Introduction
Acute lung injury (ALI) is a severe type of lung
disease that is associated with a high mortality rate in critically
ill patients (1,2). However, even though supportive
treatments have been developed, there is still an urgent need for
the development of novel therapeutic strategies for the treatment
of patients with ALI (3). An
excessive inflammatory response in the lung tissues is a main risk
factor for the initiation and development of ALI (4). Therefore, the effective inhibition
of inflammation may be beneficial for improving the current
therapeutic approaches for the management of ALI.
Lipopolysaccharide (LPS), the active component of
bacterial endotoxin, is an important pathogenic factor in the
progression of ALI (5,6). It has been reported that LPS can
induce extensive damage and the inflammatory reaction of
neutrophils, and has been widely used to mimic ALI in vitro
and in vivo (7,8). A number of studies have demonstrated
that the LPS-induced inflammatory response is regulated by
Toll-like receptor 4 (TLR4) (9).
In ALI, TLR4 triggers the activation of the nuclear factor (NF)-κB
signaling pathway and promotes the secretion of pro-inflammatory
cytokines, including tumor necrosis factor (TNF)-α, interleukin
(IL)-1β and IL-6, finally resulting in damage to the lung tissue
(10-13). Extensive research has revealed
that the silencing of TLR4 can reduce the expression of the
intracellular adaptor protein, myeloid differentiation primary
response 88 (MyD88), and can decrease NF-κB activity, leading to
the inhibition of pro-inflammatory cytokines in ALI (14). Thus, the proper regulation of the
TLR4/NF-κB pathway may prove to be an effective strategy with which
to supress the development of ALI.
MicroRNAs (miRNAs or miRs) are a family of short,
small, non-coding RNAs (an average size of 22 nucleotides), which
negatively regulate target gene expression through either
translation repression or RNA degradation (15). The potential regulatory role of
miRNAs in lung diseases via the regulation of crucial factors or
key pathways, has been widely reported, including in ALI (16). For example, Ling et al
demonstrated that the inhibition of miR-494 attenuated the
inflammatory response in rats with ALI through the NAD(P)H quinone
dehydrogenase 1 (NQO1)-mediated nuclear factor erythroid 2-related
factor 2 (Nrf2) signaling pathway (17). Another study demonstrated that the
enforced expression of miR-125b attenuated the development of ALI
using the LPS-induced model (18). These studies suggest that
targeting miRNAs may be beneficial for the suppression of the
development of ALI. Recently, miR-93, a member of the miR-17
family, was reported to be involved in the regulation of the
inflammatory response in various types of tissue injury and
diseases (19-21), in addition to its anti-tumor
effects. However, little attention has been paid to the function of
miR-93 in the inflammatory response during ALI.
Thus, the present study investigated the expression
of miR-93 in a mouse model of ALI induced by LPS. The role of
miR-93 in the regulation of the inflammatory response during ALI
was further investigated. The findings presented herein suggest
that miR-93 may prove to be a novel therapeutic strategy for
ALI.
Materials and methods
Animals
All animal experiments were performed according to
the guidelines of the Chinese Council on Animal Care and ethical
approval was obtained for the use of animals prior to the
commencement of the study from the Ethics Committee of the West
China Second University Hospital, Sichuan University. Male BALB/c
mice (weighing 18-22 g) were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. The BALB/c mice were housed under
standard conditions (12-h light/dark cycle, temperature of 25-27°C
and a humidity of ~40%) with free access to food and water
throughout the duration of the experiments. All mice were randomly
divided into 4 groups (n=6/group) as follows: i) The control group;
ii) the LPS group; iii) the LPS + agomir-93 group; and iv) the LPS
+ agomir-NC group. In the control group, mice were injected
intravenously (via the tail vein) with an intra-tracheal
instillation of 2 mg/kg normal saline (NS); in the LPS group, mice
were injected intravenously (via the tail vein) with an
intra-tracheal instillation of 2 mg/kg LPS; in the agomir-93 or
agomir negative control (NC) groups, mice were injected
intravenously (via the tail vein) with agomir-93 or agomir NC [8
mg/kg (22), RiboBio Co., Ltd.]
at 24 h prior to the injection of 2 mg/kg LPS. Pentobarbital sodium
(50 mg/kg, intraperitoneal injection) was used for anesthesia prior
to each surgical procedure, and all efforts were made to minimize
animal suffering. No mice were found dead during the process of
anesthesia. If an animal reached the defined humane endpoints [a
body weight loss of >15% in 1-2 days or an overall body weight
loss of >20%, or displayed obvious signs of suffering (e.g.,
lethargy, squinted eyes, dehydration or hunched back)], it was
humanely euthanized. Sacrifice was performed by an intraperitoneal
injection of pentobarbital sodium (50 mg/kg) followed by cervical
dislocation, and mortality was confirmed by observation (23) and the lung tissues and
bronchoalveolar lavage fluid (BALF) were then collected for
subsequent analysis.
In addition, the survival experiments were performed
in 4 groups of mice (n=10/group) (LPS, Control, LPS + agomir-93 and
LPS + agomir-NC). If an animal reached the defined humane endpoints
mentioned above, it was humanely euthanized. The remaining mice
were humanely euthanized 96 h later. The survival rate of the mice
was observed from 0 to 96 h using the Kaplan Meier method.
RT-qPCR analysis
Total RNA was isolated from the lung tissues or BALF
using the miRNeasy mini kit (Qiagen, Inc.). The reverse
transcription of miR-93 was performed using the miScript II RT kit
and the reverse transcription kit (Invitrogen; Thermo Fisher
Scientific, Inc.), respectively. miR-93 expression was measured
using Exiqon SYBR-Green Master Mix (Exiqon) on a Light Cycler
instrument (Bio-Rad Laboratories, Inc.). The reaction mixtures were
denatured at 95°C for 3 min, followed by 40 two-step cycles of 95°C
for 10 sec and 60°C for 30 sec. The primers for RT-qPCR analysis
were as follows: miR-93 forward, 5′-AGG CCC AAA GTG CTG TTC GT-3′
and reverse, 5′-GTG CAG GGT CCG AGG T-3′; U6 forward, 5′-TGC GGG
TGC TCG CTT CGC AGC-3′ and reverse, 5′-CCA GTG CAG GGT CC GAG
GT-3′. miRNA relative expression levels were analyzed using the
2−ΔΔcq method (24)
and determined by normalization to U6.
Histopathological evaluation of lung
tissues
At 24 h after the LPS challenge, the mice were
sacrificed and the left lung tissues of mice were obtained and
fixed in in 4% (v/v) paraformaldehyde, embedded in paraffin,
sectioned at 4 µm thickness. Then, the fixed lung tissues
were stained with hematoxylin and eosin (H&E) for the
evaluation of the severity of lung injury. Images were captured
using a microscope (Nikon Corp.), and the lung injury score was
assessed by two pathologists as previously described (25). In brief, i) the thickness of
alveolar walls; ii) infiltration of inflammatory cells; iii)
hemorrhage; and iv) alveolar congestion were graded according to
the following scale: 0, no damage; 1, mild damage; 2, moderate
damage; 3, severe damage; 4, maximal damage.
Evaluation of lung permeability
Pulmonary capillary permeability was assessed using
the Evans blue (EB) dye extravasation method, as previously
described (26). Briefly, EB dye
(20 mg/kg, Sigma-Aldrich; Merck KGaA) in normal saline was injected
into the mice in each group (the Control group; the LPS group; the
LPS + agomir-93 group; the LPS + agomir-NC group; n=6/group)
through via the tail vein prior to the termination of the
experiment. The mice were sacrificed after dye injection at 2 h,
and EB dye was then extracted using formamide for 18 h at 60°C and
the absorbance was measured at 620 nm. The dye concentration was
reported as the ratio of absorbance relative to the amount of dry
lung tissue (µg/100 mg).
Lung wet/dry (W/D) ratio
The lung W/D ratio was used to calculate pulmonary
edema. The right lungs of the mice were obtained and immediately
weighted, then placed in an incubator at 80°C for 60 h to obtain
the dry weight.
Measurement of IL-6, IL-1β and TNF-α
levels
The concentrations of IL-6 (ab100712), IL-1β
(ab197742) and TNF-α (ab100747) in BALF were analyzed using ELISA
kits (Abcam) according to the kit instructions.
Immunohistochemical staining
Immunohistochemistry (IHC) and IHC scoring systems
were performed as described previously (27,28). 4-µm-thick formalin-fixed,
paraffin-embedded serial sections of lung tissue were blocked and
then incubated with a primary antibody specific for nuclear
phosphorylated (p-)p65 (1:4,000 dilution; ab53489, Abcam) in 10%
rabbit serum overnight at 4°C. The sections were then treated with
a secondary antibody conjugated to horseradish peroxidase (1:10,000
dilution; ab7090, Abcam) overnight at 4°C. After the colorimetric
reaction was completed, the sections were observed under a light
microscope (Eclipse E100, Nikon; ×40 magnification). IHC scores are
calculated as follows: IHC score=1× (number of weakly stained cells
in the field) + 2× (number of moderately stained cells in the
field) + 3× (number of intensely stained cells in the field)
(29).
Cell culture and transfection
RAW 264.7 macrophages are widely used as a model of
LPS-induced ALI, and play an essential role in the regulation of
the inflammatory response (30,31). Therefore, RAW 264.7 cells were
selected for use in an ex vivo experiment as the present
study focused on the ALI-induced inflammatory response. The
RAW264.7 cell line was obtained from ATCC. The cells were
maintained in DMEM supplemented with 10% FBS (Sigma-Aldrich; Merck
KGaA), 1% penicillin and streptomycin (Sigma-Aldrich; Merck KGaA)
at 37°C and a 5% CO2 incubator.
When the RAW264.7 cells in a 6-well plate grown to
approximately 80% confluency, miR-93 mimics (20 nmol/l) and miR-93
inhibitor (20 nmol/l) were transfected into the cells at 37°C for
24 h, using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). miR-93 mimics, miR-93 inhibitor and the
corresponding control vectors were purchased from RiboBio Co.,
Ltd.
Luciferase assay
miRNA target prediction tools, including miRanda
(https://miranda.org.uk/) and TargetScan Release
7.0 (http://targetscan.org/) were used to
search for the putative targets of miR-93. The dual-luciferase
reporter assay was performed as described previously (32). Briefly, partial sequences of TLR4
3'-UTR containing miR-93 binding sites was amplified by PCR and
cloned into the dual-luciferase reporter vector (pmirGLO; Promega
Corporation) (wild-type pmirGLO-TLR4-3'-UTR, wt). Mutation of the
predicted binding site (mutation pmirGLO-TLR4-mut-3'-UTR) was
generated using the QuikChange Site-Directed Mutagenesis kit
(Stratagene; Agilent Technologies, Inc.) according to the
manufacturer's instructions. RAW264.7 cells were co-transfected
with miR-93 mimics, miR-93 inhibitor and the luciferase reporter
plasmids using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h post-transfection, luciferase activities
were detected using the dual luciferase reporter kit (Beyotime
Institute of Biotechnology) with Renilla luciferase activity
as an internal control.
Western blot analysis
Western blot analysis was performed as previously
described (32,33). Total protein was extracted using
RIPA buffer (Beyotime Institute of Biotechnology). The extraction
and isolation of nuclear and cytoplasmic proteins were performed
using the Nuclear and Cytoplasmic Protein Extraction kit (Beyotime
Institute of Biotechnology). The concentrations of total cellular
protein were determined using a BCA assay kit (Pierce; Thermo
Fisher Scientific, Inc.). Briefly, 40 µg extracted protein
samples were transferred onto polyvinylidene difluoride (PVDF,
Millipore) membranes and then blocked with 5% skim milk at 4°C
overnight. Each membrane was then probed with primary antibodies
against TLR4 (1:2,000, ab9870), nuclear p-p65 (1:1,500, ab53489),
p65 (1:2,000, ab86299), p-IκBα (1:2,000, ab133462), IκBα (1:2,000,
ab32518), MyD88 (1:1,000, ab199247), Histone 3 (1:1,000, ab1791)
and β-actin (1:1,000, ab8226) (all from Abcam) at 4°C overnight,
followed by HRP-conjugated goat anti-rabbit IgG (1:10,000; cat. no.
ab205718; Abcam). The protein bands were developed using an ECL kit
(GE Healthcare) and blot bands were quantified using ImageJ
software version 1.46 (Rawak Software, Inc.).
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, Inc.) was
used to perform the statistical analyses. Each experiment was
performed at least 3 times. Numerical data are presented as the
means ± SD. Statistical significance between 2 groups was
determined using a Student's t-test. Comparisons among multiple
groups were performed using one-way analysis of variance (ANOVA)
with Tukey's post hoc test. The Kaplan-Meier method was performed
to calculate the survival rates, and multiple comparisons were made
using the Bonferroni method. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-93 is downregulated in mice with
ALI
As is known, the mouse model of LPS-induced ALI is
widely used to simulate the pathological conditions of lung injury
in vivo (34). Following
the injection of mice with LPS (2 mg/kg) for 24 h, lung
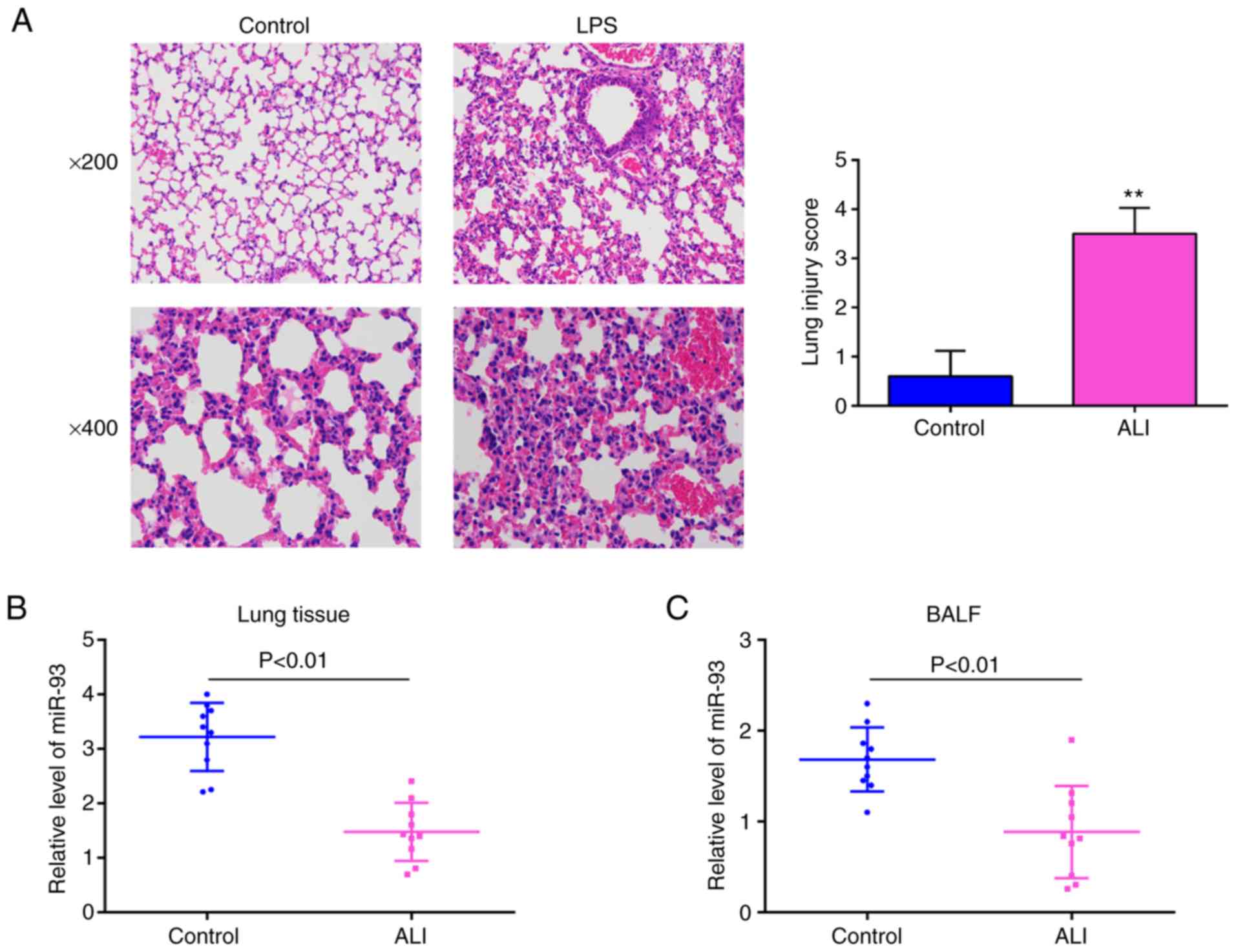
histopathological changes were evaluated. As shown in Fig. 1A, the lungs of the mice in the ALI
group displayed significant pathological changes, including obvious
inflammatory cell infiltration and widespread alveolar wall
thickness, compared with that in the control group. Moreover, lung
injury was scored using a semi-quantitative histopathological score
system, and the lung injury score was significantly increased in
the ALI group, compared with that in the control group. The
above-mentioned data indicated that the mouse model of ALI was
successfully established. Subsequently, the levels of miR-93 in the
lung tissues and in BALF from the mice with ALI were examined by
RT-qPCR. It was observed that the expression levels of miR-93 were
significantly downregulated in the lung tissues and in BALF,
compared with those in the control group (Fig. 1B and C). These data suggest that
miR-93 is involved in the pathogenesis of ALI.
miR-93 attenuates LPS-induced ALI in
mice
To further investigate the roles of miR-93 in
LPS-induced ALI, the mice were intravenously injected with
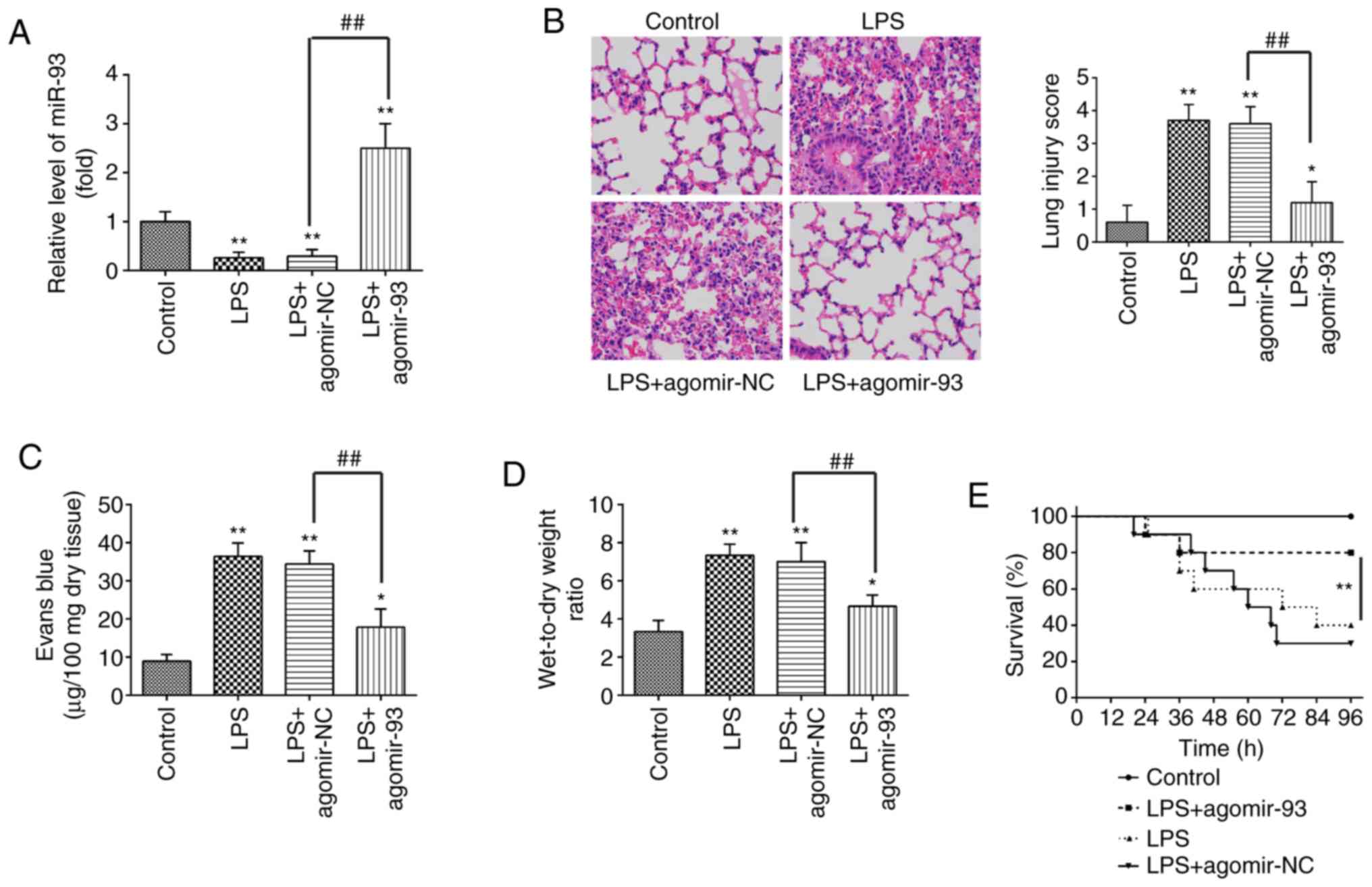
agomir-93 or agomir NC prior to LPS administration. As shown in
Fig. 2A, miR-93 expression in the
lung tissues from mice with ALI was notably increased compared with
that in the agomir NC group. Subsequently, H&E staining was
carried out to detect the inflammatory status in the lungs. The
results revealed that the agomir-93 injection markedly attenuated
the histopathological lesions, compared with those in the ALI group
(Fig. 2B). Lung micro-vascular
permeability was assessed by EB extravasation. As shown in Fig. 2C, pulmonary permeability was
significantly increased in the ALI group, compared with that in the
control group, whereas the increased pulmonary permeability was
markedly alleviated after the agomir-93 injection. The lung W/D
ratio was evaluated to indicate pulmonary edema. It was found that
the lung W/D ratio was significantly increased in the ALI group,
compared with that in the control group, whereas the increased lung
W/D ratio was markedly decreased after the agomir-93 injection
(Fig. 2D). In addition, only 40%
of the mice administered LPS survived up to 96 h, whereas 80% of
the mice that were injected with agomiR-93 survived following
exposure to LPS (Fig. 2E).
Collectively, these results indicate that agomir-93 injection was
able to improve LPS induced lung injury.
miR-93 suppresses the LPS-induced
inflammatory response in mice
Currently, ALI is recognized as an unbalanced
inflammatory response, and the inflammatory process is regulated by
multiple miRNAs (35-37). In the present study, to further
investigate whether miR-93 affects the inflammatory response
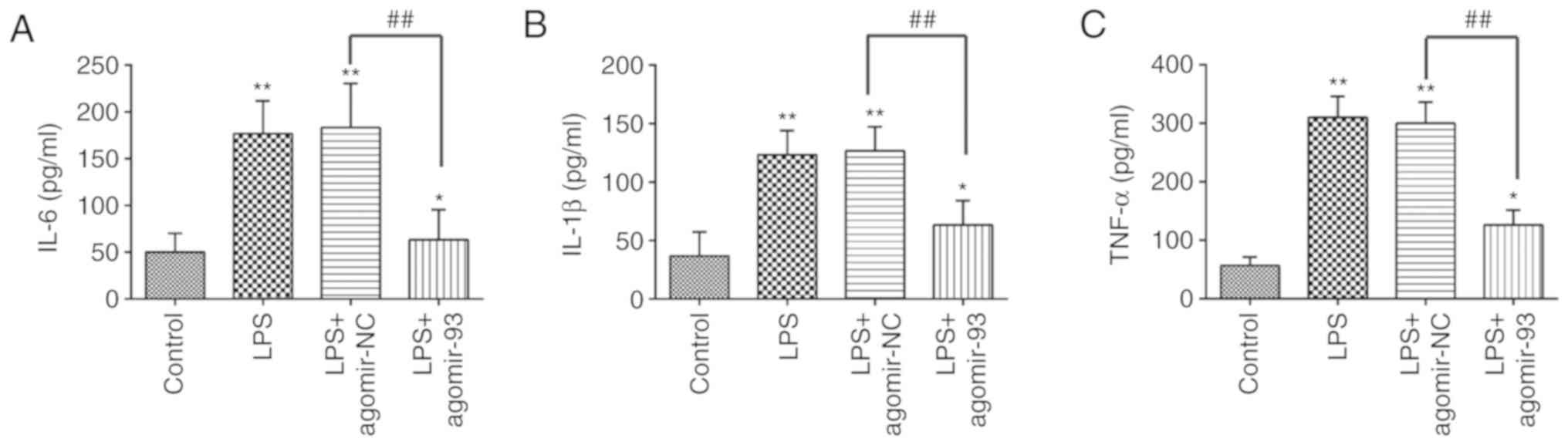
induced by LPS in mice, ELISA was performed to examine the levels
of pro-inflammatory cytokines, including IL-6, IL-1β, and TNF-α in
BALF from mice with ALI. As shown in Fig. 3, exposure to LPS resulted in a
significant increase in the production of IL-6, IL-1β and TNF-α. By
contrast, these increased levels of pro-inflammatory cytokines were
markedly attenuated by the agomir-93 injection. These data thus
suggest that the agomir-93 injection alleviated lung injury by
suppressing the inflammatory response in mice with ALI.
TLR4 is a target of miR-93 in RAW264.7
cells
According to previous research, TLR4 is a common
receptor of LPS, and plays important roles in the inflammatory
response in ALI (38-42). Through bioinformatics prediction
using TargetScan 7.0 and miRanda, the present study found that
miR-93 directly targeted the TLR4 gene and that the target
sequences were highly conserved among species (Fig. 4A and B). Notably, a previous study
demonstrated that TLR4 was a direct target of miR-93 in
chondrocytes (20). However, the
association between miR-93 and TLR4 in ALI has not yet been
clarified. To validate the possibility that TLR4 is directly
targeted by miR-93, a luciferase reporter assay was performed. As
shown in Fig. 4C, the expression
levels of miR-93 were significantly increased following
transfection with miR-93 mimics, while they were decreased by
transfection with miR-93 inhibitor in the RAW264.7 cells,
demonstrating the sufficient transfection efficacy. It was observed
that transfection with miR-93 mimics markedly inhibited the
luciferase activity in the TLR4-3'UTR wt reporter, and that the
miR-93 inhibitor induced an increased luciferase activity; however,
no changes were observed in the cells transfected with the TLR4
3'-UTR-mut plasmid (Fig. 4D),
indicating a targeting interaction between miR-93 and TLR4. In
addition, western blot analysis demonstrated that TLR4 protein
expression was decreased by transfection with miR-93 mimics,
whereas it was increased by transfection with miR-93 inhibitor in
the RAW264.7 cells (Fig. 4E). The
effect of agomir-93 on the protein expression of TLR4 protein in
vivo was also assessed by western blot analysis. It was found
that exposure to LPS significantly increased the protein expression
of TLR4 compared with the control group, whereas this promoting
effect of LPS on TLR4 expression was attenuated by agomir-93
(Fig. 4F). These results
suggest that miR-93 targets TLR4 and suppresses its expression
induced by LPS in vitro and in vivo.
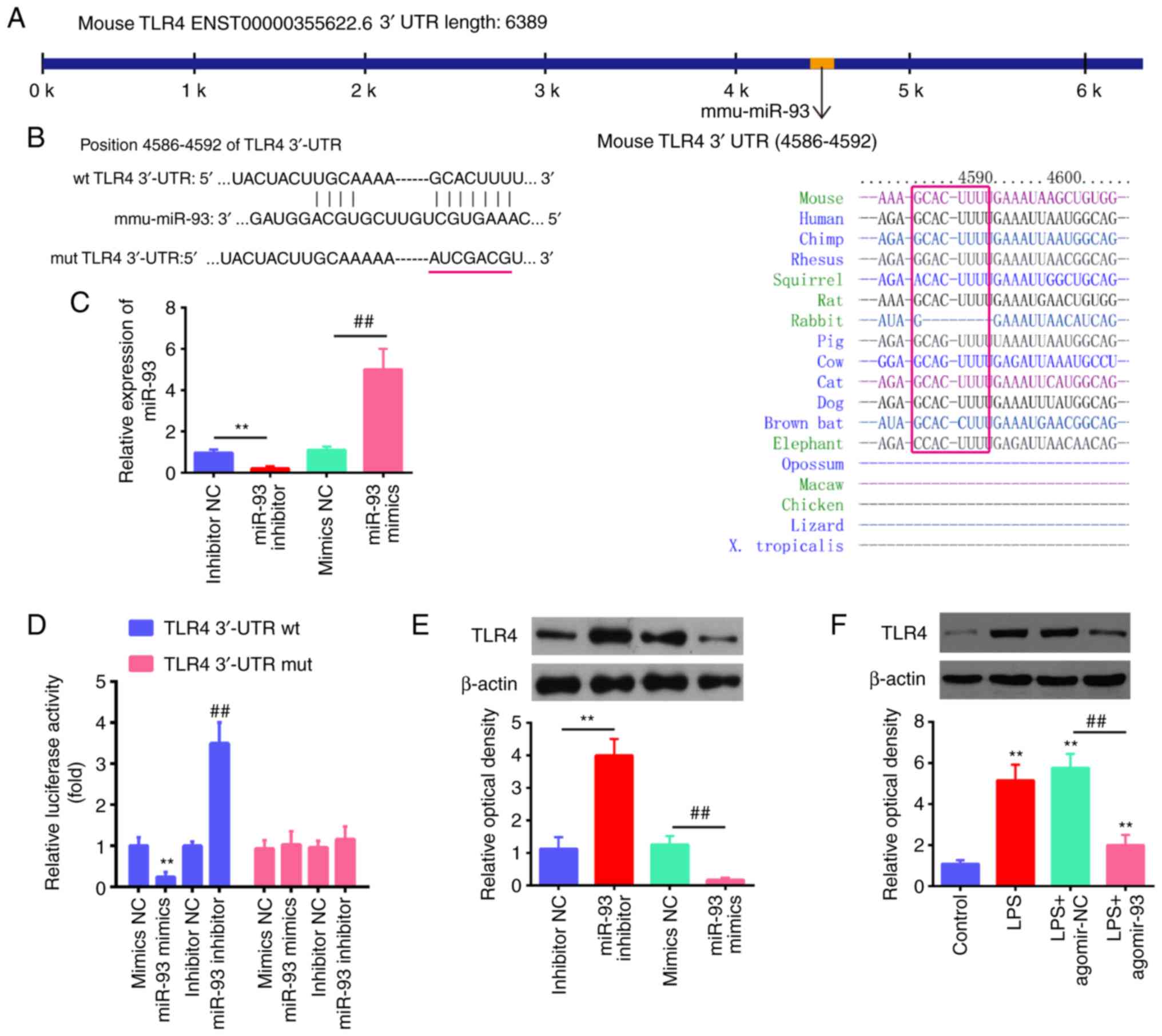 | Figure 4TLR4 is a direct target of miR-93. (A
and B) The putative binding site of miR-93 and TLR4 is shown. (C)
The expression levels of miR-93 were measured by RT-qPCR following
transfection with miR-93 mimics or miR-93 inhibitor in RAW264.7
cells. (D) Luciferase activity of RAW264.7 cells was detected by a
dual luciferase assay. Data are the means ± SD (n=3) of one
representative experiment, **P<0.01 vs. mimics NC,
##P<0.01 vs. inhibitor NC. (E) Protein expression
levels of TLR4 following transfection with miR-93 mimics or miR-93
inhibitor measured by western blot analysis. Data are the means ±
SD (n=3) of one representative experiment, **P<0.01
vs mimics NC, ##P<0.01 vs inhibitor NC. (F) Mice were
injected intravenously with agomir-93, and agomir NC (8 mg/kg) 24 h
prior to the LPS (2 mg/kg) challenge. After the LPS administration
for 24 h, the mice were sacrificed and lung tissues were then
collected for the detection of the protein expression levels of
TLR4 by western blot analysis. Data are the means ± SD (n=3) of one
representative experiment, **P<0.01 vs. control
group. ##P<0.01 vs. the LPS + agomiR-NC group. LPS,
lipopolysaccharide; ALI, acute lung injury; TLR4, Toll-like
receptor 4. |
miR-93 inhibits LPS-induced inflammatory
responses through the TLR4/MyD88/NF-κB pathway
Previous studies have reported that the NF-κB
pathway, acting as the downstream signaling effector of TLR4, plays
a crucial role in LPS-induced ALI (43,44). Thus, the present study sought to
determine whether miR-93 influences the TLR4/MyD88/NF-κB pathway in
a mouse model of ALI. The expression levels of key proteins of this
pathway, including MyD88, IκB-α, p-IκB-α, p65 and nuclear p-p65
were measured by western blot analysis. As shown in Fig. 5A and B, exposure to LPS
significantly increased the expression levels of MyD88, p-IκB-α and
nuclear p-p65, compared with those in the control group. However,
the promoting effects of LPS on the expression levels of these
proteins were markedly attenuated by agomir-93 injection. Similar
results were observed for the expression of p-p65, as determined by
IHC staining (Fig. 5C).
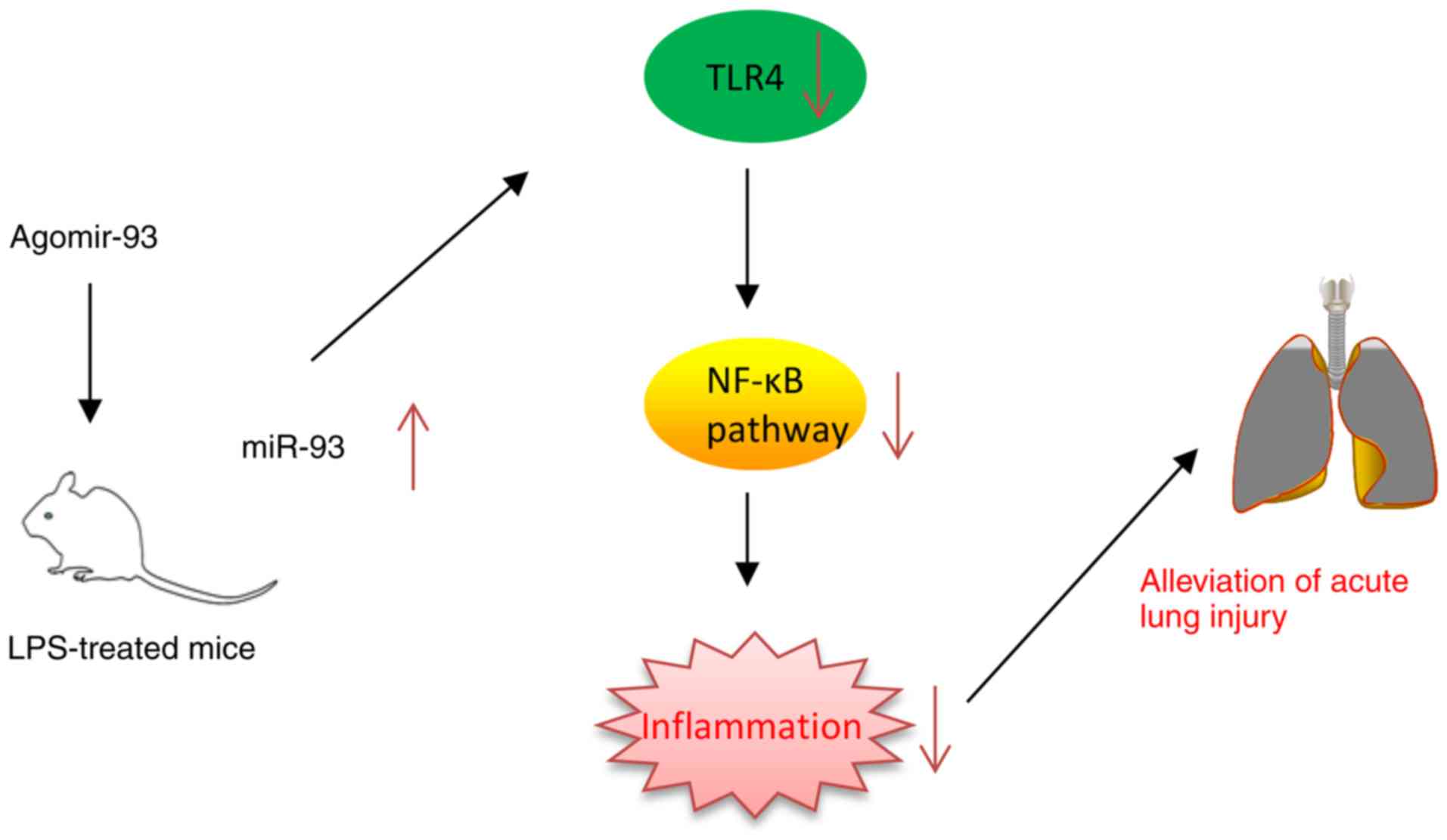
Collectively, these results suggest that miR-93 inhibited the
TLR4-mediated activation of the NF-κB pathway, and thus suppressed
the inflammatory response in LPS treated mice (Fig. 6).
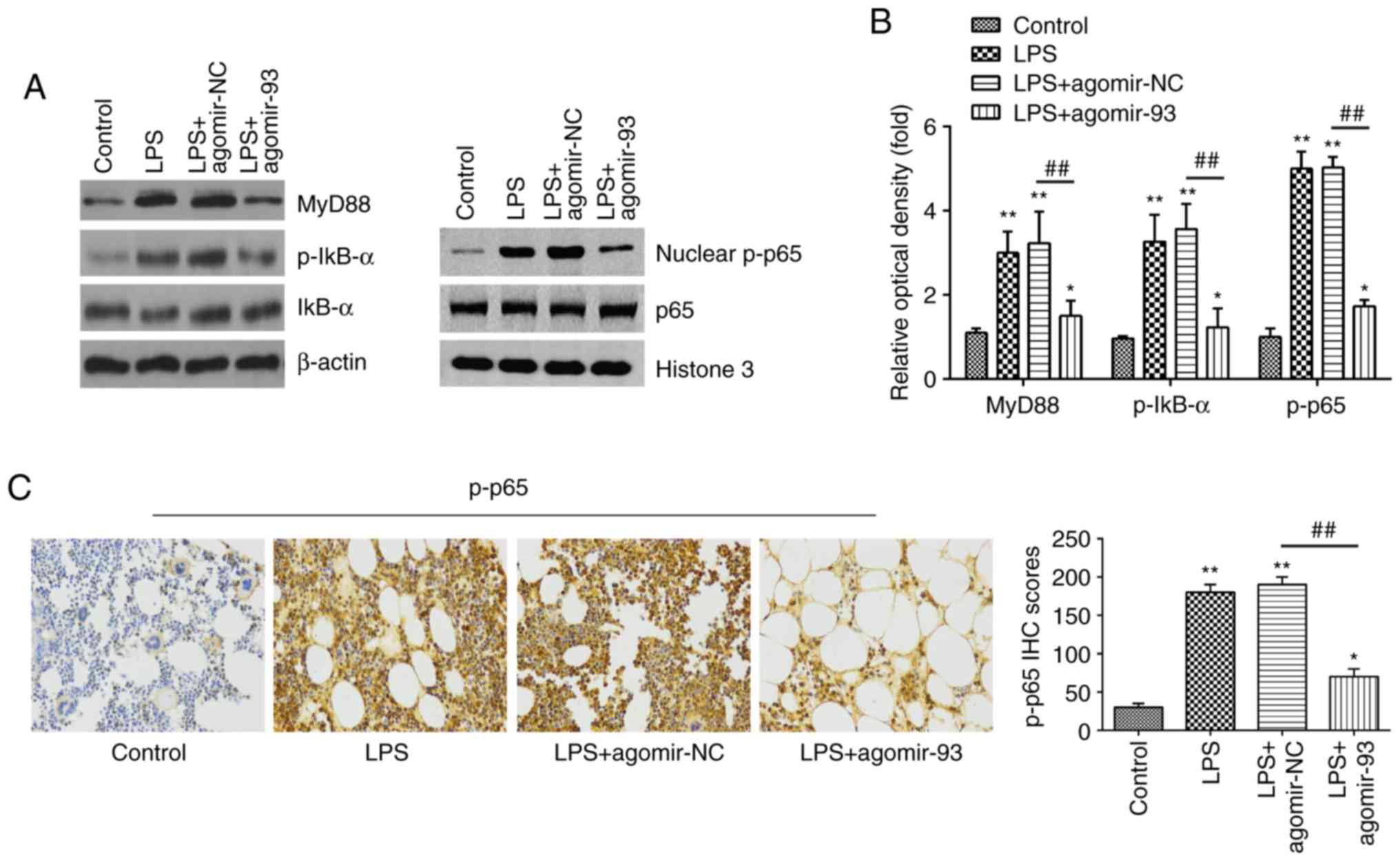 | Figure 5miR-93 inhibits the LPS-induced
inflammatory response through the TLR4/MyD88/NF-κB pathway. Mice
were injected intravenously with agomir-93, and agomir NC (8 mg/kg)
24 h prior to the LPS (2 mg/kg) challenge. After the LPS
administration for 24 h, the mice were sacrificed and lung tissues
were then collected for analysis. (A) The levels of MyD88, IκB-α,
p-IκB-α, p65 and nuclear p-p65 were measured by western blot
analysis. (B) The bands were semi-quantitatively analyzed using
Image J software, and normalized to β-actin density. (C) The
expression of nuclear p-p65 was determined by IHC (×400
magnification) and IHC scores are calculated. Data are the means ±
SD (n=3) of one representative experiment. *P<0.05,
**P<0.01 vs. control group. ##P<0.01
vs. LPS + agomiR-NC group. LPS, lipopolysaccharide; ALI, acute lung
injury; TLR4, Toll-like receptor 4; MyD88, myeloid differentiation
primary response 88. |
Discussion
In the present study, it was found that miR-93
expression was downregulated in lung tissues and BALF from mice
with LPS-induced ALI. Agomir-93 injection attenuated LPS-induced
lung injury through the suppression of the pulmonary inflammatory
response by blocking the TLR4/MyD88/NF-κB pathway in mice. On the
basis of this, miR-93 may thus serve as a potential therapeutic
target for the treatment of ALI.
A number of studies have demonstrated the
involvement of miRNAs in the regulation of the inflammatory
response in ALI. Several miRNAs, such as miR-24 and miR-27b, have
been shown to attenuate lung injury in vitro and in
vivo (45,46). miR-93 has previously been reported
to play an important role in inflammatory diseases. For example, in
a mouse model of cerebral ischemia reperfusion (CIR), miR-93
overexpression was shown to inhibit the inflammatory response by
the regulation of IRAK4 expression (21). Xu et al found that miR-93
suppressed pro-inflammatory cytokine production in a rat model of
acute ocular inflammation (47).
However, to date, the role of miR-93 in the pulmonary inflammatory
responses of ALI has not yet been extensively studied. In the
present study, it was found that miR-93 expression was
significantly downregulated in lung tissues and BALF from mice with
LPS-induced ALI. It was also observed that agomiR-93 injection
markedly attenuated LPS-induced lung damage by suppressing the
inflammatory response in mice, which indicates the
anti-inflammatory roles of miR-93 in ALI. However, the molecular
mechanisms underlying the attenuating effects of miR-93 on ALI
remain unclear.
TLR4 is a well-known pathogen recognition receptor
that has been proposed to contribute to the inflammatory response
in LPS-induced ALI, and its inhibition may provide further
therapeutic value (48). For
example, Guo et al demonstrated that the inhibition of TLR4
lessened the severity of ALI in a rat model (49). Xia et al reported that
YiQiFuMai (YQFM), re-developed based on the well-known traditional
Chinese medicinal formula, Sheng-Mai-San, improved the recovery of
lung tissue damage induced by ALI in mice through the inhibition of
the TLR4 pathway (50). Notably,
a previous study demonstrated that miR-93 inhibited chondrocyte
inflammation in osteoarthritis (OA) by targeting TLR4 (20). Another study by Tang et al
demonstrated that miR-93 protected cardiomyocytes against the
LPS-induced inflammatory response by inhibiting TLR4 expression
(51). In the present study, TLR4
was identified as a target of miR-93 in RAW264.7 cells and its
expression was negatively regulated by miR-93 in mice with ALI.
These results suggest that miR-93 may exert its anti-inflammatory
effects through the suppression of TLR4.
It is a growing consensus that the TLR4/NF-κB
signaling pathway has been implicated in the inflammatory responses
of different diseases, including ALI (41,52,53). For example, Meng et al
reported that umbelliferone (Umb) protected against LPS-induced
lung injury by alleviating the inflammatory response through the
inhibition of the TLR4/NF-κB pathway in mice (54). Previous studies have reported that
miRNAs play a regulatory role in the inflammatory response induced
by ALI through the TLR4/NF-κB pathway. For example, Yang et
al demonstrated that the upregulation of miR-140-5p inhibited
inflammatory cyto-kine production in ALI through the
TLR4/MyD88/NF-κB pathway (36).
Ju et al found that miR-27a alleviated LPS-induced lung
injury and the inflammatory response in mice by modulating this
pathway (37). Given the
important role of the TLR4/MyD88/NF-κB signaling pathway in ALI,
the present study aimed to investigate whether miR-93 attenuates
LPS-induced lung injury by regulating the TLR4/MyD88/NF-κB
signaling pathway. The findings of the present study revealed that
agomir-93 injection significantly inhibited the LPS-induced
activation of the TLR4/MyD88/NF-κB signaling pathway in
vivo. These results suggest that the protective effect of
miR-93 on LPS induced lung injury may be mediated via the
TLR4/MyD88/NF-κB signaling pathway.
In conclusion, the findings of the present study
demonstrate that miR-93 may exert protective effects against
LPS-induced ALI both in vitro and in vivo by
targeting the TLR4/MyD88/NF-κB signaling pathway. These results
suggest that miR-93 might hold promise as a potential therapeutic
target for use in the treatment of ALI.
Funding
The present study was supported by the Key R&D
projects of science and Technology Department of Sichuan Province
(grant no. 2020YFS0104).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HG and DX performed the experiments, contributed to
data analysis and wrote the manuscript. LG and XL conceptualized
the study design, contributed to data analysis and experimental
materials. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal studies were performed according to the
guidelines of the Chinese Council on Animal Care and ethical
approval was obtained for the use of animals prior to the start of
the study from the ethics committee of the West China Second
University Hospital, Sichuan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Herridge MS, Tansey CM, Matte A, Tomlinson
G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart
TE, et al: Functional disability 5 years after acute respi-ratory
distress syndrome. N Engl J Med. 364:1293–1304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mendez JL and Hubmayr RD: New insights
into the pathology of acute respiratory failure. Curr Opin Crit
Care. 11:29–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Cao J, Feng J, Wu Q and Chen BY:
A study of noninvasive positive-pressure mechanical ventilation in
the treatment of acute lung injury with a complex critical care
ventilator. J Int Med Res. 42:788–798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsushima K, King LS, Aggarwal NR, De
Gorordo A, D'Alessio FR and Kubo K: Acute lung injury review.
Intern Med. 48:621–630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deng J, Wang DX, Liang AL, Tang J and
Xiang DK: Effects of baicalin on alveolar fluid clearance and
α-ENaC expression in rats with LPS-induced acute lung injury. Can J
Physiol Pharmacol. 95:122–128. 2017. View Article : Google Scholar
|
|
6
|
Fragoso IT, Ribeiro EL, Gomes FO, Donato
MA, Silva AK, Oliveira AC, Araújo SM, Barbosa KP, Santos LA and
Peixoto CA: Diethylcarbamazine attenuates LPS-induced acute lung
injury in mice by apoptosis of inflammatory cells. Pharmacol Rep.
69:81–89. 2017. View Article : Google Scholar
|
|
7
|
Hu Y, Lou J, Mao YY, Lai TW, Liu LY, Zhu
C, Zhang C, Liu J, Li YY, Zhang F, et al: Activation of MTOR in
pulmonary epithelium promotes LPS-induced acute lung injury.
Autophagy. 12:2286–2299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsia TC and Yin MC: Post-Intake of S-Ethyl
cysteine and S-Methyl cysteine improved LPS-Induced acute lung
injury in mice. Nutrients. 8:E5072016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Porcherie A, Cunha P, Trotereau A, Roussel
P, Gilbert FB, Rainard P and Germon P: Repertoire of Escherichia
coli agonists sensed by innate immunity receptors of the bovine
udder and mammary epithelial cells. Vet Res. 43:142012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wieland CW, Florquin S, Maris NA, Hoebe K,
Beutler B, Takeda K, Akira S and van der Poll T: The
MyD88-dependent, but not the MyD88-independent, pathway of TLR4
signaling is important in clearing nontypeable haemophilus
influenzae from the mouse lung. J Immunol. 175:6042–6049. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vilahur G and Badimon L:
Ischemia/reperfusion activates myocardial innate immune response:
The key role of the toll-like receptor. Front Physiol. 5:4962014.
View Article : Google Scholar
|
|
12
|
Baker RG, Hayden MS and Ghosh S: NF-κB,
inflammation, and metabolic disease. Cell Metab. 13:11–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chuffa LG, Fioruci-Fontanelli BA, Mendes
LO, Ferreira Seiva FR, Martinez M, Fávaro WJ, Domeniconi RF,
Pinheiro PF, Delazari Dos Santos L and Martinez FE: Melatonin
attenuates the TLR4-mediated inflammatory response through MyD88-
and TRIF-dependent signaling pathways in an in vivo model of
ovarian cancer. BMC Cancer. 15:342015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng J, Zou Y, Chen J, Qin F, Chen X, Chen
X and Dai S: sTLR4/sMD-2 complex alleviates LPS-induced acute lung
injury by inhibiting pro-inflammatory cytokines and chemokine CXCL1
expression. Exp Ther Med. 16:4632–4638. 2018.PubMed/NCBI
|
|
15
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suo T, Chen GZ, Huang Y, Zhao KC, Wang T
and Hu K: MiRNA-1246 suppresses acute lung injury-induced
inflammation and apoptosis via the NF-κB and Wnt/β-catenin signal
pathways. Biomed Pharmacother. 108:783–791. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ling Y, Li ZZ, Zhang JF, Zheng XW, Lei ZQ,
Chen RY and Feng JH: MicroRNA-494 inhibition alleviates acute lung
injury through Nrf2 signaling pathway via NQO1 in sepsis-associated
acute respiratory distress syndrome. Life Sci. 210:1–8. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo Z, Gu Y, Wang C, Zhang J, Shan S, Gu
X, Wang K, Han Y and Ren T: Enforced expression of miR-125b
attenuates LPS-induced acute lung injury. Immunol Lett. 162:18–26.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hua Q, Chen Y, Liu Y, Li M, Diao Q, Xue H,
Zeng H, Huang L and Jiang Y: Circular RNA 0039411 is involved in
neodymium oxide-induced inflammation and antiproliferation in a
human bronchial epithelial cell line via sponging miR-93-5p.
Toxicol Sci. 170:69–81. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding Y, Wang L, Zhao Q, Wu Z and Kong L:
MicroRNA93 inhibits chondrocyte apoptosis and inflammation in
osteoarthritis by targeting the TLR4/NFKB signaling pathway. Int J
Mol Med. 43:779–790. 2019.
|
|
21
|
Tian F, Yuan C, Hu L and Shan S:
MicroRNA-93 inhibits inflammatory responses and cell apoptosis
after cerebral ischemia reperfusion by targeting interleukin-1
receptor-associated kinase 4. Exp Ther Med. 14:2903–2910. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu Z, Zhang C, Cheng L, Hu M, Tao H and
Song L: The microRNA miR-17 regulates lung FoxA1 expression during
lipopolysaccharide-induced acute lung injury. Biochem Biophys Res
Commun. 445:48–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carbone L, Carbone ET, Yi EM, Bauer DB,
Lindstrom KA, Parker JM, Austin JA, Seo Y, Gandhi AD and Wilkerson
JD: Assessing cervical dislocation as a humane euthanasia method in
mice. J Am Assoc Lab Anim Sci. 51:352–356. 2012.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Barreto TR, Costola-de-Souza C, Margatho
RO, Queiroz- Hazarbassanov N, Rodrigues SC, Felício LF,
Palermo-Neto J and Zager A: Repeated Domperidone treatment
modulates pulmonary cytokines in LPS-induced acute lung injury in
mice. Int Immunopharmacol. 56:43–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duan Y, Learoyd J, Meliton AY, Leff AR and
Zhu X: Inhibition of Pyk2 blocks lung inflammation and injury in a
mouse model of acute lung injury. Respir Res. 13:42012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimizu T, Kasamatsu A, Yamamoto A, Koike
K, Ishige S, Takatori H, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H
and Uzawa K: Annexin A10 in human oral cancer: Biomarker for
tumoral growth via G1/S transition by targeting MAPK signaling
pathways. PLoS One. 7:e455102012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iyoda M, Kasamatsu A, Ishigami T,
Nakashima D, Endo-Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H and
Uzawa K: Epithelial cell transforming sequence 2 in human oral
cancer. PLoS One. 5:e140822010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koyama T, Ogawara K, Kasamatsu A, Okamoto
A, Kasama H, Minakawa Y, Shimada K, Yokoe H, Shiiba M, Tanzawa H
and Uzawa K: ANGPTL3 is a novel biomarker as it activates ERK/MAPK
pathway in oral cancer. Cancer Med. 4:759–769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li C, Yang D, Cao X, Wang F, Jiang H, Guo
H, Du L, Guo Q and Yin X: LFG-500, a newly synthesized flavonoid,
attenuates lipopolysaccharide-induced acute lung injury and
inflammation in mice. Biochem Pharmacol. 113:57–69. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lei C, Jiao Y, He B, Wang G, Wang Q and
Wang J: RIP140 down-regulation alleviates acute lung injury via the
inhibition of LPS-induced PPARγ promoter methylation. Pulm
Pharmacol Ther. 37:57–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao Y, Sun F and Lei M: MiR-25 inhibits
sepsis-induced cardiomyocyte apoptosis by targetting PTEN. Biosci
Rep. 38:BSR201715112018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Q, Xu HX, Li JP, Wang S, Fu Z, Jia J,
Wang L, Zhu ZF, Lu R and Yao Z: Growth differentiation factor 15
induces growth and metastasis of human liver cancer stem-like cells
via AKT/GSK-3β/β-catenin signaling. Oncotarget. 8:16972–16987.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang C, Zeng L, Zhang T, Liu J and Wang W:
Casticin inhibits lipopolysaccharide-induced acute lung injury in
mice. Eur J Pharmacol. 789:172–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alghetaa H, Mohammed A, Sultan M, Busbee
P, Murphy A, Chatterjee S, Nagarkatti M and Nagarkatti P:
Resveratrol protects mice against SEB-induced acute lung injury and
mortality by miR-193a modulation that targets TGF-β signalling. J
Cell Mol Med. 22:2644–2655. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang Y, Liu D, Xi Y and Li J, Liu B and Li
J: Upregulation of miRNA-140-5pinhibits inflammatory cytokines in
acute lung injury through the MyD88/NF-κB signaling pathway by
targeting TLR4. Exp Ther Med. 16:3913–3920. 2018.PubMed/NCBI
|
|
37
|
Ju M, Liu B, He H, Gu Z, Liu Y, Su Y, Zhu
D, Cang J and Luo Z: MicroRNA-27a alleviates LPS-induced acute lung
injury in mice via inhibiting inflammation and apoptosis through
modulating TLR4/MyD88/NF-κB pathway. Cell Cycle. 17:2001–2018.
2018. View Article : Google Scholar :
|
|
38
|
Yang Y, Yang F, Yu X, Wang B, Yang Y and
Zhou X, Cheng R, Xia S and Zhou X: MiR-16 inhibits NLRP3
inflammasome activation by directly targeting TLR4 in acute lung
injury. Biomed Pharmacother. 112:1086642019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dong Z and Yuan Y: Accelerated
inflammation and oxidative stress induced by LPS in acute lung
injury: Lotanhibition by ST1926. Int J Mol Med. 41:3405–3421.
2018.PubMed/NCBI
|
|
40
|
Hu X, Liu S, Zhu J and Ni H: Dachengqi
decoction alleviates acute lung injury and inhibits inflammatory
cytokines production through TLR4/NF-κB signaling pathway in vivo
and in vitro. J Cell Biochem. 120:8956–8964. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu JX, Li X, Yan FG, Pan QJ, Yang C, Wu
MY, Li G and Liu HF: Protective effect of forsythoside B against
lipopolysaccharide-induced acute lung injury by attenuating the
TLR4/NF-κB pathway. Int Immunopharmacol. 66:336–346. 2019.
View Article : Google Scholar
|
|
42
|
Deng G, He H, Chen Z, OuYang L, Xiao X, Ge
J, Xiang B, Jiang S and Cheng S: Lianqinjiedu decoction attenuates
LPS-induced inflammation and acute lung injury in rats via
TLR4/NF-κB pathway. Biomed Pharmacother. 96:148–152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang F, Zhu M, Jiang N, Zhang M, Feng L
and Jia X: Paeonol ameliorates lipopolysaccharides-induced acute
lung injury by regulating TLR4/MyD88/NF-κB signaling pathway.
Pharmazie. 74:101–106. 2019.PubMed/NCBI
|
|
44
|
Wang D, Wang X, Tong W, Cui Y, Li X and
Sun H: Umbelliferone alleviates lipopolysaccharide-induced
inflammatory responses in acute lung injury by down-regulating
TLR4/MyD88/NF-κB signaling. Inflammation. 42:440–448. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin Y and Yang Y: MiR-24 inhibits
inflammatory responses in LPS-induced acute lung injury of neonatal
rats through targeting NLRP3. Pathol Res Pract. 215:683–688. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huang Y, Huang L, Zhu G, Pei Z and Zhang
W: Downregulated microRNA-27b attenuates lipopolysaccharide-induced
acute lung injury via activation of NF-E2-related factor 2 and
inhibition of nuclear factor κB signaling pathway. J Cell Physiol.
234:6023–6032. 2019. View Article : Google Scholar
|
|
47
|
Xu Y, Jin H, Yang X, Wang L, Su L, Liu K,
Gu Q and Xu X: MicroRNA-93 inhibits inflammatory cytokine
production in LPS-stimulated murine macrophages by targeting IRAK4.
FEBS Lett. 588:1692–1698. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
He X, Qian Y, Li Z, Fan EK, Li Y, Wu L,
Billiar TR, Wilson MA, Shi X and Fan J: TLR4-Upregulated IL-1β and
IL-1RI promote alveolar macrophage pyroptosis and lung inflammation
through an autocrine mechanism. Sci Rep. 6:316632016. View Article : Google Scholar
|
|
49
|
Guo S, Jiang K, Wu H, Yang C, Yang Y, Yang
J, Zhao G and Deng G: Magnoflorine ameliorates
lipopolysaccharide-induced acute lung injury via suppressing NF-κB
and MAPK activation. Front Pharmacol. 9:9822018. View Article : Google Scholar
|
|
50
|
Xia Y, S D, Jiang S, Fan R, Wang Y, Wang
Y, Tang J, Zhang Y, He RL, Yu B and Kou J: YiQiFuMai lyophilized
injection attenuates particulate matter-induced acute lung injury
in mice via TLR4-mTOR-autophagy pathway. Biomed Pharmacother.
108:906–913. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tang B, Xuan L, Tang M, Wang H, Zhou J,
Liu J, Wu S, Li M, Wang X and Zhang H: MiR-93-3p alleviates
lipopolysaccharide-induced inflammation and apoptosis in H9c2
cardiomyocytes by inhibiting toll-like receptor 4. Pathol Res
Pract. 214:1686–1693. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun XJ, Li XQ, Wang XL, Tan WF and Wang
JK: Sevoflurane inhibits nuclear factor-κB activation in
lipopolysaccha-ride-induced acute inflammatory lung injury via
toll-like receptor 4 signaling. PLoS One. 10:e01227522015.
View Article : Google Scholar
|
|
53
|
Bai S, Zhou H and Wu L: Bone marrow
stromal cells improved functional recovery in spinal cord injury
rats partly via the Toll-like receptor-4/nuclear factor-κB
signaling pathway. Exp Ther Med. 17:444–448. 2019.PubMed/NCBI
|
|
54
|
Meng X, Liu J, Wang H, Chen P and Wang D:
MicroRNA-126-5p downregulates BCAR3 expression to promote cell
migration and invasion in endometriosis. Mol Cell Endocrinol.
494:1104862019. View Article : Google Scholar : PubMed/NCBI
|