Introduction
Diabetes mellitus is a common chronic endocrine
disorder characterized by hyperglycemia, resulting from insulin
resistance or insufficiency. Currently, it is estimated that there
are 382 million individuals with diabetes worldwide, which is
predicted to increase by 55% by 2035 (1). Recently, cognitive impairment was
identified as a potential complication of diabetes and a major
pathway developing into dementia (2). Mijnhout et al proposed a
specific term diabetes encephalopathy to clarify the
characteristics of this disorder (3).
The exact pathophysiology of diabetic encephalopathy
is not yet completely understood. However, it is likely that
vascular disease, hypoglycemia, insulin resistance and
hyperglycemia play significant roles in the development of diabetic
encephalopathy (4). Previous
studies by the authors have demonstrated that the hippocampus in a
diabetes model manifests dysfunction in modulating inflammation,
oxidative response and synaptic plasticity (5-7).
The direct glucose toxicity caused by hyperglycemia in neurons is
mainly due to increased intracellular glucose oxidation, which
leads to the excessive production of free radicals (8,9).
Oxidative stress has been implicated in the development of diabetic
complications, including diabetic retinopathy, nephropathy,
peripheral neuropathy, and cardiovascular disease (10-13). Due to the high requirement of
oxygen and the existence of abundant unsaturated lipids, the brain
is vulnerable to free radical attacks, which leads to neuronal cell
death, autophagy and inflammation (14,15). A substantial amount of evidence
has revealed that enhanced oxidative stress in types 1 and 2
diabetes mediates diabetic neuropathy and cognitive deficits
(16-19). Nicotinamide adenine
dinucleotidephosphate (NADPH) oxidase is a major source of
superoxide, which is a precursor of reactive oxygen species (ROS)
(20). Increased NADPH oxidase
(NOX) activity underlines the free radical overproduction in
oxidative stress (21). The major
mechanism of cells combating oxidative injury is the management of
the nuclear factor erythroid 2-related factor 2/antioxidant
responsive element (Nrf2/ARE) signaling pathway, which regulates
the transcription and expression of multiple phase II antioxidant
enzymes, including heme oxygenase-1 (HO-1) and NAD(P)H: Quinone
oxidoreductase-1 (NQO1) (22).
Troxerutin, a trihydroxyethylated derivative of the
natural bioflavonoid, rutin, is extracted from Sophora
japonica. It has previously been demonstrated that troxerutin
possesses a variety of beneficial activities, including
antioxidant, anti-inflammatory and anti-thrombotic abilities
(23). Moreover, troxerutin has
been shown to exert a protective effect against D-gal-induced
inflammation in diabetic cardiomyopathy (24). Studies on the protective effects
of troxerutin against oxidative stress-induced diabetes-associated
cognitive decline are rare. The present study aimed to i) explore
the protective effects of troxerutin on cognitive decline in rats
with streptozotocin (STZ)-induced diabetes; ii) investigate the
link between troxerutin treatment and oxidative stress in the
hippocampus; and iii) determine the role of NOX activity and the
Nrf2/ARE signaling pathway in balancing cell homeostasis.
Materials and methods
Animals
A total of 50 male specific-pathogen free (SPF)
Sprague-Dawley rats (weighing, 150-170 g) at the age of 10 weeks,
were purchased from Beijing Vital River Laboratory Animal
Technology. The justification of male rats was based on the
relatively longer distance between the urethral orifice and
genitals compared with female rats. The animals were housed in
individual cages under a controlled environment (12:12 h day/night
cycle; temperature, 22±1°C) with free access to food and water. All
experimental procedures followed the Guide for the Care and Use of
Laboratory Animals. All protocols for animal treatment were
approved by the Animal Ethics Committee of Hebei Medical
University. The animals were allowed to acclimatize to the
laboratory conditions for 2 weeks prior to the experiment.
Drugs
Troxerutin was purchased from Shanghai Jingchun
Biotechnology Co., Ltd. α-lipoic acid (LA) was purchased from Stada
Arzneimittel AG.
Experimental design
The rat model of diabetes was established by a
single intraperitoneal injection of STZ (Sigma-Aldrich; Merck) at
doses of 60 mg/kg for 12-h fasted rats at the age of 12 weeks. STZ
is a glucosamine-nitrosourea compound and alkylating agent that is
particularly toxic to islet β-cells. STZ is widely used to induce
type 1 diabetes in mice or rats by using a large dose on an empty
stomach. A single intravenous injection of STZ into fasted rats at
dose of 50-75 mg/kg body weight will cause the necrosis of β-cells
followed by β-cell loss and the atrophy of islets (25). It will then lead to a decrease in
insulin levels, hyperglycemia and an increase in serum free-fatty
acids. STZ was dissolved in 0.1 mmol/l sodium citrate buffer (pH
4.4). The rats in the normal control group received an
intraperitoneal injection of sodium citrate buffer. The blood
glucose levels of the caudal vein were measured after 72 h using a
portable glucose meter (ACCU-CHEK Performa, Roche Diagnostics). The
rat model of diabetes was successfully established once the blood
glucose levels exceeded 16.7 mmol/l for 2 consecutive days. Body
weight and fasting blood glucose (FBG) levels of the rats were
recorded once each week. At 4 weeks after the STZ injection, 40
rats with diabetic cognitive dysfunction and 10 normal control rats
were well prepared and evaluated.
The diabetic rats were randomly divided into 4
groups (n=10 in each group) as follows: i) The diabetes control
group (DC group), in which diabetic rats were left without any
treatment; ii) diabetes and saline-treated group (DN group), in
which diabetic rats were intraperitoneally injected with saline;
iii) diabetes with LA treatment group (DL group), in which diabetic
rats were injected intraperitoneally with LA at doses of 60
mg/kg/day; iv) diabetes with troxerutin treatment group (DT group),
in which diabetic rats were injected intraperitoneally with
troxerutin at doses of 150 mg/kg/day. Moreover, the normal control
rats were injected intraperitoneally with physical saline at the
same volume as the NC group (n=10). The treatment was administered
once a day for 6 weeks.
Morris water maze test
Spatial learning and memory in each group were
analyzed using the Morris water maze for 5 consecutive days
following the 6-week intervention. The Morris water maze test is a
convenient and classical method with which to assess cognitive
function in rodents. The apparatus was composed of a circular water
pool (150 cm in diameter and 60 cm high), containing various
prominent visual cues, with opaque water kept at 24-26°C. A
transparent platform was hidden 1 cm below the water surface in one
quadrant. For each trial, the latency to escape from water of each
rat was calculated. A probe test, in which the hidden platform was
removed, was conducted after the last trial on training day 5. The
probe test was performed and the rats were allowed to swim freely
for 60 sec with the platform absent. The time spent in the target
quadrant and the times of platform crossing were measured. All data
were recorded using a visual tracking system (SuperMaze software,
Shanghai Xinruan Information Technology, Co., Ltd.).
Biochemical analysis
Rats were sacrificed following anesthesia with 10%
chloral hydrate (300 mg/kg, intraperitoneal injection). The rats
were anaesthetized within 5 min after the chloral hydrate
intraperitoneal injection. The anesthetized states of the rats were
evaluated by the reaction of rats to external stimuli, such as
clamping the root of the rat's tail. There were no signs of
peritonitis, pain or discomfort following the intraperitoneal
injection. The rats were then sacrificed by intracardiac puncture.
The death of rats was confirmed by cardiac arrest. The brains of
the rats were carefully and rapidly removed and washed with cold
physiological saline. The hippocampus was immediately separated
from the cerebrum on an iced plate and then stored at −80°C for use
in subsequent experiments. The hippo-campal tissue was made into a
tissue homogenate in ice-cold physiological saline solution, and
centrifuged (1,000 × g, 4°C, 10 min) to remove particulates. The
obtained supernatant was assayed. Superoxide dismutase (SOD)
activity, and the malondi-aldehyde (MDA) and glutathione (GSH)
levels were measured using a SOD Activity Assay kit, MDA assay kit
and GSH assay kit supplied by Nanjing Jiancheng Bio-engineering
according to the manufacturer's instructions.
Reverse transcription-quantitative
PCR
Total RNA was isolated from the hippocampal tissues
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
and reverse transcribed into cDNA using the Takara RNA PCR kit
(AMV) (Takara Biotechnology Co., Ltd.). Specific primers for the
target transcripts listed in Table
I were synthesized (Invitrogen; Thermo Fisher Scientific,
Inc.). mRNA expression was quantified using the SYBR-Green PCR kit
(SYBR® Premix Ex Taq™ II; Takara Biotechnology Co.,
Ltd.). The real-time detection was performed using the ABI prism
7900 RT-PCR System (Applied Biosystems). The results were
normalized to the β-actin expression levels and the quantities of
gene expression were calculated by the 2−ΔΔCq method
(26). All experiments were
performed in triplicate. The primers of the target genes are listed
in Table I.
 | Table IPrimers used for RT-qPCR. |
Table I
Primers used for RT-qPCR.
| Gene name | Forward (5′)
primer | Reverse (3′)
primer |
|---|
| gp91phox |
5′-CCTAAGATAGCGGTTGATGG-3′ |
5′-GACTTGAGAATGGATGCGAA-3′ |
| p47phox |
5′-GTCAGATGAAAGCAAAGCGA-3′ |
5′-CATAGTTGGGCTCAGGGTCT-3′ |
| p22phox |
5′-TCTGGGAGCAACACCTTGGAAAC-3′ |
5′-AGAAGCCAAGTGAGGCCGAAGAG-3′ |
| Nrf2 |
5′-GCGGCAACTTTATTCTTCCCTCT-3′ |
5′-AGTCCCATTCACAAAAGACAAACA-3′ |
| HO1 |
5′-GAAACAAGCAGAACCCAGTCTATG-3′ |
5′-CAGCAGCTCAGGATGAGTACCTC-3′ |
| NQO1 |
5′-CTGTGACAGCAAAATGAACGAGG-3′ |
5′-ATAGCAACAAGTGGGTGGAGGAT-3′ |
| β-actin |
5′-GGCATGGACTGTGGTCATGA-3′ |
5′-TTCACCACCATGGAGAAGGC-3′ |
Western blot analysis
Hippocampal samples (100 mg) were homogenized and
lysed on ice with cell lysis buffer (Ding Changsheng
Biotechnology). The homogenates were centrifuged at 2,000 × g or 5
min at 4°C. The supernatants were then collected. Nuclear
extraction reagent (Pierce; Thermo Fisher Scientific, Inc.) was
used for the separation of the cytosolic extract (cytosol) and
nuclear extract (nucleus). The protein concentration was determined
using the Bradford assay (Pierce; Thermo Fisher Scientific, Inc.).
Equal amounts of protein (20 µg) were separated by 12%
SDS-polyacrylamide gel electrophoresis, and transferred onto PVDF
membranes. The membranes were blocked with 5% non-fat milk for 2 h
and incubated overnight at 4°C with primary antibodies (rabbit
anti-HO-1, Epitomics, cat. no. 2322-1, 1:800; anti-gp91phox, Santa
Cruz Biotechnology, Inc., cat. no. sc-130543, 1:500; anti-p47phox,
EMD Millipore, cat. no. 07-502, 1:500; anti-p22phox, Santa Cruz
Biotechnology, Inc., cat. no. sc-130550, 1:200; anti-NQO1,
Epitomics, cat. no. 2558-1, 1:1,000; anti-Nrf2, Epitomics, cat. no.
2178-1, 1:500; anti-β-actin, Santa Cruz Biotechnology, Inc., cat.
no. sc-69879, 1:2,000; anti-histone H3, Santa Cruz Biotechnology,
Inc., cat. no. sc-517576, 1:1,000). The membranes were then
incubated with goat anti-rabbit secondary antibody conjugated with
horseradish peroxidase (cat. no. 7074, 1:15,000; Cell Signaling
Technology, Inc.) for 1 h at room temperature. Immunoreactive
proteins were detected using enhanced chemiluminescence (Applygen
Technologies Inc.). The quantification of the detected bands was
performed using ImageJ software 3.0 (NIH). The data were
standardized using β-actin (internal control of cytosolic protein)
or histone H3 (internal control of nuclear protein).
Statistical analysis
All data are expressed as the means ± SD. The data
of the Morris water maze were analyzed by repeated measures ANOVA
and Tukey's test as the post hoc test. The other data were analyzed
by one-way ANOVA followed by the Tukey's test. All statistical
analyses were performed using SPSS 23 software (SPSS, Inc.). A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Effects of troxerutin on body weight and
blood glucose level
Movement, drinking, eating, urine volume and the fur
color of the NC rats were normal. By contrast, the diabetic rats
exhibited polyuria, polydipsia, weight loss and a dull fur color.
As shown in Table II,
hyperglycemia persisted in the diabetic rats after the STZ
injection. The rats in the DC group was characterized by
significantly increased FBG levels and a reduced body weight
compared with those in the NC group (F(4,45)=130.22, P<0.05;
F(4,45)=33.59, P<0.05). The other
diabetic groups exhibited no statistically significant differences
in FBG and body weight compared with the DC group (P>0.05).
Thus, troxerutin and LA treatment had no marked effect on the FBG
level and body weight of the diabetic rats.
 | Table IIEffects of troxerutin on body weights
and blood glucose levels in diabetic rats. |
Table II
Effects of troxerutin on body weights
and blood glucose levels in diabetic rats.
| Group | Body weight
(g) | Fasting blood
glucose levels (mmol/l) |
|---|
| NC | 383.56±12.14 | 7.63±0.24 |
| DC | 268.67±8.41a | 28.29±0.88a |
| DN | 244.10±4.38a | 29.25±1.01a |
| DL | 263.72±6.74a | 30.54±0.84a |
| DT |
252.21±10.14a | 27.77±1.05a |
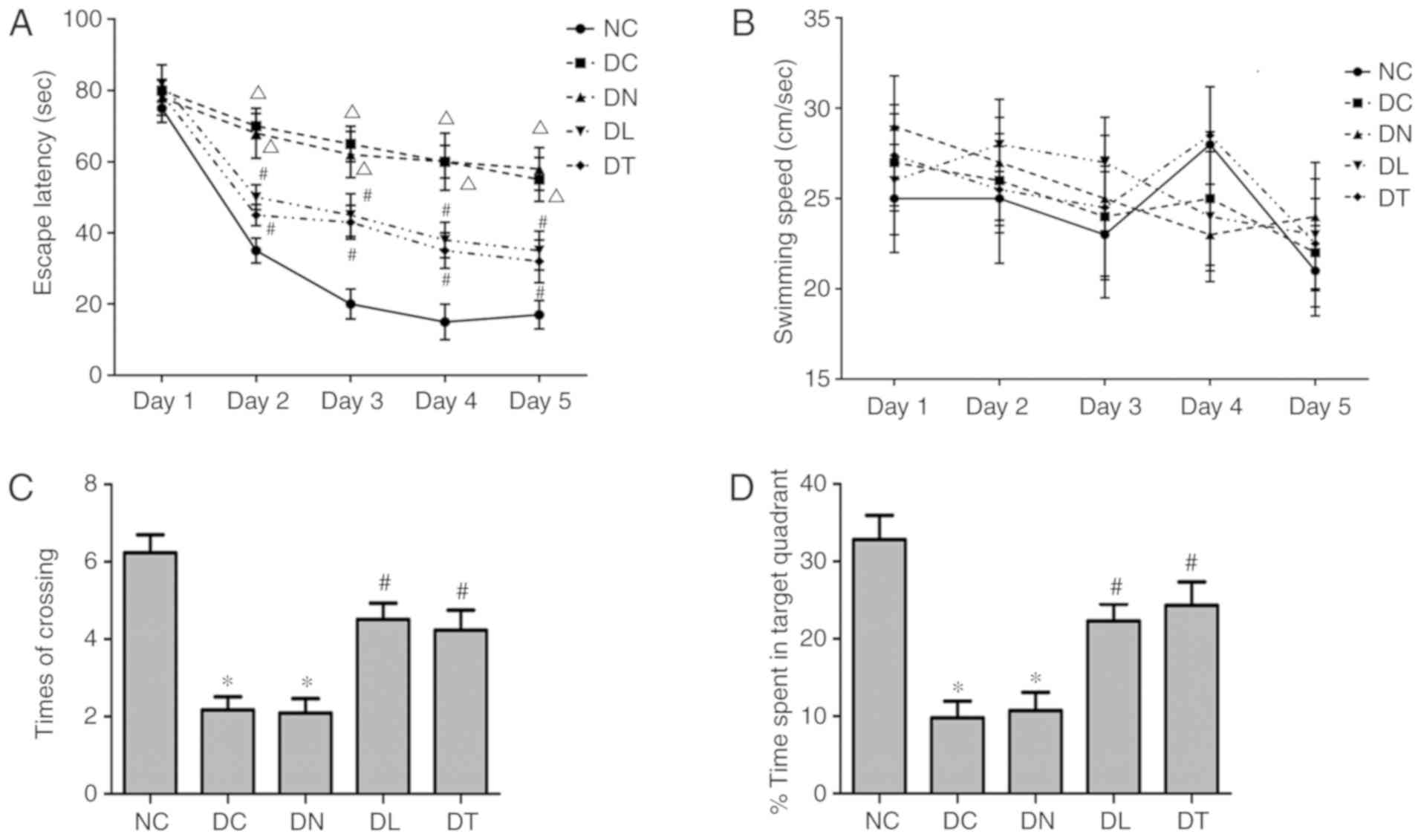
Effects of troxerutin on diabetes-induced
cognitive deficits
Repeated measures ANOVA was used to compare the data
between each group (Fig. 1A). The
escape latency of DC and DN group increased significantly compared
with the NC group (P<0.01). The escape latency of the DT and DL
group decreased significantly compared with the DC group
(P<0.05). No significant differences were observed in escape
latency between the rats treated with troxerutin and LA
(P>0.05).
In a probe trial of the water maze test, the times
of crossing the platform area (Fig.
1C) and percentage of time spent in the target quadrant
(Fig. 1D) were significantly
decreased in the DC rats compared with the NC rats. These
performances were significantly improved by troxerutin or LA
treatment (F(4,45)=21.74, P<0.05;
F(4,45)=129.46, P<0.05
respectively), with no significant differences observed between the
DT and DL groups (P>0.05). No significant differences were also
observed in the swimming speed among the 5 groups (P>0.05,
Fig. 1B), indicating that the
motor deficits of rats did no contribute to the differences in
escape latency, times of crossing and time spent in the target
quadrant.
Effects of troxerutin on oxidative stress
markers in the hippocampus of diabetic rats
As shown in Fig.
2, an increased SOD activity (F(4,25)=427.22, P<0.01) and GSH
concentration (F(4,25)=74.25, P<0.01), alongside
reduced MDA (F(4,25)=189.12, P<0.01) levels were
observed in the DL and DT groups, compared with the DC and DN
groups.
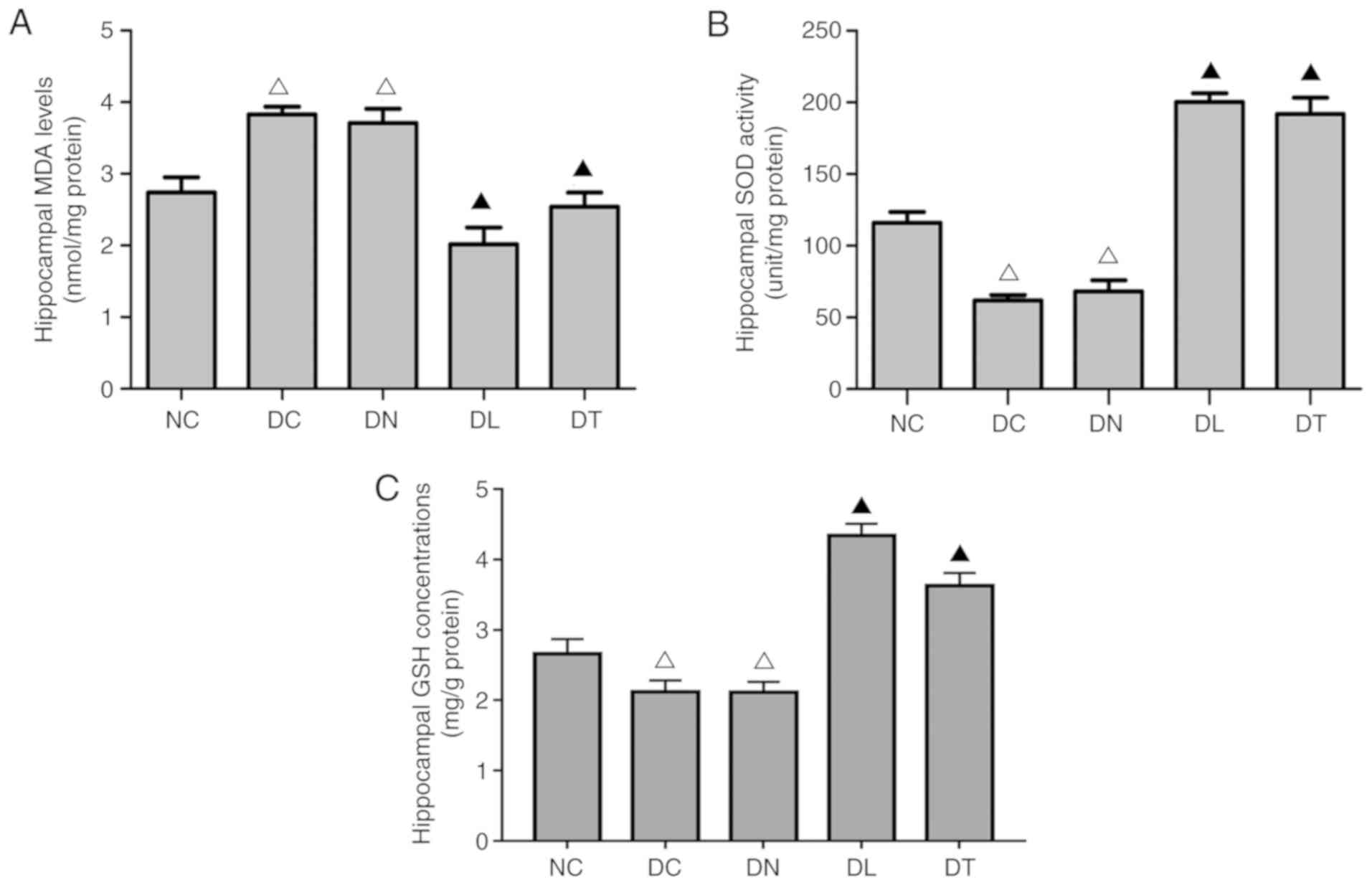 | Figure 2Effects of troxerutin treatment on
(A) MDA levels, (B) SOD activities and (C) GSH concentration in the
hippocampus of rats with STZ-induced diabetes. Data are presented
as the means ± SD, n=3. ΔP<0.01 compared to NC group;
▲P<0.01 compared to DC group. STZ, streptozotocin; NC
group, normal blood glucose control rats; DC group, diabetic rats
without any treatment; DN group, diabetes with saline treated
group; DL group, diabetes with LA treated groups; DT group,
diabetes with troxerutin treated group; MDA, malondialdehyde; SOD,
superoxide dismutase; GSH, glutathione. |
Effects of troxerutin on the expression
of NOX subunits in the hippocampus of diabetic rats
RT-qPCR and western blot analysis were performed to
measure the expression of NOX subunits in the hippocampus,
including gp91phox, p47phox and p22phox. As illustrated in Fig. 3E-G, STZ-induced diabetes increased
the mRNA expression of gp91phox, p47phox and p22phox in the DC and
DN groups, while troxerutin treatment attenuated the augmentation
of gp91phox, p47phox and p22phox expression in the diabetic rats
(F(4,25)=12.18, P<0.01;
F(4,25)=7.44, P<0.05;
F(4,25)=137.25, P=0.01, respectively).
In addition, the cytosolic protein fractions of gp91phox, p47phox
and p22phox were significantly increased in the diabetic groups.
Following troxerutin treatment, all these levels were decreased
compared with the samples from the DC group (F(4,25)=55.50, P<0.01;
F(4,25)=6.59, P<0.05;
F(4,25)=60.58, P<0.01) (Fig. 3A-D).
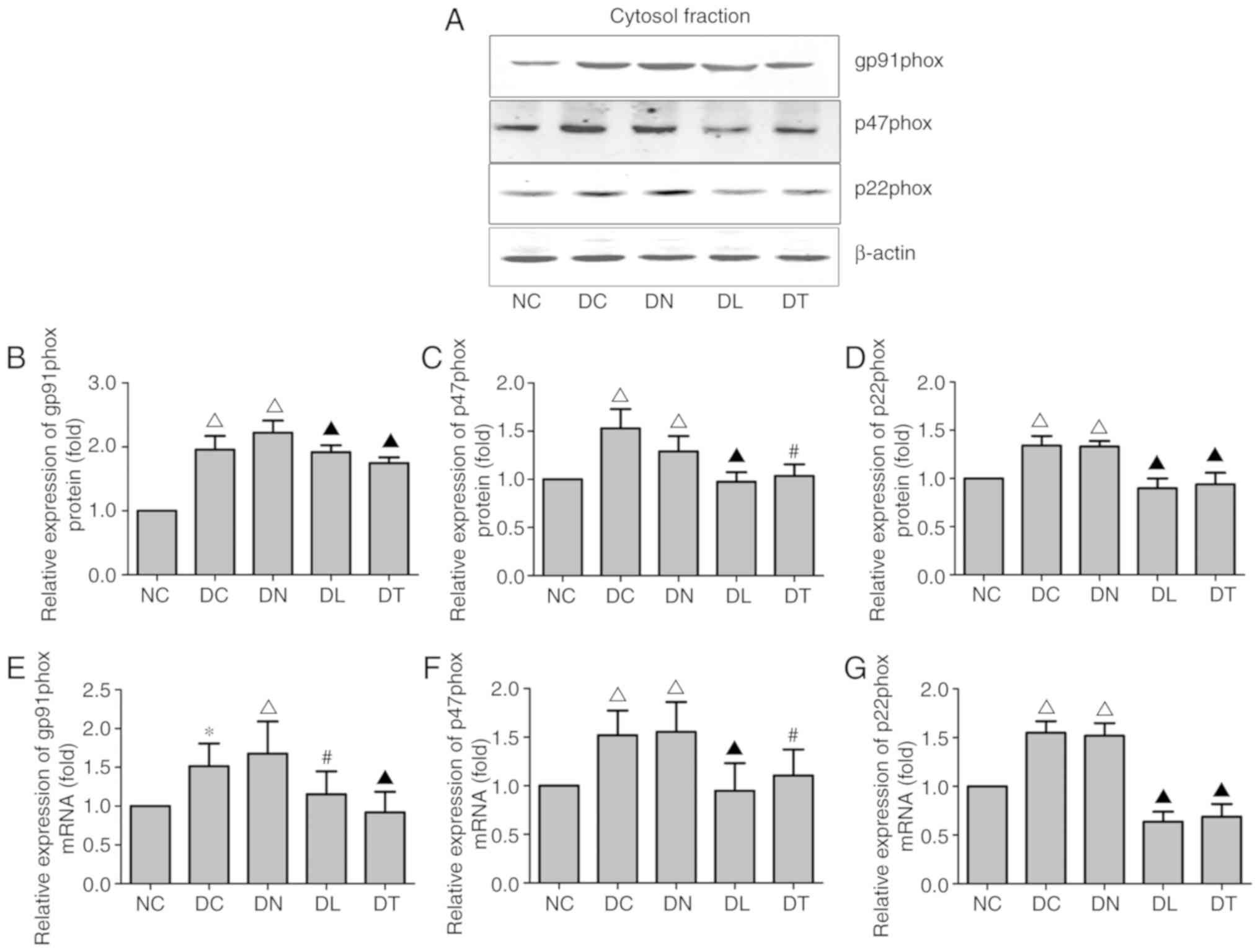 | Figure 3Effects of troxerutin treatment on
the expression levels of NOX subunits in rats with STZ-induced
diabetes. (A) Representative western blot for gp91phox, p47phox and
p22ohox in the cytosolic protein fraction of hippocampal tissue.
β-actin was used as a loading control. (B-D) The relative protein
expression levels of NOX subunits are expressed as the ratio to
β-actin. (E-G) Relative mRNA levels of NOX subunits. Experiments
were performed on 3 independent hippocampal preparations (n=3).
*P<0.05 compared to NC; ΔP<0.01
compared to NC; #P<0.05 compared to DC;
▲P<0.01 compared to DC group. STZ, streptozotocin; NC
group, normal blood glucose control rats; DC group, diabetic rats
without any treatment; DN group, diabetes with saline treated
group; DL group, diabetes with LA treated groups; DT group,
diabetes with troxerutin treated group; MDA, malondialdehyde; SOD,
superoxide dismutase; GSH, glutathione. |
Effects of troxerutin on the Nrf2/ARE
signaling pathway in the hippocampus of diabetic rats
To determine the effects of troxerutin on the
activation of the Nrf2/ARE signaling pathway in the model of
diabetes-induced cognitive deficits, the hippocampal expression
levels of Nrf2 and two well-known downstream phase II antioxidant
enzymes, HO-1 and NQO1, were measured by western blot analsyis and
RT-qPCR. As illustrated in Fig. 4H
and I, STZ-induced diabetes significantly decreased the NQO1
and HO-1 mRNA expression levels. Troxerutin treatment resulted in a
noticeably increased mRNA expression of NQO1 and HO-1 compared with
the DC group (F(4,25)=72.53, P<0.01;
F(4,25)=6.95, P<0.05,
respectively).
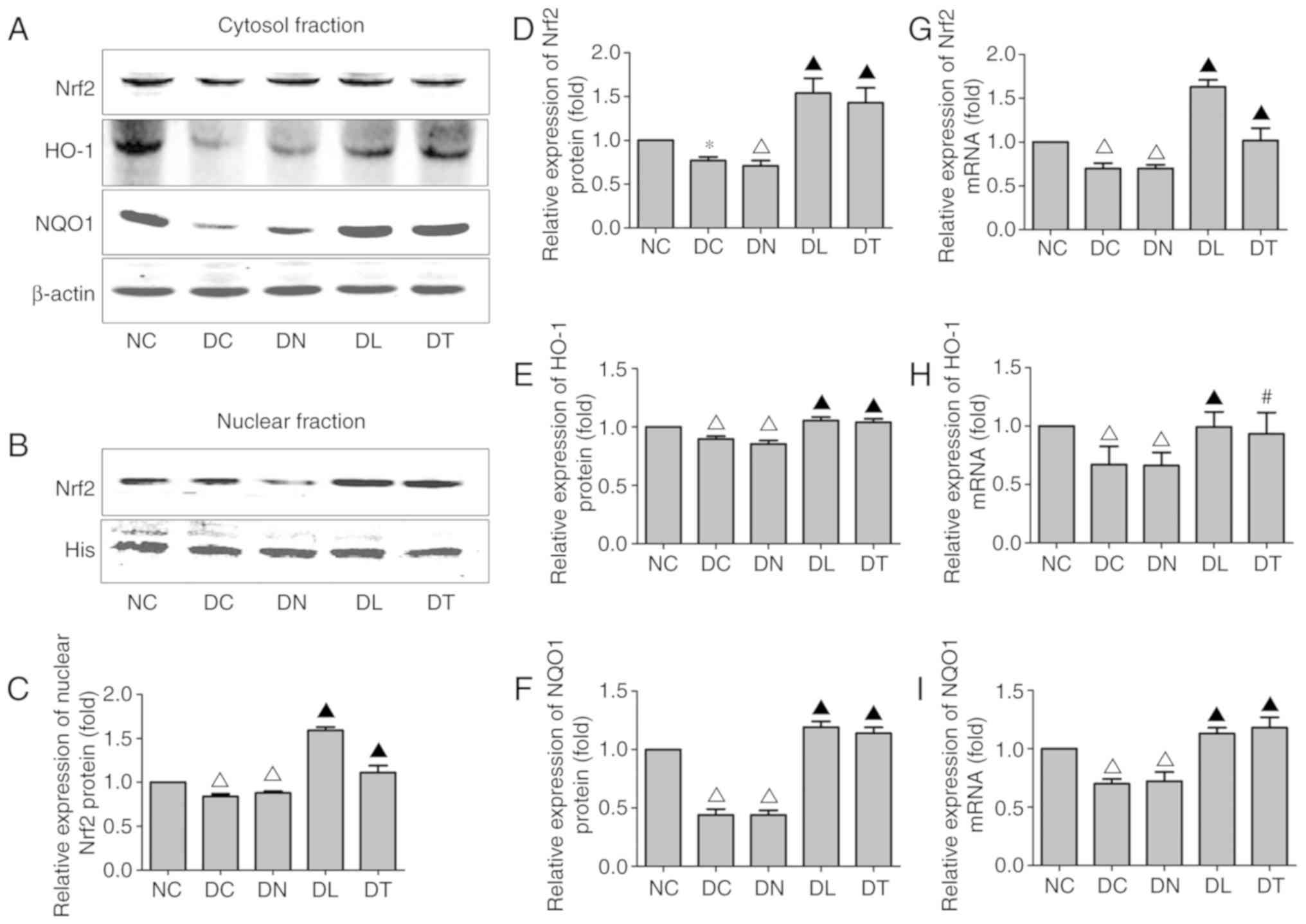 | Figure 4Effects of troxerutin treatment on
the activation of the Nrf2/ARE signaling pathway. (A)
Representative western blot of Nrf2, HO-1 and NQO1 in the cytosolic
protein fraction of hippocampal tissue. (B) Representative western
blot for Nrf2 in the nuclear fraction of hippocampal tissue. The
relative protein expression of (C) nuclear Nrf2 and (D) cytosolic
Nrf2, as well as the (G) mRNA level of Nrf2 were analyzed. Relative
mRNA and cytosolic protein levels of (E and H) HO-1 and (F and I)
NQO1 are expressed as the ratio to β-actin. Experiments were
performed on 3 independent hippocampal preparations (n=3).
*P<0.05 compared to NC; ΔP<0.01
compared to NC; #P<0.05 compared to DC;
▲P<0.01 compared to DC group. NC group, normal blood
glucose control rats; DC group, diabetic rats without any
treatment; DN group, diabetes with saline treated group; DL group,
diabetes with LA treated groups; DT group, diabetes with troxerutin
treated group; MDA, malondialdehyde; SOD, superoxide dismutase;
GSH, glutathione. |
As regards the cytosolic protein fractions, the NQO1
and HO-1 levels were markedly decreased in the DC group compared
with those in the NC group. Troxerutin resulted in noticeably
increased cytosolic protein fractions of NQO1 and HO-1 compared
with those in the DC group (F(4,25)=86.86, P<0.01;
F(4,25)=96, P<0.01) (Fig. 4A, E and F).
In addition, STZ-induced diabetes significantly
inhibited the nuclear translocation of Nrf2. This inhibitory effect
of Nrf2 was reversed by troxerutin. Consistent with the changes
observed in Nrf2 cytosolic and nuclear protein expression
(F(4,25)=52.35, P<0.01;
F(4,25)=159.36, P<0.01) (Fig. 4A-D), the mRNA level of Nrf2 was
markedly increased in the DT group compared with the DC group
(F(4,25)=69.62, P<0.01) (Fig. 4G). These results collectively
demonstrate that troxerutin plays a protective role in the
suppression of oxidative stress via the regulation of Nrf2/ARE
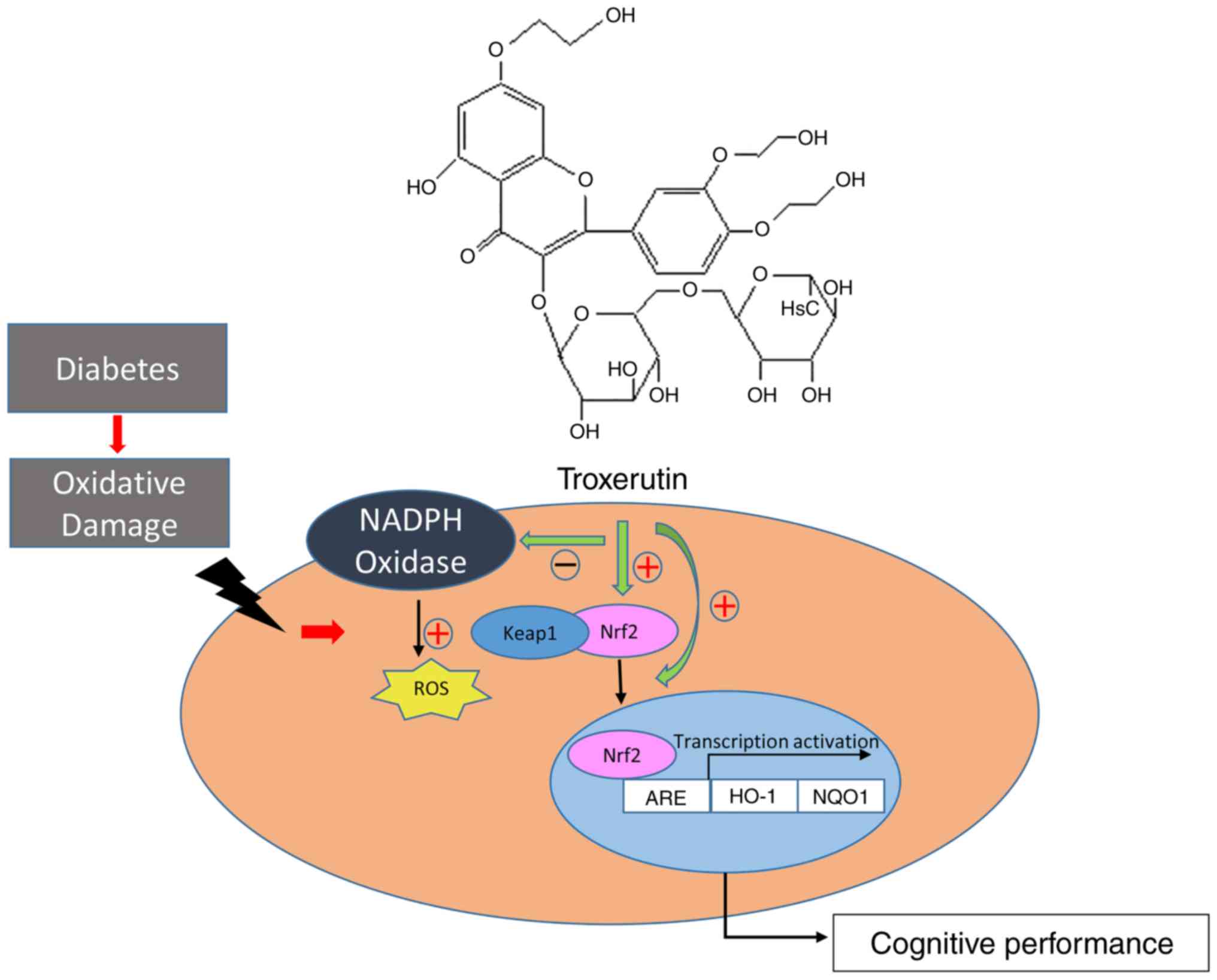
signaling pathway. In Fig. 5, the
schematic diagram demonstrates the mechanism of troxerutin in
relieving the oxidative stress in hippocampus of diabetic rats.
Discussion
Diabetes mellitus is associated with an increased
risk of dementia (2). The
diabetes- associated cognitive decline is an increasing
complication of diabetes (27).
Accumulating studies have indicated that oxidative stress and
inflammation response induced by chronic hyperglycemia can trigger
the degenerative pathways leading to diabetes-associated cognitive
decline (11,19,28,29). In the present study, it was
demonstrated that troxerutin exerted protective effects against
cognitive impairment in rats with STZ-induced diabetes. The rats
with STZ-induced diabetes exhibited typically increased blood
glucose levels and a decreased body weight, as well as cognitive
decline at the age of 22 weeks. Troxerutin treatment alleviated
diabetes-induced oxidative damage, learning and memory loss in the
hippocampus. At the molecular level, the administration of
troxerutin reduced the activity of NOX, which is a major source of
ROS. Moreover, it also demonstrated that troxerutin enhanced the
Nrf2/ARE signaling pathway in hippocampus of STZ-induced diabetic
rats, regulating the expression of HO-1 and NQO1.
LA(1,2-dithiolane-3-pentanoic) is a natural dithiol
compound synthesized from octanoic acid, which has been reported to
have antioxidant activities. It is also a potent antioxidant
inhibiting ROS-induced damaging effects. The present study selected
LA as a positive control for its ability to scavenge ROS (30). The selection of troxerutin was
based on its outstanding pharmacological potentials in
cardiovascular disease and cognitive deficits (24,31-33). Its role in protecting against
diabetes-associated cognitive decline, however, remains unclear. It
is well known that the Morris water maze is a gold criterion for
spatial learning and memory in rodents (34). In the present study, an increased
escape latency, decreased times of crossing the platform area and
the percentage of time spent in the target quadrant suggested that
diabetes significantly impaired learning and memory performance,
which is consistent with the findings of previous studies (23,35). It was found that troxerutin
treatment significantly improved the cognitive function of diabetic
rats.
The elevation of the MDA level was assessed as an
index for lipid peroxidation-induced oxidative damage. In addition,
the activity of the antioxidant enzyme, SOD, was measured to
evaluate the antioxidant response in hippocampus of diabetic rats.
GSH is a major component of redox regulation. It has been shown in
a previous study that the GSH concentration in the hippo-campus of
STZ-treated rats is reduced at hyperglycemia, but is normalized
upon acute glycemic restoration to control levels (36).
In the present study, the increase in the MDA levels
together with a decrease in SOD and GSH levels suggest that chronic
hyperglycemia induced cognitive impairment through oxidative stress
occurring in the hippocampus. Troxerutin decreased the MDA level,
and increased SOD activity and the GSH level in the hippocampus of
diabetic rats. Consequently, it ameliorated the diabetes-associated
cognitive decline by reducing oxidative stress and enhancing
antioxidant defenses in the hippocampus. Notably, LA exerted more
potent suppressive effects on the MDA level, which is consistent
with the findings of previous studies on the effects of LA on lipid
peroxidation (30,37).
NOX is a prooxidant enzyme that catalyzes the
oxidation of NADPH, playing an important role in generating
super-oxide, particularly in neuronal pathologies. Indeed, previous
studies have demonstrated that superoxide production by NOX
underlies cognitive impairment following cerebral ischemia and
traumatic brain injury (38,39). NOX consists of membrane subunit
proteins (p22phox and gp91phox) and cytosolic subunit proteins
(p47phox, p67phox, p40phox). The cytosolic subunit proteins were
significantly elevated with the progression of Alzheimer's disease
(40). The increased expression
of NOX membrane subunit p22phox underlies long-term memory loss
induced by ROS overproduction in anesthetic exposure (41). Moreover, the knockdown of NOX
subunit p47phox has been shown to increase spatial learning and
memory in mice (42). The
findings of the present study are in agreement with those of
previous studies, demonstrating that troxerutin exerts antioxidant
effects by reducing gp91phox, p47phox and p22phox expression levels
in the hippocampus of diabetic rats. These data indicated that the
upregulation of NOX in the hippo-campus and the increase in
NOX-associated redox pathways may participate in the early
pathogenesis and contribute to the progression of
diabetes-associated cognitive decline.
The Nrf2/ARE signaling pathway plays a key role in
regulating transcription of the genes encoding endogenous
antioxidant enzymes, such as NO-1, NQO1 and SOD (43). The present study demonstrated that
the intracellular Nrf2 mRNA and protein levels in hippocampus
increased with troxerutin followed STZ-induced hyperglycemia. When
cells are exposed to free radicals, Nrf2 disassociates from its
cytosolic inhibitor, Keap-1 and translocates to the nucleus, where
it combines with the ARE (the promoter region of many phase II
enzymes) (44-46). The results indicated that
increased nuclear Nrf2 protein along with elevated HO-1 and NQO1
expression may be the underlying mechanism of troxerutin mitigating
diabetes-associated cognitive decline.
Additionally, it was found LA treatment exerted more
porent effects on promoting Nrf2 transcription and nuclear
translocation. Although the diabetic rats benefited more from LA
treatment, no statistically significant differences were observed
in spatial learning and memory performances between the LA- and
troxerutin-treated diabetic rats. This may due to other biological
activities of troxerutin, such as anti-inflammatory,
anti-thrombotic and anti-fibrinolytic effects.
In conclusion, the present study demonstrates that
troxerutin prevents diabetes-associated cognitive decline in rats
with STZ-induced diabetes by suppressing oxidative stress through
the inhibition of NOX and the Nrf2/ARE signaling pathway. However,
the present study only performed experiments on an animal model.
The precise conclusions drawn warrant further verification in in
vitro cell experiments. These findings however suggest that
troxerutin may have therapeutic potential for use in protecting
against cognitive dysfunction resulting from diabetes. Further
studies are required to explore the interaction between NOX and the
Nrf2/ARE signaling pathway.
Abbreviations:
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
LA
|
α-lipoic acid
|
|
STZ
|
streptozotocin
|
|
Nrf2
|
nuclear factor-E2-related factor-2
|
|
HO-1
|
heme oxygenase-1
|
|
NQO1
|
NAD(P) H:quinone oxidoreductase
|
|
ARE
|
antioxidant response element
|
|
Keap1
|
kelch-like ECH associating protein
1
|
|
NADPH
|
nicotinamide adenine dinucleotide
phosphate
|
Acknowledgments
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Hebei, China (grant no. H2018206320) and
Health and Traditional Medicine Administration Scientific Research
Plan of Hebei, China (grant no. 2019119).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
The corresponding author SZ contributed to the
design and conception of the study. MG contributed to the design of
the study, performed the experiments, analyzed the data and
prepared the manuscript for writing. YK established the animal
model and performed the behavioral test. LZ contributed the
experimental design and manuscript reviewing. HL and CQ performed
the molecular biological analysis. XL and LL were involved in data
analysis and figure editing. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
All experimental procedures followed the Guide for
the Care and Use of Laboratory Animals. All protocols for animal
treatment were approved by the Animal Ethics Committee of Hebei
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guariguata L, Whiting DR, Hambleton I,
Beagley J, Linnenkamp U and Shaw JE: Global estimates of diabetes
prevalence for 2013 and projections for 2035. Diabetes Res Clin
Pract. 103:137–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koekkoek PS, Kappelle LJ, van den Berg E,
Rutten GE and Biessels GJ: Cognitive function in patients with
diabetes mellitus: Guidance for daily care. Lancet Neurol.
14:329–340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mijnhout GS, Scheltens P, Diamant M,
Biessels GJ, Wessels AM, Simsek S, Snoek FJ and Heine RJ: Diabetic
encephalopathy: A concept in need of a definition. Diabetologia.
49:1447–1448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Biessels GJ and Despa F: Cognitive decline
and dementia in diabetes mellitus: Mechanisms and clinical
implications. Nat Rev Endocrinol. 14:591–604. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao M, Ji S, Li J and Zhang S:
DL-3-n-butylphthalide (NBP) ameliorates cognitive deficits and
CaMKII-mediated long-term potentiation impairment in the
hippocampus of diabetic db/db mice. Neurol Res. 41:1024–1033. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Zhang S, Zhang L, Wang R and Wang M:
Effects of L-3-n-butylphthalide on cognitive dysfunction and NR2B
expression in hippocampus of streptozotocin (STZ)-induced diabetic
rats. Cell Biochem Biophys. 71:315–322. 2015. View Article : Google Scholar
|
|
7
|
Zhang SY, Ji SX, Bai XM, Yuan F, Zhang LH
and Li J: L-3-n-butylphthalide attenuates cognitive deficits in
db/db diabetic mice. Metab Brain Dis. 34:309–318. 2019. View Article : Google Scholar
|
|
8
|
Muriach M, Flores-Bellver M, Romero FJ and
Barcia JM: Diabetes and the brain: Oxidative stress, inflammation,
and autophagy. Oxid Med Cell Longev. 2014:1021582014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cobley JN, Fiorello ML and Bailey DM: 13
reasons why the brain is susceptible to oxidative stress. Redox
Biol. 15:490–503. 2008. View Article : Google Scholar
|
|
10
|
Yang H, Jin X, Kei Lam CW and Yan SK:
Oxidative stress and diabetes mellitus. Clin Chem Lab Med.
49:1773–1782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elsherbiny NM, Zaitone SA, Mohammad HMF
and El-Sherbiny M: Renoprotective effect of nifuroxazide in
diabetes-induced nephropathy: Impact on NFκB, oxidative stress, and
apoptosis. Toxicol Mech Methods. 28:467–473. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aboualizadeh E, Ranji M, Sorenson CM,
Sepehr R, Sheibani N and Hirschmugl CJ: Retinal oxidative stress at
the onset of diabetes determined by synchrotron FTIR widefield
imaging: Towards diabetes pathogenesis. Analyst. 142:1061–1072.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ravassa S, Beaumont J, Huerta A, Barba J,
Coma-Canella I, González A, López B and Díez J: Association of low
GLP-1 with oxidative stress is related to cardiac disease and
outcome in patients with type 2 diabetes mellitus: A pilot study.
Free Radic Biol Med. 81:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mecocci P, Boccardi V, Cecchetti R,
Bastiani P, Scamosci M, Ruggiero C and Baroni M: A long journey
into aging, brain aging, and Alzheimer's disease following the
oxidative stress tracks. J Alzheimers Dis. 62:1319–1335. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maciejczyk M, Żebrowska E and Chabowski A:
Insulin resistance and oxidative stress in the brain: What's new?
Int J Mol Sci. 20:8742019. View Article : Google Scholar
|
|
16
|
Kim J, Cho CH, Hahn HG, Choi SY and Cho
SW: Neuroprotective effects of N-adamantyl-4-methylthiazol-2-amine
against amyloid β-induced oxidative stress in mouse hippocampus.
Brain Res Bull. 128:22–28. 2017. View Article : Google Scholar
|
|
17
|
Tian X, Liu Y, Ren G, Yin L, Liang X, Geng
T, Dang H and An R: Resveratrol limits diabetes-associated
cognitive decline in rats by preventing oxidative stress and
inflammation and modulating hippocampal structural synaptic
plasticity. Brain Res. 1650:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Samarghandian S, Azimi-Nezhad M and Samini
F: Ameliorative effect of saffron aqueous extract on hyperglycemia,
hyperlipidemia, and oxidative stress on diabetic encephalopathy in
streptozotocin induced experimental diabetes mellitus. Biomed Res
Int. 2014:9208572014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gomaa AA, Makboul RM, Al-Mokhtar MA and
Nicola MA: Polyphenol-rich Boswellia serrata gum prevents cognitive
impairment and insulin resistance of diabetic rats through
inhibition of GSK3β activity, oxidative stress and pro-inflammatory
cytokines. Biomed Pharmacother. 109:281–292. 2019. View Article : Google Scholar
|
|
20
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu XF, Wang L, Xiang G, Lei W and Feng YF:
Angiogenesis impairment by the NADPH oxidase-triggered oxidative
stress at the bone-implant interface: Critical mechanisms and
therapeutic targets for implant failure under hyperglycemic
conditions in diabetes. Acta Biomater. 73:470–487. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang S, Li H, Zhang L, Li J, Wang R and
Wang M: Effects of troxerutin on cognitive deficits and glutamate
cysteine ligase subunits in the hippocampus of
streptozotocin-induced type 1 diabetes mellitus rats. Brain Res.
1657:355–360. 2017. View Article : Google Scholar
|
|
24
|
Najafi M, Noroozi E, Javadi A and
Badalzadeh R: Anti-arrhythmogenic and anti-inflammatory effects of
troxerutin in ischemia/reperfusion injury of diabetic myocardium.
Biomed Pharmacother. 102:385–391. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goyal SN, Reddy NM, Patil KR, Nakhate KT,
Ojha S, Patil CR and Agrawal YO: Challenges and issues with
streptozotocin-induced diabetes-A clinically relevant animal model
to understand the diabetes pathogenesis and evaluate therapeutics.
Chem Biol Interact. 244:49–63. 2016. View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Rawlings AM, Sharrett AR, Schneider AL,
Coresh J, Albert M, Couper D, Griswold M, Gottesman RF, Wagenknecht
LE, Windham BG and Selvin E: Diabetes in midlife and cognitive
change over 20 years: A cohort study. Ann Intern Med. 161:785–793.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chung CC, Pimentel D, Jor'Dan AJ, Hao Y,
Milberg W and Novak V: Inflammation-associated declines in cerebral
vasore-activity and cognition in type 2 diabetes. Neurology.
85:450–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ward R and Ergul A: Relationship of
endothelin-1 and NLRP3 inflammasome activation in HT22 hippocampal
cells in diabetes. Life Sci. 159:97–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi XC, Jin A, Sun J, Yang Z, Tian JJ, Ji
H, Yu HB, Li Y, Zhou JS, Du ZY and Chen LQ: α-lipoic acid
ameliorates n-3 highly-unsaturated fatty acids induced lipid
peroxidation via regulating antioxidant defenses in grass carp
(Ctenopharyngodon idellus). Fish Shellfish Immunol. 67:359–367.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu J, Wu DM, Zheng YL, Hu B, Cheng W,
Zhang ZF and Li MQ: Troxerutin counteracts domoic acid-induced
memory deficits in mice by inhibiting CCAAT/enhancer binding
protein β-mediated inflammatory response and oxidative stress. J
Immunol. 190:3466–3479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu J, Wu DM, Zheng ZH, Zheng YL, Hu B and
Zhang ZF: Troxerutin protects against high cholesterol-induced
cognitive deficits in mice. Brain. 134:783–797. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu Y and Zheng G: Troxerutin protects
against diabetic cardio-myopathy through NF-κB/AKT/IRS1 in a rat
model of type 2 diabetes. Mol Med Rep. 15:3473–3478. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morris R: Developments of a water-maze
procedure for studying spatial learning in the rat. J Neurosci
Methods. 11:47–60. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang S, Yuan L, Zhang L, Li C and Li J:
Prophylactic use of troxerutin can delay the development of
diabetic cognitive dysfunction and improve the expression of Nrf2
in the hippo-campus on STZ diabetic rats. Behav Neurol.
2018:86785392018. View Article : Google Scholar
|
|
36
|
Duarte JM, Carvalho RA, Cunha RA and
Gruetter R: Caffeine consumption attenuates neurochemical
modifications in the hippocampus of streptozotocin-induced diabetic
rats. J Neurochem. 111:368–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Uchendu C, Ambali SF, Ayo JO and Esievo
KAN: Chronic co-exposure to chlorpyrifos and deltamethrin
pesticides induces alterations in serum lipids and oxidative stress
in Wistar rats: Mitigating role of alpha-lipoic acid. Environ Sci
Pollut Res Int. 25:19605–19611. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi GX, Wang XR, Yan CQ, He T, Yang JW,
Zeng XH, Xu Q, Zhu W, Du SQ and Liu CZ: Acupuncture elicits
neuroprotective effect by inhibiting NAPDH oxidase-mediated
reactive oxygen species production in cerebral ischaemia. Sci Rep.
5:179812015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ferreira AP, Rodrigues FS, Della-Pace ID,
Mota BC, Oliveira SM, Velho Gewehr Cde C, Bobinski F, de Oliveira
CV, Brum JS, Oliveira MS, et al: The effect of NADPH-oxidase
inhibitor apocynin on cognitive impairment induced by moderate
lateral fluid percussion injury: Role of inflammatory and oxidative
brain damage. Neurochem Int. 63:583–593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ansari MA and Scheff SW: NADPH-oxidase
activation and cognition in Alzheimer disease progression. Free
Radic Biol Med. 51:171–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun Z, Satomoto M, Adachi YU, Kinoshita H
and Makita K: Inhibiting NADPH oxidase protects against long-term
memory impairment induced by neonatal sevoflurane exposure in mice.
Br J Anaesth. 117:80–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Walton JC, Selvakumar B, Weil ZM, Snyder
SH and Nelson RJ: Neuronal nitric oxide synthase and NADPH oxidase
interact to affect cognitive, affective, and social behaviors in
mice. Behav Brain Res. 256:320–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ahmed SM, Luo L, Namani A, Wang XJ and
Tang X: Nrf2 signaling pathway: Pivotal roles in inflammation.
Biochim Biophys Acta Mol Basis Dis. 1863:585–597. 2017. View Article : Google Scholar
|
|
44
|
Shalaby YM, Menze ET, Azab SS and Awad AS:
Involvement of Nrf2/HO-1 antioxidant signaling and NF-κB
inflammatory response in the potential protective effects of
vincamine against methotrexate-induced nephrotoxicity in rats:
Cross talk between nephrotoxicity and neurotoxicity. Arch Toxicol.
93:1417–1431. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang L, Guo Y, Wang H, Zhao L, Ma Z, Li
T, Liu J, Sun M, Jian Y, Yao L, et al: Edaravone reduces Aβ-induced
oxidative damage in SH-SY5Y cells by activating the Nrf2/ARE
signaling pathway. Life Sci. 221:259–266. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ren B, Yuan T, Diao Z, Zhang C, Liu Z and
Liu X: Protective effects of sesamol on systemic oxidative
stress-induced cognitive impairments via regulation of Nrf2/Keap1
pathway. Food Funct. 9:5912–5924. 2018. View Article : Google Scholar : PubMed/NCBI
|