Introduction
Breast cancer is a complex and heterogeneous disease
that is considered the leading cause of cancer-related death among
females worldwide (1). In the
past decades, advances in molecular biology have contributed to
significant improvements in the diagnosis and classification of
breast cancer (2,3). Surgery, radiotherapy and
chemotherapy (hormone and targeted) have shown remarkable survival
benefits in patients with breast cancer (4). Approximately 30% of breast tumors
overexpress human epidermal growth factor receptor 2 (HER-2)
(5). These cells are
characterized by high rates of cell proliferation, metastasis and
low overall survival (5). The
humanized HER-2 monoclonal antibody trastuzumab, also known as
Herceptin, targets HER-2 and has been approved by the United States
Food and Drug Administration (6).
Trastuzumab drug has become the standard treatment for patients
with HER-2-positive cancer in early or advanced breast cancer
(7). Trastuzumab treatment has
led to a marked improvement in the outcomes of patients with
HER-2-positive breast cancer (8).
It was reported that trastuzumab markedly inhibits tumor
proliferation, angiogenesis and metastasis via downregulation of
HER-2 (9-12). Although this antibody has led to
optimal stratification of breast cancer, while the prognosis of
patients with HER-2 breast cancer remains poor (13,14).
MicroRNAs (miRs/miRNAs) are small regulatory RNAs
that modulate the expression of their target genes (15). Several miRNAs have shown
preferentially conserved interactions with the majority of human
mRNAs; they can influence specific developmental processes and the
progression of several diseases (16). Notably, miRNAs are well known for
their roles in regulating cell growth, which is critical to cancer
development (17). Several
studies have investigated the combination of miRNAs with
trastuzumab for the treatment of patients with HER-2-positive
breast cancer. It was reported that miR-16 (18), miR-26a, miR-30b (1), miR-141-3p (19), miR-770-5p (20) and miR-205-5p (21) are involved in mediating
trastuzumab response in HER-2-positive breast cancer. Moreover,
repression of miR-135b-5p was reported to promote metastasis of
early-stage breast cancer (22),
whereas it was also shown to enhance the antitumor effect of
doxorubicin in breast cancer cells by targeting anterior gradient 2
(23). The present study
hypothesized that miR-135b-5p may be associated with regulating the
sensitivity of breast cancer cells to trastuzumab. Therefore, the
focus of the current study was to investigate of the expression of
miR-135b-5p, which was markedly down-regulated in HER-2-positive
breast cancer cells. In addition, the mechanism by which
miR-135b-5p improved trastuzumab sensitivity was investigated.
Materials and methods
Cell culture and transfection
HER-2-positive breast cancer cells BT-474 and
SK-BR-3 and the normal breast epithelial cell line MCF-10A were
obtained from the American Type Culture Collection. Both cell lines
were grown in Roswell Park Memorial Institute medium (Thermo Fisher
Scientific, Inc.), which was supplemented with 10% fetal bovine
serum (Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin. The cells were maintained at 37°C in a
humidified atmosphere at 5% CO2.
miR-135b-5p agomir (5′-UAU GGC UUU UUA UUC CUG UGU
GA-3′) and its negative control (NC; 5′-UUU GUA CUA CAC AAA AGU ACU
G-3′) were synthesized and purchased from Guangzhou RiboBio Co.,
Ltd. The cells were transfected with 50 nM miR-135b-5p agomir and
its NC (50 nM) using Lipofectamine 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 24 h according to the manufacturer's
protocol. Cells in the blank group were not given any
treatment.
pcDNA3.1-cyclin D2 was purchased from Shanghai
GenePharma Co., Ltd. BT-474 cells were transiently transfected with
2 µg/ml pcDNA3.1-NC or 2 µg/ml pcDNA3.1-cyclin D2
using Lipofectamine™ 3000 (Invitrogen; Thermo Fisher Scientific,
Inc.) for 48 h according to the manufacturer's instructions.
Cell viability
The cell viability of BT-474 and SK-BR-3 cells was
evaluated using Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) according to the manufacturer's protocol. The
cells were seeded into 96-well plates at a density of
5×104 cells/ml overnight. Subsequently, the cells were
treated with 10 nM miR-135b-5p agomir or agomir NC for 48 h.
Finally, 10 µl CCK-8 (5 mg/ml) solution was added to the
culture medium in each well and the cells were incubated for 2 h at
37°C. The absorbance was measured at a wavelength of 450 nm using a
multifunctional microplate reader (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
PCR(RT-qPCR)
miR-135b-5p levels were quantified using RT-qPCR.
Total RNA was extracted from BT-474, SK-BR-4 or MCF-10A cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. cDNA synthesis was
performed using a High Capacity cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) with miRNA-specific primers. The following
specific primers were used for RT: U6, 5′-AAC GCT TCA CGA ATT TGC
GT′-3′ and miR-135b-5p, 5′-CTC AAC TGG TGT CGT GGA GTC GGC AAT TCA
GTT GAG TCA CAT AG-3′. qPCR was performed on an Applied Biosystems
7500 Real-Time PCR machine (Thermo Fisher Scientific, Inc.) with
the EnTurbo™ SYBR Green PCR SuperMix (ELK Biotechnology Co., Ltd.).
The following primer pairs were used for the qPCR: miR-135b-5p
forward, 5′-AGC TAT GGC TTT TCA TTC CTA TG-3′ and reverse, 5′-CTC
AAC TGG TGT CGT GGA GTC -3′; U6 forward, 5′-CTC GCT TCG GCA GCA CAT
-3′ and reverse, 5′-AAC GCT TCA CGA ATT TGC GT-3; GAPDH forward,
5′-CGG ACC AAT ACG ACC AAA TCC G-3′ and reverse, 5′-AGC CAC ATC GCT
CAG ACA CC-3′; and cyclin D2 forward, 5′-GAA CTC GAG GAG AGC CAT
CT-3′ and reverse, 5′-AGT TCG AAT CTG CAC CGT AG-3′. The relative
level of miR-135b-5p was normalized to U6, while the relative level
of cyclin D2 normalized to GAPDH according to the 2−ΔΔCq
method (24). miRNA RT-qPCR
primer sets (one RT primer and one pair of qPCR primers) specific
for miR-135b-5p, and mRNA RT-qPCR primers specific for cyclin D2
were obtained from Genecreate. Cyclin D2 levels were analyzed by
RT-qPCR in BT-474 breast cancer cells transfected with miR-135b-5p
agomir for 24 and 48 h.
Detection of apoptosis and cell cycle
analysis
Apoptosis induction was analyzed using the Annexin
V-FITC apoptosis detection kit (BD Biosciences) according to the
manufacturer's protocol. The cells were harvested and washed with
PBS twice. Subsequently, they were stained with propidium iodide
(PI) and Annexin V. Following 15 min of incubation in the dark at
room temperature, cell apoptosis was detected by flow cytometry
(FACSCalibur; BD Biosciences) using the CellQuest Pro software
(version 3.3; BD Biosciences).
For cell cycle analysis, the cells were harvested
and resuspended at 1×106 cells/ml in modified Krishan's
buffer (0.1% sodium citrate, 0.3% NP-40, 0.02 mg/ml RNase and 0.05
mg/ml PI). The stained cells were detected by flow cytometry
(FACSCalibur; BD Biosciences) using CellQuest Pro software (version
3.3; BD Biosciences).
Transwell assay
Migration and invasion assays were performed using a
24-well Transwell chamber (Sigma-Aldrich; Merck KGaA) with or
without a Matrigel-coated membrane. The cells
(5×104/well) were plated in serum-free medium in the
upper chamber with a non-coated membrane (24-well insert,
8-µm pore size) in order to assess migration, whereas a
matrigel-coated membrane was used for the invasion assay. The lower
chamber was filled with medium containing 10% fetal bovine serum
(Thermo Fisher Scientific, Inc.). After 24 h of incubation, the
cells on the upper surface were removed by a cotton swab and the
cells on the lower surface were stained with 10% w/v aqueous
Giemsa's solution (VWR International, LLC) for 30 min at room
temperature. The stained cells were imaged and counted under a
fluorescence microscope (Nikon Eclipse Ti-E; Nikon Corporation) in
five randomly selected fields at ×200 magnification.
Dual-luciferase reporter assay
TargetScan (http://www.targetscan.org/vert_71/) and miRWalk
(http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/)
online tools were used to predict target genes of miR-135b-5p. The
predicted miR-135b-5p binding sequence (wild-type; WT) or a
mismatch sequence (mutant type) in the 3′-untranslated region
(3′-UTR) of cyclin D2 mRNA were synthesized and cloned separately
into the multiple cloning site of the luciferase miRNA expression
reporter vector (Promega Corporation). The sequences of these
synthesized oligonucleotides are as follows: Forward WT: 5′-GGC UCA
GGU UUU GAG AAG CCA UC-3′ and mutant 5′-GGC UCA GGU UUU GAG GUU AAG
GC-3′. Subsequently, cells were co-transfected with pRL-TK-cyclin
D2-WT or pRL-TK-cyclin D2-MT plasmid and miR-135b-5p agomir using
Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.).
Additionally, cells were co-transfected with pRL-TK-cyclin D2-WT or
pRL-TK-cyclin D2-MT plasmid and NC using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), which was considered
as the vector-control group. Meanwhile, cells transfected with
pRL-TK-cyclin D2-WT or pRL-TK-cyclin D2-MT plasmid alone was
considered as the control group. Following transfection, the cells
were cultured for 6 h at 37°C and the transfection medium was
removed and replenished with DMSO. At 48 h post-transfection,
luciferase activity was measured using the dual-luciferase reporter
assay system (Promega Corporation) and normalized to Renilla
luciferase activity.
Western blotting
Total protein was extracted from the cells with
lysis buffer (Cell Signaling Technology, Inc.). The lysates were
centrifuged at 10,000 × g for 10 min at 4°C. The protein
concentration was determined using a bicinchoninic acid protein
quantification kit (Promega Corporation). Equal amounts of cell
extracts (30 µg/well) were subjected to 10% SDS-PAGE and
transferred to PVDF membranes (EMD Millipore) for anti-body
blotting. The membranes were blocked with 5% bovine serum albumin
(Thermo Fisher Scientific, Inc.) for 1 h at room temperature and
subsequently incubated with β-actin (1:1,000; dilution, cat. no.
ab179467), Bax (1:1,000; cat. no. ab32503), cleaved caspase 3
(1:1,000; cat. no. ab2303), Bcl-2 (1:1,000; cat. no. ab59348),
cyclin D2 (1:1,000; cat. no. ab207604), p27kip1(1:1,000;
cat. no. ab32034), cyclin E1 (1:1,000; cat. no. ab33911),
phosphorylated (p)-Akt (1:1,000; cat. no. 38449) and Akt (1:1,000;
cat. no. ab8850) primary antibodies (all from Abcam) overnight at
4°C. Subsequently, the membranes were incubated with a horseradish
peroxidase-conjugated anti-mouse (1:5,000; cat. no. ab97040; Abcam)
or an anti-rabbit secondary antibody (1:5,000; cat. no. ab7090;
Abcam) at room temperature for 1 h. Protein bands were visualized
using an enhanced chemiluminescence reagent kit (GE Healthcare) on
a Tanon 5200 Chemiluminescent imaging system (Tanon Science and
Technology Co., Ltd.) according to the manufacturer's protocol.
ImageJ software (v1.8.0.112; National Institutes of Health) was
used for quantification of protein expression with β-actin as the
reference protein.
Tumor xenograft model
A total of 24 female BALB/c nude mice (18-20 g, 4-5
weeks) were obtained from Vital River. The animals were housed at a
temperature of 23±1°C, and a humidity of 50-60% for 4 days in a
12-h light/dark cycle prior to the initiation of the experiment.
For induction of anesthesia, the mice were anesthetized by
isoflurane (3% v/v) inhalation. Nude mice were inoculated with
17β-estradiol pellets 48 h before implantation of BT-474 cells.
Subsequently, BT-474 cells (5×106 cells/ml in 100
µl PBS mixed with Matrigel matrix) were subcutaneously
injected into the mice. Animal health and behavior were monitored
twice a day. All animal procedures were approved by the Seventh
People's Hospital of Shanghai University of Traditional Chinese
Medicine Committee in June 2019. The National Institute of Health
Guide for the Care and Use of Laboratory Animals was followed
(25). The mice were randomly
divided into four groups (n=6) when the tumor volume reached 100
mm3: Control, miR-135b-5p agomir, trastuzumab and
trastuzumab + miR-135b-5p agomir. The tumor volume was estimated
according to the following equation: Volume= Length x
Width2/2. Trastuzumab (10 mg/kg; ChemBest), miR-135b-5p
agomir (50 nM) or the combination treatment of trastuzumab with
miR-135b-5p agomir were administered as follows: Trastuzumab (10
mg/kg) was administered intraperitoneally once per week
whilemiR-135b-5p agomir was directly injected into the implanted
tumor at a dose of 50 nM (in 20 µl PBS) per mouse twice a
week. After 3 weeks of treatment, the mice were euthanized using
CO2 at a displacement rate of 20% of the chamber
volume/min (CO2 flow rate, 2.5 l/min). The tumors were
removed and weighted. The duration of the experiment is 6 weeks,
and no mice died during the experimental process. Humane endpoints
were determined as previously described (26). Criteria for judging the death of
animals is continuous no spontaneous breathing for 2-3 min and no
blink reflex.
Immunohistochemistry (IHC) assay
The tumor tissues were fixed in 4% paraformaldehyde
for 48 h at room temperature, embedded in paraffin and cut into
4-µm sections. Deparaffinized tissue sections were incubated
with Ki67 monoclonal antibody (1:500; cat. no. ab15580; Abcam)
over-night at 4°C. Subsequently, the sections were incubated with
biotinylated goat anti-rabbit immunoglobulin G (1:1,000; cat. no.
ab6721; Abcam) at room temperature for 30 min. IHC reactions were
visualized by using the IHC detection system (EnVision kit; Dako;
Agilent Technologies, Inc.). The images were captured under a
fluorescence microscope (Nikon Eclipse Ti-E; Nikon Corporation) at
×200 magnification. ImageJ software (v1.8.0.112; National
Institutes of Health) was used for quantification of protein
expression.
Statistical analysis
All experiments were repeated three times and the
results are expressed as the mean ± SD. One-way ANOVA followed by
Tukey's post hoc test was performed to analyze differences among
multiple groups (>2 groups). Statistical analysis was performed
using GraphPad Prism (version 7; GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-135b-5p agomir enhances the
anti-proliferative effect of trastuzumab in HER-2-positive breast
cancer cells
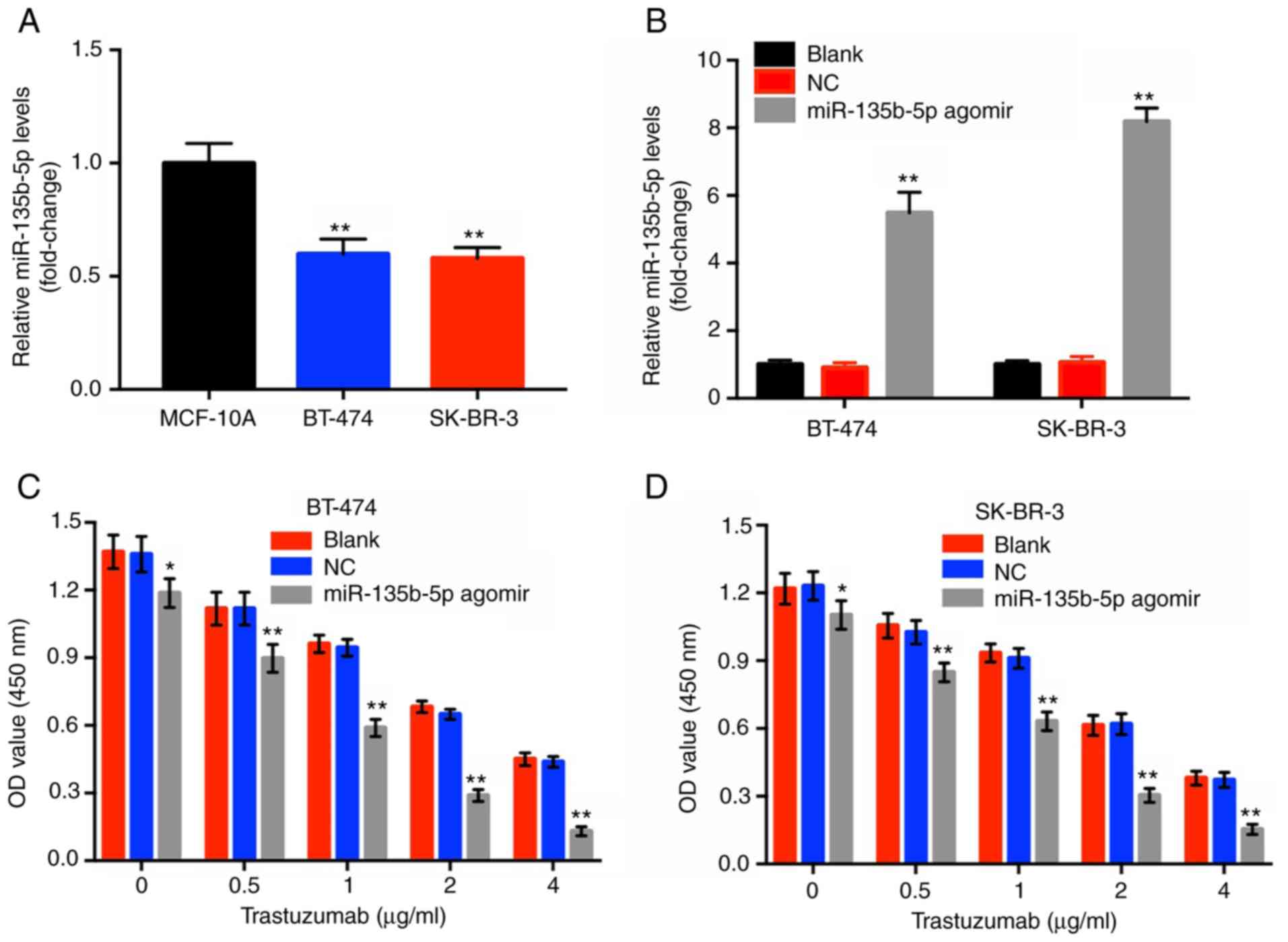
To investigate the effects of miR-135b-5p agomir in
BT-474 and SK-BR-3 cells, RT-qPCR assay was performed. The results
indicated that the levels of miR-135b-5p in BT-474 and SK-BR-3
cells were significantly lower compared within the normal breast
epithelial cell line MCF-10A (Fig.
1A). Moreover, miR-135b-5p agomir caused a significant
upregulation in miR-135b-5p expression in these two breast cancer
cell lines compared with the blank group (Fig. 1B). Subsequently, a CCK-8 assay was
performed to investigate the cytotoxic effects of trastuzumab or of
the combination treatment (trastuzumab plus miR-135b-5p agomir) in
HER-2-positive breast cancer cells. Trastuzumab alone inhibited the
proliferation of HER-2-positive breast cancer cells in a
dose-dependent manner, which was significantly enhanced in the
presence of miR-135b-5p agomir compared with the blank groups
(Fig. 1C and D). Since
trastuzumab (1 µg/ml) combined with miR-135b-5p agomir
induced ~50% inhibition of cellular growth, this dose was applied
for subsequent cell assays. In addition, BT-474 cells were more
sensitive to the combination treatment compared with SK-BR-3 cells
and therefore this cell line was employed in the following
experiments. The results suggested that miR-135b-5p significantly
increased the anti-proliferative effect of trastuzumab in
HER-2-positive breast cancer cells.
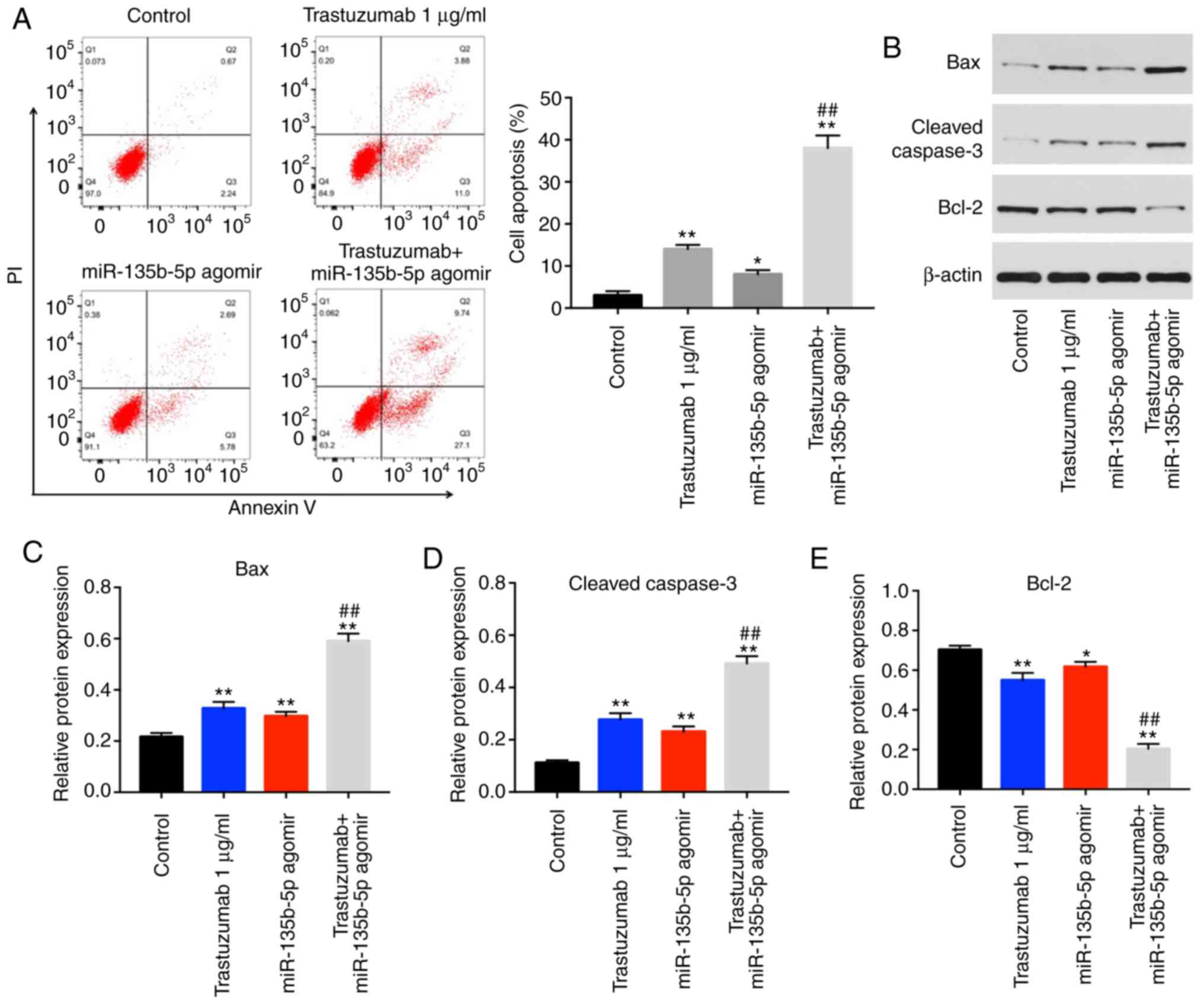
Trastuzumab-induced apoptosis is enhanced
by miR-135b-5p agomir in BT-474 cells
Annexin V/PI staining was performed to detect the
mechanism by which miR-135b-5p enhanced the anti-proliferative
effect of trastuzumab. The results indicated that trastuzumab or
miR-135b-5p agomir treatment significantly induced cell apoptosis
compared with the control group (Fig.
2A). In addition, trastuzumab-induced apoptosis was
significantly enhanced by miR-135b-5p agomir in BT-474 cells
(Fig. 2A). Moreover, the
expression levels of apoptotic-associated proteins were examined in
BT-474 cells by western blotting. The combination of miR-135b-5p
agomir with trastuzumab significantly increased the expression
levels of cleaved caspase 3 and Bax in BT-474 cells compared cells
treated miR-135b-5p agomir or trastuzumab alone (Fig. 2B-D). Furthermore,
trastuzumab-induced Bcl-2 downregulation was significantly enhanced
in the presence of miR-135b-5p agomir (Fig. 2E). Taken together, the data
demonstrated that miR-135b-5p agomir could enhance
trastuzumab-induced apoptosis via activation of the intrinsic
apoptotic pathway.
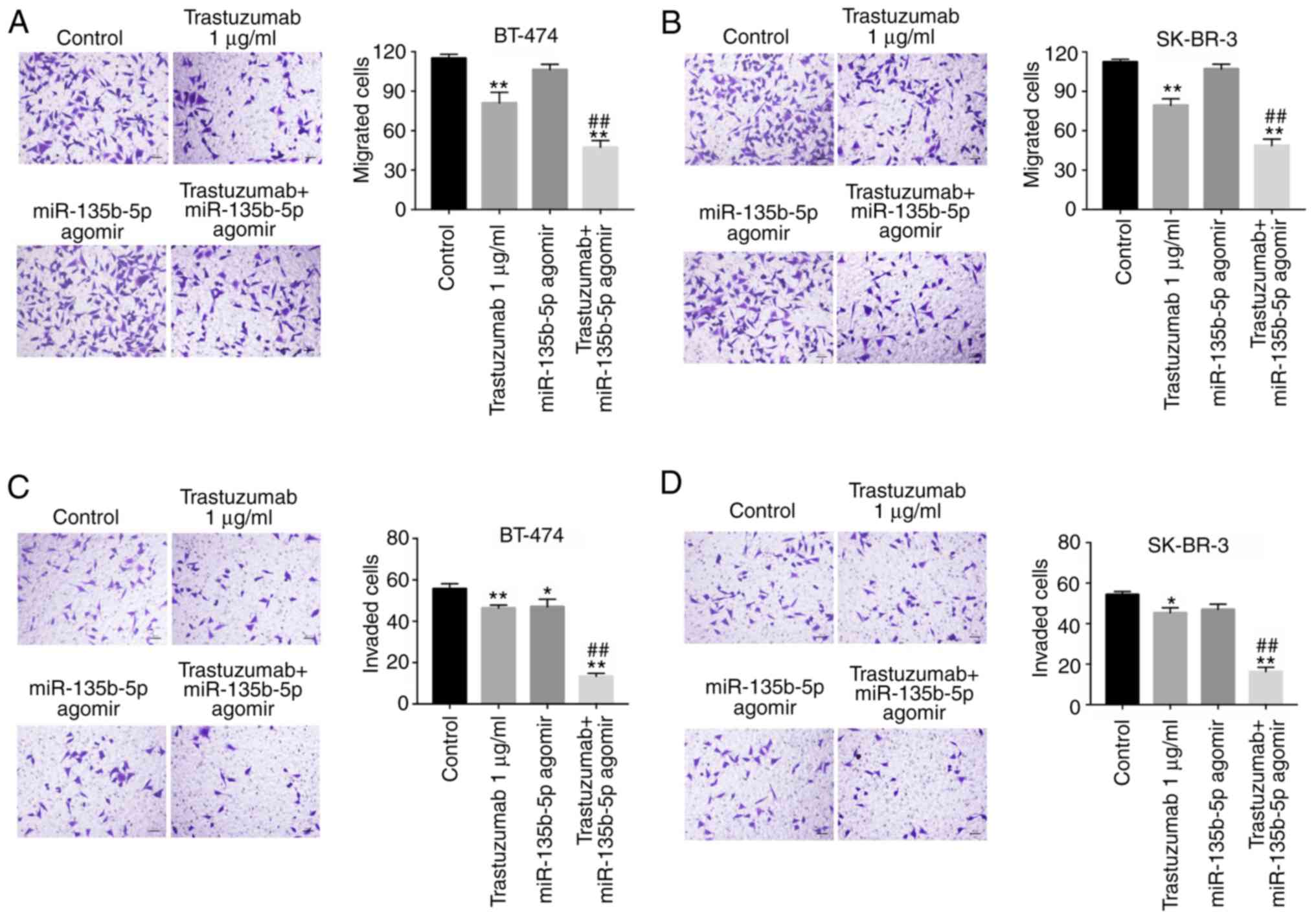
Anti-migration and anti-invasion effects
of trastuzumab is enhanced by miR-135b-5p agomir
Following the initial investigation of the effects
of miR-135b-5p on trastuzumab-induced apoptosis, its effectson the
inhibition breast cancer cell migration and invasion were also
investigated. Transwell assays indicated that trastuzumab
significantly inhibited migration and invasion abilities in BT-474
and SK-BR-3 cells compared with the control group (Fig. 3A-D). Furthermore, the inhibitory
effects of trastuzumab on the migration and invasion of BT-474 and
SK-BR-3 cells were significantly enhanced in the presence of
miR-135b-5p agomir (Fig. 3A-D).
These results suggested that miR-135b-5p agomir could increase the
anti-metastatic effects of trastuzumab in HER-2-positive breast
cancer cells.
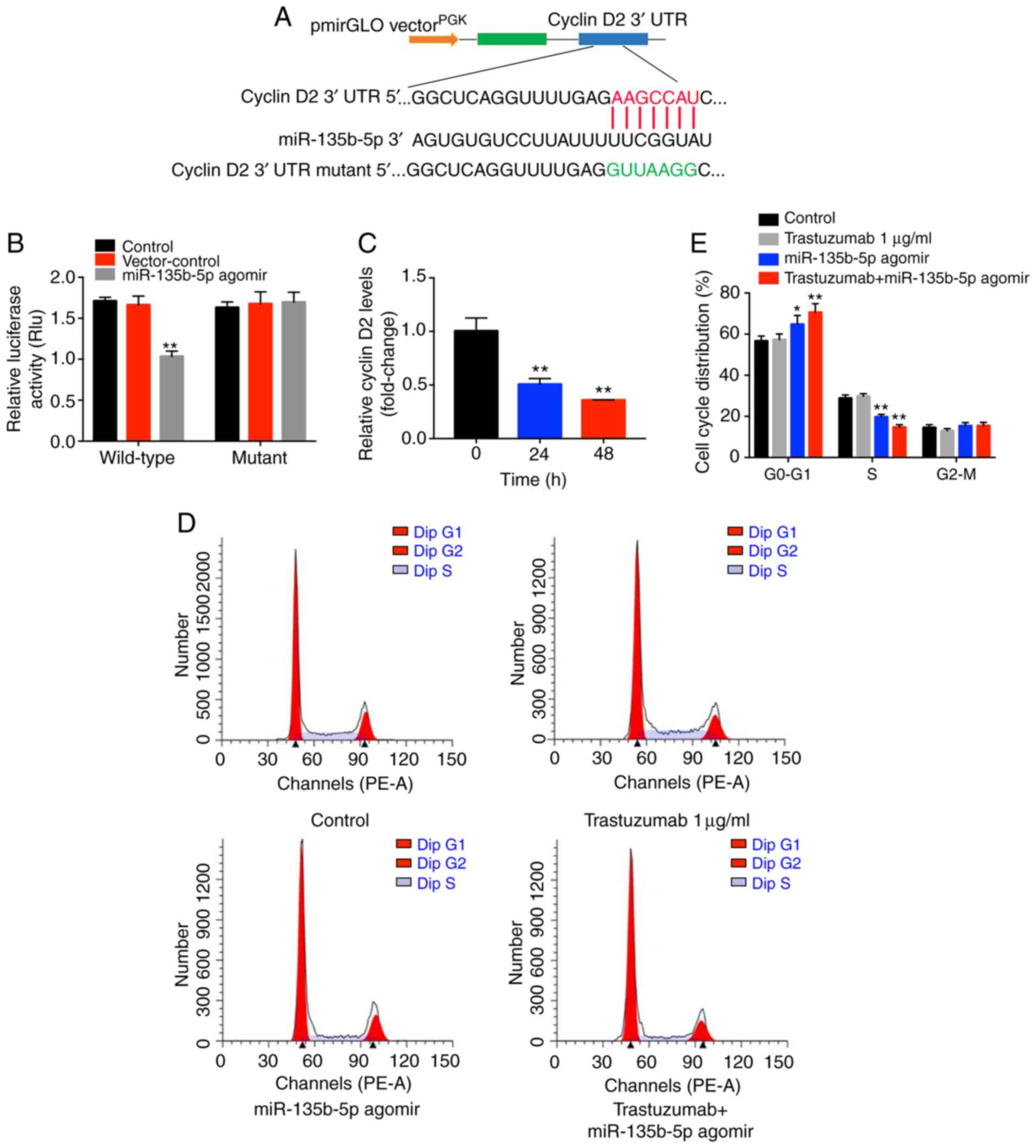
Cyclin D2 is a direct target of
miR-135b-5p in BT-474 breast cancer cells
To investigate the underlying mechanisms by which
miR-135b-5p agomir increased the antitumor effects of trastuzumab
in breast cancer cells, TargetScan and miRWalk online tools were
used. A putative miR-135b-5p-binding site was identified at a
specific position in the 3′-UTR of cyclin D2 mRNA. To further
confirm the direct interaction between miR-135b-5p and cyclin D2
mRNA, the predicted binding site of miR-135b-5p to cyclin D2 mRNA
was cloned into a luciferase reporter vector. miR-135b-5p agomir
significantly inhibited luciferase activity compared with the
control group (Fig. 4B),
suggesting that it could interact directly with the 3′-UTR of
cyclin D2 mRNA. In contrast to these observations, miR-135b-5p
agomir did not affect the luciferase activity of the mutated cyclin
D2. In addition, the qPCR data indicated that cyclin D2 mRNA
synthesis was significantly inhibited when the cells were treated
with miR-135b-5p agomir, and cyclin D2 levels decreased over time
following agomir transfection (Fig.
4C). Taken together, the data indicated that cyclin D2 was a
direct target of miR-135b-5p in BT-474 breast cancer cells.
Since cyclin D2 mRNA was demonstrated to directly
bind to miR-135b-5p, the cell cycle ofmiR-135b-5p agomir-treated
cells was examined. miR-135b-5p-overexpressing BT-474 cells
displayed a significant increase in the percentage of cells in the
G0/G1-phase but significantly decreased proportions in S-phase
cells compared with controls, while trastuzumab exhibited no
effects (Fig. 4D and E). In
addition, western blot analysis illustrated that trastuzumab
treatment had no effect on the expression levels of cyclin D2,
p27kip1 and cyclin E1 in BT-474 cells. However,
miR-135b-5p agomir or combination treatment significantly inhibited
the expression levels of cyclin D2 and cyclin E1 and significantly
increased p27kip1 levels in BT-474 cells compared with
the control group (Fig. 5A-D).
Moreover, trastuzumab treatment significantly downregulated the
expression of phosphorylated (p)-Akt in BT-474 cells, while
overexpression of miR-135b-5p did not affect p-Akt activation in
BT-474 cells (Fig. 5A and E).
Interestingly, trastuzumab-induced p-Akt downregulation was
enhanced in the presence of miR-135b-5p agomir (Fig. 5A and E). Meanwhile, overexpression
of miR-135b-5p did not show any significant effect on the
expression of cyclin D1 in BT-474 cells (Fig. 5F and G). Taken together, the data
demon-strated that miR-135b-5p agomir could induce
G0/G1 arrest in BT-474 cells by directly
binding to cyclin D2. Furthermore, the overexpression efficiency of
cyclin D2 was confirmed by western blotting (Fig. 5H and I). Overexpression of cyclin
D2 significantly reversed the growth-inhibitory activity of
miR-135b-5p in combination with trastuzumab in BT-474 cells
(Fig. 5J). These data indicated
that miR-135b-5p agomir enhanced the anti-proliferative effects of
trastuzumab in BT-474 cells via downregulation of cyclin D2.
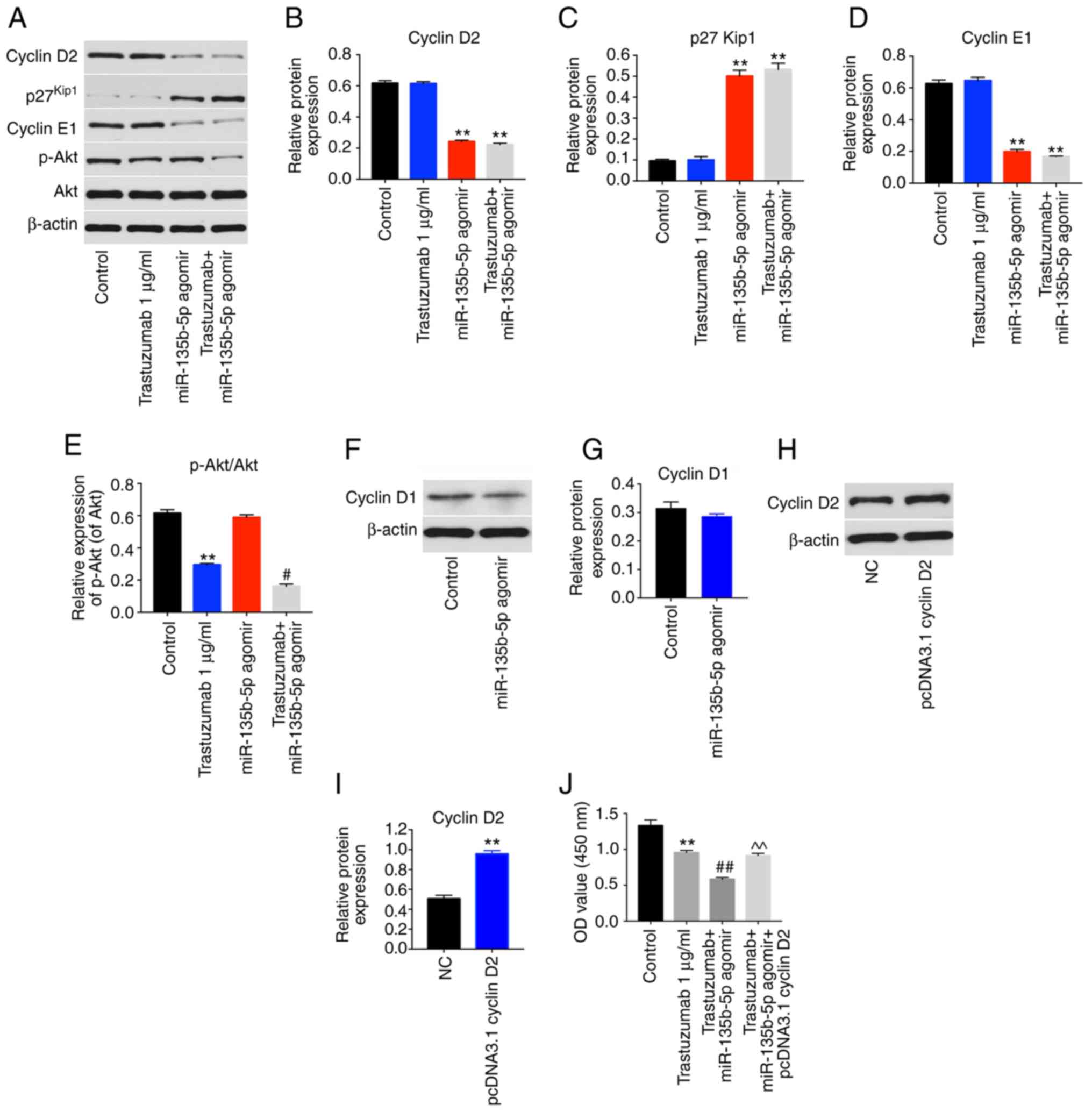 | Figure 5miR-135b-5p agomir regulates cyclin
D2, p27Kip1 and cyclin E1 signaling pathways. BT-474
cells were transfected with 1 µg/ml trastuzumab, 50 nM
miR-135b-5p agomir or a combination of trastuzumab and miR-135b-5p
agomir for 48 h. (A) Expression levels of cyclin D2,
p27Kip1, cyclin E1 and p-Akt in BT-474 cells were
detected with western blotting. The relative levels of (B) cyclin
D2, (C) p27Kip1, (D) cyclin E1 and (E) p-Akt were
quantified by normalizing to β-actin levels. (F) BT-474 cells were
transfected with miR-135b-5p agomir or NC for 48 h. The levels of
cyclin D1 in BT-474 cells was detected using western blotting. (G)
Quantification of cyclin D1 expression. (H) BT-474 cells were
transfected with NC or pcDNA 3.1-cyclin D2 for 48 h. (I) The level
of cyclin D2 in BT-474 cells was detected with western blotting.
(J) BT-474 cells were transfected with pcDNA 3.1-cyclin D2 and
miR-135b-5p agomir for 48 h in the presence of trastuzumab. The
cell viability was detected using a Cell Counting Kit-8 assay.
**P<0.01 vs. control; #P<0.05 and
##P<0.01 vs. trastuzumab; ^^P<0.01 vs.
trastuzumab + miR-125b-3p agomir. p-Akt, phosphorylated Akt; miR,
microRNA; OD, optical density; NC, negative control. |
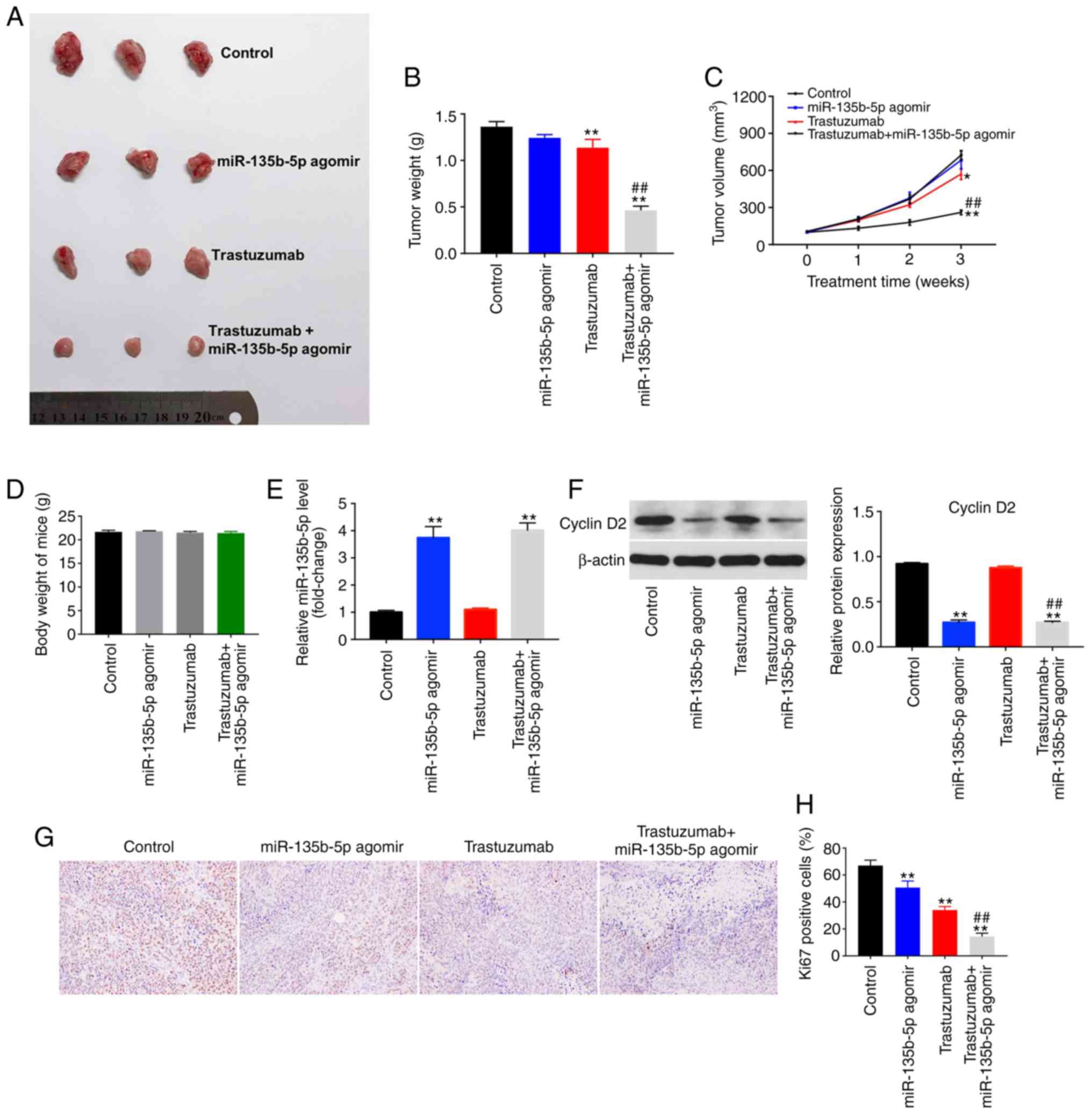
miR-135b-5p agomir enhances the antitumor
effect of trastuzumab in vivo
To explore whether miR-135b-5p could increase the
antitumor effects of trastuzumab in vivo, a breast cancer
xenograft model was established. The results indicated that
trastuzumab treatment alone was able to decrease tumor size
compared with controls (Fig. 6A).
Tumor weight and tumor volume were significantly reduced in the
combination treatment group compared with mice treated with
trastuzumab or miR-135b-5p agomir alone (Fig. 6A-C). However, miR-135b-5p agomir
alone showed no significant difference on tumor volume. In
addition, the body weight changes indicated the safety and
tolerability of the combination treatment (Fig. 6D). Furthermore, the expression
levels of miR-135b-5p was significantly upregulated in the
miR-135b-5p agomir or combination treatment group compared with the
control group, indicating that miR-135b-5p is stably expressed in
tumor tissues (Fig. 6E).
Additionally, trastuzumab had no effect on cyclin D2 expression in
tumor tissues, whereas the level of cyclin D2 significantly
decreased in the presence of miR-135b-5p agomir compared with
controls (Fig. 6F). IHC assay
indicated that miR-135b-5p agomir or trastuzumab treatment
significantly inhibited proliferation in tumor tissues, compared
with the control group. As expected, combination treatment
significantly inhibited proliferation in tumor tissues compared
with the trastuzumab treatment group (Fig. 6G and H). These data were
consistent with the in vitro results. Taken together, these
results confirmed that miR-135b-5p agomir could enhance the
antitumor effects of trastuzumab in vivo.
Discussion
In the present study, the combined antitumor effects
of trastuzumab with miR-135b-5p agomir were investigated in
vitro and in vivo. miR-135b-5p agomir enhanced the
antitumor effect of trastuzumab in breast cancer cells in
vitro and in vivo. A previous study reported that
overexpression of miR-135b-5p suppressed migration and invasion in
breast cancer (22). The present
results indicated that miR-135b-5p agomir suppressed proliferation,
migration and invasion in BT-474 and SK-BR-3 HER-2-positive breast
cancer cells, consistent with previous reports (27,28). Agomir is a type of specially
labeled and chemically modified double-stranded microRNA that
regulates the biological function of target genes by mimicking
endogenous microRNA (29).
Compared with common miRNA mimics, miRNA agomir has a higher
affinity for the cell membrane (29). In addition, agomir is especially
suitable for animal in vivo experiments and have higher
stability (30). These data
showed that miR-135b-5p exerted anti-proliferation, anti-migration
and anti-invasion effects in HER-2-positive breast cancer cells.
Moreover, miR-135b-5p agomir potentiated the antitumor effect of
trastuzumab by inducing apoptosis. In addition, the inhibition of
breast cell migration and invasion by trastuzumab was enhanced by
miR-135b-5p agomir transfection. Tang et al (31) indicated that overexpression of
miR-200c increased drug sensitivity to trastuzumab in
HER-2-positive breast cancer cells, consistent with the data
reported here. miR-135b-5p agomir enhanced the antitumor effect of
trastuzumab in vivo and in vitro.
The target predicting databases TargetScan and
miRWalk were used to investigate the mechanism by which miR-135b-5p
increases the antitumor effect of trastuzumab. Cyclin D2 was
identified as a potential target of miR-135b-5p. Cyclin D2 protein
is involved in the regulation of the cell cycle at the point of
transition from G1 to DNA synthesis (32). Zhou et al (33) indicated that overexpression of
miR-206 blocked G1/S transition and inhibited the proliferation of
breast cancer cells via downregulation of cyclin D2 expression. In
the present study, miR-135b-5p agomir suppressed the proliferation
of breast cancer cells by inhibiting cyclin D2 expression, which
was consistent with the previous study. Zhang et al
(23) reported that miR-135b-5p
enhanced doxorubicin-sensitivity of ER-positive breast cancer cells
by targeting anterior gradient 2. The differences noted on the
targeting of proteins may be associated with the breast cancer
subtypes, namely estrogen (ER) or HER-2, which are upstream
regulators of miR-135b-5p. Therefore, in addition to ER positive
breast cancer, miR-135b also plays a role as a regulator of
HER-2-positive breast cancer. These findings suggested that
miR-135b-5p may act as regulator in different types of breast
cancer.
Overexpression of ErbB2 (HER-2) leads to the
potentiation of cyclin E-Cdk2 activity by sequestration of the
cyclin-dependent kinase inhibitor p27kip1 (10). A previous study reported that
trastuzumab inhibited mitogen-activated protein kinase and PI3K/AKT
pathways, leading to cell cycle arrest (34). However, in the present study, the
HER-2 antibody trastuzumab exhibited limited effects on the
expression of cyclin D2 or p27Kip1, while miR-135b-5p
acted as a regulator of cyclin D2 and p27Kip1 expression
levels. The correlation between p27Kip1 expression and
the PI3K/Akt pathway in the combination treatment of miR-135b-5p
with trastuzumab can be further explored in future studies. In
addition, a number of miRNAs have been shown to suppress cyclin D2
expression in various tumor types (35-38), including miR-133, miR-203, miR-204
and miR-206. To the best of our knowledge, the present study was
the first to demonstrate that miR-135b-5p directly suppressed
cyclin D2 expression in HER-2-positive breast cancer.
However, the present study had several limitations.
The present study indicated that miR-770-5p agomir in combination
with trastuzumab treatment blocked the migration and invasion
capacities of HER-2-positive breast cancer cells in vitro.
However, the correlation between miR-770-5p agomir in combination
with trastuzumab and breast cancer metastasis in vivo is
still unclear and need to be investigated. In addition, the present
study provided evidence to support the anti-cancer properties of
miR-135b-5p, but little is known regarding the clinical
significance of miR-135b-5p in human breast cancer. Therefore, the
clinical application value of miR-135b-5p in breast cancer should
be investigated in the future. Moreover, the present study only
detected the expression of p-Akt in HER-2-positive breast cancer
cells. However, expression of other proteins associated with the
PI3K/Akt pathway, such as PI3K and mTOR should be investigate using
western blotting to demonstrate whether miR-135b-5p agomir enhances
the antitumor effect of trastuzumab in HER-2-positive breast cancer
cells via the PI3K/Akt pathway. Meanwhile, rescue experiments are
needed to further prove whether miR-135b-5p agomir could enhance
the antitumor effect of trastuzumab in HER-2-positive breast cancer
cells via targeting cyclin D2.
In conclusion, miR-135b-5p agomir enhanced the
anti-tumor effect of trastuzumab in BT-474 cells in vitro
and in vivo. miR-135b-5p induced cell cycle arrest by
binding to cyclin D2. Therefore, the combination of miR-135b-5p
agomir with trastuzumab may be a potential strategy for the
treatment of patients with HER-2-positive breast cancer.
Acknowledgments
Not applicable.
Funding
The study was supported by the Pudong New Area
Science and Technology Development Fund (grant no. PKJ2017-Y12),
Important Weak Subject of the Pudong New Area Health and Family
Planning Commission (grant no. PWZbr2017-01) and the National
Natural Science Foundation Youth Project (grant no. 81702422).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and YQ made major contributions to the
conception, design and manuscript drafting of this study. PC, QL
and HS were responsible for data acquisition, data analysis, data
interpretation and manuscript revision. XJ made substantial
contributions to conception and design of the study and revised the
manuscript critically for important intellectual content. All
authors agreed to be accountable for all aspects of the work. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the Seventh
People's Hospital of Shanghai University of Traditional Chinese
Medicine Committee. National Institutes of Health Guide for the
Care and Use of Laboratory Animals was followed strictly.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tormo E, Adam-Artigues A, Ballester S,
Pineda B, Zazo S, González-Alonso P, Albanell J, Rovira A, Rojo F,
Lluch A and Eroles P: The role of miR-26a and miR-30b in
HER2+ breast cancer trastuzumab resistance and
regulation of the CCNE2 gene. Sci Rep. 7:413092017. View Article : Google Scholar
|
|
2
|
Akram M, Iqbal M, Daniyal M and Khan AU:
Awareness and current knowledge of breast cancer. Biol Res.
50:332017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Merino Bonilla JA, Torres Tabanera M and
Ros Mendoza LH: Breast cancer in the 21st century: From early
detection to new therapies. Radiologia. 59:368–379. 2017.In
English, Spanish. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim YJ, Jung SY and Kim K: Survival
benefit of radiotherapy after surgery in de novo stage IV breast
cancer: A population-based propensity-score matched analysis. Sci
Rep. 9:85272019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nguyen PL, Taghian AG, Katz MS, Niemierko
A, Raad RF, Boon WL, Bellon JR, Wong JS, Smith BL and Harris JR:
Breast cancer subtype approximated by estrogen receptor,
progesterone receptor, and HER-2 is associated with local and
distant recurrence after breast-conserving therapy. J Clin Oncol.
26:2373–2378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chavez-Blanco A, Perez-Sanchez V,
Gonzalez-Fierro A, Vela-Chavez T, Candelaria M, Cetina L, Vidal S
and Dueñas-Gonzalez A: HER2 expression in cervical cancer as a
potential therapeutic target. BMC Cancer. 4:592004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baselga J and Swain SM: Novel anticancer
targets: Revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer.
9:463–475. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horton J: Trastuzumab use in breast
cancer: Clinical issues. Cancer Control. 9:499–507. 2002.
View Article : Google Scholar
|
|
9
|
Sarup JC, Johnson RM, King KL, Fendly BM,
Lipari MT, Napier MA, Ullrich A and Shepard HM: Characterization of
an anti-p185HER2 monoclonal antibody that stimulates receptor
function and inhibits tumor cell growth. Growth Regul. 1:72–82.
1991.PubMed/NCBI
|
|
10
|
Lane HA, Beuvink I, Motoyama AB, Daly JM,
Neve RM and Hynes NE: ErbB2 potentiates breast tumor proliferation
through modulation of p27(Kip1)-Cdk2 complex formation: Receptor
overexpression does not determine growth dependency. Mol Cell Biol.
20:3210–3223. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Petit AM, Rak J, Hung MC, Rockwell P,
Goldstein N, Fendly B and Kerbel RS: Neutralizing antibodies
against epidermal growth factor and ErbB-2/neu receptor tyrosine
kinases down-regulate vascular endothelial growth factor production
by tumor cells in vitro and in vivo: Angiogenic implications for
signal transduction therapy of solid tumors. Am J Pathol.
151:1523–1530. 1997.PubMed/NCBI
|
|
12
|
Sliwkowski MX, Lofgren JA, Lewis GD,
Hotaling TE, Fendly BM and Fox JA: Nonclinical studies addressing
the mechanism of action of trastuzumab (Herceptin). Semin Oncol.
26:60–70. 1999.PubMed/NCBI
|
|
13
|
Van Swearingen AED, Siegel MB, Deal AM,
Sambade MJ, Hoyle A, Hayes DN, Jo H, Little P, Dees EC, Muss H, et
al: LCCC 1025: A phase II study of everolimus, trastuzumab, and
vinorelbine to treat progressive HER2-positive breast cancer brain
metastases. Breast Cancer Res Treat. 171:637–648. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding K, Wu Z, Li X, Sheng Y, Wang X and
Tan S: LMO4 mediates trastuzumab resistance in HER2 positive breast
cancer cells. Am J Cancer Res. 8:594–609. 2018.PubMed/NCBI
|
|
15
|
Tan H, Huang S, Zhang Z, Qian X, Sun P and
Zhou X: Pan-Cancer analysis on microRNA-associated gene activation.
EBioMedicine. 43:82–97. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartel DP: Metazoan MicroRNAs. Cell.
173:20–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schneider A, Victoria B, Lopez YN,
Suchorska W, Barczak W, Sobecka A, Golusinski W, Masternak MM and
Golusinski P: Tissue and serum microRNA profile of oral squamous
cell carcinoma patients. Sci Rep. 8:6752018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Venturutti L, Cordo Russo RI, Rivas MA,
Mercogliano MF, Izzo F, Oakley RH, Pereyra MD, Proietti CJ,
Yankilevich P, Roa JC, et al: miR-16 mediates trastuzumab and
lapatinib response in ErbB-2-positive breast and gastric cancer via
its novel targets CCNJ and FUBP1. Oncogene. 35:6189–6202. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song W, Wu S, Wu Q, Zhou L, Yu L, Zhu B
and Gong X: The microRNA-141-3p/CDK8 pathway regulates the
chemosensitivity of breast cancer cells to trastuzumab. J Cell
Biochem. 120:14095–14106. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Noyan S, Gurdal H and Gur Dedeoglu B:
Involvement of miR-770-5p in trastuzumab response in HER2 positive
breast cancer cells. PLoS One. 14:e02158942019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Cola A, Volpe S, Budani MC, Ferracin M,
Lattanzio R, Turdo A, D'Agostino D, Capone E, Stassi G, Todaro M,
et al: miR-205-5p-mediated downregulation of ErbB/HER receptors in
breast cancer stem cells results in targeted therapy resistance.
Cell Death Dis. 6:e18232015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pu T, Shen M, Li S, Yang L, Gao H, Xiao L,
Zhong X, Zheng H, Liu Y, Ye F and Bu H: Repression of miR-135b-5p
promotes metastasis of early-stage breast cancer by regulating
downstream target SDCBP. Lab Invest. 99:1296–1308. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Xia F, Zhang F, Cui Y, Wang Q,
Liu H and Wu Y: miR-135b-5p enhances doxorubicin-sensitivity of
breast cancer cells through targeting anterior gradient 2. J Exp
Clin Cancer Res. 38:262019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
National Institutes of Health: Guide for
the Care and Use of Laboratory Animals. The National Academies
Press; Washington, DC: pp. p2462011
|
|
26
|
Workman P, Aboagye EO, Balkwill F, Balmain
A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DAH,
Glennie MJ, et al: Guidelines for the welfare and use of animals in
cancer research. Br J Cancer. 102:1555–1577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Le XF, Almeida MI, Mao W, Spizzo R, Rossi
S, Nicoloso MS, Zhang S, Wu Y, Calin GA and Bast RC Jr: Modulation
of MicroRNA-194 and cell migration by HER2-targeting trastuzumab in
breast cancer. PLoS One. 7:e411702012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lulli V, Buccarelli M, Martini M, Signore
M, Biffoni M, Giannetti S, Morgante L, Marziali G, Ilari R,
Pagliuca A, et al: miR-135b suppresses tumorigenesis in
glioblastoma stem-like cells impairing proliferation, migration and
self-renewal. Oncotarget. 6:37241–37256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao M, Cai J, Cai L, Jia J, Xie L, Zhu Y,
Huang B, Jin D and Wang Z: Let-7e sensitizes epithelial ovarian
cancer to cisplatin through repressing DNA double strand break
repair. J Ovarian Res. 10:242017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Krützfeldt J, Rajewsky N, Braich R, Rajeev
KG, Tuschl T, Manoharan M and Stoffel M: Silencing of microRNAs in
vivo with 'antagomirs'. Nature. 438:685–689. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang H, Song C, Ye F, Gao G, Ou X, Zhang L
and Xie X and Xie X: miR-200c suppresses stemness and increases
cellular sensitivity to trastuzumab in HER2+ breast
cancer. J Cell Mol Med. 23:8114–8127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Montagnoli A, Fiore F, Eytan E, Carrano
AC, Draetta GF, Hershko A and Pagano M: Ubiquitination of p27 is
regulated by Cdk-dependent phosphorylation and trimeric complex
formation. Genes Dev. 13:1181–1189. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou J, Tian Y, Li J, Lu B, Sun M, Zou Y,
Kong R, Luo Y, Shi Y, Wang K and Ji G: miR-206 is down-regulated in
breast cancer and inhibits cell proliferation through the
up-regulation of cyclinD2. Biochem Biophys Res Commun. 433:207–212.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vu T and Claret FX: Trastuzumab: Updated
mechanisms of action and resistance in breast cancer. Front Oncol.
2:622012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu WF, Wang HM, Lu BC, Zhang GZ, Ma HM and
Wu ZY: miR-206 inhibits human laryngeal squamous cell carcinoma
cell growth by regulation of cyclin D2. Eur Rev Med Pharmacol Sci.
19:2697–2702. 2015.
|
|
36
|
Wu X, Zeng Y, Wu S, Zhong J, Wang Y and Xu
J: miR-204, down-regulated in retinoblastoma, regulates
proliferation and invasion of human retinoblastoma cells by
targeting cyclin D2 and MMP-9. FEBS Lett. 589:645–650. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pan JL, Yuan DZ, Zhao YB, Nie L, Lei Y,
Liu M, Long Y, Zhang JH, Blok LJ, Burger CW and Yue LM:
Progesterone-induced miR-133a inhibits the proliferation of
endometrial epithelial cells. Acta Physiol (Oxf). 219:683–692.
2017. View Article : Google Scholar
|
|
38
|
Zhao S, Han J, Zheng L, Yang Z, Zhao L and
Lv Y: MicroRNA-203 regulates growth and metastasis of breast
cancer. Cell Physiol Biochem. 37:35–42. 2015. View Article : Google Scholar : PubMed/NCBI
|