Introduction
Human adipose-derived mesenchymal stem cells
(hADSCs), a newly recognized subpopulation of mesenchymal stem
cells (MSCs), have the ability to differentiate into bone,
cartilage, muscle and adipose tissue (1,2),
and have thus been suggested for use as potential seed cells
clinically (3,4). Osteogenic differentiation, a key
function of hADSCs, plays a fundamental role in bone formation and
remodeling. The elucidation of the molecular mechanisms of hADSCs
osteogenic differentiation could promote the development of
regenerative therapies for benign and malignant bone diseases.
Several regulatory pathways, including the PI3K/AKT (5,6),
Wnt signaling (7) and
transforming growth factor-β (TGF-β)/bone morphogenic protein (BMP)
signaling pathways (8) have been
implicated in the regulation of osteoblastic differentiation;
however, the regulatory effects of the molecular pathways has yet
to be investigated.
MicroRNAs (miRNAs or miRs) are a family of short,
small, non-coding RNAs (an average size of 22 nucleotides), which
negatively regulate target gene expression through either
translation repression or RNA degradation (9,10).
Increasing evidence suggests that the osteoblastic differentiation
of hADSCs is widely regulated by temporarily-expressed miRNAs. For
instance, Luzi et al. demonstrated that miR-26a inhibited
the osteogenesis of hADSCs by suppressing the translation of SMAD1,
a well-known osteogenic marker (11). Li et al. demonstrated that
miR-154-5p regulated the osteogenic differentiation of hADSCs under
tensile stress by targeting Wnt11 (12). Moreover, the overexpression of
miR-196a targeting HOXC8 has been shown to enhance osteogenic
differentiation through the downregulation of hADSC proliferation
(13). Despite these studies
demonstrating the function of miRNA in the osteogenic
differentiation of hADSCs, a limited number of studies have
investigated the mechanisms of action of miRNAs during the
osteogenic differentiation of hADSCs (14,15).
In the present study, the differential expression of
miRNAs was investigated using miRNA microarray analysis during the
osteogenic differentiation of hADSCs. Moreover, the biological
functions and mechanisms of action of miR-29b in the osteogenic
differentiation of hADSCs were examined. The findings presented
herein provide a better understanding of the molecular mechanisms
underlying the complex processes of hADSCs in osteoblastic
differentiation.
Materials and methods
Cells and cell culture
Adipose tissues were obtained from 3 healthy donors
undergoing tumescence liposuction (age range, 32-52 years; 2 males
and 1 female) who underwent surgery at the Shanghai General
Hospital of Nanjing Medical University between January, 2018 and
April, 2018. Clinical and biochemical examinations confirmed that
these subjects did not suffer from acute inflammation, cancers,
endocrine diseases or infectious diseases. The hADSCs were isolated
as previously described (16).
hADSCs were cultured in basal MSC culture medium (bM) [Dulbecco's
modified Eagle's medium (DMEM), 10% FCS penicillin/streptomycin,
L-glutamine] (Lonza Group, Ltd.). The cells from the 3 donors were
pooled together for all experiments and the experiments were
performed 3 times. The present study was approved by the Ethics
Committee of Shanghai General Hospital of Nanjing Medical
University (IRB approval no. 2018-0032). Informed consent was
obtained from all patients prior to study inclusion.
hADSC differentiation and staining
analysis
hADSCs were grown in basal MSC culture medium at
37°C for 2 days, followed by differentiation into osteoblasts,
adipocytes and chondrocytes. For osteogenic differentiation, the
culture medium was exchanged for osteogenic differentiation medium
(odM) [DMEM, 100 nM dexamethasone, L-glutamine, 50 mM ascorbate, 1%
penicillin/streptomycin, mesangial cell growth supplement (MCGS)
and 10 mM β-lycerophosphate] (Lonza Group, Ltd.). The medium was
changed every 3 days during the course of incubation (21 days) and
the cells were harvested and analyzed at 7, 14 and 21 days. Cells
cultured in bM medium served as a control. Alizarin Red S staining
was performed to assess the occurrence of calcium phosphate
deposition. Briefly, hADSCs were washed twice with PBS and fixed
with 4% paraformaldehyde for 10 min. The fixed cells were stained
with 500 µl of Alizarin Red solution (Sigma-Aldrich; Merck KGaA)
for 5 min at room temperature. For adipogenic differentiation, the
hADSCs were induced using the StemPro adipogenesis differentiation
kit media (Gibco; Thermo Fisher Scientific, Inc.). The
differentiation medium was changed every 3 days. After 21 days, Oil
Red O staining was performed for the assessment of lipid/fatty acid
droplets in adipocytes. Briefly, the fixed cells as above were
stained with filtered 0.6 mg/ml Oil Red O solution for 30 min at
room temperature. For chondrogenic differentiation, the hADSCs were
induced using an MSC Chondrogenic Differentiation kit (Cyagen
Bioscience, Inc.) according to the manufacturer's protocol. The
differentiation medium was changed every 3 days. After 21 days,
Alcian Blue staining was performed to assess the chondrogenic
differentiation potential of hADSCs. Briefly, cells were rinsed in
PBS and then fixed in methanol for 30 min, following Alcian blue
staining for 30 min at room temperature. All these images were
viewed by a microscopy at ×400 magnification.
Alkaline phosphatase (ALP) activity
The osteoblast phenotype was evaluated by
determining ALP activity. Briefly, hADSCs were collected and lysed
with RIPA buffer, and the total protein concentration was
determined with Pierce BCA Protein Assay kit (Thermo Fisher
Scientific). Relative ALP activity was normalized by the protein
concentration. Then, the ALP activity of the hADSCs was detected
with the Colorimetric Alkaline Phosphatase Assay kit (BioVision),
according to the manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
miRNA was extracted from the hADSCs using an
miRNeasy extraction kit (Qiagen, Inc.) according to the
manufacturer's instructions. cDNA was synthesized using TaqMan™
MicroRNA Reverse Transcription kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.). To quantify the mRNA levels of
runt-related transcription factor 2 (Runx2), osteopontin (OPN),
osteocalcin (OCN) and bone sialoprotein (BSP), total RNA was
isolated using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
cDNA was synthesized using the PrimeScript RT reagent kit (Takara).
Quantitative PCR was performed using a standard protocol from the
SYBR-Green PCR kit (Toyobo) on an ABI 7500 thermocycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.). All reactions were
performed in triplicate or duplicate. The relative levels of miRNAs
and mRNA in cells were normalized to U6 and GAPDH, respectively.
95°C for 10 min followed by 45 cycles consisting of 95°C for 15
sec, 60°C for 30 sec and 68°C for 60 sec were amplification
conditions. The sequences of the primers were as follows: miR-29b
forward, 5′-GGGGTAGCACCATTTGAA-3′ and reverse, 5′-TGC GTG TCG TGG
AGTC-3′; U6 forward, 5′-GCT TCG GCA GCA CAT ATA CTA AAAT-3′ and
reverse, 5′-CGC TTC ACG AAT TTG CGT GTCAT-3′; Runx2 forward, 5′-GTC
TCA CTG CCT CTC ACTTG-3′ and reverse, 5′-CAC ACA TCT CCT CCC
TTCTG-3′; OCN forward, 5′-ACA GAC AAG TCC CAC ACA GCAGC-3′ and
reverse, 5′-TGA AGG CTT TGT CAG ACT CAG GGC-3′; BSP forward, 5′-GCC
AGA GGA GCA ATC ACCAA-3′ and reverse, 5′-CAG GCT GGA GGT TCA
CTGGT-3′; GAPDH forward, 5′-GTC TCA CTG CCT CTC ACTTG-3′ and
reverse, 5′-GTG GTG AAG ACG CCA GTGGA-3′; OPN forward, 5′-TTG GCT
TTG CAG TCT CCT GCGG-3′ and reverse, 5′-AGG CAA GGC CGA ACA GGC
AAA-3′ and GAPDH forward, 5′-CGA GCC ACA TCG CTC AGACA-3′. The
relative expression of each gene was calculated using the
2−ΔΔCq method (17).
Microarray assay
Total RNA was isolated using a miRNAeasy mini kit
(Qiagen, Inc.), followed by labeling and hybridization with the
miRCURYTM LNA Array (v.16.0, Exiqon), which contained capture
probes targeting all miRNAs for human, mouse and rats registered in
the Sanger miRBase 21.0 database (www.mirbase.org/). The procedure and imaging processes
were as described previously (18).
Cell transfection
Agomir-29b, antagomir-29b, phosphatase and tensin
homolog (PTEN) overexpression plasmid and negative controls were
all provided by RiboBio Co., Ltd. When the hADSCs in the 12-well
plate grew to approximately 80% confluency, agomir-29b (0.1 nmol),
antagomir-29b (0.1 nmol), or 2 µg pcDNA-PTEN were transfected into
the cells at 37°C for 24 h, using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.).
Luciferase reporter assay
TargetScan Release 7.0 (http://targetscan.org/), Miranda (http://miranda.org.uk) and PicTar (https://pictar.mdc-berlin.de) were used to search for
the putative targets of miR-34a.pGL3-PTEN wild-type (Wt) or
pGL3-PTEN mutant type (mut) were co-transfected with agomir-29b
(0.1 nmol), antagomir-29b (0.1 nmol) into 293T cells (ATCC) in
24-well plates (2×105/well) using Lipofectamine 2000
(Invitrogen). At 24 h post-transfection, the double luciferase
activities were analyzed using the Dual-Luciferase Reporter Assay
system (Promega Corp.). The pRL-TK plasmid (Promega Corp.) was used
as a normalizing control.
Western blot analysis
Western blot analysis was performed as previously
described (19). Briefly, the
cells were lysed with RIPA buffer, and the total protein
concentration was determined with Pierce BCA Protein Assay kit
(Thermo Fisher Scientific). A total of 40 µg protein samples were
separated by 12% SDS-PAGE, and then transferred onto polyvinylidene
difluoride (Millipore) membranes at 200 mA for 1 h on ice. After
blocking with 5% skim milk for 2 h at 4°C overnight, the membranes
were incubated with the following primary antibodies: Anti-PTEN
(cat. no. 9188, Cell Signaling Technology, Inc. 1:1,000),
anti-Runx2 (cat. no. 12556, Cell Signaling Technology, Inc.
1:1,000), anti-OPN (cat. no. ab75285, Abcam, 1:1,000), anti-OCN
(cat. no. ab13420, Abcam, 1:1,000), anti-BSP (cat. no. ab52128,
Abcam, 1:1,000), anti-AKT (cat. no. 4691, Cell Signaling
Technology, Inc. 1:1,000), phosphorylated-AKT (cat. no. 9611, Cell
Signaling Technology, Inc. 1:1,000), glycogen synthase kinase
(GSK)-3β (cat. no. 12456, Cell Signaling Technology, Inc. 1:1,000),
phosphorylated-GSK-3β (Ser9) (cat. no. 8466, Cell Signaling
Technology, Inc. 1:1,000), β-catenin (cat. no. 8480, Cell Signaling
Technology, Inc. 1:1,000), phosphorylated-β-catenin (cat. no. 4176,
Cell Signaling Technology, Inc. 1:1,000) at 4°C overnight. After
washing with PBST, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibody (1:2,000; cat. no. 7074;
Cell Signaling Technology, Inc.) for 1 h at room temperature.
Finally, antibody labeling was detected by enhanced
chemiluminescence (Thermo Fisher Scientific, Inc.). Band intensity
was evaluated using ImageJ v1.48 U software (National Institutes of
Health).
Statistical analysis
All data were presented as the means ± standard
deviation (SD). The comparisons among data were calculated by
one-way ANOVA followed by Tukey's post hoc test by using SPSS
software, version 13.0 (SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-29b is upregulated during hADSC
osteogenic differentiation
To assess the potential of hADSCs to differentiate
into multiple lineages, several conventional analyses were
conducted following the induction of osteogenesis, adipogenesis and
chondrogenesis of hADSCs. As shown in Fig. 1A, hADSCs were able to
differentiate into osteogenic cells (Alizarin Red S staining),
adipocytes (Oil Red O staining) and chondrocytes (Alcian blue
staining), which suggested that the hADSCs successfully differented
into 3 different lineages. Alizarin Red S staining was performed to
visualize calcium deposition in osteoblasts at 7, 14 and 21 days
following the induction of osteogenesis. It was found that calcium
deposition in osteoblasts was significantly increased, and these
promoting effects were time-dependent (Fig. 1B). Moreover, the osteoblast
phenotype was evaluated by determining ALP activity. The results
revealed that ALP activity was also increased in a time-dependent
manner (Fig. 1C). Several
osteogenic markers, (Runx2 and BSP as early marker genes), (OPN and
OCN as late marker genes) were also examined at the genomic level.
As expected, the expression levels of these osteogenic markers were
markedly increased in a time-dependent manner (Fig. 1D-G), suggesting that the
osteogenic differentiation of hADSCs was induced successfully.
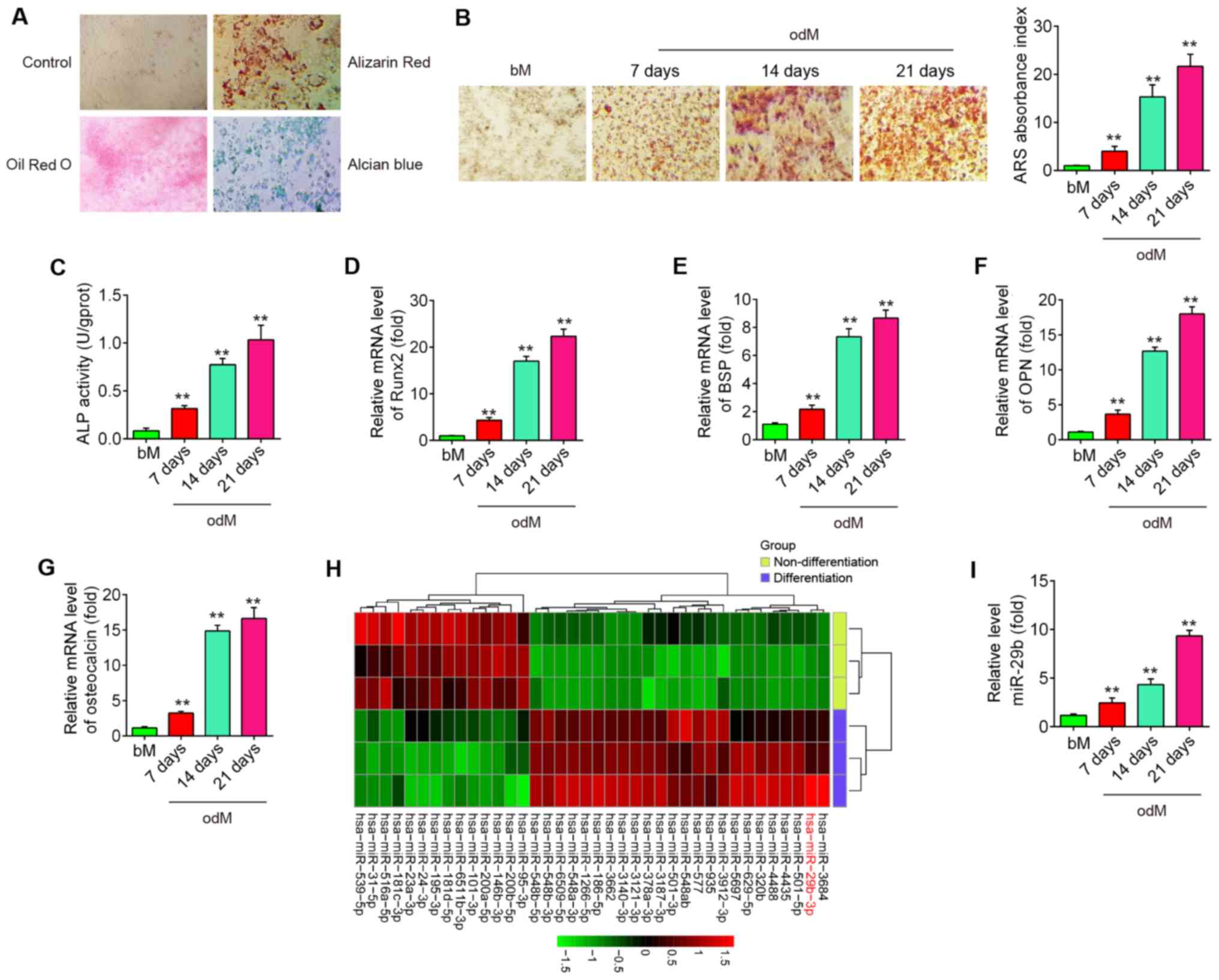 | Figure 1miR-29b is downregulated during hADSC
differentiation. (A) Photograph morphology of hADSCs following Oil
Red O, Alizarin Red S or Alcian Blue staining following 21 days of
culture in normal, adipogenic, osteogenic or chondrogenic
differentiation medium. Magnification, ×200. (B) Alizarin Red S
staining at 7, 14 and 21 days following osteogenic differentiation.
(C) Quantitative assessment of ALP activity by colorimetric
alkaline phosphatase assay at 7, 14 and 21 days after osteogenic
differentiation. RT-qPCR analysis was used to assess the mRNA
expression levels of the osteogenic differentiation markers (D)
Runx2, (E) BSP, (F) OPN and (G) osteocalcin at 7, 14 and 21 days
following osteogenic differentiation. (H) Heatmap of miRNA profiles
represents the differentially expressed miRNAs between
differentiated and undifferentiated hADSCs. Green indicates low
expression levels; red indicates high expression levels. (I)
RT-qPCR analysis was used to assess the expression levels of
miR-29b on days 7, 14 and 21 post-induction. Data are represented
as the means ± SD of 3 independent experiments.
**P<0.01 vs. bM group. hADSC, human adipose derived
mesenchymal stem cell; Runx2, runt-related transcription factor 2;
BSP, bone sialoprotein; OPN, osteopontin; bM, basal mesenchymal
stem cell culture medium. |
Previous studies have suggested that miRNAs play a
role in osteogenic differentiation (20,21). In the present study, using
microarray assay, it was found that the levels of 24 miRNAs were
increased and those of 14 miRNAs were decreased in the
differentiated cells compared with the undifferentiated control
cells (Fig. 1H). Of the
upregulated miRNAs, miR-29b expression displayed the most
alteration in this array. Of relevance, miR-29b has been identified
as a key regulator of the osteogenic differentiation of MSCs by
targeting anti-osteogenic factors or modulating bone extracellular
matrix proteins in a mineralogenic cell system (ABSa15) or in MC3T3
cells (22,23). However, whether miR-29b affects
the osteogenic differentiation of hADSCs remains unclear. To
further verify the expression of miR-29b, the expression of miR-29b
was quantified by RT-qPCR at different time points during the
osteogenesis process. As shown in Fig. 1I, the results revealed that
miR-29b expression was increased in a time-dependent manner
compared with the undifferentiated control cells. These results
suggest that miR-29b may play an important role in the osteogenic
differentiation of hADSCs.
Overexpression of miR-29b promotes the
osteogenic differentiation of hADSCs
To determine whether miR-29b affects the osteogenic
differentiation of hADSCs, the hADSCs was transfected with
agomir-29b, agomir-negative control (agomir NC), antagomir-29b and
antagomir NC during osteogenic differentiation. As shown in
Fig. 2A, miR-29b expression was
significantly increased (or decreased) in the hADSCs following
agomir-29b (or antagomir-29b) transfection, compared with the
agomir (or antagomir) NC group. At 16 h after transfection, the
medium was exchanged for osteogenic medium and the effects of
miR-29b on the hADSCs induced to differentiate into osteoblasts
were evaluated. It was observed that the overexpression of miR-29b
significantly increased the mineralization of the extracellular
matrix and ALP activity, while miR-29b inhibition produced the
opposite results (Fig. 2B and C).
Additionally, supporting the above-mentioned observations,
agomir-29b increased the expression of bone-specific markers, such
as Runx2, BSP, OPN and OCN at the mRNA and protein level, whereas
the expression of bone-specific markers was significantly
suppressed by antagomir-29b infection (Fig. 2D-H). More importantly, the effects
of miR-29b on the tri-lineage differentiation potential of hADSCs
were examined. As shown in Fig.
2I, miR-29b upregulation led to a significant increase in the
adipogenic and chondrogenic differentiation of hADSCs, while
miR-29b downregulation exerted an opposite effect, which is
consistent with the findings of a previous study (24). These data suggest that miR-29b
plays a regulatory role in the adipogenic and chondrogenic
differentiation of hADSCs. Notably, previous studies have paid
increasing attention to the osteogenic differentiation of hADSCs as
an effective treatment for musculoskeletal disorders, such as
osteoarthritis (OA) (25) and
rheumatoid arthritis (RA) (26).
Therefore, the present study focused on the progression of the
osteogenic differentiation of hADSCs regulated by miR-26. In the
future, the authors aim to further investigate the mechanisms
underlying the regulation of the adipogenic and chondrogenic
differentiation of hADSCs by miR-29b.
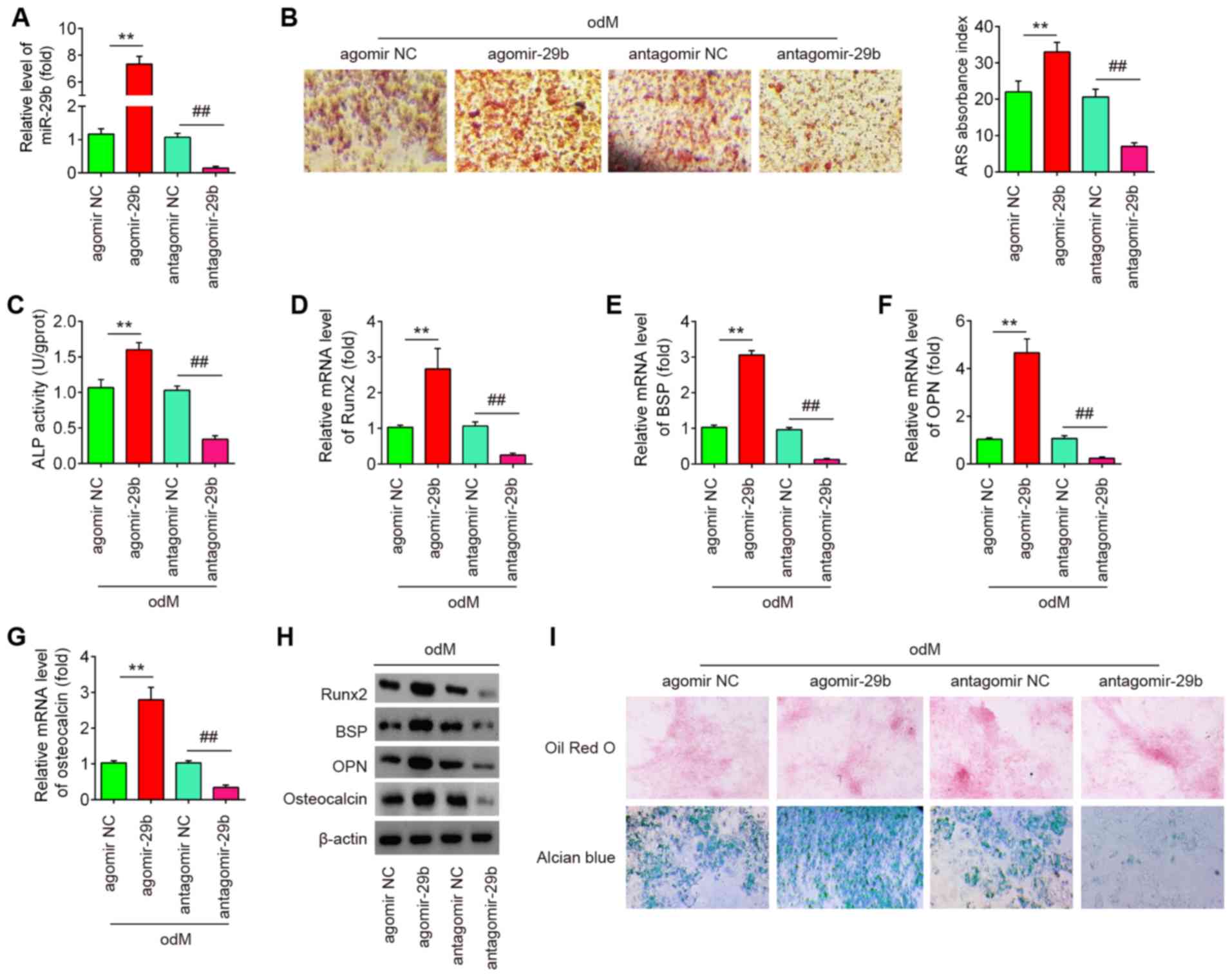 | Figure 2Overexpression of miR-29b promotes
the osteogenic differentiation of hADSCs. hADSCs were transfected
with agomir-29b, agomir NC, antagomir-29b and antagomir NC. At 48 h
following transfection, cells were induced with differentiation
medium. (A) RT-qPCR analysis was used to assess the expression
levels of miR-29b 48 h after transfection. (B) Osteogenic
differentiation was determined by staining with Alizarin Red S on
day 21 post-induction. (C) Quantitative assessment of ALP activity
by colorimetric alkaline phosphatase assay on day 21
post-induction. RT-qPCR analysis was used to assess the mRNA
expression levels of the osteogenic differentiation markers (D)
Runx2, (E) BSP, (F) OPN and osteocalcin (G) on day 21
post-induction. (H) Western blot analysis was used to assess the
protein expression levels of the osteogenic differentiation markers
(Runx2, BSP, OPN and osteocalcin). (I) Photograph morphology of
hADSCs following Oil Red O, Alizarin Red S or Alcian blue staining
after 21 days of culture in normal, adipogenic, osteogenic or
chondrogenic differentiation medium. Magnification, x200. Data were
represented as the means ± SD of 3 independent experiments.
**P<0.01 vs. agomir NC group; ##P<0.01
vs. antagomir NC group. hADSC, human adipose derived mesenchymal
stem cell; Runx2, runt-related transcription factor 2; BSP, bone
sialoprotein; OPN, osteopontin. |
PTEN is a direct target of miR-29b
Through bioinformatics online programs, such as
TargetScan, miRanda and PicTar, PTEN, a negative regulator of the
AKT/mTOR signaling pathway, was found to have a putative target
site of miR-29b in its 3′-UTR (Fig.
3A). To validate the possibility that PTEN was directly
targeted by miR-29b, a luciferase reporter assay was performed.
Luciferase reporter assay revealed that miR-29b mimics markedly
inhibited the luciferase activity in the constructs containing the
wild-type binding site of PTEN-3′UTR, and that the miR-29b
inhibitor caused an increased luciferase activity. However, the
luciferase activity of the constructs containing the mutant binding
site exhibited no difference upon miR-29b expression compared with
the negative control (Fig. 3B).
Subsequently, to further validate the suppressive effect of miR-29b
on PTEN, the expression of PTEN was measured in the hADSCs by
western blot analysis. As shown in Fig. 3C, the PTEN levels were
significantly downregulated following transfection with agomir-29b,
whereas they were increased following transfection with
antagomir-29b (Fig. 3C). In
addition, the expression of PTEN decreased in a time-dependent
manner during the osteogenic differentiation of hADSCs, as
determined by western blot analysis (Fig. 3D). These results indicated that
miR-29b can bind to PTEN through specific sites and can
downregulate PTEN expression during osteogenic differentiation.
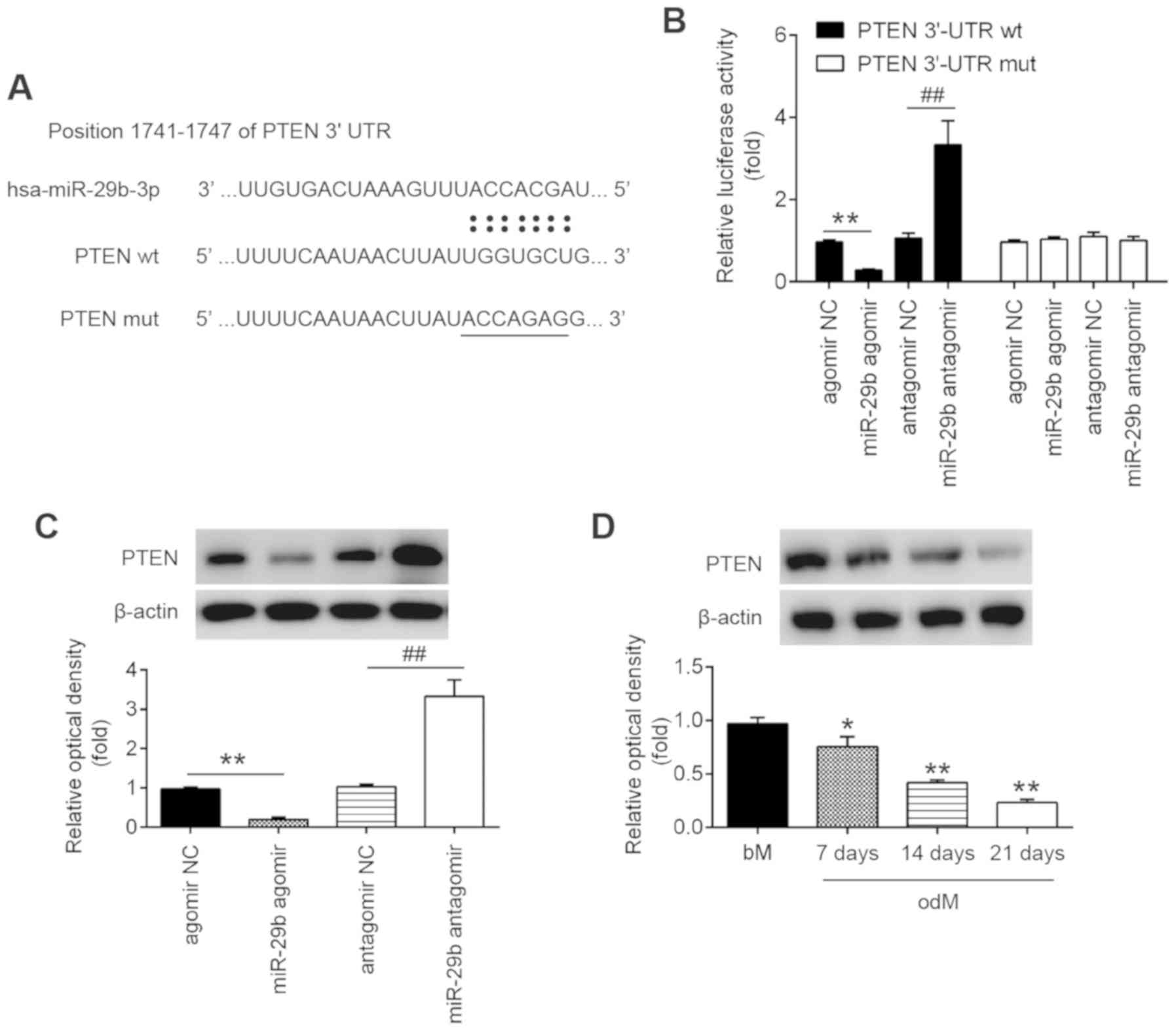 | Figure 3PTEN is a direct target of miR-29b.
(A) Putative binding sites of between miR-29b and PTEN. (B) hADSCs
were co-transfected with lucif-erase reporter constructs containing
wt or mut PTEN 3′-UTR and agomir-29b, agomir NC, antagomir-29b, and
antagomir NC and luciferase activity was then detected (n=3). Data
are represented as the means ± SD of 3 independent experiments.
**P<0.01 vs. agomir NC group; ##P<0.01
vs. antagomir NC group. (C) Western blot analysis was used to
assess the protein expression levels of PTEN in hADSCs following
transfection with agomir-29b, agomir NC, antagomir-29b, and
antagomir NC (n=3). Data are represented as the means ± SD of 3
independent experiments. **P<0.01 vs. agomir NC
group; ##P<0.01 vs. antagomir NC group. (D) Western
blot analysis was used to assess the protein expression levels of
PTEN in hADSCs at days 7, 14 and 21 post-induction. Data are
represented as the means ± SD of 3 independent experiments.
*P<0.05, **P<0.01 vs. bM group. PTEN,
phosphatase and tensin homolog; hADSC, human adipose derived
mesenchymal stem cell; bM, basal mesenchymal stem cell culture
medium. |
miR-29b activates the AKT/β-catenin
signaling pathway through the downregulation of PTEN
expression
PTEN is an important upstream regulator of the AKT
signaling pathway that has been implicated as an important
regulator of osteoblast differentiation in MSCs (27,28). However, the association between
miR-29b and the PTEN/Akt pathway in the osteogenic differentiation
of hADSCs remains unclear. As shown in Fig. 4, the introduction of agomir-29b
into the hADSCs significantly suppressed the expression of PTEN,
but promoted p-AKT protein expression. As is already known, the
AKT/GSK-3β/β-catenin signaling pathway is important for MSC
differentiation, including osteogenic differentiation (6,29).
Therefore, the present study further investigated whether miR-29b
affects the AKT/GSK-3β/β-catenin signaling pathway during the
osteogenic differentiation of hADSCs. As shown in Fig. 4, the overexpression of miR-29b in
the hADSCs induced the expression of phospho-GSK3β and
phospho-β-catenin compared with the cells infected with agomir-NC.
These results suggest that miR-29b promotes the osteogenic
differentiation of hADSCs via the PTEN/AKT/GSK-3β/β-catenin
pathway.
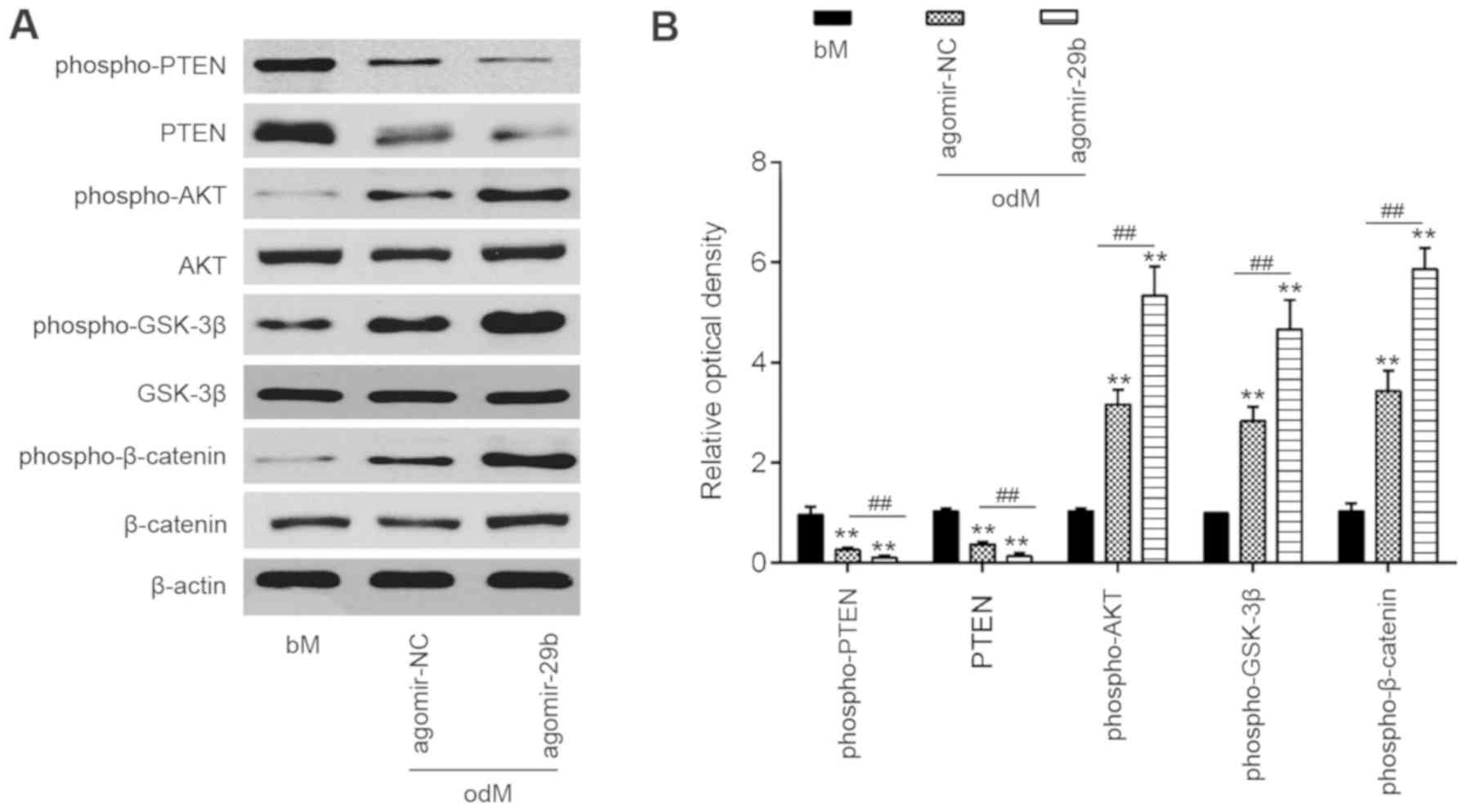 | Figure 4miR-29b activates the AKT/β-catenin
signaling pathway by downregulating PTEN expression. hADSCs were
transfected with agomir-29b and agomir NC, following the induction
of osteogenic differentiation. (A) Western blot analysis was used
to assess the protein expression levels of PTEN, phospho-AKT, AKT,
phospho-GSK-3β, GSK-3β, phospho-β-catenin and β-catenin. β-actin
was used as an internal control. (B) The bands were
semi-quantitatively analyzed using ImageJ software, normalized to
β-actin density. Data represent the means ± SD of 3 independent
experiments. **P< 0.01 vs. bM group,
##P< 0.01 vs. agomir NC group. PTEN, phosphatase and
tensin homolog; hADSC, human adipose derived mesenchymal stem cell;
GSK-3β, glycogen synthase kinase 3β. |
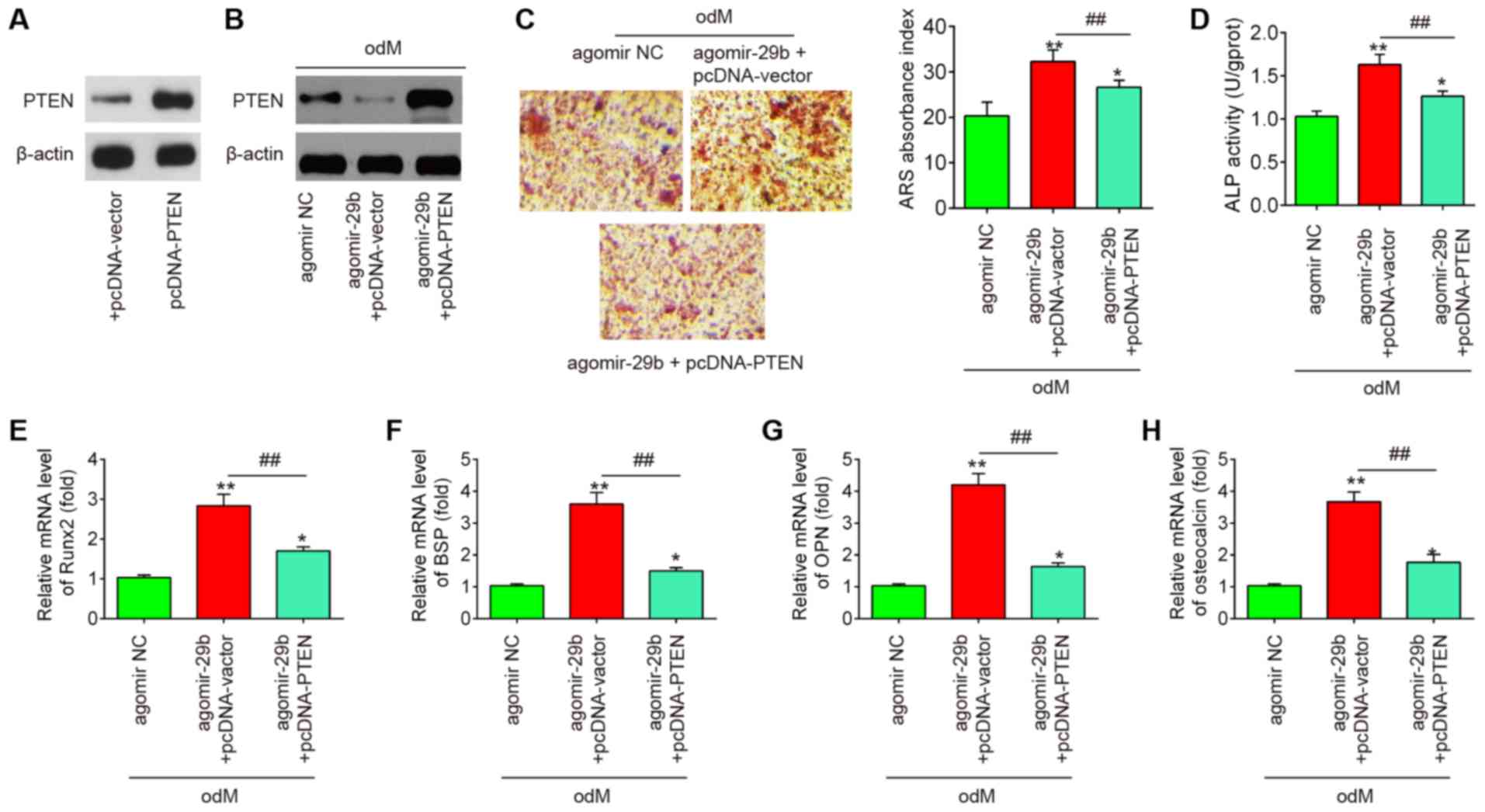
PTEN reverses the promoting effects of
miR-29b on the osteogenic differentiation of hADSCs
To further investigate whether PTEN is involved in
the miR-29b-mediated osteogenic differentiation of hADSCs, the
hADSCs were co-transfected with agomir-29b and pcDNA-PTEN during
osteogenic differentiation. It was found that the protein
expression of PTEN was significantly increased in the hADSCs when
they were transfected with the pcDNA-PTEN plasmid (Fig. 5A). As was expected, the protein
expression of PTEN was also significantly increased in the
miR-29b-overexpressing hADSCs when they were transfected with the
pcDNA-PTEN plasmid (Fig. 5B).
Subsequently, the ALP activity, the changes in matrix
mineralization and the levels of osteogenic marker genes were
assessed by ALP staining, Alizarin Red S staining and RT-qPCR
assays, respectively. The results revealed that the upregulation of
miR-29b by agomir-29b significantly enhanced the ALP activity and
the mineralization of extracellular matrix, and led to an increase
in Runx2, OPN, OCN and BSP mRNA expression, whereas the promoting
effects of agomir-29b were reversed by the overexpression of PTEN
(Fig. 5C-H). Collectively, these
data suggest that miR-29b promotes the osteogenic differentiation
of hADSCs by targeting PTEN.
Discussion
In the present study, miR-29b expression was found
to be significantly upregulated during the osteogenic
differentiation of hADSCs. Further experiments demonstrated that
miR-29b promoted the osteogenic differentiation of hADSCs through
the activation of the AKT/β-catenin signaling pathway via the
downregulating PTEN. These findings provide novel insight into the
mechanisms underlying osteogenic differentiation and contribute to
development of therapies for bone defects by targeting miRNAs.
Dozens of miRNAs have been demonstrated to modulate
numerous biological processes, including the differentiation of
hADSCs (30,31). For example, Huang et al.
demonstrated that the overexpression of miR-22 promoted the
osteogenic differentiation and inhibited the adipogenic
differentiation of hADSCs by suppressing HDAC6 protein expression
(32). Zeng et al.
revealed that miR-100 targeting bone morphogenetic protein receptor
type II (BMPR2) inhibited the osteogenic differentiation of hADSCs
(33). In the present study,
using microarray assay, miR-29b was found to be significantly
upregulated during the osteogenic induction of hADSCs, and this
effect was time-dependent. Previous studies have indicated that
miR-29b is aberrantly expressed during the osteoblast
differentiation of murine calvaria-derived preosteoblasts (MC3T3)
and acts as a positive regulator of the osteogenic differentiation
of MC3T3 cells (15,22,23). However, these studies were
performed on the MC3T3 osteoblast cell line. Improvements in the
knowledge of the characteristics of miRNAs in osteogenesis provide
an important step for their application in translational
investigations of bone tissue engineering and bone disease.
Therefore, the present study determine whether miR-29b plays a
similar role in the osteogenic differentiation of hADSCs and
further examined its potential molecular mechanisms. It was found
that the overexpression of miR-29b promoted the mineralized
deposition and ALP activity of hADSCs, whereas the inhibition of
miR-29b suppressed these processes. The expression profiles of
osteogenic markers (Runx2, BSP, OPN and OCN) in the hADSCs further
confirmed this finding. These data indicate that miR-29b functions
as a positive regulator of hADSC osteogenesis.
Various studies have indicated that miRNAs regulate
MSC osteogenesis by acting on the corresponding genes that regulate
osteogenic differentiation (1,34).
PTEN has been previously reported to function as a key
transcription factor on controlling osteoblast functions (35-37). For example, Shen et al.
found that the loss of PTEN in bone marrow-derived MSCs (BMSCs) led
to a decreased osteogenic differentiation potential by increasing
the proliferative capability (38). Liu et al. demonstrated that
PTEN deletion mutants increased osteoblast activity and bone
mineral density (39). In the
present study, using bioinformatic analysis via TargetScan and
miRanda, PTEN was identified as one of the potential targets of
miR-29b, which is consistent with the findings of a previous study
(40). It was also indicated that
miR-29b suppressed the expression of PTEN during the osteogenic
differentiation of hADSCs. Therefore, it was hypothesized that the
miR-29b/PTEN axis may play a key role in hADSC osteogenesis. As was
expected, it was demonstrated that the promoting effects of
agomir-29b on ALP activity, matrix mineralization and the levels of
osteogenic marker genes were reversed by the overexpression of
PTEN. These data suggest that miR-29b promotes the osteogenic
differentiation of hADSCs by suppressing the expression of
PTEN.
To date, a variety of signaling pathways that
regulate osteogenic differentiation have been discovered. Among
these, the AKT/β-catenin pathway is considered to be a critical
negative regulator of the osteogenic differentiation of MCS
(41,42). Previous studies have demonstrated
that the activation of AKT is sufficient to activate the β-catenin
signaling pathway, whereupon β-catenin in itself was previously
demonstrated to potentiate osteogenesis (43). Given the fact that PTEN can
restrict AKT (44,45), it is possible that miR-29b
regulates the osteogenic differentiation of hADSCs via the
AKT/β-catenin pathway. In the present study, it was proven that the
upregulation of miR-29b promoted the expression levels of
phospho-AKT, phospho-GSK3β and phospho-β-catenin, suggesting that
miR-29b can activate the AKT/GSK-3β/β-catenin signaling pathway.
Therefore, these results indicate that miR-29b can activate the
AKT/mTOR pathway by suppressing PTEN expression, thereby promoting
the osteogenic differentiation of hADSCs.
In conclusion, the present study demonstrated that
miR-29b promoted the osteogenic differentiation of hADSCs, at least
partly through the PTEN/AKT/GSK-3β/β-catenin signaling pathway.
These observations provide preclinical data supporting the
potential application of hADSCs engineered with miR-29b in curing
bone-related diseases.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
TX and SD performed the experiments, contributed to
data analysis and wrote the manuscript. TX and SD analyzed the
data. JT conceptualized the study design, contributed to data
analysis and experimental materials. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shanghai General Hospital of Nanjing Medical
University (IRB approval no. 2018-0032). Informed consent was
obtained from all patients prior to study inclusion.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deng Y, Zhou H, Zou D, Xie Q, Bi X, Gu P
and Fan X: The role of miR-31-modified adipose tissue-derived stem
cells in repairing rat critical-sized calvarial defects.
Biomaterials. 34:6717–6728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin CY, Chang YH, Li KC, Lu CH, Sung LY,
Yeh CL, Lin KJ, Huang SF, Yen TC and Hu YC: The use of ASCs
engineered to express BMP2 or TGF-β3 within scaffold constructs to
promote calvarial bone repair. Biomaterials. 34:9401–9412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Locke M, Feisst V and Dunbar PR: Concise
review: Human adipose-derived stem cells: Separating promise from
clinical need. Stem Cells. 29:404–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gadkari R, Zhao L, Teklemariam T and
Hantash BM: Human embryonic stem cell derived-mesenchymal stem
cells: An alternative mesenchymal stem cell source for regenerative
medicine therapy. Regen Med. 9:453–465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song F, Jiang D, Wang T, Wang Y, Lou Y,
Zhang Y, Ma H and Kang Y: Mechanical stress regulates osteogenesis
and adipogenesis of rat mesenchymal stem cells through
PI3K/Akt/GSK-3β/β-catenin signaling pathway. Biomed Res Int.
2017:60274022017. View Article : Google Scholar
|
|
6
|
Wu X, Zheng S, Ye Y, Wu Y, Lin K and Su J:
Enhanced osteogenic differentiation and bone regeneration of
poly(lactic-co-glycolic acid) by graphene via activation of
PI3K/Akt/GSK-3β/β-catenin signal circuit. Biomater Sci.
6:1147–1158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saidak Z, Le Henaff C, Azzi S, Marty C, Da
Nascimento S, Sonnet P and Marie PJ: Wnt/β-catenin signaling
mediates osteoblast differentiation triggered by peptide-induced
α5β1 integrin priming in mesenchymal skeletal cells. J Biol Chem.
290:6903–6912. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen JC and Jacobs CR: Mechanically
induced osteogenic lineage commitment of stem cells. Stem Cell Res
Ther. 4:1072013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luzi E, Marini F, Sala SC, Tognarini I,
Galli G and Brandi ML: Osteogenic differentiation of human adipose
tissue-derived stem cells is modulated by the miR-26a targeting of
the SMAD1 transcription factor. J Bone Miner Res. 23:287–295. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Hu C, Han L, Liu L, Jing W, Tang W,
Tian W and Long J: MiR-154-5p regulates osteogenic differentiation
of adipose-derived mesenchymal stem cells under tensile stress
through the Wnt/PCP pathway by targeting Wnt11. Bone. 78:130–141.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim YJ, Bae SW, Yu SS, Bae YC and Jung JS:
miR-196a regulates proliferation and osteogenic differentiation in
mesenchymal stem cells derived from human adipose tissue. J Bone
Miner Res. 24:816–825. 2009. View Article : Google Scholar
|
|
14
|
Chen S, Zheng Y, Zhang S, Jia L and Zhou
Y: Promotion effects of miR-375 on the osteogenic differentiation
of human adipose-derived mesenchymal stem cells. Stem Cell Reports.
8:773–786. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng S, Gao D, Gao C, Wei P, Niu M and
Shuai C: MicroRNAs regulate signaling pathways in osteogenic
differentiation of mesenchymal stem cells (Review). Mol Med Rep.
14:623–629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boquest AC, Shahdadfar A, Brinchmann JE
and Collas P: Isolation of stromal stem cells from human adipose
tissue. Methods Mol Biol. 325:35–46. 2006.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Mei LL, Wang WJ, Qiu YT, Xie XF, Bai J and
Shi ZZ: miR-125b-5p functions as a tumor suppressor gene partially
by regulating HMGA2 in esophageal squamous cell carcinoma. PLoS
One. 12:e01856362017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang MY, Lin J and Kui YC: MicroRNA-345
suppresses cell invasion and migration in non-small cell lung
cancer by directly targeting YAP1. Eur Rev Med Pharmacol Sci.
23:2436–2443. 2019.PubMed/NCBI
|
|
20
|
Fang S, Deng Y, Gu P and Fan X: MicroRNAs
regulate bone development and regeneration. Int J Mol Sci.
16:8227–8253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang P, Xiong Q, Ge W and Zhang L: The
role of microRNAs in osteoclasts and osteoporosis. RNA Biol.
11:1355–1363. 2014. View Article : Google Scholar
|
|
22
|
Roberto VP, Tiago DM, Silva IA and Cancela
ML: MiR-29a is an enhancer of mineral deposition in bone-derived
systems. Arch Biochem Biophys. 564:173–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Hassan MQ, Jafferji M, Aqeilan RI,
Garzon R, Croce CM, van Wijnen AJ, Stein JL, Stein GS and Lian JB:
Biological functions of miR-29b contribute to positive regulation
of osteoblast differentiation. J Biol Chem. 284:15676–15684. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mayer U, Benditz A and Grässel S: miR-29b
regulates expression of collagens I and III in chondrogenically
differentiating BMSC in an osteoarthritic environment. Sci Rep.
7:132972017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang W and Cao W: Treatment of
osteoarthritis with mesenchymal stem cells. Sci China Life Sci.
57:586–595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baharlou R, Ahmadi-Vasmehjani A, Faraji F,
Atashzar MR, Khoubyari M, Ahi S, Erfanian S and Navabi SS: Human
adipose tissue-derived mesenchymal stem cells in rheumatoid
arthritis: Regulatory effects on peripheral blood mononuclear cells
activation. Int Immunopharmacol. 47:59–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng YB, Li X, Li ZY, Zhao J, Yuan XB, Ren
Y, Cui ZD, Liu YD and Yang XJ: microRNA-21-promotes osteogenic
differentiation of mesenchymal stem cells by the PI3K/β-catenin
pathway. J Orthop Res. 33:957–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen GY, Ren H, Huang JJ, Zhang ZD, Zhao
WH, Yu X, Shang Q, Qiu T, Zhang YZ, Tang JJ, et al: Plastrum
testudinis extracts promote BMSC proliferation and osteogenic
differentiation by regulating let-7f-5p and the TNFR2/PI3K/AKT
signaling pathway. Cell Physiol Biochem. 47:2307–2318. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie Q, Wang Z, Zhou H, Yu Z, Huang Y, Sun
H, Bi X, Wang Y, Shi W, Gu P and Fan X: The role of
miR-135-modified adipose-derived mesenchymal stem cells in bone
regeneration. Biomaterials. 75:279–294. 2016. View Article : Google Scholar
|
|
30
|
Hu R, Li H, Liu W, Yang L, Tan YF and Luo
XH: Targeting miRNAs in osteoblast differentiation and bone
formation. Expert Opin Ther Targets. 14:1109–1120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho HH, Shin KK, Kim YJ, Song JS, Kim JM,
Bae YC, Kim CD and Jung JS: NF-kappaB activation stimulates
osteogenic differentiation of mesenchymal stem cells derived from
human adipose tissue by increasing TAZ expression. J Cell Physiol.
223:168–177. 2010.PubMed/NCBI
|
|
32
|
Huang S, Wang S, Bian C, Yang Z, Zhou H,
Zeng Y, Li H, Han Q and Zhao RC: Upregulation of miR-22-promotes
osteogenic differentiation and inhibits adipogenic differentiation
of human adipose tissue-derived mesenchymal stem cells by
repressing HDAC 6-protein expression. Stem Cells Dev. 21:2531–2540.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zeng Y, Qu X, Li H, Huang S, Wang S, Xu Q,
Lin R, Han Q, Li J and Zhao RC: MicroRNA-100 regulates osteogenic
differentiation of human adipose-derived mesenchymal stem cells by
targeting BMPR2. FEBS Lett. 586:2375–2381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei J, Shi Y, Zheng L, Zhou B, Inose H,
Wang J, Guo XE, Grosschedl R and Karsenty G: miR-34s inhibit
osteoblast proliferation and differentiation in the mouse by
targeting SATB2. J Cell Biol. 197:509–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nielsen-Preiss SM, Silva SR and Gillette
JM: Role of PTEN and Akt in the regulation of growth and apoptosis
in human osteoblastic cells. J Cell Biochem. 90:964–975. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Zhang L, Yan M and Zheng X:
Inhibition of phosphatidylinositol 3-kinase causes cell death in
rat osteoblasts through inactivation of Akt. Biomed Pharmacother.
61:277–284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kawamura N, Kugimiya F, Oshima Y, Ohba S,
Ikeda T, Saito T, Shinoda Y, Kawasaki Y, Ogata N, Hoshi K, et al:
Akt1 in osteoblasts and osteoclasts controls bone remodeling. PLoS
One. 2:e10582007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shen Y, Zhang J, Yu T and Qi C: Generation
of PTEN knockout bone marrow mesenchymal stem cell lines by
CRISPR/Cas9-mediated genome editing. Cytotechnology. 70:783–791.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu X, Chen T, Wu Y and Tang Z: Role and
mechanism of PTEN in adiponectin-induced osteogenesis in human bone
marrow mesenchymal stem cells. Biochem Biophys Res Commun.
483:712–717. 2017. View Article : Google Scholar
|
|
40
|
Lin X, Zhou X, Liu D, Yun L, Zhang L, Chen
X, Chai Q and Li L: MicroRNA-29 regulates high-glucose-induced
apoptosis in human retinal pigment epithelial cells through PTEN.
In. Vitro Cell Dev Biol Anim. 52:419–426. 2016. View Article : Google Scholar
|
|
41
|
Sen B, Styner M, Xie Z, Case N, Rubin CT
and Rubin J: Mechanical loading regulates NFATc1 and beta-catenin
signaling through a GSK3beta control node. J Biol Chem.
284:34607–34617. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Day TF, Guo X, Garrett-Beal L and Yang Y:
Wnt/beta-catenin signaling in mesenchymal progenitors controls
osteoblast and chondrocyte differentiation during vertebrate
skeletogenesis. Dev Cell. 8:739–750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qu ZH, Zhang XL, Tang TT and Dai KR:
Promotion of osteogenesis through beta-catenin signaling by
desferrioxamine. Biochem Biophys Res Commun. 370:332–337. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Steck PA, Pershouse MA, Jasser SA, Yung
WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T,
et al: Identification of a candidate tumour suppressor gene, MMAC1,
at chromosome 10q23.3 that is mutated in multiple advanced cancers.
Nat Genet. 15:356–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Whang YE, Wu X, Suzuki H, Reiter RE, Tran
C, Vessella RL, Said JW, Isaacs WB and Sawyers CL: Inactivation of
the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer
through loss of expression. Proc Natl Acad Sci USA. 95:5246–5250.
1998. View Article : Google Scholar : PubMed/NCBI
|