Introduction
Myelin is a membrane structure surrounding the axon
of nerve cells that acts as an insulating sheath to prevent the
mutual interference between nerve impulses conducted by various
nerve fibers, but also acts by increasing the impulse conduction
velocity of nerve fibers (1).
Therefore, myelin plays an important role in the function of the
nervous system. Demyelinating diseases of the central nervous
system, such as multiple sclerosis, may destroy the myelin sheath,
leading to its rupture, with subsequent clearance of myelin debris
by phagocytes, such as microglia, a process referred to as
demyelination. These changes markedly affect the function of axons
and neurons in diseased tissues, and promote the development of
central nervous system (CNS) diseases (2). The myelin sheath of the CNS is
mainly composed of oligodendrocytes derived from oligodendrocyte
precursor cells (OPCs). OPCs are widely distributed in the gray and
white matter of the adult brain, and play an important role in the
regeneration and repair of myelin in chronic demyelinating diseases
(3,4). However, the inflammatory reaction
and oxidative stress occurring in these diseases often adversely
affect the proliferation and differentiation of OPCs and affect the
regeneration and repair of the myelin sheath (4-6).
Furthermore, when these diseases occur, some inflammatory cells are
activated and accumulate in the lesion sites, releasing a large
number of inflammatory factors, including interleukin (IL)-1β,
tumor necrosis factor-α and IL-11. These inflammatory factors can
affect the formation of the myelin sheath by regulating the
functional activities of oligodendrocytes (7-9).
Among those, IL-1β can promote oligodendrocyte apoptosis (8). Additionally, IL-1β has been reported
to impede OPC recruitment during chronic cerebral hypoperfusion
(10). However, the molecular
mechanisms underlying the damaging effects of IL-1β on OPCs remain
unclear.
A possible mechanism underlying endogenous OPC
injury is the dysregulation of various microRNAs. MicroRNA(miR)-219
has been reported to play a critical role in coupling
differentiation to proliferation arrest in the oligodendrocyte
lineage, enabling the rapid transition from proliferating OPCs to
myelinating oligodendrocytes (11). Stroke can downregulate the
expression of miR-9 and miR-200 to stimulate the upregulation of
serum response factor and, thus, regulate OPC differentiation
(12). Overexpression of miR-146a
promotes oligodendrogenesis in stroke (13). miR-202-3p, an intracellular target
of IL-1β, has been reported to be expressed in glioma (14). However, the functional role of
miR-202-3p in the regulation of OPC proliferation and
differentiation remains unclear.
In light of these observations, it was hypothesized
that IL-1β modulates the expression of miR-202-3p in OPCs, and that
this modulation, if present, is responsible for the damaging
effects of IL-1β on OPC proliferation and differentiation.
Materials and methods
Reagents
Recombinant rat IL-1β (cat. no. 501-RL) and the
IL-1β receptor inhibitor IL-1Ra (cat. no. 1545-RA) were purchased
from R&D Systems, Inc.; the β-catenin inhibitor XAV939 (cat.
no. X3004) and bromodeoxyuridine (BrdU) incorporation ELISA kit
(cat. no. 11647229001) for cell proliferation detection were
purchased from Sigma-Aldrich; Merck KGaA. Anti-platelet-derived
growth factor receptor (PDGFR)-α (cat. no. ab203491, dilution
1:500), anti-β-catenin (cat. no. ab32572, dilution 1:5,000),
anti-glioma-related oncogene homolog (Gli)-1 (cat. no. ab49314,
dilution 1:1,000), anti-GAPDH (cat. no. ab9485, dilution 1:2,500),
anti-myelin basic protein (MBP, cat. no. ab7349, dilution 1:200)
and anti-oligodendrocyte transcription factor (Olig)2 (cat. no.
ab42453, dilution 1:200) antibodies were purchased from Abcam.
Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG
antibody (cat. no. A16116, dilution 1:2,000) was purchased from
Thermo Fisher Scientific, Inc. miR-202-3p mimic, negative scramble
control (NC), blank siRNA and Gli1 siRNA were synthesized by
Guangzhou RiboBio Co., Ltd. The transfection reagent Lipofectamine
2000, Alexa Fluor 488 and Alexa Fluor 568 were purchased from
Invitrogen; Thermo Fisher Scientific, Inc. The BCA kit for protein
detection, DAPI and RIPA lysis buffer were purchased from Shanghai
Beyotime Biotechnology Co., Ltd. Primer sequences were synthesized
by Shanghai Sangon Biotech Co., Ltd.
Isolation and culture of OPCs
As described previously (10), primary OPCs were isolated from 20
Sprague-Dawley rats (1-2 days old) of SPF grade, provided by the
Laboratory Animal Center of Chengdu Medical College. All animal
procedures were performed according to the National Institutes of
Health Guide for the Care and Use of Laboratory Animals (NIH
Publication no. 85-23, revised 1996) and approved by the
Institutional Animal Care and Use Committee at Chengdu Medical
College (Chengdu, China). Briefly, the rats were anesthetized by
hypothermia (chilling on ice for ~5 min) and sacrificed by
decapitation. After the animals were sacrificed, their brains were
removed and cerebral cortices were harvested, minced and digested
in 0.25% trypsin solution for 15 min at 37°C. Dissociated cells
were adjusted to 1.2×109 cells/l, plated in the flasks,
and cultured for ~10 days in an incubator with 5% CO2 at
37°C. After the OPCs were >70% confluent, the flasks were placed
in an orbital laboratory shaker (model SHKE2000; Thermo Fisher
Scientific, Inc.) at 37°C and shaken for 1 h at a speed of 1.03 × g
to remove microglia. After the medium was replaced by fresh medium,
the flasks continued to be shaken overnight (20 h). The medium was
collected, plated on a 10-cm culture dish, and cultured for 1 h at
37°C for adherence of astrocytes and microglia. To eliminate
contaminating astrocytes and microglia, the non-adherent cells were
collected to obtain the purified OPCs. Purified OPCs were
identified by immunofluorescence detection of PDGFR-α. When the
purity of PDGFR-α-positive cells was >95%, cultured OPCs were
selected for drug treatment.
For the observation of OPC proliferation, purified
OPCs were replated in DMEM containing 2% B27, 10 ng/ml PDGF-AA and
10 ng/ml basic fibroblast growth factor (OPC proliferation medium)
on 96-well plates. For the observation of OPC differentiation,
purified OPCs were replated in DMEM containing 10 ng/ml ciliary
neurotrophic factor and 15 nmol/l T3 thyroid hormone (OPC
differentiation medium) on a 6-well plate coated with
poly-L-lysine.
Drug treatment of OPCs
Before the proliferation or differentiation
experiments, OPCs were randomly divided into the normal control,
IL-1β, IL-1Ra, XAV939, blank siRNA, Gli1 siRNA, negative control
(NC) transfection and miR-202-3p transfection groups. The normal
control group cells were cultured in medium without any drugs for 4
days. Cells in the other groups were cultured in medium containing
30 ng/ml recombinant rat IL-1β, with or without IL-1Ra (10 ng/ml
for the IL-1Ra group), XAV939 (0.05 µmol/l for the XAV939
group), Gli1 siRNA (50 nmol/l for the Gli1 siRNA group), or
miR-202-3p mimics (30 nmol/l for the miR-202-3p transfection group)
for 4 days. The blank siRNA and NC transfection groups were
cultured in medium containing 30 ng/ml IL-1β with blank siRNA or
Lipofectamine 2000, respectively. After 4 days of drug treatment,
OPCs were collected for analysis. The doses of IL-1Ra and XAV939
were selected according to previous reports (15,16).
Detection of OPC proliferation
The BrdU incorporation method was used to detect OPC
proliferation. One day before the completion of drug treatment,
BrdU was added into each well at a final concentration of 10
µmol/l, and the culture was continued for 24 h at 37°C.
After being fixed with FixDenat for 30 min at room temperature,
OPCs were incubated with the anti-BrdU-POD antibody at room
temperature for 90 min, followed by substrate reaction solution for
30 min. After the reaction was terminated with a termination
reaction solution, the absorbance value at 450 nm (A450) of each
well was detected using a Synergy 2 Multi-Mode Microplate Reader
(BioTek Instruments, Inc.).
Detection of the proportion of
MBP-positive cells
The double immunofluorescence staining method was
used to detect the expression of MBP and Olig2. The proportion of
MBP-positive cells among cultured cells was used as the
differentiation index of OPCs based on a previous study (17). After the completion of drug
treatment, the OPCs attached to the coverglass were fixed with 10%
formalin at room temperature for 30 min, treated with 0.1% Triton-X
for 20 min, and blocked with 1.5% goat serum for 1 h. OPCs were
then incubated with primary antibodies (anti-MBP and anti-Olig2) at
4°C overnight followed by the fluorescent secondary antibodies
(Alexa Fluor 568-labeled anti-rabbit IgG and Alexa Fluor
488-labeled anti-mouse IgG) at room temperature for 1 h. After the
nuclei were counterstained with DAPI, the images were collected and
analyzed using a Nikon ECLIPSE Ti-S fluorescent microscope at a
magnification of ×200 (Nikon Corporation). At least 20 fields were
counted for each sample. As MBP is expressed in mature
oligodendrocytes and Olig2 is expressed in all developmental stages
from OPCs to mature oligodendrocytes, the percentage of
MBP-positive cells in the total number of Olig2-positive cells
[(MBP+/Olig2+)%] was considered as the
indicator of OPC differentiation in the present study.
Western blot analysis
The protein expression of β-catenin and Gli1 was
detected by western blotting. After drug treatment, the cells of
each group were collected and total protein in OPCs was extracted
with RIPA lysis buffer. The protein concentration was detected by
the BCA protein detection kit. Proteins (40 µg) were
separated by 10% SDS-PAGE and transferred to a PVDF membrane
(Invitrogen; Thermo Fisher Scientific, Inc.). The membrane was
blocked with 5% skimmed milk for 1 h. Subsequently, the membranes
were incubated overnight at 4°C with anti-β-catenin, anti-Gli1 and
anti-GAPDH. The membranes were incubated for 1 h at room
temperature with HRP-conjugated anti-IgG. Enhanced
chemiluminescence reagent was added to display the band of target
proteins. Images of the protein bands were digitally captured and
analyzed with ImageJ software (version 1.52v, National Institutes
of Health). The relative expression of target proteins was
expressed as the ratio of target proteins to the GAPDH protein.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
RT-qPCR analysis was used to detect the expression
of miR-202-3p. After drug treatment, total RNA was extracted from
OPCs with TRIzol reagent kit (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA concentration was measured by NanoDrop ND-1000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.). Subsequently, cDNA was synthesized by the TaqMan microRNA
Reverse Transcription kit (Invitrogen; Thermo Fisher Scientific,
Inc.), and the amplification reaction was completed in the MX3005p
PCR instrument (Agilent Technologies, Inc.). For miR-202-3p
amplification, the amplification conditions included denaturation
at 95°C for 3 min, followed by 35 cycles at 95°C for 10 sec, and
60°C for 30 sec. Primer sequences were as follows: miR-202-3p
sense, 5′-CTC CAG AGA UAG UAG AG C CT-3′ and antisense, 5′-CTC AAC
CAC CAT CAC CTG AC A GA-3′; and internal reference sense, 5′-CTC
GCT TCG GAG CAC A-3′ and antisense, 5′-AAC GCT TCA CGA ATT GCG
T-3′. The relative expression of miR-202-3p was expressed as
miR-202-3p/U6.
Statistical analysis
All quantitative data are expressed as mean ±
standard error of the mean. Statistical analysis was performed
using one-way ANOVA, followed by Tukey's post hoc test. P<0.05
was considered to indicate statistically significant
differences.
Results
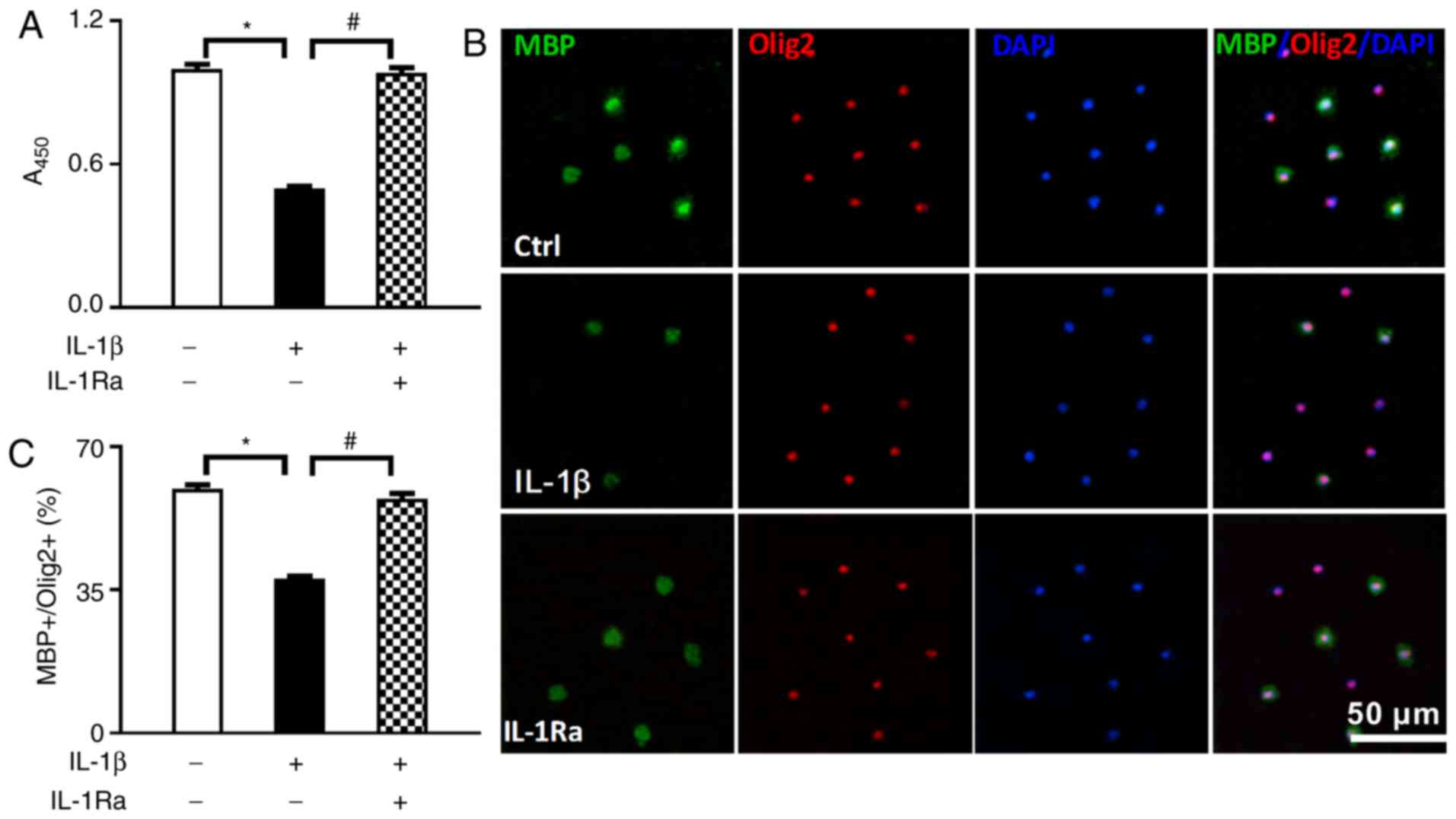
IL-1β inhibits the proliferation and
differentiation of OPCs
To observe the effects of IL-1β on OPC
proliferation, the BrdU incorporation method was applied and cell
proliferation was expressed as A450 of each well. As shown in
Fig. 1A, when compared with the
normal control group, IL-1β treatment significantly inhibited the
proliferation and viability of OPCs (P<0.05), which was reversed
by the IL-1β receptor inhibitor IL-1Ra (P<0.05).
To observe the effects of IL-1β on OPC
differentiation, double immunofluorescence staining was applied to
detect the expression of MBP and Olig2. The percentage of
MBP-positive cells in the total number of Olig2-positive cells
[(MBP+/Olig2+)%] was considered as the index
of OPC differentiation. As shown in Fig. 1B, the cytoplasm in MBP-positive
cells appeared as bright green, and the nuclei in Olig2-positive
cells were red. As shown in Fig.
1C, when compared with the normal control group, IL-1β
treatment significantly decreased the differentiation index of OPCs
(P<0.05), which was reversed by the IL-1β receptor inhibitor
IL-1Ra (P<0.05).
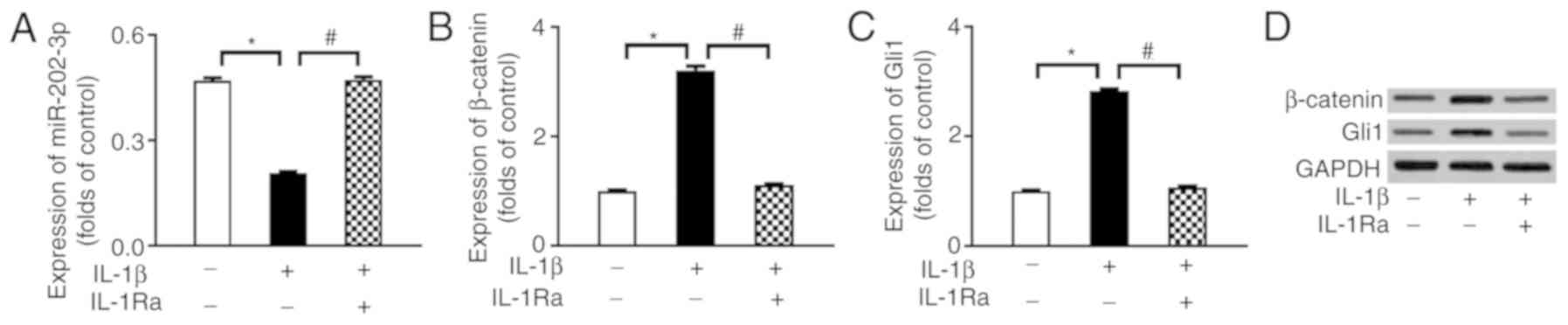
IL-1β modulates the expression of the
miR-202-3p/ β-catenin/Gli1 axis in OPCs
To investigate the possible role of the
miR-202-3p/β-catenin/Gli1 axis in IL-1β suppression of OPC
proliferation and differentiation, the expression of the
miR-202-3p/β-catenin/Gli1 axis was determined by western blotting
and RT-qPCR analysis. As shown in Fig. 2, when compared with the normal
control group, IL-1β treatment significantly decreased the
expression of miR-202-3p (P<0.05) and increased the protein
expression of β-catenin and Gli1 (P<0.05), all of which were
reversed by the IL-1β inhibitor IL-1Ra (P<0.05).
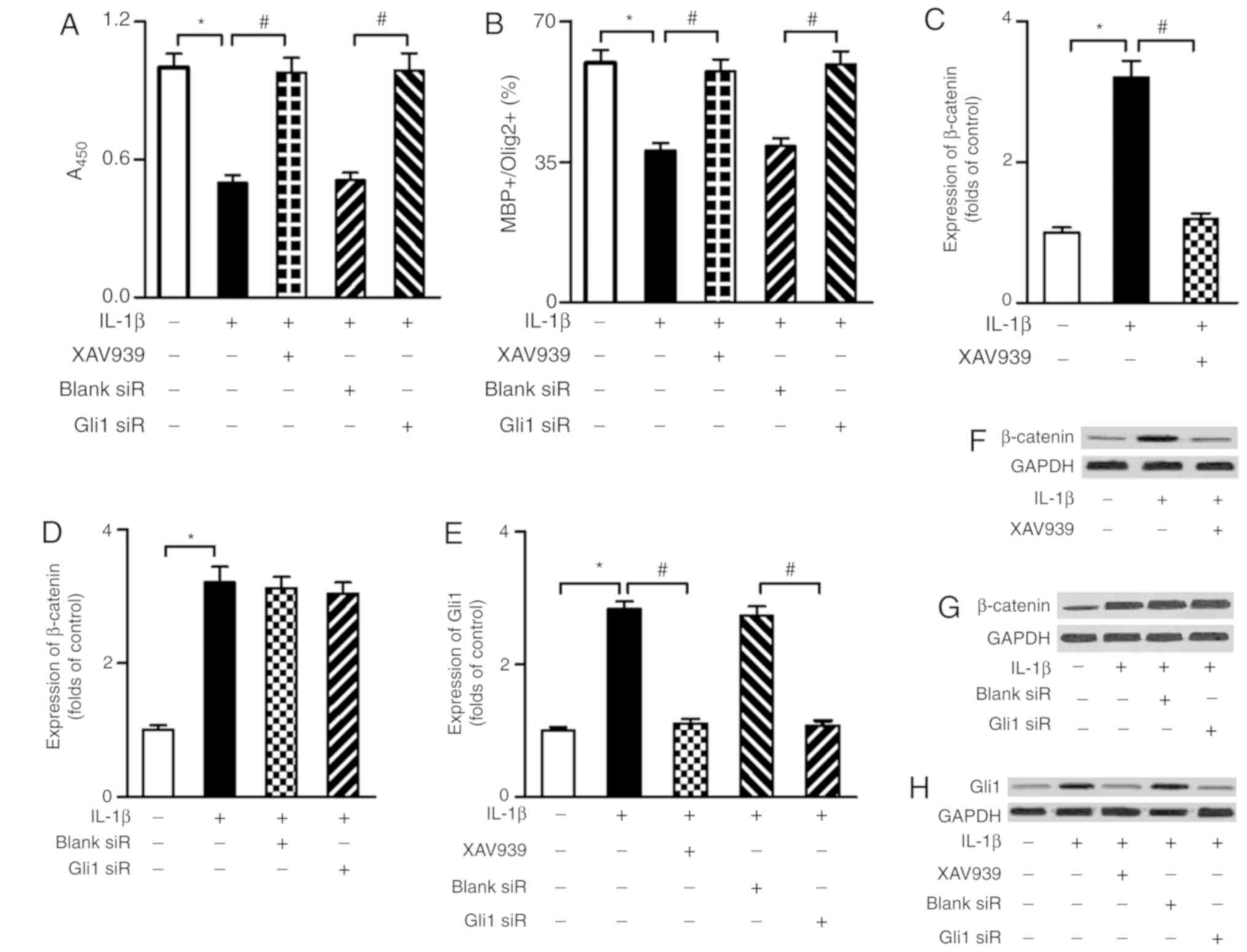
Inhibition of the β-catenin/Gli1 pathway
improves the proliferation and differentiation of OPCs
To evaluate the role of the β-catenin/Gli1 pathway
in IL-1β-mediated suppression of OPC proliferation and
differentiation, the β-catenin inhibitor XAV939 and Gli1 siRNA were
used in the present study. Knockdown of Gli1 protein was validated
by western blot analysis (Fig.
S1). As shown in Fig. 3, when
compared with the IL-1β group, the β-catenin inhibitor XAV939
markedly improved the proliferation and differentiation of OPCs
(P<0.05) and decreased the expression of Gli1 (P<0.05).
Furthermore, when compared with the blank siRNA group, the Gli1
siRNA group exhibited enhanced proliferation activity and higher
differentiation index of OPCs (P<0.05). However, the
transfection of Gli1 siRNA into OPCs did not affect β-catenin
expression in response to IL-1β stimulation.
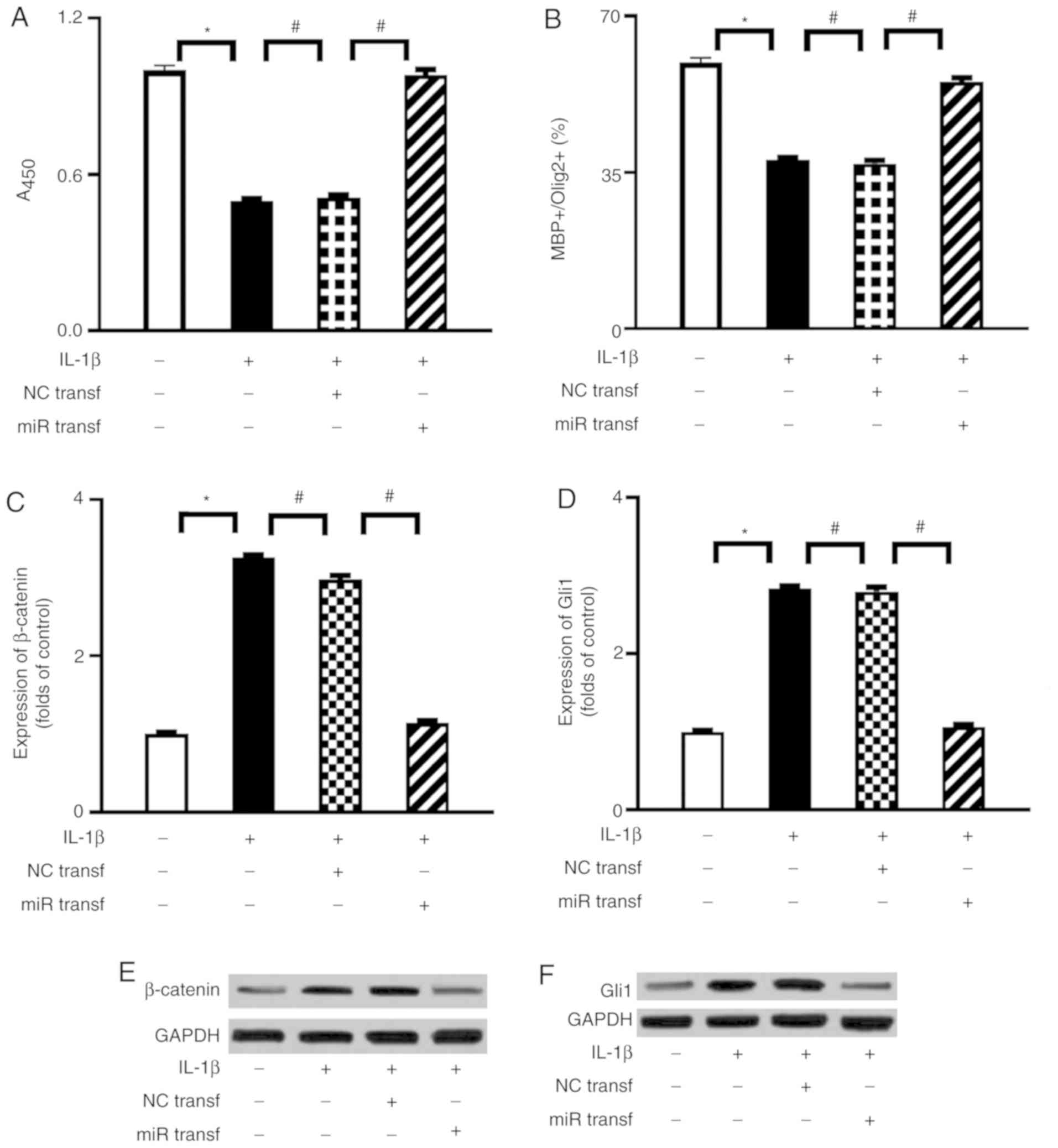
Activation of miR-202-3p improves the
proliferation and differentiation of OPCs through downregulation of
the β-catenin/Gli1 pathway
To evaluate a possible interaction between
miR-202-3p and the β-catenin/Gli1 pathway in IL-1β-mediated
suppression of OPC proliferation and differentiation, miR-202-3p
mimic transfection was performed in the present study. Successful
transfection of miR-202-3p mimic was validated by RT-qPCR analysis
(Fig. S1). As shown in Fig. 4, when compared with the NC
transfection group, miR-202-3p mimic transfection significantly
enhanced the proliferation activity and differentiation index of
OPCs and attenuated the expression of β-catenin and Gli1 under
IL-1β stimulation.
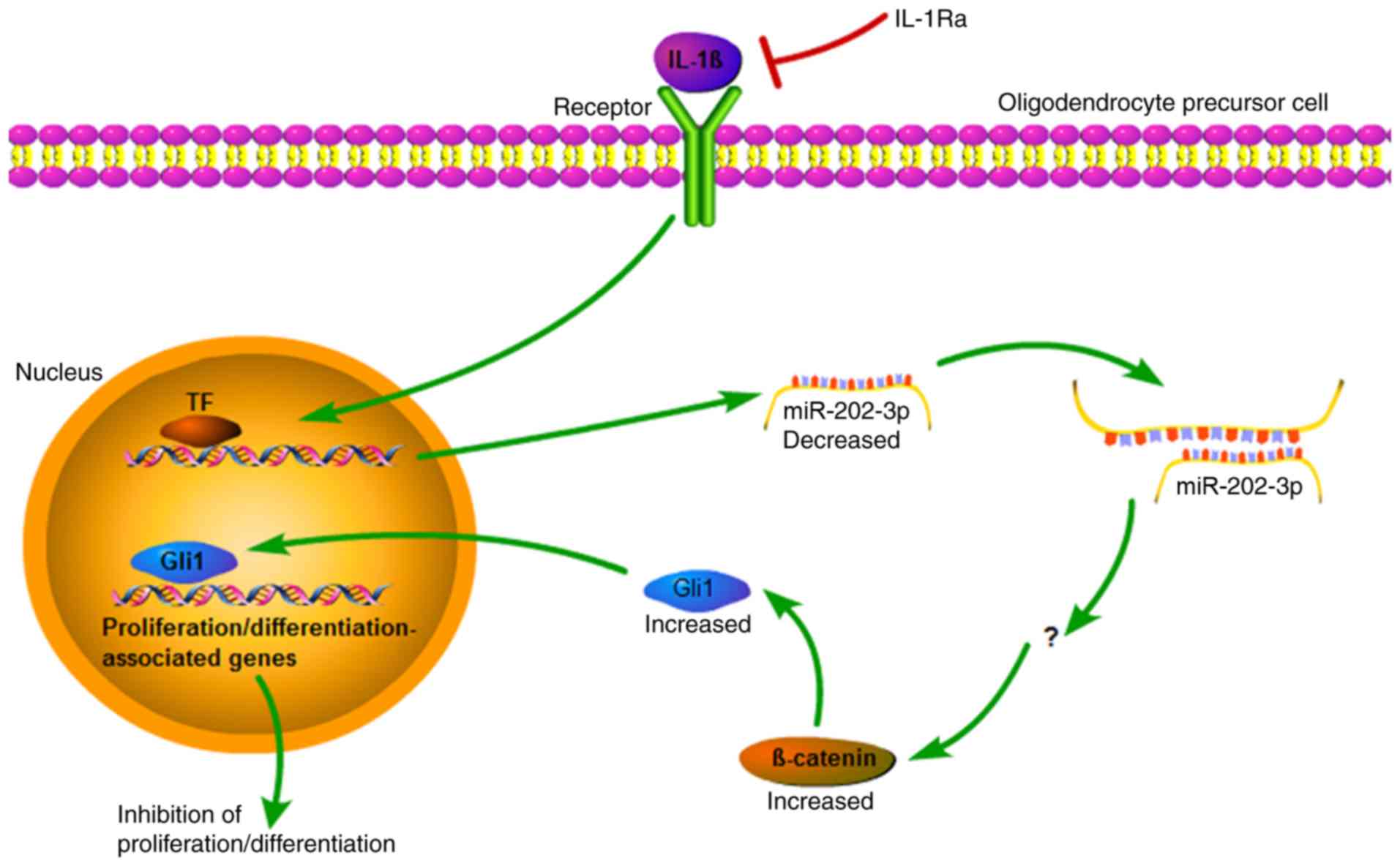
Discussion
The present study demonstrated that the inflammatory
cytokine IL-1β modulated the expression of the miR-202-3p/
β-catenin/Gli1 axis in cultured OPCs via its specific receptor. In
addition, modulation of the miR-202-3p/β-catenin/Gli1 axis mediated
IL-1β suppression of OPC proliferation and differentiation. A
schematic illustration of the proposed mechanisms underlying the
damaging effects of IL-1β on OPCs is presented in Fig. 5.
One of the main findings of the present study was
that IL-1β attenuated the proliferation and differentiation of OPCs
via activation of the β-catenin/Gli1 pathway. Canonical
Wnt/β-catenin signaling has been implicated in the regulation of
cell proliferation, differentiation, migration, and the development
of axon, dendrites and synapses in the CNS (18,19). Dysregulation of Wnt/β-catenin
signaling has been linked to demyelinating diseases of the CNS,
such as multiple sclerosis (20-22). However, the results from different
scientific groups are controversial. Some demonstrated that
activation of Wnt/β-catenin signaling may mediate failure of
remyelination in chronic demyelinating diseases (20,21), whereas others demonstrated that
the downregulation of Wnt/β-catenin signaling inhibits the
myelination process (21,22). A possible explanation for these
contradictory observations is that canonical Wnt signaling may
interact with other pathways, such as the Hedgehog pathway. Gli1, a
key factor of the Hedgehog pathway (23), can be expressed by OPCs (24). γ-catenin (also referred to as
plakoglobin), a partial functional homolog of β-catenin, has been
reported to be upregulated by Gli1 in gastric cancer cells
(25). Contrary to γ-catenin,
β-catenin has been demonstrated to upregulate the expression of
Gli1 (26,27). Although a high degree of sequence
homology exists in the central armadillo repeat regions between the
two catenins, there is little homology in their N- and C-termini
(28). The differences in the N-
and C-termini between the catenins are considered to be linked to
the failure of γ-catenin to fully make up for β-catenin loss in
mediating Wnt signaling (29).
Therefore, structural differences between them may explain why the
two catenins can be differentially regulated by Gli1. However,
whether the β-catenin/Gli1 pathway in OPCs is regulated in response
to IL-1β stimulation remains unknown. As shown in Fig. 2, the expression of β-catenin and
Gli1 in OPCs were upregulated in response to IL-1β treatment.
Conversely, the β-catenin inhibitor XAV939 markedly decreased the
expression of Gli1. Therefore, these findings suggest that
β-catenin acts as an upstream regulator of Gli1. Subsequently, it
was hypothesized that IL-1β modulates the proliferation and
differentiation of OPCs via the β-catenin/Gli1 pathway. The results
of the present study demonstrated that IL-1β suppressed the
proliferation and differentiation of OPC and upregulated the
expression of the β-catenin/Gli1 pathway, both of which were
reversed by IL-1β receptor inhibition. Treatment with the β-catenin
inhibitor XAV939 or Gli1 siRNA attenuated IL-1β-induced suppression
of the proliferation and differentiation of OPCs. Treatment with
XAV939 downregulated the expression of Gli1. Thus, activation of
the β-catenin/Gli1 pathway may mediate the adverse effects of IL-1β
on OPC proliferation and differentiation.
miRNAs are small non-coding RNA molecules that
negatively control the expression of target genes and play key
roles in animal development, cell proliferation, differentiation
and metabolism. Recent researches have proposed that several miRNAs
regulate the function of OPCs. miR-219 and miR-146a promote OPC
differentiation and enhance remyelination in demyelinating diseases
(30,31). By contrast, miR-26b, miR-9 and
miR-200 inhibit OPC differentiation (12,32). IL-1β has been reported to regulate
miR-372 in human neural stem cells (33), miR-147b in human astrocytes
(34) and miR-202-3p in human
nucleus pulposus cells (35).
However, whether miR-202-3p mediates the damaging effects of IL-1β
on OPCs remains unknown. The present study was therefore designed
to investigate the possible role of miR-202-3p in IL-1β-induced
suppression of OPC proliferation and differentiation. The results
demonstrated that IL-1β markedly decreased the expression of
miR-202-3p, increased the expression of β-catenin and Gli1, and
inhibited the proliferation and differentiation of OPCs. By
contrast, miR-202-3p mimic transfection significantly attenuated
the expression of β-catenin and Gli1 and enhanced the proliferation
and differentiation of OPCs under IL-1β stimulation. Thus, the
downregulation of miR-202-3p may mediate the effects of IL-1β on
OPCs. Additionally, a previous study demonstrated that miR-202-3p
directly suppresses the expression of Gli1 to regulate the
proliferation and apoptosis of gastric cancer cells (25). In the present study, miR-202-3p
mimic transfection significantly enhanced the proliferation
activity and differentiation index of OPCs and attenuated the
expression of β-catenin and Gli1 under IL-1β stimulation. However,
these data do not rule out the possibility that miR-202-3p directly
suppresses the expression of Gli1 to regulate the proliferation and
differentiation of OPCs. Interestingly, lipoprotein
receptor-related protein 6 (LRP6) and cyclin D1 of the
Wnt/β-catenin signaling pathway have been also identified as direct
targets of miR-202-3p (36). Both
LRP6 and cyclin D1 have been implicated in the differentiation of
OPCs (37). Thus, the results of
the present and other studies suggest that miR-202-3p regulates
OPCs by targeting the β-catenin/Gli1 pathway at multiple levels.
The precise mechanism underlying miR-202-3p regulation of the
β-catenin/Gli1 pathway remains elusive and must be further
elucidated in the future.
In conclusion, the present study investigated the
possible role of the miR-202-3p/β-catenin/Gli1 axis in
IL-1β-induced effects on OPCs. To the best of our knowledge, the
present study is the first to demonstrate that IL-1β can prevent
the proliferation and differentiation of OPCs through modulation of
the miR-202-3p/β-catenin/Gli1 axis. As the failure of endogenous
OPCs to differentiate into myelinating oligoden-drocytes is
considered to be implicated in the demyelinating diseases of the
CNS, such as multiple sclerosis, these findings raise the
possibility that the miR-202-3p/β-catenin/Gli1 axis may be of value
as a therapeutic target for multiple sclerosis.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by the Research Fund
of Chengdu Medical College (grant no. CYZ19-21).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YaL and XD conceived and designed the research. YaL,
LL, YoL, QY and BR performed the experiments. YoL and LL analyzed
the data. YaL, LL and XD wrote the paper. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were performed according to
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (NIH Publication no. 85-23, revised 1996) and
approved by the Institutional Animal Care and Use Committee at
Chengdu Medical College (Chengdu, China).
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Baumann N and Pham-Dinh D: Biology of
oligodendrocyte and myelin in the mammalian central nervous system.
Physiol Rev. 81:871–927. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohno N and Ikenaka K: Axonal and neuronal
degeneration in myelin diseases. Neurosci Res. 139:48–57. 2019.
View Article : Google Scholar
|
|
3
|
Skaper SD: Oligodendrocyte precursor cells
as a therapeutic target for demyelinating diseases. Prog Brain Res.
245:119–144. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nait-Oumesmar B, Decker L, Lachapelle F,
Avellana-Adalid V, Bachelin C and Baron-Van Evercooren A:
Progenitor cells of the adult mouse subventricular zone
proliferate, migrate and differentiate into oligodendrocytes after
demyelination. Eur J Neurosci. 11:4357–4366. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu Y, Yu Z, Xie M, Wang W and Luo X: Hv1
proton channel facilitates production of ROS and pro-inflammatory
cytokines in microglia and enhances oligodendrocyte progenitor
cells damage from oxygen-glucose deprivation in vitro. Biochem
Biophys Res Commun. 498:1–8. 2018. View Article : Google Scholar
|
|
6
|
Mallucci G, Peruzzotti-Jametti L,
Bernstock JD and Pluchino S: The role of immune cells, glia and
neurons in white and gray matter pathology in multiple sclerosis.
Prog Neurobiol. 127-128:1–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takahashi JL, Giuliani F, Power C, Imai Y
and Yong VW: Interleukin-1beta promotes oligodendrocyte death
through glutamate excitotoxicity. Ann Neurol. 53:588–595. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cai Z, Lin S, Pang Y and Rhodes PG: Brain
injury induced by intracerebral injection of interleukin-1beta and
tumor necrosis factor-alpha in the neonatal rat. Pediatr Res.
56:377–384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Taveggia C, Melendez-Vasquez C,
Einheber S, Raine CS, Salzer JL, Brosnan CF and Johan GR:
Interleukin-11 potentiates oligodendrocyte survival and maturation,
and myelin formation. J Neurosci. 26:12174–12185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie D, Shen F, He S, Chen M, Han Q, Fang
M, Zeng H, Chen C and Deng Y: IL-1β induces hypomyelination in the
periventricular white matter through inhibition of oligodendrocyte
progenitor cell maturation via FYN/MEK/ERK signaling pathway in
septic neonatal rats. Glia. 64:583–602. 2016. View Article : Google Scholar
|
|
11
|
Dugas JC, Cuellar TL, Scholze A, Ason B,
Ibrahim A, Emery B, Zamanian JL, Foo LC, McManus MT and Barres BA:
Dicer1 and miR-219 Are required for normal oligodendrocyte
differentiation and myelination. Neuron. 65:597–611. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Buller B, Chopp M, Ueno Y, Zhang L, Zhang
RL, Morris D, Zhang Y and Zhang ZG: Regulation of serum response
factor by miRNA-200 and miRNA-9 modulates oligodendrocyte
progenitor cell differentiation. Glia. 60:1906–1914. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu XS, Chopp M, Pan WL, Wang XL, Fan BY,
Zhang Y, Kassis H, Zhang RL, Zhang XM and Zhang ZG: MicroRNA-146a
promotes oligodendrogenesis in stroke. Mol Neurobiol. 54:227–237.
2017. View Article : Google Scholar
|
|
14
|
Yang J, Fan B, Zhao Y and Fang J:
MicroRNA-202 inhibits cell proliferation, migration and invasion of
glioma by directly targeting metadherin. Oncol Rep. 38:1670–1678.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Y, Zhang J, Wang L, Chen Y, Wan Y, He
Y, Jiang L, Ma J, Liao R, Zhang X, et al: Interleukin-1β impedes
oligodendrocyte progenitor cell recruitment and white matter repair
following chronic cerebral hypoperfusion. Brain Behav Immun.
60:93–105. 2017. View Article : Google Scholar
|
|
16
|
Shih Y, Ly PTT, Wang J and Pallen CJ:
Glial and neuronal protein tyrosine phosphatase Alpha (PTPα)
regulate oligodendrocyte differentiation and myelination. J Mol
Neurosci. 62:329–343. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Diao HJ, Low WC, Milbreta U, Lu QR and
Chew SY: Nanofiber-mediated microRNA delivery to enhance
differentiation and maturation of oligodendroglial precursor cells.
J Control Release. 208:85–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Masuda T and Ishitani T: Context-dependent
regulation of the β-catenin transcriptional complex supports
diverse functions of Wnt/β-catenin signaling. J Biochem. 161:9–17.
2017. View Article : Google Scholar
|
|
19
|
He CW, Liao CP and Pan CL: Wnt signalling
in the development of axon, dendrites and synapses. Open Biol.
8:1801162018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vallee A, Vallee JN, Guillevin R and
Lecarpentier Y: Interactions between the canonical WNT/Beta-catenin
pathway and ppar gamma on neuroinflammation, demyelination, and
remyelination in multiple sclerosis. Cell Mol Neurobiol.
38:783–795. 2018. View Article : Google Scholar
|
|
21
|
Xie C, Li Z, Zhang GX and Guan Y: Wnt
signaling in remyelination in multiple sclerosis: Friend or foe?
Mol Neurobiol. 49:1117–1125. 2014. View Article : Google Scholar
|
|
22
|
Fancy SP, Baranzini SE, Zhao C, Yuk DI,
Irvine KA, Kaing S, Sanai N, Franklin RJM and Rowitch DH:
Dysregulation of the Wnt pathway inhibits timely myelination and
remyelination in the mammalian CNS. Genes Dev. 23:1571–1585. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruiz i Altaba A, Mas C and Stecca B: The
Gli code: An information nexus regulating cell fate, stemness and
cancer. Trends Cell Biol. 17:438–447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lelievre V, Ghiani CA, Seksenyan A,
Gressens P, de Vellis J and Waschek JA: Growth factor-dependent
actions of PACAP on oligodendrocyte progenitor proliferation. Regul
Pept. 137:58–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao Y, Li C, Wang M, Su L, Qu Y, Li J, Yu
B, Yan M, Yu Y, Liu B and Zhu Z: Decrease of miR-202-3p expression,
a novel tumor suppressor, in gastric cancer. PLoS One.
8:e697562013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Lin P, Wang Q, Zheng M and Pang L:
Wnt3a-regulated TCF4/β-catenin complex directly activates the key
Hedgehog signalling genes Smo and Gli1. Exp Ther Med. 16:2101–2107.
2018.PubMed/NCBI
|
|
27
|
Noubissi FK, Yedjou CG, Spiegelman VS and
Tchounwou PB: Cross-Talk between Wnt and Hh signaling pathways in
the pathology of basal cell carcinoma. Int J Environ Res Public
Health. 15:14422018. View Article : Google Scholar :
|
|
28
|
Zhurinsky J, Shtutman M and Ben-Ze'ev A:
Plakoglobin and beta-catenin: Protein interactions, regulation and
biological roles. J Cell Sci. 113:3127–3139. 2000.PubMed/NCBI
|
|
29
|
Wickham RJ, Alexander JM, Eden LW,
Valencia-Yang M, Llamas J, Aubrey JR and Jacob MH: Learning
impairments and molecular changes in the brain caused by β-catenin
loss. Hum Mol Genet. 28:2965–2975. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Zhang ZG, Lu M, Zhang Y, Shang X
and Chopp M: MiR-146a promotes oligodendrocyte progenitor cell
differentiation and enhances remyelination in a model of
experimental autoimmune encephalomyelitis. Neurobiol Dis.
125:154–162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan HB, Chen LX, Qu XB, Ren CL, Wu XX,
Dong FX, Zhang BL, Gao DS and Yao RQ: Transplanted
miR-219-overexpressing oligodendrocyte precursor cells promoted
remyelination and improved functional recovery in a chronic
demyelinated model. Sci Rep. 7:414072017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng L, Wang C, Yao F, Li Z, Liu W and
Jing J: MicroRNA-26b inhibits oligodendrocyte precursor cell
differentiation by targeting adrenomedullin in spinal cord injury.
J Cell Physiol. 235:2429–2440. 2020. View Article : Google Scholar
|
|
33
|
Zhou W, Yuan T, Gao Y, Yin P, Liu W, Pan
C, Liu Y and Yu X: IL-1β-induces NF-κB and upregulates microRNA-372
to inhibit spinal cord injury recovery. J Neurophysiol.
117:2282–2291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van Scheppingen J, Mills JD, Zimmer TS,
Broekaart DWM, Iori V, Bongaarts A, Anink JJ, Lyer AM, Korotkov A,
Jansen FE, et al: miR147b: A novel key regulator of interleukin 1
beta-mediated inflammation in human astrocytes. Glia. 66:1082–1097.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi C, Wu L, Lin W, Cai Y, Zhang Y, Hu B,
Gao R, Im HJ, Yuan W, Ye X and van Wijnen AJ: MiR-202-3p regulates
interleukin-1beta-induced expression of matrix metallopro-teinase 1
in human nucleus pulposus. Gene. 687:156–165. 2019. View Article : Google Scholar
|
|
36
|
Yang C, Yao C, Tian R, Zhu Z, Zhao L, Li
P, Chen H, Huang Y, Zhi E, Gong Y, et al: miR-202-3p regulates
sertoli cell proliferation, synthesis function, and apoptosis by
targeting LRP6 and cyclin D1 of Wnt/β-catenin signaling. Mol Ther
Nucleic Acids. 14:1–19. 2019. View Article : Google Scholar
|
|
37
|
Chew LJ, Shen W, Ming X, Senatorov VV Jr,
Chen HL, Cheng Y, Hong E, Knoblach S and Gallo V: SRY-box
containing gene 17 regulates the Wnt/β-catenin signaling pathway in
oligodendro-cyte progenitor cells. J Neurosci. 31:13921–13935.
2011. View Article : Google Scholar : PubMed/NCBI
|