Introduction
Pancreatic cancer (PaCa) is gastrointestinal type of
cancer with a very high malignancy and is one of the leading causes
of cancer-related mortality worldwide (1). Due to the high recurrence rate and
rapid tumor growth, the prognosis of patients with PaCa is poor,
and the 5-year survival rate is as low as 10% (2), which is a major obstacle towards the
improvement of patient survival. Previous studies have demonstrated
that the loss or low expression of Bcl-2/adenovirus
E1B-19kDa-interacting protein 3 (BNIP3) in PaCa leads to
chemotherapeutic resistance and a worse prognosis (3,4).
BNIP3 is a member of the Bcl-2 family and has the potential to
facilitate cell apoptosis and evoke cell death and autophagic cell
death. Recently, it has been demonstrated that BNIP3 is regulated
by p21 and induces cell cycle arrest and cell apoptosis in PaCa
(5) and that BNIP3 participates
in cell apoptosis via the modulation of the activation of the 5′
AMP-activated protein kinase (AMPK) pathway (6). A previous study reported that
mitogen-activated protein kinase (MAPK) was involved in the cell
apoptosis of PaCa, suggesting its association with PaCa progression
(7).
Moreover, bioinformatics software have predicted the
complementary base pairs between miR-27a-3p and BNIP3, suggesting a
potential binding site between them. MicroRNAs (miRNAs or miRs) are
a type of non-coding RNA (of approximately 22 nucleotides) that
negatively regulate gene expression in various biological
processes, and the dysregulation of miRNAs has been implicated in
numerous human disorders (8). For
instance, miR-27a has been shown to be aberrantly expressed in
several types of cancer. As previously reported, the expression of
miR-27a is associated with clinical pathological parameters,
including tumor size, lymph node metastasis and distant metastasis
in breast cancer; further-more, a high expression of miR-27a is
related to poor the overall survival of patients with breast cancer
(9). In addition, the
downregulation of miR-27a has been shown to suppress cell growth
and migration in PaCa (10). The
expression of miR-27a-3p in peripheral blood mononuclear cells
(PBMCs) has also been demonstrated to be a potential marker for
PaCa screening, serving as a promising strategy for the accurate
diagnosis of this disease (11).
As an identified tumor suppressor, lncRNA DGCR5 has
been revealed to be downregulated in hepatocellular carcinoma and
lung cancer, and the overexpression of DGCR5 contributes to the
repression of cell proliferation, migration and invasion, and
promotes the cell apoptosis of these cancers (12,13). Regrettably, the role of DGCR5 in
PaCa has not been focused on and explored yet. The prediction of
complementary base pairs between DGCR5 through bioinformatics
analysis has been a breakthrough, and it indicates the potential
binding and targeting effects between them. Therefore, it was
hypothesized that lncRNA DGCR5 may also play an important role in
the progression of PaCa by interacting with miR-27a-3p and that
miR-27a-3p may also affect the growth or development of PaCa by
targeting BNIP3, a hypothesis which is explored in the present
study.
Materials and methods
Clinical samples
The present study was approved by the Medical Ethics
Committee of the Affiliated Hospital of Medical School, Ningbo
University. All the patients agreed and signed the documented
informed consent for tissue donation for study prior to sample
collection.
PaCa tissues and adjacent normal tissues (n=20) were
obtained from patients with PaCa in the Affiliated Hospital of
Medical School, Ningbo University, and the clinical and
pathological characteristics of the patients are depicted in
Table I. The tissue samples were
frozen in liquid nitrogen and immediately stored at -80°C prior to
analysis.
 | Table IClinical and pathological
characteristics of the patients included in the present study. |
Table I
Clinical and pathological
characteristics of the patients included in the present study.
| Characteristic | No. of patients |
|---|
| Newly diagnosed | |
| Yes | 18 |
| No | 2 |
| Age, years | |
| <60 | 15 |
| ≥60 | 5 |
| Sex | |
| Male | 14 |
| Female | 6 |
| Tumor size, cm | |
| <3 | 7 |
| ≥3 | 13 |
| Histological
grade | |
| Well/moderate | 8 |
| Poor | 12 |
| TNM stage | |
| I-II | 7 |
| III-IV | 13 |
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from PaCa and adjacent
tissues and cell lines using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's standard
procedures. The reverse transcription of total RNA was performed
using the cDNA Reverse Transcription kit (Applied Biosystems) to
synthesize cDNA. Quantitative PCR was performed on an ABI Prism
7900HT system (Applied Biosystems) with the SYBR Select Master Mix
(Applied Biosystems) to quantify gene expression. Primers were
synthesized with Sangon Biotech. β-actin and U6 served as
endogenous controls. Finally, the 2−∆∆Cq method was used
to determine the relative expression levels (14). The primer sequences were as
follows: DGCR5 forward, 5′-CAC GAG TGT AGT GCC CAG TT-3′ and
reverse, 5′-GGT CAG GGA CCT TTG TCG TT-3′; miR-27a-3p forward,
5′-GGC TTA GCT GCT TGT GA-3′ and reverse, 5′-GAA CAT GTC TGC GTA
TCT C-3′; BNIP3 forward, 5′-GCC CAC CTC GCT CGC AGA CAC-3′ and
reverse, 5′-CAA TCC GAT GGC CAG CAA ATG AGA-3′; β-actin forward,
5′-AAG TAC TCC GTG TGG AT C GG-3′ and reverse, 5′-ATG CTA TCA CCT
CCC CTG TG-3′; and U6 forward, 5′-CTC GCT TCG GCA GCA CA-3′ and
reverse, 5′-AAC GCT TCA CGA ATT TGC GT-3′. The PCR thermocycling
conditions were as follows: PCR reactions were carried out for 40
cycles that comprised a denaturation step at 95°C for 15 sec, an
annealing step at 62°C for 45 sec and an extension step at 72°C for
30 sec.
Western blot analysis
Western blot analysis was performed to examine the
protein levels. The tissues and cells were lysed in lysis buffer
(Beyotime Institute of Biotechnology) containing protease
inhibitors. The protein concentration was measured using a Bradford
protein assay kit (Bio-Rad Laboratories, Inc.), and the proteins
were then separated by 10% SDS-PAGE with an electrophoresis system
(Bio-Rad Laboratories, Inc.). Following separation, the proteins
were transferred to a poly-vinylidene difluoride (PVDF) membrane
(Invitrogen; Thermo Fisher Scientific, Inc.). After blocking in
Tris-buffered saline (TBS) containing 5% skimmed milk for 1 h at
room temperature, the membrane was incubated with primary
antibodies against BNIP3 (1:500, sc-56167; Santa Cruz
Biotechnology, Inc.), p-p38 (1:1,000, 4511; Cell Signaling
Technology, Inc.), p38 (1:1,000, 8690; Cell Signaling Technology,
Inc.), Bcl-2 (1:1,000, 15071; Cell Signaling Technology, Inc.),
cleaved caspase-3 (1:1,000, 9661; Cell Signaling Technology, Inc.)
and β-actin (1:500, sc-47778; Santa Cruz Biotechnology, Inc.) at
4̊C over-night. The membrane was then incubated with HRP-bounded
antibodies (1:10,000, 31460; Pierce, Thermo Fisher Scientific,
Inc.) for 2 h at room temperature. The target proteins were
visualized using an ECL Plus Western Blot Substrate (Thermo Fisher
Scientific, Inc.). The protein levels were normalized to β-actin
and densitometric analysis was performed using Image J software (v.
1.52r; National Institutes of Health).
Cells and cell culture
The human PaCa cell lines, SW1990 and PANC-1
[American Type Culture Collection (ATCC)], and the normal human
pancreatic duct epithelial cell line, HPDE6-C7 (ATCC), were
cultured in standard DMEM containing 10% fetal bovine serum (FBS,
Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml of penicillin, and
100 µg/ml of streptomycin (HyClone; GE Healthcare Life
Sciences) and maintained at 37°C in a humidified atmosphere with 5%
CO2.
Prediction of DGCR5 and miR-27a-3p
binding sites
Bioinformatics software (LncBase Predicted v.2)
predicted that DGCR5 and miR-27a-3p had binding sites. The specific
method was to enter the following website: http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2/index-predicted,
and then entered the names of DGCR5 and miR-27a-3p,
respectively.
RNA immunoprecipitation (RIP)
RNA immunoprecipitation (RIP) was performed using
the RNA-Binding Protein Immunoprecipitation kit (EMD Millipore).
The SW1990 cells were lysed in lysis buffer, and the cell lysis
solutions were incubated with argonaute 2 (AGO2) antibody (1:1,000,
ab32381, Abcam)or normal mouse IgG at 4̊C for 4 h. RNA-protein
complexes were immunoprecipitated with protein A agarose beads, and
RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). IP-western blot analysis was used to detect the
protein level of AGO2, and RT-qPCR was used to quantify the DGCR5
and miR-27a-3p level.
RNA pull-down assay
RNA pull-down assay was conducted to determine the
interaction between DGCR5 and miR-27a-3p. Briefly, the DNA probe
complementary to DGCR5 was synthesized and biotinylated using
GenePharma Co., Ltd. RNA pull-down assay was performed using a
Magnetic RNA-Protein Pull-Down kit (Thermo Fisher Scientific, Inc.)
according to company specifications. The RNA-binding protein
complexes were washed and eluted for western blot or RT-qPCR
analysis.
Cell transfection
The miR-27a-3p mimic, pre-negative control (pre-NC),
miR-27a-3p inhibitor, and negative control (NC) were purchased from
GenePharma for cell transfection. Cells (3×105/well)
were seeded in a 6-well plate and cultured for 24 h prior to
transfection. The cells were transfected with miR-27a-3p
mimics/inhibitor or NC mimics/inhibitor (50 nM) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for induction. After 24 h, the efficiency of cell
transfection was examined by RT-qPCR and western blot analysis. The
siRNA for BNIP3 and its control group si-control were synthesized
by GenePharma. Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to transfect si-BNIP3 and si-control (50
nM) into cells, respectively. The interference sequences of BNIP3
and its control were as follows: si-BNIP3, 5′-GGA ATT AAG TCT CCG
ATT A-3′; si-control, 5′-UUC UUC GAA CGU GUC ACG UTT-3′.
Prediction of miR-27a-3p and BNIP3
binding sites
Bioinformatics software (microrna.org) predicted that miR-27a-3p and BNIP3 had
binding sites. The specific method was to enter the following
website: http://www.microrna.org/microrna/home.do, and then
entered miR-27a-3p name.
Dual luciferase reporter assay
Dual luciferase reporter assay was used to examine
the interaction between miR-27a-3p and BNIP3. The WT BNIP3 3′-UTR
or Mut BNIP3 3′-UTR was inserted into the pmirGLO vector (Promega
Corp.) to establish the recombinant plasmid. The recombinant
plasmids miR-27a-3p mimic (or pre-NC), miR-27a-3p inhibitor, or NC
were co-transfected into 293 cells (Procell) using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). The luciferase
activity was detected using the dual luciferase reporter assay
system (Promega Corp.) according to manufacturer instructions,
followed by normalizing to Renilla luciferase activity.
Plasmid construction
The pcDNA3.1 was used to construct the pcDNA-DGCR5
expression plasmids, and the vector pcDNA was used as a control,
and they were transfected into SW1990 and PANC-1 cell lines using
Lipofectamine 2000. In brief, the target gene DGCR5 was amplified
using PCR, and the product was purified with gel extraction. The
product of double enzyme digestion was then ligated with T4 DNA
ligase (Takara), and the purified product and pcDNA3.1 were
integrated into the pcDNA-DGCR5 recombinant plasmid. The
recombinant pcDNA-DGCR5 was cloned into E. coli, and
positive clones were selected and amplified. The recombinant
plasmids were extracted from positive clones and transfected into
PaCa cells following identification with restriction analysis and
sequence analysis (Sangon Biotech).
Flow cytometric (FCM) analysis
The apoptosis of SW1990 and PANC-1 cells was
determined by FCM analysis using an Annexin V-FITC/propidium iodide
(PI) cell apoptosis detection kit (Abcam). Cells (1×106)
were collected and washed twice with phosphate-buffered saline
(PBS) and suspended in 500 ml of binding buffer. Annexin V-FITC was
added, and the cells were then incubated for 10 min at room
temperature. The cells were subsequently stained using 5 µl
PI for 20 min. The stained cells were analyzed with FCM and the
FlowJo V10 software (Tree Star, Inc.).
Establishment of a nude mouse model of
PaCa
All animal experiments were approved by the Medical
Ethics Committee of the Affiliated Hospital of Medical School,
Ningbo University. A total of 12 BALB/C nude mice (5 weeks old)
were purchased from the Shanghai Lab Animal Research Center. The
mice were placed in a temperature- and humidity-controlled
environment and subjected to a 12-h light and dark cycle. All mice
always had free access to water and food, and animal health and
behavior were monitored every 24 h. Mice were randomly divided into
the pcDNA group (n=6) and the pcDNA-DGCR5 group (n=6). Mice in each
group were inoculated with 3×106 SW1990 cells
transfected with pcDNA or pcDNA-DGCR5, respectively, and cells in
0.2 ml of PBS were injected subcutaneously into the right axilla of
each mouse, as previously described (15). Tumor volume was measured every 7
days and calculated by using the following formula: V=(L x W2)/2,
in which V refers to volume, and L and W represent the longest and
shortest diameters, respectively. After 5 weeks, mice were
euthanized by an intraperitoneal injection of 150 mg/kg
pentobarbital sodium, and the mice were examined to determine
whether they did not breathe spontaneously for 2-3 min and
exhibited no blinking reflex.
Statistical analysis
The data are presented as the mean values ± standard
deviation (SD). SPSS 21.0 (SPSS, Inc.), and GraphPad Prism
(GraphPad Software) software were used to conduct the analyses. A
Student's t-test with 2-tailed value in GraphPad Prism was used for
2-group comparisons, while one-way ANOVA with the Bonferroni
correction was used for multi-group comparisons. Pearson's
correlation coefficient analysis was used to examine the
correlation between lncRNA DGCR5, miR-27a-3p, and BNIP3, and the r
value was obtained directly by GraphPad Prism software and was used
to evaluate the correlation of 2 variables. Differences were
considered statistically significant at P<0.05.
Results
Expression of lncRNA DGCR5, miR-27a-3p
and BNIP3 in PaCa tissues
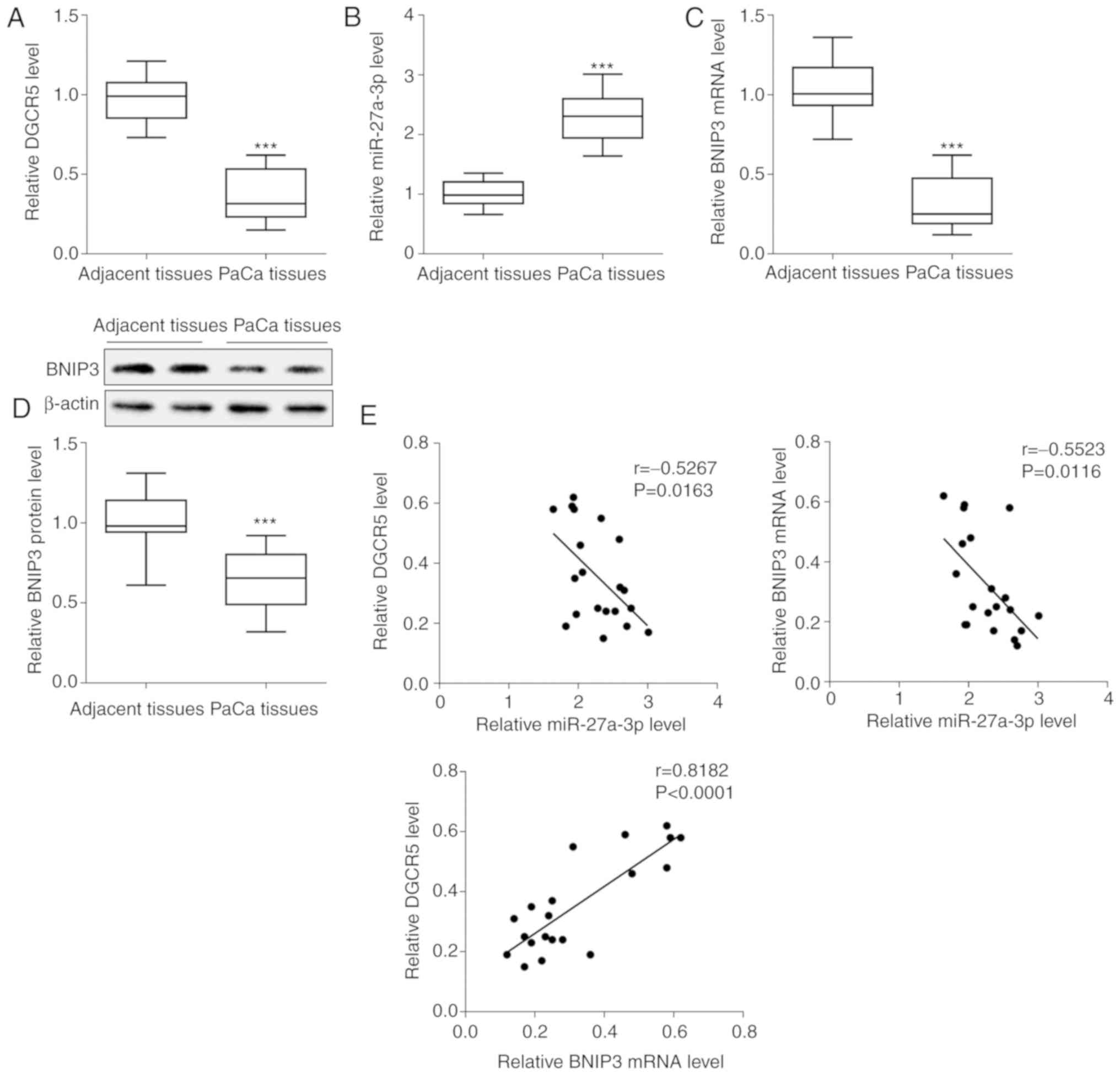
PaCa (n=20) tissues and adjacent tissues (n=20) were
isolated from patients, and the expression levels of related genes
were determined. The results demonstrated that DGCR5 expression was
downregulated in the PaCa tissues compared with the adjacent normal
tissues (Fig. 1A), whereas
miR-27a-3p expression was upregulated (Fig. 1B). The mRNA and protein expression
levels of BNIP3 were downregulated in the PaCa tissues (Fig. 1C and D). The expression of BNIP3
was tested in all 20 pairs of clinical tissues, and the most
representative 2 pairs from 20 pairs of clinical tissues were
selected to display the results of western blot analysis. In
addition, the correlation between lncRNA DGCR5, miR-27a-3p and
BNIP3 we analyzed. It was found that miR-27a-3p negatively
correlated with either lncRNA DGCR5 or BNIP3, whereas lncRNA DGCR5
positively correlated with BNIP3 (Fig. 1E).
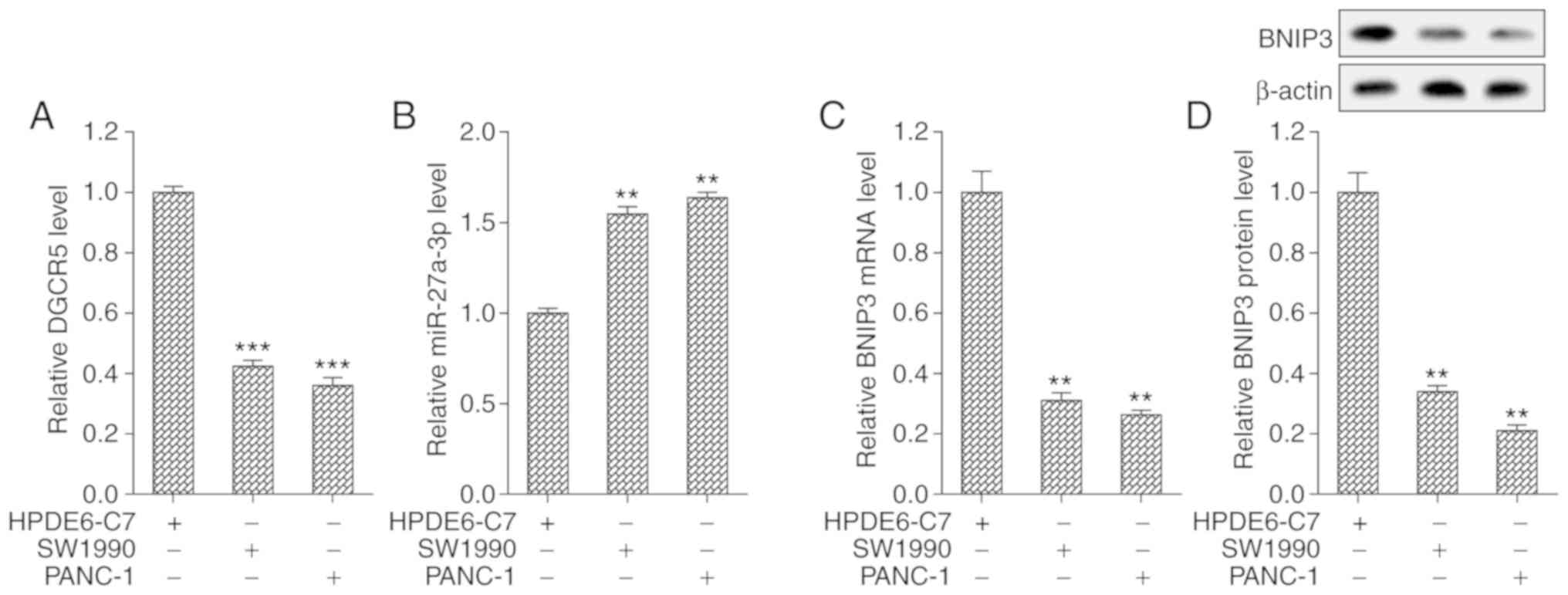
Expression of lncRNA DGCR5, miR-27a-3p
and BNIP3 in PaCa cells
In previous preliminary experiments, the changes in
the expression of DGCR5, miR-27a-3p and BNIP3 were detected in the
following 4 cancer cells: PANC-1, SW1990, BXPC-3 and H6C7,
respectively, and the results revealed that the changes in
expression were most pronounced in the PANC-1 and SW1990 cell lines
(data not shown). Therefore, for the present study, the PANC-1 and
SW1990 cell lines were selected for further research, and this was
also consistent with the conclusion of a previous study (7). The expression levels of DGCR5,
miR-27a-3p and BNIP3 were examined in PaCa cell lines (SW1990 and
PANC-1) and the normal human pancreatic duct epithelial cell line
(HPDE6-C7). Compared with the HPDE6-C7 cells, DGCR5 expression was
downregulated in the PaCa cells (Fig.
2A), whereas miR-27a-3p expression was upregulated (Fig. 2B). The mRNA and protein expression
levels of BNIP3 were downregulated in the PaCa cells (Fig. 2C and D).
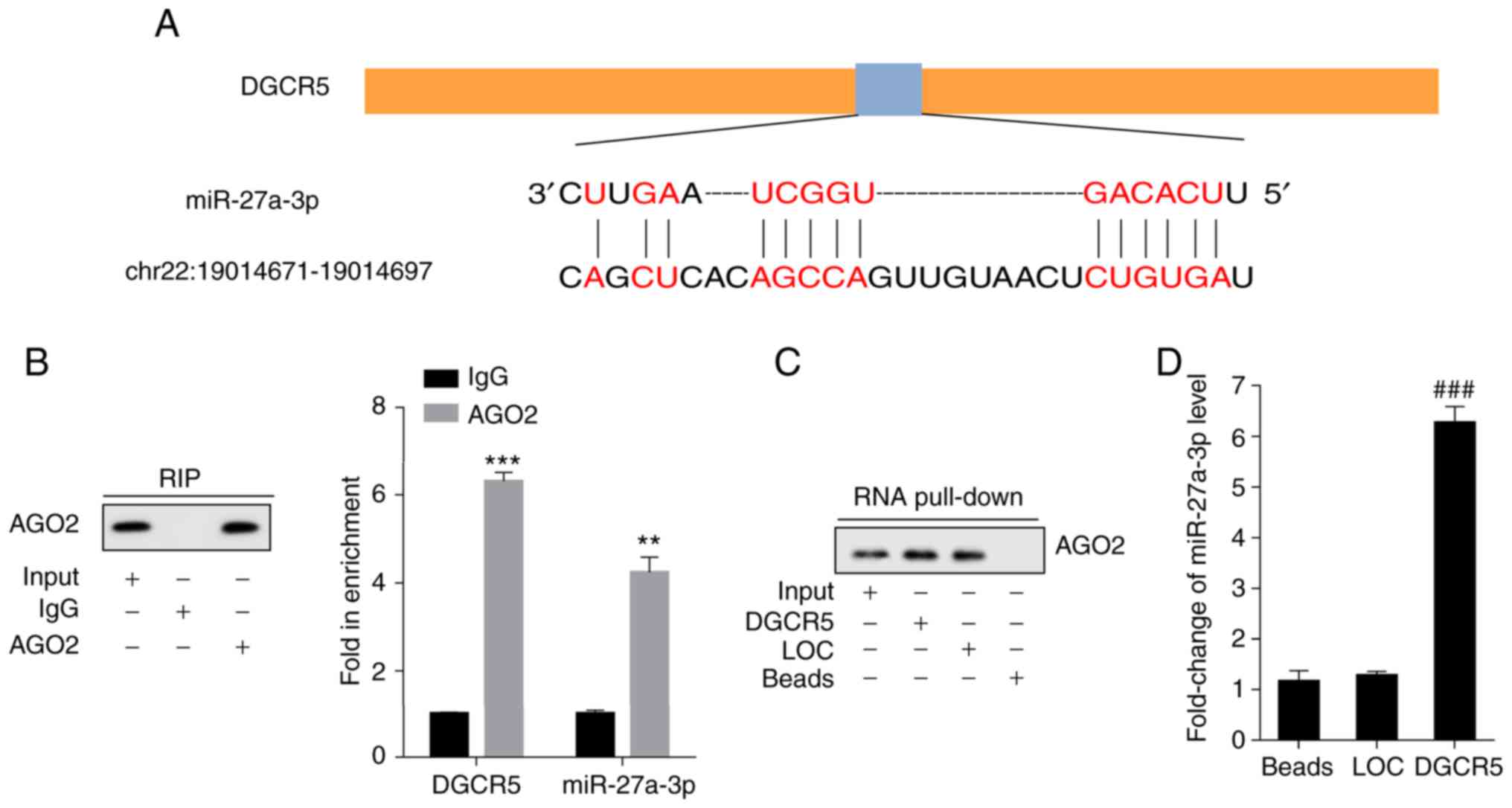
DGCR5 functions as a decoy of
miR-27a-3p
The binding sites of DGCR5 and miR-27a-3p were
predicted using bioinformatics software (LncBase Predicted v.2)
(Fig. 3A). The combination
conditions and interaction between DGCR5 and miR-27a-3p were
determined with RIP and RNA pull-down assays, respectively. AGO2
antibody was used in the RIP assay, and it was detected using
IP-western blot analysis. RT-qPCR was performed to examine the
expression levels of DGCR5 and miR-27a-3p. High levels of DGCR5 and
miR-27a-3p were detected with the AGO2 antibody (Fig. 3B). Using RNA pull-down assay, AGO2
in the pull-down complex of DGCR5 was examined by western blot
analysis (Fig. 3C). The results
revealed that miR-27a-3p was enriched in the pull-down complex of
DGCR5 (Fig. 3D).
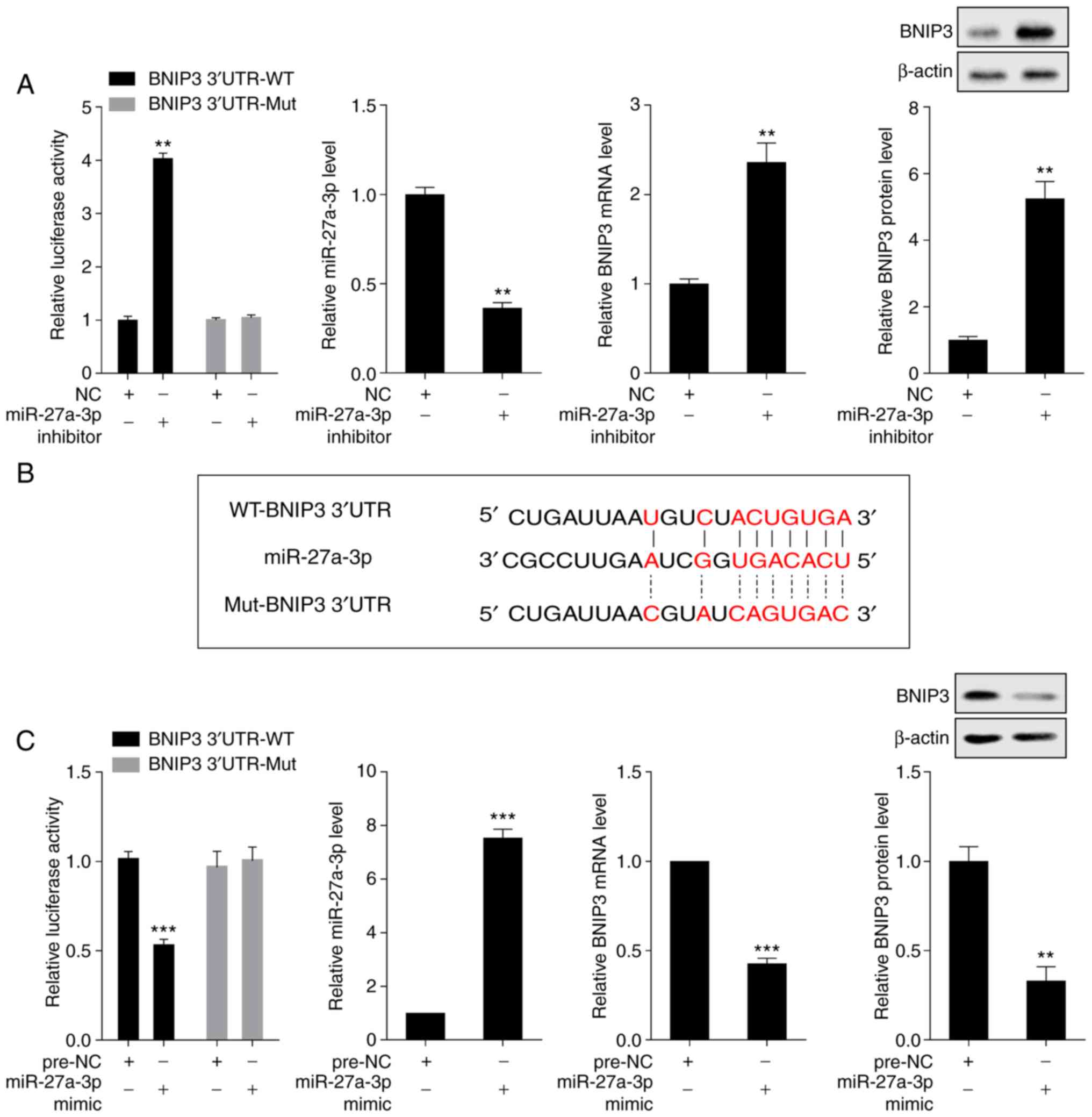
BNIP3 is negatively regulated by
miR-27a-3p
The interaction between miR-27a-3p and BNIP3 was
detected by dual luciferase reporter assay. Compared with the NC
group, transfection with miR-27a-3p inhibitor increased the
luciferase activity of the luciferase reporter gene containing
BNIP3 3'UTR-WT and downregulated the expression of miR-27a-3p;
furthermore, it upregulated the mRNA and protein expression levels
of BNIP3, whereas transfection with miR-27a-3p inhibitor exerted no
effect on the luciferase activity of the luciferase reporter gene
containing BNIP3 3'UTR-Mut (Fig.
4A). The binding sites of miR-27a-3p and the WT-BNIP3 3' UTR
were predicted using bioinformatics software (microrna.org) (Fig.
4B). Compared with the pre-NC group, transfection with
miR-27a-3p mimic decreased the luciferase activity of the
luciferase reporter gene containing BNIP3 3'UTR-WT and upregulated
the expression of miR-27a-3p; however, it downregulated the mRNA
and protein expression levels of BNIP3, whereas transfection with
miR-27a-3p mimic exerted no effect on the luciferase activity of
the luciferase reporter gene containing BNIP3 3'UTR-Mut (Fig. 4C).
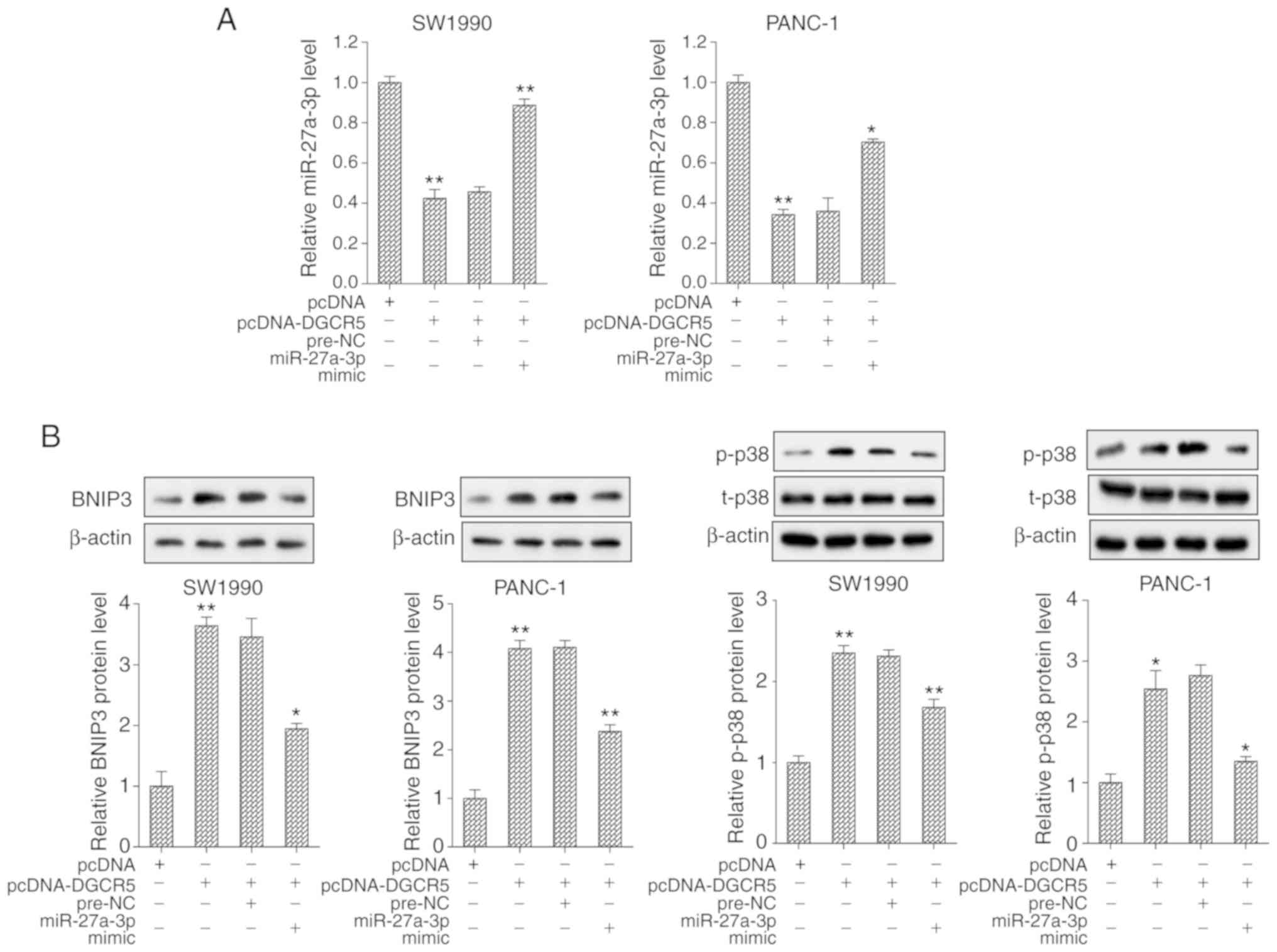
DGCR5 regulates BNIP3 and the p38 MAPK
pathway through miR-27a-3p
For cell transfection, the SW1990 and PANC-1 cells
were divided into 4 groups as follows: pcDNA, pcDNA-DGCR5,
pcDNA-DGCR5 + pre-NC and pcDNA-DGCR5 + miR-27a-3p mimic. The
results revealed that the expression of miR-27a-3p was
significantly downregulated and the expression of BNIP3 protein was
upregulated in cells transfected with pcDNA-DGCR5; however, these
effects were reversed following transfection with miR-27a-3p mimic
(Fig. 5). Furthermore,
transfection with pcDNA-DGCR5 promoted the phosphorylation of p38;
however, transfection with miR-27a-3p mimic suppressed the
phosphorylation level of p38 (Fig.
5B). In addition, the effects of BNIP3 on p38 phosphorylation
and the MAPK signaling pathway were further explored. si-BNIP3 and
pcDNA-BNIP3 were transfected into the PANC-1 and SW1990 cell lines,
respectively. From the results of western blot analysis, it was
found that the phosphorylation of p38 was reduced and the MAPK
signaling pathway was inhibited following the interference of
BNIP3; however, the overexpression of BNIP3 produced the opposite
effect (Fig. S1).
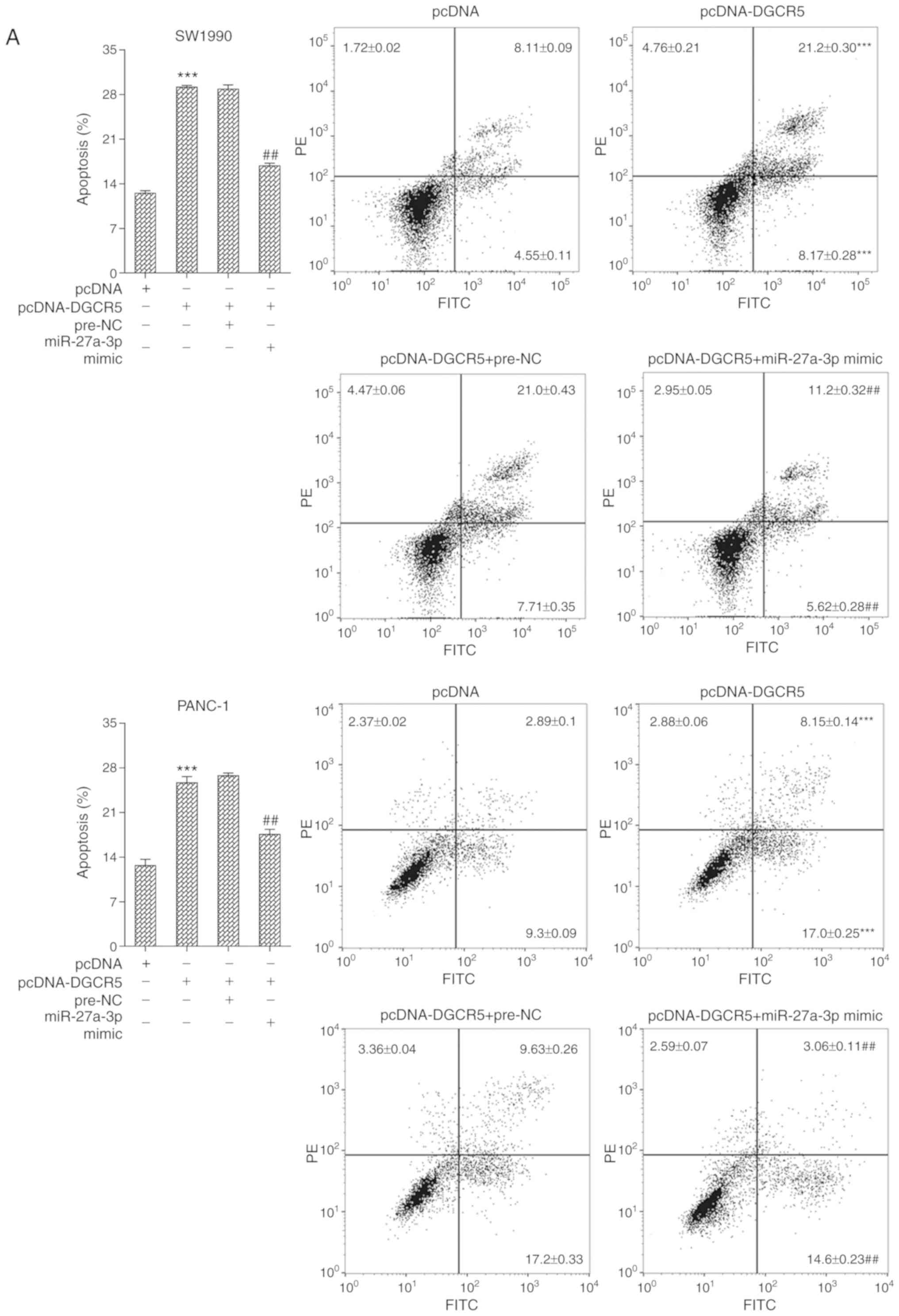
DGCR5 promotes the apoptosis of PaCa
cells via the miR-27a-3p/BNIP3 pathway
For cell transfection, the SW1990 and PANC-1 cells
were divided into 4 groups as follows: pcDNA, pcDNA-DGCR5,
pcDNA-DGCR5 + pre-NC and pcDNA-DGCR5 + miR-27a-3p mimic. FCM
analysis was used to examine the apoptosis of the SW1990 and PANC-1
cells. The results revealed that transfection with pcDNA-DGCR5
promoted cell apoptosis; however, this effect was reversed
following transfection with miR-27a-3p mimic (Fig. 6A). In addition, the results of
western blot analysis demonstrated that transfection with
pcDNA-DGCR5 upregulated the protein level of cleaved caspase-3 and
downregulated the protein level of Bcl-2, whereas these effects
were reversed following transfection with miR-27a-3p mimic
(Fig. 6B).
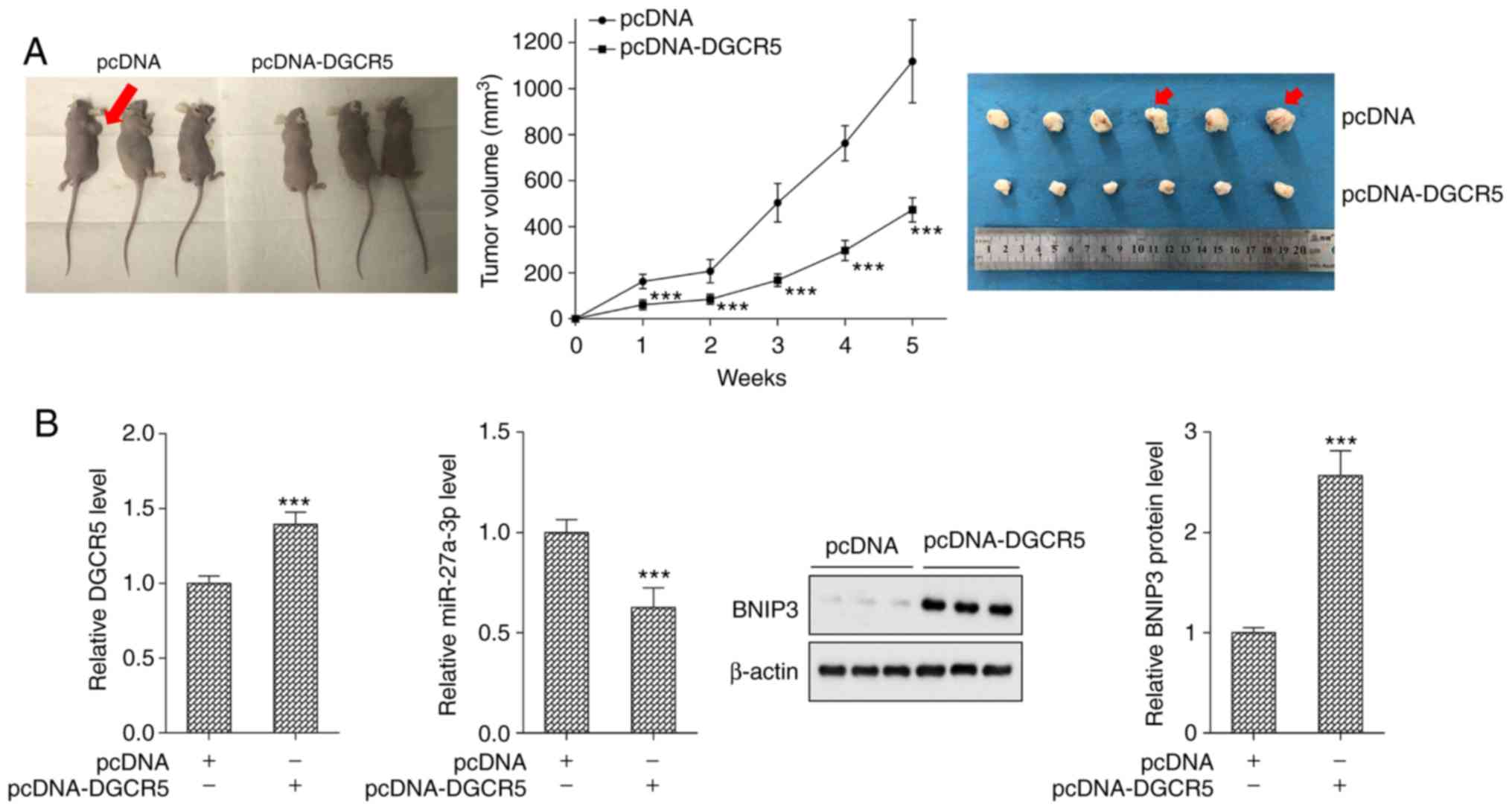
DGCR5 inhibits tumor growth in a mouse
model of PaCa
The tumor volumes of the nude mouse model of PaCa
with pcDNA (n=6) and pcDNA-DGCR5 (n=6) were detected. Compared with
the pcDNA group (n=6), tumor growth was significantly inhibited in
the pcDNA-DGCR5 group (Fig. 7A).
Compared with the pcDNA group, the expression of DGCR5 was
significantly upregulated, that of miR-27a-3p was down-regulated,
and the protein level of BNIP3 was upregulated in the pcDNA-DGCR5
group (Fig. 7B).
Discussion
The present study mainly explored the interactions
between lncRNA DGCR5, miR-27a-3p and BNIP3 in PaCa tissues and
cells and examined their effects on cell apoptosis and tumor
growth. Specifically, the results of the present study demonstrated
that DGCR5 expression was downregulated in PaCa, and that DGCR5
upregulated BNIP3 and activated the p38 MAPK signaling pathway
through miR-27a-3p to promote PaCa cell apoptosis, thereby
suppressing tumor growth. The findings of the present study may
provide important insight for the development of novel therapies
and improving the prognosis of PaCa.
With high mortality and low diagnosis and recovery
rates, PaCa is known as an aggressive and lethal type of cancer,
and has been considered a medical challenge that has attracted
considerable attention in etiology exploration and therapy
development (16). A deeper
understanding of the molecular alterations shows promise for the
future development of more targeted and safer therapeutic therapies
for the diagnosis and treatment of patients with PaCa. lncRNAs
refer to a type of non-coding RNA with approximately 200
nucleotides, which have emerged as pivotal regulators of the
pathogenesis and progression of various diseases (17). The abnormal expression of lncRNAs
and the interaction between lncRNAs and miRNAs have been shown to
be associated with a variety of cancers. As previously reported,
lncRNA DGCR5 is involved in the regulation of the proliferation,
migration and invasion of lung cancer by targeting miR-1180
(13), exhibiting its anti-tumor
role in the development and progression of lung cancer. In the
present study, DGCR5 expression was downregulated in PaCa, and
DGCR5 exerted antitumor effects by promoting cell apoptosis, which
has been identified as playing an antitumor role in PaCa for the
first time. In addition, further research found that miR-27a-3p was
a novel target of DGCR5 and was negatively regulated by DGCR5 to
facilitate cell apoptosis and tumor growth in PaCa.
As the essential regulators of gene expression, the
abnormal expression of miRNAs has been reported in various types of
cancer (18). As has been
reported, the expression of miR-27a is upregulated in human ovarian
cancer, and it plays a crucial role in cell growth and metastasis
(19). In PaCa, miR-27a also
functions as an oncogene by regulating the growth, colony formation
and migration of PaCa cells (20). Furthermore, miR-27a-3p also
exhibits oncogenicity in glioma, esophageal cancer and gastric
cancer by promoting cell proliferation via different targets
(21-23). Considering the recommendation of
using combined serum CA19-9 and miR-27a-3p in PBMC to diagnose PaCa
(11), it was initially
demonstrated herein that miR-27a-3p was downregulated in PaCa and
that miR-27a-3p mimic contributed to the suppression of cell
apoptosis through the downregulation of BNIP3. These results were
in accordance with those of previous research that revealed that
grape seed proanthocyanidins extract suppressed PaCa cell growth by
downregulating the expression of miR-27a (10), recon-firming the tumor-promotion
effect of miR-27a in PaCa.
The adverse effect of the loss or decreased
expression of BNIP3 in PaCa has been previously well-illustrated in
terms of chemoresistance and a poor prognosis (24). Silencing followed by the
methylation of the BNIP3 gene occur in a significant proportion of
cancer cases, particularly in PaCas (25). In the present study, it was
demonstrated that BNIP3 was negatively regulated by miR-27a-3p and
occupied an important position in enhancing cell apoptosis and PaCa
progression, exhibiting its pivotal and characteristic role in
suppressing tumors. Moreover, the present study also demonstrated
that the cell apoptosis-promoting effect of BNIP3 was achieved by
targeting MAPK, verifying the activating effect of BNIP3 on MAPK
and underlining the key role of MAPK in promoting cell apoptosis
(26).
In conclusion, the present study highlighted the
role of lncRNA DGCR5 in PaCa for the first time, at least to the
best of our knowledge. Furthermore, the interactions between DGCR5,
miR-27a-2p and BNIP3 were investigated. In particular, the results
indicated that DGCR5 upregulate BNIP3 expression and activated the
p38 MAPK pathway via miR-27a-3p, which promoted cell apoptosis in
PaCa, thereby inhibiting tumor growth. The present study emphasized
the roles of DGCR5 and miR-27a-3p in PaCa development and
progression, providing innovative insight into the optimal
management of its treatment and prognosis. However, it should be
noted that a limitation of this study was the lack of H&E
staining to identify the successful construction of animal models
of PaCa, and the authors aim to continue to further investigations
in this matter in the future.
Supplementary Data
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
XL designed the study, performed the experiments and
was the major contributor to the writing of the manuscript. SZ
participated in the experiments and assisted in the writing of the
manuscript. TF collected data and performed the statistical
analysis. XF made substantial contributions to the conception,
design and critical revision of the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Affiliated Hospital of Medical School, Ningbo
University. All the patients agreed and signed the documented
informed consent for tissue donation for study before sample
collection. All animal experiments were approved by the Medical
Ethics Committee of the Affiliated Hospital of Medical School,
Ningbo University.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Singh D, Upadhyay G, Srivastava RK and
Shankar S: Recent advances in pancreatic cancer: Biology,
treatment, and prevention. Biochim Biophys Acta. 1856:13–27.
2015.PubMed/NCBI
|
|
2
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Erkan M, Kleeff J, Esposito I, Giese T,
Ketterer K, Büchler MW, Giese NA and Friess H: Loss of BNIP3
expression is a late event in pancreatic cancer contributing to
chemoresistance and worsened prognosis. Oncogene. 24:4421–4432.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akada M, Crnogorac-Jurcevic T, Lattimore
S, Mahon P, Lopes R, Sunamura M, Matsuno S and Lemoine NR:
Intrinsic chemore-sistance to gemcitabine is associated with
decreased expression of BNIP3 in pancreatic cancer. Clin Cancer
Res. 11:3094–3101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manu KA, Chai TF, Teh JT, Zhu WL, Casey PJ
and Wang M: Inhibition of isoprenylcysteine
carboxylmethyltransferase induces cell-cycle arrest and apoptosis
through p21 and p21-regulated BNIP3 induction in pancreatic cancer.
Mol Cancer Ther. 16:914–923. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bujak AL, Crane JD, Lally JS, Ford RJ,
Kang SJ, Rebalka IA, Green AE, Kemp BE, Hawke TJ, Schertzer JD and
Steinberg GR: AMPK activation of muscle autophagy prevents
fasting-induced hypoglycemia and myopathy during aging. Cell Metab.
21:883–890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang FT, Peng JF, Cheng WJ, Zhuang YY,
Wang LY, Li CQ, Tang J, Chen WY, Li YH and Zhang SN: MiR-143
targeting TAK1 attenuates pancreatic ductal adenocarcinoma
progression via MAPK and NF-κB pathway in vitro. Dig Dis Sci.
62:944–957. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mørk S, Pletscher-Frankild S, Palleja Caro
A, Gorodkin J and Jensen LJ: Protein-driven inference of
miRNA-disease associations. Bioinformatics. 30:392–397. 2014.
View Article : Google Scholar
|
|
9
|
Tang W, Zhu J, Su S, Wu W, Liu Q, Su F and
Yu F: MiR-27 as a prognostic marker for breast cancer progression
and patient survival. PLoS One. 7:e517022012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma J, Fang B, Zeng F, Pang H, Ma C and Xia
J: Grape seed proanthocyanidins extract inhibits pancreatic cancer
cell growth through down-regulation of miR-27a expression. Zhong
Nan Da Xue Xue Bao Yi Xue Ban. 40:46–52. 2015.In Chinese.
PubMed/NCBI
|
|
11
|
Wang WS, Liu LX, Li GP, Chen Y, Li CY, Jin
DY and Wang XL: Combined serum CA19-9 and miR-27a-3p in peripheral
blood mononuclear cells to diagnose pancreatic cancer. Cancer Prev
Res (Phila). 6:331–338. 2013. View Article : Google Scholar
|
|
12
|
Huang R, Wang X, Zhang W, Zhangyuan G, Jin
K, Yu W, Xie Y, Xu X, Wang H and Sun B: Down-regulation of LncRNA
DGCR5 correlates with poor prognosis in hepatocellular carcinoma.
Cell Physiol Biochem. 40:707–715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen EG, Zhang JS, Xu S, Zhu XJ and Hu HH:
Long non-coding RNA DGCR5 is involved in the regulation of
proliferation, migration and invasion of lung cancer by targeting
miR-1180. Am J Cancer Res. 7:1463–1475. 2017.PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Ye Y, Chen J, Zhou Y, Fu Z, Zhou Q, Wang
Y, Gao W, Zheng S, Zhao X, Chen T and Chen R: High expression of
AFAP1-AS1 is associated with poor survival and short-term
recurrence in pancreatic ductal adenocarcinoma. J Transl Med.
13:1372015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohammed S, Van Buren G II and Fisher WE:
Pancreatic cancer: Advances in treatment. World J Gastroenterol.
20:9354–9360. 2014.PubMed/NCBI
|
|
17
|
Li X, Wu Z, Fu X and Han W: lncRNAs:
Insights into their function and mechanics in underlying disorders.
Mutat Res Rev Mutat Res. 762:1–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer-A brief overview. Adv Biol Regul. 57:1–9.
2015. View Article : Google Scholar
|
|
19
|
Xu L, Xiang J, Shen J, Zou X, Zhai S, Yin
Y, Li P, Wang X and Sun Q: Oncogenic MicroRNA-27a is a target for
genistein in ovarian cancer cells. Anticancer Agents Med Chem.
13:1126–1132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma Y, Yu S, Zhao W, Lu Z and Chen J:
miR-27a regulates the growth, colony formation and migration of
pancreatic cancer cells by targeting Sprouty2. Cancer Lett.
298:150–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu W, Liu M, Peng X, Zhou P, Zhou J, Xu K,
Xu H and Jiang S: miR-24-3p and miR-27a-3p promote cell
proliferation in glioma cells via cooperative regulation of MXI1.
Int J Oncol. 42:757–766. 2013. View Article : Google Scholar
|
|
22
|
Wu XZ, Wang KP, Song HJ, Xia JH, Jiang Y
and Wang YL: MiR-27a-3p promotes esophageal cancer cell
proliferation via F-box and WD repeat domain-containing 7 (FBXW7)
suppression. Int J Clin Exp Med. 8:15556–15562. 2015.PubMed/NCBI
|
|
23
|
Zhou L, Liang X, Zhang L, Yang L, Nagao N,
Wu H, Liu C, Lin S, Cai G and Liu J: MiR-27a-3p functions as an
oncogene in gastric cancer by targeting BTG2. Oncotarget.
7:51943–51954. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mahon PC, Baril P, Bhakta V, Chelala C,
Caulee K, Harada T and Lemoine NR: S100A4 contributes to the
suppression of BNIP3 expression, chemoresistance, and inhibition of
apoptosis in pancreatic cancer. Cancer Res. 67:6786–6795. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee H and Paik SG: Regulation of BNIP3 in
normal and cancer cells. Mol Cells. 21:1–6. 2006.PubMed/NCBI
|
|
26
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|