Introduction
Myocardial infarction (MI) as a result of
cardiovascular diseases is a leading cause of death and disability
world-wide (1). MI caused by
temporary or permanent occlusion of the main coronary arteries may
lead to a reduction in the blood supply to the cardiac muscle
(2). Significant levels of
cardiomyocyte apoptosis are an important physiological feature of
MI, which can be triggered by hypoxia (3). Ultimately, MI may promote heart
failure due to decreased contraction of the myocardium (4). Although the treatment of MI has
mark-edly improved, the mortality and morbidity rates are still far
from satisfactory (5). Therefore,
the identification of potential therapeutic agents against MI, as
well as the elucidation of its underlying mechanisms, is
critical.
Icariside II (ICS II) is a flavonol glycoside that
can be isolated from Herba Epimedii, which has commonly been
used in Traditional Chinese Medicine for thousands of years
(6). ICS II has been reported to
possess anti-inflammatory, antitumor and anti-osteoporotic
properties (7). In recent years,
ICS II has been found to exert various cardioprotective effects,
such as the alleviation of myocardial fibrosis and diabetic
cardiomyopathy (8,9). However, the specific roles and
related molecular mechanisms of ICS II in hypoxia-induced H9c2
cells remain elusive.
MicroRNAs (miRNAs/miRs) are a group of endogenous,
noncoding small RNAs that play important roles in numerous
pathological and physiological processes (10). Accumulating evidence has revealed
that miRNAs play an essential role in the pathophysiological
consequences of MI (11,12), and several potential agents
isolated from herbs have been found to exert their pharmacological
activities through the regulation of miRNAs (13,14). miR-7-5p is a multifaceted miRNA
that participates in the development of various types of cancer
(15). It is also able to protect
cardiomyocytes against ischemia/reperfusion (I/R) injury by
targeting Poly [ADP-ribose] polymerase 1 (16). However, whether miR-7-5p is also
involved in regulating the functions of ICS II in MI was previously
unknown.
In the current study, H9c2 rat cardiomyocytes were
cultured under hypoxic conditions to produce an MI injury model.
Then, the effects of ICS II on the hypoxia-induced injury of H9c2
cells, and its possible molecular mechanisms, were investigated.
The results indicate that ICS II protects H9c2 cells from
hypoxia-induced injury via the regulation of the miR-7-5/BTG
anti-proliferation factor (BTG2) axis and the PI3K/Akt signaling
pathway.
Materials and methods
Cell culture and treatment
Rat cardiomyocyte H9c2 cells were purchased from the
American Type Culture Collection. H9c2 cells were maintained in
DMEM (Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum,
100 U/ml penicillin and 100 µg/ml streptomycin (all Gibco;
Thermo Fisher Scientific, Inc.) at 37°C (5% CO2) in a
humidified atmosphere. To establish the hypoxia-induced injury
model, cells were exposed to hypoxic conditions (93% N2,
2% O2 and 5% CO2) for 24 h; cells incubated
under normoxic conditions (95% air and 5% CO2) were used
as a control. ICS II (purity >98% by HPLC) was purchased from
Nanjing Zelang Medical Technology Corporation Ltd. ICS II was
dissolved in DMSO (Sigma-Aldrich; Merck KGaA) to a concentration of
10 mM and stored at -80°C. ICS II was diluted with complete medium
to achieve the different designated concentrations. Cells were
treated with different concentrations of ICSII (2, 4 and 8
µM) at 37°C for 24 h, and then subjected to different types
of analysis. LY294002 (Sigma-Aldrich; Merck KGaA), an inhibitor of
the PI3K/Akt pathway, was stored at −20°C and diluted in complete
medium at a final concentration of 10 µM. Where appropriate,
cells were co-treated with LY294002 (10 µM) and ICSII (8
µM) at 37°C for 24 h, and subsequently analyzed
experimentally. The same concentration of DMSO was applied to cells
as a control. All other routine chemicals were obtained from
Sigma.
Transfection
miR-7-5p mimics, miR-7-5p inhibitor and a negative
control miR (NC) were all synthesized by Suzhou GenePharma Co., Ltd
and used at a concentration of 100 nM. The full-length BTG2
sequence was inserted into a pcDNA 3.1 plasmid (Suzhou GenePharma
Co., Ltd.), and the empty vector was used as a negative control.
The cells were transfected with the corresponding vectors using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Reverse
transcription-quantitative (RT-q) PCR and western blot analysis
were performed to determine transfection efficacy. Subsequent
experimentation was performed 24 h after the transfection.
Cell viability assay
The Cell Counting Kit-8 (CCK-8; Beijing Solarbio
Science & Technology Co., Ltd.) was used to assess cell
viability according to the manufacturer's protocol. H9c2 cells were
seeded into 96-well plates at a density of 5×103
cells/well and cultured at 37°C overnight. The cells were then
cultured under hypoxic conditions for 24 h (as afore-mentioned),
which was followed by a further 24-h incubation at 37°C under
normoxic conditions. After reoxygenation, 10 µl CCK-8
solution was added to each well, followed by a 4-h incubation
period. The absorbance at 450 nm was measured using a microplate
reader (Bio-Rad Laboratories, Inc.).
Cell migration and invasion assays
The migration of H9c2 cells was assessed by chamber
migration assays, using two-chamber-Transwell 24-well cell culture
plates (Corning, Inc.) with 8-µm polycarbonate filters.
Briefly, hypoxic injury was induced in H9c2 cells as
aforementioned, following treatment with ISC II where appropriate;
the cells were then resuspended in 200 µl serum-free media,
and then seeded into the upper chambers of the inserts within a
24-well plate (1×105 cells/well); 500 µl complete
medium was added to the lower compartment as a chemoattractant.
After culture for 48 h at 37°C, the migrated cells in the lower
chamber were fixed with ethanol, stained with crystal violet at
room temperature for 5 min, and counted under an inverted light
microscope at ×200 magnification (Olympus Corporation). For the
invasion assays, the inserts were precoated with Matrigel (BD
Biosciences) at room temperature for 1 h. Each experiment was
performed in triplicate.
Apoptosis assay
The percentage of apoptotic cells was measured using
the FITC-Annexin V Apoptosis Detection kit (BD Bioscience)
according to the manufacturer's instructions. The cells were
analyzed using a FACScan flow cytometer (BD Bioscience) with FlowJo
v10.0.7 software (Tree Star, Inc.).
RNA purification and RT-qPCR
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Reverse
transcription of the RNA was performed using the PrimeScript™ RT
reagent kit (Takara Biotechnology Co., Ltd.) and qPCR was performed
with the TaqMan™ MicroRNA Reverse Transcription kit and TaqMan™
Universal Master Mix (Applied Biosystems; Fisher Scientific, Inc.).
GAPDH and U6 were used as internal controls for BTG2 and miR-7-5p,
respectively. The reverse transcription primer for miR-7-5p was
5′-GTC GTA TCC AGT GCA GGG TCC GAG GTG CAC TGG ATA CGA CAC AAC
AA-3′. The qPCR primers were as follows: miR-7-5p forward, 5′-TGG
AAG ACT AGT GAT TTT-3′ and reverse, 5′-CTC AAC TGG TGT CGT G-3;
BTG2 forward, 5′-CTG GAG GAG AAC TGG CTG TC-3′ and reverse, 5′-AAA
ACA ATG CCC AAG GTC TG-3′; U6 forward, 5′-GCT TCG GCA GCA CAT ATA
CTA AAA T-3′ and reverse, 5′-CGC TTC ACG AAT TTG CGT GTC AT-3′; and
GAPDH forward, 5′-TGA CCA CAG TCC ATG CCA TC-3′ and reverse, 5′-GAC
AAG CTT CCC GTT CTC AG-3′. The qPCR conditions were as following:
30 sec at 94°C, followed by 40 cycles of 5 sec at 94°C, 10 sec at
55°C, 10 sec at 72°C, and a final extension of 10 min at 72°C. The
expression levels in tissues and cells were calculated using the
2−ΔΔCq method (17).
miRNA microarray analysis
Total cellular RNA was purified from cells cultured
under hypoxic conditions using TRIzol® reagent and the
RNeasy mini kit (Qiagen GmbH) according to the manufacturer's
instructions. The RNA samples were quantified using a NanoDrop
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.), and then labeled using the miRCURY™ Hy3™/Hy5™ Power
labelling kit (Qiagen GmbH). The labelled samples were hybridized
to a microarray (NimbleGen Systems, Inc.) for 16-20 h, and the
array sections were scanned using the GenePix 4000B micro-array
scanner (Axon Instruments). The results were read and analyzed
using GenePix Pro V6.0 (Molecular Devices, LLC). A heat map diagram
was used to display the data for two-way hierarchical clustering of
genes and samples.
Bioinformatic analysis
TargetScan bioinformatics software (www.targetscan.org) was used to predict the potential
target of miR-7-5p.
Dual-luciferase reporter assay
A BTG2 3'-unranslated region (UTR) cDNA fragment,
which contained the miR-7-5p binding site, was subcloned into a
pmirGIO dual luciferase miRNA target expression plasmid (Promega
Corporation) to create a luciferase reporter vector (BTG2-wt). A
BTG2 3'-UTR that was mutated at the miR-7-5p complementary site was
generated (BTG2-mut) using the GeneArt™ Site-directed Mutagenesis
System (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions, and was also inserted into a pmirGIO
reporter plasmid. H9c2 cells were subsequently co-transfected with
the both reporter plasmid and miR-7-5p mimics or mimic NC, and
luciferase activity was subsequently detected using the
Dual-luciferase Reporter Assay system (Promega Corporation).
Transfection was performed using Lipofectamine® 2000
according to the manufacturer's protocol. Luciferase activity was
measured 24 h after transfection and Renilla luciferase
activity was used to normalize that of firefly luciferase.
Lactate dehydrogenase (LDH) release
detection
The release of LDH was measured using the LDH
Cytotoxicity Assay kit (Beyotime Institute of Biotechnology)
according to the manufacturer's instructions.
Western blot analysis
Total protein was extracted from cells using RIPA
lysis buffer and quantified using the Caspase-3 Assay kit
(Fluorometric) (both Beyotime Institute of Biotechnology). Equal
amounts of protein (20 µg) were resolved using 12% SDS-PAGE
gels and then transferred onto PVDF membranes (EMD Millipore).
After blocking with 5% skim milk, the membranes were incubated with
primary antibodies overnight at 4°C, which was followed by probing
with ant-mouse IgG, HRP-linked antibody (cat. no. 7076) or
anti-rabbit HRP-linked antibody (cat. no. 7074) (1:5,000, Cell
Signaling Technology, Inc.) at room temperature for 1 h. The
protein bands were visualized using an ECL detection system (EMD
Millipore), and the intensity of the protein bands was quantified
using ImageJ Software (National Institutes of Health). The
following primary antibodies were purchased from Cell Signaling
Technology, Inc.: Bcl-2 (cat. no. 15071), Bax (cat. no. 14796),
GAPDH (cat. no. 5174), caspase-3 (cat. no. 9662), caspase-9 (cat.
no. 9502), phosphorylated (p)-PI3K (cat. no. 4228), PI3K (cat. no.
3011), p-Akt (cat. no. 9614) and Akt (cat. no. 2966). The BTG2
antibody (cat. no. ab85051) was purchased from Abcam. All primary
antibodies were diluted at a ratio of 1:1,000 in 5% BSA.
Statistical analysis
The data are presented as the means ± standard
deviation from three independent repeated experiments. Statistical
analysis was performed using GraphPad 7.0 statistical software
(GraphPad Software, Inc.), and the significant differences between
groups were deter-mined by one-way analysis of variance followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
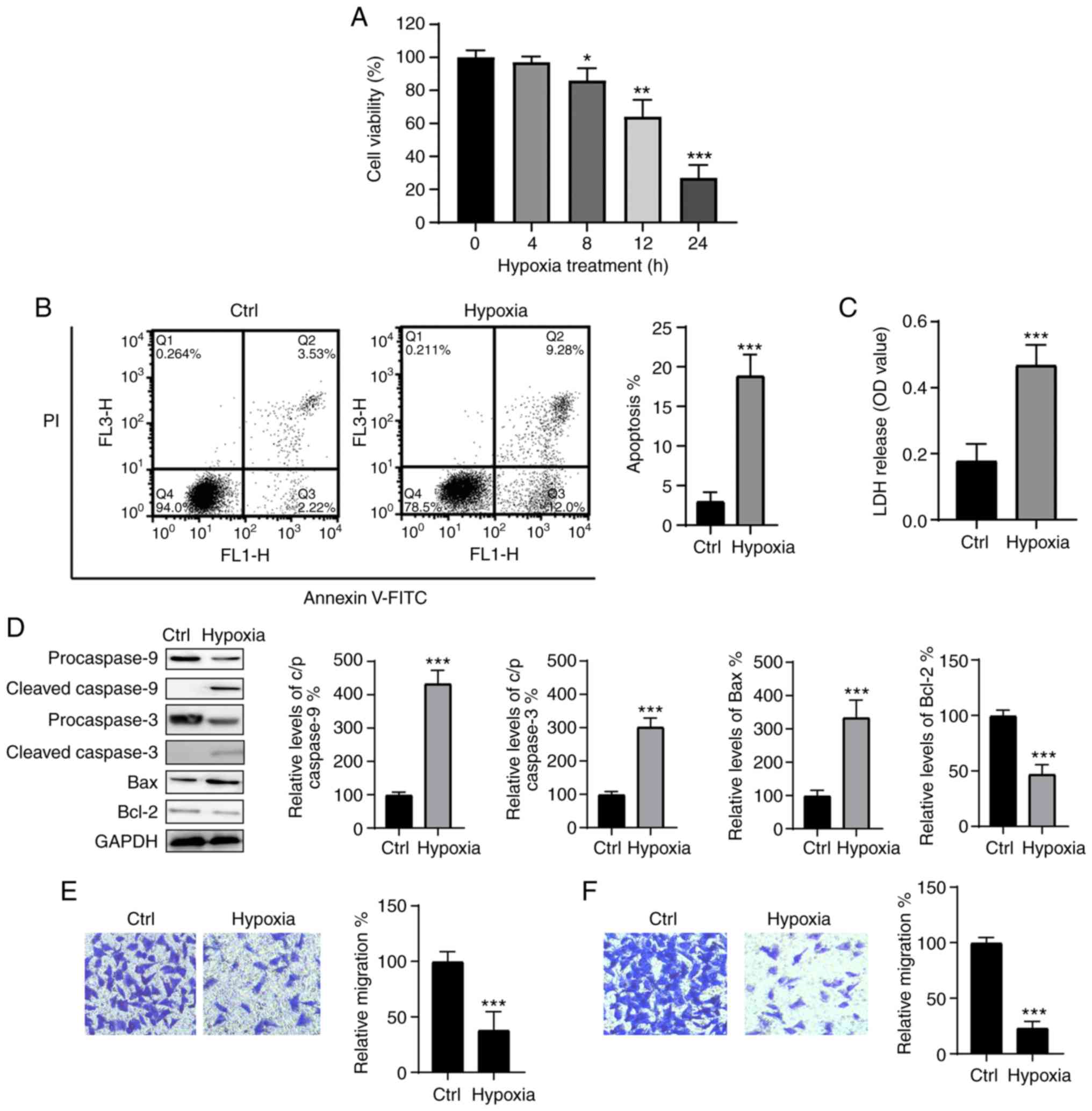
Hypoxia induces myocardial injury in H9c2
cells
To produce a hypoxia-induced injury model, H9c2
cells were exposed to hypoxic conditions for 4, 8, 12 and 24 h, and
cellular viability was assessed using the CCK-8 assay. As indicated
in Fig. 1A, hypoxia reduced the
viability of H9c2 cells in a time-dependent manner. The levels of
apoptosis were also detected following culture under hypoxic
conditions for 24 h and were significantly increased when compared
with those of the control group (Fig.
1B). These results were further supported by an LDH assay,
which confirmed that hypoxia treatment significantly promoted LDH
release (Fig. 1C). Furthermore,
compared with the control, hypoxia treatment led to the
downregulation of the anti-apoptotic protein Bcl-2, and
upregulation of the proapoptotic protein Bax; the cleavage of
caspase-3 and -9 was also observed following exposure to hypoxic
conditions (Fig. 1D). The
migration and invasion abilities of H9c2 cells were also
significantly decreased following culture under hypoxic conditions
(Fig. 1E and F). Therefore, it
was confirmed that hypoxia results in damage to H9c2 cells, and
that an in vitro hypoxia cell model was successfully
generated.
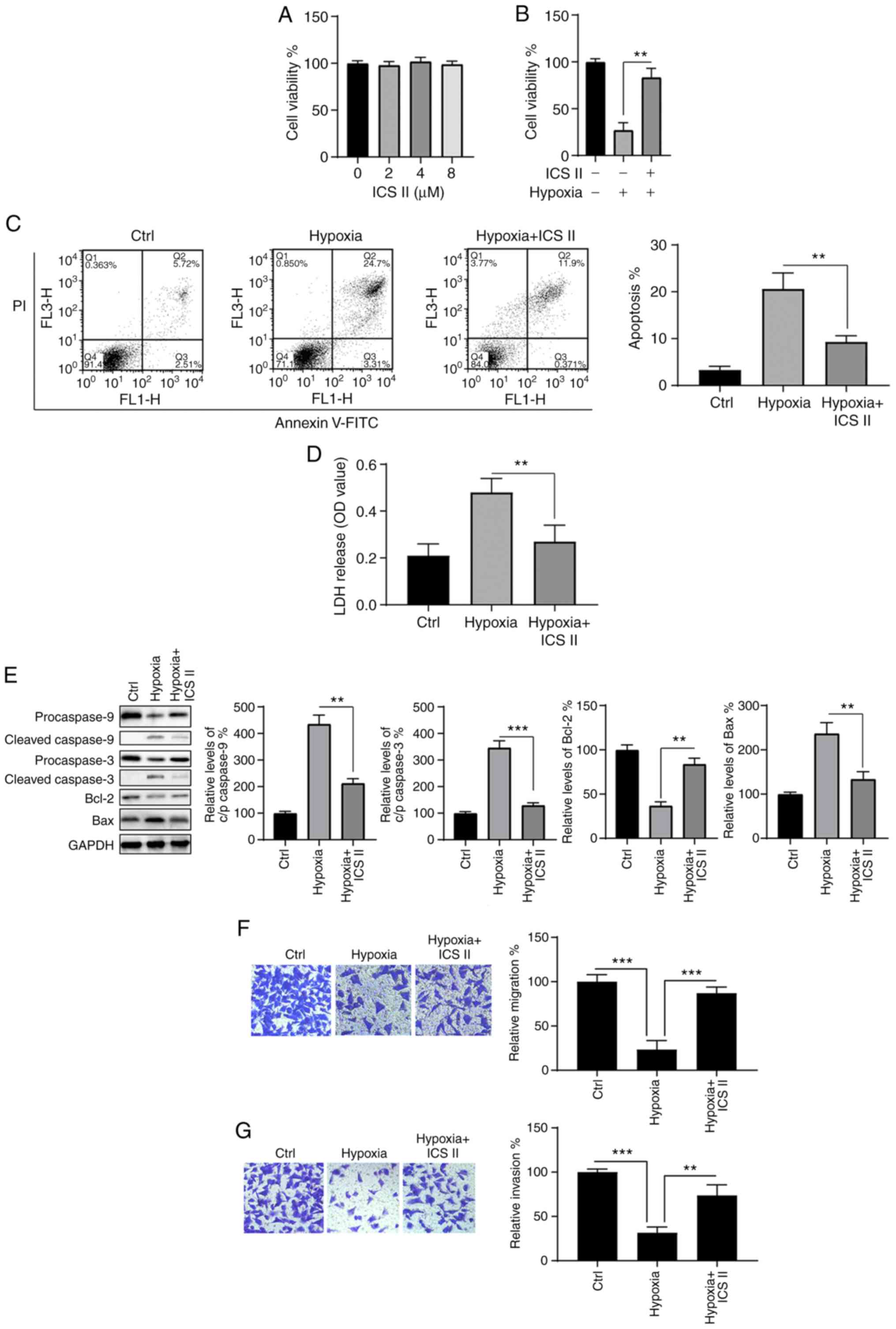
ICS II protects H9c2 cells from
hypoxia-induced injury
The effects of ICS II on hypoxia-induced injury in
H9c2 cells were investigated. To determine a suitable dosage, cells
were incubated with various concentrations of ICS II (2, 4 and 8
µM) for 24 h, and cell viability was then assessed. As shown
in Fig. 2A, there were no
significant differences in cell viability among cells treated with
different concentrations of ICS II. As 2-8 µM exerted
neither pro- nor anti-survival effects on H9c2 cells under normoxic
conditions, 8 µM was selected as the dose of ICS II for
subsequent experiments.
The protective effects of ICS II on H9c2 cells under
hypoxic conditions were then investigated. CCK-8 assay results
indicated that the hypoxia-induced decrease in cell viability was
notably mitigated by ICS II pretreatment (P<0.01; Fig. 2B). Similarly, the hypoxia-induced
increase in apoptosis was significantly reversed (P<0.001;
Fig. 2C), and hypoxia-induced LDH
release was significantly inhibited (P<0.01; Fig. 2D) by ICS II pretreatment. Western
blot analysis revealed that hypoxia induced alterations in Bax,
Bcl-2, cleaved caspase-3 and -9, and that these results were
significantly reversed by ICS II treatment (P<0.05; Fig. 2E). Furthermore, ICS II treatment
markedly increased the migration and invasion capacities of H9c2
cells under hypoxic conditions (Fig.
2F and G). These data indicate that ICS II provides protection
against hypoxia-associated injury in H9c2 cells.
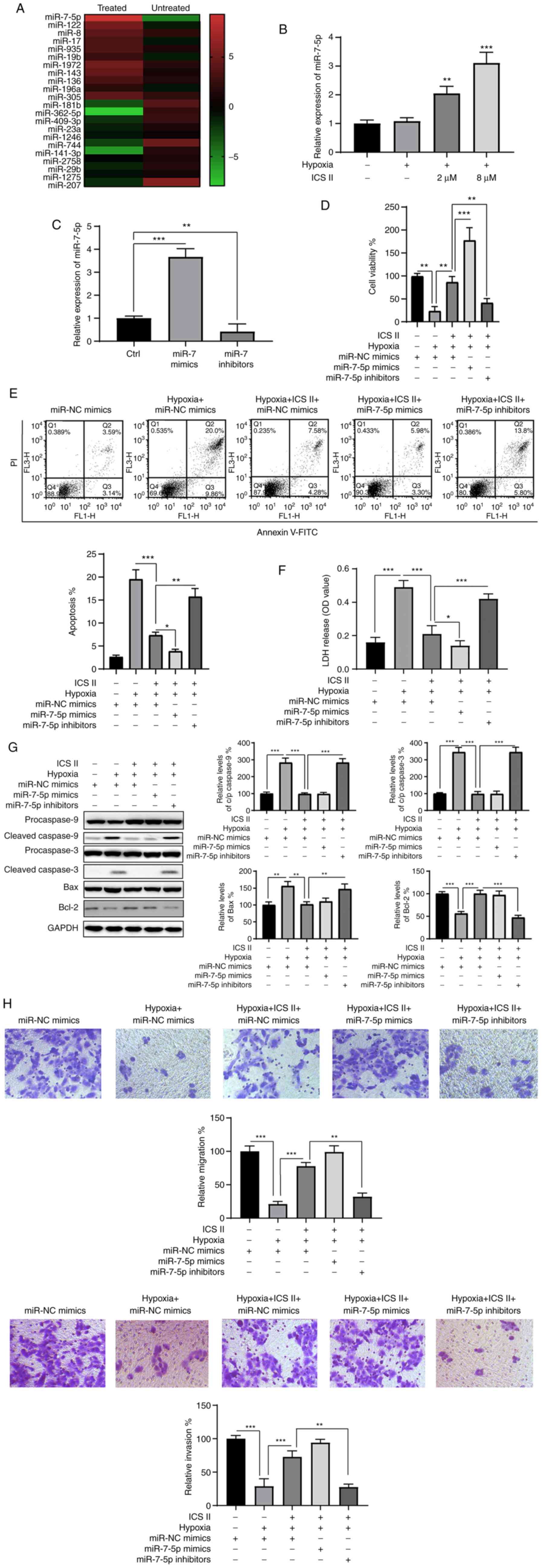
ICS II attenuates hypoxia-induced injury
in H9c2 cells by upregulating miR-7-5p expression
An miRNA chip array was used to determine whether
the protective effects of ICS II were exerted via the regulation of
miRNAs. In total, 11 upregulated and 11 downregulated miRNAs were
identified following ICS II treatment of H9c2 cells under hypoxic
conditions, of which miR-7-5p was the most upregulated (Fig. 3A). The levels of miR-7-5p
following ICS II treatment were then verified using RT-qPCR. The
results showed that treatment with ICS II markedly increased
miR-7-5p expression (Fig. 3B),
indicating the involvement of miR-7-5p in the protective effects of
ICS II. To investigate the role of miR-7-5p in this setting,
miR-7-5p mimics and inhibitors were transfected into H9c2 cells. As
indicated in Fig. 3C, miR-7-5p
levels were significantly increased following transfection with
miR-7-5p mimics (P<0.001), and notably decreased by the miR-7-5p
inhibitor (P<0.01) compared with the control cells. The
transfected cells were then treated with ICS II prior to exposure
to hypoxic conditions. The effects of ICS II pretreatment were
significantly abrogated by inhibiting miR-7-5p, which is indicated
by decreased cell viability (P<0.01; Fig. 3D), increased apoptosis (P<0.01;
Fig. 3E) and increased LDH
release (P<0.001; Fig. 3F).
Conversely, miR-7-5p mimics enhanced the protective effects of ICS
II, showing increased cell viability (P<0.001; Fig. 3C), decreased apoptosis (P<0.01;
Fig. 3E) and decreased LDH
release (P<0.05; Fig. 3F).
Moreover, western blot analysis confirmed that the protective
effect of ICS II against hypoxia-induced injury relied on the
upregulation of miR-7-5p (Fig.
3G), since caspase-3 cleavage was rescued by inhibiting
miR-7-5p in the presence of ICS II under hypoxic conditions. ICS II
was also demonstrated to restore the migration and invasive
capacities of H9c2 cells under hypoxic conditions via miR-7-5p,
since inhibition of miR-7-5p blocked this effect (Fig. 3H). These data indicate that ICS II
provides protective effects against hypoxia in H9c2 cells, at least
partly via the upregulation of miR-7-5p.
miR-7-5p directly targets BTG2, which
reverses the effect of miR-7-5p on H9c2 cells exposed to hypoxic
conditions
Using TargetScan, BTG2 was identified as a potential
target for miR-7-5p (Fig. 4A,
left). Luciferase reporter assays confirmed the association between
miR-7-5p and BTG2 (Fig. 4B); the
luciferase activity of BTG2-wt was significantly inhibited in
miR-7-5p-transfected cells, while that of the BTG2-mut remained
unaltered. Furthermore, the mRNA and protein levels of BTG2 were
significantly decreased and increased in the miR-7-5p mimic and
inhibitor groups, respectively (Fig.
4B and C). These observations suggest a negative regulatory
relationship between miR-7-5p and BTG2.
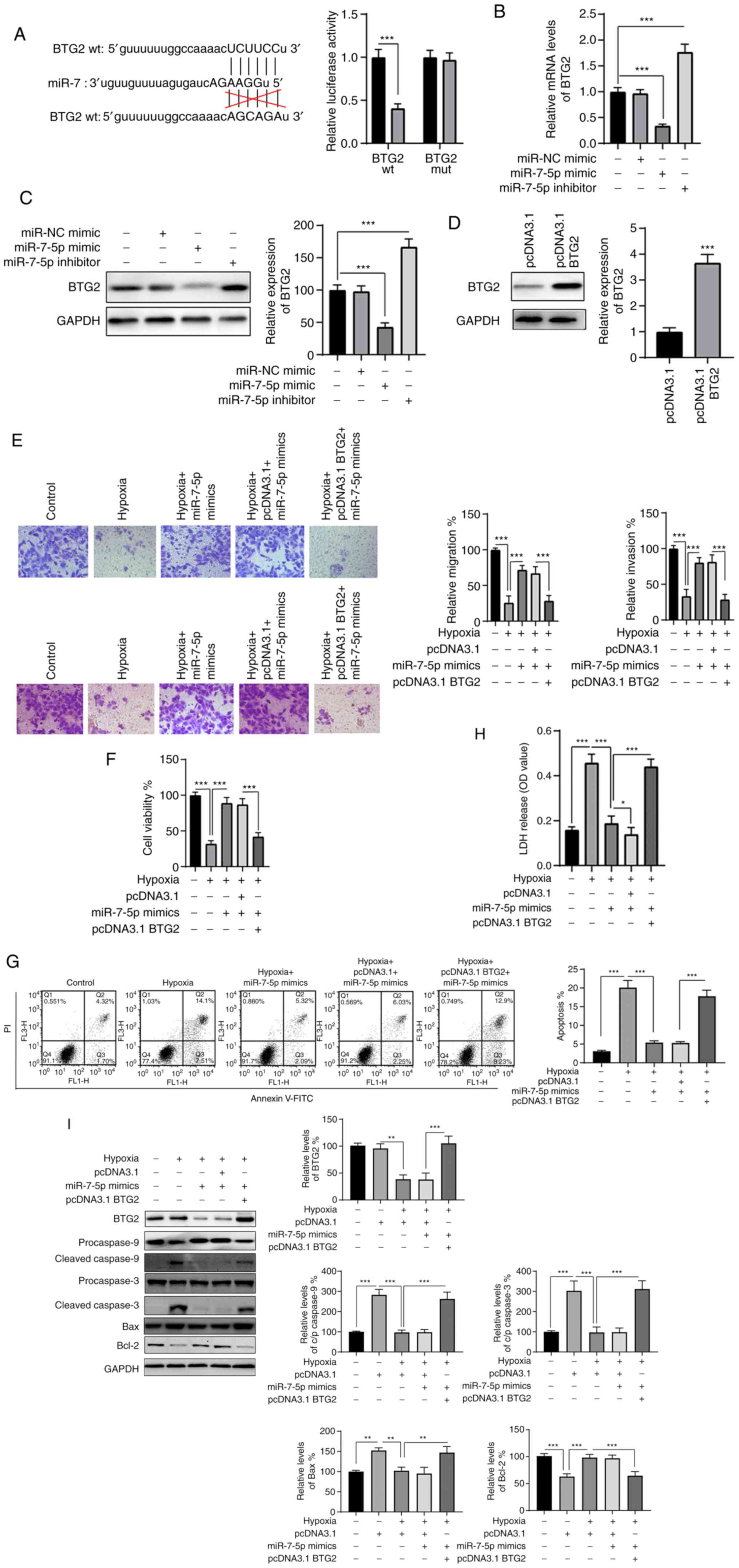 | Figure 4BTG2 is a direct target of miR-7-5p.
(A) The putative binding site between BTG2 and miR-7-5p was
predicted and the association was analyzed by dual luciferase
reporter assay. After transfection, the (B) mRNA and (C) protein
expression levels of BTG2 were measured using reverse
transcription-quantitative PCR and western blotting, respectively.
(D) H9c2 cells were transfected as indicated, and the protein
levels of BTG2 were measured by western blotting. Cells were
treated as indicated, and (E) migration and (F) invasion capacities
were assayed. Cells were treated as indicated, and the (F)
viability, (G) rates of apoptosis and (H) release of LDH were
assayed. (I) Total cellular lysates were subjected to the western
blot analysis. Data are presented as the mean ± SD of three
independent experiments. *P<0.05,
**P<0.01 and ***P<0.001. BTG2, BTG
anti-proliferation factor; miR/miRNA, micro RNA; WT, wild type;
MUT, mutant; LDH, lactate dehydrogenase. |
BTG2 was then ectopically expressed to investigate
whether miR-7-5p affected hypoxia-induced injury in H9c2 cells via
the regulation of BTG2. As indicated in Fig. 4D, western blot analysis showed
that the protein level of BTG2 was successfully overexpressed in
H9c2 cells via transfection with pcDNA3.1 BTG2 (P<0.001).
Further experiments revealed that the effects of miR-7-5p on
migration and invasion under hypoxic conditions were blocked by
BTG2 overexpression (Fig. 4E).
Functional experiments indicated that miR-7-5p overexpression
retained cell viability (P<0.001; Fig. 4F), reduced apoptosis (P<0.001;
Fig. 4G), reduced LDH release
(P<0.001; Fig. 4H),
downregulated cleaved caspase-3/-9 and pro-apoptotic Bax, and
upregulated Bcl-2 (Fig. 4I) in
hypoxia-treated cells. However, the effects of miR-7-5p
overexpression on cells treated under hypoxic conditions were
obviously abrogated by BTG2 overexpression (P<0.001; Fig. 4F-I). These results indicate that
the overexpression of BTG2 reverses the effect of miR-7-5p on
hypoxia-treated H9c2 cells.
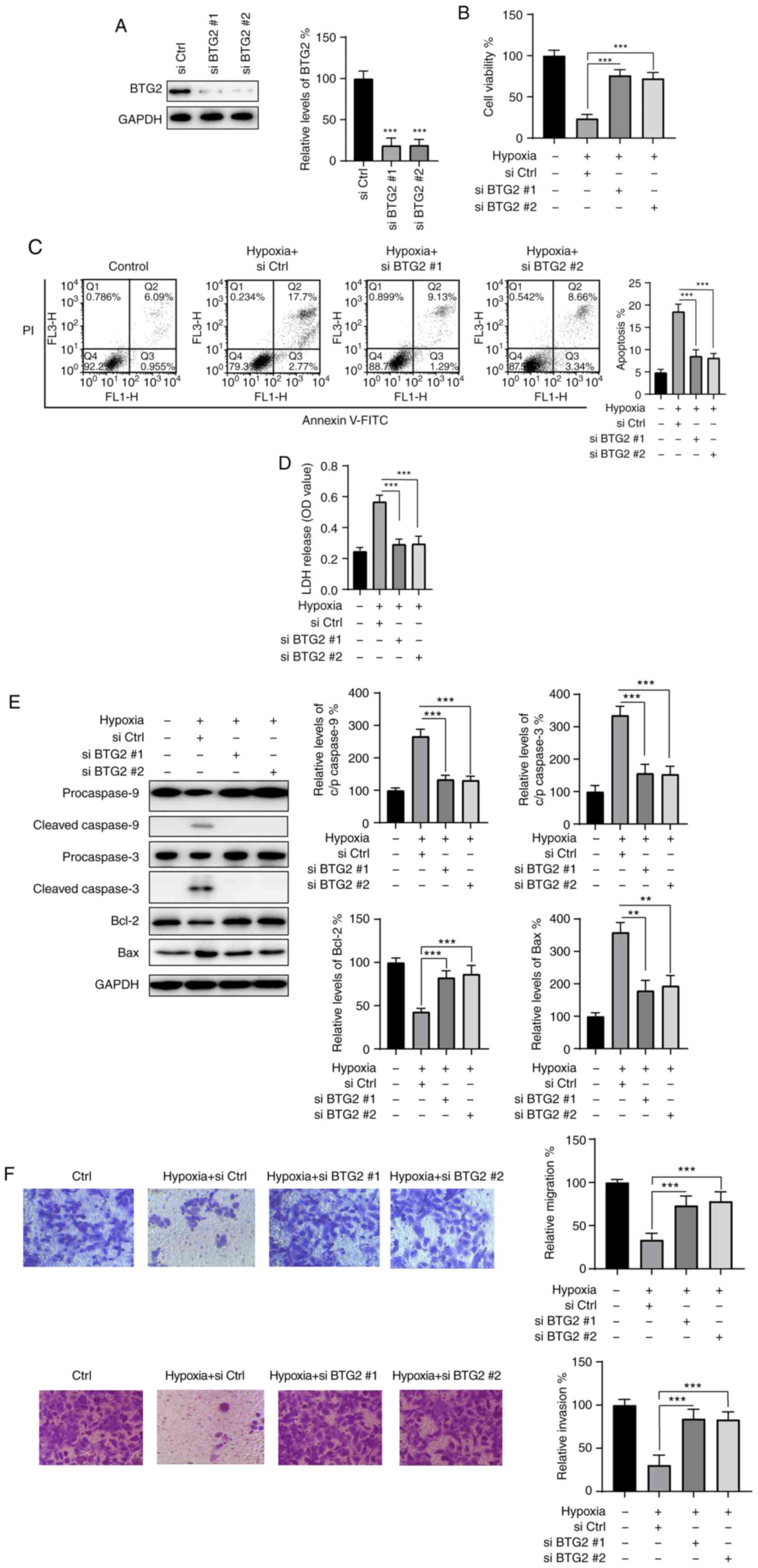
BTG2 knockdown protects H9c2 cells from
hypoxia-induced injury
To further confirm the role of BTG2 in the
protective effects of ICS II, siRNAs were used to knock down BTG2
in H9c2 cells. As indicated in Fig.
5A, two siRNAs targeting BTG2 (siBTG2 #1 and #2) were
transfected into H9c2 cells for 24 h, which resulted in a
significant decrease in BTG2 expression compared with the control
group (siCtrl); 24 h post-transfection, H9c2 cells were exposed to
hypoxic conditions for another 24 h, and cell viability was
assayed. BTG2 knockdown under hypoxic conditions markedly enhanced
the viability of H9c2 cells compared with that of the control group
(Fig. 5B). Moreover, knockdown of
BTG2 successfully reduced apoptosis and LDH release caused by
hypoxia (Fig. 5C and D). In
addition, western blotting also confirmed that the activation of
caspase-3 and -9, the downregulation of Bcl-2 and the upregulation
of Bax caused by hypoxia were reversed by BTG2 knockdown. Knocking
down BTG2 also restored the migration and invasion abilities of
H9c2 cells under hypoxic conditions (Fig. 5F). Taken together, these data
suggest that BTG2 may be an important regulator in hypoxia-induced
injury of H9c2 cells.
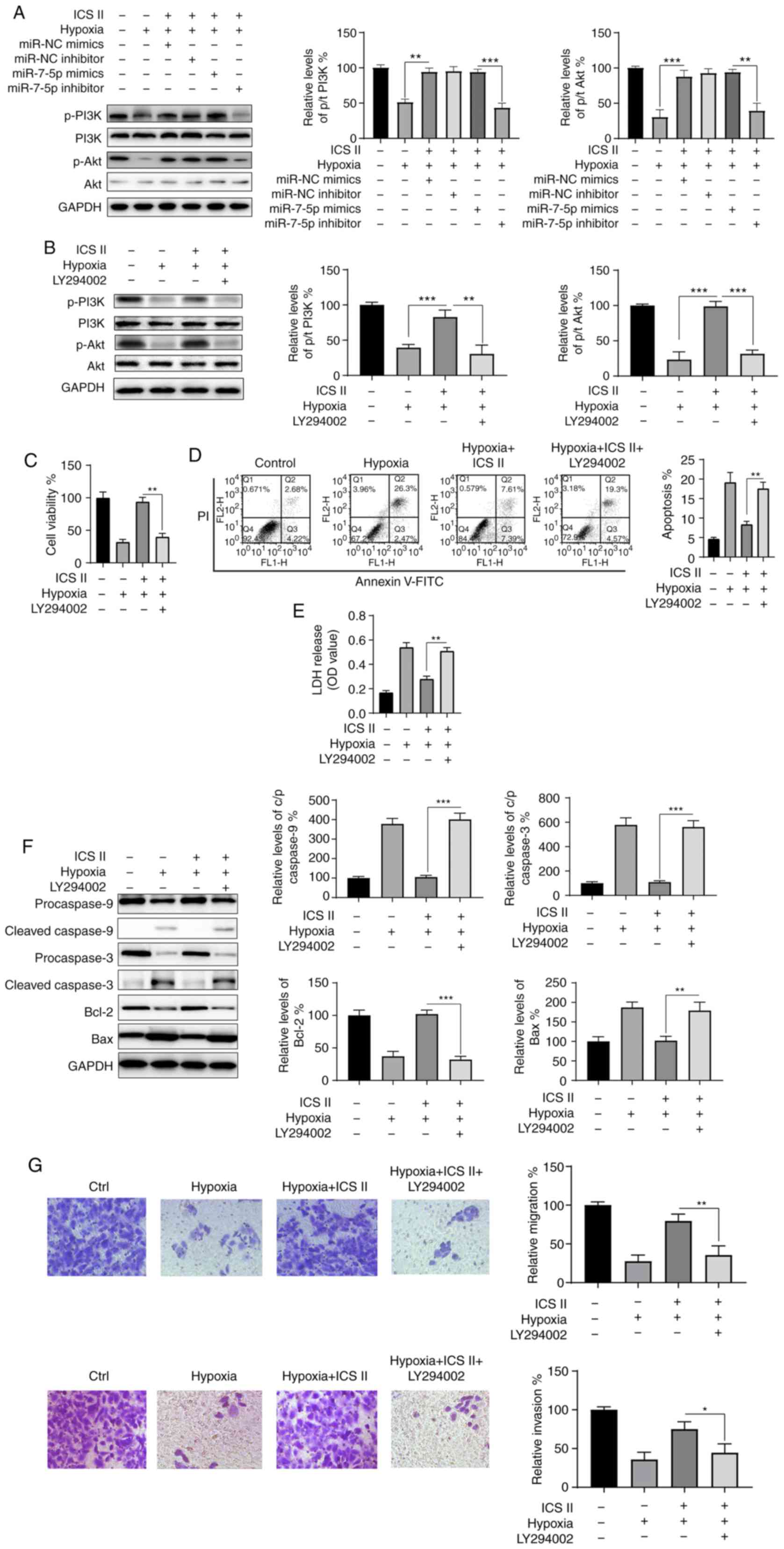
ICS II exerts its protective effect by
activating the PI3K/Akt signaling pathway
Mounting evidence indicates that the PI3K/Akt
signaling pathway plays an essential protective role in
cardiomyoblasts. Therefore, the potential effects of ICS II on the
PI3K/Akt signaling pathway were investigated. As indicated in
Fig. 5A, p-PI3K and p-Akt levels
were notably decreased in H9c2 cells exposed to hypoxic conditions,
compared with those of the control group; there was little change
in the levels of total PI3K and Akt. Treatment with ICS II or
overexpression of miR-7-5p mimics restored the expression of p-PI3K
and p-Akt, indicating the activation of the PI3K/Akt signaling
pathway (Fig. 6A). However, the
phosphorylation levels of PI3K and Akt were decreased in the
presence of hypoxia, ICS II and miR-7-5p inhibitor combined
(Fig. 6A). These findings
indicate that ICS II activates the PI3K/Akt signaling pathway via
the upregulation of miR-7-5p under hypoxic conditions. To further
investigate the function of the PI3K/Akt signaling pathway in the
protective effect of ICS II, cells were treated with LY294002, a
specific PI3K/Akt inhibitor. As shown in Fig. 6B, LY294002 successfully inhibited
the activation of the PI3K/Akt signaling pathway. LY294002 also
abrogated the protective effects of ICS II on the viability of H9c2
cells under hypoxic conditions (Fig.
6C). LY249002 also promoted apoptosis and the release of LDH,
which was induced by hypoxia in the presence of ICS II (Fig. 6D and E). Moreover, the activation
of caspase-3 and -9, a decrease in Bcl-2 and an increase in Bax
caused by hypoxia were also caused by LY294002 (Fig. 6F). Furthermore, the effects of ICS
II on the migration and invasion of H9c2 cells under hypoxic
conditions could be blocked by LY294002 (Fig. 6G). Collectively, these data
suggest that ICS II treatment activates the PI3K/Akt signaling
pathway, which is essential for the survival of H9c2 cells under
hypoxic conditions.
Discussion
In the present study, the protective effects of ICS
II on hypoxia-induced cardiomyocyte injury were investigated, as
well as the potential underlying molecular mechanisms. Hypoxic
exposure was found to markedly decrease the viability of rat H9c2
cardiomyocytes and to induce apoptosis. ICS II protected H9c2 cells
against hypoxia-induced apoptosis and a loss in viability by
upregulating the expression of miR-7-5p. In addition, BTG2 was
identified as a direct target of miR-7-5p, and its overexpression
abrogated the protective effects of miR-7-5p in a hypoxia-induced
injury model. Furthermore, ICS II was also shown to activate the
PI3K/Akt signaling pathway, which is essential for the survival of
H9c2 cells under hypoxic conditions.
ICS II is a flavonol glycoside isolated from
Herba Epimedii that has been found to exhibit antitumor,
anti-inflammatory, neuroprotective and antidiabetic activities
(18-20). However, there are few reports
about the effects of ICS II on hypoxia-induced cell injury. A
previous study showed that ICS II could attenuate myocardial
fibrosis by inhibiting the NF-kB and transforming growth
factor-β1/Smad2 signaling pathways (8). Another study indicated that ICS II
ameliorated diabetic cardiomyopathy in streptozotocin-induced
diabetic rats (9). However, the
effects of ICS II on hypoxic-induced cardiomyocyte injury remained
elusive. In the present study, hypoxia-induced injury was
significantly abrogated by ICS II, presenting with increased
viability and reduced apoptosis. In line with previous studies,
these findings further confirmed the cardioprotective effects of
ICS II.
To elucidate the potential underlying molecular
mechanisms of ICS II protection on hypoxia-induced H9c2 cell
injury, the relationship between ICS II and miR-7-5p was
investigated in hypoxia-treated H9c2 cells. miR-7-5p is a miRNA
that is evolutionarily conserved from parasites to mammals.
miR-7-5p has been found to act as a tumor suppressor in various
types of cancer, such as bladder cancer, pancreatic cancer and
melanoma (21-23). Only a few studies have
investigated the functions of miR-7-5p in cardiomyopathy, but it
has been found to protect cardiomyocytes from I/R-induced injury
(16). In addition, a recent
study also revealed that the inhibition of miR-7-5p contributed to
myocardial I/R injury (24). In
line with these reports, the present study revealed that inhibiting
miR-7-5p expression notably reversed the protective effects of ICS
II in a hypoxia-induced H9c2 cell injury model.
BTG2 is a member of the BTG/TOB gene family
(25) that BTG2 has been
identified as a tumor suppressor in various cancer types (26). A previous study found that the
downregulation of BTG2 via miR-21 could protect cardiomyocytes
against doxorubicin-induced apoptosis (27). In the present study,
bioinformatics and luciferase gene reporter assays showed that BTG2
was a direct target of miR-7-5p in H9c2 cells. Moreover, BTG2
reversed the protective effect of miR-7-5p on hypoxia-induced
injury in H9c2 cells. In addition, BTG2 knockdown emulated the
effects of ICS II treatment by providing protective effects to H9c2
cells against hypoxia-induced injury. These findings indicate that
BTG2 may be an important regulator in MI, and that the effect of
ICS II on the expression of miR-7-5p may also rely on the
regulation of BTG2. Notably, it was also found that hypoxia
treatment could lead to the upregulation of BTG2 (28); there-fore, further studies are
required to further investigate the role of BTG2 in hypoxia.
An abundance of evidence has shown that activation
of the PI3K/Akt pathway is able to protect the heart from I/R
injury, as well as enhance the survival of cardiomyocytes (29,30). Consistent with the findings of the
present study, ICS II activated the Akt signaling pathway and
thereby exerted protective effects on osteoblasts (31). However, another study reported
that ICS II inhibited the PI3K/Akt signaling pathway in non-small
cell lung cancer cells. From the results of the present study, it
was proposed that ICS II is able to both activate and inhibit the
PI3K/Akt signaling pathway depending on the cell type involved,
though further study is required to confirm this hypothesis. In
line with a previous study, miR-7-5p was also shown to trigger the
activation of the PI3K/Akt signaling pathway (32). Notably, in another recent study,
it was shown that silencing BTG2 contributed to the activation of
the PI3K/Akt pathway, which is in accordance with the findings of
the present study (33).
To the best of our knowledge, the present study was
the first to investigate the effects of ICS II on hypoxia-treated
H9c2 cells. The results revealed an in vitro protective
effect of ICS II on cardiomyocytes. The present study also revealed
that miR-7-5p was a downstream factor responsive to ICS II, and
that ICS II may protect H9c2 cells from hypoxia-induced injury via
the upregulation of miR-7-5p. Furthermore, BTG2 was identified as a
direct target gene of miR-7-5p, and BTG2 over-expression reversed
the effects of miR-7-5p on hypoxia-treated H9c2 cells, while BTG2s
knockdown provided protective effects. Finally, the present study
demonstrated that ICS II treatment also led to the activation of
the PI3K/Akt pathway, which is essential for the survival of H9c2
cells under hypoxic conditions. More clinical and/or in vivo
experimentation is required to support these findings.
Acknowledgments
Not applicable.
Funding
The present study was supported by the key fund of
the Jiangxi Science and Technology Bureaucracy (grant no.
20171BBG70016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DH was involved in the conception of the study and
writing the original manuscript draft. YG was involved in study
conception and design, and critical revision of the manuscript. DW
was involved in study conception and design, and data acquisition.
JZ, QL and JL analyzed and interpreted the data and drafted the
manuscript. SL took part in data acquisition and revising the
manuscript for important intellectual content. ZY and BZ took part
in study conception and design, as well as supervision, project
administration, funding acquisition and writing (reviewing and
editing). All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Townsend N, Wilson L, Bhatnagar P,
Wickramasinghe K, Rayner M and Nichols M: Cardiovascular disease in
Europe Epidemiological update 2016. Eur Heart J. 37:3232–3245.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Graham RM, Frazier DP, Thompson JW, Haliko
S, Li H, Wasserlauf BJ, Spiga MG, Bishopric NH and Webster KA: A
unique pathway of cardiac myocyte death caused by hypoxia-acidosis.
J Exp Biol. 207:3189–3200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kemp CD and Conte JV: The pathophysiology
of heart failure. Cardiovasc Pathol. 21:365–371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambrosy AP, Fonarow GC, Butler J, Chioncel
O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN
and Gheorghiade M: The global health and economic burden of
hospitalizations for heart failure: Lessons learned from
hospitalized heart failure registries. J Am Coll Cardiol.
63:1123–1133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie F, Wu CF, Lai WP, Yang XJ, Cheung PY,
Yao XS, Leung PC and Wong MS: The osteoprotective effect of Herba
epimedii (HEP) extract in vivo and in vitro. Evid Based Complement
Alternat Med. 2:353–361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen M, Wu J, Luo Q, Mo S, Lyu Y, Wei Y
and Dong J: The anticancer properties of Herba epimedii and its
main bioactive componentsicariin and icariside II. Nutrients.
8:E5632016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fu S, Li YL, Wu YT, Yue Y, Qian ZQ and
Yang DL: Icariside II attenuates myocardial fibrosis by inhibiting
nuclear factor-κB and the TGF-β1/Smad2 signalling pathway in
spontaneously hypertensive rats. Biomed Pharmacother. 100:64–71.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Peng C, Xia J, Zhang W, Tian L,
Tian Y, Yang X and Cao Y: Effects of icariside II ameliorates
diabetic cardiomyopathy in streptozotocin-induced diabetic rats by
activating Akt/NOS/NF-κB signaling. Mol Med Rep. 17:4099–4105.
2018.
|
|
10
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boon RA and Dimmeler S: MicroRNAs in
myocardial infarction. Nat Rev Cardiol. 12:135–142. 2015.
View Article : Google Scholar
|
|
13
|
Gu Y, Liang Z, Wang H, Jin J, Zhang S, Xue
S, Chen J, He H, Duan K, Wang J, et al: Tanshinone IIA protects
H9c2 cells from oxidative stress-induced cell death via
microRNA-133 upregulation and Akt activation. Exp Ther Med.
12:1147–1152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang G, Wu H, Hu Y, Li J and Li Q:
Gastrodin inhibits glutamate-induced apoptosis of PC12 cells via
inhibition of CaMKII/ASK-1/p38 MAPK/p53 signaling cascade. Cell Mol
Neurobiol. 34:591–602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalinowski FC, Brown RA, Ganda C, Giles
KM, Epis MR, Horsham J and Leedman PJ: microRNA-7: A tumor
suppressor miRNA with therapeutic potential. Int J Biochem Cell
Biol. 54:312–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li B, Li R, Zhang C, Bian HJ, Wang F, Xiao
J, Liu SW, Yi W, Zhang MX, Wang SX, et al: MicroRNA-7a/b protects
against cardiac myocyte injury in ischemia/reperfusion by targeting
poly(ADP-ribose) polymerase. PLoS One. 9:e900962014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Song J, Feng L, Zhong R, Xia Z, Zhang L,
Cui L, Yan H, Jia X and Zhang Z: Icariside II inhibits the EMT of
NSCLC cells in inflammatory microenvironment via down-regulation of
Akt/NF-κB signaling pathway. Mol Carcinog. 56:36–48. 2017.
View Article : Google Scholar
|
|
19
|
Khan M, Maryam A, Qazi JI and Ma T:
Targeting apoptosis and multiple signaling pathways with icariside
II in cancer cells. Int J Biol Sci. 11:1100–1112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao J, Deng Y, Yin C, Liu Y, Zhang W, Shi
J and Gong Q: Icariside II, a novel phosphodiesterase 5 inhibitor,
protects against H2 O2 -induced PC12 cells
death by inhibiting mitochondria-mediated autophagy. J Cell Mol
Med. 21:375–386. 2017. View Article : Google Scholar
|
|
21
|
Li J, Qiu M, An Y, Huang J and Gong C:
miR-7-5p acts as a tumor suppressor in bladder cancer by regulating
the hedgehog pathway factor Gli3. Biochem Biophys Res Commun.
503:2101–2107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu W, Wang Y, Zhang D, Yu X and Leng X:
MiR-7-5p functions as a tumor suppressor by targeting SOX18 in
pancreatic ductal adenocarcinoma. Biochem Biophys Res Commun.
497:963–970. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giles KM, Brown RA, Ganda C, Podgorny MJ,
Candy PA, Wintle LC, Richardson KL, Kalinowski FC, Stuart LM, Epis
MR, et al: microRNA-7-5p inhibits melanoma cell proliferation and
metastasis by suppressing RelA/NF-κB. Oncotarget. 7:31663–31680.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zou L, Ma X, Lin S, Wu B, Chen Y and Peng
C: Long noncoding RNA-MEG3 contributes to myocardial
ischemia-reperfusion injury through suppression of miR-7-5p
expression. Biosci Rep. 39:BSR201902102019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Buanne P, Corrente G, Micheli L, Palena A,
Lavia P, Spadafora C, Lakshmana MK, Rinaldi A, Banfi S, Quarto M,
et al: Cloning of PC3B, a novel member of the PC3/BTG/TOB family of
growth inhibitory genes, highly expressed in the olfactory
epithelium. Genomics. 68:253–263. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuniati L, Scheijen B, van der Meer LT and
van Leeuwen FN: Tumor suppressors BTG1 and BTG2: Beyond growth
control. J Cell Physiol. 234:5379–5389. 2019. View Article : Google Scholar :
|
|
27
|
Tong Z, Jiang B, Wu Y, Liu Y, Li Y, Gao M,
Jiang Y, Lv Q and Xiao X: MiR-21 protected cardiomyocytes against
doxorubicin-induced apoptosis by targeting BTG2. Int J Mol Sci.
16:14511–14525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leszczynska KB, Foskolou IP, Abraham AG,
Anbalagan S, Tellier C, Haider S, Span PN, O'Neill EE, Buffa FM and
Hammond EM: Hypoxia-induced p53 modulates both apoptosis and
radiosensitivity via AKT. J Clin Invest. 125:2385–2398. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bell RM and Yellon DM: Bradykinin limits
infarction when administered as an adjunct to reperfusion in mouse
heart: The role of PI3K, Akt and eNOS. J Mol Cell Cardiol.
35:185–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Keyes KT, Xu J, Long B, Zhang C, Hu Z and
Ye Y: Pharmacological inhibition of PTEN limits myocardial infarct
size and improves left ventricular function postinfarction. Am J
Physiol Heart Circ Physiol. 298:H1198–H1208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu W, Mao L, Ji F, Chen F, Wang S and Xie
Y: Icariside II activates EGFR-Akt-Nrf2 signaling and protects
osteoblasts from dexamethasone. Oncotarget. 8:2594–2603. 2017.
View Article : Google Scholar :
|
|
32
|
Liu ML, Zhang Q, Yuan X, Jin L, Wang LL,
Fang TT and Wang WB: Long noncoding RNA RP4 functions as a
competing endogenous RNA through miR-7-5p sponge activity in
colorectal cancer. World J Gastroenterol. 24:1004–1012. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Z, Wang Y, Xiong J, Cui F, Wang L and
Peng H: NUSAP1 knockdown inhibits cell growth and metastasis of
non-small-cell lung cancer through regulating BTG2/PI3K/Akt
signaling. J Cell Physiol. 235:3886–3893. 2019. View Article : Google Scholar : PubMed/NCBI
|