room
temperature and were stained with anti-mouse antibodies against
zonular occludens-1 (1:1,000; ZO-1; Novus Biologicals, Ltd.; cat.
no. NBP1-85047) and occludin (1:200; Abcam; cat. no. ab216327) in
2.5% BSA for overnight at 4°C, and then with the secondary antibody
goat anti-rabbit IgG (H+L) with a FITC fluorescent label (1:1,000;
GeneCopoeia; cat. no. L147A) or Cy-3 tag (1:1,000; Boster
Biological Technology; cat. no. BA1032) in 2.5% BSA for 2 h at room
temperature. Sections were examined under a fluorescence microscope
(magnification, ×20). Quantitative analysis was conducted using
Image J software (National Institutes of Health; version 1.46).
Immunohistochemistry
ZO-1 and occludin anti-mouse antibodies were diluted
with PBS at a ratio of 1:100; and PBS was used as a negative
control for overnight at 4°C. Subsequently, eight fields of view
were randomly observed under a fluorescence microscope
(magnification, ×40), and the percentage of positive cells and the
intensity of staining was scored. The percentage of stained cells
in the field of view was scored as follows: <5%, 0 point; 5-25%,
1 point; 26-50%, 2 points; 51-75%, 3 points; and >75%, 4 points.
The staining intensity was scored as follows: No staining, 0 point;
light yellow staining, 1 point; brown staining, 2 points; and tan
staining, 3 points. The two scores were then multiplied to
calculate the immunoreactivity score.
Western blotting
For western blotting, ~100 mg distal colonic tissue
was weighed and cut into pieces. The tissue was then grinded with
liquid nitrogen and added to 1 ml ice-cold PBS in a round bottom
flask. The tissue was homogenized at a speed of 90.56 × g and
transferred to a 1.5 ml EP tube. The homogenate liquid was washed
twice with PBS containing PMSF (Beijing Solarbio Science &
Technology, Co., Ltd.) and then centrifuged at 16,099 × g for 5 min
at 4°C. The supernatant was removed and 500 μl protein lysis
buffer (Beyotime Institute of Biotechnology; cat. no. P0013) was
added. After the tissue was lysed on ice for 2 h, it was
centrifuged at 16,099 × g for 30 min at 4°C. The supernatant was
collected and mixed with 5X loading buffer (Beyotime Institute of
Biotechnology) and then the protein was denatured at 100̊C for 10
min. Protein concentration was detected using a BCA kit (Beyotime
Institute of Biotechnology) and 30 μg protein was separated
on a 10% SDS-PAGE gel before being transferred to PVDF membranes
(Thermo Fisher Scientific, Inc.). Membranes were blocked with 4%
BSA-TBST for 90 min and probed overnight at 4°C with primary
antibodies in 5% BSA against the following ZO-1 (1:2,500; Novus
Biologicals, Ltd.; cat. no. NBP1-85047), occludin (1:1,000; Abcam;
cat. no ab216327), ERK1/2 (1:1,000; Abways Technology; cat. no.
CY1066), phosphorylated (p)-Erk1/2 (1:1,000; Abways Technology;
cat. no. CY6190), JNK (1:1,000; Abways Technology; cat. no.
AB3296), p-JNK (1:1,000; Abways Technology; cat. no. CY6315), p38
(1:1,000; Abways Technology; cat. no. AB3374) and p-p38 (1:1,000;
Abways Technology; cat. no. CY6390). GAPDH (1:1,000; Santa Cruz
Biotechnology, Inc.; cat. no. sc-166574) was used as a loading
control. Then, the membranes were incubated with an anti-rabbit HRP
secondary antibody (1:1,000; Jackson ImmunoResearch Laboratories,
Inc.; cat. no. 111-005-003) for 2 h at room temperature and bands
were visualized using super ECL detection reagent (Shanghai Yeasen
Biotechnology, Co., Ltd.; cat. no. 36208ES60). Quantitative
analysis was conducted using Image J software (National Institutes
of Health; version 1.46), and the ratio of phosphorylated
protein/total protein was evaluated.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 software (GraphPad Software, Inc.). The data are
presented as the mean ± standard deviation. Significant differences
for between multiple groups were analyzed by one-way ANOVA followed
by Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference. Each experiment was repeated
a minimum of three times.
Results
Effects of decitabine treatment on
intestinal pathology and histopathology in mice
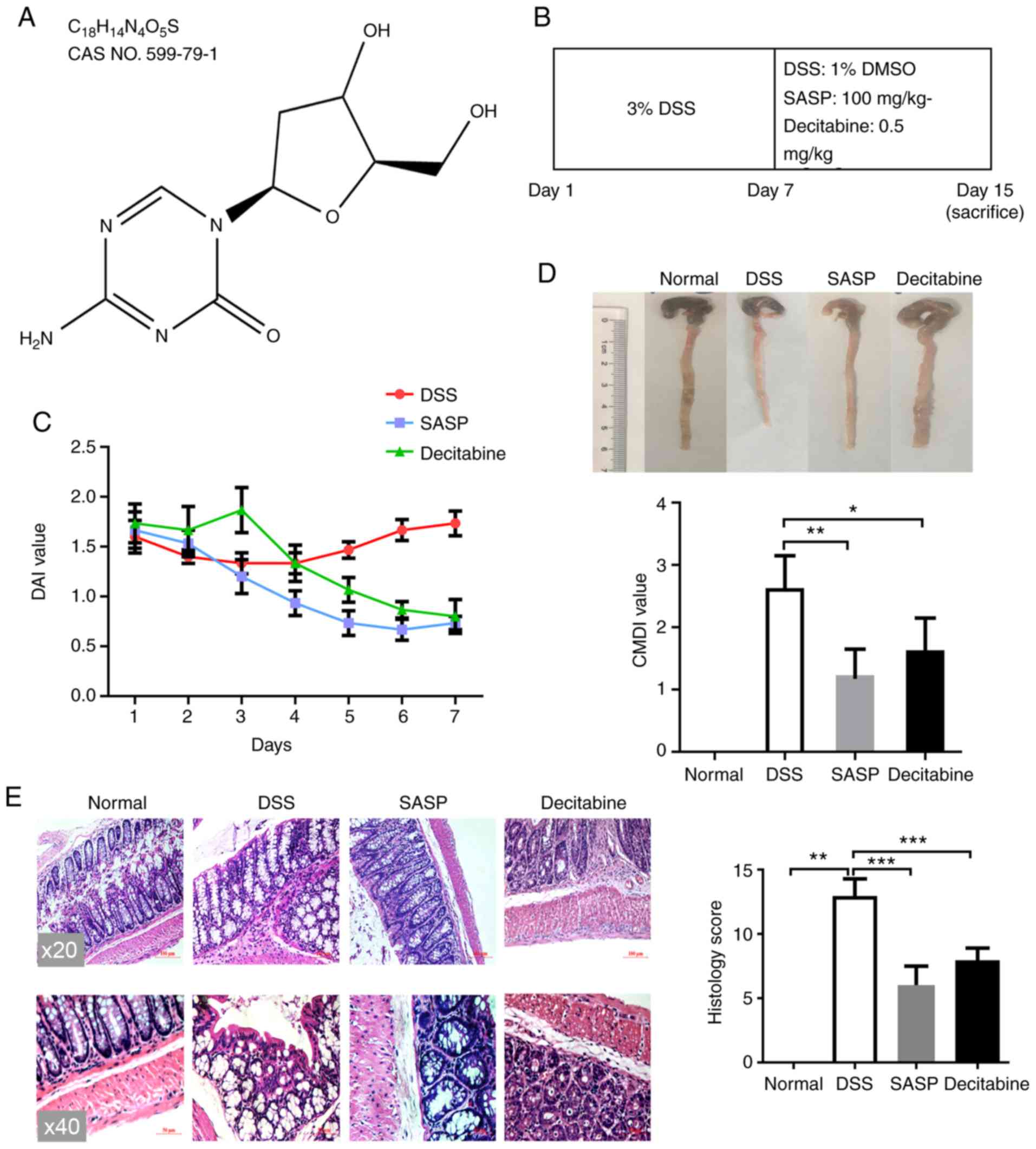
Decitabine, also known as 5-aza-2′-deoxycytidine, is
a natural adenosine analog of 2′-deoxycytidine (17), and its structure is presented in
Fig. 1A. Apart from the normal
control group, all other BALB/c mice were given 3% DSS for 7
consecutive days to establish an experimental colitis model. On the
8th day, decitabine (0.5 mg/kg), SASP (100 mg/kg) or placebo (1%
DMSO) were intraperitoneally injected for a further 7 consecutive
days. The recommended clinical dose of decitabine is 15
mg/m2 (18). The
Meeh-Rubner formula (19)
calculates the body surface area of mice. The body surface area of
20 g mice is ~0.67 dm2 (20). Thus, the mice were
intraperitoneally injected with decitabine at a dose of 5 mg/kg,
which was only used in the preliminary experiments. A total of one
week after the first treatment with decitabine, two mice in the
decitabine group died; therefore, the administration concentration
was reduced by ten times to 0.5 mg/kg (Fig. 1B).
The DAI was used to evaluate the therapeutic effect
of decitabine on DSS-induced IBD. No mice died during the modeling
process. After 24 h of modeling, which was after the animals were
treated with DSS, the animals developed symptoms such as loose
stools, severe perianal contamination, reduced food intake and
weight loss. During the whole administration period, the DAI of the
normal control group was 0. Following the last day of DSS
administration, the mice in the model group slightly recovered over
the following three days but then their condition began to decline
on the fourth day; they ate and drank less, the body weight
declined and they had loose stools with mucus and pus. In the SASP
group, the general condition of the mice gradually improved, and
the DAI score gradually decreased. After 7 days of treatment with
SASP, the symptoms of IBD were relieved and the mean DAI score was
only 0.73 points. On day 4 of treatment with decitabine, food
intake increased and the extent of loose stools was reduced.
Compared with the model group, the body weight was increased in the
decitabine group. After 7 days of treatment with decitabine, the
mean average DAI score reached 0.8. (Fig. 1C).
From the results of colon length measurement, the
colon length of the model group was shorter than that of the normal
control group. The shortening of the colon length in mice was
relieved in the SASP and decitabine groups. The general morphology
of the colon and the CMDI score demonstrated that the normal
control group had a smooth colon surface and a clear texture. In
the DSS alone group, the surface of the intestine was uneven, and
the phenomenon of inflammatory congestion and edema. In the model
group, severe inflammatory hyperemia and edema were observed in the
colon and the CMDI score was significantly higher compared the
normal control group (P<0.01). Local hyperemia and edema were
observed in the decitabine intervention group, but mainly
concentrated in the distal colon, and the CMDI score was
significantly lower compared with in the model group (P<0.05;
Fig. 1D).
Histopathological observation and the CHPI score
demonstrated that there was no obvious inflammatory cell
infiltration in the normal control group, and the intact mucosal
epithelium was well arranged. In the model group, the colonic
mucosa was exfoliated, the gland structure was severely damaged,
the gland was disordered and a large number of lymphocytes and
neutrophils were infiltrated, and the CHPI score was significantly
higher compared with the normal group (P<0.01). The infiltration
of inflammatory cells, hyperplasia and edema in was alleviated in
the SASP group, and the CHPI score was significantly reduced
compared with the model group (P<0.001). In the decitabine
group, the intestinal mucosa of the mice was intact, inflammatory
cell infiltration was low and the CHPI score was significantly
decreased compared with the model group (P<0.001; Fig. 1E).
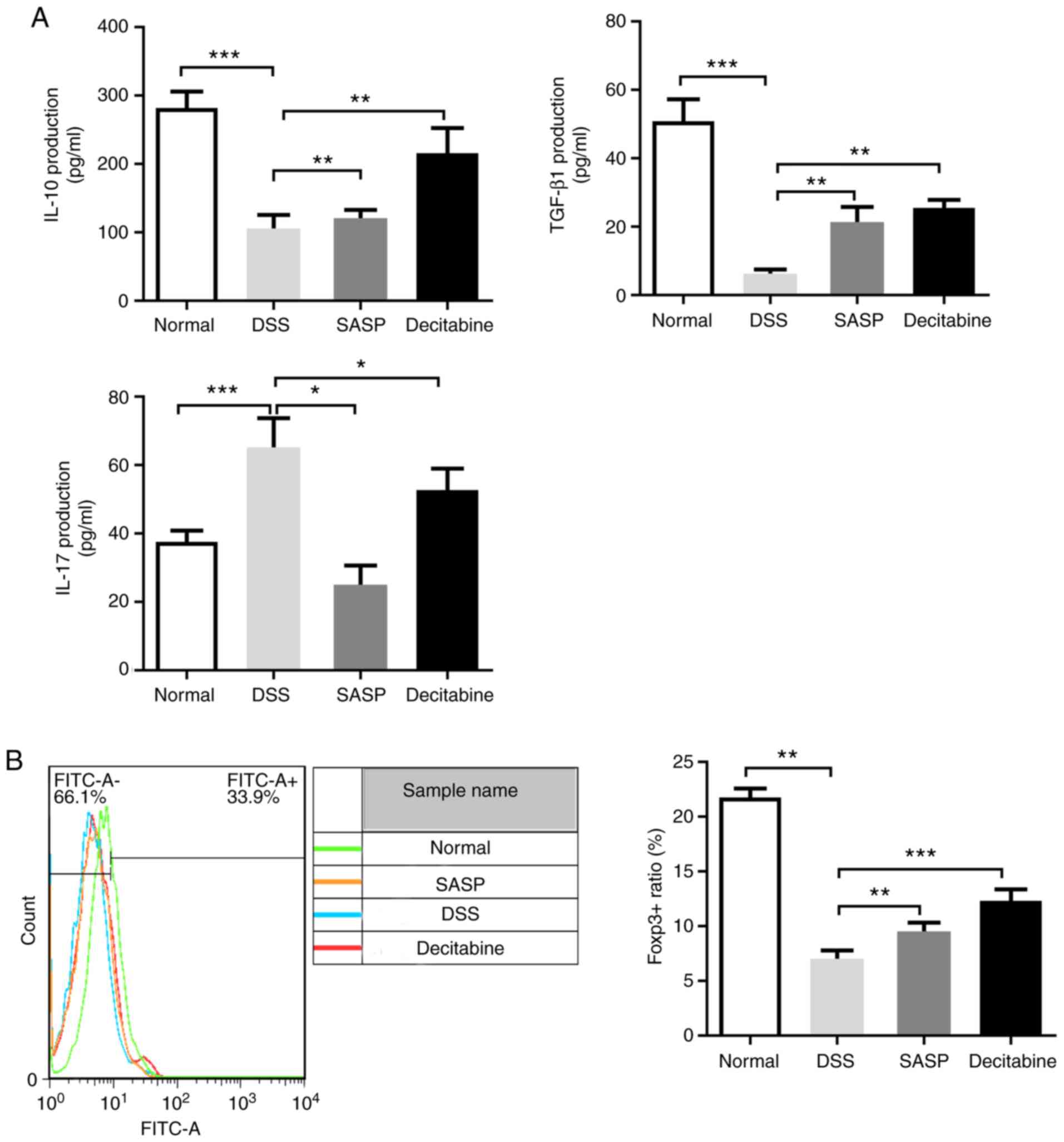
Expression of regulatory T cell
(Treg)-associated cytokines IL-17, TGF-β and IL-10 in mice colon
tissue
Tregs are important for the homeostasis of the
immune system (21).
CD4+ CD25+ Tregs are a subset of Tregs that
maintain immune tolerance through direct contact with effector T
cells and secretion of TGF-β, IL-10 and other cytokines (22). Compared with the normal control
group, the level of cytokine IL-17 in the colon tissue of the model
group was significantly increased, while the levels of cytokines
IL-10 and TGF-β were significantly decreased (P<0.001). Compared
with the model group, the level of IL-17 in the SASP group was
significantly lower (P<0.05) and the IL-10 level was
significantly increased (P<0.01). Compared with the model group,
the TGF-β levels were significantly increased (P<0.001). There
was a significant increase of IL-10 in the decitabine group
compared with the model group (P<0.01; Fig. 2A).
In the present study, Foxp3 expression in the spleen
of the model group was significantly decreased compared with the
normal control group (P<0.01), while treatments with SASP or
decitabine resulted in significant increases in Foxp3 expression
(P<0.01 and P<0.001, respectively; Fig. 2B).
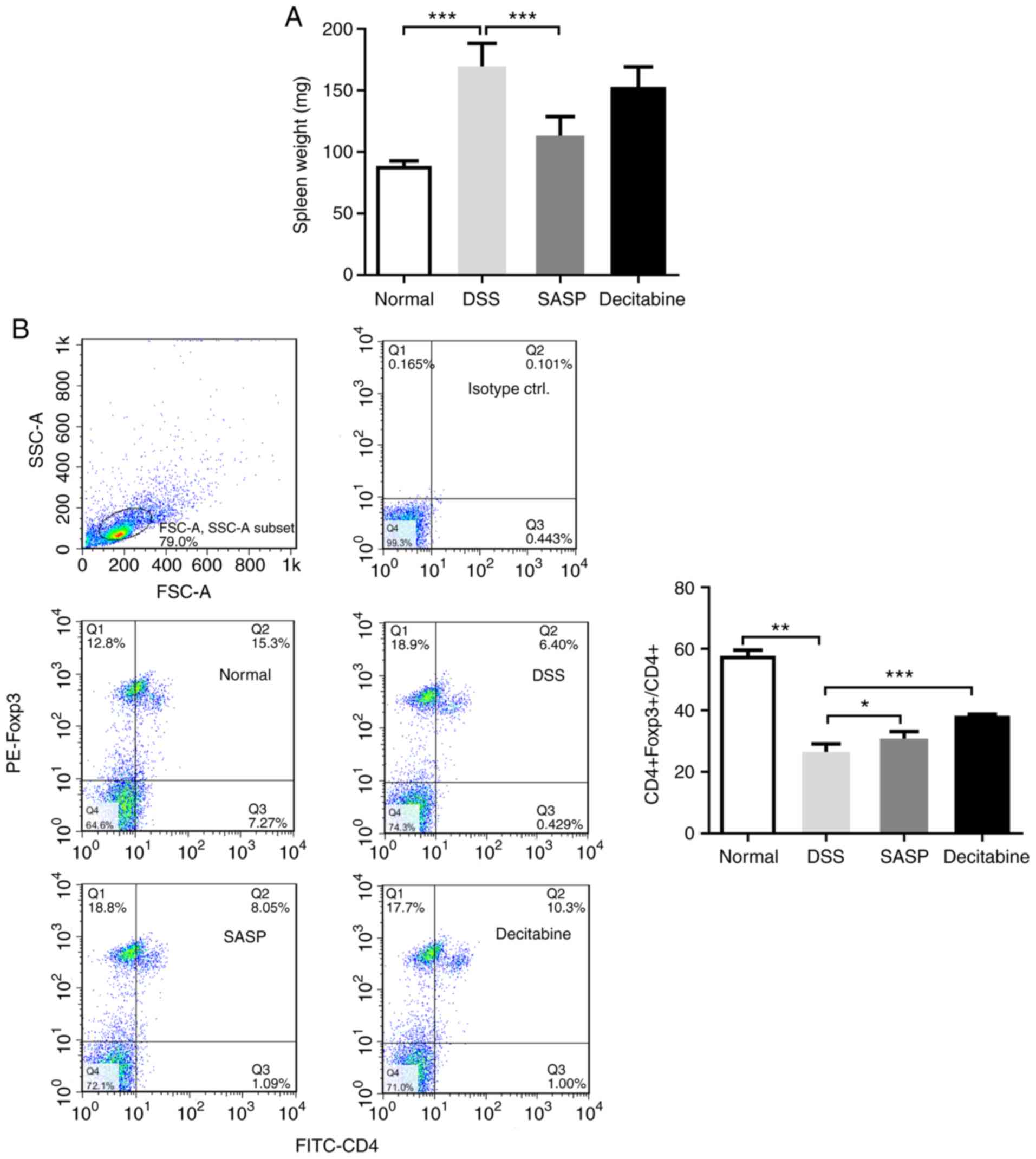
Decitabine treatment increases the
proportion of CD4+ Foxp3+ T cells in
CD4+ T cells
DSS can promote edema and inflammation, and
significantly increases the weight of mice spleens (P<0.001)
(23). SASP reduced the weight of
the mice spleens compared with the model group (P<0.001). In
addition. decitabine reduced the weight of the mice spleen, but it
was not statistically significant compared with the model group
(Fig. 3A).
Compared with the normal control group, DSS
significantly reduced the proportion of CD4+
Foxp3+ T cells in CD4+ T cells in the spleen
of mice (P<0.01). Compared with the model group, the proportion
of CD4+ Foxp3+ T cells in CD4+ T
cells was significantly increased following 7 days of
intraperitoneal injection of decitabine (P<0.001; Fig. 3B). This indicates that
intraperitoneal injection of decitabine can promote the
re-expression of Foxp3 in naive T cells of mice spleen, and
increase the proportion of CD4+ Foxp3+ T
cells in CD4+ T cells, suggesting that the use of
methyltransferase inhibitors in vivo can play a role in
demethylation and amplify Tregs.
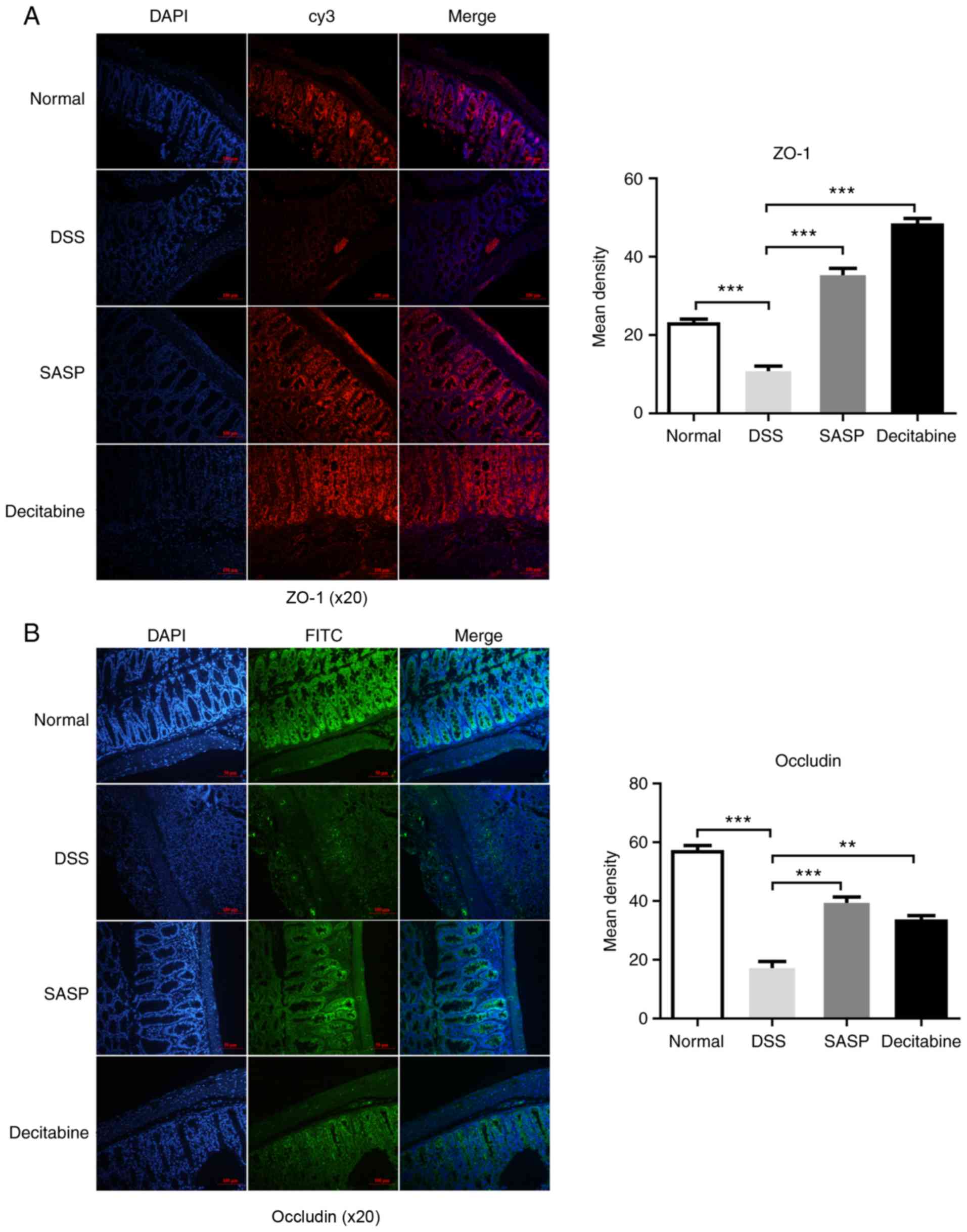
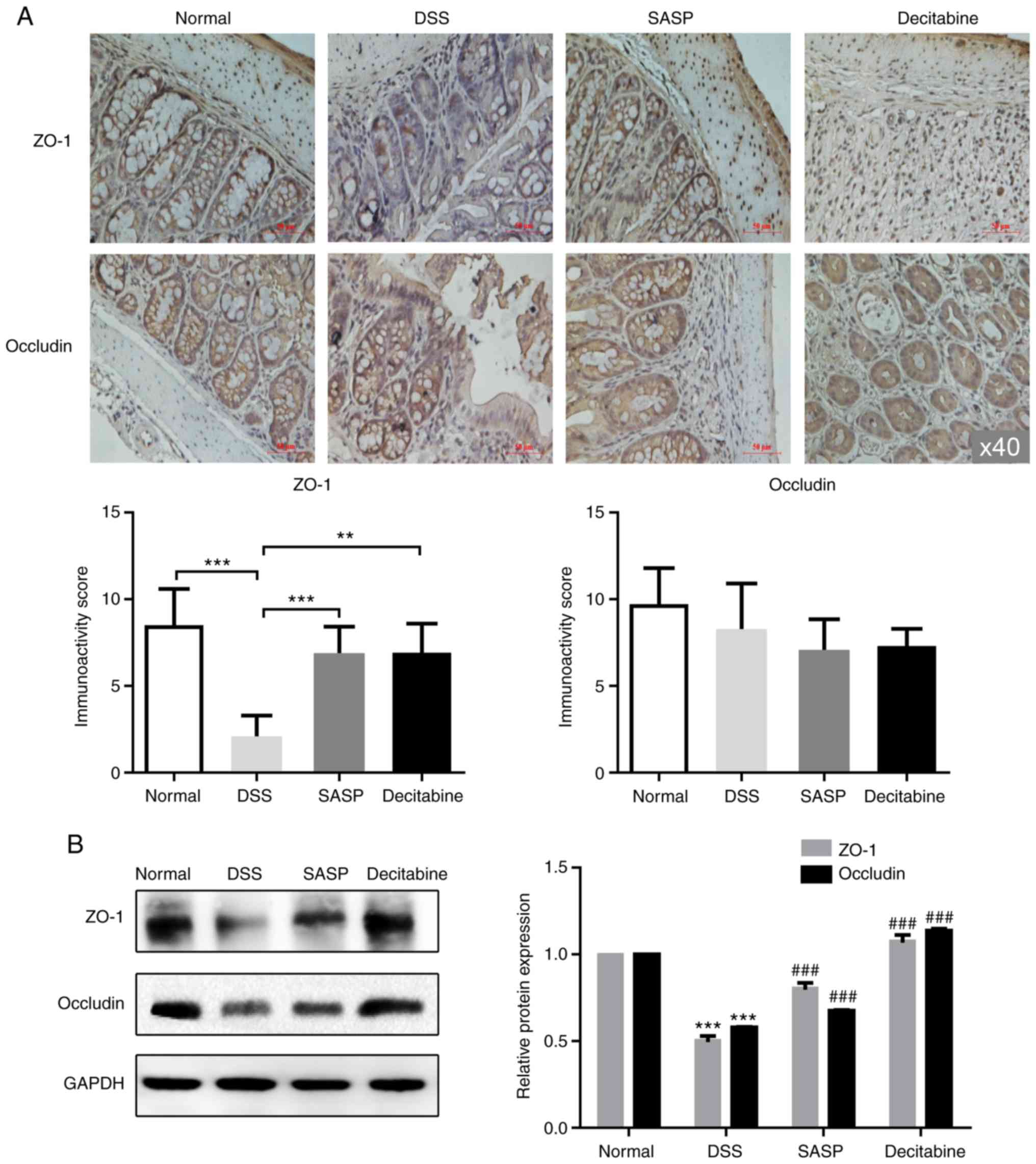
Expression and distribution of ZO-1 and
occludin proteins in the colon tissue of mice
After the mice were sacrificed, the fluorescence
staining of ZO-1 and occludin in the colonic mucosa of the normal
control group was continuously distributed at the junction of the
colon cells, and the edges were smooth and the fluorescence scope
was wide. The mice in the model group demonstrated both a decreased
fluorescence intensity and discontinuity distribution of tight
junction proteins in the cell apical membrane. Compared with the
model group, the tight junction proteins in SASP group and
decitabine group were continuously distributed in the apical
membrane of intestinal epithelial cells, the edges were smooth, and
the fluorescence area and intensity were increased (Fig. 4A and B). The immunohistochemistry
demonstrated that the intestinal epithelial structure of the normal
control group was intact, while in the model group the tight
junction structure and microvilli were destroyed; the gap between
cells was widened and the cells were vacuolated. As observed by
immunofluorescence, the expression of ZO-1 was significantly lower
in the model group compared with the normal control group, and
significantly higher in the SASP (P<0.001) and decitabine groups
(P<0.001) compared with the model group (Fig. 4A). As observed by
immunofluorescence, the expression of Occludin was significantly
lower in the model group compared with the normal control group,
and significantly higher in the SASP (P<0.001) and decitabine
groups (P<0.01) compared with the model group (Fig. 4B). However, differences in
occludin expression among all groups were not statistically
significant (Fig. 5A). The levels
of ZO-1 and occludin in colon tissue of each group were detected by
western blotting. The results demonstrated that the levels of ZO-1
and occludin in the model group were significantly lower compared
with those in the normal control group (P<0.001), indicating
impaired colon barrier function. Compared with the model group, the
expression levels of ZO-1 and occludin were significantly higher in
the SASP and decitabine groups (P<0.001; Fig. 5B).
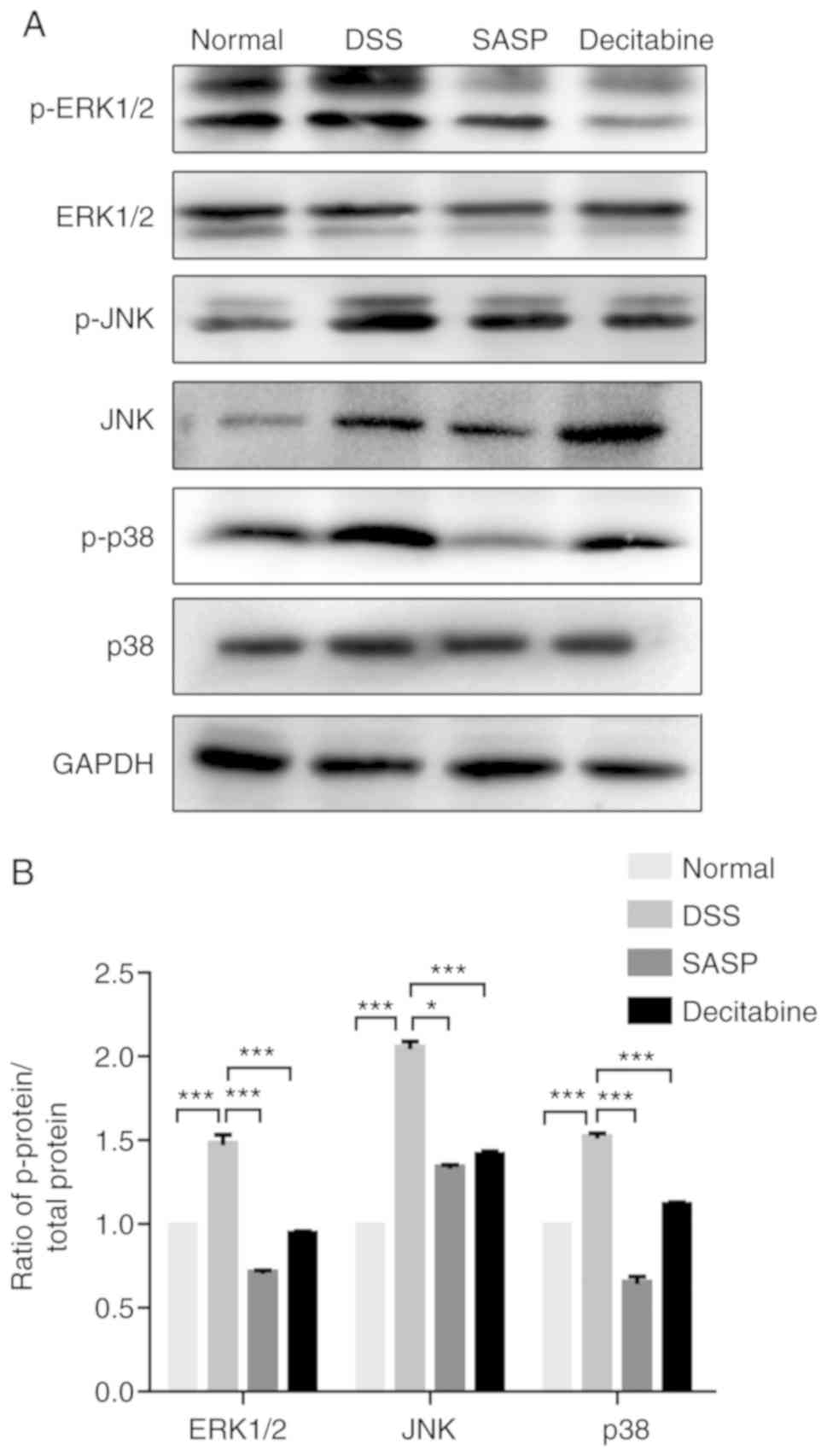
Effect of decitabine on DSS-induced MAPK
signaling pathway in colonic tissues of mice
The expression levels of ERK1/2, JNK and p38 are
presented in Fig. 6A. MAPK
signaling is an important signaling system that utilizes a
step-by-step phosphorylation process to amplify signals into the
nucleus, regulate the activity of transcription factors and the
expression of corresponding genes, and then cause cellular
responses (24). It was
determined that decitabine not only affected the expression of
ERK1/2, JNK and P38 in the MAPK pathway, but also p-ERK1/2, p-JNK
and p-p38. The phosphorylation of ERK1/2, JNK and p38 in each group
is presented in Fig. 6B. Compared
with the normal control group, the phosphorylation of ERK1/2, JNK
and p38 protein in the model group were significantly increased
(P<0.001), and significantly decreased after SASP (P<0.05)
and decitabine (P<0.001) intervention. Phosphorylation of
ERK1/2, JNK and p38 proteins represents the activation state of
MAPK (25), and decitabine
inhibits phosphorylation of ERK1/2, JNK and p38 in colon tissues,
indicating that it can exert anti-inflammatory effects by
inhibiting the activation of the MAPK signaling.
Discussion
UC is a chronic inflammatory disease of the rectum
and colon that increases the risk of CRC in patients (26). UC is the predominant form of
chronic IBD characterized by persistent inflammation in the mucosa
and submucosa of the rectum and colon. UC is associated with the
destruction of immune tolerance and leads to pathological
inflammation (27).
The present study successfully established a
DSS-induced colitis mouse model. The mice in the model group
exhibited mucus, pus and bloody stools, weight loss. Gross
morphology observation demonstrated obvious shortening of the
colon, thickening of the intestinal wall and stenosis of the
intestine. Congestion and edema were observed in the intestinal
mucosa. A large amount of bloody secretions were observed in the
intestine, accompanied by different degrees of erosion and ulcer
formation. Under the microscope, the present study observed obvious
congestion and edema, extensive large-scale erosion, deep ulcer
formation, and disordered arrangement of crypts. A typical amount
of colonic inflammation, such as lymphocyte and neutrophil
infiltration, was observed in the mucosa and submucosa, indicating
successful modeling. Subsequently, after 7 days of decitabine
treatment, signs of diarrhea, mucus, pus and bloody stools in
colitis mice were relieved, intestinal mucosal inflammation was
reduced, and DAI, gross morphology and histological scores were
significantly lower compared with the model group. These results
indicate that decitabine has a good clinical effect on DSS-induced
experimental colitis in mice.
Tregs are a subset of CD4+ T lymphocytes
with inhibitory activity, which play an important role in
controlling immune responses (28). A study has shown that IBD is
associated with a decrease or dysfunction of Tregs (29). Effector T cells can induce
intestinal hyperimmune response due to the lack of
immunosuppressive regulation of Tregs, ultimately leading to
intestinal mucosal damage (30).
Tregs mainly secrete cytokines such as IL-4, IL-10 and TGF-β1
(31). TGF-β, IL-2, IL-2R and
IL-10 gene knockout mice can achieve spontaneous colitis due to the
decrease of Tregs. Their intestinal tract exhibits crypt
destruction, crypt abscess, submucosal inflammatory cell
infiltration and other characteristics (32). Foxp3 is a major transcription
factor that controls the development and function of Tregs. If
Tregs (Foxp3+ CD4+ T cells) predominate over
other pro-inflammatory CD4+ T cell subsets, the lesions
are less severe (33). DNA
methyltransferase inhibitor (DNMTi) 5-azacytidine and its
derivative 5-aza 2′-deoxy-cytidine (decitabine) demonstrated
immunomodulatory potential in vitro and in vivo, and
induce demethylation of the Foxp3 gene (11). The application of DNMTi to induce
Foxp3 expression has been extensively studied in mice (34). A previous study reported that
decitabine is an inhibitor of DNMT, which blocks DNA methylation of
the 5′-untranslated region promoter of Foxp3 and increases
expression of Foxp3 in CD4+ Foxp3- cells
(35). Other preclinical studies
have demonstrated that in vitro decitabine administration
increases the population and immunosuppressive function of
Foxp3+ regulatory T cells (36). Landman et al (34) demonstrated that DNA
methyltransferase can inhibit Th1 polarization in CD4+
CD25high FOXP3+ Tregs. Kehrmann et al
(37) also reported that
5-aza-2′-deoxycytidine can induce Tregs. Epigenetic regulation of
Foxp3 by DNMT can supply stable functional Tregs (28). Therefore, the present study
suggested that decitabine can increase Tregs in vivo to
treat UC. It was identified that the function of decitabine is
dose-dependent. For decitabine, 'dual mechanism' refers to the
inhibition of cell proliferation at high doses, and gene
re-expression mediated by DNA hypomethylation at low doses, which
affects cell differentiation and tumor suppression (38). The current study investigated
whether 0.5 mg/kg decitabine could affect intestinal barrier
function in mice with IBD by regulating naive T cell transformation
in vivo. It was demonstrated that in 0.5 mg/kg
decitabine-treated enteritis mice, the inflammatory response was
inhibited, the release of the pro-inflammatory factor IL-17 was
reduced and the release of the tumor suppressor IL-10 was
promoted.
A decreased number of Tregs or dysfunction triggers
IBD, meanwhile Th17 cells and their secreted cytokines also play an
important role in the pathogenesis of IBD (39). Induction of Th17 differentiation
leads to increased expression of IL-17 and results in decreased
IL-10 expression (40). The
highly expressed cytokine IL-17, which is activated by a Th17 and
Tregs imbalance, synergizes with pro-inflammatory cytokines, such
as TNF-α and IL-1β, leading to a worsening of colitis (41). Therefore, inhibition of Th17 cell
differentiation is considered a potential therapy for IBD (42). IL-17 is a major effector molecule
of Th17 cells that exerts inflammatory effects. Neutralizing
antibodies and small molecule inhibitors against IL-17 in different
colitis animal models may exhibit different therapeutic effects
(32). IL-10 is mainly secreted
by Tregs and exerts an anti-inflammatory effect (43). IL-10 knockout and IL-10 receptor
knockout mice spontaneously induce colitis (35). The present study identified that
intraperi-toneal injection of decitabine can promote the
re-expression of Foxp3 in naive T cells in mice spleen, and
increase the proportion of CD4+ Foxp3+ T
cells in CD4+ T cells, indicating that the use of DNMTi
in vivo can play a role in demethylation and amplify Tregs.
It also promotes IL-10 and TGF-β and inhibits IL-17 expression.
The main connections between intestinal epithelial
cells are tight junctions (44).
Tight junctions are the determinant of intestinal barrier function;
they can prevent antigens and microorganisms in the intestinal
cavity from entering the body (45). The integrity of intestinal
epithelial tight junctions is critical in IBD (44). The clinical symptoms of IBD are
caused by intestinal inflammation and consequent dysfunction,
including impaired absorption function and intestinal barrier
function (46,47). ZO-1 and occcludin are the main
proteins constituting tight junctions, and changes in the levels of
ZO-1 and occcludin suggest a change of intestinal mucosal barrier
function to a certain extent (48). Immunofluorescence in the present
study revealed tight junction proteins in the decitabine group were
distributed in the apical membrane of intestinal epithelial cells,
with a continuous distribution and increased fluorescence
intensity. Immunohistochemical results indicated that decitabine
treatment can repair tight junction structures and microvilli,
reduce intercellular gaps and cell vacuolation. Compared with the
normal control group, the levels of ZO-1 and occludin in the
intestinal epithelial cells of the model group were significantly
decreased, indicating that the colon barrier function was impaired.
Compared with the model group, the expression levels of ZO-1 and
occludin in the SASP and decitabine groups were enhanced.
MAPK signaling is closely associated with the
pathogenesis of UC, and its activation is considered to be one of
the major factors leading to the release of cytokines and
inflammatory mediators in UC (49). The MAPK pathway is an important
signal transduction pathway involved in numerous biological
functions. Abnormal activation of the MAPK pathway is closely
associated with the overexpression of inflammatory cytokines and is
also an important indicator for the occurrence and development of
UC (50,51). The MAPK family includes members
such as ERK1, ERK2, JNK and p38 (52). Intervention of MAPK signaling can
inhibit normal colonic epithelial cell apoptosis, accelerate
colonic inflammatory cell apoptosis and inhibit the production of
inflammatory cytokines, which is considered to be a potential
anti-inflammatory molecular target for the treatment of UC
(53). Phosphorylation of ERK1/2,
JNK and p38 proteins represents the activation state of MAPK, and
decitabine inhibited phosphorylation of ERK1/2, JNK and p38 in
colon tissues, indicating that decitabine can exert
anti-inflammatory effects by inhibiting the activation of the MAPK
signaling.
In conclusion, the present study demonstrated that
decitabine enhances the ratio of CD4+ Foxp3+
T cells to CD4+ T cells by promoting the re-expression
of Foxp3 in naive T cells in mice spleens, and amplifies Tregs,
thereby repairing the colonic barrier of mice. Decitabine inhibits
IL-17 expression in DSS-induced colitis mice and induces IL-10 and
TGF-β, and thereby inhibits inflammation, which may be caused by
inhibiting Th17 cell differentiation and promoting Treg
differentiation. In addition, decitabine can also participate in
anti-inflammatory effects by inhibiting the activation of the MAPK
signaling.
Funding
This study was supported by a grant from the
Shanghai Minhang Hospital (grant no. 2018MHJC06).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL, PZ and YC participated in the design of the
study, interpretation of the results and review of the manuscript.
CS, SL, XM and XY conducted the experiments. XM analyzed the data
and wrote the manuscript. CS applied for the funds. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The animal research was approved by the Ethics
Committee of Minhang Hospital, Shanghai, China [Medical Ethics
Committee (2018) Approval no. 2].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Yu SM and Kim SJ: 5-Azacytidine regulates
matrix metalloproteinase-9 expression, and the migration and
invasion of human fibrosarcoma HT1080 cells via PI3-kinase and
ERK1/2 pathways. Int J Oncol. 49:1241–1247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Woo JK, Choi S, Kang JH, Kim DE, Hurh BS,
Jeon JE, Kim SY and Oh SH: BMC Complement Altern Med. 16:4982016.
View Article : Google Scholar
|
|
3
|
Kim MW, Choi S, Kim SY, Yoon YS, Kang JH
and Oh SH: Allyl isothiocyanate ameliorates dextran sodium
sulfate-induced colitis in mouse by enhancing tight junction and
mucin expression. Int J Mol Sci. 19:E20252018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hartnett L and Egan LJ: Inflammation, DNA
methylation and colitis-associated cancer. Carcinogenesis.
33:723–731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Molodecky NA, Soon IS, Rabi DM, Ghali WA,
Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema
HW and Kaplan GG: Increasing incidence and prevalence of the
inflammatory bowel diseases with time, based on systematic review.
Gastroenterology. 142:46–54e42. 2012. View Article : Google Scholar
|
|
6
|
Ramos GP and Papadakis KA: Mechanisms of
disease: Inflammatory bowel diseases. Mayo Clin Proc. 94:155–165.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scarpa M, Scarpa M, Castagliuolo I, Erroi
F, Kotsafti A, Basato S, Brun P, D'Incà R, Rugge M, Angriman I and
Castoro C: Aberrant gene methylation in non-neoplastic mucosa as a
predictive marker of ulcerative colitis-associated CRC. Oncotarget.
7:10322–10331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harris RA, Nagy-Szakal D, Mir SA, Frank E,
Szigeti R, Kaplan JL, Bronsky J, Opekun A, Ferry GD, Winter H and
Kellermayer R: DNA methylation-associated colonic mucosal immune
and defense responses in treatment-naive pediatric ulcerative
colitis. Epigenetics. 9:1131–1137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Müller S, Fritz Y and Wagenknecht HA:
Control of energy transfer between pyrene- and perylene-nucleosides
by the sequence of DNA-templated supramolecular assemblies.
ChemistryOpen. 9:389–392. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hollenbach PW, Nguyen AN, Brady H,
Williams M, Ning Y, Richard N, Krushel L, Aukerman SL, Heise C and
MacBeth KJ: A comparison of azacitidine and decitabine activities
in acute myeloid leukemia cell lines. PLoS One. 5:e90012010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu CH, Wu CJ, Chan CC, Nguyen DT, Lin KR,
Lin SJ, Chen LC, Yen JJ and Kuo ML: DNA methyltransferase inhibitor
promotes human CD4(+)CD25(h)FOXP3(+) regulatory T lymphocyte
induction under suboptimal TCR stimulation. Front Immunol.
7:488eCollection 2016. 2016. View Article : Google Scholar
|
|
12
|
Vargas-Robles H, Castro-Ochoa KF,
Citalán-Madrid AF and Schnoor M: Beneficial effects of nutritional
supplements on intestinal epithelial barrier functions in
experimental colitis models in vivo. World J Gastroenterol.
25:4181–4198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sann H, Erichsen J, Hessmann M, Pahl A and
Hoffmeyer A: Efficacy of drugs used in the treatment of IBD and
combinations thereof in acute DSS-induced colitis in mice. Life
Sci. 92:708–718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Andújar I, Recio MC, Giner RM,
Cienfuegos-Jovellanos E, Laghi S, Muguerza B and Ríos JL:
Inhibition of ulcerative colitis in mice after oral administration
of a polyphenol-enriched cocoa extract is mediated by the
inhibition of STAT1 and STAT3 phosphorylation in colon cells. J
Agric Food Chem. 59:6474–6483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishiyama Y, Kataoka T, Yamato K, Taguchi
T and Yamaoka K: Suppression of dextran sulfate sodium-induced
colitis in mice by radon inhalation. Mediators Inflamm.
2012:2396172012. View Article : Google Scholar
|
|
16
|
Samak G, Chaudhry KK, Gangwar R, Narayanan
D, Jaggar JH and Rao R: Calcium/Ask1/MKK7/JNK2/c-Src signalling
cascade mediates disruption of intestinal epithelial tight
junctions by dextran sulfate sodium. Biochem J. 465:503–515. 2015.
View Article : Google Scholar :
|
|
17
|
Qin J, Yu B, Moyer AM, Nowsheen S, Liu T,
Qin S, Zhuang Y, Liu D, Lu SW, Kalari KR, et al: DNA
methyltransferase expression in triple-negative breast cancer
predicts sensitivity to decitabine. J Clin Invest. 128:2376–2388.
2018. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kantarjian H, Issa PJ, Rosenfeld CS,
Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C,
Ravandi F, et al: Decitabine improves patient outcomes in
myelodysplastic Syndromes: Results of a phase III randomized study.
Cancer. 106:1794–1803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheung MC, Spalding PB, Gutierrez JC,
Balkan W, Namias N, Koniaris LG and Zimmers TA: Body surface area
prediction in normal, hypermuscular, and obese mice. J Surg Res.
153:326–331. 2009. View Article : Google Scholar
|
|
20
|
Jansen YJL, Verset G, Schats K, Van Dam
PJ, Seremet T, Kockx M, Van Laethem JB and Neyns B: Phase I
clinical trial of decitabine (5-aza2′-deoxycytidine) administered
by hepatic arterial infusion in patients with unresectable
liver-predominant metastases. ESMO Open. 4:e0004642019. View Article : Google Scholar
|
|
21
|
Sakaguchi S, Miyara M, Costantino CM and
Hafler DA: FOXP3+ regulatory T cells in the human immune system.
Nat Rev Immunol. 10:490–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Danikowski KM, Jayaraman S and Prabhakar
BS: Regulatory T cells in multiple sclerosis and myasthenia gravis.
J Neuroinflammation. 14:1172017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nunes NS, Kim S, Sundby M, Chandran P,
Burks SR, Paz AH and Frank JA: Temporal clinical, proteomic,
histological and cellular immune responses of dextran sulfate
sodium-induced acute colitis. World J Gastroenterol. 24:4341–4355.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Furumoto Y, Nunomura S, Terada T, Rivera J
and Ra C: The FcepsilonRIbeta immunoreceptor tyrosine-based
activation motif exerts inhibitory control on MAPK and IkappaB
kinase phosphorylation and mast cell cytokine production. J Biol
Chem. 279:49177–49187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marchesi N, Thongon N, Pascale A,
Provenzani A, Koskela A, Korhonen E, Smedowski A, Govoni S,
Kauppinen A, Kaarniranta K and Amadio M: Autophagy stimulus
promotes early hur protein activation and p62/SQSTM1 protein
synthesis in ARPE-19 cells by triggering Erk1/2, p38(MAPK), and JNK
kinase pathways. Oxid Med Cell Longev. 2018:4956080. 2018.
View Article : Google Scholar
|
|
26
|
Munkholm P: The incidence and prevalence
of colorectal cancer in inflammatory bowel disease. Aliment
Pharmacol Ther. 18:1–5. 2003. View Article : Google Scholar
|
|
27
|
Hou H, Cao R, Quan M, Sun Y, Sun H, Zhang
J, Li B, Guo L and Song X: Rapamycin and fingolimod modulate
Treg/Th17 cells in experimental autoimmune encephalomyelitis by
regulating the Akt-mTOR and MAPK/ERK pathways. J Neuroimmunol.
324:26–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu CJ, Yang CY, Chen YH, Chen CM, Chen LC
and Kuo ML: The DNA methylation inhibitor 5-azacytidine increases
regulatory T cells and alleviates airway inflammation in
oval-bumin-sensitized mice. Int Arch Allergy Immunol. 160:356–364.
2013. View Article : Google Scholar
|
|
29
|
Smids C, Horjus Talabur, Horje CS,
Drylewicz J, Drylewicz J, Roosenboom B, Groenen MJM, van Koolwijk
E, van Lochem EG and Wahab PJ: Intestinal T cell profiling in
inflammatory bowel disease: Linking T cell subsets to disease
activity and disease course. J Crohns Colitis. 12:465–475. 2018.
View Article : Google Scholar
|
|
30
|
Kaskow BJ and Baecher-Allan C: Effector T
cells in multiple sclerosis. Cold Spring Harb Perspect Med.
8:a0290252018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ruitenberg EJ and Elgersma A: Response of
intestinal globule leucocytes in the mouse during a Trichinella
spiralis infection and its independence of intestinal mast cells.
Br J Exp Pathol. 60:246–251. 1979.PubMed/NCBI
|
|
32
|
Fitzpatrick LR: Inhibition of IL-17 as a
pharmacological approach for IBD. Int Rev Immunol. 32:544–555.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jacquemin C, Augusto JF, Scherlinger M,
Gensous N, Forcade E, Douchet I, Levionnois E, Richez C, Lazaro E,
Duffau P, et al: OX40L/OX40 axis impairs follicular and natural
Treg function in human SLE. JCI Insight. 3:1221672018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Landman S, Cruijsen M, Urbano PCM, Huls G,
van Erp PEJ, van Rijssen E, Joosten I and Koenen HJPM: DNA
methyltransferase inhibition promotes th1 polarization in human
CD4(+) CD25(high) FOXP3(+) regulatory T cells but does not affect
their suppressive capacity. J Immunol Res. 2018:49739642018.
View Article : Google Scholar
|
|
35
|
Fedorak RN, Gangl A, Elson CO, Rutgeerts
P, Schreiber S, Wild G, Hanauer SB, Kilian A, Cohard M, LeBeaut A
and Feagan B: Recombinant human interleukin 10 in the treatment of
patients with mild to moderately active Crohn's disease. The
Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group.
Gastroenterology. 119:1473–1482. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Polansky JK, Kretschmer K, Freyer J,
Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H and
Huehn J: DNA methylation controls Foxp3 gene expression. Eur J
Immunol. 38:1654–1663. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kehrmann J, Tatura R, Zeschnigk M,
Probst-Kepper M, Geffers R, Steinmann J and Buer J: Impact of
5-aza-2′-deoxycytidine and epigallocatechin-3-gallate for induction
of human regulatory T cells. Immunology. 142:384–395. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jabbour E, Issa JP, Garcia-Manero G and
Kantarjian H: Evolution of decitabine development: Accomplishments,
ongoing investigations, and future strategies. Cancer.
112:2341–2351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duan L, Rao X, Braunstein Z, Toomey AC and
Zhong J: Role of incretin axis in inflammatory bowel disease. Front
Immunol. 8:17342017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cui Y, Hu Q, Gu Y, Sheng L, Wu K, Shi J,
Tan Y, Fu H, Liu L, Fu S, et al: Decitabine facilitates the
generation and immunosuppressive function of regulatory gammadeltaT
cells derived from human peripheral blood mononuclear cells.
Leukemia. 27:1580–1585. 2013. View Article : Google Scholar
|
|
41
|
Granet C and Miossec P: Combination of the
pro-inflammatory cytokines IL-1, TNF-alpha and IL-17 leads to
enhanced expression and additional recruitment of AP-1 family
members, Egr-1 and NF-kappaB in osteoblast-like cells. Cytokine.
26:169–177. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gizinski AM, Fox DA and Sarkar S:
Pharmacotherapy: Concepts of pathogenesis and emerging treatments.
Co-stimulation and T cells as therapeutic targets. Best Pract Res
Clin Rheumatol. 24:463–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brinkhoff A, Sieberichs A, Engler H, Dolff
S, Benson S, Korth J, Schedlowski M, Kribben A, Witzke O and Wilde
B: Pro-Inflammatory Th1 and Th17 cells are suppressed during human
experimental endotoxemia whereas anti-inflammatory IL-10 producing
T-cells are unaffected. Front Immunol. 9:11332018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Awad WA, Hess C and Hess M: Enteric
pathogens and their toxin-induced disruption of the intestinal
barrier through alteration of tight junctions in chickens. Toxins
(Basel). 9. pp. E602017, View Article : Google Scholar
|
|
45
|
Okumura R and Takeda K: Roles of
intestinal epithelial cells in the maintenance of gut homeostasis.
Exp Mol Med. 49:e3382017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Parikh K, Antanaviciute A, Fawkner-Corbett
D, Jagielowicz M, Aulicino A, Lagerholm C, Davis S, Kinchen J, Chen
HH, Alham NK, et al: Colonic epithelial cell diversity in health
and inflammatory bowel disease. Nature. 567:49–55. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
O'Callaghan AA and Corr SC: Establishing
boundaries: The relationship that exists between intestinal
epithelial cells and gut-dwelling bacteria. Microorganisms.
7:E6632019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vanhove W, Nys K, Arijs I, Cleynen I,
Noben M, De Schepper S, Van Assche G, Ferrante M and Vermeire S:
Biopsy-derived intestinal epithelial cell cultures for
pathway-based stratification of patients with inflammatory bowel
disease. J Crohns Colitis. 12:178–187. 2018. View Article : Google Scholar
|
|
49
|
Setia S, Nehru B and Sanyal SN:
Upregulation of MAPK/Erk and PI3K/Akt pathways in ulcerative
colitis-associated colon cancer. Biomed Pharmacother. 68:1023–1029.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
You BH, Chae HS, Song J, Ko HW, Chin YW
and Choi YH: α-Mangostin ameliorates dextran sulfate sodium-induced
colitis through inhibition of NF-κB and MAPK pathways. Int
Immunopharmacol. 49:212–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shematorova EK, Shpakovski DG, Chernysheva
AD and Shpakovski GV: Molecular mechanisms of the juvenile form of
batten disease: Important role of MAPK signaling pathways
(ERK1/ERK2, JNK and p38) in pathogenesis of the malady. Biol
Direct. 13:192018. View Article : Google Scholar
|
|
53
|
Zhao HM, Huang XY, Zhou F, Tong WT, Wan
PT, Huang MF, Ye Q and Liu DY: Si shen wan inhibits mRNA expression
of apoptosis-related molecules in p38 MAPK signal pathway in mice
with colitis. Evid Based Complement Alternat Med. 2013:4320972013.
View Article : Google Scholar : PubMed/NCBI
|