Introduction
Intervertebral disc degeneration (IDD) is closely
related to lower back pain induced by inflammatory responses, nerve
ingrowth in degenerated intervertebral discs and degradation of the
extracellular matrix (ECM), which cause subsequent changes in
spinal biomechanics. IDD can have a large impact on the livelihood
of patients and their economic development (1). IDD disrupts the distribution and
density of the aged bone tissues in vertebrae (2). Nucleus pulposus (NP) mesenchymal
stem cells (MSCs) can promote the regeneration of intervertebral
discs due to their endogenous repair function (3). Furthermore, since a loss of NP cells
and the ECM are the primary causes of IDD, and MSC differentiation
is conducive to intervertebral disc regeneration, the ability of
NPMSCs to form cartilage can help to protect patients with IDD
(4). MSCs reduce stress-mediated
NP cell apoptosis, thus relieving the severity of IDD (5). Currently, IDD, which is associated
with the accumulation of injuries and aging, is a health threat to
millions of individuals worldwide (6). In this context, biomarkers for early
stage diagnosis and novel therapeutic strategies for IDD are
urgently needed. As stem cell transplantation is an effective
therapy for IDD (7), the present
study focused on the underlying mechanisms of NPMSCs, as well as
their roles in cartilage differentiation and apoptosis, to develop
novel intervention strategies.
Sirtuins (SIRTs) are actively involved in preventing
cell senescence and prolonging the lifespan of an organism by
modulating various cell biological processes, including apoptosis,
transcription and angiogenic metabolism (8). Suppression of SIRT activity is
related to abnormalities in physiological development, such as
short stature, bone fragility and craniofacial dimorphisms, whereas
SIRT over-expression protects bones from damage caused by aging or
immobilization (9). As a member
of the SIRT family, SIRT1, whose dysfunction may trigger skeletal
diseases, directly acts on bone cells and cartilage, and it plays
an important role in maintaining bone homeostasis and growth
(10). Additionally, activated
SIRT1 in MSCs restores the osteoblastic bone formation (11). Moreover, recently it has been
reported that both monocyte chemoattractant protein 1 (MCP1) and
chemokine receptor 2 (CCR2) are overexpressed in the spinal cord of
patients with cancer-induced bone pain (CIBP), indicating a severe
and long-lasting CIBP (12). MCP1
is expressed at high levels in obese children, who are vulnerable
to fractures and osteoporosis (13) Additionally, MCP1 is secreted at
higher levels in ankylosing spondylitis MSCs than it is in normal
MSCs (14). High levels of CCR2
is present in the peripheral blood of patients with osteoarthritis
(15). Moreover, increased CCR2
expression has been observed in mice with bone fractures, and it
further exaggerates bone fracture and its complications (16). In a recent study, the crosstalk of
MCP1 and CCR2 is increased in response to diverse stimuli,
potentially resulting in severe osteoclastogenesis (13). Based on accumulating evidence,
SIRT1 knockout increases MCP1 expression in individuals with
various diseases, such as atherosclerosis and disc degeneration,
thus further worsening the severity of the symptoms (17,18). Therefore, a reasonable hypothesis
is that the interaction between SIRT1 and the MCP1/CCR2 axis
represents a useful target in IDD treatment. Thus, in the present
study a series of experiments were conducted to verify the
hypothesis.
Materials and methods
Separation and culture of NPMSCs
Between May 2016 and July 2019, 20 patients with
lumbar disc herniation (LDH; 12 men and 8 women, with the age
ranging from 60-75 years) and 10 patients with lumbar vertebral
fracture (LVF; 8 men and 2 women, with the age ranging from 20-25
years) who received an operation at The Sixth Medical Centre of PLA
General Hospital were enrolled in this study for the collection of
NP samples. All 20 patients with LDH were diagnosed with IDD
through magnetic resonance imaging prior to the operation and a
pathological examination post-operation. Additionally, the 10
patients with LVF were treated with urgent surgical decompression,
discectomy and fusion with internal fixation within 24 h after
trauma. NP samples were collected from the patients with LVF during
the operation and served as controls for the samples from patients
with LDH.
The annulus fibrosus of the samples was transected
using a scalpel to expose the NP, and then the NP tissues were
removed. NP tissue samples were minced and washed in PBS, and then
centrifuged at 500 × g for 5 min at room temperature, after which
the supernatant was discarded. Next, the NP cells were detached by
incubating the samples with 0.25% trypsin at 37°C for 20 min and
centrifuged at 500 × g at room temperature for 5 min to discard the
supernatant. Next, the NP cells were treated with 0.25% collagen II
at 37°C for 4 h, followed by centrifugation at 500 × g for 5 min at
room temperature, following which the supernatant was discarded.
The cell suspension was seeded in a cell culture flask with
Dulbecco's modified Eagle's medium (DMEM)/F12 (Thermo Fisher
Scientific, Inc.) containing 15% fetal bovine serum (FBS; Beijing
Solarbio Science & Technology Co., Ltd.). The flask was placed
in a 37°C incubator with 5% CO2. The medium was replaced
every 3 or 4 days. Cell growth was observed under an inverted
microscope (Olympus Corporation). At ~day 15, the fast-growing
cells aggregated into a mass, requiring ongoing subculture. Then,
adherent cells were detached with 0.25% trypsin and subcultured at
a ratio of 1:2. Passage 2 NPMSCs exhibiting good growth under a
microscope were collected for further experiments.
Identification of NPMSCs
After detachment in 0.25% trypsin, the passage 2
NPMSCs were incubated with fluorescein isothiocyanate
(FITC)-conjugated primary antibodies (all from Abcam) against CD34
(1:50; cat. no. ab78165), CD45 (1:50; cat. no. ab27287), CD73
(1:50; cat. no. ab54217) and CD90 (1:50; cat. no. ab124527) at 4°C
for 45 min in the dark. An appropriate negative control (NC) was
established for each tube. After washing with PBS three times,
cells were centrifuged at 400 × g for 5 min at 4°C, resuspended in
50 µl PBS, and verified using a flow cytometer (BD
FACSCanto™ II) and BD FACSDiva™ 6.0 software (BD Biosciences).
The passage 2 NPMSCs were cultured in osteogenic
cartilage differentiation medium (low-glucose DMEM supplemented
with 0.01% dexamethasone, 10 mmol/l β-sodium glycerophosphate, 50
µg/ml vitamin, 1% penicillin-streptomycin, 1% glutamine and
10% FBS), lipogenic differentiation medium (high-glucose DMEM
supplemented with 0.1% dexamethasone, 0.1% isobutylmethylxanthine,
1% penicillin-streptomycin, 1% glutamine, 10 µmol/l insulin
and 10% FBS) or chondroblast differentiation medium (DMEM/F12
supplemented with 0.01% dexamethasone, 10 ng/ml transforming growth
factor-β1, 50 mg/l vitamin, 6.25 µg/ml transferrin, 1%
penicillin-streptomycin, 1% glutamine and 10% FBS), all purchased
from Beijing Solarbio Science & Technology Co., Ltd. The medium
was replaced every 3 days. Blank controls with no inducer added to
the medium were also prepared.
After a 3-week induction of adipogenic and
osteoplastic differentiation, cells were fixed with 4%
paraformaldehyde for 15 min at room temperature and stained with
alizarin red for 20 min and oil red O for 10 min. After a 3-week
induction of chondrogenic differentiation, cell microspheres were
collected, fixed with 4% paraformaldehyde for 15 min at room
temperature, embedded in paraffin, cut into 4 µm sections
and stained with alcian blue for 30 min at room temperature. Next,
these sections were observed under an optical microscope and
photographed. Alcian blue, alizarin red and oil red O were all
purchased from Beyotime Institute of Biotechnology.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Passage 2 NPMSCs were seeded into 96-well plates at
a density of 1×103 cells/well and incubated in a 37°C
incubator with a 5% CO2 atmosphere. Then, 20 µl
MTT solution (5 mg/ml; Beijing Solarbio Science & Technology
Co., Ltd.) was added to 3 different wells on days 2, 4, 6, 8 and
10, and cells were incubated at 37°C for an additional 4 h.
Subsequently, the solution in each well was replaced with 150
µl DMSO and incubated at 37°C for 30 min. Then, the optical
density value of each well was recorded at 492 nm using a
microplate reader (ThermoMax; Molecular Devices, LLC).
Experimental groups and treatments
Cells were assigned into the following groups: i)
Blank group, NPMSCs from patients with LVF without any treatment;
ii) LDH group, NPMSCs from patients with LDH without any treatment;
iii) resveratrol (RES) group, 50 µmol/l RES (SIRT1 agonist)
was added to NPMSCs from patients with LDH; iv) MCP1 group, 50
ng/ml MCP1 was added to NPMSCs from patients with LDH; v) RS102895
group, 10 µmol/l RS102895 (CCR2 inhibitor) was added to
NPMSCs from patients with LDH; and vi) RES + MCP1 group, 50
µmol/l RES and 50 ng/ml MCP1 were added to NPMSCs from
patients with LDH. RES, MCP1 and RS102895 were all purchased from
Beyotime Institute of Biotechnology.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
A TRIzol kit (Beijing Dingguo Changsheng
Biotechnology Co., Ltd.) was utilized to extract total RNA from
cells and tissue homogenates in each group. RNA concentration and
purity were measured, and the extracted RNA was reverse transcribed
into cDNAs using a ReverTra Ace® qPCR RT Master Mix kit
(Toyobo Life Science). The conditions of reverse transcription
were: Genomic DNA eraser at 42°C for 2 min, 37°C for 15 min and
85°C for 5 sec. PCR was conducted using SYBR Premix Ex Taq™ II
(Takara Bio, Inc.) with β-actin as an internal reference. PCR
conditions were as follows: Pre-denaturation at 95°C for 5 min;
then 40 cycles of denaturation at 95°C for 10 sec and annealing at
60°C for 20 sec; and final extension at 72°C for 10 sec. Relative
gene expression was measured using the 2−∆∆Cq method
(PMID:11846609). The primer sequences are listed in Table I.
 | Table IPrimer sequences used for
RT-qPCR. |
Table I
Primer sequences used for
RT-qPCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| 4-Oct | F:
GTATTCAGCCAAACGACCATCT |
| R:
GCTTCCTCCACCCACTTCT |
| Nanog | F:
ACCCCGTTTCACTGTGTTAGC |
| R:
GACGGCAGCCAAGGTTATTAAA |
| Collagen II | F:
AAGAAGCACATCTGGTTTGGA |
| R:
CAGTGGACAGTAGACGGAGGA |
| Aggrecan | F:
CGAGAATCAAATGGAGCCG |
| R:
CACAACACCTTTCACCACGAC |
| Sox-9 | F:
GACAACTTTACCAGTTTCGGTC |
| R:
GAGGGAAAACAGAGAACGAAAC |
| MCP-1 | F:
TCTCTTCCTCCACCACTATGCA |
| R:
GGCTGAGACAGCACGTGGAT |
| CCR2 | F:
GGAATCTTCTTCATTATCCTCCTGAC |
| R:
TGACTACACTTGTTATTACCCCAAAGG |
| SIRT1 | F:
CCAGAACATAGACACGCTGGAAC |
| R:
CTCCTCGTACAGCTTCACAGTCA |
| β-actin | F:
TGGCTGGCCGGGACCTGACTGA |
| R:
CGCGCCGTGGCCATCTCCTG |
Western blot analysis
Total proteins were extracted from cells using a
protein extraction kit (Beijing Solarbio Science & Technology
Co., Ltd.) and tissue homogenates in each group and the
concentrations were measured by a bicinchoninic acid protein assay
kit (Beyotime Institute of Biotechnology). Proteins (20 g) were
loaded onto 6-15% gels and resolved via SDS-PAGE, and then
transferred Onto polyvinylidene fluoride membranes (Beyotime
Institute of Biotechnology). Next, the membranes were blocked with
5% skimmed milk powder for 1 h at room temperature and incubated
with primary antibodies (all purchased from Abcam) against MCP1
(1:2,000; cat. no. ab25124), CCR2 (1:1,000; cat. no. ab203128),
aggrecan (1:100; cat. no. ab3778), collagen II (1:1,000; cat. no.
ab34712), Sry-related HMG box (Sox)-9 (1:1,000; cat. no. ab185966),
Bcl-2 (1:1,000; cat. no. ab32124), Bax (1:1,000; cat. no. ab32503),
cleaved caspase-3 (1:1,000; cat. no. ab2302), matrix
metalloproteinase (MMP)-13 (1:3,000; cat. no. ab39012), tissue
inhibitor of metalloproteinase 1 (TIMP-1; 1:1,000; cat. no.
ab38978) and SIRT1 (1:2,000; cat. no. ab110304) at 4°C overnight.
Afterwards, the membranes were incubated with a horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G antibody
(1:2,000; cat. no. ab205718) at room temperature for 1 h. Following
incubation, the membranes were developed using enhanced
chemiluminescence reagent (Beijing Solarbio Science &
Technology Co., Ltd.) and visualized using BioSpectrum gel imaging
system (Bio-Rad Laboratories, Inc.). The protein bands were
analyzed by Image-Pro Plus 6.0 (Media Cybernetics, Inc.). GAPDH
(1:1,000; cat. no. ab8245) served as the internal reference, and
the ratio of the gray value of the target protein to GAPDH was
recorded as the relative protein expression.
Flow cytometry detection of Annexin
V-FITC/propidium iodide (PI) staining
After detachment using 0.25% trypsin followed by
washing with PBS, NPMSCs were resuspended in 500 µl binding
buffer, and then were treated with 5 µl Annexin V-FITC and 5
µl PI. After a 15-min reaction at room temperature, the
mixture was detected and analyzed using a BD FACSCalibur™ flow
cytometer and BD FACSDiva™ 6.0 software (BD Biosciences). All the
steps mentioned above were performed according to the instructions
of the Annexin V-FITC/PI double-staining cell apoptosis detection
kit (Nanjing KeyGen Biotech Co., Ltd.).
Rat model experimental groups and
treatments
A total of 40 healthy 8-week old Sprague-Dawley rats
(weight, 220-250 g), purchased from Jinan University, Guangzhou,
Guangdong, China [SYXK (Guangdong) 2017-0174], were raised under
standard conditions at a temperature of 25±2°C, 45% relative
humidity, controlled 12 h light/dark cycles, and free access to
drinking water and food. One week later, they were subjected to
experiments.
The rats were numbered by body weight and randomly
assigned into the control group, the IDD group, the lentivirus (LV)
NC group and the LV-SIRT1 group, with 10 rats per group. The normal
rats in the control group received no treatment. Rats in the IDD
group, the LVNC group and the LV-SIRT1 were subjected to a coccyx
puncture to establish rat IDD models. Briefly, 60 mg/kg
pentobarbital sodium was intraperitoneally injected into rats for
anesthesia, and then the 5 and 6th (Co5-6) and 6 and 7th (Co6-7)
coccygeal vertebrae of the rat intervertebral discs were located
using a por table X-ray machine (Shanghai Xianwei Photoelectric
Technology Co., Ltd.). Afterwards, 22 G needles were used to pierce
the Co5-6 and Co6-7, with the tips inserted perpendicular to rat
tails until they had completely punctured through the other side
(5-mm depth, 360° rotation for 5 sec) (19). Subsequently, 200,000 U/kg
penicillin sodium salt (Shandong Lukang Record Pharmaceutical Co.,
Ltd.) was injected into each rat daily. When the models were
successfully established, a rat NPMSC suspension (2×106
cells/ml) that had been transfected with 1×108 TU/ml
(MOI=10) LV NC or LV-SIRT1 was injected into rats in the LVNC group
or the LV-SIRT1 group, respectively. The follow-up experiments were
conducted 4 weeks after transfection. Both the construction and
packaging of LV NC and LV-SIRT1 (lentivirus vector of SIRT1) were
conducted by Shanghai GeneChem Co., Ltd.
Tissue sample collection
Four weeks after the aforementioned operation, all
rats were euthanatized with an intraperitoneal injection of
pentobarbital sodium (800 mg/kg) (20). Afterwards, intervertebral disc
tissues were immediately removed. Tissues from 6 rats in each group
were fixed with 4% paraformaldehyde overnight, embedded in paraffin
and sectioned (4 µm) for staining, and the tissues from the
other 4 rats were used to prepare tissue homogenates.
Hematoxylin and eosin (H&E)
staining
The paraffin-embedded sections were routinely
dewaxed, dehydrated, stained with hematoxylin (Beijing Solarbio
Science & Technology Co., Ltd.) for 3 min at room temperature,
washed, and differentiated in 1% acid alcohol for 15 sec. Next,
these sections were stained with eosin (Beijing Solarbio Science
& Technology Co., Ltd.) for 2 min at room temperature and
observed under an optical microscope (Olympus Corporation).
TdT-mediated dUTP nick-end labeling
(TUNEL) staining
The paraffin-embedded sections were routinely
dewaxed and dehydrated before apoptosis detection using a TUNEL kit
(Shanghai Yeasen Biotechnology Co., Ltd.), according to the
manufacturer's instructions, followed by incubation with TdT buffer
at 37°C in the dark for 60 min. Next, the cell nuclei were stained
with 10 µg/ml DAPI (Beyotime Institute of Biotechnology) and
incubated for 5 min at room temperature in the dark. Subsequently,
the sections were sealed with anti-fluorescence quenching sealing
agent (cat. no. 0100-01; SouthernBiotech) and 10 fields were
randomly selected to observe TUNEL-positive cells under a
fluorescence microscope.
Statistical analysis
SPSS v21.0 (IBM Corp.) was employed for data
analysis. Kolmogorov-Smirnov tests were performed to determine the
normal distribution of the data. The data are presented as the mean
± standard deviation. For data comparing two groups, two-tailed t
tests were used, whereas one-way ANOVA and two-way ANOVA were used
to compare data between multiple groups followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
NPMSC identification and the differential
expression of SIRT1 and genes related to the MCP1/CCR2 axis between
NPMSCs from patients with LVF and LDH
Elongated spindle-shaped passage 2 NPMSCs from
patients with LVF and LDH were observed under the optical
microscope (Fig. 1A). According
to the flow cytometry results, NPMSCs of the blank group and the
LDH group expressed CD73 and CD90, but not CD34 and CD45 (Fig. 1B). Alizarin red, oil red O and
alcian blue staining revealed the potential of NPMSCs in the blank
group and the LDH group to undergo adipogenic, osteoplastic and
chondrogenic differentiation (Fig.
1C-E). The MTT assay showed that the proliferation of NPMSCs
was slower in the LDH group than in the blank group (P<0.01;
Fig. 1F). RT-qPCR revealed that
the expression of Oct4 and Nanog mRNA in NPMSCs was lower in the
LDH group compared with in the blank group (both P<0.01;
Fig. 1G). Compared with the blank
group, the LDH group exhibited decreased expression of SIRT1 mRNA
and protein, but increased expression of MCP1 and CCR2 mRNA and
protein (Fig. 1H and I).
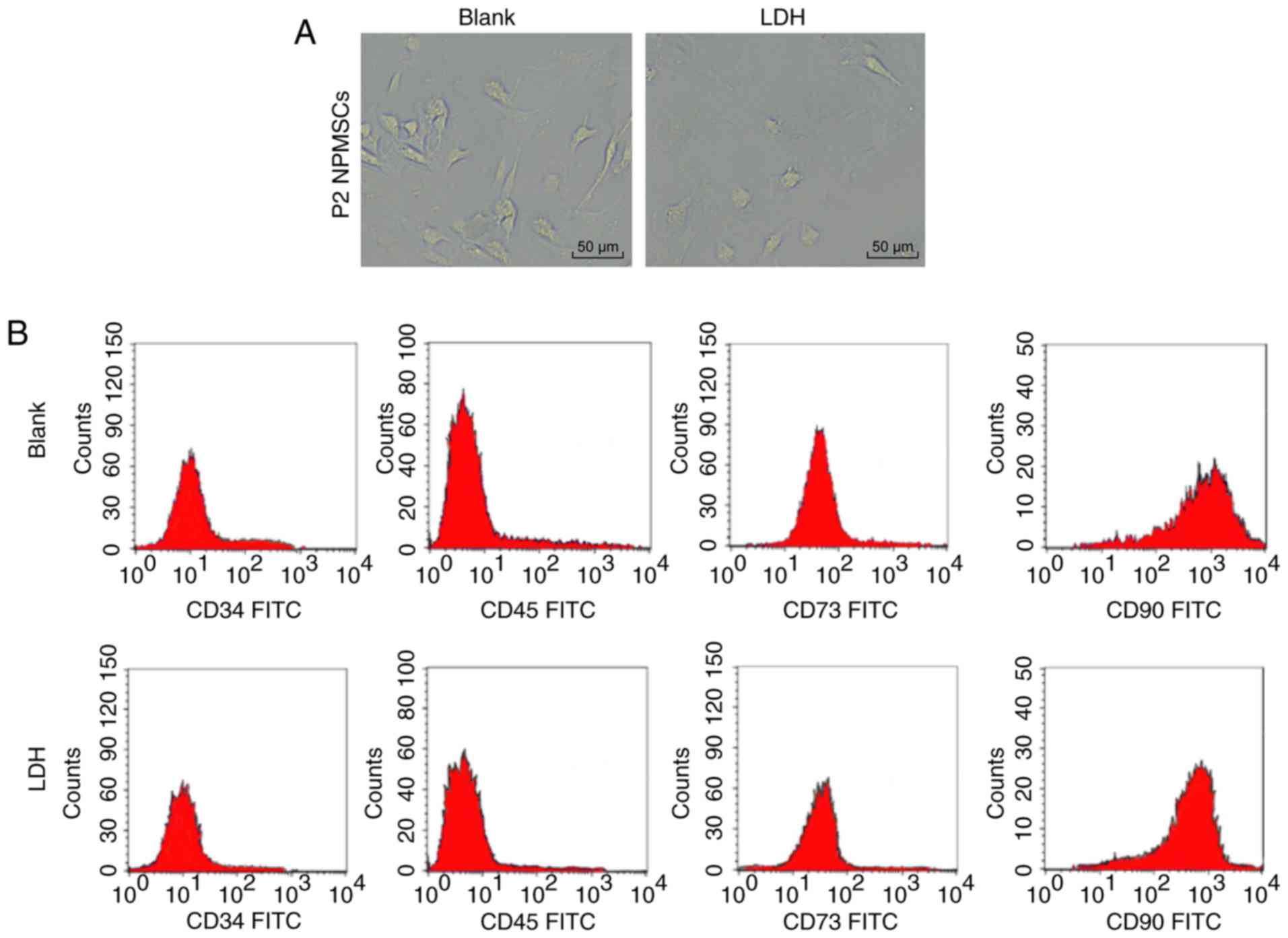 | Figure 1Roles of NPMSCs in IDD, as well as
decreased SIRT1 expression and increased expression of genes
related to the MCP1/CCR2 axis in LDH were verified. (A) Elongated
spindle-shaped passage 2 NPMSCs from patients with LVF and LDH were
observed under an optical microscope. (B) Flow cytometry revealed
the expression of CD73 and CD90, but not CD34 and CD45, in NPMSCs
from the blank group and the LDH group. (C) Alizarin red, (D) oil
red O and (E) alcian blue staining revealed the potential of NPMSCs
in the blank group and the LDH group to undergo adipogenic,
osteoplastic and chondrogenic differentiation. (F) According to the
results of the MTT assay, NPMSCs in the LDH group proliferated
faster than NPMSCs in the blank group. (G) RT-qPCR revealed lower
expression of Oct4 and Nanog mRNA in NPMSCs from the LDH group
compared with in the blank group. (H and I) Compared with the blank
group, the LDH group displayed lower mRNA and protein expression of
SIRT1 and higher mRNA and protein expression of MCP1 and CCR2,
based on the results of RT-qPCR and western blot analyses. Two-way
ANOVA was used to analyze data in panels F-I. Tukey's multiple
comparisons test was applied as the post hoc test. n=3.
**P<0.01. NPMSC, nucleus pulposus mesenchymal stem
cell; SIRT, Sirtuin; MCP, monocyte chemoattractant protein; CCR,
chemokine receptor; LVF, lumbar vertebral fracture; LDH, lumbar
disc herniation; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction. |
Overexpression of SIRT1 and
downregulation of the MCP1/CCR2 axis promote NPMSC cartilage
differentiation but inhibit NPMSC apoptosis
Compared with the LDH group, the RES group displayed
increased expression of SIRT1 mRNA and protein, the MCP1 group
exhibited increased expression of MCP1 and CCR2 mRNA and protein,
whereas in the RS102895 group CCR2 mRNA and protein expression
decreased (all P<0.05; Fig.
2A-D). Compared with the LDH group, RES and RS102895 groups
exhibited increased expression levels of aggrecan, collagen II and
Sox-9 mRNA and protein, but the MCP1 group displayed decreased
expression levels of aggrecan, collagen II and Sox-9 mRNA and
protein (all P<0.01; Fig. 2E and
F). According to the flow cytometry results, compared with the
LDH group, apoptosis was decreased in the RES and RS102895 groups,
and increased in the MCP1 group (all P<0.05; Fig. 2G). Compared with the LDH group,
the expression levels of Bax, cleaved caspase-3 and MMP13 protein
in the RES group decreased, whereas Bcl-2 and TIMP-1 expression
increased. The expression levels of Bax, Bcl-2, cleaved caspase-3
and TIMP-1 protein in the RS102895 groups increased compared with
the LDH group, and MMP13 protein expression decreased. The MCP1
group presented the opposite results (all P<0.05; Fig. 2H).
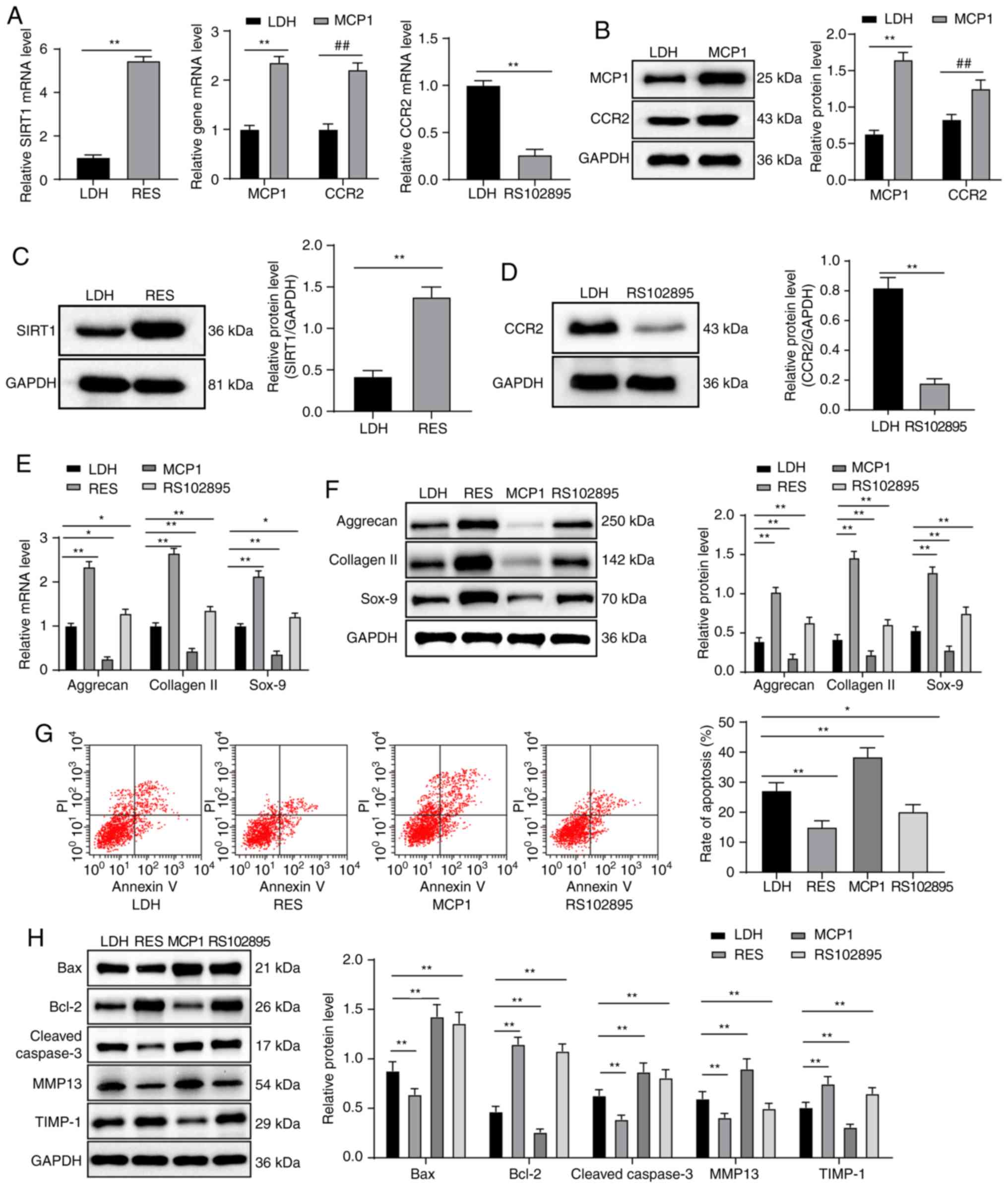 | Figure 2Overexpression of SIRT1 and
downregulation of the MCP1/CCR2 axis increases NPMSC cartilage
differentiation and inhibits NPMSC apoptosis. (A-D) According to
RT-qPCR and western blot analyses, the RES group displayed
increased mRNA and protein expression of SIRT1, the MCP1 group
exhibited increased mRNA and protein expression of MCP1 and CCR2,
and the RS102895 group presented decreased mRNA and protein
expression of CCR2 compared with the LDH group. (E and F) Compared
with the LDH group, the RES and RS102895 groups displayed increased
mRNA and protein expression of aggrecan, collagen II and Sox-9, and
the MCP1 group exhibited decreased mRNA and protein expression of
aggrecan, collagen II and Sox-9, as shown by the results of RT-qPCR
and western blot analyses. (G) According to the flow cytometry
results, apoptosis was decreased in the RES and RS102895 groups but
increased in the MCP1 group compared with the LDH group. (H)
Western blot analysis showed decreased expression levels of Bax,
cleaved caspase-3 and TIMP-1 and increased levels of Bcl-2 and
MMP13 in the RES and RS102895 groups compared with the LDH group;
the opposite results were observed in the MCP1 group. Data in
panels A, C, D and E were analyzed using t tests, data in panels A,
B, E, F and H were analyzed using two-way ANOVA, and data in panel
G were analyzed using one-way ANOVA. Tukey's multiple comparisons
test was applied as the post hoc test. n=3. *P<0.05
and **P<0.01; ##P<0.01. SIRT, Sirtuin;
MCP, monocyte chemoattractant protein; CCR, chemokine receptor;
NPMSC, nucleus pulposus mesenchymal stem cell; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; LDH, lumbar
disc herniation; RES, resveratrol; Sox, Sry related HMG box; Bax,
Bcl-2-associated X; TIMP1, tissue inhibitor of metalloproteinase
1. |
Overexpression of SIRT1 downregulates the
MCP1/CCR2 axis
Lower expression levels of MCP1 and CCR2 mRNA and
protein were observed in the RES group compared with in the LDH
group. Higher expression levels of MCP1 and CCR2 mRNA and protein
were observed in the RES + MCP1 compared with in the RES group, but
expression was lower compared with in the MCP1 group (all
P<0.01; Fig. 3A and B).
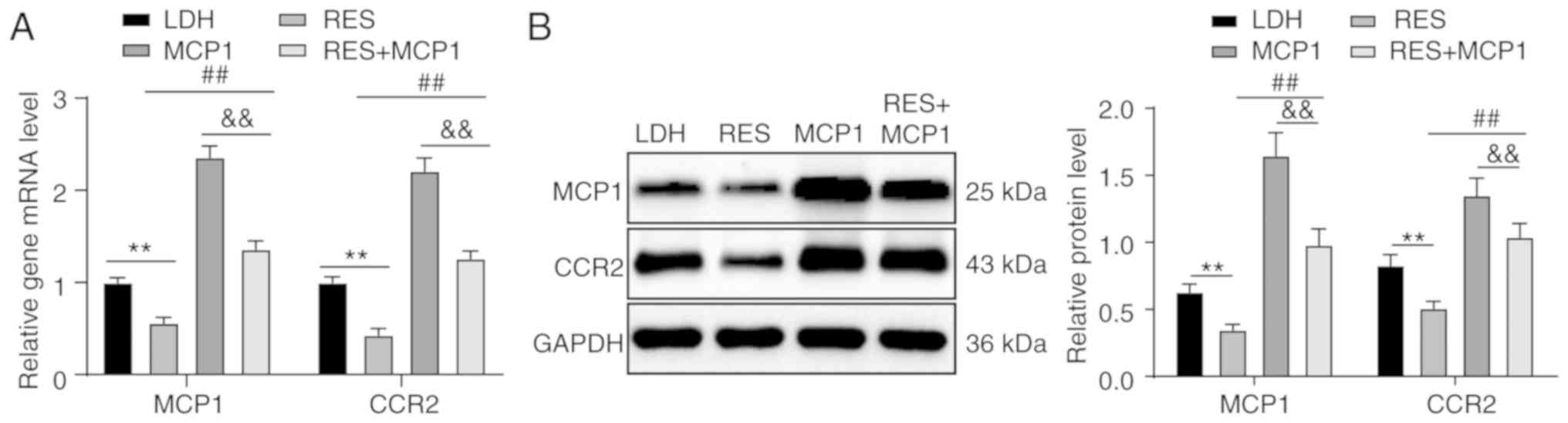 | Figure 3Overexpression of SIRT1 downregulates
the MCP1/CCR2 axis. According to (A) RT-qPCR and (B) western blot
analyses, increased mRNA and protein expression of MCP1 and CCR2
were observed in the RES group, and higher mRNA and protein
expression of MCP1 and CCR2 were observed in the RES + MCP1 group
compared with in the RES group, but the levels were lower than
those of the MCP1 group. Two-way ANOVA, followed by Tukey's
multiple comparisons test as the post hoc test, were used to
determine statistical significance. n=3. **P<0.01;
##P<0.01; &&P<0.01. SIRT,
Sirtuin; MCP, monocyte chemoattractant protein; CCR, chemokine
receptor; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; RES, resveratrol; LDH, lumbar disc herniation. |
MCP1 reverses the progression of NPMSC
cartilage differentiation and the inhibition of NPMSC apoptosis
induced by SIRT1 overexpression
Compared with the RES group, the RES + MCP1 group
showed decreased mRNA and protein expression of aggrecan, collagen
II and Sox-9, increased cell apoptosis, decreased expression of
Bcl-2 and TIMP-1, and increased expression of Bax, cleaved
caspase-3 and MMP13 (all P<0.05; Fig. 4A-D).
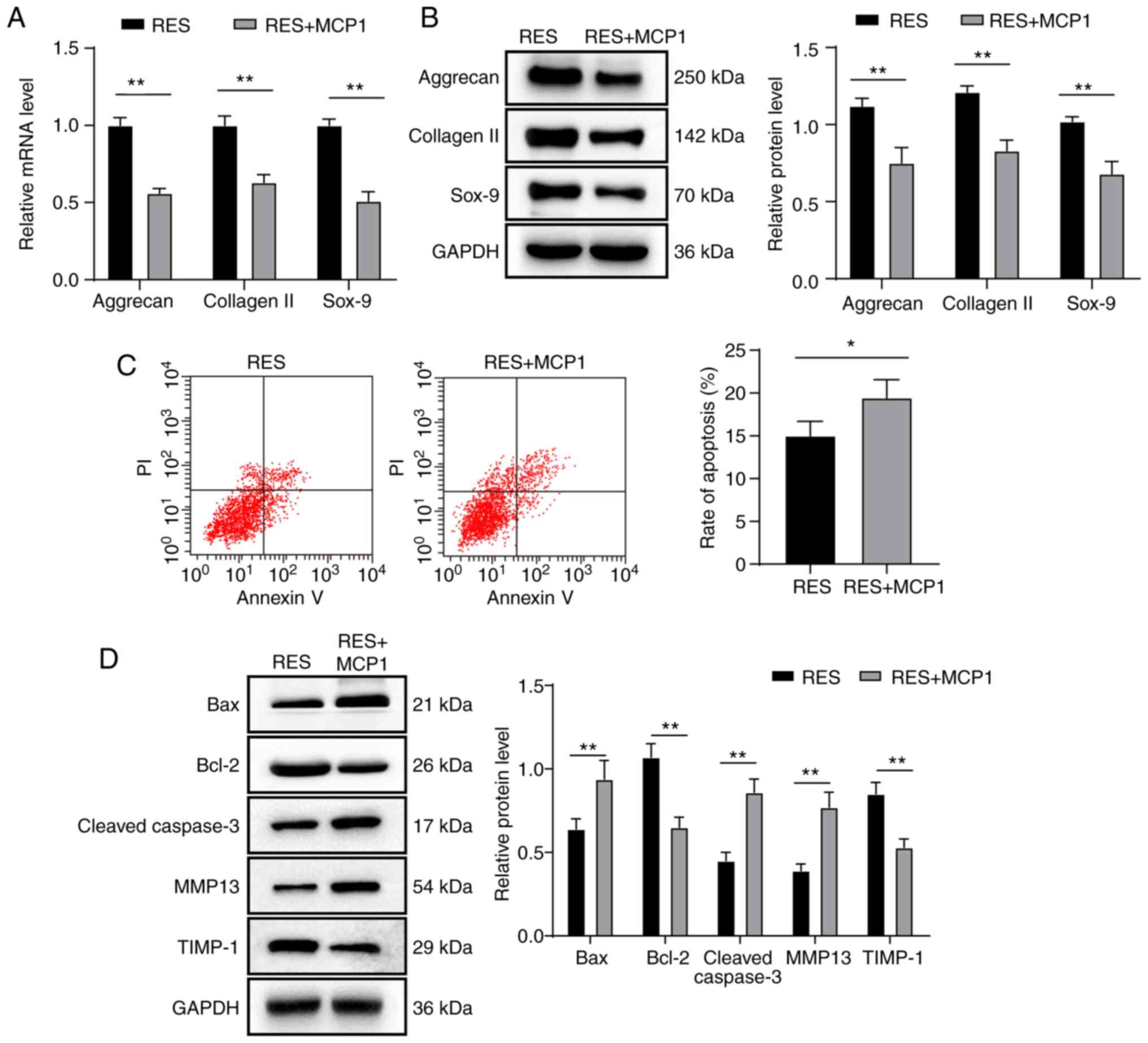 | Figure 4MCP1 reverses the progression of
NPMSC cartilage differentiation and the inhibition of NPMSC
apoptosis induced by SIRT1 overexpression. (A-D) Compared with the
RES group, the RES + MCP1 group displayed decreased mRNA and
protein expression levels of aggrecan, collagen II and Sox-9, lower
levels of cell apoptosis, decreased protein expression of Bcl-2 and
TIMP-1, and increased protein expression of Bax, cleaved caspase-3
and MMP13, based on the results of (A) RT-qPCR, (B and D) western
blotting and (C) flow cytometry. Data in panel C were analyzed
using a t test; data in panels A, B and D were analyzed using
two-way ANOVA. Tukey's multiple comparisons test was applied as the
post hoc test. n=3. *P<0.05 and
**P<0.01. MCP, monocyte chemoattractant protein;
NPMSC, nucleus pulposus mesenchymal stem cell; SIRT, Sirtuin; RES,
resveratrol; Sox, Sry related HMG box; Bcl-2, B-cell lymphoma-2;
TIMP1, tissue inhibitor of metalloproteinase 1; Bax,
Bcl-2-associated X; MMP, matrix metalloproteinase; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
Transplantation of rat NPMSCs
overexpressing SIRT1 relieves IDD in rats by downregulating the
MCP1/CCR2 axis
In the IDD group, mRNA and protein expression levels
of MCP1 and CCR2 increased, and expression of SIRT1 mRNA and
protein decreased. In contrast, in the LV NC and LV-SIRT1 groups,
mRNA and protein expression levels of MCP1 and CCR2 decreased, and
expression of SIRT1 mRNA and protein increased compared with IDD
rats (all P<0.05; Fig. 5A and
B). H&E staining revealed a normal intervertebral space and
orderly arrangement of the annulus fibrosus in normal rats of the
control group. Meanwhile, in IDD rats, intervertebral spaces were
narrowed, the annulus fibrosus was disordered and the NP matrix was
decreased. When rat NPMSCs or rat NPMSCs overexpressing SIRT1 were
transplanted, the annulus fibrosus displayed an orderly arrangement
and the intervertebral structure had recovered notably (Fig. 5C). Lower mRNA and protein
expression levels of aggrecan, collagen II and Sox-9 were observed
in IDD rats compared with in rats of the control group, and those
changes were reversed by the transplantation of NPMSCs or NPMSCs
overexpressing SIRT1 (all P<0.01; Fig. 5D and E). TUNEL staining revealed
increased apoptosis in IDD rats compared with rats in the control
group, but apoptosis was reduced after rat NPMSC transplantation
and decreased even further when rat NPMSCs overexpressing SIRT1
were transplanted (all P<0.01; Fig. 5F). Compared with the control
group, the IDD group displayed increased protein expression levels
of Bax and cleaved caspase-3, but decreased protein expression of
Bcl-2. These changes were reversed by the transplantation of rat
NPMSCs or rat NPMSCs overexpressing SIRT1 (all P<0.05; Fig. 5G).
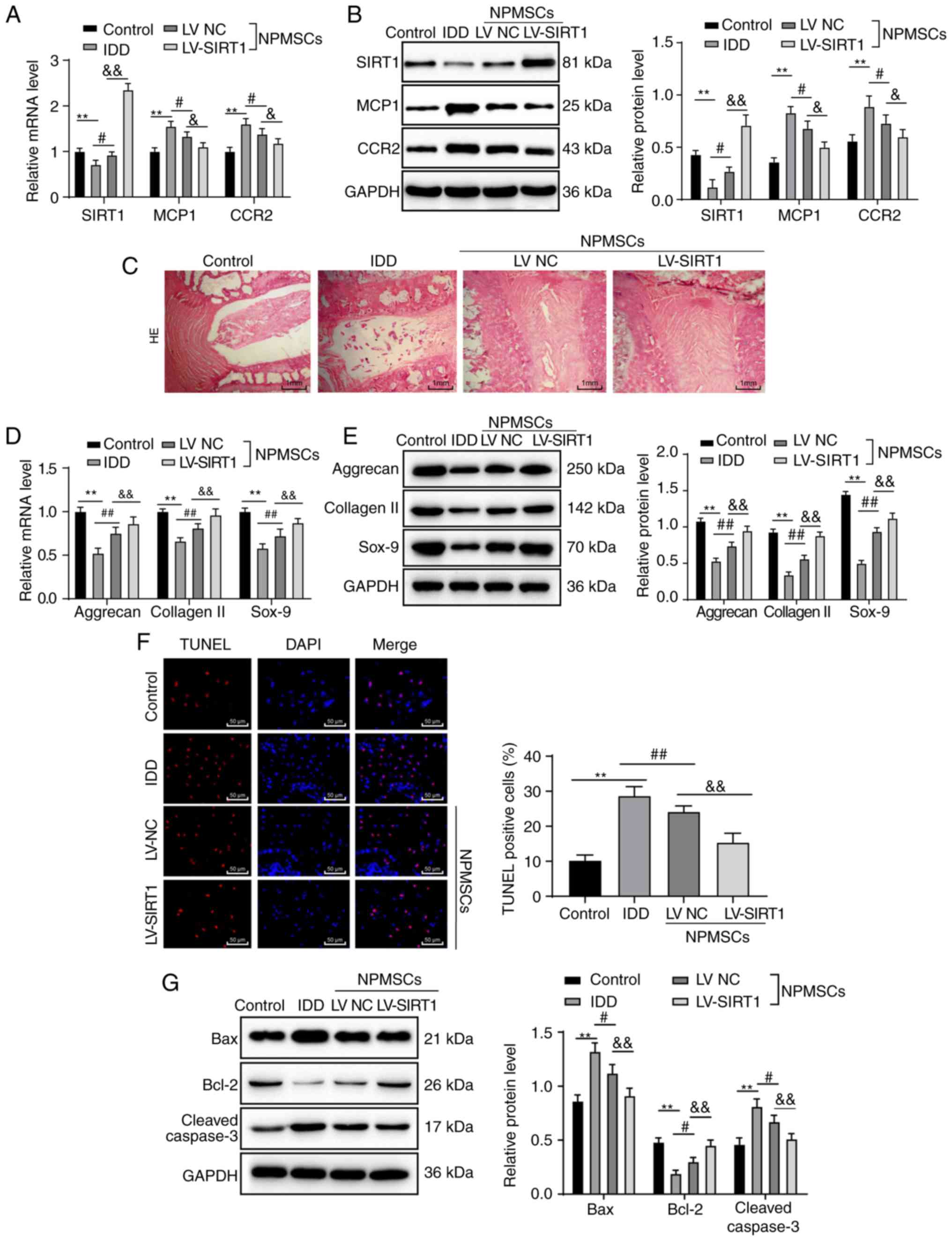 | Figure 5Transplantation of rat NPMSCs
overexpressing SIRT1 relieves IDD in rats by downregulating the
MCP1/CCR2 axis. (A) RT-qPCR and (B) western blot analyses showed
increased mRNA and protein expression of MCP1 and CCR2, and reduced
mRNA and protein expression of SIRT1 in the IDD group compared with
the control group. In contrast, the mRNA and protein expression of
MCP1 and CCR2 decreased and mRNA and protein expression of SIRT1
increased in the IDD rats after transplantation of the NPMSCs or
SIRT1-overexpressing NPMSCs, n=4. (C) H&E staining revealed an
amelioration of the disordered intervertebral structure after the
transplantation of NPMSCs or SIRT1-overexpressing NPMSCs, n=6.
Based on the (D) RT-qPCR and (E) western blot analyses, lower mRNA
and protein expression levels of aggrecan, collagen II and Sox-9
were observed in IDD rats compared with in rats of the control
group, n=4. (F) TUNEL staining revealed increased cell apoptosis in
IDD rats compared with rats in the control group, which was reduced
by NPMSC transplantation and even further decreased after the
transplantation of SIRT1-overexpressing NPMSCs, n=6. (G) Western
blotting showed higher protein expression of Bax and cleaved
caspase-3 and lower expression of Bcl-2 in the IDD group compared
with in the control group, and these changes were reversed by the
transplantation of NPMSCs or SIRT1-overexpressing NPMSCs, n=4. Data
in panel F were analyzed using one-way ANOVA; data in panels A, B,
C, D, E and G were analyzed using two-way ANOVA. Tukey's multiple
comparisons test was applied as the post hoc test.
**P<0.01; #P<0.05 and
##P<0.01; &P<0.05 and
&&P<0.01. NPMSC, nucleus pulposus mesenchymal
stem cell; IDD, intervertebral disc degeneration; SIRT, Sirtuin;
MCP, monocyte chemoattractant protein; CCR, chemokine receptor;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; HE, hematoxylin-eosin; Sox, Sry related HMG box; TUNEL,
tdT-mediated dUTP nick-end labeling; Bcl-2, B-cell lymphoma-2; Bax,
Bcl-2-associated X. |
Discussion
As one of the most common human spinal conditions,
IDD is primarily characterized by the degeneration of NP cells
(21). NPMSC proliferation,
stemness preservation and colony formation are decreased, whereas
apoptosis is increased in individuals with IDD (22). Additionally, SIRT1 exerts a
protective effect on IDD, and its expression is downregulated in
the degenerating disc (23).
Although multiple studies on IDD and SIRT1 or IDD and NPMSCs have
been conducted, only a few have focused on the interaction among
these three factors, and even fewer have analyzed possible
signaling pathways. Therefore, the present study aimed to identify
some novel therapies for IDD based on SIRT1. These data indicated
that SIRT1 increased NPMSC differentiation into cartilage and
reduced apoptosis by downregulating the MCP1/CCR2 axis.
First, an MTT assay and RT-qPCR found that compared
with the normal NPMSCs, NPMSCs from LDH patients exhibited
expression of CD73 and CD90, but not CD34 and CD45, decreased
expression of Oct4 and Nanog, and decreased NPMSC proliferation
rate and SIRT1 expression. As reported by Liu et al
(24), IDD stem cells exhibit
high CD73 and CD90 expression, low CD34 and CD45 expression, and
downregulated expression of genes related to stem cells, including
Oct4 and Nanog, which is consistent with the present findings.
Nevertheless, NPMSC proliferation and viability decrease in
response to hypoxia-triggered IDD (25). In a previous study, SIRT1
expression decreased in IDD cells (26). Additionally, MCP1 and CCR2
expression increased in subjects with LDH compared with healthy
subjects in the present study. It has been demonstrated that MCP1
is activated in NP cells, and it can exacerbate vertebral erosion
(27). Additionally, high
expression of MCP1 indicates an increase in spinal pressure pain
sensitivity and overall pain ratings (28). CCR2 is mainly expressed in neurons
and macrophages, and it is responsible for cumulative pain
hypersensitivity resulting from autologous NP implantation in
patients with LDH (29). In the
present study, SIRT1 overexpression increased differentiation of
NPMSCs into cartilage, but inhibited cell apoptosis. NPMSCs
alleviate IDD by differentiating into cells that closely resemble
NP cells and improving the function of disc cells (25). Meanwhile, the degeneration of
cartilage end plates induces IDD (26). When SIRT1 was upregulated, the
expression of aggrecan, collagen II and Sox-9 also increased in the
present study. This is consistent with a previous finding that
activated SIRT1 can reverse the degeneration of aggrecan and
collagen II (21). Moreover, the
apoptosis rate of NPMSCs decreased after SIRT1 overexpression,
which was accompanied by decreased expression of Bax, cleaved
caspase-3 and MMP13, and increased expression of TIMP-1 and Bcl-2.
According to the findings of He et al (30), overexpression of SIRT1 reduces
chondrocyte apoptosis and ECM degeneration in subjects with
osteoarthritis (OA) by increasing Bcl-2 expression and inhibiting
Bax expression. Furthermore, in spinal cord injuries, when SIRT1 is
upregulated, cleaved caspase-3 expression was reduced, suggesting a
negative association between these two proteins (31). Fujita et al (32) revealed that overexpression of
SIRT1 in chondrocytes suppresses OA gene expression, such as
MMP-13.
In addition, the present study revealed that MCP1
reversed the progression of NPMSC differentiation into cartilage
and inhibited SIRT1-induced cell apoptosis. It was previously
reported that in NPMSCs, MCP1 expression is notably reduced
(33). SIRT1 overexpression
downregulates MCP1, whereas the loss of SIRT1 increases MCP1
expression, indicating a negative association between these two
proteins (34). The present study
demonstrated that after MCP1 treatment TIMP-1 expression decreased.
In a recent study, MCP1 expression decreased, whereas TIMP-1
expression increased in cells treated with canagliflozin (35). Moreover, in the present study the
transplantation of SIRT1-overexpressing NPMSCs relieved IDD in rats
by downregulating the MCP1/CCR2 axis. The transplantation of MSCs
into patients with IDD could alleviate IDD by inhibiting NP cell
apoptosis (36). According to a
previous study, SIRT1 maintains MSC stemness, thus mitigating
age-related skeletal disorders, such as osteoporosis (37). Overall, SIRT1 represents a
potential target for the treatment of IDD.
In summary, the present study supported the
hypothesis that SIRT1 induces cartilage differentiation and
inhibits the apoptosis of NPMSCs in individuals with IDD by
inhibiting the MCP1/CCR2 axis. These results reveal a novel
theoretical approach for IDD treatment. However, this study simply
describes preclinical research. Although these findings provide
therapeutic implications for IDD treatment, the experimental
results and effective application in clinical practice require
further validation. Future studies will further explore the
underlying mechanisms of other targets of SIRT1 by focusing on the
identification of reliable therapeutic targets for IDD and the
application of the results of the present study in clinical
settings for IDD treatment.
Acknowledgments
Not applicable.
Funding
This study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81772399).
Availability of data and materials
All the data generated or analyzed during the
present study are included in this published article.
Authors' contributions
DR is the guarantor of the integrity of the entire
study and contributed to the conception and design of this study.
XO contributed to the definition of intellectual content, clinical
studies, experimental studies, data analysis and manuscript
preparation and editing. JY and XO contributed to the literature
search. XO and CW contributed to data acquisition. XB, CW and JY
were responsible for the statistical analyses. DR contributed to
the manuscript review. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved and supervised by the ethics
committee of The Sixth Medical Centre of PLA General Hospital. All
subjects provided signed informed consent. The protocol was also
approved by the Institutional Animal Care and Use Committee of The
Sixth Medical Centre of PLA General Hospital. Significant efforts
were made to minimize both the number of animals used and their
respective suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Loibl M, Wuertz-Kozak K, Vadala G, Lang S,
Fairbank J and Urban JP: Controversies in regenerative medicine:
Should inter-vertebral disc degeneration be treated with
mesenchymal stem cells? JOR Spine. 2:e10432019. View Article : Google Scholar
|
|
2
|
Kaiser J, Allaire B, Fein PM, Lu D,
Jarraya M, Guermazi A, Demissie S, Samelson EJ, Bouxsein ML and
Morgan EF: Correspondence between bone mineral density and
intervertebral disc degeneration across age and sex. Arch
Osteoporos. 13:1232018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li XC, Wang MS, Liu W, Zhong CF, Deng GB,
Luo SJ and Huang CM: Co-culturing nucleus pulposus mesenchymal stem
cells with notochordal cell-rich nucleus pulposus explants
attenuates tumor necrosis factor-α-induced senescence. Stem Cell
Res Ther. 9:1712018. View Article : Google Scholar
|
|
4
|
Li H, Wang J, Li F, Chen G and Chen Q: The
influence of hyper-osmolarity in the intervertebral disc on the
proliferation and chondrogenic differentiation of nucleus
pulposus-derived mesenchymal stem cells. Cells Tissues Organs.
205:178–188. 2018. View Article : Google Scholar
|
|
5
|
Chen S, Zhao L, Deng X, Shi D, Wu F, Liang
H, Huang D and Shao Z: Mesenchymal stem cells protect nucleus
pulposus cells from compression-induced apoptosis by inhibiting the
mitochondrial pathway. Stem Cells Int. 2017:98431202017. View Article : Google Scholar
|
|
6
|
Mohanty S and Dahia CL: Defects in
intervertebral disc and spine during development, degeneration, and
pain: New research directions for disc regeneration and therapy
Wiley. Interdiscip Rev Dev Biol. 8:e3432019. View Article : Google Scholar
|
|
7
|
Jezierska-Wozniak K, Barczewska M, Habich
A, Wojtacha P, Badowska W, Maksymowicz W and Wojtkiewicz J: The
feasibility of the CD271+ and CD271-mesenchymal stromal cell
enrichment toward nucleus pulposus-like cells. Folia Histochem
Cytobiol. 55:114–123. 2017. View Article : Google Scholar
|
|
8
|
Lee SH, Lee JH, Lee HY and Min KJ: Sirtuin
signaling in cellular senescence and aging. BMB Rep. 52:24–34.
2019. View Article : Google Scholar :
|
|
9
|
Bradley EW, Carpio LR, van Wijnen AJ,
McGee-Lawrence ME and Westendorf JJ: Histone deacetylases in bone
development and skeletal disorders. Physiol Rev. 95:1359–1381.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Almeida M and Porter RM: Sirtuins and
FoxOs in osteoporosis and osteoarthritis. Bone. 121:284–292. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun W, Qiao W, Zhou B, Hu Z, Yan Q, Wu J,
Wang R, Zhang Q and Miao D: Overexpression of Sirt1 in mesenchymal
stem cells protects against bone loss in mice by FOXO3a
deacetylation and oxidative stress inhibition. Metabolism.
88:61–71. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Ni H, Li H, Deng H, Xu LS, Xu S,
Zhen Y, Shen H, Pan H and Yao M: Nuclear factor kappa B regulated
monocyte chemoattractant protein-1/chemokine CC motif receptor-2
expressing in spinal cord contributes to the maintenance of
cancer-induced bone pain in rats. Mol Pain.
14:17448069187886812018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Faienza MF, D'Amato G, Chiarito M,
Colaianni G, Colucci S, Grano M, Corbo F and Brunetti G: Mechanisms
involved in childhood obesity-related bone fragility. Front
Endocrinol (Lausanne). 10:2692019. View Article : Google Scholar
|
|
14
|
Xie Z, Wang P, Li J, Li Y, Wang S, Wu X,
Sun S, Cen S, Su H, Deng W, et al: MCP1 triggers monocyte
dysfunctions during abnormal osteogenic differentiation of
mesenchymal stem cells in ankylosing spondylitis. J Mol Med (Berl).
95:143–154. 2017. View Article : Google Scholar
|
|
15
|
Arkestal K, Mints M, Enocson A, Linton L,
Marits P, Glise H, Andersson J and Winqvist O: CCR2 upregulated on
peripheral T cells in osteoarthritis but not in bone marrow. Scand
J Immunol. 88:e127222018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Kang S, Zou D, Zhan L, Li Z, Zhu W
and Su H: Bone fracture pre-ischemic stroke exacerbates ischemic
cerebral injury in mice. PLoS One. 11:e01538352016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hui X, Zhang M, Gu P, Li K, Gao Y, Wu D,
Wang Y and Xu A: Adipocyte SIRT1 controls systemic insulin
sensitivity by modulating macrophages in adipose tissue. EMBO Rep.
18:645–657. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang X, Wei J, He Y, Jing T, Li Y, Xiao Y,
Wang B, Wang W, Zhang J and Lin R: SIRT1 inhibition promotes
atherosclerosis through impaired autophagy. Oncotarget.
8:51447–51461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han B, Zhu K, Li FC, Xiao YX, Feng J, Shi
ZL, Lin M, Wang J and Chen QX: A simple disc degeneration model
induced by percutaneous needle puncture in the rat tail. Spine
(Phila Pa 1976). 33:1925–1934. 2008. View Article : Google Scholar
|
|
20
|
Zatroch KK, Knight CG, Reimer JN and Pang
DS: Refinement of intraperitoneal injection of sodium pentobarbital
for euthanasia in laboratory rats (Rattus norvegicus). BMC Vet Res.
13:602017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song Y, Wang Z, Liu L, Zhang S, Zhang H
and Qian Y: 1,4-Dihydropyridine (DHP) suppresses against oxidative
stress in nucleus pulposus via activating sirtuin-1. Biomed
Pharmacother. 121:1095922020. View Article : Google Scholar
|
|
22
|
Liu Y, Li Y, Huang ZN, Wang ZY, Nan LP,
Wang F, Zhou SF, Wang JC, Feng XM and Zhang L: The effect of
intervertebral disc degenerative change on biological
characteristics of nucleus pulposus mesenchymal stem cell: An in
vitro study in rats. Connect Tissue Res. 60:376–388. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo J, Shao M, Lu F, Jiang J and Xia X:
Role of Sirt1 plays in nucleus pulposus cells and intervertebral
disc degeneration. Spine (Phila Pa 1976). 42:E757–E766. 2017.
View Article : Google Scholar
|
|
24
|
Liu J, Tao H, Wang H, Dong F, Zhang R, Li
J, Ge P, Song P, Zhang H, Xu P, et al: Biological behavior of human
nucleus pulposus mesenchymal stem cells in response to changes in
the acidic environment during intervertebral disc degeneration.
Stem Cells Dev. 26:901–911. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang H, Chen S, Huang D, Deng X, Ma K and
Shao Z: Effect of compression loading on human nucleus
pulposus-derived mesenchymal stem cells. Stem Cells Int.
2018:14812432018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou N, Lin X, Dong W, Huang W, Jiang W,
Lin L, Qiu Q, Zhang X, Shen J, Song Z, et al: SIRT1 alleviates
senescence of degenerative human intervertebral disc cartilage
endo-plate cells via the p53/p21 pathway. Sci Rep. 6:226282016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu Z, Huang P, Chong Y, George SK, Wen B,
Han N, Liu Z, Kang L and Lin N: Nucleus pulposus cells derived
IGF-1 and MCP-1 enhance osteoclastogenesis and vertebrae disruption
in lumbar disc herniation. Int J Clin Exp Pathol. 7:8520–8531.
2014.
|
|
28
|
Palada V, Ahmed AS, Finn A, Berg S,
Svensson CI and Kosek E: Characterization of neuroinflammation and
periphery-to-CNS inflammatory cross-talk in patients with disc
herniation and degenerative disc disease. Brain Behav Immun.
75:60–71. 2019. View Article : Google Scholar
|
|
29
|
Zhu X, Cao S, Zhu MD, Liu JQ, Chen JJ and
Gao YJ: Contribution of chemokine CCL2/CCR2 signaling in the dorsal
root ganglion and spinal cord to the maintenance of neuropathic
pain in a rat model of lumbar disc herniation. J Pain. 15:516–526.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He DS, Hu XJ, Yan YQ and Liu H: Underlying
mechanism of Sirt1 on apoptosis and extracellular matrix
degradation of osteoarthritis chondrocytes. Mol Med Rep.
16:845–850. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao H, Chen S, Gao K, Zhou Z, Wang C,
Shen Z, Guo Y, Li Z, Wan Z, Liu C and Mei X: Resveratrol protects
against spinal cord injury by activating autophagy and inhibiting
apoptosis mediated by the SIRT1/AMPK signaling pathway.
Neuroscience. 348:241–251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujita N, Matsushita T, Ishida K, Kubo S,
Matsumoto T, Takayama K, Kurosaka M and Kuroda R: Potential
involvement of SIRT1 in the pathogenesis of osteoarthritis through
the modulation of chondrocyte gene expressions. J Orthop Res.
29:511–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suvakov S, Cubro H, White WM, Tobah YSB,
Weissgerber TL, Jordan KL, Zhu XY, Woollard JR, Chebib FT, Milic
NM, et al: Targeting senescence improves angiogenic potential of
adipose-derived mesenchymal stem cells in patients with
preeclampsia. Biol Sex Differ. 10:492019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Wang P, Yang X, Wang W, Zhang J, He
Y, Zhang W, Jing T, Wang B and Lin R: SIRT1 inhibits inflammatory
response partly through regulation of NLRP3 inflammasome in
vascular endo-thelial cells. Mol Immunol. 77:148–156. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nasiri-Ansari N, Dimitriadis GK,
Agrogiannis G, Perrea D, Kostakis ID, Kaltsas G, Papavassiliou AG,
Randeva HS and Kassi E: Canagliflozin attenuates the progression of
atherosclerosis and inflammation process in APOE knockout mice.
Cardiovasc Diabetol. 17:1062018. View Article : Google Scholar
|
|
36
|
Cheng X, Zhang G, Zhang L, Hu Y, Zhang K,
Sun X, Zhao C, Li H, Li YM and Zhao J: Mesenchymal stem cells
deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus
cell apoptosis and reduce intervertebral disc degeneration. J Cell
Mol Med. 22:261–276. 2018. View Article : Google Scholar
|
|
37
|
Lin CH, Li NT, Cheng HS and Yen ML:
Oxidative stress induces imbalance of adipogenic/osteoblastic
lineage commitment in mesenchymal stem cells through decreasing
SIRT1 functions. J Cell Mol Med. 22:786–796. 2018.
|



















