Introduction
Although a fetus attains only half of its genetic
material from the mother, maternal-fetal tolerance allows
implantation of the fetus within the womb and the establishment of
a temporary immune system during fetal development (1,2).
The underlying molecular mechanism remains unclear; however, three
hypotheses of maternal immunological tolerance have been proposed:
i) Physical separation of fetal and maternal tissues; ii) antigenic
immaturity of fetal tissues; and iii) immunological inertness
(tolerance) of maternal tissues towards fetal alloantigens
(3). However, these hypotheses do
not fully explain the underlying mechanisms. It has been
hypothesized trophoblast cells from the outer layer of a blastocyst
act as an interface layer and provide nutrients and a constant
blood supply to the embryo. Furthermore, trophoblast cells develop
to form an important part of the placenta, particularly through
their interactions with decidua and placental villi (4).
Estrogen is vital for the female reproductive
development and the three major naturally occurring forms are:
Estrone (E1), 17 β-Estradiol (E2), Estriol (E3). During pregnancy,
E2 and E3 serum levels have been reported to increase 100 and 1,000
times, respectively, while estrogen markedly decreases the levels
of regulatory T-cells (Tregs) following parturition (5). During pregnancy, placenta cells
produce E3. Estrogen also has a function in the immune system;
however, its underlying molecular mechanism remains to be fully
understood.
Indoleamine 2,3-dioxygenase (IDO) is an essential
enzyme in tryptophan catabolism. It degrades amino acid
L-tryptophan into kynurenine, which depletes tryptophan and
subsequently suppresses T-cell activity (6,7).
The molecular mechanisms protecting the fetus include trophoblast
cells, major histocompatibility complex, T cell apoptosis,
suppression of cell proliferation and IDO-mediated tryptophan
catabolism (8). It has been
demonstrated that IDO is expressed by human trophoblasts and
decidual cells, and is activated at the maternal-fetal interface
(9-11). Toxic effects of tryptophan
metabolites are responsible for inhibition of T cell proliferation
and Tregs, and it has been suggested that IDO may be associated
with the T helper (Th)1/Th2 cell balance (6,12,13). Both direct and indirect analyses
have reported that IDO is associated with human maternal fetal
interface and the immune system (14,15).
Transforming growth factor β (TGF-β) is a
multifunctional signal transducer, which is associated with cell
apoptosis, cell cycle and differentiation, and has also been
implicated in tumor growth (16).
A previous study reported that IDO expression increased in mouse
plasmacytoid dendritic cells (pDCs) treated with TGF-β (17). Furthermore, in chorionic villi and
decidua tissues of healthy pregnant women, both TGF-β and IDO are
expressed at high levels and TGF-β expression is positively
correlated with IDO expression (11). Thus, the current study
hypothesized that TGF-β may upregulate IDO expression, and that
increased levels of estrogen may induce IDO expression in chorionic
villi and decidual tissues by upregulating TGF-β expression in
pregnant women.
Materials and methods
Participants and samples
A total of 40 healthy pregnant women who underwent
legal termination of pregnancy at the Affiliated Hospital of
Guizhou Medical University (Guiyang, China) between April 2015 and
October 2017 were recruited in the present study. The mean age of
participants was 27.56±6.27 years, while gestational age was
57.23±7.42 days. All participants presented with normal embryonic
development determined by ultrasonic examination, cases with
abnormalities of the reproductive system were identified and
excluded (18). The exclusion
criteria were as follows: Participant with history of
endometriosis, and chronic diseases associated with chronic
hypertension, kidney disease and diabetes. The development of a
healthy fetus was confirmed via ultrasonic examination, with no
detectable uterine abnormality. All participants provided voluntary
consent to pregnancy termination.
Tissue culture
Decidua and chorionic villi tissues were identified
by morphology. Chorionic villi and decidua tissue samples collected
under aseptic conditions were immediately placed in 0.1 M sterile
PBS (pH 7.2), transferred to ice within 10 min and subsequently
washed twice with PBS. Tissue samples were either preserved at
-80°C prior to western blot analysis or cut into small pieces (≤1
mg wet weight) for tissue culture. The samples prepared for tissue
culture were processed within 40 min of collection. The chorionic
villi and decidua pieces were washed twice with F-12 nutrient
mixture (F-12)/DMEM (Invitrogen; Thermo Fisher Scientific, Inc.),
centrifuged at 1,200 × g for 5 min at room temperature within less
than 40 min, and cultured in F-12/DMED supplemented with 10% FBS
(Gibco, Thermo Fisher Scientific, Inc) and 1%
penicillin-streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) in a CO2 incubator (Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2.
Treatment of villi and decidua
Villi and decidua cultures were plated in six-well
plates at a density of 5×105 cells/well (Corning Inc.)
and cultured in phenol red-free DMED/Ham's F-12 (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin-streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. Villi and decidua cells were prepared for
immunofluorescence (IF) and a part of western blotting experiments.
TGF-β1 powder (R&D Systems, Inc.) was dissolved in PBS at three
concentrations (0.01, 0.5 and 0.1 ng/ml, respectively) and
incubated with the chorionic villi and decidua tissue sections for
48 h at 37°C with 5% CO2. Fulvestrant was purchased from
Selleck Chemicals, diluted in DMED/Ham's F-12 and stored at 4°C. E2
and E3 were diluted in ethyl alcohol (all Sigma-Aldrich; Merck
KGaA). An equal concentration of ethyl alcohol alone was used in
the control group. Sections of chorionic villi or decidua were
incubated in medium containing 10 ng/ml E2 or 1 µg/ml E3,
prior to addition of 0.1, 1 and 10 µg/ml fulvestrant for 12
h at 37°C with 5% CO2.
IF staining
For immunofluorescence staining, villi and decidua
cells were treated with 10 mg/ml E2, 1 µg/ml E3 or 1
µg/ml fulvestrant for 48 h, then fixed in PBS supplemented
with 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) for 15 min,
prior to treatment in PBS supplemented with 0.5% Triton X-100 for
15 min. Cells were incubated with PBS containing 4% bovine serum
albumin (Gibco; Thermo Fisher Scientific, Inc.) at room temperature
for 30 min and subsequently washed three times with PBS (5
min/wash) Tissue samples were incubated with the following primary
antibodies: IDO rabbit anti-human (1:1,000; cat. no. 86630; Cell
Signaling Technology, Inc.) and TGF-β rabbit anti human (1:500;
cat. no. 3709; Cell Signaling Technology, Inc.) overnight at 4°C.
Following the primary antibody incubation, membranes were incubated
with isothiocyanate-conjugated donkey anti-rabbit FITC (1:1,000;
F8070; Beijing Solarbio Science & Technology Co., Ltd.) and
Cy® 3 Immunoglobulin G donkey anti-rabbit (1:1,000;
S1050; Beijing Solarbio Science & Technology Co., Ltd.)
secondary antibodies for 1 h at room temperature. All antigen were
retrieval Once store at 4°C, staining cells were wash with PBS
reagent 3 times every 5 mins. Nuclei were stained with DAPI (cat.
no. D1306; Invitrogen; Thermo Fisher Scientific, Inc.) for 3 min at
room temperature, mounted onto slides and confocal dishes, and
observed under a confocal microscope (LSM 710; Carl Zeiss AG).
Images were captured using a 63X numerical aperture objective.
Western blotting
Western blot analysis was performed as previously
described (10). Briefly, total
protein was extracted from chorionic villi tissues, decidua tissues
or cultured isolated cells using ice-cold RIPA (Sigma-Aldrich;
Merck KGaA). Tissue samples or isolated cells were treated with
protease inhibitors at 4°C for 30 min (Sigma Aldrich) and proteins
were isolated followed by centrifugation at 11,000 × g for 20 min
at 4°C (repeated twice). Proteins were separated via SDS-PAGE on 10
or 15% gel. The separated proteins were subsequently transferred
onto PVDF membranes (PerkinElmer, Inc.) at 200 mA for 1.5 h. After
blocking for 1 h in 5% non-fat milk, the membranes were incubated
with the following primary antibodies: Anti-human IDO monoclonal
antibody (1:3,000; cat. no. 86630; Cell Signaling Technology,
Inc.), TGF-β polyclonal antibody (1:3,000; cat. no. 3709; Cell
Signaling Technology, Inc.), or human GAPDH polyclonal antibody
(1:6,000; cat. no. GTX100118; GeneTex, Inc.) for 3 h at room
temperature. Membranes were washed three time with tris-buffered
saline with Tween-20. Following the primary antibody incubation,
membranes were incubated with horseradish peroxidase-labeled donkey
anti-rabbit immunoglobulin G antibody (Cell Signaling Technology,
Inc.) for 1 h at room temperature. Protein bands were visualized
using the ECL kit (PerkinElmer, Inc.) followed by autoradiography
(GenoSwns1880, 3300018-7Q; Clinx Science Instruments Co. Ltd.). All
experiment were performed in triplicate.
Statistical analysis
All statistical analyses were performed using
GraphPad (version 6.0; GraphPad Software, Inc.) and SPSS (23.0;
IBM, Corp.) and presented as the mean ± standard error of the mean.
Two independent groups were compared using Pearson's correlation
analysis. One-way ANOVA followed by Student-Newman-Keuls post hoc
test or Turkey's test were used to assess protein expression
following western blot analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
IDO and TGF-β expression in chorionic
villi and decidua tissues at early stages of pregnancy
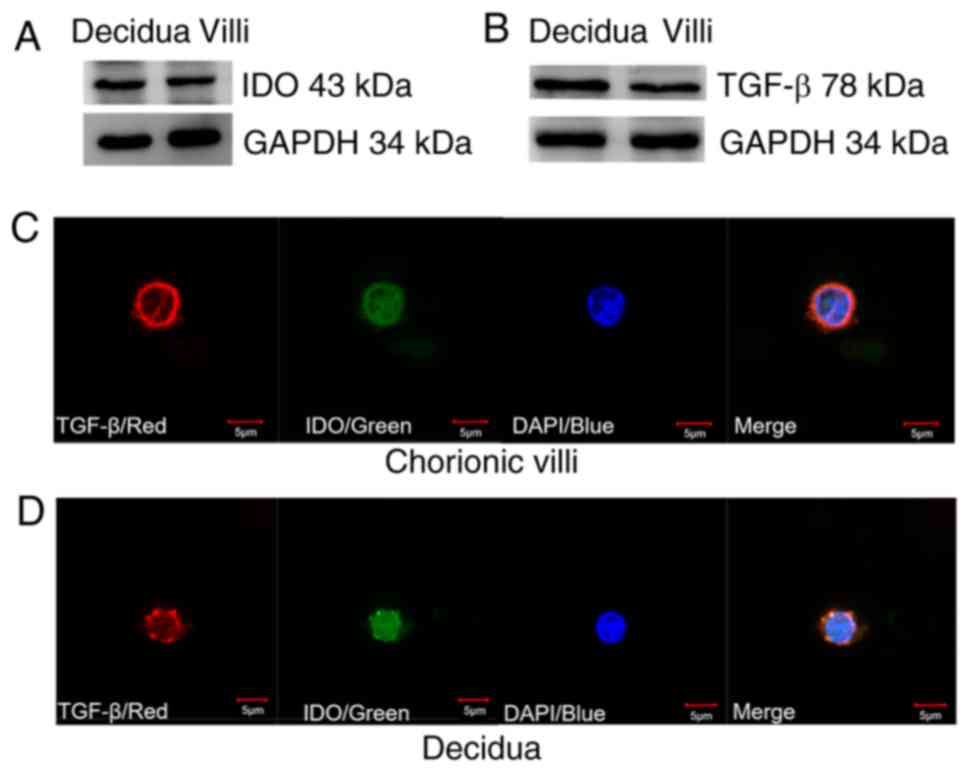
The western blot analysis demonstrated that IDO
(Fig. 1A) and TGF-β (Fig. 1B) were expressed in both villi and
decidua tissue samples. IF staining indicated that TGF-β was
predominantly expressed in the cytoplasm of villi and decidua cells
as indicated by red fluorescence, while IDO was identified in both
the cytoplasm and nucleus of villi and decidua cells as indicated
by green fluorescence (Fig. 1C and
D). Furthermore, villi and decidua cells exhibited a similar
cell size and IDO and TGF-β expression levels.
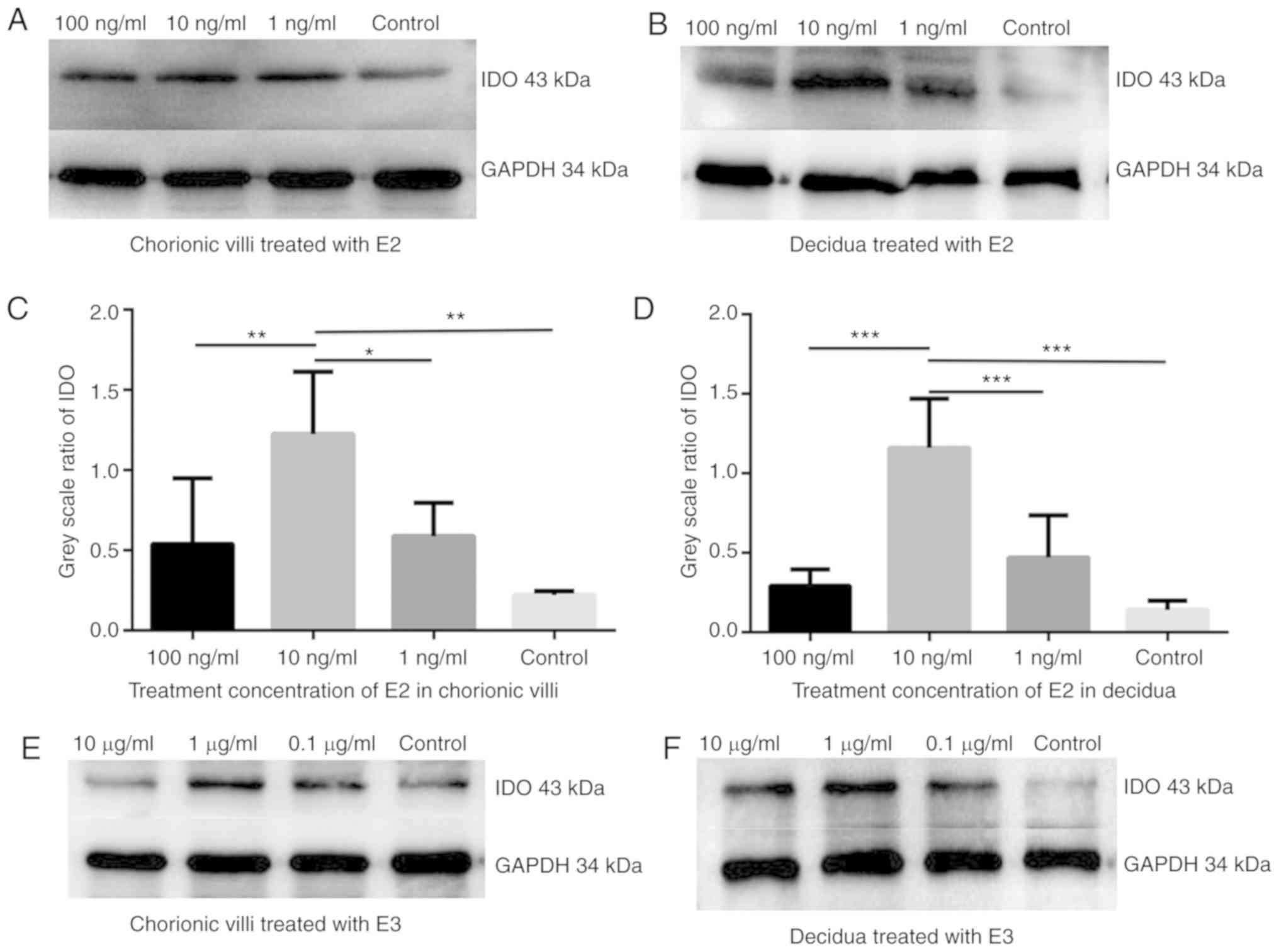
E2 and E3 induce IDO expression in
chorionic villi and decidua
In order to confirm whether estrogen affected IDO
expression, the chorionic villi and decidua sections were cultured
in the medium containing 100, 10 or 1 ng/ml E2 or 10, 1 and 0.1
µg/ml E3 for 48 h. Western blot analysis demonstrated that
IDO expression increased in chorionic villi (Fig. 2A, C, E and G) and decidua
(Fig. 2B, D, F and H) cultured in
the medium containing E2 (Fig.
2A-D) or E3 (Fig. 2E-H)
compared with the control group. The greatest increase in IDO
expression was detected in sections of chorionic villi and decidua
cultured in the medium containing 10 ng/ml of E2 or in villi and
decidua cultured in 1 µg/ml of E3.
E2 and E3 induce TGF-β expression in
chorionic villi and decidua
The effect of estrogen on TGF-β expression was
determined in the chorionic villi and decidua sections. Western
blot analysis illustrated that TGF-β expression increased in
chorionic villi and decidua tissues cultured in the medium
containing 10 ng/ml E2 (Fig. 2I)
or 1 µg/ml E3 (Fig. 2J)
compared with control group.
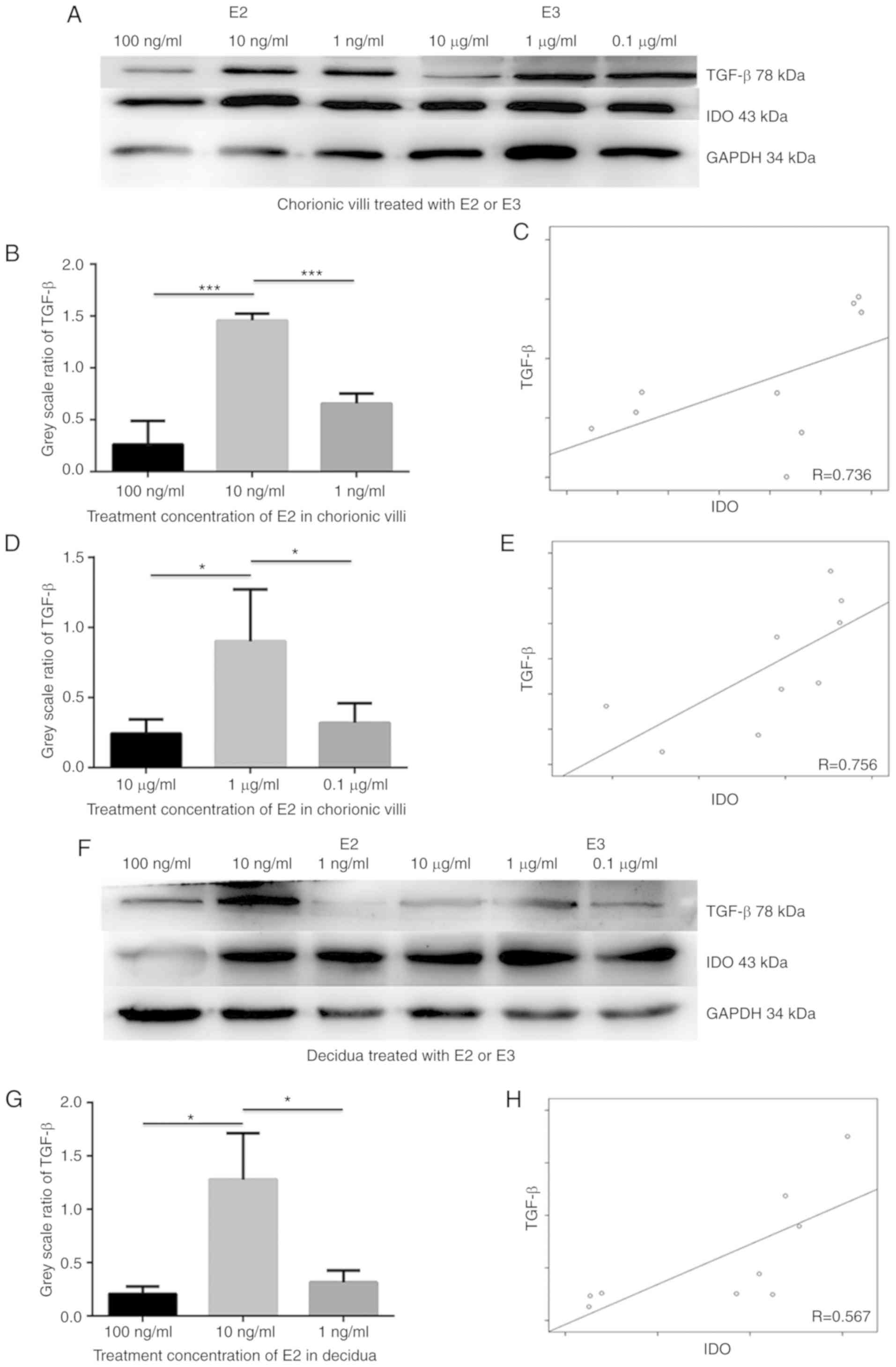
E2 and E3 induce IDO expression via TGF-β
in chorionic villi and decidua
To elucidate the underlying molecular mechanism by
which estrogen induces IDO expression, chorionic villi and decidua
cells were incubated in medium containing 100, 10 and 1 ng/ml E2 or
10, 1 and 0.1 µg/ml E3 for 48 h. Western blot analysis
indicated that both IDO and TGF-β expression levels increased in 10
ng/ml of E2 and 1 µg/ml of E3 groups compared with the other
treatment concentrations, and that TGF-β expression was positively
associated with IDO levels in sections of chorionic villi (Fig. 3C and E; R=0.736 and 0.756) and
decidua (Fig. 3H and J; R=0.567
and 0.714) cultured in the medium containing E2 (Fig. B-C) or E3
(Fig. D-E). The greatest increase in IDO and TGF-β expression
levels was observed in sections of chorionic villi (Fig. 3A, B and D) and decidua (Fig. 3F, G and I) cultured in the medium
containing 10 ng/ml E2 (Fig. 3A, B, F
and G) or 1 µg/ml E3 (Fig.
3A, D, F and I). The results demonstrated that the TGF-β
expression was positively associated with IDO expression in
cultured chorionic villi and decidua in the presence of estrogen,
suggesting that estrogen may simultaneously upregulate TGF-β and
IDO expression.
In order to determine whether TGF-β induced the
expression of IDO, sections of chorionic villi and decidua were
incubated in the medium containing 0.01, 0.5 and 0.1 ng/ml TGF-β
for 48 h. Western blot analysis demonstrated that IDO expression
increased in a dose-dependent manner in both chorionic villi
(Fig. 3K and M) and decidua
(Fig. 3L and N) cultured in the
medium containing TGF-β. Taken together, these results suggest that
TGF-β may upregulate the expression of IDO and that estrogen may
induce IDO expression via TGF-β.
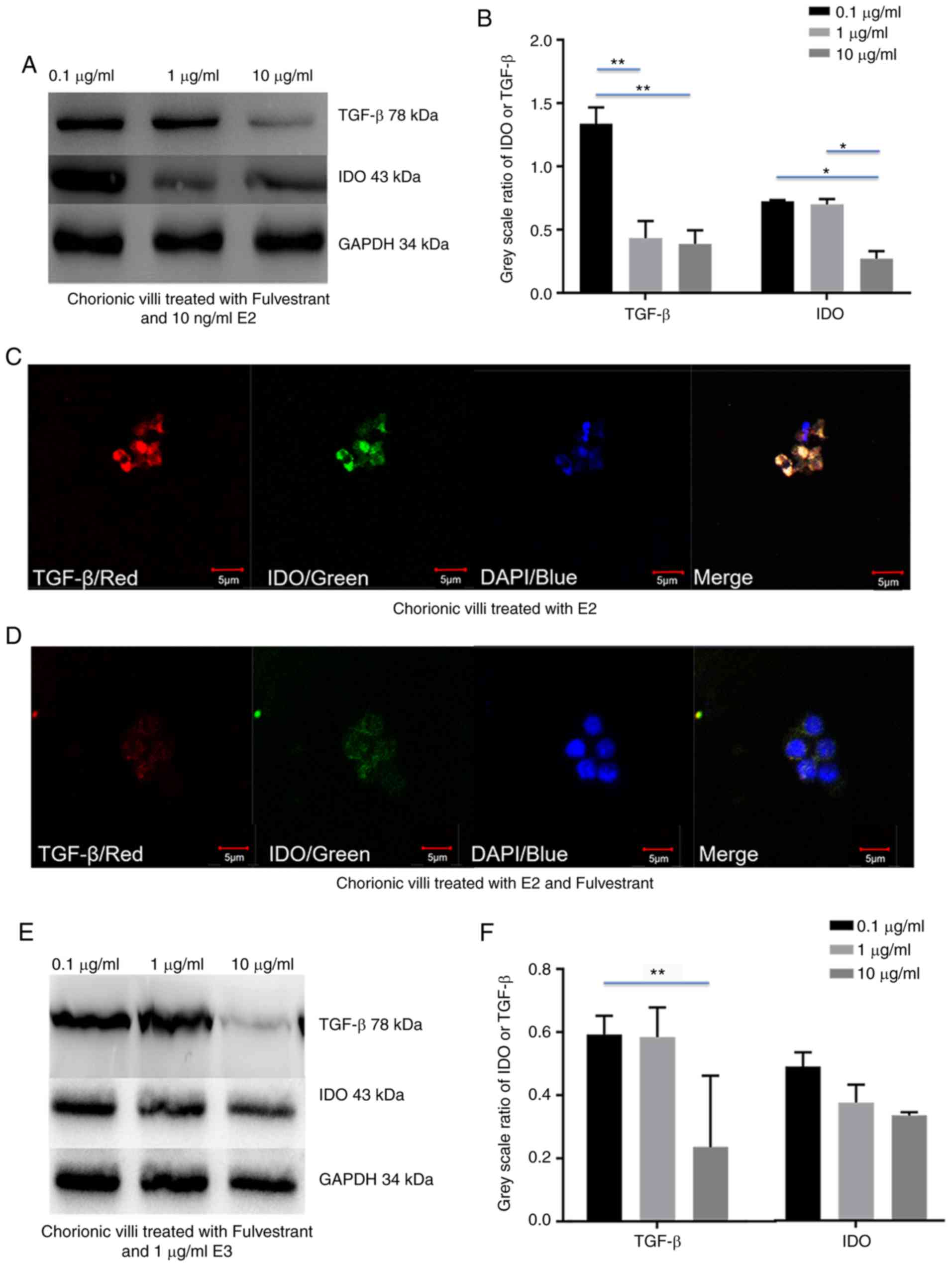
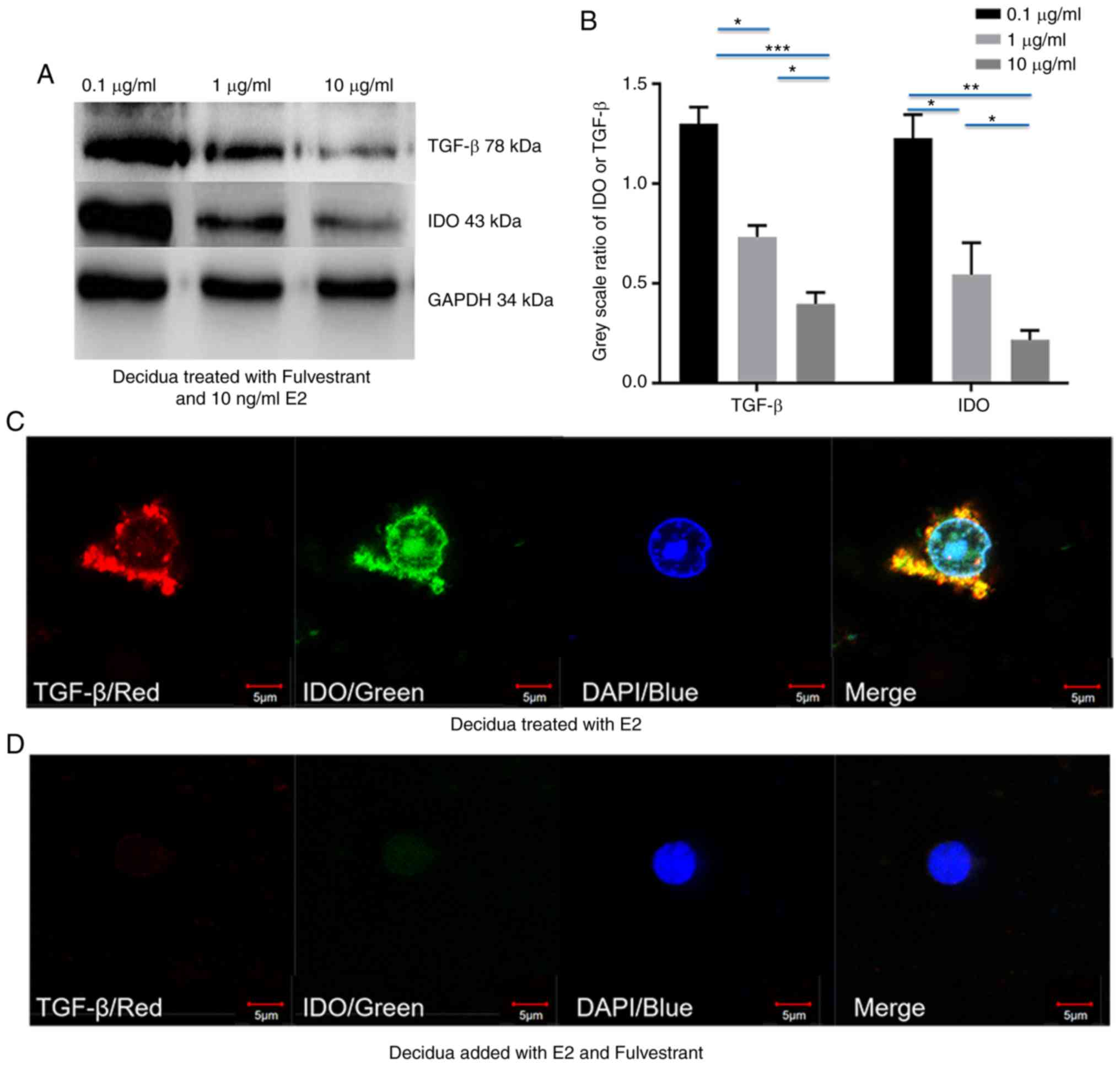
Fulvestrant decreases estrogen-depended
upregulation of IDO by inhibiting TGF-β expression in chorionic
villi and decidua
To validate whether estrogen induced IDO expression
via TGF-β in chorionic villi and decidua, chorionic villi or
decidua cells were incubated in medium containing 10 ng/ml E2 or 1
µg/ml E3, prior to addition of 0.1, 1 and 10 µg/ml
fulvestrant for 12 h. Western blot analysis demonstrated that both
IDO and TGF-β expression decreased with the increased fulvestrant
dosage in chorionic villi (Fig.
4A-H) and decidua (Fig.
5A-H), cultured in the medium containing 10 ng/ml E2 and 0.1, 1
or 10 µg/ml fulvestrant (Figs.
4A and B, and 5A and B), 1
µg/ml E3 added with 0.1, 1 or 10 µg/ml fulvestrant
(Figs. 4E and F, and 5E and F), respectively. For IF staining,
chorionic villi or decidua were treated with 10 ng/ml E2 (Figs. 4C and 5C) or 10 ng/ml E2 and 1 µg/ml
fulvestrant (Figs. 4D and
5D), 1 µg/ml E3 (Figs. 4G and 5G) or 1 µg/ml E3 and 1
µg/ml fulvestrant (Figs.
4H and 5H), respectively. The
greatest decrease in IDO and TGF-β expression was observed in
sections of chorionic villi (Fig. 4A
and E) and decidua (Fig. 5A and
E) cultured in the medium containing 10 ng/ml of E2 and 10
µg/ml fulvestrant (Figs.
4A and 5A), or in medium
containing 1 µg/ml of E3 and 10 µg/ml fulvestran
(Figs. 4E and 5E). Overall, the results demonstrated
that fulvestrant had the ability to decreased estrogen-depended
upregulation of IDO, partly by inhibiting TGF-β expression.
Discussion
IDO is considered the main protein that protects
embryos from the maternal immune system. During pregnancy, IDO is
secreted by placental syncytiotrophoblasts, cytotrophoblasts,
decidual cells and maternal monocyte-macrophages, which inhibit the
T-lymphocyte reaction and mediate the immune tolerance to the fetus
via tryptophan depletion and defective tryptophan catabolism
(19). Previous studies have
predominantly focused on investigating IDO expression and function
(20). It has been reported that
interferon-γ (IFN-γ) secreted by infiltrating leukocytes may
increase IDO expression in endometrial stromal cells (21); however, little is known about the
regulation of IDO expression at the maternal-fetal interface.
Estrogen affects the development of reproductive
capabilities, and high E2 and E3 secretion levels have been
observed during the gestation period. Estrogen-mediated IDO
expression in monocyte-derived dendritic cells has been reported to
limit T-cell proliferation and Th1/Th2 cytokine production in
patients with multiple sclerosis (22). During pregnancy, the mean cord
serum concentration of E2 is ~7.5 ng/ml (range, 4-13 ng/ml) and the
mean cord serum concentration of E3 is 0.3 µg/ml (range,
0.2-0.5 µg/ml) (23). It
has been reported that during pregnancy, estradiol may enhance the
production of interleukin (IL)-10 and maintain Th2 cytokine
expression (24). The results of
the present study indicated that E2 and E3 may induce IDO
expression, and demonstrated that IDO expression increased most
notably when E2 and E3 were administered at concentrations similar
to those normally observed in pregnant women. However, further
studies are required to fully elucidate the molecular mechanism by
which estrogen induces IDO expression at the maternal-fetal
interface. The IDO expression induced by IFN-γ may explain the
immunoregulatory effects of this enzyme in acute inflammation;
however, animal experiments indicated that long-term IDO expression
in noninflammatory contexts is driven by TGF-β, as demonstrated in
mouse pDCs treated with TGF-β (17).
Our previous study demonstrated that TGF-β
expression was positively associated with IDO expression in
chorionic villi and decidua tissues of healthy pregnant women
(11). In the present study, IDO
expression increased in chorionic villi and decidua cultured in the
medium containing TGF-β. Thus, the current results support the
hypothesis that TGF-β may upregulate the expression of IDO in
chorionic villi and decidua.
TGF-β is closely associated with tissue remodeling
events and reproductive processes, and is known to be abundantly
expressed in the endometrium, decidua and chorionic villi. This
protein is a vital regulator of endometrial and placental
development and functions. TGF-β affects several modulatory effects
on endometrium, such as preparation events for implantation,
interactions with preimplantation embryos, and promoting pre- and
post-implantation embryo development. TGF-β is also known to have
several modulatory effects on trophoblast cells, such as inhibition
of proliferation and invasiveness, and stimulation of
differentiation by inducing multinucleated cell formation (25,26)
It has been reported that estrogen can upregulate
TGF-β expression. A previous study demonstrated that estrogen
effected TGF-β1 expression in mouse endometrium by stimulating its
expression in the stroma cells and inhibiting its expression in
glandular epithelium (27).
Another study reported that E2 expression significantly increased
in response to TGF-β1 secretion in cultured dermal fibroblasts
following wound healing (27).
Certain studies reported that estrogen reduced TGF-β expression in
endometrial carcinoma; however, these studies were based on a
different abnormal cell growth mechanism of endometrial cancer
cells and hormone release (28,29). The results of the present study
demonstrated that TGF-β may increase the expression of IDO in a
dose-dependent manner, and that TGF-β was highly expressed in
chorionic villi and decidua tissues of normal pregnant women.
During pregnancy, ovaries induce placenta
cell-mediated production of high concentrations of estrogen and
progesterone. Abnormally low levels of estrogen and progesterone
can lead to a miscarriage, while abnormally high levels may disturb
the function of ovaries and cause cancer or early menopause. The
mean cord serum concentration of E2 is ~7.5 ng/ml (range, 4-13
ng/ml) and the mean cord serum concentration of E3 is 0.3
µg/ml (range, 0.2-0.5 µg/ml) during pregnancy
(30). For our study, the 10
ng/ml E2 and 1 µg/ml E3 is more suitable for increased TGF-β
and IDO expression, one of the causes is by simulation of pregnancy
women uterus micro-environment in vitro, for another
possibility is about the toxicological effect of estrogen and
progesterone added in cultured tissues. Since high concentrations
of estrogen and high TGF-β expression levels were simultaneously
observed in chorionic villi and decidua tissues, it unlikely that
estrogen downregulates TGF-β expression. Fulvestrant functions as
an antagonist of estrogen and is used to treat postmenopausal
patients with hormone receptor-positive advanced breast cancer
(31). The results of the current
study showed that estrogen could upregulate TGF-β expression in
chorionic villi and decidua tissues cultured in medium containing
E2 or E3. Furthermore, this effect was reversed by fulvestrant, an
inhibitor of estrogen receptor.
The results of the present study demonstrated that
IDO was expressed in chorionic villi and decidua tissues, and that
TGF-β may upregulate IDO expression in these tissues. Furthermore,
the results indicated that estrogen during pregnancy may induce
maternal-fetal tolerance by upregulation of IDO via TGF-β, E2 and
E3. The current study had certain limitations. A wider range of E2
and E3 does will be necessary to confirm the colclusions.
Furthermore, Pearson's correlation analysis of the association
between the maternal E1, E2 and E3 levels in chorionic villi and
decidua tissues and peripheral blood TGF-β/IDO expression levels
was not performed. A future study will explore the downstream and
upstream signaling pathways of TGF-β, and other molecules that may
be involved in estrogen signaling pathways, such as Smad3 and
Stat3. The protein expression levels of TGF-β and IDO receptors,
and the receptors of estrogen and progesterone should also be
determined in future studies.
In conclusion, the current results indicated that
there may be an association between estrogen and TGF-β and IDO
expression levels in chorionic villi and decidua tissues.
Inhibition of estrogen signaling decreased TGF-β and IDO expression
levels, suggesting that estrogen may upregulate IDO expression via
TGF-β. The current findings may be used in future clinical research
to support the understanding of the molecular mechanism of
maternal-fetal immunological tolerance. The current results may
also be used for the development of effective treatment for
autoimmune diseases, including transplant rejection and immune
infertility (32,33).
Abbreviations:
|
E1
|
estrone
|
|
E2
|
17β-estradiol
|
|
E3
|
estriol
|
|
IDO
|
indoleamine 2,3-dioxygenase
|
|
IFN-γ
|
interferon-γ
|
|
pDC
|
plasmacytoid dendritic cell
|
|
Th
|
T helper
|
|
TGF-β
|
transforming growth factor-β
|
|
Tregs
|
regulatory T cells
|
Acknowledgments
The authors thank Dr Fang Yu (Clinical Laboratory
Center, Affiliated Hospital of Guizhou Medical University, Guizhou,
China) for helpful suggestions regarding the experiments.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81360452).
Availability of data and materials
The data sets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
JW, GH, SZ, ZC, ZX, LW and FS conceived and designed
the study. JW and PL analyzed the data. JW, ZC and MY performed the
experiments. YW, LY, YT, HZ and JW recruited patients, collected
the chorionic villi and decidua samples, and analyzed patient
information. JW and GH wrote the manuscript. SZ was responsible for
the acquisition of funding. All authors agreed with manuscript
results and conclusions and approved the final manuscript.
Ethics approval and consent to
participate
The current study was conducted according to the
principles expressed in the Declaration of Helsinki. The study was
approved by the Ethics Committee of the Affiliated Hospital of
Guizhou Medical University (Guizhou, China). All patients who have
undergone voluntary termination of pregnancy provided written
informed consent for the collection of samples and subsequent
analysis.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Trowsdale J and Betz AG: Mother's little
helpers: Mechanisms of maternal-fetal tolerance. Nat Immunol.
7:241–246. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tafuri A, Alferink J, Möller P, Hämmerling
GJ and Arnold B: T cell awareness of paternal alloantigens during
pregnancy. Science. 270:630–633. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hogarth PJ: Immunological aspects of
foeto-maternal relations in lower vertebrates. J Reprod Fertil
Suppl. 3(Suppl 3): S15–S27. 1968.
|
|
4
|
Kaufmann P, Huppertz B and Frank HG: The
fibrinoids of the human placenta: Origin, composition and
functional relevance. Ann Anat. 178:485–501. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiong YH, Yuan Z and He L: Effects of
estrogen on CD4(+) CD25(+) regulatory T cell in peripheral blood
during pregnancy. Asian Pac J Trop Med. 6:748–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Terness P, Bauer TM, Röse L, Dufter C,
Watzlik A, Simon H and Opelz G: Inhibition of allogeneic T cell
proliferation by indoleamine 2,3-dioxygenase-expressing dendritic
cells: Mediation of suppression by tryptophan metabolites. J Exp
Med. 196:447–457. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mellor AL, Baban B, Chandler P, Marshall
B, Jhaver K, Hansen A, Koni PA, Iwashima M and Munn DH: Cutting
edge: Induced indoleamine 2,3 dioxygenase expression in dendritic
cell subsets suppresses T cell clonal expansion. J Immunol.
171:1652–1655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kudo Y, Boyd CA, Spyropoulou I, Redman CW,
Takikawa O, Katsuki T, Hara T, Ohama K and Sargent IL: Indoleamine
2,3-dioxygenase: Distribution and function in the developing human
placenta. J Reprod Immunol. 61:87–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Curti A, Trabanelli S, Salvestrini V,
Baccarani M and Lemoli RM: The role of indoleamine 2,3-dioxygenase
in the induction of immune tolerance: Focus on hematology. Blood.
113:2394–2401. 2009. View Article : Google Scholar
|
|
10
|
Ligam P, Manuelpillai U, Wallace EM and
Walker D: Localisation of indoleamine 2,3-dioxygenase and
kynurenine hydroxylase in the human placenta and decidua:
Implications for role of the kynurenine pathway in pregnancy.
Placenta. 26:498–504. 2006. View Article : Google Scholar
|
|
11
|
Liu W, Huang Y, Huang G, Zhou C, Zeng X,
Zhao S, Wu L, Zhou H, Wu Q and Dai L: Relationship of SOCS3 and
TGF-β with IDO expression in early pregnancy chorionic villi and
decidua. Exp Ther Med. 14:4817–4824. 2017.PubMed/NCBI
|
|
12
|
Fallarino F, Grohmann U, Vacca C, Bianchi
R, Orabona C, Spreca A, Fioretti MC and Puccetti P: T cell
apoptosis by trypto-phan catabolism. Cell Death Differ.
9:1069–1077. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frumento G, Rotondo R, Tonetti M, Damonte
G, Benatti U and Ferrara GB: Tryptophan-derived catabolites are
responsible for inhibition of T and natural killer cell
proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med.
196:459–468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hönig A, Rieger L, Kapp M, Sütterlin M,
Dietl J and Kämmerer U: Indoleamine 2,3-dioxygenase (IDO)
expression in invasive extravillous trophoblast supports role of
the enzyme for materno-fetal tolerance. J Reprod Immunol. 61:79–86.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang G, Zeng Y, Liang P, Zhou C, Zhao S,
Huang X, Wu L and He X: Indoleamine 2,3-dioxygenase (IDO)
downregulates the cell surface expression of the CD4 molecule. Int
J Mol Sci. 13:10863–10879. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Biernacka A, Dobazewski M and
Frangogiannis NG: TGF-β signaling in fibrosis. Growth Factor.
29:196–202. 2011. View Article : Google Scholar
|
|
17
|
Pallotta MT, Orabona C, Volpi C, Vacca C,
Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M,
Bicciato S, et al: Indoleamine 2,3-dioxygenase is a signaling
protein in long-term tolerance by dendritic cells. Nat Immunol.
12:870–878. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
ESHRE Guideline Group on RPL; Bender Atik
R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, Nelen
W, Peramo B, Quenby S, et al: ESHRE guideline: Recurrent pregnancy
loss. Hum Reprod Open. 2018:hoy0042018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kudo Y: The role of placental indoleamine
2,3-dioxygenase in human pregnancy. Obstet Gynecol Sci. 56:209–216.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mei J, Li MQ, Ding D, Li DJ, Jin LP, Hu WG
and Zhu XY: Indoleamine 2,3-dioxygenase-1 (IDO1) enhances survival
and invasiveness of endometrial stromal cells via the activation of
JNK signaling pathway. Int J Clin Exp Pathol. 6:431–444.
2013.PubMed/NCBI
|
|
21
|
Honig A, Rieger L, Dietl J and Kämmerer U:
Mechanisms regulating the expression of indoleamine 2,3-dioxygenase
during decidualization of human endometrium. Hum Reprod.
19:2683–2684. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu WH, Lu CZ, Huang YM, Link H and Xiao
BG: A putative mechanism on remission of multiple sclerosis during
pregnancy: Estrogen-induced indoleamine 2,3-dioxygenase by
dendritic cells. Mult Scler. 13:33–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Troisi R, Potischman N, Johnson CN,
Roberts JM, Lykins D, Harger G, Markovic N, Siiteri P and Hoover
RN: Estrogen and androgen concentrations are not lower in the
umbilical cord serum of pre-eclamptic pregnancies. Cancer Epidemiol
Biomarkers Prev. 12(11 Pt 1): 1268–1270. 2003.PubMed/NCBI
|
|
24
|
Matalka KZ: The effect of estradiol, but
not progesterone, on the production of cytokines in stimulated
whole blood, is concentration-dependent. Neuro Endocrinol Lett.
24:185–191. 2003.PubMed/NCBI
|
|
25
|
Xuan YH, Choi YL, Shin YK, Ahn GH, Kim KH,
Kim WJ, Lee HC and Kim SH: Expression of TGF-beta signaling
proteins in normal placenta and gestational trophoblastic disease.
Histol Histopathol. 22:227–234. 2007.
|
|
26
|
Jones RL, Stoikos C, Findlay JK and
Salamonsen LA: TGF-beta superfamily expression and actions in the
endometrium and placenta. Reproduction. 132:217–232. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stevenson S, Nelson LD, Sharpe DT and
Thornton MJ: 17beta-estradiol regulates the secretion of TGF-beta
by cultured human dermal fibroblasts. J Biomater Sci Polym Ed.
19:1097–1109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lecanda J, Parekh TV, Gama P, Lin K,
Liarski V, Uretsky S, Mittal K and Gold LI: Transforming growth
factor-beta, estrogen, and progesterone converge on the regulation
of p27Kip1 in the normal and malignant endometrium. Cancer Res.
67:1007–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ito L, Hanyu A, Wayama M, Goto N, Katsuno
Y, Kawasaki S, Nakajima Y, Kajiro M, Komatsu Y, Fujimura A, et al:
Estrogen inhibits transforming growth factor beta signaling by
promoting Smad2/3 degradation. J Biol Chem. 285:14747–14755. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Noyola-Martinez N, Halhali A and Barrera
D: Steroid hormones and pregnancy. Gynecol Endocrinol. 35:376–384.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Xu B, Wang W, Zhai X and Chen X:
Efficacy and safety of fulvestrant in postmenopausal patients with
hormone receptor-positive advanced breast cancer: A systematic
literature review and meta-analysis. Breast Cancer Res Treat.
171:533–544. 2018. View Article : Google Scholar
|
|
32
|
Gao J, Deng F and Jia W: Inhibition of
indoleamine 2,3-dioxy-genase enhances the therapeutic efficacy of
immunogenic chemotherapeutics in breast cancer. J Breast Cancer.
22:196–209. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishi M, Yoshikawa K, Higashijima J,
Tokunaga T, Kashihara H, Takasu C, Ishikawa D, Wada Y and Shimada
M: The impact of indoleamine 2,3-dioxygenase (IDO) expression on
Stage III gastric cancer. Anticancer Res. 38:3387–3392. 2018.
View Article : Google Scholar : PubMed/NCBI
|