Introduction
Impaired wound healing is a major complication of
diabetes mellitus (DM) and despite the associated risks, treatment
strategies for diabetic-related wounds are limited. Endothelial
dysfunction is an indicator of diabetes-induced macrovascular
complications (1,2). Vascular differentiation at injury
sites affects the speed of wound healing, while the homing and
angiogenic differentiation of endothelial progenitor cells (EPCs)
are critical for successful wound healing (3). EPCs are immature endothelial cells
that can proliferate and differentiate to promote new blood vessel
formation at injury sites. EPCs can also secrete various angiogenic
and vasoactive factors to enhance angiogenesis (4-6).
Endothelial dysfunction is closely related to the occurrence and
development of vascular disease and is also an intrinsic cause of
impaired wound healing (7). It
has been suggested that the dysregulation of EPC phenotypes and
functions in patients with DM may be attributed to the aberrant
signaling of cytokines and other molecules. Indeed, it has been
reported that high glucose levels promote EPC dysfunction and
induce EPC apoptosis (8). Through
inflammatory responses and the NADPH oxidase (NOX)-mediated
overproduction of reactive oxygen species (ROS), hyperglycemia (HG)
alone can induce alterations in gene expression and cellular
behaviors in DM (9,10). However, the specific mechanisms of
HG-induced endothelial cell injury are unclear.
CircRNAs are closed circular RNA molecules that
function as competitive endogenous RNAs by regulating transcription
and blocking the miRNA-mediated inhibition of target genes
(11). CircRNAs are implicated in
a number of diseases and their differential expression plays a
crucial role in disease development processes (12). It has been reported that circRNAs
participate in the regulation of insulin secretion and diabetes
pathogenesis (13). The
expression of circRNA hsa_circ0054633 and circRNA Cdr1 in
peripheral blood can be used as a diagnostic biomarker for both
type 2 DM (T2DM) and pre-diabetes (14,15). The expression of circRNA
circANKRD36 has been shown to be upregulated in peripheral blood
leukocytes, and this increased expression is associated with
chronic inflammation in T2DM (16). It has been reported that the
expression of hsa_circ_0058092 is downregulated in patients with DM
(17); however, the role of this
circRNA in HG-induced EPC damage remains unclear. Thus, using in
vitro approaches, the present study aimed to identify the
mechanisms through which the decreased expression of
hsa_circ_0058092 induces EPC damage under HG conditions.
Materials and methods
EPC characterization and isolation
Peripheral blood from healthy volunteers was diluted
twice in phosphate-buffered saline (PBS) and the solution was
gently layered over 4 ml of lymphocyte separation liquid
(Sigma-Aldrich; Merck KGaA). The present study was approved by the
Ethics Committee of the Tenth People's Hospital of Tongji
University after obtaining informed patient consent
(SHDSYY-2019-3322). The tubes were centrifuged at 800 × g for 30
min at 4°C. Peripheral blood mononuclear cells (PBMCs) at the
interface were then transferred to a new tube and washed with PBS
by centrifugation at 400 × g for 5 min at 4°C. EPCs were cultured
from PBMCs in 24-well plates (5×106 cells/well) in
endothelial cell basal medium-2 (Lonza Group, Ltd.). The cells were
cultured continuously for 10 days and then utilized for co-culture
experiments. Subsequently, CD133+ EPCs were selected
using CD133-coupled magnetic microbeads (Miltenyi Biotech)
according to standard processing procedures. Following isolation,
CD133+ EPCs were expanded in endothelial cell basal
medium-2 as previously described (17).
To analyze CD14, CD45, kinase insert domain receptor
(KDR) and CD34 expression, the EPCs were incubated at 4°C with a
biotinylated anti-rat IgG (H1L) horse antibody (1:200) (cat. no.
AI-2001, Vector Laboratories) for 12 h and (1:200) FITC-conjugated
streptavidin (cat. no. 9013-20-1, Caltag Laboratories) for 1 h.
Following treatment, the EPCs were fixed in 1% paraformaldehyde.
Quantitative analyses were performed using FlowJo software (FlowJo,
LLC) and a FACSCalibur flow cytometer.
Reagent generation and cell
treatment
FOXO3 overexpression vector (using pcDNA3.1 vector),
miR-217 mimic/inhibitor and hsa_circ_0058092 overexpression vector
(using pcDNA3.1 vector) were generated by GenePharm Co. Ltd. The
EPCs were maintained at approximately 40% confluence and cells were
transfected with the different vectors (50 ng) using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h before
treatment with high glucose (30 mM). An Empty pcDNA3.1 vector was
used as the control for FOXO3 and hsa_circ_0058092 overexpression.
The negative controls for miR-217 mimic/inhibitor (50 ng) (were
provided by GenePharm Co. Ltd.) were also transfected into EPCs
using Lipofectamine 2000 for 48 h prior to treatment with high
glucose (30 mM).
In vitro tube formation assay. In
vitro
Matrigel tube formation assays were performed to
determine the angiogenic activity of EPCs (18). Briefly, the EPCs (5×104
cells) transfected with or without hsa_circ_0058092 overexpression
vector or siFOXO3 vector were seeded in Matrigel-coated 48-well
plates with or without HG treatment for 12 h. After this period,
tubular EPC structures were examined under a microscope (Axioplan 2
imaging E, Carl Zeiss). The total number of tubes, which served as
a measure of in vitro angiogenesis, were scanned and
quantified from 3 random fields of view in each well at ×100
magnification.
Cell proliferation assay
The cell counting kit-8 (CCK-8; Invitrogen; Thermo
Fisher Scientific, Inc.) was used to assess EPC proliferation
following standard protocols. Briefly, 2×104 EPCs were
seeded in 100 µl of DMEM in 96-well plates. Cell viability
was measured 0, 24, 48 and 72 h after seeding by the addition of 10
µl of CCK-8 solution to the wells using Thermo Scientific
Microplate Reader (Thermo Fisher Scientific, Inc.).
Western blot analysis
Protein was extracted from the EPCs using RIPA lysis
buffer (Sigma-Aldrich; Merck KGaA). Protein concentrations were
quantified using the BCA Protein Assay kit (Vigorous Biotechnology
Beijing Co. Ltd.). Proteins were then resolved by sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE, 12%
concentrated adhesive and 4% separated adhesive) and transferred to
nitrocellulose membranes (EMD Millipore). The membranes were
blocked in non-fat milk (5%) before being incubated with the
primary antibodies NOX1 (1:200, cat. no. sc-130543, Santa Cruz
Biotechnology, Inc.) and NOX4 (1:200, cat. no. sc-518092, Santa
Cruz Biotechnology, Inc.) at 4°C for 12 h and horseradish
peroxidase-coupled secondary antibodies IgG (1:200, cat. no.
sc-516102, Santa Cruz Biotechnology, Inc.) at 4°C for 4 h.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:200, cat. no.
sc-365062, Santa Cruz Biotechnology, Inc.) was used as an internal
control and detected using an ECL detection kit (cat. no. SL100309,
SignaGen).
RNA extraction and RT-qPCR
RNA extractions were performed using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to a
previously described protocol (19). The pTRUEscript First Strand cDNA
Synthesis kit (Aidlab) was used for cDNA synthesis with 2X
SYBR-Green qPCR mix. cDNA was used for RT-qPCR detection and
reactions were conducted on an ABI 7900HT sequence detection system
(Thermo Fisher Scientific, Inc.). Data were processed using the
2−ΔΔCq method (20).
The primers utilized to for PCR were as follows:
hsa_circ_0058092 expression forward, 5′-GAA TAA TCA GAA GAG
CGA GCC-3′ and reverse, 5′-GTC TGG ACC AAT GTT GGT GAA TCG-3′;
miR-217 forward, 5′-CGC AGA TAC TGC ATC AGG AA-3′ and
reverse, 5′-CTG AAG GCA ATG CAT TAG GAA CT-3′; FOXO3
forward, 5′-GCG TGC CCT ACT TCA AGG ATA AG-3′ and reverse, 5′-GAC
CCG CAT GAA TCG ACT ATG-3′; U6 forward, 5′-CTC GCT TCG GCA
GCA CA-3′ and reverse, 5′-AAC GCT TCA CGA ATT TGC GT-3′; and
GAPDH forward, 5′-GCA CCG TCA AGG CTG AGA AC-3′ and reverse,
5′-GGA TCT CGC TCC TGG AAG ATG-3′.
Migration assay
For cell migration analysis, the EPCs were placed
into a Transwell upper chamber (8-µm pore membrane, BD
Biosciences) at a density of 1×105 cells in 200
µl of serum-free medium. Complete EPC medium (500 µl)
was added to the bottom chamber. Following 1 day of culture (at
37°C in a humidified atmosphere with 5% CO2), the EPCs
in the bottom chamber were fixed in 4% paraformaldehyde, stained
with 0.1% crystal violet (cat. no. C8470-5, Beijing Solaibao
Technology Co. Ltd.) at room temperature for 20 min, and counted
using a microscope (Axioplan 2 imaging E, Carl Zeiss).
Bioinformatics analyses
The Circular RNA Interactome websites for circRNA
and miRNA (https://circinteractome.nia.nih.gov/) were used to
predict interactions. The target sites between miR-217 and FOXO3
3′UTR were predicted using the TargetScan web-based tool
(http://www.targetscan.org/vert_71/).
Enzyme-linked immunosorbent assay (ELISA)
for the determination of the levels of soluble inflammatory
cytokines
Cytokine interleukin (IL)-6, tumor necrosis factor
(TNF)-α and IL-1β concentrations were quantified in the
supernatants from EPC cultures using commercially available ELISA
kits (Shanghai Senxiong Technology Industry Co., Ltd.).
Supernatants were stored at -80°C prior to analysis following
standard procedures. Standards and samples were assayed in
triplicate. The OD450 nm was calculated by subtracting
background readings and plotting standard curves using a Thermo
Scientific Microplate Reader (Thermo Fisher Scientific, Inc.).
Flow cytometry
Flow cytometry was used to define the EPC apoptotic
rates. The integrative application of propidium iodide (PI) and
Annexin V (AV)-FITC (cat. no. 40302ES20, Yeasen) was used to
differentiate the viable cells from apoptotic or necrotic cells.
The cells were washed twice and adjusted to a concentration of
1×106 cells/ml in cold D-Hank's buffer. PI (10
µl) and AV-FITC (10 µl) were added to 100 µl
of cell suspension and incubated for 15 min at room temperature in
the dark. Finally, 400 µl of the binding buffer were added
to each sample without washing, and then analyzed by flow cytometry
(D2060R, ACEA NovoCyte, Agilent Technologies, Inc.). The
experiments were performed at least 3 times.
Dual luciferase reporter assay
Reporter plasmids were generated by inserting the
FOXO3 3′UTR sequence or circRNA into the pGL3 plasmid (Promega
Corp.). Reporter plasmids and miR-217 mimics were co-transfected
into 293T cells (from the Cell Bank of the Chinese Academy of
Sciences, Shanghai) using Lipofectamine 2000. Following culture at
37°C in a humidified atmosphere with 5% CO2 for 2 days,
Firefly and Renilla luciferase activities were detected
using the Dual Luciferase Reporter Assay System (Promega Corp.)
following standard procedures.
Statistical analysis
Data are expressed as the means ± standard deviation
(SD). GraphPad Prism software, version 5.0 (GraphPad, Inc.) was
used to compare differences between groups. The differences between
groups were assessed using one-way variance analysis with Tukey's
post hoc test (compared with all pairs of columns). P-values ≤0.05
was considered to indicate a statistically significant
difference.
Results
Overexpression of hsa_circ_0058092
reverses HG-induced EPC damage
Human peripheral blood-derived EPCs were isolated to
characterize the role of hsa_circ_0058092 in HG-induced vascular
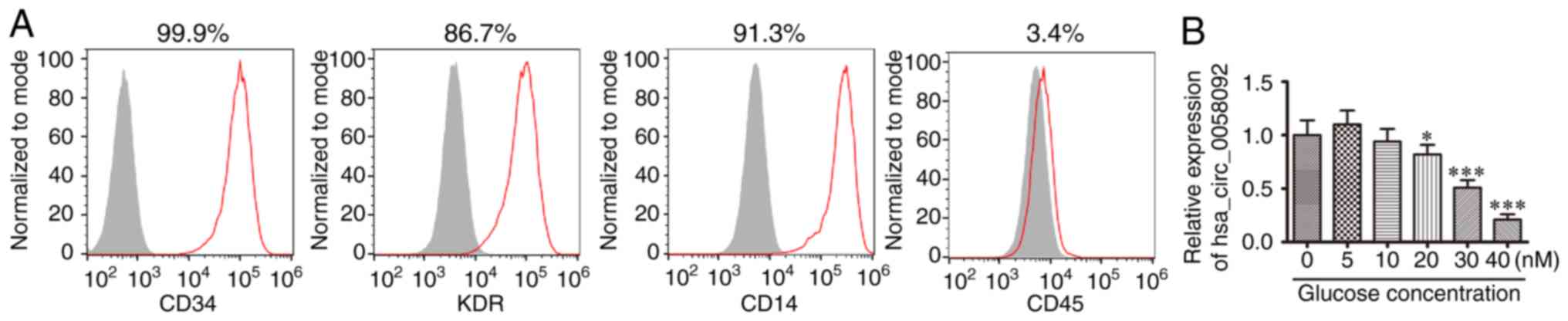
endothelial cell injury. These EPCs were positive for expression of
CD14, KDR and CD34, but not for the expression of the leukocyte
marker, CD45 (Fig. 1A). These
observations suggested that the isolated cells were indeed EPCs, as
previously demonstrated (21).
The EPCs were then incubated in 0-40 mM glucose for 24 h. RT-qPCR
quantification verified that hsa_circ_0058092 expression decreased
with the increasing glucose concentrations (Fig. 1B) and 30 mM glucose was selected
as the concentration for HG used in subsequent experiments.
To ascertain whether hsa_circ_0058092 plays a
protective role in EPCs under HG conditions, a hsa_circ_0058092
overexpression plasmid was constructed and transfected into EPCs.
The expression of hsa_circ_0058092 increased significantly at 48 h
following transfection with the overexpression plasmid (Fig. 2A). The results of CCK-8 assay
revealed that HG conditions suppressed the proliferation of the
EPCs, while the overexpression of hsa_circ_0058092 partially
restored the proliferative capacity of the EPCs under HG conditions
(Fig. 2B). Western blot analysis
revealed that HG conditions increased the expression of the
oxidative stress-related proteins, NOX1 and NOX4; however, the
overexpression of hsa_circ_0058092 suppressed the expression of
both these proteins (Fig. 2C).
This observation suggested that hsa_circ_0058092 regulated
HG-induced oxidative stress. The EPC apoptotic rates were
determined using Annexin V/PI staining at 1 day following exposure
to HG. The data indicated that the overexpression of
hsa_circ_0058092 suppressed the HG-induced apoptosis of EPCs
(Fig. 2D and E).
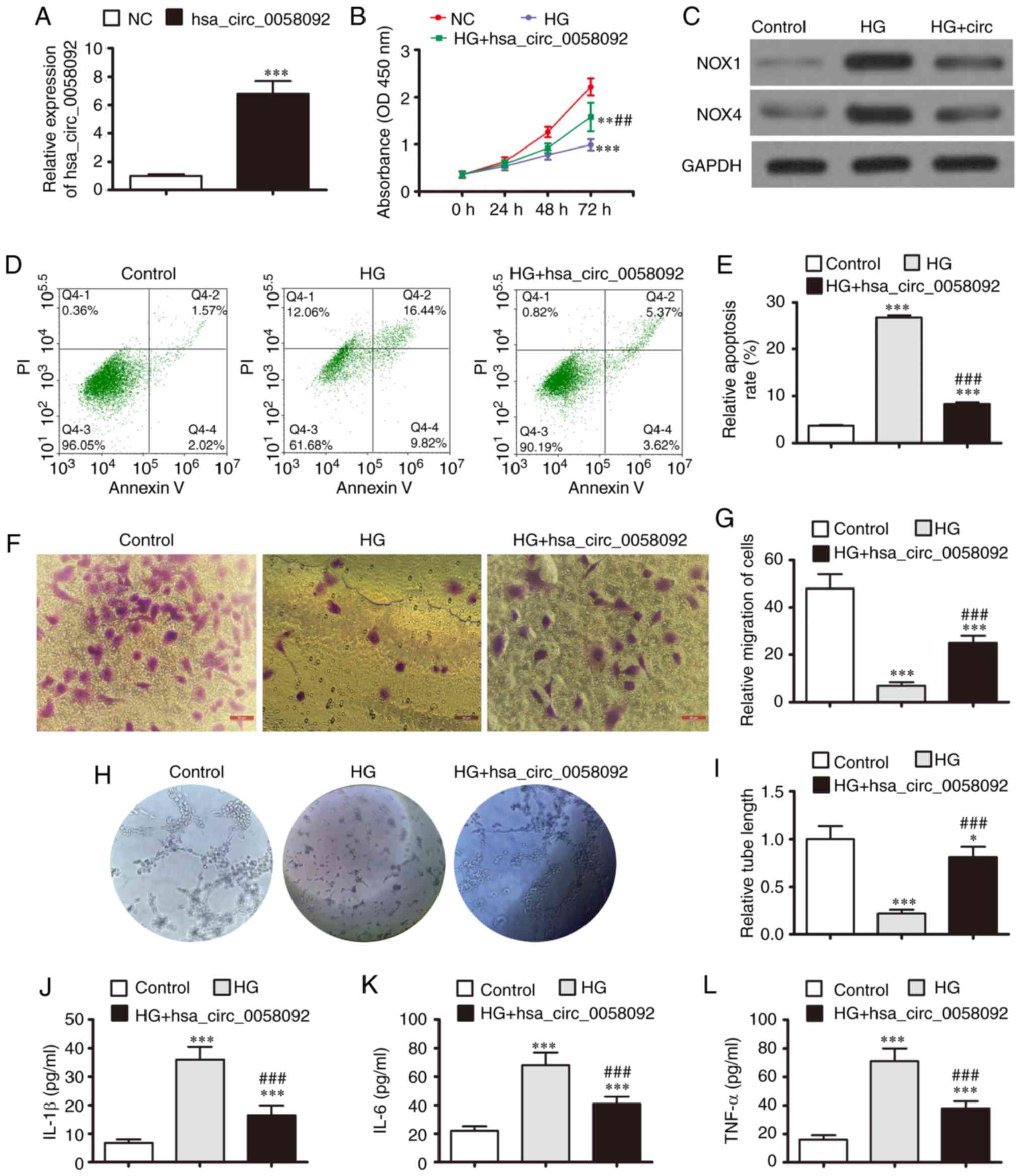 | Figure 2Overexpression of hsa_circ_0058092
reverses EPC damage induced by 30 mM glucose (HG). (A) RT-qPCR
detection for the expression of hsa_circ_0058092 in EPCs following
transfection with hsa_circ_0058092 overexpression vector. Data are
presented as the means ± SD. ***P<0.001 vs. normal
control (NC). (B) CCK-8 assay for the proliferation of EPCs. Data
are presented as the means ± SD. **P<0.01,
***P<0.001 vs. NC; ##P<0.01 vs. HG. (C)
Expression of the oxidative stress proteins, NOX1 and NOX4,
measured by western blot analysis. GAPDH served as an internal
control. (D and E) Apoptosis of EPCs determined by Annexin V/PI
staining 24 h following HG induction. Data are presented as the
means ± SD. ***P<0.001 vs. NC;
###P<0.001 vs. HG. (F and G) Transwell assays for the
migration of EPCs. Data are presented as the means ± SD.
***P<0.001 vs. NC; ###P<0.001 vs. HG.
Scale bar, 95 µm. (H and I) EPC tube formation capabilities
were measured (magnification, 200). Data are presented as the means
± SD. *P<0.05, ***P<0.001 vs. NC;
###P<0.001 vs. HG. (J-L) Levels of the inflammatory
cytokines, IL-1β, IL-6 and TNF-α, were measured by ELISA. Data are
presented as the means ± SD. ***P<0.001 vs. NC;
###P<0.001 vs. HG. EPCs, endothelial progenitor
cells; HG, high glucose. |
Transwell migration experiments revealed that EPC
migration was inhibited under HG conditions; however, migration was
restored by the overexpression of hsa_circ_0058092 (Fig. 2F and G). The capacity of EPCs to
form tubes was also analyzed. Tube formation was decreased under HG
conditions and was restored by the overexpression of
hsa_circ_0058092 (Fig. 2H and I).
The levels of the inflammatory cytokines, IL-6, TNF-α and IL-1β,
were then measured by ELISA. The results revealed that the
HG-induced expression of inflammatory cytokines was suppressed by
the overexpression of hsa_circ_0058092 (Fig. 2J-L). In summary, these results
demonstrated that the overexpression of hsa_circ_0058092 promoted
EPC survival, proliferation, migration and tube formation under HG
conditions. However, the specific regulatory mechanisms underlying
these effects need to be determined.
miR-217 is a target of
hsa_circ_0058092
Increasing evidence has indicated that circRNAs
regulate gene expression by targeting miRNAs (22). In the present study,
bioinformatics analyses were performed to predict direct
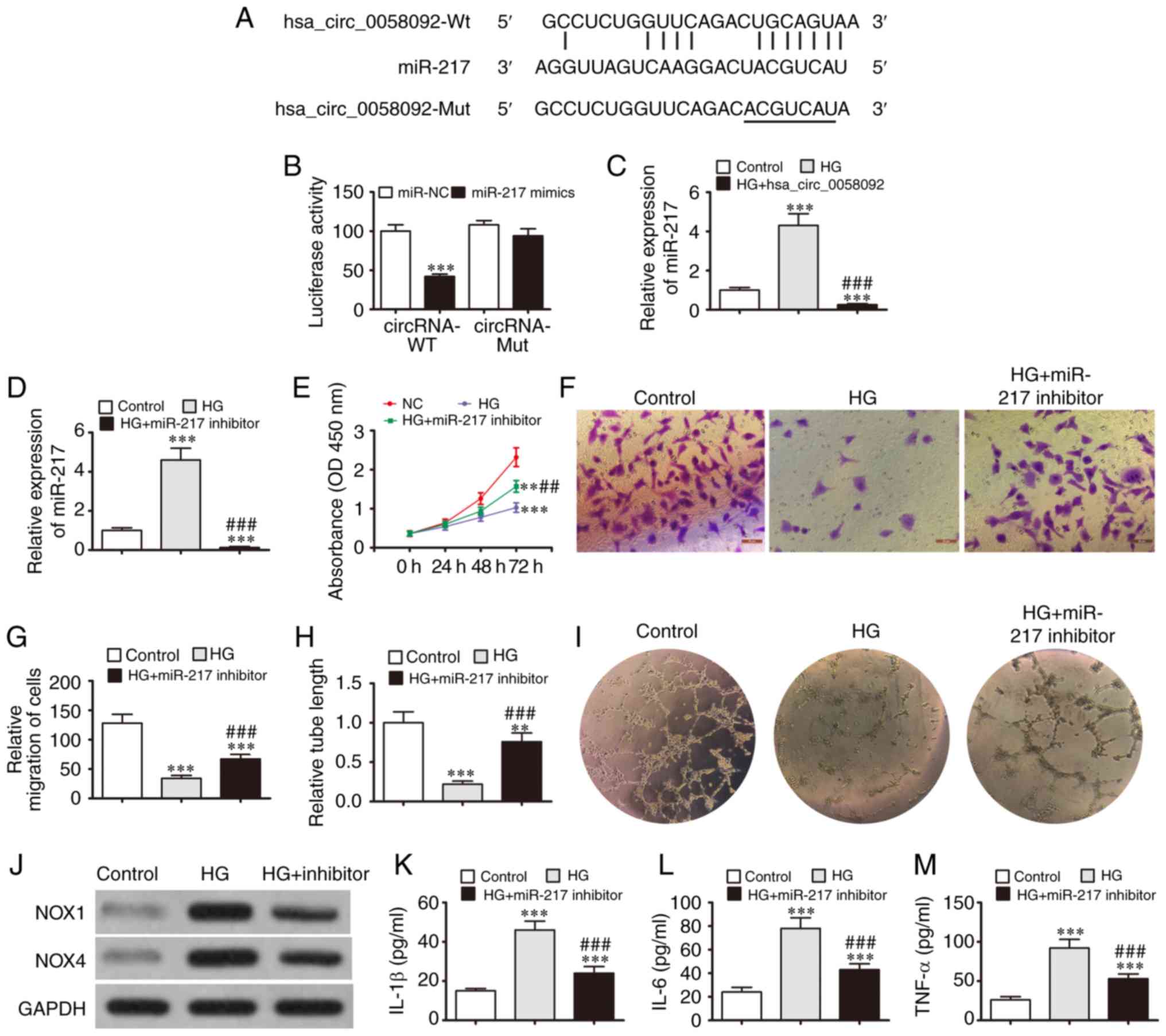
interactions between miR-217 and hsa_circ_0058092 (Fig. 3A). A luciferase reporter assay
revealed that miR-217 inhibited luciferase activity in the cells
transfected with the wild-type hsa_circ_0058092 luciferase reporter
vector. Luciferase activity was not affected in the cells
transfected with a mutated hsa_circ_0058092 luciferase reporter
vector. This suggests that miR-217 is a target of hsa_circ_0058092
(Fig. 3B). RT-qPCR analysis
revealed that HG conditions increased the expression of miR-217,
while the overexpression of hsa_circ_0058092 suppressed the
HG-induced expression of miR-217 (Fig. 3C). This further confirmed that
miR-217 is a target of hsa_circ_0058092.
To determine whether miR-217 exerts a regulatory
effect on EPCs under HG conditions, the EPCs were pre-treated with
an miR-217 inhibitor. The results revealed that the HG-induced
expression of miR-217 was suppressed in the cells that were
pre-treated with the miR-217 inhibitor (Fig. 3D). The results of CCK-8 assay also
revealed that HG suppressed EPC proliferation, while miR-217
inhibition partly restored the EPC proliferative capacity under HG
conditions (Fig. 3E). Transwell
migration experiments also demonstrated that the HG-mediated
inhibition of EPC migration was restored by the downregulation of
miR-217 (Fig. 3F and G). The EPC
tube formation capacity was also decreased under HG conditions and
was recovered by the downregulation of miR-217 (Fig. 3H and I). Western blot analysis
also revealed that HG increased the expression of NOX4 and NOX1,
while miR-217 inhibition prevented the increase in NOX1 and NOX4
protein expression (Fig. 3J). In
addition, the HG-induced expression of TNF-α, IL-1β and IL-6 was
suppressed by the downregulation of miR-217 (Fig. 3K-M). Taken together, these results
demonstrate that miR-217 facilitates the damaging effects of HG on
EPC proliferation, migration, tube formation and survival.
FOXO3 is a target of miR-217
Bioinformatics analysis also predicted that miR-217
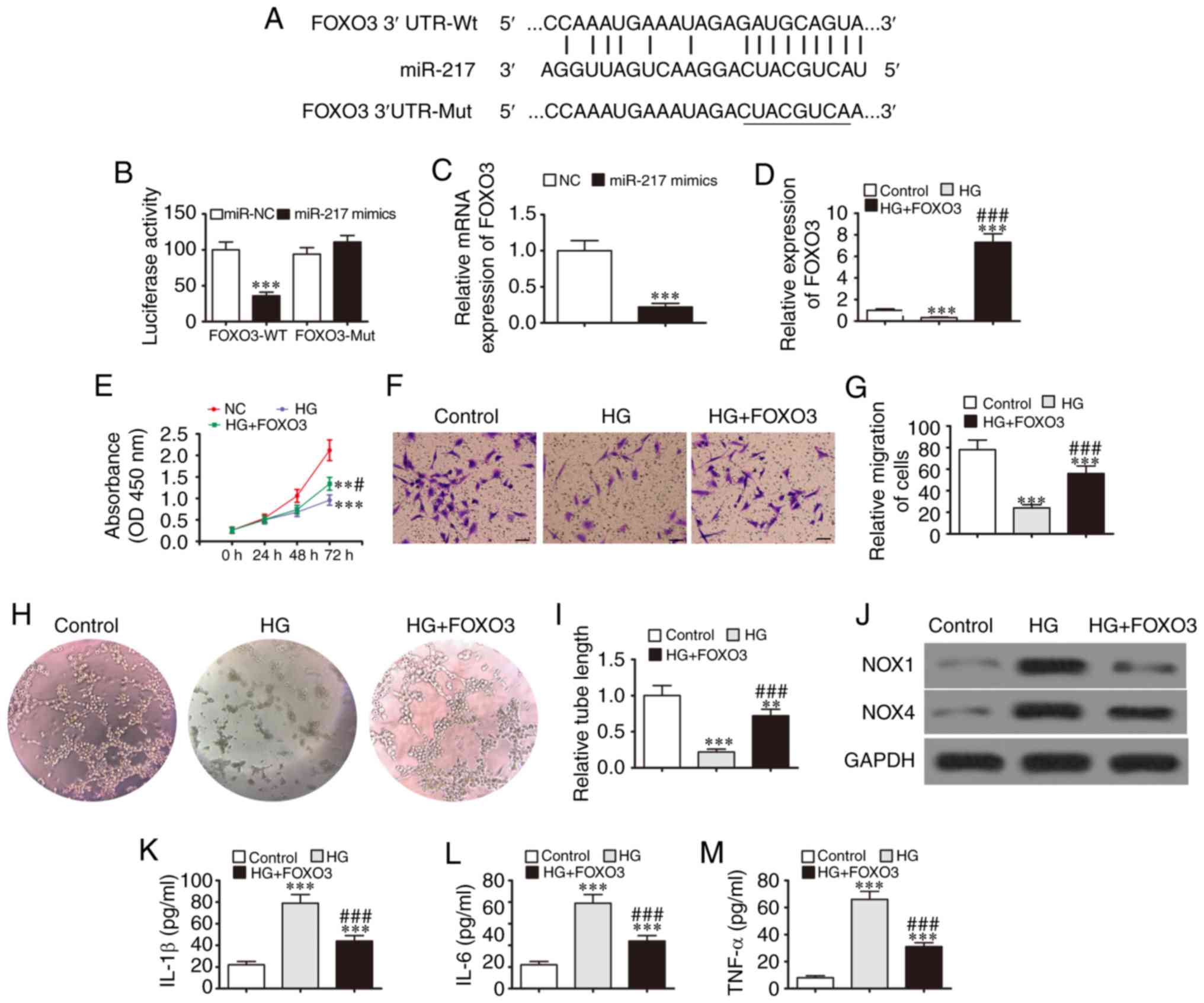
directly interacts with the FOXO3 3′UTR (Fig. 4A). A luciferase reporter assay
revealed that miR-217 inhibited luciferase activity in cells
transfected with a wild-type FOXO3 luciferase reporter vector.
However, the luciferase activity was not affected in cells
transfected with a mutated FOXO3 luciferase reporter vector,
suggesting that FOXO3 is a target of miR-217 (Fig. 4B). An RT-qPCR assay revealed that
the overexpression of miR-217 suppressed FOXO3 expression (Fig. 4C), further confirming that FOXO3
is a target of miR-217.
To determine whether FOXO3 exerts a regulatory
effect on EPC under HG conditions, a FOXO3 overexpression vector
was constructed and transfected into EPCs prior to HG induction.
The data verified that the HG conditions decreased FOXO3
expression, and this expression was recovered and upregulated
following transfection with the FOXO3 overexpression vector
(Fig. 4D). The results of CCK-8
revealed that FOXO3 overexpression restored the EPC proliferative
capacity under HG conditions (Fig.
4E). Transwell migration assays also revealed that the
inhibitory effects of HG on EPC migration were blocked by the
overexpression of FOXO3 (Fig. 4F and
G). The EPC tube formation capacity was also decreased under HG
conditions and this decrease was blocked by the overexpression of
FOXO3 (Fig. 4H and I). Western
blot analysis revealed that NOX4 and NOX1 expression was increased
under HG conditions, while FOXO3 overexpression prevented the
increase in NOX4 and NOX1 protein expression (Fig. 4J). In addition, the HG-induced
expression of the inflammatory cytokine, TNF-α, IL-1β and IL-6, was
suppressed by the overexpression of FOXO3 (Fig. 4K-M). Taken together, these data
indicate that the overexpression of FOXO3 exerted a protective
effect on EPC survival, proliferation, migration and tube formation
under HG conditions.
Protective effects of hsa_circ_0058092 on
EPC proliferation, migration and angiogenic differentiation are
reversed by the overexpression of miR-217 or downregulation of
FOXO3
The present study then aimed to identify an
interactive association between hsa_circ_0058092, miR-217 and FOXO3
in relation to EPC proliferation, migration and angiogenic
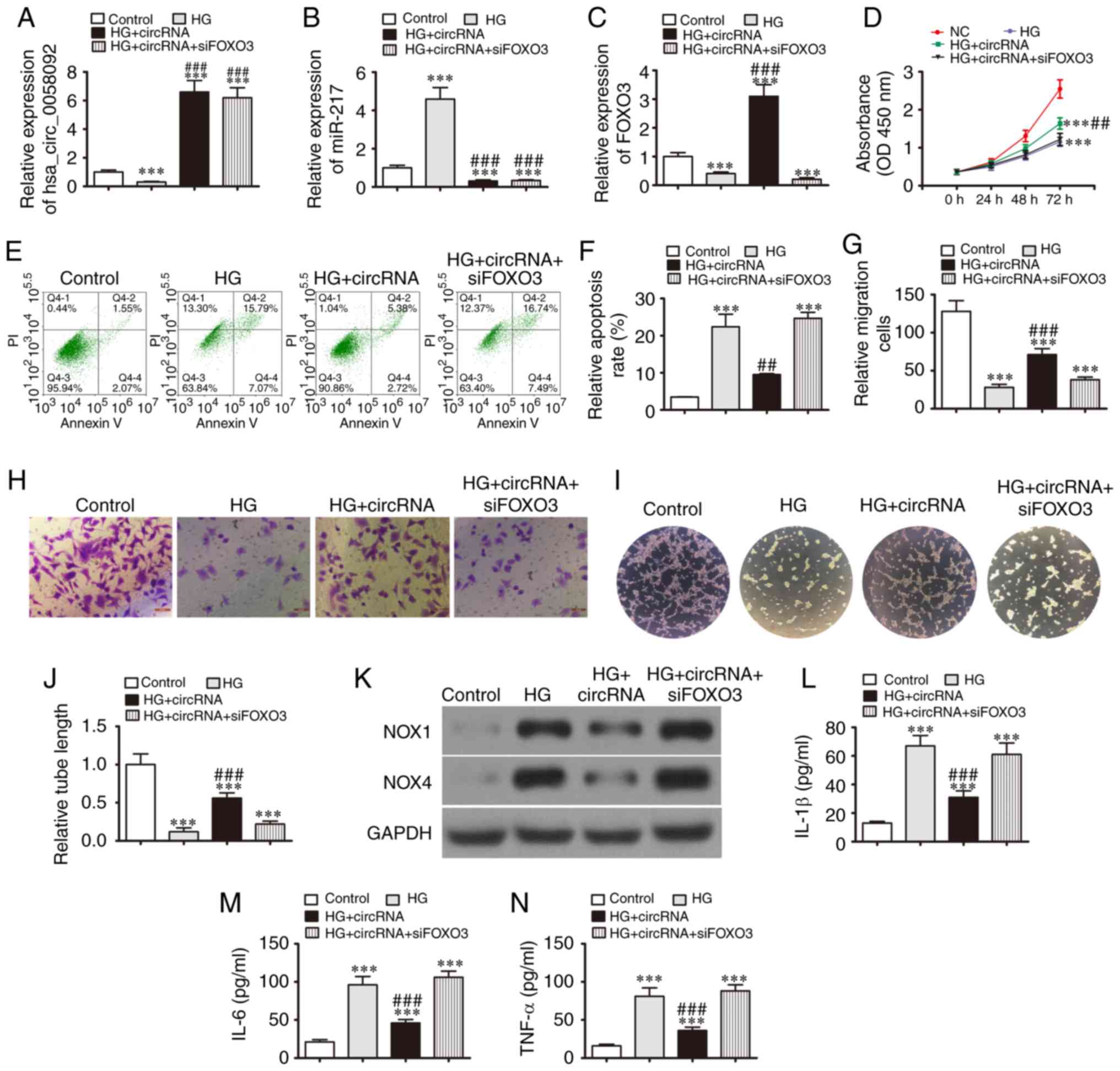
differentiation. The EPCs were transfected with an hsa_circ_0058092
overexpression vector or FOXO3 siRNA prior to induction with HG.
The results of RT-qPCR demonstrated that induction with HG promoted
the expression of miR-217, whereas it suppressed the expression of
hsa_circ_0058092 and FOXO3. The over-expression of hsa_circ_0058092
downregulated miR-217 and upregulated FOXO3 expression. FOXO3
silencing had no effect on the expression of hsa_circ_0058092 or
miR-217 (Fig. 5A-C). These
results indicated that hsa_circ_0058092 regulated the expression of
FOXO3 by miR-217 adsorption. The results of CCK-8 assay
demonstrated that the overexpression of hsa_circ_0058092 restored
the proliferative capacity of the EPCs under HG conditions, while
FOXO3 silencing suppressed the protective effects of
hsa_circ_0058092 (Fig. 5D). EPC
apoptosis was assessed by Annexin V/PI staining at 24 h following
HG induction. The data revealed that the overexpression of
hsa_circ_0058092 protected the EPCs against HG-induced apoptosis,
while the silencing of FOXO3 again suppressed the protective
effects of hsa_circ_0058092 (Fig. 5E
and F). The results of Transwell migration assays also revealed
that the overexpression of hsa_circ_0058092 protected against the
HG-induced inhibition of EPC migration, while FOXO3 silencing
suppressed the protective effects of hsa_circ_0058092 (Fig. 5G and H). The overexpression of
hsa_circ_0058092 protected against the HG-induced impairment of EPC
tube formation capacity. However, FOXO3 downregulation suppressed
the protective effects of hsa_circ_0058092 on EPC tube formation
(Fig. 5I and J). Western blot
analysis also revealed that the overexpression of hsa_circ_0058092
prevented the HG-mediated induction of NOX4 and NOX1 proteins,
while FOXO3 silencing suppressed the protective effects of
hsa_circ_0058092 on oxidative stress in EPCs (Fig. 5K). Finally, the overexpression of
hsa_circ_0058092 prevented the HG-induced increase in the levels of
the inflammatory cytokines, TNF-α, IL-1β and IL-6, and the
protective effects of hsa_circ_0058092 were attenuated following
the FOXO3 of silencing under HG conditions (Fig. 5L-N). Taken together, these data
suggest that hsa_circ_0058092 downregulates miR-217, which in turn
upregulates FOXO3 expression and ultimately protects against
HG-induced EPC damage.
Discussion
HG induces the impaired function of circulating
progenitor cell populations (23,24). Although the precise underlying
mechanisms remain unknown, the production of excessive ROS occurs
due to the elevated levels of vascular NOX. Persistent HG can also
induce the expression of inflammatory cytokines, which further
damages EPCs (25,26). EPC injury suppresses cellular
capacity for migration and angiogenic differentiation, which
ultimately results in delayed wound healing (27,28). Therefore, in order to improve
wound healing, it is necessary to identify the factors that
regulate these microenvironments.
The present study observed a decreased expression of
hsa_circ_0058092 under HG conditions. Previous research has
indicated that certain circRNAs are aberrantly expressed in
patients with T2DM and that these molecules may influence
angiogenic mechanisms by regulating endothelial cell migration,
proliferation and tube formation (29). The present study found that the
upregulation of hsa_circ_0058092 protected against HG-induced
damage to EPC proliferation, migration and tube formation.
It has been indicated that circRNAs function as
miRNA sponges (30). The present
study observed that miR-217 was a target of hsa_circ_0058092. It
has been demonstrated that miR-217 serum levels are upregulated in
patients with diabetic foot ulcers when compared to healthy
controls (31). The suppression
of miR-217 protects against HG-induced podocyte injury and insulin
resistance by restoring phosphatase and tensin homologue-mediated
autophagy pathways (32). The
present study also found that the downregulation of miR-217
suppressed the HG-induced damage to EPCs by recovering cell
survival, proliferation, migration and tube formation capacities.
The luciferase reporter assay confirmed than an interactive
association existed between hsa_circ_0058092 and miR-217.
Bioinformatics analysis also predicted that miR-217
interacts with FOXO3 3′UTR to suppress FOXO3 at the
post-transcriptional level. It has been previously found that FOXO3
expression is reduced under HG conditions (33). The present study found that the
overexpression of FOXO3 protected against HG-induced damage in EPC
cell survival, proliferation, migration and tube formation
capacity. Moreover, FOXO3 silencing suppressed the protective
effects of hsa_circ_0058092 with respect to HG-induced damage to
EPCs. FOXO proteins are transcription factors that participate in
several cellular processes, such as immune cell homeostasis,
cytokine production, cell proliferation and anti-oxidative stress
mechanisms. The downregulation of FOXO3 inhibits cell
proliferation, invasion and migration (34). FOXO3 is also critical for
endothelial cell survival under HG conditions and has been
implicated in the development of diabetes-induced retinal vascular
endothelial cell injury (33).
The present study observed that the downregulation of FOXO3 under
HG conditions played an important role in the induction of EPC
damage.
In conclusion, the present study observed that
hsa_circ_0058092 expression was downregulated and that the
decreased expression of this circRNA was associated with EPC damage
under HG conditions. Although the complexity of the in vivo
cellular crosstalk cannot be replicated in in vitro
experiments, the data from the present study revealed that the
overexpression of hsa_circ_0058092 protected against HG-induced
damage to EPC angiogenesis and migration, and that these protective
effects were regulated via the miR-217/FOXO3 signaling pathway.
These results provide novel insight into the management and
treatment of endothelial dysfunction in DM.
Abbreviations:
|
circRNAs
|
circular RNAs
|
|
miR-217
|
microRNA-217
|
|
miRNAs
|
microRNAs
|
|
EPCs
|
endothelial progenitor cells
|
|
FOXO3
|
forkhead box O 3
|
|
ROS
|
reactive oxygen species
|
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
JC, FZ, and WH performed research, processing of
figures/images, providing materials and analyzed results. YW and ML
designed the research and drafted the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Tenth People's Hospital of Tongji University after
obtaining informed patient consent (SHDSYY-2019-3322).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barakat A, Nakao S, Zandi S, Sun D,
Schmidt-Ullrich R, Hayes KC and Hafezi-Moghadam A: In contrast to
Western diet, a plant-based, high-fat, low-sugar diet does not
exacerbate retinal endothelial injury in streptozotocin-induced
diabetes. FASEB J. 33:10327–10338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bin-Jaliah I, Hewett PW, Al-Hashem F,
Haidara MA, Kader DHA, Morsy MD and Al-Ani B: Insulin protects
against type 1 diabetes mellitus-induced aortopathy associated with
the inhibition of biomarkers of vascular injury in rats. Arch
Physiol Biochem. 1–7. Jun 28–2019.Epub ahead of print. View Article : Google Scholar
|
|
3
|
Zhang R, Liu J, Yu S, Sun D, Wang X, Fu J,
Shen J and Xie Z: Osteoprotegerin (OPG) promotes recruitment of
endothelial progenitor cells (EPCs) via CXCR4 signaling pathway to
improve bone defect repair. Med Sci Monit. 25:5572–5579. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dvorin EL, Wylie-Sears J, Kaushal S,
Martin DP and Bischoff J: Quantitative evaluation of endothelial
progenitors and cardiac valve endothelial cells: Proliferation and
differentiation on poly-glycolic acid/poly-4-hydroxybutyrate
scaffold in response to vascular endothelial growth factor and
transforming growth factor beta1. Tissue Eng. 9:487–493. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rethineswaran VK, Kim YJ, Jang WB, Ji ST,
Kang S, Kim DY, Park JH, Van LTH, Giang LTT, Ha JS, et al:
Enzyme-aided extraction of fucoidan by AMG augments the
functionality of EPCs through regulation of the AKT/Rheb signaling
pathway. Mar Drugs. 17:3922019. View Article : Google Scholar :
|
|
6
|
Jin H, Zhang Z, Wang C, Tang Q, Wang J,
Bai X, Wang Q, Nisar M, Tian N, Wang Q, et al: Melatonin protects
endothelial progenitor cells against AGE-induced apoptosis via
autophagy flux stimulation and promotes wound healing in diabetic
mice. Exp Mol Med. 50:1–15. 2018. View Article : Google Scholar
|
|
7
|
Gao J, Zhao G, Li W, Zhang J, Che Y, Song
M, Gao S, Zeng B and Wang Y: MiR-155 targets PTCH1 to mediate
endothelial progenitor cell dysfunction caused by high glucose. Exp
Cell Res. 366:55–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosso A, Balsamo A, Gambino R, Dentelli P,
Falcioni R, Cassader M, Pegoraro L, Pagano G and Brizzi MF: p53
Mediates the accelerated onset of senescence of endothelial
progenitor cells in diabetes. J Biol Chem. 281:4339–4347. 2006.
View Article : Google Scholar
|
|
9
|
Kadam S, Kanitkar M, Dixit K, Deshpande R,
Seshadri V and Kale V: Curcumin reverses diabetes-induced
endothelial progenitor cell dysfunction by enhancing MnSOD
expression and activity in vitro and in vivo. J Tissue Eng Regen
Med. 12:1594–1607. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sosale B, Chandrashekara S, Aravind SR,
Renuka P and Anupama KR: Influence of cytokine status on insulin
resistance and circulating endothelial progenitor cells in type 2
diabetes mellitus. Cytokine. 99:179–185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cortes-Lopez M and Miura P: Emerging
functions of circular RNAs. Yale J Biol Med. 89:527–537.
2016.PubMed/NCBI
|
|
12
|
Huang G, Li S, Yang N, Zou Y, Zheng D and
Xiao T: Recent progress in circular RNAs in human cancers. Cancer
Lett. 404:8–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu H, Wu S, Zhu Y, Ye M, Shen J, Liu Y,
Zhang Y and Bu S: Hsa_circRNA_0054633 is highly expressed in
gestational diabetes mellitus and closely related to glycosylation
index. Clin Epigenetics. 11:222019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Z, Li X, Jian D, Hao P, Rao L and Li
M: Hsa_circ_0054633 in peripheral blood can be used as a diagnostic
biomarker of pre-diabetes and type 2 diabetes mellitus. Acta
Diabetol. 54:237–245. 2017. View Article : Google Scholar :
|
|
15
|
Xu H, Guo S, Li W and Yu P: The circular
RNA Cdr1as, via miR-7 and its targets, regulates insulin
transcription and secretion in islet cells. Sci Rep. 5:124532015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang Y, Wang X, Li W, Han J, Jin J, Su F,
Zhang J, Huang W, Xiao F, Pan Q and Zou L: Screening of circular
RNAs and validation of circANKRD36 associated with inflammation in
patients with type 2 diabetes mellitus. Int J Mol Med. 4:1865–1874.
2018.
|
|
17
|
Wang H, She G, Zhou W, Liu K, Miao J and
Yu B: Expression profile of circular RNAs in placentas of women
with gestational diabetes mellitus. Endocr J. 66:431–441. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li WD, Zhou DM, Sun LL, Xiao L, Liu Z,
Zhou M, Wang WB and Li XQ: LncRNA WTAPP1 promotes migration and
angio-genesis of endothelial progenitor cells via MMP1 through
MicroRNA 3120 and Akt/PI3K/autophagy pathways. Stem Cells.
36:1863–1874. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Z, Huang W, Wang X, Wang T, Chen Y,
Chen B, Liu R, Bai P and Xing J: Circular RNA CEP128 acts as a
sponge of miR-145-5p in promoting the bladder cancer progression
via regulating SOX11. Mol Med. 24:402018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Wu H, Chen Z, Chen JZ, Xie J and Xu B:
Resveratrol improves tube formation in AGE-induced late endothelial
progenitor cells by suppressing syndecan-4 shedding. Oxid Med Cell
Longev. 2018:90459762018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen S, Wu Y, Chen J, Xie Z, Huang K, Wang
G, Yang Y, Ni W, Chen Z, Shi P, et al: CircSERPINE2 protects
against osteoarthritis by targeting miR-1271 and ETS-related gene.
Ann Rheum Dis. 78:826–836. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loomans CJ, de Koning EJ, Staal FJ,
Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B,
Rabelink TJ and van Zonneveld AJ: Endothelial progenitor cell
dysfunction: A novel concept in the pathogenesis of vascular
complications of type 1 diabetes. Diabetes. 53:195–199. 2004.
View Article : Google Scholar
|
|
24
|
Kränkel N, Adams V, Linke A, Gielen S,
Erbs S, Lenk K, Schuler G and Hambrecht R: Hyperglycemia reduces
survival and impairs function of circulating blood-derived
progenitor cells. Arterioscler Thromb Vasc Biol. 25:698–703. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lontchi-Yimagou E, Sobngwi E, Matsha TE
and Kengne AP: Diabetes mellitus and inflammation. Curr Diab Rep.
13:435–444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karam BS, Chavez-Moreno A, Koh W, Akar JG
and Akar FG: Oxidative stress and inflammation as central mediators
of atrial fibrillation in obesity and diabetes. Cardiovasc
Diabetol. 16:1202017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boniakowski AE, Kimball AS, Jacobs BN,
Kunkel SL and Gallagher KA: Macrophage-mediated inflammation in
normal and diabetic wound healing. J Immunol. 199:17–24. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Salazar JJ, Ennis WJ and Koh TJ: Diabetes
medications: Impact on inflammation and wound healing. J Diabetes
Complications. 30:746–752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang SJ, Chen X, Li CP, Li XM, Liu C, Liu
BH, Shan K, Jiang Q, Zhao C and Yan B: Identification and
characterization of circular RNAs as a new class of putative
biomarkers in diabetes retinopathy. Invest Ophthalmol Vis Sci.
58:6500–6509. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin CJ, Lan YM, Ou MQ, Ji LQ and Lin SD:
Expression of miR-217 and HIF-1α/VEGF pathway in patients with
diabetic foot ulcer and its effect on angiogenesis of diabetic foot
ulcer rats. J Endocrinol Invest. 42:1307–1317. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun J, Li ZP, Zhang RQ and Zhang HM:
Repression of miR-217 protects against high glucose-induced
podocyte injury and insulin resistance by restoring PTEN-mediated
autophagy pathway. Biochem Biophys Res Commun. 483:318–324. 2017.
View Article : Google Scholar
|
|
33
|
Chen Y, Wang Y, Jiang Y, Zhang X and Sheng
M: High-glucose treatment regulates biological functions of human
umbilical vein endothelial cells via sirt1/FOXO3 pathway. Ann
Transl Med. 7:1992019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li W and Jiang H: Up-regulation of miR-498
inhibits cell proliferation, invasion and migration of
hepatocellular carcinoma by targeting FOXO3. Clin Res Hepatol
Gastroenterol. 44:29–37. 2020. View Article : Google Scholar
|