Introduction
Head and neck cancers involve the formation of
tumors originating from any tissue or organ in the head and neck,
apart from the eyes, brain, ears, thyroid and esophagus, including
neck tumors, otolaryngological tumors, and oral and maxillofacial
tumors. Head and neck cancer is the 6th most common malignancy
worldwide (1,2), and, 90% of cases are head and neck
squamous cell carcinoma (HNSCC) cases. With the development of
surgery, chemotherapy, radiation therapy, targeted therapy and
multidisciplinary sequential therapy, the quality of life of
patients with HNSCC has improved to a certain extent. However, the
5-year survival rate of patients with HNSCC remains low compared to
that of patients with other malignant cancers, such as cervical and
breast cancers (3). Recurrence,
distant metastasis and drug resistance are the main obstacles to
the treatment of oral squamous carcinoma (4). In recent years, immunotherapy has
provided new treatment options for patients with head and neck
cancers (5). Nonetheless, the
lack of a proper model linking immunology research with clinical
diagnosis and treatment strategies severely hampers the development
of novel antitumor immunotherapies and drugs (6).
4-Nitroquinoline-1-oxide (4-NQO) is a potent
chemical carcinogen. It has been extensively used in both rats and
mice (7-9) and is useful for studies on the
mechanisms and progression of HNSCC (10,11). Long-term repeated exposure to
carcinogens is widely considered to be one of the most common risk
factors of HNSCC. The 4-NQO-induced tumor model requires several
months to establish, and the process is similar to the process of
human HNSCC. 4-NQO can cause NADPH-catalyzed DNA damage. NADPH
quinone oxidoreductase is abundantly expressed in the tongue
mucosa. As a result, the progression of tumors induced by 4-NQO can
aptly mimic the progression of human cancers, and the mouse model
of 4-NQO-induced tumors may be an ideal tool for the study of the
molecular mechanisms of HNSCC. Additionally, such a mouse model
provides a platform for investigating the molecular mechanisms of
and therapeutic strategies for HNSCC. However, the mouse model of
4-NQO-induced tumors is time-consuming to establish and cannot be
easily repeated.
Immunotherapy is a significant part of malignancy
therapy, which highlights the importance of research on the
mechanisms of tumor immunology for successful clinical translation
(12). However, obstacles to such
research exist; for example, there only a limited number of proper
animal models for immunological research in vivo, and
experiments in vitro have limitations that can cause drug
resistance and relapse (13). As
a result, a reliable mouse model is urgently required for tumor
immunology studies (14).
Microsatellites (MSs) are ubiquitous in the human
genome and are mostly located in the non-coding regions of genes,
and the proximal telomere regions of chromosomes. Currently, it is
considered that MSs play an important role in maintaining genomic
stability and regulating gene expression. Microsatellite
instability (MSI) is characterized by the existence of different
numbers of replicated units of the same MS locus between different
individuals or between normal and abnormal tissues in the same
individual. The loss of heterozygosity (LOH) is characterized by
the loss of a normal allele from a region of one chromosome of a
pair, which allows a defective allele on the homologous chromosome
to cause clinical manifestations. LOH is generally associated with
tumor suppressor genes (such as p53), which inhibit the occurrence
of malignant tumors when both alleles are present. When one allele
is clearly abnormal or missing (and the other is already inactive)
and no longer inhibits malignancy, normal cells become malignant.
However, the mechanisms of genetic aberrations, such as MSI and LOH
remain unclear in HNSCC. According to the method of LOH analysis
described previously (15), the
degree of LOH was determined to exclude the possibility that clones
may have lost neoantigen-generating mutations and the results
revealed that there were no LOH events at the genomic positions of
the neoantigens, suggesting that no neoantigens were lost owing to
LOH.
In the present study, the tumorigenic HNSCC cell
line 'JC1' and the tumorigenicity-enhanced cell line 'JC1-2' were
established. Transplanted tumors derived from JC1 cells could only
grow in immunodeficient nude mice, while tumors derived from JC1-2
cells could grow in immunocompetent C57BL/6 mice. Next-generation
sequencing (NGS) technologies were used to characterize the JC1 and
JC1-2 cells, and it was verified that both cell lines had an MS
stability (MSS) phenotype and a responsive interferon (IFN)-γ
pathway. Orthotopic and heterotopic mouse tumor models of JC1-2
cells were established and more intense immune responses were
observed in the microenvironment of the orthotopic model. This
syngeneic model may thus enable the better delineation of
interactions between HNSCC and lymphocytes and the exploration of
potential therapeutic targets for HNSCC.
Materials and methods
Animals and primary culture of the HNSCC
cell lines
All C57BL/6 mice used in the present study (n=58;
weighing 16-22 g, 6 weeks old) had ad libitum access to
sterile food and water and were maintained in a stable environment
under constant temperature and humidity (22±5°C, 60±3% humidity)
with a 12-h light-dark cycle. In the experiments, all mice were
female to avoid the antigenic diversity resulting from any sex
differences. 4-NQO (Sigma-Aldrich; Merck KGaA) at a concentration
of 120 µg/ml (we selected 80, 100 and 120 µg/ml as
the preliminary experimental dose of 4-NQO, due to the high tumor
formation rate and low mouse death rate, 120 µg/ml was
selected) was added to the drinking water of the mice (n=20) for 20
weeks (the animal bedding and water were changed every week). In
total, 3 of these 20 mice (15%) died during this experiment
(possibly due to drug toxicity) (16), and the surviving animals were
observed for an additional 4 weeks prior to sacrifice. All the mice
were observed and weighed once every 3 days to monitor their
health. Tumors were measured once a week. Once the volumes of
tumors were >1,000 mm3, the mice were euthanized with
100% compressed CO2 gas at a flow rate of 20% chamber
vol/min. In addition, 5 C57BL/6 mice were provided with normal
drinking water as the normal controls (to observe the appearances
and pathological manifestations of normal mucosal tissues). A
strict criterion for humane endpoints was used as follows: i)
weight loss >15% for 72 h; ii) severe dehydration; iii) sluggish
behaviors (inability to eat or drink); iv) arching back or lateral
decubitus; and v) inability to move normally as the tumor was too
large (>1,000 mm3) or for other reasons. Once a mouse
was found to exhibit any of the above symptoms, it would be
euthanized with 100% compressed CO2 gas at a flow rate
of 20% chamber vol/min for 7 min. The death of the mice was
verified by the assurance of cessation of respiratory and
cardiovascular movements by observation at room air for at least 10
min. All animal experiments were approved by and performed in
accordance with the guidelines of the Shanghai Jiao Tong University
School of Medicine.
After 24 weeks, all mice were sacrificed, and oral
tumors (mostly on the tongue, some were on the cheeks and mouth
floor) were collected from the mice for further cell line
establishment. Fresh tongue tumor samples were selected and cut
into sections (0.5 mm3), after which they were incubated
at 37°C. A limiting dilution assay was applied to screen
mono-clonal HNSCC cells, and the largest colonies were kept for
further culture. After 20 passages, the murine HNSCC cell lines
were considered to have been established. A total of 15 athymic
nude mice were used to examine the tumorigenicity of 3 cell lines.
Cell morphology was examined under a microscope (ZEISS Axioscope
5). The 3 HNSCC cell lines, named JC1, JC2 and JC3, were then
established, and one of these, termed JC1, was selected for further
analysis due to its enhanced proliferative ability and
tumorigenicity in immunodeficient nude mice (data not shown).
JC1 cells were incubated with 4-NQO (0.1
µg/ml) for 24 h and the treated cells were then inoculated
into immunocompetent C57BL/6 mice. Successfully growing tumors
(tumor formation rate, approximately 30%) were collected, primary
culture was applied, and a new cell line, termed JC1-2, was
established.
Following the anesthetization of the mice with
pentobarbital (Sigma-Aldrich; Merck KGaA, diluted in saline, 75
mg/kg injected intraperitoneally), 5×106 JC1-2 cells
were injected carefully into the cheeks and underneath the skin of
the backs of the mice using a 27-gauge needle to establish
orthotopic and heterotopic tumor models. A total of 12 C57BL/6 mice
were involved and sacrificed when the tumor dimension was >1,000
mm3. The mouse melanoma cell line B16 (TCM 2), mouse
colorectal cancer cell line CT26 (TCM37) and the mouse breast
cancer cell line 4T1 (TCM32) were purchased from the Chinese
Academy of Sciences Cell Bank/Stem Cell Bank. The mouse squamous
cell line, SCC7, was a generous gift from Professor Zhuang Liu
[Institute of Functional Nano and Soft Materials (FUNSOM),
Collaborative Innovation Center of Suzhou Nano Science and
Technology, Soochow University]. A total of 1×106 cells
were injected carefully underneath the skins of the backs of the
mice (B16 cells, C57BL/6 mice; CT26 and 4T1 cells, BALB/c mice;
SCC7 cells, C3H mice) using a 27-gauge needle to establish
trans-planted tumor models (3, 6-week-old female mice weighing
16-22 g were used for each different type of mouse). All mice were
provided with ad libitum access to sterile food and water
and were maintained in a stable environment under constant
temperature and humidity (22±5°C, 60±3% humidity) with a 12-h
light-dark cycle. Tumors were measured once a week. Once the
volumes of tumors were >1,000 mm3, the mice were
euthanized with 100% compressed CO2 gas at a flow rate
of 20% chamber vol/min for 7 min. The death of the mice was
verified by the assurance of cessation of respiratory and
cardiovascular movements by observation at room air for at least 10
min. All tumor samples were fixed with formalin and embedded in
paraffin for further staining.
Immunohistochemical and
immunofluorescence staining
Murine tumor samples were fixed with
paraformaldehyde (Sangon Biotech) and embedded in paraffin. The
slides (3-µm-thick) of the formalin-fixed paraffin-embedded
(FFPE) samples were then used for staining. Slides were incubated
with primary antibodies (Abs) against the following proteins at
37°C for 1 h: Anti-pan cytokeratin (CK-Pan; 1:400 dilution, ab7753,
Abcam), Ki-67 (1:400 dilution, 9449s, Cell Signaling Technology,
Inc.) and vimentin (1:1,000 dilution, 10366-1-AP, Proteintech)
β-2-microglobulin (B2m; 1:4,000 dilution, ab218230, Abcam) and with
HRP-conjugated goat anti-rabbit IgG secondary antibody (1:250,
ab7090, Abcam) at 37°C for 30 min. The HNSCC samples were stained
with hematoxylin (abs9139 Absin Bioscience, Inc.) and eosin
(abs9222, Absin Bioscience, Inc.) (H&E). Normal tongues from
the control group were used as control samples. All IHC images were
examined with a microscope (ZEISS Axioscope 5).
After the slides were stained with primary
(β-2-microglobulin, B2m; 1:4,000 dilution, ab218230, Abcam; 45 min,
37°C) and secondary antibodies (1:250, ab7090, Abcam; 30 min,
37°C), a tyramide (TSA)-conjugated fluorophore (NEL791001KT, Perkin
Elmer; T20950, Life Technologies; Thermo Fisher Scientific, Inc.)
was added to the slides at a 1:100 dilution in amplification buffer
(NEL791001KT, Perkin Elmer). The slides were incubated for 10 min
at room temperature and then washed with PBS 3 times. Finally, the
slides were stained with DAPI (40728ES50, Yeasen) and incubated in
37°C for 3 min. The slides were imaged by Zeiss Axio Scan Z1 and
analyzed using ZEISS imaging software ZEN lite.
Exome and RNA sequencing and data
analysis
Total DNA was isolated from the JC1 and JC1-2 cells
using an AllPrep DNA/RNA Mini kit (Qiagen GmbH). Library
preparations and sequencing were performed at Shanghai
Biotechnology Corporation. Syngeneic mouse normal mucosal exomes
and JC1 and JC1-2 cell samples were collected and sequenced with an
Illumina HiSeq 2500 platform. The variant analysis was performed
using GATK developed by Broad Institute which primarily focuses on
SNPs and INDELs (17,18). For RNA sequencing, total RNA was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and the quality was verified. An nCounter
Analysis System (NanoString Technologies) was used to screen for
significantly differentially expressed genes. The purified mRNA was
subsequently fragmented into sizes of 200-500 bp. An Illumina
HiSeqTM 2000 was used for cDNA library paired-end sequencing. The
cDNA libraries were subjected to library quantification prior to
cluster generation. Paired end 2,650 bp sequencing runs were
performed to align the cDNA sequences to the mouse mm9 reference
genome. Potential mutated peptides resulting from non-synonymous
mutations were analyzed to predict their binding affinity to the
major histocompatibility complex class I (Mhc-I) alleles H-2Kb and
H-2Db. A binding affinity <500 nM was considered to indicate
strong binding. A mutation was defined as expressed if the
normalized counts of the corresponding gene were >10. The
pheatmap package in R (version 3.2.0) was employed to conduct the
bidirectional hierarchical clustering. String v11.0 (https://string-db.org/) and CytoScape (http://www.cytoscape.org/) were used to illustrate
functional interactions among the differential expression genes
(DEGs). The Gene Ontology (GO, http://geneontology.org/) and Kyoto Encyclopedia of
Genes and Genomes (KEGG, https://www.genome.jp/kegg/) databases were used for
further pathway and function enrichment analysis. Protein-protein
analyses were performed to reveal the network of the differentially
expressed genes based on the interactions among the genes, proteins
and compounds included in the KEGG database. Lollipops-v1.3.2
(https://github.com/pbnjay/lollipops/releases/tag/v1.3.2)
was used to show point locations of specific genes in the genomic
region.
Co-culture of JC1-2 cells and
splenocytes
A total of 6 female C57BL/6 mice were euthanized
with 100% compressed CO2 gas and splenocytes were
isolated. The spleens were ground repeatedly and filtered twice.
Following suspension and centrifugation at 400 x g at 4°C for 10
min, the cells were washed twice with PBS and centrifuged at 400 x
g at 4°C for 15 min to isolate the mononuclear cell layer.
For indirect co-culture experiments, 1.0 µm
pore size Millicell Hanging Cell Culture Inserts (EMD Millipore)
were placed on top of the JC1-2 cells that had been previously
plated. The splenocytes were seeded onto the insert at a density of
1×107 cells per insert. The JC1-2 cells had no direct
contact with the splenocytes when the inserts were used in this
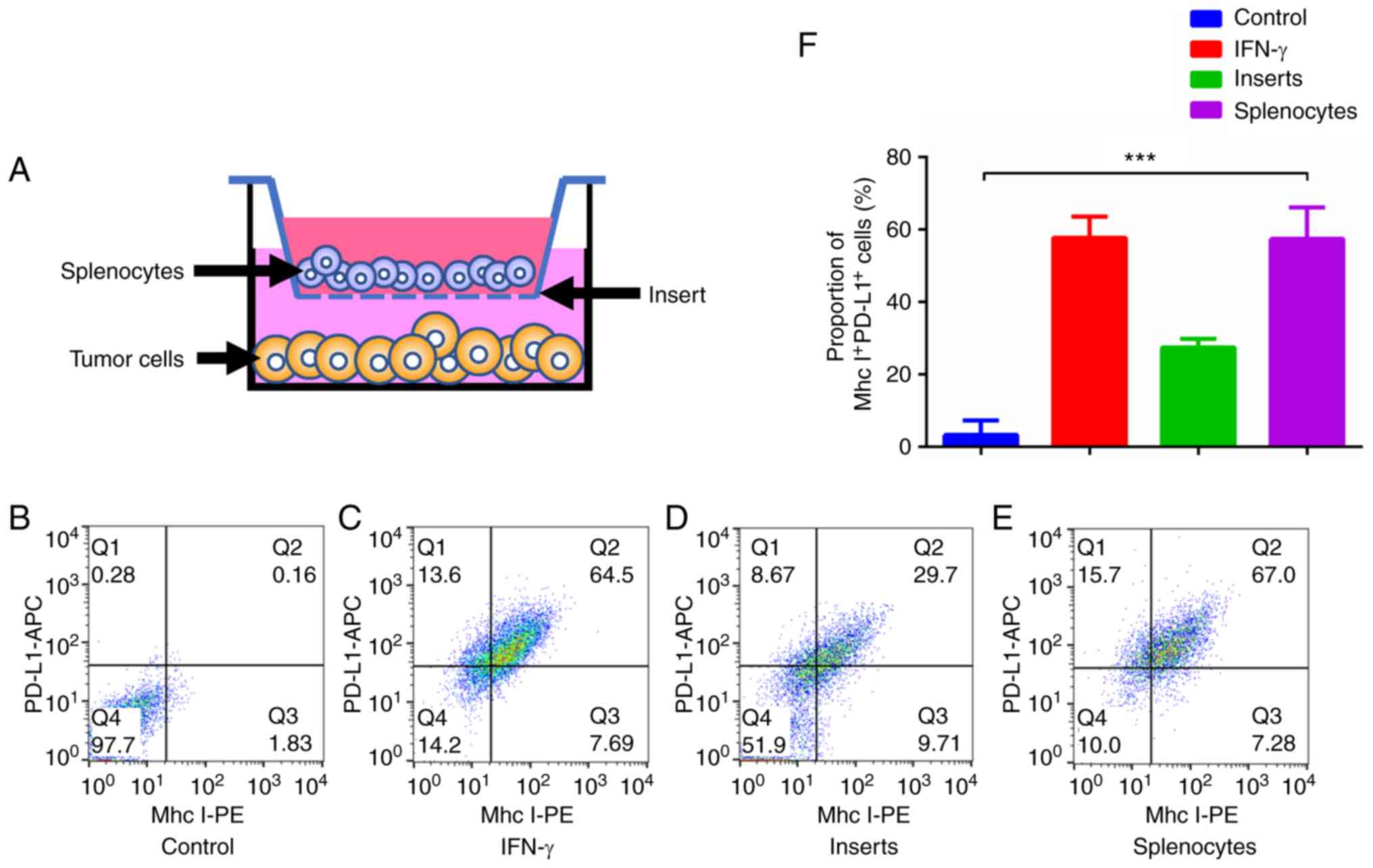
manner (Fig. 3A). IFN-γ
(Sigma-Aldrich; Merck KGaA) was used at a concentration of 10 ng/ml
for 48 h.
In direct co-culture experiments, the splenocytes
(1×107 cells per well) were directly added to JC1-2
cells (5×105 cells per well). A BD IMag™ Mouse T
Lymphocyte Enrichment Set-DM kit (BD Biosciences) was used to
isolate CD3+ T lymphocytes and other non-CD3+
splenocytes. CD3+ T lymphocytes and non-CD3+
splenocytes were added at a density of 1×107 cells per
well.
JC1-2 cells were digested and resuspended at a
concentration of 1×106 cells/ml. All plates were
incubated at 37°C for 6 h to allow cell adherence, and the culture
medium was then discarded and replaced with 1 ml of fresh medium.
After 4 days, all wells contents were collected for analysis.
Flow cytometry and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis of Mhc I and PD-L1 expression in JC1-2 cells
JC1-2 cells were collected, 1×106 cells
were suspended, and 2 µl of Fc block (BD Biosciences) was
added. The cells were washed with PBS twice, resuspended in 1 ml of
PBS with 1% FBS and then incubated at 37°C for 45 min with
antibodies against PE-MHC-I (562004, BD Biosciences) and APC-PD-L1
(564715, BD Biosciences). The cells were again washed with PBS and
resuspended in 500 µl of BD stain buffer, and the different
positive cells were analyzed with a BD FACSCanto flow cytometer (BD
Biosciences). To examine the cell cycle of JC1-2 cells, the cells
were incubated at 4°C for 20 min with Propidium Iodide Staining
Solution (556463, BD Biosciences) prior to flow cytometry.
A total 1×106 cells were harvested for
RT-qPCR analysis. Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and cDNA was
synthesized using SuperScript IV (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's instructions. The
mRNA expression of Mhc-I was measured by RT-qPCR using the
StepOnePlus™ Real-Time PCR System (4376600; Thermo Fisher
Scientific, Inc.) using SYBR-Green (Takara Biotechnology Co., Ltd.)
technology. The thermocycling conditions were as follows: 95°C for
30 sec, then 30 cycles of 95°C for 5 sec, 60°C for 30 sec). The
Mhc-I primer sequences were as follows: Forward, 5′-AGG ACA TGG AGC
TTG TGG AGA CC-3′ and reverse, 5′-TGT TGG AGA CAG TGG ATG GA G
GA-3′. GAPDH was used as an internal control. The EGFR primer
sequences were as follows: Forward, 5′-TCC TGA TTG GTG CTGTGC GAT
TC-3′ and reverse, 5′-CTG GCA GTT CTC CTC TCC TCC TC-3′. The GAPDH
primer sequences were as follows: Forward, 5′-GGT TGT CTC CTG CGA
CTT CA-3′ and reverse, 5′-TGG TCC AGG GTT TCT TAC TCC-3′. The
average cycle threshold (Ct) value of each group was calculated,
and the Ct value of the control group was subtracted to determine
the ΔCt value. The mRNA expression levels of Mhc-I and PD-L1 mRNA
was calculated by using the 2−ΔΔCq method (19). The values for the control group
were set as 1, and the values for the other groups were calculated
as the fold changes relative to the control values.
Multiplex fluorescent
immunohistochemistry (IHC) for immune cell markers in tumor
samples
Murine tumor samples were fixed with
paraformaldehyde (Sangon Biotech). After the slides were stained
with primary (CD3, 1:200 dilution, ab16669, Abcam; CD8, 1:400
dilution, 98941, Cell Signaling Technology, Inc.; and PD-1, 1:400
dilution, ab214421, Abcam; 45 min, 37°C) and secondary antibodies
(30 min, 37°C), a tyramide (TSA)-conjugated fluorophore
(NEL791001KT, PerkinElmer; T20950, Life Technologies) was added to
the slides at a 1:100 dilution in amplification buffer
(NEL791001KT, PerkinElmer). The slides were incubated for 10 min at
room temperature and then washed with PBS 3 times. This step was
followed by heat-mediated Ag stripping [0.1 M glycine (G2879,
Sigma-Aldrich; Merck KGaA), adjusted to pH 10 using NaOH (795429,
Sigma-Aldrich; Merck KGaA) and 0.5% Tween] to remove the primary
antibody for labeling with the appropriate primary antibody.
Finally, slides were added DAPI (40728ES50, Yeasen) and incubated
in 37°C for 3 min. The slides were imaged by Zeiss Axio Scan Z1 and
analyzed using ZEISS imaging software ZEN lite.
Statistical analysis
The data are presented as the means ± SD. One-way or
two-way analysis of variance (ANOVA) followed by Tukey's post hoc
test was performed to identify any significant differences. A
computer-based statistical package (SPSS, version 22.0) was
utilized for the analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Successful establishment of mouse models
of 4-NQO-induced HNSCC and murine HNSCC cell lines
All the tumor samples from the mice in the
4-NQO-induced tumor model were positive for CK and Ki-67, which
confirmed that the tumors were of epithelial origin (Fig. S1A). Considering that the
4-NQO-induced mouse tumor models are time-consuming to establish
and unrepeatable, more convenient and replicable models are
required. In the present study, mouse tumor samples were collected
and primary culture was applied to acquire murine-derived HNSCC
cell lines. All the 3 cell lines exhibited tumorigenicity in
athymic nude mice (data not shown). However, the transplanted
tumors of all 3 cell lines could not grow in immunocompetent
C57BL/6 mice (n>10). Once inoculated into C57BL/6 mice, the
tumors shrank and disappeared by approximately 3 to 4 weeks.
One of these cell lines, termed JC1, was selected
for further research as it exhibited better proliferative and
migratory ability than the other cell lines (data not shown). To
enhance the tumorigenicity of the JC1 cells, chemical induction was
performed with the use of 4-NQO. The induced cells were then
inoculated into immunocompetent C57BL/6 mice and tumors that grew
stably in the immunocompetent mice were selected. The tumorigenic
cell line with the optimal proliferative and migratory ability,
JC1-2, was established for further research (Fig. S1B). The doubling time of the
JC1-2 cells was 14.6 h (data not shown). The distribution of the
cell cycle was also detected (Fig.
S1C).
Immunogenomic and transcriptomic
characterization of JC1 and JC1-2 cells
To better understand the molecular basis of the
differences in tumorigenicity between the JC1 and JC1-2 cell lines,
whole-exome sequencing and single nucleotide polymorphism (SNP)
array analysis were performed. Copy number variations, LOH, somatic
mutation single-nucleotide variants (SNVs) and insertions and
deletions (indels) were identified. The 2 cell lines exhibited
mostly diploid genomes, with some regions of amplifications and
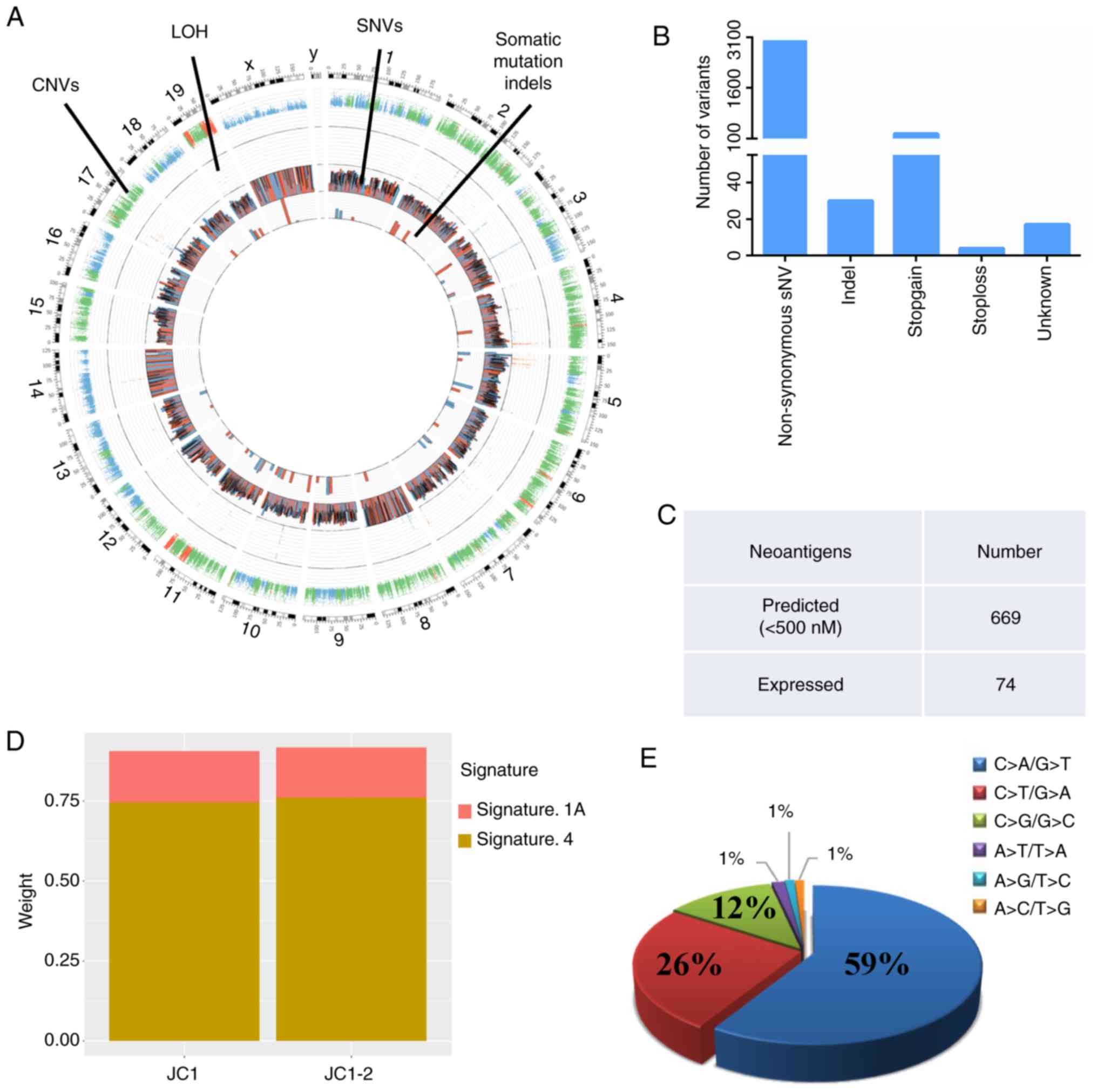
deletions (Fig. 1A). A total of
5,871 somatic mutations were identified in the JC1-2 cell line, of
which 3,010 were non-synonymous and 30 were indels (Fig. 1B).
Tumor neoantigens originate from tumor-specific DNA
alterations, do not exist in the normal genome, and can be
presented and recognized by T lymphocytes. Identified potential
neoantigens can be used to create synthetic vaccines and induce or
expand neoantigen-specific T cells for combination with adjuvant
and checkpoint blockade therapy (20). Therefore, in the present study,
the numbers of predicted and expressed neoantigens were identified,
which allowed us to better understand the immunogenicity of the
JC1-2 cells. In the JC1-2 cells, 669 neoantigens were predicted to
strongly bind to the C57BL/6 Mhc I molecules, H2-Kb and H2-Db, at
<500 nM, and of these, 74 were expressed (Fig. 1C).
A previously published mutational signature
classification system (21,22) was used to identify the JC1-2 cell
line. As shown by the results, JC1-2 was most similar to Signature
1A and Signature 4 (Fig. 1D). One
mutational characterization of Signature 4 [which is exhibited in
35% of HNSCC cases (22)] is a
high ratio of C>A/G>T transversions (21,22). In addition, a high frequency of
C>A/G>T transversions is considered to be a mutagen
fingerprint of tobacco smoking (23,24). The trans-versions were analyzed in
the JC1-2 cells, and the majority of the transversions were
C>A/G>T (59%) (Fig. 1E).
This may be since the JC1-2 cells originated from tumors induced by
the chemical mutagen, 4-NQO. As a result, the JC1-2 cells exhibited
similarities with patients HNSCC who are smokers, which accounts
for the vast majority of patients with HNSCC, and may be a
preclinical model for this group of patients.
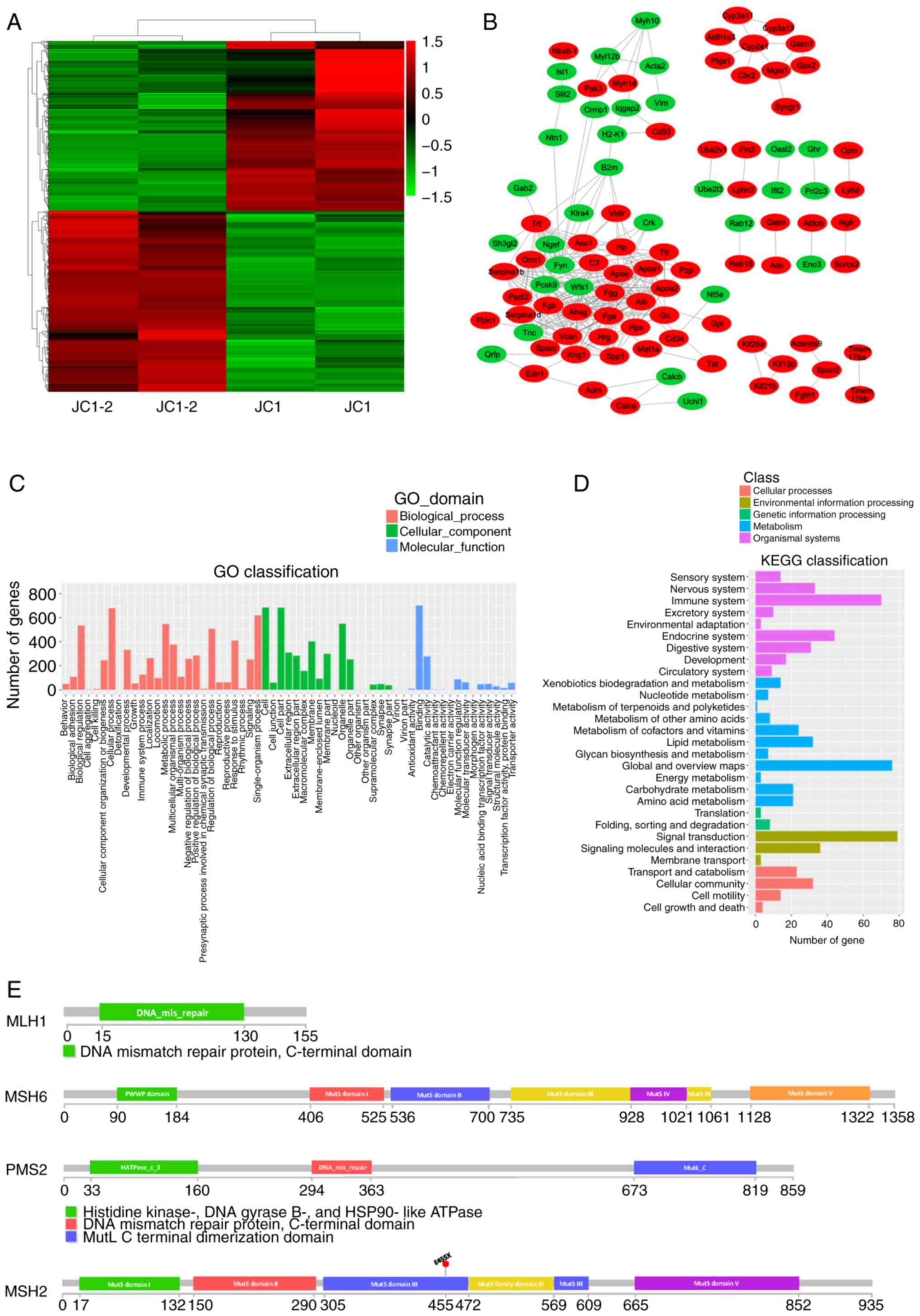
The JC1 and JC1-2 cell lines exhibited clear
distinctions (Fig. 2A). A
gene-action-network was established for the key genes that were
suggested to be significant by GO analysis. The most significantly
differentially expressed genes were selected, and a protein-protein
interaction (PPI) network between the JC1 and JC1-2 cells was
established (Fig. 2B). In the
network, genes related to antigen processing and presentation (B2m)
and H2-K1, fibrinogen encoding (Fga, Fgb and Fgg), fat
transportation (Apoa1 and Apoa2) and the regulation of blood plasma
colloid osmotic pressure (Alb) were found at central positions;
these findings matched well with the significantly enriched
pathways and functions of the differentially expressed genes
classified by GO and KEGG analyses, which were associated with the
immune system and metabolic processes (Fig. 2C and D). In addition, the observed
lower expression levels of B2m and H2-K1 in the JC1 cells (Fig.
S1D) suggested that the enhanced tumorigenicity of the JC1-2 cells
may be due to reasons other than defects in the antigen processing
machinery (APM) system.
Several markers have been reported to be predictive
of the effectiveness of immune checkpoint inhibitor (ICI) therapy,
such as MSI, PD-L1 expression and tumor mutation burden (TMB). It
has been reported that patients with MSI-exhibiting colorectal
cancer (CRC) have better prognoses and a longer survival time than
those with MSS-exhibiting CRC (25-27). The mismatch repair (MMR) system is
a security system that can repair DNA base mismatches. It plays
roles in the restoration of normal nucleotide sequences in DNA
molecules containing mismatched bases and can recognize and direct
the repair of nucleotide mismatches derived from DNA polymerase
errors. If the MMR system does not operate normally, mutations
accumulate, which results in the pathogenesis and progression of
some familial and sporadic cancers. Functional defects in the MMR
system often lead to MSI (28).
The present study analyzed mutations of 4 MMR genes in the JC1-2
cells: MLH1, MSH2, MSH6 and PMS2 (Fig. 2E). Only an MSH2 E455X nonsense
mutation was found. Thus, the characterization of the genomic and
transcriptomic landscapes of the HNSCC cell lines, JC1 and JC1-2,
demonstrated that the JC1-2 cell line is a model cell line for
MSS-exhibiting or MMR-proficient (pMMR) HNSCC.
The responsive IFN-γ pathway in the JC1-2
cell line render it a serviceable model for tumor immunology
It is well accepted that the immune system plays an
important role in tumor progression. Due to the differences in
tumor formation rates between nude and C57BL/6 mice (data not
shown), it was hypothesized that the immune system may play an
important role in JC1-2 cell-derived transplanted tumor
progression. CD8+ T cells cannot recognize tumor
antigens to perform specific cell killing unless the APM system is
functional. The IFN-γ pathway of tumor cells is indispensable for
the antitumor function of immune cells (29). To determine whether the IFN-γ
pathway is responsive to IFN-γ in the JC1-2 cells, the
IFN-γ-treated cells were compared to co-cultured cells. The results
revealed that the levels of Mhc-I and PD-L1 were elevated to
similar degrees in the IFN-γ-treated cells and splenocyte
co-culture cells as in the control group, which indicated that the
JC1-2 cells were responsive to IFN-γ. The inserts groups (Fig. 3A) did not differ as significantly
as the above 2 groups, which indicated that direct contact was
necessary for tumor antigen recognition (Fig. 3B-F).
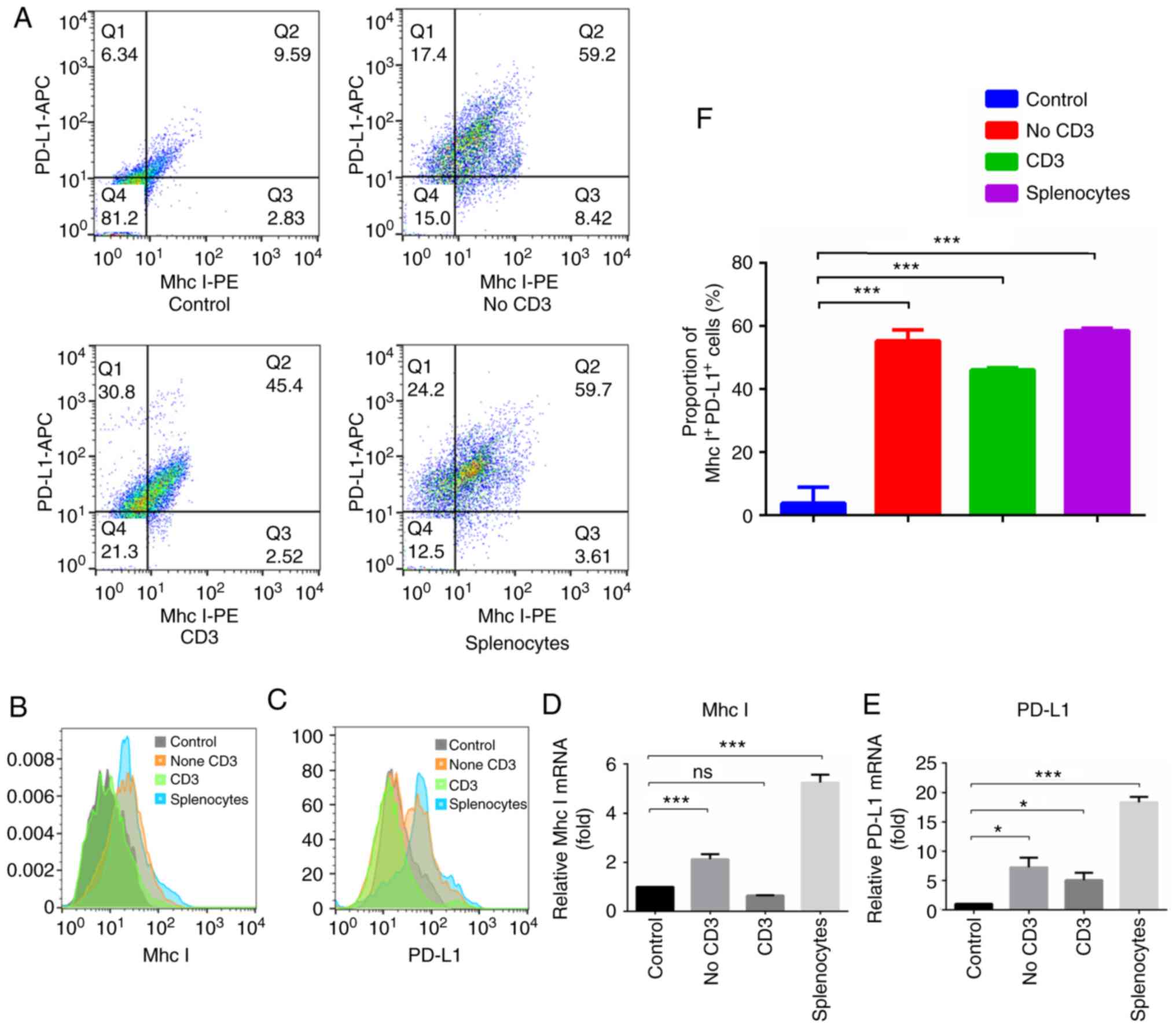
To further identify the effects of different
subgroups of immune cells on tumor cells, flow cytometry was
performed to observe the changes in the expression of Mhc-I and
PD-L1 in JC1-2 cells following co-culture. The results revealed
that Mhc-I and PD-L1 expression in the cells co-cultured with total
splenocytes was significantly higher than that in the control
cells; Mhc-I and PD-L1 expression in the cells co-cultured with
non-CD3+ cells was also higher than that in the control
cells, while that in cells co-cultured with CD3+ T cells
was not (Fig. 4A-C). The results
of RT-qPCR were in accordance with these findings (Fig. 4D and E), which indicated that
non-CD3+ cells in splenocytes play an important role in
the expression of PD-L1 and Mhc-I in JC1-2 cells.
Epidermal growth factor receptor (EGFR) is
overexpressed in up to 90% of HNSCC cases and has been proven to be
an effective target for HNSCC therapeutic strategies. EGFR is
crucial to squamous cells and to signaling through the Ras-MAPK,
PI3K-PTEN-AKT and phospholipase C path-ways (30). The present study found that EGFR
expression in cells co-cultured with total splenocytes also
differed notably from that in the control group (Fig. S1E and
F).
Comparison of the tumor immune
microenvironment between the orthotopic and heterotopic models
using multiplex fluorescent IHC
Multiplex fluorescent IHC was used to assess the
landscape of the JC1-2 tumor immune microenvironment. Compared with
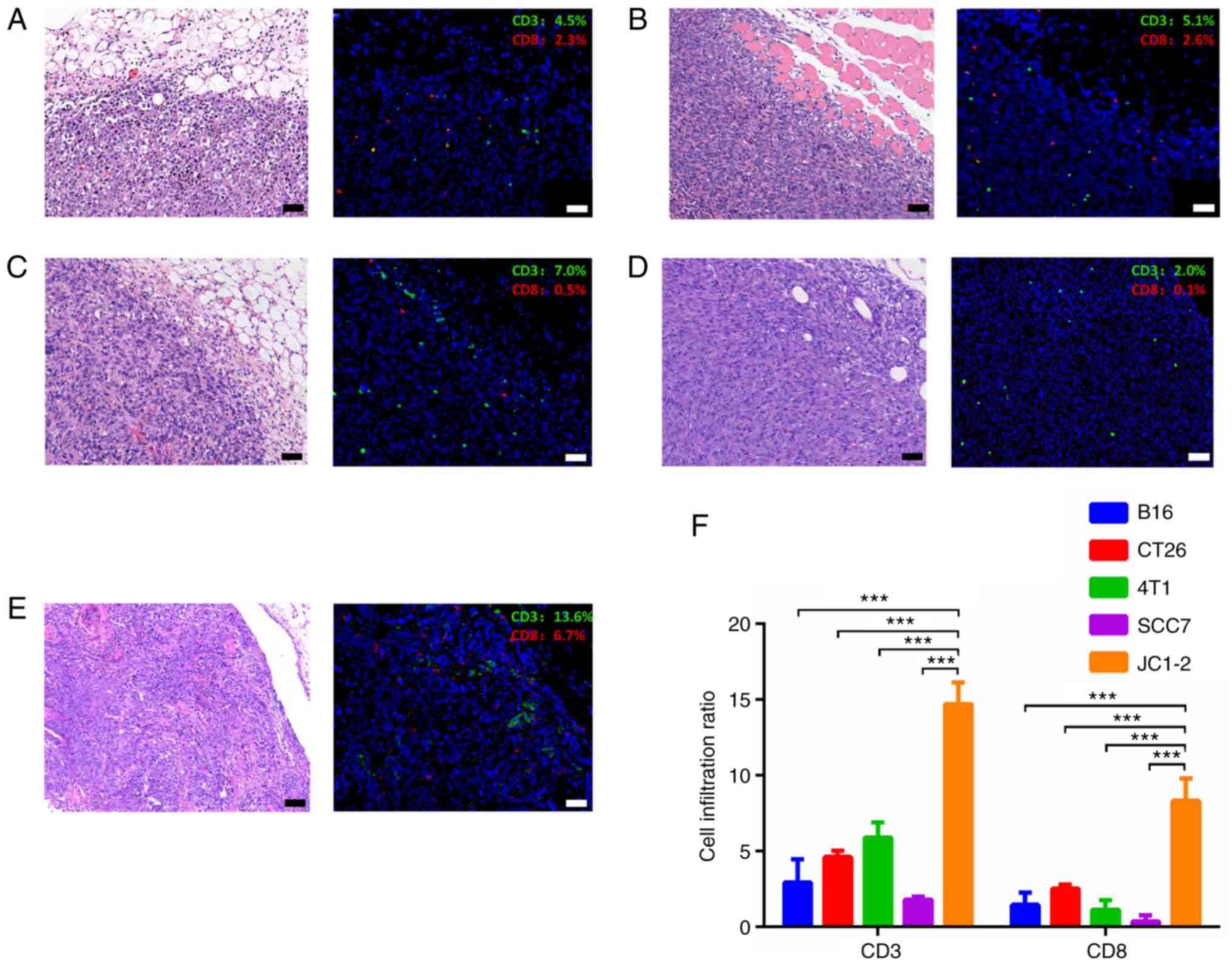
other widely used syngeneic model tumors, such as B16, CT26, 4T1
and SCC7 transplanted tumors, the JC1-2-formed tumors exhibited a
greater number of CD3+ T lymphocytes in the tumor
microenvironment, most of which were CD8+ cytotoxic
cells (Fig. 5).
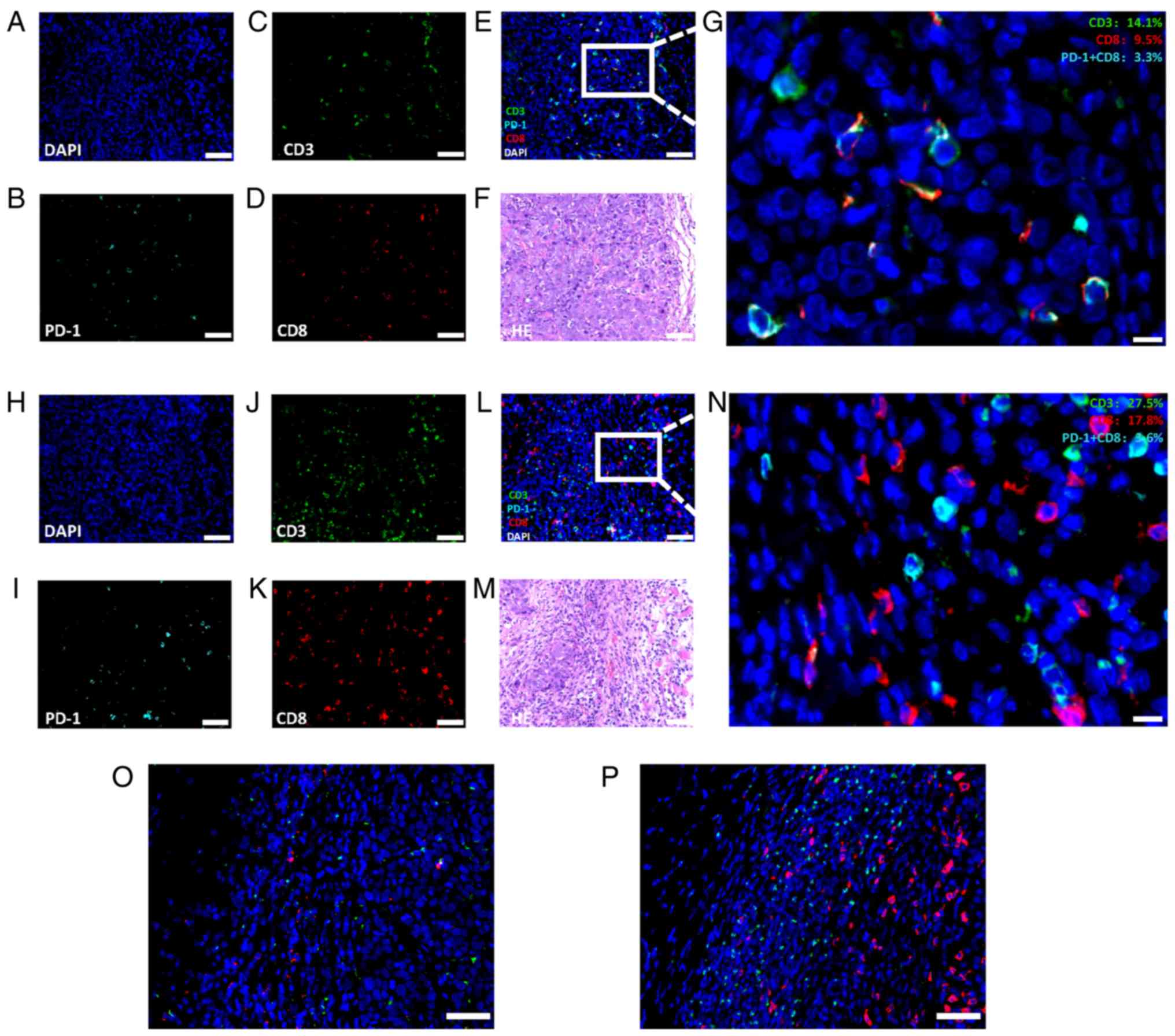
Both orthotopic and heterotopic models were
established, and the tumor samples were stained for CD3, CD8 and
PD-1. Of note, there were distinct differences between tumors from
the subcutaneous and buccal mucosae (Fig. 6A-N). A greater amount of
CD8+ T cells existed in the orthotopic buccal tumors
than in the heterotopic subcutaneous tumors, indicating that the
immune responses were more intense in orthotopic model.
PD-1+CD8+ tumor-infiltrating lymphocytes
(TILs) are considered to form the basis of PD-1/PD-L1 immunotherapy
(31,32). In the present study, the amount of
CD3+ and CD8+ TILs in the orthotopic tumors
was greater than that in the heterotopic tumors, which indicated
that the orthotopic tumor model was a better selection for
immunotherapy evaluation. Additionally, a higher B2m expression was
found in the orthotopic model than in the heterotopic model
(Fig. 6O and P) and the majority
of B2m-positive cells, were immune cells, which verified that the
immune response was more intense in the orthotopic model.
Through multiplex fluorescent IHC and H&E
staining, the immune microenvironments of HNSCC tumors were
examined. The imaging results revealed that the tumors formed from
JC1-2 cells were 'inflamed tumors' with abundant immune-subset
cells within the intratumoral and peritumoral microenvironment.
Such findings are rare in current experimental tumor models and
indicate that this model may be a useful tool for further studies
on immunotherapy. It could be used as an experimental model for
screening or development of anticancer drugs and for verification
of related molecular mechanisms. With the help of multiplex IHC,
the JC1-2 tumor model may be used as a reliable platform that
exhibits an intratumoral immune microenvironment appropriate for
further immunotherapy research on HNSCC patients who are
smokers.
Discussion
Mice are often used as animal models to examine the
tumor microenvironment and to verify the related molecular
mechanisms in order to enhance our understanding of the occurrence
and progression of HNSCC and for the development of therapeutic
strategies (33). A crucial
aspect of a preclinical model is that it mimics human cancer
development. Due to its time-consuming and unrepeatable natures of
the current models, we performed primary culture to establish an
HNSCC cell line model. The resulting syngeneic murine tumor model
is fully immune-competent and will be particularly useful in the
evaluation of immunooncology agents, as it can be used to study the
generation of antitumor immune responses and does not require the
adoptive transfer of immune populations. Additionally, this cell
line model is more reliable than existing models due to its
repeatability and easy operation.
In the present study, the originally established
cell line JC1 was cultured in vitro and subjected to
repeated passaging and monoclonal screening. Under such conditions,
alterations in some biological and genetic characteristics (such as
immunogenicity) can occur in this cell line. As a result, the JC1
cells exhibited tumorigenicity in immunodeficient nude mice, but
could not grow into tumors in immunocompetent C57BL/6 mice (data
not shown). In addition, it has been reported that in chronic
infections and cancer, T cells tend to become non-functional (at
which point they are termed exhausted T cells) and express several
inhibitory receptors because of persistent exposure to antigens
(34). In the present study,
compared to transplanted tumors, the 4-NQO-induced tumors exhibited
a more immunosuppressive microenvironment, which may explain why
the JC1 cells can only grow into tumors in nude mice. Fortunately,
the JC1-2 cell line established through chemical induction
exhibited tumorigenicity in immunocompetent C57BL/6 mice.
Through NGS, it was found that the HNSCC cell lines
that was established herein may be of great value as alternatives
in clinical trials for a specific group of patients. It was
verified that both JC1 and JC1-2 cells exhibited MSS phenotype.
Notably, HNSCC cell lines with MSI have been reported to exhibit
decreased sensitivity to chemotherapeutic drugs. LOH is a common
mechanism of inactivation of tumor-suppressor genes, which might be
related to chemotherapy resistance. Further sequencing and analysis
of the genome or the gene expression patterns of JC1-2 cells or
transplanted tumors could reveal matched clinical patient subgroups
whose tumors may have similar biological behaviors and key gene
mutations. Hence, this mouse model, and this approach of model
establishment, could be of great significance for early diagnosis
of HNSCC, direction of therapeutic strategies and prediction of the
antitumor efficacy of immunotherapy.
Differences between JC1 and JC1-2 cells in CNVs,
somatic number variations, LOH, base changes and substitutions,
enriched functions and pathways for significantly differentially
expressed genes and protein-protein interactions were revealed by
the present findings. However, further bioinformatics analyses are
warranted to explore the potential factors affecting the
tumorigenicity of these tumor cells. In particular, the most
significantly differentially expressed genes should be investigated
as they could be potential therapeutic targets for HNSCC.
By comparing JC1-2 cells cultured alone and
co-cultured with different subgroups of splenocytes, it was
confirmed that the gene expression of tumor cells can be
significantly altered through coculture with immune cells in
vitro. Non-CD3+ cells include several subgroups of
immune cells, such as dendritic cells (DCs), macrophages and
natural killer (NK) cells, which play significant roles in antigen
processing and, antigen presentation and exhibit strong killing
effects on tumor cells. The results suggest that
non-CD3+ cells are important components in the
interactions between tumor cells and the immune system. The killing
effects of CD8+ cells towards tumor cells are based on
the proper functioning of the Mhc I APM. Mhc peptide complexes can
be recognized by T cell receptors (TCRs), thus leading to TCR
activation and tumor-killing effects. An intact Mhc I APM is
indispensable for immunocytes responses to tumors. The expression
levels of Mhc I in untreated JC1 and JC1-2 cells were low (Fig.
S1D); however, Mhc expression was increased on JC1-2 cells
stimulated with IFN-γ and splenocytes, indicating that experiments
in vitro may not reflect the real interactions between
immune cells and tumor cells. Thus, in vivo tumor models
were deemed necessary. B2m was identified to stabilize the
synthesized Mhc I- peptide complex so that it could be expressed on
the cell surface. IT was found that H2-K1 and H2-D1 expression was
even higher in the JC1-2 cells than in the JC1 cells, which
suggested that enhanced immunogenicity and tumorigenicity of JC1-2
cells may be due to some reasons except the defect of the antigen
processing machinery (APM) system. In the present study, clear
differences in B2m expression were revealed between orthotopic and
heterotopic tumor models. Further studies should focus on the
immune escape mechanism. Generally, the JC1-2 cell line had a
responsive IFN-γ pathway and thus may potentially be suitable for
drug screening and immunotherapy evaluation in vitro. In
addition, the JC1-2 cell line may also be an appropriate model for
exploration of the mechanisms of interactions between tumor cells
and immune cells in vitro.
The CD8+ T effector cell population is
believed to be a major immune cell population in antitumor adaptive
immunity and to represent a significant independent prognostic
factor (35), but other types of
cells, such as macrophages, DCs and B cells, are also indispensable
for effective presentation, recognition and tumor killing. Under
normal conditions, the immune system reacts to exogenous antigens
that carry danger signals, leading to the proliferation of
antigen-specific CD8+ T cells and/or CD4+
helper cells. As a result, the proliferation of antigen-specific T
cells and the apoptosis of regulatory T cells are markedly reduced,
and thus, suppress tumor growth (36,37). Upon IFN-γ stimulation, PD-L1 is
expressed on T cells and other immune cells (38). It was hypothesized that tumor
anti-gens cannot be recognized and presented normally without the
APM; thus, the progression of malignancy is greatly influenced by
whether an integrated immune system exists. The transplantation of
tumors into immunodeficient nude mice may therefore not be an
appropriate method for tumor immunology research. Tumors
transplanted into immunocompetent mice better reflect the
biological behaviors and genetic characteristics of tumors in
humans than those transplanted into immunodeficient mice. In
addition, it is important to explore the functionality of different
subgroups of immune cells. In the present study, some functional
immune cells were identified, such as
CD8+PD-1+ cytotoxic T cells (CTLs). The
present study however, did not analyze the functional and spatial
characteristics of these cells in detail; such an analysis may
provide additional information for studies on immunotherapy and the
tumor microenvironment that will enable the enhanced under-standing
of the progression of malignancy. Combined with multiplex IHC, this
cell line model can also be a useful tool for potential
immunotherapy target screening, pharmacodynamics evaluation, and
the investigation of the immune microenvironment of HNSCC. JC1-2
cells provide a more reliable preclinical model than existing
models for biomarker investigation, drugs targeting screening and
immunotherapy optimization.
Tumor heterogeneity has long been one of the most
significant reasons for tumor metastasis, recurrence and drug
resistance (39). IHC is an
important auxiliary method for clinical pathologic diagnosis. Since
a number of immunotherapies can benefit from biomarkers, techniques
such as multiplex IHC, which enables clear visualization of
multiple markers on a single slide, have been widely utilized.
Multiplex IHC on human tumor tissues has been carried out for a
long time and its process has been continuously optimized (40,41). However, similar methods have
rarely been carried out on murine tumor tissues as epitopes, such
as CD4 and CD8 were not to be sufficiently detected in
formalin-fixed paraffin-embedded (FFPE) samples until recently
(42). Therefore, flow cytometry
has long been the gold standard for the analysis of TILs in the
tumor microenvironment (43).
However, flow cytometry cannot provide exact dimensional
information, which has been proven to be significant in the
diagnosis and prognosis of malignancies. Multiplex IHC provides
more quantitative and spatial information on the tumor
microenvironment than flow cytometry and is of great value for
multitarget combination therapy.
Notably, the formation rate of JC1-2 transplanted
tumors in immunocompetent mice was not 100% and the growth rates of
the tumors were relatively slower than those of tumors in widely
used syngeneic models, such as B16 and SCC7 models; these findings
indicate that JC1-2 cells may have relatively high immunogenicity.
Different syngeneic mice exhibited different degrees of
tumorigenicity (immune responses) upon JC1-2 inoculation, which
resembles the clinical situation in which individuals exhibit
different responses to the same therapeutic regimen. The JC1-2 cell
line model may be an excellent tool for the observation of
early-stage HNSCC (particularly as regards 'inflamed' tumors, which
severely lack preclinical models) and an appropriate model for
studying the tumorigenesis and progression of HNSCC. The abundant
PD-1+ T cells in the tumor microenvironments of JC1-2
trans-planted tumors suggest that the JC1-2 tumor model may have a
positive response to PD-1/PD-L1 blockade. Further research
evaluating drug safety and efficacy is required to verify the
previous results in vitro, and target screening and
pharmaco-dynamic evaluation is also warranted.
The syngeneic murine tumor model established in the
present study has the ease of use, since JC1-2 cells can be rapidly
and reproducibly expanded in large numbers. It can also be used for
research on the impacts of immune cells on tumor development and
antitumor immune responses and for the evaluation of
immunotherapies. This syngeneic murine tumor model may prove to be
of great value for diagnosis and for the evaluation of novel
immunotherapies.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by The Ninth
People's Hospital affiliated to the Shanghai Jiao Tong University
School of Medicine supported by the National Natural Science
Foundation of China (grant no. 81902749) and the China Postdoctoral
Science Foundation (grant no. 2017M610264).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
YF and GT made substantial contributions to the
conception and design of the study, as well as in the acquisition,
analysis, or interpretation of data for the study. JL and ZZ made
substantial contributions to the conception and design of the study
and together with the other authors, gave the final approval of the
version to be published and agreement to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved. KX was involved in the drafting of the
study or revising it critically for important intellectual content,
as well as in the acquisition, analysis, or interpretation of data
for the study. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by and
performed in accordance with the guidelines of the Shanghai Jiao
Tong University School of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
De Moerlooze L, Spencer-Dene B, Revest JM,
Hajihosseini M, Rosewell I and Dickson C: An important role for the
IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in
mesenchymal-epithelial signalling during mouse organogenesis.
Development. 127:483–492. 2000.PubMed/NCBI
|
|
2
|
Kang H, Kiess A and Chung CH: Emerging
biomarkers in head and neck cancer in the era of genomics. Nat Rev
Clin Oncol. 12:11–26. 2015. View Article : Google Scholar
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beenken A and Mohammadi M: The FGF family:
Biology, pathophysiology and therapy. Nat Rev Drug Discov.
8:235–253. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rothschild U, Muller L, Lechner A,
Schlösser HA, Beutner D, Läubli H, Zippelius A and Rothschild SI:
Immunotherapy in head and neck cancer-scientific rationale, current
treatment options and future directions. Swiss Med Wkly.
148:w146252018.
|
|
6
|
Gould SE, Junttila MR and de Sauvage FJ:
Translational value of mouse models in oncology drug development.
Nat Med. 21:431–439. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Visscher SA, Witjes MJ, van der Vegt B,
de Bruijn HS, van der Ploegvan den Heuvel A, Amelink A, Sterenborg
HJ, Roodenburg JL and Robinson DJ: Localization of liposomal mTHPC
formulations within normal epithelium, dysplastic tissue, and
carcinoma of oral epithelium in the 4NQO-carcinogenesis rat model.
Lasers Surg Med. 45:668–678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miki K, Orita Y, Gion Y, Takao S, Ohno K,
Takeuchi M, Ito T, Minoura A, Tachibana T, Marunaka H, et al:
Tumor-associated macrophages in the development of
4-nitro-quinoline-1-oxide-induced tongue squamous cell carcinoma in
a mouse model. Oncology. 93:204–212. 2017. View Article : Google Scholar
|
|
9
|
Young MR: Use of carcinogen-induced
premalignant oral lesions in a dendritic cell-based vaccine to
stimulate immune reactivity against both premalignant oral lesions
and oral cancer. J Immunother. 31:148–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tseng SH, Yang CC, Yu EH, Chang C, Lee YS,
Liu CJ, Chang KW and Lin SC: K14-EGFP-miR-31 transgenic mice have
high susceptibility to chemical-induced squamous cell tumorigenesis
that is associating with Ku80 repression. Int J Cancer.
136:1263–1275. 2015. View Article : Google Scholar
|
|
11
|
Miki K, Orita Y, Gion Y, Takao S, Ohno K,
Takeuchi M, Ito T, Hanakawa H, Tachibana T, Marunaka H, et al:
Regulatory T cells function at the early stage of tumor progression
in a mouse model of tongue squamous cell carcinoma. Cancer Immunol
Immunother. 65:1401–1410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y: Cancer immunotherapy: Harnessing
the immune system to battle cancer. J Clin Invest. 125:3335–3337.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu X, Horner JW, Paul E, Shang X, Troncoso
P, Deng P, Jiang S, Chang Q, Spring DJ, Sharma P, et al: Effective
combinatorial immunotherapy for castration-resistant prostate
cancer. Nature. 543:728–732. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olson B, Li Y, Lin Y, Liu ET and Patnaik
A: Mouse models for cancer immunotherapy research. Cancer Discov.
8:1358–1365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Efremova M, Rieder D, Klepsch V,
Charoentong P, Finotello F, Hackl H, Hermann-Kleiter N, Löwer M,
Baier G, Krogsdam A and Trajanoski Z: Targeting immune checkpoints
potentiates immunoediting and changes the dynamics of tumor
evolution. Nat Commun. 9:322018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang XH, Knudsen B, Bemis D, Tickoo S and
Gudas LJ: Oral cavity and esophageal carcinogenesis modeled in
carcinogen-treated mice. Clin Cancer Res. 10:301–313. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DePristo MA, Banks E, Poplin R, Garimella
KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA,
Hanna M, et al: A framework for variation discovery and genotyping
using next-generation DNA sequencing data. Nat Genet. 43:491–498.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
McKenna A, Hanna M, Banks E, Sivachenko A,
Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly
M and DePristo MA: The Genome Analysis Toolkit: A MapReduce
framework for analyzing next-generation DNA sequencing data. Genome
Res. 20:1297–1303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alexandrov LB, Nik-Zainal S, Wedge DC,
Campbell PJ and Stratton MR: Deciphering signatures of mutational
processes operative in human cancer. Cell Rep. 3:246–259. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alexandrov LB, Nik-Zainal S, Wedge DC,
Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A,
Børresen-Dale AL, et al: Signatures of mutational processes in
human cancer. Nature. 500:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun S, Schiller JH and Gazdar AF: Lung
cancer in never smokers-a different disease. Nat Rev Cancer.
7:778–790. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Le Calvez F, Mukeria A, Hunt JD, Kelm O,
Hung RJ, Tanière P, Brennan P, Boffetta P, Zaridze DG and Hainaut
P: TP53 and KRAS mutation load and types in lung cancers in
relation to tobacco smoke: Distinct patterns in never, former, and
current smokers. Cancer Res. 65:5076–5083. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Collura A, Lagrange A, Svrcek M, Marisa L,
Buhard O, Guilloux A, Wanherdrick K, Dorard C, Taieb A, Saget A, et
al: Patients with colorectal tumors with microsatellite instability
and large deletions in HSP110 T17 have improved response to
5-fluorouracil-based chemotherapy. Gastroenterology. 146:401–411.
2014. View Article : Google Scholar
|
|
26
|
Tajima A, Hess MT, Cabrera BL, Kolodner RD
and Carethers JM: The mismatch repair complex hMutS alpha
recognizes 5-fluorouracil-modified DNA: Implications for
chemosensitivity and resistance. Gastroenterology. 127:1678–1684.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hemminki A, Mecklin JP, Jarvinen H,
Aaltonen LA and Joensuu H: Microsatellite instability is a
favorable prognostic indicator in patients with colorectal cancer
receiving chemo-therapy. Gastroenterology. 119:921–928. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baretti M and Le DT: DNA mismatch repair
in cancer. Pharmacol Ther. 189:45–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He
Q, Chen T, Roszik J, Bernatchez C, Woodman SE, et al: Loss of IFN-γ
pathway genes in tumor cells as a mechanism of resistance to
anti-CTLA-4 therapy. CELL. 167:397–404. 2016. View Article : Google Scholar
|
|
30
|
Hynes NE and Lane HA: ERBB receptors and
cancer: The complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mattox AK, Lee J, Westra WH, Pierce RH,
Ghossein R, Faquin WC, Diefenbach TJ, Morris LG, Lin DT, Wirth LJ,
et al: PD-1 expression in head and neck squamous cell carcinomas
derives primarily from functionally anergic CD4(+) TILs in the
presence of PD-L1(+) TAMs. Cancer Res. 77:6365–6374. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou G, Sprengers D, Boor PPC, Doukas M,
Schutz H, Mancham S, Pedroza-Gonzalez A, Polak WG, de Jonge J,
Gaspersz M, et al: Antibodies against immune checkpoint molecules
restore functions of tumor-infiltrating T cells in hepatocellular
carcinomas. Gastroenterology. 153:1107–1119. 2017. View Article : Google Scholar
|
|
33
|
Bi Y, Deng J, Murry DJ and An G: A
whole-body physiologically based pharmacokinetic model of gefitinib
in mice and scale-up to humans. AAPS J. 18:228–238. 2016.
View Article : Google Scholar :
|
|
34
|
Wherry EJ and Kurachi M: Molecular and
cellular insights into T cell exhaustion. Nat Rev Immunol.
15:486–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Topalian SL, Taube JM, Anders RA and
Pardoll DM: Mechanism-driven biomarkers to guide immune checkpoint
blockade in cancer therapy. Nat Rev Cancer. 16:275–287. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karwacz K, Bricogne C, MacDonald D, Arce
F, Bennett CL, Collins M and Escors D: PD-L1 co-stimulation
contributes to ligand-induced T cell receptor down-modulation on
CD8+ T cells. EMBO Mol Med. 3:581–592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen G, Huang AC, Zhang W, Zhang G, Wu M,
Xu W, Yu Z, Yang J, Wang B, Sun H, et al: Exosomal PD-L1
contributes to immunosuppression and is associated with anti-PD-1
response. Nature. 560:382–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Flies DB and Chen L: The new B7s: Playing
a pivotal role in tumor immunity. J Immunother. 30:251–260. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vermeer DW, Coppock JD, Zeng E, Lee KM,
Spanos WC, Onken MD, Uppaluri R, Lee JH and Vermeer PD: Metastatic
model of HPV+ oropharyngeal squamous cell carcinoma demonstrates
heterogeneity in tumor metastasis. Oncotarget. 7:24194–24207. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stack EC, Wang C, Roman KA and Hoyt CC:
Multiplexed immunohistochemistry, imaging, and quantitation: A
review, with an assessment of Tyramide signal amplification,
multispectral imaging and multiplex analysis. Methods. 70:46–58.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Feng Z, Puri S, Moudgil T, Wood W, Hoyt
CC, Wang C, Urba WJ, Curti BD, Bifulco CB and Fox BA: Multispectral
imaging of formalin-fixed tissue predicts ability to generate
tumor-infiltrating lymphocytes from melanoma. J Immunother Cancer.
3:472015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Feng Z, Jensen SM, Messenheimer DJ, Farhad
M, Neuberger M, Bifulco CB and Fox BA: Multispectral imaging of T
and B cells in murine spleen and tumor. J Immunol. 196:3943–3950.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu J, Yuan Y, Chen W, Putra J,
Suriawinata AA, Schenk AD, Miller HE, Guleria I, Barth RJ, Huang YH
and Wang L: Immune-checkpoint proteins VISTA and PD-1
nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci
USA. 112:6682–6687. 2015. View Article : Google Scholar : PubMed/NCBI
|