Introduction
Cisplatin (CDDP) is a divalent platinum compound
with broad-spectrum anticancer activity that synergizes with a
variety of antitumor drugs without cross-resistance, making it a
first-line anticancer drug (1).
CDDP is commonly used for the treatment of various cancers
including bladder cancer, lung cancer, ovarian cancer and other
soft tissue tumors. In children, CDDP is also effective for the
treatment of various solid tumors (2,3).
However, CDDP has serious side effects, including irreversible
hearing loss, especially in juvenile patients treated with CDDP and
radiotherapy for at least six months (4-6).
The mechanism of CDDP-induced ototoxicity remains to
be elucidated. CDDP could induce apoptosis of cochlear cells, which
led to hearing loss (7-9). Several studies also showed that CDDP
increased NADPH oxidase 3 (NOX 3) expression in the cochlea, which
increased the production of reactive oxygen species (ROS) (10,11). ROS directly attacked the cochlear
cell membrane and produced the toxic lipid peroxidation product
4-hydroxynonenal (4-HNE), which might also account for cochlear
cell apoptosis (12). A study
showed that CDDP-induced ototoxicity in the cochlea was related to
calcium (Ca2+) over-load (13). As an important second messenger,
Ca2+ is involved in various functions, including
auditory signal transduction (14). Moreover, maintenance of a low
intracellular Ca2+ concentration in the normal resting
state plays an important role in sustaining homeostasis (15). Abnormal influx of free
Ca2+ in cells (referred to as Ca2+ overload)
can cause structural damage and disruption of intracellular
metabolism of hair cells (HCs), eventually leading to apoptosis of
HCs (16). In the cochlea,
Ca2+ overload was related to high expression of the
transient receptor potential vanilloid receptor 1 (TRPV1) (17). TRP channels are a family of
non-selective cation ion channels that control important cellular
functions and signaling pathways (18). TRP channels are also important
targets for a wide range of drugs, such as menthol, which adjust
human TRP melastatin 8 (hTRPM8)-induced signaling, and could
alleviate hyperglycemia in alloxantreated mice (a model of type 1
diabetes) or reverse muscle atrophy in dexamethasone-treated mice
(a model of muscle wasting) (19). TRP channel activation can lead to
changes in Ca2+ homeostasis, resulting in long lasting
modulation of Ca2+ levels (20,21). TRPV1 is expressed in many organs,
including the heart, kidney, brain, dorsal root ganglions and
sensory neurons (22). TRPV1
plays a critical role in the maintenance of cellular homeostasis,
particularly in cochlear cells (23). Studies have suggested that CDDP
induced Ca2+ overload by increasing TRPV1 expression,
and that Ca2+ subsequently activates calpain, a neutral
cysteine protease, to cause cochlear apoptosis (24). However, the association between
Ca2+ overload and TRPV1 remains to be confirmed.
Ursolic acid (UA) is a plant-derived triterpene
compound with strong antioxidant effects (25-27). UA is the main ingredient of
several traditional Chinese herbal medicines used in the treatment
of liver diseases and in lowering blood lipid (28,29). In PC12 cells, UA could assert its
neuroprotective effects by inhibiting ROS production induced by
amyloid beta peptide (30,31).
In addition, UA could capture oxygen free radicals in the P450
monoamine oxidase system and liver microsome, thus exerting a
strong inhibitory effect on lipid peroxidation (32). However, to the best of our
knowl-edge, there are currently no reports on the anti-apoptotic
and anti-oxidative effects of UA on ototoxicity induced by CDDP.
The present study evaluated the protective effects of UA on
CDDP-induced ototoxicity and the underlying mechanisms were
investigated. It was hypothesized that UA could protect against
CDDP-induced ototoxicity by inhibiting oxidative stress and
TRPV1-mediated Ca2+-signaling in the cochlear cells.
Materials and methods
Animal and experimental protocols
A total of 48 BALB/c mice (3 days) were purchased
from the Animal Experimental Center, Jinzhou Medical University,
China. A total of 48 BALB/c mice (20-22 g, 4 weeks, male and
female) with normal Preyer's reflexes were purchased from the
Animal Experimental Center, Dalian Medical University, China
[license no. SCXK (Liao) 2008-0002]. All animal studies complied
with the regulations for the Management of Laboratory Animals
published by the Ministry of Science and Technology of the People's
Republic of China, and all animal protocols were approved by the
Animal Care and Use Committee of Jinzhou Medical University. All
mice were fed a standard commercial diet and housed at an ambient
temperature of 20-24°C with a relative humidity of 50±5% under a
12/12 h light-dark cycle in a specific pathogen-free facility
during the experiment. All animals were allowed 1 week to acclimate
before treatment and then were randomly divided into four groups
(n=12 in each group) and treated for five days as follows: i)
Normal control group, where the mice received physiological saline
[0.5 ml/100 g, intra-peritoneally (i.p)]; ii) UA group, where the
mice received UA (purity ≥98%; cat. no. N1823, APExBIO Technology
LLC; 80 mg/kg/day, i.p); iii) CDDP group, where the mice received
CDDP (Sigma-Aldrich; Merck KGaA; 4.5 mg/kg/day, i.p,) and iv) UA
plus CDDP (UA+CDDP) group, where the mice received UA (80
mg/kg/day, i.p) followed by CDDP (4.5 mg/kg/day, i.p) 30 min later
each day (Fig. 1A).
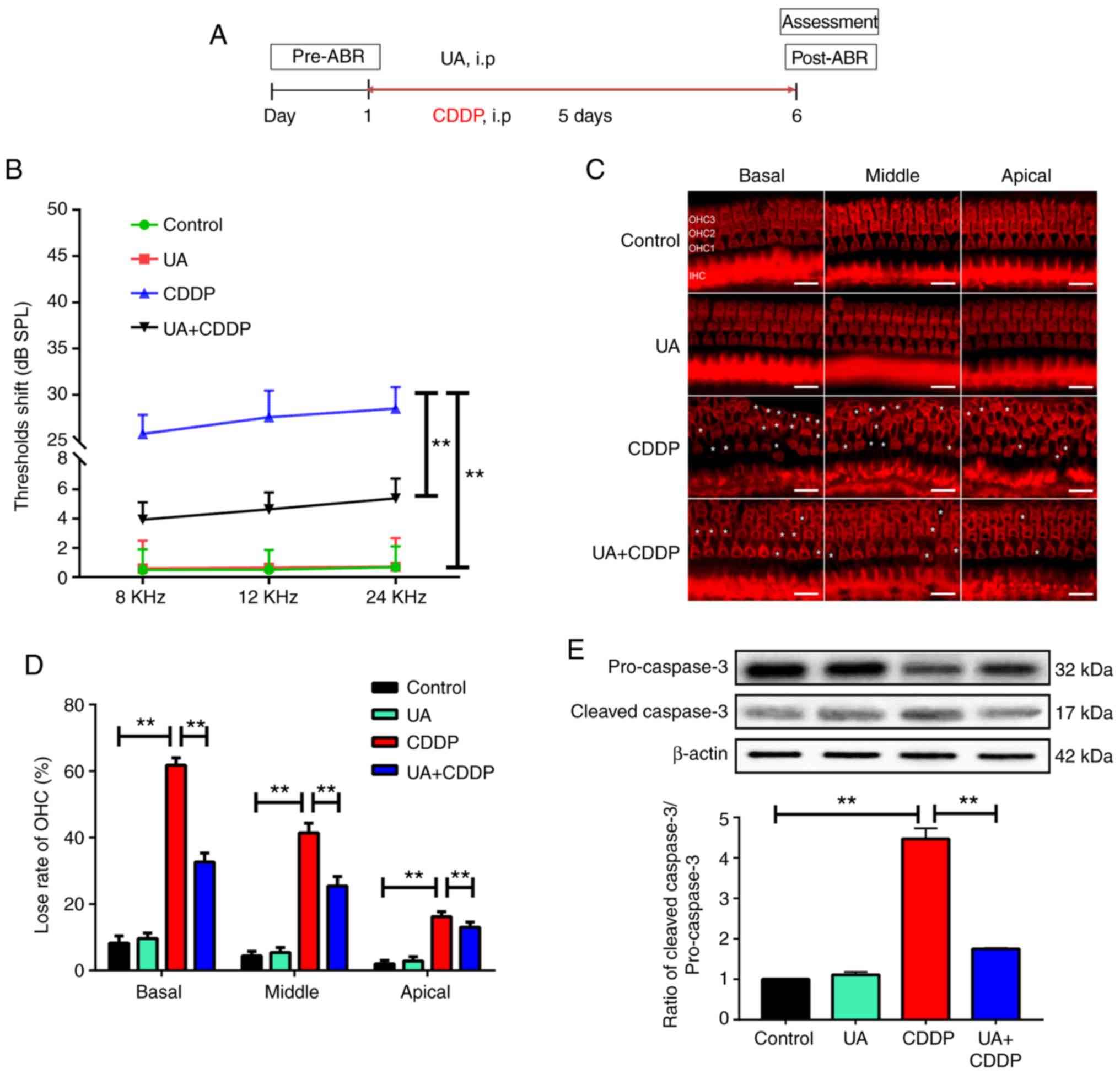 | Figure 1UA prevents CDDP-induced hearing loss
and cochlea apoptosis in mice. (A) The flow chart of the
experimental protocol. (B) ABR results at tone bursts of 8, 12, 24
kHz. n=12 mice for each group. **P<0.01. (C) UA
prevented CDDP-induced OHC loss. OHCs were labeled by
tetramethylrhodamine-phalloidin staining in the basilar membrane.
White asterisks indicate cell loss. Scale bar, 20 µm. OHCs
exhibited regional variation in their susceptibility to cisplatin,
with gradual severity of hair cell loss from the apical turn to the
basal turn. Mice receiving UA + CDDP demonstrated fewer OHC loss.
(D) The numbers of intact hair cells on the apical, middle and
basal turns. The graph shows quantitative differences of OHC loss
at apical, middle and basal turns of the cochlear basilar membrane
The UA + CDDP group exhibited significantly fewer hair cell loss in
comparison compared with the CDDP group. n=3 ears for each group.
**P<0.01 (E) Western blot analysis was performed to
detect protein expression levels of cleaved caspase-3 and caspase-3
in cochlea, with β-actin as a loading control. n=6 ears for each
group. Mean values were obtained from n=3 independent experiments.
**P<0.01. ABR, auditory brainstem response; UA,
ursolic acid; i.p, intraperitoneal; CDDP, cisplatin; OHC, outer
hair cell; SPL, sound pressure levels. |
During the treatment period, the weight of the
experimental animals was measured every day (Fig. S1). Although CDDP is toxic, the
survival rate of mice is 100% during the administration (33).
Measurement of auditory brainstem
response (ABR)
For the analysis of the auditory threshold, ABR was
recorded 1 day before and 5 days after related drug treatment with
tone bursts of 8, 12 and 24 kHz using the Smart EP & OAE
auditory evoked potential recording system (Intelligent Hearing
Systems). The mice were anesthetized using pentobarbital sodium (90
mg/kg) and kept warm with a heating pad during ABR recording. A
subdermal (active) needle electrode was inserted at the vertex,
while ground and reference electrodes were inserted subdermally in
the loose skin beneath the pinnae of opposite ears. The technique
used to record ABRs has been previously described in detail
(34). The ABR waveforms were
averaged over a 10 msec time window using the Smart EP & OAE
auditory evoked potential recording system software (Launch Pad
2.32 HIS program). The sound intensity was varied at 5 dB intervals
near the hearing threshold. The differences in ABR thresholds shift
for each frequency between the starting and the terminal points of
the experimental time course were noted. The threshold was
determined off-line by two independent, experimentally blinded
observers based on the ABR records. In addition, the mice were
euthanized, and double cochleae were removed for further
analysis.
Outer hair cell (OHC) counting
Immediately after completion of the ABR test, the
animals were killed by cervical dislocation under anesthesia with
an intraperitoneal injection of pentobarbital sodium (90 mg/kg). An
array of criteria was used to verify the death of animals,
including absence of respiration and heartbeat and loss of corneal
and palpebral reflexes.
The temporal bones were removed, and the cochlea was
immersed in 4% paraformaldehyde and incubated overnight at 4°C. The
cochlea was decalcified in 4% EDTA for 7 days at 4°C. Subsequently,
the basilar membrane was dissected under a dissecting microscope,
and the stria vascularis and tectorial membrane were removed. The
basilar membrane was then stained with
phalloidin-tetramethylrhodamine (TRITC; Sigma-Aldrich; Merck KGaA)
for 20 min at 20-24°C and protected from light. The specimens were
then rinsed three times with 0.01 M phosphate-buffered saline (PBS;
pH 7.4) and then mounted on slides. Images were obtained using the
TCS-SP5 II laser-scanning confocal microscope (Leica Biosystems
GmbH). Outer hair cells were counted from the captured images using
a fluorescent BX41 microscope (Olympus Corporation) at ×200
magnification in 20 consecutive fields from the apex to the basal
turn along the entire length of the cochlear epithelium. The
percentages of outer hair cell loss in each 0.5-mm length of
epithelium were plotted as a function of the cochlear length as a
cytocochleogram (34).
Immunohistochemical staining of paraffin
sections of the cochlea
After the last auditory brainstem response test,
five mice from each group were decapitated under abdominal
anesthesia using pentobarbital sodium (90 mg/kg), and the temporal
bones were removed immediately. The round and oval windows were
opened, cochleae were perfused with 40 g/l paraformaldehyde (pH
7.4), and the specimens were immersed in the 4% paraformaldehyde
for 24 h at 4°C. Following decalcification in 40 g/l EDTA solution
(pH 7.4) for 5 days at 4°C, the cochleae were dehydrated with
ethanol, cleared with xylene and embedded in paraffin wax. Serial
sections (5-µm thickness) were prepared, dewaxed, rehydrated
in descending alcohol series, rinsed with distilled water and
treated with high pressure (125°C; 103 kPa; 3 min) to retrieve
antigens. Endogenous peroxidase activity was blocked with 3 g/l
H2O2 for 10 min at 20-24°C, followed by a PBS
wash. After incubation with normal goat serum (cat. no. C0265;
Beyotime Institute of Biotechnology) for 15 min at 20-24°C, tissue
sections were stained overnight at 4°C with primary antibodies for
TRPV1 (1:500; cat. no. ab6166; Abcam) and calpain 2 (1:1,000; cat.
no. sc-373967; Santa Cruz Biotechnology, Inc.). After washing with
PBS, sections were stained with horseradish
peroxidase-conjugated-biotinylated goat anti-rabbit immunoglobulin
G (IgG) secondary anti-body (1:1,000; cat. no. ab205718; Abcam) for
1 h at 20-24°C. Following washing with PBS, color was developed
with 0.05% 3,3'-diaminobenzidine for 3 min at 20-24°C. Sections
were counterstained with hematoxylin for 10 min at 20-24°C,
dehydrated with gradient concentrations of ethanol, vitrified with
xylene and mounted with neutral gum. PBS was used as a negative
control throughout the study. The sections were observed by using a
BX41 fluorescent microscope at ×400 magnification (Olympus
Corporation). Relative optical intensity was determined using
Image-Pro Plus 6.0 software (Media Cybernetics, Inc.).
Western blot analysis
Total cochlea tissue was homogenized in RIPA buffer
(cat. no. P0013B; Beyotime Institute of Biotechnology) containing
protease inhibitor cocktail tablets and phosphatase inhibitors
cocktail (Roche Diagnostics GmbH). The tissue homogenate was
sonicated for 30 sec and centrifuged at 14,000 × g at 4°C for 30
min to extract the supernatant. Protein concentrations were
determined using a bicinchoninic acid kit. The protein samples (50
µg/lane) were separated using SDS-PAGE (7.5% for TRPV1 and
calpain 2; 10% for 4-HNE and procaspase 3 and 12.5% for cleaved
caspase 3). Following electrophoresis, the proteins were
transferred onto nitrocellulose membranes (Thermo Fisher
Scientific, Inc.). The membranes were then blocked with 10% bovine
serum albumin for 1 h at 4°C and incubated overnight at 4°C with
the following primary antibodies: Anti-TRPV1 (1:500; cat. no.
ab6166; Abcam), anti-calpain 2 (1:1,000; cat. no. sc-373967; Santa
Cruz Biotechnology, Inc.), anti-4-HNE (1:800; cat. no. ab48506;
Abcam), anti-cleaved caspase-3 (1:1,000; cat. no. ab13847; Abcam)
and anti-caspase-3 (1:1,000; cat. no. sc-7272; Santa Cruz
Biotechnology, Inc.). After washing three times with TBS-0.05%
Tween-20, membranes were incubated with IRDye 800CW goat
anti-rabbit IgG secondary antibody (1:10,000; cat. no. 925-32211;
LI-COR Biosciences.) for 1 h at 4°C. Following extensive washing,
the immunoreactive bands were visualized by ECL. The Odyssey CLx
Infrared imaging system (LI-COR Biosciences) was used to analyze
the optical density value of immunoreactive bands. β-actin
(1:1,000; cat. no. sc-47778; Santa Cruz Biotechnology, Inc.) was
used as a loading control.
Organotypic cultures of postnatal organ
of Corti
The organ culture procedures were modified from a
previously published report (35). In brief, Cochlear explants were
isolated from newborn 3-day-old BALB/c mice. BALB/c pups were
euthanized following antisepsis using 75% ethanol. The two otic
capsules were extracted from surrounding tissue and immersed
ice-cold Hank's balanced salt solution (HBSS; cat. nos. CC014 and
CC016; M&C Gene Biotechnology). The lateral wall tissues (stria
vascularis and spiral ligament) and the auditory nerve bundle were
micro-dissected for sterile dissection under a binocular microscope
(Olympus SZX7; Olympus Corporation). The explants were carefully
placed onto a prepared culture dish containing a 15-µl
polymerized drop of rat tail collagen in 1 ml culture medium
consisting of DMEM/F12 (Invitrogen; Thermo Fisher Scientific,
Inc.), 1% bovine serum albumin (Invitrogen; Thermo Fisher
Scientific, Inc.), and 10 U/ml penicillin G. Following 4 h of
incubation (37°C, 5% CO2), an additional 1 ml medium was
added to submerge the explants.
Measurement of Ca2+ levels in
OHCs
Cell-permeable Ca2+ indicator Fluo-4 AM
(cat. no. F14201; Invitrogen; Thermo Fisher Scientific, Inc.) were
used to monitor levels of intracellular Ca2+, based on
the literature (36). Following
immersion in HBSS without Ca2+ and Mg2+ for
20 min at 20-24°C, the organ explants were immersed in dye (cat.
no. F14201; Invitrogen; Thermo Fisher Scientific, Inc.) for 25 min
following Fluo4-AM loading at 24°C. Subsequently, the explants were
fixed in 4% paraformaldehyde for 30 min at 20-24°C. Phalloidin
(1:200) was applied for 1 h at 20-24°C and protected from light.
For measurement of Fluo-4 Ca2+ signals, organs were
imaged under a BX41 microscope at ×400 magnification (Olympus
Corporation) with epifluorescence, and the images were obtained
using a TCS-SP5II laser-scanning confocal micro-scope (×400
magnification; Leica Biosystems GmbH) in 20 consecutive fields.
Immunofluorescence for assessment of
4-HNE
Preparation of cochlear paraffin sections
(5-µm thickness), deparaffinization, rehydration, antigen
retrieval and blocking nonspecific binding were performed as
described in a previous study (34). Tissue sections were incubated with
rabbit anti-4-HNE antibody (1:200; cat. no. ab46545; Abcam),
followed by Cy3-labeled goat anti-rabbit IgG secondary antibody
(1:500; cat. no. A0516; Beyotime Institute of Biotechnology)
incubation for 1 h at 24°C. Immunofluorescence images were obtained
via TCS-SP5II laser-scanning confocal microscopy (×400
magnification; Leica Biosystems GmbH). Relative optical intensity
was determined using Image-Pro Plus 6.0 software (Media
Cybernetics, Inc.).
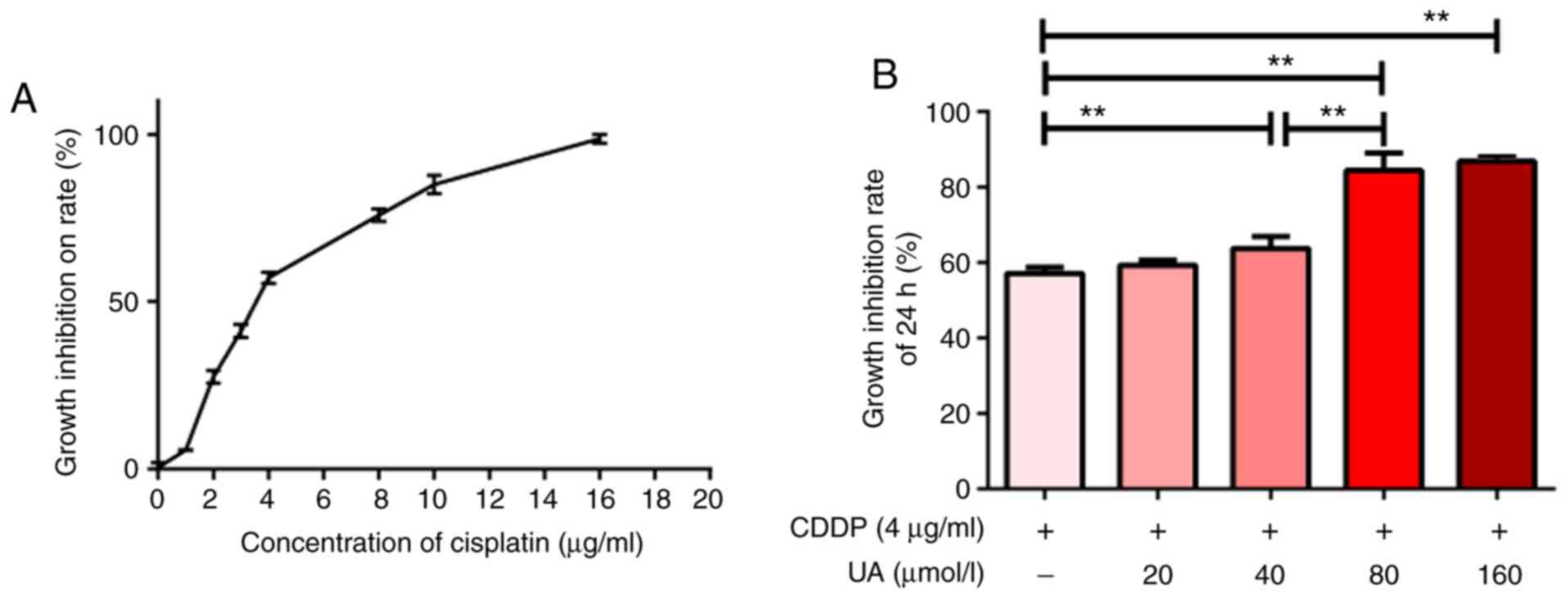
Measurement of cell growth inhibition by
MTT assay
The human ovarian cancer cell line SKOV3 (Peking
Union Medical College, Beijing) were seeded at a density of
3×103 cells/well in 96-well plates and treated with
different concentrations (1, 2, 3, 4, 8, 10 and 16 µg/ml) of
CDDP. Each concentration treatment was repeated in six separate
wells. Cells treated with RPMI 1640 media (100 ul; Sigma-Aldrich;
Merck KGaA) alone acted as a control. The cells were incubated at
37°C with 5% CO2 for 24 h, and MTT reagent (20
µl; 5 mg/ml in PBS; Sigma-Aldrich; Merck KGaA) was added to
each well and incubated at 37°C with 5% CO2 for 4 h. The
absorbance in each well was measured at a wavelength of 570 nm
using an enzyme-linked immunoassay microplate reader. According to
the tumor cell growth inhibition rate, 4 µg/ml of CDDP was
determined as optimal inhibitory concentration. Subsequently, SKOV3
cells were seeded at a density of 3×103 cells/well in a
96-well plate and treated with CDDP (4 µg/ml). Different
concentrations (20, 40, 80 and 160 mol/l) of UA were added later.
Each concentration of UA treatment was repeated in six separate
wells. The cells were incubated at 37°C with 5% CO2 for
24 h, and MTT reagent (20 µl; 5 mg/ml in PBS; Sigma-Aldrich;
Merck KGaA) was added to each well and incubated at 37°C with 5%
CO2 for 4 h. The absorbance in each well was measured at
a wavelength of 570 nm using an enzyme-linked immunoassay
microplate reader (BioTek Instruments, Inc.).
Statistical analysis
Data are presented as the mean ± SD and were
analyzed using SPSS 17.0 software (SPSS, Inc.). Intergroup
differences in mean values were compared by one-way ANOVA followed
by Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
UA prevents CDDP-induced hearing loss and
hair cell loss in vivo
To investigate whether UA could prevent CDDP-induced
hearing loss, the ABR test was performed on mice. In the control
group, ABR thresholds at 8, 12, and 24 kHz were observed to be
stable (0.48±1.43, 0.50±1.37 and 0.67±1.44 dB, respectively).
However, ABR threshold shifts in the CDDP group were significantly
increased at 8, 12 and 24 kHz (25.78±2.06, 27.59±2.86 and
28.51±2.32 dB, respectively), compared with the control group
(P<0.01). In the UA + CDDP group, ABR threshold shifts
significantly reduced at 8, 12 and 24 kHz (P<0.01; 3.93±1.19,
4.63±1.16 and 5.38±1.36 dB, respectively), compared with the CDDP
group. In the UA group, UA treatment alone did not affect the ABR
threshold shifts compared with the control group. These results
suggested that UA can mitigate CDDP-induced hearing loss in mice
(Fig. 1B).
To further determine the in vivo protective
effects of UA against CDDP, TRITC staining of OHCs was performed on
the basilar membrane of mouse cochlea and the number of surviving
OHCs was counted. In the control and UA groups, OHCs on the basilar
membrane appeared normal in structure and the staining pattern was
homogeneous. Moreover, the loss rate of OHCs at each turn of the
basilar membrane was <10%. In the CDDP group, the stereocilia of
OHCs were characterized by loss and disorder. OHCs exhibited
regional variation in their susceptibility to cisplatin, with
gradual severity of OHC loss from the apical to the basal turn, and
loss rates of OHCs at apical, middle and basal turns reaching 16,
41 and 61%, respectively, which was significantly higher compared
with the control group (P<0.01; Fig. 1C and D). However, compared with
the CDDP group, animals receiving UA + CDDP demonstrated
significantly fewer OHC loss, especially at basal and middle turns,
with the loss rate of OHCs at apical, middle and basal turns
reaching 13, 25 and 32%, respectively (P<0.01; Fig. 1C and D). These results suggested
that UA effectively protects against CDDP-induced OHC structural
damage. Numerous studies showed that CDDP mainly damaged OHCs,
while the damage of inner hair cells was weak (8). Hence, the present study focused on
the outer hair cell change in and inner hair cell counting was not
investigated.
Western blot analysis showed that the CDDP group
expressed significantly higher levels of cleaved caspase-3 protein
(a marker for apoptosis) compared with the control group
(P<0.01; Fig. 1E). By
contrast, cleaved caspase-3 expression levels in the UA + CDDP
group were significantly lower compared with the CDDP group
(P<0.01; Fig. 1E). However,
cleaved caspase-3 expression levels in the UA group were not
significantly different compared with the control group (Fig. 1E). Again, these results are
consistent with the notion that UA can prevent the auditory loss
observed in mice treated with CDDP.
UA inhibits CDDP-induced activation of
the TRPV1/Ca2+/calpain 2 signaling pathway in the
cochleae
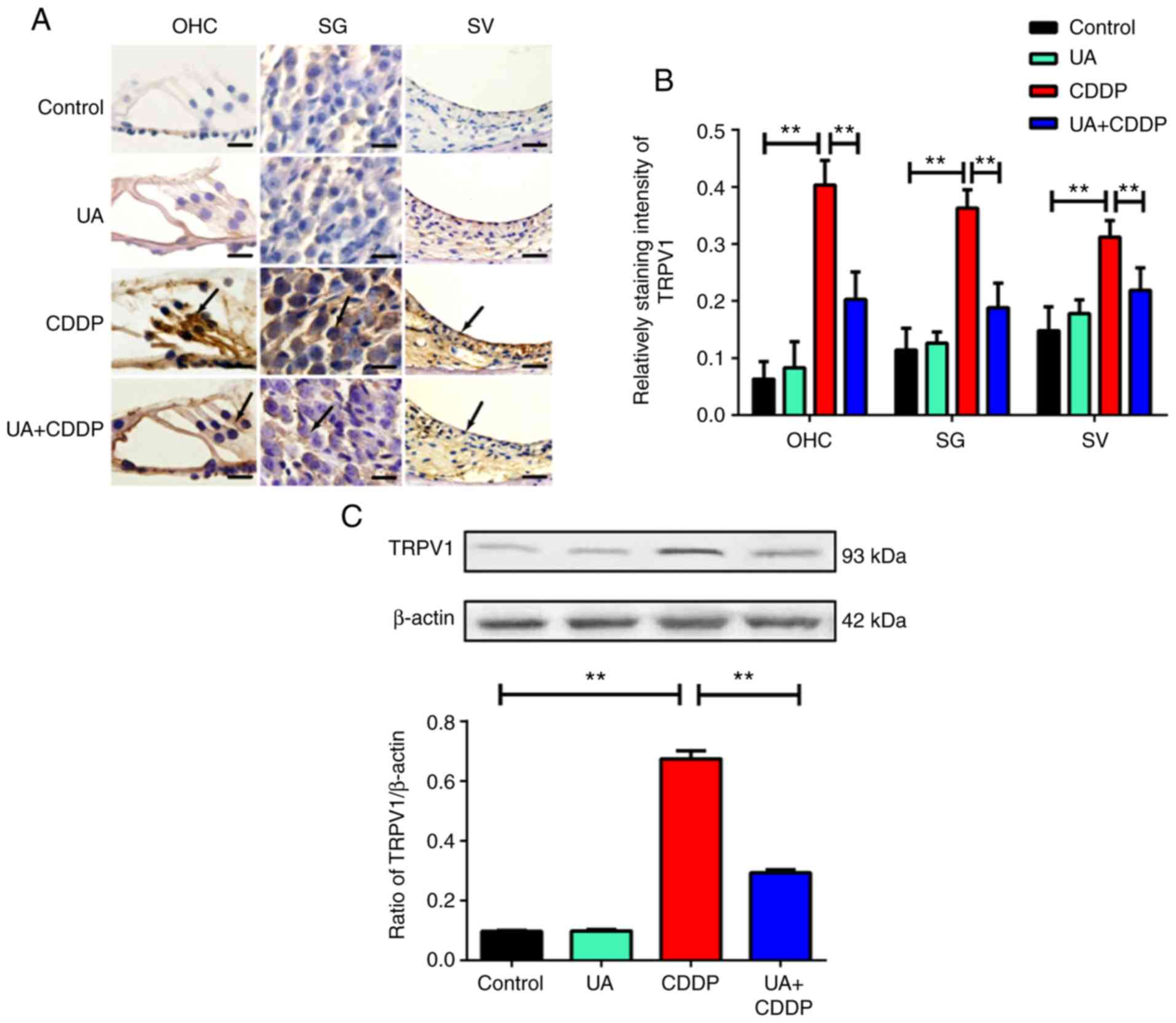
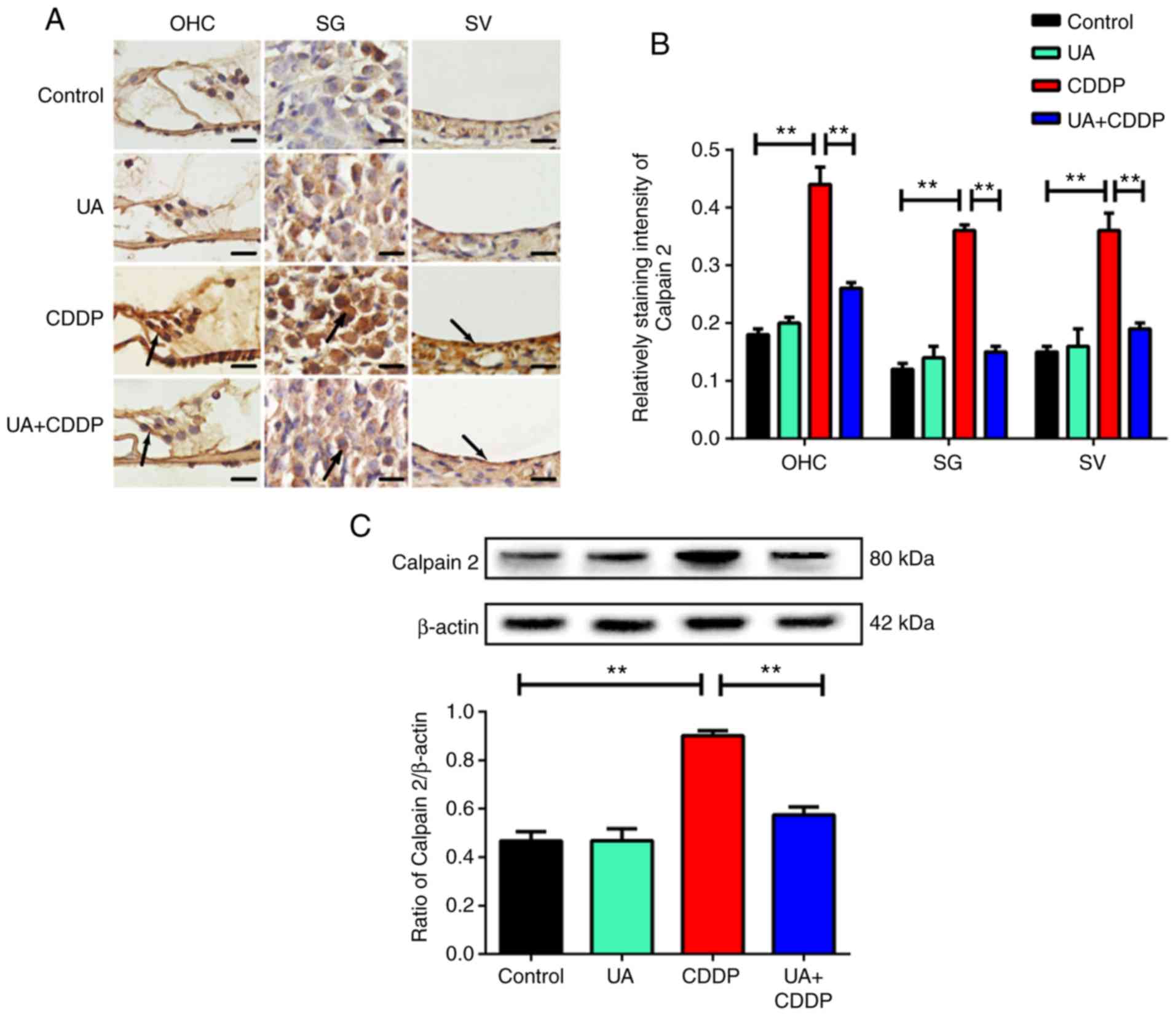
Based on immunohistochemistry results (Figs. 2A and 3A), TRPV1 and calpain 2 were uniformly
distributed on the OHCs, spiral ganglion (SG), and stria vascularis
(SV) of the cochlea in the control group. By contrast, TRPV1 and
calpain 2 staining was unevenly distributed in the CDDP group, and
the expression levels of these proteins significantly increased
compared with the control group (P<0.01; Figs. 2B and 3B). In the UA + CDDP group, TRPV1 and
calpain 2 staining was uniformly distributed, and expression
significantly decreased compared with the CDDP group (P<0.01,
respectively; Figs. 2B and
3B). UA treatment alone did not
affect TRPV1 and calpain 2 expression in the mouse cochlea
(Figs. 2A and B and 3A and B). These results were consistent
with the results of western blot analysis (P<0.01; Figs. 2C and 3C).
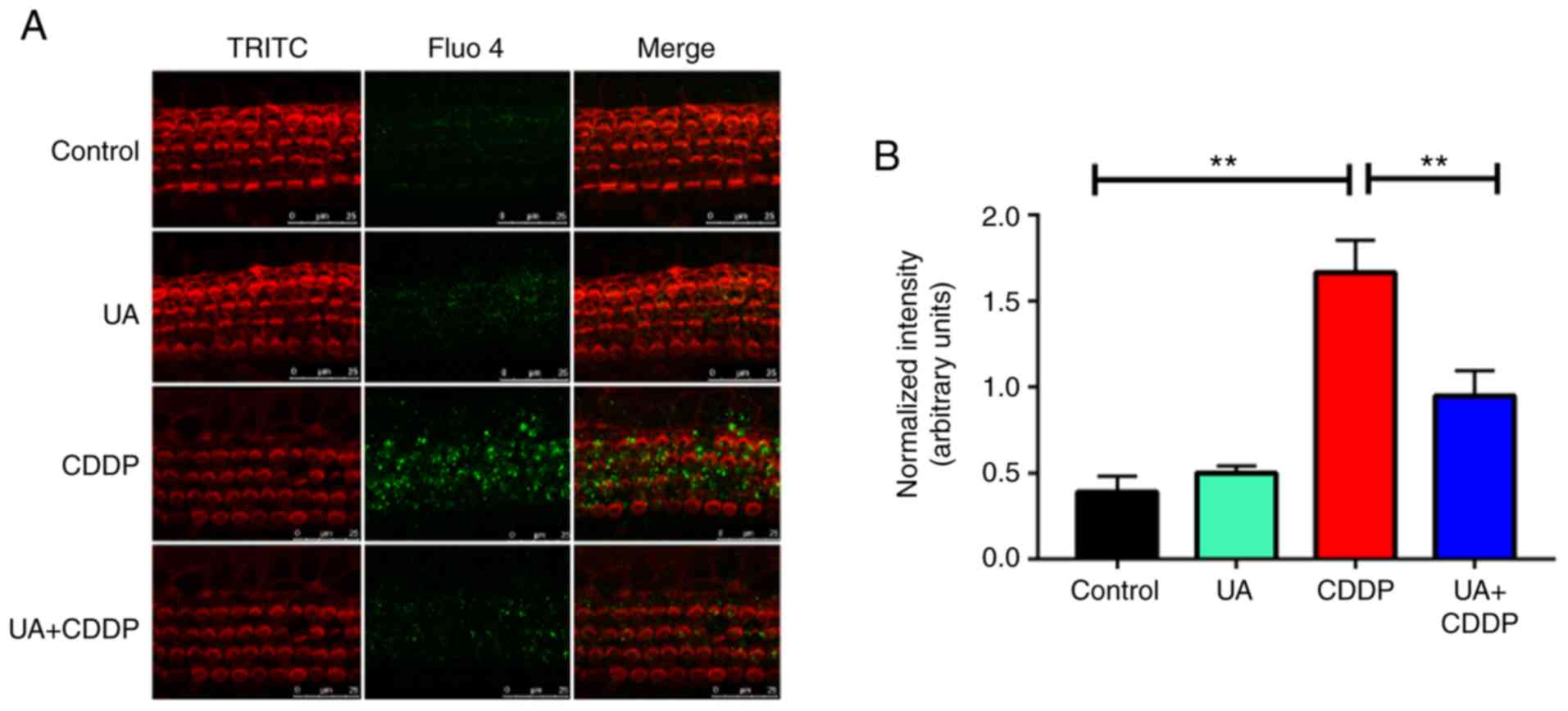
In the control group, Ca2+ staining was
uniformly distributed in OHCs of the basement membrane (Fig. 4A). In the CDDP group,
Ca2+ staining was unevenly distributed (Fig. 4A), and its intensity significantly
increased compared with the control group (P<0.01; Fig. 4B). In the UA + CDDP group,
Ca2+ staining was more uniformly distributed (Fig. 4A), with significantly lower
intensity compared with the CDDP group (P<0.01; Fig. 4B). The results suggested that CDDP
could cause Ca2+ overload, and that UA could effectively
inhibit Ca2+ influx in mice cochleae.
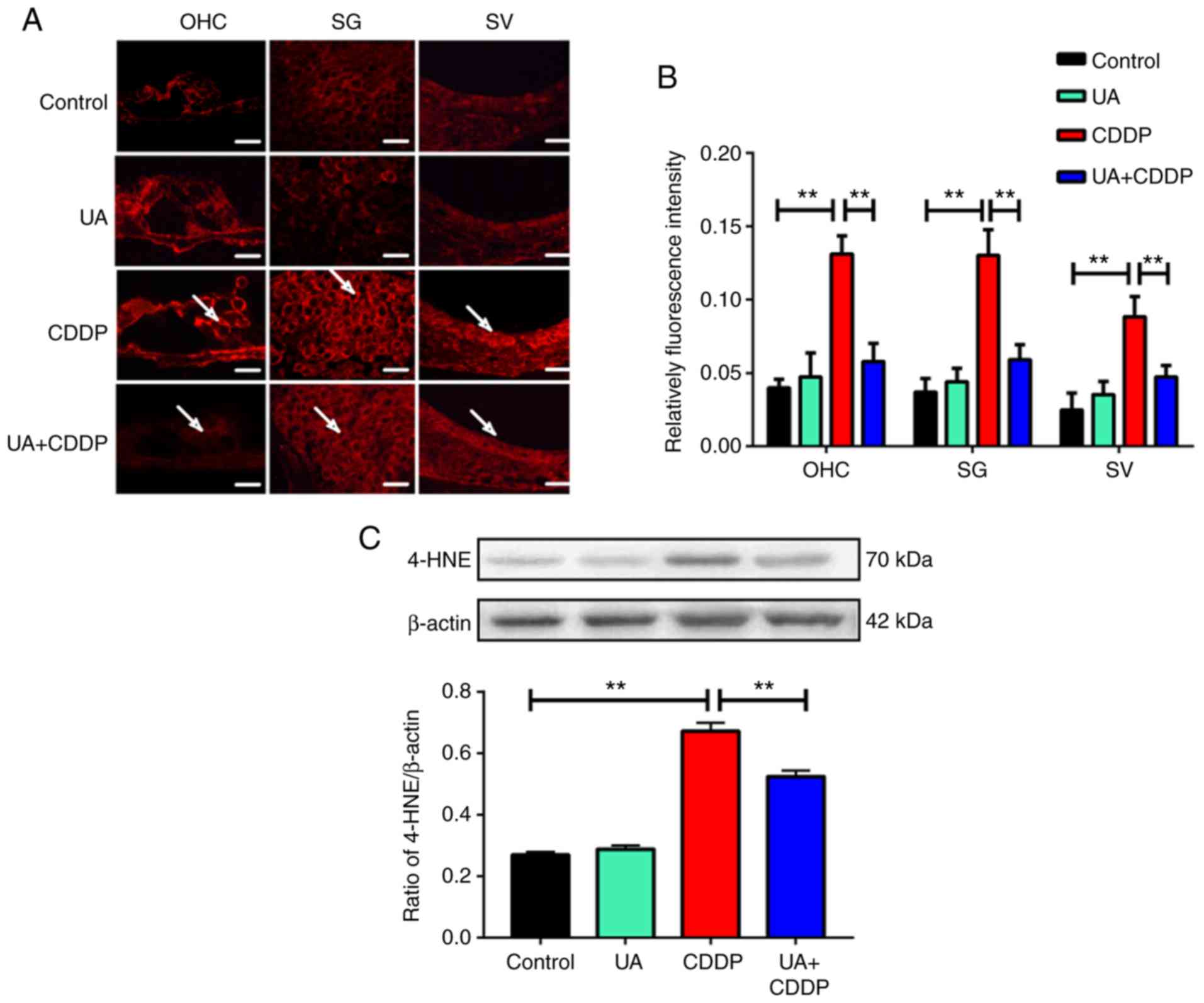
UA alleviates oxidative stress in mice
with CDDP-induced ototoxicity
To investigate whether UA could alleviate oxidative
stress, the levels of 4-HNE were analyzed. Based on
immunofluorescence results, CDDP significantly enhanced production
of 4-HNE in the OHCs, SG, and SV of mouse cochlea compared with the
control group (P<0.01; Fig. 5A and
B). However, 4-HNE production was significantly inhibited in
the UA + CDDP group compared with the CDDP group (P<0.01;
Fig. 5A and B). Moreover,
compared with the control group, UA treatment alone did not affect
4-HNE production in the mouse cochleae (Fig. 5B). These results were confirmed by
western blot analysis of 4-HNE levels (P<0.01; Fig. 5C). The results suggested that UA
could prevent an increase in oxidative stress-related markers in
the cochlea of mouse treated with CDDP.
The effect of UA on the antitumor effect
of CDDP
Since CDDP is used as an anticancer therapeutic, it
was investigated whether UA co-treatment would impair its antitumor
effects. MTT assay was performed to evaluate the viability of the
human ovarian cancer cell line SKVO3 in response to CDDP, UA, and
UA + CDDP. While CDDP inhibited the growth of SKVO3 cells in a
dose-dependent manner (Fig. 6A),
growth inhibition of SKVO3 cells was significantly enhanced
following UA + CDDP (4 g/ml) treatment. Moreover, UA inhibited the
growth of SKOV3 cells in a dose-dependent manner (P<0.01;
Fig. 6B), suggesting that UA
enhanced the antitumor effect of CDDP in vitro.
Discussion
UA is a triterpenoid compound found in >60
varieties of plants, including bearberry, Chinese elder herb and
hawthorn (37). The therapeutic
effects of UA include sedative, anti-inflammatory, anti-ulcer and
anti-diabetic effects (38,39). A previous study demonstrated that
UA significantly alleviated hydrogen peroxide-induced apoptosis
against HEI-OC1 cells in vitro (40). In the present study, UA was found
to inhibit CDDP-induced cochlear OHC damage, which in turn
significantly attenuated hearing loss at low and high frequencies
in mice. Therefore, based on the present findings, it was proposed
that UA could provide therapeutic benefits for CDDP-induced
ototoxicity.
TRPV1 channels are highly permeable to intracellular
calcium influx, and overexpression of TRPV1 induces Ca2+
overload and the activation of calpains, especially calpain 2
(41). Activation of calpain 2
might mediate caspase-3-dependent apoptosis of cochlear cells by
down-regulating Bcl-xl and up-regulating Bax (42-45). The TRPV1 channel was previously
reported to play a critical role in CDDP-induced ototoxicity
(46). Moreover, several studies
have shown that TRPV1 expression is significantly increased in OHC,
SG and SV in the cochlea in response to CDDP treatment (47-49). The present study found that UA
decreased the levels of CDDP-mediated TRPV1 protein expression in
the cochlear OHC, SG, and SV. In addition, CDDP dysregulated
Ca2+ homeostasis in the cochlea, which is accompanied by
increased expression of calpain 2. UA was previously shown to
alleviate coughing via TRPV1 channel desensitization (50). Whether a similar mechanism can
account for inhibition of CDDP-mediated TRPV1 protein expression by
UA remains to be elucidated. TRPV1 is also activated by heat
(>42°C), capsaicin, low extracellular pH and oxidative stress
products (51). Thus, the
antioxidant activity of UA might inhibit activation of the TRPV1
channel by reducing oxidative stress products in the cochlea. The
present results suggested that UA significantly blocked
CDDP-induced Ca2+ overload in hair cells and also
inhibited calpain 2 expression. These results suggested an
association between the TRPV1 channel and the
Ca2+/calpain signaling pathway, which might explain the
decrease in CDDP-induced Ca2+ influx observed in the
cochlea following UA treatment.
Oxidative stress is considered to be an important
cause of CDDP-induced hearing loss (52). The main source of ROS is NADPH
oxidase (NOX) (53). CDDP induced
expression of NOX3 in the cochlea, resulting in the formation of
ROS, and consequently lipid peroxidation of the cochlea (10). In the present study, the levels of
4-HNE, a highly toxic lipid peroxidation product of aldehydes
(54), were observed to increase
as a result. High 4-HNE levels can induce the release of cytochrome
c from the mitochondria by activating the mitogen-activated
protein kinase/JNK cell death signal cascade (55). In the cytoplasm, cytochrome
c activates caspase-9 and caspase-3, eventually leading to
apoptosis of cochlear cells (56,57). Studies have shown that UA exerts
significant antioxidant effects, inhibiting excessive free radical
production by upregulation of endothelial nitric oxide synthase
expression and downregulation of inducible nitric oxide synthase
and NOX expression (58-60). In addition, UA might play an
antioxidant role by enhancing free radical scavenging (61). Consistent with these studies, the
present study found that UA inhibited the production of the highly
toxic lipid peroxidation product 4-HNE in the cochlea, indicating
that UA could inhibit CDDP-induced lipid peroxidation.
Antioxidants such as tiopronin, resveratrol, and
vitamin E can alleviate inner ear damage caused by CDDP (62-64). However, most of these antioxidants
have been found to attenuate the antitumor effects of CDDP, making
them a poor therapeutic option for reducing CDDP-induced
ototoxicity (65). Previous
research has shown that UA can exert antitumor effects by promoting
cancer cell apoptosis, inhibiting tumor angiogenesis and reducing
cytotoxicity, and that it might have therapeutic effects on several
tumors, including gastric, liver and ovarian cancers (66-68). The present study found that UA
enhanced CDDP-induced tumor cell apoptosis in vitro. It was
hypothesized that UA may complement cisplatin in its antitumor
effect. These results suggested the potential of UA and CDDP
co-treatment for the treatment of solid tumors.
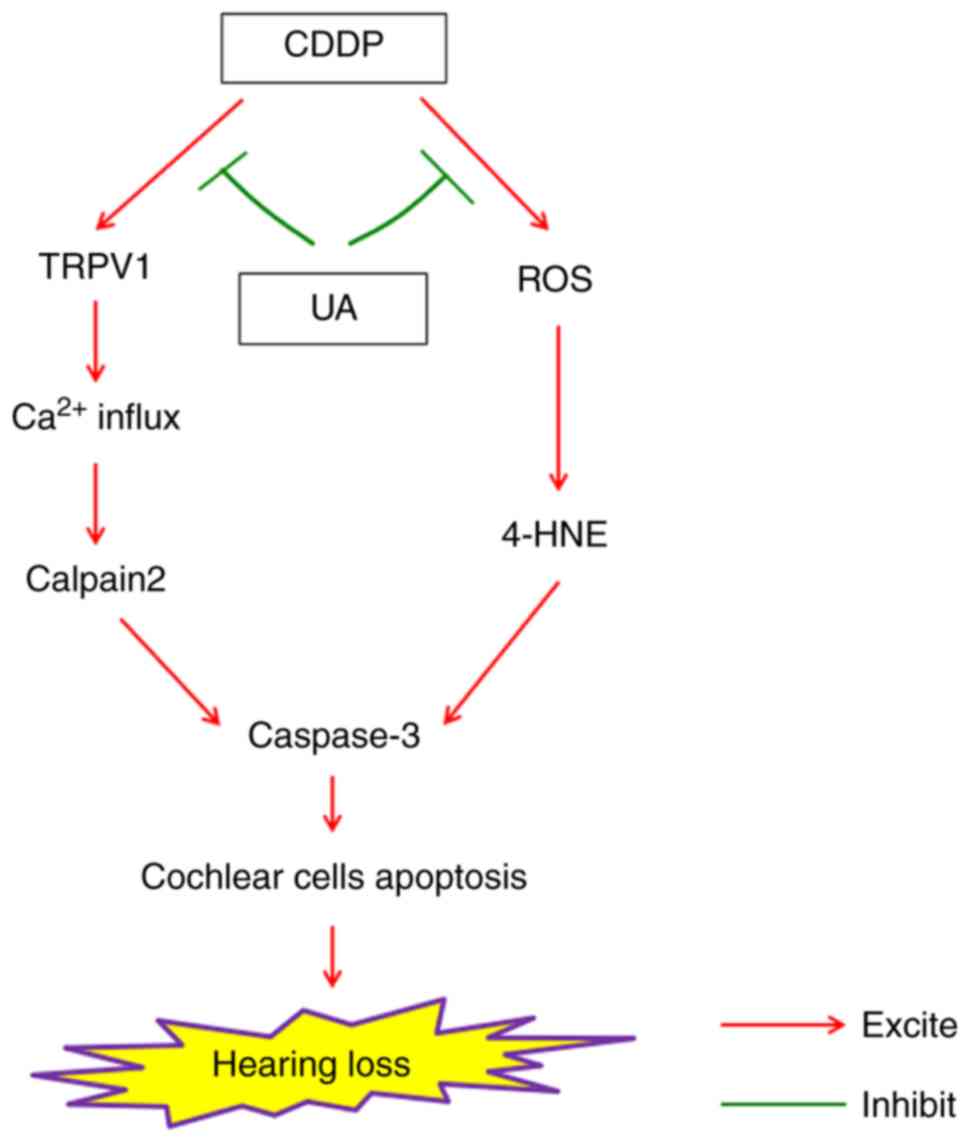
In conclusion, the present study found a role in the
TRPV1/Ca2+/calpain signaling pathway, which may
facilitate CDDP-induced hearing loss. UA can reduce the extent of
calcium overload and oxidative stress, and subsequently decrease
the induction of apoptotic pathways associated with OHC loss
(Fig. 7). These results suggested
that UA can function as a lead compound for the pharmacological
control of drug-induced ototoxicity.
Supplementary Data
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81674036 and
81704127), the Liaoning Natural Science Foundation (grant nos.
2019-ZD-0832 and 20180550402), the Doctoral Scientific Research
Foundation of Liaoning Province (grant no. 20170520045) and College
Students' Innovation and Entrepreneurship Training Program (grant
nos. 201710160000214, 201710160000154 and 201910160112).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YD was responsible for the study design and
implementation, data analysis and manuscript preparation. TX
interpreted the data and wrote the manuscript. YT, TM, DQ, YW and
DB were responsible for data collection and statistical analysis.
YL, LY, SL and AW played an important role in study design and
guidance and were responsible for the revision of the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal studies complied with the regulations for
the Management of Laboratory Animals published by the Ministry of
Science and Technology of the People's Republic of China, and all
animal protocols were approved by the Animal Care and Use Committee
of Jinzhou Medical University
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
We thank Professor Ming-Sheng Zhou from the
Department of Physiology, Shenyang Medical College, China for
helpful discussions and comments.
References
|
1
|
Basu A, Bhattacharjee A, Samanta A and
Bhattacharya S: An oxovanadium(IV) complex protects murine bone
marrow cells against cisplatin-induced myelotoxicity and DNA
damage. Drug Chem Toxicol. 40:359–367. 2017. View Article : Google Scholar
|
|
2
|
Vera O, Jimenez J, Pernia O,
Rodriguez-Antolin C, Rodriguez C, Sanchez Cabo F, Soto J, Rosas R,
Lopez-Magallon S, Esteban Rodriguez I, et al: DNA methylation of
miR-7 is a mechanism involved in platinum response through MAFG
overexpression in cancer cells. Theranostics. 7:4118–4134. 2017.
View Article : Google Scholar :
|
|
3
|
van As JW, van den Berg H and van Dalen
EC: Different infusion durations for preventing platinum-induced
hearing loss in children with cancer. Cochrane Database Syst Rev.
7:CD0108852018.PubMed/NCBI
|
|
4
|
Klumpers MJ, Coenen MJ, Gidding CE and Te
Loo DM: The role of germline variants in chemotherapy outcome in
brain tumors: A systematic review of pharmacogenetic studies.
Pharmacogenomics. 18:501–513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pang J, Xiong H, Zhan T, Cheng G, Jia H,
Ye Y, Su Z, Chen H, Lin H, Lai L, et al: Sirtuin 1 and autophagy
attenuate cisplatin-induced hair cell death in the mouse cochlea
and zebrafish lateral line. Front Cell Neurosci. 12:5152019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ryu NG, Moon IJ, Chang YS, Kim BK, Chung
WH, Cho YS and Hong SH: Cochlear implantation for profound hearing
loss after multimodal treatment for neuroblastoma in children. Clin
Exp Otorhinolaryngol. 8:329–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Youm I, West MB, Li W, Du X, Ewert DL and
Kopke RD: siRNA-loaded biodegradable nanocarriers for therapeutic
MAPK1 silencing against cisplatin-induced ototoxicity. Int J Pharm.
528:611–623. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo X, Bai X, Li L, Li J and Wang H:
Forskolin protects against cisplatin-induced ototoxicity by
inhibiting apoptosis and ROS production. Biomed Pharmacother.
99:530–536. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martín-Saldaña S, Palao-Suay R, Aguilar
MR, Ramírez-Camacho R and San Román J: Polymeric nanoparticles
loaded with dexamethasone or α-tocopheryl succinate to prevent
cisplatin-induced ototoxicity. Acta Biomater. 53:199–210. 2017.
View Article : Google Scholar
|
|
10
|
Mukherjea D, Jajoo S, Sheehan K, Kaur T,
Sheth S, Bunch J, Perro C, Rybak LP and Ramkumar V: NOX3 NADPH
oxidase couples transient receptor potential vanilloid 1 to signal
transducer and activator of transcription 1-mediated inflammation
and hearing loss. Antioxid Redox Signal. 14:999–1010. 2011.
View Article : Google Scholar :
|
|
11
|
Kim SJ, Park C, Lee JN and Park R:
Protective roles of fenofibrate against cisplatin-induced
ototoxicity by the rescue of peroxisomal and mitochondrial
dysfunction. Toxicol Appl Pharmacol. 353:43–54. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fetoni AR, Eramo SL, Paciello F, Rolesi R,
Podda MV, Troiani D and Paludetti G: Curcuma longa (curcumin)
decreases in vivo cisplatin-induced ototoxicity through heme
oxygenase-1 induction. Otol Neurotol. 35:e169–e177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harris MS, Gilbert JL, Lormore KA,
Musunuru SA and Fritsch MH: Cisplatin ototoxicity affecting
cochlear implant benefit. Otol Neurotol. 32:969–972. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ceriani F, Hendry A, Jeng JY, Johnson SL,
Stephani F, Olt J, Holley MC, Mammano F, Engel J, Kros CJ, et al:
Coordinated calcium signalling in cochlear sensory and non-sensory
cells refines afferent innervation of outer hair cells. EMBO J.
38:e998392019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hegedűs L, Zámbó B, Pászty K, Padányi R,
Varga K, Penniston JT and Enyedi Á: Molecular diversity of plasma
membrane Ca2+ transporting ATPases: Their function under
normal and pathological conditions. Adv Exp Med Biol.
1131:2020.
|
|
16
|
Wang X, Zhu Y, Long H, Pan S, Xiong H,
Fang Q, Hill K, Lai R, Yuan H and Sha SH: Mitochondrial calcium
transporters mediate sensitivity to noise-induced losses of hair
cells and cochlear synapses. Front Mol Neurosci. 11:4692018.
View Article : Google Scholar
|
|
17
|
Brito R, Sheth S, Mukherjea D, Rybak LP
and Ramkumar V: TRPV1: A potential drug target for treating various
diseases. Cells. 3:517–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Samanta A, Hughes TET and Moiseenkova-Bell
VY: Transient receptor potential (TRP) channels. Subcell Biochem.
87:141–165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bai P, Liu Y, Xue S, Hamri GC, Saxena P,
Ye H, Xie M and Fussenegger M: A fully human transgene switch to
regulate therapeutic protein production by cooling sensation. Nat
Med. 25:1266–1273. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Övey IS and Naziroğlu M: Homocysteine and
cytosolic GSH depletion induce apoptosis and oxidative toxicity
through cytosolic calcium overload in the hippocampus of aged mice:
Involvement of TRPM2 and TRPV1 channels. Neuroscience. 284:225–233.
2015. View Article : Google Scholar
|
|
21
|
Kaneko Y and Szallasi A: Transient
receptor potential (TRP) channels: A clinical perspective. Br J
Pharmacol. 171:2474–2507. 2014. View Article : Google Scholar :
|
|
22
|
Lima B, Sánchez M, Luna L, Agüero MB,
Zacchino S, Filippa E, Palermo JA, Tapia A and Feresin GE:
Antimicrobial and anti-oxidant activities of Gentianella
multicaulis collected on the Andean Slopes of San Juan Province,
Argentina. Z Naturforsch C J Biosci. 67:29–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaur T, Borse V, Sheth S, Sheehan K, Ghosh
S, Tupal S, Jajoo S, Mukherjea D, Rybak LP and Ramkumar V:
Adenosine A1 receptor protects against cisplatin ototoxicity by
suppressing the NOX3/STAT1 inflammatory pathway in the cochlea. J
Neurosci. 36:3962–3977. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marwaha L, Bansal Y, Singh R, Saroj P,
Bhandari R and Kuhad A: TRP channels: Potential drug target for
neuropathic pain. Inflammopharmacology. 24:305–317. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jabeen M, Ahmad S, Shahid K, Sadiq A and
Rashid U: Ursolic acid hydrazide based organometallic complexes:
Synthesis, characterization, antibacterial, antioxidant, and
docking studies. Front Chem. 6:3432018. View Article : Google Scholar
|
|
26
|
Iqbal J, Abbasi BA, Ahmad R, Mahmood T,
Kanwal S, Ali B, Khalil AT, Shah SA, Alam MM and Badshah H: Ursolic
acid a promising candidate in the therapeutics of breast cancer:
Current status and future implications. Biomed Pharmacother.
108:752–756. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Silva FS, Oliveira PJ and Duarte MF:
Oleanolic, ursolic, and betulinic acids as food supplements or
pharmaceutical agents for type 2 diabetes: Promise or illusion? J
Agric Food Chem. 64:2991–3008. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wan SZ, Liu C, Huang CK, Luo FY and Zhu X:
Ursolic acid improves intestinal damage and bacterial dysbiosis in
liver fibrosis mice. Front Pharmacol. 10:13212019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim GH, Kan SY, Kang H, Lee S, Ko HM, Kim
JH and Lim JH: Ursolic acid suppresses cholesterol biosynthesis and
exerts anti-cancer effects in hepatocellular carcinoma cells. Int J
Mol Sci. 20:47672019. View Article : Google Scholar :
|
|
30
|
Yoon JH, Youn K, Ho CT, Karwe MV, Jeong WS
and Jun M: p-Coumaric acid and ursolic acid from Corni fructus
attenuated β-amyloid (25-35)-induced toxicity through regulation of
the NF-κB signaling pathway in PC12 cells. J Agric Food Chem.
62:4911–4916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hong SY, Jeong WS and Jun M: Protective
effects of the key compounds isolated from corni fructus against
β-amyloid- induced neurotoxicity in PC12 cells. Molecules.
17:10831–10845. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Misra RC, Sharma S, Sandeep, Garg A,
Chanotiya CS and Ghosh S: Two CYP716A subfamily cytochrome P450
mono-oxygenases of sweet basil play similar but nonredundant roles
in ursane- and oleanane-type pentacyclic triterpene biosynthesis.
New Phytol. 214:706–720. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
DeBacker JR, Harrison RT and Bielefeld EC:
Cisplatin-induced threshold shift in the CBA/CaJ, C57BL/6J, BALB/cJ
mouse models of hearing loss. Hear Res. 387:1078782020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu S, Xu T, Wu X, Lin Y, Bao D, Di Y, Ma
T, Dang Y, Jia P, Xian J, et al: Pomegranate peel extract
attenuates D-galactose-induced oxidative stress and hearing loss by
regu-lating PNUTS/PP1 activity in the mouse cochlea. Neurobiol
Aging. 59:30–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matt T, Ng CL, Lang K, Sha SH, Akbergenov
R, Shcherbakov D, Meyer M, Duscha S, Xie J, Dubbaka SR, et al:
Dissociation of antibacterial activity and aminoglycoside
ototoxicity in the 4-monosubstituted 2-deoxystreptamine apramycin.
Proc Natl Acad Sci USA. 109:10984–10989. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Spinelli KJ and Gillespie PG: Monitoring
intracellular calcium ion dynamics in hair cell populations with
Fluo-4 AM. PLoS One. 7:e5.18742012. View Article : Google Scholar
|
|
37
|
Woźniak Ł, Skąpska S and Marszałek K:
Ursolic acid-a penta-cyclic triterpenoid with a wide spectrum of
pharmacological activities. Molecules. 20:20614–20641. 2015.
View Article : Google Scholar
|
|
38
|
Jinhua W: Ursolic acid: Pharmacokinetics
process in vitro and in vivo, a mini review. Arch Pharm (Weinheim).
352:e18002222019. View Article : Google Scholar
|
|
39
|
Xu HL, Wang XT, Cheng Y, Zhao JG, Zhou YJ,
Yang JJ and Qi MY: Ursolic acid improves diabetic nephropathy via
suppression of oxidative stress and inflammation in
streptozotocin-induced rats. Biomed Pharmacother. 105:915–921.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu HH, Hur JM, Seo SJ, Moon HD, Kim HJ,
Park RK and You YO: Protective effect of ursolic acid from Cornus
officinalis on the hydrogen peroxide-induced damage of HEI-OC1
auditory cells. Am J Chin Med. 37:735–746. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bao D, Zhao W, Dai C, Wan H and Cao Y: H89
dihydrochloride hydrate and calphostin C lower the body temperature
through TRPV1. Mol Med Rep. 17:1599–1608. 2018.
|
|
42
|
Huang KF, Ma KH, Chang YJ, Lo LC, Jhap TY,
Su YH, Liu PS and Chueh SH: Baicalein inhibits matrix
metalloproteinase 1 expression via activation of TRPV1-Ca-ERK
pathway in ultra-violet B-irradiated human dermal fibroblasts. Exp
Dermatol. 28:568–575. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Berekméri E, Deák O, Téglás T, Sághy É,
Horváth T, Aller M, Fekete Á, Köles L and Zelles T: Targeted
single-cell electroporation loading of Ca indicators in the mature
hemicochlea preparation. Hear Res. 371:75–86. 2019. View Article : Google Scholar
|
|
44
|
Chang L and Wang A: Calpain mediated
cisplatin-induced ototoxicity in mice. Neural Regen Res.
8:1995–2002. 2013.PubMed/NCBI
|
|
45
|
Yu L, Tang H, Jiang XH, Tsang LL, Chung YW
and Chan HC: Involvement of calpain-I and microRNA34 in kanamycin-
induced apoptosis of inner ear cells. Cell Biol Int. 34:1219–1225.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bhatta P, Dhukhwa A, Sheehan K, Al Aameri
RFH, Borse V, Ghosh S, Sheth S, Mamillapalli C, Rybak L, Ramkumar V
and Mukherjea D: Capsaicin protects against cisplatin ototoxicity
by changing the STAT3/STAT1 ratio and activating cannabinoid (CB2)
receptors in the cochlea. Sci Rep. 9:41312019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mukherjea D, Jajoo S, Whitworth C, Bunch
JR, Turner JG, Rybak LP and Ramkumar V: Short interfering RNA
against transient receptor potential vanilloid 1 attenuates
cisplatin-induced hearing loss in the rat. J Neurosci.
28:13056–13065. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rybak LP, Mukherjea D, Jajoo S, Kaur T and
Ramkumar V: siRNA-mediated knock-down of NOX3: Therapy for hearing
loss? Cell Mol Life Sci. 69:2429–2434. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sheth S, Mukherjea D, Rybak LP and
Ramkumar V: Mechanisms of cisplatin-induced ototoxicity and
otoprotection. Front Cell Neurosci. 11:3382017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang Y, Sreekrishna K, Lin Y, Huang L,
Eickhoff D, Degenhardt C and Xu T: Modulation of transient receptor
potential (TRP) channels by chinese herbal extracts. Phytother Res.
25:1666–1670. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang S, Wang S, Asgar J, Joseph J, Ro JY,
Wei F, Campbell JN and Chung MK: Ca2+ and calpain
mediate capsaicin-induced ablation of axonal terminals expressing
transient receptor poten-tial vanilloid 1. J Biol Chem.
292:8291–8303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
González-García JA, Nevado J,
García-Berrocal JR, Sánchez-Rodríguez C, Trinidad A, Sanz R and
Ramírez-Camacho R: Endogenous protection against oxidative stress
caused by cisplatin: Role of superoxide dismutase. Acta
Otolaryngol. 130:453–457. 2010. View Article : Google Scholar
|
|
53
|
Peng X, Yang Y, Tang L, Wan J, Dai J, Li
L, Huang J, Shen Y, Lin L, Gong X and Zhang L: Therapeutic benefits
of apocynin in mice with lipopolysaccharide/D-galactosamine-induced
acute liver injury via suppression of the late stage pro-apoptotic
AMPK/JNK pathway. Biomed Pharmacother. 125:1100202020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang Y, Xu K, Kerwin T, LaManna JC and
Puchowicz M: Impact of aging on metabolic changes in the Ketotic
rat brain: Glucose, oxidative and 4-HNE metabolism. Adv Exp Med
Biol. 1072:21–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang L, He T, Wan B, Wang X and Zhang L:
Orexin A ameliorates HBV X protein-induced cytotoxicity and
inflam-matory response in human hepatocytes. Artif Cells Nanomed
Biotechnol. 47:2003–2009. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hsu CL, Hong BH, Yu YS and Yen GC:
Antioxidant and anti-inflammatory effects of Orthosiphon aristatus
and its bioactive compounds. J Agric Food Chem. 58:2150–2156. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yang EJ, Moon JY, Lee JS, Koh J, Lee NH
and Hyun CG: Acanthopanax koreanum fruit waste inhibits
lipopolysaccha-ride-induced production of nitric oxide and
prostaglandin E2 in RAW 264.7 macrophages. J Biomed Biotechnol.
2010:7157392010. View Article : Google Scholar
|
|
58
|
Mu H, Liu H, Zhang J, Huang J, Zhu C, Lu
Y, Shi Y and Wang Y: Ursolic acid prevents doxorubicin-induced
cardiac toxicity in mice through eNOS activation and inhibition of
eNOS uncoupling. J Cell Mol Med. 23:2174–2183. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang C, Wang C, Li W, Wu R, Guo Y, Cheng
D, Yang Y, Androulakis IP and Kong AN: Pharmacokinetics and
pharmacodynamics of the triterpenoid ursolic acid in regulating the
antioxidant, anti-inflammatory, and epigenetic gene responses in
rat leukocytes. Mol Pharm. 14:3709–3717. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yu SS, Chen B, Huang CK, Zhou JJ, Huang X,
Wang AJ, Li BM, He WH and Zhu X: Ursolic acid suppresses
TGF-β1-induced quiescent HSC activation and transformation by
inhibiting NADPH oxidase expression and Hedgehog signaling. Exp
Ther Med. 14:3577–3582. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Antônio E: Poly(lactic acid) nanoparticles
loaded with ursolic acid: Characterization and in vitro evaluation
of radical scavenging activity and cytotoxicity. Mater Sci Eng C
Mater Biol Appl. 71:156–166. 2017. View Article : Google Scholar
|
|
62
|
Fetoni AR, Sergi B, Ferraresi A, Paludetti
G and Troiani D: Protective effects of alpha-tocopherol and
tiopronin against cisplatin-induced ototoxicity. Acta Otolaryngol.
124:421–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chirtes F and Albu S: Prevention and
restoration of hearing loss associated with the use of cisplatin.
Biomed Res Int. 2014:9254852014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Luo J, Hu YL and Wang H: Ursolic acid
inhibits breast cancer growth by inhibiting proliferation, inducing
autophagy and apoptosis, and suppressing inflammatory responses via
the PI3K/AKT and NF-κB signaling pathways in vitro. Exp Ther Med.
14:3623–3631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chang HY, Chen CJ, Ma WC, Cheng WK, Lin
YN, Lee YR, Chen JJ and Lim YP: Modulation of pregnane X receptor
(PXR) and constitutive androstane receptor (CAR) activation by
ursolic acid (UA) attenuates rifampin-isoniazid cytotoxicity.
Phytomedicine. 36:37–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kim SH, Jin H, Meng RY, Kim DY, Liu YC,
Chai OH, Park BH and Kim SM: Activating hippo pathway via Rassf1 by
ursolic acid suppresses the tumorigenesis of gastric cancer. Int J
Mol Sci. 20:47092019. View Article : Google Scholar :
|
|
67
|
Wang WJ, Sui H, Qi C, Li Q, Zhang J, Wu
SF, Mei MZ, Lu YY, Wan YT, Chang H and Guo PT: Ursolic acid
inhibits proliferation and reverses drug resistance of ovarian
cancer stem cells by downregulating ABCG2 through suppressing the
expression of hypoxia-inducible factor-1α in vitro. Oncol Rep.
36:428–440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhou M, Yi Y, Liu L, Lin Y, Li J, Ruan J
and Zhong Z: Polymeric micelles loading with ursolic acid enhancing
anti-tumor effect on hepatocellular carcinoma. J Cancer.
10:5820–5831. 2019. View Article : Google Scholar : PubMed/NCBI
|